Abstract
Background
It is not clear whether the pattern of kidney function decline in patients with chronic kidney disease (CKD) may relate to outcomes after reaching end-stage renal disease (ESRD). We hypothesize that an abrupt decline in kidney function prior to ESRD predicts early death after initiating maintenance hemodialysis.
Study Design
Prospective cohort study
Setting & Participants
The Chronic Renal Insufficiency Cohort (CRIC) Study enrolled men and women with mild to moderate CKD. For this study, we studied 661 subjects who developed chronic kidney failure that required hemodialysis initiation.
Predictors
The primary predictor was the presence of abrupt decline in kidney function prior to ESRD. We incorporated annual estimated glomerular filtration rates (eGFRs) into a mixed effects model to estimate subject-specific eGFR at three months prior to initiation of hemodialysis. Abrupt decline was defined as having an extrapolated eGFR ≥30 ml/min/1.73 m2 at that time point.
Outcomes
All-cause mortality within one year after initiating hemodialysis.
Measurements
Multivariable Cox proportional hazards.
Results
Among 661 CKD patients initiating hemodialysis, 56 (8.4%) had abrupt pre-dialysis decline in kidney function, and 69 died within one year after initiating hemodialysis. After adjustment for demographics, cardiovascular disease, diabetes, and cancer, abrupt decline in kidney function was associated with a threefold higher risk of death within the first year of ESRD (adjusted HR, 3.09; 95% CI, 1.65-5.76).
Limitations
Relatively small number of outcomes; infrequent (yearly) eGFR determinations; lack of more granular clinical data.
Conclusions
Abrupt decline in kidney function prior to ESRD occurred in a significant minority of incident hemodialysis patients and predicted early death in ESRD.
Keywords: kidney function, disease trajectory, estimated glomerular filtration rate (eGFR), eGFR decline, hemodialysis, mortality, end-stage renal disease (ESRD), transition to ESRD, renal replacement therapy (RRT) initiation, Chronic Renal Insufficiency Cohort (CRIC)
Healthy People 2020 lists as one of its objectives to “reduce the number of deaths in dialysis patients within the first 3 months of initiation of renal replacement therapy,”1 underscoring a major but not well-understood problem of early mortality in ESRD.2-5 Most prior studies looking at early death among incident dialysis patients have focused primarily on factors observed after dialysis initiation, such as type of vascular access and characteristics of dialysis prescription such as dose and ultrafiltration.2, 4, 6 This is because studies of incident dialysis patients typically begin prospective data collection only after initiation of dialysis.2-7 On the flip side, studies of CKD have ended follow-up at the start of ESRD,8, 9 making it difficult to ascertain whether what transpired prior to dialysis initiation may be associated with outcomes afterwards.7, 10, 11 The potential importance of clinical course and management before dialysis initiation was highlighted in a recent study which demonstrated that strict blood pressure control during the time patients had non−dialysis-dependent CKD was associated with lower risk of mortality after dialysis initiation.12
We hypothesize that the manner by which patients with earlier stages of CKD transition to ESRD has implications for survival after dialysis initiation. Recent studies have described heterogeneous patterns of kidney function decline prior to ESRD, including a significant proportion of patients who had a rapid or “abrupt” decline in glomerular filtration rate (GFR) before beginning renal replacement therapy.13, 14 Those who transition abruptly may be less informed and prepared for renal replacement therapy, and may be more likely to rely on the use of dialysis catheters. If there was an intervening episode of severe (dialysis-requiring) acute kidney injury, these patients may also be more debilitated at initiation of dialysis, having recently survived a major illness. Patients with decompensated heart failure may develop increased venous congestion and decreased kidney perfusion (“cardio-renal syndrome”), leading to accelerated loss of kidney function, rapid transition to dialysis initiation, and early death. We therefore hypothesize that an abrupt pattern of kidney function decline may be a risk factor for a higher death rate observed among maintenance dialysis patients, especially in the first year on dialysis.
In this study, we use data from an ongoing observational study of CKD, the Chronic Renal Insufficiency Cohort (CRIC) study, to quantify the proportion of incident hemodialysis patients who had an abrupt decline in kidney function immediately prior to hemodialysis initiation, and to investigate whether this pattern of abrupt decline is associated with early death in ESRD.
METHODS
Study Population
The CRIC Study is an ongoing multicenter observational cohort which initially enrolled 3939 adult participants with CKD. The study design, methods, and baseline characteristics of participants were described previously.15-17 Briefly, seven clinical centers (from thirteen recruitment sites) recruited men and women aged 21-74 years whose estimated GFR (eGFR) was 20-70 ml/min/1.73 m2 at baseline.18 The original participants were enrolled from June 2003 to August 2008.
The CRIC participants had serum creatinine and cystatin C measurements at baseline and annually during follow-up. Serum creatinine values were calibrated to isotope-dilution mass spectrometry-traceable standards,19-21 and cystatin C was standardized to correct for drift over time when using different calibrator and reagent lots.21 The eGFR was calculated using an internally derived CRIC equation based on age, sex, race, standardized serum creatinine, and cystatin C.21 The study was approved by the institutional review boards from participating centers (University of California, San Francisco, Committee on Human Research approval #10-04231) and is in accordance with the Declaration of Helsinki. All study participants provided written informed consent.
We included only incident hemodialysis patients and excluded patients who transitioned to ESRD by initiating peritoneal dialysis or receiving a kidney transplant. We chose to focus only on hemodialysis patients because fates and determinants of death within the first year from the initiation of dialysis likely differ by the modality of renal replacement therapy, and hemodialysis is by far the most common initial modality in the United States. Most of the literature regarding high rates of early death in incident ESRD has been based among hemodialysis patients. It is less likely that patients who transition to ESRD abruptly would be initiated on peritoneal dialysis or would have received a preemptive kidney transplant. Only study participants with at least one CRIC visit (with an eGFR determined during that visit) prior to 3 months before dialysis initiation were included. Figure 1 illustrates the disposition of study participants from entry into the CRIC Study, initiation of hemodialysis, to the end of follow-up.
