Abstract
Objective
To use a Rehabilomics framework to evaluate relations between post-TBI depression (PTD) and potential associated factors, including antidepressant use, on cognitive recovery following severe TBI.
Participants
Severe TBI survivors (n=154), recruited from a level 1 trauma center.
Design
Prospective cohort study with assessments at 6 and 12 months post-injury.
Main Measures
Patient Health Questionnaire-9 (PTD symptoms); cognitive composite score from a neuropsychological assessment battery (cognitive impairment); and Functional Independence Measure–Cognition (FIM-Cog, self-reported functional cognition).
Results
Individuals with and without PTD did not differ with respect to cognitive impairment. However, antidepressant use, regardless of PTD status, was associated with cognitive impairment. Individuals with PTD reported lower FIM-Cog scores at both time-points compared to those without PTD. In a post-hoc longitudinal analysis, individuals with late-onset PTD had worse cognitive impairment.
Conclusion
These results suggest antidepressant use impairs cognition among individuals without PTD. Also, PTD does not directly affect cognitive impairment but may affect functional cognitive limitations through self-evaluation and apathy/motivation factors.
Keywords: traumatic brain injury, depression, cognition, Rehabilomics, antidepressants, international classification of functioning
Introduction
Traumatic brain injury (TBI) is increasingly recognized as a chronic health condition with multiple accompanying physical, cognitive, and neurobehavioral impairments or limitations. Mood changes and cognitive impairments greatly diminish quality of life and can impact return to work or school following TBI1–5. Post-TBI depression (PTD) is a common neurobehavioral complication; during the first year of recovery, individuals with TBI are 10 times more likely to experience a depressive episode when compared to the annual rate of depression in the general population (53%6 compared to 6%7) and are at greater risk decades later for recurring depressive symptoms8. One study found depression to be a better predictor of continued disability after TBI9 than injury-related cognitive impairments. Clinically, the overlapping symptomatology of PTD and TBI-related cognitive impairments (e.g., reduced processing speed, poor concentration, memory difficulty, and increased fatigue) may complicate differential diagnoses as well as treatment. As a result, an increased understanding about the relationship between depression and cognitive impairments post-TBI, especially following more severe injuries, is important for understanding individual functional recovery and developing effective personalized treatments.
In populations without TBI, individuals with depression often have accompanying cognitive impairments that may be due to common underlying neuropathology10. Meta-analyses indicate uninjured individuals with depression frequently have working memory impairments and reduced cognitive flexibility; individuals with depression also complain of memory problems and difficulty concentrating11. While individuals with depression may report several deficits on tasks where cognitive control is necessary to focus memory (e.g. those with additional attentional components or time limitations)13,14. Cognitive control, or the ability to direct cognitive processing in the context of ever-changing goals and distractions, is important for functional cognition (cognitive processes required for every-day life), as performance generally requires reacting to changing and distracting environments. Further, cognitive control is particularly susceptible to mood and motivational factors 15. Individuals with depressive symptoms can also have an increased bias to attend to negative stimuli16, with some evidence of this phenomenon occurring in mild TBI17. Such an increased negative attentional bias, combined with reduced cognitive control, may increase severity and duration of depressive symptoms.
Similar to individuals with depression in the general population, survivors of TBI commonly exhibit significant memory, cognitive control, and attentional limitations18–21. In addition, previous studies suggest poorer cognition among individuals with depressive symptoms compared to individuals with no depression following TBI22,23, though this association has not been consistently reported 24. Similarly, studies consistently demonstrate that individuals with depressive symptoms following TBI have more functional cognitive limitations than those without depressive symptoms25–27. Apathy, insomnia, psychomotor agitation or retardation, and fatigue are reported frequently after TBI, even among individuals without depression, and these symptoms can influence cognition28–30.
Our group has developed a research framework called Rehabilomics31 to improve understanding of complex health conditions for those with disability (e.g. TBI) that involve multiple complications with overlapping symptoms, interactions with individual risk factors, and diversity in response to rehabilitation. Rehabilomics methodologies utilize the World Health Organization’s International Classification of Functioning, Disability and Health (ICF) framework to explore the concepts of biological susceptibility, genetic variation, and epigenetic factors as environmental and personal factors that affect functional recovery and treatment outcomes. This framework expands the ICF’s definition of personal and environmental factors to incorporate personal genetics and potential genetic modifiers from the environment, while still preserving the original ICF conceptualization where there are complex interactions among impairments, functional limitations, and daily participation that are unique to each individual. By accounting for these multiple factors that may affect rehabilitation-relevant outcomes, the Rehabilomics framework aims to improve prediction and personalized approaches to optimize outcome32,33. Consistent with this framework, we describe cognitive impairment among individuals with PTD and operationalize our understanding about the relationships between PTD, cognitive impairment, and functional cognition using ICF constructs. We also examined personal and environmental factors like pre-morbid mood disorders, injury severity, and antidepressant use associated with PTD and cognition, as studies suggest that remission of depressive symptoms with antidepressant treatment can lead to improved cognition following TBI34.
The primary purpose of this study was to evaluate interrelationships between cognitive impairment (measured by neuropsychological tests), functional cognitive limitations (measured by FIM-Cog), and PTD in the first year following TBI.
Methods
Participants
Participants in this study, which was approved by the University of Pittsburgh’s Institutional Review Board, were screened as a part of a larger study examining outcomes after TBI among individuals receiving care at inpatient and/or outpatient clinics within the University of Pittsburgh Medical Center (UPMC). Based on medical records review, participants sustained a non-penetrating TBI with evidence of intracranial injury on Computed Tomography (CT). As part of standardized neurocritical care, Glasgow Coma Scale (GCS) assessments were completed by neurosurgical physicians. To meet criteria for inclusion, participants had to have a GCS within the first 8 hours of admission of <9 after resuscitation and while off of paralytics and sedation, indicating a severe initial level of injury. Trained research staff reviewed exclusion criteria including: cardiac arrest prior to admission, documented prolonged hypoxia or hypotension prior to admission, or penetrating TBI. Participants reported on herein are a subset of those in a larger study investigating possible biomarkers and genetic factors related to individual recovery following TBI.
Injury severity was described using the best Glasgow Coma Scale (GCS) obtained within the first 24 hours post-injury. The GCS is the standard tool for measuring injury severity after trauma. However, the “best GCS” has been shown to have greater sensitivity in discriminating later cognitive outcomes, compared to immediate GCS which may be complicated by substance use or other factors3,35. Demographic information including age, sex, education, and information regarding prescribed antidepressant medications at both 6 and 12 months was collected by chart review as well as through participant or caregiver interviews (see Table 1 for a list of antidepressant medications considered in this study). In addition, participants taking atypical anti-psychotics were excluded from associations with cognition due to known negative effects of these medications on cognitive functioning post-TBI36,37. A pre-injury history of mood disorders including depression, bipolar disorder, and anxiety was established primarily by self-report (Has a physician ever diagnosed you with any of the following conditions?) and in some cases, chart review (reviewing patient history reports for diagnoses including mood disorders).
Table 1.
