Abstract
Purpose
Ionizing radiation (IR) generates reactive oxygen species (ROS), which cause DNA double-strand breaks (DSBs) that are responsible for cytogenetic alterations. Because antioxidants are potent ROS scavengers, we determined whether the vitamin E isoform γ-tocotrienol (GT3), a radio-protective multifunctional dietary antioxidant, can suppress IR-induced cytogenetic damage.
Methods
We measured DSB formation in irradiated primary human umbilical vein endothelial cells (HUVECs) by quantifying the formation of γ-H2AX foci. Chromosomal aberrations (CAs) were analyzed in irradiated HUVECs and in the bone marrow cells of irradiated mice by conventional and fluorescence-based chromosome painting techniques. Gene expression was measured in HUVECs with quantitative reverse transcriptase polymerase chain reaction (qRT-PCR).
Results
GT3 pretreatment reduced DSB formation in HUVECS, and also decreased CAs in HUVECs and mouse bone marrow cells after irradiation. Moreover, GT3 increased expression of the DNA-repair gene RAD50 and attenuated radiation-induced RAD50 suppression.
Conclusions
GT3 attenuates radiation-induced cytogenetic damage, possibly by affecting RAD50 expression. GT3 should be explored as a therapeutic to reduce the risk of developing genetic diseases after radiation exposure.
Key Words: chromosomal aberrations, DNA repair, endothelial cells, gamma-tocotrienol, ionizing radiation
Introduction
Ionizing radiation (IR) compromises the redox state of cells and generates reactive oxygen species (ROS) in excess. ROS are highly reactive and damage DNA, as well as various cellular components. Mammalian cells exposed to 1 Gy of IR accumulate approximately 1000 single-strand breaks, 40 double-strand breaks (DSBs), and 3000 damaged DNA bases (1). DSBs are serious lesions that can cause numerous structural chromosomal aberrations (CAs) such as sister chromatid unions, chromatid- or chromosome-type breaks, and dicentric or ring chromosomes (2). Such CAs are particularly deleterious and are associated with the progression and development of several diseases, including cancer (3). To date, CAs have been found in all major tumor types and are also associated with mental illness, congenital heart disease, and respiratory problems (4–6). This suggests that CAs are not only a hallmark of cancer but also a cytogenetic signature of various pathophysiological conditions. While CAs can arise spontaneously, radiation is a major factor that induces CAs.
DNA-repair proteins such as RAD50 mitigate CAs by repairing DSBs. Curiously, radiation suppresses RAD50 gene expression in mouse white blood cells for up to 48 h after irradiation (7). RAD50 is part of the MRN complex which consists of meiotic recombination 11 (MRE11), RAD50, and Nijmegen breakage syndrome 1 (NBS1) proteins. The primary function of the MRN complex is to recognize DSBs, bind the damaged DNA, and initiate repair by regulating the homologous recombination pathway and the alternate-non-homologous end- joining cascade with support from other DNA-repair proteins (8).
The type and frequency of radiation-induced CAs vary with radiation dose, dose rate, and the quality of irradiation. CAs may be evident in metaphase chromosomes soon after irradiation, or they may appear several generations later. This delay is a sign of genomic instability and is a hallmark of cancer (9). Similarly, IR is a major risk factor for endothelial dysfunction (10), and genetically unstable endothelial cells can directly stimulate cancer metastases (11,12). Thus, both cancer and endothelial dysfunction may benefit from therapies that reduce radiation-induced oxidative stress.
Antioxidants suppress DSBs and CAs in irradiated human lymphocytes (13,14). The vitamin E isoform γ-tocotrienol (GT3) is a natural dietary antioxidant and a potent radio-protector, with the largest dose reduction factor among all the natural products identified to date (15,16). GT3 protects the endothelials cells that line the blood and lymphatic vessels of higher eukaryotes (17), regulates a number of signaling pathways that mitigate radiation damage (18), and also protects mice from a lethal dose of radiation (16). Although we do not fully understand how GT3 protects against radiation damage, studies by various groups, including our own, suggest that GT3 may regulate apoptotic signals, cytokine production, cholesterol biosynthesis, or progenitor cell mobilization (17,19–21). Moreover, gene-expression profiles suggest that GT3 affects a number of genes known to be important for the DNA damage response (18).
Here, we demonstrate that GT3 pretreatment decreases the frequency of radiation-induced DSBs and CAs both in vitro and in vivo. We also show that GT3 stabilizes RAD50 expression after irradiation. These findings suggest that GT3 is a potent radio-protector that can protect against radiation-induced cytogenetic damage.
Materials and Methods
Materials
GT3 was obtained from Yasoo Health Inc. (Johnson City, TN). The EGM-2 Bullet Kit and HEPES-buffered saline solution used for cell culture were obtained from Lonza (Walkersville, MD). All other reagents and media were obtained from Sigma (St. Louis, MO).
Cell Culture and Animals
Primary human umbilical vein endothelial cells (HUVECs) (American Type Culture Collection, Manassas, VA) were cultured in tissue culture vessels with EGM-2 Bullet Kit in 5% CO2 incubator at 37°C. The cells were passaged every 2 to 3 days with standard aseptic techniques, and experiments were performed using cells from passages 3 to 6.
All animal studies were carried out in strict accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The animal protocol was approved by the Institutional Animal Care and Use Committee of the University of Arkansas for Medical Sciences. Female C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME, stock no. 000664) were held in quarantine for 1 week after arrival. All animals were housed in conventional cages in a standard air conditioned animal facility at 20 ± 2°C, with 10–15 hourly cycles of fresh air, and a 12–12-h day-night light cycle. Animals had free access to water and chow (Envigo Teklad diet 7012, Madison, WI). All animals were humanely euthanized with CO2 in an appropriate chamber.
