Abstract
Objectives
The aim of this study was to examine skeletal muscle mitochondria content, oxidative capacity, and the expression of key mitochondrial dynamics proteins in patients with heart failure with preserved ejection fraction (HFpEF) and to determine potential relationships with measures of exercise performance.
Background
Multiple lines of evidence indicate that severely reduced peak exercise oxygen uptake (peak VO2) in older patients with HFpEF, is related to abnormal skeletal muscle oxygen utilization. Mitochondria are key regulators of skeletal muscle metabolism, however little is known about how these organelles are affected in HFpEF.
Methods
Vastus lateralis skeletal muscle citrate synthase activity, and the expression of porin and regulators of mitochondrial fusion were examined in older patients with HFpEF (n=20) and healthy, age-matched controls (n=17).
Results
In HFpEF patients compared to age-matched healthy controls, mitochondrial content assessed by Porin expression was 46% lower (p-value= 0.01), citrate synthase activity was 29% lower (p= 0.01), and Mfn2 expression was 54% lower (p= <0.001). Expression of Porin was significantly positively correlated with both peak VO2 and 6 minute walk distance (r=0.48, p=0.003 and r=0.33, p=0.05, respectively). Expression of Mfn2 was also significantly positively correlated with both peak VO2 and 6 minute walk distance (r=0.40, p=0.02 and r=0.37, p=0.03 respectively).
Conclusion
These findings suggest that skeletal muscle oxidative capacity, mitochondrial content, and mitochondrial fusion are abnormal in older patients with HFpEF and may contribute to their severe exercise intolerance.
Keywords: heart failure, preserved ejection fraction, exercise, aging, mitochondria, skeletal muscle
INTRODUCTION
Heart failure with preserved left ventricular ejection fraction (HFpEF) is the most prevalent form of heart failure (HF) and is nearly unique to older adults, particularly older women (1, 2). The primary manifestation of chronic stable HFpEF is severe exercise intolerance measured objectively as reduced peak exercise oxygen uptake (peak VO2) (3). Multiple recent reports indicate that, in addition to underlying cardiac dysfunction, ‘non-cardiac’ factors contribute to severe exercise intolerance in HFpEF. Previously, our group has reported that reduced cardiac output accounts for only 50% of the markedly reduced peak oxygen uptake (peak VO2) in HFpEF patients (4), suggesting a significant role for peripheral factors. Endurance exercise training significantly improves peak VO2 in clinically stable older individuals with HFpEF; however, a majority of the improvement is mediated by non-cardiac factors, presumably skeletal muscle function (5, 6). Although we and others have found significant abnormalities in central arterial stiffness and their relation to exercise intolerance, we reported that arterial stiffness and conduit arterial endothelial dysfunction do not improve with exercise training in HFpEF (7). Altogether, these studies suggest that in HFpEF, skeletal muscle alterations contribute to exercise intolerance and that improvements in skeletal muscle function contribute to the beneficial effects of exercise training for these patients.
Several lines of evidence indicate that older adults with HFpEF have altered skeletal muscle metabolism. We have reported that older HFpEF patients have abnormal skeletal muscle oxygen utilization and that this is related to their severely reduced peak VO2 (8). Using magnetic resonance spectroscopy in HFpEF patients, Bhella et al found reduced skeletal muscle oxidative metabolism (9). More recently, Dhakal et al, using invasive hemodynamic monitoring during exercise, showed that oxygen extraction was significantly reduced in HFpEF and was a major contributor to their reduced peak VO2 (10). While HFpEF patients have a lower percent lean mass, the increase in peak exercise VO2 relative to lean mass is lower in HFpEF compared to healthy controls (8). Using skeletal muscle biopsies, we recently showed that HFpEF patients have a decreased number of type I oxidative fibers compared to healthy controls (11). Taken together, these data suggest the hypothesis that skeletal muscle mitochondrial dysfunction contributes to impaired skeletal muscle aerobic metabolism in patients with HFpEF.
To examine this hypothesis, we measured the expression of key mitochondrial proteins in skeletal muscle biopsy specimens from patients with HFpEF and healthy, age-matched, controls (HC). We measured the expression of Mitofusins 1 and 2 (Mfn1 and Mfn2), proteins localized to the mitochondrial outer membrane that play an essential role in the fusion of these organelles. Mitofusins, in particular Mfn2, play an important role in mitochondrial quality control by mediating complementation of organelles and the elimination of dysfunctional mitochondria by autophagy (12, 13). A potential difference in skeletal muscle mitochondrial content was determined by analysis of Porin expression. Further validation was provided by the measurement of citrate synthase activity, a biomarker widely recognized as the most reliable indicator of skeletal muscle oxidative capacity and mitochondrial content (14). Finally, we examined the relationships of these mitochondrial parameters with measures of exercise intolerance, peak VO2 and 6-minute walk distance.
METHODS
Participants
As previously described in studies from our laboratory (3, 4, 6, 15–17), and in accord with the 2013 ACC/AHA recommendations (18), HFpEF was defined as symptoms and signs of HF according to the National Health and Nutrition Examination Survey HF clinical score of ≥3 and the criteria of Rich et al (19, 20), preserved resting left ventricular systolic function (ejection fraction ≥50%, and no segmental wall motion abnormalities), and no significant ischemic or valvular heart disease, pulmonary disease, anemia, or other disorder that could explain the patients’ symptoms (3, 4, 16). HFpEF subjects were ≥ 60yrs of age at study entry. Age-matched, sedentary HC subjects were recruited and screened and excluded if they had any chronic medical illness, were on any chronic medication, had current complaints or an abnormal physical examination (including blood pressure ≥ 140/90 mmHg), had abnormal results on the screening tests (including electrocardiogram, exercise echocardiogram, and spirometry), or regularly undertook vigorous exercise (8, 21). The protocol was approved by the Wake Forest School of Medicine institutional review board, and all participants provided written, informed consent.
Exercise Testing
As previously described (3, 22), exercise testing was performed on a treadmill using the modified Naughton protocol for the HFpEF subjects and using the modified Bruce protocol for the HC subjects. Expired gas analysis was conducted using a commercially available system (CPX-2000 and Ultima; MedGraphics; Minneapolis, MN) that was calibrated before each test with a standard gas of known concentration and volume. Breath-by-breath gas exchange data were measured continuously during exercise and averaged every 15 seconds, and peak values were averaged from the last two 15-second intervals during peak exercise. A six-minute walk test was performed using the method of Guyatt (23).
