Abstract
GnRH neurons are regulated by hypothalamic kisspeptin neurons. Recently, galanin was identified in a subpopulation of kisspeptin neurons. Although the literature thoroughly describes kisspeptin activation of GnRH neurons, little is known about the effects of galanin on GnRH neurons. This study investigated whether galanin could alter kisspeptin signaling to GnRH neurons. GnRH cells maintained in explants, known to display spontaneous calcium oscillations, and a long-lasting calcium response to kisspeptin-10 (kp-10), were used. First, transcripts for galanin receptors (GalRs) were examined. Only GalR1 was found in GnRH neurons. A series of experiments was then performed to determine the action of galanin on kp-10 activated GnRH neurons. Applied after kp-10 activation, galanin 1–16 (Gal1–16) rapidly suppressed kp-10 activation. Applied with kp-10, Gal1–16 prevented kp-10 activation until its removal. To determine the mechanism by which galanin inhibited kp-10 activation of GnRH neurons, Gal1–16 and galanin were applied to spontaneously active GnRH neurons. Both inhibited GnRH neuronal activity, independent of GnRH neuronal inputs. This inhibition was mimicked by a GalR1 agonist but not by GalR2 or GalR2/3 agonists. Although Gal1–16 inhibition relied on Gi/o signaling, it was independent of cAMP levels but sensitive to blockers of G protein-coupled inwardly rectifying potassium channels. A newly developed bioassay for GnRH detection showed Gal1–16 decreased the kp-10-evoked GnRH secretion below detection threshold. Together, this study shows that galanin is a potent regulator of GnRH neurons, possibly acting as a physiological break to kisspeptin excitation.
Reproductive success relies upon the integration of physiological and environmental cues. GnRH neurons are the final output in the central nervous system, relaying signals to the pituitary that then act upon the ovaries. Estrogen (E2) feedback from the ovaries to the central nervous system is one of the most important signals coming from the periphery to keep the hypothalamic-pituitary-gonadal axis tuned. E2 feedback is critically dependent on E2 receptor (ER)α; however, GnRH neurons lack ERα and receive E2 signals from upstream E2-sensitive cell populations.
Galanin is a brain-gut neuropeptide widely distributed in the brain (rat [1], human [2], and mouse [3]). Galanin gene expression (4) and immunoreactivity (5) are regulated by E2. Many neuronal cell types producing classical neurotransmitters or neuropeptides coexpress galanin (6). GnRH neuronal population is one of them (7, 8). GnRH neurons also receive inputs from fibers immunoreactive for galanin (rat [7], human [9], mouse [10]). The number of galanin fibers onto GnRH neurons increases at puberty (11), with E2 treatment in ovariectomized female rats (12) or with preoptic area grafts restoring cycles in hypogonadal female mice (13). Supporting the putative integration of galanin inputs, GnRH neurons express the galanin receptor (GalR)1 (14–16); however, how GnRH neurons process galanin signals remains unclear (16). Recently, galanin has been identified in a subpopulation of kisspeptin neurons, a critical ERα expressing input to GnRH neurons (10, 17). Whether galanin impacts the kisspeptin-evoked activation of GnRH neurons is unknown.
This report shows that primary GnRH neurons maintained in explants expressed GalR1, not GalR2 or GalR3, and that galanin 1–16 (Gal1–16) rapidly suppresses the kisspeptin-10 (kp-10)-induced calcium responses of GnRH neurons and prevents calcium responses during coapplication. Both the full-length galanin peptide and its truncated form, Gal1–16, inhibit spontaneous intracellular calcium ([Ca2+]i) oscillations. The inhibition was independent of excitatory inputs and could be mimicked with a GalR1-specific agonist but not GalR2- or GalR2/3-specific agonists. Although the downstream signaling pathway relies on the activation of Gi/o protein, intracellular levels of cAMP do not mediate the inhibition. Galanin inhibits GnRH neurons by activating G protein-coupled inwardly rectifying potassium (GIRK) channels. Using gonadotrophs as biosensors for GnRH showed that Gal1–16 also decreased kp-10-induced GnRH secretion. These data provide evidence for a physiological break, galanin, to the long-term excitation mediated by kisspeptin.
Materials and Methods
Nasal explants
Explants were cultured as previously described (18, 19). Briefly, embryonic day 11.5 embryos (undetermined sex) were obtained from timed pregnant NIH Swiss mice. Nasal pits were dissected under aseptic conditions in Gey's balanced salt solution (Life Technologies, Inc) supplemented with glucose (Sigma Chemical Co). One embryo generates one single explant. Explants were adhered onto coverslips by a plasma (Cocalico Biologicals)/thrombin (Sigma) clot and maintained at 37°C in a defined serum-free medium (SFM) in a humidified atmosphere with 5% CO2. On culture day 3, SFM was replaced by fresh SFM containing fluorodeoxyuridine (80μM; Sigma) for 3 days to inhibit proliferation of dividing olfactory neurons and nonneuronal explant tissue. On culture day 6, and every 2 days afterward, the medium was changed with fresh SFM. Explants were used between 6–11 days in vitro (Figure 1A) and at 14–15 days in vitro. All procedures were approved by National Institute of Neurological Disorders and Stroke, Animal Care and Use Committee and performed in accordance with National Institutes of Health guidelines.
Figure 1.

GnRH neurons maintained in nasal explants express GalR1. A, Schematic representation (left side) and low magnification of a nasal explant (right side) obtained from E11.5 mouse and maintained for 9 days in vitro and then immunolabeled for GnRH (brown). NPE, nasal pit epithelium; NMC, nasal midline cartilage. GnRH neurons (dots in schematic representation and arrows in image) migrate from NPE and follow olfactory axons to the NMC and off the explant into the periphery; scale bar, 200 μm. B, Explants and single cells positive for GnRH transcripts (bottom panel) were positive for GalR1 (top panel). Although explants were positive for GalR2 and GalR3, single cells were not (middle panels). C, High magnification of GnRH neurons recorded with calcium imaging. Calcium imaging recordings were performed on GnRH cells maintained for 8–11 days in vitro. The phenotype of cells, identified by their bipolar morphology (left panel) and loaded with a calcium-sensitive dye Calcium Green 1-AM (middle panel), was confirmed by immunocytochemistry after recording (right panel); scale bar, 50 μm.
Dissociated pituitary cells
Pituitaries (3–5/dissociation) from adult female NIH Swiss mice were collected in ice-cold HEPES-buffered Medium 199 (Invitrogen), supplemented with 10% heat-deactivated normal horse serum (Gibco). Ca2+/Mg2+-free Earle's Balanced Salt Solution (EBSS) with 0.3% BSA (Gemini), streptomycin/penicillin mixture, glutamine, and vitamin mix (Invitrogen) was used throughout the procedure. After several washes in 1.26mM Ca supplemented EBSS, posterior lobes were removed and the anterior lobes chopped into 4–5 fragments and incubated on a shaker in 10× trypsin-EDTA solution (Sigma) supplemented with 0.3% BSA and diluted at a final concentration of 4-mg/mL trypsin with 1.26mM Ca2+ EBSS, at 37°C for 18 minutes. After several washes with deoxyribonuclease (Sigma) containing 1.26mM Ca2+/Mg2+-free EBSS then trypsin inhibitor (Gibco), pituitary fragments were transferred into Ca2+/Mg2+-free EBSS containing 2mM, then 1mM EDTA. The fragments were then transferred in Ca2+/Mg2+-free EBSS containing deoxyribonuclease for mechanical tissue disruption (on ice). After a brief centrifugation, the dissociated pituitary cells were resuspended in bicarbonate-buffered Medium 199 (Invitrogen), supplemented with 10% heat-deactivated normal horse serum, adhered to a poly-L-lysine coverslip (Corning), and allowed to recover for 24–48 hours before use.
PCR on single cells and total explants
Poly(A) amplified cDNA libraries were created from individual GnRH cells from explants (20). The phenotype and the quality of each single cell cDNA pool was confirmed by PCR for GnRH (Figure 1B), ribosomal protein L19, and β-tubulin (21). The same method was used to generate cDNA libraries from whole explants (20). Primers were then designed in the 3′-untranslated region of the genes galanin, GalR1, GalR2, and GalR3, and potassium inwardly-rectifying channel subfamily J member 3 (Kcnj3) (aka inward-rectifier potassium ion channel Kir 3.1) within the 350 base pairs before the poly adenylation site. All designed primers were screened using BLAST to ensure specificity. For each reaction, 30.5-μL H2O, 5-μL 10× PCR buffer, 4-μL 25mM MgCl2, 5-μL deoxynucleotide mix (Life Technologies; 25 μL of each 100mM deoxynucleotide, 900-μL H2O), 2-μL 6.25μM forward primer, 2-μL 6.25μM reverse primer, and 0.5-μL AmpliTaq Gold (Life Technologies) were added to 1-μL template cDNA. PCR was performed as following: initial 10-minute denaturation (94°C), 40 cycles with denaturation 30 seconds (94°C), annealing 30 seconds (55°C) and extension 2 minutes (72°C), followed by a 10-minute postelongation at 72°C. For galanin on single cells, a nested PCR was used, ie, 1 μL of the product was run a second time with a second set of primers. Amplified products were run on a 1.5% agarose gel. Specific bands of the predicted size were observed in control total brain, whereas no bands were seen in water (negative control). The sequences of the primers used are in Table 1.
Table 1.
