Abstract
Objective
To evaluate trajectories of and predictors for changes in upper extremity (UE) function in women (n=396) during the first year following breast cancer treatment.
Design
Prospective, longitudinal assessments of shoulder range of motion (ROM), grip strength, and perceived interference of function were performed prior to and for one year following surgery. Demographic, clinical, and treatment characteristics were evaluated as predictors of postoperative function.
Results
Women were 54.9 years (SD 11.6) of age and 64% were white. Small, but statistically significant reductions in shoulder ROM were found on the affected side over 12 months (p<0.001). Predictors of inter-individual differences in ROM at the one month assessment were: ethnicity, neoadjuvant chemotherapy, type of surgery, axillary lymph node dissection (ALND), and preoperative ROM. Predictors of inter-individual differences in changes over time in postoperative ROM were: living alone, type of surgery, ALND, and adjuvant chemotherapy. Declines in mean grip strength from prior to through one-month after surgery were small and not clinically meaningful. Women with greater preoperative breast pain interference scores had higher postoperative interference scores at all postoperative assessments.
Conclusion
Some of the modifiable risk factors identified in this study can be targeted for intervention to improve UE function in these women.
Keywords: upper extremity, mobility, function, grip strength, range of motion, breast cancer
INTRODUCTION
Over half of the women treated for invasive breast cancer will face one or more long-term upper extremity (UE) impairments.1 Lymphedema, pain, weakness, and limitations in shoulder range of motion (ROM) are linked to decreases in UE function and quality of life (QOL).1–5 Predictors of reductions in UE function after breast cancer treatment include older age, greater body mass index (BMI), having a mastectomy versus breast conserving surgery (BCS), having an axillary lymph node dissection (ALND), and receipt of radiation therapy and chemotherapy (CTX).1,6 Identification of additional predictors, particularly modifiable preoperative predictors, could have a significant impact on recovery of function following breast cancer treatment.
Changes in UE function are evaluated by self-report and through assessments of ROM and strength. In a prospective study of 115 women with Stage 0–III breast cancer,6 reductions in flexion and abduction ROM were found in 60% of the women 1 month after breast cancer surgery. Among the 10% of women whose ROM reductions persisted at 12 months, characteristics associated with these reductions included a higher number of positive lymph nodes, older age, and BMI >25 kg/m2. In a study that evaluated the impact of early postoperative shoulder function on shoulder function at 12 months post treatment for Stage I to IIb breast cancer,1 external rotation and combined abduction–external rotation at 6 weeks were the strongest predictors of external rotation and combined abduction–external rotation at 12 months. Similarly, degree of shoulder abduction at 6 weeks was an independent predictor of shoulder abduction at 12 months. Importantly, 24% of the women in this study had a clinically meaningful restriction in shoulder abduction ROM (i.e., ≥20 degrees) on their treated versus their untreated side at 12 months.
Upper extremity strength is commonly measured by assessment of grip strength.7 Sagen et al.8 found an 11% bilateral reduction in grip strength from preoperative levels to 2.5 years following surgery, in women who had an ALND. In a prospective evaluation of changes in grip strength in women taking aromatase inhibitors or tamoxifen,9 musculoskeletal pain complaints were increased and grip strength was decreased in both groups at 12 months.
However, no information is available on the trajectories of change in UE function following breast cancer treatment and the predictors of inter-individual differences in these trajectories. Particularly missing is the impact of preoperative UE function (i.e., shoulder ROM) on postoperative UE function. Identification of modifiable preoperative characteristics that can be targeted for early intervention, even before surgery, may minimize the occurrence and severity of treatment-related UE impairments. Therefore, the purposes of this prospective, longitudinal study were to evaluate for changes in UE function (i.e., shoulder ROM, grip strength, and functional interference due to breast pain) in women during the first year following breast cancer treatment and to evaluate for demographic and clinical characteristics that predicted inter-individual differences in these trajectories.
MATERIALS AND METHODS
Patients and Settings
This study is part of a larger prospective longitudinal study that evaluated for neuropathic pain and lymphedema in a sample of women who underwent breast cancer surgery. The methods for this study are described in detail elsewhere.10–13 Patients were recruited from Breast Care Centers located in a Comprehensive Cancer Center, two public hospitals, and four community practices. Patients were eligible to participate if they: were adult women (>18 years) who would undergo unilateral breast cancer surgery on one breast; were able to read, write, and understand English; agreed to participate; and gave written informed consent. Patients were excluded if they were having bilateral breast cancer surgery or had distant metastases at the time of diagnosis. Of the 516 patients approached, 410 were enrolled (response rate 79.5%), and 396 completed the study. The major reasons for refusal were: too busy, overwhelmed with the cancer diagnosis, or insufficient time available to do the assessment prior to surgery.
Subjective measures
The demographic questionnaire obtained information on age, marital status, education, ethnicity, employment status, living situation, activity level, and financial status. The Self-Administered Comorbidity Questionnaire (SCQ) was used to assess comorbidities.14 The Karnofsky Performance Status (KPS) scale was used to evaluate functional status (i.e., range from 30 (I feel severely disabled and need to be hospitalized) to 100 (I feel normal; I have no complaints or symptoms)).15
The breast pain functional interference scale was adapted from the Brief Pain Inventory (BPI).16 This valid and reliable scale was used to evaluate the extent to which breast pain interfered with the patient’s ability to function.17,18 In addition to the original eight items on the BPI interference scale (i.e., general activity, mood, walking ability, normal work, relations with other people, sleep, enjoyment of life, sexual activity), nine activities from the work of Tasmuth et al.,19,20 were evaluated (i.e., ability to sleep on the operated side, ability to touch the site, ability to reach out in front, ability to carry things, ability to get up from bed, ability to do handicrafts, ability to drive a car, ability to write, and ability to reach overhead). Patients were asked to evaluate the degree to which pain interfered with each of these activities during the past week, using a 0 (does not interfere) to 10 (completely interferes) numeric rating scale (NRS). Scores on these nine individual UE items were averaged to obtain an UE total interference score that ranged from 0 to 10. Higher scores indicate greater interference with function.
Objective Measures
Active ROM was assessed using a goniometer and standardized procedures reported by Norkin and White.21 Bilateral shoulder abduction and flexion were assessed with the patient supine. Two measurements were taken for each motion and a mean obtained. Grip strength was assessed using a Jamar hydraulic hand dynamometer (Patterson Medical, Bolingbrook, IL). Patients stood with the arm held down at their side and were instructed to squeeze with maximal effort. Three trials for each extremity were done and a mean grip score was calculated. BMI was calculated from height and weight, which were assessed at enrollment and at each postoperative assessment.
Study Procedures
The study was approved by the Committee on Human Research at the University of California, San Francisco and by the Institutional Review Boards at each of the study sites. During the patient’s preoperative visit, a clinician explained the study to the patient, determined the patient’s willingness to participate, and introduced the patient to the research nurse. The research nurse determined patient eligibility. After providing written informed consent, patients completed the study questionnaires. The research nurse then performed the objective measurements. Disease and treatment information were abstracted from the patients’ medical records. The research nurse met with the patients either in their home or in the clinic at 1, 2, 3, 4, 5, 6, 8, 10, and 12 months after surgery. During each of the study visits, patients completed the study questionnaires and had the objective measures assessed by the research nurse.
Data analysis
Descriptive statistics and frequency distributions were generated for demographic and clinical characteristics using SPSS Statistics for Windows, Version 22.0 (IBM Corporation, Armonk, NY). Comparisons of shoulder ROM and grip strength between the preoperative and the 12-month assessments were done using paired t-tests. Differences between ROM on the unaffected and affected sides were calculated to determine how many women had interlimb differences of ≥20 degrees at 12 months. This degree of change is associated with impairment in activities of daily living.1,22 Differences in proportions were evaluated using the McNemar’s test.
Hierarchical linear modeling (HLM), based on full maximum likelihood estimation, was done using the software developed by Raudenbush and colleagues.23 With HLM, the repeated measures for ROM, grip strength, and breast pain interference scores are nested within individuals and the analysis of change in these outcomes has two levels: within person variability (Level 1) and between persons variability (Level 2).23 Separate HLM analyses were done to evaluate for changes over time in each outcome for the at-risk limb (i.e., the arm on the operated side). Each HLM analysis proceeded in two stages. First, within person variability (Level 1) in the outcome measure over time was examined. Four Level 1 models were compared, which represented 1) change over time (i.e., time effect), 2) change at a constant rate (i.e., linear time effect), 3) change at a rate that accelerates or decelerates over time (i.e., quadratic effect), and 4) change in the pattern of acceleration/deceleration (i.e., cubic effect). The Level 1 model was constrained to be unconditional (i.e., no predictors) and likelihood ratio tests were used to determine the best model.