Skeletal muscle biopsy
As previously described, skeletal muscle biopsies were performed in the early morning after an overnight fast (24–26). Subjects were asked to refrain from taking aspirin, non-steroidal anti-inflammatory drugs, and other compounds that may affect bleeding, platelets, or bruising for the week prior to the biopsy, and to refrain from any strenuous activity for at least 36 hours prior to the biopsy. Muscle was obtained from the vastus lateralis using the percutaneous needle biopsy technique with a University College Hospital needle under local anesthesia with 1% lidocaine (27). There were no medical complications or other reported adverse events from the procedure.
Visible blood and connective tissue were removed from muscle specimens, and portions for Western blot and enzyme analyses were partitioned for freezing. Muscle portions used for mitochondrial analyses were stored at −80°C prior to homogenization and analysis.
Protein Expression
Expression of mitochondrial proteins was determined by Western blotting. Frozen skeletal muscle biopsy samples (10–15 mg) were homogenized with stainless steel beads using a BBX24 Bullet Blender (Next Advance, Averill Park, NY) and lysed with radioimmunoprecipitation assay (RIPA). Equal amounts of total protein, determined by BCA protein assay (Thermo Scientific, Rockford, IL), were separated by electrophoresis in Laemmli buffer on 12% polyacrylamide-SDS gels (Invitrogen, Carlsbad, CA). The samples were electrophoretically transferred to nylon polyvinyl difluoride (PVDF) membrane and the blots were incubated with commercially-available primary antibodies to Mfn1 (1:1000), Mfn2 (1:1000), porin (1:1000), and GAPDH (1:2000) (Abcam, Cambridge, MA). Appropriate horseradish peroxidase (HRP)-conjugated secondary antibodies were added and immunoreactive products were visualized using the Supersignal West-Pico chemiluminescent reagent (Thermo Scientific, Rockford, IL). Kaleidoscope markers (BioRad, Hercules, CA) were used to monitor protein transfer and Magic Markers (Invitrogen, Carlsbad, CA) were used for molecular weight approximations. The density of each immunoreactive product was quantified using a Kodak imaging system (Kodak, Rochester, NY). Densitometry values for Mfn1 and Mfn2 were normalized to Porin in order to account for differences in mitochondrial content. Measurement of Porin was normalized to GAPDH.
Citrate Synthase Activity
The activity of a mitochondrial enzyme, citrate synthase, in skeletal muscle homogenates was measured using the Citrate Synthase Assay Kit (Sigma, CS0720). Measurements of citrate synthase activity were carried out at room temperature in 0.2 ml of assay medium containing 20µg of muscle homogenate in the presence of saturating concentrations of acetyl-coenzyme A (0.3 mM), dithionitrobenzoic acid (0.1 mM) and oxaloacetate (0.5 mM). The reaction was initiated by adding 0.5 mM oxaloacetate and monitored by measuring the increase in absorbance at 412 nm due to thionitrobenzoic acid (TNB) formation using a SpectraMax Microplate Reader (UV/Vis) and SoftMax Pro software (Molecular Devices). Citrate synthase activities were expressed as nmol/min/mg protein using molar extinction coefficient of TNB, ε412=13.6/mM/cm.
Statistical Methods
Intergroup (HFpEF vs. HC) comparisons of participant characteristics were made by independent samples t-tests for continuous variables, by Fisher’s exact tests for binomial variables, and by Chi-square tests for general categorical variables. To ensure confidence in our findings, comparisons of exercise capacity variables between groups were made by analysis of covariance, adjusting for gender. Comparisons of mitochondrial protein expression between groups were made initially by independent samples t-test and then by analysis of covariance, adjusting for gender, BMI, and race. Sex, BMI, and race were selected because of prior data suggesting their influence on mitochondrial function, in addition to the intergroup imbalances observed. The tests for group differences were made by the Type III sum of squares test for removal. The final analysis was conducted as a single test with subsequent adjustments. Logarithmic transformation was performed for Porin which was highly skewed. The relationships between mitochondrial protein expression variables and exercise capacity (peak VO2, six-minute walk distance) were assessed by Spearman correlations. For all analyses, a two-tailed p-value of <0.05 was required for significance. All statistical tests were conducted using SAS version 9.1.3 .
RESULTS
Subject Characteristics
The patients had characteristics typical of chronic, stable HFpEF with NYHA class II-III symptoms, and abnormal Doppler LV diastolic function compared to HC (Table 1). A history of chronic systemic hypertension was present in 95% of HFpEF patients. HFpEF and HC were well matched for age; however, there was a trend for a greater number of women in the HFpEF group. No participants in the HFpEF or HC groups had a history, signs, or symptoms of thyroid dysfunction. Although body weight, fat mass, percent body fat, and body mass index were higher in HFpEF compared to HC, lean mass was similar. Multiple population-based studies and clinical trials have reported significantly higher BMI in HFpEF patients compared to the general population (28–32). To account for the differences in gender, BMI, and race, adjustments for these variables are included in the comparisons of mitochondrial parameters.
Table 1.
Participant Characteristics
| HFpEF (N=20) | HC (N=17) | p-value | |
|---|---|---|---|
| Age (years) | 68.2 ± 6.0 | 71.2 ± 7.7 | 0.18 |
| Women | 12 (60%) | 5 (29%) | 0.10 |
| White | 11 (55%) | 17 (100%) | 0.002 |
| Height (cm) | 167 ± 8 | 173 ± 8 | 0.02 |
| Weight (kg) | 101 ± 17 | 83 ± 20 | 0.005 |
| Body mass index (kg/m2) | 36.2 ± 4.9 | 27.4 ± 5.3 | <0.001 |
| Total Fat Mass (kg, by DEXA) | 39.7 ± 9.6 | 24.2 ± 10.6 | <0.001 |
| Total Lean Mass (kg, by DEXA) | 56.0 ± 11.5 | 56.8 ± 11.2 | 0.84 |
| Percent Body Fat, % | 40.5 ± 7.9 | 28.3 ± 7.5 | <0.001 |
| Systolic blood pressure, mmHg | 132 ± 14 | 125 ± 9 | 0.07 |
| Diastolic blood pressure, mmHg | 79 ± 9 | 75 ± 6 | 0.16 |
| Ejection Fraction, % | 61 ± 5 | 58 ± 5 | 0.07 |
| Lateral mitral annulus velocity (e’, cm/s) | 7.3 ± 1.8 | 9.1 ± 1.8 | 0.004 |
| Early mitral flow velocity / e’ | 10.0 ± 2.5 | 7.5 ± 1.4 | <0.001 |
| Diastolic Filling Pattern | |||
| Normal | 0 (0%) | 13 (76%) | <0.001 |
| Impaired relaxation | 19 (95%) | 4 (24%) | |
| Pseudonormal | 1 (5%) | 0 (0%) | |
| Restrictive | 0 (0%) | 0 (0%) | |
| History of hypertension | 19 (95%) | -- | -- |
| Diabetes mellitus | 5 (25%) | -- | -- |
| New York Heart Association class | |||
| II | 14 (70%) | -- | -- |
| III | 6 (30%) | -- | -- |
| Medications | |||
| Diuretics | 15 (75%) | -- | -- |
| Angiotensin converting enzyme inhibitors |
9 (45%) | -- | -- |
| Beta blockers | 8 (40%) | -- | -- |
| Calcium channel blockers | 5 (25%) | -- | -- |
HFpEF, Heart failure and preserved ejection fraction; HC, Healthy age-matched control; DEXA, Dual-Energy X-ray absorptiometry; Values are Mean ± SD, or number (%).
