Abstract
Introduction
Traumatic brain injury (TBI) frequently results in impaired cognition, a function that can be modulated by monoaminergic signaling. Genetic variation among monoaminergic genes may affect post-TBI cognitive performance. The vesicular monoamine transporter 2 (VMAT2) gene may be a novel source of genetic variation important for cognitive outcomes post-TBI given VMAT2’s role in monoaminergic neurotransmission.
Objective
Evaluate associations between VMAT2 variability and cognitive outcomes post-TBI.
Methods
We evaluated 136 white adults with severe TBI for variation in VMAT2 using a tagging single nucleotide polymorphism (tSNP) approach (rs363223, rs363226, rs363251, and rs363341). We show genetic variation interacts with assessed cognitive impairment [cognitive composite T-scores (Comp-Cog)] to influence functional cognition [Functional Independence Measure Cognitive subscale (FIM-Cog)] 6 and 12 months post-injury.
Results
Multivariate analyses at 6-months post-injury showed rs363226 genotype was associated with Comp-Cog (p=0.040) and interacted with Comp-Cog to influence functional cognition (p<0.001). G-homozygotes had the largest cognitive impairment, and their cognitive impairment had the greatest adverse effect on functional cognition.
Discussion
We provide the first evidence that genetic variation within VMAT2 is associated with cognitive outcomes following TBI. Further work is needed to validate this finding and elucidate mechanisms by which genetic variation affects monoaminergic signaling, mediating differences in cognitive outcomes.
Keywords: Traumatic Brain Injury (TBI), vesicular monoamine transporter type 2 (VMAT2), genetic polymorphism, cognition, depression
Introduction
Traumatic brain injury (TBI) is a major cause of morbidity and mortality worldwide. At least 10 million injuries serious enough to result in death or hospitalization occur annually [1], leaving many with persistent symptoms that result in functional impairment and low levels of satisfaction with quality of life [2]. Individuals recovering from all levels of injury severity frequently experience both acute and chronic cognitive deficits that impair communication skills, memory, attention, executive function, and the ability to problem-solve [3–5]. Many also experience post-TBI depression (PTD), which is the most common neurobehavioral complication post-TBI [6]. While some studies have suggested that individuals with TBI and PTD demonstrate poorer cognition compared to those without PTD [7, 8], other studies have shown no effect of PTD on cognition [9, 10]. However, it has been demonstrated that symptoms post-TBI that overlap with PTD can affect functional cognition [11–16]. Early identification of individuals at risk for cognitive deficits and impaired functional cognition would allow for earlier interventions and increase our understanding of the development of these complications.
Monoaminergic neurotransmission, which includes dopamine (DA) and serotonin (5-HT) signaling, modulates executive function and mood in the general population [17, 18]. In addition, polymorphisms within multiple monoaminergic genes appear to be associated with disparate cognitive performance in healthy individuals [18]. For example, Met/Met carriers within the Val158Met polymorphism for the gene encoding catechol-O-methyltransferase (COMT), an enzyme responsible for prefrontal cortex degradation of DA, demonstrated superior performance in sustained attention relative to Val/Val carriers [19, 20]. Genetic variation within the Taq1A polymorphism near the DRD2 receptor gene has been associated with variable cognitive flexibility in a task-switching test [21]. Similarly, a 7-repeat variant allele within the DA DRD4 receptor gene has been associated with higher accuracy in a go/no-go task and with improved cognitive flexibility [22]. Also, variation in the HTR2A gene (encodes the serotonin 2A receptor) has been associated with altered response inhibition as measured by a go/no-go task [23]. These studies demonstrate that variability in cognitive task performance among healthy human individuals is associated with detectable genetic variation within monoaminergic genes, providing a basis for exploring cognitive differences related to other monoaminergic genes, such as VMAT2.
TBI appears to induce changes in dopaminergic systems, as both animal and human studies have identified a series of temporally specific alterations in DA neurotransmission that occur after TBI [24]. Multiple clinical studies show that striatal DA transporter binding is decreased among individuals up to one year after severe TBI, even in cases where no anatomical evidence of direct striatal injury exists [25, 26]. A transient decrease in striatal D1 receptors immediately after injury, followed by a subsequent return to pre-injury levels has also been reported in experimental TBI [27]. Also, TBI can increase tyrosine hydroxylase, the rate-limiting enzyme in catecholamine synthesis, in the rat frontal cortex at 28 days post-injury [28]. Interestingly, DA receptors are also widely expressed in brain regions known to be commonly damaged after TBI such as the frontal cortex and striatum, which are important for cognitive function [29–31].
The vesicular monoamine transporter-2 (VMAT2) is a vesicular membrane protein responsible for transporting monoamine neurotransmitters from the cytosol into synaptic vesicles for subsequent storage and synaptic release [32]. VMAT2, encoded in the gene SLC18A2 (referred to here as the VMAT2 gene), is expressed in monoaminergic neurons within the central nervous system [33]. To our knowledge, the role of VMAT2 in post-TBI recovery has yet to be investigated. Functionally, VMAT2’s activity is of particular importance for DA handling; this transporter provides vesicular DA immediately available for neurotransmission and neuromodulation [34], protects DA against oxidation/degradation, and protects neurons against damage secondary to intracellular DA oxidation products, as cytosolic DA is toxic to the cell [35]. Some studies reveal significant associations between VMAT2 polymorphisms and disease states such as alcohol dependence [36], tardive dyskinesia [37], and Parkinson’s Disease [38–40], supporting the concept that genetic variation in VMAT2 may influence clinically relevant outcomes.
Using a Rehabilomics approach, which incorporates the concepts of personal biology and genetics into an established biopsychosocial model of health condition [41], allows us to identify mechanisms and biomarkers of cognitive outcomes post-TBI, which may have significant prognostic and personalized medical management value. To date there have been no known investigations into the clinical significance of genetic variation within VMAT2 on TBI recovery. Using a tagging SNP (tSNP) approach, we hypothesized that genetic variability within VMAT2 would be associated with cognitive deficits post-TBI. Given our previous work linking both cognitive deficits and depression to functional cognition [10], we then conducted post-hoc analyses to explore whether or not these cognitive deficits, in conjunction with VMAT2 gene variation and PTD, would be associated with impaired functional cognition.
Methods
Participants
The Institutional Review Board at the University of Pittsburgh approved this study. Participants included 136 patients recruited from a level 1 trauma center, aged 16–75, who sustained a non-penetrating TBI with an initial GCS<8 and positive findings on admission head CT. Analyses were limited to White individuals to remove potential population stratification effects by race on genetic associations, as there was not a large enough representation of other races to perform stratified analyses. Participants were enrolled and consented as a part of a larger prospective cohort study examining relationships between genetics and outcomes for patients with moderate to severe TBI.
Assessments
TBI severity was determined using the Glasgow Coma Scale (GCS), a widely used measure of injury severity that is sensitive to outcome across a variety of TBI populations [42–45]. While initial GCS was used as a measure of inclusion, the best GCS within 24 hours of injury was used for analysis to limit the influence of alcohol, sedatives or paralytics on the scoring; best GCS in 24 hours has been shown to be sensitive to differences in survivor based outcomes [46, 47]. Additional demographic information including age, sex, and years of education and antidepressant use at time of assessment (documented use within 1 month prior to assessment) was obtained through chart abstraction and/or participant and family interviews after enrollment.
Cognitive impairment was quantified using a cognitive composite T-score (Comp-Cog), as a measure of general cognitive function. Comp-Cog was our primary outcome of interest. Past research suggests that composite measures are a practical means of evaluating general cognitive function in terms of recovery and performance, with increased consistency attained by aggregating multiple tests [48–52]. For the cognitive composite T-score, 8 neuropsychological tests were administered to participants, targeting cognitive domains of attention, executive function, memory, and verbal fluency. A composite score was generated, with higher scores reflecting better cognition, for each subject that completed at least one test in each of the four cognitive domains. The raw scores from each test were converted into a T-score norm-based for appropriate metrics as indicated by the test publisher (i.e. education, age, sex, race). T-scores were averaged within each domain to create domain sub-scores. The domain sub-score average was considered the cognitive composite score. Comp-Cog T-scores have a mean of 50 and standard deviation of 10, with a score below 40 suggesting below average performance.
