Abstract
Background
Cognitive deficits occur in a subset of individuals with obesity. Deficits can be reversed with bariatric surgery, though cognitive recovery is not equally exhibited across patients. Recent work shows obesity during adolescence portends medical complications in adulthood; it is unknown if obesity in adolescence predicts adult cognition or cognitive recovery following weight loss surgery.
Objectives
The current study examines the relationship between weight history and cognitive function in obese adults undergoing bariatric surgery.
Setting
Academic medical centers with bariatric care services.
Methods
Seventy-eight bariatric surgery patients (mean age=43.2) enrolled in an ancillary study to the Longitudinal Assessment of Bariatric Surgery (LABS) project completed a questionnaire recalling weight history at age 18. Cognitive testing was completed pre-operatively and at 12-month follow-up.
Results
Weight status at age 18 was linked to performance in several aspects of cognition. Higher BMI at age 18 predicted poorer pre-operative verbal fluency (B=−0.26, p=0.045) as well as post-operative cognitive recovery in attention (B=−0.30, p=0.01) at 12 month follow-up.
Conclusions
Higher BMI at age 18 predicts verbal fluency performance in adults with obesity, as well as post-operative recovery of attention following bariatric surgery. The mechanisms underlying this connection are not fully clear, though findings may reflect effects of obesity on the brain during a crucial period of neural maturation, or duration of obesity and cumulative impact of comorbidities on cognition. Future work examining possible causal factors involved in these relationships is needed.
Keywords: cognition, obesity, bariatric, weight history
Introduction
Adolescent obesity is a looming public health crisis; recent data suggest nearly one fifth of adolescents are obese and rates continue to climb(1). Excess weight during adolescence presents a health risk not only during youth, but also predicts medical complications later in life(2). Severe obesity at age 18 confers higher risk of multiple adverse health outcomes in adulthood, including venous edema, kidney dysfunction, polycystic ovary syndrome, respiratory conditions, diabetes, and hypertension(3).
In addition to medical conditions associated with obesity, cognitive difficulty is present in many individuals with obesity(4). While mechanisms are not fully understood, medical conditions common in obesity, including sleep apnea(5), hypertension(6), and type 2 diabetes(7) (T2DM) are all linked to reduced cognition. Cognitive deficits are reversible with bariatric surgery(4); however, cognitive recovery is not equally exhibited across patients(8). In light of recent work(3), adolescent history of obesity could confer higher risk of reduced cognition and poorer cognitive recovery following bariatric surgery in adulthood. Risk factors for reduced cognitive improvement following bariatric surgery must be identified, as cognitive ability predicts poorer postoperative adherence(9) and weight loss outcomes(10).
The current study examines the relationship between adolescent weight status and adult cognitive function in adults undergoing bariatric surgery. Cognitive testing and a weight history questionnaire were completed pre-operatively. Cognitive testing was repeated 12 months after surgery. We hypothesized that obesity at age 18 would predict poorer cognition and reduced post-operative cognitive improvement in obese adults completing bariatric surgery.
Materials and Methods
Study Design and Participants
Seventy-eight bariatric surgery patients were recruited from a multi-site National Institutes of Health (NIH) study examining effects of bariatric surgery on neurocognitive function. All were part of the Longitudinal Assessment of Bariatric Surgery (LABS) parent project(11). Seventy-six patients completed Roux en Y gastric bypass, while two had gastric banding. Participants were English-speaking, first time bariatric surgery candidates scheduled for surgery (thus met clinical, medical, and weight criteria for surgical eligibility), ages 20–70 years. Exclusion criteria included past or present neurological disorder (e.g., dementia, seizures) or injury (e.g., stroke, moderate or severe head injury), past or present severe psychiatric illness (e.g., schizophrenia, bipolar disorder), past or present alcohol or drug abuse (defined by DSM-IV criteria), any history of a learning disorder or developmental disability (defined by DSM-IV criteria), or impairment of any sensory function sufficient to preclude cognitive testing. The study included all participants with complete cognitive and weight data. See Table 1 for sample characteristics.
Table 1.
Sample Characteristics
| Pre-Operative | 12-Months | |
|---|---|---|
| Age, mean (SD) | 43.22 (10.50) | – |
| Gender, % female | 83.3 | – |
| Race, % Caucasian | 93.6 | – |
| Type 2 diabetes mellitus, % present | 37.2 | 17.6 (n =74) |
| Hypertension, % present | 41.0 | 40.5 (n = 74) |
| Sleep apnea, % present | 37.1 | 17.8 (n = 73) |
| Body mass index, mean (SD) kg/m2 | 46.69 (5.29) | 30.66 (5.39) |
| Total cholesterol, mean (SD) mg/dl | 177.4 (38.43) | 157.9 (26.53) |
| HDL, mean (SD) mg/dl | 41.74 (9.11) | 57.4 (12.26) |
| LDL, mean (SD) mg/dl | 104.47 (33.31) | 82.60(23.00) |
| Glucose, mean (SD) mg/dl | 107.60 (28.36) | 92.9 (18.22) |
| Insulin, mean (SD) | 26.49 (24.02) | 7.4 (7.45) |
| Cystatin C, mean (SD) | 0.9 (0.28) | 0.8 (0.34) |
Note. Mean (SD) BMI at 18 = 28.39 (6.48); sample size for medical comorbidities is reduced due to missing data.
