Abstract
Steroid sex hormones and genetic sex regulate the phenotypes of motivated behaviors and relevant disorders. Most studies seeking to elucidate the underlying neuroendocrine mechanisms have focused on how 17β-estradiol modulates the role of dopamine in striatal brain regions, which express membrane-associated estrogen receptors. Dopamine action is an important component of striatal function, but excitatory synaptic neurotransmission has also emerged as a key striatal substrate and target of estradiol action. Here, we focus on excitatory synaptic input onto medium spiny neurons (MSNs) in the striatal region nucleus accumbens core (AcbC). In adult AcbC, miniature excitatory postsynaptic current (mEPSC) frequency is increased in female compared with male MSNs. We tested whether increased mEPSC frequency in female MSNs exists before puberty, whether this increased excitability is due to the absence of estradiol or testosterone during the early developmental critical period, and whether it is accompanied by stable neuron intrinsic membrane properties. We found that mEPSC frequency is increased in female compared with male MSNs before puberty. Increased mEPSC frequency in female MSNs is abolished after neonatal estradiol or testosterone exposure. MSN intrinsic membrane properties did not differ by sex. These data indicate that neonatal masculinization via estradiol and/or testosterone action is sufficient for down-regulating excitatory synaptic input onto MSNs. We conclude that excitatory synaptic input onto AcbC MSNs is organized long before adulthood via steroid sex hormone action, providing new insight into a mechanism by which sex differences in motivated behavior and other AbcC functions may be generated or compromised.
17β-estradiol action and genetic sex are emerging as important variables for nervous system function beyond the brain regions directly associated with reproduction (1–3). One example is motivated behavior, reward, and relevant disorders (4). Across humans and rodent animal models, behavioral data show significant sex differences in motivation and reward-based tasks, and also differing phenotypes between sexes in response to drugs of abuse (5, 6). These sex differences in behavior are reflected in key underlying neural substrates such as the striatal brain regions, including the nucleus accumbens core (AcbC). The AcbC is a nexus region that plays a prominent role in controlling behaviors related to both natural and pathological reinforcers, select motivational and emotional processes, and mood disorders (7, 8). All of these exhibit sex differences in phenotype and/or incidence (9, 10). The AcbC expresses membrane-associated estrogen receptors α and β, GPER-1, aromatase (the enzyme that converts the steroid sex hormone testosterone to estradiol), and few if any nuclear hormone receptors (11–13). Thus, this region is an excellent neuroendocrine model system for assessing how noncanonical estrogen signaling modulates neuronal function.
Most research on the role of estradiol in the AcbC and the striatum in general has focused on dopaminergic neurotransmission (14, 15). Dopamine is an important component of striatal function, but another critical player in the AcbC is excitatory glutamatergic synaptic transmission onto medium spiny neurons (MSNs) (16–18). MSNs are the predominant output neuron in the AcbC. MSNs project outside of the AcbC, and are capable of influencing motor and cognitive behaviors (8, 19). They receive multiple glutamatergic inputs, from the hippocampus, amygdala, prefrontal cortex, and thalamus, in addition to dopaminergic and GABAergic inputs (20, 21). Excitatory synaptic input onto AcbC MSNs is essential for generating action potential output and regulating synaptic plasticity (22–27). These excitatory synaptic inputs onto MSNs are implicated in not only motivated behavior and reward activity, but also disorders such as drug addiction, inhibitory control, and depression. In adult rats and humans, excitatory synapse number and markers are increased in female compared with male AcbC (28–31). This includes MSN dendritic spine density (32), which is elevated in females, and is sensitive to acute estradiol exposure (33, 34). Functionally, in the AcbC adult female MSNs receive greater frequencies of miniature excitatory synaptic currents (mEPSCs) compared with male MSNs (29).
Here, we test 3 specific hypotheses regarding excitatory synaptic input onto AcbC MSNs: whether increased mEPSC frequency in female MSNs exists before puberty, whether it is organized via early steroid sex hormone exposure, and whether it is accompanied by stable intrinsic electrophysiological properties. Testing each of these hypotheses makes an important contribution to understanding how functional sex differences in the neural substrate of the AcbC are either formed or interpreted. Testing whether increased mEPSC frequency exists before puberty directly addresses whether this AcbC feature is sexually differentiated early in development, or perhaps during a different developmental critical period such as puberty. Testing whether AcbC excitatory synaptic input is organized by early steroid sex hormones will take a strong step in determining the underlying mechanism, which is of particular interest given that the AcbC expresses membrane-associated estrogen receptors. Finally, testing whether MSN intrinsic electrophysiological properties differ by sex will determine whether the interpretation of these changes should focus primarily on the excitatory synaptic inputs onto MSNs or additionally include homeostatic and/or synergistic alterations in MSN intrinsic properties.
To test these hypotheses we employed an electrophysiological approach, using whole-cell patch clamp recordings of MSNs in acute brain slices of rat AcbC. First, we tested whether increased excitatory input onto female MSNs is established before puberty by measuring mEPSC properties. We found that mEPSC frequency is increased in female compared with male prepubertal rats. Second, we tested whether masculinization/defeminization via exposure to estradiol or testosterone during the neonatal critical period blocks increased mEPSC frequency in females. MSNs from females exposed to estradiol or testosterone as neonates lacked increased mEPSC frequency, indicating that neonatal masculinization/defeminization is sufficient to permanently down-regulate excitatory synaptic input onto MSNs. Third, we tested whether sex differences in excitatory synaptic input are accompanied by stable intrinsic electrophysiological properties. Intrinsic membrane properties did not differ by sex during prepuberty. Overall, these findings indicate that sex differences in excitatory synaptic input in the AcbC are organized by steroid sex hormones long before adulthood. This provides new insight by which sex differences in motivated behavior and other nucleus accumbens functions may be generated or compromised. This is an indication that brain regions expressing membrane-associated estrogen receptors are sensitive to organizational hormone action.
Materials and Methods
Animals
All animal protocols were approved by Institutional Animal Care and Use Committee at North Carolina State University. Female (n = 36) and male (n = 27) Sprague Dawley CD IGS rats were born from timed-pregnant females purchased from Charles River (Charles River). Rats were housed with their littermates and dams in a temperature- and light-controlled room (23°C, 40% humidity, 12-hour light, 12-hour dark cycle) at the Biological Resource Facility of North Carolina State University. Age at recording ranged from postnatal day (P)16 to P23 and was matched between sexes (mean ± SEM: male, P19 ± 1; female, P19 ± 1). Animals were not weaned before experimental use. All cages were washed polysulfone bisphenol A free and were filled with bedding manufactured from virgin hardwood chips (Beta Chip; NEPCO) to avoid the endocrine disruptors present in corncob bedding (35–37). Soy protein-free rodent chow (2020X; Teklad) and glass-bottle provided water were available ad libitum. In select experiments, on P0, P1, and P2, pups received a sc injection (0.05 mL) of either 5% dimethyl sulfoxide in sesame oil (vehicle), or 5% dimethyl sulfoxide and oil containing 100-μg estradiol (males and females) or testosterone (females only), following masculinization/defeminization techniques adapted from previously published protocols (38, 39).
Acute brain slice preparation
Brain slices for electrophysiological recordings were prepared as previously described (40). Briefly, rats were deeply anesthetized with isoflurane gas and killed by decapitation. The brain was then dissected rapidly into ice-cold, oxygenated sucrose artificial cerebrospinal fluid (ACSF) containing 75mM sucrose, 1.25mM NaH2PO4, 3mM MgCl2, 0.5mM CaCl2, 2.4mM Na pyruvate, and 1.3mM ascorbic acid from Sigma-Aldrich, and 75mM NaCl, 25mM NaHCO3, 15mM dextrose, 2mM KCl from Fisher. The osmolarity of the sucrose ACSF was 295–305 mOsm, and the pH was between 7.2 and 7.4. Coronal brain slices (300 μm) were prepared using a vibratome and then incubated in regular ACSF containing 126mM NaCl, 26mM NaHCO3, 10mM dextrose, 3mM KCl, 1.25mM NaH2PO4, 1mM MgCl2, and 2mM CaCl2 (295–305 mOsm; pH 7.2–7.4) for 30 minutes at 30°C–35°C, and then at least 30 minutes at room temperature (21°C–23°C). Slices were stored submerged in room temperature, oxygenated ACSF for up to 5 hours after sectioning in a large volume bath holder.
Electrophysiological recording
Slices were allowed to rest at least 1 hour after sectioning. They were then placed in a Zeiss Axioscope equipped with IR-DIC optics, a Dage IR-1000 video camera, and ×10 and ×40 lenses with optical zoom, and superfused with oxygenated ACSF heated to approximately 28°C (male, 28.50 ± 0.81°C; female, 28.21 ± 1.31°C; P > .05). Whole-cell patch-clamp recordings were used to record the electric properties of MSNs in the AcbC (Figure 1), with glass electrodes (7–11 MΩ) containing 115mM K D-gluconate, 8mM NaCl, 2mM EGTA, 2mM MgCl2, 2mM MgATP, 0.3mM NaGTP, 10mM phosphocreatine from Sigma-Aldrich, and 10mM HEPES from Fisher (285 mOsm; pH 7.2–7.4). Signals were amplified, filtered (2 kHz), and digitized (10 kHz) with a MultiClamp 700B amplifier attached to a Digidata 1550 system and a personal computer using pClamp 10 software. Membrane potentials were corrected for a calculated liquid junction potential of −13.5 mV. Using previously described procedures (41), recordings were first made in current clamp to assess neuronal electrophysiological properties. MSNs were identified by their medium-sized somas, the presence of a slow ramping subthreshold depolarization in response to low-magnitude positive current injections, an action potential amplitude >20 mV, a hyperpolarized resting potential more negative than −65 mV, inward rectification, and prominent spike afterhyperpolarization (42, 43).
