Abstract
The Prostate Outreach Project (POP) provided free prostate cancer (PCa) education and early detection to medically underserved communities. POP recruited participants in medically underserved communities. PCa education and detection events occurred in POP locations (static) or natural gathering places (mobile) within the community. PCa education was delivered by video and evaluated using a questionnaire. Screening consisted of serum prostate-specific antigen and digital rectal examination. A navigated follow-up strategy was utilized to provide medical care for participants with abnormal screening examinations (ASE). POP recruited 4,420 men, 62.8% (2,667) were African American (AA). Most participants had a high school education and no prior screening. Fifty-four percent (2,159) were uninsured and 41% (1,811) had no access to a physician. PCa knowledge increased following the educational video. Prostate-specific antigen levels were elevated in 9.8% (436), while 6.9% (233) had an abnormal digital rectal examination. Follow-up among 609 men with ASE was successful in 40% (244), despite a navigated approach. Overall, 3.3% (144) cancers were diagnosed among the POP with AA participants exhibiting a significantly higher incidence. Recruitment, education, and PCa testing among a medically underserved cohort was successful. However, failure to follow through on ASE could contribute to maintaining the disparity in PCa outcomes noted among AAs and the medically underserved if not addressed.
Keywords: prostate cancer screening, medically underserved African Americans
Introduction
African American (AA) men have the highest incidence and mortality from prostate cancer (PCa) in the United States and this alarming trend has persisted for more than 30 years. Overall PCa mortality, however, has decreased by 39% in the prostate-specific antigen (PSA) era with an average annual decrease of 3.5% to 3.7% among both U.S. White and Black male populations (Jemal et al., 2013). Racial and ethnic minorities are often overrepresented in cancer diagnosis, yet they make up a disproportionately low percentage of participants in cancer-screening trials (Andriole et al., 2009; Pinsky et al., 2008). Men from low socioeconomic backgrounds are at a higher risk for having increased PCa burden and lower utilization of PCa-screening services (Fedewa, Etzioni, Flanders, Jemal, & Ward, 2010; Hoffman et al., 2001; Miller et al., 2009). In Texas, two racial and ethnic minorities (AA and Hispanic men) had a lower incidence of digital rectal examination (DRE) performed as well as a lower likelihood of being diagnosed with early stage PCa, yet higher likelihood of being diagnosed with late-stage PCa when compared with White men (American Cancer Society [ACS], High Plains Division, Inc., 2008). To address this health disparity among medically underserved racial and ethnic groups, there must be increased education and awareness about PCa. The University of Texas MD Anderson Cancer Center Prostate Outreach Project (POP) was initiated in April 2003, and worked with community leaders to provide free PCa education and early detection services to medically underserved communities in Houston, Texas. The goal was to enhance access toward early detection of PCa while promoting informed decisions regarding testing. In this prospective study, the efficacy of the POP program along the continuum of recruitment education, early detection, and follow-up was evaluated to determine its impact as a potential strategy to reduce prostate cancer mortality among AA and other medically underserved groups.
Method
Participants
The POP prospectively accrued participants from April 2003 through September 2009. The initial participants targeted were AA from medically underserved communities in Houston (as well as surrounding Harris County) aged 40 to 70 years. Hispanic, Asian, and Caucasian men within the same or neighboring medically underserved communities were also later included due to the perceived need of such services. The term medically underserved refers to special populations who may be underinsured or uninsured people, possess low levels of education, live within rural and inner city populations, are unemployed, or those with low socioeconomic status (Haynes & Smedley, 1999). Those meeting the above definition were from communities where the median level of education was high school and the median annual household income was $20,000 to $50,000. Community information was obtained from the 2000 census.
Recruitment Strategies
POP received institutional review board approval to conduct the prospective study. Advertisement of the free education and screening events were promoted through mass media outlets such as magazines, radio, and flyers within a variety of community sites (churches, grocery stores, barbershops, community centers). Study recruitment occurred in two phases. In the “static” phase from April 2003 to August 2004, participants were invited to either a general hospital or a community health center that primarily served a large proportion of AA of low socioeconomic status. Men were solicited via flyers to make an appointment to attend free POP screening events located at either location. Interested community organizations scheduled sessions to discuss POP objectives, PCa information, and to schedule education/detection sessions. Screening events were held Tuesday evenings and Saturday mornings. The second “mobile” phase of the POP occurred from September 2004 to September 2009 and employed a refurbished bus to visit various locations. These mobile education/detection sessions occurred where participants were already congregated for preplanned activities such as church health fairs, barbershops, grocery stores, and homeless shelters.
Education/Early Detection Procedures
Figure 1 reports a diagram of participant involvement.
Figure 1.