Measures
Neurocognitive Function
The previously described(12) IntegNeuro battery assessed cognitive function across multiple domains. This computerized cognitive battery is completed in 45–60 minutes and yields standardized scores via comparison of raw scores to the Brain Resource International Database, a large archive of healthy persons without significant medical or psychiatric history. Standardized scores control for age, gender, and estimated premorbid intellectual functioning measured via the Spot the Word task (Brain Resource Company, Ltd). Psychometric properties of this test range from acceptable to good, including convergent validity with standardized neuropsychological measures (r=0.53 to r=0.77) and test-retest reliability (r=0.52 to r=0.89)(12,13). Alternate forms were utilized to reduce possible practice effects. The respective tests included were grouped according to cognitive domains demonstrated in prior factor analytic work of Integneuro subtests(14).
Attention
Verbal Interference: This task taps into the ability to inhibit automatic and irrelevant responses and mimics a Stroop task. Participants are presented with the names of different colors, one at a time. Below each colored word is a response pad with the four possible words displayed in black and in fixed format. The subject is required to first read the color names printed in black ink as fast as possible, assessing attention and processing speed (word trial). Participants then must identify the color of each word printed in a different color, assessing inhibitory control (color-word trial). Performance for the word and color-words trials served as dependent variables.
Executive Function
Maze Task: This is a computerized maze task that assesses executive function. Participants are presented with a grid (8×8 matrix) of circles and asked to identify the hidden path through the grid. Distinct auditory and visual cues are presented for correct and incorrect responses. The trial ends when the subject completed the maze twice without error or after 10 minutes has elapsed. Total errors served as the dependent variable.
Memory
Verbal List-learning: Participants are read a list of 12 words a total of 4 times and asked to recall as many words as possible after each trial. Following presentation and recall of a distraction list, participants are then asked to recall words from the original list. Participants are asked to freely recall the learned list after a 20-minute delay and then perform a recognition trial comprised of target words and distracter items. Total words recalled upon the brief and long delay, as well as total words recognized after a delay served as the dependent variables.
Verbal Fluency
Letter and Animal Fluency: Two fluency tasks were given; letter fluency and animal fluency. For letter fluency, participants are asked to rapidly generate as many words as possible beginning with a given letter of the alphabet in 60 seconds. A different letter is used for each of the three trials. Total number of correct words produced across the three trials served as the dependent variable. For animal fluency, participants are asked to generate as many animal names as possible in 60 seconds. Total number of correct animal names generated served as the dependent variable.
Weight History
The Cincinnati Weight History Questionnaire(15) (CWHQ) ascertained height and weight status at age 18. This 20-item questionnaire asks the adult participant to self-report weight and height at past time points, including age 18. Research shows moderate sensitivity for accuracy of height and weight recall(15). BMI was calculated using kg/m2; and defined by standard cutoffs: healthy weight–BMI <25; overweight–25 to <30; class 1 obese–BMI 30 to <35; class 2 obese–BMI 35 to <40; and class 3 obese–BMI >40.
Demographics, Clinical, and Medical Characteristics
A previously described self-report questionnaire(11,16) was used to collect demographic status (e.g., age, gender, race) at the time of LABS enrollment. Medical variables were ascertained through participant self-report, medical record review, patient interview, and physical examination.
Procedures
Procedures were approved by appropriate Institutional Review Boards. Participants provided written informed consent prior to study involvement. All measures were completed pre-operatively, within 30 days of surgery; cognitive testing was repeated 12 months after surgery.
Statistical Analyses
Neuropsychological dependent variable raw scores were transformed to T-scores (M=50, SD=10) using IntegNeuro normative data accounting for age, gender, and premorbid intelligence. Composites from individual test T-scores were computed for attention, executive function, memory, and verbal fluency in accordance with cognitive domains based on factor analytic work of the Integneuro subtests(14). Cognitive impairment was defined as T-score <35 (1.5 SD below the mean). Descriptive statistics characterized sample weight and cognitive status.
