Abstract
Estrogens are important regulators of bone mass and their effects are mainly mediated via estrogen receptor (ER)α. Central ERα exerts an inhibitory role on bone mass. ERα is highly expressed in the arcuate (ARC) and the ventromedial (VMN) nuclei in the hypothalamus. To test whether ERα in proopiomelanocortin (POMC) neurons, located in ARC, is involved in the regulation of bone mass, we used mice lacking ERα expression specifically in POMC neurons (POMC-ERα−/−). Female POMC-ERα−/− and control mice were ovariectomized (OVX) and treated with vehicle or estradiol (0.5 μg/d) for 6 weeks. As expected, estradiol treatment increased the cortical bone thickness in femur, the cortical bone mechanical strength in tibia and the trabecular bone volume fraction in both femur and vertebrae in OVX control mice. Importantly, the estrogenic responses were substantially increased in OVX POMC-ERα−/− mice compared with the estrogenic responses in OVX control mice for cortical bone thickness (+126 ± 34%, P < .01) and mechanical strength (+193 ± 38%, P < .01). To test whether ERα in VMN is involved in the regulation of bone mass, ERα was silenced using an adeno-associated viral vector. Silencing of ERα in hypothalamic VMN resulted in unchanged bone mass. In conclusion, mice lacking ERα in POMC neurons display enhanced estrogenic response on cortical bone mass and mechanical strength. We propose that the balance between inhibitory effects of central ERα activity in hypothalamic POMC neurons in ARC and stimulatory peripheral ERα-mediated effects in bone determines cortical bone mass in female mice.
Estrogens are important endocrine regulators of skeletal growth and maintenance (1–3). Estrogen deficiency, induced by ovariectomy (OVX) in animal models, or after menopause in humans, results in reduces trabecular bone mineral density (BMD) as well as cortical bone mass, but estrogen substitution restores both bone compartments (4–6). The physiological effects of estrogens are mainly exerted via the 2 classic nuclear estrogen receptors (ERs), ERα and ERβ, which are ligand-activated transcription factors. The effects of estradiol (E2) on bone mass are predominantly mediated via ERα, but the ERα activity can be slightly modulated by ERβ in female mice (7–10). Bone loss caused by estrogen deficiency is restored by local injections of E2, demonstrating that peripheral estrogen action is important for bone regulation (11). Furthermore, using a variety of conditional gene-targeted mouse models, the importance of ERα in both osteoblasts and osteoclasts for the peripheral effects of estrogens on bone has been confirmed (8, 12). Thus, it is well established that estrogens exert stimulatory ERα-mediated peripheral effects on bone mass in female mice.
Bone is traditionally considered to be regulated by hormones, autocrine/paracrine signals, and mechanical loading. It is now recognized that the regulation of bone also involves the central nervous system. It has been known for long time that bone is an innervated tissue containing both efferent and afferent fibers in bone marrow and the periosteum (13). However, the first clear evidence that central signaling affects bone mass was the finding that leptin-deficient mice had high bone mass and that this phenotype was reversed by intracerebroventricular injections of leptin (14). In contrast, peripheral leptin treatment increases bone mass, suggesting that leptin has opposite peripheral vs central effects on bone mass (14–16). Several neurotransmitters have been shown to be involved in bone regulation, including serotonin. Central serotonin has been suggested to enhance bone mass (17). The notion that peripheral serotonin has a negative influence on bone mass, suggesting that this neurotransmitter may have opposite peripheral vs central effects, is supported by some but not others (18, 19). Taken together, it is now well established that neuronal signaling is important for the regulation of bone mass (20) and that the central and peripheral effects on bone mass sometimes are opposite.
The hypothalamus is a major regulator of fat mass and energy homeostasis and several recent studies indicate that specific hypothalamic neurons are also involved in the regulation of bone mass (20–24). Interestingly, it is reported that the transcription factor activator protein-1 in hypothalamic proopiomelanocortin (POMC) neurons, located in the arcuate nucleus (ARC), is involved in the regulation of bone mass (24).
The central nervous system is a target for estrogens and nuclear ERs are widely distributed in the brain (25). Central ERα has been reported to exert an inhibitory role on bone mass, partly counteracting the peripheral stimulatory effect of estrogen (5). However, the primary target cell for this central inhibitory effect of estrogen on bone mass is unknown. ERα expression is high in ARC and the ventromedial nucleus (VMN) in the hypothalamus (26). Within ARC, ERα is abundantly expressed in POMC neurons but not in neuropeptide Y (NPY) or agouti-related protein (AgRP) neurons (27).