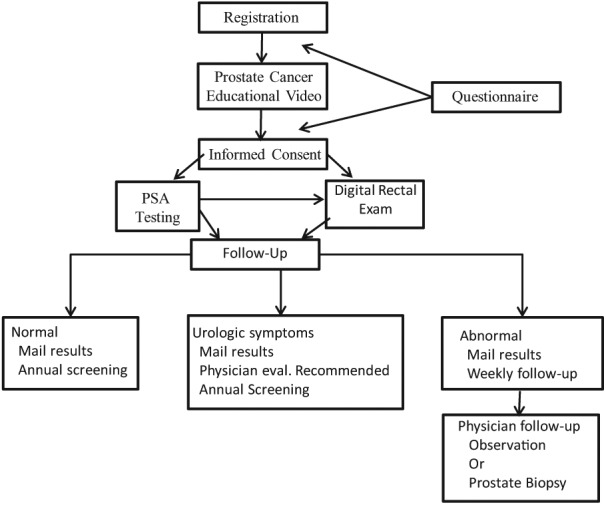
Prostate Outreach Project: Methodology.
Note. PSA = prostate-specific antigen. Prostate Outreach Project subject flow and methods.
Regardless of the recruitment strategy, participants completed a registration form that included demographics and items related to health access/socioeconomic status. They also completed a medical history form that included clinical history (i.e., other cancers, PCa cancer screening and family history, urologic symptoms, exposures, comorbid illness) and the American Urological Association Symptom Score (Barry et al., 1992). The POP educated men about PCa using a video format with the objective of comparing baseline performance on a knowledge survey before and after watching a prostate cancer educational video. The video titled Listen Up II: Prostate Cancer Awareness through Education (http://www3.mdanderson.org/streams/FullVideoPlayer.cfm?xml=prostate/config/KnowAboutProstate_102_cfg) reviewed information on PCa prevention, the risks and benefits of early detection testing utilizing serum PSA and DRE, as well as treatment options for PCa and complications. The video content was developed in collaboration with a multidisciplinary panel that included medical oncologists, radiation oncologists, urologists, medical illustrators, and members of the institution’s department of public education. The content was written at a 10th-grade Flesch–Kincaid reading level and incorporated early detection guidelines published by the ACS (Smith, Cokkindes, & Eyre, 2004). The video also served to inform underserved men about the risks and benefits of early detection for PCa utilizing serum PSA and DRE testing. Voice-over narration was available in Spanish, Chinese, and Vietnamese to facilitate education of diverse populations. A 13-item survey was developed that included 10 questions modified from (Ashford et al., 2001). The answers were reviewed in the video; see Supplementary Figure 1 (available online at http://ajmh.sagepub.com/content/by/supplemental-data). Participants were instructed to complete the survey, watch the Listen Up II video and then retake the same survey to compare test performance and efficacy of the video as an educational tool.
PCa Early Detection Testing
PCa early detection services were subsequently offered free of charge to eligible participants who signed an informed consent to participate in the study.
Subsequent to the blood draw for serum PSA testing study, participants had a DRE performed. Some participants did not complete one or both tests due to medical conditions, patient refusal, physician availability to perform DRE, or not meeting screening criteria. A serum PSA value greater than 2.5 ng/mL was considered abnormal as several studies reported that the incidence of PCa was similar among those with serum PSA values between 2.4 and 3.9 as those with serum PSA values greater than 4 ng/mL (Babaian et al, 2001; Catalona, Smith, & Ornstein, 1997; Thompson et al., 2004). Induration or frank nodules identified during DRE were considered abnormal. Thus, either an abnormal serum PSA level or DRE were considered an abnormal screening exam (ASE) and were indications for further evaluation and potential prostate biopsy.
Follow-Up
Patients with an ASE were encouraged to have follow-up care with their local physician, at the MD Anderson Cancer Center or affiliated hospitals. DRE results were provided to patients at the time of testing. Participants with an ASE received a registered letter within 7 to 10 days describing their serum PSA value, options for follow-up care, and notification that they would be contacted for follow-up from the POP staff (see Figure 1). Patients without medical insurance were instructed to contact a POP coordinator for enrollment in the Harris Health System Gold Card Program, which provides reduced cost (or free) health care services to low-income residents of Harris County at Harris hospitals and clinics. Attempts to contact patients with an ASE and move them to an endpoint of a physician evaluation occurred for at least 6 months before they were classified as lost to follow-up. Telephone calls and mailed reminders were used to communicate with participants. Patients with normal test results and no other symptoms or concerns were encouraged to share their screening results with their primary care provider and return for screening in 1 year.
Evaluation
The evaluation of the POP program components included analysis of (a) recruitment, (b) education, (c) PCa test results, (d) follow-up with recommendations among participants with an ASE, and (e) PCa detected among participants. The PCa endpoint included those men diagnosed with PCa whether or not they were diagnosed subsequent to POP follow-up. Among noncompliant POP participants diagnosed outside of the program, the Texas Cancer Registry (TCR) was utilized to identify PCa cases in the state from April 2003 to December 2011. Subsequent to institutional review board approval, specific POP data fields were provided to the TCR.
Subsequently, the TCR returned a data file of POP participants who had a diagnosis of PCa.