Primer Sequences
| Gene (NCBI Reference Sequence) | Primers Sequences (5′-3′) | Annealing Temperature | Product Size |
|---|---|---|---|
| Galanin (NM_010253.3) | |||
| PCR number 1 | GCACCTTAAAGAGGCCGGG | 55°C | 185 bp |
| GCAGAGAACAGACGATTGGC | |||
| Nested PCR number 2 | GCACCTTAAAGAGGCCGGG | 55°C | 171 bp |
| ATTGGCTTGAGGAGTTGGCA | |||
| GalR1 (NM_008082.2) | GTTAGAGAGAGGAGGGCGGA | 55°C | 319 bp |
| ACTGGCCTGAAAATGTGGAT | |||
| GalR2 (NM_010254.4) | GGGGGTCATGCTTGGATCTT | 55°C | 162 bp |
| TGATGGCTGGCTTTGTTCCT | |||
| GalR3 (NM_015738.2) | CCTCGCGGTTGGAGTATGGA | 55°C | 153 bp |
| TTGGTGACATTCTACTGCCCA | |||
| Kcnj3 (Kir3.1, GIRK1) (NM_008426.2) | AGGAGTGAAAAACCTGACGAGA | 55°C | 256 bp |
| ACCATATATCATCCAAACCAGGACA | |||
| GnRH (NM_008145.2) | CTGATGGCCGGCATTCTACTGC | 65°C | 221 bp |
| CCAGAGCTCCTCGCAGATCCC |
Calcium imaging
Experiments were performed as previously described (22). Briefly, Calcium Green-1 AM (Life Technologies) was dissolved at 2.7mM in 80% dimethyl sulfoxide and 20% pluronic F-127 (Life Technologies), diluted at 13.5μM in SFM and aliquoted. Explants were incubated in warm loading solution for 20 minutes at 37°C in a 5% CO2 humidified incubator. After washes in fresh SFM, explants were mounted in a perfusion chamber (Warner Instruments) and continuously perfused at a rate of 300 μL/min. Calcium Green-1 was visualized using an inverted Nikon microscope, through a ×20 fluorescence objective and a charge-coupled device camera (QImaging) connected to a computer. Time-lapse recording was piloted by iVision imaging software (Scanalytics, Inc) and pictures acquired every 2 seconds. Excitation wavelengths were provided with a medium-width excitation bandpass filter at 465–495 nm, and emission was monitored through a 40-nm bandpass centered on 535 nm. Calcium imaging recordings were divided into periods. For single drug paradigms, the treatment period was preceded by a control period in SFM and followed by a washout period. For multiple drug paradigms, when possible, drugs were sequentially added after the SFM control period. When a drug required a long pretreatment (ie, Cs), recordings started during the 10 last minutes of the pretreatment to obtain a pretreatment baseline. All recordings were terminated with a 40mM KCl stimulation to ensure the viability of the cells. The changes of fluorescence over time were measured in single GnRH neurons a posteriori with iVision and analyzed with MATLAB (Mathworks) as previously described (22). The phenotype of the cells defined as regions of interest was confirmed using a chromogenic immunocytochemistry against GnRH (Figure 1C).
For dissociated pituitary cells, the same loading method and recording set up described above were used. Concurrent with Calcium Green-1 loading of pituitary cells, 8–10 explants were randomly pooled into 2 groups of 4–5 and incubated with kp-10 (10nM) alone or kp-10+Gal1–16 (10nM) for 1 hour (1 mL per explant). Bacitracin (20μM) was added to the media to reduce the degradation of GnRH. After 1 hour, the media were collected and immediately used. Pituitary cells were continuously perfused with SFM containing kp-10 and Gal1–16. Bacitracin was also added to this SFM to maintain the composition of the solutions throughout the paradigm. After a 4-minute control period, the cells were exposed for 2 minutes to media conditioned with explants+kp-10+Gal1–16. After a 9-minute washout period, the cells were exposed for 2 minutes to media conditioned with explants+kp-10. After a 9-minute washout period, the cells were exposed for 2 minutes to media containing kp-10+Gal1–16+D-Trp6-GnRH (10nM) to identify functional gonadotrophs (schematic in figure 8 below). In some experiments, the 2 conditioned media were applied in reverse order (see figure 8 below). To identify cells responding to D-Trp6-GnRH ie, gonadotrophs (see figure 8 below), image arithmetic was performed a posteriori in iVision. The sum of the 10 last frames before D-Trp6-GnRH was generated. To account for the delay in perfusion, the sum of 10 frames was generated 30 seconds after the beginning of the application of D-Trp6-GnRH. The first image was subtracted from the second to highlight responding cells. The changes of fluorescence over time were measured in the responding cells in iVision and analyzed with MATLAB. The area under the curve (AUC) was calculated for the 2 minutes before the exposure to treatment, control period (SFM+kp-10+Gal1–16), and for the 2 minutes of the exposure to treatment (conditioned SFM from explants treated with kp-10+Gal1–16 or conditioned SFM from explants treated with kp-10 alone). The AUC was also calculated during the exposure to SFM+kp-10+Gal1–16+D-Trp6-GnRH. The 2-minute control period before each treatment was used to adjust the baseline of the subsequent treatment period.
Chromogenic immunocytochemistry for GnRH
Explants were fixed for 30 minutes with PBS containing 4% formaldehyde at room temperature. After a few washes in PBS, explants were incubated in a blocking solution (10% normal horse serum and 0.3% Triton X-100) for 1 hour, washed several times in PBS, and incubated at 4°C overnight in the primary antibody (1:3000; SW-1). The next day, explants were washed in PBS, incubated for 1 hour with biotinylated secondary rabbit antibodies (1:500 in PBS/0.3% Triton X-100; Vector Laboratories, Inc), washed in PBS, and processed for avidin-biotin-horseradish peroxidase/3′3-diaminobenzidine cytochemistry (Figure 1C, right panel).
Fluorescent immunocytochemistry for galanin and GnRH
Explants were fixed for 30 minutes with PBS containing 4% formaldehyde at room temperature. After a few washes in PBS, explants were incubated in a blocking solution (10% normal horse serum and 0.3% Triton X-100) for 1 hour, washed several times in PBS. After 2 15-minute blocks with avidin and biotin, respectively (Vector Laboratories, Inc), explants were incubated at 4°C for 2 nights in the first primary antibody (galanin, 1:1000; Hyde). The next day, explants were washed in PBS, incubated for 1 hour with biotinylated secondary rabbit antibodies (1:500 in PBS/0.3% Triton X-100; Vector Laboratories, Inc), washed in PBS, and incubated for 1 hour with avidin conjugated with Alexa Fluor 555 (1:2000; Life Technologies). After several washes in PBS, explants were rapidly fixed with PBS containing 4% formaldehyde, washed, and incubated at 4°C for 2 nights in the second primary antibody (GnRH, F1D3C5, 1:4000; generous gift from Dr A. Karande, Department of Biochemistry, Indian Institute of Science, Bangalore, India). The next day, explants were washed in PBS, incubated for 1 hour with secondary mouse antibodies conjugated with Alexa Fluor 488 (1:500; Life Technologies). After several washes in PBS and water, explants were coverslipped with an antifade mounting solution (Electron Microscopy Sciences).
Drugs
Gal1–16 (nonspecific GalR agonist) was purchased from RBI. Galanin (rat, mouse) (nonspecific GalR agonist) and Gal2–11 (GalR2/3-specific GalR agonist) were purchased from American Peptide Co. M15 (nonspecific GalR antagonist) was purchased from Bachem. M40 (nonspecific GalR antagonist), M617 (GalR1-specific GalR agonist), M1145 (GalR2-specific GalR agonist), (−)-bicuculline (BIC) chloride (an A-type γ-aminobutyric acid [GABA] receptor antagonist), D-(−)-2-amino-5-phosphonopentanoic acid (AP5) (a N-methyl-D-aspartate receptor antagonist), 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) (an α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid/kainate receptor antagonist), proglumide (PRG) (nonspecific cholecystokinin (CCK) receptor antagonist), kp-10 (G protein-coupled receptor GPR54 agonist) were purchased from Tocris. Forskolin (FSK), pertussis toxin (PTX), 3-isobutyl-1-methylxanthine (IBMX), Cs chloride, Ba chloride, and D-Trp6-GnRH were purchased from Sigma-Aldrich.
Statistical analysis
Comparisons of the frequencies of calcium oscillations between 2 recording periods were done using paired Student t tests. Comparisons of the magnitudes of inhibition between 2 groups of cells were done with unpaired t tests. Significant differences were defined by a P < .05. χ2 tests were used to compare the percentage of cells responding to kp-10 between different experiments. In results section and figures, frequencies of calcium oscillations are expressed as mean ± SEM, n and N represent the number of cells and explants recorded, respectively.
For gonadotrophs, comparisons of AUC (expressed in arbitrary units [a.u.]) were done with one-way ANOVA followed by a Sidak's multiple comparisons test. In results section, n and N represent the number of gonadotrophs and the number of pituitary cultures recorded, respectively.
Results
Transcripts for GalRs are detected in GnRH neurons
Transcripts for GalR1, GalR2, and GalR3 were detected in all cDNAs tested that were generated from total explants (n = 7–8 explants tested/transcripts). Single cell analysis was performed on cDNAs generated from single GnRH neurons maintained in explants for 7 days. Transcripts for GalR1 were detected in 5/5 GnRH neurons. Neither transcripts for GalR2 or GalR3 were detected in GnRH neurons (n = 5 each) (Figure 1B).
Galanin rapidly suppresses kp-10-evoked activation of GnRH neurons
Literature reports indicate potent electrical responses to kp-10 in GnRH neurons from adult mouse brain slices (23), adult mouse in vivo preparation (24), and from organotypic nasal explants (25). Kisspeptin neurons have been shown to coexpress galanin in mice (10, 17), and GnRH neurons have been shown to respond to galanin (16). The interaction between kp-10 and galanin was assessed in GnRH neurons maintained in explants. As previously described (25), a 3-minute exposure to kp-10 (10nM) evoked a long-lasting rise in [Ca2+]i levels in most GnRH neurons; approximately 73% (35/48; kp-10 only group) (Figure 2A) and approximately 74% (31/42; kp-10/Gal1–16 group; P > .05, χ2 test) (Figure 2B). In the kp-10/Gal1–16 group, the subsequent 10-minute application of Gal1–16 (10nM, agonist for GalR1, GalR2, and GalR3), rapidly suppressed the kp-10-induced [Ca2+]i plateau in 23/31 GnRH neurons responding to kp-10 (Figure 2B), bringing them to silent (9/23; upper trace) or active (14/23; lower trace) baseline levels. Notably, the Gal1–16 suppression of the kp-10 plateau only lasted during the application of Gal1–16, indicating Gal1–16 was able to “hold quiet” GnRH neurons activated by kp-10 but not reverse the signaling pathway triggered by kp-10 (Figure 2B, superscript letter). In the reverse experiment, when coapplied with kp-10, Gal1–16 was able to prevent GnRH neurons from being activated by kp-10 (only 8/60 responded to kp-10; Gal1–16/Gal1–16+kp-10 group) (Figure 2C) compared with kp-10 only group (P < .05, χ2 test). This suppression lasted until Gal1–16 was removed. The 10-minute application of Gal1–16 had no effect on the number of responding cells to 3-minute kp-10 (34/41; Gal1–16/kp-10 group) (Figure 2D) compared with kp-10 only group (P > .05, χ2 test).