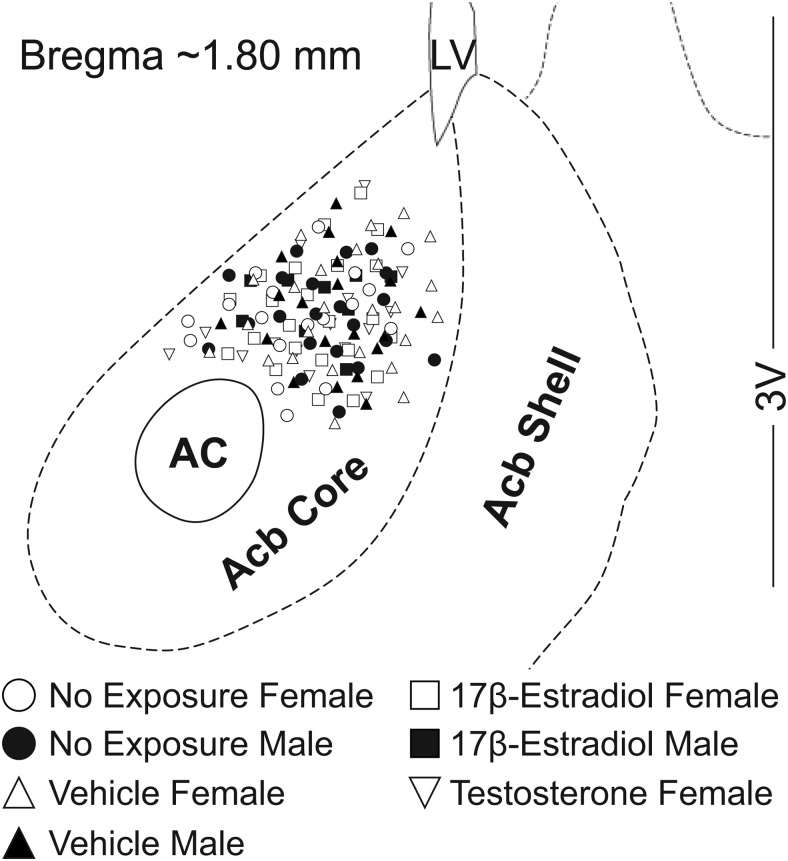
Figure 1.
Location of whole-cell patch clamped MSNs in the AcbC. No exposure males and females represent MSNs recorded from animals not manipulated as neonates. Vehicle, 17β-estradiol, and Testosterone males and females represent MSNs recorded from animals receiving neonatal injections of vehicle or hormone.
In a subset of recordings from unexposed animals, and from all animals exposed to neonatal injections, oxygenated ACSF containing both the GABAA receptor antagonist picrotoxin (PTX) (150μM; Fisher) and the voltage-gated sodium channel blocker tetrodotoxin (TTX) (1μM; Abcam Biochemicals) was applied to the bath to abolish inhibitory postsynaptic current events and action potentials, respectively. Once depolarizing current injection no longer generated an action potential, MSNs were voltage clamped at −70 mV and mEPSCs were recorded for at least 5 minutes. In a different set of experiments, before TTX exposure the paired-pulse ratio was assessed using a protocol adapted from published work in the AcbC (29, 44). EPSCs were evoked using a concentric bipolar stimulating electrode placed approximately 200 μm from the recording electrode in the AcbC. Paired pulses were elicited at 50-millisecond interspike interval, and with a 10-second intersweep interval. At least 6 sweeps were recorded. In all experiments input/series resistance was monitored for changes and cells were excluded if resistance changed more than 25%.
Data recording and analysis
Intrinsic electrophysiological properties and action potential characteristics were analyzed using pClamp 10. After break-in, the resting membrane potential was first allowed to stabilize approximately 1–2 minutes, as in Ref. 45. At least 3 series of depolarizing and hyperpolarizing current injections were applied to elicit basic neurophysiological properties (41). Most properties measured followed the definitions of Ref. 41, which were drawn from those of Perkel and coworkers (46–49). For each neuron, measurements were made of at least 3 action potentials generated from minimal current injections. These measurements were then averaged to generate the reported action potential measurement for that neuron. For action potential measurements, only the first generated action potential was used unless more action potentials were required to meet the standard 3 action potentials per neuron. Action potential threshold was defined as the first point of sustained positive acceleration of voltage (δ2V/δt2) that was also more than 3 times the SD of membrane noise before the detected threshold (50). The slope of the linear range of the evoked firing rate to positive current curve (FI slope) was calculated from the first current stimulus which evoked an action potential to the first current stimulus that generated an evoked firing rate that persisted for at least 2 consecutive current stimuli. Input resistance in the linear, nonrectified range was calculated from the steady-state membrane potential in response to −0.02-nA hyperpolarizing pulses. Rectified range input resistance and percent inward rectification was calculated as described previously, with rectified range input resistance measured using the most hyperpolarizing current injected into the MSN (42). The membrane time constant was calculated by fitting a single exponential curve to the membrane potential change in response to −0.02-nA hyperpolarizing pulses. Membrane capacitance was calculated using the following equation: capacitance = membrane time constant/input resistance. Sag index was used to assess possible sex differences in hyperpolarization-induced “sag” (ie, IH current) (46). Sag index is the difference between the minimum voltage measured during the largest hyperpolarizing current pulse and the steady-state voltage deflection of that pulse, divided by the steady-state voltage deflection. Thus, a cell with no sag would have a sag index of 0, whereas a cell whose maximum voltage deflection is twice that of the steady-state deflection would have a sag index of 1. Cells with considerable sag typically have an index of more than or equal to 0.1.
Frequency, amplitude, and decay of mEPSCs were analyzed off-line using Mini Analysis (Synaptosoft, http://www.synaptosoft.com/MiniAnalysis/). Recordings were filtered (1 kHz), mEPSC threshold was set at a minimum value of 5 pA, and accurate event detection was validated by visual inspection. Paired-pulse ratio was calculated by dividing the absolute magnitude of the second evoked EPSC in the 50-millisecond interval pair over that of the first.
Statistics
Experiments were analyzed as appropriate with 2-tailed t tests, Mann-Whitney U tests, or one- or two-way ANOVAs with Bonferroni post hoc tests (Prism, SigmaPlot 11.0; Systat Software). An a priori outlier analysis was performed and values falling more than 3 SDs from the mean were excluded from analysis. Distributions were analyzed for normality using the D'Agostino and Pearson omnibus normality test (Prism version 5.00; GraphPad Software). P < .05 were considered a priori as significant. Data are presented as mean ± SEM.
Results
We recorded 52 MSNs from prepubertal (P16–P21) male and 67 MSNs from prepubertal female rat AcbC (Figure 1). MSNs compose most neurons in the AcbC, express membrane-associated estrogen receptorα, estrogen receptorβ, and GPER-1 (11), and project outside of the brain region to various targets. Several sex differences are noted in MSN electrophysiological properties across striatal regions and developmental properties (29, 41, 51, 52). Specific to the AcbC, in adult rats, female MSNs receive increased excitatory synaptic input compared with male MSNs, and this input is sensitive to cocaine exposure (28, 29). It is unknown whether this sex difference is organized before puberty.
Increased mEPSC frequency onto female MSNs is established before puberty
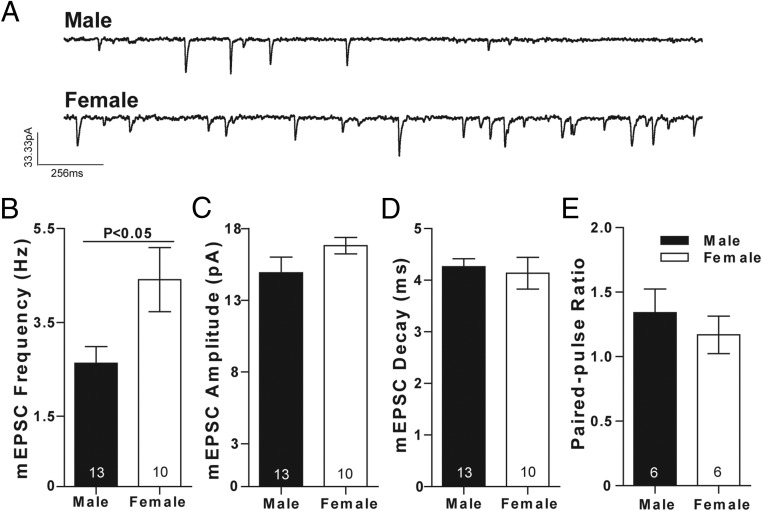
We tested this hypothesis by recording from male and female prepubertal rat MSNs in acute brain slices of the AcbC. MSNs were voltage-clamped at −70 mV, and mEPSCs were recorded in the presence of 1μM TTX and 150μM PTX to block sodium channel-dependent action potentials and GABA receptors, respectively (Figure 2A). mEPSC frequency was higher in female compared with male MSNs (Figure 2B). No sex differences were found in mEPSC amplitude (Figure 2C) or decay (Figure 2D). This suggests that female MSNs receive an increased number excitatory synapses compare with male MSNs. However, differences in mEPSC frequency can also be generated by differences in presynaptic release probability. To test whether release probability differed by sex, we assessed paired pulse ratios in a subset of male and female MSNs. MSNs were voltage clamped at −70 mV, and pairs of EPSCs were elicited at intervals of 50 milliseconds in the presence of PTX. No sex differences were found in paired-pulse ratio (Figure 2E). These data indicate that increased excitatory synaptic input onto female MSNs is organized before puberty and is likely not due to changes in presynaptic release probability.
Figure 2.
mEPSC frequency is increased in prepubertal female compared with male MSNs. A, mEPSCs recorded in male (upper panel) and female (lower panel) AcbC MSNs. MSNs were voltage clamped at −70 mV and recorded in the presence of TTX and PTX to block voltage-gated sodium channels and GABAergic synaptic activity, respectively. B, mEPSC frequency is increased in female compared with male MSNs. C, mEPSC amplitude does not differ by sex. D, mEPSC decay does not differ by sex. E, Paired-pulse ratio does not differ by sex. Experiments depicted without P values do not show significantly differences (P > .05); complete statistical information is in Table 1.