Statistical Analysis
Baseline characteristics were summarized using descriptive statistics and bar plots. Associations between categorical variables were assessed using chi-square tests or Fisher’s exact tests, as appropriate. Differences in continuous variables between two groups of participants were evaluated using Wilcoxon rank-sum tests. p Values less than .05 were deemed as statistically significant. All statistical analyses were performed using SAS 9.3. Percentages and numbers reported throughout the article represent known numerator and denominator values while censoring missing information. For data that were reported in a figure or table, the percentage and the numerator were provided in the article. For data not reported in a table or figure, the respective percentage, numerator, and denominators were provided.
Results
Participants
Table 1 provides descriptive data on the 4,420 men who were recruited over a 6-year period. The median age was 52 years (range, 27-84). AA or men of African descent composed 62.8% (2,667) of participants. Most participants had the equivalent of a high school education/technical school degree or less. One half of participants 53.6% (2,159) were uninsured and 41% (1,811) did not have access to a physician. The majority had no prior history of testing for PCa. Asian and Hispanic men were less likely to have had prior screening for PCa; 77% (272/353) and 72.9% (315/432) of men, respectively when compared with Caucasian or AA men 47.5% (48/101) and 49.8% (469/941), respectively. Thirty percent (709) of participants had a family history of PCa.
Table 1.
POP Subject Characteristics.
| Variable | Levels | Total |
|---|---|---|
| Education | Advanced degree | 371 (8.7%) |
| Bachelor’s degree | 569 (13.3%) | |
| Associate’s degree | 647 (15.1%) | |
| Technical school certificate | 409 (9.5%) | |
| GED/High School | 1,475 (34.4%) | |
| 9th grade to 11th grade | 472 (11%) | |
| 8th grade or less | 341 (8%) | |
| Missing | 136 | |
| Insurance | No | 2,159 (53.6%) |
| Yes | 1,867 (46.4%) | |
| Missing | 394 | |
| Race | Caucasian | 307 (7.2%) |
| African American | 2,667 (62.8%) | |
| Asian American | 137 (3.2%) | |
| Hispanic | 682 (16.1%) | |
| Native American | 8 (0.2%) | |
| Middle Eastern | 7 (0.2%) | |
| Asian | 401 (9.4%) | |
| Other | 40 (0.9%) | |
| Missing | 171 | |
| Physician | No | 1,811 (41%) |
| Yes | 2,609 (59%) | |
| Abnormal PSA (within 1 year) | No | 4,080 (96.4%) |
| Yes | 153 (3.6%) | |
| Missing | 187 | |
| Abnormal rectal exam (within 1 year) | No | 4,050 (95%) |
| Yes | 214 (5%) | |
| Missing | 156 | |
| Blood in Urine (within 1 year) | No | 4,027 (94%) |
| Yes | 257 (6%) | |
| Missing | 136 | |
| Burning with urination (within 1 year) | No | 3,898 (90.9%) |
| Yes | 390 (9.1%) | |
| Missing | 132 | |
| Prostate infection (within 1 year) | No | 4,129 (96.2%) |
| Yes | 164 (3.8%) | |
| Missing | 127 | |
| Have you ever been screened | No | 1,325 (62.8%) |
| Yes | 784 (37.2%) | |
| Missing | 2,311a | |
| AUA score | Mild (0-7) | 2,932 (72.4%) |
| Moderate (8-19) | 848 (20.9%) | |
| Severe (>19) | 268 (6.6%) | |
| Missing | 372 | |
| PCa family history | No | 1,635 (69.8%) |
| Yes | 709 (30.2%) | |
| Missing | 2,076a |
Note. GED = general educational development; AUA = American Urological Association; PCa = prostate cancer; POP = Prostate Outreach Project; PSA = prostate-specific antigen.
Question added to later in the course of study recruitment percentages were calculated using known numerator and denominator values, while the total number of missing information was censored from the percentiles.
Recruitment Strategies
Supplementary Figure 2a (available online at http://ajmh.sagepub.com/content/by/supplemental-data) reports the distribution of recruitment strategies between the static and mobile phases of recruitment as evidenced by how men came to learn about the POP events. For both phases, the promotion via mass media and word of mouth was often reported as the mechanism by which participants heard about the event. During the mobile phase, the percentage of men hearing about an event through the church increased to 17.4% (669) from 8.4% (48) as churches partnered with POP to promote screening onsite (see Supplementary Figure 2a-2b; available online at http://ajmh.sagepub.com/content/by/supplemental-data). A higher screening ratio was observed during the mobile phase compared with the static phase (p < .0001; see Supplementary Figure 2c; available online at http://ajmh.sagepub.com/content/by/supplemental-data). Participants recruited through the static phase were more likely to have a history of abnormal PSA test within the past year (p = .0004), have a history of hematuria within the past year (p = .0007), have history of burning sensation with urination (p = .0002), report a prostate infection (p = .03), and have a greater incidence of a positive family history of PCa compared with participants recruited from the mobile phase (p = .006; see Supplementary Table 1; available online at http://ajmh.sagepub.com/content/by/supplemental-data).
