Abstract
The venoms of spiders and scorpions contain a variety of chemical compounds. Antimicrobial peptides (AMPs) from these organisms were first discovered in the 1990s. As of May 2015, there were 42 spider’s and 63 scorpion’s AMPs in the Antimicrobial Peptide Database (http://aps.unmc.edu/AP). These peptides have demonstrated broad or narrow-spectrum activities against bacteria, fungi, viruses, and parasites. In addition, they can be toxic to cancer cells, insects and erythrocytes. To provide insight into such an activity spectrum, this article discusses the discovery, classification, structure and activity relationships, bioinformatics analysis, and potential applications of spider and scorpion AMPs. Our analysis reveals that, in the case of linear peptides, spiders use both glycine-rich and helical peptide models for defense, whereas scorpions use two distinct helical peptide models with different amino acid compositions to exert the observed antimicrobial activities and hemolytic toxicity. Our structural bioinformatics study improves the knowledge in the field and can be used to design more selective peptides to combat tumors, parasites, and viruses.
Keywords: Anticancer peptides, anti-HIV peptides, antimalarial peptides, antimicrobial peptides, bioinformatics, scorpions, spiders, structural biology
1. Introduction
Animal venoms are excellent sources of potential antimicrobial substances [1,2]. In particular, there are over 40,000 species of known poisonous arthropods. Spiders and scorpions, both belonging to arachnids, are an important part of arthropods. However, less than 150 arthropod species have been investigated so far and only a few of them have been characterized for antimicrobial activity [3].
Various compounds have been identified from the venom of spiders and scorpions. Interested readers may refer to excellent reviews [4–9]. In the past two decades, there were also interests in evaluating antimicrobial activity of the toxic peptides from spiders or scorpions. Such peptides with known amino acid sequence and demonstrated antimicrobial activity are regarded as antimicrobial peptides (AMPs) and collected into the Antimicrobial Peptide Database (APD). By the time this manuscript was written, there were 42 spider’s and 63 scorpion’s AMPs [10,11]. Traditionally, these peptides are classified into three families: α-helix, β-sheet, and rich in amino acids (e.g., glycines, prolines, or tryptophans) [12]. This article deals with AMPs from both spiders and scorpions with a goal of elucidating the common theme in the design of these toxic peptides. Following this Introduction section, Section 2 describes the timeline of peptide discovery. Section 3 discusses peptide classification. Subsequently in Section 4, three-dimensional structures of spider and scorpion AMPs are overviewed. Various activities of spider and scorpion AMPs are described in Sections 5 and 6, respectively. Since these peptides tend to be toxic to mammalian cells, we have analyzed them by bioinformatics in Section 7. Finally, we provide perspectives for future peptide discovery and potential applications of these molecules. Our studies shed light on antimicrobial and toxic activities of the venom AMPs from spiders and scorpions that tend to use different amino acids and 3D structures.
2. Discovery of spider and scorpion antimicrobial peptides
The isolation and characterization of AMPs from scorpions and spiders was initiated in the 1990s. As shown in Table 1, lycotoxins I and II are the first spider AMPs isolated from the venom of the wolf spider Lycosa carolinensis by reverse-phase liquid chromatography [13]. These peptides are proposed to play a dual role in assisting in the predatory process (as toxins) as well as in protecting the animals from infection (as antimicrobials). In 2002, cupiennins and oxyopinins were characterized [14, 15]. In 2011, Oxyopinin 4a (Oxt 4a), another member of oxyopinins [16], was also isolated chromatographically from the venom of the lynx spider Oxyopes takobius. Different from other oxyopinins, Oxt 4a is a unique peptide with a disulfide bond between Cys4 and Cys10. This is reminiscent of the Rana box structure frequently found in amphibian peptides that can make the peptide more stable [17]. Russian scientists continued to document additional helical AMPs from spiders. While Budnik et al. reported a family of lycocitins from Lycosa singoriensis in 2004 [18], Kozlov et al. discovered a family of latarcins from Lachesana tarabaevi in 2006 by chromatographic isolation as well as prediction from the spider venom expressed sequence tag data [19]. In 2012, both lycosin-I [20] and rondonin [21] were established as spider AMPs. Of note, antifungal rondonin, a 10-residue short peptide, is generated by cleaving a protein that delivers oxygen in the haemolymph of chelicerates.
Table 1.
Discovery timeline of antimicrobial peptides from spiders1
| Year | α-Helical peptides | β-Sheet peptides | Rich in glycines | Total peptides per year |
|---|---|---|---|---|
| 1998 | Lycotoxins I & II | 2 | ||
| 2000 | Gomesin (2S–S) | 1 | ||
| 2002 | Cupiennins 1a-1d; Oxyopinins 1, 2a-2d |
9 | ||
| 2003 | Acanthoscurrins 1 & 2 (Gly-rich) |
2 | ||
| 2004 | Lycocitins 1 & 2 | Psalmopeotoxins I & II (3S–S) |
4 | |
| 2006 | Latarcins | 12 | ||
| 2008 | Cyto-insectotoxin 1a |
1 | ||
| 2010 | LyeTx I | Ctenidins (Gly-rich) | 4 | |
| 2011 | Oxyopinin 4a (1S- S) |
Oh-defensin (3S–S) | 2 | |
| 2012 | Lycosin I; Rondonin |
Juruin (3S–S) | 3 | |
| 2013 | latartoxin 1a (4S–S); Spiderine OtTx1a (5S–S) |
2 |
Data obtained from http://aps.unmc.edu/AP on May 15, 2015. The number of disulfide bonds, if there is any, is indicated between parentheses.
Meanwhile, several AMPs from the β-sheet family were also discovered. In 2000, gomesin, a small peptide with 18 amino acids (aa), was isolated from the hemocytes of the tarantula spider Acanthoscurria gomesiana [22]. This two-stranded β-sheet is stabilized by two disulfide bonds. Four years later, two psalmopeotoxins were found with three disulfide bonds [23]. During 2011 and 2012, oh-defensin and juruin were also established to have three disulfide bonds [24, 25]. There are even more disulfide bonds in LtTx-1a (four S-S bonds) and OtTx1a (five S-S bonds) [26, 27]. In addition, five glycine-rich AMPs were discovered from spiders in 2003 and 2010, respectively [28, 29].
The discovery timeline for scorpion AMPs is given in Table 2. The first scorpion AMP was reported in 1993. This scorpion defensin was isolated from Leiurus quinquestriatus based on the striking structural similarity between insect defensins and scorpion toxins [30]. In 1996, three more scorpion peptides were reported [31]. Androctonin consists of two disulfide bonds, while buthinin and androctonus possess three disulfide bonds. During 2004 and 2007, more three disulfide-linked AMPs were found. These include charybdotoxin, opiscorpine, and heteroscorpine [32–34]. In 2008, BmK AS was characterized to have four disulfide bonds [35]. Additional peptides with four disulfide bonds were subsequently identified. Among them, bactridines 1 and 2 were demonstrated to be antimicrobial [36].
Table 2.
Discovery timeline of antimicrobial peptides from scorpions1
| Year | α-Helical peptides | β-Sheet peptides | Total AMPs per year |
|---|---|---|---|
| 1993 | scorpion defensin (3S–S) | 1 | |
| 1996 | Androctonin (2S–S); Buthinin (3S- S); Androctonus (3S–S) |
3 | |
| 2000 | Hadrurin | Scorpine (3S–S) | 2 |
| 2001 | Pandinins 1 & 2; IsCT1 | 3 | |
| 2002 | Opistoporins 1&2; IsCT2; Parabutoporin |
4 | |
| 2004 | Bmkb1; BmKn2 | Charybdotoxin (3S–S); Opiscorpine 1 (3S–S) |
4 |
| 2007 | Heteroscorpine (3S–S) | 1 | |
| 2008 | Mucroporin | BmK AS (4S–S) | 2 |
| 2009 | Imcroporin; Meucin-13; Meucin-18; |
Bactridines 1 & 2 (4S–S) | 5 |
| 2010 | Im-1; Meucin-24; Meucin-25; StCT1 |
4 | |
| 2011 | Vejovine; Ctriporin; Hp1090 | 3 | |
| 2012 | StCT2; HsAp1; VmCT1; VmCT2; AamAP2; BmKbpp |
7 | |
| 2013 | UyCT1; UyCT2; UyCT3; UyCT5; Css54; Pantinins 1–3; TsAP-1, TsAP-2; Hp1036; Hp1239 |
12 | |
| 2014 | Hp1404; Heterins 1 & 2; Spiniferin |
4 | |
| 2015 | AaeAP1; AaeAP2; Cm38; Stigmurin |
4 |
Data obtained from http://aps.unmc.edu/AP on May 15, 2015. The number of disulfide bonds, if there is any, is indicated between parentheses.
In the helical family, hadrurin was first isolated in two chromatographic steps from the Mexican scorpion Hadrurus aztecus in 2000 [37]. Between 2001 and 2004, pandinins, IsCTs, opistoporin, Bmkb1, and BmKn2 were also characterized [38–42]. Since 2008, there have been reports for new helical AMPs from scorpions every year (Table 2). AaeAP1, Cm38, and stigmurin are the newest scorpion peptides reported in 2015 [43–45].
3. Scorpion and spider peptide classification
Recently, a unified classification system has been proposed for peptides based on polypeptide chain connection patterns [46]. This classification can be applied to spider and scorpion AMPs. In this unified classification, AMPs are separated into four large classes. The first class consists of linear peptides (class-LL). Examples are spider lycotoxins and latarcins, as well as scorpion hadrurin and IsCT. The second class comprises sidechain-sidechain connected peptides (class-SS), usually using cysteines to form disulfide bonds. Examples have been found in both spiders (e.g., gomesin and oh-defensins) and scorpions (scorpion defensin and scorpine). The third class contains sidechain-backbone linked peptides (class-SB). The last class is made of backbone-backbone connected circular peptides (class-BB). Although the APD contains AMPs of the third and fourth classes identified from other organisms [11], such members from spiders or scorpions are awaited to be discovered.
The peptides in each class can be further classified in different ways. For example, Zeng et al. has classified linear scorpion peptides (referred to as non-disulfide-bridged peptides, NDBP) into six subfamilies based on a set of mixed criteria, including activity, peptide chain length, and structural similarity [47]. In their classification, subfamilies 1, 2, and 6 are function-based, while subfamilies 3–5 are length-based. Alternatively, one can classify the linear peptides (class-LL) based on one criterion at a time (visit http://aps.unmc.edu/AP/class.php). Thus, scorpion or spider AMPs can be classified as antibacterial, antiviral, antifungal, antiparasitic, and anticancer peptides (see Sections 5 and 6) based on biological activity. Here natural peptides with unknown functions can be placed in the “unclassified” group. One may also classify linear AMPs based on peptide size. In the case of scorpions, there are 9 long AMPs (51–100 aa), 19 intermediate AMPs (21–50 aa), and 34 short AMPs (10–20 aa). Scorpion and spider peptides may also be classified based on peptide structure (below).