Figure 1. Derivation of analytic cohort and mortality outcomes.
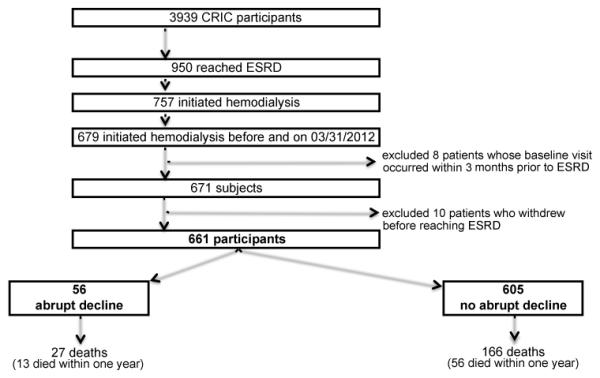
Diagram shows the exclusions applied from the initial CRIC population to our final cohort of 661 subjects; and the breakdown of the final cohort by abrupt kidney function decline status.
Abbreviations: CRIC, Chronic Renal Insufficiency Cohort. ESRD, end-stage renal disease.
Predictor Variable
Our primary predictor was an abrupt decline in kidney function prior to initiating hemodialysis. Abrupt decline was defined as having a predicted eGFR ≥30 ml/min/1.73m2 at three months prior to the initiation of dialysis. We applied this definition of abrupt kidney function decline to be consistent with prior studies in the literaure.13, 22 Most clinicians would likely agree that progressing from an eGFR ≥30 ml/min/1.73m2 to ESRD within a three-month window represents a fairly “abrupt” transition.
In order to predict eGFR at three months prior to the initiation of dialysis, we first fit a linear mixed effects model using eGFR values (baseline and yearly, up until three months prior to the date of dialysis initiation) as the outcome. The only predictor in the model was time, and both random intercepts and slopes were included. We then used this model to extrapolate patient-specific extrapolated eGFRs at three months prior to the initiation of hemodialysis to determine whether each patient met criterion for pre-dialysis abrupt eGFR decline.
Outcome Variables
The primary outcome was all-cause death during the first year after initiating hemodialysis. A secondary outcome was all-cause death at any time after dialysis initiation up to the end of follow-up in March 2012. Deaths in the CRIC study were tracked in multiple ways including report from next of kin, retrieval of death certificates or obituaries, review of hospital records, and periodic searches in the Social Security Administration’s Death Master File. Dialysis status was ascertained by self-report, review of medical records and periodic cross-linkage with US Renal Data System (USRDS).
Covariates
Demographic variables were collected at CRIC baseline visits. For this study, race-ethnicity was defined as follows: Hispanic, non-Hispanic white, non-Hispanic black, and non-Hispanic other. Cardiovascular disease was defined from participants’ self-report of any of the following at baseline and/or during follow-up: coronary artery disease, heart failure, stroke, and peripheral vascular disease. Diabetes mellitus was defined as self-reported use of diabetes medications, or having a fasting glucose ≥ 126 mg/dL or non-fasting glucose ≥ 200 mg/dL (at baseline and/or during follow-up). History of cancer was self-reported for within the 5 years prior to CRIC enrollment and/or during any follow-up visit. Cardiovascular disease, diabetes, and cancer were all time-updated during follow-up until the latest study visit at or before three months prior to dialysis initiation.
Heart failure events were identified from hospitalization discharge codes (up to 30) and adjudicated by at least two study physicians through review of medical records using specific guidelines on documented symptoms, physical examination findings, and radiographic/hemodynamic data.23, 24 Adjudicated valid heart failure events during follow-up that occurred within 3 months prior to dialysis initiation were also included in final adjusted models.
Hospitalizations within 3 months prior to dialysis initiation were also ascertained using a combination of participants’ self-report during twice yearly questionnaires as well as routine searches through hospital databases.
Additional variables pertaining to pre-dialysis care and ESRD were obtained from the ESRD Medical Evidence Report (Centers for Medicare & Medicaid Services [CMS]-2728) forms from cross-linkage with USRDS: type of first dialysis access, presence/duration of nephrologist care before ESRD, primary cause of ESRD, and measures of serum creatinine, hemoglobin, and albumin at the time of dialysis initiation. Due to the large number of patients with missing data on these variables (as well as known weak validity of the duration of nephrology care variable25), we did not adjust for these variables in our main analyses.
Statistical Analysis
Analyses were performed using SAS 9.3 (SAS Institute Inc, Cary, N.C.). Characteristics of the participants who had abrupt kidney function decline before hemodialysis initiation were compared to those without such a decline, with one-way ANOVA for continuous variables and chi-squared test for categorical variables.
We performed Cox proportional hazards regression models to examine the association between abrupt decline in kidney function before dialysis initiation (as defined above) and all-cause death after dialysis initiation. Participants’ follow-up time in the Cox models began on the date of dialysis initiation. In the first Cox model examining one-year mortality, follow-up time ended at death, withdrawal from study, one year after date of dialysis initiation, or end of follow-up on March 31, 2012. In a second Cox model examining mortality until the end of follow-up, we did not censor at one year after dialysis initiation. Unadjusted models were first performed, followed by adjustment for age, sex, race/ethnicity, prior cardiovascular disease, diabetes, and cancer. We then further adjusted for heart failure events occurring during follow-up that were within 3 months prior to dialysis initiation to evaluate its role as a potential mediator or confounder of any observed association with abrupt kidney function decline and death after hemodialysis initiation.