Antidepressant categories and distribution within population.
| Type | Generic | 6 months | 12 months |
|---|---|---|---|
| Selective Serotonin Reuptake Inhibitors (SSRI) | Fluoxetine | 1 | 3 |
| Citalopram | 7 | 5 | |
| Sertraline | 3 | 4 | |
| Escitalopram | 15 | 9 | |
| Paroxetine | 3 | 2 | |
| Serotonin Antagonist and Reuptake Inhibitor (SARI) | Trazodone | 4 | 3 |
| Serotonin-Norepinephrine Reuptake Inhibitors (SNRI) | Duloxetine | 1* | 4* |
| Venlafaxine | 6 | 2 | |
| Norepinephrine –Dopamine Reuptake Inhibitors (NDRI) | Bupropion | 1** | 1 |
| Noradrenergic and Specific Serotonergic Antidepressant (NaSSA) | Mirtazapine | 1 | 1 |
Cognitive Assessment
Self-perceived Cognitive Functioning
Participants’ functional cognitive limitations were assessed using the FIM-Cog38 at both 6 and 12 months after injury. FIM-Cog has five components: expression, comprehension, social interaction, problem-solving, and memory. Each component is rated from one to seven, with a 5 or lower indicating a need for caregiver assistance. The sum of these five components yields the FIM-Cog Score. In the current study, this measure was assessed by trained research staff primarily through in-person interview and was rated primarily based on subject self-report of functioning.
Neuropsychological Assessment and Overall Cognitive Composite
To examine cognitive impairment, nine standard neuropsychological tests were administered at 6 and 12 months post-injury to assess processing speed, visual and verbal memory, attention, language fluency, and executive functions: Trail Making Tests A and B39, the digit span sub-test from the Wechsler Adult Intelligence Scale-R40, the Rey-Osterreith Complex Figure Test Delayed Recall41, the Controlled Oral Word Association (COWA)42, the Delis-Kaplan Executive Function Systems (DKEFS) Verbal Fluency43, the Stroop Task44, the Wisconsin Card Sorting Task (WCST)45 and the California Verbal Learning Test-II (CVLT-II46). Alternate, equivalent forms of the CVLT were used at 6 and 12 months to minimize practice effects from repeated administration. Raw scores from each test were converted into T-scores using appropriate metrics (i.e. education, age, sex, race) based on available norms indicated by the test manufacturer.
This battery was examined at the individual test level and as a part of a cognitive composite (similar to published studies47). For the purposes of data analysis, an overall cognitive composite score was the primary outcome measure for analysis, and this composite score was generated from selected component scores of eight of the nine neuropsychological tests. The composite components included were chosen to best represent domains of interest and to balance the number of domains used in the analysis, allowing evaluation of overall cognitive impairment as a function of included domains. This approach resulted in the exclusion of one test, as explained below. As noted above, we targeted four cognitive domains for analysis (memory, attention, language fluency, and executive functioning) and used specific subtest scores from each of the neuropsychological tests to assess each of these domains. Two subtests from each domain were selected, and their T-scores were averaged to create domain-specific averages. To calculate the cognitive composite score, participants had to complete at least one test in each domain. Mean values across domain sub-scores were calculated to obtain the overall cognitive composite score.
Specifically, we used the following subtest scores as measures within each domain for the composite: for attention, the scores from Trails A and Digit Span; for memory, we chose the long delayed recall score from the Rey-Osterreith Complex Figure Test and the long delay free recall score from the CVLT-II. Language fluency was measured by averaging the animal naming subtest of the COWA and letter fluency from the DKEFS. Executive functioning was measured via the score of Trail Making Test B and the interference score from the Stroop. As we had three tests within the executive functioning domain, the Wisconsin Card Sorting Task was excluded from the executive functioning component of the overall cognitive composite so as not to bias the composite within the executive function domain.
Depression Symptom Assessment
At 6 and 12 months, the presence or absence of depression symptoms was evaluated using the Patient Health Questionnaire-9 (PHQ-9), a brief self-report symptom inventory based on the 9 DSM-IV diagnostic criteria for Major Depressive Disorder (MDD). The PHQ-9 has been validated as a depression assessment after TBI48 that can reliably discriminate between chronic TBI and depression symptoms49. On the PHQ-9, participants rate how often over the last two weeks they have experienced symptoms of depression using a Likert scale ranging from 0 (none) to 3 (nearly every day). Participants were grouped as “depressed” vs. “non-depressed” using the PHQ-9 questions as they map to DSM diagnostic criteria (previously described and validated in TBI48). To be categorized as depressed (PTD), individuals responded positively to at least five symptom questions on the PHQ-9, with at least one pertaining to a cardinal symptom of MDD (anhedonia or depressed mood). Higher total scores (PHQ-9 Total) reflect a greater number of and/or greater severity of depressive symptoms, with the maximum score being 27. PTD severity categories were defined as previously described50 based on PHQ-9 total: none (0–4), mild (5–9), moderate (10–14), moderate severe (15–19), severe (+20). In addition, individuals with PHQ-9 data collected at both six and 12 months after injury were categorized with longitudinal PTD subtypes as none (no PTD at 6 or 12 months), transient (PTD at 6 months only), late-onset (PTD at 12 months only), and persistent (PTD at 6 and 12 months).
Statistical Analysis
Data analysis was conducted using Statistical Analysis Software (version 9.4; SAS Institute). Descriptive analyses included means and standard deviations and/or medians for continuous and ordinal variables such as age, GCS, and education. Frequencies were calculated for categorical variables such as sex and antidepressant use. Demographic and relevant clinical information was assessed for relationships with cognitive impairment using Student’s t-tests or ANOVA to compare means. Non-parametric tests (Mann-Whitney and Kruskal-Wallis) were employed when appropriate. Pearson’s or Spearman’s rho (r) correlations were used to assess relationships between two continuous variables. Multivariate linear regression models were used to assess factors influencing cognitive impairment or functional cognitive limitations. Target variables and covariates were entered into the model and removed in a backwards step-wise fashion when p>0.2 to generate final models.
Results
Specific cohort demographics are shown in Table 2. Participants had a GCS (best in 24 hrs) of 3–15 (mean GCS, 7.7 ± 2.8, median=7). Participants were aged 16–72 (mean age 34.1±13.8 years) and 18.9% of participants were women. At 6 months post-injury, 38.3% had PTD, while 30.3% had PTD at 12 months. Although not statistically significant, those with PTD tended to have a higher mean age compared to those without PTD (p=0.061). Participants with PTD at 12 months tended to have a higher GCS compared to those with no PTD (p=0.057). Those with premorbid mood disorders had significantly higher PTD rates at both 6 (27.9% versus 6.0%, p=0.002) and 12 months (31.4% versus 10.0%, p=0.006). At 6 months, 51% of participants with PTD were on antidepressants while only 26.1% of participants with no PTD were on antidepressants (p=0.007). At 12 months, 40.0% of participants with PTD were taking an antidepressant, compared to 25.0% of those with no PTD (p=0.110). It is important to note that the percentage of individuals on antidepressants did not differ by depression severity category (data not shown).
Table 2.