Figure 2.

Gal1–16 suppresses kp-10-induced activation of GnRH neurons. A, Representative calcium imaging recordings with 3-minute kp-10 (10nM). B, Gal1–16 (10nM) suppressed kp-10-induced calcium response of GnRH neurons during the time of application. The asterisk indicates the return of the kp-10-induced response. C, When coapplied with Gal1–16 (10nM), kp-10 (10nM) did not induce calcium response until the removal of Gal1–16. D, The preexposure to Gal1–16 had no effect on the kp-10-induced calcium response; y-scale bar, 200 U and x-scale bar, 2 minutes.
Exogenous galanin inhibits GnRH neuronal activity
[Ca2+]i oscillations occur spontaneously in GnRH neurons (26), concomitant with bursts of action potentials (27). The frequency of [Ca2+]i oscillations in GnRH neurons was used to assess GnRH neuronal activity. After a control period (SFM, 5 min), explants were superfused with Gal1–16 (100nM). Gal1–16 inhibited GnRH neuronal activity (P < .05, paired t test) (Table 2, row a). Based on the efficiency observed after kp-10, lower doses were tested. Both Gal1–16 (30nM) and Gal1–16 (10nM) inhibited GnRH neuronal activity (Table 2, rows b and c [P < .05, paired t test], and Figure 3A). Gal1–16 (10nM) was the dose of Gal1–16 chosen for the rest of the experiments. Using the same model system, earlier work (28) showed that GnRH pulse were present by 7 days in vitro, but amplitude increased between 7 and 14 days in vitro. To verify the inhibitory effect of Gal1–16 persisted, the experiment with Gal1–16 (10nM) was performed on GnRH cells maintained for 14 days in vitro. Inhibition was still observed (P < .05, paired t test) (Table 2, row cc).
Table 2.
Frequencies of Calcium Oscillations in GnRH Neurons
| Paradigms | Period 1 (Peaks/min) | Period 2 (Peaks/min) | Period 3 (Peaks/min) | Period 4 (Peaks/min) | Cells (n) | Explants (N) | |
|---|---|---|---|---|---|---|---|
| a | SFM–Gal1–16 (100nM)–SFM | 2.33 ± 0.12 | 1.27 ± 0.14a | 1.74 ± 0.15a | n/a | 77 | 3 |
| b | SFM–Gal1–16 (30nM)–SFM | 2.24 ± 0.12 | 0.74 ± 0.09a | 1.76 ± 0.12a | n/a | 72 | 3 |
| c | SFM–Gal1–16 (10nM)–SFM | 2.07 ± 0.12 | 1.09 ± 0.10a | 1.63 ± 0.13a | n/a | 71 | 4 |
| d | SFM–galanin–SFM | 2.54 ± 0.08 | 1.19 ± 0.11a | 2.13 ± 0.11a | n/a | 114 | 3 |
| e | SFM–AAB–AAB + Gal1–16–SFM | 1.61 ± 0.16 | 0.80 ± 0.13a | 0.51 ± 0.10a | 1.64 ± 0.14 | 38 | 3 |
| f | SFM–AAB + PRG–AAB + PRG + Gal1–16–SFM | 1.87 ± 0.10 | 0.93 ± 0.10a | 0.64 ± 0.09a | 1.68 ± 0.11 | 68 | 3 |
| g | SFM–M617–SFM | 1.91 ± 0.14 | 0.90 ± 0.12a | 1.74 ± 0.13a | n/a | 52 | 3 |
| h | SFM–M1145–SFM | 1.81 ± 0.11 | 1.57 ± 0.11a | 1.93 ± 1.12a | n/a | 65 | 3 |
| i | SFM–Gal2–11–SFM | 1.67 ± 0.12 | 1.78 ± 0.09 | 1.80 ± 0.10 | n/a | 79 | 3 |
| j | SFM–AAB–AAB + M617–SFM | 1.92 ± 0.12 | 1.05 ± 0.11a | 0.40 ± 0.08a | 1.28 ± 0.12 | 64 | 3 |
| k | PTX pretreated (2–3 h): SFM–Gal1–16–SFM | 1.77 ± 0.10 | 1.83 ± 0.11 | 1.68 ± 0.10 | n/a | 82 | 3 |
| l | SFM–FSK–FSK + Gal1–16–SFM | 1.81 ± 0.10 | 2.37 ± 0.08a | 1.37 ± 0.11a | 1.77 ± 0.10 | 100 | 3 |
| m | SFM–IBMX–IBMX + Gal1–16–SFM | 1.91 ± 0.08 | 2.16 ± 0.07 | 1.05 ± 0.07a | 1.49 ± 0.08 | 168 | 3 |
| n | TPNQ pretreated (50 min): TPNQ–TPNQ + Gal1–16 | 2.27 ± 0.11 | 1.36 ± 0.12a | n/a | n/a | 79 | 3 |
| o | Cs pretreated (20 min): Cs–Cs + Gal1–16 | 1.25 ± 0.06 | 1.05 ± 0.05a | n/a | n/a | 71 | 3 |
| p | Ba pretreated (10 min): Ba–Ba + Gal1–16 | 0.95 ± 0.07 | 0.71 ± 0.05a | n/a | n/a | 80 | 3 |
| cc | SFM–Gal1–16–SFM | 2.39 ± 0.13 | 0.47 ± 0.07a | 1.02 ± 0.15a | n/a | 67 | 4 |
| gg | SFM–M617–SFM | 1.95 ± 0.16 | 0.59 ± 0.11a | 1.38 ± 0.20a | n/a | 39 | 4 |
Galanin rat (10nM), Gal1–16 (10nM), M617 (10nM), M1145 (10nM), Gal2–11 (10nM), AAB (20μM BIC, 10μM CNQX, and 10μM AP5), PRG (100nM), PTX (250ng/mL), FSK (1μM), IBMX (100μM), Cs (5mM), Ba (400μM), and TPNQ (100nM). Data are expressed in mean ± SEM, n cells, N explants. Rows above the thick line contain data from 7-day in vitro explants; rows below the thick line contain data from 14-day in vitro explants. Rows cc and gg are repetitions at 14 days in vitro of rows c and g, respectively.
Significant difference compared with the previous period (per 2 with per 1) or (per 3 with per 2) (Student's t test; P < .05).
Figure 3.
Galanin inhibits GnRH neuronal activity via a signaling pathway downstream GalR1. A, Representative calcium imaging recordings with the full-length peptide, galanin (left panel). Galanin (10nM), rapidly and reversibly, inhibited GnRH neurons. B, Representative calcium imaging recordings with a truncated form of galanin, Gal1–16. Similar to galanin, Gal1–16 (10nM) inhibited GnRH neurons. C, Representative calcium imaging recordings with M617, GalR1-specific agonist. M617 (10nM) was also able to inhibit GnRH neurons; y-scale bar, 200 U and x-scale bar, 2 minutes. Bar graphs (mean ± SEM) on the right panel of A–C represent the quantification of GnRH neuronal activity (in peaks/min) of all cells in that experiment group. Asterisks indicate significant difference with the pretreatment period (SFM). WO, drug washout period.
Galanin is a 29-amino acid long peptide. To ensure that both truncated and full-length peptide displayed the same biological activity, GnRH neurons were exposed to galanin (rat, mouse, 10nM). Similar to Gal1–16, galanin inhibited GnRH neuronal activity (Table 2, row d [P < .05, paired t test], and Figure 3B). Notably, the magnitude of the inhibition with galanin was greater than the magnitude of the inhibition with Gal1–16 (galanin: −1.34 ± 0.10 peaks/min [n = 114; N = 3]; Gal1–16: −0.99 ± 0.11 peaks/min [n = 71; N = 4]; P < .05, unpaired t test).
Activation of GalR1 mediates galanin inhibition
Galanin can bind to 3 different receptors, GalR1, GalR2, and GalR3 (6). To determine which receptor was responsible for the decrease in GnRH neuronal activity, 3 different peptides were tested: M617 (GalR1-specific agonist) (29), M1145 (GalR2-specific agonist) (30), and Gal2–11 (also known as AR-M1896; GalR2/3-specific agonist) (6). The application of M617 (10nM) inhibited the frequency of [Ca2+]i oscillations (Table 2, row g [P < .05, paired t test], and Figure 3C). The magnitude of inhibition with Gal1–16 or M617 was similar (Gal1–16: −0.99 ± 0.11 peaks/min [n = 71; N = 4]; M617: −1.01 ± 0.13 peaks/min [n = 52; N = 3]; P > .05, unpaired t test). Although M1145 (10nM, GalR2 agonist) evoked an inhibition of GnRH neuronal activity (P > .05, paired t test) (Table 2, row h), its magnitude was much smaller than with Gal1–16 (Gal1–16: −0.99 ± 0.11 [n = 71; N = 4]; M1145: −0.24 ± 0.07 [n = 65; N = 3]; P < .05, unpaired t test). Gal2–11 (10nM, GalR2/3 agonist) had no significant effect on the frequency of [Ca2+]i oscillations in GnRH neurons (P > .05, paired t test) (Table 2, row i). Involvement of GalR1 using M617 was verified on GnRH cells maintained for 15 days in vitro (P < .05, paired t test) (Table 2, row gg).