The second stage of the HLM analysis examined inter-individual differences (Level 2) in the trajectories of each of the outcomes by modeling the individual change parameters (i.e., intercept, linear, quadratic, cubic) as a function of the proposed predictors. A list of predictors for each outcome can be found in online supplemental Tables S1 and S2. To improve estimation efficiency and construct a parsimonious model, exploratory Level 2 analyses were done in which each potential predictor was assessed to see if it would result in a better fitting model if it alone was added as a Level 2 predictor. Predictors with a t-value of ≤2.0, which indicates a lack of a significant effect, were dropped from subsequent model testing.23 All of the potentially significant predictors from the exploratory analyses were entered into the model to predict each individual change parameter. Only predictors that maintained a significant contribution in conjunction with other variables were retained in the final model. Statistical significance was set at a p-value of <0.05. Once the significant predictors were identified, they were categorized for graphical display to illustrate their effects on the dependent variable. Continuous predictor variables were dichotomized using the convention of 1 standard deviation above and below the mean value for the study participants.
The intercepts reflect the predicted mean values of the outcomes at the one-month postoperative assessment and the trajectories reflect the changes in the postoperative outcomes during the year following breast cancer surgery. In each of the figures, the preoperative value for the UE outcome is plotted as a dotted line across the 12 months of the study.
RESULTS
Patient characteristics
Of the 396 women who completed the study, 64% were White, 89% were right handed, and 47% had their cancer on the side of their dominant limb. Eighty percent had breast-conserving surgery (BCS) and 20% had a mastectomy. Forty-four percent of the women in this study had Stage II disease or higher (Table 1).
Table 1.
Patient characteristics at enrollment (unless otherwise indicated) n = 396
| Characteristic | Number (%) | Mean (SD) |
|---|---|---|
| Age (years) | 54.9 (11.6) | |
| Body mass index (kilogram/meter2) | 26.8 (6.2) | |
| Karnofsky Performance Status score | 93.2 (10.3) | |
| Symptom Comorbidity Questionnaire score | 4.3 (2.8) | |
| Years of education | 15.7 (2.7) | |
| Employed | 189 (47.7%) | |
| Ethnicity | ||
| Caucasian – White | 255 (64.2%) | |
| Asian or Pacific Islander | 50 (12.6%) | |
| Black | 39 (9.8%) | |
| Other | 36 (9.1%) | |
| Hispanic | 17 (4.3%) | |
| Married or partnered | 164 (41.4%) | |
| Living alone | 94 (23.7%) | |
| Affected side | ||
| Right | 188 (47.2%) | |
| Left | 209 (52.8%) | |
| Right handed | 355 (89.4%) | |
| Affected side = Dominant side | 186 (47.0%) | |
| Exercised on a regular basis prior to surgery | 275 (69.1%) | |
| Stage of cancer at diagnosis | ||
| Stage 0 | 73 (18.3%) | |
| Stage 1 | 151 (37.9%) | |
| Stage IIA and IIB | 141 (35.4%) | |
| Stage IIIA–IV | 33 (8.3%) | |
| Received neoadjuvant chemotherapy | 79 (19.9%) | |
| Type of surgery | ||
| Breast conserving surgery | 318 (80.3%) | |
| Mastectomy | 77 (19.7%) | |
| Sentinel node biopsy | 327 (82.6%) | |
| Axillary lymph node dissection | 147 (37.1%) | |
| Number of nodes removed | 5.8 (6.7) | |
| Surgical drain placed at time of surgery | 158 (39.9%) | |
| Received adjuvant chemotherapy during first year | 136 (34.3%) | |
| Received adjuvant radiation therapy during first year | 280 (70.7%) | |
| Underwent reconstruction at time of surgery | 86 (21.7%) | |
| Underwent reconstruction during first year | 46 (11.6%) | |
| Underwent re-excision or mastectomy during first year | 119 (30.1%) | |
| Prior surgery to either arm unrelated to cancer | 25 (6.3%) | |
| Prior surgery to either hand unrelated to cancer | 32 (8.1%) | |
| Prior injury to either arm | 90 (22.7%) | |
| Prior injury to either hand | 89 (22.4%) | |
| Used non-steroidal anti-inflammatory drugs postoperatively | 16 (4.0%) | |
| Received physical therapy during 1st year | 95 (24.0%) | |
| Breast pain interference score (0–10 scale) | 0.3 (1.0) | |
SD: tandard deviation
Preoperative and 12 month postoperative comparisons of ROM and grip strength
Comparisons between preoperative and 12-month postoperative values for shoulder ROM and for grip strength were performed bilaterally (Table 2). Mean shoulder flexion and abduction ROM of the affected limb were slightly lower at 12 months compared to preoperative levels.
Table 2.
Comparison of shoulder ROM and grip at preoperative and 12 month postoperative visits
| Range of motion (in degrees) | Pre-operative | 12 months post-operative | Difference in means (95% CI) | Significance (p-value) | |
|---|---|---|---|---|---|
| Shoulder abduction (degrees) | |||||
| Unaffected side | Mean (SD) | 150.4 (18.7) | 152.3 (18.2) | 1.9 (0.1, 3.8) | 0.04 |
| Affected side | Mean (SD) | 149.5 (20.2) | 144.0 (24.2) | −5.5 (−7.8. −3.2) | < 0.001 |
| Shoulder flexion (degrees) | |||||
| Unaffected side | Mean (SD) | 166.7 (9.7) | 166.9 (10.0) | 0.2 (−0.8, 1.1) | 0.74 |
| Affected side | Mean (SD) | 166.0 (10.3) | 163.9 (11.9) | −2.2 (−3.3, −1.1) | < 0.001 |
| Grip (kilograms) | |||||
| Unaffected side | Mean (SD) | 23.8 (5.7) | 24.3 (5.6) | 0.5 (0.2, 0.9) | 0.003 |
| Affected side | Mean (SD) | 23.6 (5.5) | 24.0 (5.9) | 0.4 (−0.02, 0.8) | 0.06 |
Affected side – side of breast cancer
Paired t tests were used to evaluated differences in means
ROM: range of motion SD: standard deviation
However, 16.6% of the women had ≥20 degrees of restriction in shoulder abduction ROM on their affected side compared to their unaffected side at 12 months, compared to only 7.8% preoperatively (p< 0.001). Similarly, more women had ≥20 degrees restriction of shoulder flexion at 12 months compared to preoperatively (4.0% versus 1.8% respectively), although this difference did not reach statistical significance (p=0.057).
Hierarchical linear modeling
The first stage of the HLM analysis was performed for each outcome to evaluate for changes over time in abduction, flexion, grip strength, and breast pain interference on the affected side over the 12-month postoperative period. Linear, quadratic, and cubic models were estimated. For each outcome, the final estimate of the fixed effects determined that a cubic model fit the data best (Tables 3 and 4). In the second phase of the HLM analyses, variability in the trajectories of change in each outcome based on specific demographic, clinical, and treatment characteristics were evaluated. Exploratory analyses were conducted with the predictors listed in the online supplemental Tables S1 and S2. Predictors that maintained a significant contribution in combination with the other variables were retained in the final models (Tables 3 and 4).
Table 3.