Irradiation and Drug Treatment
Mice were irradiated with a single, total-body radiation dose (3 Gy) in a Shepherd Mark I model 25 137Cs irradiator (J. L. Shepherd & Associates, San Fernando, CA). The average dose rate was 0.98 Gy/min and was corrected for decay. The animals were placed in a well-ventilated rotating chamber composed of T-6061 aluminum with a gold-anodized coating. The chamber was divided into eight equal “pie slice” compartments with a well-ventilated Plexiglas lid (J.L. Shepherd & Associates). Animals were injected subcutaneously into the loose skin over the neck with a single dose of GT3 or vehicle (5% Tween-80 in saline) 24 h before irradiation. All radiation experiments were performed in the morning to minimize diurnal effects. HUVECs were irradiated in T25 tissue-culture flasks (Corning, NY) or chambered slides (Nunc Lab-Tek, St. Louis, MO). HUVECs were treated with 0–5 μM of GT3 or vehicle (DMSO) prior to irradiation. Radiation doses used for the various studies were carefully selected based on experimental endpoints and knowledge regarding cellular radio-sensitivity.
γ-H2AX Detection
HUVECs were grown in 4-well chambered slides overnight and treated with GT3 or vehicle (DMSO) for 1 h or 24 h before irradiation. Cells were washed with PBS and fixed with 4% paraformaldehyde 1 h after irradiation. The fixed cells were washed, permeabilized with 0.3% Triton-X for 15 min at room temperature, quenched with 1% glycine, and blocked with 4% BSA in 1X TBST overnight at 4°C. The cells were incubated with primary antibody (1:600 dilution; monoclonal Anti-phospho-Histone H2AX (Ser139), clone JBW301; Millipore, Temecula, CA) for 3 h at room temperature. After washing 3 times with PBS, secondary antibody (Alexa Fluor 594 F (ab') 2 fragment of goat anti-mouse IgG; Invitrogen, Grand Island, NY) was added, and the cells were incubated for 1 h at room temperature in the dark. Finally, the cells were washed with PBS and stained with Hoechst solution to visualize nuclei. γ-H2AX foci were detected with a Zeiss AxioPlan microscope. γ-H2AX foci were not counted in apoptotic cells. The apoptotic cells, particularly cells in late apoptotic stages, were identified by their highly compromised nuclear membrane.
Preparation of Metaphase Spreads
For in vitro experiments, HUVECs were treated with 5 μl/ml colcemid (Invitrogen, Grand Island, NY) 24 h before cell harvest. HUVECs were collected by trypsinization and washed 2 times with PBS. For in vivo experiments, mice were given 100 μl of 0.05% colchicine intraperitoneally 2 h before bone marrow harvest. Bone marrow cells were collected from the tibias and femurs of both hind legs by flushing with ice cold PBS containing 2% FBS. The cell suspension was layered onto equal volume (3 ml) of Histopaque-1083 (Sigma-Aldrich, St. Louis, MO) in a 15-ml tube. The tubes were centrifuged at 400 g for 30 min at room temperature. The buffy coat, containing bone marrow mononuclear cells, was collected and washed with PBS. HUVECs and mouse bone marrow mononuclear cells were then treated with hypotonic solution (0.56% KCl solution) for 15 min and fixed in methanol-acetic acid (3:1). Fixed cells were applied to precleaned, wet glass slides and air-dried overnight at room temperature.
Chromosome Staining Techniques
Two techniques were used to examine CAs in metaphase spreads. Giemsa staining: Metaphase spreads were prepared as described above and air dried at room temperature overnight. Slides were immersed in Giemsa staining solution (Fisher Scientific) for 20 min, rinsed in distilled water to remove excess stain, and air-dried for 24 h before scoring at 100x magnification under bright field on a Zeiss microscope. Multiple fluorescence in situ hybridization (mFISH) painting: Whole-chromosome DNA probes (Applied Spectral Imaging, Carlsbad, CA) were used to label human and mouse chromosomes 1, 2, and 3, according to the manufacturer’s instructions. Metaphase spreads were prepared 24 h before mFISH painting. The slides were treated with 2X SSC buffer at room temperature for 2 min, then dehydrated in a series of ethanol solutions (70%, 80%, and 100%) for 2 min each. Chromosomes were denatured by placing the slides in pre-warmed (72°C) denaturation solution (70% formamide in 2X SSC) for 60–80 s, then immediately passed through a series of cold ethanol solutions (70%, 80%, and 100%) and air-dried. mFISH probe (10 μl) was denatured at 80°C for 7 min and applied to the slides. The slides were covered with glass cover slips, sealed with rubber cement, and incubated overnight at 37°C in a humidified CO2 incubator. The coverslips were removed, and the slides were washed with 0.4X SSC at 74°C for 5 min and placed in washing solution-II (4X SSC with 0.1% Tween-20) for 2 min. Finally, the slides were counter-stained with 10 μl of DAPI containing anti-fade (Vector Laboratories, Burlingame, CA) and sealed with a coverslip.
qRT-PCR
HUVECs were washed twice with cold calcium- and magnesium-free PBS. Total RNA was extracted with the RNeasy Plus Mini Kit (Qiagen, Valencia, CA), and each sample was treated with TURBO DNase (Ambion, Grand Island, NY). RNA was quantified with an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA). Samples with an RNA integrity number (RIN) of 10 were used to produce cDNA. Although in a typical in vitro experiment RIN value varies between 7.5 and 10, in the current study we used samples with RIN value 10 to ensure reproducibility and accuracy of the gene expression study. cDNA was generated from 2 μg of RNA using the high-capacity cDNA Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA). qRT-PCR was performed with the ABI Prism 7000 Sequence Detection System (Applied Biosystems). Standard real-time PCR (50°C for 2 min; 95°C for 10 min; 50 cycles of 95°C for 15 s, 60°C for 60 s) was performed with TaqMan 2X Universal PCR Master Mix (Applied Biosystems). Relative mRNA expression was calculated with the comparative CT method (2-∆∆CT) and normalized to 18S rRNA for each sample.