Gal1–16 inhibition is independent of GABAergic and glutamatergic inputs
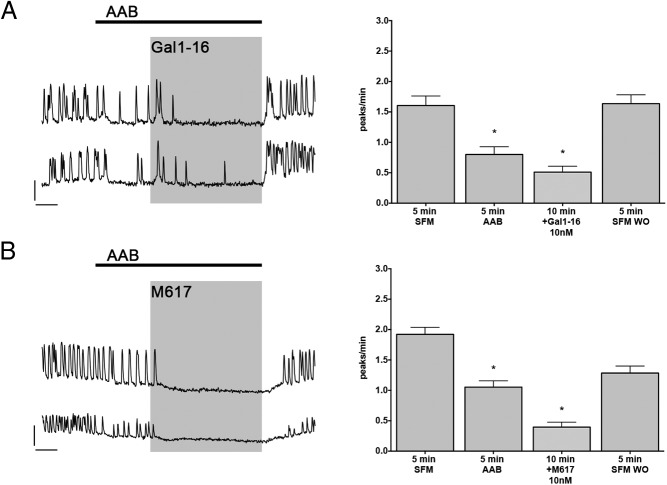
The major excitatory inputs of GnRH neurons in explants are GABAergic and glutamatergic (31). Literature reports found presynaptic action of Gal1–16 on excitatory inputs (32). Thus, the effects of Gal1–16 were reevaluated after isolation of GnRH neurons from GABAergic and glutamatergic inputs with amino acid blockers (AAB) (20μM BIC, 10μM CNQX, and 10μM AP5). As previously described, AAB reduced the frequency of [Ca2+]i oscillations. The subsequent application of Gal1–16 still reduced the frequency of [Ca2+]i oscillations (Table 2, row h [paired t test, P < .05], and Figure 4A). M617 inhibition (GalR1 agonist) also persisted after blocking amino acid inputs (Table 2, row j [P > .05, paired t test], and Figure 4B). These data indicated that galanin inhibition of GnRH neurons was independent of amino acid inputs.
Figure 4.
Galanin inhibition is independent of major excitatory inputs. A, Representative calcium imaging recordings with AAB (20μM BIC, 10μM CNQX, and 10μM AP5) and Gal1–16. Blocking the major excitatory inputs, GABAergic and glutamatergic, reduced GnRH neuronal activity. However, subsequent application of Gal1–16 (10nM) inhibited GnRH neurons further, indicating a direct effect. B, Representative calcium imaging recordings with AAB and M617. Similar to Gal1–16, M617 (10nM) was also able to inhibit GnRH neurons after AAB, confirming a postsynaptic effect mediated by GalR1; y-scale bar, 200 U and x-scale bar, 2 minutes. Bar graphs (mean ± SEM) on the right panel of A and B represent the quantification of GnRH neuronal activity (in peaks/min) of all cells in that experiment group. Asterisks indicate significant difference with the pretreatment period (SFM or AAB).
CCK inputs are not required for Gal1–16 inhibition
Explants contain CCKergic fibers that directly regulate GnRH neuronal activity (31, 33), and literature reports modulatory effects of galanin on CCKergic fibers (34). To determine whether galanin-induced inhibition could be mediated through CCKergic inputs, the effect of Gal1–16 was reevaluated after blocking simultaneously amino acid inputs with AAB and CCKergic inputs with PRG, a CCK receptor antagonist (100nM). The combination of AAB+PRG reduced the frequency of [Ca2+]i oscillations but did not prevent galanin inhibition of GnRH neurons (P > .05, paired t test) (Table 2, row f). Together, the data are consistent with a direct inhibitory effect of galanin on GnRH neurons.
Gal1–16 inhibition is dependent on a signaling pathway downstream Gi/o protein
An exclusive Gi/o-type G protein coupling for GalR1 has been reported (6). Explants were treated with PTX (250 ng/mL) for more than 3 hours to uncouple Gi/o proteins from its receptors. After PTX, Gal1–16 failed to inhibit GnRH neurons (Table 2, row k [P > .05, paired t test], and Figure 5A). The PTX sensitivity of the galanin inhibition confirmed the coupling of the GalR1 with Gi/o protein.
Figure 5.
Galanin inhibition downstream GalR1 relies on Gi/o coupling but not on cAMP intracellular levels. A, Representative calcium imaging recordings with Gal1–16 (10nM) after a more than 2–3 hours of pretreatment with PTX (250 ng/mL). Uncoupling G protein-coupled receptors with PTX prevented Gal1–16 inhibition. However, preexposure to adenylyl activator (FSK; 1μM) (B) or phosphodiesterase inhibitor (IBMX; 100μM) (C) did not prevent the Gal1–16 inhibition; y-scale bar, 200 U and x-scale bar, 2 minutes. Bar graphs (mean ± SEM) on the right panel of A–C represent the quantification of GnRH neuronal activity (in peaks/min) of all cells in that experiment group. Asterisks indicate significant difference with the pretreatment period (SFM, FSK, or IBMX).
Reduction of cAMP is not sufficient to trigger Gal1–16 inhibition
Increase in cAMP stimulates GnRH neuronal activity (22, 27). To test whether it is a decrease in cAMP, that follows the Gi/o-mediated inhibition of adenylyl cyclase, that triggers the galanin inhibition, explants were treated with FSK, activator of adenylyl cyclase (FSK; 1μM) or IBMX, an inhibitor of phosphodiesterase (IBMX; 100μM), before application of Gal1–16. Although both FSK and IBMX increased the frequency of [Ca2+]i oscillations, neither FSK or IBMX were able to prevent the Gal1–16 inhibition of GnRH neurons (Table 2, rows l and m [P < .05, paired t test], and Figure 5, B and C).
GIRK channels are involved in the Gal1–16 inhibition
PTX-sensitive activation of GIRK channels have been reported for all 3 GalRs (6). Previous data from explants describe GIRK channels-mediated inhibition sensitive to tertiapin-Q (TPNQ) (35). Explants were incubated for 1 hour with TPNQ (100nM) before exposure to Gal1–16. The Gal1–16 inhibition persisted after this pretreatment and in the presence of TPNQ (Table 2, row n [P < .05, paired t test], and Figure 6A). The experiment was repeated with Cs or Ba, 2 nonspecific blockers of inwardly rectifying potassium (Kir) channels, replacing TPNQ. Because the maximal inhibition of GIRK-mediated currents with Cs and Ba is obtained after 30 and 20 minutes, respectively (36), explants were incubated with Cs (5mM) or Ba (400μM) before exposure to Gal1–16. Neither exposure to Cs nor Ba fully blocked the Gal1–16 inhibition (Table 2, row o and p [P < .05, paired t test], and Figure 6, B and C). However, the magnitude of inhibition, was significantly reduced by Cs and Ba (Gal1–16: −0.99 ± 0.11 peaks/min [n = 71; N = 4]; Cs: −0.22 ± 0.05 peaks/min [n = 71; N = 3] [P < .05, unpaired t test]; Ba: −0.28 ± 0.06 peaks/min [n = 80; N = 3] [P < .05, unpaired t test]). Together, these data confirmed galanin inhibition was mediated by GIRK channels, sensitive to Cs and Ba but insensitive to TPNQ. Previous data indicated that neuropeptide Y mediated inhibition was sensitive to TPNQ and showed that GnRH neurons did not express kcnj6 (Kir3.2 or GIRK2) or kcnj5 (Kir3.4 or GIRK4). In contrast, kcnj9 (Kir3.3 or GIRK3) was detected in most GnRH neurons (35). Here, via single cell PCR analysis, we show the presence of kcnj3 (Kir3.1 or GIRK1) in 5/5 GnRH neurons tested.
Figure 6.
Galanin inhibition is sensitive to GIRK channel blockers. A, Representative calcium imaging recordings with Gal1–16 (10nM) after a 1-hour pretreatment with TPNQ (100nM). TPNQ, specific GIRK1/4 blocker, did not prevent the Gal1–16 inhibition. However, both 30-minute Cs (5mM) (B) and 20-minute Ba (400μM) (C), broad spectrum inward rectifying potassium channel blockers, blunted the galanin inhibition; y-scale bar, 200 U and x-scale bar, 2 minutes. Bar graphs (mean ± SEM) on the right panel of A–C represent the quantification of GnRH neuronal activity (in peaks/min) of all cells in that experiment group. Asterisks indicate significant difference with the pretreatment period (TPNQ, Cs, or Ba).
M15 exhibits partial agonist activity, inhibiting GnRH neurons
Single cells analysis by nested PCR on 7 days GnRH neurons detected transcripts for galanin in 3/5 GnRH neurons, confirming previous work showing galanin coexpressing GnRH cells as well as galanin non-GnRH-expressing cells in explants (8). Immunocytochemistry did not detect galanin positive cell bodies, but showed the presence of fibers immunoreactive for galanin in close apposition with GnRH neurons (Figure 7A).
Figure 7.

GalR inhibitor M15 (galantide) acts as a partial agonist on GnRH neurons. A, Fluorescent immunocytochemical labeling showing a galanin fiber (red) near GnRH neurons (green); scale bar, 10 μm. B, Representative calcium imaging recordings with AAB and M15 (10nM). After blocking the major excitatory inputs, GABAergic and glutamatergic, and CCKergic inputs with PRG (100nM), M15 potently inhibited GnRH neuronal activity showing a direct effect on GnRH neurons. C, Representative calcium imaging recordings with M15 (10nM) followed by Gal1–16 (10nM). Once GnRH neurons were inhibited with M15, Gal1–16 inhibition was residual. D, Representative calcium imaging recordings with Gal1–16 (10nM) followed by M15 (10nM). After the Gal1–16 inhibition, the M15 inhibition remained potent, indicating a strong affinity of M15 for GalR1. E, Representative calcium imaging recordings with Gal1–16 (10nM) after a 30-minute pretreatment with Cs (5mM). Similar to the Gal1–16 inhibition, the M15 inhibition was sensitive to the GIRK channel blocker, Cs, indicating the activity of M15 as partial agonist; y-scale bar, 200 U and x-scale bar, 2 minutes. Bar graphs (mean ± SEM) on the right panel of A–C represent the quantification of GnRH neuronal activity (in peaks/min) of all cells in that experiment group. Asterisks indicate significant difference with the pretreatment period (SFM, AAB+PRG, M15, Gal1–16, or Cs).