Hierarchical linear models for shoulder abduction and shoulder flexion ROM (degrees)
| Abduction | Coefficient (SE) | |
|---|---|---|
|
| ||
| Variables | Unconditional Model | Final Model |
| Fixed effects | ||
| Intercept | 128.20 (1.56) b | 127.97 (1.21) b |
| Time a (linear rate of change) | 7.76 (0.80) b | 7.96 (0.71) b |
| Time a (quadratic rate of change) | −1.40 (0.16) b | −1.44 (0.15) b |
| Time a (cubic rate of change) | 0.07 (0.01) b | 0.08 (0.01) b |
| Time invariant covariates | ||
| Intercept | ||
| Ethnicity (Non-white) | −6.66 (1.73) b | |
| Neoadjuvant chemotherapy | −5.28 (2.26) c | |
| Type of surgery | −30.54 (3.10) b | |
| Axillary dissection | −11.32 (2.64) b | |
| Shoulder abduction (pre-operative, affected side) | 0.64 (0.04) b | |
| Linear | ||
| Lived alone at enrollment | 3.86 (1.35) c | |
| Type of surgery | 15.01 (1.83) b | |
| Axillary dissection | 3.45 (1.51) c | |
| Adjuvant chemotherapy during 1styear | 3.71 (1.23) c | |
| Quadratic | ||
| Lived alone at enrollment | −0.94 (0.32) c | |
| Type of surgery | −2.38 (0.39) b | |
| Axillary dissection | −0.66 (0.32) c | |
| Adjuvant chemotherapy during 1st year | −0.86 (0.30) c | |
| Cubic | ||
| Lived alone at enrollment | 0.05 (0.02) c | |
| Type of surgery | 0.11 (0.02) b | |
| Axillary dissection | 0.04 (0.02) c | |
| Adjuvant chemotherapy during 1st year | 0.05 (0.02) c | |
| Variance components | ||
| In intercept | 815.74 b | 435.74 b |
| In linear rate | 142.21 b | 90.38 b |
| In quadratic fit | 5.07 b | 3.41 b |
| In cubic fit | 0.01 b | 0.01 b |
| Goodness-of-fit deviance (parameters estimated) | 26350.367 (15) | 25981.557 (32) |
| Model comparison χ2 (df) | 368.81 (17) b | |
| Flexion | Coefficient (SE) | |
|---|---|---|
|
| ||
| Variables | Unconditional Model | Final Model |
| Fixed effects | ||
| Intercept | 154.56 (0.98) b | 154.49 (0.78) b |
| Time a (linear rate of change) | 4.50 (0.48) b | 4.53 (0.43) b |
| Time a (quadratic rate of change) | −0.76 (0.10) b | −0.77 (0.09) b |
| Time a (cubic rate of change) | 0.04 (0.01) b | 0.04 (0.01) b |
| Time invariant covariates | ||
| Intercept | ||
| Years of education | 0.37 (0.17) c | |
| Exercised preoperatively | 3.02 (0.91) b | |
| Neoadjuvant chemotherapy | −3.23 (1.16) c | |
| Breast pain functional interference score | −1.67 (0.46) b | |
| Axillary dissection | −2.19 (1.03) c | |
| Surgical drain placed | −14.72 (1.64) b | |
| Shoulder flexion (pre-operative, affected side) | 0.61 (0.04) b | |
| Linear | ||
| Lived alone at enrollment | 1.89 (0.77) c | |
| Type of surgery | 2.82 (0.99) c | |
| Surgical drain placed | 5.35 (1.01) b | |
| Adjuvant chemotherapy during 1st year | 1.88 (0.71) c | |
| Quadratic | ||
| Lived alone at enrollment | −0.44 (0.19) c | |
| Type of surgery | −0.67 (0.25) c | |
| Surgical drain placed | −0.67 (0.22) c | |
| Adjuvant chemotherapy during 1st year | −0.45 (0.18) c | |
| Cubic | ||
| Lived alone at enrollment | 0.03 (0.01) c | |
| Type of surgery | 0.04 (0.02) c | |
| Surgical drain placed | 0.03 (0.01) c | |
| Adjuvant chemotherapy during 1st year | 0.02 (0.01) c | |
| Variance components | ||
| In intercept | 321.92 b | 185.43 b |
| In linear rate | 49.24 b | 33.70 b |
| In quadratic fit | 1.59 b | 1.17 b |
| In cubic fit | 0.004 b | 0.003 b |
| Goodness-of-fit deviance (parameters estimated) | 23226.56 (15) | 22862.41 (24) |
| Model comparison χ2 (df) | 364.15 (19) b | |
Time was coded 0 for initial visit post operative visit;
p < 0.001;
p < 0.05
df: degrees of freedom; SE: standard error
Table 4.
Hierarchical linear models of grip strength and breast pain interference
| Grip strength (pounds) | Coefficient (SE) | |
|---|---|---|
|
| ||
| Variables | Unconditional Model | Final Model |
| Fixed effects | ||
| Intercept | 23.13 (0.30) b | 23.13 (0.16) b |
| Time a (linear rate of change) | 0.43 (0.10) b | 0.46 (0.08) b |
| Time a (quadratic rate of change) | −0.07 (0.03) c | −0.09 (0.02) b |
| Time a (cubic rate of change) | 0.004 (0.002) c | 0.005 (0.001) b |
| Time invariant covariates | ||
| Intercept | ||
| Age | −0.05 (0.01) b | |
| Karnofsky Performance Status score | 0.04 (0.01) c | |
| Grip strength (pre-operative, affected side) | 0.81 (0.03) b | |
| Linear | ||
| Adjuvant chemotherapy during 1st year | 0.04 (0.17) d | |
| Quadratic | ||
| Adjuvant chemotherapy during 1st year | −0.07 (0.04) d | |
| Cubic | ||
| Adjuvant chemotherapy during 1st year | 0.005 (0.002) c | |
| Variance components | ||
| In intercept | 30.66 b | 7.31 b |
| In linear rate | 0.59 c | 0.88 b |
| In quadratic fit | 0.08 c | 0.04 b |
| In cubic fit | 0.0004 c | 0.0001 b |
| Goodness-of-fit deviance (parameters estimated) | 15943.08 (15) | 14142.35 (21) |
| Model comparison χ2 (df) | 1800.73 (6) b | |
| Breast pain functional interference | Coefficient (SE) | |
|---|---|---|
|
| ||
| Variable | Unconditional Model | Final Model |
| Fixed effects | ||
| Intercept | 2.67 (0.14) b | 2.58 (0.12) b |
| Time a (linear rate of change) | −0.84 (0.09) b | −0.86 (0.08) b |
| Time a (quadratic rate of change) | 0.15 (0.02) b | 0.16 (0.02) b |
| Time a (cubic rate of change) | −0.008 (0.001) b | −0.008 (0.001) b |
| Time invariant covariates | ||
| Intercept | ||
| Age | −0.02 (0.01) b | |
| Ethnicity (Non-white) | 0.94 (0.26) b | |
| Karnofsky Performance Status score | −0.02 (0.01) c | |
| Self-Administered Comorbidity Questionnaire score | 0.09 (0.03) c | |
| Stage of cancer at time of diagnosis | −0.02 (0.01) c | |
| Breast pain function interference score at enrollment | 0.26 (0.08) b | |
| Reconstruction at time of initial surgery | 0.64 (0.21) c | |
| Linear | ||
| Ethnicity (Non-white) | −0.47 (0.17) c | |
| Type of surgery | −0.57 (0.20) c | |
| Surgical drain placed | 0.24 (0.17) d | |
| Any re-excision in the 1st year | 0.41 (0.15) c | |
| Quadratic | ||
| Ethnicity (Non-white) | 0.09 (0.04) c | |
| Type of surgery | 0.16 (0.05) c | |
| Surgical drain placed | −0.10 (0.04) c | |
| Any re-excision in the 1st year | −0.07 (0.04) d | |
| Cubic | ||
| Ethnicity (Non-white) | −0.004 (0.003) d | |
| Type of surgery | −0.01 (0.004) c | |
| Surgical drain placed | 0.01(0.003) c | |
| Any re-excision in the 1st year | 0.003 (0.003) d | |
| Variance components | ||
| In intercept | 3.77 b | 2.71 b |
| In linear rate | 0.32 b | 0.24 b |
| In quadratic fit | 0.002 b | 0.001 b |
| In cubic fit | * | * |
| Goodness-of-fit deviance (parameters estimated) | 4582.51 (11) | 4460.45 (30) |
| Model comparison χ2 (df) | 122.06 (19) b | |
Time was coded 0 for initial visit post operative visit;
p ≤ 0.001;
p < 0.05;
non-significant;
random error not free to vary
df: degrees of freedom; SE: standard error
Shoulder Abduction
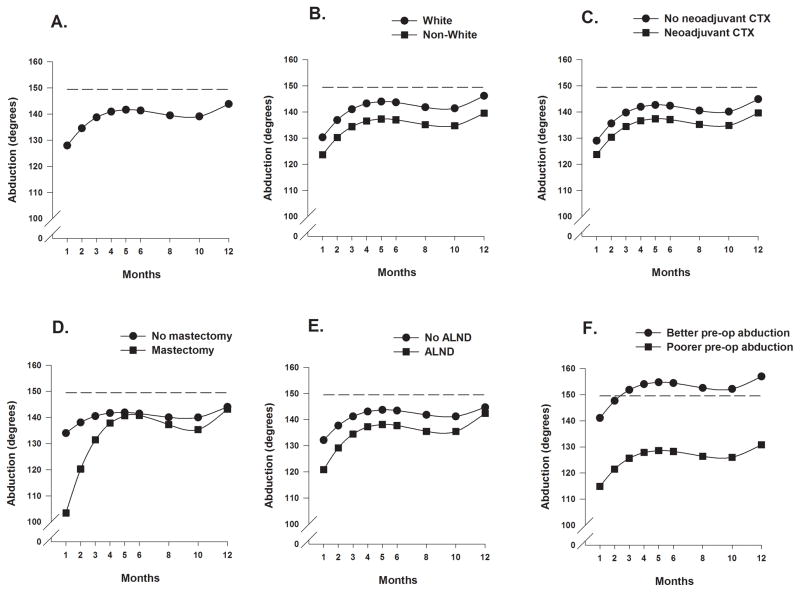
Estimates of the unconditional cubic change model for shoulder abduction ROM are presented in Table 3. As Figure 1A depicts, the trajectory for abduction over the 12-months showed an increase from months 1 through 5, a modest decline between months 5 and 8, and then a gradual increase beginning at month 10 to the maximum predicted value at month 12.