Demographic description of study population.
| Total Population | 6 Months | 12 Months | |||||
|---|---|---|---|---|---|---|---|
| None (n=71) | PTD (n=44) | p value | None (n=83) | PTD (n=36) | p value | ||
| Age, mean±STD | 34.1±13.8 | 32.9±13.8 | 36.1±13.5 | 0.061 | 34.4±13.8 | 36.5±13.3 | 0.153 |
| GCS, median | 7 | 7 | 8 | 0.493 | 7 | 8 | 0.057 |
| Sex, # (%) Males | 177 (83.9) | 61 (85.9) | 33 (75.0) | 0.146 | 67 (80.7) | 27 (75.0) | 0.482 |
| Race, # (%) Caucasian | 192 (91.9) | 67 (94.4) | 40 (90.9) | 0.485 | 77 (92.8) | 32 (88.9) | 0.493 |
| Education, mean±STD | 13.0±1.9 | 13.1±1.9 | 12.7±1.9 | 0.178 | 13.1±1.8 | 12.7±2.0 | 0.246 |
| Premorbid Mood Disorders, # (%) | 4 (6.0) | 12 (27.9) | 0.002 | 8 (10.0) | 11 (31.4) | 0.006 | |
| Antidepressant Use, # (%) | 18 (26.1) | 22 (51.0) | 0.007 | 20 (25.0) | 14 (40.0) | 0.110 | |
STD, Standard Deviation; PTD, Post-TBI Depression; GCS, Glasgow Coma Scale
Cross-sectional associations with cognitive impairment
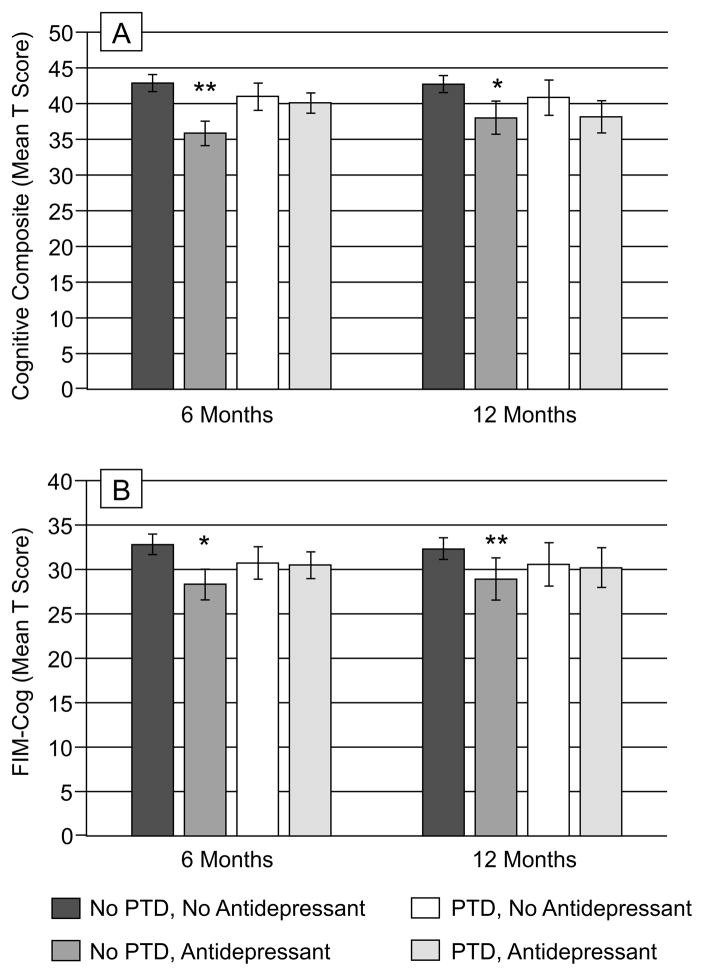
We examined cognitive composite total scores by both PTD and antidepressant use in order to understand possible interactions with global cognitive impairment. Figure 1A shows cognitive composite scores by PTD and antidepressant use at 6 and 12 months post-TBI. Among participants with no PTD, those on antidepressants had significantly worse cognitive impairment at 6 months (p=0.002) than those not on antidepressants, and scores were significantly worse than both groups with PTD (no antidepressant use, p=0.033; antidepressant use, p=0.036). There were no significant effects at 12 months. Of particular importance, the relation between antidepressant use and cognition was not moderated by severity of PTD (data not shown). This interaction between PTD and antidepressant use at 6 months tended to be associated with our cognitive composite score model after adjusting for other covariates (Table 3), meeting criteria of p<0.2 to remain in the model, including, age, GCS, and education. At 12 months, only age, GCS, and education were associated with cognitive composites.
Figure 1.
(A) Cognitive composite scores (T score on y-axis) are shown in groups of participants based on PTD and antidepressant use at 6 and 12 months post-injury. At 6 months, participants with no PTD, but who are on antidepressants perform worse on cognitive composite measures, compared to all other groups (**compared to no PTD, no antidepressant use, p=0.002, compared to PTD, no antidepressant use, p=0.033, compared to PTD, antidepressant use, p=0.036). At 12 months, within participants with no PTD, antidepressant use was associated with poorer scores on the overall composite (*p=0.027). (B) Functional cognition scores (mean score on y-axis) are shown in groups of participants based on PTD and antidepressant use at 6 and 12 months post-injury. At 6 and 12 months, participants with no PTD, but who are on antidepressant, have lower functional cognition compared to no PTD, no antidepressant use (*p<0.0001, **p=0.008).
Table 3.
Linear regression models for overall cognitive composites at 6 and 12 months post-injury.
| Variable | Beta | Standard Error | t value | p value |
|---|---|---|---|---|
| 6 Months | ||||
| Age | −0.09871 | 0.05415 | −1.82 | 0.0716 |
| GCS | 0.73400 | 0.25554 | 2.87 | 0.0051 |
| Education | 0.75151 | 0.36925 | 2.04 | 0.0448 |
| PTD | −1.20868 | 1.89645 | −0.64 | 0.5255 |
| Antidepressant Use | −5.85912 | 2.08453 | −2.81 | 0.0061 |
| PTD*Antidepressant Use | 5.05606 | 3.06514 | 1.65 | 0.1025 |
| 12 months | ||||
| Age | −0.14271 | 0.06421 | −2.22 | 0.0297 |
| GCS | 0.93176 | 0.31603 | 2.95 | 0.0044 |
| Education | 0.95049 | 0.51361 | 1.85 | 0.0688 |
GCS, Glasgow Coma Scale; PTD, Post-traumatic brain injury depression
As these cognitive composite models suggested there were no effects of PTD on cognitive impairment, post-hoc analysis of individual neuropsychological tests was conducted to determine if there were underlying associations with specific cognitive testing components (Supplementary Table 1). Both those with and those without PTD had comparable cognitive performance at 6 months post-TBI. At 12 months, participants with PTD had higher T-scores on the Rey immediate copy test (p=0.027) compared to participants with no PTD, but no other significant associations were found at 12 months.
Furthermore, a secondary analysis was conducted with linear regression models examined for each individual neuropsychological test (Supplementary Table 2). As age, GCS, premorbid mood disorders, and antidepressant use were associated with PTD, we examined PTD associations after adjusting for these variables to determine if covariates differentially predicted PTD association with individual cognitive domains. However, as age and premorbid mood disorders were not consistent contributors to the models, they were omitted. While PTD was not associated with individual neuropsychological tests, antidepressant use was a consistent predictor. Antidepressant use was associated with worse scores on the CVLT, DKEFS Category Total, Rey delayed copy, and COWA at 6 months (p<0.05 all comparisons), and the Stroop Word, Trails A, and Trails B at 12 months (p<0.05 all comparisons). GCS was also a consistent predictor of impaired performance on multiple neuropsychological tests.
