Abstract
Glycerol and dimethyl sulfoxide (DMSO) are commonly used cryoprotectants in cellular systems, but due to the challenges of measuring the properties of surface-bound solvent, fundamental questions remain regarding the concentration, interactions, and conformation of these solutes at lipid membrane surfaces. We measured the surface water diffusivity at gel-phase dipalmitoylphosphatidylcholine (DPPC) bilayer surfaces in aqueous solutions containing ≤7.5 mol. % of DMSO or glycerol using Overhauser dynamic nuclear polarization. We found that glycerol similarly affects the diffusivity of water near the bilayer surface and that in the bulk solution (within 20%), while DMSO substantially increases the diffusivity of surface water relative to bulk water. We compare these measurements of water dynamics with those of equilibrium forces between DPPC bilayers in the same solvent mixtures. DMSO greatly decreases the range and magnitude of the repulsive forces between the bilayers, whereas glycerol increases it. We propose that the differences in hydrogen bonding capability of the two solutes leads DMSO to dehydrate the lipid head groups, while glycerol affects surface hydration only as much as it affects the bulk water properties. The results suggest that the mechanism of the two most common cryoprotectants must be fundamentally different: in the case of DMSO by decoupling the solvent from the lipid surface, and in the case of glycerol by altering the hydrogen bond structure and intermolecular cohesion of the global solvent, as manifested by increased solvent viscosity.
INTRODUCTION
Glycerol and dimethyl sulfoxide (DMSO) are the two most commonly used cryoprotectants in cellular systems.1,2 While both solutes colligatively lower the freezing temperature of bulk water, it is understood that their specific molecular interactions with lipid bilayers and surface-bound water also play a key role in the mechanisms of cryoprotection. Such interactions are not fully understood, despite numerous studies comparing their empirical effectiveness in cellular cryopreservation.3–7 By utilizing concurrent surface water diffusivity and equilibrium surface forces measurements, the present work suggests that the two molecules influence membrane hydration differently, with DMSO effectively desolvating the surface and glycerol strengthening surface hydration, but only as much as glycerol increases the bulk water viscosity. Similar comparisons on surface hydration between DMSO and glycerol have been raised in studies on model peptides, largely through neutron scattering measurements,8,9 but direct comparisons of the effects of these cryoprotectants on lipid membrane systems remain absent.
Interactions between DMSO and water, as well as between glycerol and water, in bulk solution are well characterized. Through a number of IR and dielectric spectroscopy, neutron scattering, and molecular dynamics simulation studies, both glycerol and DMSO have been shown to readily form hydrogen bonds with water.10–14 DMSO can participate in 2 hydrogen bonds with water,15 and glycerol participates in 6-12.14,13 For both solutes, the bonds between solute and water are longer lived than water-water or solute-solute hydrogen bonds.15,16 Unsurprisingly, both solvents are fully miscible with water. Because of the particularly favorable interactions with water, aqueous mixtures of both solutes display a minimum in freezing point much lower than that of the pure components, with DMSO– and glycerol–water mixtures reaching a minimum of −140 °C and −45 °C, respectively (both at ∼30 mol. % solute).17,18 This is consistent with both solutes being effective viscogens, with DMSO increasing the solution viscosity by 72.5% and glycerol by 131% at 0.075 mole fraction of solute.19,20
How DMSO and glycerol differentially influence membrane surface hydration is more difficult to probe. Most membrane studies have focused on the zwitterionic phosphatidylcholine (PC) head group. At mole fractions of solute <0.3, DMSO decreases the solvent gap thickness between lamellae of DPPC multilamellar vesicles,21–23 whereas glycerol increases it.24 Moreover, DMSO increases the chain melting temperature,25 and glycerol leaves it unaffected.24 These phenomena suggest that DMSO decreases interlipid repulsion (both laterally and between bilayers), and glycerol either increases the repulsion or has little effect on the lipid–water interface. For DMSO, the decrease in repulsion is attributed largely to surface dehydration, which was recently illustrated in detail.26,27 However, the molecular interpretation for the glycerol results remained unclear.