Figure 1.
Figure 1A displays the trajectory for average shoulder abduction range of motion over the 12-month postoperative period using an unconditional model. Figures 1B through 1F display the adjusted change curves of average shoulder abduction range of motion over 12 months by (B) ethnicity (White or Non-white), (C) receipt of neoadjuvant chemotherapy (yes/no), (D) type of surgery (mastectomy or breast conserving surgery), (E) receipt of axillary lymph node dissection (yes/no), and (F) level of preoperative shoulder abduction range of motion (better/poorer calculated as on 1 standard deviation above/below the mean preoperative shoulder abduction range of motion).
As shown in the final model in Table 3 and Figures 1B through 1F, five variables predicted inter-individual differences in the intercept for shoulder abduction ROM. Being non-White, having received neoadjuvant CTX, having a mastectomy versus BCS, having an ALND, and having poorer preoperative shoulder abduction were associated with greater reductions in shoulder abduction at the one-month assessment.
Four variables predicted inter-individual differences in the trajectories of shoulder abduction ROM over the 12-month postoperative period were: type of breast cancer surgery (Figure 1D), ALND (Figure 1E), lived alone at enrollment (Supplemental Figure 1A), and receipt of adjuvant CTX (Supplemental Figure 1B) during the first year.
Women who lived alone demonstrated a greater improvement in abduction ROM to month 4, at which time a decline in ROM occurred to month 10. From months 10 to 12, their abduction improved and at month 12 was essentially the same as the women who did not live alone. Women who had a mastectomy versus BCS had greater reductions in ROM at month one that improved by 5 months to levels seen in the women with BCS. While both groups demonstrated reductions in abduction between months 5 and 10, these reductions were greater in the women with mastectomy. Women who had an ALND demonstrated less abduction ROM than women who did not have an ALND. Both groups demonstrated gradual improvements between months 1 to 6, followed by a slight reduction between months 6 and 10, and then improvements to similar values by 12 months. While no differences in the one-month values were found, the women who received adjuvant CTX demonstrated greater increases in shoulder abduction during the first 4 months. Between months 5 and 10, their abduction ROM declined and gradually improved by month 12, to levels seen in the women who did not receive adjuvant CTX.
Shoulder Flexion
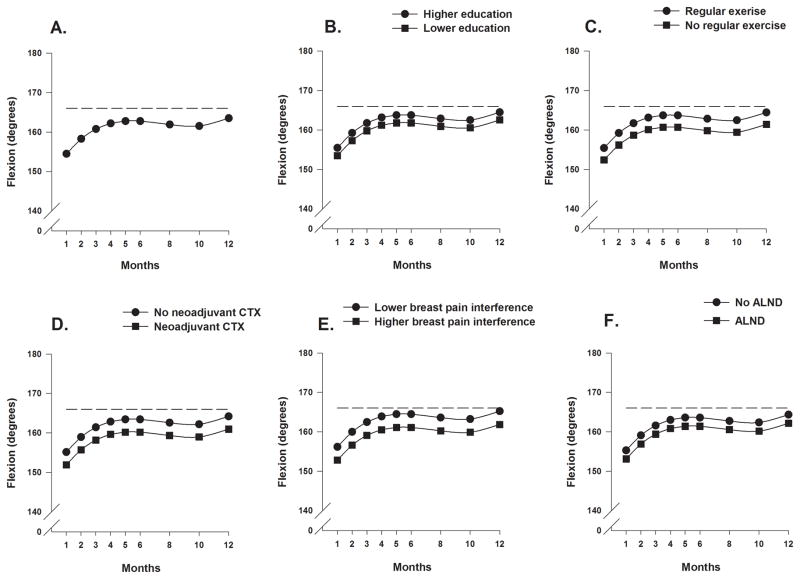
Estimates of the unconditional cubic change model for shoulder flexion ROM are presented in Table 3. As seen in Figure 2A, the trajectory for flexion over the 12-months showed an increase from months 1 through 5, a modest decline between months 5 and 8, followed by a gradual increase beginning at month 10 to the maximum predicted value at month 12.
Figure 2.
Figure 2A displays the trajectory for average shoulder flexion range of motion over the 12-month postoperative period using an unconditional model. Figures 2B through 2F display the adjusted change curves of average shoulder flexion range of motion over 12 months by (B) education (higher/lower calculated as 1 standard deviation above/below the mean preoperative years of education), (C) participation in regular exercise (yes/no), (D) receipt of neoadjuvant chemotherapy (yes/no), (E) breast pain interference score (higher/lower calculated as 1 standard deviation above/below the mean preoperative breast pain interference scores), and (F) receipt of axillary lymph node dissection (yes/no).
As shown in the final model in Table 3 and Figures 2B through 2F, and 3A and 3B, seven variables predicted inter-individual differences in the intercept for shoulder flexion ROM. Fewer years of education, decreased participation in exercise preoperatively, receipt of neoadjuvant CTX, higher breast pain interference scores at enrollment, having an ALND, placement of a surgical drain, and having poorer preoperative shoulder flexion were associated with decreased shoulder flexion ROM at the one-month assessment.
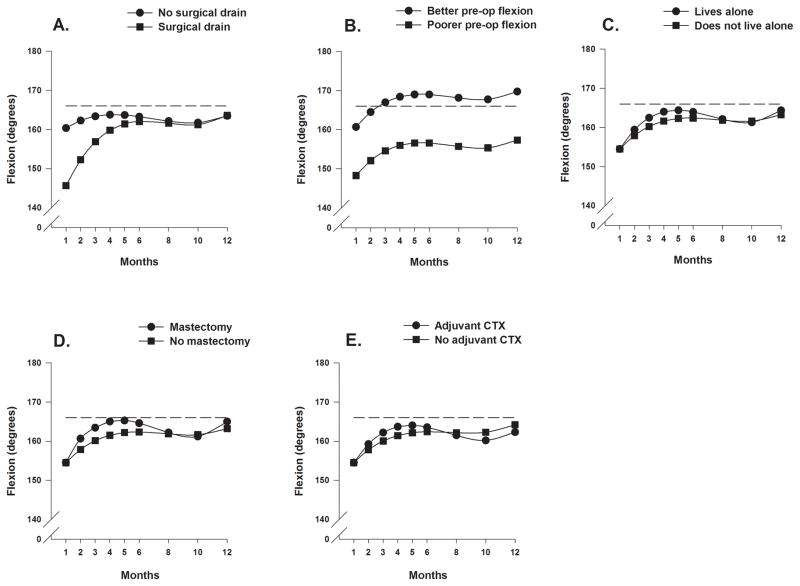
Figure 3.
Figure 3 displays the adjusted change curves of average shoulder flexion range of motion over 12 months by (A) placement of surgical drain (yes/no) and (B) level of preoperative shoulder flexion ROM (better/poorer calculated as 1 standard deviation above/below the mean preoperative shoulder flexion range of motion), (C) living alone (yes/no), (D) type of surgery (mastectomy or breast conserving surgery), and (E) receipt of adjuvant chemotherapy (yes/no).,
As shown in Figures 3A, and 3C through 3E, the four variables that predicted inter-individual differences in the trajectories of shoulder flexion ROM were: lived alone, type of breast cancer surgery, placement of a surgical drain, and receipt of adjuvant CTX during the first year. The trajectories of shoulder flexion predicted by living alone and receipt of CTX are similar to those for shoulder abduction. Women with mastectomy and women with BCS had similar shoulder flexion at one month but the women with mastectomy had greater improvements between months 1 and 4. ROM declined in this group between months 5 and 10 and then gradually improved and slightly surpassed that for the women with BCS. The women who had a surgical drain placed at the time of surgery demonstrated greater reductions in shoulder flexion at 1 month with gradual and consistent improvements to month 6 at which time trajectories were similar for women with and without drain placement.