Neonatal exposure to estradiol eliminates increased mEPSC frequency onto female MSNs
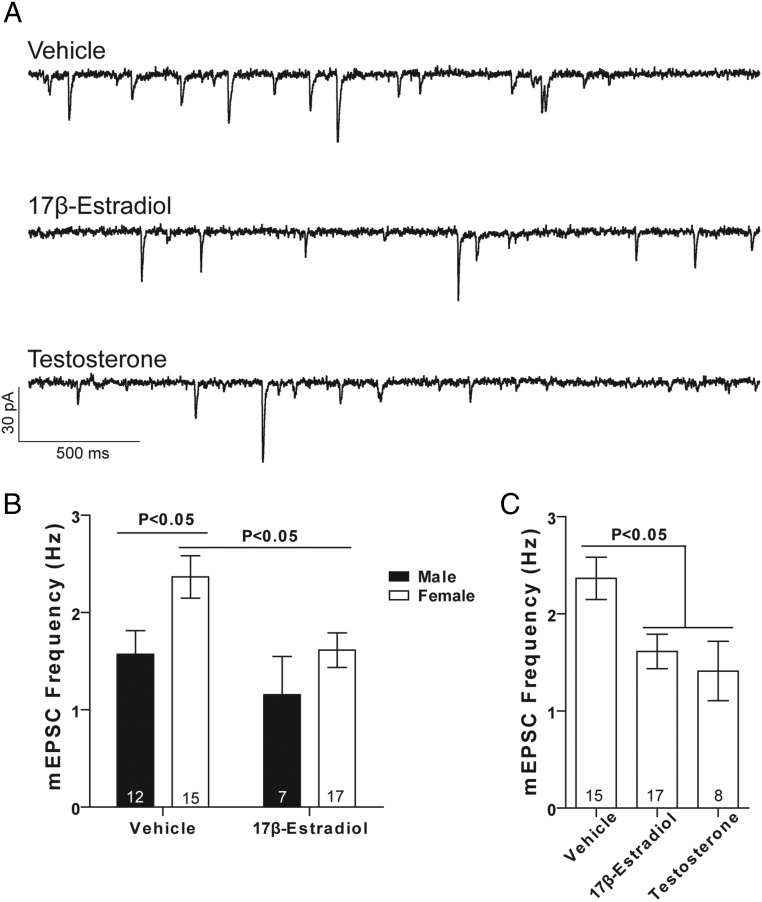
Increased mEPSC frequency in female MSNs before puberty indicates that sex differences in excitatory synaptic input are established early in life. This could potentially be generated by the organizing action of early steroid sex hormone exposure in males, by a genetic program independent of hormone exposure, or via environmental influences. To differentiate between these 3 different mechanisms, we first verified that sc neonatal vehicle injections did not disrupt sex differences in mEPSC frequency. We injected male and female rat pups with vehicle solution on P0, P1, and P2. We made acute brain slices between P16 and P21, and recorded MSNs to measure mEPSC frequency (Figure 3A). Increased mEPSC frequency was observed in female MSNs compared with male MSNs (Figure 3B), as in animals with no injections. We then followed the same experimental timeline, but instead performed neonatal injections of estradiol in females and males. We reasoned that if neonatal exposure to masculinizing/defeminizing levels of estradiol decreases mEPSC frequency in males, then a comparable hormone exposure in females should also decrease mEPSC frequency. We further reasoned that additional exposure to estradiol in males should have no further effects on mEPSC frequency, given that in male animals this process would normally already be occurring.
Figure 3.
Early hormone exposure regulates mEPSC frequency. A, mEPSCs recorded from MSNs in prepubertal female animals exposed as neonates to: (upper panel) vehicle, (middle panel) 17β-estradiol, and (lower panel) testosterone. B, Neonatal exposure to estradiol decreases mEPSC frequency in prepubertal female MSNs to levels found in male MSNs. C, In MSNs recorded from prepubertal females, neonatal exposure to testosterone decreases mEPSC frequency to levels indistinguishable from animals exposed to estradiol. The P value within each subpanel indicates statistical significance; complete statistical information is in Table 2.
Neonatal estradiol exposure eliminated increased mEPSC frequency in female MSNs (Figure 3B). In MSNs from males exposed to estradiol, mEPSC frequency was indistinguishable from MSNs in males not exposed to estradiol. In contrast to animals not exposed to sc injections, subtle sex and/or estradiol differences were detected in mEPSC amplitude or decay, with female MSNs displaying increased mEPSC amplitude and decay (see table 2 below). These results support the hypothesis that early estradiol exposure decreases excitatory synaptic input onto MSNs, a process that would normally only occur in males.
Neonatal exposure to testosterone eliminates increased mEPSC frequency onto female MSNs
The precursor hormone to estradiol is testosterone. In male rodents, testosterone is secreted by the testes both in utero and in neonates. In the brain, testosterone can then either act on androgen receptors, or be aromatized into estradiol and act on estrogen receptors. Given this action on estrogen receptors, we reasoned that neonatal exposure of females to testosterone should also decrease mEPSC frequency. To test this, we followed the same experimental timeline as above, but this time with neonatal injections of testosterone. Neonatal testosterone exposure eliminated increased mEPSC frequency in female MSNs (frequency, 1.4 ± 0.3 Hz; amplitude, 15.17 ± 0.91 pA; decay, 3.44 ± 0.25 ms). Overall mEPSC frequency was not different from that in MSNs recorded from females exposed to estradiol (Figure 3C). This demonstrates that exposure to either estradiol or its precursor hormone, testosterone, is sufficient to decrease excitatory synaptic input onto female MSN to a masculine-like phenotype.
Membrane excitability and action potential properties do not differ by sex
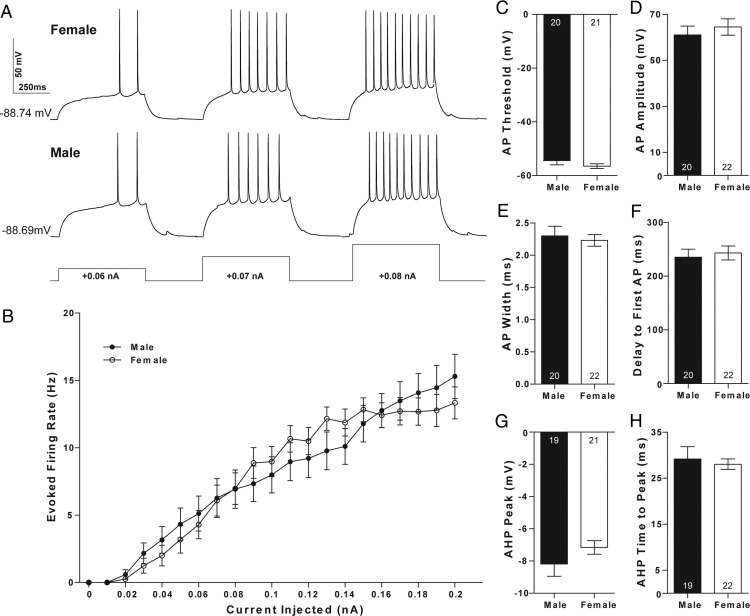
MSNs can exhibit significant differences in intrinsic membrane electrophysiological properties in multiple experimental paradigms (18, 53, 54). For example, intrinsic excitability is increased in female compared with male MSNs in prepubertal rat caudate-putamen (41). Thus, in order to properly interpret the significance of increased mEPSC frequency in females, it is necessary to also test the hypothesis that the intrinsic membrane properties of female MSNs differ from those recorded from males. To accomplish this, we injected a series of positive and negative currents into MSNs recorded from prepubertal male and female rats not manipulated as neonates to comprehensively assess intrinsic membrane electrophysiological properties before mEPSC assessment. Overall, MSN electrophysiological properties were very similar to those reported in previously published studies, including a slow ramping subthreshold depolarization in response to low-magnitude positive current injections, inward rectification, and a hyperpolarized resting potential (Figure 4A) (42, 43, 55).
Figure 4.
Action potential properties of MSNs do not differ by sex. A, Voltage response of a prepubertal female (upper panel) and male (lower panel) MSN to a series of positive current injections. B, No sex differences were detected in action potential firing rates evoked by positive current injection. No sex differences were detected in (C) action potential threshold, (D) action potential amplitude, (E) action potential width, (F) the delay to first action potential, (G) action potential afterhyperpolarization peak, and (H) action potential afterhyperpolarization time to peak. The P value of all experiments was P > .05; complete statistical information is in Table 1.
MSN excitability did not differ by sex in AcbC MSNs, as assessed by analyzing the action potential firing rates evoked by depolarizing current injection (Figure 4B), and by comparing the slopes of the evoked firing rate with positive current curve (FI slope) (Table 1). Similarly, no sex differences were detected in MSN action potential properties (Table 1). This included action potential threshold (Figure 4C), amplitude (Figure 4D), and width (Figure 4E). No sex difference was detected in the delay to first action potential (Figure 4F), which reflects the magnitude of the slowly inactivating A-current responsible for the slow ramping subthreshold depolarization found in MSNs (56). Finally, unlike caudate-putamen MSNs, no sex differences were detected in the action potential afterhyperpolarization peak (Figure 4G) or time to afterhyperpolarization peak (Figure 4H). These data indicate that MSN excitability and action potential properties do not differ by sex, suggesting that female MSNs do not exhibit a compensatory response in evoked action potential number or action potential properties to increased mEPSC frequency.
Table 1.
Electrophysiological Properties of MSNs in Prepubertal Male and Female Rat AcbC
| Property | Male | Female | Statistics (t/U, P) |
|---|---|---|---|
| Resting membrane potential (mV) | −83.09 ± 1.83 (20) | −86.52 ± 1.16 (21) | 1.60; 0.12 |
| Input resistance (MΩ) | 343.00 ± 50.48 (19) | 247.70 ± 22.60 (21) | 166, 0.37 |
| Time constant of the membrane (ms) | 23.98 ± 2.51 (19) | 19.87 ± 1.40 (20) | 1.45; 0.16 |
| Capacitance (pF) | 79.08 ± 6.50 (20) | 84.99 ± 6.56 (21) | 186; 0.54 |
| Rectified range input resistance (MΩ) | 195.90 ± 21.79 (19) | 152.90 ± 10.93 (20) | 1.79; 0.08 |
| % inward rectification (%) | 62.34 ± 3.09 (20) | 66.10 ± 2.01 (22) | 182; 0.35 |
| Sag index | 0.008 ± 0.002 (20) | 0.007 ± 0.001 (22) | 219; 0.99 |
| AP threshold (mV) | −54.74 ± 1.40 (20) | −56.50 ± 0.88 (21) | 1.08; 0.29 |
| AP amplitude (mV) | 61.11 ± 3.45 (20) | 64.48 ± 3.41 (22) | 0.69; 0.49 |
| AP width (ms) | 2.30 ± 0.14 (20) | 2.23 ± 0.09 (22) | 0.41; 0.69 |
| AHP peak (mV) | −8.14 ± 0.81 (19) | −7.16 ± 0.420 (21) | 1.11; 0.27 |
| AHP time to peak (ms) | 29.19 ± 2.69 (19) | 28.00 ± 1.30 (22) | 0.42; 0.68 |
| Delay to first AP (ms) | 234.20 ± 15.26 (20) | 242.8 ± 12.48 (22) | 0.44, 0.66 |
| Rheobase (nA) | 0.06 ± 0.01 (20) | 0.07 ± 0.01 (22) | 0.43, 0.67 |
| FI slope (Hz/nA) | 231.50 ± 20.22 (17) | 233.55 ± 17.66 (22) | 0.08, 0.94 |
| mEPSC frequency (Hz) | 2.60 ± 0.35 (13) | 4.41 ± 0.68 (10) | 2.52, 0.02 |
| mEPSC amplitude (pA) | 14.52 ± 1.12 (13) | 16.81 ± 0.57 (10) | 1.66, 0.11 |
| mEPSC decay (ms) | 4.20 ± 0.18 (13) | 4.14 ± 0.31 (10) | 0.20, 0.84 |
Values are mean ± SEM. Numbers in parentheses indicate sample size. The sag index is unitless. Significant differences are indicated with bold font. Recordings were made from prepubertal male and female rats that did not receive injections as neonates. AP, action potential; AHP, afterhyperpolarization.