4. Three-dimensional structure
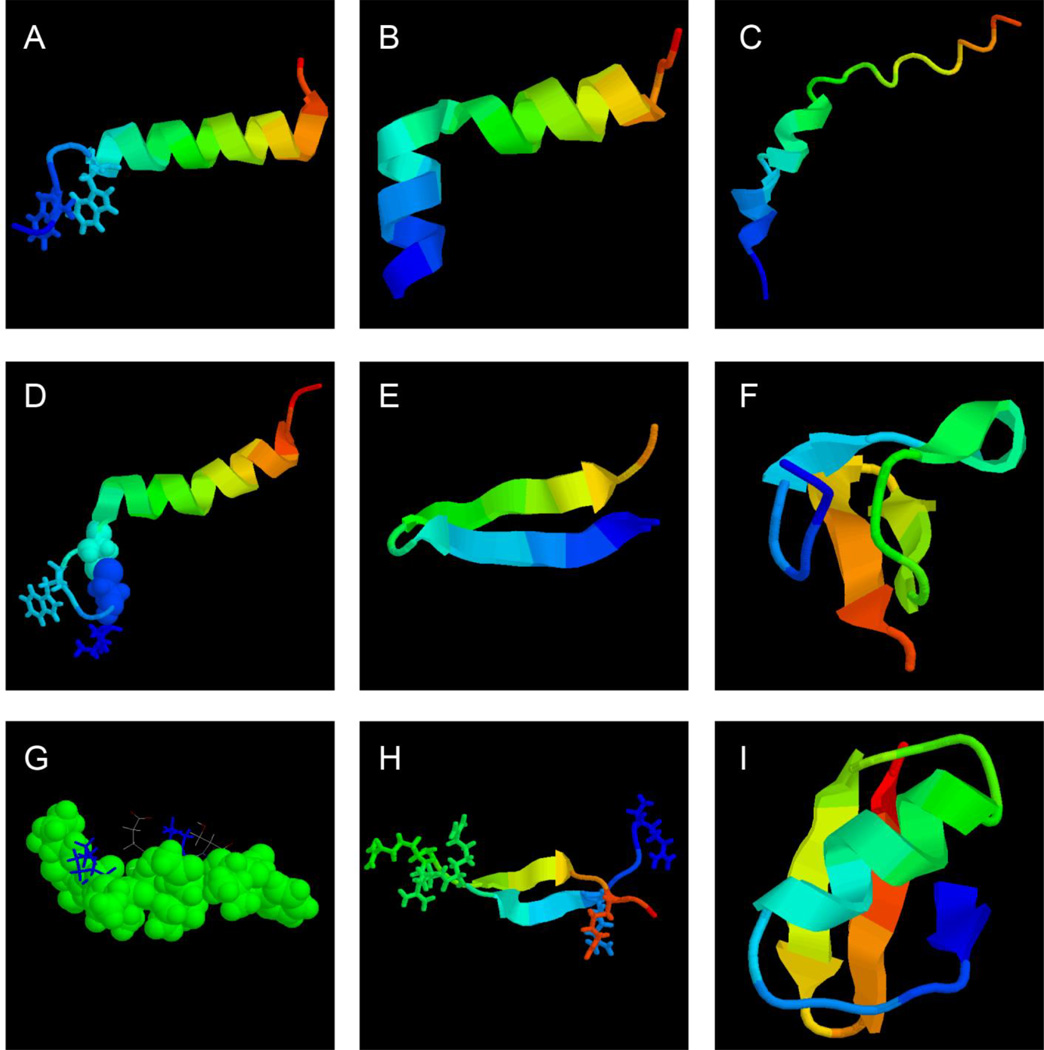
We have analyzed the 3D structures of spider and scorpion AMPs annotated in the APD database. Of the 42 spider AMPs, only seven have known 3D structures [six with coordinates in the Protein Data Bank (PDB) at http://www.rcsb.org/pdb/home/home.do]. In the case of scorpion AMPs, nine out of 63 have 3D structures (only four deposited into the PDB). These structures (Figure 1) were all determined by two-dimensional 1H nuclear magnetic resonance (2D NMR) spectroscopy [48]. Usually, linear peptides do not have an ordered structure in water, and become structured in membrane-mimetic environments. Commonly used models include organic solvent trifluoroacetic acid (TFE), micelles of sodium dodecylsulfate (SDS) and dodecylphosphocholine (DPC) [48]. Another unique model is dioctanoyl phosphatidylglycerol (D8PG) [49–51]. The known 3D structures of AMPs can be classified into four families: α-helices, β-sheets, mixed αβ structures, and non-αβ structures (no α or β structure) [52]. Of the seven known spider structures, five are α-helical (Figure 1, A–D) [16,53–55] and two are β-sheet peptides (Figure 1, E and F) [56,57]. The NMR structure of spider lyeTx I [55] was not found in the PDB. In the case of scorpions, there are seven helical peptides (IsCT, pandinin-1, pandinin-2, meucin-13, meucin-18, meucin-24, and ctriporin), one (androctonin) [58] from the β-sheet family (Figure 1H) and one (charybdotoxin) from the αβ family (Figure 1I). The structure of IsCT [59] in the space-filling model is presented in Figure 1G. The structure of meucin-24 is another typical amphipathic helix [60]. (Structures of other helical peptides were not deposited into the PDB). These structures provide useful models to understand the activity of the peptides.
Figure 1.
Structural biology of spider (A–F) and scorpion (G–I) antimicrobial peptides. Shown are the NMR structure of spider (A) latarcin 1 (PDB ID: 2PCO), (B) latarcin 2a (PDB ID: 2G9P), (C) cupiennin 1a (PDB ID: 2K38), (D) oxyopinin 4a (PDB ID: 2L3I), (E) gomesin (PDB ID: 1KFP), (F) psalmopeotoxin I (PDB ID: 1×5V), scorpion (G) IsCT (PDB ID: 1T51), (H) androctonin (PDB ID: 1CZ6), and (I) charybdotoxin (PDB ID: 2CRD), respectively. In these structural views, the peptide chain is colored from the N (blue) to the C-terminus (red). To emphasize the broad hydrophobic surface of IsCT in panel G, as well as the hydrophobic nature of sulfurs involved in the disulfide bond in panel D, they are displayed in space-filling models (G). The figure was generated using the software RasMol (https://www.umass.edu/microbio/rasmol/). See the text for further details.
5. Spider antimicrobial peptides
Of the 42 spider peptide entries in the APD database, 35 are known to be antibacterial and 20 can inhibit fungi. Only one is known to be antiviral, while three are known to have antiparasitic activity. In addition, two peptides show anticancer properties.
5.1. Antibacterial activity
(1) Helical peptides
Lycotoxins were predicted to have helical structures. They were initially shown to be active against Escherichia coli at the minimal inhibitory concentration (MIC) of 10–20 µM [13]. Interestingly, database screening revealed high potency of lycotoxin 1 against community-associated methicillin-resistant Staphylococcus aureus (MRSA) USA300 (MIC 3.1 µM) [61]. Latarcins (1, 2a, 3a, 3b, 4a, 4b, 5, 6a, and 7) constitute another family of helical peptides purified from the venom of the spider Lachesana tarabaevi [19]. While Ltc6a and Ltc7 showed no inhibitory activity till 70 µM, other peptides produced lytic effects on cells of diverse origins (Gram-positive and Gram-negative bacteria, erythrocytes, and yeast) at micromolar concentrations. To understand the structural basis of membrane targeting, both circular dichroism (CD) and NMR were utilized to determine the structure of two select peptides. SDS micelles were selected for high-resolution structural determination by NMR based on similar CD spectra of Ltc1 in SDS micelles and anionic lipid vesicles [53]. While Ltc1 adopted an uninterrupted amphipathic helix covering residues 8–23 (Figure 1A), Ltc2a adopted a helix-hinge-helix structure with the N-terminus being more hydrophobic (Figure 1B). Note that the non-helical N-terminus of Ltc1 contains two aromatic tryptophans (W3 and W7), which might facilitate membrane anchoring (Figure 1A). Interestingly, only Ltc2a is able to disrupt lipid bilayers based on vesicle dye release and 31P NMR studies, whereas Ltc1 can only cause a lesion on membranes [53].
Cupiennins were isolated from the American wandering spider Cupiennius salei. Cupiennin 1a (35 aa) possesses a hydrophobic content of 48% and net charge of +8 (due to lysines). In addition to antimicrobial activities, this peptide also displayed insecticidal activity, which might be helpful for capturing the prey. Like hadrurin [37], synthesis of cupiennin 1a in all L- or D-amino acids led to similar activity, implying membrane targeting rather than the use of chiral targets such as receptors [62]. NMR studies [54] revealed a helix-hinge-helix structure (Figure 1C). Such a structure is responsible for antimicrobial activity of the peptide against E. coli, Pseudomonas aeruginosa, S. aureus, and Enterococcus faecalis at very low concentrations (0.31–5.00 µM) [14, 62]. In particular, it has a more hydrophobic N-terminus characterized by three aromatic phenylalanines (Table 3). These aromatic residues may facilitate membrane targeting of the peptide. In the case of 13-residue amphibian aurein 1.2 and its analogs, the two aromatic rings of F3 and F13 account for ∼50% of the exposed hydrophobic surface. Indeed, both aromatic rings directly interact with lipid chains according to the NMR observations [63]. Remarkably, this effect can be extended from amphibian skin AMPs to human skin cathelicidin. In LL-37 encoded by the only human cathelicidin gene, the four aromatic rings (F5, F6, F17, and F27) all directly interact with anionic phosphatidyglycerol chains [64]. Of note is that in the APD, shorter amphibian AMPs tend to have higher contents of phenylalanines (Wang G, unpublished). For example, temporin-SHf, the shortest temporin, contains as high as 50% phenylalanines [65]. However, peptide activity is not determined by one residue such as phenylalanines [66], but by the overall membrane perturbation potential defined by the peptide surface for membrane targeting [63].
Table 3.
Amino acid sequences of selected antimicrobial peptides from spiders and scorpions1
| Species /structure |
Peptide name | Amino acid sequence | Length | Net charge |
Pho% |
|---|---|---|---|---|---|
| Spiders | |||||
| α | Cupiennin 1a | GFGALFKFLAKKVAKTVAKQAA KQGAKYVVNKQME |
35 | +8 | 48% |
| α | Latarcin 1 | SMWSGMWRRKLKKLRNALKK KLKGE |
25 | +9 | 36% |
| α | Latarcin 2a | GLFGKLIKKFGRKAISYAVKKA RGKH |
26 | +9 | 38% |
| α | Oxyopinin 4a | GIRCPKSWKCKAFKQRVLKRLL AMLRQHAF |
30 | +9 | 50% |
| α | LyeTx I |
IWLTALKFLGKNLGKHLAKQQL AKL |
25 | +6 | 52% |
| β | Gomesin | QCRRLCYKQRCVTYCRGR | 18 | +6 | 33% |
| β | Psalmopeotoxin I | ACGILHDNCVYVPAQNPCCRGL QCRYGKCLVQV |
33 | +3 | 48% |
| Scorpions | |||||
| α | IsCT | ILGKIWEGIKSLF | 13 | +2 | 53% |
| α | Meucin-13 | IFGAIAGLLKNIF | 13 | +2 | 69% |
| α | Pandinin 1 | GKVWDWIKSAAKKIWSSEPVSQ LKGQVLNAAKNYVAEKIGATPT |
44 | +4 | 40% |
| α | Pandinin 2 |
FWGALAKGALKLIPSLFSSFSKK D |
24 | +3 | 50% |
| α | Meucin-18 | FFGHLFKLATKIIPSLFQ | 18 | +2 | 55% |
| α | Meucin-24 | GRGREFMSNLKEKLSGVKEKMK NS |
24 | +4 | 25% |
| α | Ctriporin | FLWGLIPGAISAVTSLIKK | 19 | +3 | 57% |
| β | Androctonin | RSVCRQIKICRRRGGCYYKCTNR PY |
25 | +8 | 28% |
Data from the APD (http://aps.unmc.edu/AP). Peptide hydrophobic content is indicated with Pho%.
LyeTx I was isolated from the venom of Lycosa erythrognatha. It is active against both Gram-positive and negative bacteria such as S. aureus and E. coli. The MIC spans 4–8 µM. It showed a relatively low level of hemolysis (50% lysis at 130 µM). NMR structural determination found a helical region for residues 6–25 when bound to DPC micelles [55].
Oxyopinins are the largest linear cationic amphipathic peptides (37–48 aa) isolated from the crude venom of the wolf spider Oxyopes kitabensis. CD analyses revealed helical conformations for the five Oxyopinins (1, 2a-2d) [15]. Oxt 4a, isolated from a different species Oxyopes takobius, is strongly active against Bacillus subtilis, E. coli, Pseudomonas fluorescens (MIC 0.5–1 µM), and S. aureus (MIC 10 µM) [16]. Oxt 4a is also highly toxic to human erythrocytes (EC50 7 µM). NMR studies revealed a helical structure for residues 12–25 in complex with DPC micelles (Figure 1D). The N-terminal hydrophobic moiety is proposed to be important for peptide cytotoxicity [67].
Eight linear cationic peptides with cytolytic and insecticidal activity, designated cyto-insectotoxins (CITs), were identified from the Lachesana tarabaevi spider venom. CIT 1a (69 aa), a helical peptide, showed antibacterial activity against Gram-positive and Gram-negative bacteria at micromolar concentrations as well as toxicity against insects [68].
There are additional spider peptides predicted to have helical structures. For example, Budnik et al. isolated lycocitins 1 and 2 from the venom glands of the wolf spider Lycosa singoriensis. Both peptides can inhibit the growth of Gram-positive (S. aureus, B. subtilis) and Gram-negative (E. coli) bacteria at micromolar concentrations [18]. We may assume that these peptides also use the amphipathic helix to attack invading bacteria.