Repeated measures analysis of variance (ANOVA) examined BMI changes and pre- to post-operative cognitive changes for each domain. Separate three-step hierarchical regression analyses determined whether BMI at age 18 predicted pre-operative cognitive function in each domain (i.e., attention, executive function, memory, and verbal fluency). History of hypertension, T2DM, and sleep apnea were entered in block 1 for all analyses to account for their known cognitive influence. Block 2 included a difference score between current age and 18 in order to control (to the extent possible) for duration of obesity. BMI at age 18 was entered in block 3. These same analyses were repeated to test whether BMI at age 18 predicted cognitive changes following surgery, except these analyses included baseline cognition in block 1 and the dependent variable was cognitive function at 12-months. Analyses were repeated with BMI at age 18 dichotomized into obese (BMI ≥30) or not obese (BMI<30) entered in block 3.
Results
Weight and Medical Status
See Table 1 for participant demographic and medical characteristics. Average reported BMI at age 18 was 28.4 (SD=6.48) kg/m2, placing the sample in the overweight range at that time. According to standard BMI cutoffs, 34.6% of the sample fell into the healthy weight BMI category at age 18, 25.6% were overweight, 20.5% class 1 obese, 14.1% class 2 obese, and 5.1% class 3 obese.
Average BMI at the pre-operative time point was 46.7 (SD=5.29) kg/m2, falling into the class 3 obese range, and representing a significant increase from age 18, F(1,77)=604.22, p<0.001. BMI 12 months after surgery almost returned to BMI at age 18, with average 30.9 (SD=5.37) kg/m2, representing significant weight loss from the pre-operative visit, F(1,77)=1046.19, p<0.001. While greater BMI at age 18 was associated with higher preoperative BMI, r(76)=0.39, p<0.001, no significant association between BMI at 18 and postoperative weight status was found. Comorbid medical conditions were prevalent pre-surgery, notably type 2 diabetes mellitus (37.2%), hypertension (41.0%), and sleep apnea (37.1%). These analyses did not detect a relationship between obesity at age 18 and T2DM [F(1, 75) = 0.42, p = 0.52], hypertension [F(1, 75) = 1.00, p = 0.32], nor sleep apnea [F(1, 75) = 1.89, p = .17] at the time of pre-surgical cognitive testing when adjusting for age.
Pre- and Post-Operative Cognitive Function
Prevalence of cognitive impairment is presented in Table 2. Impairments were most common in aspects of memory and verbal fluency. Overall rates of cognitive impairment decreased following surgery (Figure 1). Repeated measures ANOVA showed significant pre- to post-operative improvements in attention, F(1,77)=14.01, p<0.001, executive function F(1,77)=24.67, p<0.001, and memory F(1,77)=44.89, p<0.001, but not verbal fluency F(1,77)=0.69, p>0.05. Pre-operative BMI did not predict pre- or post-operative cognitive function in any of the domains, p>0.05 for all.
Table 2.
Neuropsychological Test Performance (T-scores)
| Baseline Mean (SD) | Baseline % T- score < 35 | 12-Month Mean (SD) | 12-Month % T - score < 35 | |
|---|---|---|---|---|
| ATTENTION | ||||
| Verbal Interference Word | 54.20 (9.87) | 6.4 | 55.86 (15.52) | 1.3 |
| Verbal Interference Color-Word | 54.29 (13.49) | 7.7 | 62.64 (11.60) | 1.3 |
| EXECUTIVE FUNCTION | ||||
| Maze Errors | 50.78 (11.69) | 7.7 | 55.05 (10.04) | 5.1 |
| MEMORY | ||||
| Short-Delayed Free Recall | 47.39 (10.25) | 10.3 | 53.63 (9.76) | 1.3 |
| Long-Delayed Free Recall | 47.10 (10.76) | 12.8 | 54.43 (9.17) | 1.3 |
| Recognition | 42.94 (9.74) | 16.7 | 48.96 (9.71) | 6.4 |
| VERBAL FLUENCY | ||||
| Verbal Fluency | 46.76 (11.42) | 16.7 | 47.49 (10.51) | 11.5 |
| Animal Fluency | 50.57 (10.87) | 5.1 | 51.14 (10.83) | 5.1 |
Figure 1.
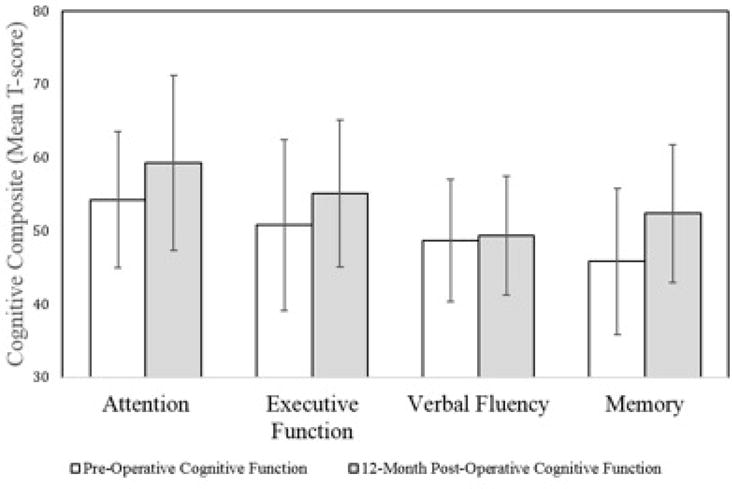
Y-axis represents T-score values. Bars plot the mean composite T-score for each cognitive domain; error bars represent standard deviations. There was a significant difference for all composites, except verbal fluency.