Exercise performance
In accord with prior reports, HFpEF patients had severely reduced peak VO2 compared to HC (Table 2) (33, 34). This was evident despite similar peak exercise respiratory exchange ratio in HFpEF versus HC, which was ≥1.12 in both groups, indicating exhaustive exercise effort. There was a trend for lower peak HR in HFpEF versus HC. The 6-minute walk distance was also significantly less in HFpEF compared to HC.
Table 2.
Cardiorespiratory and hemodynamic responses during peak treadmill exercise and distance walked in 6 minutes
| HFpEF | HC | HFpEF | HC | p-value | |
|---|---|---|---|---|---|
| Raw Data | Adjusted data* | ||||
| Peak Pulmonary oxygen uptake, ml/kg/min | 15.8 ± 2.7 | 26.5 ± 6.9 | 16.0 ± 1.1 | 26.0 ± 1.3 | <0.001 |
| Peak Pulmonary oxygen uptake, ml/min | 1574 ± 333 | 2147 ± 584 | 1638 ± 78 | 2013 ± 87 | 0.003 |
| Ventilatory anaerobic threshold, ml/min | 1064 ± 279 | 1284 ± 383 | 1106 ± 60 | 1198 ± 67 | 0.32 |
| Peak Carbon dioxide production, ml/min | 1767 ± 427 | 2463 ± 711 | 1841 ± 103 | 2311 ± 115 | 0.005 |
| Peak Respiratory exchange ratio | 1.12 ± 0.11 | 1.15 ± 0.09 | 1.12 ± 0.02 | 1.14 ± 0.03 | 0.58 |
| Peak Heart rate, beats/min | 142 ± 20 | 152 ± 18 | 141 ± 4 | 153 ± 5 | 0.09 |
| Peak Systolic blood pressure, mmHg | 173 ± 19 | 182 ± 20 | 173 ± 4 | 182 ± 5 | 0.21 |
| Peak Diastolic blood pressure, mmHg | 79 ± 12 | 76 ± 7 | 79 ± 2 | 77 ± 3 | 0.61 |
| 6-minute walk distance, feet | 1419 ± 201 | 1858 ± 284 | 1428 ± 55 | 1840 ± 61 | <0.001 |
HFpEF, Heart failure and preserved ejection fraction; HC, Healthy age-matched control. Raw data are presented as Mean ± SD
Adjusted for gender and presented as least square means ± SE; p-value corresponds to adjusted data.
Expression of Mitochondrial Proteins
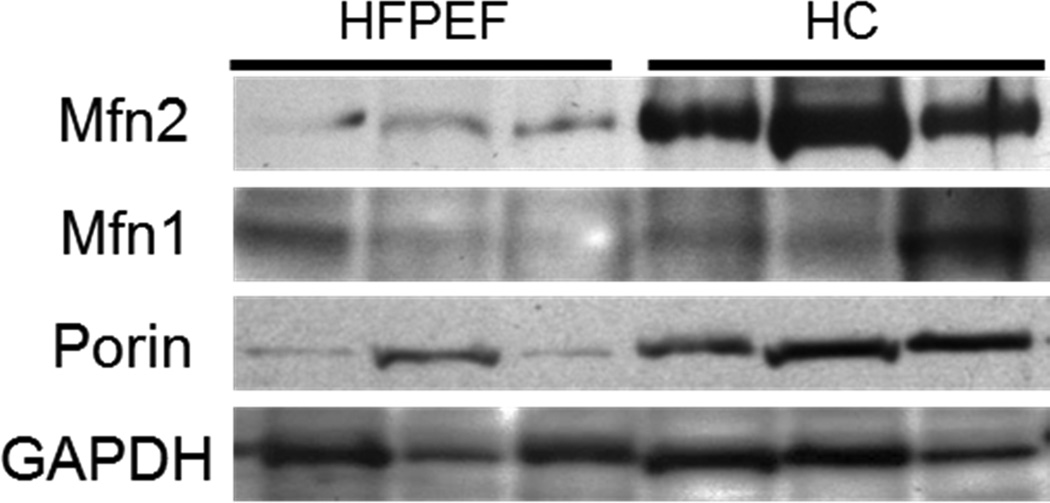
Protein expression of Porin and Mfn2 (normalized to Porin) were significantly lower (p=0.01 and p=<0.001 respectively) in skeletal muscle tissue of patients with HFpEF compared to HC (Table 3). Normalization of mitofusins to Porin, rather than gapdh, was appropriate because these protein reside on the mitochondrial outer membrane. Normalization to Porin accounts for the difference in mitochondrial content, which would exacerbate the observed difference on Mfn2 expression. The lower Mfn2 expression in HFpEF patients remained statistically significant after adjustments for gender (p=<0.001), BMI (p=0.03), race (p=0.002), or gender, BMI, and race (p=0.03). Decreased Porin expression in HFpEF skeletal muscle remained significant when adjusting for gender alone (p=0.004), race alone (p=0.003) and a strong trend for significance when adjusted for gender, BMI and race (p=0.07). Expression of Mfn1 was not significantly different in HFpEF skeletal muscle compared to HC. No significant differences were found with subgroup analysis comparing the effects of gender. Representative western blot images from three participants from each group are presented in figure 1.
Table 3.
Mitochondrial Characteristics
| HFPEF | HC | p-value | p-value† | p-value‡ | p-value# | p-value* | |
|---|---|---|---|---|---|---|---|
| Mfn1 / porin | 1.64 ± 0.23 | 2.13 ± 0.25 | 0.16 | 0.21 | 0.32 | 0.30 | 0.52 |
| Mfn2 / porin | 1.15 ± 0.20 | 2.52 ± 0.30 | <0.001 | <0.001 | 0.03 | 0.002 | 0.03 |
| Porin / gapdh | 1.24 ± 0.29 | 2.29 ± 0.45 | 0.01 | 0.004 | 0.21 | 0.003 | 0.07 |
| Citrate Synthase (nmol/min/mg) | 98.0 ± 7.6 | 137.8 ± 13.3 | 0.01 | 0.04 | 0.005 | 0.06 | 0.053 |
HFPEF, Heart failure and preserved ejection fraction; HC, Healthy age-matched control. Raw data are presented as Mean ± SE with unadjusted p-value;
p-value adjusted for gender
p-value adjusted for body mass index
p-value adjusted for race
p-value adjusted for race, gender, and BMI; all p-values shown for Porin/gapdh are shown following logarithmic transformation.