The 8 cognitive tests included: the Rey-Osterrieth Complex Figure Test, Delis-Kaplan Executive Function System Fluency sub-test, Trailmaking Tests (parts A and B), Controlled Oral Word Association Test, California Verbal Learning Test – Long Delay Free Recall, Digit Span Test, and Stroop Test. Digit Span, a sub-test from the Wechsler Adult Intelligence Scale-R, requires individuals to repeat a sequence of numbers both forward and backward as a measure of memory and attention [53]. The Controlled Oral Word Association Test is used for assessing verbal fluency and the ability to generate words that begin with a specific letter or belong to a specific category (animals being the category used) within one minute [54]. Letter Fluency from the Delis-Kaplan Executive Function Systems (DKEFS) [55] battery tests were administered to assess semantic and phonemic fluency. The Trail Making Test parts A & B is a paper-and-pencil test that measures attention, visual searching, and mental flexibility. Part A requires a subject to consecutively connect circles numbered in successive order, while Part B requires a subject to consecutively connect circles while alternating between numbers and letters. Performance for both tasks is measured using time (in seconds) to complete each part [56]. The Stroop Neuropsychological Screening Test measures selective attention and cognitive flexibility by testing an individual’s ability to meet changing cognitive demands and to suppress habitual responses in favor of more effortful ones [57].
Functional cognition was assessed using the Functional Independence Measure Cognitive subscale (FIM-Cog). The FIM-Cog rates functional cognition in the domains of comprehension, expression, social interaction, problem-solving and memory on an ordinal scale of 1–7, with higher scores indicating greater functional independence [58].
PTD was assessed as a moderator of cognitive outcomes post-TBI using the Patient Health Questionnaire (PHQ-9) [59], a self-report of frequency of the 9 symptoms of the Diagnostic and Statistical Manual of Mental Disorders, edition IV (DSM-IV) criteria for depression from 0 (not at all) to 3 (nearly every day) over the previous 2 weeks. The PHQ-9 has excellent sensitivity and specificity as a screen for major depressive disorder [59] and minimizes false positive reports in both TBI and non-TBI populations [60]. PTD status was dichotomized as “Depressed” or “Not Depressed” based on previously established criteria of a total of five symptoms endorsed, including at least one of the cardinal symptoms of depression (depressed mood, lack of interest) for a categorization of “Depressed” [61].
Genotyping
A tagging SNP (tSNP) approach was taken to explore potential genetic variability within the VMAT2 gene and its association with outcome. tSNPs, each representing unique blocks of DNA within the VMAT2 gene, were identified and selected based on the concept of linkage disequilibrium (LD), where LD refers to the degree of nonrandom association between alleles at 2 genetic loci; higher LD suggest that 2 alleles are more likely to be present together in the population. tSNPs covering VMAT2, and a 1-kb region flanking DNA 5’ and 3’ were selected using HapMap Database build 36. tSNP selection criteria were set at r2 = 0.80 and a minor allele frequency of 0.20 or more to allow for robust evaluation of heterozygote status [62] and potential associations with cognitive deficits.
Subjects were genotyped for four tagging SNPs including VMAT2, rs363223, rs363226, rs363341, and rs363251. For each subject, DNA was extracted from whole blood or cerebral spinal fluid. Whole blood was collected in EDTA vacutainer tubes and genomic DNA was isolated using a salting out protocol [63]. SNPs for the VMAT2 gene were genotyped using the iPLEX MassArray platform (Sequenom, San Diego, CA, U.S.A). Duplicate samples and in-house controls were used for quality control.
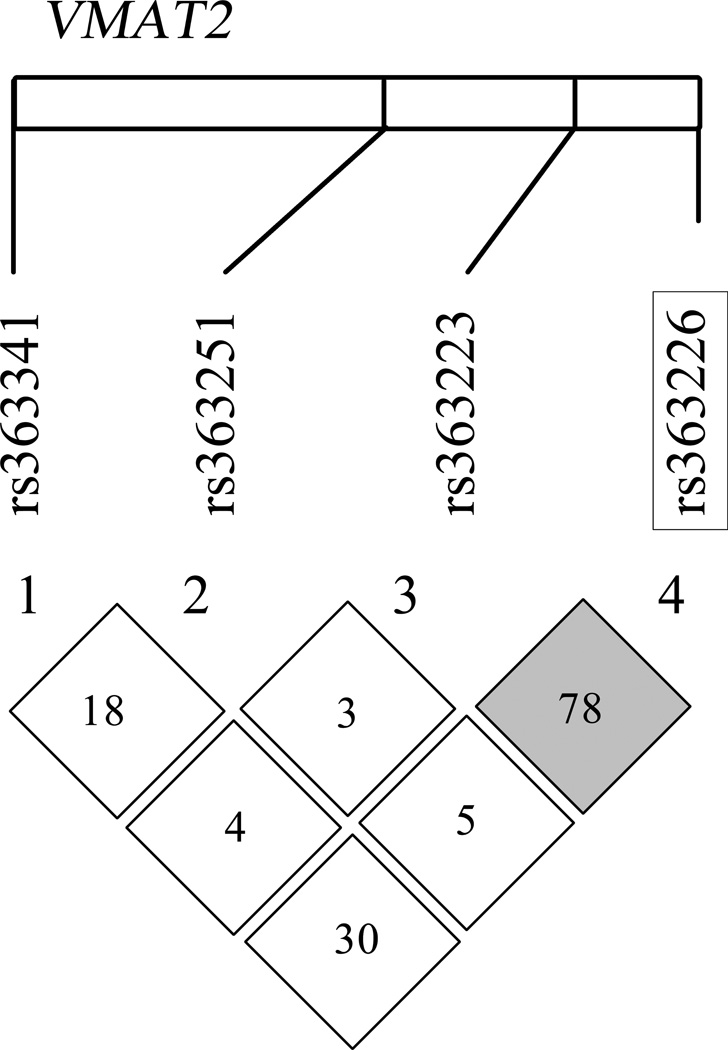
Hardy-Weinberg equilibrium was verified for all SNPs, indicating genotype distributions were within the expected proportions. Information regarding the degree of linkage disequilibrium (LD) between SNPs in this study was obtained using HapMap database build 36 (http://hapmap.ncbi.nlm.nih.gov). LD and D’ were examined within the specific study population using Haploview[64] software to generate a genetic map for VMAT2 showing the relative relationships and LD between the tSNPs examined. (Broad Institute Cambridge, MA, U.S.A; see Figure 1)
Figure 1.
Targeted single nucleotide polymorphisms (SNPs) within the VMAT2 gene on chromosome 10 are shown here as mapped on the white rectangle. Linkage disequilibrium (LD) between the 4 SNPs examined (calculated LDs using Haploview v.4.2) is represented as the numbers in each square between each pair of SNPs (D’). Grey squares indicate high LD and white squares indicate low LD based on algorithms calculated within Haploview. Significant SNP in multiple regression is outlined in black box
Statistical Analysis
Data analysis was performed with SAS (Cary, NC; version 9.4). Descriptive statistics across SNP genotypes were calculated using mean and standard deviation for continuous variables including age, GCS, education, with frequencies reported for sex (Table 1). SNP genotype was used to screen for SNPs potentially associations with cognitive composite at 6 and 12 months using Plink analysis toolset (http://pngu.mgh.harvard.edu/~purcell/plink/). Due to the observed correlation among the SNPs of interest, as evidenced by LD from examination using Haploview (described above), it was likely the number of truly independent tests would be less than the number of SNPs analyzed. Therefore, the minimum number of effective tests (Meff) was calculated for the VMAT2 gene [65], and based on the 4 SNPs selected for genotyping, the Meff was determined to be 2. To correct for multiple SNP comparisons during screening procedures, and to adjust for multiple time points of cognitive composite assessment, a Bonferroni correction was applied to the initial screening level of significance (α=0.05), using the Meff as the number of tests, and using the number of outcome time points. Bivariate statistics were then compared to a corrected level of significance of α=0.0125 to account for multiple comparisons. SNPs that were significant when compared to the corrected alpha level were further examined for their relationships with cognitive performance using multivariable linear regression, using age, GCS, education, sex, PTD, and antidepressant use as relevant covariates predicting cognitive outcomes in our previous analyses [10]. A backward selection method was performed to systematically remove nonsignificant variables and create the most parsimonious model. Variables remained in multivariable models if p<0.2. Partial eta squared effect sizes are presented to indicate the percentage of variance in outcome explained by each covariate once variance from all other covariates is accounted for.