Statistical Analysis
Statistical analyses were performed with GraphPad software (La Jolla, CA). For experiments with only one parameter under consideration, p values were determined by an unpaired Student’s t test. The standard error for CAs was calculated by √a/A, where a was the number of aberration under consideration and A was the total number of cells analyzed (22). Z tests were used to test differences in the frequency of CAs before and after exposure to IR, as described elsewhere (23). Results were considered statistically significant when p < 0.05.
Results
GT3 Suppresses Radiation-Induced DSB Formation in Primary Human Endothelial Cells
Because GT3 has strong antioxidant properties and protects endothelial cells from radiation damage (15,17), we proposed that GT3 pretreatment would suppress radiation-induced DSBs in HUVECs. To determine whether GT3 was able to suppress DSBs in HUVECs, we assayed the formation of γ-H2AX foci as an indicator of DSBs after irradiation. Previous gene expression profiling study from our group show that GT3 treatment for 24 h substantially alters genetic pathways known to be of critically important in the regulation of cellular responses of radiation exposure, including the responses to DNA damage stimuli in HUVECs (18). However, a recent study demonstrate GT3 treatment for 1 h considerably suppresses AKT phosphorylation, another critical marker of DSBs formation, in HUVECs (24). Therefore, we decided to treat HUVECs with GT3 either for 1 h or 24 h to assess its ability to modify radiation-induced γH2AX foci formation. HUVECs were treated with either 5 μM GT3 or vehicle (DMSO) 24 h before irradiation with 0.2 Gy of γ-rays. γ-H2AX foci were quantified by microscopy 1 h after irradiation (a low dose of radiation was used to minimize counting error). γ-H2AX foci were almost undetectable in non-irradiated cells treated with vehicle or GT3 (Fig. 1a I, II, b). Irradiation induced γ-H2AX foci in both the vehicle- (3.8 ± 0.07) and GT3-treated groups (2.7 ± 0.23) but the GT3-treated cells contained significantly (p = 0.01) fewer γ-H2AX foci (Fig. 1a III, VI, b). Further, to determine the ability of GT3 in suppressing residual DSBs that are persisted several hours after irradiation, we treated HUVECs with 5 μM of GT3 for 1 h, then exposed to two different radiation doses 3 Gy and 5 Gy, and further incubated for 29 h before immunofluorescence staining for γH2AX foci. We observed GT3 also effectively suppressed frequency of persistent DSB formation in primary human endothelial cells after 3 Gy (3.4 ± 0.19 in vehicle treated group vs 2.0 ± 0.46 in GT3 treated group) and 5 Gy (4.4 ± 0.31 in vehicle treated group vs 3.2 ± 0.23 in GT3 treated group) irradiation (Fig. 1c).
Fig. 1.
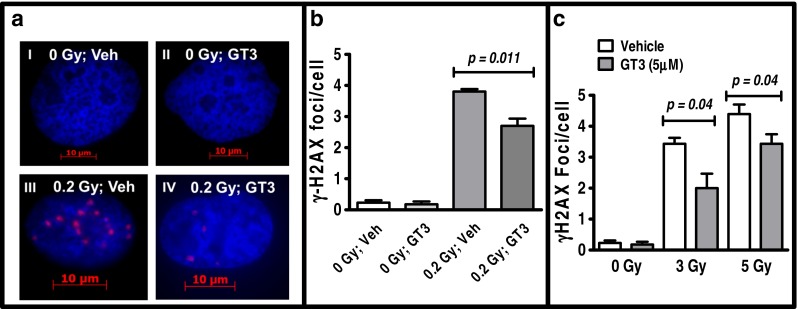
GT3 decreases the number of radiation-induced γ-H2AX foci (red) in HUVEC nuclei (DAPI stain, blue). (a) HUVECs were treated with (I) vehicle (Veh, DMSO) for 24 h or (II) 5 μM GT3 for 24 h without irradiation, or (III) pretreated with vehicle or (IV) 5 μM GT3 24 h before 0.2 Gy irradiation. Images were taken 1 h after irradiation. (b) Data from (a) are expressed as γ-H2AX foci per cell for each treatment. (c) To determine the efficacy of GT3 in suppressing persistent DSBs, HUVECs were treated with GT3 or vehicle for 1 h, exposed to 0, 3 and 5 Gy of radiation and further incubated for 29 h before γH2AX immunofluorescence staining. At least 200 nuclei were counted per treatment group. The data are presented as the mean ± SEM of 3 to 6 independent assays and p values were determined by an unpaired Student’s t test.
GT3 Decreases Chromosomal Aberrations in Irradiated Primary Human Endothelial Cells
Next, we asked whether GT3 pretreatment could decrease CAs in irradiated endothelial cells. HUVECs were irradiated with 5 Gy, and CAs were visualized with Giemsa staining and mFISH analysis with probes specific for human chromosomes 1, 2, and 3. CAs were scored as chromatid-type breaks, sister chromatid unions, chromatid-type exchanges, dicentric and ring chromosomes, double minutes, and chromosome-type breaks. The percentage of radiation-induced CAs was lower in HUVECs pretreated with GT3 (115.6 ± 13.4) than in the vehicle-treated group (176.3 ± 15.2), as detected by Giemsa staining (Table I). Likewise, the number of radiation-induced CAs in chromosomes 1, 2, and, 3 was lower in HUVECs pretreated with GT3 (90.5 ± 11.1) than in the vehicle-treated group (128.4 ± 10.5) (Table II). Thus, GT3 effectively decreased DSBs and CAs in HUVECs.