M15, or galantide, has been described as an antagonist to the neuronal actions of galanin (37). To determine whether endogenous galanin was exerting a constitutive inhibitory input onto GnRH neurons, M15 (10nM) was applied after blocking amino acid, and CCKergic inputs. Unexpectedly, M15 reduced the frequency of [Ca2+]i oscillations in GnRH neurons (Table 3, row a [P < .05, paired t test], and Figure 7B). When M15 was applied before Gal1–16, Gal1–16 still decreased the frequency of [Ca2+]i oscillations in GnRH neurons (Table 3, row b [P < .05, paired t test], and Figure 7C). However, when Gal1–16 was applied before M15, M15 decreased the frequency of [Ca2+]i oscillations in GnRH neurons further (Table 3, row c [P < .05, paired t test], and Figure 7D). Notably, the inhibition of M15 after Gal1–16 (-0.60 ± 0.09 peaks/min; n = 52, N = 3) was greater than the inhibition of Gal1–16 after M15 (−0.12 ± 0.04 peaks/min; n = 61, N = 3; P < .05, unpaired t test). Similar data were obtained with M40, another GalR antagonist (data not shown).
Table 3.
Frequencies of Calcium Oscillations in GnRH Neurons
| Paradigms | Period 1 (Peaks/min) | Period 2 (Peaks/min) | Period 3 (Peaks/min) | Period 4 (Peaks/min) | Cells (n) | Explants (N) | |
|---|---|---|---|---|---|---|---|
| a | SFM–AAB + PRG–AAB + PRG + M15–SFM | 2.35 ± 0.10 | 1.52 ± 0.12a | 0.63 ± 0.08a | 1.53 ± 0.12 | 101 | 3 |
| b | SFM–M15–M15 + Gal1–16–SFM | 1.90 ± 0.09 | 0.67 ± 0.10a | 0.55 ± 0.11a | 1.33 ± 0.11 | 61 | 3 |
| c | SFM–Gal1–16–Gal1–16 + M15–SFM | 2.28 ± 0.13 | 1.36 ± 0.15a | 0.75 ± 0.13a | 1.32 ± 0.15 | 52 | 3 |
| d | PTX pretreated (2–3 h): SFM–M15–SFM | 1.91 ± 0.09 | 1.49 ± 0.08a | 1.78 ± 0.09a | n/a | 134 | 4 |
| e | Cs pretreated (20 min): Cs–Cs + M15 | 0.77 ± 0.05 | 0.85 ± 0.06 | n/a | n/a | 57 | 3 |
Gal1–16 (10nM), AAB (20μM BIC, 10μM CNQX, and 10μM AP5), PRG (100nM), PTX (250ng/mL), Cs (5mM), and M15 (10nM). Data are expressed in mean ± SEM, n cells, N explants.
Significant difference compared with the previous period (per 2 with per 1) or (per 3 with per 2) (Student's t test; P < .05).
To determine whether M15 was triggering the same pathway as Gal1–16, explants were incubated with PTX (250 ng/mL) for more than 3 hours or with Cs (5mM) for 30 minutes before exposure to M15 (Table 3, rows d and e, and Figure 7E). Under these 2 paradigms, the magnitude of inhibition with M15 was significantly reduced (M15: −1.23 ± 0.10 peaks/min; PTX/M15: −0.4 ± 0.07 peaks/min [P < .05, unpaired t test]; Cs/M15: 0.08 ± 0.05 peaks/min [P < .05, unpaired t test]), indicating that M15 used the same pathway as Gal1–16 and that M15 was acting as a partial agonist for GalR1. Under these circumstances, determining whether endogenous galanin was released and constitutively repressing GnRH neuronal activity was not possible.
kp-10-evoked GnRH secretion is diminished by Gal1–16
To have an on-line readout of GnRH secretion, we took advantage of the natural ability of gonadotrophs to respond to GnRH and established a protocol that allows one to use them as biosensors to determine whether Gal1–16 had an effect on kp-10-evoked GnRH secretion (Figure 8). Four explants (9 d in vitro) were treated with kp-10 10nM+Gal1–16 10nM for 1 hour (KG), whereas 4 other explants were treated with kp-10 10nM only (K). The first media failed to evoke a response in gonadotrophs (AUC before KG: 2058 ± 642 a.u.; AUC with KG:1554 ± 578 a.u.; n = 19, N = 3; ANOVA, P > .05) (Figure 8C), whereas the second did (AUC before K: 2036 ± 443 a.u.; AUC with K:3759 ± 721 a.u; ANOVA, P < .05). When the same media were applied in the reverse order, the first media evoked a response in gonadotrophs (AUC before K: 2351 ± 577 a.u.; AUC with K:3333 ± 559 a.u.; n = 8, N = 1; ANOVA, P < .05) whereas the second did not (AUC before KG: 1121 ± 251 a.u.; AUC with KG:1825 ± 828 a.u; ANOVA, P > .05) (Figure 8D). The experiment was repeated with 15 days in vitro old explants (Figure 8E). Five explants were treated with kp-10+Gal1–16 for 1 hour (KG), whereas 5 other explants were treated with kp-10 only (K). The first media failed to evoke a response in gonadotrophs (AUC before KG: 2230 ± 494 a.u.; AUC with KG:1884 ± 533 a.u.; n = 47, N = 3; ANOVA, P > .05) whereas the second did (AUC before K: 2142 ± 261 a.u.; AUC with K:3788 ± 443 a.u; ANOVA, P < .05). Together, our data show that the GnRH content of media from kp-10+Gal1–16 treated explants was below the threshold of detection of gonadotrophs, whereas the GnRH content of media from kp-10 only treated explants was above, indicating that Gal1–16 blocked or at least decreased the kp-10-induced secretion of GnRH neurons.
Figure 8.
Galanin reduces kp-10-evoked GnRH secretion. A, Schematic of paradigm employed to measure GnRH secretion using dissociated anterior pituitary cells, specifically represents C and E. Anterior pituitary cells were continuously superfused with SFM containing kp-10 (10nM) and Gal1–16 (10nM), although the source varied. The SFM was either control SFM+kp-10+Gal1–16 (white box), SFM conditioned for 1 hour by explants+kp-10+Gal1–16 (hatched boxes \\\) or SFM conditioned for 1 hour by explants+kp-10 and supplemented with Gal1–16 (hatched boxes ///). All recordings terminated after application of D-Trp6-GnRH (10nM) to SFM. Washout periods are symbolized by time break circles. B, Functional gonadotrophs were distinguished from unidentified pituitary cells with D-Trp6-GnRH (10nM). Pituitary cells are shown in bright field (left, 70-μm wide box), on an average image of a 2-minute period before (black arrow on A, middle) and a 2-minute period during D-Trp6-GnRH challenge (white arrow on A, right). The arrows point at a responding cell. C, Representative calcium imaging recordings of gonadotrophs exposed for 2 minutes to SFM conditioned by explants (9 d in vitro) and challenged with D-Trp6-GnRH. The AUC was calculated for each 2-minute period and plotted on the right. D, The paradigm displayed in A and used in C was reversed to test the accuracy of the method. Representative calcium imaging recordings are shown on the left and the calculated AUC is plotted on the right. E, The paradigm displayed in A and used in C was repeated using SFM conditioned by older explants (15 d in vitro). Representative calcium imaging recordings are shown on the left, and the calculated AUC is plotted on the right (y-scale bars, 200 U and x-scale boxes, 2 minutes).
Discussion
This study investigated the potential crosstalk between kisspeptin and galanin signals to GnRH neurons maintained in explants (18). Primary GnRH neurons in this model system display many adult-like features (reviewed in Refs. 26, 38). One of the most important characteristics of cells maintained in explants, compared with acute adult brain slices, is cellular integrity between the cell soma and processes, known to impact both cell behavior and responsiveness (39). We report here that galanin inhibits spontaneous GnRH neuronal activity and kisspeptin-induced GnRH neuronal activity. The inhibition, mediated by GalR1, is direct and dependent on activation of GIRK channels (Figure 9). Using the physiological response of gonadotrophs to GnRH, we show that the addition of Gal1–16 to GnRH neurons reduces kp-10-evoked GnRH secretion. Together, these data suggest that galanin inhibition balances kisspeptin excitation in GnRH neurons and, as such, can modulate reproductive function.
Figure 9.

Schematic of the signaling pathway activated by galanin (Gal) in GnRH neurons. Upon the binding of Gal to the GalR1, the β/γ-subunit of the dissociated G protein activates GIRK channels. The potassium efflux hyperpolarizes the GnRH neuron and consequently reduces GnRH neuronal activity.
Although mRNA GalR3 does not seem to be well represented in the brain (40), mRNA for both GalR1 (1) and GalR2 are widely distributed (41). The presence of mRNA for GalR1 in GnRH neurons has been reported (14–16). However, the presence of mRNA for GalR2 is more controversial. In ewes, immunocytochemistry suggests GnRH and GalR2 are never coexpressed (42), whereas in female mice, RT-PCR suggests the presence of GalR2 in a small subpopulation of GnRH neurons (16). Our RT-PCR data and pharmacological data (discussed below) support only the presence of GalR1 on GnRH neurons maintained in our model system.