Cross-sectional associations with functional cognition
Antidepressant use, PTD, and cognitive impairment effects on functional cognition were then investigated. In Figure 1B, participants with no PTD who were also taking antidepressants had worse FIM-Cog scores compared to participants with no PTD who were not on antidepressants (6 months, p<0.0001; 12 months, p=0.008). In Table 4, multivariate regression models predicting functional cognition were examined. At 6 months, race, cognitive composites scores, and the interaction between PTD and antidepressant use remained in the model. At 12 months, age, GCS, cognitive composites, and PTD predicted functional cognition. There were no significant interactions at 12 months.
Table 4.
Linear regression models for functional cognition (FIM-Cog Total) at 6 and 12 months post-injury.
| Variable | Beta | Standard Error | t value | p value |
|---|---|---|---|---|
| 6 Months | ||||
| Race | 1.25500 | 0.49164 | 2.55 | 0.0123 |
| Cognitive Composite | 0.25148 | 0.03707 | 6.78 | <0.0001 |
| PTD | −1.30494 | 0.72604 | −1.80 | 0.0755 |
| Antidepressant Use | −1.97104 | 0.83523 | −2.36 | 0.0204 |
| PTD*Antidepressant Use | 1.80275 | 1.18158 | 1.53 | 0.1305 |
| 12 months | ||||
| Age | −0.05916 | 0.02740 | −2.16 | 0.0348 |
| GCS | 0.23572 | 0.13792 | 1.71 | 0.0925 |
| Cognitive Composite | 0.29447 | 0.04607 | 6.39 | <0.0001 |
| PTD | −1.03001 | 0.73845 | −1.39 | 0.1681 |
GCS, Glasgow Coma Scale; PTD, Post-traumatic brain injury depression
Post-hoc analyses of associations with individual FIM-Cog components are reported in Supplementary Table 1. At 6 months, FIM-Memory was lower in participants with PTD (p=0.029). At 12 months, FIM-Memory, FIM-Problem-Solving, and FIM-Social Interaction were lower in participants with PTD (p<0.02 all comparisons).
Longitudinal PTD Associations with Cognition
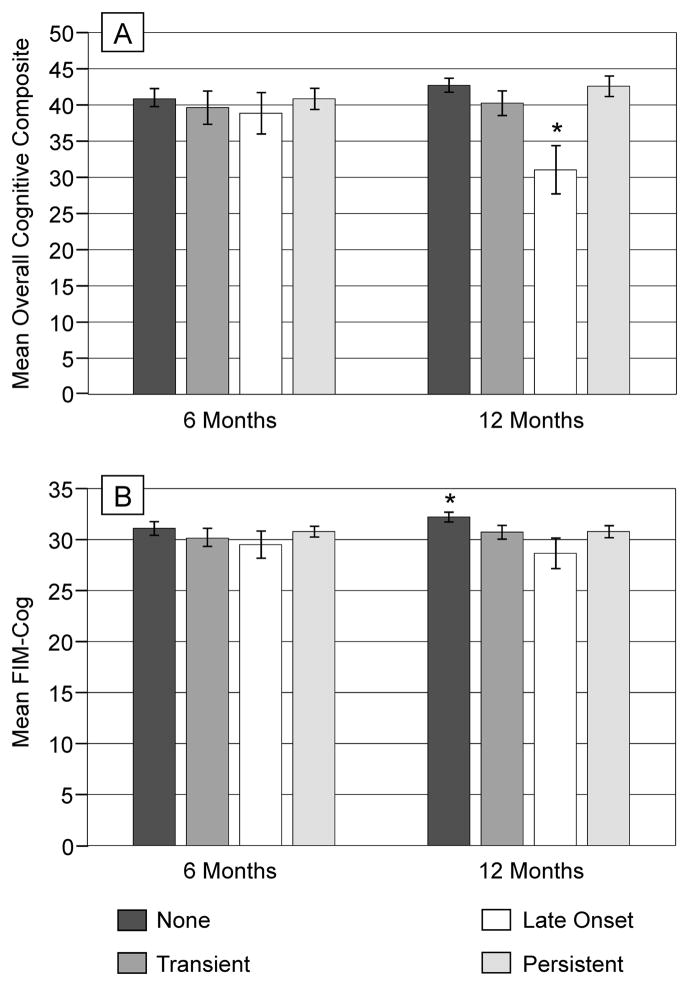
PTD subtype was evaluated for associations with both cognitive impairment and functional cognition for those with PHQ-9 data at both 6 and 12 months post-injury n=98) (Figure 2). Individuals with late-onset of PTD had worse cognitive impairment at 12 months compared to those with no PTD or persistent PTD (p=0.026), but this relationship was not evident at 6 months. Individuals who never experienced PTD had the highest FIM-Cog scores at 6 and 12 months, and these scores were significantly higher than those of all other groups at 12 months (p<0.01).
Figure 2.
(A) Cognitive composite scores (mean T score on y-axis) at 6 and 12 months are shown based on PTD subtypes. At 12 months, participants with late-onset PTD, had significantly worse overall cognitive composite scores compared to those with no PTD (*p=0.026) and persistent PTD (*p=0.026). (B) Functional cognition scores (mean score on y-axis) at 6 and 12 months are shown based on PTD subtypes. At 6 months, participants who never experienced any PTD had significantly higher scores compared to those with late-onset PTD (*p=0.035). At 12 months, participants who never experienced any PTD had significantly higher scores compared to those with any PTD subtype (*p<0.01 for all comparisons).
Discussion
In this study, we explored how cognition may be susceptible to effects of post-TBI depression (PTD) and the additional potential relations among PTD, cognitive impairment (as measured by standard neuropsychological testing), and functional cognition (as measured by self-report, FIM-Cog), and antidepressant use. We found no compelling evidence that cognitive impairment is associated with PTD, yet individuals with PTD had significantly greater functional cognitive limitations than those without PTD. However, cognitive impairment was associated with antidepressant use. Specifically, individuals on antidepressants performed worse on neuropsychological tests, even when correcting for injury severity and PTD.
Multiple studies have suggested that individuals with PTD have worse cognition than individuals without PTD22,23,25–27. However, many of these studies examined raw neuropsychological data and did not correct for individual demographic or clinical differences in their populations (e.g. age, injury severity, medication use)22,23. Thus, we examined whether depressive symptoms were associated with differences in cognition, after adjusting scores for age, education, sex, and race using normative data from healthy populations. As age, sex, education, and race can all affect standard neuropsychological evaluations, the previous literature may overestimate cognitive deficits in individuals with PTD. Previous studies suggest older age as a contributing factor to PTD23, potentially confounding cognitive associations.