There have been conflicting conclusions reached regarding the partitioning and molecular behavior of both solutes near the membrane surface. Neutron scattering measurements have shown a slight enrichment of DMSO near the surface,21 which appears to be in agreement with IR measurements that suggest association of the partially charged atoms of DMSO (i.e., the positive sulfur and the negative oxygen) with either of the oppositely charged head group moieties of the phospholipid head group (i.e., the positive amine/choline and the negative phosphate).28 In contrast, recent simulations have shown a ∼50% depletion of DMSO near the membrane surface,29 which has been explained through a stronger preference of DMSO for water than the lipid. Differential vapor pressure and calorimetry measurements suggest that glycerol is preferentially excluded from the surface, and that its binding is thermodynamically unfavorable,30 while simulations have shown a substantial enrichment.31
EXPERIMENTAL
Preparation of lipid vesicle samples
The lipid stocks were prepared by dissolving dry lipids in chloroform–methanol (4:1, v:v) and mixing at the desired proportions. The solvent was then evaporated under a stream of nitrogen. The traces of solvent were removed by evacuating the samples under vacuum for 24 h. The dried lipids were then rehydrated in an aqueous solution of the desired DMSO or glycerol concentration at a temperature 20 °C above the gel-fluid lipid phase transition temperature with gentle vortexing for 1 h. The large unilamellar vesicle (LUV) samples were prepared by the extrusion method using filters with a 200 nm pore diameter (Avanti Polar Lipids, Alabaster, AL). The samples were prepared 24 h before measurements.
ODNP and EPR experiments
For the ODNP and EPR measurements, a phospholipid spin probe TEMPO-PC, 1,2-dioleoyl-sn-glycero-3-phospho(TEMPO)choline (Avanti Polar Lipids, Alabaster, AL), in which a nitroxide radical is attached on the choline moiety, was used. The concentrations of spin probes and lipids were 670 μM and 32 mM, respectively (a ratio of 48:1, unlabeled:labeled lipids). The samples were studied without adjusted pH. During ODNP experiments, the center field of the nitroxide hyperfine transition line (as measured with EPR) was pumped continuously by microwave irradiation at 9.8 GHz, while the 1H NMR signal was recorded.32 All the experiments were performed at 25 °C under air flow to minimize microwave heating.
SFA experiments