PCa Knowledge Assessment
Supplementary Figure 1 (available online at http://ajmh.sagepub.com/content/by/supplemental-data) reports the results of PCa knowledge assessment based on responses to 10 questions asked in the prevideo or postvideo watching period. This assessment was conducted among 719 men between March 2006 and March 2009. Adequate knowledge of PCa was demonstrated when participants correctly answered 8 out of 10 knowledge questions. This prevideo threshold was only achieved in 17.5% (126) men. However, post video, the threshold was achieved in 58.8% (399/678) men who took the survey representing over a threefold increase in knowledge among the participants. Factors associated with adequate baseline knowledge of PCa prior to watching the educational tool were: AA (p = .001), education level greater than high school (p < .001), positive family history of PCa (p = .03), possession of insurance (p = .01), and prior PCa testing (p < .001). At least 40% (288) or more of participants exhibited correct baseline knowledge regarding age of onset of PCa, family history, lifetime risk of developing the disease, urinary symptoms, prior tests for PCa, and age for AA to be tested.
PCa Testing
Supplementary Table 2 (available online at http://ajmh.sagepub.com/content/by/supplemental-data) provides early detection testing results among the 4,420 men. Overall, 98% (4,327) men had a serum PSA test. The mean, median, and range of serum PSA levels were 2.3 ng/mL, 0.8 ng/mL, and 0.1 ng/mL to 2,639 ng/mL, respectively. Ten percent of men (436) had a serum PSA ≥ 2.5 ng/mL. DRE was performed in 76.4% (3,375) men with 6.9% (233) identified as abnormal. Among patients having both a serum PSA test and a DRE, 85% (2,795) men were normal with 15% (493) men having either an abnormal DRE or PSA test (see Figure 2; see Supplementary Table 2; available online at http://ajmh.sagepub.com/content/by/supplemental-data).
Figure 2.
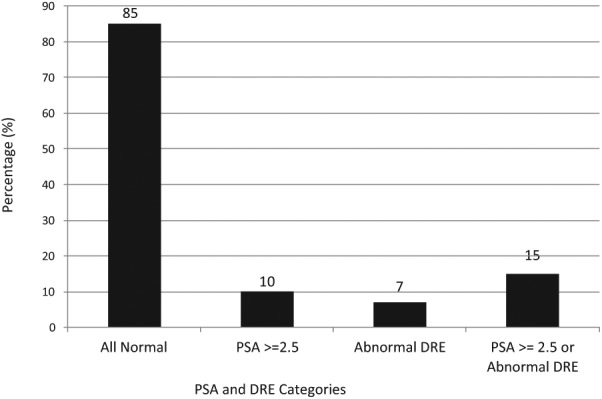
Distribution of test results among the cohort of 4,420 men.
Note. PSA = prostate-specific antigen; DRE = digital rectal examination. Serum PSA level ≥ 2.5 ng/mL or abnormal DRE considered indication for further evaluation.
Correlates of an abnormal DRE included race, p < .0001 (Asian, Other, AA abnormal = 7%-11% vs. Caucasian = 2.7%). Correlates of an abnormal serum PSA level included race, p < .0001 (Asian, Latin American, Other abnormal = 13%-21% vs. Latin American or AA, respectively, 6%-8.9% abnormal), burning with urination p = .003, and prostate infection within the year, p = .0001 (data not reported). Among men who had never been screened, AA men exhibited higher serum PSA levels than other racial/ethnic groups, p < .0001 (see Supplementary Table 3; available online at http://ajmh.sagepub.com/content/by/supplemental-data). When comparing serum PSA levels by race/ethnic group and whether or not participants had been previously screened, PSA levels were only significantly higher among the unscreened AA cohort: mean 4.59 ng/mL versus 1.18 ng/mL, p = .0001 (see Supplementary Table 3; available online at http://ajmh.sagepub.com/content/by/supplemental-data).