Table 1.
VMAT2 Associations with Demographic Variables
| SNP | Genotype | Age1 | Sex2 | GCS3 | Education1 |
|---|---|---|---|---|---|
| rs363223 | AA (n=39) | 29.9 ± 12.0 | 79.5% (31) | 7 | 12.9 ± 1.9 |
| AG (n=68) | 37.3 ± 14.6 | 85.3% (58) | 7 | 12.8 ± 1.7 | |
| GG (n=27) | 32.0 ± 12.5 | 77.8% (21) | 7 | 13.0 ± 1.9 | |
| p value | 0.043 | 0.544 | 0.421 | 0.506 | |
| rs363226 | GG (n=14) | 35.3 ± 13.9 | 78.6% (11) | 7 | 12.3 ± 1.0 |
| CG (n=51) | 35.7 ± 14.8 | 84.3% (43) | 7 | 12.9 ± 1.8 | |
| CC (n=68) | 32.5 ± 13.0 | 80.9% (55) | 7 | 13.0 ± 1.9 | |
| p value | 0.666 | 0.848 | 0.430 | 0.857 | |
| rs363251 | GG (n=20) | 35.2 ± 15.6 | 75.0% (15) | 7 | 13.0 ± 1.8 |
| AG (n=64) | 36.2 ± 14.6 | 82.8% (53) | 8 | 12.9 ± 1.9 | |
| AA (n=47) | 30.3 ± 10.7 | 85.1% (40) | 7 | 12.8 ± 1.6 | |
| p value | 0.171 | 0.579 | 0.210 | 0.849 | |
| rs363341 | TT (n=8) | 31.4 ± 10.6 | 87.5% (7) | 9.5 | 12.7 ± 2.2 |
| TC (n=56) | 35.2 ± 14.7 | 87.5% (49) | 7 | 12.9 ± 1.6 | |
| CC (n=71) | 33.5 ± 13.4 | 77.5% (55) | 7 | 13.0 ± 1.9 | |
| p value | 0.936 | 0.353 | 0.385 | 0.600 |
Age and education reported mean ± standard deviation.
Percent male reported, (# male).
Glasgow Coma Scale (GCS) reported as median value for best GCS score in the first 24 hours after admission. Bold and italics signify significant values, p<0.05.
Post-hoc analyses were then completed using multivariable linear regression with FIM-Cog as the outcome of interest, to explore how genotype and cognitive composite score interact to influence functional cognition at those time points in which selected SNPs were significantly associated with cognitive composite scores. PTD was also included as a relevant covariate, given previous findings that PTD significantly affects functional cognition after TBI [10]. Partial eta squared effect sizes are also presented.
Results
Population Description
This study included 136 participants with severe TBI. Of these participants, 82.4% (112) were male, mean (±SEM) age was 34.1±1.2 years (range 17–71), median best GCS in 24 hours was 7, and mean (±SEM) education was 13.0±0.16 years. Demographic information by genotype for each of the four SNPs is shown in Table 1. There was a significant difference in age by rs363223 genotype (p=0.043). There were no other significant differences in demographics or antidepressant use by genotype for any of the examined SNPs.
VMAT2 associations with Comp-Cog
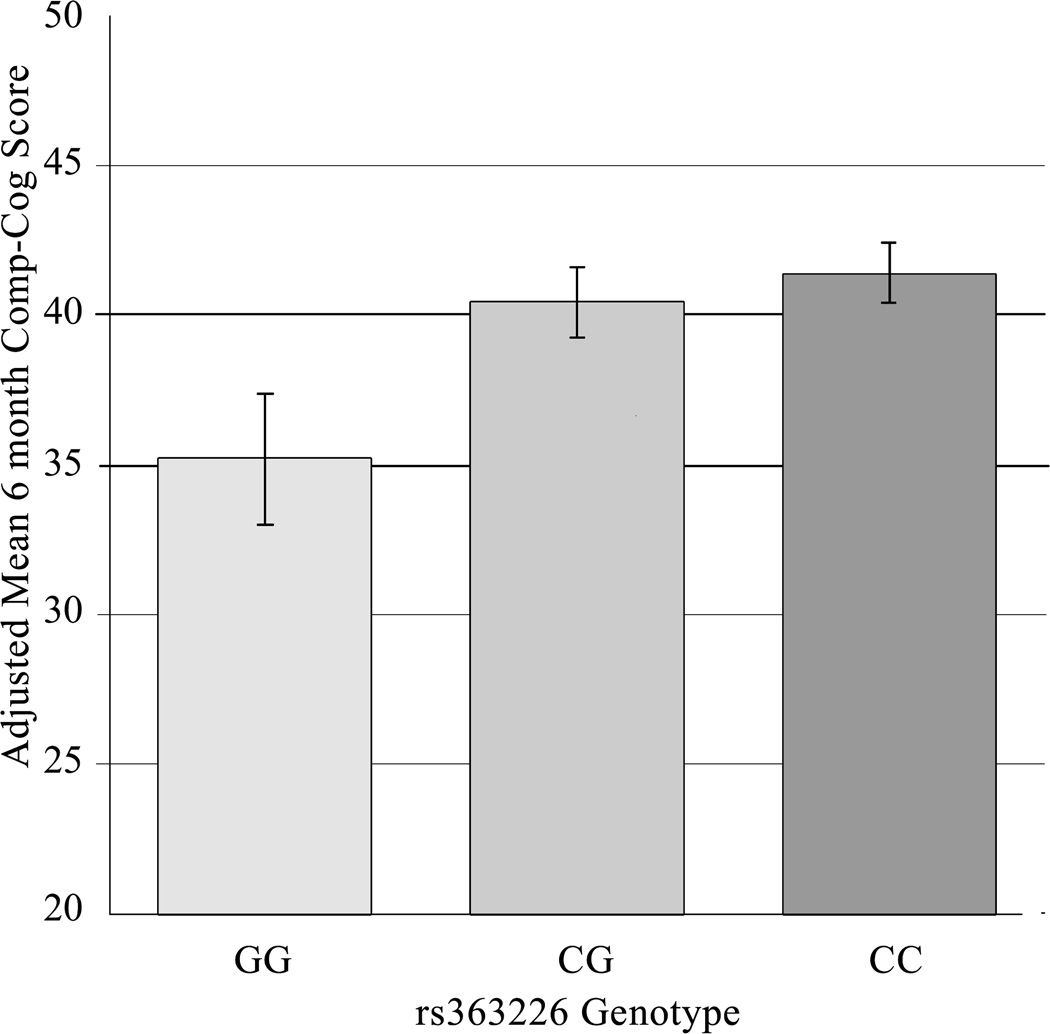
Table 2 presents Plink analyses of Comp-Cog outcome versus SNP genotype following a Bonferroni adjustment for multiple comparisons. The p-value for the association between rs363226 genotype and 6-month Comp-Cog (0.00584) was less than the threshold α level (0.0125). There were no statistically significant associations with 12-month Comp-Cog. Thus, a multiple linear regression model was constructed to assess the relationship between this tSNP and 6-month cognitive impairment after controlling for other covariates (Table 3A). In the final model, significant predictors included rs363226 genotype, age, and GCS. The raw mean ± SEM (unadjusted T-scores) 6-month post-injury Comp-Cog scores for each of the GG, CG, and CC genotype groups were 33.8 ± 3.0, 40.5 ± 1.2, and 41.9 ± 1.0 respectively, where C-carriers had cognitive composite scores at or near the cut off for clinical impairment (T-score=40). Figure 2 shows the adjusted means ± SEM derived from the linear regression model (controlling for covariates) and that the GG group had significantly lower cognitive composite scores from both the CG group (p=0.036) and CC group (p=0.012), while the CG and CC groups did not significantly differ from each other (p=0.528). Genotype alone accounted for 7% of the total variance in Comp-Cog outcome.
Table 2.