In SFA experiments the absolute distance between back-silvered mica surfaces in a cross-cylinder geometry is measured with interferometry, and the force, F, measured by the deflection of a cantilever spring.33 Before each SFA experiment, the two mica surfaces were brought into contact in air to calibrate the absolute zero of separation distance. Next, the mica surfaces were coated with the lipid bilayers using Langmuir-Blodgett deposition and transferred under water to the SFA, where the desired solution was injected between the surfaces for force measurements. The bilayers in the SFA experiments were composed of an inner leaflet monolayer of DPPE (deposited at 28 mN/m surface pressure, 0.42 nm2 per lipid) and an outer leaflet monolayer of DPPC (19 mN/m, 0.52 nm2). The single bilayer thickness, To, was calculated from SFA measurements of the thickness of two DPPE monolayers in air, and two DPPC monolayers in air, both at the same surface pressure as the bilayers in the force measurements. All measurements were done at 22 °C.
RESULTS AND DISCUSSION
Herein, we present measurements on gel-phase DPPC lipid membranes in DMSO–water or glycerol–water solutions with varying solute mole fractions between 0 (pure water) and 0.075. First, we present the translational water diffusivity near the surface of large unilamellar vesicles, a property which has been shown to correlate with the hydrated volume of lipid head groups.26 We further measured bulk water diffusivities in the presence of either solute in order to compare with the trends in surface water diffusivities. We employed Overhauser dynamic nuclear polarization (ODNP),34 a relaxometry technique that probes water dynamics within ∼1 nm of nitroxide spin labels that are either in bulk solution (as 4-amino-TEMPO) or attached to the lipid head groups of unilamellar vesicles (as PC-TEMPO).27
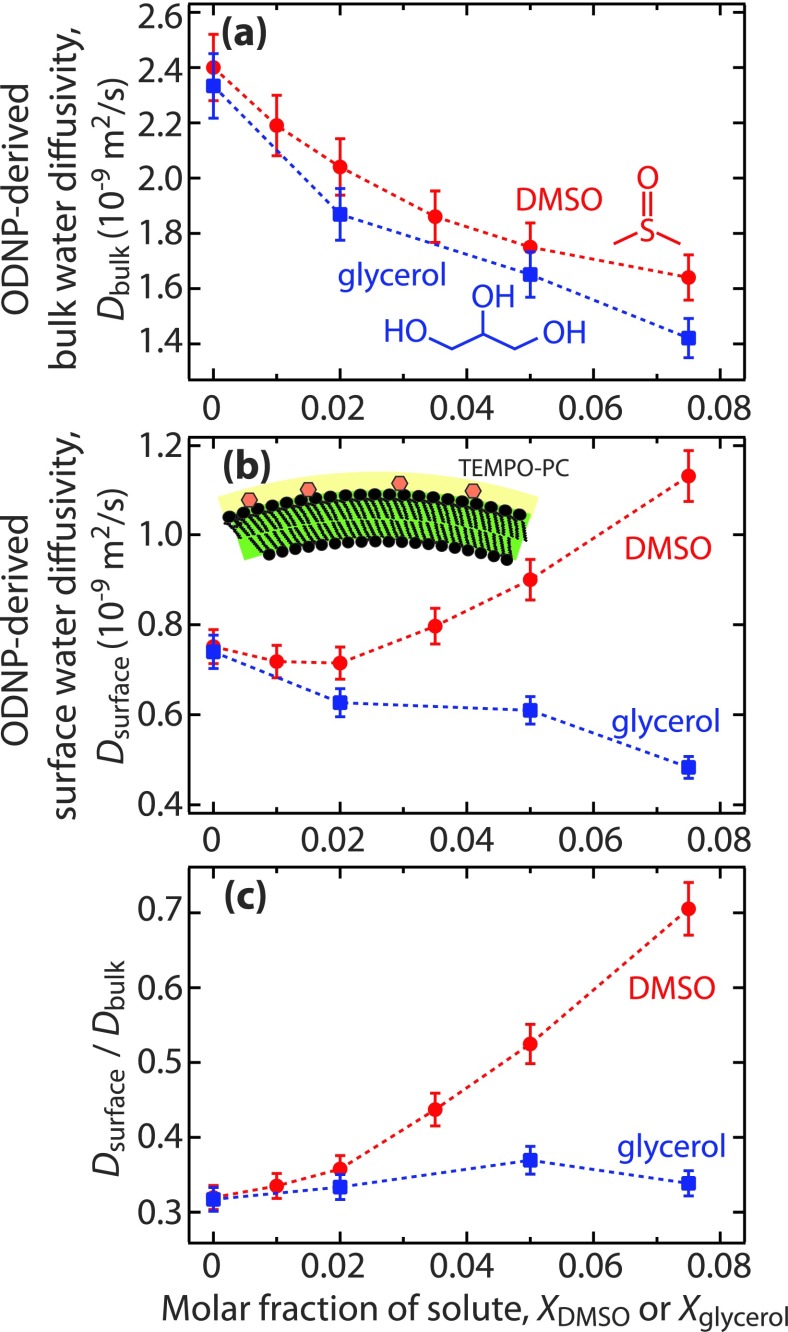
The ODNP measurement-derived water diffusivities in bulk solutions, Dbulk, are shown in Fig. 1(a). Both DMSO and glycerol monotonically decrease Dbulk, which is not surprising, as both additives are known to monotonically increase bulk viscosity over this concentration range.19,20 However, the similarity between the two curves is noteworthy, given that the increase in solution viscosity is ∼2 times greater for glycerol than DMSO in this range of solute concentration (by mole fraction).19,20 This suggests that ODNP-derived diffusivities measured at picosecond time scales linearly correlate with, but are not equivalent to, the macroscopic viscosity effect of DMSO and glycerol. However, the trends in surface water diffusivity values, Dsurface, shown in Fig. 1(b) differ markedly between the two solutes. When going from pure water to a solute mole fraction, Xglycerol or XDMSO, of 0.075, glycerol decreases Dsurface by 37% and DMSO increases Dsurface by 51%. The ratio Dsurface/Dglycerol, shown in Fig. 1(c), represents how the solutes impact the hydration dynamics at the surface relative to that in the bulk. This ratio grows substantially with XDMSO, whereas it is relatively independent of Xglycerol.
FIG. 1.