(2) β-sheet peptides
Gomesin (18 aa) showed a variety of antimicrobial activities. Not only has it good inhibitory activity against Gram-positive, Gram-negative bacteria, fungi, yeast, parasites, gomesin can also kill several cancer cells at very low concentrations [11,36–38]. As shown in Figure 1E, it has a two-stranded β-sheet structure [56]. Because of these properties, there is great interest in engineering gomesin, which is described in the application section.
Psalmopeotoxin I (also called PcFk1) adopts a three-stranded β-sheet (Figure 1F) [57]. Importantly, this peptide inhibited malaria-causing Plasmodium falciparum at 1.59 µM [23].
(3) Rich in glycines
The APD collected five glycine-rich small proteins (>10 kDa) from spiders. Acanthoscurrins 1 & 2 were isolated from hemocytes of the spider Acanthoscurria gomesiana [28]. These peptides are only made of seven kinds of amino acids: glycines (72–73%), leucines (12%), tyrosines (7%), lysines (4–5%), arginines (2%), valines (1%) and aspartic acids (1%). Acanthoscurrins had marked activity against E. coli (MIC 2.3–5.6 µM). However, no activity could be detected against the Gram-positive strain in the range of concentration investigated (up to 5.6 µM) [28]. Another family of Gly-rich peptides is composed of three ctenidins found in the hemocytes of Cupiennius salei [29]. Ctenidins are linear, cationic peptides with a net charge of +5. These ctenidins are sequence deletion variants. Relative to ctenidin-1, ctenidin-3 had an insertion of two glycines at positions 19 and 20 and one glycine deletion from the C-terminus, while ctenidin-2 is the shortest due to the deletion of the sequence GGGLGGGQGG. Antimicrobial activity assays against S. aureus and E. coli were conducted using a mixture of these peptides. The amino acid composition of ctenidins resembles those of acanthoscurrins. However, acanthoscurrins exhibit 1.7 times more hydrophobic amino acid residues (mainly Leu) than the ctenidins, but 4 times less Asp [29]. We predict that these Gly-rich peptides may not adopt an ordered structure prior to binding to its target yet to be elucidated.
5.2. Antifungal activity
There are 20 spider peptides with known activity against fungi. Among them, 12 are helical peptides, one β-sheet peptide, and two rich in glycines. Another five have unknown structure.
(1) α-helical peptides
Lycotoxins are only weakly inhibitory to Candida glabrata at 100–150 µM [13]. LyeTx I, 84% similar to lycotoxin 1 in sequence, is active against yeasts Candida krusei (MIC 26 µM) and Cryptococcus neoformans (MIC 13 µM) [55]. Latarcins (1, 2a, 3a, 3b, 4a, 4b, 5, 6a, and 7) were found to produce lytic effects on Pichia pastoris and Saccharomyces cerevisiae at micromolar concentrations [19]. Lycosin-I, a venom peptide from the spider Lycosa singoriensis, displays strong ability to inhibit growth of fungi: Penicillium sp., Aspergillus flavus (MIC 3.1 µM), Beauveria bassiana (6.2 µM), Candida albicans, and S. cerevisiae (50 µM) [69]. Lycocitins 1 and 2 (18 aa) differ only at the second position of the peptide, Lys in lycocitin 1 and Arg in lycocitin 2, respectively. These two peptides are highly potent against C. albicans (MICs in the range of 1.25–2.51 µM) [13], making them potential templates for designing antifungal peptides.
(2) β-sheet peptides
Gomesin can inhibit the growth of C. neoformans and, at the antifungal concentration, it was not toxic to human brain cells. In synergy with fluconazole, gomesin better inhibited fungal growth and enhanced the antimicrobial activity of brain phagocytes [22,56,70,71].
(3) Rich in glycines
Two Gly-rich peptides, acanthoscurrins 1 and 2, had marked activity against C. albicans (MIC 1.15–2.3 µM) [28].
5.3. Antiviral activity
Virucidal activities of spider peptides have not yet widely explored. Only the Ltc1 peptide was demonstrated to exhibit significant inhibitory effects on dengue NS2B–NS3pro and virus replication in the infected cells (HepG2 cells) [72].
5.4. Antiparasitic activity
Three spider peptides are active against parasites. They are gomesin, psalmopeotoxin I (PcFK1) and psalmopeotoxin II (PcFK2), which are all disulfide-linked AMPs (Table 1). Synthetic gomesin was found to be highly effective in killing the parasite Leishmania amazonensis at 2.5 µM. The activity against the parasite L. amazonensis depends on the peptide concentration [22]. In vitro, PcFK1 and PcFK2 can inhibit the intra-erythrocyte stage of Plasmodium falcipatum (IC50 1.1–1.59 µM). These peptides could therefore be promising templates for the development of antimalarial drugs [23].
5.5. Anticancer activity
Gomesin induced necrotic cell death and was cytotoxic to SH-SY5Y and PC12 cells. The mechanism of cytotoxicity implies calcium entry through L-type calcium channels, activation of MAPK/ERK, PKC and PI3K signaling as well as the generation of reactive oxygen species [73]. Lycosin-I (24 aa), a venom peptide from the spider Lycosa singoriensis, displayed strong ability to inhibit cancer cell growth in vitro and could effectively suppress tumor growth in vivo. Mechanistically, it activates the mitochondrial death pathway to sensitize cancer cells for apoptosis, and up-regulates p27 to inhibit cell proliferation [20].
5.6. Hemolytic activity
Spider peptides are not only active against microorganism but also toxic to mammalian cells. Some of these peptides are highly toxic. For example, oxyopinin 4a was able to cause 50% lysis (HC50) of human erythrocytes at 7 µM [16]. Another peptide Ltc2a also exhibited a strong hemolytic activity, whereas Ltc1 and Ltc5 were moderately hemolytic [19]. Some spider peptides, however, are less hemolytic. Gerardo Corzo et al. used the blood cells of guinea pigs, pigs, and sheep to test the hemolysis of oxyopinins (e.g., Oxki1 to Oxki2). The hemolytic activities of Oxki1, Oxki2, and pindinin 1 in these erythrocytes were lower compared with that of pindinin 2 [15]. Lycosin-I showed only 10% hemolysis at 100 µM [42,69]. Gomesin only caused 25% lysis of human erythrocytes at 100 µM [22]. Different from latarcins 1 and 2, Sergey A. et al. found that rabbit erythrocytes were highly resistant to latarcins 3a, 3b, 4a, 4b, 6a, and 7. Only 20% lysis was obtained at 120 µM of Ltc3a, Ltc3b, or Ltc4a [19]. Peptide hemolytic activity of cupiennin 1a was found to be related to negatively charged sialic acids on the outer leaflet of red blood cells [62]. The low hemolytic ability of disulfide-bonded small gomesin (18 aa) makes it attractive for peptide engineering.
6. Scorpion antimicrobial peptides
Of a total of 63 entries in the APD database, 56 scorpion AMPs are antibacterial. Furthermore, 22 are known to inhibit fungi, while five are antiviral. Three are known to have antiparasitic activity. In addition, six peptides show anticancer properties.
6.1. Antibacterial activity
As of May 2015, 21 scorpion peptides are annotated as “helix” in the APD database (seven determined by NMR and 14 determined by CD). Some of these antibacterial peptides are rather short (13–19 residues, Table 3). These include IsCT, IsCT2, meucin-13, meucin-18, BmKn2, VmCT1, VmCT2, StCT2, hp1404, stigmurin, and ctriposin [74]. IsCT (13 aa) is a short Cys-free scorpion peptide with a helical structure (Figure 1G) [59]. It has a net charge of +2 as a result of C-terminal amidation. IsCT demonstrated antimicrobial activity against both Gram-positive and Gram-negative bacteria [39]. IsCT2 shared high homology with IsCT. IsCT2 showed broad activity spectra against microbes (Gram-positive and negative bacteria as well as fungi) and relatively weak hemolytic activity to sheep red blood cells [40]. Pantinin-1, pantinin-2 and pantinin-3, with 13–14 aa, were identified from the scorpion Pandinus imperator [41]. All of the three peptides possess stronger activities against Gram-positive bacteria than Gram-negative bacteria. Meucin-13 and meucin-18 were isolated from a cDNA library of the venom gland of the scorpion Mesobuthus eupeus [75]. They showed sequence similarity to amphibian 13-residue temporins and 18-residue brevinins, respectively. These peptides are highly potent against Gram-positive and Gram-negative bacteria. The amphipathic helices determined by NMR spectroscopy are responsible for such activities. Meucin-18, with a longer hydrophobic surface, was more potent and hemolytic than meucin-13.
BmKn2, isolated from Buthus martensii Kasch, is an α-helical peptide with no disulfide-bridge. Chemically synthetized BmKn2 displayed potent activity against 18 clinical isolates of N. gonorrhoeae [76]. StCT2 are active against S. aureus, including antibiotic-resistant strains such as MRSA [77]. Css54 has typical α-helix secondary structures in hydrophobic mimicking environments. It can inhibit the growth of both E. coli and S. aureus (MIC 12.5 µg/ml). At the same time, Css54 was evaluated in the presence of antibiotics used for the treatment of tuberculosis, isoniazid, rifampicin, pyrazinamide and ethambutol. The best combined effect was found with rifampicin [78]. VmCT1 and VmCT2 were isolated from the venom glands of Vaejovis mexicanus. These 13-residue short peptides share important amino acid sequence similarities among themselves, but their biological activities vary dramatically. They showed broad-spectrum antibacterial activity. Interestingly, ctriporin, Hp1404, and stigmurin are solely active against Gram-positive bacteria [45,79,80]. Hp1404, isolated from Heterometrus petersii, is an amphipathic α-helical peptide with activity against Gram-positive bacteria including MRSA. Hp1404 can penetrate the membrane of S. aureus at a low concentration, and disrupts the cellular membrane directly at high concentrations. In vivo, hp1404 can improve the survival rate of the MRSA infected balb-c mice in the peritonitis model [80]. Stigmurin was selected based on a transcriptomic analysis of the Brazilian yellow scorpion Tityus stigmurus venom gland. This newly found peptide exhibited antibacterial and antifungal activity, with MICs ranging from 8.7 to 69.5 µM [45].
Different from the above short peptides, several peptides are much longer with 44–56 amino acids. These include opistoporins 1 and 2, pandinin 1, vejovine, and Im-1. Opistoporins 1 and 2 differ by only one amino acid: F34 in opistoporins 2 and L34 in opistoporins 1 (OP1). In the same concentration range, OP1 was more active against Gram-negative bacteria (1.3–25 µM) than Gram-positive bacteria. Pandinin 1 and Im-1 [40] can kill both Gram-positive and negative bacteria. Vejovine (47 aa), however, is primarily active against Gram-negative bacteria (P. aeruginosa, Klebsiella pneumoniae, E. coli, Enterobacter cloacae and Acinetobacter baumanii) with MICs 4.4–50 µM [81]. The N-terminal eight amino acids are essential for antimicrobial activity. Additional scorpion antibacterial peptides with 3D structure not yet determined are listed in Table 4.
Table 4.