To formally test the differential effect of time on the association between abrupt kidney function decline and mortality after dialysis initiation, we also added an interaction term, [abrupt decline*time], to the final adjusted model (for death until end of follow-up) and performed a proportionality of hazards test.
RESULTS
Clinical Characteristics From Baseline to Hemodialysis Initiation
Among the 661 CRIC participants in our analytic cohort, 56 (8.4%) met criteria for abrupt decline in kidney function within 3 months before initiating hemodialysis (Figure 1). Baseline characteristics of those with or without abrupt kidney function declines are shown in Table 1. The abrupt decline group tended to have a higher proportion of men, but there were no significant differences in age or race-ethnicity. Baseline eGFR at entry into CRIC was higher in the abrupt decline group versus non-abrupt decline group (mean, 42.4 versus 31.7 ml/min/1.73m2; P<0.001), while both groups had considerable proteinuria at baseline. The abrupt decline group appeared to have higher prevalence of cardiovascular disease, diabetes, and malignancy compared to the non-abrupt decline group, though differences between groups did not reach statistical significance. Both groups had similarly high hospitalization rates within the last 3 months prior to hemodialysis initiation (56.6% overall), but those with abrupt eGFR decline were more likely to have had at least one heart failure event within the last 3 months before dialysis initiation compared with those without abrupt eGFR decline (29% versus 13.4%; P=0.002).
Table 1.
Baseline and time-updated characteristics of incident HD patients in CRIC: abrupt vs. non-abrupt kidney function decline before HD initiation.
| All | Abrupt Decline |
Non-abrupt
Decline |
||
|---|---|---|---|---|
| (N=661) | (n=56) | (n=605) | P | |
| Baseline Patient Characteristics | ||||
| Age at study enrollment, y | 56.3 ±11.3 | 57.1 (11.7) | 56.3 ±11.3 | 0.6 |
| Female sex | 256 (38.7%) | 15 (26.8%) | 241 (39.8%) | 0.06 |
| Race / ethnicity | 0.1 | |||
| Hispanic | 127 (19.2%) | 16 (28.6%) | 111 (18.3%) | |
| Non-Hispanic Black | 360 (54.5%) | 30 (53.6%) | 330 (54.5%) | |
| Non-Hispanic White | 149 (22.5%) | 10 (17.9%) | 139 (23%) | |
| Non-Hispanic Other | 25 (3.78%) | 0 (0%) | 25 (4.1%) | |
| Baseline eGFR (ml/min/1.73m2) | 32.6 ±10.4 | 42.4 ±8.9 | 31.7 ±10.0 | <0.001 |
| Baseline UPCR, mg/mg, n=628 | 2.9 ±3.9 | 3.2 ±5.1 | 2.9 ±3.8 | 0.6 |
| Annual Income level | 0.4 | |||
| ≤$20,000 | 309 (46.7%) | 33 (58.9%) | 276 (45.6%) | |
| $20,001 - $50,000 | 143 (21.6%) | 11 (19.6%) | 132 (21.8%) | |
| $50,001 - $100,000 | 80 (12.1%) | 4 (7.1%) | 76 (12.6%) | |
| >$100,000 | 28 (4.2%) | 1 (1.8%) | 27 (4.5%) | |
| Don't wish to answer | 101 (15.3%) | 7 (12.5%) | 94 (15.5%) | |
| Graduated high school or beyond | 450 (68.1%) | 32 (57.1%) | 418 (69.1%) | 0.07 |
| Time-Updated Comorbidities and Characteristics Up to 3 Mo Before HD Initiation | ||||
| Cardiovascular Disease | 333 (50.4%) | 32 (57.1%) | 301 (49.8%) | 0.3 |
| Diabetes | 466 (70.5%) | 44 (78.6%) | 422 (69.8%) | 0.2 |
| Diagnosed or treated for any cancer since 5 y before enrollment |
62 (9.4%) | 8 (14.3%) | 54 (8.9%) | 0.2 |
| Most recent SBP, mmHg (n=660) | 144.4 (26.9) | 146.7 (26.2) | 144.2 (27.0) | 0.5 |
| Most recent DBP, mmHg (n=660) | 72.2 (14.8) | 75.7 (16.0) | 71.9 (14.7) | 0.07 |
| Most recent UPCR, mg/mg (n=644) | 3.6 (4.0) | 3.8 (5.0) | 3.6 (3.9) | 0.7 |
| Extrapolated eGFR at 3 mo before HD initiation (ml/min/1.73m2) |
19.3 (7.4) | 34.7 (4.8) | 17.8 (5.8) | <0.001 |