(a) Diffusivity of water in the bulk, Dbulk, in the absence of lipid vesicles, (b) water diffusivity, Dsurface, at the surface of DPPC large unilamellar vesicles, and (c) the ratio of Dsurface and Dbulk at various molar fractions of DMSO (XDMSO) and glycerol (Xglycerol) at 25 °C. The error bars represent the standard deviation of the parameters estimated from the fitting.
Electron paramagnetic resonance (EPR) measurements have been used previously to show that there is no change in the mobility of the lipid head groups for XDMSO ≤ 0.075 .27 Above XDMSO = 0.075, the mobility of lipid head groups decreases significantly. Analogous measurements with glycerol (Fig. S1 of the supplementary material32) confirmed that head group mobility is also independent of Xglycerol when Xglycerol ≤ 0.075. Extended ODNP parameters are shown in Section S2 of the supplementary material.32
While the ODNP measurements give no explicit information regarding the concentration or orientation of either solute at the interface, they clearly suggest that the glycerol–water and water–water interactions are of similar energies at the surface and in the bulk in glycerol–water solutions. In contrast, DMSO disproportionately weakens the molecular cohesion energies involving water near the surface, implying specific interactions of DMSO with the lipid head groups.
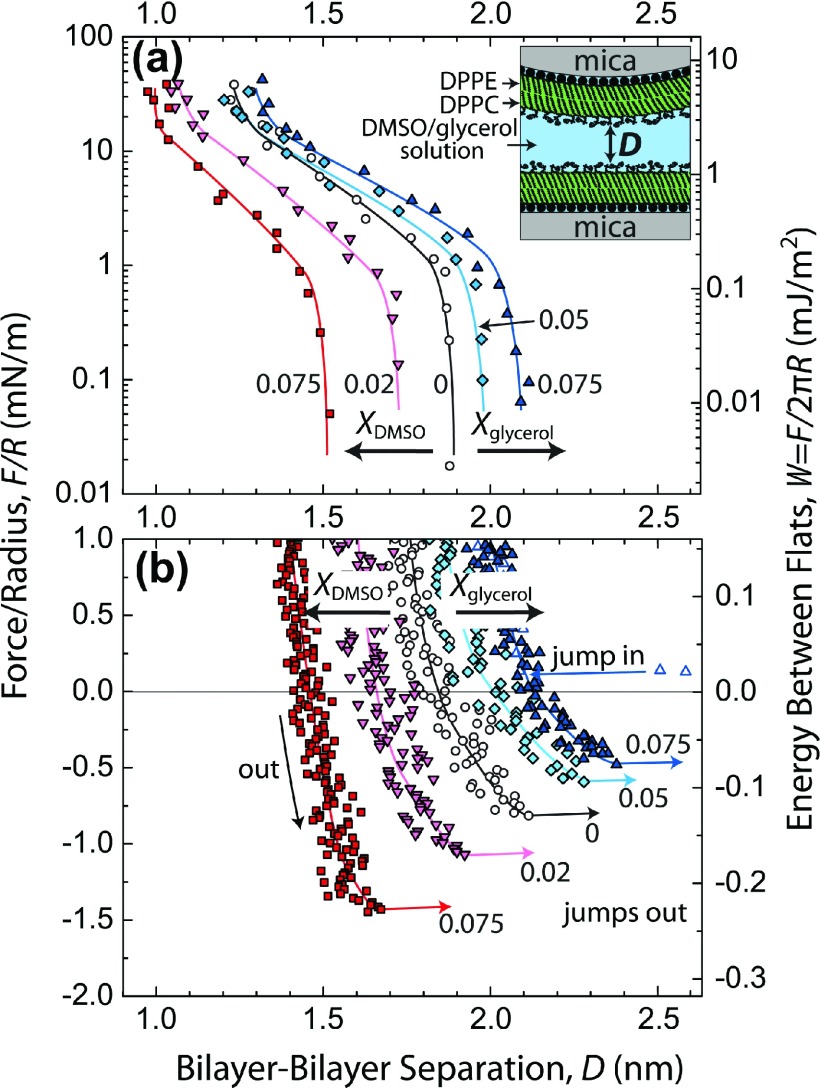
Measurements of the equilibrium interaction force, F, as a function of bilayer–bilayer separation, D, between supported DPPC membranes in DMSO–water and glycerol–water mixtures are shown in Fig. 2. Bilayer–bilayer force profiles generally contain additive contributions from two opposing forces, which are an attractive van der Waals force and a repulsive steric-hydration force, and whose balance determines the equilibrium bilayer–bilayer separation. We examined these forces at small separations, at D < 2.5 nm, at which range the forces contain information on solvation and head group ordering. Measurements were made using a surface forces apparatus (SFA), wherein two molecularly smooth and back-silvered mica sheets are mounted on cylindrical disks of radius R and coated with lipid bilayers using Langmuir-Blodgett deposition. Further details of the surface preparation and measurement of D can be found elsewhere.26,33
FIG. 2.