POP Subject Follow-up
About 14% (609) of screened participants had an abnormal screening event. Of the 40% of men (245) who had adequate follow-up, 47% (116) underwent a prostatic biopsy, the other 53% (129) men underwent continued observation (see Table 2). Those having a biopsy were significantly more likely to have an elevated serum PSA greater than 4.0 ng/dL (p = .01) as well as a history of an elevated PSA within the past year (p < .00) compared with an abnormality on DRE (p = .89; data not reported). Among the POP participants who were actually biopsied, 47% (55) were reported to have PCa and the remainder benign findings (see Table 2). Participants with a positive biopsy of the prostate were more likely to have a history of burning on urination within the past year (p = .02; data not reported). Among the 129 participants who had adequate follow-up, the decision to postpone biopsy was made by either the patient or physician. These participants were less likely than those who underwent biopsy to have had a history of an abnormal serum PSA level within the past year (p = .001) and also less likely to have an elevated serum PSA level at the time of screening (p = .01; data not reported). About 60% (364) men were lost to follow-up after an abnormal screening result after lack of response to repeated measures of contact over 6 months (see Table 2). Participants who had follow-up were more likely to be AA race versus others (p < .001), had a physician (p < .001), had insurance (p < .001). In addition those with a higher likelihood of follow-up were more likely to be recruited from the static POP locations versus the mobile program (p = .001). Among mobile phase participants, those recruited via church events versus community centers and grocery stores were more likely to follow-up with POP (p = .02; see Supplementary Table 4; available online at http://ajmh.sagepub.com/content/by/supplemental-data). Based on a limited experience with additional repeat contact attempts after being categorized as lost to follow-up, several themes emerged as to reasons for lack of follow-up: these included participants stating they were unaware of being lost to follow-up, incorrect contact information, language barriers, and preference to follow-up with other physicians.
Table 2.
Prostate Cancer Status by Follow-Up Grouping.
| Prostate cancer status according to POP follow-up and Texas Cancer Registry Data | ||
|---|---|---|
| POP follow-up status | POP abnormal test (N = 609) | POP abnormal reported to TCR as PCa |
| Prostate Biopsy—Cancer | 55 | 47a |
| Prostate Biopsy—No cancer | 61 | 10b |
| No biopsy performed | 129 | 7b |
| Lost to follow-up | 364 | 40b |
| No immediate follow-up indicatedc | POP normal tests 3,811 | POP normal reported to TCR as PCa 32b |
Note. DRE = digital rectal examination; PCa = prostate cancer; POP = Prostate Outreach Project; TCR = Texas Cancer Registry. Those with abnormal screening examination results (abnormal DRE and/or abnormal PSA) were recommended to follow-up with their primary care provider for discussion about undergoing a prostatic biopsy to rule out clinically significant prostate cancer.
Eight PCA diagnosed via POP were not reported to TCR.
PCA diagnosed outside of POP, 6 months to follow-up period and reported to TCR.
POP participants with normal serum PSA (<2.5 ng/mL) and normal DRE.
PCa Detection
Overall, 3.3% (144) participants were diagnosed with PCa (see Supplementary Table 5; available online at http://ajmh.sagepub.com/content/by/supplemental-data). Utilizing the TCR database to correlate POP participants who were diagnosed either within the POP follow-up program or outside of the program was informative (see Table 2; see Supplementary Table 5; available online at http://ajmh.sagepub.com/content/by/supplemental-data). Of the cases of PCa diagnosed within the POP, 85% (47) were reported to the TCR. However, of the total cases of PCa only 38% (55) were diagnosed within the POP framework. Thus, most of the men who were diagnosed were reported to have PCa via mechanisms outside of the program at a median time of 31.2 months (0.3-98.8 months) from the date of their POP detection event. Table 2 and Supplementary Table 5 (available online at http://ajmh.sagepub.com/content/by/supplemental-data) provide a breakdown of the subsequent cancer diagnosis according to POP follow-up status via cases reported to TCR. Of note, 0.8% (32/3,811) participants with initial normal testing results were subsequently reported to have cancer during the follow-up period. Overall, participants with an abnormal DRE or serum PSA test were significantly more likely to be diagnosed with PCa (p < .0001) as were AA participants when compared with men of other racial groups (p = .002; see Supplementary Table 5; available online at http://ajmh.sagepub.com/content/by/supplemental-data).
Discussion
To our knowledge, the POP represents the largest education and early detection experience reported to date among AA and the medically underserved. Our goal was to promote PCA awareness while capturing baseline data about PCa education, early detection, and follow-up among such populations recruited in the community setting. POP was initiated in set locations in two largely AA communities that were easily accessible. However, men who did attend sessions at the static POP location appeared motivated as evidenced by an increased incidence of prior abnormal serum PSA levels, prostate-related symptoms (i.e., dysuria, hematuria, prostate infection) or family history of PCa.
Use of a mobile strategy resulted in a fourfold increase in participation. This is consistent with the observations of Weinrich, Boyd, Bradford, Mossa, and Weinrich (1998) and Powell et al. (1997) who observed that recruitment of AA men, educational assessment, and PCa screening were most successful when conducted simultaneously at natural gathering places such as work and church sites. Giri et al. (2009) previously reported that the “show rate” for evaluation was adversely affected by AA race, lower education level, not being married/partnered, being unemployed, lower income, and having no family history of PCa while using a model which separated initial recruitment from evaluation. Similar adverse risk factors were common to the POP population.