Figure 1.
Representative western blot bands from 3 patients with HFPEF and 3 healthy controls (HC). For each protein, images were obtained from the same blot and exposure.
Citrate Synthase activity
Citrate synthase activity was significantly lower (p=0.01) in patients with HFpEF compared to HC (Table 3). The difference remained significant with individual adjustments for gender and BMI, and a strong trend for significance when adjusted for race (p=0.06) and for gender, BMI, and race (p=0.053).
Relationships between skeletal muscle mitochondria and exercise performance
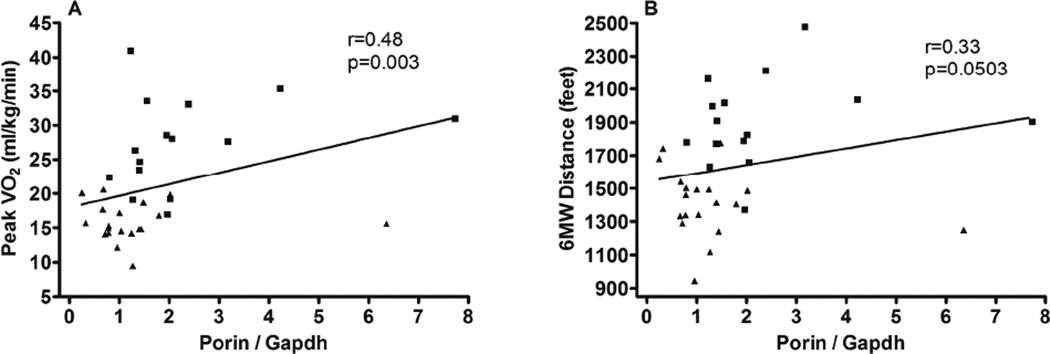
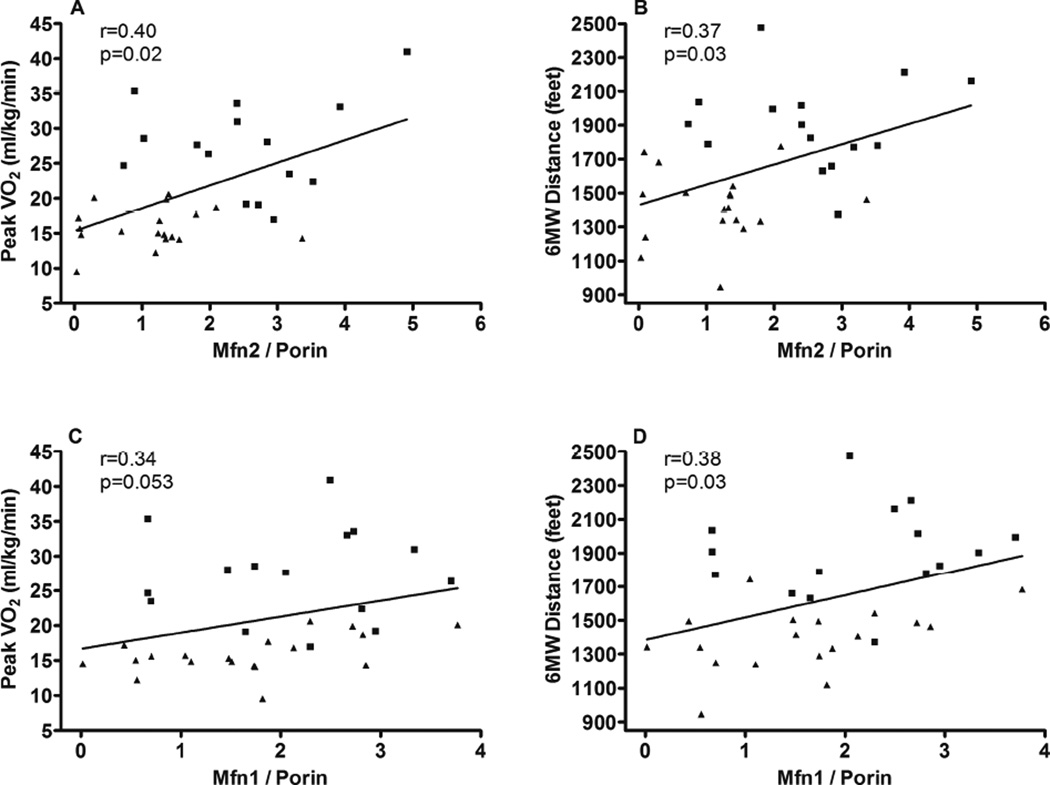
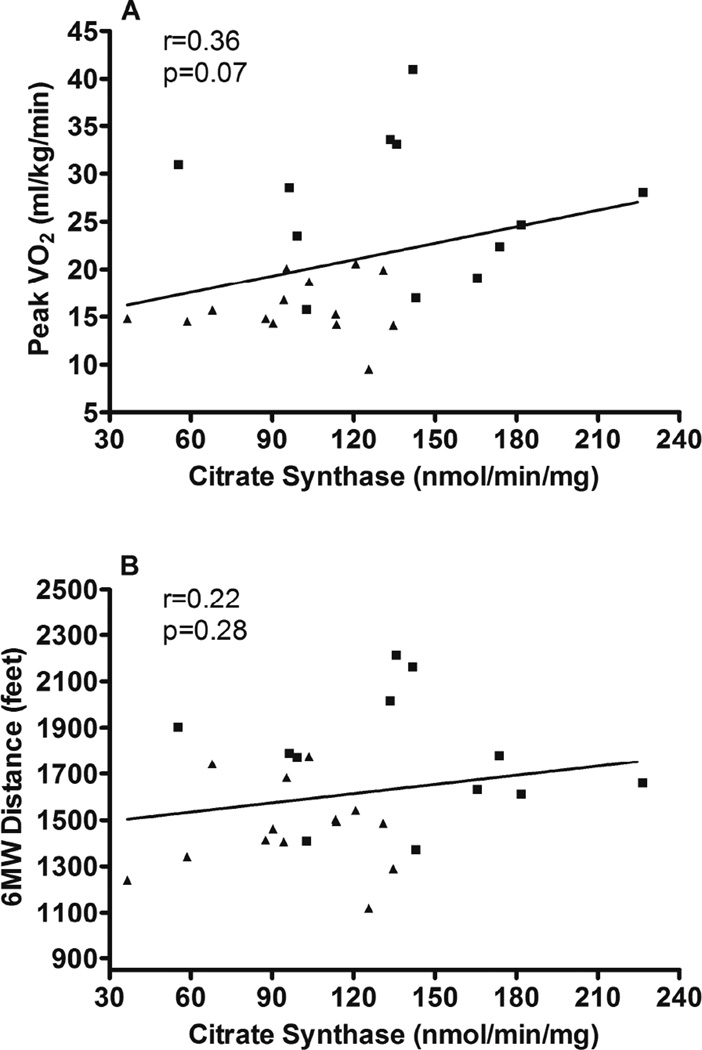
Expression of mitochondrial proteins and citrate synthase activity were compared with aerobic power (i.e. peak VO2) and endurance (six-minute walk distance, 6MWD) (Table 4). With all subjects combined, Porin expression was significantly positively associated with both peak VO2 and 6MWD (Figure 2). The expression of Mfn2 was positively associated with both measures of exercise performance (Figure 3). Mfn1 was significantly associated with 6MWD with a trend (p=0.053) for a positive association with peak VO2. There was a trend toward a relationship (p=0.07) between citrate synthase activity and peak VO2 (Figure 4).