Grip strength
Estimates of the unconditional cubic change model for grip strength are presented in Table 4. The trajectory for grip strength over the 12-month postoperative period illustrates a small increase in grip strength between months 1 and 4 (Supplemental Figure 2A). The trajectory plateaus between months 4 and 6 with a minimal decline between months 6 and 10 followed by a small increase.
As shown in the final model in Table 4 and Supplemental Figures 2B through 2D, three variables predicted inter-individual differences in the intercept for grip strength. Older age, having a lower KPS score, and having poorer preoperative grip strength were associated with decreased grip strength at the one-month assessment.
The only variable that predicted inter-individual differences in the grip strength trajectory was receipt of adjuvant CTX during the first year (Supplemental Figure 2E). Women who had adjuvant CTX demonstrated poorer grip strength, with a progressively greater decline between months 3 and 10.
Functional interference related to breast pain
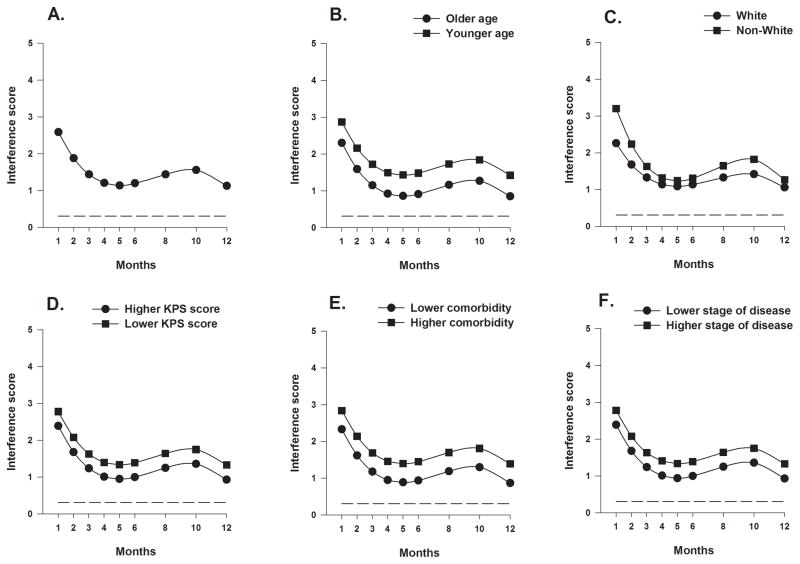
Estimates of the unconditional cubic change model for the mean functional interference score related to breast pain are presented in Table 4. As shown in Figure 4A, the trajectory for the average functional interference score over the 12-month postoperative period shows improved scores between months 1 and 5, gradually worsening of the scores between months 6 and 10, and then improvements again from months 10 to 12, with the lowest scores (reflecting least interference) at months 5 and 12.
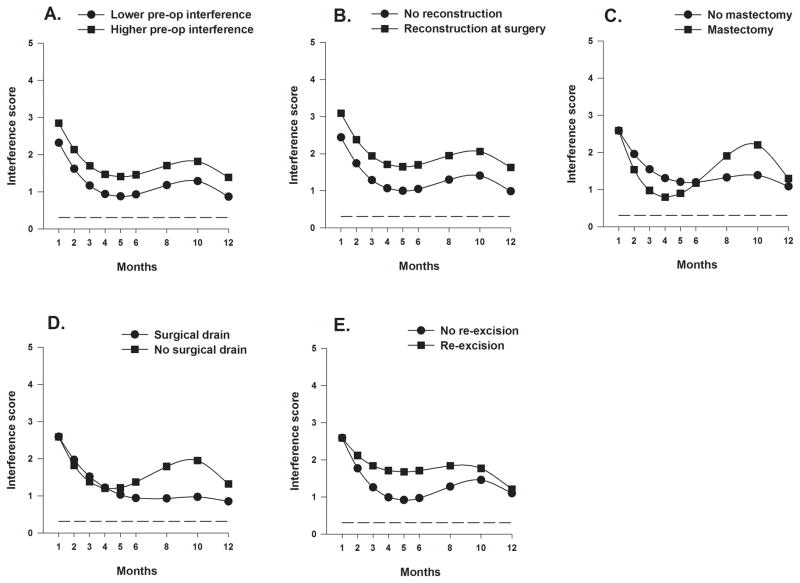
Figure 4.
Figure 4A displays the trajectory for average breast pain interference scores over the 12-month postoperative period using an unconditional model. Figures 4B through 4F display the adjusted change curves of average breast pain interference scores over 12 months by (B) age (older/younger calculated as 1 standard deviation above/below the mean preoperative age), (C) ethnicity (White or Non-white), (D) Karnofsky Performance Status (KPS) score (higher/lower calculated as 1 standard deviation above/below the mean preoperative KPS score), (E) self-reported comorbidity questionnaire score (SCQ) (higher/lower calculated as 1 standard deviation above/below the mean preoperative SCQ score), and (F) stage of disease (higher/lower).
As shown in the final model in Table 4 and Figures 4B through 4F and Figures 5A and 5B, seven variables predicted inter-individual differences in the intercept for breast pain functional interference. Younger age, being non-White, having a lower KPS score, a higher SCQ score, a higher disease stage at time of diagnosis, having a higher preoperative breast pain functional interference score, and having reconstruction at the time of surgery were associated with higher breast pain functional interference scores at the one month assessment.
Figure 5.
Figure 5 displays the adjusted change curves of average breast pain interference scores over 12 months by (A) breast pain interference score (higher/lower calculated as 1 standard deviation above/below the mean preoperative breast pain interference scores), (B) breast reconstruction at time of breast cancer surgery (yes/no), (C) type of surgery (mastectomy or breast conserving surgery), (D) placement of surgical drain (yes/no), and (E) any re-excision (yes/no).
As shown in Figures 4C, and 5C through 5E, the four variables that predicted inter-individual differences in the trajectories of interference scores over the 12-month postoperative period were ethnicity, type of breast cancer surgery, placement of a surgical drain, and re-excision during the first year. Non-White women demonstrated higher interference scores than White women across all assessments, with the largest differences seen between months 1 to 3 and 8 to 10, when both groups demonstrated relatively higher scores. Despite similar scores at the one-month assessment, women who had mastectomy demonstrated a greater improvement (drop) in the interference scores compared to women with BCS, until month 4. At this time, scores for women post mastectomy increased and were notably higher than for women with BCS, whose scores had essentially plateaued. This trajectory was similar to that seen for the women who did not have a drain placed at the time of surgery. Scores for the women who did and did not undergo re-excision were the same at 1 month but scores were higher for the women who underwent re-excision in each subsequent assessment until month 12 at which time scores were similar.
Summary of predictors
Table 5 provides a summary of the predictors for each of the outcomes, for comparative purposes.
Table 5.
Significant predictors for each outcome
| Characteristics | Shoulder abduction ROM | Shoulder flexion ROM | Grip Strength | Breast Pain Function Interference |
|---|---|---|---|---|
| Demographic | ||||
| Age | I | I | ||
| Lived alone at enrollment | S | S | ||
| Years of education | I | |||
| Ethnicity (non-white) | I | I, S | ||
| Clinical | ||||
| Exercised regularly prior to surgery | I | |||
| Self-administered comorbidity questionnaire score | I | |||
| Karnofsky Performance Scale score | I | I | ||
| Stage of disease at time of diagnosis | I | |||
| Neoadjuvant chemotherapy | I | I | ||
| Type of surgery (mastectomy, BCS) | I, S | S | S | |
| Axillary lymph node dissection | I, S | I | ||
| Breast reconstruction at time of surgery | I | |||
| Breast re-excision during the year after surgery | S | |||
| Surgical drain placed | I, S | S | ||
| Adjuvant chemotherapy in first year | S | S | S | |
| Shoulder abduction ROM (affected) at enrollment | I | |||
| Shoulder flexion ROM (affected) at enrollment | I | |||
| Grip strength (affected) at enrollment | I | |||
| Breast pain functional interference score at enrollment | I | I | ||
BCS: breast conserving surgery; I: intercept; ROM: range of motion; S: slope
DISCUSSION
To our knowledge, this study is the first to use HLM to model trajectories of UE function after breast cancer treatment and to evaluate the impact of preoperative function on postoperative function. Since one of the goals of this analysis was to identify risk factors for impaired UE function, the discussion is organized by predictors: treatment-related predictors, demographic and clinical predictors, and finally modifiable preoperative predictors.