Education
While controversy exists as to the benefit of screening for PCa, there is consensus that on an individual level, the risks and benefits of PCa detection utilizing PSA should be discussed with men prior to ordering the test (Andriole et al., 2009; Moyer, 2012; Schröder et al., 2009). Furthermore, the ACS and others have proposed the elements of such a discussion to facilitate informed decision making (Miller et al., 2009;). Among our medically underserved cohort, over 40% of participants had no primary physician. A community-based program that incorporated an educational component prior to testing would be valuable to provide medically underserved men with an opportunity to make an informed decision about PCa testing and receive testing should they desire. Among our cohort, only 17% of participants exhibited adequate baseline knowledge. Magnus (2004) also noted that only 19% of a multiethnic cohort of men in Florida were able to answer 80% of PCa knowledge questions correctly. Baseline knowledge was correlated with factors similar to the POP cohort and other studies (Magnus, 2004; Smith, Dehaven, Grundig, & Wilson, 1997; Weinrich et al., 2004).
Using a pre/post video test format demonstrated that PCa knowledge increased significantly among study participants as the percentage of men that correctly answered 8 of 10 questions correctly increased. Prior studies have also reported that such a format can be utilized among men with varying literacy levels, does not lengthen appointment times, can change a participant’s mind with respect to their desire for testing, and that knowledge may be retained (Gattellari & Ward, 2005; Ross, Ashford, Bleechington, Dark, & Erwin, 2010; Ruthman & Ferrans, 2004, Volk, Spann, Cass, & Hawley, 2003). Participant knowledge appeared to improve on certain survey questions but not with others, whereas the video content was written at a 10th-grade reading level health literacy was not specifically measured in our study. Recently, Kilbridge et al. (2009) reported that commonly utilized PCa terms such as incontinence, erection, and impotent were not understood among an indigent AA population.
Early Detection, Follow-Up, and Cancer Incidence
Early detection testing was highly successful in this contemporary community-acquired screening experience among a sizable multiethnic, largely AA, medically underserved cohort. Among the men having both screening tests, 15% of participants had an abnormal ASE. Two prior studies carried out in AA cohorts in the United States revealed that the incidence of an ASE was 14.9% to 18% using a serum PSA cutoff of 4 ng/mL with the higher value noted in an older cohort of men (mean age 59.5 years; Shelton et al., 2005; Smith, Bullock, Catalona, & Herschman, 1996). Given that our serum PSA cutoff was lower (i.e., 2.5 ng/mL), one might have expected a higher incidence of ASEs. However, this is likely explained by the relatively young age of our participants (median = 52 years) along with the lower percentage of AA participants in our cohort. Additionally, among men who had never been screened, serum PSA levels among the AA cohort were significantly higher than all the other groups. This suggests the possibility of finding more asymptomatic PCa among the previously unscreened AA cohort, especially given the extreme range in values noted.
Frequent communication was made to POP participants with ASE; yet 40% (245) of participants were compliant with follow-up and saw a physician. Specific data indicating why each patient did not have a prostatic biopsy is not available but some common themes include, “wanting to follow or repeat the serum PSA later” or that a repeat DRE was identified as normal by their physician. In addition, men who were biopsied had higher serum PSA levels that were more likely to be >4 ng/mL. Similar findings were noted by Catalona et al. (1997) who previously noted that only 36% of eligible participants with serum PSA levels between 2.6 ng/mL and 4 ng/mL elected to undergo a prostate biopsy.
Despite our follow-up efforts, over half were lost to follow-up. The patient navigator approach was thought to be the missing link between vulnerable populations and improved health care outcomes and utilization, but the POP’s unexpected lost to follow-up rate highlights the variable effectiveness of follow-up strategies with regard to health care delivery to medically underserved populations. One veterans affairs study reported that one third of patients with abnormal PCA screening results had incomplete follow-up, even within a relatively equal access health care system. Correlates of incomplete follow-up in this largely Caucasian population included veterans affairs center location, whether a copay was required, urologic symptoms, the number of prior PSA tests, and the level of serum PSA (Zeliadt, Hoffman, Etzioni, Ginger, & Lin, 2010). Some similar variables were visible within our population.
In general, the participants screened at static locations were more symptomatic than those tested during the mobile program and this may have led to their enhanced follow-up. In addition, those participants not having a physician were more likely to receive follow-up within POP. This is consistent with our informal observation that participants with a physician were often likely to follow-up via their physicians and thus did not feel the need to participate further. Surprisingly, some patients reported incorrect contact information leading to our inability to follow-up with a portion of the participants with abnormal tests. Myers et al. (2000) previously reported a “low intention to adhere” to follow-up among a cohort of AA men, reasons included physical discomfort with exam, concerns about cost and detection of cancer.