| Median | 18.5 [14.4 - 23.3] | 33.2 [31.4 – 36.0] | 17.9 [13.8 - 21.9] | <0.001 |
| Events Before HD Initiation | ||||
| Hospitalized within last 3 mo before HD | 374 (56.6%) | 34 (60.7%) | 340 (56.2%) | 0.5 |
| HF events within last 3 mo before HD | 97 (14.7%) | 16 (28.6%) | 81 (13.4%) | 0.002 |
| No. of HF events within last 3 mo before HD | <0.001 | |||
| 0 | 564 (85.3%) | 40 (71.4%) | 524 (86.6%) | |
| 1 | 72 (10.9%) | 8 (14.3%) | 64 (10.6%) | |
| 2 | 21 (3.2%) | 6 (10.7%) | 15 (2.5%) | |
| ≥3 | 4 (0.6%) | 2 (3.6%) | 2 (0.3%) | |
| Characteristics at Time of HD Initiation | ||||
| Age, y | 59.7 (11.6) | 59.8 (12.5) | 59.7 (11.6) | 0.9 |
| eGFR, ml/min/1.73m2 (n=493) | 10.4 (4.4) | 13.0 (5.1) | 10.1 (4.2) | <0.001 |
| Median | 9.8 [7.3 - 12.8] | 12.9 [9.8 - 16.6] | 9.6 [7.2 - 12.5] | <0.001 |
| Hemoglobin, g/dl (n=446) | 9.6 (1.6) | 9.8 (1.9) | 9.6 (1.6) | 0.6 |
| Serum albumin, g/dl (n=353) | 3.2 (0.7) | 2.8 (0.7) | 3.3 (0.6) | <0.001 |
| Ascribed etiology of ESRDa (n= 502) | 0.004 | |||
| Diabetes Type 2 | 260 (51.8%) | 22 (47.8%) | 238 (52.2%) | |
| HTN, unspecified with kidney failure | 148 (29.5%) | 13 (28.3%) | 135 (29.6%) | |
| Diabetes Type 1 | 18 (3.59%) | 2 (4.3%) | 16 (3.5%) | |
| Tubular necrosis without recovery | 1 (0.20%) | 1 (2.2%) | 0 (0%) | |
| Time under care of a nephrologist (n=473) | <0.001 | |||
| >12 months | 149 (31.5%) | 7 (17.9%) | 142 (32.7%) | |
| 6-12 months | 86 (18.2%) | 5 (12.8%) | 81 (18.7%) | |
| <6 months | 84 (17.8%) | 3 (7.7%) | 81 (18.7%) | |
| No | 91 (19.2%) | 17 (43.6%) | 74 (17.1%) | |
| Unknown | 63 (13.3%) | 7 (17.9%) | 56 (12.9%) | |
| First access type used (n=467) | 0.002 | |||
| Catheter | 324 (69.4%) | 32 (84.2%) | 292 (68.1%) | |
| Fistula | 104 (22.3%) | 2 (5.3%) | 102 (23.8%) | |
| Graft | 30 (6.4%) | 1 (2.6%) | 29 (6.8%) | |
| Other | 9 (1.9%) | 3 (7.9%) | 6 (1.4%) | |
| Duration of f/u from study enrollment to ESRD (y) |
3.3 (1.9) | 2.6 (1.8) | 3.4 (1.9) | 0.003 |
| Median | 3.1 [1.8 - 4.7] | 2.1 [1.1 - 4.2] | 3.2 [1.9 - 4.8] | 0.002 |
Note: Values for categorical variables are given as number (percentage); values for continuous variables, as mean ±standard deviation or median [interquartile range].
Abbreviations: CRIC, Chronic Renal Insufficiency Cohort Study. DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HD, hemodialysis; HF, heart failure; ESRD, end-stage renal disease.SBP, systolic blood pressure; UPCR, urine protein-creatinine ratio; HTN, hypertension; f/u, follow-up
Only the top three etiologies along with “Tubular necrosis without recovery” listed.
Figure 2 illustrates the aggregate kidney function trajectories for patients who were classified as having abrupt decline versus those who were not. Shown are individual annual CRIC eGFRs (blue triangle), individual extrapolated eGFRs at 3 months prior to hemodialysis initiation (red star), and individual eGFRs at hemodialysis initiation (red circle). In addition, group mean curves and 95% confidence intervals (CIs; shaded region) and prediction intervals (dashed lines) are shown.
Figure 2a and 2b. Aggregate eGFR plots for patients with abrupt eGFR decline (2a, upper) and non-abrupt eGFR decline (2b, lower) before dialysis initiation.
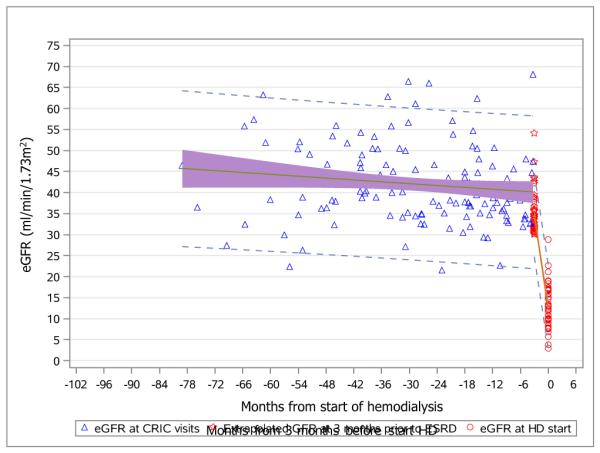
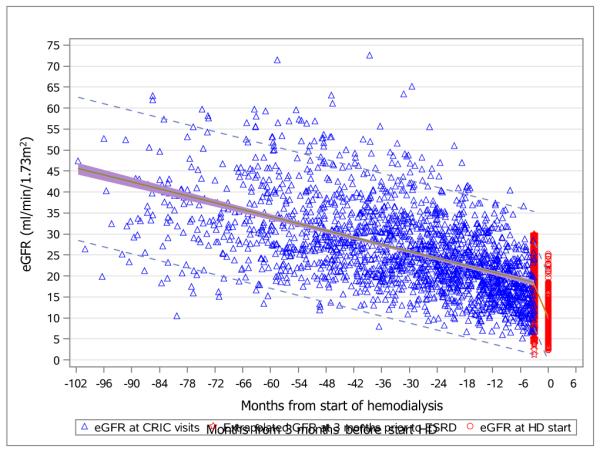
Annual eGFR values at CRIC visits (blue triangles); extrapolated eGFR values at 3 months before initiation of hemodialyis (red stars); actual eGFR values at the initiation of hemodialysis (red circles).
Group mean curves (green lines); 95% confidence intervals (shaded regions); prediction intervals (dashed lines).