Scorpion antimicrobial peptides with potential helical structure
| Name | Scientific name |
Continent | length | Activity | Ref |
|---|---|---|---|---|---|
| Hadrurin |
Hadrurus aztecus |
Mexico, N. America |
41 |
S. thyphi; K. pneumonia; E. cloacae; P. aeruginosa; E. coli; S. marscences |
37 |
| TsAP-1; TsAP-2 |
Tityus serrulatus |
Brazil, S. America | 17 | S. aureus; E. coli; C. albicans | 93 |
| AamAP1; AamAP2 |
Androctonus amoreuxi |
N. Africa | 18 | S. aureus; E. coli; C. albicans | 96 |
| AaeAP1; AaeAP2 |
Androctonus aeneas |
Africa | 19 | S. aureus; E. coli; C. albicans | 43 |
| Cm38 |
Centruroides margaritatus |
Central America | 19 | K. pneumonia | 44 |
| Bmkb1; BmKn2 |
Buthus martensii Kasch |
China, Asia | 18 |
S. aureus; M. luteus; B. subtilis; B. thuringiensis; P. aeruginosa; N. gonorrhoeae |
42 |
| Mucroporin | Lychas mucronatus | Asia | 17 | S. aureus, B. thuringiensis, B. subtilis | 87 |
| Imcroporin | Isometrus maculates |
Tropical area | 17 | MRSA, B. thuringiensis, S. aureus, B. subtilis |
95 |
| StCT1 |
Scorpiops tibetanus |
China, Asia | 14 |
S. aureus , B. subtilis , M. luteus, B. thuringiensis, E. coli , P. aeroginosa, MRSA |
118 |
| BmKbpp |
Mesobuthus martensii Karsch |
China, Asia | 47 |
B. cinerea, F. culmorum, N. crassa, E. coli; H. influenza, K. pneumonia, S. enterica, P. aeruginosa , S. marcescens, B. subtilis , L. monocytogenes, M. luteus , E. faecalis |
119 |
| BmK AS |
Buthus martensii Karsch |
China, Asia | 66 |
S. aureusS. typhimuriumE. coliP. aeruginosa |
35 |
| HsAp |
Heterometru s spinifer |
Asia | 29 |
E. coli, P. putida, P. fluorescens, K. oxytoca, E. cloacae subsp, S. enterica, S. aureus , B. magaterium, B. thuringiensis , C. tropicalis , |
94 |
| heterin-1; |
Heterometru s spinifer |
China, Asia | 43 |
S. aureus , B. megaterium , M. luteus , E. coli , P. fluorences, P. putida , E. cloacae , S. enterica |
122 |
| heterin-2; |
Heterometru s spinifer |
China, Asia | 24 |
S. aureus , B. megaterium , M. luteus , E. coli , P. fluorences, P. putida , K. oxytoca |
122 |
| spiniferin |
Heterometru s spinifer |
China, Asia | 13 |
B. megaterium, M. luteus, E. coli |
122 |
| UyCT |
Urodacus yaschenkoi |
Australia | 14 |
S. aureus, E. Coli, P. aeruginosa |
120,121 |
| Opistoporin 1 |
Opistophtalm us carinatus |
S. Africa | 44 |
E. coli, S. marcescens, P. aeruginosa, K. pneumonia, S. choleraesuis, H. influenza, B. subtilis, L. monocytogenes, M. luteus, E. faecalis, S. aureus, S. pneumonia, N. asteroides |
74 |
| Opistoporin 2 |
Opistophtalm us carinatus |
S. Africa | 44 | NA | 74 |
| Scorpine |
Pandinus imperator |
Africa | 75 | B. subtilis, K. pneumonia, | 82 |
| Css54 |
Centruroide s suffusus suffusus |
25 | 78 | ||
| Pantinin-1 Pantinin-2 Pantinin-3 |
Pandinus imperator |
China, Asia | 14 |
S. aureus, B. magaterium, M. luteus, VRE, MRSA, E. coli, P. putida, K. oxytoca, E. cloacae, S. enterica, C. tropicalis |
41 |
| Hp1036 Hp1239 |
Heterometru s petersii |
China, Asia | 13 | HSV | 71 |
Androctonin (25 aa) was isolated from the hemolymph of unchallenged scorpions of the species Androctonus australis [31]. It can inhibit both bacteria and fungi. Like spider gomesin, scorpion androctonin consists of a two-stranded β-sheet (Figure 1H) [58]. Interestingly, the arginines are located at the terminal or turn regions of androctonin. Such a feature may allow the peptide to agglutinate bacteria. There are also other disulfide-bonded peptides that have the potential to form a β-sheet structure. Buthinin is a 34-residue antibacterial peptide with three disulfide bridges. It was isolated from the hemolymph of unchallenged scorpions of the species Androctonus australis [31]. Scorpion defensin, isolated from Leiurus quinquestriatus, shows a remarkably high degree of sequence homology with an insect defensin [30]. Heteroscorpine-1 (HS-1) is a peptide from the crude venom of the Thai giant scorpion Heterometrus laoticus. It exhibited good activity in disc diffusion assays against B. subtilis, K. pneumoniae, P. aeruginosa and S. aureus. Its sequence show high homology to scorpine [32]. Bactridines with four disulfide bridges were isolated from the Tityus discrepans scorpion venom. Bactridines had high antibacterial activity against a wide range of Gram-positive and Gram-negative bacteria. Complete bacterial growth inhibition occurred at concentrations from 20 to 80 µM depending on the bacteria and peptide tested [36]. Scorpine was isolated from the venom of the West Africa scorpion Pandinus imperator, with antibacterial activity and a potent inhibitory effect on the ookinete (ED50 0.7 µM) and gamete (ED50 10 µM) stages of the Plasmodium berghei development. Scorpine (75 aa) has a unique amino acid sequence. While its N-terminal segment is similar only to some cecropins, the C-terminal region, with tree-disulfide bonds, more resembles defensins [82].
Charybdotoxin is a scorpion toxin known for a long time. It consists of a 3-stranded β-sheet packed with a helix (Figure 1I) [83]. This protein fold is further stabilized by three disulfide bonds. It was initially known as a blocker of the single Ca2+-activated K+ channel from mammalian skeletal muscles [84]. However, its antimicrobial activity was not demonstrated until 2004 by Young and Yeaman who identified this AMP based on the conserved γ-core motif [33].
6.2. Antifungal activity
Helical peptides, such as opistoporin1, parabutoporin and pantinin-2, can inhibit the growth of fungi [74]. Meucin-13 and meucin-18 exhibited extensive cytolytic effects on fungi and yeasts [75]. Stigmurin exhibited both antibacterial and antifungal activity with MICs ranging from 8.7 to 69.5 µM [45]. The MICs of ctriporin against C. albicans are 5 to 20 µg/ml [85].
Androctonin, a 25-residue β-sheet peptide with two disulfide bridges, is active against fungi [31]. Zhu and Tytgat isolated four homologs of scorpine (named opiscorpines 1–4) from the African scorpion Opistophthalmus carinatus venom gland. Although antifungal activity for the intact molecule of opiscorpine 1 has not been demonstrated, a synthetic fragment (Jan-f3) corresponding to the N-terminal 35 amino acids was shown to be active against fungi Fusarium culmorum and Fusarium oxysporum (IC50 8.8 and 10 µM) [34].
6.3. Antiviral activity
Scorpion peptides can also have antiviral activity. For example, Hp1090 was screened from the venomous gland cDNA library of the scorpion Heterometrus petersii. This peptide inhibited hepatitis C virus (HCV) infection with an IC50 of 5.0 µM. Furthermore, Hp1036 and Hp1239 inhibited herpes simplex virus type 1 (HSV-1) infection in vitro [86]. In addition, a peptide variant of mucroporin [87] (Mucroporin-M1), but not the original peptide, showed virucidal activity against four RNA viruses (measles viruses, SARS-CoV, influenza H5N1 and H1N1) [88, 89]. Chen et al. designed Kn2–7 based on BmKn2 by making three amino acid changes G3K, A4R, and S10R. Kn2–7 was found to be more potent against HIV-1 (EC50 2.76 µg/ml) but showed low cytotoxicity to host cells [90]. This is in line with the previous results from the database-guided peptide design. Wang et al. showed that increasing positive charges, especially arginines, enhanced anti-HIV activity of several AMPs [91,92]. Therefore, it is possible to improve the antiviral activity of AMPs.
6.4. Antiparasitic activity
Scorpion AMPs, including meucin-24, meucin-25 and scorpine, are also active against parasites [60]. Meucin-24 and meucin-25 effectively inhibited the development of Plasmodium berghei ookinetes in the concentration range from 10 to 20 µM. At 20 µM, meucin-24 and meucin-25 inhibited this parasite by 40% and 50%, respectively, higher than several well-characterized antiparasitic AMPs such as gomesin [22]. Importantly, 10 µM meucin-24 and meucin-25 significantly reduced the parasite density of P. falciparum, which is directly responsible for human malaria [60]. Scorpine could completely inhibit both fecundation and ookinete formation at 50 and 3 µM, respectively. The calculated ED50 were 10 µM for the gamete and 0.7 µM for the ookinete stages of development [82].
6.5. Anti-cancer activity
Some scorpion AMPs have also been tested for anticancer activity. AaeAP1 and AaeAP2 can inhibit the proliferation of four different human cancer cell lines (H4600, MB435s, MCF-7, and PC3) at concentrations ranging between 10−4 and 10−9 M. At a concentration of 10−5 M (equivalent to approximately 22 mg/L), both peptides caused more than 85% inhibition of the cell proliferation. But their effects were somewhat more selective on certain cell lines at much lower concentrations, indicating that a general cytotoxic effect was not responsible for all of the observed effects [42]. TsAP-1 is effective against human cancer cell lines of H157 and H838, while TsAP-2 can inhibit the growth of all five human cancer lines (H157, H838, MCF-7, PC3 and U251-MG). Notably, Lys residue substitutions of both peptides enhanced their potency against the five cancer cell lines with TsAP-S2 being the most potent (IC50 in the range of 0.83 and 2.0 µM) [93].
6.6. Hemolysis activity
Like spider peptides, most of the scorpion AMPs are also hemolytic. In particular, BmKn1, BmKn2, meucin-13, meucin-18 [41], hadrurin [37], VmCT1, and VmCT2 [56] are highly hemolytic (50–100% lysis < 30 µM). In addition, Css54 [78] caused 80% lysis at 30 µM, while HsAp induced 95%-100% hemolysis at concentrations 3.2–6.4 µM [94]. Pandinin 2 also demonstrated strong hemolytic activity against sheep erythrocytes [41]. It is likely that high hydrophobicity, at least of those short peptides, is responsible for toxicity [52].
Some scorpion peptides are moderately hemolytic (HC50 ∼50 µM). For example, imcroporin did not achieve 50% lysis at 50 µg/ml [95]. Parabutoporin, opistoporin, and IsCT are less hemolytic than melittin, a well-known lytic peptide from insects. IsCT demonstrated over 50% hemolysis of sheep red blood cells at 75 µM [39].
A few scorpion AMPs, however, showed poor hemolytic activity (HC50 ≥100 µM). Vejovine caused 50% hemolysis of human erythrocytes at 100 µM [81]. AamAP1 is less hemolytic than AamAP2. At 100 µM, AamAP1 caused ∼28% hemolysis, while AamAP2 disrupted 50%. This marked difference in hemolytic activity could be attributed to the overall hydrophobic content of the peptides: 61% for AamAP1 and 64% for AamAP2 in the APD database. A change of H8 to K8 made AamAP1 more hemolytic and more antimicrobial [96]. A new scorpion peptide, stigmurin, also showed a low hemolytic activity (22% lysis at 140 µM). This is consistent with cytotoxicity obtained using other mammalian cell lines. Stigmurin caused 50% killing (IC50) of human cervical cancer cells (SiHa) and African green monkey kidney epithelial (Vero E6) cell lines at 118 and 150 µM, respectively [45].
7. Spiders and scorpions use different molecular design strategies for cytotoxicity
Potential cytotoxicity is one of the main problems for making practical use of AMPs. To provide insight into the molecular basis of cytotoxicity, we analyzed the toxic AMPs from spiders or scorpions collected in the APD database [10,11]. To facilitate the comparison, we selected toxic peptides from the helical family, which is dominant for both spiders and scorpions (Tables 1 & 2). The amino acid composition for these peptides was analyzed using the APD tool [10] and the results are summarized in Table 5. Because hydrophobic amino acids are known to be significant for peptide toxicity [52], we have summed the contents of all hydrophobic amino acids (L, I, V, A, M, F, W, C). While these scorpion AMPs have on average a hydrophobic content of 47%, spider AMPs have a low hydrophobic content of 41%. To be more precise, we further divided the toxic peptides from both spiders and scorpions into two groups: short and long. In the case of spiders, this split does not have an effect on hydrophobic content (Table 5). However, the two groups for scorpions are rather distinct. While the long group (average peptide length 45) has an average hydrophobic content of 40%, the short group (average length 15.8) possesses a much higher hydrophobic content of 57.6%. Thus, we may classify these toxic peptides into two families: family I with a high hydrophobic content (only in scorpions) and family II with ∼40% hydrophobic amino acids. Based on this, we may attribute the toxic effects of short scorpion AMPs to high hydrophobic contents as found for the toxic peptides in the entire APD database [52]. A search of the APD reveals 110 AMPs with a length between 10–15 aa and hydrophobic content between 60–70%. 82 of these peptides originate from frogs, 13 from insects, and 10 from scorpions [10,11]. In particular, crabrolin was also isolated from the venom of European insect hornet [97]. Thus, such a peptide design is shared by both invertebrates (e.g., insects and scorpions) and vertebrates (e.g., amphibians). The evolutional relationship of scorpion AMPs with select vertebrates has been shown previously by Gao B et al. [75]. Structurally, such peptides tend to have a broad hydrophobic surface (usually incorporated with aromatic phenylalanines) (Table 5), rendering them more effective in membrane disruption [98].