Table 4.
Relationships of mitochondrial measures with Peak VO2 and 6 minute walk distance
| Peak VO2 | 6MWD | |
|---|---|---|
| Porin / gapdh | R=0.48, p=0.003 | R=0.33, p=0.05 |
| Mfn1 / porin | R=0.34, p=0.05 | R=0.38, p=0.03 |
| Mfn2 / porin | R=0.40, p=0.02 | R=0.37, p=0.03 |
| Citrate Synthase Activity (nmol/min/mg) | R=0.37, p=0.07 | R=0.22, p=0.28 |
Peak VO2, peak oxygen uptake; 6MWD, 6 minute walk distance; R; Spearman correlation coefficient; p, 2 tailed p-value.
Figure 2.
Regression analysis (Spearman) comparing Vastus lateralis Porin expression with peak VO2 and 6 minute walk distance. Both mitochondrial markers were normalized to the housekeeping protein, GAPDH. HFpEF participants are designated by triangles and HC participants are designated by squares.
Figure 3.
Regression analysis (Spearman) comparing Vastus lateralis Mfn1 and Mfn2 expression with peak VO2 and 6 minute walk distance. Both mitochondrial markers were normalized to the expression of Porin to account for differences in mitochondrial content. HFpEF participants are designated by triangles and HC participants are designated by squares.
Figure 4.
Regression analysis (Spearman) comparing Vastus lateralis citrate synthase activity with peak VO2 and 6 minute walk distance. HFpEF participants are designated by triangles and HC participants are designated by squares.
When analyses were performed only within the HFpEF group, the relationships became non-significant, except for Porin and Mfn1, where there remained a trend with 6 minute walk distance (r=0.33; p=0.16, and r=0.37, p=0.11, respectively); there also remained a trend for Mfn1 with peak VO2 (r=0.38; p=0.10).
Effects of statin use
By definition, no participants in the HC group were taking statin medications. Among the participants with HFpEF, 11 were on statins and 9 were not. Analyses comparing the effects of statin use on mitochondrial outcomes indicate that there was no effect on Mfn2 expression (p=0.67) and no effect of citrate synthase activity (p=0.94).
DISCUSSION
To our knowledge, this study provides the first report of skeletal muscle mitochondrial alterations in patients with HFpEF, the most common form of HF in older persons. The major new finding of this study is that compared to healthy controls, patients with HFpEF exhibit lower vastus lateralis mitochondrial content and oxidative capacity as reported by citrate synthase activity and porin expression. The relationships of these mitochondrial parameters with measures of exercise capacity indicate that these deficits may contribute to severely reduced exercise tolerance. Additionally, the mitochondrial fusion regulator, Mfn2, was significantly decreased in HFpEF and may also contribute to exercise intolerance.
The findings presented in this report are supported by multiple lines of evidence, including a recent study reporting that mitochondrial density, measured in the soleus muscle of a rat model of HFpEF, was reduced compared to controls (35). Further support is provided by our previous report that, compared to age-matched healthy subjects, older HFpEF patients have reduced peak exercise arteriovenous-oxygen difference (A-VO2diff), which is an important contributor to their severely reduced peak VO2 (33). In addition, non-cardiac peripheral factors were also a major contributor to improved peak VO2 after endurance training (6). Using 31phosphate magnetic resonance spectroscopy, Bhella et al reported that during static leg exercise, HFpEF patients had impaired skeletal muscle oxidative metabolism versus healthy controls(9). Recently, we showed that in older HFpEF patients compared to healthy age-matched controls, the slope of the relationship of peak VO2 with percent leg lean mass was markedly reduced in HFpEF versus age-matched healthy controls, suggesting that skeletal muscle hypoperfusion or impaired oxygen utilization may play an important role in limiting exercise performance in HFpEF(8). Further, using skeletal muscle biopsies, we showed that HFpEF patients had fewer type I oxidative fibers compared to healthy controls (11).
Studies performed in healthy older adults provide further evidence linking mitochondrial bioenergetics with physical function. Coen et. al, has reported that the respiratory capacity of muscle fibers and maximal phosphorylation capacity are associated with peak VO2 and walk speed in older adults (36). Similarly, we have reported that walk speed in older adults is also positively associated with the function of isolated skeletal muscle mitochondria, assessed as respiratory control (25).
Our data are also supported by previous reports of multiple skeletal muscle mitochondrial abnormalities, including reduced citrate synthase activity, in patients with HF with reduced EF (HFrEF) and their relation to exercise intolerance in those patients (37, 38). A study by Drexler and colleagues reported over 20 years ago that in patients with HFrEF, skeletal muscle mitochondrial morphology is disrupted and characterized by a reduction in mitochondrial volume and surface density (39). Due to its key role in mitochondrial fusion (40), reduced Mfn2 expression may mediate changes in mitochondrial morphology associated with HF. The Drexler study found that the alterations in mitochondrial morphology in HFrEF were significantly correlated with peak VO2. Similarly, we find that in HFpEF, Mfn2 expression is correlated to both peak exercise peak VO2 and 6MWD, indicating a potential role for impaired mitochondrial fusion in associated impaired exercise tolerance during sub-maximal (as occurs during activities of daily living) as well as maximal exercise.
The mean BMI of our HFpEF patients and HC reflects that of population-based studies and clinical trials which indicate that approximately 80% of older HFpEF patients are overweight or obese, twice the general older population (41). Excess regional adipose tissue may impair skeletal muscle function and reduce mitochondrial density (42, 43); hence, the potential effects of obesity on skeletal muscle mitochondria are important to consider in the interpretation of the present results. While an earlier study has reported that Mfn2 mRNA is decreased in the skeletal muscle of obese individuals (44), a more recent study has shown that protein content of Mfn2 is unaffected (45). In our study, controlling for BMI did not alter the statistical difference between Mfn2 expression in patients with HFpEF and HC. The difference in Porin in HFpEF patients compared to HC was weaker statistically (p=0.21) after controlling for BMI, suggesting a role for body mass / adioposity in mitochondrial content and biogenesis in HFpEF.