Abbreviations: eGFR, estimated glomerular filtration rate. CRIC, Chronic Renal Insufficiency Cohort.
At the time of dialysis initiation, the two groups were similar in age and hemoglobin levels, but the abrupt decline group at higher eGFR at time of hemodialysis initiation (13 versus 10 ml/min/1.73m2) and lower serum albumin levels. The top three diagnoses listed as the primary cause of ESRD were the same in both groups: (in order) diabetes type 2, hypertension, diabetes type 1. The abrupt decline group had a shorter interval (median, 2.1 years) from study enrollment to dialysis initiation compared with the non-abrupt decline group (median, 3.2 years; Table 1).
A total of 467 (70.7%) participants had available data on both initial dialysis access and duration of nephrology care prior to dialysis initiation. A greater proportion of those who had abrupt eGFR decline initiated dialysis with a catheter versus those without abrupt eGFR decline (Table 1). Lack of nephrology care was more commonly reported in the abrupt eGFR decline group.
Mortality Outcomes
Overall, 193 participants (29.2% of the cohort) died after initiating hemodialysis, among whom 69 died within the first year, with 13 (23%) among those with abrupt decline and 56 (9.3%; P<0.001) among those without abrupt decline (Figure 1). Deaths at any time after hemodialysis initiation were also more frequent in the abrupt decline group (48% versus 27.4% in non-abrupt decline group; P=0.005). Among those who died during follow-up, the overall median time from dialysis initiation to death (among those who died during follow-up) was 1.67 years (Table 2). Kaplan-Meir survival curves are shown in Figure 3, highlighting that the differential rates of death is particularly pronounced soon after hemodialysis initiation. .
Table 2.
Mortality in incident HD patients in CRIC: abrupt vs. non-abrupt kidney function decline before HD initiation.
| All (N=661) |
Abrupt Decline (n=56) |
Non-abrupt Decline (n=605) |
P | |
|---|---|---|---|---|
| First year mortality | ||||
| Died within 1 y after ESRD | 69 (10.4%) | 13 (23.2%) | 56 (9.3%) | <0.001 |
| Died within first 6 wk | 21 | 7 | 14 | |
| Died in 6-12 wk | 12 | 3 | 9 | |
| Died in 12 wk-6 mo | 14 | 2 | 12 | |
| Died in 6 mo-1 y | 22 | 1 | 21 | |
| HR (95% CI) for death within 1 y after ESRD |
||||
| Unadjusted | -- | 2.79 (1.52-5.09) | 1.00 (reference) | <0.001 |
| Adjusted for age, sex, race-ethnicity, CVD, DM, cancer |
-- | 3.09 (1.65-5.76) | 1.00 (reference) | <0.001 |
| Adjusted for above + HF events within 3 mo before HD initiation |
-- | 3.00 (1.59-5.65) | 1.00 (reference) | <0.001 |
| Mortality until end of follow-up | ||||
| Died during follow-up | 193 (29.2%) | 27 (48.2%) | 166 (27.4%) | 0.005 |
| HR (95% CI) for death during follow-up | ||||
| Unadjusted | -- | 1.69 (1.13-2.54) | 1.00 (reference) | 0.01 |
| Adjusted for age, sex, race-ethnicity, CVD, DM, cancer |
-- | 1.76 (1.15-2.67) | 1.00 (reference) | 0.009 |
| Adjusted for above + HF events within 3 mo before HD initiation |
-- | 1.71 (1.12-2.61) | 1.00 (reference) | 0.01 |
| Time from ESRD to death, y (n=193) | 2.0 ±1.7 | 1.9 ±2.2 | 2.0 ±1.6 | 0.9 |
| Median | 1.7 [0.5 – 3.0] | 1.3 [0.1 - 3.3] | 1.7 [0.6 – 3.0] | 0.2 |
Note: Values for categorical variables are given as number or number (percentage); values for continuous variables, as mean ±standard deviation or median [interquartile range].
Abbreviations: CI, confidence interval; CRIC, Chronic Renal Insufficiency Cohort Study; CVD, cardiovascular disease; ESRD, end-stage renal disease ; HD, hemodialysis; HF, heart failure; HR, hazard ratio; DM, diabetes mellitus
Figure 3.
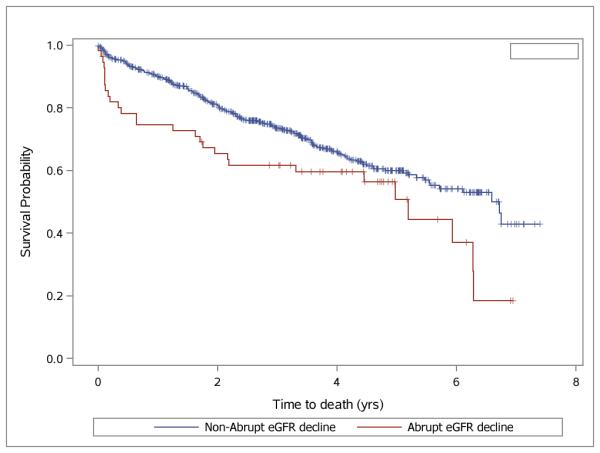
Kaplan-Meier plot for survival after onset of ESRD for patients classified as having had abrupt eGFR decline (red) vs. non-abrupt eGFR decline (blue).
Abbreviations: ESRD, end-stage renal disease. eGFR, estimated glomerular filtration rate.