Forces, F, measured with the SFA between mica-supported gel-phase DPPC bilayers in DMSO–water and glycerol–water mixtures at 22 °C and pH 6.0 ± 0.2. Solid, colored lines were drawn by hand. (a) Static measurements of repulsive forces on a semilog plot. (b) Adhesion forces measured upon slow separation of the surfaces. Measurements were made at XDMSO and Xglycerol values of 0, 0.02, 0.05, and 0.075, and the trends were consistent, but due to overlap between force curves, only certain data series are shown here for clarity.
Repulsive forces, corresponding to F > 0, are displayed in Fig. 2(a), and show that DMSO monotonically decreases the range of the interbilayer steric-hydration repulsion, whereas glycerol clearly increases the repulsion. The range and steepness of these repulsive forces are indicative of the hydrated excluded volume of the lipid head groups, which has previously been addressed quantitatively.26 The attractive forces at F < 0 are shown in Fig. 2(b), in which the most negative values of F correspond to the adhesion force. Based on the dielectric properties of their aqueous mixtures, both DMSO and glycerol should theoretically weaken the attractive van der Waals force by 30% when going from pure water to a mole fraction of 0.075 (by comparing the magnitudes of the van der Waals forces at any particular D value. Calculations are shown in Section S2 of the supplementary material32). This makes the observation of an overall increase in the inter-bilayer adhesion forces with DMSO the more unambiguous. DMSO is substantially dehydrating the head groups to cause a weakening of the repulsive forces that results in a net increase in the adhesion force. In contrast, glycerol clearly increases the range of the repulsion (Fig. 2(a)) which brings the adhesion separation to a distance where the van der Waals force is weaker, which leads to lower net adhesion forces (Fig. 2(b)).
It is worth noting that if either solute were to be depleted from the interface, one would observe a depletion attraction that would give rise to ∼100 times stronger adhesion.26 Given that our data demonstrate a lack of a depletion attraction (and fits well with just a van der Waals attractive force), we can deduce that the solute is neither repelled from the surface, nor completely expelled upon compression.
Collectively, the ODNP and SFA data show that glycerol strengthens the intermolecular cohesion of both bulk and interfacial water in a similar fashion, and that glycerol is both isotropically oriented and equally partitioned near the lipid membrane surface as in the bulk. DMSO, in contrast, strengthens solvent–solvent cohesion in the bulk, but weakens it at the lipid membrane surface, indicating that DMSO must be net anisotropically oriented at the surface.
Although a direct comparison of the effect of DMSO and glycerol on lipid membrane surface hydration has not been reported to date, comparative studies were carried out with the hydrophobic model peptide, NALMA, where neutron scattering measurements suggest that DMSO disrupts the hydration shell and glycerol leaves it unaffected (compared to the hydration shell of NALMA in pure water).8,9 Furthermore, in those studies it was observed that glycerol is depleted from the surface. This “preferential hydration” is also thought to occur in glycerol–water solutions of lysozyme35,36 and a number of proteins,37 but we suggest this is not the case here in the lipid membrane system.
We hypothesize that the above differences between glycerol and DMSO stem essentially from water’s similarity to glycerol at the molecular level, and dissimilarity to DMSO, both in terms of hydrogen bonding number and orientation. Glycerol and water further share a hydroxyl functionality and the ability to both accept and donate hydrogen bonds. Moreover, glycerol is highly flexible (containing only single bonds) and can thus adapt readily to the hydrogen bond structure of the surrounding water.38 Glycerol and water perhaps interact with lipid head groups in a similar fashion, and thus one would expect glycerol to partition equally between the bulk and the surface. By doing so, glycerol enhances and strengthens the hydrogen bond network of surface and bulk water similarly. DMSO instead competes with the head group for favorable interactions with water, and thereby decreases the head group hydrated volume.