Our study demonstrates that when neuropsychological test scores are corrected for demographic factors, injury severity, and antidepressant use, PTD is not associated with cognitive impairment when examined in a cross-sectional manner. The importance of these covariates suggests the need for more Rehabilomics-based studies examining individual differences (e.g. genetic differences) in PTD development and/or post-TBI cognitive impairment, response to treatment (e.g. antidepressant medications, whether prescribed to treat depression or to manage other post-TBI symptoms), and functional limitations resulting from post-TBI changes and treatment side effects. Identifying early markers of susceptibility to poor outcome and/or response to treatment could improve clinical management through effective triage and efficient resource allocation.
Our null findings are not likely due to a lack of impairment as all participants, on average, performed greater than one standard deviation below average on standard neuropsychological assessments, and thus, were considered to have at least mild cognitive impairments. However, the use of this brief battery of neuropsychological tests, and the formulation of composite scores, tends to identify gross differences in impairment. A more comprehensive neuropsychological test battery may provide a more detailed picture of specific cognitive impairments and their discrete associations with depression. In addition, nuanced cognitive testing, using distractors or increasing levels of difficulty may mirror more clearly the demands of daily functioning and reveal cognitive differences associated with PTD, even after correction for demographic factors. Future prospective studies are needed to examine the effects of cognitive demand and cognitive control on cognition performance in the setting of PTD.
While there were no associations between PTD and cognitive impairment from cross-sectional analysis, there were significant associations between PTD subtype (longitudinal analysis) and cognitive impairment. In this study, late-onset PTD was associated with worse cognitive impairment. As a post-hoc exploration, this finding was surprising and requires further study. Understanding the longitudinal relationship between PTD and cognitive impairment and function will be especially important, given the evidence of spontaneous recovery from PTD51. Future studies using serial measures of depression and cognition, along with measures of environmental and life-related stressors, will likely provide the temporal discrimination needed to understand these relationships.
While some studies demonstrate that individuals with depressive symptomatology following TBI have increased functional cognitive limitations or complaints25–27, other studies report no relationship24. We found no PTD-associated cognitive impairments, but did confirm a significant impact of PTD on functional cognitive limitations. In the context of Rehabilomics and using the ICF framework, we hypothesized that PTD would worsen cognitive impairment. We instead found that impairments in cognition and mood (PTD) co-occurred and independently influenced functional cognition. This finding suggests that emotional issues (e.g. apathy, anxiety) can be present in individuals with PTD such that, despite these individuals having comparatively similar cognitive impairment, they can still report more functional limitations compared to those without PTD. Those with PTD may have more difficulty compensating for cognitive impairment than their non-PTD counterparts, and thus, require greater assistance with daily cognitive tasks. Alternatively, those with PTD may be more aware of their cognitive deficits, and thus, their self-report of daily functioning may be more accurate.
Of note, the FIM is a self-report measure of functional performance, capturing what individuals report actually doing in their daily life, not what they are capable of performing. Our findings suggest that those who develop PTD may have cognitive capacity comparable to individuals without PTD, but as a result of depressive symptomatology, this capacity does not translate into similar functional performance. Thus, our findings reflect a discrepancy between cognitive ability and functional cognitive limitations among individuals with PTD. Individuals with PTD have been previously reported to complain more of subjective cognitive difficulties than those without PTD52, suggesting that the relationship between subjective cognitive complaints and neuropsychological measures is confounded by PTD status. Therefore, those who complain of cognitive difficulties may be expected to perform poorly on neuropsychological tests and exhibit greater testing anxiety. However, these effects were likely minimal in our cohort, as participants who reported worse cognitive functioning did not differ with regard to cognitive impairments.
More likely, PTD affects motivation, effort, or distractibility, each of which can exacerbate functional cognitive limitations. Symptoms like apathy, insomnia, psychomotor agitation or retardation, and fatigue are reported frequently after TBI and can influence cognitive function28,53. This overlapping symptomatology could greatly influence functional cognition without manifesting in cognitive impairments. Importantly, these overlapping symptoms can also make identification of PTD difficult, though the PHQ-9 can differentiate between cognitive symptoms and PTD48. One study suggested that functional performance (measured with an ecologically valid test like the Multiple Errands Test) can differ for individuals with pure cognitive impairments compared to those with suboptimal effort54, making this test a potentially useful tool in future studies. Identifying relationships among mood, emotional/behavioral symptoms, and cognitive difficulties also may help delineate unique risk profiles for those with PTD presenting with or without cognitive deficits.
Severity of injury has not been associated consistently with development of PTD. Many researchers support the hypothesis that PTD is due to increased awareness of deficits55, citing studies that show an increase in depressive symptoms in subjects with less severe injuries22,56 where there is likely a heightened awareness of TBI-related difficulties. Individuals with an increased awareness of TBI-related deficits may have greater stress related to their recovery process. This finding is consistent with a biological stress depression model57 for PTD. Our study adds some support to this hypothesis, as individuals with PTD tended to have higher GCS scores even within this cohort of individuals with severe TBI. Similar to previous studies23, those with PTD tended to be older than their non-PTD counterparts. Increasing age has consistently been found to be a risk factor for depressive symptomatology, especially in the context of neurological disorders58.
Antidepressant use was associated with cognitive impairment across cognitive domains and also with increased functional cognitive limitations. For some measures, like CVLT-II scores at 6 months, antidepressant use was associated with nearly a one standard deviation difference in cognitive impairment. Notably, anti-depressants had variable associations with neuropsychological tests. This phenomenon could be due to different etiology of PTD at 6 or 12 months or to changes in cognitive recovery. Also, it is unclear if study participants who are not depressed, but are on antidepressants, were previously depressed or were being treated for other common complications post-TBI, like sleep disturbances30 where antidepressants like trazodone can be effective. We were not able to capture reasons for antidepressant use, but it will be critical for future studies to evaluate antidepressants and their use in TBI populations.
The relationship between antidepressant use and cognitive recovery after TBI is still unclear. In a study of individuals with moderate to severe TBI, sertraline did not improve cognition when administered early in recovery (first three months) and demonstrated a possible negative effect (though this finding was not statistically significant)59. In animal models of TBI, fluoxetine increased hippocampal neurogenesis without any improvement in memory60,61. While future studies will need to examine the relationship of depression remittance to cognition in a priori designed studies, one study in a mild TBI population suggested that antidepressant treatment, with remittance of depression, actually improves cognition34. Although there is some additional support for this finding62, other studies have not reported similar improvement63. It is important to note that while antidepressant treatment is clinically common for PTD, there is rather limited evidence regarding efficacy with respect to remittance of depressive symptoms64–66. Thus, if antidepressants compromise cognitive recovery, this effect may contribute to the reduced efficacy of antidepressants observed with PTD. Also, these initial findings suggest that understanding antidepressant effects on cognition post-TBI may be an important consideration when deciding if/when it is appropriate to prescribe antidepressants for individuals with PTD.