ERα is a critical player for E2 feedback to brain (43, 44). Because GnRH neurons lack ERα (45, 46), this feedback must rely on E2-sensitive cell populations upstream of GnRH neurons. Kisspeptin neurons are potent activators of GnRH neurons (47). Virtually all kisspeptin neurons express ERα and kisspeptin gene expression fluctuates with circulating E2 (48). Recently, galanin has been identified in a subpopulation of kisspeptin neurons (10, 17). Previous work showed that GnRH cells in explants (25) and adult slices (24, 49) exhibited similar responses to kisspeptin. In agreement with our earlier data, transient exposure to kp-10 was able to initiate a long lasting response in GnRH neurons. Gal1–16 induced a rapid suppression of the kp-10-induced activation of GnRH neurons. Notably, when coapplied with kp-10, Gal1–16 prevented activation of GnRH neurons until its removal. The potent inhibitory action of galanin on GnRH neurons was supported by the action of galanin on spontaneous GnRH neuronal activity as well. Both the full-length and the truncated form of galanin, Gal1–16, inhibited spontaneous GnRH neuronal activity. The inhibitory response is in contrast to galanin-induced depolarization of GnRH neurons that was reported in acute brain slices (16). We can hypothesize that this difference could arise from the nature of the in vitro preparation itself (slices vs explants) or from the maturity of the GnRH neurons recorded (adult vs embryonic). For the first hypothesis, in Todman et al (16), 50% of the GnRH neuronal pools were reported to express GalR1, whereas 25% expressed GalR2 transcript. Notably, 55% of the single GnRH cells recorded responded to galanin with depolarization. The response recorded in GnRH neurons certainly correlates best with the proportion of GalR1 transcript Todman et al (16) found in their GnRH cDNA pools. However, a link between GalR1 and excitation is not supported in the literature. All GalR1 have been linked to a Gi/o coupling, whereas GalR2 has been also linked to Gq/11 (reviewed in Ref. 6). In many preparations, galanin inhibition is mediated by GalR1 (arcuate nucleus [50] and substantia gelatinosa neurons [32]), whereas galanin excitation is mediated by GalR2 (dentate gyrus neurons [51], adrenocortical cells [52], and substantia gelatinosa neurons [32]). Although galanin has been shown to increase GnRH release from arcuate nucleus/median eminence fragments, the effect was indirect (53). Thus, one must infer that the excitatory effect recorded by Todman et al (16), was due to GalR2. With respect to GalR2 expression, one cannot exclude the second hypothesis, that a developmental change may occur in GalR subtypes on GnRH neurons and the persistence of inhibition with Gal1–16 and M617 in 14-day in vitro cannot exclude it. It is known that galanin and GalR are highly expressed in embryonic stem cells (54), galanin is present in tissues originating from mesenchyme and neural crest (55) and in the nasal placode (8), and that galanin can act developmentally as a neuronal trophic factor (56). Notably, the signaling pathway of GalR2, via Rho-family GTPase signaling, affecting cytoskeletal integrity and dynamics, makes it a better candidate for developmentally regulated mechanisms such as neurite outgrowth (57). However, the literature supports a role for both receptor subtypes in neurodevelopment, neuroprotection (58, 59) and synaptic transmission (60, 61).
To insure that GnRH neuronal activity was inhibited by galanin, and identify the signaling pathway, pharmacological studies with GalR1-, GalR2-, and GalR2/3-specific agonists were performed. Only the GalR1-specific agonist elicited an effect, mimicking Gal1–16 inhibition of GnRH neurons, corroborating the absence of GalR2 and GalR3 and suggesting a direct effect of galanin via GalR1 on GnRH neurons. In fact, the inhibitory action of Gal1–16 and the GalR1-specific agonist on GnRH neurons after blockade of GABAergic, glutamatergic, and CCKergic inputs further supports the direct action of galanin on GnRH neurons via GalR1. The PTX data strengthen this argument, with the Gi/o-dependent signaling pathway commonly associated with GalR1 (reviewed in Ref. 6). Notably, the downstream signaling pathway underlying inhibition did not rely upon intracellular levels of cAMP. This observation is in agreement with previous work showing that an increase in cAMP evoked by an adenylyl cyclase stimulator leads to excitation dependent on the activation of protein kinase A (27) but a decrease in cAMP evoked by an adenylyl cyclase inhibitor did not reciprocally lead to inhibition (27). Some studies suggest a role for potassium channels in galanin inhibition (29, 50, 62) and our data with Cs and Ba indicate that GIRK channels were mediating the galanin induced inhibition of GnRH neuronal activity. This observation is also supported by the literature (reviewed in Ref. 6). Earlier work indicated that neuropeptide Y-mediated inhibition of GnRH neurons (35), also dependent on GIRK channels, was sensitive to TPNQ. Here, we found that the galanin-mediated inhibition was not. This difference might be due to the composition of the GIRK channels. GIRK channels are tetrameric and their configuration defines their sensitivity to blockers. Previous data showed that GnRH neurons express GIRK3, but not GIRK2 or GIRK4 (35). Here, we showed that GnRH neurons also express GIRK1. Because GIRK1 cannot form functional homotetramers (63), this observation leads to only 2 possible configurations GIRK1/3, sensitive to TPNQ, and GIRK3/3, insensitive to TPNQ (64). A preferential subcellular colocalization between GalR1 and GIRK3/3 could explain the resistance of the galanin response to TPNQ. Taken together, these data indicate that galanin inhibition of GnRH neuronal activity is, mediated by GalR1, is direct, and dependent on the activation of GIRK channels.
M15 (or galantide) has been described and commonly used as a nonspecific antagonist for GalRs (6, 37), blocking postsynaptic action of galanin (16, 50). However, we observed an agonistic action of M15 on GnRH neurons. In fact, this pharmacological feature has been reported. In magnocellular neurosecretory cells from the supraoptic nucleus, both galanin and M15 evoked a hyperpolarization (62). In arcuate nucleus neurons, both galanin and M15 acted presynaptically to induce a depression of synaptic transmission; antagonization of galanin by M15 was not consistently observed (65). Previous data from GnRH neurons showed that M15 had no effect on its own but blocked galanin excitation (16). However, because only the excitatory effect of galanin was observed by Todman et al (16), and as previously discussed, probably mediated by GalR2 subtype, the agonistic activity of M15 may be limited to GalR1 due to its higher affinity (66, 67). These data are also consistent with the GalR1 being distal to the cell body, which may not be present after slice preparations but maintained in explants, allowing us to identify the partial agonistic action of M15.
A positive relationship between GnRH firing rate and GnRH/LH secretion is clear, with in vivo stimulation of GnRH neurons evoking GnRH/LH secretion (68). Compounds known to directly excite GnRH neurons in vitro like kp-10 (25, 49) also evoke GnRH/LH secretion (69). However, under basal conditions, the correlation between changes in the frequencies of cellular events recorded (in vitro [26, 70] or in vivo [24]), and GnRH/LH pulses has been elusive, and even less is known about the effects of inhibitory modulators of GnRH neuronal activity on GnRH secretion.
In the present report, galanin clearly inhibits single GnRH neurons. In previous reports, galanin increased LH release in ovariectomized female rats, supplemented with E2 and progesterone (71) and from male median eminence-arcuate nucleus explants (53), whereas M15 decreased spontaneous GnRH release from median eminence-arcuate nucleus explants from OVX+E2+P rats (72). To elucidate the physiological relationship between changes in GnRH neuronal activity and GnRH secretion, the present study uses the natural ability of gonadotrophs to respond to GnRH. Using this bioassay, we show that Gal1–16 reduces kp-10-stimulated GnRH secretion. In rat, dissociated gonadotrophs has been shown to reliably respond to 50pM GnRH (∼59 pg/mL) (73) and gonadotrophs in acute pituitary slices from mouse were found to respond to as low as 10pM GnRH (12 pg/mL) (74, 75). In mouse, 10nM kp-10 stimulation of median eminence-arcuate nucleus explants for 1 hour evoked approximately 12- to 16-pg GnRH/mL (76) and 100nM kp-10 stimulation of nasal explants for 1 hour evoked approximately 8-pg GnRH/mL (28). In the present report, combining 4–5 explants per experiment enabled us to reach the expected detection threshold of gonadotrophs for GnRH and testing the collected media in multiple pituitary cultures allowed us to overcome the intrinsic variability of the response of gonadotrophs to GnRH (73–75). One caveat of this bioassay is that the active compounds used to stimulate GnRH neurons might also have an effect on gonadotrophs, ie, galanin is a known paracrine modulator of gonadotrophs by lactotrophs (77). To circumvent the effects of pharmacological compounds added to the explants, altering gonadotroph responses acutely, the compounds were added chronically to gonadotrophs throughout the experiment, ie, the concentrations of kp-10 and Gal1–16 were constant throughout all recording periods.
The results of the present study suggest a modulatory mechanism of the kisspeptin response of GnRH neurons by kisspeptinergic inputs via a coexpressed peptide, galanin. Although neither galanin nor GalR1 knockout mice display gross fertility impairment (78, 79), galanin modulation could impact the onset of puberty and/or terminating the GnRH/LH surge, which to our knowledge were not examined in these mice models. The cascade of galanin tuning on GnRH neuronal activity may even go further than a direct modulation of kisspeptin responses as the inhibitory actions of galanin have expanded with the ability of the GalRs to form heteromers with other G protein-coupled receptors (α2-adrenoreceptors, D2-dopaminergic receptors, 5HT1A-serotoninergic receptors, Y1-neuropeptide Y receptors) and thereby modulate their responses as well (reviewed in Ref. 80). Thus, the physiological role of galanin upon GnRH neurons and reproductive function needs to be reevaluated.
Acknowledgments
We thank Dr Milos Rokic for his help with pituitary dissociation and Dr Stanko Stojilkovic for his help with calcium imaging of dissociated pituitary cells.
This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Neurological Disorders and Stroke Grant ZIA NS002824-25.
Disclosure Summary: The authors have nothing to disclose.
Appendix
Antibody Table
Table 4.
Antibody Table
| Peptide/Protein Target | Name of Antibody | Manufacturer, Catalog Number, and/or Name of Individual Providing the Antibody | Species Raised in; Monoclonal or Polyclonal | Dilution Used |
|---|---|---|---|---|
| GnRH | SW-1 | Susan Wray | Rabbit; polyclonal | 1:3000 |
| GnRH | F1D3C5 | A. Karande | Mouse; monoclonal | 1:4000 |
| Galanin | James Hyde | Rabbit; polyclonal | 1:1000 |
Footnotes
- AAB
- amino acid blocker
- AP5
- D-(−)-2-amino-5-phosphonopentanoic acid
- a.u.