Table I.
Percentages of CAs in HUVECs. Aberration Percentage ± SE (Total Number of Aberration Observed) in Metaphase Spreads of HUVECs After Various Treatment As Detected By Conventional Giemsa Solid Staining. Standard Errors on the Aberration Percentage were Calculated By √a/A, As Described in Material and Method Section. The Abbreviations Used for CAs Are As Follows CTB, Chromatid-Type Break; SCU, Sister Chromatid Union; CTE, Chromatid-Type Exchange; DIC/R, Dicentric/Ring; DIMN, Double Minutes; CSB, Chromosome-Type Break
| Treatment | Normal metaphase | Aberrant metaphase | CTB | SCU | CTE | DIC/R | DIMN | CSB | Total aberration |
|---|---|---|---|---|---|---|---|---|---|
| Vehicle; 0 Gy | 99.4 ± 8.0 (155) | 0.6 ± 0.6 (1) | 0.6 ± 0.6 (1) | 0.0 ± 0.0 (0) | 0.0 ± 0.0 (0) | 0.0 ± 0.0 (0) | 0.0 ± 0.0 (0) | 0.0 ± 0.0 (0) | 0.6 ± 0.6 (1) |
| GT3; 0 Gy | 100.0 + 10.0 (100) | 0.0 + 0.0 (0) | 0.0 + 0.0 (0) | 0.0 + 0.0 (0) | 0.0 + 0.0 (0) | 0.0 + 0.0 (0) | 0.0 + 0.0 (0) | 0.0 + 0.0 (0) | 0.0 + 0.0 (0) |
| Vehicle; 5 Gy | 31.6 ± 6.4 (24) | 68.4 ± 9.5 (52) | 114.5 ± 12.3 (87) | 9.2 ± 3.5 (7) | 3.9 ± 2.3 (3) | 21.1 ± 5.3 (16) | 2.6 ± 1.9 (2) | 25.0 ± 5.7 (19) | 176.3 ± 15.2 (134) |
| GT3; 5 Gy | 48.4 ± 8.7 (31) | 51.6 ± 9.0 (33) | 85.9 ± 11.6 (55) | 6.3 ± 3.1 (4) | 0.0 ± 0.0 (0) | 12.5 ± 4.4 (8) | 0.0 ± 0.0 (0) | 10.9 ± 4.1 (7) | 115.6 ± 13.4 (74) |
Table II.
Percentages of CAs in HUVEC Chromosomes 1, 2, and 3. Aberration Percentage ± SE (Total Number of Aberration Observed) in the Metaphase Spreads of HUVECs After Various Treatment As Detected by mFISH Using Whole Chromosome DNA Probe for Human Chromosome 1, 2, and 3. Standard Errors on the Aberration Percentage Were Calculated By √a/A, As Described in Material And Method Section
| Treatment | Normal metaphase | Aberrant metaphase | Chr-1 | Chr-2 | Chr-3 | Total aberrations |
|---|---|---|---|---|---|---|
| Vehicle; 0Gy | 97.1 ± 7.6 (165) | 2.9 ± 1.3 (5) | 0.6 ± 0.6 (1) | 1.2 ± 0.8 (2) | 1.8 ± 1.0 (3) | 3.5 ± 1.4 (6) |
| GT3; 0Gy | 96.5 ± 7.5 (167) | 3.5 ± 1.4 (6) | 1.2 ± 0.8 (2) | 0.6 ± 0.6 (1) | 1.7 ± 1.0 (3) | 3.5 ± 1.4 (6) |
| Vehicle; 5Gy | 38.8 ± 5.8 (45) | 61.2 ± 7.3 (71) | 49.1 ± 6.5 (57) | 35.3 ± 5.5 (41) | 44.0 ± 6.2 (51) | 128.4 ± 10.5 (149) |
| GT3; 5Gy | 52.7 ± 8.4 (39) | 47.3 ± 8.0 (35) | 39.2 ± 7.3 (29) | 21.6 ± 5.4 (16) | 29.7 ± 6.3 (22) | 90.5 ± 11.1 (67) |
GT3 Decreases Chromosomal Aberrations in Irradiated Mice
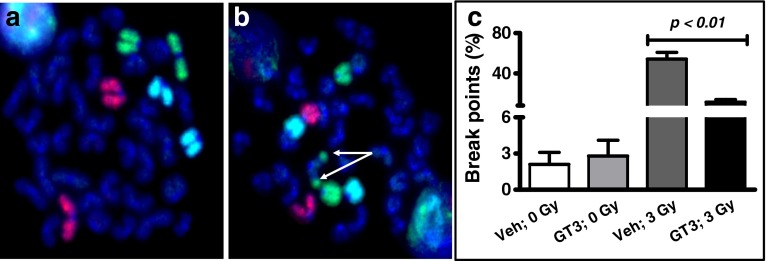
Considering that GT3 effectively decreased CAs in vitro, we predicted that GT3 would decrease CAs in mice after irradiation. Mice were administered GT3 (100 μl at dose of 200 mg/kg) subcutaneously 24 h before 3 Gy of total-body irradiation, and bone marrow mononuclear cells were collected 24 h or 30 days after irradiation. We used a relatively lower dose (3 Gy) for the in vivo study than for the in vitro chromosomal aberration assay (5 Gy) because lymphocytes are more radiosensitive than HUVECs. Giemsa staining detected CAs in mouse bone marrow cells 24 h after irradiation (Fig. 2b, arrows), compared to the non-irradiated control (Fig. 2a). However, GT3 suppressed CAs in the irradiated bone marrow cells both 24 h (p = 0.0001) and 30 days (p = 0.007) after irradiation (Fig. 2c). GT3 treatment also significantly (p < 0.01) decreased radiation-induced CAs involving mouse chromosomes 1, 2, and 3 24 h after irradiation (Fig. 3). Thus, GT3 effectively decreased CAs in vivo for up to 30 days after total-body irradiation.