MSN membrane properties do not differ by sex
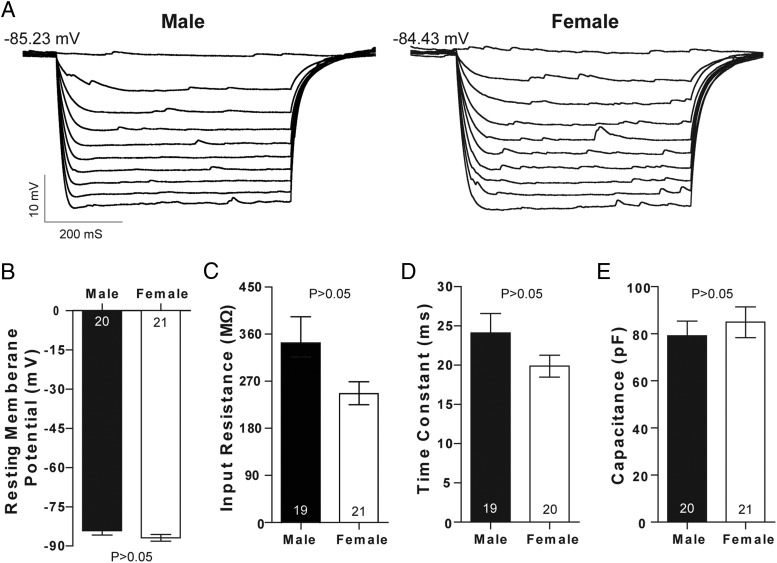
We then tested the hypothesis that intrinsic membrane electrophysiological properties differed by sex, including input resistance, τ, and capacitance. Overall, these MSN properties did not differ by sex (Figure 5A). This includes the resting membrane potential (Figure 5B), the input resistance in the nonrectified range (Figure 5C), and the time constant of the membrane (τ) (Figure 5D). Capacitance also did not differ by sex, consistent with previous work that detected no sex differences in AcbC neuron soma size (Figure 5E) (57).
Figure 5.
Passive MSN electrophysiological properties do not significantly differ by sex. A, Voltage response of a prepubertal male (left) and female (right) MSN to a series of negative current injections. No sex differences were detected in (B) resting membrane potential, (C) input resistance in the nonrectified range, D) the time constant of the membrane (τ), and (E) capacitance. The P value of all experiments was P > .05; complete statistical information is in Table 1.
We then analyzed inward rectification. Inward rectification in response to hyperpolarizing current is a prominent feature of MSNs (42, 58) and is thought to help stabilize MSN resting membrane potential. Although there were no significant differences in inward rectification (P = .549), we note that mean inward rectification values appeared to be more pronounced in female compared with male MSNs when a current-voltage curve was plotted (Figure 6A). We thus employed more rigorous assessments of inward rectification, including the input resistance of the rectified range (Figure 6B), and percent inward rectification (Figure 6C). Neither of these analyses detected sex differences. Supporting this conclusion, MSNs recorded from animals exposed to vehicle or estradiol as neonates showed no differences in current-voltage properties (Figure 7A), including rectified range input resistance (Figure 7B) and input resistance in the nonrectified range (Figure 7C). Collectively, these data indicate that MSN input resistance properties show no or minimal differences by sex, including both the rectified and nonrectified ranges.
Figure 6.
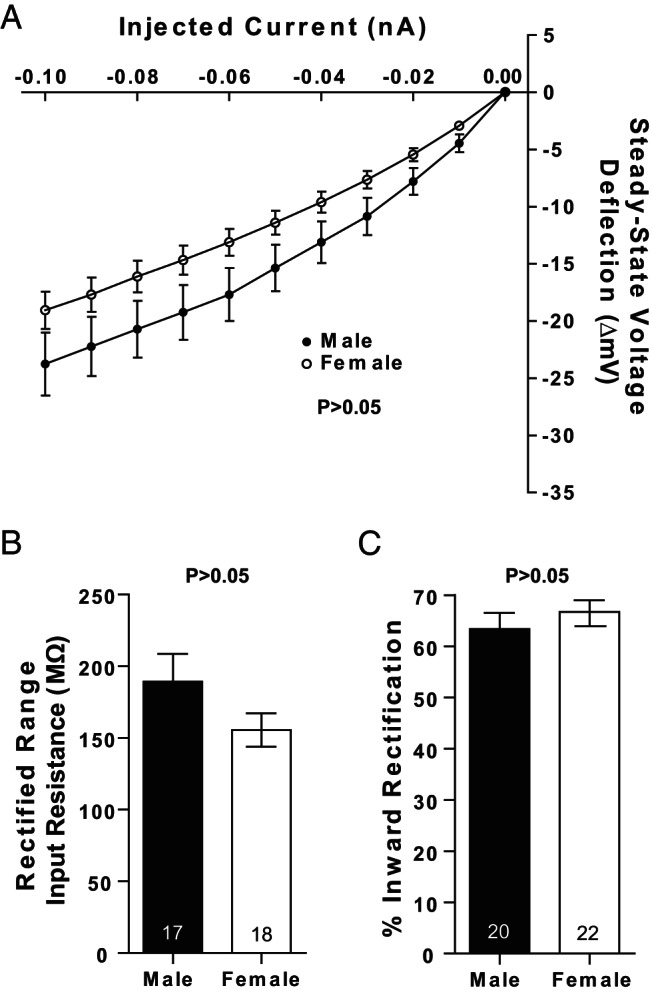
Input resistance in the rectified range does not significantly differ by sex. A, No sex differences were detected in input resistance in either the linear or rectified range in MSNs recorded from prepubertal animals. No sex differences were detected in (B) rectified range input resistance and (C) percent inward rectification. The P value of all experiments was P > .05; complete statistical information is in Table 1.
Figure 7.
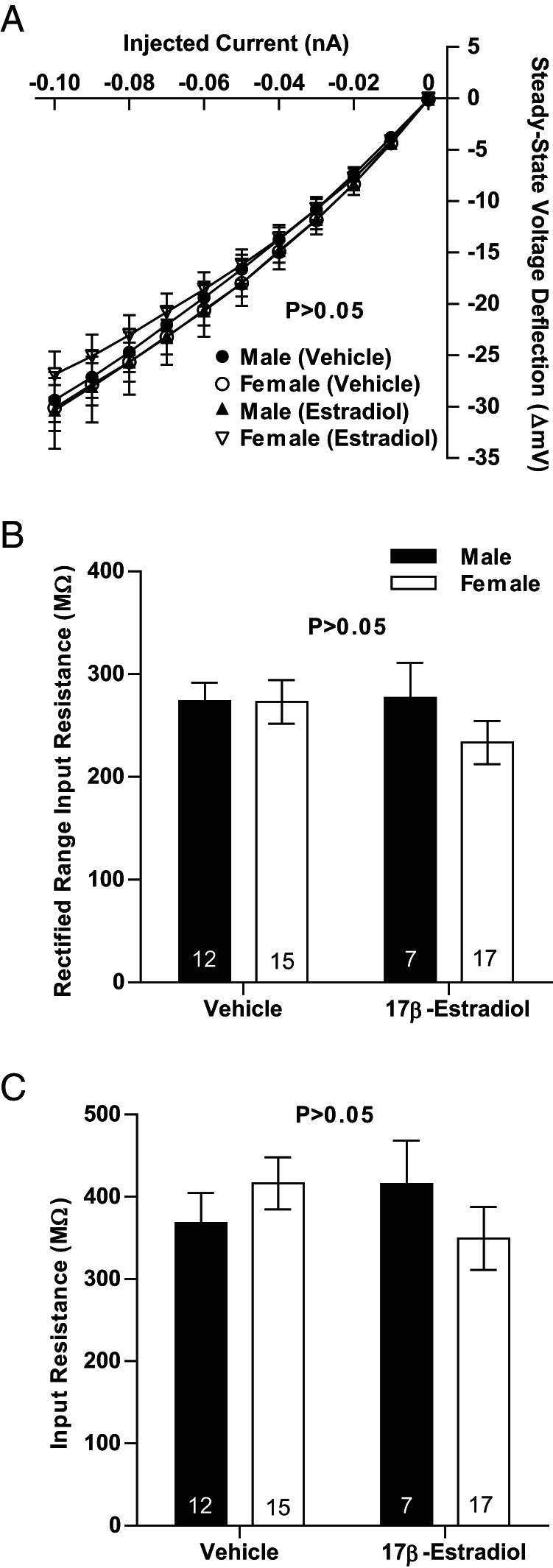
Input resistance in the rectified and nonrectified ranges does not vary by neonatal hormone exposure. A, No differences were detected in input resistance in either the linear or rectified range in MSNs recorded from prepubertal animals exposed to vehicle or estradiol as neonates. B, Neonatal exposure to estradiol does not modify rectified range input resistance. C, Neonatal exposure to estradiol does not modify input resistance in the nonrectified range. The P value of all experiments was P > .05.
Regarding other properties, MSNs recorded from animals exposed to vehicle or estradiol as neonates showed no differences between experimental groups in the time constant of the membrane (P > .05, vehicle-exposed males, 22.6 ± 4.1 ms; vehicle-exposed females, 27.0 ± 1.8 ms; estradiol-exposed males, 24.5 ± 3.5 ms; estradiol-exposed females, 24.2 ± 3.0 ms), and capacitance (P > .05, vehicle-exposed males, 58.3 ± 5.3 pF; vehicle-exposed females, 68.5 ± 5.5 pF; estradiol-exposed males, 59.1 ± 4.2 pF; estradiol-exposed females, 68.0 ± 5.8 pF). Similarly, MSNs recorded from animals exposed to vehicle or estradiol as neonates showed no differences between experimental groups in the slopes of the evoked firing rate to positive current curve (P > .05; FI slope; vehicle-exposed males, 181.0 ± 15.4 Hz/nA; vehicle-exposed females, 247.1 ± 22.5 Hz/nA; estradiol-exposed males, 277.8 ± 36.7 Hz/nA; estradiol-exposed females, 229.4 ± 26.0 Hz/nA). However, overall an interaction was detected between estradiol exposure and sex (F = 5.4, P = .0245). This is difficult to interpret as the factors sex or estradiol exposure alone were not significant (sex: F = 0.16, P > .05, estradiol exposure: F = 2.65, P > .05).