BMI at 18 and Pre-Operative Cognitive Function
See Table 3 for regression analyses examining the association between BMI at age 18 and adult cognitive function. BMI at age 18 emerged as a significant but modest independent predictor of pre-operative verbal fluency, B=−0.26, p=0.045, after controlling for T2DM, hypertension, sleep apnea, and difference in current age from 18 years old. For each 1 kg/m2 increase in BMI at age 18, verbal fluency test performance decreased by 0.26 units. There was no effect for BMI at age 18 as a dichotomized variable (obese or not obese) on verbal fluency performance, p>0.05. There were no significant findings for BMI at age 18 and pre-operative attention, executive function, or memory, p>0.05 for both.
Table 3.
Results of Regression Analyses Examining the Associations between BMI at Age 18 and Adult Cognitive Function (Pre-Operative and 12-Month Post-Operative)
| Pre-Operative Cognitive Function | R2 | Model F (p) | Δ R2 | F Δ (p) |
|---|---|---|---|---|
| Attention | ||||
| Block 1 | ||||
| T2DM, HTN, sleep apnea | 0.02 | 0.53 (0.66) | – | – |
| Block 2 | ||||
| 0.15 | ||||
| Age difference | 0.43 (0.79) | 0.00 | ||
| (0.70) | ||||
| Block 3 | 0.02 | |||
| 0.03 | 0.40 (0.85) | 0.00 | 0.31 | |
| BMI 18 years | (0.58) | |||
| Executive function | ||||
| Block 1 | ||||
| T2DM, HTN, sleep apnea | 0.06 | 1.59 (0.20) | – | – |
| Block 2 | ||||
| Age difference | 1.20 (0.32) | 0.09 | ||
| Block 3 | 0.06 | 0.00 | (0.77) | |
| 0.06 | 0.97 (0.45) | 0.00 | 0.08 | |
| BMI 18 years | (0.77) | |||
| Memory | ||||
| Block 1 | ||||
| T2DM, HTN, sleep apnea | 0.03 | 0.72 (0.54) | – | – |
| Block 2 | ||||
| 0.17 | ||||
| Age difference | 0.03 | 0.58 (0.68) | 0.00 | |
| (0.68) | ||||
| Block 3 | ||||
| 0.03 | 0.47 (0.80) | 0.00 | 0.04 | |
| BMI 18 years | (0.84) | |||
| Verbal Fluency | ||||
| Block 1 | ||||
| T2DM, HTN, sleep apnea | 0.01 | 0.29 (0.83) | – | – |
| Block 2 | ||||
| 0.92 | ||||
| Age difference | 0.02 | 0.45 (0.77) | 0.01 | |
| (0.34) | ||||
| Block 3 | ||||
| 0.08 | 1.21 (0.31) | 0.05 | 4.17 | |
| BMI 18 years | (0.045) | |||
| 12-Month Post-Operative Cognitive Function | ||||
| Attention | ||||
| Block 1 | ||||
| BL Attention, T2DM, HTN, sleep apnea | 0.17 | 3.70 (0.01) | – | – |
| Block 2 | ||||
| 0.20 | ||||
| Age difference | 0.17 | 2.97 (0.02) | 0.00 | |
| (0.66) | ||||
| Block 3 | ||||
| 0.24 | 3.74 (<0.01) | 0.07 | 6.48 | |
| BMI 18 years | (0.01) | |||
| Executive function | ||||
| Block 1 | ||||
| BL Executive function, T2DM, HTN, sleep apnea | 0.59 | 26.23 (<0.01) | – | – |
| Block 2 | ||||
| 0.13 | ||||
| Age difference | 0.59 | 20.76 (<0.01) | 0.00 | |
| (0.73) | ||||
| Block 3 | ||||
| 0.59 | 17.07 (<0.01) | 0.00 | 0.03 | |
| BMI 18 years | (0.87) | |||
| Memory | ||||
| Block 1 | ||||
| BL Memory, T2DM, HTN, sleep apnea | 0.22 | 5.16 (<0.01) | – | – |
| Block 2 | ||||
| 1.19 | ||||
| Age difference | 0.23 | 4.37 (<0.01) | 0.01 | |
| (0.28) | ||||
| Block 3 | ||||
| 0.25 | 3.91 (<0.01) | 0.02 | 1.45 | |
| BMI 18 years | (0.23) | |||
| Verbal Fluency | ||||
| Block 1 | ||||
| BL Verbal Fluency, T2DM, HTN, sleep apnea | 0.56 | 23.37 (<0.01) | – | – |
| Block 2 | ||||
| 0.75 | ||||
| Age difference | 0.57 | 18.79 (<0.01) | 0.01 | |
| (0.39) | ||||
| Block 3 | ||||
| BMI 18 years | 0.57 | 15.44 (<0.01) | 0.00 | 0.00 |
Note. BL = baseline; T2DM = type 2 diabetes mellitus; HTN = hypertension; BMI = body mass index; BMI is continuous; age difference = difference between current age and 18.