The major limitations of our study include the high percentage of participants who were lost to follow-up, as well as incomplete ascertainment for the reasons for the lack of follow-up. As a result, the estimates of PCa among our population may be underestimated. Notwithstanding the studies limitations, the POP experience with recruitment of medically underserved AA and other populations for the purpose of PCa education and detection represents the largest yet reported and reveals that: (a) men were receptive to undergoing an education session as well as the collection of blood and DRE testing in the community setting and that (b) baseline PCa knowledge was enhanced utilizing a video format to inform men about PCa as well as the risks and benefits of PCa testing. However, an unexpected finding was a disconnect between willingness to be educated and screened for PCa and follow through with recommendations for participants with an ASE. Potential solutions likely to improve follow-up outcomes of POP include (a) utilization of mechanisms to ensure validity of participant’s contact information in addition to the (b) availability of point of care PSA testing (Karim et al., 2007). With accurate collection of participants’ contact information (through verification processes such as a driver’s license or photo ID) and availability of PSA results during the initial encounter, physicians and patient navigators could potentially address the socioeconomic and psychosocial factors that may affect a participant’s intention to follow-up with appropriate medical management. For patients with their own physicians, ensuring that their physicians are also involved in the follow-up process would assist with providing enhanced continuity of care. In the end, given that PCa early detection benefits with respect to cancer mortality are mediated at least in part via earlier detection of clinically significant cancers and effective treatment (Schröder et al., 2009). Our data highlight a significant gap in the continuum of care between detection and treatment that could continue the disparity in PCa outcomes among medically underserved men including AA if not addressed.
Supplementary Material
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Grants from the Centers for Disease Control and Prevention, Department of Health and Human Services (U48 CCU06195155, H75 CCH624031), the Texas Cancer Council (05-19,06-19), The University of Texas MD Anderson Cancer Center, Procter & Gamble Company, as well as philanthropic support from the TEX US TOO Prostate Cancer Support Group. Omotola Ashorobi was supported by a cancer prevention fellowship from the National Cancer Institute R25E CA56452.
References
- American Cancer Society, High Plains Division, Inc. (2008). 2008 Texas cancer facts & figures. Austin, TX: Author; Retrieved from http://www.texascancer.info/pdfs/factsandfigs2008.pdf [Google Scholar]
- Andriole G. L., Crawford E. D., Grubb R. L., Buys S. S., Chia D., Church T. R. (2009). Mortality results from a randomized prostate-cancer screening trial. New England Journal of Medicine, 360, 1310-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashford A. R., Albert S. M., Hoke G., Cushman L. F., Miller D. S., Bassett M. (2001). Prostate carcinoma knowledge, attitudes, and screening behavior among African-American men in Central Harlem, New York City. Cancer, 91, 164-172. [DOI] [PubMed] [Google Scholar]
- Babaian R. J., Johnston D. A., Naccarato W., Ayala A., Bhadkamkar V. A., Fritsche H. A., Jr. (2001). The incidence of prostate cancer in a screening population with a serum prostate specific antigen between 2.5 and 4.0 ng/mL: Relation to biopsy strategy. Journal of Urology, 165, 757-760. [PubMed] [Google Scholar]
- Barry M., Fowler F. J., O’Leary M. P., Bruskewitz R. C., Holtgrewe H. L., Mebust W. K. (1992). The American Urological Association symptom index for benign prostatic hyperplasia: The Measurement Committee of the American Urological Association. Journal of Urology, 148, 1549-1557. [DOI] [PubMed] [Google Scholar]
- Catalona W. J., Smith D. S., Ornstein D. K. (1997). Prostate cancer detection in men with serum PSA concentrations of 2.6 to 4.0 ng/mL and benign prostate examination: Enhancement of specificity with free PSA measurements. Journal of the American Medical Association, 277, 1452-1455. [PubMed] [Google Scholar]
- Fedewa S. A., Etzioni R., Flanders W. D., Jemal A., Ward E. M. (2010). Association of insurance and race/ethnicity with disease severity among men diagnosed with prostate cancer, National Cancer Database 2004-2006. Cancer Epidemiology, Biomarkers & Prevention, 19, 2437-2444. [DOI] [PubMed] [Google Scholar]
- Gattellari M., Ward J. E. (2005). A community-based randomized controlled trial of three different educational resources for men about prostate cancer screening. Patient Education & Counseling, 57, 168-182. [DOI] [PubMed] [Google Scholar]
- Giri V. N., Coups E. J., Ruth K., Goplerud J., Raysor S., Kim T. Y. (2009). Prostate cancer early detection program recruitment methods and show rates in men at high risk. Journal of Urology, 182, 2212-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes M. A., Smedley B. D. (1999). The unequal burden of cancer: An assessment of NIH research and programs for ethnic minorities and the medically underserved. Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- Hoffman R. M., Gilliland F. D., Eley J. W., Harlan L. C., Stephenson R. A., Standford J. L. (2001). Racial and ethnic differences in advanced-stage prostate cancer: The Prostate Cancer Outcomes Study. Journal of the National Cancer Institute, 93, 388-395. [DOI] [PubMed] [Google Scholar]