Discussion
These studies make 3 significant findings. First, increased excitatory synaptic input, as indicated by increased mEPSC frequency, is present in female compared with male MSNs before puberty. Second, neonatal exposure to steroid sex hormones blocks increased excitatory synaptic input onto MSNs. Third, MSN intrinsic membrane properties do not differ by sex before puberty. These data indicate that hormone action during early development is a mechanism by which sex differences in excitatory synaptic input in the AcbC are induced. This provides a route by which sex differences in motivated behavior and other AcbC functions are generated, and also illustrates a potential vulnerability of these processes to endocrine disruption. Broadly, this positions the AcbC as an excellent neuroendocrine model system for elucidating how estradiol changes neuronal electrophysiology properties in an important brain region that expresses nonnuclear hormone receptors.
Alterations in excitatory synaptic transmission play a strong role in mediating AcbC function in a variety of contexts (17, 18, 32, 59–62). For example, extensive research has been devoted to the role of AcbC excitatory synaptic input in the response to drugs of abuse, which shows phenotypic sex differences (5, 6). At least one part of the mechanism underlying these sex differences are elevated excitatory synapse number in adult female compared with male MSNs in the AcbC (28–30). Data from prepubertal animals with no neonatal manipulations support and extend this basic model. mEPSC frequency is increased in female compared with male MSNs and is accompanied by comparable paired-pulse ratios, mEPSC amplitude and decay.
We also found no significant differences in AcbC MSN membrane properties. This indicates that female AcbC MSN membrane properties do not seem to exhibit homeostatic and/or synergistic plasticity to increased excitatory synaptic input in this context. This focuses the working model towards neuroanatomical inputs onto MSNs, and reduces the probability that increased excitatory synaptic input onto MSNs is a compensatory mechanism related to alterations in MSN intrinsic excitability. Rather, these findings argue that there are fundamental differences in the local neuroanatomy of the AcbC between males and females that are sculpted very early in development. These differences include at least excitatory synaptic input (28–30) and dopaminergic input (63), which then perhaps together more strongly converge onto female AcbC MSNs to produce sex and/or individual differences in motivated behaviors. Indeed, there is evidence that female AcbC MSNs feature an increased proportion of large head dendritic spines near dopaminergic terminals than male MSNs (30). These natural sex differences then consequently produce sex differences in AcbC disorders such as the responsiveness to drugs of abuse.
A finding that augments this basic model of increased synapse number in female AcbC is that animals exposed to vehicle or estradiol as neonates showed differences not only in mEPSC frequency, but also in mEPSC amplitude, and mEPSC decay (Table 2). Given these results, we cannot rule out that sex differences in excitatory input encompass more than just synapse number. Although this caveat is not directly supported by the above-cited studies or data from animals not injected as neonates, it is important to acknowledge that MSN excitatory synapses are malleable in other contexts. For example, in adult caudate-putamen, estradiol interacts with dopaminergic inputs to induce excitatory synaptic plasticity (64). Estradiol can also regulate dopaminergic and GABAergic transmission in striatal regions (15, 65–68). Other examples of modulation in AcbC excitatory input includes AMPA subunit composition and number, N-methyl-D-aspartate receptors and silent synapse formation, and Glutamate receptor A2 subunit incorporation (17, 18, 59, 60, 69). It is also possible that there was a vehicle and/or injection stress effect on these animals that modified genetic sex and/or hormone influences on excitatory synaptic input. Although increased mEPSC frequency in female MSN compared with male MSN was maintained in animals receiving neonatal injections of vehicle solution, the overall magnitude of mEPSC frequency was decreased in males and females receiving neonatal injections compared with those that did not. This may be due to a stress effect from either neonatal handling and/or sc injections. The nucleus accumbens is linked with stress, anxiety, depression and the prefrontal cortex and limbic systems (20, 70, 71). Beyond the nucleus accumbens, perinatal stress in particular has been linked to masculinization of behavior and relevant biological substrates in a number of contexts across species (72–75) and can potentially disrupt the perinatal testosterone surge in males (76, 77). With these considerations, we conclude that estradiol and testosterone exposure are sufficient to permanently organize sex differences in excitatory synapse number, but that under some circumstances, there is the potential for additional effects on excitatory synaptic transmission.
Table 2.
mEPSC Properties of AcbC MSNs Recorded From Prepubertal Male and Female Rats Exposed to Vehicle or Estradiol as Neonates
| mEPSC Property | Neonate Exposure | Male | Female | Statistics (F, P) |
|---|---|---|---|---|
| Frequency (Hz) | Vehicle | 1.57 ± 0.24 (12) | 2.37 ± 0.22 (15) | Interaction: 0.46, 0.50 |
| Estradiol | 1.16 ± 0.39 (7) | 1.61 ± 0.18 (17) | Sex: 6.42, 0.01 | |
| Hormone: 5.59, 0.02 | ||||
| Amplitude (pA) | Vehicle | 11.63 ± 0.31 (12) | 12.90 ± 0.52 (15) | Interaction: 0.01, 0.93 |
| Estradiol | 14.02 ± 1.13 (7) | 15.36 ± 0.81 (17) | Sex: 4.36, 0.04 | |
| Hormone: 8.84, 0.01 | ||||
| Decay (ms) | Vehicle | 2.94 ± 0.24 (12) | 3.75 ± 0.24 (15) | Interaction: 1.09, 0.30 |
| Estradiol | 3.17 ± 0.34 (7) | 3.98 ± 0.20 (17) | Sex: 11.29, 0.002 | |
| Hormone: 0.01, 0.91 |
Values are mean ± SEM. Numbers in parentheses indicate sample size. Data from animals unexposed to steroid sex hormones as neonates are located in Table 1. Select membrane properties analyzed in these experiments are presented in Results.
AcbC excitatory synapse appear more sensitive to both the organizational and activational effects of steroid sex hormones than those in the caudate-putamen or nucleus accumbens shell (Table 3) (28–31, 33, 34, 41, 52, 78). Regarding organizational action, this includes the data presented here, which show that neonatal estradiol or testosterone exposure permanently suppresses mEPSC frequency in female MSN. These data support the hypothesis that sex differences in mEPSC frequency are generated via the classical organizational/aromatization hypothesis (79–81). In this model, plasma testosterone levels are elevated in utero and in neonatal male rodents. Testosterone can act directly on androgen receptors, or be metabolized into estradiol via aromatase. Estradiol can then stimulate either nuclear or membrane-associated estrogen receptors to induce permanent changes in cellular phenotypes (38, 82). Estrogens and androgens can then act either independently or together during the perinatal period to induce sexual differentiation. This organizational/aromatization process is not exclusive. Both epigenetic/environmental and genetic sex influences independent of gonadal sex have been implicated in other aspects of striatal sexual differentiation (71, 83–85).
Table 3.
Development of Sex Differences in MSN Electrophysiology Across Striatal Regions
| Electrophysiological Property | Developmental Stage | Caudate-Putamen | AcbC | Nucleus Accumbens Shell |
|---|---|---|---|---|
| Intrinsic excitability | Prepuberty | ♀ > ♂ | ♀ = ♂ | ♀ = ♂ |
| adult | ? | ? | ? | |
| Excitatory synaptic Input | Prepuberty | ♀ = ♂ | ♀ > ♂ | ♀ = ♂ |
| adult | ? | ♀ > ♂ | ♀ = ♂ (?) |
Please see Discussion section for relevant citations.
The data presented here establish that either estradiol or testosterone exposure during the neonatal period is sufficient to sexually differentiate excitatory synaptic transmission onto AcbC MSN. The experiments and interpretation presented here primarily focus on estradiol action and the potential action of testosterone aromatized to estradiol. This is because androgen receptor expression in the nucleus accumbens seems to be sparse (86–88), that there does not seem to be androgen receptor immunoreactivity in nucleus accumbens axons or dendrites as detected by light microscopy (89), that testosterone exposure does not rapidly increase dopamine release in the nucleus accumbens (90), and because androgen-sensitive reward seems to be mediated in the nucleus accumbens shell, not the AcbC (91). However, in another striatal region, the caudate-putamen, the relatively low immunocytochemical expression of androgen receptor does vary by estrous cycle in the rat (92), and an exhaustive search for genomic androgen receptor expression has not been performed in the AcbC across estrous cycle in the adult rat. It is also important to note that there is evidence for membrane androgen receptor action playing a role in androgen-sensitive reward (93), and that the definitive electron microscopy studies necessary to determine membrane androgen receptor expression have not to our knowledge been performed in the AcbC. Thus, although we favor the hypothesis that testosterone is acting through an estrogenic metabolite, it is important to note that the experiments presented here do not exclude the possibility that androgen receptor action is also by itself sufficient to masculinize/defeminize excitatory synaptic transmission onto AcbC MSN. To definitively test this hypothesis, future experiments will need to expose males to an aromatase inhibitor during the perinatal period, and/or expose females to a nonaromatizable androgen such as DHT during the perinatal period. Also, several brain regions that directly or indirectly interact with the nucleus accumbens are also potentially sensitive to androgens, including, for example, the ventral tegmental area, prefrontal cortex, and basolateral amygdala (94–96). These regions are also sensitive to estrogen action, so another potential future direction should be to isolate the locus of steroid sex hormone action.
The data presented here do not differentiate between prenatal and postnatal hormone action in either males or females, other than elucidating that sex differences in excitatory synaptic input are established before puberty. Establish the exact developmental period for hormone action is important avenue for future experiments because prenatal and postnatal hormone exposure can act in combination to sculpt neural substrate and behavior (97, 98). One recent example is that both prenatal and postnatal hormone programming influences the sexually differentiation of the GnRH surge in sheep (99). We also note that the perinatal period is not the only period in which steroid sex hormones can induce organizational changes in neural substrate. For example, hormone action during the pubertal period can play a significant role in altering the properties of brain regions and related behaviors (100). In the case of the AcbC, it has previously been demonstrated by Woolley and coworkers (29) that sex differences in excitatory synapse are present in adulthood. These findings, in combination with data presented here, suggest that puberty is not necessary for sexual differentiation of excitatory synaptic input into the AcbC. With that said, these data do not exclude puberty from playing a modulatory role, especially perhaps in regulating the magnitude of the sex difference in excitatory synaptic input or its potential for synaptic plasticity. It is also certainly possible that puberty could play a role in organizing neural properties not addressed by this study. One example is the sex difference in overproduction and then elimination of D1 and D2 dopamine receptors during the peripubertal period, which differs by sex (101–103). Another potential example is MSN intrinsic membrane properties, which have not yet been examined in adult animals (Table 3).
Regarding temporary, activational actions, acute estradiol exposure decreases AcbC MSN spine density in adult females (33, 34). This is blocked by mGluR inhibitors (33), as is estradiol's acute actions on cocaine self-administration (104), and is not reliably detected in other striatal regions. This is interesting given that the caudate-putamen, nucleus accumbens shell, and AcbC contain the same neuron types. Likewise, all of the striatal regions show no or minimal sex differences in gross volume, neuron morphology or density (57, 105), and all express membrane-associated estrogen receptor-α or estrogen receptor-β, GPER-1, aromatase, and few if any nuclear estrogen receptors (11–13, 51, 106–111).