BMI at age 18 and Post-Operative Cognitive Function
Table 3 also presents hierarchical regression analyses examining the predictive validity of BMI at age 18 on post-operative cognitive changes. After controlling for baseline attention, T2DM, hypertension, sleep apnea, and the age difference score, BMI at 18 years of age predicted attention 12 months post-operatively, B=−0.30, p=0.01. For each 1 kg/m2 increase in BMI at age 18, a 0.30 unit reduction in attention/executive function performance occurred 12 months post-operatively. With BMI at age 18 dichotomized into obese or not obese, those who were obese at age 18 exhibited a lower degree of improvement in attention 12 months after surgery FΔ=6.55, R2Δ=0.07, B=−0.30, p=0.01 relative to non-obese counterparts, even after adjustment for medical comorbidities and age difference from 18. No significant findings emerged for executive function, memory, or verbal fluency, p>0.05 for all. See Figure 2.
Figure 2.
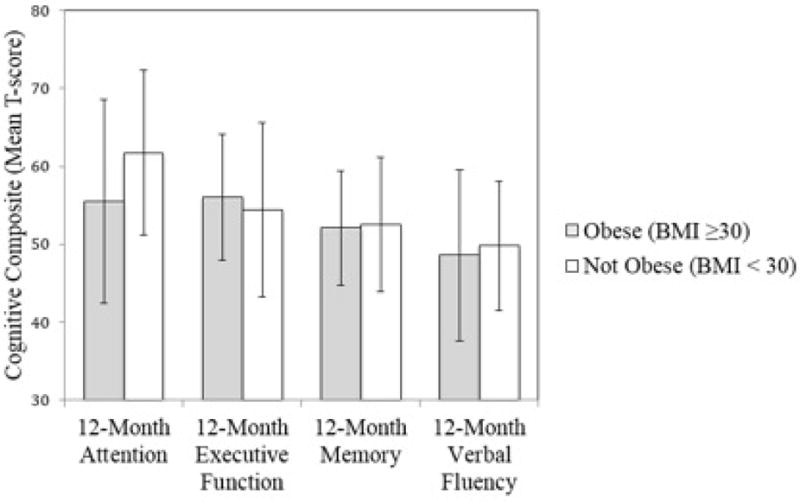
Y-axis represents T-score values. Bars plot the mean composite T-score for each cognitive domain 12 months after surgery; error bars represent standard deviations. There was a significant between BMI group difference for 12-month attention after adjustment for baseline hypertension, type 2 diabetes mellitus, sleep apnea, and baseline attention as well as duration of time since age 18; as shown, participants who were obese at the age of 18 exhibited lower attention performance post-surgery relative to their non-obese counterparts. No significant differences emerged for the other cognitive domains. BMI = body mass index.
Discussion
This study examined the relationship between adolescent weight status and adult cognitive function in obese adults prior to and 12 months following bariatric surgery. Elevated BMI in adolescence predicted poorer pre- and post-operative performance in aspects of cognition. History of elevated weight in adolescence predicted poorer pre-operative verbal fluency function and poorer post-operative improvement in attention following surgery. Higher adolescent weight status predicted higher pre-operative BMI, but there was no association between adolescent weight status and post-operative weight loss 12 months following surgery. Post-operative differences in cognition between groups (i.e., those with adolescent history of obesity and those without) thus cannot be accounted for by differences in post-operative weight loss outcomes, and may represent a fixed impairment due to obesity history that is not overcome in the short-term (1 year) following surgery. This work is the first to demonstrate that adolescent obesity increases risk for cognitive impairment in obese adults, and suggests it is also linked to diminished cognitive resilience following weight loss surgery.
Findings support our hypothesis that adolescent history of obesity confers greater cognitive risk in adults with obesity. Although the current work is correlational in nature, there are two reasons to believe that obesity during adolescence could affect adult cognitive outcomes. The first is a cumulative effect. Childhood obesity is linked to obesity in adulthood(17), suggesting a role for duration of obesity and associated comorbidities. Longer duration of diabetes may increase the likelihood of adverse neural outcomes, such as functional hypoglycemia leading to hippocampal damage(18) or chronic hyperglycemia leading to macroangiopathy and microvascular remodeling(19,20). Analogously, longer duration of obesity (with associated metabolic dysfunction) could exert deleterious central nervous system effects. We did not have access to continuous BMI data from adolescence throughout adulthood for this sample, thus do not have precise information regarding true duration of obesity. However, as these effects are found even after controlling for the length of time since age 18, the current findings argue against duration of obesity and cumulative effects of these comorbidities as the sole contributors to these relationships.