- arbitrary unit
- AUC
- area under the curve
- BIC
- bicuculline
- [Ca2+]i
- intracellular calcium
- CCK
- cholecystokinin
- CNQX
- 6-cyano-7-nitroquinoxaline-2,3-dione
- E2
- estrogen
- ER
- E2 receptor
- FSK
- forskolin
- GABA
- γ-aminobutyric acid
- Gal1–16
- galanin 1–16
- GalR
- galanin receptor
- GIRK
- G protein-coupled inwardly rectifying potassium
- IBMX
- 3-isobutyl-1-methylxanthine
- Kir
- inwardly rectifying potassium
- kp-10
- kisspeptin-10
- PRG
- proglumide
- PTX
- pertussis toxin
- SFM
- serum-free medium
- TPNQ
- tertiapin-Q.
References
- 1. Skofitsch G, Jacobowitz DM. Quantitative distribution of galanin-like immunoreactivity in the rat central nervous system. Peptides. 1986;7:609–613. [DOI] [PubMed] [Google Scholar]
- 2. Gentleman SM, Falkai P, Bogerts B, Herrero MT, Polak JM, Roberts GW. Distribution of galanin-like immunoreactivity in the human brain. Brain Res. 1989;505:311–315. [DOI] [PubMed] [Google Scholar]
- 3. Pérez SE, Wynick D, Steiner RA, Mufson EJ. Distribution of galaninergic immunoreactivity in the brain of the mouse. J Comp Neurol. 2001;434:158–185. [DOI] [PubMed] [Google Scholar]
- 4. Kaplan LM, Gabriel SM, Koenig JI, et al. Galanin is an estrogen-inducible, secretory product of the rat anterior pituitary. Proc Natl Acad Sci USA. 1988;85:7408–7412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gabriel SM, Koenig JI, Kaplan LM. Galanin-like immunoreactivity is influenced by estrogen in peripubertal and adult rats. Neuroendocrinology. 1990;51:168–173. [DOI] [PubMed] [Google Scholar]
- 6. Lang R, Gundlach AL, Holmes FE, et al. Physiology, signaling, and pharmacology of galanin peptides and receptors: three decades of emerging diversity. Pharmacol Rev. 2015;67:118–175. [DOI] [PubMed] [Google Scholar]
- 7. Merchenthaler I, Lopez FJ, Negro-Vilar A. Colocalization of galanin and luteinizing hormone-releasing hormone in a subset of preoptic hypothalamic neurons: anatomical and functional correlates. Proc Natl Acad Sci USA. 1990;87:6326–6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Key S, Wray S. Two olfactory placode derived galanin subpopulations: luteinizing hormone-releasing hormone neurones and vomeronasal cells. J Neuroendocrinol. 2000;12:535–545. [DOI] [PubMed] [Google Scholar]
- 9. Dudas B, Merchenthaler I. Three-dimensional representation of the neurotransmitter systems of the human hypothalamus: inputs of the gonadotrophin hormone-releasing hormone neuronal system. J Neuroendocrinol. 2006;18:79–95. [DOI] [PubMed] [Google Scholar]
- 10. Porteous R, Petersen SL, Yeo SH, et al. Kisspeptin neurons co-express met-enkephalin and galanin in the rostral periventricular region of the female mouse hypothalamus. J Comp Neurol. 2011;519:3456–3469. [DOI] [PubMed] [Google Scholar]
- 11. Rajendren G, Li X. Galanin synaptic input to gonadotropin-releasing hormone perikarya in juvenile and adult female mice: implications for sexual maturity. Brain Res Dev Brain Res. 2001;131:161–165. [DOI] [PubMed] [Google Scholar]
- 12. Rajendren G, Gibson MJ. A confocal microscopic study of synaptic inputs to gonadotropin-releasing hormone cells in mouse brain: regional differences and enhancement by estrogen. Neuroendocrinology. 2001;73:84–90. [DOI] [PubMed] [Google Scholar]
- 13. Rajendren G. Increased galanin synapses onto activated gonadotropin-releasing hormone neuronal cell bodies in normal female mice and in functional preoptic area grafts in hypogonadal mice. J Neuroendocrinol. 2002;14:435–441. [DOI] [PubMed] [Google Scholar]
- 14. Mitchell V, Bouret S, Prévot V, Jennes L, Beauvillain JC. Evidence for expression of galanin receptor Gal-R1 mRNA in certain gonadotropin releasing hormone neurones of the rostral preoptic area. J Neuroendocrinol. 1999;11:805–812. [DOI] [PubMed] [Google Scholar]
- 15. Dufourny L, Skinner DC. Distribution of galanin receptor 1-immunoreactive neurons in the ovine hypothalamus: colocalization with GnRH. Brain Res. 2005;1054:73–81. [DOI] [PubMed] [Google Scholar]
- 16. Todman MG, Han SK, Herbison AE. Profiling neurotransmitter receptor expression in mouse gonadotropin-releasing hormone neurons using green fluorescent protein-promoter transgenics and microarrays. Neuroscience. 2005;132:703–712. [DOI] [PubMed] [Google Scholar]
- 17. Kalló I, Vida B, Deli L, et al. Co-localisation of kisspeptin with galanin or neurokinin B in afferents to mouse GnRH neurones. J Neuroendocrinol. 2012;24:464–476. [DOI] [PubMed] [Google Scholar]
- 18. Fueshko S, Wray S. LHRH cells migrate on peripherin fibers in embryonic olfactory explant cultures: an in vitro model for neurophilic neuronal migration. Dev Biol. 1994;166:331–348. [DOI] [PubMed] [Google Scholar]
- 19. Klenke U, Taylor-Burds C. Culturing embryonic nasal explants for developmental and physiological study. Curr Protoc Neurosci. 2012;Chapter 3:Unit 3.25.1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kramer PR, Krishnamurthy R, Mitchell PJ, Wray S. Transcription factor activator protein-2 is required for continued luteinizing hormone-releasing hormone expression in the forebrain of developing mice. Endocrinology. 2000;141:1823–1838. [DOI] [PubMed] [Google Scholar]
- 21. Giacobini P, Kopin AS, Beart PM, Mercer LD, Fasolo A, Wray S. Cholecystokinin modulates migration of gonadotropin-releasing hormone-1 neurons. J Neurosci. 2004;24:4737–4748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Constantin S, Wray S. Gonadotropin-releasing hormone-1 neuronal activity is independent of hyperpolarization-activated cyclic nucleotide-modulated channels but is sensitive to protein kinase a-dependent phosphorylation. Endocrinology. 2008;149:3500–3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu X, Lee K, Herbison AE. Kisspeptin excites gonadotropin-releasing hormone neurons through a phospholipase C/calcium-dependent pathway regulating multiple ion channels. Endocrinology. 2008;149:4605–4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Constantin S, Iremonger KJ, Herbison AE. In vivo recordings of GnRH neuron firing reveal heterogeneity and dependence upon GABAA receptor signaling. J Neurosci. 2013;33:9394–9401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Constantin S, Caligioni CS, Stojilkovic S, Wray S. Kisspeptin-10 facilitates a plasma membrane-driven calcium oscillator in gonadotropin-releasing hormone-1 neurons. Endocrinology. 2009;150:1400–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jasoni CL, Romanò N, Constantin S, Lee K, Herbison AE. Calcium dynamics in gonadotropin-releasing hormone neurons. Front Neuroendocrinol. 2010;31:259–269. [DOI] [PubMed] [Google Scholar]
- 27. Constantin S, Wray S. Gonadotropin-releasing hormone-1 neuronal activity is independent of cyclic nucleotide-gated channels. Endocrinology. 2008;149:279–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Constantin S, Caraty A, Wray S, Duittoz AH. Development of gonadotropin-releasing hormone-1 secretion in mouse nasal explants. Endocrinology. 2009;150:3221–3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lundstrom L, Sollenberg U, Brewer A, et al. A galanin receptor subtype 1 specific agonist. Int J Pept Res Ther. 2005;11:17–27. [Google Scholar]
- 30. Runesson J, Saar I, Lundström L, Järv J, Langel U. A novel GalR2-specific peptide agonist. Neuropeptides. 2009;43:187–192. [DOI] [PubMed] [Google Scholar]
- 31. Constantin S, Klenke U, Wray S. The calcium oscillator of GnRH-1 neurons is developmentally regulated. Endocrinology. 2010;151:3863–3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yue HY, Fujita T, Kumamoto E. Biphasic modulation by galanin of excitatory synaptic transmission in substantia gelatinosa neurons of adult rat spinal cord slices. J Neurophysiol. 2011;105:2337–2349. [DOI] [PubMed] [Google Scholar]
- 33. Giacobini P, Wray S. Cholecystokinin directly inhibits neuronal activity of primary gonadotropin-releasing hormone cells through cholecystokinin-1 receptor. Endocrinology. 2007;148:63–71. [DOI] [PubMed] [Google Scholar]
- 34. Meister B, Hulting AL, Uvnäs-Moberg K, Hökfelt T. Galanin stimulates the release of cholecystokinin from nerve fibres in the pituitary neurointermediate lobe. Neuroreport. 1993;4:631–634. [DOI] [PubMed] [Google Scholar]
- 35. Klenke U, Constantin S, Wray S. Neuropeptide Y directly inhibits neuronal activity in a subpopulation of gonadotropin-releasing hormone-1 neurons via Y1 receptors. Endocrinology. 2010;151:2736–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Meneses D, Mateos V, Islas G, Barral J. G-protein-coupled inward rectifier potassium channels involved in corticostriatal presynaptic modulation. Synapse. 2015;69:446–452. [DOI] [PubMed] [Google Scholar]
- 37. Bartfai T, Bedecs K, Land T, et al. M-15: high-affinity chimeric peptide that blocks the neuronal actions of galanin in the hippocampus, locus coeruleus, and spinal cord. Proc Natl Acad Sci USA. 1991;88:10961–10965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Constantin S. Physiology of the gonadotrophin-releasing hormone (GnRH) neurone: studies from embryonic GnRH neurones. J Neuroendocrinol. 2011;23:542–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Constantin S, Piet R, Iremonger K, et al. GnRH neuron firing and response to GABA in vitro depend on acute brain slice thickness and orientation. Endocrinology. 2012;153:3758–3769. [DOI] [PubMed] [Google Scholar]