Although all striatal regions express membrane-associated estrogen receptors in MSNs, it is possible that there is differential expression across other cell types and terminals. Electron microscopy studies detected membrane-associated estrogen receptor-α and GPER-1 expression in dopaminergic terminals in the nucleus accumbens, but not the caudate-putamen (11, 106). We also note that AcbC MSNs comprise at least 2 subtypes which show differential efferent projections and dopamine receptor and neuropeptide expression (112). It is unknown at this point which MSN subtypes express estrogen receptors and show sex differences. We suspect that both MSN subtypes express estrogen receptors, given the relatively high expression of membrane-associated estrogen receptors in the AcbC and caudate-putamen (11, 106, 113), the robust sex difference in mEPSC properties (28–30), and the broad sensitivity of AcbC MSNs to estradiol (33, 34). Future experiments should address this, perhaps using adeno-associated viruses (114), retrograde labeling (115) or transgenic mice (116, 117). One drawback of using transgenic mice to test neuroendocrine questions is that neural sex differences vary by strain. An example is the volume of the sexually dimorphic nucleus of the medial preoptic area (118). Sex ratios in sexually dimorphic nucleus of the medial preoptic area volume vary by mouse strain (119), and do not necessarily match those detected in other species, including rats, primates, and humans (120). Along with differences between mice strains, we also note that there are species differences between mice and rats in other neuroendocrine-relevant metrics as well (121).
Another candidate hypothesis explaining the sensitivity of the AcbC to estradiol is that the ontogeny of estrogen receptor and/or aromatase expression might differ between striatal regions during critical developmental periods. Expression of estrogen receptor and aromatase cDNA has been confirmed in neonatal striatum (108, 109). This technique does not necessarily differentiate between nuclear and membrane-associated estrogen action, given that GPER-1 is encoded by a different gene than other estrogen receptors (122), and that estrogen receptors α/β can be directed to the membrane via posttranscriptional modifications (123, 124). The underlying assumption of this possibility is that the locus of estrogen action is in the AcbC itself. Alternatively, estradiol may be acting in an afferent brain region to the AcbC to induce a transsynaptic hormone effect, as previously demonstrated in a songbird striatal region (125). This could perhaps involve release of a growth factor such as brain-derived neurotrophic factor, which has been implicated separately in estradiol-induced and AcbC-relevant plasticity (126, 127). For this alternative hypothesis to be viable, the AcbC must receive an estrogen and/or androgen-sensitive projection that other striatal regions do not, and exhibit a different developmental pattern in sex differences in excitatory synaptic input onto MSN compared with other striatal regions (Table 3). Neuroanatomical studies show that the AcbC receives a different pattern of glutamatergic afferents than the caudate-putamen or nucleus accumbens shell (20, 24). The AcbC has been shown to be differentially involved not only in locomotor behaviors (128, 129), but also other maternal, social, reward, learning, sensorimotor, and sex-related behaviors compared with other striatal regions such as the caudate-putamen and nucleus accumbens shell (128–136). These hypotheses, along with elucidating the identity of the underlying estrogen-sensitive receptor(s), should be addressed in future experiments.
Acknowledgments
We thank Jaime Willett, Caitlin Hauser, and Dr Heather Patisaul for their support.
This work was supported by North Carolina State University Start-Up Funds (J.M.) and North Carolina State Faculty Research and Professional Development Grant (J.M.), and National Institutes of Health grants R01MH109471 (J.M.) and P30ES025128 (Center for Human Health and the Environment).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AcbC
- nucleus accumbens core
- ACSF
- artificial cerebrospinal fluid
- GABA
- gamma-Aminobutyric acid
- GPER-1
- G Protein-Coupled Estrogen Receptor 1
- mEPSC
- miniature excitatory synaptic current
- MSN
- medium spiny neuron
- P
- postnatal day
- PTX
- picrotoxin
- TTX
- tetrodotoxin.
References
- 1. Cahill L. Fundamental sex difference in human brain architecture. Proc Natl Acad Sci USA. 2014;111:577–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McCarthy MM, Arnold AP, Ball GF, Blaustein JD, De Vries GJ. Sex differences in the brain: the not so inconvenient truth. J Neurosci. 2012;32:2241–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. de Vries GJ, Forger NG. Sex differences in the brain: a whole body perspective. Biol Sex Differ. 2015;6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yoest KE, Cummings JA, Becker JB. Estradiol, dopamine and motivation. Cent Nerv Syst Agents Med Chem. 2014;14:83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Becker JB, Koob GF. Sex differences in animal models: focus on addiction. Pharmacol Rev. 2016;68:242–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carroll ME, Anker JJ. Sex differences and ovarian hormones in animal models of drug dependence. Horm Behav. 2010;58:44–56. [DOI] [PubMed] [Google Scholar]
- 7. Salgado S, Kaplitt MG. The nucleus accumbens: a comprehensive review. Stereotact Funct Neurosurg. 2015;93:75–93. [DOI] [PubMed] [Google Scholar]
- 8. Yager LM, Garcia AF, Wunsch AM, Ferguson SM. The ins and outs of the striatum: role in drug addiction. Neuroscience. 2015;301:529–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McLean CP, Anderson ER. Brave men and timid women? A review of the gender differences in fear and anxiety. Clin Psychol Rev. 2009;29:496–505. [DOI] [PubMed] [Google Scholar]
- 10. Young E, Korszun A. Sex, trauma, stress hormones and depression. Mol Psychiatry. 2010;15:23–28. [DOI] [PubMed] [Google Scholar]
- 11. Almey A, Milner TA, Brake WG. Estrogen receptors in the central nervous system and their implication for dopamine-dependent cognition in females. Horm Behav. 2015;74:125–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Foidart A, Harada N, Balthazart J. Aromatase-immunoreactive cells are present in mouse brain areas that are known to express high levels of aromatase activity. Cell Tissue Res. 1995;280:561–574. [DOI] [PubMed] [Google Scholar]
- 13. Stanic D, Dubois S, Chua HK, et al. Characterization of aromatase expression in the adult male and female mouse brain. I. Coexistence with oestrogen receptors α and β, and androgen receptors. PLoS One. 2014;9:e90451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29:36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Di Paolo T. Modulation of brain dopamine transmission by sex steroids. Rev Neurosci. 1994;5:27–41. [DOI] [PubMed] [Google Scholar]
- 16. Gipson CD, Kalivas PW. More cocaine-more glutamate-more addiction. Biol Psychiatry. 2014;76:765–766. [DOI] [PubMed] [Google Scholar]
- 17. Grueter BA, Rothwell PE, Malenka RC. Integrating synaptic plasticity and striatal circuit function in addiction. Curr Opin Neurobiol. 2012;22:545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wolf ME. The Bermuda Triangle of cocaine-induced neuroadaptations. Trends Neurosci. 2010;33:391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Smith RJ, Lobo MK, Spencer S, Kalivas PW. Cocaine-induced adaptations in D1 and D2 accumbens projection neurons (a dichotomy not necessarily synonymous with direct and indirect pathways). Curr Opin Neurobiol. 2013;23:546–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Groenewegen HJ, Wright CI, Beijer AV, Voorn P. Convergence and segregation of ventral striatal inputs and outputs. Ann NY Acad Sci. 1999;877:49–63. [DOI] [PubMed] [Google Scholar]
- 21. Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev. 2004;27:765–776. [DOI] [PubMed] [Google Scholar]
- 22. Mulder AB, Hodenpijl MG, Lopes da Silva FH. Electrophysiology of the hippocampal and amygdaloid projections to the nucleus accumbens of the rat: convergence, segregation, and interaction of inputs. J Neurosci. 1998;18:5095–5102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. O'Donnell P, Grace AA. Synaptic interactions among excitatory afferents to nucleus accumbens neurons: hippocampal gating of prefrontal cortical input. J Neurosci. 1995;15:3622–3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Britt JP, Benaliouad F, McDevitt RA, Stuber GD, Wise RA, Bonci A. Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron. 2012;76:790–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Papp E, Borhegyi Z, Tomioka R, Rockland KS, Mody I, Freund TF. Glutamatergic input from specific sources influences the nucleus accumbens-ventral pallidum information flow. Brain Struct Funct. 2012;217:37–48. [DOI] [PubMed] [Google Scholar]
- 26. Sesack SR, Carr DB, Omelchenko N, Pinto A. Anatomical substrates for glutamate-dopamine interactions: evidence for specificity of connections and extrasynaptic actions. Ann NY Acad Sci. 2003;1003:36–52. [DOI] [PubMed] [Google Scholar]
- 27. Stuber GD, Sparta DR, Stamatakis AM, et al. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475:377–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Forlano PM, Woolley CS. Quantitative analysis of pre- and postsynaptic sex differences in the nucleus accumbens. J Comp Neurol. 2010;518:1330–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wissman AM, McCollum AF, Huang GZ, Nikrodhanond AA, Woolley CS. Sex differences and effects of cocaine on excitatory synapses in the nucleus accumbens. Neuropharmacology. 2011;61:217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wissman AM, May RM, Woolley CS. Ultrastructural analysis of sex differences in nucleus accumbens synaptic connectivity. Brain Struct Funct. 2012;217:181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sazdanovic M, Slobodanka M, Zivanovic-Macuzic I, et al. Sexual dimorphism of medium-sized neurons with spines in human nucleus accumbens. Arch Biol Sci Belgrade. 2013;65:1149–1155. [Google Scholar]
- 32. Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47(suppl 1):33–46. [DOI] [PubMed] [Google Scholar]
- 33. Peterson BM, Mermelstein PG, Meisel RL. Estradiol mediates dendritic spine plasticity in the nucleus accumbens core through activation of mGluR5. Brain Struct Funct. 2015;2415–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Staffend NA, Loftus CM, Meisel RL. Estradiol reduces dendritic spine density in the ventral striatum of female Syrian hamsters. Brain Struct Funct. 2011;215:187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mani SK, Reyna AM, Alejandro MA, Crowley J, Markaverich BM. Disruption of male sexual behavior in rats by tetrahydrofurandiols (THF-diols). Steroids. 2005;70:750–754. [DOI] [PubMed] [Google Scholar]