Another possible explanation is an adverse impact of obesity on the brain during a critical period. Adolescence represents a unique stage of neural development in which the frontal lobes, key to attention, executive functions, and verbal production(21), have not reached full maturation. Obesity during adolescence could influence neurodevelopmental processes. A recent animal study showed elevated BMI during childhood/adolescence was associated with synaptic abnormalities in later life, despite normal weight throughout adulthood(22). Similarly, adolescent obesity in humans is associated with cognitive impairment and structural and functional brain alterations(23–25). Alosco and colleagues (2014) recently demonstrated a link between higher BMI and reduced gray matter volume of the frontal and limbic brain regions—structures mediating cognitive abilities affected in this sample—in healthy children and adolescents(26). The link demonstrated in the current study could thus reflect alteration of typical neural maturation due to early onset obesity interfering with brain development during this critical period.
Clinical treatment in bariatric surgery should consider key aspects of cognition that are directly linked to adherence behavior and weight loss outcomes. The current study suggests that those with elevated body mass in adolescence show greater pre-operative impairment in verbal fluency and poorer post-operative recovery in higher order attentional abilities, difficulties that could impact daily functioning. The attentional task that showed poorer post-operative recovery assessed ability to quickly process information and inhibit a learned response in favor of a novel response. This cognitive ability could play a role in post-operative behaviors related to making new dietary and physical activity choices that are consistent with post-operative lifestyle recommendations rather than resorting to learned behavioral patterns that contributed to obesity. For example, it is possible that an individual with this difficulty might rely upon old, well-learned habits when needing to quickly decide (e.g., in the case of hunger) whether to choose a high fat/calorie food rather than making a healthier choice, which may be a more novel response. Although further work is needed to determine the best way to address such cognitive limitations, these individuals may need greater support throughout the preoperative preparation and post-bariatric anticipatory guidance to achieve the best weight loss and medical outcomes. Regularly screening for adolescent obesity among bariatric surgery candidates may provide insight into this possibility. Additionally, although the strength of the relationships noted in the current study is modest, on the heels of recent work demonstrating that excess weight during adolescence predicts obesity later in life(17) as well as negative medical outcomes during youth and in adulthood(2,3) the current study contributes to accumulating evidence of the potential harmful effects of obesity early in life. This highlights the importance of early interventions to prevent childhood and adolescent obesity linked to lifelong patterns of poorer physical and cognitive outcomes.
Of note, cognitive effects of adolescent obesity emerged even when controlling for history of common medical factors impacting cognition, including T2DM, sleep apnea, and hypertension. This may argue for an independent contribution of adolescent obesity, or other medical factors not accounted for. As recently reviewed(27), factors extending beyond the obesity-related comorbidities examined in the current manuscript, including presence of psychological factors, such as depressive or eating disorders, individual differences in fitness and cerebral perfusion, subclinical metabolic dysregulation such as reduced kidney or liver function, or impaired peripheral glucoregulation, differences in appetetive neurohormones, or even inflammation could play a role in degree of cognitive impairment associated with obesity and in recovery following bariatric surgery. Research demonstrates post-operative improvement in these variables following bariatric surgery, and accumulating evidence links these factors to neurocognitive performance(27) as well. Variability in presence of and improvement in such factors may thus underlie cognitive outcomes.
Although our hypothesis that history of adolescent obesity would confer greater cognitive risk in the obese adult was confirmed, given the correlational nature of this work, the possibility of another moderating variable is not excluded. For example, higher adolescent BMI was associated with a higher pre-operative adult BMI in the current study; as such, we cannot rule out that the relationships noted in the current study are in part driven by the higher pre-operative BMI of those individuals. Similarly, socioeconomic status, access to education, and environment are linked both to obesity(28,29) and cognitive outcomes(30), thus could also underlie the relationships noted in the current work.
Additionally, this work relies on self-reported history, which may be susceptible to errors of bias and recall(31), though participants from the current study describe similar weight histories compared to past work(3), demonstrating consistency across studies. Further, as this study was conducted ancillary to the larger LABS project, the limitations inherent to this larger project, such as variability in clinical approaches utilized(11), are present in this study as well, and could contribute to study findings. Another limitation lies in our sample size. Those with severe obesity in adolescence might show greater cognitive difficulty than a general obese group; however, with the small total sample, less than 20% of which had history of severe obesity, stratified analyses of obesity classes was not possible. Similarly, the relatively small number of men in the sample did not allow comparison to determine if sex differences in exist. Future work will need to determine the extent to which moderating variables might exert an influence on the current findings and examine contribution of individual differences (e.g., severity of obesity, sex differences). In addition to incorporation of such variables, larger samples and longitudinal methods monitoring BMI from adolescence through adulthood will be needed. Such designs may begin to address underlying mechanisms of these cognitive effects.