Bivariate Analysis of Genotype vs Cognitive Impairment
| Genotype Grouping |
Comp-Cog | ||
|---|---|---|---|
| 6m | 12m | ||
| rs363223 | n | 105 | 69 |
| AA v GA v GG | 0.851 | 0.980 | |
| rs363226 | n | 105 | 69 |
| GG v CG v CC | 0.00584 | 0.323 | |
| rs363251 | n | 105 | 70 |
| AA v GA v GG | 0.634 | 0.468 | |
| rs363341 | n | 106 | 70 |
| CC v TC v TT | 0.684 | 0.693 | |
Bold and italicized values signify p-values less than alpha (0.0125).
Table 3.
Multivariable models for cognitive associations with VMAT2.
| A. Multiple Linear Regression of rs363226 and Cognitive Composite at 6 Months | ||||||
|---|---|---|---|---|---|---|
| Variable | Parameter Estimate |
Standard Error |
t Value | P value | 95% Confidence Interval |
Partial η2 |
| rs363226 genotype (CC) | 6.197 | 2.405 | 2.577 | 0.012 | (1.42, 10.98) | .070 |
| rs363226 genotype (CG) | 5.211 | 2.443 | 2.133 | 0.036 | (0.36, 10.98) | |
| rs363226 genotype (GG) | 0 | - | - | - | - | |
| Age | −0.133 | 0.053 | −2.486 | 0.015 | (−0.24, −0.027) | .065 |
| EDU | 0.714 | 0.387 | 1.845 | 0.068 | (−0.055, 1.48) | .037 |
| GCS | 0.779 | 0.260 | 2.993 | 0.004 | (0.26, 1.30) | .091 |
| B. Multiple Linear Regression of rs363226 and FIM-Cog at 6 Months | ||||||
|---|---|---|---|---|---|---|
| Variable | Parameter Estimate |
Standard Error |
t value | p value | 95% Confidence Interval |
Partial η2 |
| rs363226 genotype | 7.766 | 1.715 | 4.529 | <0.001 | (4.36, 11.18) | .020 |
| PTD | −0.833 | 0.544 | −1.530 | 0.130 | (−1.92, 0.25) | .029 |
| Antidepressant Use | −1.345 | 0.571 | −2.356 | 0.021 | (−2.48, −0.21) | .065 |
| Comp-Cog Score | 0.705 | 0.1 | 7.03 | <0.001 | (0.51, 0.91) | .510 |
| SNP*Comp-Cog interaction | −0.184 | 0.043 | −4.237 | <0.001 | (−0.27, −0.097) | .176 |
n (GG) = 11, n (CG) = 37, n (CC) = 47. Regression model shows GG genotype as reference group for rs363226 genotype variable. Sex, Post-traumatic depression (PTD), and antidepressant use were removed by backward selection. EDU=years of education; GCS=Glasgow Coma Scale
n (GG)=10, n (CG)=36, n (CC)=45. Sex, GCS, education, and age were removed by backward selection. PTD=Post-traumatic depression; SNP=single nucleotide polymorphism; Comp-Cog=Composite Cognition
Figure 2. Adjusted Mean 6-Month Comp-Cog Score by rs363226 Genotype.
Adjusted mean Comp-Cog scores are predicted values derived from the regression model (Table 3A), which controls for covariates. Standard error bars are shown. Mean (95% confidence interval) Comp-Cog scores for genotype groups were as follows: GG=35.2 (30.9, 39.5), CG=40.4 (38.1, 42.7), and CC=41.42 (39.4, 43.5). The GG group average was significantly different from the CG (p=0.036) and CC (p=0.012) group averages. CG and CC groups did not significantly differ (p=0.528)
VMAT2 modulates Comp-Cog Associations with FIM-Cog
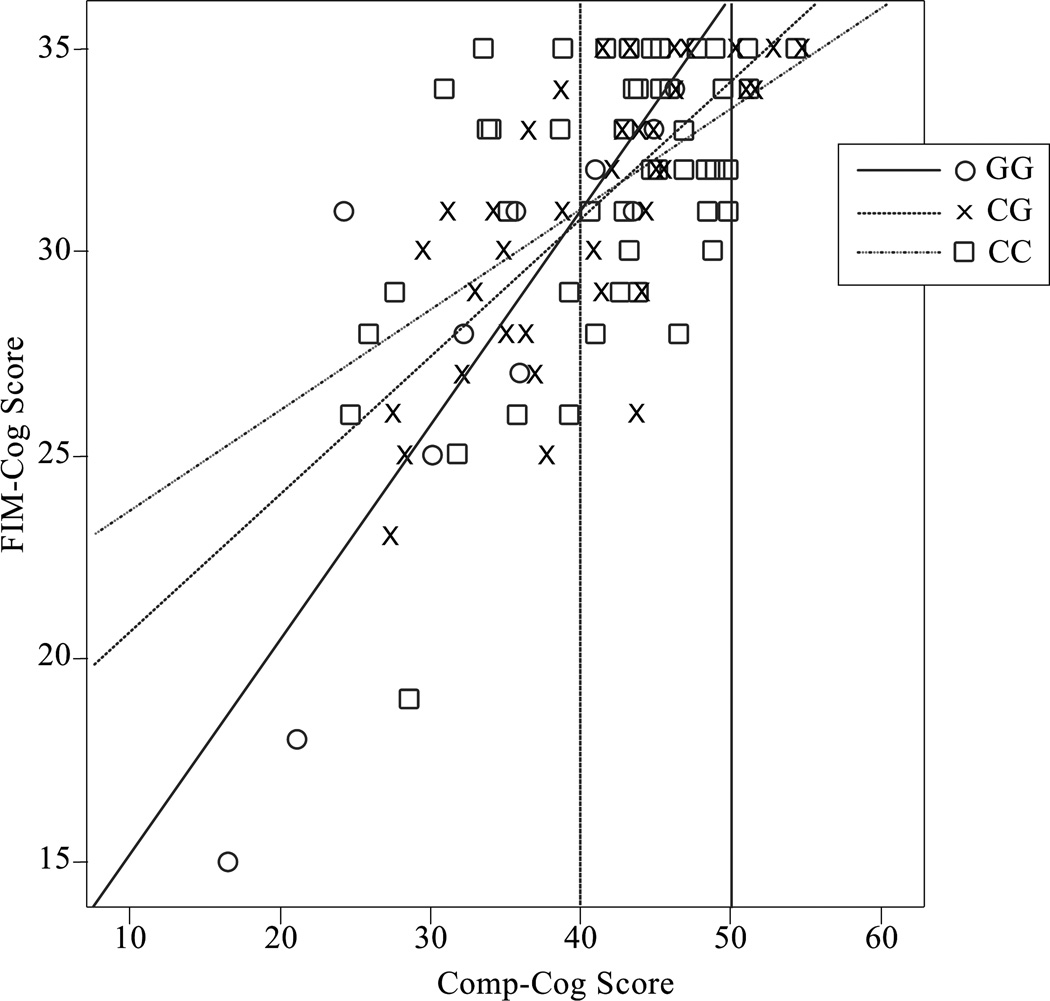
Table 3B shows the multiple linear regression model for functional cognition (FIM-Cog) scores at 6 months. In this model, significant predictors included antidepressant use and an interaction between rs363226 genotype and Comp-Cog. While Comp-Cog alone accounted for the majority of the variance in FIM-Cog (51%), its interaction with genotype accounted for an additional 17.6% of the variance in FIM-Cog. The mean ±SEM 6-month post-injury FIM-Cog scores of the GG, CG, and CC genotype groups were 25.8±2.5, 28.7±1.2, and 29.3±1.0 respectively.. Figure 3 presents a descriptive plot of the genotype by cognitive impairment interaction. Of note, those with a Comp-Cog T-score below 40 (the threshold for clinically relevant cognitive impairment), deficits in FIM-Cog become more pronounced for the GG genotype as Comp-Cog declines compared to the CG (p<0.001) and CC (p=0.019) genotype, and for the CG genotype compared to the CC genotype (p=0.040).
Figure 3. 6-Month FIM-Cog vs Comp-Cog by rs363226 Genotype.
Descriptive plot of individual 6-month FIM-Cog scores versus Comp-Cog score, labeled by rs363226 genotype. Best fit lines are shown for each genotype group, which have R2 values of 0.722 (GG), 0.514 (CG), and 0.262 (CC). Vertical lines were added to denote an average Comp-Cog score (50) and one standard deviation below average (40), which marks the threshold for mild cognitive impairment.