Fig. 2.
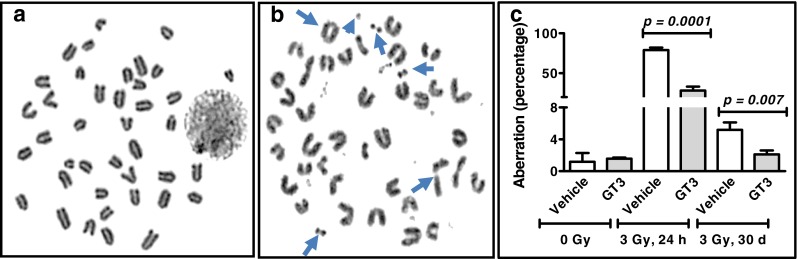
GT3 decreases radiation-induced CAs in mouse bone marrow cells. CAs were assayed by Giemsa solid staining in mouse bone marrow metaphase spreads from (a) non-irradiated (b) or irradiated mice (CAs indicated by arrows). (c) Mice (n = 3) were pretreated with vehicle or GT3 (200 mg/kg) 24 h before 3 Gy of total body irradiation. CAs were scored in 200–600 metaphase spreads 24 h and 30 days after irradiation. Standard deviation represents interindividual (n = 3) variability. An unpaired t test was performed to determine p values.
Fig. 3.
GT3 decreases radiation-induced CAs in mouse chromosomes 1 (red), 2 (green), and 3 (blue). CAs were assayed by mFISH in metaphase spreads from (a) non-irradiated or (b) irradiated mice (CAs indicated by arrows). (c) Mice (n = 3) were pretreated with vehicle or GT3 (200 mg/kg) 24 h before 3 Gy of total-body irradiation and pooled bone marrow samples were assayed by mFISH. CAs were scored in 100–300 metaphase spreads and the standard error was calculated by √a/A as described in materials and method section.
GT3 Increases RAD50 Expression
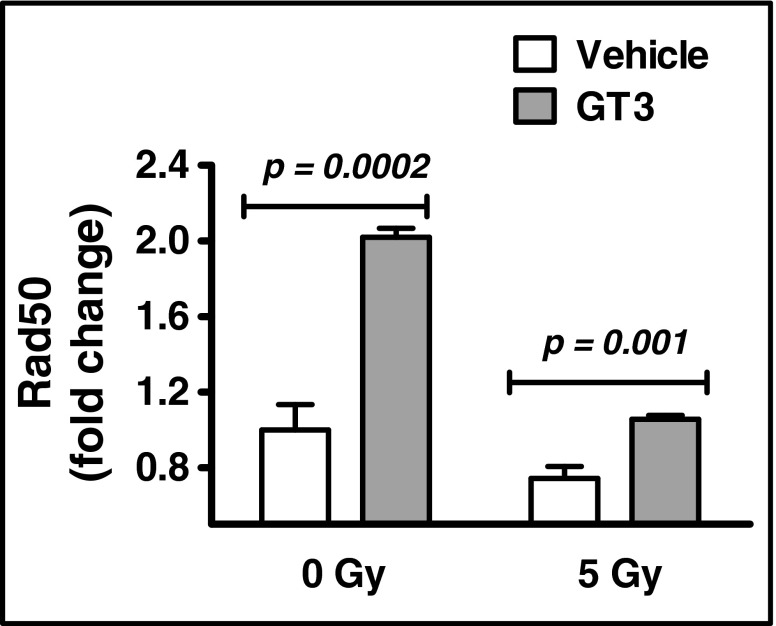
Because GT3 decreased the incidence of DSBs in vitro, we reasoned that GT3 may affect expression of the DSB-repair protein RAD50. RAD50 expression in GT3-treated HUVECs was roughly two-fold higher than in vehicle-treated cells (Fig. 4). Irradiation is known to decrease RAD50 expression, and this was, indeed, the case for both GT3- and vehicle-treated cells (Fig. 4); however, GT3 maintained RAD50 expression at a level similar to that observed in non-irradiated, vehicle-treated controls (Fig. 4). These findings suggest that GT3 restricts radiation-induced cytogenetic damage, possibly by modulating RAD50 expression.
Fig. 4.
GT3 modulates RAD50 expression. HUVECs were treated with vehicle or GT3 (5 μM) for 1 h before 0 Gy and 5 Gy irradiation, cells were further incubated for 23 h before harvest. RAD50 mRNA was measured relative to 18S rRNA with Taqman assay. The data are presented as the mean ± SD of three independent assays.
Discussion
Radiation generates ROS that often cause DSBs. Studies from various groups, including our own, suggest that GT3 detoxifies ROS, thereby expecting it to reduce DSB formation. Here, we demonstrate that GT3 suppresses DSB formation in irradiated HUVECs. Specifically, GT3 reduced the number of γ-H2AX foci, a strong indicator of DSBs, by roughly 29% in HUVECs. We suggest several mechanisms to explain these findings. GT3 may regulate mitochondrial activity, transcription factors, or endothelial cell surface receptors. For example, GT3 alleviates ROS by preventing mitochondrial dysfunction in primary rabbit renal tubular cells exposed to tert-Butyl hydroperoxide (25). With respect to transcription factors, GT3 decreases ROS in human oral keratinocytes exposed to chemotherapy by stabilizing Nrf2 activation. Nrf2 is a transcription factor that regulates cellular antioxidant pathways (26). Further, we recently demonstrated that GT3 increases the expression and activity of the endothelial surface receptor thrombomodulin (TM) (27). The lectin-like domain of TM activates Nrf2-dependent antioxidant pathways, substantially decreasing serum and renal ROS levels in a mouse model of type-2 diabetic nephropathy (28). Moreover, TM forms a complex with the coagulation factor thrombin to generate activated protein C (APC) in the blood (29). Among its many properties, APC prevents glucose-induced oxidative stress in endothelial cells (30). Thus, our data indicate that GT3 protects HUVECs from ROS-mediated DSBs, and it may do so in a number of ways.