- 36. Markaverich B, Mani S, Alejandro MA, et al. A novel endocrine-disrupting agent in corn with mitogenic activity in human breast and prostatic cancer cells. Environ Health Perspect. 2002;110:169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Villalon Landeros R, Morisseau C, Yoo HJ, Fu SH, Hammock BD, Trainor BC. Corncob bedding alters the effects of estrogens on aggressive behavior and reduces estrogen receptor-α expression in the brain. Endocrinology. 2012;153:949–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Meitzen J, Grove DD, Mermelstein PG. The organizational and aromatization hypotheses apply to rapid, nonclassical hormone action: neonatal masculinization eliminates rapid estradiol action in female hippocampal neurons. Endocrinology. 2012;153:4616–4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cao J, Mickens JA, McCaffrey KA, Leyrer SM, Patisaul HB. Neonatal bisphenol A exposure alters sexually dimorphic gene expression in the postnatal rat hypothalamus. Neurotoxicology. 2012;33:23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dorris DM, Hauser CA, Minnehan CE, Meitzen J. An aerator for brain slice experiments in individual cell culture plate wells. J Neurosci Methods. 2014;238:1–10. [DOI] [PubMed] [Google Scholar]
- 41. Dorris DM, Cao J, Willett JA, Hauser CA, Meitzen J. Intrinsic excitability varies by sex in pre-pubertal striatal medium spiny neurons. J Neurophysiol. 2015;113:720–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Belleau ML, Warren RA. Postnatal development of electrophysiological properties of nucleus accumbens neurons. J Neurophysiol. 2000;84:2204–2216. [DOI] [PubMed] [Google Scholar]
- 43. O'Donnell P, Grace AA. Physiological and morphological properties of accumbens core and shell neurons recorded in vitro. Synapse. 1993;13:135–160. [DOI] [PubMed] [Google Scholar]
- 44. Spencer S, Brown RM, Quintero GC, et al. α2δ-1 signaling in nucleus accumbens is necessary for cocaine-induced relapse. J Neurosci. 2014;34:8605–8611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mu P, Moyer JT, Ishikawa M, et al. Exposure to cocaine dynamically regulates the intrinsic membrane excitability of nucleus accumbens neurons. J Neurosci. 2010;30:3689–3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Farries MA, Meitzen J, Perkel DJ. Electrophysiological properties of neurons in the basal ganglia of the domestic chick: conservation and divergence in the evolution of the avian basal ganglia. J Neurophysiol. 2005;94:454–467. [DOI] [PubMed] [Google Scholar]
- 47. Farries MA, Perkel DJ. Electrophysiological properties of avian basal ganglia neurons recorded in vitro. J Neurophysiol. 2000;84:2502–2513. [DOI] [PubMed] [Google Scholar]
- 48. Farries MA, Perkel DJ. A telencephalic nucleus essential for song learning contains neurons with physiological characteristics of both striatum and globus pallidus. J Neurosci. 2002;22:3776–3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Meitzen J, Weaver AL, Brenowitz EA, Perkel DJ. Plastic and stable electrophysiological properties of adult avian forebrain song-control neurons across changing breeding conditions. J Neurosci. 2009;29:6558–6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Baufreton J, Atherton JF, Surmeier DJ, Bevan MD. Enhancement of excitatory synaptic integration by GABAergic inhibition in the subthalamic nucleus. J Neurosci. 2005;25:8505–8517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mermelstein PG, Becker JB, Surmeier DJ. Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. J Neurosci. 1996;16:595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Willett JA, Will TR, Hauser CA, Dorris DM, Cao J, Meitzen J. No evidence for sex differences in the electrophysiological properties and excitatory synaptic input onto nucleus accumbens shell medium spiny neurons. eNeuro. 2016;3:pii:ENEURO.0147–0115.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ishikawa M, Mu P, Moyer JT, et al. Homeostatic synapse-driven membrane plasticity in nucleus accumbens neurons. J Neurosci. 2009;29:5820–5831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Surmeier DJ, Plotkin J, Shen W. Dopamine and synaptic plasticity in dorsal striatal circuits controlling action selection. Curr Opin Neurobiol. 2009;19:621–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ma YY, Cepeda C, Chatta P, Franklin L, Evans CJ, Levine MS. Regional and cell-type-specific effects of DAMGO on striatal D1 and D2 dopamine receptor-expressing medium-sized spiny neurons. ASN Neuro. 2012;4.pii:e00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nisenbaum ES, Xu ZC, Wilson CJ. Contribution of a slowly inactivating potassium current to the transition to firing of neostriatal spiny projection neurons. J Neurophysiol. 1994;71:1174–1189. [DOI] [PubMed] [Google Scholar]
- 57. Meitzen J, Pflepsen KR, Stern CM, Meisel RL, Mermelstein PG. Measurements of neuron soma size and density in rat dorsal striatum, nucleus accumbens core and nucleus accumbens shell: differences between striatal region and brain hemisphere, but not sex. Neurosci Lett. 2011;487:177–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mermelstein PG, Song WJ, Tkatch T, Yan Z, Surmeier DJ. Inwardly rectifying potassium (IRK) currents are correlated with IRK subunit expression in rat nucleus accumbens medium spiny neurons. J Neurosci. 1998;18:6650–6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Huang YH, Schlüter OM, Dong Y. Silent synapses speak up: updates of the neural rejuvenation hypothesis of drug addiction. Neuroscientist. 2015;21:451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Terrier J, Lüscher C, Pascoli V. Cell-type specific insertion of GluA2-lacking AMPARs with cocaine exposure leading to sensitization, cue-induced seeking, and incubation of craving. Neuropsychopharmacology. 2016;41:1779–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Roitman MF, Na E, Anderson G, Jones TA, Bernstein IL. Induction of a salt appetite alters dendritic morphology in nucleus accumbens and sensitizes rats to amphetamine. J Neurosci. 2002;22:RC225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Meisel RL, Mullins AJ. Sexual experience in female rodents: cellular mechanisms and functional consequences. Brain Res. 2006;1126:56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cummings JA, Jagannathan L, Jackson LR, Becker JB. Sex differences in the effects of estradiol in the nucleus accumbens and striatum on the response to cocaine: neurochemistry and behavior. Drug Alcohol Depend. 2014;135:22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tozzi A, de Iure A, Tantucci M, et al. Endogenous 17β-estradiol is required for activity-dependent long-term potentiation in the striatum: interaction with the dopaminergic system. Front Cell Neurosci. 2015;9:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Becker JB. Direct effect of 17 β-estradiol on striatum: sex differences in dopamine release. Synapse. 1990;5:157–164. [DOI] [PubMed] [Google Scholar]
- 66. Hu M, Watson CJ, Kennedy RT, Becker JB. Estradiol attenuates the K+-induced increase in extracellular GABA in rat striatum. Synapse. 2006;59:122–124. [DOI] [PubMed] [Google Scholar]
- 67. Walker QD, Rooney MB, Wightman RM, Kuhn CM. Dopamine release and uptake are greater in female than male rat striatum as measured by fast cyclic voltammetry. Neuroscience. 2000;95:1061–1070. [DOI] [PubMed] [Google Scholar]
- 68. Xiao L, Becker JB. Effects of estrogen agonists on amphetamine-stimulated striatal dopamine release. Synapse. 1998;29:379–391. [DOI] [PubMed] [Google Scholar]
- 69. Kourrich S, Rothwell PE, Klug JR, Thomas MJ. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J Neurosci. 2007;27:7921–7928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. [DOI] [PubMed] [Google Scholar]
- 71. Hodes GE, Pfau ML, Purushothaman I, et al. Sex differences in nucleus accumbens transcriptome profiles associated with susceptibility versus resilience to subchronic variable stress. J Neurosci. 2015;35:16362–16376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Barrett ES, Swan SH. Stress and androgen activity during fetal development. Endocrinology. 2015;156:3435–3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kaiser S, Kruijver FP, Swaab DF, Sachser N. Early social stress in female guinea pigs induces a masculinization of adult behavior and corresponding changes in brain and neuroendocrine function. Behav Brain Res. 2003;144:199–210. [DOI] [PubMed] [Google Scholar]
- 74. Knapp R, Marsh-Matthews E, Vo L, Rosencrans S. Stress hormone masculinizes female morphology and behaviour. Biol Lett. 2011;7:150–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kranendonk G, Hopster H, Fillerup M, Ekkel ED, Mulder EJ, Taverne MA. Cortisol administration to pregnant sows affects novelty-induced locomotion, aggressive behaviour, and blunts gender differences in their offspring. Horm Behav. 2006;49:663–672. [DOI] [PubMed] [Google Scholar]
- 76. Ward IL, Weisz J. Maternal stress alters plasma testosterone in fetal males. Science. 1980;207:328–329. [DOI] [PubMed] [Google Scholar]
- 77. Ward IL, Weisz J. Differential effects of maternal stress on circulating levels of corticosterone, progesterone, and testosterone in male and female rat fetuses and their mothers. Endocrinology. 1984;114:1635–1644. [DOI] [PubMed] [Google Scholar]
- 78. Bayless DW, Daniel JM. Sex differences in myelin-associated protein levels within and density of projections between the orbital frontal cortex and dorsal striatum of adult rats: implications for inhibitory control. Neuroscience. 2015;300:286–296. [DOI] [PubMed] [Google Scholar]
- 79. Feder HH, Whalen RE. Feminine behavior in neonatally castrated and estrogen-treated male rats. Science. 1965;147:306–307. [DOI] [PubMed] [Google Scholar]
- 80. McCarthy MM, Arnold AP. Reframing sexual differentiation of the brain. Nat Neurosci. 2011;14:677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. [DOI] [PubMed] [Google Scholar]
- 82. Levin ER. Minireview: extranuclear steroid receptors: roles in modulation of cell functions. Mol Endocrinol. 2011;25:377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Chen X, Grisham W, Arnold AP. X chromosome number causes sex differences in gene expression in adult mouse striatum. Eur J Neurosci. 2009;29:768–776. [DOI] [PubMed] [Google Scholar]
- 84. Ghahramani NM, Ngun TC, Chen PY, et al. The effects of perinatal testosterone exposure on the DNA methylome of the mouse brain are late-emerging. Biol Sex Differ. 2014;5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Forger NG, Strahan JA, Castillo-Ruiz A. Cellular and molecular mechanisms of sexual differentiation in the mammalian nervous system. Front Neuroendocrinol. 2016;40:67–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294:76–95. [DOI] [PubMed] [Google Scholar]
- 87. Stumpf WE, Sar M. Steroid hormone target sites in the brain: the differential distribution of estrogin, progestin, androgen and glucocorticosteroid. J Steroid Biochem. 1976;7:1163–1170. [DOI] [PubMed] [Google Scholar]