Interestingly, skeletal muscle Mfn2 has been associated with a number of metabolic abnormalities. In mice, genetic ablation of Mfn2 leads to the development of impaired glucose tolerance, hyperinsulinemia, and insulin resistance (46). In humans, mRNA levels of Mfn2 have been positively correlated with glucose disposal rate (44). Mfn2 depletion in HFpEF may play a role in impaired skeletal muscle metabolism as well as insulin resistance, and glucose intolerance associated with this disease (47). Importantly, Mfn2 plays an important role in mitochondrial quality control by mediating complementation of organelles and the elimination of dysfunctional mitochondria by autophagy (40, 48, 49). Low expression of Mfn2 may lead to the accumulation of dysfunctional organelles within the mitochondrial network, leading to reduced overall oxidative phosphorylation capacity.
Limitations
This study was limited to mitochondrial measures that could be performed on 10–15mg samples of frozen skeletal muscle tissue that were available. Future studies, with access to larger, fresh samples at the time of biopsy, will enable the examination of mitochondrial function by muscle fiber respirometry, and the preparation of tissues for imaging of mitochondria using electron microscopy and for gene expression. Such studies will be able to determine the bioenergetic and morphological consequences of the low citrate synthase activity and low Mfn2 expression we observed patients with HFpEF. This study specifically focused on citrate synthase because this enzyme has been shown to be decreased in HFrEF (37). A recent review of manuscripts published between 1983 and 2013, reported that changes in peak VO2 are highly correlated with measures of skeletal muscle citrate synthase activity (50). Furthermore, the authors report that changes in whole body oxidative capacity also matched changes in muscle citrate synthase activity.
We selected healthy control subjects who were sedentary since habitual level of physical activity can influence skeletal muscle function. Since we did not use formal survey assessments of habitual physical activity, we cannot ensure that there weren’t subtle intergroup differences that influenced our results. However, similar mitochondrial abnormalities were present in animal models of HF and HFpEF where physical activity was able to be controlled (35). Furthermore, it is known that the mitochondrial abnormalities in HFrEF, which are similar to those we observed in our HFpEF patients, are independent of level of habitual phyical activity and cardiac output (38, 51).
Although relationships of mitochondrial function variables with exercise capacity became less significant when examined within the HFpEF group only, the most appropriate analyses to address the question of mechanisms accounting for differences in exercise capacity between HFpEF and controls is with the groups combined; this also allows a larger range for correlations and a larger sample size.
Since we did not test a control group with hypertension but not heart failure, we cannot determine whether a portion of the intergroup differences in mitochondrial content and biogenesis that we observed was mediated partly by this common comorbidity.
Future directions
The results of this study warrant multiple lines of future investigation. We have previously reported that the capillary-to-fiber ratio is decreased in patients with HFpEF and that these alterations were associated with their decreased peak VO2 (26). This study highlights the need to better understand the relative contributions of oxygen supply versus utilization in the impairment of skeletal muscle metabolism in patients with HFpEF. Future studies can also examine the effects of interventions, such as exercise training, on skeletal muscle mitochondrial bioenergetics in patients with HFpEF. Alterations in mitochondrial oxidative phosphorylation may underlie our previous observation that the increase in peak VO2 after exercise training is primarily due to improved A-VO2diff (6). In a rat model of HFpEF, exercise training prevented the reduction of skeletal muscle citrate synthase activity (35). In healthy humans, exercise training has also been shown to increase the expression of Mfn2 (52, 53). Mitochondria, and Mfn2 in particular, represent potentially important targets for intervention since they can underlie skeletal muscle defects associated with a number of the primary symptoms of HFpEF. Interventions that rescue Mfn2 expression, prevent Mfn2 decline, or promote Mfn2 function may have important implications in the treatment of HFpEF. Understanding the role of mitochondria in HFpEF-associated exercise intolerance and its improvement with intervention could lead to the development of novel treatments for exercise intolerance in HFpEF with strategies specifically designed to improve skeletal muscle metabolism.
CONCLUSION
These findings suggest that skeletal muscle oxidative capacity, mitochondrial content, and mitochondrial fusion are abnormal in older patients with HFpEF and may contribute to their severe exercise intolerance.
Clinical Perspectives.
Recent studies have reported that alterations in skeletal muscle metabolism are major contributors to exercise intolerance in patients with HFpEF. This study provides the first evidence that skeletal muscle mitochondrial defects are present in patients with HFpEF and are related to key measures of exercise intolerance. Understanding the role of mitochondria in HFpEF may uncover potential new targets for the development of therapeutic interventions.
Translational Outlook.
The relationship of low citrate synthase activity and low Mfn2 expression with mitochondrial function and skeletal muscle metabolism in patients with HFpEF remains to be determined. In addition, future studies should examine the role of mitochondria in interventions that have been shown to improve exercise capacity in patients with HFpEF (e.g. exercise training). The results of these studies will inform on whether targeting mitochondria can effectively improve the exercise capacity of patients with HFpEF.
Acknowledgments
Grant Support: This work was supported by National Institutes of Health grants R01AG18915, 5R01-AG020583 and 3R01-AG020583-09S1, and the Wake Forest Claude D. Pepper Older Americans Independence Center (P30-AG21332). Dr. Kitzman is the Kermit Glenn Phillips II Chair in Cardiovascular Medicine at the Wake Forest School of Medicine. Dr. Haykowsky is the Inaugural Moritz Chair in Geriatric Nursing in the College of Nursing and Health Innovation at the University of Texas at Arlington.
Abbreviations
- ACC
American College of Cardiology
- AHA
American Heart Association
- A-VO2diff
arterio-venous oxygen difference
- BMI
body mass index
- DEXA
dual energy x-ray absorptiometry
- EF
ejection fraction
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- HC
healthy age-matched control
- HF
heart failure
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- HRP
horseradish peroxidase
- LV
left ventricle
- Mfn1
mitofusin 1
- Mfn2
mitofusin 2
- mRNA
messenger ribonucleic acid
- NYHA
New York Heart Association
- PVDF
polyvinyl difluoride
- RIPA
radioimmunoprecipitation assay
- TNB
thionitrobenzoic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
Dr. Kitzman serves as a consultant for Relypsa, Corvia Medical, Abbvie, Merck, Bayer, GSK, and Regeneron, and owns stock in Gilead Sciences and Relypsa. No other authors have conflicts of interest to declare.