Improperly repaired DSBs induce CAs that are visible in metaphase chromosomes. We found that GT3 decreases CAs in both irradiated HUVECs and primary mouse bone marrow cells. It is not entirely clear how GT3 protects cells from induction of CAs after radiation exposure, but we offer one possible explanation. GT3 induces the production of granulocyte colony stimulating factor (G-CSF) in mice (31) and evidence suggests that G-CSF reduces chromosomal translocations in patients with cancer (32). Specifically, G-CSF prevented translocations between the long arms of chromosomes 8 and 21 in patients with acute myeloid leukemia (32). The role of G-CSF may not be so straightforward, however. G-CSF was shown to cause aneuploidy in healthy individuals (33), although a separate study found no such evidence (34).
Regardless of the specific mechanisms involved, data suggest that various vitamin E isoforms, including GT3, protect against cytogenetic damage in humans. For example, α-tocopherol decreases the frequency of chromatid-type breaks and sister chromatid exchanges in lymphocytes from people with Fanconi anemia (35,36). Similarly, α-tocopherol decreases the accumulation of CAs during G2 phase in Down’s syndrome patients (37). Finally, a commercially available, tocotrienol-rich vitamin E derivative decreases sister chromatid exchanges in elderly human subjects (38). Thus, multiple vitamin E isoforms could be used to prevent radiation-induced cytogenetic damage in endothelial cell.
Finally, intracellular DNA-repair enzymes are critically important for repairing cytogenetic damage. Radiation damage activates the cellular DNA-repair machinery that identifies DSBs and recruits DNA-repair proteins. Previously, we found that GT3 modulates as many as 27 gene clusters in HUVECs involved in DNA damage (18). Here, we show that GT3 increases RAD50 expression and attenuates the radiation-induced suppression of RAD50. These findings corroborate a previous study by Li et al. that demonstrated that Rad50 expression was suppressed for up to 48 h in mouse white blood cells after 8-Gy irradiation (7). Experimental evidence suggests that not only is RAD50 required for efficient DNA repair after irradiation (39) but also that its deficiency makes early embryonic cells hypersensitive to IR (40).
Conclusion
The present studies demonstrate that GT3 pretreatment decreases radiation-induced DSB and CA formation in HUVECs. GT3 also reduces acute and delayed CAs in irradiated mice and increases RAD50 expression in HUVECs. We conclude that GT3 suppresses radiation-induced cytogenetic damage and possibly GT3-mediated alteration in Rad50 expression plays some role in limiting cytogenetic damage after radiation; however, further investigation is required to determine whether GT3 regulates additional DNA-repair proteins. GT3 may, therefore, be a useful therapeutic to protect against radiation-induced genetic diseases.
Acknowledgments and Disclosures
This study was supported by the National Institutes of Health (P20 GM109005 (MH-J), R37 CA71382 (MH-J), and R01 CA148679 [MB]), the US Veterans Administration (MH-J), the Arkansas Space Grant Consortium through the National Aeronautics and Space Administration (NNX15AK32A [RP]), and the National Space Biomedical Research Institute (RE03701) through NCC 9-58 (MB). This publication was made possible, in part, by the Arkansas INBRE program, supported by funding from NIH/NIGMS (P20 GM103429, formerly P20RR016460 [AB]) and the National Center for Research Resources (NCRR - P20RR016460, NIGMS - P20 GM103429) from the National Institutes of Health, (AB). These agencies had no role in the study design, data analysis, or the preparation of this manuscript.
This manuscript was edited by Kerry L. Evans from the Office of Grants and Scientific Publications at the University of Arkansas for Medical Sciences. Assistance with metaphase chromosome preparation by Joeline Brown and Hemant Kemkar is gratefully acknowledged.