Table 5.
Amino acid sequence analysis of toxic helical peptides from spiders and scorpions1
| Organism | Spiders | Scorpions | ||
|---|---|---|---|---|
| Peptide group | 5 Short (24–28 aa) | 5 Long (35–48 aa) | 7 Short (13–24 aa) | 4 Long (44–47 aa) |
| Examples | Lycosin-I; Latarcins |
Oxyopinins | IsCT2, VmCT2, Meucin-13 |
Opistoporin 1, Parabutoporin |
| Average length |
26.6 | 39.2 | 15.8 | 45.0 |
| Pho% | 42.1% | 40.3% | 57.63% | 39.97% |
| Abundant hydrophobic residues |
L 10.5%; F 9%; A 9% |
L 8.2%; F 9.2%; A 10.2% |
I 13.5%; L 15.3%; F 15.3% |
L 8.3%; A 13.3%; I/V 11.1% |
| Net charge | +8.4 | +8.0 | +1.43 | +4.75 |
| Lysine% | 24.8% | 21.4% | 10.8% | 17.2% |
| Arginine% | 9.8% | 5.1% | 0% | 1.1% |
Only toxic peptides from the helical family were split into short and long groups and analyzed. Both peptide number and length are indicated for each group.
Then, what about the spider AMPs? These peptides have a moderate hydrophobic content but an unusual high content of lysines at 23% on average (Table 5). Figure 1 (panels A–D) shows a variety of two-domain structural models, where the N-terminal region varies. It can be an aromatic region (Figure 1A), a helix (Figure 1, B and C), or a disulfide-bond linked loop (Figure 1D), which could modulate the exact mechanism of action of the peptide [53]. The C-terminal region is usually helical (Figure 1, A–D). We propose that the C-terminal region with multiple basic amino acids is deployed mainly for the purpose of molecular recognition, whereas the N-terminal hydrophobic region is primarily for membrane anchoring. A hydrophobic cluster exists at the N-terminus of several spider peptides (Table 3, bold), which can also be important for hemolysis. Indeed, specific mutations in the N-terminal hydrophobic region of latarcins 2a and 5 reduced hemolytic activity (>36 µM reported) [99]. Likewise, substitutions of the two aromatic residues F2 and F6 at the N-terminus of cupiennin 1a reduced peptide insecticidal activity as well as hemolytic activity (>40 µM) [67]. Note that a toxic hydrophobic cluster may not be always located at the N-termini of natural AMPs. While human cathelicidin LL-37 contains an N-terminal hydrophobic helix separated from the major antimicrobial region by a hydrophilic serine at position 9 [64,100], BMAP-27 has a hydrophobic region at the C-terminus. The resulting peptide showed a reduced hemolytic activity when the C-terminal hydrophobic segment of BMAP-27 was truncated [101]. All these examples further reinforce the general strategy for reducing cytotoxicity of membrane-targeting AMPs by reducing hydrophobicity [52,102].
8. Perspectives and therapeutic potential
The venoms of spiders and scorpions are rich in antimicrobial substances. Linear and helical peptides are dominant in the known spider and scorpion AMPs (Tables 1 & 2). Our analysis reveals two primary structural models for helical peptides. The first helical model is longer, allowing for two structural and functional regions (Figure 1, A–D), while the second helical model is short, leading to a broad hydrophobic surface with few basic residues (Figure 1G). Long helical peptides rich in basic amino acids usually have a broad activity spectrum, while those short helices poor in basic amino acids tend to be active primarily against Gram-positive bacteria such as S. aureus [45,79,80,103]. These structural models explain the dominance of helical structures in spiders and scorpions and shed light on their cytotoxicity. It remains to be discovered whether spiders use short helical peptides as designed for scorpions (Figure 1G). However, spiders deploy Gly-rich peptides (>70%), which have not yet been reported for scorpions. These spider peptides are primarily active against Gram-negative bacteria such as E. coli. Whether scorpions have deployed Gly-rich peptides remains to be seen. In addition, sidechain-linked defensin-like peptides have been discovered in both spiders (Figure 1, E and F) and scorpions (Figure 1, H and I). Some of these peptides such as charybdotoxin play a dual role: antimicrobial and channel-blocking [33,84]. Finally, both sidechain-backbone connected and circular AMPs have not yet been found in spiders or scorpions. Future studies may reveal whether spiders and scorpions also use such structural scaffolds for defense.
Beyond structural scaffolds, amino acid composition of AMPs is clearly important. Boman classified AMPs into α-helical, β-sheet, and rich in amino acids such as prolines and glycines [12]. The amino acid composition of Gly-rich peptides clearly differs from those peptides with helical conformations. Our analysis of amphibian peptides, which usually form helical conformations, revealed that alanines, glycines, leucines, and lysines are four frequently occurring amino acids (>10%). This was obtained in 2009 from an analysis of 398 frog AMPs [11]. Consistently, we found the same four frequently occurring amino acids although the number of frog AMPs has reached the current 927 in the APD. Thus, these four amino acids are critical to determine the helical conformation of the peptides [52]. Interestingly, our database-guided ab initio peptide design using only glycines, leucines, lysines, and serines led to a peptide that primarily killed Gram-positive bacteria such as MRSA [98], whereas the Gly-rich peptides from spiders only inhibited the growth of Gram-negative bacteria [28]. In helical AMPs, glycines appear to play an important role in modulating peptide selectivity. Tossi and colleagues found that the placement of a flexible glycine at position 7 of helical peptides improved selectivity (i.e., less hemolytic) [104]. Furthermore, a computer-designed selective AMP also contained several glycines [105]. Based on the abundant amino acids in the APD, Gellman and colleagues applied the flexible glycine idea to reducing hemolytic ability of peptide mimics [106]. In the case of β-sheet peptides, they almost always contain disulfide bonds (varying from 1 to 8 in the APD). Our amino acid composition analysis of the 298 defensins in the APD revealed that cysteines, glycines, and arginines are frequently occurring amino acids, which differ from those found in helical AMPs. All the three residues are known to be essential for structure and activity of defensins [52]. The typical pattern of cysteines in such defensin-like AMPs has been used to guide the identification of additional defensin genes in the human genome [107]. Yeaman and colleagues advanced this by discovering the γ-core motif in nearly all disulfide-bonded β-sheet structures [33]. They could link this motif to antimicrobial activity. Such a link guided the authors to test antimicrobial activity of existing γ-core motif-containing proteins such as charybdotoxin originally not identified as AMPs [33]. All these results indicate the importance of amino acid composition in determining structure and activity of AMPs (for a systematic summary, please refer to ref. [103]).
The identification of novel AMPs opens the door to potential applications. Spider lycotoxin 1 was found to be highly potent against MRSA [61]. Meucin-24 and meucin-25 showed a narrow-spectrum activity against P. falciparum, making them interesting templates for developing antimalarial peptides [60]. It is demonstrated that anti-HIV activity of scorpion peptides [90], as well as other AMPs, can be improved [91,92]. Gomesin is a wide-spectrum peptide. Its small size and folded β-sheet structure makes it attractive as a template for engineering antifungal, anticancer or antimalarial peptides. The two disulfide bonds of gomesin are essential for antibacterial activity, hemolysis and serum stability [108,109]. To reduce peptide cytotoxicity, linear forms of gomesin have been designed. [D-Thr(2,6,11,15), Pro(9)]-Gm is the best cysteine-free analog of gomesin with a higher therapeutic index and good activity against C. albicans [110]. The systemic use of the linearized peptide, however, may be limited due to reduced serum stability [111]. As a different strategy, the peptide can be made more stable by forming a peptide bond between the N and C-termini of gomesin. This peptide may become useful for systemic treatment of cancer or malaria [112].
To avoid the potential problems with direct use of AMPs, therapeutic peptides may also be expressed at a needed site. Lazarez et al. have demonstrated that expression of spider peptides could prevent Chlamydia infection [46]. In the HEK293 cell line model, expression of cytoinsectotoxin 1a (induced by 0.02 µg/ml doxycycline) was found to be most effective (85% inhibition). Since spider AMPs also have insecticidal activity, Hughes et al. designed a method to utilize this effect [113]. The peptide was expressed inside the yeast that was ingested by the insects. At day 1, those alanine mutants at positions 6–9 or 12–15 displayed armyworm killing, and the mutants at positions 7–10 achieved 80–100% insect-killing at day 2. Likewise, expression of the same peptide in tobacco made the plant more resistant to bacteria, as well as insects such as the corn earworm and cigarette beetle [114].
Finally, it could be a useful strategy to combine AMPs with commercial antibiotics. As examples, Garcia et al. found the best effect in the treatment of tuberculosis when spider peptide Css54 was used in combination with rifampicin [78]. Barbosa and colleagues observed a synergistic effect between gomesin and fluconazole in inhibiting fungal growth [71]. Because pathogen killing can be achieved at lower doses in the case of combination therapy, this practice would reduce potential cytotoxicity and production cost when AMPs are administered at high doses. Because of this, combination therapy is actively pursued (reviewed in refs. [115–117]). The synergistic effects observed for spider or scorpion AMPs laid a solid basis for us to develop such a treatment.
Acknowledgements
This study was supported by the grants from the Nebraska Research Initiative and NIAID/NIH R01AI081975 to GW. XW is a visiting professor sponsored by the China Scholarship Council.
Footnotes
Conflict of Interest
None declared.