CONCLUSIONS
Our results indicate that while DMSO and glycerol are the two most common cryoprotectants, the manner in which they associate with lipid membranes and interfacial water to afford protection upon freezing is distinctly different: DMSO decouples the solvent from the lipid surface entirely, whereas glycerol uniformly alters the hydrogen bond structure of the solvent so as not to strain the membrane upon crystallization of freezing water.
Acknowledgments
We acknowledge support by the National Institute of General Medical Sciences of the National Institutes of Health under Award No. 1R01GM116128-01 and the Materials Research Science and Engineering Centers (MRSEC) Program of the National Science Foundation Award No. DMR 1121053.
REFERENCES
- 1.Lovelock J. E., Biochim. Biophys. Acta 11, 28 (1953). 10.1016/0006-3002(53)90005-5 [DOI] [PubMed] [Google Scholar]
- 2.Lovelock J. E. and Bishop M. W. H., Nature 183, 1394 (1959). 10.1038/1831394a0 [DOI] [PubMed] [Google Scholar]
- 3.Anchordoguy T. J., Rudolph A. S., Carpenter J. F., and Crowe J. H., Cryobiology 24, 324 (1987). 10.1016/0011-2240(87)90036-8 [DOI] [PubMed] [Google Scholar]
- 4.McGann L. E., Cryobiology 15, 382 (1978). 10.1016/0011-2240(78)90056-1 [DOI] [PubMed] [Google Scholar]
- 5.Surewicz W. K., Chem. Phys. Lipids 34, 363 (1984). 10.1016/0009-3084(84)90010-0 [DOI] [Google Scholar]
- 6.Pribor D., Cryobiology 12, 309 (1975). 10.1016/0011-2240(75)90004-8 [DOI] [PubMed] [Google Scholar]
- 7.Bickis I. J., Kazaks K., Finn J. J., and Henderson I. W. D., Cryobiology 4, 1 (1967). 10.1016/S0011-2240(67)80180-9 [DOI] [PubMed] [Google Scholar]
- 8.Malardier-Jugroot C., Bowron D. T., Soper A. K., Johnson M. E., and Head-Gordon T., Phys. Chem. Chem. Phys. 12, 382 (2010). 10.1039/B915346B [DOI] [PubMed] [Google Scholar]
- 9.Russo D., Chem. Phys. 345, 200 (2008). 10.1016/j.chemphys.2007.08.001 [DOI] [Google Scholar]
- 10.Soper A. K. and Luzar A., J. Chem. Phys. 97, 1320 (1992). 10.1063/1.463259 [DOI] [Google Scholar]
- 11.Soper A. K., Facility I., and Ox D., J. Phys. Chem. 100, 1357 (1996). 10.1021/jp951783r [DOI] [Google Scholar]
- 12.Cabral J. T., Luzar A., Teixeira J., and Bellissent-Funel M.-C., J. Chem. Phys. 113, 8736 (2000). 10.1063/1.1315333 [DOI] [Google Scholar]
- 13.Puzenko A., Hayashi Y., Ryabov Y. E., Balin I., Feldman Y., Kaatze U., and Behrends R., J. Phys. Chem. B 109, 6031 (2005). 10.1021/jp0445122 [DOI] [PubMed] [Google Scholar]
- 14.Dashnau J. L., V Nucci N., Sharp K. A., and Vanderkooi J. M., J. Phys. Chem. B 110, 13670 (2006). 10.1021/jp0618680 [DOI] [PubMed] [Google Scholar]
- 15.Luzar A. and Chandler D., J. Chem. Phys. 98, 8160 (1993). 10.1063/1.464521 [DOI] [Google Scholar]
- 16.Chen C., Li W. Z., Song Y. C., and Yang J., J. Mol. Struct.: THEOCHEM 916, 37 (2009). 10.1016/j.theochem.2009.09.007 [DOI] [Google Scholar]
- 17.Havemeyer R. N., J. Pharm. Sci. 55, 851 (1966). 10.1002/jps.2600550822 [DOI] [PubMed] [Google Scholar]
- 18.Lane L. B., Ind. Eng. Chem. 17, 924 (1925). 10.1021/ie50189a017 [DOI] [Google Scholar]
- 19.LeBel R. G. and Goring D. A. I., J. Chem. Eng. Data 7, 100 (1962). 10.1021/je60012a032 [DOI] [Google Scholar]
- 20.Cheng N., Ind. Eng. Chem. Res. 47, 3285 (2008). 10.1021/ie071349z [DOI] [Google Scholar]