In our primary analysis examining one-year mortality (Table 2), having had abrupt decline in kidney function was associated with a nearly three-fold increase in risk of death within the first year after hemodialysis initiation (unadjusted hazard ratio [HR], 2.79; 95% CI, 1.52-5.09). Adjustment for demographics, cardiovascular disease, diabetes, and cancer did not attenuate this risk in one-year mortality (adjusted HR, 3.09; 95% CI, 1.65-5.76). Further adjustment for heart failure events within the three months prior to dialysis initiation did not alter the increased hazards of death within a year associated with abrupt eGFR decline (adjusted HR, 3.00; 95% CI, 1.59-5.65).
In our secondary analysis examining mortality until the end of follow-up, abrupt eGFR decline was also associated with death at any time following dialysis initiation (unadjusted HR, 1.69; 95% CI, 1.13-2.54). Sequential adjustment for demographic characteristics, comorbidities, and heart failure events did not significantly change this association (Table 2). There was a significant interaction between abrupt kidney function decline and follow-up time (P=0.02), confirming that the increased risk of death associated with abrupt decline in kidney function weakens over time.
DISCUSSION
In this longitudinal study of 661 subjects followed up from non−dialysis-dependent CKD through ESRD, we estimated that 1 in 12 (8%) incident hemodialysis patients had an abrupt decline in kidney function from eGFR ≥30 ml/min/1.73m2 within three months before initiating hemodialysis. This abrupt decline in kidney function was associated with an approximately threefold increased relative rate of death within one year of initiating dialysis, even after adjustment for demographics and known cardiovascular disease, diabetes, and cancer.
The proportion of patients estimated to have had abrupt transition to hemodialysis-dependent ESRD in this study is consistent with the few prior reports in selected samples in the United States.13, 14, 22 A prior case series using data from two outpatient dialysis units in San Francisco showed that approximately one in ten incident hemodialysis patients had a documented eGFR >30 ml/min/1.73m2 within three months prior to dialysis initiation.13 A larger study using Veterans Affairs data identified four groups of incident dialysis patients with distinctive patterns of eGFR trajectory, including 3.1% who had a “catastrophic eGFR loss” within six months or less starting from above 60 ml/min/1.73m2.14 These rates are lower than another report of 100 incident hemodialysis patients from four Wisconsin-Mayo Clinic dialysis units which found 34% of patients had eGFR ≥30 ml/min/1.73m2 on or before the 90th day preceding initiation of first dialysis.22 One advantage of the current analysis over prior studies13, 14, 22, 26 is that we relied on eGFRs obtained as part of a structured research protocol, thus reducing ascertainment bias which may be present in studies which relied on serum creatinine measured as part of clinical care, as some patients may not have had serum creatinine measured prior to onset of ESRD, while other, more unstable patients may have had more serum creatinine measurements. In addition, our study was multi-centered, national in scope, and more balanced in terms of gender and racial-ethnic representation.13, 22
An important strength of our current study is that there was systematic capture of cardiovascular disease, diabetes, and cancer as well as adjudication of heart failure events. Notably, there were no differences in several key clinical parameters such as proteinuria among those who did or did not have abrupt decline; and the association between abrupt transition to ESRD and early death after maintenance hemodialysis initiation was not attenuated after adjusting for important demographic and clinical covariates. These findings suggest a possible causal relationship and support further investigation to better understand potentially modifiable reasons for abrupt transition in order to devise successful interventions to prevent abrupt declines in eGFR as one possible way to reduce rates of early death after dialysis initiation.
To the best of our knowledge, only two prior studies have directly examined the association between the pattern of eGFR loss during CKD with death after ESRD.14 Using Veterans Affairs clinical data, O’Hare and colleagues found that, compared with those with slow progression of CKD, patients with the most rapid loss of kidney function before ESRD had a 2.34-fold increase in adjusted risk of death within six months of dialysis initiation and a 1.95-fold increase from six to twelve months of follow-up.14 Haapio and colleagues used a database of all adult incident ESRD patients in Finland to show that patients with the steepest tertile of eGFR slope (from within one year prior to dialysis or transplantation) had a 1.7-fold increased risk of death after ESRD, though the association became non-significant after adjusting for ESRD etiology, comorbidities, presence of renin-angiotensin-system blockers, BMI, albumin, and hemoglobin.26 Our interpretation of the totality of the literature is that abrupt declines close to initiation of dialysis matters more than eGFR slope and the impact may be greatest for early mortality after initiation of dialysis for various reasons.
It is very plausible that abrupt transition to dialysis-dependent ESRD is causally linked to increased risk of death since those who transition abruptly may be less educated and prepared for renal replacement therapy, and may be more likely to rely on the use of dialysis catheters. Indeed, we noted that use of a dialysis catheter as access was more common in the abrupt decline group. A study using data from the USRDS showed that initiating hemodialysis via a catheter was associated with an adjusted HR of 2.73 of early death within weeks 7 through 12 after dialysis initiation (versus initiating via a fistula).5 Studies have also shown that earlier and more frequent nephrology care in the period leading to dialysis initiation were associated with reduced risk of death after ESRD.5, 27
Our study has important clinical and public health implications. If treating nephrologists are able to identify one or more pre-dialysis eGFR values ≥30 ml/min/1.73m2 prior to the three months before hemodialysis initiation and recognize this pattern of abrupt decline, they may be able to infer a higher risk of early death for individual patients. This added risk may help inform decisions in the critical early period on dialysis, such as frequency of visits and goals of care discussions. From a policy standpoint, it may be prudent to not subject all patients who transitioned to dialysis-dependence in this abrupt manner to the same quality measures, such as laboratory performance measures by the CMS’s Quality Improvement Program.28
Our study has the following limitations. First, since eGFR was determined in the CRIC protocol only yearly (versus, say, every 90 days), we needed to utilize a mixed effects regression to predict whether eGFR was ≥30 ml/min/1.73m2 at the three-month mark before dialysis initiation. Nevertheless, our ascertainment of the proportion of ESRD cases preceded by abrupt transition was comparable to prior studies that had more frequent measurements.13, 14 In addition, those we classified as having abrupt decline were at higher risk of early death after dialysis initiation, similar to what was found in a prior study that had more frequent eGFR measurements to classify trajectory.14 Second, the number of outcomes (deaths) was relatively small which led to wide CIs. Because we were missing information from the ESRD Medical Evidence Report (CMS-2728) form on nearly a third of the study participants, we had insufficient sample size to adequately explore whether the increase risk of early death was mediated in part by use of a catheter. For example, in the subgroup of 467 ESRD patients with information on vascular access from the 2728 form, abrupt transition was associated with a higher risk of early death but was not statistically significant with wide CIs (unadjusted HR, 2.04; 95% CI, 0.86-4.86). We lacked more granular clinical data to better characterize the causes for abrupt kidney function decline in individual patients; we also lacked more detailed information on the exact circumstances/location of hemodialysis initiation (emergency admission vs. elective admission vs. outpatient initiation). We also lacked data on additional comorbidities such as psychiatric illness and neurologic diseases (besides stroke).