References
- 1.Bouzid W, Verdenaud M, Klopp C, Ducancel F, Noirot C, Vétillard A. De Novo sequencing and transcriptome analysis for Tetramorium bicarinatum: a comprehensive venom gland transcriptome analysis from an ant species. BMC Genomics. 2014;15:987–1002. doi: 10.1186/1471-2164-15-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.König E, Zhou M, Wang L, Chen T, Bininda-Emonds OR, Shaw C. Antimicrobial peptides and alytesin are co-secreted from the venom of the Midwife toad, Alytes maurus (Alytidae, Anura): implications for the evolution of frog skin defensive secretions. Toxicon. 2012;6:967–981. doi: 10.1016/j.toxicon.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 3.Vlisidou I, Wood W. Drosophila blood cells and their role in immune responses. FEBS J. 2015;8:1368–1382. doi: 10.1111/febs.13235. [DOI] [PubMed] [Google Scholar]
- 4.Aili SR, Touchard A, Escoubas P, Padula MP, Orivel J, Dejean A, Nicholson GM. Diversity of peptide toxins from stinging ant venoms. Toxicon. 2014;92:166–78. doi: 10.1016/j.toxicon.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 5.Ortiz E, Gurrola GB, Schwartz EF, Possani LD. Scorpion venom components as potential candidates for drug development. Toxicon. 2015;93:125–35. doi: 10.1016/j.toxicon.2014.11.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao Z, Di Z, Wu Y, Li W. Overview of scorpion species from China and their toxins. Toxins. 2014;6:796–815. doi: 10.3390/toxins6030796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harrison PL, Abdel-Rahman MA, Miller K, Strong PN. Antimicrobial peptides from scorpion venoms. Toxicon. 2014;88:115–137. doi: 10.1016/j.toxicon.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Almaaytah A, Albalas Q. Scorpin venom peptides with no disulfide bridges: A review. Peptides. 2014;51:35–45. doi: 10.1016/j.peptides.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 9.Gao L, Zhang J, Feng W, Bao N, Song D, Zhu BC. Pharmacological characterisation of spider antimicrobial peptides Protein Pept. Lett. 2005;12:507–511. doi: 10.2174/0929866054395806. [DOI] [PubMed] [Google Scholar]
- 10.Wang Z, Wang G. APD: the antimicrobial peptide database. Nucleic Acids Res. 2004;32:D590–D592. doi: 10.1093/nar/gkh025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang G, Li X, Wang Z. APD2: the updated antimicrobial peptide database and its application in peptide design. Nucleic Acids Res. 2009;37:D933–D937. doi: 10.1093/nar/gkn823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boman HG. Antibacterial peptides: basic facts emerging concepts. J. Int. Med. 2003;254:197–215. doi: 10.1046/j.1365-2796.2003.01228.x. [DOI] [PubMed] [Google Scholar]
- 13.Yan L, Adams ME. Lycotoxins antimicrobial peptides from venom of the wolf spider Lycosa carolinensis . J. Biol. Chem. 1998;273:2059–2066. doi: 10.1074/jbc.273.4.2059. [DOI] [PubMed] [Google Scholar]
- 14.Kuhn-Nentwig L, Muller J, Schaller J, Walz A, Dathe M, Nentwig W. Cupiennin 1, a new family of highly basic antimicrobial peptides in the venom of the spider Cupiennius salei (Ctenidae) J. Biol Chem. 2002;13:11208–11216. doi: 10.1074/jbc.M111099200. [DOI] [PubMed] [Google Scholar]
- 15.Corzo G, Villegas E, Gómez-Lagunas F, Possani LD, Belokoneva OS, Nakajima T. Oxyopinins, large amphipathic peptides isolated from the venom of the wolf spider Oxyopes kitabensis with cytolytic properties positive insecticidal cooperativity with spider neurotoxins. J. Biol. Chem. 2002;26:23627–23637. doi: 10.1074/jbc.M200511200. [DOI] [PubMed] [Google Scholar]
- 16.Dubovskii PV, Vassilevski AA, Samsonova OV, Egorova NS, Kozlov SA, Feofanov AV, Arseniev AS, Grishin EV. Novel lynx spider toxin shares common molecular architecture with defense peptides from frog skin. J. FEBS. 2011;278:4382–4393. doi: 10.1111/j.1742-4658.2011.08361.x. [DOI] [PubMed] [Google Scholar]
- 17.Abraham P, George S, Kumar KS. Novel antibacterial peptides from the skin secretion of the Indian bicoloured frog Clinotarsus curtipes . Biochimie. 2014;97:144–51. doi: 10.1016/j.biochi.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Budnik BA, Olsen JV, Egorov TA, Anisimova VE, Galkina TG, Musolyamov AK, Grishin EV, Zubarev RA. De novo sequencing of antimicrobial peptides isolated from the venom glands of the wolf spider Lycosa singoriensis . J. Mass Spectrom. 2004;39:193–201. doi: 10.1002/jms.577. [DOI] [PubMed] [Google Scholar]
- 19.Kozlov SA, Vassilevski AA, Feofanov AV, Surovoy AY, Karpunin DV, Grishin EV. Latarcins, antimicrobial and cytolytic peptides from the venom of the spider Lachesana tarabaevi (Zodariidae) that exemplify biomolecular diversity. J. Biol. Chem. 2006;30:20983–20992. doi: 10.1074/jbc.M602168200. [DOI] [PubMed] [Google Scholar]
- 20.Liu Z, Deng M, Xiang J, Ma H, Hu W, Zhao Y, Li DW, Liang S. A novel spider peptide toxin suppresses tumor growth through dual signaling pathways. J. Curr Mol Med. 2012;12:1350–1360. doi: 10.2174/156652412803833643. [DOI] [PubMed] [Google Scholar]
- 21.Riciluca KC, Sayegh RS, Melo RL, Silva PI., Jr Rondonin an antifungal peptide from spider (Acanthoscurria rondoniae) haemolymph. Results Immunol. 2012;2:66–71. doi: 10.1016/j.rinim.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silva PI, Jr, Daffre S, Bulet P. Isolation and characterization of Gomesin an 18-residue cysteine-rich defense peptide from the spider Acanthoscurria gomesiana hemocytes with sequence similarities to horseshoe crab antimicrobial peptides of the Tachyplesin family. J. Biol. Chem. 2000;43:33464–33470. doi: 10.1074/jbc.M001491200. [DOI] [PubMed] [Google Scholar]
- 23.Choi SJ, Parent R, Guillaume C, Deregnaucourt C, Delarbre C, Ojcius DM, Montagne JJ, Célérier ML, Phelipot A, Amiche M, Molgo J, Camadro JM, Guette C. Isolation and characterization of Psalmopeotoxin I and II: two novel antimalarial peptides from the venom of the tarantula Psalmopoeus cambridgei . FEBS Lett. 2004;572:109–117. doi: 10.1016/j.febslet.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 24.Zhao H, Kong Y, Wang H, Yan T, Feng F, Bian J, Yang Y, Yu H. A defensin-like antimicrobial peptide from the venoms of spider Ornithoctonus hainana . J. Peptide. Sci. 2011;7:540–544. doi: 10.1002/psc.1370. [DOI] [PubMed] [Google Scholar]
- 25.Ayroza G, Ferreira IL, Sayegh RS, Tashima AK, da Silva Junior PI. Juruin: an antifungal peptide from the venom of the amazonian pink toe spider, Avicularia juruensis, which contains the inhibitory cysteine knot motif. Frontiers Microbiol. 2012;3:1–10. doi: 10.3389/fmicb.2012.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuzmenkov AI, Fedorova IM, Vassilevski AA, Grishin EV. Cysteine-rich toxins from Lachesana tarabaevi spider venom with amphiphilic C-terminal segments. Biochim. Biophys. Acta. 2013;1828:724–31. doi: 10.1016/j.bbamem.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 27.Vassilevski AA, Sachkova MY, Ignatova AA, Kozlov SA, Feofanov AV, Grishin EV. Spider toxins comprising disulfide-rich and linear amphipathic domains: a new class of molecules identified in the lynx spider Oxyopes takobius . J. FEBS. 2013;280:6247–6261. doi: 10.1111/febs.12547. [DOI] [PubMed] [Google Scholar]
- 28.Lorenzini DM, da Silva PI, Jr, Fogaça AC, Bulet P, Daffre S. Acanthoscurrin: a novel glycine-rich antimicrobial peptide constitutively expressed in the hemocytes of the spider Acanthoscurria gomesiana . Dev. Comp. Immunol. 2003;27:781–791. doi: 10.1016/s0145-305x(03)00058-2. [DOI] [PubMed] [Google Scholar]
- 29.Baumann T, Kämpfer U, Schürch S, Schaller J, Largiadèr C, Nentwig W, Kuhn-Nentwig L. Ctenidins: antimicrobial glycine-rich peptides from the hemocytes of the spider Cupiennius salei . Cell. Mol. Life Sci. 2010;67:2787–2798. doi: 10.1007/s00018-010-0364-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cociancich S, Goyffon M, Bontems F, Bulet P, Bouet F, Menez A, Hoffmann J. Purification and characterization of a scorpion defensin, a 4kDa antibacterial peptide presenting structural similarities with insect defensins and scorpion toxins. Biochem. Biophys. Res. Commun. 1993;194:17–22. doi: 10.1006/bbrc.1993.1778. [DOI] [PubMed] [Google Scholar]
- 31.Ehret-Sabatier L, Loew D, Goyffon M, Fehlbaum P, Hoffmann JA, van Dorsselaer A, Bulet P. Characterization of novel cysteine-rich antimicrobial peptides from scorpion blood. J. Biol Chem. 1996;47:29537–29544. doi: 10.1074/jbc.271.47.29537. [DOI] [PubMed] [Google Scholar]
- 32.Uawonggul N, Thammasirirak S, Chaveerach A, Arkaravichien T, Bunyatratchata W, Ruangjirachuporn W, Jearranaiprepame P, Nakamura T, Matsuda M, Kobayashi M, Hattori S, Daduang S. Purification and characterization of Heteroscorpine-1 (HS-1) toxin from Heterometrus laoticus scorpion venom. Toxicon. 2007;49:19–29. doi: 10.1016/j.toxicon.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 33.Yount NY, Yeaman MR. Multidimensional signatures in antimicrobial peptides. Proc. Natl Acad. Sci. 2004;101:7363–8. doi: 10.1073/pnas.0401567101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu S, Tytgat J. The scorpine family of defensins: gene structure, alternative polyadenylation and fold recognition. Cell. Mol. Life Sci. 2004;61:1751–1763. doi: 10.1007/s00018-004-4149-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shao JH, Wang YQ, Wu XY, Jiang R, Zhang R, Wu CF, Zhang JH. Cloning, expression, and pharmacological activity of BmK AS, an active peptide from scorpion Buthus martensii Karsch. Biotechnol. Lett. 2008;30:23–29. doi: 10.1007/s10529-007-9499-y. [DOI] [PubMed] [Google Scholar]
- 36.Díaz P, D'Suze G, Salazar V, Sevcik C, Shannon JD, Sherman NE, Fox JW. Antibacterial activity of six novel peptides from Tityus discrepans scorpion venom A fluorescent probe study of microbial membrane Na+ permeability changes. Toxicon. 2009;54:802–817. doi: 10.1016/j.toxicon.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 37.Torres-Larios A, Gurrola GB, Zamudio FZ, Possani LD. Hadrurin, a new antimicrobial peptide from the venom of the scorpion Hadrurus aztecus . Eur. J. Biochem. 2000;267:5023–5031. doi: 10.1046/j.1432-1327.2000.01556.x. [DOI] [PubMed] [Google Scholar]
- 38.Corzo G, Escoubas P, Villegas E, Barnham KJ, He W, Norton RS, Nakajima T. Characterization of unique amphipathic antimicrobial peptides from venom of the scorpion Pandinus imperator . J. Biochemical. 2001;359:35–45. doi: 10.1042/0264-6021:3590035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dai L, Yasuda A, Naoki H, Corzo G, Andriantsiferana M, Nakajima T. IsCT, a novel cytotoxic linear peptide from scorpion Opisthacanthus madagascariensis . Biochem. Biophys. Res. Commun. 2001;286:820–825. doi: 10.1006/bbrc.2001.5472. [DOI] [PubMed] [Google Scholar]
- 40.Dai L, Corzo G, Naoki H, Andriantsiferana M, Nakajima T. Purification, structure-function analysis, and molecular characterization of novel linear peptides from scorpion Opisthacanthus madagascariensis . Biochem. Biophys. Res. Commun. 2002;293:1514–1522. doi: 10.1016/S0006-291X(02)00423-0. [DOI] [PubMed] [Google Scholar]