Functional cognition was influenced by multiple covariates in our study. At 6 months, covariate effects were found for cognitive composite scores, in addition to a trend for antidepressant use to be associated with greater functional limitations. As individuals on antidepressants who are not depressed also experience greater cognitive impairment, it is not clear if the functional cognition limitations experienced by these individuals are due to an increase in their underlying cognitive impairment or to an independent interaction with PTD*antidepressant use. At 12 months, there was no interaction, and antidepressant use was no longer a significant predictor in the model. The differences in 6- and 12-month models may reflect changing recovery patterns after TBI, but may also be influenced by sample size differences. One caveat when interpreting functional cognition models is that individuals on antidepressants or with reported depression receive an automatic reduction by one point on the Social Interaction subscale of the FIM-Cog. Thus, the FIM-Cog total is expected to be associated with antidepressant use by at least one point, yet we see differences greater than 1 point. At 6 months, it is difficult to assess the effect of antidepressant use alone on FIM-Cog, due to its interactions with PTD. Also, at 6 months race is still a contributing factor, which could be related to racial disparities in response to antidepressant use67, though this possibility cannot be evaluated in this study.
There are some important limitations to consider in this study. Our sample consists mostly of individuals with severe TBI. Further investigation of our observations across TBI severities is needed. This study suggests special considerations about antidepressant use following TBI, but the implications of this finding are limited because it is not clear how antidepressant use directly affects cognitive impairment or functioning post-TBI. Future treatment studies may benefit from a study design that evaluates cognition before and after antidepressant administration, in addition to documenting the reason(s) for antidepressant administration. Furthermore, pre-morbid mood disorder status was collected by self-report; future studies will need to evaluate how pre-morbid mood disorders influence post-TBI recovery patterns as this study suggests important implications. Another limitation is that our measure of functional cognition was self-report. It is important to recognize that there is likely an association between self-awareness and capacity to report functional limitations embedded in an individual’s responses to FIM items. As such, individuals with measured impairments on neuropsychological testing may also have self-awareness deficits, thus (inaccurately) reporting fewer functional limitations. Future studies that include self-awareness measures and caregiver report of functional limitations may help disentangle this issue.
This study suggests that, while functional cognition is greatly affected by PTD, a number of other factors must be considered in understanding the relationship of PTD to outcome in individual cases. In fact, this work suggests that there are important interactions among pharmacological treatment, cognitive impairment, and depressive symptoms that can affect functional cognition in dynamic ways. Understanding the timing of these interactions will be important for future studies designed to modify current treatment approaches with antidepressants post-TBI. Within the Rehabilomics framework, it is also important to consider biological or genetic effects that could be associated with PTD development, antidepressant use, or functional recovery. It will be important to examine how biomarkers (inflammation68, neurotrophins69) or genetics (serotonin transporter70,71 and related genes72) that have previously shown relationships to PTD might influence PTD-cognition interactions. In addition, multicenter data collection in TBI, such as in the TBI Model Systems73, may allow evaluation of relationships between PTD and cognition using additional instruments like the Brief Test of Adult Cognition by Telephone74 (a common data element75) in order to examine relationships between cognition and depression post-TBI across instruments and in larger sample sizes. Incorporating personal factors, complications, and other individual difference variables in designing personalized treatment algorithms across large datasets will likely lead to more effective depression treatment following TBI. Finally, assessing functional cognition through ecologically valid performance-based measures such as the Multiple Errands Test76–78 rather than self- or caregiver-report may reveal whether the observations reported here are related to functional cognitive limitations or are merely an artifact of self-reporting in the context of cognitive impairment and PTD.
Supplementary Material
Acknowledgments
Research/Grant Support:
This research was supported by Department of Defense (W81XWH-07-1-0701), National Institute on Disability and Rehabilitation Research (NIDRR H133A120087, and National Institute of Health (R01HD048162) and the UPMC Rehabilitation Institute.
Footnotes
Conflict of Interest Statement: The authors declare no conflicts of interest.
References
- 1.Fleming J, Tooth L, Hassell M, Chan W. Prediction of community integration and vocational outcome 2–5 years after traumatic brain injury rehabilitation in Australia. Brain Inj BI. 1999;13:417–431. doi: 10.1080/026990599121476. [DOI] [PubMed] [Google Scholar]
- 2.Juengst S, Skidmore E, Arenth PM, Niyonkuru C, Raina KD. Unique contribution of fatigue to disability in community-dwelling adults with traumatic brain injury. Arch Phys Med Rehabil. 2013;94:74–79. doi: 10.1016/j.apmr.2012.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cifu DX, et al. Acute predictors of successful return to work 1 year after traumatic brain injury: a multicenter analysis. Arch Phys Med Rehabil. 1997;78:125–131. doi: 10.1016/s0003-9993(97)90252-5. [DOI] [PubMed] [Google Scholar]
- 4.Yasuda S, Wehman P, Targett P, Cifu D, West M. Return to work for persons with traumatic brain injury. Am J Phys Med Rehabil Assoc Acad Physiatr. 2001;80:852–864. doi: 10.1097/00002060-200111000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Juengst SB. Self Awareness and Community Integration after Traumatic Brain Injury. University of Pittsburgh; 2012. [Google Scholar]
- 6.Bombardier CH, et al. Rates of Major Depressive Disorder and Clinical Outcomes Following Traumatic Brain Injury. JAMA. 2010;303:1938–1945. doi: 10.1001/jama.2010.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kessler RC, Chiu WT, Demler O, Walters EE. Prevalence, Severity, and Comorbidity of 12-Month DSM-IV Disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koponen S, et al. Axis I and II psychiatric disorders after traumatic brain injury: a 30-year follow-up study. Am J Psychiatry. 2002;159:1315–1321. doi: 10.1176/appi.ajp.159.8.1315. [DOI] [PubMed] [Google Scholar]