Discussion
To date, no studies have investigated the relationship between genetic variation within the VMAT2 gene and any outcome following TBI. The purpose of our analysis was to investigate associations between genetic variability in four intronic tSNPs in the VMAT2 gene with cognitive outcomes following TBI. We found that genetic variability in VMAT2 SNP rs363226 is associated with cognitive recovery at 6 months post-injury, but not at 12 months post-injury.
Individuals with the GG genotype had significantly more cognitive impairment at 6-months post-injury than C-allele carriers, suggesting that G-homozygosity portends a higher risk for cognitive impairment while C-carriers have some relative degree of protection (better cognitive performance). Further, G-homozygotes on average crossed the threshold into mild cognitive impairment status, indicating a clinically meaningful difference in outcome based on genotype. Also of note, PTD and antidepressant use did not remain as significant predictors of cognitive impairment in the multivariate model. This finding is consistent with some previous studies that did not find a significant an effect of depression on cognition post-TBI [9]. However, PTD – and perhaps antidepressant use specifically [10] – remains an important factor for consideration with regard to functional cognition. The magnitude of the effect that cognitive impairment has on functional cognition may depend upon rs363226 genotype; as cognition declines, functional cognition declines more sharply for G-homozygotes than for C-carriers, yet it declines similarly for CG-heterozygotes compared to C-homozygotes. Notably, the relationship between cognitive impairment and functional cognition by genotype group diverges below a FIM-Cog of ~30, a score that indicates at least one FIM-Cog domain requires some degree of assistance, and below a Comp-Cog T-score of ~40 (cognitive impairment threshold), suggesting the moderating effect of rs363226 genotype on the relationship between cognitive impairment and functional cognition emerges only below the level of clinically apparent deficits. Additionally, our findings suggest that the putative influence of rs363226 genotype on functional cognition post-TBI is independent of depression status or treatment. Executive function (comprised of abilities such as problem-solving, planning, organization, information retrieval, categorization, and abstraction) and motivation (a process of generating, directing, and sustaining goal-directed behaviors) may also impact functional cognition, and it is possible that these domains are differentially impacted following TBI based on an individual’s genetics. It also conceivable that post-injury differences in adaptive or compensatory skills for G-homozygotes relative to C-carriers lead to greater functional dependence even across similar levels of cognitive impairment. Independent results validation is necessary to ensure these findings are not due to potential outliers in the relatively small G-homozygote group, but the data indicate that G-homozygotes may be a particularly vulnerable population for poor cognitive outcomes post-TBI.
The lack of significant findings between VMAT2 and 12 month cognitive outcomes could be due to multiple factors. Other work from our group suggests temporal genetic associations with depression and post-traumatic seizure development after TBI [66]. Importantly, TBI induces a global hypo-methylation state [67, 68], a phenomenon that can moderate gene variation effects on injury effects outcomes over time due to transient epigenetic remarking that likely occurs during TBI recovery. Also, temporal VMAT2 gene effects on post-TBI cognitive outcomes, wherein larger effects are observed more proximal to injury (e.g. 6 months), may be due to evolving, environmental and biopsychosocial factors that increasingly may contribute to post-TBI cognition. Additionally, there may be more natural recovery with cognition as time since injury increases. These effects could reduce the magnitude of genotype group differences later after injury, thus requiring larger samples to detect significant differences in cognitive performance.
The tSNP rs363226 is a relatively unexplored source of variation in the genome, and its functional implications are unknown. We found a comparable minor allele frequency for the rs363226 G-allele to that previously reported [69]. rs363226 is located in an area of high linkage disequilibrium with rs363223 (Figure 1), which did not show significant bivariate associations with the Comp-Cog outcome. It is possible that the intronic tSNP rs363226 may regulate transcription of VMAT2 through epigenetic or transcription factor binding site regulation [70]. Alterations in VMAT2 transcription could greatly affect monoamine function and modify cognitive outcomes post-TBI.
A number of factors influencing how DA levels and VMAT2 activity affect monoamingeric neurotransmission may be, at least in part, responsible for differential outcomes between individuals following TBI. DA transporter blockers like cocaine and DA enhancers like amphetamine influence VMAT2 DA uptake kinetics and expression, which suggests cytoplasmic DA levels may actually directly regulate VMAT2 function [71, 72]. Also, DA is highly susceptible to oxidation into toxic DA-quinones and reactive oxygen species, and the resultant cellular oxidative stress can impart irreversible neuronal damage and cell death [73, 74]. Impaired DA storage into synaptic vesicles may initiate alterations in protein function and neuronal lipid dynamics, eventually leading to neurodegeneration [73]. Further studies assessing gene dosage, pharmacology, and aging in mice support the notion that reduced VMAT2 gene expression or function can cause increased susceptibility to neurodegeneration and neurotoxicity [74–76]. In rats, it also has been demonstrated that VMAT2 interacts with tyrosine hydroxylase and aromatic amino acid decarboxylase, the enzymes responsible for DA synthesis [77]; this coupling can affect DA levels in the local area surrounding the synaptic vesicle membrane and allow for more efficient transport into synaptic vesicles [33]. In addition, immobilization stress, used extensively as a model with which to determine the effects of stress on brain function, can increase cytosolic DA levels and oxidative damage to DA neurons in vivo in mice [78]. Biopsychosocial stress following TBI, by inducing increased cytosolic DA levels, may therefore further insult dopaminergic neurons in subjects with relatively impaired VMAT2 activity. Thus, genetic variation that results in impaired VMAT2 function, reduced expression, or altered dynamics with other proteins may lead to selective vulnerability of dopaminergic neurons to neurodegeneration after TBI.
In addition to preclinical studies outlining how DA systems are dysfunctional after TBI [24], clinical evidence exists that genetic variability within DA system genes is linked with variations in DA system pathology, and that this genetic variability affects DA system impairment after TBI. For example, previous work shows that sex and DAT1 genotype interact to influence CSF DA levels observed during the first week after severe TBI [79]. Also, DAT expression in caudate and putamen is preferentially decreased in association with both DAT1 and DRD2 receptor genotype in subjects one year after severe injury [80, 81]. From this, we may surmise that reduced DAT expression helps maximize DA neurotransmission in the setting of dysfunctional DA state. Further studies may incorporate concurrent DAT and VMAT2 imaging and genetics to determine the functional consequences of VMAT2 genetic variation on both DAT and VMAT2 expression after TBI.
It is important to mention that tSNP rs363226 did not significantly differ in its distribution across age, sex, GCS, or education, all of which are factors that have been demonstrated to affect post-TBI outcomes, including cognition [82–90]. Our findings revealed age to be a significant predictor for greater cognitive impairment; likely explanations include age-related exacerbation of secondary injury mechanisms [89], other premorbid medical conditions and functional impairments, and limitations with cognitive reserve often observed in older individuals with TBI [91]. Of note, the effect of age on cognitive impairment was small; in contrast, the difference in cognitive impairment between G-homozygotes and C-carriers in our cohort was substantial, roughly the equivalent of a 45 year age difference in cognitive performance. Also, age remained a TBI specific prediction factor outside of any age related adjustments made when scoring individuals neuropsychological test performance. GCS was also a significant predictor of cognitive impairment at 6 months post-injury, suggesting that cognitive performance does map to early severity markers, even among an injury population with primarily severe levels of TBI.
Our findings are associative, implicating regions of genetic variability that appear to play some role in the neurobiology of cognitive recovery. Future work is needed to determine the molecular mechanisms by which the genetic variants implicated in this study influence recovery in the context of TBI. Additionally, FIM-Cog is self- or caregiver-reported, which may yield variable responses in the setting of post-TBI cognitive deficits or PTD. Assessing functional cognition with an ecologically valid performance-based measure that captures higher levels of cognitive function, such as the Multiple Errands Test [92, 93], could be a more reliable measure of functional cognition used in future studies to confirm the findings reported in this study. Lastly, given the role of 5-HT in cognition and mood [17, 18] and VMAT2’s role in 5-HT signaling [32, 33], it would be interesting to explore post-TBI outcomes in light of serotonergic systems. For example, 5-HT 1A receptor agonism with buspirone has been shown to enhance cognitive performance post-TBI in rats [94], and variation in the serotonin transporter gene has been associated with mood outcomes post-TBI in adults [95]. Exploring the 5-HT 1A receptor and 5-HT transporter, as well as other serotonergic targets, in conjunction with VMAT2 may provide additional understanding of the putative effect of VMAT2 variation on cognitive outcomes post-TBI.