We have developed a mouse model with specific Cre-mediated ERα inactivation in POMC neurons in ARC (POMC-ERα−/− mice) and have recently shown that these mice display hyperphagia but normal energy expenditure and fat distribution (26). In addition, we have in a separate mouse model used adeno-associated viral (AAV) gene silencing of ERα in hypothalamic VMN, resulting in obesity and increased visceral fat deposition (28). Collectively, our previous findings indicate that ERα expressed in VMN and POMC neurons in ARC provide the coordinated control of food intake, energy expenditure, and fat distribution (27). The skeletal phenotype was not evaluated in our previous studies. We here tested the hypothesis that ERα in POMC neurons of ARC and/or ERα in VMN are involved in the regulation of bone mass.
Materials and Methods
POMC-ERα−/− mice
The generation of mice with specific inactivation of ERα in hypothalamic POMC neurons (POMC-ERα−/− mice) has previously been described (26). Briefly, ERαlox/lox mice were crossed with POMC-Cre transgenic mice, to generate mice lacking ERα in POMC neurons (POMC-ERα−/−) and their control littermates (ERαlox/lox). We have previously confirmed the specificity of ERα deletion in hypothalamic POMC neurons in POMC-ERα−/− mice (26). For assessment of E2 response, 6-month-old female POMC-ERα−/− and control mice were OVX followed by treatment with E2 (0.5 μg/d, 60-d release, SE-121; Innovative Research of America) or corresponding vehicle pellet. The mice were fed with standard phytoestrogen-free chow (2916; Harlan-Teklad). Animal care was in accordance with institutional guidelines, and the study was approved by the local ethical committee.
High-resolution microcomputed tomography (μCT)
High-resolution μCT analyses were performed on femurs using a Skyscan 1172 μCT (Bruker micro-CT). The femurs were imaged with an x-ray tube voltage of 50 kV and a current of 201 μA, with a 0.5-mm aluminum filter. The scanning angular rotation was 180°, and the angular increment was 0.70°. The isotropic voxel size was 4.48 μm. NRecon (version 1.6.9) was employed to perform the reconstruction after the scans. In the femurs, the trabecular bone proximal to the distal growth plate was selected for analyses within a conforming volume of interest (cortical bone excluded) commencing at a distance of 538.5 μm from the growth plate and extending a further longitudinal distance of 134.5 μm in the proximal direction. Cortical measurements were performed in the diaphyseal region of the femur starting at a distance of 3.59 mm from the growth plate and extending a further longitudinal distance of 134.5 μm in the proximal direction. For BMD analysis, the equipment was calibrated with ceramic standard samples.
Three-point bending
The 3-point bending test (span length 5.5 mm, loading speed 0.155 mm/s) at the midtibia was made by the Instron universal testing machine (Instron 3366; Instron Corp). Based on the recorded load deformation curves, the biomechanical parameters (maximal load, stiffness, and absorbed energy) were acquired from raw-files produced by Bluehill 2 software v2.6 (Instron), with custom-made Excel macros (29).
Dynamic histomorphometry
Tibia were fixed in Bürckhardt's fixative, dehydrated in 70% ethanol, and embedded in plastic (L R White Resin; Agar Scientific). For the measurement of dynamic parameters, the mice were double-labeled with calcein, which was injected (ip) 1 and 8 days before termination. Dynamic histomorphometric analyses of cortical bone were carried out on 20-μm-thick transverse cross-sectional section from the middiaphyseal region of the tibia.
Fourier transform infrared (FTIR) microspectroscopy
Humerus was fixed in Bürckhardt's fixative, dehydrated in 70% ethanol and embedded in Technovit 9100 (Heraeus-Kulzer); 4-μm-thick longitudinal sections were cut with a microtome and placed on IR transparent barium fluoride windows.
The spatial molecular bone composition was assessed by means of FTIR microspectroscopy (30, 31) at beamline D7, MAX IV Laboratory, Lund University, Sweden, using a Hyperion 3000 microscope and a Bruker IFS66/v FTIR spectrometer. Measurements were conducted in transmission mode, using spectral resolution of 4 cm−1 and 64 repeated scans together with a focal plane array detector that covered 341 × 341 μm (64 × 64 elements, resulting in 5.32-μm pixels). Data was collected in the wavenumber range of 4000–800 cm−1. Two areal measurements of the full cross-section of the cortex between 1/3 and 2/3 of the middiaphysis was made on each sample. During preprocessing, the spectra were cut to 2000–900 cm−1, the average Technovit spectrum was normalized and subsequently subtracted from the bone spectra and all peaks of interest were linearly baseline corrected following earlier established protocols (32, 33).
For each measurement point, the mineral-to-matrix ratio (phosphate [900–1200 cm−1]/amide I [1585–1720 cm−1]) was calculated to estimate the degree of mineralization (30, 34). Moreover, collagen maturity estimating the degree of immature to mature collagen cross-links (1660/1690 cm−1) (35) and crystallinity estimating the perfection of the hydroxylapatite crystals (1030 cm−1/1020 cm−1) (36, 37) were determined.