However, our study had multiple strengths. We had repeated measures of eGFR obtained through a structured, timed protocol to allow for modeled trajectories of eGFR decline not otherwise biased by the number of clinic visits or laboratory measurements. There was also systematic capture of important demographic and comorbidity variables. The CRIC study has excellent long-term follow-up and comprehensive ascertainment of ESRD and death as well as interim heart failure events, with low rates of withdrawal and loss to follow-up.
Our study supports that the manner of transition from CKD to maintenance dialysis could significantly affectt post-ESRD outcomes, with abrupt decline in kidney function in the 3 months before initiating dialysis being associated with higher risk of early death. Additional efforts are needed to identify factors responsible for this rapid decline, and whether efforts to prevent or slow abrupt decline in eGFR may improve survival after initiating dialysis.
ACKNOWLEDGEMENTS
The CRIC Study Investigators are Lawrence J. Appel, MD, MPH, Harold I. Feldman, MD, MSCE, Alan S. Go, MD, Jiang He, MD, PhD, John W. Kusek, PhD, James P. Lash, MD, Akinlolu Ojo, MD, PhD, Mahboob Rahman, MD, and Raymond R. Townsend, MD.
The authors thank the participants, investigators, and staff of the CRIC Study for their time and commitment.
Support: Funding for the CRIC Study was obtained under a cooperative agreement from the National Institute of Diabetes and Digestive and Kidney Diseases (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this work was supported in part by: the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award (CTSA) National Institutes of Health (NIH)/National Center for Advancing Translational Sciences (NCATS) UL1TR000003, Johns Hopkins University UL1 TR-000424, University of Maryland General Clinical Research Center M01 RR-16500, Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the NCATS component of the NIH and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research UL1TR000433, University of Illinois at Chicago CTSA UL1RR029879, Tulane University Translational Research in Hypertension and Renal Biology P30GM103337, Kaiser Permanente Northern California NIH/National Center for Research Resources University of California, San Francisco−Clinical & Transitional Science Institute UL1 RR-024131. This study was additionally supported by K23DK100468 and KL2TR000143 (Dr RK Hsu), K24DK092291 (Dr C-y Hsu), K23DK088865 (Dr Bansal). The funding sources did not have any role in the study design, collection, analysis, interpretation of data, writing of report, and decision to submit the report for publication.
Footnotes
N SECTION:
A List of the CRIC Study Investigators appears in the Acknowledgements.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: Dr Go has received a research grant from Astra-Zeneca. The other authors declare that they have no other relevant financial interests.
Contributions: Research idea and study design: RKH, BC, JAR, HIF, ASG, JH, JWK, JPL, AO, RRT, C-yH; data acquisition: BC, JAR, AHA, HIF, ASG, JH, JWK, JPL, AO, RRT, C-yH; data analysis/interpretation: RKH, BC, JAR, AHA, NB, HIF, ASG, JH, EJH, JWK, JPL, AO, JHS, RRT, MZ, C-yH; statistical analysis: RKH, BC, JAR, C-yH; supervision or mentorship: JAR, AHA, HIF, ASG, JH, JWK, JPL, AO, RRT, C-yH; Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. RKH takes responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Peer Review: Evaluated by 3 external peer reviewers, a Statistical Editor, a Co-Editor, and the Editor-in-Chief.