Conclusions
The current study demonstrates that adolescent weight history predicts pre- and postoperative cognition in obese adults completing bariatric surgery. Although mechanisms for this adverse impact are unclear, cumulative effects of longstanding obesity or interference with a critical period of brain maturation are possible. Future work examining mechanisms and how these factors predict future weight loss outcomes is needed.
Acknowledgments
This research was supported by NIH grants DK075119 (Dr. Gunstad), U01-DK066557, to the Data Coordinating Center; U01-DK66667, to Columbia University; U01-DK66568, to the University of Washington, in collaboration with General Clinical Research Center grant M01RR-00037; U01-DK66471, to the Neuropsychiatric Research Institute; U01-DK66526, to East Carolina University; U01-DK66585, to the University of Pittsburgh Medical Center; and U01-DK66555, to Oregon Health and Science University), and U01DK072493 (Teen-LABS consortium). The authors acknowledge the use of LABS data as the sole contribution of the LABS consortium.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: Dr. Inge reports personal fees from NPS Pharma, personal fees from Sanofi Corporation, and grants from Ethicon Endosurgery, outside the submitted work. Dr. Devlin reports grants from National Institutes of Health, during the conduct of the study, and personal fees from UpToDate, outside the submitted work. Dr. Crosby reports personal fees from Health Outcomes Solutions, outside the submitted work. Dr. Spitznagel, Mr. Alosco, Mrs. Rochette, Dr. Strain, Dr. Mitchell, and Dr. Gunstad have nothing to disclose.
References
- 1.Skinner AC, Skelton JA. Prevalence and trends in obesity and severe obesity among children in the United States, 1999–2012. JAMA Pediatr. 2014;168:561–566. doi: 10.1001/jamapediatrics.2014.21. [DOI] [PubMed] [Google Scholar]
- 2.Yarnell JW, Patterson CC, Thomas HF, Sweetnam PM. Comparison of weight in middle age, weight at 18 years, and weight change between, in predicting subsequent 14 year mortality and coronary events: Caerphilly Prospective Study. J Epidemiol Community Health. 2000;54:344–348. doi: 10.1136/jech.54.5.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inge TH, King WC, Jenkins TM, et al. The effect of obesity in adolescence on adult health status. Pediatrics. 2013;132:1098–1104. doi: 10.1542/peds.2013-2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gunstad J, Strain G, Devlin MJ, et al. Improved Memory Function 12 Weeks after Bariatric Surgery. Surg Obes Relat Dis. 2011;7:465–472. doi: 10.1016/j.soard.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gelir E, Basaran C, Bayrak S, et al. Electrophysiological assessment of the effects of obstructive sleep apnea on cognition. PLoS One. 2014;9:e90647. doi: 10.1371/journal.pone.0090647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gasecki D, Kwarciany M, Nyka W, Narkiewicz K. Hypertension, brain damage, and cognitive decline. Curr Hypertens Rep. 2013;15:547–58. doi: 10.1007/s11906-013-0398-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts RO, Knopman DS, Przybelski SA, et al. Association of type 2 diabetes with brain atrophy and cognitive impairment. Neurology. 2014;82:1132–1141. doi: 10.1212/WNL.0000000000000269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alosco ML, Spitznagel MB, Strain G, et al. Family History of Alzheimer’s Disease Limits Improvement in Cognitive Function after Bariatric Surgery. SAGE Open Medicine. 2014;2 doi: 10.1177/2050312114539477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spitznagel MB, Galioto R, Limbach K, Gunstad J, Heinberg L. Cognitive function is linked to adherence to bariatric postoperative guidelines. Surg Obes Relat Dis. 2013;9:580–585. doi: 10.1016/j.soard.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spitznagel MB, Alosco M, Galioto R, et al. The role of cognitive function in postoperative weight loss outcomes: 36-month follow-up. Obes Surg. 2014;24:1078–1084. doi: 10.1007/s11695-014-1205-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belle SH, Berk PD, Courcoulas AP, et al. Safety and efficacy of bariatric surgery: Longitudinal Assessment of Bariatric Surgery. Surg Obes Relat Dis. 2007;3:116–126. doi: 10.1016/j.soard.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paul RH, Lawrence J, Williams LM, Richard CC, Cooper N, Gordon E. Preliminary validity of “integneuro”: a new computerized battery of neurocognitive tests. The Int J Neurosci. 2005;115:1549–1567. doi: 10.1080/00207450590957890. [DOI] [PubMed] [Google Scholar]