Abbreviations
- APC
Activated protein C
- CAs
Chromosomal aberrations
- DMSO
Dimethyl sulfoxide
- DSBs
DNA double strand breaks
- EPCR
Endothelial protein C receptor
- G-CSF
Granulocyte colony stimulating factor
- GT3
γ-tocotrienol
- Gy
Gray unit
- HUVECs
Human umbilical vein endothelial cells
- IR
Ionizing radiation
- mFISH
Multiple fluorescence in situ hybridization
- qRT-PCR
Quantitative reverse transcriptase polymerase chain reaction
- Rad50
RAD50 homolog, double strand break repair protein
- ROS
Reactive oxygen species
- TM
Thrombomodulin
References
- 1.Ward JF. DNA damage produced by ionizing radiation in mammalian cells: identities, mechanisms of formation, and reparability. Prog Nucleic Acid Res Mol Biol. 1988;35:95–125. doi: 10.1016/S0079-6603(08)60611-X. [DOI] [PubMed] [Google Scholar]
- 2.Pfeiffer P, Goedecke W, Obe G. Mechanisms of DNA double-strand break repair and their potential to induce chromosomal aberrations. Mutagenesis. 2000;15:289–302. doi: 10.1093/mutage/15.4.289. [DOI] [PubMed] [Google Scholar]
- 3.Solomon E, Borrow J, Goddard AD. Chromosome aberrations and cancer. Science. 1991;254:1153–1160. doi: 10.1126/science.1957167. [DOI] [PubMed] [Google Scholar]
- 4.MacIntyre DJ, Blackwood DH, Porteous DJ, Pickard BS, Muir WJ. Chromosomal abnormalities and mental illness. Mol Psychiatry. 2003;8:275–287. doi: 10.1038/sj.mp.4001232. [DOI] [PubMed] [Google Scholar]
- 5.Trevisan P, Zen TD, Rosa RF, Silva JN, Koshiyama DB, Paskulin GA, et al. Chromosomal abnormalities in patients with congenital heart disease. Arq Bras Cardiol. 2013;101:495–501. doi: 10.5935/abc.20130204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pravit J. Bronchoscopic findings in Down syndrome children with respiratory problems. J Med Assoc Thai. 2014;97(Suppl 6):S159–S163. [PubMed] [Google Scholar]
- 7.Li MJ, Wang WW, Chen SW, Shen Q, Min R. Radiation dose effect of DNA repair-related gene expression in mouse white blood cells. Med Sci Monit. 2011;17:BR290–BR297. doi: 10.12659/MSM.881976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stracker TH, Petrini JH. The MRE11 complex: starting from the ends. Nat Rev Mol Cell Biol. 2011;12:90–103. doi: 10.1038/nrm3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Negrini S, Gorgoulis VG, Halazonetis TD. Genomic instability--an evolving hallmark of cancer. Nat Rev Mol Cell Biol. 2010;11:220–228. doi: 10.1038/nrm2858. [DOI] [PubMed] [Google Scholar]
- 10.Soloviev AI, Tishkin SM, Parshikov AV, Ivanova IV, Goncharov EV, Gurney AM. Mechanisms of endothelial dysfunction after ionized radiation: selective impairment of the nitric oxide component of endothelium-dependent vasodilation. Br J Pharmacol. 2003;138:837–844. doi: 10.1038/sj.bjp.0705079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akino T, Hida K, Hida Y, Tsuchiya K, Freedman D, Muraki C, et al. Cytogenetic abnormalities of tumor-associated endothelial cells in human malignant tumors. Am J Pathol. 2009;175:2657–2667. doi: 10.2353/ajpath.2009.090202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franses JW, Drosu NC, Gibson WJ, Chitalia VC, Edelman ER. Dysfunctional endothelial cells directly stimulate cancer inflammation and metastasis. Int J Cancer. 2013;133:1334–1344. doi: 10.1002/ijc.28146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brand M, Sommer M, Ellmann S, Wuest W, May MS, Eller A, et al. Influence of Different Antioxidants on X-Ray Induced DNA Double-Strand Breaks (DSBs) Using gamma-H2AX Immunofluorescence Microscopy in a Preliminary Study. PLoS One. 2015;10 doi: 10.1371/journal.pone.0127142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dusinska M, Kazimirova A, Barancokova M, Beno M, Smolkova B, Horska A, et al. Nutritional supplementation with antioxidants decreases chromosomal damage in humans. Mutagenesis. 2003;18:371–376. doi: 10.1093/mutage/geg002. [DOI] [PubMed] [Google Scholar]
- 15.Xu Z, Hua N, Godber JS. Antioxidant activity of tocopherols, tocotrienols, and gamma-oryzanol components from rice bran against cholesterol oxidation accelerated by 2,2′-azobis(2-methylpropionamidine) dihydrochloride. J Agric Food Chem. 2001;49:2077–2081. doi: 10.1021/jf0012852. [DOI] [PubMed] [Google Scholar]
- 16.Ghosh SP, Kulkarni S, Hieber K, Toles R, Romanyukha L, Kao TC, et al. Gamma-tocotrienol, a tocol antioxidant as a potent radioprotector. Int J Radiat Biol. 2009;85:598–606. doi: 10.1080/09553000902985128. [DOI] [PubMed] [Google Scholar]
- 17.Berbee M, Fu Q, Boerma M, Pathak R, Zhou D, Kumar KS, et al. Reduction of radiation-induced vascular nitrosative stress by the vitamin E analog gamma-tocotrienol: evidence of a role for tetrahydrobiopterin. Int J Radiat Oncol Biol Phys. 2011;79:884–891. doi: 10.1016/j.ijrobp.2010.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berbee M, Fu Q, Boerma M, Sree KK, Loose DS, Hauer-Jensen M. Mechanisms underlying the radioprotective properties of gamma-tocotrienol: comparative gene expression profiling in tocol-treated endothelial cells. Genes Nutr. 2012;7:75–81. doi: 10.1007/s12263-011-0228-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kulkarni S, Singh PK, Ghosh SP, Posarac A, Singh VK. Granulocyte colony-stimulating factor antibody abrogates radioprotective efficacy of gamma-tocotrienol, a promising radiation countermeasure. Cytokine. 2013;62:278–285. doi: 10.1016/j.cyto.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 20.Parker RA, Pearce BC, Clark RW, Gordon DA, Wright JJ. Tocotrienols regulate cholesterol production in mammalian cells by post-transcriptional suppression of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. J Biol Chem. 1993;268:11230–11238. [PubMed] [Google Scholar]