REFERENCES
- 1.USDHHS . CDC Office of Disease Prevention and Health Promotion; Atlanta, GA: 2010. Healthy People 2020. [Google Scholar]
- 2.Bradbury BD, Fissell RB, Albert JM, et al. Predictors of early mortality among incident US hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS) Clinical journal of the American Society of Nephrology : CJASN. 2007;2(1):89–99. doi: 10.2215/CJN.01170905. [DOI] [PubMed] [Google Scholar]
- 3.Robinson BM, Zhang J, Morgenstern H, et al. Worldwide, mortality risk is high soon after initiation of hemodialysis. Kidney Int. 2014;85(1):158–165. doi: 10.1038/ki.2013.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan KE, Maddux FW, Tolkoff-Rubin N, Karumanchi SA, Thadhani R, Hakim RM. Early outcomes among those initiating chronic dialysis in the United States. Clinical journal of the American Society of Nephrology : CJASN. 2011;6(11):2642–2649. doi: 10.2215/CJN.03680411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foley RN, Chen SC, Solid CA, Gilbertson DT, Collins AJ. Early mortality in patients starting dialysis appears to go unregistered. Kidney Int. 2014;86(2):392–398. doi: 10.1038/ki.2014.15. [DOI] [PubMed] [Google Scholar]
- 6.Eckardt KU, Gillespie IA, Kronenberg F, et al. High cardiovascular event rates occur within the first weeks of starting hemodialysis. Kidney Int. 2015;88(5):1117–1125. doi: 10.1038/ki.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bao Y, Dalrymple L, Chertow GM, Kaysen GA, Johansen KL. Frailty, dialysis initiation, and mortality in end-stage renal disease. Arch Intern Med. 2012;172(14):1071–1077. doi: 10.1001/archinternmed.2012.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Appel LJ, Wright JT, Greene T, et al. Long-term effects of renin-angiotensin system- blocking therapy and a low blood pressure goal on progression of hypertensive chronic kidney disease in African Americans. Arch Intern Med. 2008;168(8):832–839. doi: 10.1001/archinte.168.8.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klahr S, Levey AS, Beck GJ, et al. The effects of dietary protein restriction and blood- pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. N Engl J Med. 1994;330(13):877–884. doi: 10.1056/NEJM199403313301301. [DOI] [PubMed] [Google Scholar]
- 10.Plantinga LC, Fink NE, Levin NW, et al. Early, intermediate, and long-term risk factors for mortality in incident dialysis patients: the Choices for Healthy Outcomes in Caring for ESRD (CHOICE) Study. Am J Kidney Dis. 2007;49(6):831–840. doi: 10.1053/j.ajkd.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 11.Abbott KC, Glanton CW, Trespalacios FC, et al. Body mass index, dialysis modality, and survival: analysis of the United States Renal Data System Dialysis Morbidity and Mortality Wave II Study. Kidney Int. 2004;65(2):597–605. doi: 10.1111/j.1523-1755.2004.00385.x. [DOI] [PubMed] [Google Scholar]
- 12.Ku E, Glidden DV, Johansen KL, et al. Association between strict blood pressure control during chronic kidney disease and lower mortality after onset of end-stage renal disease. Kidney Int. 2015;87(5):1055–1060. doi: 10.1038/ki.2014.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee P, Johansen K, Hsu CY. End-stage renal disease preceded by rapid declines in kidney function: a case series. BMC Nephrol. 2011;12:5. doi: 10.1186/1471-2369-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Hare AM, Batten A, Burrows NR, et al. Trajectories of Kidney Function Decline in the 2 Years Before Initiation of Long-term Dialysis. Am J Kidney Dis. 2012;59(4):513–522. doi: 10.1053/j.ajkd.2011.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feldman HI, Appel LJ, Chertow GM, et al. The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and Methods. J Am Soc Nephrol. 2003;14(7 Suppl 2):S148–153. doi: 10.1097/01.asn.0000070149.78399.ce. [DOI] [PubMed] [Google Scholar]
- 16.Lash JP, Go AS, Appel LJ, et al. Chronic Renal Insufficiency Cohort (CRIC) Study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol. 2009;4(8):1302–1311. doi: 10.2215/CJN.00070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischer MJ, Go AS, Lora CM, et al. CKD in Hispanics: Baseline characteristics from the CRIC (Chronic Renal Insufficiency Cohort) and Hispanic-CRIC Studies. Am J Kidney Dis. 2011;58(2):214–227. doi: 10.1053/j.ajkd.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 19.Joffe M, Hsu CY, Feldman HI, et al. Variability of creatinine measurements in clinical laboratories: results from the CRIC study. Am J Nephrol. 2010;31(5):426–434. doi: 10.1159/000296250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levey AS, Coresh J, Greene T, et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53(4):766–772. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 21.Anderson AH, Yang W, Hsu CY, et al. Estimating GFR among participants in the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2012;60(2):250–261. doi: 10.1053/j.ajkd.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Onuigbo MA, Onuigbo NT, Musso CG. Syndrome of rapid onset end stage renal disease in incident Mayo Clinic chronic hemodialysis patient. Indian J Nephrol. 2014;24(2):75–81. doi: 10.4103/0971-4065.127886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bansal N, Hyre Anderson A, Yang W, et al. High-sensitivity troponin T and N-terminal pro-B-type natriuretic peptide (NT-proBNP) and risk of incident heart failure in patients with CKD: the Chronic Renal Insufficiency Cohort (CRIC) Study. J Am Soc Nephrol. 2015;26(4):946–956. doi: 10.1681/ASN.2014010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu KD, Yang W, Go AS, et al. Urine neutrophil gelatinase-associated lipocalin and risk of cardiovascular disease and death in CKD: results from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2015;65(2):267–274. doi: 10.1053/j.ajkd.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim JP, Desai M, Chertow GM, Winkelmayer WC. Validation of reported predialysis nephrology care of older patients initiating dialysis. J Am Soc Nephrol. 2012;23(6):1078–1085. doi: 10.1681/ASN.2011080871. [DOI] [PubMed] [Google Scholar]
- 26.Haapio M, Helve J, Kurimo P, Forslund T, Grönhagen-Riska C, Finne P. Decline in glomerular filtration rate during pre-dialysis phase and survival on chronic renal replacement therapy. Nephrol Dial Transplant. 2012;27(3):1157–1163. doi: 10.1093/ndt/gfr423. [DOI] [PubMed] [Google Scholar]
- 27.Singhal R, Hux JE, Alibhai SM, Oliver MJ. Inadequate predialysis care and mortality after initiation of renal replacement therapy. Kidney Int. 2014;86(2):399–406. doi: 10.1038/ki.2014.16. [DOI] [PubMed] [Google Scholar]
- 28.Centers for Medicare & Medicaid Services (CMS) HHS Medicare Program; End-Stage Renal Disease Prospective Payment System, and Quality Incentive Program. Final Rule. Fed Regist. 2015;80(215):68967–69077. [PubMed] [Google Scholar]