Treatment-related predictors
The treatment predictors of postoperative function were type of surgery, ALND, breast reconstruction at time of surgery, placement of a surgical drain, and breast re-excision during the year after surgery. Only 20% of the women in this study underwent mastectomy, lower than national trends.24,25 Compared to BCS, receipt of mastectomy was associated with significant decrements in shoulder abduction at one month, as well as across the 12-month trajectories for abduction, flexion, and breast pain interference with function. At the one-month assessment, women who had a mastectomy demonstrated markedly less abduction compared to women who had BCS, but by 12 months abduction was similar between groups. Reductions of >20 degrees are associated with loss of UE function22 and reductions in QOL.26,27 Women who had a mastectomy had ~30 degrees less abduction ROM at one month and did not regain this motion until 5 months postoperatively. In contrast, mastectomy was not associated with one-month differences in flexion ROM, but only with differences in the trajectories of flexion over the first 6 months. However, differences in flexion between mastectomy and BCS groups were not clinically meaningful at <5 degrees at all time points. Our findings contrast with those from a prospective longitudinal study6 that found an association between mastectomy and loss of flexion ROM (p=0.015) but not with loss of abduction (p=0.058) at 12 months following breast cancer surgery.
While type of surgery did not predict breast pain interference scores at the one-month assessment, it did predict the 12-month trajectory for these scores, which were highly variable for the women who had mastectomy. Of the 78 women who had a mastectomy, 47% had reconstruction between months 1 and 12, compared to only 3% of women with BCS (p<0.001), which may have contributed to the greater variability in the breast pain interference scores in women with mastectomy.
Thirty-seven percent of the women in this study had an ALND. Having an ALND versus SLNB was associated with 11 degrees less abduction at one month. While this change meets the criteria for a minimal detectable change in shoulder abduction ROM, between group differences at 12 months were not clinically meaningful.28 Differences in shoulder flexion were not clinically meaningful (<3 degrees) at any time point.28 Our findings of limited shoulder abduction are similar to previous reports6,29 that found an association between ALND and limitations in shoulder ROM. In one study,30 having an ALND was associated with statistically significant reductions in shoulder ROM at one year. At the 7-year follow-up,1 restriction in ROM, particularly in abduction, persisted in women who had ALND compared to those who had a SLNB. In another prospective study,8 women who had an ALND had more UE impairments at 2.5 years than women who had a SLNB. However, the differences in ROM between the groups at 2.5 years were not statistically significant.
Placement of a surgical drain was required for 94% of the women who had mastectomy and 27% of the women who had BCS. Drains are an important component of surgical management to prevent accumulation of fluid. Women who had a drain placed during surgery had 15 degrees less shoulder flexion at one month than the women who did not require a surgical drain. However by 6 months, this difference was negligible. It is possible that restriction in shoulder flexion could results from soft tissue changes at the lateral thorax and chest wall. Interestingly, beginning at month 5, women who had a surgical drain demonstrated better functional interference scores. Of the women who did not have a surgical drain, 77% of them received radiation therapy during the first year (compared to 61% of the women who had a drain, p<0.001) and 35% underwent re-excision (compared to 23% of the women who had a surgical drain, p=0.01). This finding suggests that women who have surgical drains require referral to physical therapy.
Twenty-two percent of the patients had breast reconstruction at the time of surgery. This procedure was associated with higher interference ratings at one month and these differences were consistent across time and persisted even at 12 months. Not surprisingly, breast re-excision during the first year (30.1% of women) was associated with higher interference scores between months 1 and 12. However by the 12-month assessment, these scores were only slightly higher in the women who required re-excision.
In this study, 20% of women received neoadjuvant CTX. These women had 5 degrees less shoulder abduction ROM at all of the postoperative assessments than the women who did not receive neoadjuvant CTX. In addition, neoadjuvant CTX predicted only minimal reductions in shoulder flexion at one-month (<4 degrees). Receipt of adjuvant CTX (20%) predicted the 12-month trajectories for shoulder abduction and flexion ROM. These differences were not clinically meaningful at any point and were essentially nil by month 12. However, differences in trajectories for grip strength between women who did and did not receive adjuvant CTX were more pronounced. Women receiving adjuvant CTX demonstrated less grip strength beginning at month 3. Even by 12 months, although increasing, grip strength did not reach the values for women who had not received CTX. This finding may be related to neuropathic pain associated with CTX agents or musculoskeletal complaints associated with hormonal therapies.9
Demographic and Clinical Predictors
The need for more aggressive treatment may have contributed to ethnic differences in shoulder abduction ROM and breast pain interference scores. More non-White women than White women had Stage II to IV cancer (51% compared to 40%, p=0.03) and more had an ALND (44% versus 33%, p=0.03). Non-White woman consistently had ~8 degrees less shoulder abduction ROM and had higher breast pain interference scores than White women across all postoperative assessments. Higher stage of disease was also associated with higher breast pain interference scores at the one-month assessment.
In terms of age, younger women had higher pain interference scores across all time points. However, no age differences were found in the trajectories of breast pain interference scores. This finding is consistent with previous results from our group31 and others32 that younger patients are at higher risk for more severe persistent pain following breast cancer surgery.
In addition, older women had weaker grip at the one-month assessment. Normative values for grip strength for women between the ages of 55 and 59 are reported as 27.2 kg (left), and 29.9 kg (right).33 Between 50 and 54 years, normative values are 28.8 kg (right) and 30.9 kg (left). Mean age for the women in this study was 54.9 years and mean preoperative grip strength was 23.8 kg and 23.6 kg on the unaffected and affected sides, respectively. Our results are lower than these healthy norms across all time points. Since poorer grip strength is associated with higher risk for future disability,34,35 grip strength may provide a simple and useful postoperative screening tool.
At the time of enrollment, 23.7% of the women in this study reported living alone. While living alone did not predict differences in shoulder ROM at one month, it did predict the trajectories for both shoulder abduction and flexion. While differences in trajectories for abduction ROM were more marked, the patterns were similar. Women who lived alone demonstrated slightly greater improvements in ROM until month 5 they had gradual declines in ROM. By month 12, ROM was essentially the same between women who did and did not alone. It is possible that women who lived alone used their arm more for activities of daily living, which resulted in early improvement in their ROM, but this finding needs to be verified in future studies.
The KPS scale is organized in 10 unit increments with 100 representing normal performance, 90 being able to carry on normal activity, 80 being normal activity with effort, 70 being able to cares for self, and so on. While differences in grip strength were associated with KPS scores, these differences were small. The difference in grip strength between women who were 1 SD above and 1 SD below the mean preoperative KPS scores was 0.88 kg at one month. This difference represents a small standardized effect size of 0.15 which was consistent across all assessments. Differences in breast pain functional interference scores at one month were also associated with KPS scores. Breast pain functional interference scores were very low prior to surgery, indicating little interference with function. However, at one month and over the year following surgery, breast pain interference scores were consistently higher (greater interference) in women with lower preoperative KPS scores. Better self-report of performance preoperatively is associated with less functional interference due to breast pain postoperatively. Higher levels of comorbidity were associated with worse breast pain interference scores one month following surgery. These differences were consistent and persisted across time.
Modifiable Preoperative Predictors
Enrollment values for shoulder abduction ROM, shoulder flexion ROM, grip strength, and breast pain functional interference score each predicted their respective functional outcomes at one-month post surgery, but did not predict the trajectories of change over the subsequent assessments. Not surprisingly, lower preoperative function predicted lower postoperative function.
Preoperative ROM was a strong predictor of one-month postoperative ROM for both abduction and flexion. Each degree more of preoperative ROM was associated with 0.64 degrees more abduction ROM at one month and 0.61 degrees more flexion ROM at one month. To further evaluate this association, ROM outcomes were dichotomized using the convention of 1 standard deviation above and below the mean preoperative ROM values. One month postoperatively, the women who were ≥1 SD (20.2 degrees) below the preoperative mean for abduction demonstrated an average 28 degrees less abduction than the women who were 1 SD above the mean. Women who were ≥1 SD (10.3) below the preoperative mean for flexion demonstrated an average of 12 degrees less flexion postoperatively than the women who were 1 SD above the preoperative mean. Patients with less ROM prior to surgery had greater reductions following surgery, which did not return to the pre-surgical levels even by 12 months.
Not surprisingly, preoperative grip strength predicted one-month postoperative grip strength. Additionally, lower grip strength scores at one month were seen in older women and in women with lower KPS scores. The decline in mean grip strength from pre-surgery to one-month post surgery was very small and not clinically meaningful and grip strength remained fairly stable over time. While differences in grip strength reached statistical significance, they did not reach the threshold for a clinically meaningful difference36 and may be accounted for, in part, by hand dominance.