While the results of this study are both interesting and novel, there are some limitations to this investigation to consider. Our sample size is modest, particularly for 12 month outcomes, and larger sample sizes are needed to validate the results reported here, to assess race stratification, and to evaluate a broader range of injury severity for generalizability of these results across various subpopulations, and to explore effects of VMAT2 on cognitive outcomes over time. Additionally, while model assumptions were satisfied, G-homozygotes made up only 11 of the 95 participants. Given that the minor allele G-homozygotes had significantly worse outcomes, a larger sample size is required validate our findings. While our sample is small compared to standards for a large population-based genetics study, we believe that numerous aspects of this study support its importance and scientific rigor. First, TBI is a very specialized clinical population, with a relatively low incidence compared to other clinical populations (e.g. many psychiatric conditions) and may not present the same capacity for recruitment and phenotyping as a more general population. Pilot data to screen for and identify likely genetic factors contributing to post-TBI outcomes is needed in order to support future larger-scale genetic studies in TBI that will require significant time and resources to conduct effectively. Second, we have employed rigorous statistical methods to minimize potential type 1 errors. Finally, while we have screened tagging SNPs without known functional variants associated with the DNA blocks that these tSNPs represent, there is substantial literature supporting the link between DA function and cognition that provides foundational theoretical support for exploring VMAT2 as a likely genetic contributor to post-TBI cognitive outcomes. While the single gene approach was appropriate to the current study, our recent work focuses on including VMAT2 as one of multiple contributing genes that assesses neurobiological contributions to cognitive dysfunction after TBI [96]. Also, our study does not consider gene*gene or gene*environment interactions.
In conclusion, the associations between VMAT2 and impairments in cognition and functional cognition suggest that VMAT2 genetic variability may be a viable biomarker to contextualize within the Rehabilomics framework as representing a potentially important neurobiological link to cognition and multidimensional TBI outcomes.
Acknowledgements
The project described was supported by NIH 5T35AT005933-02, NIH R01 HD048162-02, NIH R01NR008424, NIH 5P01NS030318-16, NIDRR H133A120087, DODW81XWH-071-0701. The authors would like to thank the UPMC Trauma Registry for their support with some elements of data collection.
Footnotes
Conflict of Interest Statement:
The authors declare no conflicts of interest.
References
- 1.Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21(5):375–378. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Marshall S, et al. Clinical practice guidelines for mild traumatic brain injury and persistent symptoms. Can Fam Physician. 2012;58(3):257–267. [PMC free article] [PubMed] [Google Scholar]
- 3.Cristofori I, Levin HS. Traumatic brain injury and cognition. Handb Clin Neurol. 2015;128:579–611. doi: 10.1016/B978-0-444-63521-1.00037-6. [DOI] [PubMed] [Google Scholar]
- 4.Thurman DJ, et al. Traumatic brain injury in the United States: A public health perspective. J Head Trauma Rehabil. 1999;14(6):602–615. doi: 10.1097/00001199-199912000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Arciniegas DB, Held K, Wagner P. Cognitive Impairment Following Traumatic Brain Injury. Curr Treat Options Neurol. 2002;4(1):43–57. doi: 10.1007/s11940-002-0004-6. [DOI] [PubMed] [Google Scholar]
- 6.Bombardier CH, et al. Rates of major depressive disorder and clinical outcomes following traumatic brain injury. Jama. 2010;303(19):1938–1945. doi: 10.1001/jama.2010.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jorge RE, et al. Major depression following traumatic brain injury. Arch Gen Psychiatry. 2004;61(1):42–50. doi: 10.1001/archpsyc.61.1.42. [DOI] [PubMed] [Google Scholar]
- 8.Rapoport MJ, et al. Cognitive impairment associated with major depression following mild and moderate traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2005;17(1):61–65. doi: 10.1176/jnp.17.1.61. [DOI] [PubMed] [Google Scholar]
- 9.Satz P, et al. Depression, cognition, and functional correlates of recovery outcome after traumatic brain injury. Brain Inj. 1998;12(7):537–553. doi: 10.1080/026990598122313. [DOI] [PubMed] [Google Scholar]
- 10.Failla MD, et al. Effects of Depression and Antidepressant Use on Cognitive Deficits and Functional Cognition following Severe Traumatic Brain Injury. J Head Trauma Rehabil. doi: 10.1097/HTR.0000000000000214. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bornstein RA, Miller HB, van Schoor JT. Neuropsychological deficit and emotional disturbance in head-injured patients. J Neurosurg. 1989;70(4):509–513. doi: 10.3171/jns.1989.70.4.0509. [DOI] [PubMed] [Google Scholar]
- 12.Rapoport M, et al. The role of injury severity in neurobehavioral outcome 3 months after traumatic brain injury. Neuropsychiatry Neuropsychol Behav Neurol. 2002;15(2):123–132. [PubMed] [Google Scholar]
- 13.Gfeller JD, Chibnall JT, Duckro PN. Postconcussion symptoms and cognitive functioning in posttraumatic headache patients. Headache. 1994;34(9):503–507. doi: 10.1111/j.1526-4610.1994.hed3409503.x. [DOI] [PubMed] [Google Scholar]
- 14.Andersson S, Bergedalen AM. Cognitive correlates of apathy in traumatic brain injury. Neuropsychiatry Neuropsychol Behav Neurol. 2002;15(3):184–191. [PubMed] [Google Scholar]
- 15.Larson EB, Zollman FS. The effect of sleep medications on cognitive recovery from traumatic brain injury. J Head Trauma Rehabil. 2010;25(1):61–67. doi: 10.1097/HTR.0b013e3181c1d1e1. [DOI] [PubMed] [Google Scholar]
- 16.Bushnik T, Englander J, Wright J. Patterns of fatigue and its correlates over the first 2 years after traumatic brain injury. J Head Trauma Rehabil. 2008;23(1):25–32. doi: 10.1097/01.HTR.0000308718.88214.bb. [DOI] [PubMed] [Google Scholar]
- 17.Ruhe HG, Mason NS, Schene AH. Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: a meta-analysis of monoamine depletion studies. Mol Psychiatry. 2007;12(4):331–359. doi: 10.1038/sj.mp.4001949. [DOI] [PubMed] [Google Scholar]
- 18.Logue SF, Gould TJ. The neural and genetic basis of executive function: attention, cognitive flexibility, and response inhibition. Pharmacol Biochem Behav. 2014;123:45–54. doi: 10.1016/j.pbb.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blasi G, et al. Effect of catechol-O-methyltransferase val158met genotype on attentional control. J Neurosci. 2005;25(20):5038–5045. doi: 10.1523/JNEUROSCI.0476-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stefanis NC, et al. Effect of COMT Val158Met polymorphism on the Continuous Performance Test, Identical Pairs Version: tuning rather than improving performance. Am J Psychiatry. 2005;162(9):1752–1754. doi: 10.1176/appi.ajp.162.9.1752. [DOI] [PubMed] [Google Scholar]
- 21.Stelzel C, et al. Frontostriatal involvement in task switching depends on genetic differences in d2 receptor density. J Neurosci. 2010;30(42):14205–14212. doi: 10.1523/JNEUROSCI.1062-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kramer UM, et al. ADHD candidate gene (DRD4 exon III) affects inhibitory control in a healthy sample. BMC Neurosci. 2009;10:150. doi: 10.1186/1471-2202-10-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nomura M, et al. Involvement of a polymorphism in the 5-HT2A receptor gene in impulsive behavior. Psychopharmacology (Berl) 2006;187(1):30–35. doi: 10.1007/s00213-006-0398-z. [DOI] [PubMed] [Google Scholar]
- 24.Bales JW, et al. Persistent cognitive dysfunction after traumatic brain injury: A dopamine hypothesis. Neurosci Biobehav Rev. 2009;33(7):981–1003. doi: 10.1016/j.neubiorev.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donnemiller E, et al. Impaired dopaminergic neurotransmission in patients with traumatic brain injury: a SPECT study using 123I-beta-CIT and 123I-IBZM. Eur J Nucl Med. 2000;27(9):1410–1414. doi: 10.1007/s002590000308. [DOI] [PubMed] [Google Scholar]
- 26.Wagner AK, et al. The influence of genetic variants on striatal dopamine transporter and D2 receptor binding after TBI. J Cereb Blood Flow Metab. 2014;34(8):1328–1339. doi: 10.1038/jcbfm.2014.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henry JM, et al. Cerebral trauma-induced changes in corpus striatal dopamine receptor subtypes. J Invest Surg. 1997;10(5):281–286. doi: 10.3109/08941939709032167. [DOI] [PubMed] [Google Scholar]