References
- 1.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 2.Kitzman DW, Gardin JM, Gottdiener JS, et al. Importance of heart failure with preserved systolic function in patients > or = 65 years of age CHS Research Group. Cardiovascular Health Study. Am J Cardiol. 2001;87:413–419. doi: 10.1016/s0002-9149(00)01393-x. [DOI] [PubMed] [Google Scholar]
- 3.Kitzman DW, Little WC, Brubaker PH, et al. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA. 2002;288:2144–2150. doi: 10.1001/jama.288.17.2144. [DOI] [PubMed] [Google Scholar]
- 4.Haykowsky MJ, Brubaker PH, John JM, Stewart KP, Morgan TM, Kitzman DW. Determinants of exercise intolerance in elderly heart failure patients with preserved ejection fraction. J Am Coll Cardiol. 2011;58:265–274. doi: 10.1016/j.jacc.2011.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fleg JL, Cooper LS, Borlaug BA, et al. Exercise training as therapy for heart failure: current status and future directions. Circ Heart Fail. 2015;8:209–220. doi: 10.1161/CIRCHEARTFAILURE.113.001420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haykowsky MJ, Brubaker PH, Stewart KP, Morgan TM, Eggebeen J, Kitzman DW. Effect of endurance training on the determinants of peak exercise oxygen consumption in elderly patients with stable compensated heart failure and preserved ejection fraction. J Am Coll Cardiol. 2012;60:120–128. doi: 10.1016/j.jacc.2012.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kitzman DW, Brubaker PH, Herrington DM, et al. Effect of endurance exercise training on endothelial function and arterial stiffness in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single-blind trial. J Am Coll Cardiol. 2013;62:584–592. doi: 10.1016/j.jacc.2013.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haykowsky MJ, Brubaker PH, Morgan TM, Kritchevsky S, Eggebeen J, Kitzman DW. Impaired aerobic capacity and physical functional performance in older heart failure patients with preserved ejection fraction: role of lean body mass. J Gerontol A Biol Sci Med Sci. 2013;68:968–975. doi: 10.1093/gerona/glt011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhella PS, Prasad A, Heinicke K, et al. Abnormal haemodynamic response to exercise in heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13:1296–1304. doi: 10.1093/eurjhf/hfr133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dhakal BP, Malhotra R, Murphy RM, et al. Mechanisms of exercise intolerance in heart failure with preserved ejection fraction: the role of abnormal peripheral oxygen extraction. Circ Heart Fail. 2015;8:286–294. doi: 10.1161/CIRCHEARTFAILURE.114.001825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haykowsky MJ, Kouba EJ, Brubaker PH, Nicklas BJ, Eggebeen J, Kitzman DW. Skeletal muscle composition and its relation to exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Cardiol. 2014;113:1211–1216. doi: 10.1016/j.amjcard.2013.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Twig G, Hyde B, Shirihai OS. Mitochondrial fusion, fission and autophagy as a quality control axis: The bioenergetic view. Biochim Biophys Acta. 2008;1777:1092–1097. doi: 10.1016/j.bbabio.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Twig G, Shirihai OS. The interplay between mitochondrial dynamics and mitophagy. Antioxid Redox Signal. 2011;14:1939–1951. doi: 10.1089/ars.2010.3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larsen S, Nielsen J, Hansen CN, et al. Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J Physiol. 2012;590:3349–3360. doi: 10.1113/jphysiol.2012.230185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haykowsky MJ, Herrington DM, Brubaker PH, Morgan TM, Hundley WG, Kitzman DW. Relationship of flow-mediated arterial dilation and exercise capacity in older patients with heart failure and preserved ejection fraction. J Gerontol A Biol Sci Med Sci. 2013;68:161–167. doi: 10.1093/gerona/gls099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kitzman DW, Brubaker PH, Morgan TM, Stewart KP, Little WC. Exercise training in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single-blind trial. Circ Heart Fail. 2010;3:659–667. doi: 10.1161/CIRCHEARTFAILURE.110.958785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scott JM, Haykowsky MJ, Eggebeen J, Morgan TM, Brubaker PH, Kitzman DW. Reliability of peak exercise testing in patients with heart failure with preserved ejection fraction. Am J Cardiol. 2012;110:1809–1813. doi: 10.1016/j.amjcard.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 19.Rich MW, Beckham V, Wittenberg C, Leven CL, Freedland KE, Carney R. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med. 1995;333:1190–1195. doi: 10.1056/NEJM199511023331806. [DOI] [PubMed] [Google Scholar]
- 20.Schocken DD, Arrieta MI, Leaverton PE, Ross EA. Prevalence and mortality rate of congestive heart failure in the United States. J Am Coll Cardiol. 1992;20:301–306. doi: 10.1016/0735-1097(92)90094-4. [DOI] [PubMed] [Google Scholar]
- 21.Stehle JR, Jr, Leng X, Kitzman DW, Nicklas BJ, Kritchevsky SB, High KP. Lipopolysaccharide-binding protein, a surrogate marker of microbial translocation, is associated with physical function in healthy older adults. J Gerontol A Biol Sci Med Sci. 2012;67:1212–1218. doi: 10.1093/gerona/gls178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scott JM, Haykowsky MJ, Eggebeen J, Morgan TM, Brubaker PH, Kitzman DW. Reliability of peak exercise testing in patients with heart failure with preserved ejection fraction. Am J Cardiol. 2012;110:1809–1813. doi: 10.1016/j.amjcard.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guyatt GH, Sullivan M, Thompson PJ, et al. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J. 1985;132:919–923. [PMC free article] [PubMed] [Google Scholar]
- 24.Bharadwaj MS, Tyrrell DJ, Lyles MF, Demons JL, Rogers GW, Molina AJ. Preparation and respirometric assessment of mitochondria isolated from skeletal muscle tissue obtained by percutaneous needle biopsy. J Vis Exp. 2015 doi: 10.3791/52350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tyrrell DJ, Bharadwaj MS, Van Horn CG, Kritchevsky SB, Nicklas BJ, Molina AJ. Respirometric Profiling of Muscle Mitochondria and Blood Cells Are Associated With Differences in Gait Speed Among Community-Dwelling Older Adults. J Gerontol A Biol Sci Med Sci. 2014 doi: 10.1093/gerona/glu096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kitzman DW, Nicklas B, Kraus WE, et al. Skeletal muscle abnormalities and exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Physiol Heart Circ Physiol. 2014;306:H1364–H1370. doi: 10.1152/ajpheart.00004.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicklas B, Leng I, Delbono O, et al. Relationship of physical function to vastus lateralis capillary density and metabolic enzyme activity in elderly men and women. Aging Clin Exper Res. 2008;20:302–309. doi: 10.1007/bf03324860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shah AM, Solomon SD. Phenotypic and pathophysiological heterogeneity in heart failure with preserved ejection fraction. Eur Heart J. 2012;33:1716–1717. doi: 10.1093/eurheartj/ehs124. [DOI] [PubMed] [Google Scholar]