- 21.Singh VK, Wise SY, Fatanmi OO, Scott J, Romaine PL, Newman VL, et al. Progenitors mobilized by gamma-tocotrienol as an effective radiation countermeasure. PLoS One. 2014;9 doi: 10.1371/journal.pone.0114078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee R, Nasonova E, Ritter S. Chromosome aberration yields and apoptosis in human lymphocytes irradiated with Fe-ions of differing LET. Adv Space Res. 2005;35:268–275. doi: 10.1016/j.asr.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 23.Pathak R, Sarma A, Sengupta B, Dey SK, Khuda-Bukhsh AR. Response to high LET radiation 12C (LET, 295 keV/microm) in M5 cells, a radio resistant cell strain derived from Chinese hamster V79 cells. Int J Radiat Biol. 2007;83:53–63. doi: 10.1080/09553000601085964. [DOI] [PubMed] [Google Scholar]
- 24.Siveen KS, Ahn KS, Ong TH, Shanmugam MK, Li F, Yap WN, et al. Y-tocotrienol inhibits angiogenesis-dependent growth of human hepatocellular carcinoma through abrogation of AKT/mTOR pathway in an orthotopic mouse model. Oncotarget. 2014;5:1897–1911. doi: 10.18632/oncotarget.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nowak G, Bakajsova D, Hayes C, Hauer-Jensen M, Compadre CM. gamma-Tocotrienol protects against mitochondrial dysfunction and renal cell death. J Pharmacol Exp Ther. 2012;340:330–338. doi: 10.1124/jpet.111.186882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takano H, Momota Y, Kani K, Aota K, Yamamura Y, Yamanoi T, et al. gamma-Tocotrienol prevents 5-FU-induced reactive oxygen species production in human oral keratinocytes through the stabilization of 5-FU-induced activation of Nrf2. Int J Oncol. 2015;46:1453–1460. doi: 10.3892/ijo.2015.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pathak R, Shao L, Ghosh SP, Zhou D, Boerma M, Weiler H, et al. Thrombomodulin contributes to gamma tocotrienol-mediated lethality protection and hematopoietic cell recovery in irradiated mice. PLoS One. 2015;10 doi: 10.1371/journal.pone.0122511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang SM, Ka SM, Wu HL, Yeh YC, Kuo CH, Hua KF, et al. Thrombomodulin domain 1 ameliorates diabetic nephropathy in mice via anti-NF-kappaB/NLRP3 inflammasome-mediated inflammation, enhancement of NRF2 antioxidant activity and inhibition of apoptosis. Diabetologia. 2014;57:424–434. doi: 10.1007/s00125-013-3115-6. [DOI] [PubMed] [Google Scholar]
- 29.Esmon CT. The protein C pathway. Chest. 2003;124:26S–32S. doi: 10.1378/chest.124.3_suppl.26S. [DOI] [PubMed] [Google Scholar]
- 30.Isermann B, Vinnikov IA, Madhusudhan T, Herzog S, Kashif M, Blautzik J, et al. Activated protein C protects against diabetic nephropathy by inhibiting endothelial and podocyte apoptosis. Nat Med. 2007;13:1349–1358. doi: 10.1038/nm1667. [DOI] [PubMed] [Google Scholar]
- 31.Kulkarni SS, Cary LH, Gambles K, Hauer-Jensen M, Kumar KS, Ghosh SP. Gamma-tocotrienol, a radiation prophylaxis agent, induces high levels of granulocyte colony-stimulating factor. Int Immunopharmacol. 2012;14:495–503. doi: 10.1016/j.intimp.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 32.Ferrara F, Schiavone EM, Palmieri S, Mele G, Pocali B, Scalia G, et al. Complete remission induced by G-CSF in a patient with acute myeloid leukemia with t(8;21)(q22;q22) Hematol J. 2003;4:218–221. doi: 10.1038/sj.thj.6200225. [DOI] [PubMed] [Google Scholar]
- 33.Nagler A, Korenstein-Ilan A, Amiel A, Avivi L. Granulocyte colony-stimulating factor generates epigenetic and genetic alterations in lymphocytes of normal volunteer donors of stem cells. Exp Hematol. 2004;32:122–130. doi: 10.1016/j.exphem.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 34.Hirsch B, Oseth L, Cain M, Trader E, Pulkrabek S, Lindgren B, et al. Effects of granulocyte-colony stimulating factor on chromosome aneuploidy and replication asynchrony in healthy peripheral blood stem cell donors. Blood. 2011;118:2602–2608. doi: 10.1182/blood-2011-04-348508. [DOI] [PubMed] [Google Scholar]
- 35.Pincheira J, Bravo M, Santos MJ, de la Torre C, Lopez-Saez JF. Fanconi anemia lymphocytes: effect of DL-alpha-tocopherol (Vitamin E) on chromatid breaks and on G2 repair efficiency. Mutat Res. 2001;461:265–271. doi: 10.1016/S0921-8777(00)00058-6. [DOI] [PubMed] [Google Scholar]
- 36.Khabour OF, Soudah OA, Aaysh MH. Genotoxicity assessment in iron deficiency anemia patients using sister chromatid exchanges and chromosomal aberrations assays. Mutat Res. 2013;750:72–76. doi: 10.1016/j.mrgentox.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 37.Pincheira J, Navarrete MH, de la Torre C, Tapia G, Santos MJ. Effect of vitamin E on chromosomal aberrations in lymphocytes from patients with Down’s syndrome. Clin Genet. 1999;55:192–197. doi: 10.1034/j.1399-0004.1999.550307.x. [DOI] [PubMed] [Google Scholar]
- 38.Chin SF, Hamid NA, Latiff AA, Zakaria Z, Mazlan M, Yusof YA, et al. Reduction of DNA damage in older healthy adults by Tri E Tocotrienol supplementation. Nutrition. 2008;24:1–10. doi: 10.1016/j.nut.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 39.Westmoreland J, Ma W, Yan Y, Van HK, Malkova A, Resnick MA. RAD50 is required for efficient initiation of resection and recombinational repair at random, gamma-induced double-strand break ends. PLoS Genet. 2009;5 doi: 10.1371/journal.pgen.1000656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luo G, Yao MS, Bender CF, Mills M, Bladl AR, Bradley A, et al. Disruption of mRad50 causes embryonic stem cell lethality, abnormal embryonic development, and sensitivity to ionizing radiation. Proc Natl Acad Sci U S A. 1999;96:7376–7381. doi: 10.1073/pnas.96.13.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]