Upper extremity function was measured subjectively through the breast pain functional interference score. Women with higher preoperative breast pain functional interference scores (greater interference) had consistently higher postoperative breast pain functional interference scores at all assessments.
Finally, engaging in exercise prior to surgery was associated with greater shoulder flexion ROM at one month, which persisted over the rest of the year. However, the difference was only 3 degrees, which is not clinically meaningful.
Clinical implications
Some decline in UE function following breast cancer surgery is expected. Findings from this study suggest that both measures of shoulder ROM were impaired during the first year after breast cancer treatment. We observed a modest decline in shoulder ROM between months 5 and 10, noted consistently in the trajectories of both abduction and flexion. Although, we do not know why this transient decline in ROM occurred, this finding warrants replication and investigation in future studies.
Of note, for some women these impairments persisted at 12 months. Moreover, consistent with a previous report,1 nearly 17% of the women in our study had a ≥20 degrees reduction in shoulder abduction ROM on their affected relative to their unaffected side at 12 months, compared to only 8% preoperatively. A reduction in shoulder abduction ROM of ≥20 degrees is associated with greater difficulty with household activities and hobbies, with subjective reports of disability,22 and with decrements in QOL.26,27 The risk factors associated with decrements in shoulder abduction ROM found in this study can assist clinicians to identify high risk patients so that interventions (e.g., education, exercise, manual therapy, pain management) can be initiated early in the preoperative or perioperative period.
Limitations in ROM, reduced grip strength, functional interference due to pain, and decreased participation in exercise are all targetable, modifiable risk factors for postoperative decrements in UE function. Early identification and treatment of physical impairments may mitigate their negative impact on function and QOL following cancer treatment. Currently, oncology rehabilitation follows an impairment-based model, in which treatment is initiated once impairments are identified following cancer treatment.37 In contrast, “prehabilitation” is initiated after the cancer diagnosis but before cancer treatment begins. Prehabilitation includes surveillance to identify impairments or risk factors for future impairments, and implementation of education or treatments to improve existing impairments or promote risk reduction for future impairments.37 Preoperative assessments and preoperative or early interventions have shown promising results in improving outcomes and decreasing costs5, 38 but additional high quality studies are needed.
Several study limitations deserve mention. First, 24% of the women in our study received physical therapy during the first year following breast cancer surgery. Patients were asked if at any time during the study they received physical therapy, which was treated as a dichotomous, non-varying, covariate. We did not have detailed information on the specific physical therapy procedures that the patient received prior to and following surgery. Although not a significant predictor for any of the study outcomes, it is possible that patients with greater symptomatic dysfunction sought out physical therapy, which may have influenced the study outcomes. Second, we did not evaluate other UE strength outcomes, such as shoulder strength, nor did we include assessment of functional shoulder ROM during task performance. Third, we did not include time-varying covariates in our model because the primary goal of this study was to understand the impact of demographic and clinical characteristics, and preoperative function, on postoperative functional outcomes. Finally, while we relied on previous literature and clinical experience to determine relevant predictors, additional predictors warrant evaluation in future studies.
Despite these limitations, this large, prospective, longitudinal study provides new insights on the impact of pre-existing functional limitations on post-surgical outcomes. Moreover, the use of HLM enabled an evaluation of predictors of inter-individual differences of the trajectories of these outcomes over the 12 months following surgery. The identification and assessment of modifiable risk factors, as well as the initiation of preemptive or prompt postoperative interventions, may reduce the need for more aggressive and costly rehabilitation following breast cancer surgery.5,6 This hypothesis warrants evaluation in future studies.
Supplementary Material
Figure S1. The adjusted change curves of average shoulder abduction range of motion over 12 months by (A) living alone (yes/no) and (B) receipt of adjuvant chemotherapy (yes/no).
Figure S2. The trajectory for average grip strength over the 12-month postoperative period using an unconditional model. Supplemental Figures 2B through 2F display the adjusted change curves of average grip strength over 12 months by (B) age (older/younger calculated as 1 standard deviation above/below the mean preoperative age), (C) Karnofsky Performance Status (KPS) score (higher/lower calculated as 1 standard deviation above/below the mean preoperative KPS score), (D) preoperative grip strength (better/poorer calculated as 1 standard deviation above/below the mean preoperative grip strength), and (E) receipt of adjuvant chemotherapy (yes/no).
Table S1. Potential Predictors of Intercepts (I), Linear Coefficients (LC), Quadratic Coefficients (QC), and Cubic Coefficients (CC) for shoulder abduction range of motion. Variables identified from exploratory analyses, based on t-values ≥ 2.00, are indicated by filled boxes (■).
Table S2. Potential Predictors of Intercepts (I), Linear Coefficients (LC), Quadratic Coefficients (QC), and Cubic Coefficients (CC) for grip strength and function interference. Variables identified from exploratory analyses, based on t-values ≥ 2.00, are indicated by filled boxes (■).
Footnotes
Disclosures: This study was funded by grants from the National Cancer Institute (NCI, CA107091 and CA118658). Dr. Christine Miaskowski is an American Cancer Society Clinical Research Professor and is funded by a K05 award from the NCI (CA168960). Dr. Betty Smoot is partially supported by the BIRCWH K12, Grant Number K12HD052163 NICHD/NIH, and by the National Center for Advancing Translational Sciences, NIH, through UCSF-CTSI Grant Number KL2TR000143. This project was supported by NIH/NCRR UCSF-CTSI Grant Number UL1RR024131. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The authors have no conflicts of interest related to this research or manuscript. This manuscript was not previously published. An abstract of preliminary findings was presented at the Annual BIRCWH Scholars Meeting and the Annual Interdisciplinary Women’s Health Research Symposium.
References
- 1.Kootstra JJ, Dijkstra PU, Rietman H, et al. A longitudinal study of shoulder and arm morbidity in breast cancer survivors 7 years after sentinel lymph node biopsy or axillary lymph node dissection. Breast Cancer Res Treat. 2013;139:125–134. doi: 10.1007/s10549-013-2509-y. [DOI] [PubMed] [Google Scholar]
- 2.Norman SA, Localio AR, Potashnik SL, et al. Lymphedema in breast cancer survivors: incidence, degree, time course, treatment, and symptoms. J Clin Oncol. 2009;27:390–397. doi: 10.1200/JCO.2008.17.9291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ridner SH. Quality of life and a symptom cluster associated with breast cancer treatment-related lymphedema. Support Care Cancer. 2005;13:904–911. doi: 10.1007/s00520-005-0810-y. [DOI] [PubMed] [Google Scholar]
- 4.Smoot B, Wong J, Cooper B, et al. Upper extremity impairments in women with or without lymphedema following breast cancer treatment. J Cancer Surviv. 2010;4:167–178. doi: 10.1007/s11764-010-0118-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Springer BA, Levy E, McGarvey C, et al. Pre-operative assessment enables early diagnosis and recovery of shoulder function in patients with breast cancer. Breast Cancer Res Treat. 2010;120:135–147. doi: 10.1007/s10549-009-0710-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levy EW, Pfalzer LA, Danoff J, et al. Predictors of functional shoulder recovery at 1 and 12 months after breast cancer surgery. Breast Cancer Res Treat. 2012;134(1):315–324. doi: 10.1007/s10549-012-2061-1. [DOI] [PubMed] [Google Scholar]