- 28.Yan HQ, et al. Tyrosine hydroxylase, but not dopamine beta-hydroxylase, is increased in rat frontal cortex after traumatic brain injury. Neuroreport. 2001;12(11):2323–2327. doi: 10.1097/00001756-200108080-00009. [DOI] [PubMed] [Google Scholar]
- 29.Seeman P, et al. Dopamine receptors in the central nervous system. Fed Proc. 1978;37(2):131–136. [PubMed] [Google Scholar]
- 30.Baron JC, et al. Dopaminergic receptor sites in human brain: positron emission tomography. Neurology. 1985;35(1):16–24. doi: 10.1212/wnl.35.1.16. [DOI] [PubMed] [Google Scholar]
- 31.McDonald BC, Flashman LA, Saykin AJ. Executive dysfunction following traumatic brain injury: neural substrates and treatment strategies. NeuroRehabilitation. 2002;17(4):333–344. [PubMed] [Google Scholar]
- 32.Erickson JD, et al. Distinct pharmacological properties and distribution in neurons and endocrine cells of two isoforms of the human vesicular monoamine transporter. Proc Natl Acad Sci U S A. 1996;93(10):5166–5171. doi: 10.1073/pnas.93.10.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sager JJ, Torres GE. Proteins interacting with monoamine transporters: current state and future challenges. Biochemistry. 2011;50(34):7295–7310. doi: 10.1021/bi200405c. [DOI] [PubMed] [Google Scholar]
- 34.Wimalasena K. Vesicular monoamine transporters: structure-function, pharmacology, and medicinal chemistry. Med Res Rev. 2011;31(4):483–519. doi: 10.1002/med.20187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanson GR, et al. Psychostimulants and vesicle trafficking: a novel mechanism and therapeutic implications. Ann N Y Acad Sci. 2004;1025:146–150. doi: 10.1196/annals.1316.019. [DOI] [PubMed] [Google Scholar]
- 36.Fehr C, et al. Association of VMAT2 gene polymorphisms with alcohol dependence. J Neural Transm. 2013;120(8):1161–1169. doi: 10.1007/s00702-013-0996-y. [DOI] [PubMed] [Google Scholar]
- 37.Zai CC, et al. Association study of the vesicular monoamine transporter gene SLC18A2 with tardive dyskinesia. J Psychiatr Res. 2013;47(11):1760–1765. doi: 10.1016/j.jpsychires.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 38.Brighina L, et al. Analysis of vesicular monoamine transporter 2 polymorphisms in Parkinson's disease. Neurobiol Aging. 2013;34(6):1712.e9–1712.e13. doi: 10.1016/j.neurobiolaging.2012.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang X, et al. Polymorphism in the Vesicular Monoamine Transporter 2 Gene Decreases the Risk of Parkinson's Disease in Han Chinese Men. Parkinsons Dis. 2015;2015:903164. doi: 10.1155/2015/903164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Glatt CE, et al. Gain-of-function haplotypes in the vesicular monoamine transporter promoter are protective for Parkinson disease in women. Hum Mol Genet. 2006;15(2):299–305. doi: 10.1093/hmg/ddi445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wagner AK. TBI translational rehabilitation research in the 21st Century: exploring a Rehabilomics research model. Eur J Phys Rehabil Med. 2010;46(4):549–556. [PubMed] [Google Scholar]
- 42.Wagner AK, et al. Use of injury severity variables in determining disability and community integration after traumatic brain injury. J Trauma. 2000;49(3):411–419. doi: 10.1097/00005373-200009000-00005. [DOI] [PubMed] [Google Scholar]
- 43.Wagner AK, et al. CSF Bcl-2 and cytochrome C temporal profiles in outcome prediction for adults with severe TBI. J Cereb Blood Flow Metab. 2011;31(9):1886–1896. doi: 10.1038/jcbfm.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wagner AK, et al. Acute serum hormone levels: characterization and prognosis after severe traumatic brain injury. J Neurotrauma. 2011;28(6):871–888. doi: 10.1089/neu.2010.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wagner AK, et al. The value of trauma scores: predicting discharge after traumatic brain injury. Am J Phys Med Rehabil. 2000;79(3):235–242. doi: 10.1097/00002060-200005000-00004. [DOI] [PubMed] [Google Scholar]
- 46.Udekwu P, et al. Glasgow Coma Scale score, mortality, and functional outcome in head-injured patients. J Trauma. 2004;56(5):1084–1089. doi: 10.1097/01.ta.0000124283.02605.a5. [DOI] [PubMed] [Google Scholar]
- 47.Cifu DX, et al. Acute predictors of successful return to work 1 year after traumatic brain injury: a multicenter analysis. Arch Phys Med Rehabil. 1997;78(2):125–131. doi: 10.1016/s0003-9993(97)90252-5. [DOI] [PubMed] [Google Scholar]
- 48.Dawson JD, et al. Neuropsychological predictors of driving errors in older adults. J Am Geriatr Soc. 2010;58(6):1090–1096. doi: 10.1111/j.1532-5415.2010.02872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Green RE, et al. Examining moderators of cognitive recovery trajectories after moderate to severe traumatic brain injury. Arch Phys Med Rehabil. 2008;89(12 Suppl):S16–S24. doi: 10.1016/j.apmr.2008.09.551. [DOI] [PubMed] [Google Scholar]
- 50.van Veelen NM, et al. Short term neurocognitive effects of treatment with ziprasidone and olanzapine in recent onset schizophrenia. Schizophr Res. 2010;120(1–3):191–198. doi: 10.1016/j.schres.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 51.Bell KR, et al. The effect of a scheduled telephone intervention on outcome after moderate to severe traumatic brain injury: a randomized trial. Arch Phys Med Rehabil. 2005;86(5):851–856. doi: 10.1016/j.apmr.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 52.Wagner AK, et al. Persistent hypogonadism influences estradiol synthesis, cognition and outcome in males after severe TBI. Brain Inj. 2012;26(10):1226–1242. doi: 10.3109/02699052.2012.667594. [DOI] [PubMed] [Google Scholar]
- 53.Larrabee GJ, Curtiss G. Construct validity of various verbal and visual memory tests. J Clin Exp Neuropsychol. 1995;17(4):536–547. doi: 10.1080/01688639508405144. [DOI] [PubMed] [Google Scholar]
- 54.Borkowski JG, Benton AL, Spreen O. Word fluency and brain damage. Neuropsychologia. 1967;5(2):135–140. [Google Scholar]
- 55.Delis DC, Kramer JH. California Verbal Learning Test. Psychological Corporation; 2000. [Google Scholar]
- 56.Reitan RM, Wolfson D. The Halstead-Reitan neuropsychological test battery: theory and clinical interpretation. Neuropsychology Press; 1985. [Google Scholar]
- 57.Trenerry MR. Stroop Neuropsychological Screening Test Manual. Psychological Assessment Resources; 1989. [Google Scholar]
- 58.Keith RA, et al. The functional independence measure: a new tool for rehabilitation. Adv Clin Rehabil. 1987;1:6–18. [PubMed] [Google Scholar]
- 59.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hart T, et al. A longitudinal study of major and minor depression following traumatic brain injury. Arch Phys Med Rehabil. 2012;93(8):1343–1349. doi: 10.1016/j.apmr.2012.03.036. [DOI] [PubMed] [Google Scholar]
- 61.Fann JR, et al. Validity of the Patient Health Questionnaire-9 in assessing depression following traumatic brain injury. J Head Trauma Rehabil. 2005;20(6):501–511. doi: 10.1097/00001199-200511000-00003. [DOI] [PubMed] [Google Scholar]
- 62.Comings DE, MacMurray JP. Molecular heterosis: a review. Mol Genet Metab. 2000;71(1–2):19–31. doi: 10.1006/mgme.2000.3015. [DOI] [PubMed] [Google Scholar]
- 63.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16(3):1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barrett JC, et al. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21(2):263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 65.Li J, Ji L. Adjusting multiple testing in multilocus analyses using the eigenvalues of a correlation matrix. Heredity (Edinb) 2005;95(3):221–227. doi: 10.1038/sj.hdy.6800717. [DOI] [PubMed] [Google Scholar]
- 66.Failla MD, et al. Variants of SLC6A4 in depression risk following severe TBI. Brain Inj. 2013;27(6):696–706. doi: 10.3109/02699052.2013.775481. [DOI] [PubMed] [Google Scholar]
- 67.Gao WM, et al. Immunohistochemical analysis of histone H3 acetylation and methylation--evidence for altered epigenetic signaling following traumatic brain injury in immature rats. Brain Res. 2006;1070(1):31–34. doi: 10.1016/j.brainres.2005.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Darrah SD, et al. Genetic variability in glutamic acid decarboxylase genes: associations with post-traumatic seizures after severe TBI. Epilepsy Res. 2013;103(2–3):180–194. doi: 10.1016/j.eplepsyres.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. [Accessed September 27, 2015]; ; Available from: Available at: http://www.ncbi.nlm.nih.gov/SNP/snp_ref.cgi?rs=rs363226.