- 29.Shah AM, Shah SJ, Anand IS, et al. Cardiac structure and function in heart failure with preserved ejection fraction: baseline findings from the echocardiographic study of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist trial. Circ Heart Fail. 2014;7:104–115. doi: 10.1161/CIRCHEARTFAILURE.113.000887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Redfield MM, Chen HH, Borlaug BA, et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2013;309:1268–1277. doi: 10.1001/jama.2013.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haass M, Kitzman DW, Anand IS, et al. Body mass index and adverse cardiovascular outcomes in heart failure patients with preserved ejection fraction: results from the Irbesartan in Heart Failure with Preserved Ejection Fraction (I-PRESERVE) trial. Circ Heart Fail. 2011;4:324–331. doi: 10.1161/CIRCHEARTFAILURE.110.959890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anjan VY, Loftus TM, Burke MA, et al. Prevalence, clinical phenotype, and outcomes associated with normal B-type natriuretic peptide levels in heart failure with preserved ejection fraction. Am J Cardiol. 2012;110:870–876. doi: 10.1016/j.amjcard.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haykowsky MJ, Brubaker PH, John JM, Stewart KP, Morgan TM, Kitzman DW. Determinants of Exercise Intolerance in Elderly Heart Failure Patients With Preserved Ejection Fraction. J Am Coll Cardiol. 2011;58:265–274. doi: 10.1016/j.jacc.2011.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haykowsky M, Kouba EJ, Brubaker PH, Nicklas BJ, Eggebeen J, Kitzman DW. Skeletal muscle composition and its relationship to exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Cardiol. 2014 doi: 10.1016/j.amjcard.2013.12.031. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bowen TS, Rolim NP, Fischer T, et al. Heart failure with preserved ejection fraction induces molecular, mitochondrial, histological, and functional alterations in rat respiratory and limb skeletal muscle. Eur J Heart Fail. 2015;17:263–272. doi: 10.1002/ejhf.239. [DOI] [PubMed] [Google Scholar]
- 36.Coen PM, Jubrias SA, Distefano G, et al. Skeletal Muscle Mitochondrial Energetics Are Associated With Maximal Aerobic Capacity and Walking Speed in Older Adults. J Gerontol A Biol Sci Med Sci. 2012 doi: 10.1093/gerona/gls196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sullivan MJ, Green HJ, Cobb FR. Skeletal muscle biochemistry and histology in ambulatory patients with long-term heart failure. Circulation. 1990;81:518–527. doi: 10.1161/01.cir.81.2.518. [DOI] [PubMed] [Google Scholar]
- 38.Middlekauff HR. Making the case for skeletal myopathy as the major limitation of exercise capacity in heart failure. Circulation: Heart Failure. 2010;3:537–546. doi: 10.1161/CIRCHEARTFAILURE.109.903773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Drexler H, Riede U, Munzel T, Konig H, Funke E, Just H. Alterations of skeletal muscle in chronic heart failure. Circulation. 1992;85:1751–1759. doi: 10.1161/01.cir.85.5.1751. [DOI] [PubMed] [Google Scholar]
- 40.Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol. 2003;160:189–200. doi: 10.1083/jcb.200211046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lam CS, Donal E, Kraigher-Krainer E, Vasan RS. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13:18–28. doi: 10.1093/eurjhf/hfq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Civitarese AE, Carling S, Heilbronn LK, et al. Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med. 2007;4:e76. doi: 10.1371/journal.pmed.0040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bharadwaj MS, Tyrrell DJ, Leng I, et al. Relationships between mitochondrial content and bioenergetics with obesity, body composition and fat distribution in healthy older adults. BMC Obes. 2015;2:40. doi: 10.1186/s40608-015-0070-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bach D, Naon D, Pich S, et al. Expression of Mfn2, the Charcot-Marie-Tooth neuropathy type 2A gene, in human skeletal muscle: effects of type 2 diabetes, obesity, weight loss, and the regulatory role of tumor necrosis factor alpha and interleukin-6. Diabetes. 2005;54:2685–2693. doi: 10.2337/diabetes.54.9.2685. [DOI] [PubMed] [Google Scholar]
- 45.Samjoo IA, Safdar A, Hamadeh MJ, et al. Markers of skeletal muscle mitochondrial function and lipid accumulation are moderately associated with the homeostasis model assessment index of insulin resistance in obese men. PLoS One. 2013;8:e66322. doi: 10.1371/journal.pone.0066322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sebastian D, Hernandez-Alvarez MI, Segales J, et al. Mitofusin 2 (Mfn2) links mitochondrial and endoplasmic reticulum function with insulin signaling and is essential for normal glucose homeostasis. Proc Natl Acad Sci U S A. 2012;109:5523–5528. doi: 10.1073/pnas.1108220109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aroor AR, Mandavia CH, Sowers JR. Insulin resistance and heart failure: molecular mechanisms. Heart Fail Clin. 2012;8:609–617. doi: 10.1016/j.hfc.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao T, Huang X, Han L, et al. Central role of mitofusin 2 in autophagosome-lysosome fusion in cardiomyocytes. J Biol Chem. 2012;287:23615–23625. doi: 10.1074/jbc.M112.379164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen Y, Dorn GW. PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science. 2013;340:471–475. doi: 10.1126/science.1231031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vigels-Hansen A, Andersen NB, Dela F. The relationship between skeletal muscle mitochondrial citrate synthase activity and whole body oxygen uptake adaptations in response to exercise training. International journal of physiology, pathophysiology and pharmacology. 2014;6:84–101. [PMC free article] [PubMed] [Google Scholar]
- 51.Simonini A, Long CS, Dudley GA, Yue P, McElhinny J, Massie BM. Heart failure in rats causes changes in skeletal muscle morphology and gene expression that are not explained by reduced activity. Circulation research. 1996;79:128–136. doi: 10.1161/01.res.79.1.128. [DOI] [PubMed] [Google Scholar]
- 52.Cartoni R, Leger B, Hock MB, et al. Mitofusins 1/2 and ERRalpha expression are increased in human skeletal muscle after physical exercise. J Physiol. 2005;567:349–358. doi: 10.1113/jphysiol.2005.092031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Konopka AR, Suer MK, Wolff CA, Harber MP. Markers of Human Skeletal Muscle Mitochondrial Biogenesis and Quality Control: Effects of Age and Aerobic Exercise Training. J Gerontol A Biol Sci Med Sci. 2013 doi: 10.1093/gerona/glt107. [DOI] [PMC free article] [PubMed] [Google Scholar]