- 41.Zeng XC, Zhou L, Shi W, Luo X, Zhang L, Nie Y, Wang J, Wu S, Cao B, Cao H. Three new antimicrobial peptides from the scorpion Pandinus imperator . Peptides. 2013;45:28–34. doi: 10.1016/j.peptides.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 42.Zeng XC, Wang SX, Zhu Y, Zhu SY, Li WX. Identification and functional characterization of novel scorpion venom peptides with no disulfide bridge from Buthus martensii Karsch. Peptides. 2004;25:143–150. doi: 10.1016/j.peptides.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 43.Du Q, Hou X, Wang L, Zhang Y, Xi X, Wang H, Zhou M, Duan J, Wei M, Chen T, Shaw C. AaeAP1 and AaeAP2: novel antimicrobial peptides from the venom of the scorpion, Androctonus aeneas: structural characterisation, molecular cloning of biosynthetic precursor-encoding cDNAs and engineering of analogues with enhanced antimicrobial and anticancer activities. Toxins. 2015;7:219–237. doi: 10.3390/toxins7020219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dueñas-Cuellar RA, Kushmerick C, Naves LA, Batista IF, Guerrero-Vargas JA, Pires OR, Jr, Fontes W, Castro MS. Cm38: a new antimicrobial peptide active against Klebsiella pneumonia is homologous to Cn11. Protein Pept. Lett. 2015;22:164–172. doi: 10.2174/092986652202150128143048. [DOI] [PubMed] [Google Scholar]
- 45.De Melo ET, Estrela AB, Santos EC, Machado PR, Farias KJ, Torres TM, Carvalho E, Lima JP, Silva-Júnior AA, Barbosa EG, Fernandes-Pedrosa M, de F. Structural characterization of a novel peptide with antimicrobial activity from the venom gland of the scorpion Tityus stigmurus: Stigmurin. Peptides. 2015;68:3–10. doi: 10.1016/j.peptides.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 46.Wang G. Improved methods for classification, prediction and design of antimicrobial peptides. Methods Mol. Biol. 2015;1268:43–66. doi: 10.1007/978-1-4939-2285-7_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zeng XC, Corzo G, Hahin R. Scorpion venom peptides without disulfide bridges. IUBMB Life. 2005;57:13–21. doi: 10.1080/15216540500058899. [DOI] [PubMed] [Google Scholar]
- 48.Wüthrich K. NMR of Proteins and Nucleic Acids. New York: Wiley and Sons; 1986. [Google Scholar]
- 49.Wang G, Keifer PA, Peterkofsky A. Short-chain diacyl phosphatidylglycerols: which one to choose for NMR structural determination of a membrane-associated peptide from Escherichia coli? Spectroscopy. 2004;18:257–264. [Google Scholar]
- 50.Wang G. Determination of solution structure and lipid micelle location of an engineered membrane peptide by using one NMR experiment and one sample. Biochim. Biophys. Acta. 2007;1768:3271–3281. doi: 10.1016/j.bbamem.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 51.Wang G. NMR studies of a model antimicrobial peptide in the micelles of SDS, dodecylphosphocholine, or dioctanoyl phosphatidylglycerol. Open Magn. Reson. J. 2008;1:9–15. [Google Scholar]
- 52.Wang G, editor. Antimicrobial peptide: discovery, design and novel therapeutic strategies. England: CABI; 2010. [Google Scholar]
- 53.Dubovskii PV, Volynsky PE, Polyansky AA, Karpunin DV, Chupin VV, Efremov RG, Arseniev AS. Three-dimensional structure/hydrophobicity of latarcins specifies their mode of membrane activity. Biochemistry. 2008;47:3525–3533. doi: 10.1021/bi702203w. [DOI] [PubMed] [Google Scholar]
- 54.Pukala TL, Boland MP, Gehman JD, Kuhn-Nentwig L, Separovic F, Bowie JH. Solution structure and interaction of cupiennin 1a, a spider venom peptide, with phospholipid bilayers. Biochemistry. 2007;46:3576–85. doi: 10.1021/bi062306+. [DOI] [PubMed] [Google Scholar]
- 55.Santos DM, Verly RM, Piló-Veloso D, de Maria M, de Carvalho MA, Soares BM, Diniz CG, Farias LM, Moreira DF, Frézard F, Bemquerer MP, Pimenta AM, de Lima ME. LyeTx I, a potent antimicrobial peptide from the venom of the spider Lycosa erythrognatha . Amino Acids. 2010;39:135–44. doi: 10.1007/s00726-009-0385-x. [DOI] [PubMed] [Google Scholar]
- 56.Mandard N, Bulet P, Caille A, Daffre S, Vovelle F. The solution structure of gomesin, an antimicrobial cysteine-rich peptide from the spider. Eur J. Biochem. 2002;269:1190–1198. doi: 10.1046/j.0014-2956.2002.02760.x. [DOI] [PubMed] [Google Scholar]
- 57.Pimentel C, Choi SJ, Chagot B, Guette C, Camadro JM, Darbon H. Solution structure of PcFK1, a spider peptide active against Plasmodium falciparum . Protein Sci. 2006;15:628–34. doi: 10.1110/ps.051860606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mandard N, Sy D, Maufrais C, Bonmatin JM, Bulet P, Hetru C, Vovelle F. Androctonin, a novel antimicrobial peptide from scorpion Androctonus australis: solution structure and molecular dynamics simulations in the presence of a lipid monolayer. J. Biomol. Struct. Dyn. 1999;17:367–80. doi: 10.1080/07391102.1999.10508368. [DOI] [PubMed] [Google Scholar]
- 59.Lee K, Shin SY, Kim K, Lim SS, Hahm KS, Kim Y. Antibiotic activity and structural analysis of the scorpion-derived antimicrobial peptide IsCT and its analogs. Biochem. Biophys. Res. Commun. 2004;323:712–9. doi: 10.1016/j.bbrc.2004.08.144. [DOI] [PubMed] [Google Scholar]
- 60.Gao B, Xu J, Rodriguez Mdel C, Lanz-Mendoza H, Hernández-Rivas R, Du W, Zhu S. Characterization of two linear cationic antimalarial peptides in the scorpion Mesobuthus eupeus . Biochimie. 2010;92:350–359. doi: 10.1016/j.biochi.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 61.Menousek J, Mishra B, Hanke ML, Heim CE, Kielian T, Wang G. Database screening and in vivo efficacy of antimicrobial peptides against methicillin-resistant Staphylococcus aureus USA300. Int. J. Antimicrob. Agents. 2012;39:402–406. doi: 10.1016/j.ijantimicag.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kuhn-Nentwig L, Willems J, Seebeck T, Shalaby T, Kaiser M, Nentwig W. Cupiennin 1a exhibits a remarkably broad, non-stereospecific cytolytic activity on bacteria, protozoan parasites, insects, and human cancer cells. Amino Acids. 2011;40:69–76. doi: 10.1007/s00726-009-0471-0. [DOI] [PubMed] [Google Scholar]
- 63.Wang G, Li Y, Li X. Correlation of three-dimensional structures with the antibacterial activity of a group of peptides designed based on a non-toxic bacterial membrane anchor. J. Biol. Chem. 2005;280:5803–5811. doi: 10.1074/jbc.M410116200. [DOI] [PubMed] [Google Scholar]
- 64.Wang G. Structures of human host defense cathelicidin LL-37 and its smallest antimicrobial peptide KR-12 in lipid micelles. J. Biol. Chem. 2008;283:32637–32643. doi: 10.1074/jbc.M805533200. [DOI] [PubMed] [Google Scholar]
- 65.Abbassi F, Lequin O, Piesse C, Goasdoué N, Foulon T, Nicolas P, Ladram A. Temporin-SHf, a new type of phe-rich and hydrophobic ultrashort antimicrobial peptide. J Biol Chem. 2010;285:16880–16892. doi: 10.1074/jbc.M109.097204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dennison SR, Harris F, Phoenix DA. A study on the importance of phenylalanine for aurein functionality. Protein Pept. Lett. 2009;16:1455–1458. doi: 10.2174/092986609789839340. [DOI] [PubMed] [Google Scholar]
- 67.Kuhn-Nentwig L, Sheynis T, Kolusheva S, Nentwig W, Jelinek R. N-terminal aromatic residues closely impact the cytolytic activity of cupiennin 1a, a major spider venom peptide. Toxicon. 2013;75:177–186. doi: 10.1016/j.toxicon.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 68.Vassilevski AA, Kozlov SA, Samsonova OV, Egorova NS, Karpunin DV, Pluzhnikov KA, Feofanov AV, Grishin EV. Cyto-insectotoxins a novel class of cytolytic and insecticidal peptides from spider venom. J. Biol. Chem. 2008;411:687–696. doi: 10.1042/bj20071123. [DOI] [PubMed] [Google Scholar]
- 69.Tan H, Ding X, Meng S, Liu C, Wang H, Xia L, Liu Z, Liang S. Antimicrobial potential of lycosin-I, a cationic and amphiphilic peptide from the venom of the spider Lycosa singorensis . Curr. Mol. Med. 2013;13:900–910. doi: 10.2174/15665240113139990045. [DOI] [PubMed] [Google Scholar]
- 70.Rodrigues EG, Dobroff AS, Cavarsan CF, Paschoalin T, Nimrichter L, Mortara RA, Santos EL, Fázio MA, Miranda A, Daffre S, Travassos LR. Effective topical treatment of subcutaneous murine B16F10-Nex2 melanoma by the antimicrobial peptide gomesin. Neoplasia. 2008;10:61–68. doi: 10.1593/neo.07885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nimrichter L, Rodrigues ML. Gomesin, a peptide produced by the spider Acanthoscurria gomesiana, is a potent anticryptococcal agent that acts in synergism with fluconazole. FEMS Microbiol. Lett. 2007;274:279–286. doi: 10.1111/j.1574-6968.2007.00850.x. [DOI] [PubMed] [Google Scholar]
- 72.Rothan HA, Bahrani H, Rahman NA, Yusof R. Identification of natural antimicrobial agents to treat dengue infection: In vitro analysis of latarcin peptide activity against dengue virus. BMC Microbiol. 2014;14:1–10. doi: 10.1186/1471-2180-14-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Soletti RC, del Barrio L, Daffre S, Miranda A, Borges HL, Moura-Neto V, Lopez MG, Gabilan NH. Peptide gomesin triggers cell death through L-type channel calcium influx, MAPK/ERK, PKC and PI3K signaling and generation of reactive oxygen species. Chem. Biol. Interact. 2010;186:135–143. doi: 10.1016/j.cbi.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 74.Moerman L, Bosteels S, Noppe W, Willems J, Clynen E, Schoofs L, Thevissen K, Tytgat J, Van Eldere J, Van Der Walt J, Verdonck F. Antibacterial and antifungal properties of α-helical, cationic peptides in the venom of scorpions from southern Africa. Eur. J. Biol. Chem. 2002;269:4799–4810. doi: 10.1046/j.1432-1033.2002.03177.x. [DOI] [PubMed] [Google Scholar]
- 75.Gao B, Sherman P, Luo L, Bowie J, Zhu S. Structural and functional characterization of two genetically related meucin peptides highlights evolutionary divergence and convergence in antimicrobial peptides. FASEB J. 2009;23:1230–45. doi: 10.1096/fj.08-122317. [DOI] [PubMed] [Google Scholar]
- 76.Arpornsuwan T, Buasakul B, Jaresitthikunchai J, Roytrakul S. Potent and rapid antigonococcal activity of the venom peptide BmKn2 and its derivatives against different Maldi biotype of multidrug-resistant Neisseria gonorrhoeae . Peptides. 2014;53:315–320. doi: 10.1016/j.peptides.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 77.Cao L, Li Z, Zhang R, Wu Y, Li W, Cao Z. StCT2, a new antibacterial peptide characterized from the venom of the scorpion Scorpiops tibetanus . Peptides. 2012;36:213–220. doi: 10.1016/j.peptides.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 78.Garcia F, Villegas E, Espino-Solis GP, Rodriguez A, Paniagua-Solis JF, Sandoval-Lopez G, Possani LD, Corzo G. Antimicrobial peptides from arachnid venoms and their microbicidal activity in the presence of commercial antibiotics. J. Antibiot. (Tokyo) 2013;66:3–10. doi: 10.1038/ja.2012.87. [DOI] [PubMed] [Google Scholar]
- 79.Ramírez-Carreto S, Quintero-Hernández V, Jiménez-Vargas M, Corzo G, Possani LD, Becerril B, Ortiz E. Gene cloning and functional characterization of four novel antimicrobial-like peptides from scorpions of the family Vaejovidae . Peptides. 2012;34:290–295. doi: 10.1016/j.peptides.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 80.Li Z, Xu X, Meng L, Zhang Q, Cao L, Li W, Wu Y, Cao Z. Hp1404, a new antimicrobial peptide from the scorpion Heterometrus petersii . PloS One. 2014;9:e97539. doi: 10.1371/journal.pone.0097539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hernández-Aponte CA, Silva-Sanchez J, Quintero-Hernández V, Rodríguez-Romero A, Balderas C, Possani LD, Gurrola GB. Vejovine, a new antibiotic from the scorpion venom of Vaejovis mexicanus . Toxicon. 2011;57:84–92. doi: 10.1016/j.toxicon.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 82.Conde R, Zamudio FZ, Rodríguez MH, Possani LD. Scorpine, an anti-malaria and anti-bacterial agent purified from scorpion venom. FEBS Lett. 2000;471:165–168. doi: 10.1016/s0014-5793(00)01384-3. [DOI] [PubMed] [Google Scholar]
- 83.Bontems F, Gilquin B, Roumestand C, Ménez A, Toma F. Analysis of side-chain organization on a refined model of charybdotoxin: structural and functional implications. Biochemistry. 1992;31:7756–7764. doi: 10.1021/bi00149a003. [DOI] [PubMed] [Google Scholar]