- Jemal A., Simard E. P., Dorell C., Noone A., Markowitz L. E., Kohler B. (2013). Annual report to the nation on the status of cancer, 1975-2009, featuring the burden and trends in human papillomavirus (HPV)–associated cancers and HPV vaccination coverage levels. Journal of the National Cancer Institute, 105, 175-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim O., Rao A., Emberton M., Cochrane D., Partidge M., Edwards P. (2007). Point-of-care PSA testing: An evaluation of PSA watch. Prostate Cancer and Prostatic Diseases, 10, 270-273. [DOI] [PubMed] [Google Scholar]
- Kilbridge K. L., Fraser G., Krahn M., Nelson E. M., Conaway M., Bashore R. (2009). Lack of comprehension of common prostate cancer terms in an underserved population. Journal of Clinical Oncology, 27, 2015-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnus M. (2004). Prostate cancer knowledge among multiethnic black men. Journal of the National Medical Association, 96, 650-656. [PMC free article] [PubMed] [Google Scholar]
- Miller D. C., Litwin M. S., Bergman J., Stepanian S., Connor S. E., Kwan L. (2009). Prostate cancer severity among low-income, uninsured men. Journal of Urology, 181, 579-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer V. A. (2012). Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Annals of Internal Medicine, 157, 120-134. [DOI] [PubMed] [Google Scholar]
- Myers R. E., Hyslop T., Wolf T. A., Burgh D., Kunkel E. J., Oyesanmi O. C. (2000). African-American men and intention to adhere to recommended follow-up for an abnormal prostate cancer early detection examination result. Urology, 55, 716-720. [DOI] [PubMed] [Google Scholar]
- Pinsky P. F., Ford M., Gamito E., Higgins D., Jenkins V., Lamerato L. (2008). Enrollment of racial and ethnic minorities in the prostate, lung, colorectal and ovarian cancer screening trial. Journal of the National Medical Association, 100, 291-298. [DOI] [PubMed] [Google Scholar]
- Powell I. J., Helibrun L., Littrip P. L., Franklin A., Parzuchowski J., Gelfand D. (1997). Outcome of African American men screened for prostate cancer: The Detroit Education and Early Detection Study. Journal of Urology, 158, 146-149. [DOI] [PubMed] [Google Scholar]
- Ross L., Ashford A. D., Bleechington S. J., Dark T., Erwin D. O. (2010). Applicability of a video intervention to increase informed decision making for prostate-specific antigen testing. Journal of the National Medical Association, 102, 228-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruthman J. L., Ferrans C. E. (2004). Efficacy of a video for teaching patients about prostate cancer screening and treatment. American Journal of Health Promotion, 18, 292-295. [DOI] [PubMed] [Google Scholar]
- Schröder F. H., Hugosson J., Robol M. J., Tammela T. L., Ciatto S., Nelen V. (2009). Screening and prostate-cancer mortality in a randomized European study. New England Journal of Medicine, 360, 1320-1328. [DOI] [PubMed] [Google Scholar]
- Shelton J. B., Barocas D. A., Conway F., Hart K., Nelson K., Richstone L. (2005). Prostate-specific antigen screening in a high-risk population: Lessons from the community and how they relate to large-scale population-based studies. Urology, 65, 931-936. [DOI] [PubMed] [Google Scholar]
- Smith D. S., Bullock A. D., Catalona W. J., Herschman J. D. (1996). Racial differences in a prostate cancer screening study. Journal of Urology, 156, 1366-1369. [PubMed] [Google Scholar]
- Smith G. E., Dehaven M. J., Grundig J. P., Wilson G. R. (1997). African-American males and prostate cancer: Assessing knowledge levels in the community. Journal of National Medical Association, 89, 387-391. [PMC free article] [PubMed] [Google Scholar]
- Smith R. A., Cokkindes V., Eyre H. J. (2004). American Cancer Society guidelines for the early detection of cancer. CA: A Cancer Journal for Clinicians, 54, 41-52. [DOI] [PubMed] [Google Scholar]
- Thompson I. M., Pauler D. K., Goodman P. J., Tangen C. M., Lucia M. S., Parnes H. L. (2004). Prevalence of prostate cancer among men with a prostate-specific antigen level ≤4.0 ng per milliliter. New England Journal of Medicine, 350, 2239-2246. [DOI] [PubMed] [Google Scholar]
- Volk R. J., Spann S. J., Cass A. R., Hawley S. T. (2003). Patient education for informed decision making about prostate cancer screening: A randomized controlled trial with 1-year follow-up. Annals of Family Medicine, 1, 22-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinrich S. P., Boyd M. D., Bradford D., Mossa M. S., Weinrich M. (1998). Recruitment of African Americans into prostate cancer screening. Cancer Practice, 6, 23-30. [DOI] [PubMed] [Google Scholar]
- Weinrich S. P., Seger R., Miller B. L., Davis C., Kim S., Wheeler C. (2004). Knowledge of the limitations associated with prostate cancer screening among low-income men. Cancer Nursing, 27, 442-453. [DOI] [PubMed] [Google Scholar]
- Zeliadt S. B., Hoffman R. M., Etzioni R., Ginger V. A. T., Lin D. W. (2010). What happens after an elevated PSA test: The experience of 13,591 veterans. Journal of General Internal Medicine, 25, 1205-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