- 70.Ward LD, Kellis M. HaploReg: a resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic Acids Res. 2012;40(Database issue):D930–D934. doi: 10.1093/nar/gkr917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eiden LE, Weihe E. VMAT2: a dynamic regulator of brain monoaminergic neuronal function interacting with drugs of abuse. Ann N Y Acad Sci. 2011;1216:86–98. doi: 10.1111/j.1749-6632.2010.05906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Narendran R, et al. In vivo evidence for low striatal vesicular monoamine transporter 2 (VMAT2) availability in cocaine abusers. Am J Psychiatry. 2012;169(1):55–63. doi: 10.1176/appi.ajp.2011.11010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vergo S, et al. Vesicular monoamine transporter 2 regulates the sensitivity of rat dopaminergic neurons to disturbed cytosolic dopamine levels. Brain Res. 2007;1185:18–32. doi: 10.1016/j.brainres.2007.09.028. [DOI] [PubMed] [Google Scholar]
- 74.Cruz-Muros I, et al. Deglycosylation and subcellular redistribution of VMAT2 in the mesostriatal system during normal aging. Neurobiol Aging. 2008;29(11):1702–1711. doi: 10.1016/j.neurobiolaging.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 75.Lin Z, et al. High regulatability favors genetic selection in SLC18A2, a vesicular monoamine transporter essential for life. Faseb j. 2010;24(7):2191–2200. doi: 10.1096/fj.09-140368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lin Z, et al. SLC18A2 promoter haplotypes and identification of a novel protective factor against alcoholism. Hum Mol Genet. 2005;14(10):1393–1404. doi: 10.1093/hmg/ddi148. [DOI] [PubMed] [Google Scholar]
- 77.Cartier EA, et al. A biochemical and functional protein complex involving dopamine synthesis and transport into synaptic vesicles. J Biol Chem. 2010;285(3):1957–1966. doi: 10.1074/jbc.M109.054510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim ST, et al. Immobilization stress causes increases in tetrahydrobiopterin, dopamine, and neuromelanin and oxidative damage in the nigrostriatal system. J Neurochem. 2005;95(1):89–98. doi: 10.1111/j.1471-4159.2005.03342.x. [DOI] [PubMed] [Google Scholar]
- 79.Wagner AK, et al. Sex and genetic associations with cerebrospinal fluid dopamine and metabolite production after severe traumatic brain injury. J Neurosurg. 2007;106(4):538–547. doi: 10.3171/jns.2007.106.4.538. [DOI] [PubMed] [Google Scholar]
- 80.Scanlon JM, Ricker JPJ, Conley YP, Becker C, Burkhardt JN, Dixon CE, Wagner AK. Response to Methylphenidate Administration and Dopaminergic Function in Persons with TBI and Healthy Controls. Journal of Neurotrauma. 2009;26(8):A-23. [Google Scholar]
- 81.Wagner AK, et al. Genetic variation in the dopamine transporter gene and the D2 receptor gene influences striatal DAT binding in adults with severe TBI. JCBFM. 2014 Aug;34(8):1328–1339. doi: 10.1038/jcbfm.2014.87. Epub 2014 May 21. PMID: 24849661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Renner C, et al. The influence of gender on the injury severity, course and outcome of traumatic brain injury. Brain Inj. 2012;26(11):1360–1371. doi: 10.3109/02699052.2012.667592. [DOI] [PubMed] [Google Scholar]
- 83.Utomo WK, et al. Predictors of in-hospital mortality and 6-month functional outcomes in older adults after moderate to severe traumatic brain injury. Injury. 2009;40(9):973–977. doi: 10.1016/j.injury.2009.05.034. [DOI] [PubMed] [Google Scholar]
- 84.Nelson DW, et al. Multivariate outcome prediction in traumatic brain injury with focus on laboratory values. J Neurotrauma. 2012;29(17):2613–2624. doi: 10.1089/neu.2012.2468. [DOI] [PubMed] [Google Scholar]
- 85.Ponsford JL, et al. Gender differences in outcome in patients with hypotension and severe traumatic brain injury. Injury. 2008;39(1):67–76. doi: 10.1016/j.injury.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 86.Farace E, Alves WM. Do women fare worse? A metaanalysis of gender differences in outcome after traumatic brain injury. Neurosurg Focus. 2000;8(1):e6. doi: 10.3171/foc.2000.8.1.152. [DOI] [PubMed] [Google Scholar]
- 87.Brown SB, Colantonio A, Kim H. Gender differences in discharge destination among older adults following traumatic brain injury. Health Care Women Int. 2012;33(10):896–904. doi: 10.1080/07399332.2012.673654. [DOI] [PubMed] [Google Scholar]
- 88.Ponsford J. Factors contributing to outcome following traumatic brain injury. NeuroRehabilitation. 2013;32(4):803–815. doi: 10.3233/NRE-130904. [DOI] [PubMed] [Google Scholar]
- 89.Mosenthal AC, et al. Isolated traumatic brain injury: age is an independent predictor of mortality and early outcome. J Trauma. 2002;52(5):907–911. doi: 10.1097/00005373-200205000-00015. [DOI] [PubMed] [Google Scholar]
- 90.Draper K, Ponsford J, Schonberger M. Psychosocial and emotional outcomes 10 years following traumatic brain injury. J Head Trauma Rehabil. 2007;22(5):278–287. doi: 10.1097/01.HTR.0000290972.63753.a7. [DOI] [PubMed] [Google Scholar]
- 91.Crownover J, Galang G, Wagner A. Rehabilitation Considerations for Traumatic Brain Injury in the Geriatric Population: Epidemiology, Neurobiology, Prognosis, and Management. Current Translational Geriatrics and Experimental Gerontology Reports. 2012;1(3):149–158. [Google Scholar]
- 92.Pedroli E, et al. Virtual Multiple Errands Test: reliability, usability and possible applications. Stud Health Technol Inform. 2013;191:38–42. [PubMed] [Google Scholar]
- 93.Alderman N, et al. Ecological validity of a simplified version of the multiple errands shopping test. J Int Neuropsychol Soc. 2003;9(1):31–44. doi: 10.1017/s1355617703910046. [DOI] [PubMed] [Google Scholar]
- 94.Olsen AS, et al. Traumatic brain injury-induced cognitive and histological deficits are attenuated by delayed and chronic treatment with the 5-HT1A-receptor agonist buspirone. J Neurotrauma. 2012;29(10):1898–1907. doi: 10.1089/neu.2012.2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Davidson J, Cusimano MD, Bendena WG. Post-Traumatic Brain Injury: Genetic Susceptibility to Outcome. Neuroscientist. 2015;21(4):424–441. doi: 10.1177/1073858414543150. [DOI] [PubMed] [Google Scholar]
- 96.Myrga J, et al. A dopamine pathway gene risk score for cognitive recovery following TBI: methodological considerations, preliminary findings, and interactions with sex. J Head Trauma Rehabil. doi: 10.1097/HTR.0000000000000199. In Press. [DOI] [PubMed] [Google Scholar]