- 9.Whitnall L, McMillan TM, Murray GD, Teasdale GM. Disability in young people and adults after head injury: 5–7 year follow up of a prospective cohort study. J Neurol Neurosurg Psychiatry. 2006;77:640–645. doi: 10.1136/jnnp.2005.078246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levin RL, Heller W, Mohanty A, Herrington JD, Miller GA. Cognitive deficits in depression and functional specificity of regional brain activity. Cogn Ther Res. 2007;31:211–233. [Google Scholar]
- 11.Gotlib IH, Joormann J. Cognition and depression: current status and future directions. Annu Rev Clin Psychol. 2010;6:285–312. doi: 10.1146/annurev.clinpsy.121208.131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burt DB, Zembar MJ, Niederehe G. Depression and memory impairment: a meta-analysis of the association, its pattern, and specificity. Psychol Bull. 1995;117:285–305. doi: 10.1037/0033-2909.117.2.285. [DOI] [PubMed] [Google Scholar]
- 13.Hertel PT. Relation between rumination and impaired memory in dysphoric moods. J Abnorm Psychol. 1998;107:166–172. doi: 10.1037//0021-843x.107.1.166. [DOI] [PubMed] [Google Scholar]
- 14.Hertel PT, Rude SS. Depressive deficits in memory: focusing attention improves subsequent recall. J Exp Psychol Gen. 1991;120:301–309. doi: 10.1037/0096-3445.120.3.301. [DOI] [PubMed] [Google Scholar]
- 15.Kouneiher F, Charron S, Koechlin E. Motivation and cognitive control in the human prefrontal cortex. Nat Neurosci. 2009;12:939–945. doi: 10.1038/nn.2321. [DOI] [PubMed] [Google Scholar]
- 16.Peckham AD, McHugh RK, Otto MW. A meta-analysis of the magnitude of biased attention in depression. Depress Anxiety. 2010;27:1135–1142. doi: 10.1002/da.20755. [DOI] [PubMed] [Google Scholar]
- 17.Mäki-Marttunen V, et al. Enhanced Attention Capture by Emotional Stimuli in Mild Traumatic Brain Injury. J Neurotrauma. 2014 doi: 10.1089/neu.2014.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vakil E. The effect of moderate to severe traumatic brain injury (TBI) on different aspects of memory: a selective review. J Clin Exp Neuropsychol. 2005;27:977–1021. doi: 10.1080/13803390490919245. [DOI] [PubMed] [Google Scholar]
- 19.Brooks J, Fos LA, Greve KW, Hammond JS. Assessment of executive function in patients with mild traumatic brain injury. J Trauma. 1999;46:159–163. doi: 10.1097/00005373-199901000-00027. [DOI] [PubMed] [Google Scholar]
- 20.Niogi SN, et al. Structural dissociation of attentional control and memory in adults with and without mild traumatic brain injury. Brain. 2008;131:3209–3221. doi: 10.1093/brain/awn247. [DOI] [PubMed] [Google Scholar]
- 21.Perlstein WM, Larson MJ, Dotson VM, Kelly KG. Temporal dissociation of components of cognitive control dysfunction in severe TBI: ERPs and the cued-Stroop task. Neuropsychologia. 2006;44:260–274. doi: 10.1016/j.neuropsychologia.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 22.Jorge RE, et al. Major depression following traumatic brain injury. Arch Gen Psychiatry. 2004;61:42–50. doi: 10.1001/archpsyc.61.1.42. [DOI] [PubMed] [Google Scholar]
- 23.Rapoport MJ, McCullagh S, Shammi P, Feinstein A. Cognitive Impairment Associated With Major Depression Following Mild and Moderate Traumatic Brain Injury. J Neuropsychiatry Clin Neurosci. 2005;17:61–65. doi: 10.1176/jnp.17.1.61. [DOI] [PubMed] [Google Scholar]
- 24.Satz P, et al. Depression, cognition, and functional correlates of recovery outcome after traumatic brain injury. Brain Inj BI. 1998;12:537–553. doi: 10.1080/026990598122313. [DOI] [PubMed] [Google Scholar]
- 25.Gfeller JD, Chibnall JT, Duckro PN. Postconcussion symptoms and cognitive functioning in posttraumatic headache patients. Headache. 1994;34:503–507. doi: 10.1111/j.1526-4610.1994.hed3409503.x. [DOI] [PubMed] [Google Scholar]
- 26.Bornstein RA, Miller HB, van Schoor JT. Neuropsychological deficit and emotional disturbance in head-injured patients. J Neurosurg. 1989;70:509–513. doi: 10.3171/jns.1989.70.4.0509. [DOI] [PubMed] [Google Scholar]
- 27.Rapoport M, McCauley S, Levin H, Song J, Feinstein A. The role of injury severity in neurobehavioral outcome 3 months after traumatic brain injury. Neuropsychiatry Neuropsychol Behav Neurol. 2002;15:123–132. [PubMed] [Google Scholar]
- 28.Andersson S, Bergedalen AM. Cognitive correlates of apathy in traumatic brain injury. Cogn Behav Neurol. 2002;15:184–191. [PubMed] [Google Scholar]
- 29.Bushnik T, Englander J, Wright J. Patterns of fatigue and its correlates over the first 2 years after traumatic brain injury. J Head Trauma Rehabil. 2008;23:25–32. doi: 10.1097/01.HTR.0000308718.88214.bb. [DOI] [PubMed] [Google Scholar]
- 30.Larson EB, Zollman FS. The effect of sleep medications on cognitive recovery from traumatic brain injury. J Head Trauma Rehabil. 2010;25:61–67. doi: 10.1097/HTR.0b013e3181c1d1e1. [DOI] [PubMed] [Google Scholar]
- 31.Wagner AK. TBI translational rehabilitation research in the 21st Century: exploring a Rehabilomics research model. Eur J Phys Rehabil Med. 2010;46:549–556. [PubMed] [Google Scholar]
- 32.Bilbao A, et al. The ICF: Applications of the WHO model of functioning, disability and health to brain injury rehabilitation. NeuroRehabilitation. 2003;18:239–250. [PubMed] [Google Scholar]
- 33.Wagner AK. Rehabilomics: a conceptual framework to drive biologics research. PM R. 2011;3:S28–30. doi: 10.1016/j.pmrj.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 34.Fann JR, Uomoto JM, Katon WJ. Cognitive Improvement With Treatment of Depression Following Mild Traumatic Brain Injury. Psychosomatics. 2001;42:48–54. doi: 10.1176/appi.psy.42.1.48. [DOI] [PubMed] [Google Scholar]
- 35.Udekwu P, Kromhout-Schiro S, Vaslef S, Baker C, Oller D. Glasgow Coma Scale score, mortality, and functional outcome in head-injured patients. J Trauma. 2004;56:1084–1089. doi: 10.1097/01.ta.0000124283.02605.a5. [DOI] [PubMed] [Google Scholar]
- 36.Elovic EP, Lansang R, Li Y, Ricker JH. The use of atypical antipsychotics in traumatic brain injury. J Head Trauma Rehabil. 2003;18:177–195. doi: 10.1097/00001199-200303000-00008. [DOI] [PubMed] [Google Scholar]
- 37.Phelps TI, Bondi CO, Ahmed RH, Olugbade YT, Kline AE. Divergent long-term consequences of chronic treatment with haloperidol, risperidone, and bromocriptine on traumatic brain injury-induced cognitive deficits. J Neurotrauma. 2014 doi: 10.1089/neu.2014.3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dodds TA, Martin DP, Stolov WC, Deyo RA. A validation of the functional independence measurement and its performance among rehabilitation inpatients. Arch Phys Med Rehabil. 1993;74:531–536. doi: 10.1016/0003-9993(93)90119-u. [DOI] [PubMed] [Google Scholar]
- 39.Reitan R, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation. Neuropsychology Press; 1985. [Google Scholar]
- 40.Larrabee GJ, Curtiss G. Construct validity of various verbal and visual memory tests. J Clin Exp Neuropsychol. 1995;17:536–547. doi: 10.1080/01688639508405144. [DOI] [PubMed] [Google Scholar]
- 41.Osterrieth P. The Complex Figure Copy Test. Arch Psychol. 1944;30:206–356. [Google Scholar]
- 42.Borkowski J, Benton A, Spreen O. Word fluency and brain damage. Neuropsychologia. 1967;5:135–140. [Google Scholar]
- 43.Delis DC, Kaplan E. Delis-Kaplan Executive Function System (DKEFS) The Psychological Corporation; 2001. [Google Scholar]