- 88. Fernandez-Guasti A, Swaab D, Rodríguez-Manzo G. Sexual behavior reduces hypothalamic androgen receptor immunoreactivity. Psychoneuroendocrinology. 2003;28:501–512. [DOI] [PubMed] [Google Scholar]
- 89. DonCarlos LL, Garcia-Ovejero D, Sarkey S, Garcia-Segura LM, Azcoitia I. Androgen receptor immunoreactivity in forebrain axons and dendrites in the rat. Endocrinology. 2003;144:3632–3638. [DOI] [PubMed] [Google Scholar]
- 90. Triemstra JL, Sato SM, Wood RI. Testosterone and nucleus accumbens dopamine in the male Syrian hamster. Psychoneuroendocrinology. 2008;33:386–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Frye CA, Rhodes ME, Rosellini R, Svare B. The nucleus accumbens as a site of action for rewarding properties of testosterone and its 5α-reduced metabolites. Pharmacol Biochem Behav. 2002;74:119–127. [DOI] [PubMed] [Google Scholar]
- 92. Feng Y, Weijdegård B, Wang T, et al. Spatiotemporal expression of androgen receptors in the female rat brain during the oestrous cycle and the impact of exogenous androgen administration: a comparison with gonadally intact males. Mol Cell Endocrinol. 2010;321:161–174. [DOI] [PubMed] [Google Scholar]
- 93. Sato SM, Johansen JA, Jordan CL, Wood RI. Membrane androgen receptors may mediate androgen reinforcement. Psychoneuroendocrinology. 2010;35:1063–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Aubele T, Kritzer MF. Androgen influence on prefrontal dopamine systems in adult male rats: localization of cognate intracellular receptors in medial prefrontal projections to the ventral tegmental area and effects of gonadectomy and hormone replacement on glutamate-stimulated extracellular dopamine level. Cereb Cortex. 2012;22:1799–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Finley SK, Kritzer MF. Immunoreactivity for intracellular androgen receptors in identified subpopulations of neurons, astrocytes and oligodendrocytes in primate prefrontal cortex. J Neurobiol. 1999;40:446–457. [PubMed] [Google Scholar]
- 96. Edwards HE, Burnham WM, MacLusky NJ. Testosterone and its metabolites affect afterdischarge thresholds and the development of amygdala kindled seizures. Brain Res. 1999;838:151–157. [DOI] [PubMed] [Google Scholar]
- 97. Campbell RE, Herbison AE. Gonadal steroid neuromodulation of developing and mature hypothalamic neuronal networks. Curr Opin Neurobiol. 2014;29:96–102. [DOI] [PubMed] [Google Scholar]
- 98. Whalen RE, Edwards DA. Hormonal determinants of the development of masculine and feminine behavior in male and female rats. Anat Rec. 1967;157:173–180. [DOI] [PubMed] [Google Scholar]
- 99. Jackson LM, Mytinger A, Roberts EK, et al. Developmental programming: postnatal steroids complete prenatal steroid actions to differentially organize the GnRH surge mechanism and reproductive behavior in female sheep. Endocrinology. 2013;154:1612–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Juraska JM, Sisk CL, DonCarlos LL. Sexual differentiation of the adolescent rodent brain: hormonal influences and developmental mechanisms. Horm Behav. 2013;64:203–210. [DOI] [PubMed] [Google Scholar]
- 101. Andersen SL, Rutstein M, Benzo JM, Hostetter JC, Teicher MH. Sex differences in dopamine receptor overproduction and elimination. Neuroreport. 1997;8:1495–1498. [DOI] [PubMed] [Google Scholar]
- 102. Andersen SL, Teicher MH. Sex differences in dopamine receptors and their relevance to ADHD. Neurosci Biobehav Rev. 2000;24:137–141. [DOI] [PubMed] [Google Scholar]
- 103. Andersen SL, Thompson AP, Krenzel E, Teicher MH. Pubertal changes in gonadal hormones do not underlie adolescent dopamine receptor overproduction. Psychoneuroendocrinology. 2002;27:683–691. [DOI] [PubMed] [Google Scholar]
- 104. Martinez LA, Peterson BM, Meisel RL, Mermelstein PG. Estradiol facilitation of cocaine-induced locomotor sensitization in female rats requires activation of mGluR5. Behav Brain Res. 2014;271:39–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Wong JE, Cao J, Dorris DM, Meitzen J. Genetic sex and the volumes of the caudate-putamen, nucleus accumbens core and shell: original data and a review [published online ahead of print December 14, 2015]. Brain Struct Funct. [DOI] [PubMed] [Google Scholar]
- 106. Almey A, Filardo EJ, Milner TA, Brake WG. Estrogen receptors are found in glia and at extranuclear neuronal sites in the dorsal striatum of female rats: evidence for cholinergic but not dopaminergic colocalization. Endocrinology. 2012;153:5373–5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Grove-Strawser D, Boulware MI, Mermelstein PG. Membrane estrogen receptors activate the metabotropic glutamate receptors mGluR5 and mGluR3 to bidirectionally regulate CREB phosphorylation in female rat striatal neurons. Neuroscience. 2010;170:1045–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Küppers E, Beyer C. Expression of aromatase in the embryonic and postnatal mouse striatum. Brain Res Mol Brain Res. 1998;63:184–188. [DOI] [PubMed] [Google Scholar]
- 109. Küppers E, Beyer C. Expression of estrogen receptor-α and β mRNA in the developing and adult mouse striatum. Neurosci Lett. 1999;276:95–98. [DOI] [PubMed] [Google Scholar]
- 110. Küppers E, Krust A, Chambon P, Beyer C. Functional alterations of the nigrostriatal dopamine system in estrogen receptor-α knockout (ERKO) mice. Psychoneuroendocrinology. 2008;33:832–838. [DOI] [PubMed] [Google Scholar]
- 111. Schultz KN, von Esenwein SA, Hu M, et al. Viral vector-mediated overexpression of estrogen receptor-α in striatum enhances the estradiol-induced motor activity in female rats and estradiol-modulated GABA release. J Neurosci. 2009;29:1897–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Kreitzer AC, Berke JD. Investigating striatal function through cell-type-specific manipulations. Neuroscience. 2011;198:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Almey A, Milner TA, Brake WG. Estrogen receptor α and G-protein coupled estrogen receptor 1 are localised to GABAergic neurons in the dorsal striatum. Neurosci Lett. 2016;622:118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Ferguson SM, Phillips PE, Roth BL, Wess J, Neumaier JF. Direct-pathway striatal neurons regulate the retention of decision-making strategies. J Neurosci. 2013;33:11668–11676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Planert H, Berger TK, Silberberg G. Membrane properties of striatal direct and indirect pathway neurons in mouse and rat slices and their modulation by dopamine. PLoS One. 2013;8:e57054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Ade KK, Wan Y, Chen M, Gloss B, Calakos N. An improved BAC transgenic fluorescent reporter line for sensitive and specific identification of striatonigral medium spiny neurons. Front Syst Neurosci. 2011;5:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Gong S, Zheng C, Doughty ML, et al. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. [DOI] [PubMed] [Google Scholar]
- 118. Gorski RA, Gordon JH, Shryne JE, Southam AM. Evidence for a morphological sex difference within the medial preoptic area of the rat brain. Brain Res. 1978;148:333–346. [DOI] [PubMed] [Google Scholar]
- 119. Brown AE, Mani S, Tobet SA. The preoptic area/anterior hypothalamus of different strains of mice: sex differences and development. Brain Res Dev Brain Res. 1999;115:171–182. [DOI] [PubMed] [Google Scholar]
- 120. Campi KL, Jameson CE, Trainor BC. Sexual dimorphism in the brain of the monogamous California mouse (Peromyscus californicus). Brain Behav Evol. 2013;81:236–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Bonthuis PJ, Cox KH, Searcy BT, Kumar P, Tobet S, Rissman EF. Of mice and rats: key species variations in the sexual differentiation of brain and behavior. Front Neuroendocrinol. 2010;31:341–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Srivastava DP, Evans PD. G-protein oestrogen receptor 1: trials and tribulations of a membrane oestrogen receptor. J Neuroendocrinol. 2013;25:1219–1230. [DOI] [PubMed] [Google Scholar]
- 123. Meitzen J, Luoma JI, Boulware MI, et al. Palmitoylation of estrogen receptors is essential for neuronal membrane signaling. Endocrinology. 2013;154:4293–4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Pedram A, Razandi M, Deschenes RJ, Levin ER. DHHC-7 and -21 are palmitoylacyltransferases for sex steroid receptors. Mol Biol Cell. 2012;23:188–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Brenowitz EA. Testosterone and brain-derived neurotrophic factor interactions in the avian song control system. Neuroscience. 2013;239:115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Harte-Hargrove LC, Maclusky NJ, Scharfman HE. Brain-derived neurotrophic factor-estrogen interactions in the hippocampal mossy fiber pathway: implications for normal brain function and disease. Neuroscience. 2013;239:46–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Li X, Wolf ME. Multiple faces of BDNF in cocaine addiction. Behav Brain Res. 2015;279:240–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Ito R, Robbins TW, Everitt BJ. Differential control over cocaine-seeking behavior by nucleus accumbens core and shell. Nat Neurosci. 2004;7:389–397. [DOI] [PubMed] [Google Scholar]
- 129. Pulvirenti L, Berrier R, Kreifeldt M, Koob GF. Modulation of locomotor activity by NMDA receptors in the nucleus accumbens core and shell regions of the rat. Brain Res. 1994;664:231–236. [DOI] [PubMed] [Google Scholar]
- 130. Bradley KC, Boulware MB, Jiang H, Doerge RW, Meisel RL, Mermelstein PG. Changes in gene expression within the nucleus accumbens and striatum following sexual experience. Genes Brain Behav. 2005;4:31–44. [DOI] [PubMed] [Google Scholar]
- 131. Henschen CW, Palmiter RD, Darvas M. Restoration of dopamine signaling to the dorsal striatum is sufficient for aspects of active maternal behavior in female mice. Endocrinology. 2013;154:4316–4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Peña CJ, Neugut YD, Calarco CA, Champagne FA. Effects of maternal care on the development of midbrain dopamine pathways and reward-directed behavior in female offspring. Eur J Neurosci. 2014;39:946–956. [DOI] [PubMed] [Google Scholar]
- 133. O'Connell LA, Hofmann HA. The vertebrate mesolimbic reward system and social behavior network: a comparative synthesis. J Comp Neurol. 2011;519:3599–3639. [DOI] [PubMed] [Google Scholar]
- 134. West EA, Carelli RM. Nucleus accumbens core and shell differentially encode reward-associated cues after reinforcer devaluation. J Neurosci. 2016;36:1128–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Corbit LH, Balleine BW. The general and outcome-specific forms of Pavlovian-instrumental transfer are differentially mediated by the nucleus accumbens core and shell. J Neurosci. 2011;31:11786–11794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Corbit LH, Muir JL, Balleine BW. The role of the nucleus accumbens in instrumental conditioning: evidence of a functional dissociation between accumbens core and shell. J Neurosci. 2001;21:3251–3260. [DOI] [PMC free article] [PubMed] [Google Scholar]