- 7.Neil-Sztramko SE, Kirkham AA, Hung SH, Niksirat N, Nishikawa K, Campbell KL. Aerobic capacity and upper limb strength are reduced in women diagnosed with breast cancer: a systematic review. J Physiother. 2014;60:189–200. doi: 10.1016/j.jphys.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Sagen A, Kaaresen R, Sandvik L, Thune I, Risberg MA. Upper Limb Physical Function and Adverse Effects After Breast Cancer Surgery: A Prospective 2.5-Year Follow-Up Study and Preoperative Measures. Arch Phys Med Rehabil. 2014;95:875–81. doi: 10.1016/j.apmr.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 9.Lintermans A, Van Asten K, Wildiers H, et al. A prospective assessment of musculoskeletal toxicity and loss of grip strength in breast cancer patients receiving adjuvant aromatase inhibitors and tamoxifen, and relation with BMI. Breast Cancer Res Treat. 2014;146:109–116. doi: 10.1007/s10549-014-2986-7. [DOI] [PubMed] [Google Scholar]
- 10.Miaskowski C, Cooper B, Paul SM, et al. Identification of patient subgroups and risk factors for persistent breast pain following breast cancer surgery. J Pain. 2012;13:1172–1187. doi: 10.1016/j.jpain.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kyranou M, Paul SM, Dunn LB, et al. Differences in depression, anxiety, and quality of life between women with and without breast pain prior to breast cancer surgery. Eur J Oncol Nurs. 2013;17:190–195. doi: 10.1016/j.ejon.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miaskowski C, Dodd M, Paul SM, et al. Lymphatic and angiogenic candidate genes predict the development of secondary lymphedema following breast cancer surgery. PLoS One. 2013;8:e60164. doi: 10.1371/journal.pone.0060164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Onselen C, Aouizerat BE, Dunn LB, et al. Differences in sleep disturbance, fatigue and energy levels between women with and without breast pain prior to breast cancer surgery. Breast. 2013;22:273–276. doi: 10.1016/j.breast.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The Self-Administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003;49:156–163. doi: 10.1002/art.10993. [DOI] [PubMed] [Google Scholar]
- 15.Karnofsky D. Performance scale. New York: Plenum Press; 1977. [Google Scholar]
- 16.Daut RL, Cleeland CS, Flanery RC. Development of the Wisconsin Brief Pain Questionnaire to assess pain in cancer and other diseases. Pain. 1983;17:197–210. doi: 10.1016/0304-3959(83)90143-4. [DOI] [PubMed] [Google Scholar]
- 17.Cleeland CS, Gonin R, Baez L, Loehrer P, Pandya KJ. Pain and treatment of pain in minority patients with cancer. The Eastern Cooperative Oncology Group Minority Outpatient Pain Study. Ann Intern Med. 1997;127:813–816. doi: 10.7326/0003-4819-127-9-199711010-00006. [DOI] [PubMed] [Google Scholar]
- 18.Serlin RC, Mendoza TR, Nakamura Y, Edwards KR, Cleeland CS. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain. 1995;61:277–284. doi: 10.1016/0304-3959(94)00178-H. [DOI] [PubMed] [Google Scholar]
- 19.Tasmuth T, von Smitten K, Hietanen P, Kataja M, Kalso E. Pain and other symptoms after different treatment modalities of breast cancer. Ann Oncol. 1995;6:453–459. doi: 10.1093/oxfordjournals.annonc.a059215. [DOI] [PubMed] [Google Scholar]
- 20.Tasmuth T, von Smitten K, Kalso E. Pain and other symptoms during the first year after radical and conservative surgery for breast cancer. Br J Cancer. 1996;74:2024–2031. doi: 10.1038/bjc.1996.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Norkin CC, White DJ. Measurement of Joint Motion, A Guide to Goniometry. 3. Philadelphia: FA Davis; 2003. [Google Scholar]
- 22.Voogd AC, Ververs JM, Vingerhoets AJ, Roumen RM, Coebergh JW, Crommelin MA. Lymphoedema and reduced shoulder function as indicators of quality of life after axillary lymph node dissection for invasive breast cancer. Br J Surg. 2003 Jan;90:76–81. doi: 10.1002/bjs.4010. [DOI] [PubMed] [Google Scholar]
- 23.Raudenbush S, Bryk A. Hierarchical Linear Models. Thousand Oaks, CA: Sage Publications; 2002. Applications and Data Analysis Methods. [Google Scholar]
- 24.Kummerow KL, Du L, Penson DF, Shyr Y, Hooks MA. Nationwide Trends in Mastectomy for Early-Stage Breast Cancer. JAMA Surg. 2015;150:9–16. doi: 10.1001/jamasurg.2014.2895. [DOI] [PubMed] [Google Scholar]
- 25.Anderson RT, Morris CR, Kimmick G, et al. Patterns of locoregional treatment for nonmetastatic breast cancer by patient and health system factors. Cancer. 2015;121:790–9. doi: 10.1002/cncr.29092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nesvold IL, Reinertsen KV, Fossa SD, Dahl AA. The relation between arm/shoulder problems and quality of life in breast cancer survivors: a cross-sectional and longitudinal study. J Cancer Surviv. 2011;5:62–72. doi: 10.1007/s11764-010-0156-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martins da Silva RC, Rezende LF. Assessment of impact of late postoperative physical functional disabilities on quality of life in breast cancer survivors. Tumori. 2014;100:87–90. doi: 10.1700/1430.15821. [DOI] [PubMed] [Google Scholar]
- 28.Muir SW, Corea CL, Beaupre L. Evaluating change in clinical status: reliability and measures of agreement for the assessment of glenohumeral range of motion. NAJSPT. 2010;5:98–110. [PMC free article] [PubMed] [Google Scholar]
- 29.Hidding JT, Beurskens CH, van der Wees PJ, van Laarhoven HW, Nijhuis-van der Sanden MW. Treatment related impairments in arm and shoulder in patients with breast cancer: a systematic review. PLoS One. 2014;9:e96748. doi: 10.1371/journal.pone.0096748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rietman JS, Dijkstra PU, Geertzen JH, et al. Treatment-related upper limb morbidity 1 year after sentinel lymph node biopsy or axillary lymph node dissection for stage I or II breast cancer. Ann Surg On. 2004;11:1018–1024. doi: 10.1245/ASO.2004.03.512. [DOI] [PubMed] [Google Scholar]
- 31.Miaskowski C, Paul SM, Cooper B, et al. Identification of patient subgroups and risk factors for persistent arm/shoulder pain following breast cancer surgery. Eur J Oncol Nurs. 2014;18:242–253. doi: 10.1016/j.ejon.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krok JLBT, McMillan SC. Age Differences in the Presence of Pain and Psychological Distress in Younger and Older Cancer Patients. Journal of Hospice & Palliative Care Nursing. 2013;15:107–113. [Google Scholar]
- 33.Bohannon RWPA, Massy-Westropp N, Desrosiers J, Bear-Lehman J. Reference values for adult grip strength measured with a Jamar dynamometer: a descriptive meta-analysis. Physiother. 2006;92:11–15. [Google Scholar]
- 34.Bohannon RW. Hand-grip dynamometry predicts future outcomes in aging adults. J Geriatr Phys Ther. 2008;31:3–10. doi: 10.1519/00139143-200831010-00002. [DOI] [PubMed] [Google Scholar]
- 35.Xue QL, Walston JD, Fried LP, Beamer BA. Prediction of risk of falling, physical disability, and frailty by rate of decline in grip strength: the women’s health and aging study. Arch Int Med. 2011;171:1119–1121. doi: 10.1001/archinternmed.2011.252. [DOI] [PubMed] [Google Scholar]
- 36.Nitschke JE, McMeeken JM, Burry HC, Matyas TA. When is a change a genuine change? A clinically meaningful interpretation of grip strength measurements in healthy and disabled women. J Hand Ther. 1999;12:25–30. [PubMed] [Google Scholar]
- 37.Silver JK, Baima J, Mayer RS. Impairment-driven cancer rehabilitation: an essential component of quality care and survivorship. CA CancerJ Clin. 2013;63:295–317. doi: 10.3322/caac.21186. [DOI] [PubMed] [Google Scholar]
- 38.Schmitz KH, DiSipio T, Gordon LG, Hayes SC. Adverse breast cancer treatment effects: the economic case for making rehabilitative programs standard of care. Support Care Cancer. 2014:5. doi: 10.1007/s00520-014-2539-y. epub ahead of print. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. The adjusted change curves of average shoulder abduction range of motion over 12 months by (A) living alone (yes/no) and (B) receipt of adjuvant chemotherapy (yes/no).
Figure S2. The trajectory for average grip strength over the 12-month postoperative period using an unconditional model. Supplemental Figures 2B through 2F display the adjusted change curves of average grip strength over 12 months by (B) age (older/younger calculated as 1 standard deviation above/below the mean preoperative age), (C) Karnofsky Performance Status (KPS) score (higher/lower calculated as 1 standard deviation above/below the mean preoperative KPS score), (D) preoperative grip strength (better/poorer calculated as 1 standard deviation above/below the mean preoperative grip strength), and (E) receipt of adjuvant chemotherapy (yes/no).
Table S1. Potential Predictors of Intercepts (I), Linear Coefficients (LC), Quadratic Coefficients (QC), and Cubic Coefficients (CC) for shoulder abduction range of motion. Variables identified from exploratory analyses, based on t-values ≥ 2.00, are indicated by filled boxes (■).
Table S2. Potential Predictors of Intercepts (I), Linear Coefficients (LC), Quadratic Coefficients (QC), and Cubic Coefficients (CC) for grip strength and function interference. Variables identified from exploratory analyses, based on t-values ≥ 2.00, are indicated by filled boxes (■).