- 44.Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol Exp Psychol. 1935;18:643–662. [Google Scholar]
- 45.Heaton RK. Wisconsin Card Sorting Test Manual. Psychological Assesment Resources; 1981. [Google Scholar]
- 46.Delis DC, et al. The California Verbal Learning Test. The Psychological Corporation; 2000. [Google Scholar]
- 47.Failla MD, Myrga JM, Ricker JH, Dixon CE, Wagner AK. Post-TBI cognitive performance is moderated by variation within ANKK1 and DRD2 genes. J Head Trauma Rehabil. doi: 10.1097/HTR.0000000000000118. Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fann JR, et al. Validity of the Patient Health Questionnaire-9 in assessing depression following traumatic brain injury. J Head Trauma Rehabil. 2005;20:501–511. doi: 10.1097/00001199-200511000-00003. [DOI] [PubMed] [Google Scholar]
- 49.Cook KF, et al. Do Somatic and Cognitive Symptoms of Traumatic Brain Injury Confound Depression Screening? Arch Phys Med Rehabil. 2011;92:818–823. doi: 10.1016/j.apmr.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dikmen SS, Bombardier CH, Machamer JE, Fann JR, Temkin NR. Natural history of depression in traumatic brain injury. Arch Phys Med Rehabil. 2004;85:1457–1464. doi: 10.1016/j.apmr.2003.12.041. [DOI] [PubMed] [Google Scholar]
- 52.Chamelian L, Feinstein A. The effect of major depression on subjective and objective cognitive deficits in mild to moderate traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2006;18:33–38. doi: 10.1176/jnp.18.1.33. [DOI] [PubMed] [Google Scholar]
- 53.Cantor JB, et al. Fatigue After Traumatic Brain Injury and Its Impact on Participation and Quality of Life. J Head Trauma Rehabil. 2008;23:41–51. doi: 10.1097/01.HTR.0000308720.70288.af. [DOI] [PubMed] [Google Scholar]
- 54.Castiel M, Alderman N, Jenkins K, Knight C, Burgess P. Use of the Multiple Errands Test-Simplified Version in the assessment of suboptimal effort. Neuropsychol Rehabil. 2012;22:734–751. doi: 10.1080/09602011.2012.686884. [DOI] [PubMed] [Google Scholar]
- 55.Malec JF, Brown AW, Moessner AM, Stump TE, Monahan P. A preliminary model for posttraumatic brain injury depression. Arch Phys Med Rehabil. 2010;91:1087–1097. doi: 10.1016/j.apmr.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 56.Hudak AM, Hynan LS, Harper CR, Diaz-Arrastia R. Association of depressive symptoms with functional outcome after traumatic brain injury. J Head Trauma Rehabil. 2012;27:87–98. doi: 10.1097/HTR.0b013e3182114efd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2007;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- 58.Fiske A, Wetherell JL, Gatz M. Depression in Older Adults. Annu Rev Clin Psychol. 2009;5:363–389. doi: 10.1146/annurev.clinpsy.032408.153621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baños JH, et al. Impact of early administration of sertraline on cognitive and behavioral recovery in the first year after moderate to severe traumatic brain injury. J Head Trauma Rehabil. 2010;25:357–361. doi: 10.1097/HTR.0b013e3181d6c715. [DOI] [PubMed] [Google Scholar]
- 60.Wilson MS, Hamm RJ. Effects of fluoxetine on the 5-HT1A receptor and recovery of cognitive function after traumatic brain injury in rats. Am J Phys Med Rehabil Assoc Acad Physiatr. 2002;81:364–372. doi: 10.1097/00002060-200205000-00009. [DOI] [PubMed] [Google Scholar]
- 61.Wang Y, et al. Fluoxetine increases hippocampal neurogenesis and induces epigenetic factors but does not improve functional recovery after traumatic brain injury. J Neurotrauma. 2011;28:259–268. doi: 10.1089/neu.2010.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Horsfield SA, et al. Fluoxetine’s effects on cognitive performance in patients with traumatic brain injury. Int J Psychiatry Med. 2002;32:337–344. doi: 10.2190/KQ48-XT0L-2H14-5UMV. [DOI] [PubMed] [Google Scholar]
- 63.Lee H, et al. Comparing effects of methylphenidate, sertraline and placebo on neuropsychiatric sequelae in patients with traumatic brain injury. Hum Psychopharmacol. 2005;20:97–104. doi: 10.1002/hup.668. [DOI] [PubMed] [Google Scholar]
- 64.Fann JR, Hart T, Schomer KG. Treatment for Depression after Traumatic Brain Injury: A Systematic Review. J Neurotrauma. 2009;26:2383–2402. doi: 10.1089/neu.2009.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Warden DL, et al. Guidelines for the Pharmacologic Treatment of Neurobehavioral Sequelae of Traumatic Brain Injury. J Neurotrauma. 2006;23:1468–1501. doi: 10.1089/neu.2006.23.1468. [DOI] [PubMed] [Google Scholar]
- 66.Ashman TA, et al. A randomized controlled trial of sertraline for the treatment of depression in persons with traumatic brain injury. Arch Phys Med Rehabil. 2009;90:733–740. doi: 10.1016/j.apmr.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 67.Murphy E, et al. Race, genetic ancestry and response to antidepressant treatment for major depression. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2013;38:2598–2606. doi: 10.1038/npp.2013.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Juengst SB, Kumar RG, Failla MD, Goyal A, Wagner AK. Acute Inflammatory Biomarker Profiles Predict Depression Risk Following Moderate to Severe Traumatic Brain Injury. J Head Trauma Rehabil. 2014 doi: 10.1097/HTR.0000000000000031. [DOI] [PubMed] [Google Scholar]
- 69.Failla M, Wagner A. Low serum brain-derived neurotrophic factor levels are associated with depression and lower functional cognition following TBI. 2013. [Google Scholar]
- 70.Failla MD, et al. Variants of SLC6A4 in depression risk following severe TBI. Brain Inj BI. 2013;27:696–706. doi: 10.3109/02699052.2013.775481. [DOI] [PubMed] [Google Scholar]
- 71.Graham DP, et al. Serotonin transporter genotype and mild traumatic brain injury independently influence resilience and perception of limitations in Veterans. J Psychiatr Res. 2013;47:835–842. doi: 10.1016/j.jpsychires.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lanctôt KL, et al. Genetic predictors of response to treatment with citalopram in depression secondary to traumatic brain injury. Brain Inj BI. 2010;24:959–969. doi: 10.3109/02699051003789229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Traumatic Brain Injury Model Systems. at < http://tbims.org/>.
- 74.Gavett BE, Crane PK, Dams-O’Connor K. Bi-factor analyses of the Brief Test of Adult Cognition by Telephone. NeuroRehabilitation. 2013;32:253–265. doi: 10.3233/NRE-130842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.NINDS Common Data Elements: Traumatic Brain Injury. at < http://www.commondataelements.ninds.nih.gov/TBI.aspx#tab=Data_Standards>.
- 76.Pedroli E, et al. Virtual Multiple Errands Test: reliability, usability and possible applications. Stud Health Technol Inform. 2013;191:38–42. [PubMed] [Google Scholar]
- 77.Alderman N, Burgess PW, Knight C, Henman C. Ecological validity of a simplified version of the multiple errands shopping test. J Int Neuropsychol Soc JINS. 2003;9:31–44. doi: 10.1017/s1355617703910046. [DOI] [PubMed] [Google Scholar]
- 78.Dawson JD, Uc EY, Anderson SW, Johnson AM, Rizzo M. Neuropsychological predictors of driving errors in older adults. J Am Geriatr Soc. 2010;58:1090–1096. doi: 10.1111/j.1532-5415.2010.02872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.