- 40. Mennicken F, Hoffert C, Pelletier M, Ahmad S, O'Donnell D. Restricted distribution of galanin receptor 3 (GalR3) mRNA in the adult rat central nervous system. J Chem Neuroanat. 2002;24:257–268. [DOI] [PubMed] [Google Scholar]
- 41. O'Donnell D, Ahmad S, Wahlestedt C, Walker P. Expression of the novel galanin receptor subtype GALR2 in the adult rat CNS: distinct distribution from GALR1. J Comp Neurol. 1999;409:469–481. [PubMed] [Google Scholar]
- 42. Chambers G, Whitelaw CM, Robinson JE, Evans NP. Distribution of galanin receptor-2 immunoreactive neurones in the ovine hypothalamus: no evidence for involvement in the control of gonadotrophin-releasing hormone secretion. J Neuroendocrinol. 2007;19:966–973. [DOI] [PubMed] [Google Scholar]
- 43. Couse JF, Yates MM, Walker VR, Korach KS. Characterization of the hypothalamic-pituitary-gonadal axis in estrogen receptor (ER) Null mice reveals hypergonadism and endocrine sex reversal in females lacking ERα but not ERβ. Mol Endocrinol. 2003;17:1039–1053. [DOI] [PubMed] [Google Scholar]
- 44. Cheong RY, Porteous R, Chambon P, Abrahám I, Herbison AE. Effects of neuron-specific estrogen receptor (ER) α and ERβ deletion on the acute estrogen negative feedback mechanism in adult female mice. Endocrinology. 2014;155:1418–1427. [DOI] [PubMed] [Google Scholar]
- 45. Herbison AE, Pape JR. New evidence for estrogen receptors in gonadotropin-releasing hormone neurons. Front Neuroendocrinol. 2001;22:292–308. [DOI] [PubMed] [Google Scholar]
- 46. Sharifi N, Reuss AE, Wray S. Prenatal LHRH neurons in nasal explant cultures express estrogen receptor β transcript. Endocrinology. 2002;143:2503–2507. [DOI] [PubMed] [Google Scholar]
- 47. Irwig MS, Fraley GS, Smith JT, et al. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80:264–272. [DOI] [PubMed] [Google Scholar]
- 48. Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146:3686–3692. [DOI] [PubMed] [Google Scholar]
- 49. Han SK, Gottsch ML, Lee KJ, et al. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25:11349–11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Poulain P, Decrocq N, Mitchell V. Direct inhibitory action of galanin on hypothalamic arcuate nucleus neurones expressing galanin receptor Gal-r1 mRNA. Neuroendocrinology. 2003;78:105–117. [DOI] [PubMed] [Google Scholar]
- 51. Badie-Mahdavi H, Lu X, Behrens MM, Bartfai T. Role of galanin receptor 1 and galanin receptor 2 activation in synaptic plasticity associated with 3′,5′-cyclic AMP response element-binding protein phosphorylation in the dentate gyrus: studies with a galanin receptor 2 agonist and galanin receptor 1 knockout mice. Neuroscience. 2005;133:591–604. [DOI] [PubMed] [Google Scholar]
- 52. Andreis PG, Malendowicz LK, Rebuffat P, Spinazzi R, Ziolkowska A, Nussdorfer GG. Galanin enhances corticosterone secretion from dispersed rat adrenocortical cells through the activation of GAL-R1 and GAL-R2 receptors coupled to the adenylate cyclase-dependent signaling cascade. Int J Mol Med. 2007;19:149–155. [PubMed] [Google Scholar]
- 53. Lopez FJ, Negro-Vilar A. Galanin stimulates luteinizing hormone-releasing hormone secretion from arcuate nucleus-median eminence fragments in vitro: involvement of an α-adrenergic mechanism. Endocrinology. 1990;127:2431–2436. [DOI] [PubMed] [Google Scholar]
- 54. Tarasov KV, Tarasova YS, Crider DG, Anisimov SV, Wobus AM, Boheler KR. Galanin and galanin receptors in embryonic stem cells: accidental or essential? Neuropeptides. 2002;36:239–245. [DOI] [PubMed] [Google Scholar]
- 55. Jones M, Perumal P, Vrontakis M. Presence of galanin-like immunoreactivity in mesenchymal and neural crest origin tissues during embryonic development in the mouse. Anat Rec (Hoboken). 2009;292:481–487. [DOI] [PubMed] [Google Scholar]
- 56. Wynick D, Bacon A. Targeted disruption of galanin: new insights from knock-out studies. Neuropeptides. 2002;36:132–144. [DOI] [PubMed] [Google Scholar]
- 57. Keimpema E, Zheng K, Barde SS, et al. GABAergic terminals are a source of galanin to modulate cholinergic neuron development in the neonatal forebrain. Cereb Cortex. 2014;24:3277–3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Shi TJ, Hua XY, Lu X, et al. Sensory neuronal phenotype in galanin receptor 2 knockout mice: focus on dorsal root ganglion neurone development and pain behaviour. Eur J Neurosci. 2006;23:627–636. [DOI] [PubMed] [Google Scholar]
- 59. Jungnickel SR, Yao M, Shen PJ, Gundlach AL. Induction of galanin receptor-1 (GalR1) expression in external granule cell layer of post-natal mouse cerebellum. J Neurochem. 2005;92:1452–1462. [DOI] [PubMed] [Google Scholar]
- 60. Burazin TC, Larm JA, Ryan MC, Gundlach AL. Galanin-R1 and -R2 receptor mRNA expression during the development of rat brain suggests differential subtype involvement in synaptic transmission and plasticity. Eur J Neurosci. 2000;12:2901–2917. [DOI] [PubMed] [Google Scholar]
- 61. Mazarati A, Lu X, Kilk K, Langel U, Wasterlain C, Bartfai T. Galanin type 2 receptors regulate neuronal survival, susceptibility to seizures and seizure-induced neurogenesis in the dentate gyrus. Eur J Neurosci. 2004;19:3235–3244. [DOI] [PubMed] [Google Scholar]
- 62. Papas S, Bourque CW. Galanin inhibits continuous and phasic firing in rat hypothalamic magnocellular neurosecretory cells. J Neurosci. 1997;17:6048–6056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kennedy ME, Nemec J, Corey S, Wickman K, Clapham DE. GIRK4 confers appropriate processing and cell surface localization to G-protein-gated potassium channels. J Biol Chem. 1999;274:2571–2582. [DOI] [PubMed] [Google Scholar]
- 64. Kubo Y, Adelman JP, Clapham DE, et al. International Union of Pharmacology. LIV. Nomenclature and molecular relationships of inwardly rectifying potassium channels. Pharmacol Rev. 2005;57:509–526. [DOI] [PubMed] [Google Scholar]
- 65. Kinney GA, Emmerson PJ, Miller RJ. Galanin receptor-mediated inhibition of glutamate release in the arcuate nucleus of the hypothalamus. J Neurosci. 1998;18:3489–3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Florén A, Land T, Langel U. Galanin receptor subtypes and ligand binding. Neuropeptides. 2000;34:331–337. [DOI] [PubMed] [Google Scholar]
- 67. Webling KE, Runesson J, Bartfai T, Langel U. Galanin receptors and ligands. Front Endocrinol. 2012;3:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Campos P, Herbison AE. Optogenetic activation of GnRH neurons reveals minimal requirements for pulsatile luteinizing hormone secretion. Proc Natl Acad Sci USA. 2014;111:18387–18392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Messager S, Chatzidaki EE, Ma D, et al. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA. 2005;102:1761–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Moenter SM. Identified GnRH neuron electrophysiology: a decade of study. Brain Res. 2010;1364:10–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sahu A, Crowley WR, Tatemoto K, Balasubramaniam A, Kalra SP. Effects of neuropeptide Y, NPY analog (norleucine4-NPY), galanin and neuropeptide K on LH release in ovariectomized (ovx) and ovx estrogen, progesterone-treated rats. Peptides. 1987;8:921–926. [DOI] [PubMed] [Google Scholar]
- 72. Sahu A, Xu B, Kalra SP. Role of galanin in stimulation of pituitary luteinizing hormone secretion as revealed by a specific receptor antagonist, galantide. Endocrinology. 1994;134:529–536. [DOI] [PubMed] [Google Scholar]
- 73. Tomić M, Cesnajaj M, Catt KJ, Stojilkovic SS. Developmental and physiological aspects of Ca2+ signaling in agonist-stimulated pituitary gonadotrophs. Endocrinology. 1994;135:1762–1771. [DOI] [PubMed] [Google Scholar]
- 74. Sánchez-Cárdenas C, Hernández-Cruz A. GnRH-Induced [Ca2+]i-signalling patterns in mouse gonadotrophs recorded from acute pituitary slices in vitro. Neuroendocrinology. 2010;91:239–255. [DOI] [PubMed] [Google Scholar]
- 75. Duran-Pasten ML, Fiordelisio T. GnRH-induced Ca(2+) signaling patterns and gonadotropin secretion in pituitary gonadotrophs. Functional adaptations to both ordinary and extraordinary physiological demands. Front Endocrinol. 2013;4:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. d'Anglemont de Tassigny X, Fagg LA, Carlton MB, Colledge WH. Kisspeptin can stimulate gonadotropin-releasing hormone (GnRH) release by a direct action at GnRH nerve terminals. Endocrinology. 2008;149:3926–3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Denef C. Paracrinicity: the story of 30 years of cellular pituitary crosstalk. J Neuroendocrinol. 2008;20:1–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Jacoby AS, Hort YJ, Constantinescu G, Shine J, Iismaa TP. Critical role for GALR1 galanin receptor in galanin regulation of neuroendocrine function and seizure activity. Brain Res Mol Brain Res. 2002;107:195–200. [DOI] [PubMed] [Google Scholar]
- 79. Hohmann JG, Krasnow SM, Teklemichael DN, Clifton DK, Wynick D, Steiner RA. Neuroendocrine profiles in galanin-overexpressing and knockout mice. Neuroendocrinology. 2003;77:354–366. [DOI] [PubMed] [Google Scholar]
- 80. Fuxe K, Borroto-Escuela D, Fisone G, Agnati LF, Tanganelli S. Understanding the role of heteroreceptor complexes in the central nervous system. Curr Protein Pept Sci. 2014;15:647. [DOI] [PubMed] [Google Scholar]