- 13.Williams LM, Simms E, Clark CR, Paul RH, Rowe D, Gordon E. The test-retest reliability of a standardized neurocognitive and neurophysiological test battery: “neuromaker”. Int J Neurosci. 2005;115:1605–1630. doi: 10.1080/00207450590958475. [DOI] [PubMed] [Google Scholar]
- 14.Stanek K, Strain G, Devlin M, et al. Body mass index and neurocognitive function across the adult lifespan. Neuropsychology. 2013;27:141–151. doi: 10.1037/a0031988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jenkins TM, Buncher CR, Akers R, et al. Validation of a weight history questionnaire to identify adolescent obesity. Obes Surg. 2013;23:1404–1412. doi: 10.1007/s11695-013-0901-7. [DOI] [PubMed] [Google Scholar]
- 16.Belle SH, Berk PD, Chapman WH, et al. Baseline characteristics of participants in the Longitudinal Assessment of Bariatric Surgery-2 (LABS-2) study. Surg Obes Relat Dis. 2013;9:926–935. doi: 10.1016/j.soard.2013.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freedman DS, Mei Z, Srinivasan SR, Berenson GS, Dietz WH. Cardiovascular risk factors and excess adiposity among overweight children and adolescents: the Bogalusa Heart Study. J Pediatr. 2007;150:12–17 e2. doi: 10.1016/j.jpeds.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 18.Convit A. Links between cognitive impairment in insulin resistance: an explanatory model. Neurobiol Aging. 2005;26S:S31–S35. doi: 10.1016/j.neurobiolaging.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 19.Tiehuis AM, Vincken KL, van den Berg E, et al. Cerebral perfusion in relation to cognitive function and type 2 diabetes. Diabetologia. 2008;51:1321–1326. doi: 10.1007/s00125-008-1041-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tuttolomondo A, Pinto A, Salemi G, et al. Diabetic and non-diabetic subjects with ischemic stroke: differences, subtype distribution and outcome. Nutr Metab Cardiovasc Dis. 2008;18:152–157. doi: 10.1016/j.numecd.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Lezak MD, Howieson DB, Bigler ED, Tranel D. Neuropsychological Assessment. 5th. New York, NY: Oxford University Press; 2012. [Google Scholar]
- 22.Wang J, Freire D, Knable L, et al. Childhood and adolescent obesity and long-term cognitive consequences during aging. J Comp Neurol. 2015;523:757–768. doi: 10.1002/cne.23708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bruehl H, Sweat V, Tirsi A, Shah B, Convit A. Obese Adolescents with Type 2 Diabetes Mellitus Have Hippocampal and Frontal Lobe Volume Reductions. Neurosci Med. 2011;2:34–42. doi: 10.4236/nm.2011.21005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yau PL, Castro MG, Tagani A, Tsui WH, Convit A. Obesity and Metabolic Syndrome and Functional and Structural Brain Impairments in Adolescence. Pediatrics. 2012;130:e856–e864. doi: 10.1542/peds.2012-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yau PL, Kang EH, Javier DC, Convit A. Preliminary evidence of cognitive and brain abnormalities in uncomplicated adolescent obesity. Obesity. 2014;22:1865–1871. doi: 10.1002/oby.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alosco ML, Stanek KM, Galioto R, et al. Body mass index and brain structure in healthy children and adolescents. Int J Neurosci. 2014;124:49–55. doi: 10.3109/00207454.2013.817408. [DOI] [PubMed] [Google Scholar]
- 27.Spitznagel MB, Hawkins M, Alosco M, Galioto R, Garcia S, Miller L, Gunstad J. Neurocognitive Effects of Obesity and Bariatric Surgery. Eur Eat Disord Rev. 2015 Nov;23(6):488–95. doi: 10.1002/erv.2393. [DOI] [PubMed] [Google Scholar]
- 28.Cohen AK, Rai M, Rehkopf DH, Abrams B. Educational attainment and obesity: A systematic review. Obes Rev. 2013;14:989–1005. doi: 10.1111/obr.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swinburn BA, Sacks G, Hall KD, et al. The global obesity pandemic: shaped by global drivers and local environments. Lancet. 2011;378:804–814. doi: 10.1016/S0140-6736(11)60813-1. [DOI] [PubMed] [Google Scholar]
- 30.Xu W, Yu J, Tan M, Tan L. Cognitive Reserve and Alzheimer’s Disease. Mol Neurobiol. 2015;51:187–208. doi: 10.1007/s12035-014-8720-y. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz N. Retrospective and concurrent self-reports: the rationale for real-time data capture. In: Stone AA, Shiffman S, Atienza AA, Nebeling L, editors. The science of real-time data capture. New York, NY: Oxford University Press; 2007. pp. 11–26. [Google Scholar]