- 21.Gorshkova J. E. and Gordeliy V. I., Crystallogr. Rep. 52, 535 (2007). 10.1134/S1063774507030364 [DOI] [Google Scholar]
- 22.Kiselev M. A., Lesieur P., Kisselev A. M., Grabielle-Madelmond C., and Ollivon M., J. Alloys Compd. 286, 195 (1999). 10.1016/S0925-8388(98)01006-8 [DOI] [Google Scholar]
- 23.Gordeliy V. I., Kiselev M. A., Lesieur P., Pole A. V., and Teixeira J., Biophys. J. 75, 2343 (1998). 10.1016/S0006-3495(98)77678-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDaniel R. V., McIntosh T. J., and Simon S. A., Biochim. Biophys. Acta 731, 97 (1983). 10.1016/0005-2736(83)90402-9 [DOI] [Google Scholar]
- 25.Yu Z. W. and Quinn P. J., Biophys. J. 69, 1456 (1995). 10.1016/S0006-3495(95)80015-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schrader A. M., Donaldson S. H., Song J., Cheng C.-Y., Lee D. W., Han S., and Israelachvili J. N., Proc. Natl. Acad. Sci. U. S. A. 112, 10708 (2015). 10.1073/pnas.1512325112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng C., Song J., Pas J., Meijer L., and Han S., Biophys. J. 109, 330 (2015). 10.1016/j.bpj.2015.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shashkov S. N., Kiselev M. A., Tioutiounnikov S. N., Kiselev A. M., and Lesieur P., Phys. B: Condens. Matter 271, 184 (1999). 10.1016/S0921-4526(99)00214-8 [DOI] [Google Scholar]
- 29.Dabkowska A. P., Collins L. E., Barlow D. J., Barker R., McLain S. E., Lawrence M. J., and Lorenz C. D., Langmuir 30, 8803 (2014). 10.1021/la501275h [DOI] [PubMed] [Google Scholar]
- 30.Westh P., Biophys. J. 84, 341 (2003). 10.1016/S0006-3495(03)74854-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malajczuk C. J., Hughes Z. E., and Mancera R. L., Biochim. Biophys. Acta 1828, 2041 (2013). 10.1016/j.bbamem.2013.05.010 [DOI] [PubMed] [Google Scholar]
- 32.See supplementary material at http://dx.doi.org/10.1063/1.4959904 E-JCPSA6-145-045629 for the extended EPR and ODNP protocols and results, and calculations of the theoretical Hamaker constant.
- 33.Israelachvili J. N., Min Y., Akbulut M., Alig A., Carver G., Greene W., Kristiansen K., Meyer E., Pesika N., Rosenberg K., and Zeng H., Rep. Prog. Phys. 73, 1 (2010). 10.1088/0034-4885/73/3/036601 [DOI] [Google Scholar]
- 34.Franck J. M., Pavlova A., Scott J. A., and Han S., Prog. Nucl. Magn. Reson. Spectrosc. 74, 33 (2013). 10.1016/j.pnmrs.2013.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sinibaldi R., Ortore M. G., Spinozzi F., Carsughi F., Frielinghaus H., Cinelli S., Onori G., and Mariani P., J. Chem. Phys. 126, 235101 (2007). 10.1063/1.2735620 [DOI] [PubMed] [Google Scholar]
- 36.Paciaroni A., Cinelli S., and Onori G., Biophys. J. 83, 1157 (2002). 10.1016/S0006-3495(02)75239-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gekko K. and Timasheff S. N., Biochemistry 20, 4667 (1981). 10.1021/bi00519a023 [DOI] [PubMed] [Google Scholar]
- 38.Egorov A. V., Lyubartsev A. P., and Laaksonen A., J. Phys. Chem. B 115, 14572 (2011). 10.1021/jp208758r [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- See supplementary material at http://dx.doi.org/10.1063/1.4959904 E-JCPSA6-145-045629 for the extended EPR and ODNP protocols and results, and calculations of the theoretical Hamaker constant.