- 84.Miller C, Moczydlowski E, Latorre R, Phillips M. Charybdotoxin, a protein inhibitor of single Ca2+ activated K+ channels from mammalian skeletal muscle. Nature. 1985;313:316–318. doi: 10.1038/313316a0. [DOI] [PubMed] [Google Scholar]
- 85.Fan Z, Cao L, He Y, Hu J, Di Z, Wu Y, Li W, Cao Z. Ctriporin, a new anti-methicillin-resistant Staphylococcus aureus peptide from the venom of the scorpion Chaerilus tricostatus . Antimicrob. Agents Chemother. 2011;55:5220–5229. doi: 10.1128/AAC.00369-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hong W, Li T, Song Y, Zhang R, Zeng Z, Han S, Zhang X, Wu Y, Li W, Cao Z. Inhibitory activity and mechanism of two scorpion venom peptides against herpes simplex virus type 1. Antiviral Res. 2014;102:1–10. doi: 10.1016/j.antiviral.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dai C, Ma Y, Zhao Z, Zhao R, Wang Q, Wu Y, Cao Z, Li W. Mucroporin, the first cationic host defense peptide from the venom of Lychas mucronatus . Antimicrob. Agents Chemother. 2008;52:3967–3972. doi: 10.1128/AAC.00542-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li Q, Zhao Z, Zhou D, Chen Y, Hong W, Cao L, Yang J, Zhang Y, Shi W, Cao Z, Wu Y, Yan H, Li W. Virucidal activity of a scorpion venom peptide variant mucroporin-M1 against measles, SARS-CoV and influenza H5N1 viruses. Peptides. 2011;32:1518–1525. doi: 10.1016/j.peptides.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhao Z, Hong W, Zeng Z, Wu Y, Hu K, Tian X, Li W, Cao Z. Mucroporin-M1 inhibits Hepatitis B virus replication by activating the mitogen-activated protein kinase (MAPK) pathway and down-regulating HNF4α in Vitro and in Vivo . J. Biol. Chem. 2012;36:30181–30190. doi: 10.1074/jbc.M112.370312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen Y, Cao L, Zhong M, Zhang Y, Han C, Li Q, Yang J, Zhou D, Shi W, He B, Liu F, Yu J, Sun Y, Cao Y, Li Y, Li W, Guo D, Cao Z, Yan H. Anti-HIV-1 activity of a new scorpion venom peptide derivative Kn2–7. PLoS One. 2012;7:e34947. doi: 10.1371/journal.pone.0034947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang G, Watson KM, Peterkofsky A, Buckheit RW., Jr Identification of novel human immunodeficiency virus type 1-inhibitory peptides based on the antimicrobial peptide database. Antimicrob. Agents Chemother. 2010;54:1343–1346. doi: 10.1128/AAC.01448-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang G. Natural antimicrobial peptides as promising anti-HIV candidates. Curr. Topics Pept. Protein Res. 2012;13:93–110. [PMC free article] [PubMed] [Google Scholar]
- 93.Guo X, Ma C, Du Q, Wei R, Wang L, Zhou M, Chen T, Shaw C. Two peptides, TsAP-1 and TsAP-2, from the venom of the Brazilian yellow scorpion, Tityus serrulatus: Evaluation of their antimicrobial and anticancer activities. Biochimie. 2013;95:1784–1794. doi: 10.1016/j.biochi.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 94.Nie Y, Zeng XC, Yang Y, Luo F, Luo X, Wu S, Zhang L, Zhou J. A novel class of antimicrobial peptides from the scorpion Heterometrus spinifer . Peptides. 2012;38:389–394. doi: 10.1016/j.peptides.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 95.Zhao Z, Ma Y, Dai C, Zhao R, Li S, Wu Y, Cao Z, Li W. Imcroporin a new cationic antimicrobial peptide from the venom of the Scorpion Isometrus maculates . Antimicrob. Agents Chemother. 2009;53:3472–3477. doi: 10.1128/AAC.01436-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Almaaytah A, Zhou M, Wang L, Chen T, Walker B, Shaw C. Antimicrobial/cytolytic peptides from the venom of the North African scorpion, Androctonus amoreuxi: Biochemical and functional characterization of natural peptides and a single site-substituted analog. Peptides. 2012;35:291–299. doi: 10.1016/j.peptides.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 97.Argiolas A, Pisano JJ. Isolation and characterization of two new peptides, mastoparan C and crabrolin, from the venom of the European hornet, Vespu crubro . J. Biol. Chem. 1984;259:10106–10111. [PubMed] [Google Scholar]
- 98.Mishra B, Wang G. Ab Initio design of potent anti-MRSA peptides based on database filtering technology. J. Am. Chem. Soc. 2012;134:12426–12429. doi: 10.1021/ja305644e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Polyansky AA, Vassilevski AA, Volynsky PE, Vorontsova OV, Samsonova OV, Egorova NS, Krylov NA, Feofanov AV, Arseniev AS, Grishin EV, Efremov RG. N-terminal amphipathic helix as a trigger of hemolytic activity in antimicrobial peptides: A case study in latarcins. FEBS Lett. 2009;583:2425–2428. doi: 10.1016/j.febslet.2009.06.044. [DOI] [PubMed] [Google Scholar]
- 100.Wang G, Mishra B, Epand RF, Epand RM. High-quality 3D structures shine light on antibacterial, anti-biofilm and antiviral activities of human cathelicidin LL-37 and its fragments. Biochim. Biophys. Acta. 2014;1838:2160–2172. doi: 10.1016/j.bbamem.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Skerlavaj B, Gennaro R, Bagella L, Merluzzi L, Risso A, Zanetti M. Biological characterization of two novel cathelicidin-derived peptides and identification of structural requirements for their antimicrobial and cell lytic activities. J. Biol. Chem. 1996;271:28375–28381. doi: 10.1074/jbc.271.45.28375. [DOI] [PubMed] [Google Scholar]
- 102.Li X, Li Y, Han H, Miller DW, Wang G. Solution structures of human LL-37 fragments and NMR-based identification of a minimal membrane-targeting antimicrobial and anticancer region. J. Am. Chem. Soc. 2006;128:5776–5785. doi: 10.1021/ja0584875. [DOI] [PubMed] [Google Scholar]
- 103.Mishra B, Wang G. The importance of amino acid composition in natural AMPs: an evolutional, structural, and functional perspective. Front. Immunol. 2012;3:221. doi: 10.3389/fimmu.2012.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zelezetsky I, Pag U, Sahl HG, Tossi A. Tuning the biological properties of amphipathic alpha-helical antimicrobial peptides: rational use of minimal amino acid substitutions. Peptides. 2005;26:2368–2376. doi: 10.1016/j.peptides.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 105.Juretić D, Vukicević D, Ilić N, Antcheva N, Tossi A. Computational design of highly selective antimicrobial peptides. J. Chem. Inf. Model. 2009;49:2873–2882. doi: 10.1021/ci900327a. [DOI] [PubMed] [Google Scholar]
- 106.Chakraborty S, Liu R, Hayouka Z, Chen X, Ehrhardt J, Lu Q, Burke E, Yang Y, Weisblum B, Wong GC, Masters KS, Gellman SH. Ternary nylon-3 copolymers as host-defense peptide mimics: beyond hydrophobic and cationic subunits. J. Am. Chem. Soc. 2014;136:14530–14535. doi: 10.1021/ja507576a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jia HP, Schutte BC, Schudy A, Linzmeier R, Guthmiller JM, Johnson GK, Tack BF, Mitros JP, Rosenthal A, Ganz T, McCray PB., Jr Discovery of new human beta-defensins using a genomics-based approach. Gene. 2001;263:211–218. doi: 10.1016/s0378-1119(00)00569-2. [DOI] [PubMed] [Google Scholar]
- 108.Fázio MA, Oliveira VX, Jr, Bulet P, Miranda MT, Daffre S, Miranda A. Structure-activity relationship studies of gomesin: importance of the disulfide bridges for conformation, bioactivities, and serum stability. Biopolymers. 2006;84:205–218. doi: 10.1002/bip.20396. [DOI] [PubMed] [Google Scholar]
- 109.Moraes LG, Fázio MA, Vieira RF, Nakaie CR, Miranda MT, Schreier S, Daffre S, Miranda A. Conformational and functional studies of gomesin analogues by CD, EPR and fluorescence spectroscopies. Biochim. Biophys. Acta. 2007;1768:52–58. doi: 10.1016/j.bbamem.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 110.Chan LY, Zhang VM, Huang YH, Waters NC, Bansal PS, Craik DJ, Daly NL. Cyclization of the antimicrobial peptide gomesin with native chemical ligation: influences on stability and bioactivity. Chembiochem. 2013;14:617–624. doi: 10.1002/cbic.201300034. [DOI] [PubMed] [Google Scholar]
- 111.Fázio MA, Jouvensa L, Vovelle F, Bulet P, Miranda MT, Daffre S, Miranda A. Biological and structural characterization of new linear gomesin analogues with improved therapeutic indices. Biopolymers. 2007;88:386–400. doi: 10.1002/bip.20660. [DOI] [PubMed] [Google Scholar]
- 112.Machado A, Fázio MA, Miranda A, Daffre S, Machini MT. Synthesis and properties of cyclic gomesin and analogues. J. Pept. Sci. 2012;18:588–598. doi: 10.1002/psc.2439. [DOI] [PubMed] [Google Scholar]
- 113.Hughes SR, Dowd PF, Hector RE, Panavas T, Sterner DE, Qureshi N, Bischoff KM, Bang SS, Mertens JA, Johnson ET, Li XL, Jackson JS, Caughey RJ, Riedmuller SB, Bartolett S, Liu S, Rich JO, Farrelly PJ, Butt TR, Labaer J, Cotta MA. Lycotoxin-1 insecticidal peptide optimized by amino acid scanning mutagenesis and expressed as a coproduct in an ethanologenic Saccharomyces cerevisiae strain. J. Pept. Sci. 2008;14:1039–1050. doi: 10.1002/psc.1040. [DOI] [PubMed] [Google Scholar]
- 114.Johnson ET, Dowd PF, Hughes SR. Expression of a wolf spider toxin in tobacco inhibits the growth of microbes and insects. Biotechnol. Lett. 2014;36:1735–1742. doi: 10.1007/s10529-014-1536-z. [DOI] [PubMed] [Google Scholar]
- 115.Wang G, Mishra B, Lau K, Lushnikova T, Golla R, Wang X. Antimicrobial peptides in 2014. Pharmaceuticals (Basel) 2015;8:123–150. doi: 10.3390/ph8010123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hancock RE, Scott MG. The role of antimicrobial peptides in animal defenses. Proc. Natl. Acad. Sci. U.S.A. 2000;97:8856–8861. doi: 10.1073/pnas.97.16.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zusman O, Avni T, Leibovici L, Adler A, Friberg L, Stergiopoulou T, Carmeli Y, Paul M. Systematic review and meta-analysis of in vitro synergy of polymyxins and carbapenems. Antimicrob Agents Chemother. 2013;57:5104–5111. doi: 10.1128/AAC.01230-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yuan W, Cao L, Ma Y, Mao P, Wang W, Zhao R, Wu Y, Cao Z, Li W. Cloning and functional characterization of a new antimicrobial peptide gene StCT1 from the venom of the scorpion Scorpiops tibetanus . Peptides. 2010;31:22–26. doi: 10.1016/j.peptides.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 119.Zeng XC, Wang S, Nie Y, Zhang L, Luo X. Characterization of BmKbpp, a multifunctional peptide from the Chinese scorpion Mesobuthus martensii Karsch: Gaining insight into a new mechanism for the functional diversification of scorpion venom peptides. Peptides. 2012;33:44–51. doi: 10.1016/j.peptides.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 120.Luna-Ramírez K, Sani MA, Silva-Sanchez J, Jiménez-Vargas JM, Reyna-Flores F, Winkel KD, Wright CE, Possani LD, Separovic F. Membrane interactions and biological activity of antimicrobial peptides from Australian scorpion. Biochim. Biophys. Acta. 2014;1838:2140–2148. doi: 10.1016/j.bbamem.2013.10.022. [DOI] [PubMed] [Google Scholar]
- 121.Luna-Ramírez K, Quintero-Hernández V, Vargas-Jaimes L, Batista CV, Winkel KD, Possani LD. Characterization of the venom from the Australian scorpion Urodacus yaschenkoi: Molecular mass analysis of components, cDNA sequences and peptides with antimicrobial activity. Toxicon. 2013;63:44–54. doi: 10.1016/j.toxicon.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 122.Wu S, Nie Y, Zeng XC, Cao H, Zhang L, Zhou L, Yang Y, Luo X, Liu Y. Genomic and functional characterization of three new venom peptides from the scorpion Heterometrus spinifer . Peptides. 2014;53:30–41. doi: 10.1016/j.peptides.2013.12.012. [DOI] [PubMed] [Google Scholar]