Quantitative real-time PCR analysis
Total RNA was prepared using TRIzol reagent (cortical bone; Life Technologies) or RNeasy Mini kit (hypothalamus; QIAGEN). The RNA was reverse-transcribed into cDNA, and real-time PCR analysis was performed using the ABI Prism 7000 Sequence Detection System (PE Applied Biosystems). The mRNA abundance of each gene was adjusted for the expression of 18S. Predesigned real-time PCR assays from Applied Biosystems were used for analysis of the following mRNA levels. Hypothalamus: ERβ, NPY, AgRP, α-melanocyte-stimulating hormone (αMSH), melanocortin 4 receptor (MC4R), leptin receptor, protein-tyrosin phosphatase 1B (PTP1B), and supressor of cytokine signaling 3 (SOCS3). Cortical bone: osteoprotegering, receptor activator of nuclear κB ligand, osteocalcin (OCN), class II major histocompatibility complex (MHC) transactivator (CIITA), and ERα.
Serum measurements
Serum levels of E2 (sensitivity 0.3 pg/mL), testosterone (sensitivity 4 pg/mL), and dihydrotestosterone (DHT) (sensitivity 1.6 pg/mL) were measured in a single run by gas chromatography-tandem mass spectometry. After the addition of isotope-labeled standards, steroids were extracted to chlorobutane, purified on a silica column and derivatized using pentafluorobenzylhydroxylamine hydrochloride followed by pentafluorobenzoyl chloride. Steroids were analyzed in multiple reactions monitoring mode with ammonia as reagent gas using an Agilent 7000 triple quadrupole mass spectrometer equipped with a chemical ionization source (38).
As a marker of bone resorption, serum levels of C-terminal type I collagen fragments (CTX) were assessed using an ELISA RatLaps kit (Immunodiagostic Systems Ltd) according to the manufacturer's instructions. Serum levels of OCN, a marker of bone formation, were determined with a mouse OCN immunoradiometric assay kit (Mouse Osteocalcin ELISA). Serum levels of leptin were analyzed using an ELISA (Crystal CHEM, Inc). Serum corticosterone levels were analyzed using a corticosterone RIA kit (Corticosterone RIA; Fisher Scientific).
Silencing of ERα expression in VMN using AAV vector
We used an AAV vector to achieve focused silencing of ERα in the VMN of the hypothalamus, a key center of energy metabolism. The AAV vectors were constructed based on our previous publication (28). A basic AAV vector, AAV.U6, containing U6 promoter for expression of short hairpin RNA targeting ERα (ERα-shRNA) or scramble shRNA or scramble shRNA as control, was used (Supplemental Figure 1A). Both vectors also express enhanced green fluorescent protein as a reporter to visualize transduced neurons (Vector BioLabs). We have previously shown that AAV vectors can be used to efficiently silence ERα in VMN of hypothalamus, resulting in obesity (28).
To determine the role of ERα in VMN for bone mass, we OVX C57BL/6J female mice at 10 weeks of age. One week after OVX, the mice were stereotactically injected with 2 × 1010 genomic particles (2 μL in PBS) into the VMN (anteroposterior −0.9, mediolateral ± 0.7, and dorsoventral −6.0) over 10 minutes using a 5-μL Hamilton syringe attached to a microinfusion pump. At the time of the stereotactic injections, E2 pellet (111 ng/mouse per d; 90-d release, NE-121; Innovative Research of America) was sc implanted. The animals were terminated 6 weeks after the stereotactic injections of either AAV-shRNA-ERα or AAV-shRNA-scramble. The body weights were recorded every week and at the end of the experiment excised femurs were analyzed by μCT, and the brains were analyzed by immunohistochemistry. For immunohistochemistry, mice were deeply anesthetized and perfused with 4% formalin. The brains were removed and postfixed in the same fixative over night at 4°C. After postfixation, the brains were stored in 30% sucrose at 4°C for at least 24 hours. The brains were then cut at 30 μm on a freezing microtome. Immunocytochemistry with free-floating sections was performed using established protocols (28, 39–41). Briefly, the sections were rinsed in wash buffer (0.1M Tris-HCl [pH 7.5], 0.15M NaCl, and 0.2% Triton X-100) and then blocked for 1 hour with blocking reagent (TNB; 0.1 M Tris-HCL [pH 7.5], 0.15 M NaCl, and 0.5% blocking reagent; PerkinElmer). Sections were then incubated with primary rabbit polyclonal anti-ERα (1:800, 06-935; Millipore) or goat polyclonal anti-green fluorescent protein (FITC), (1:500, ab6662; Abcam) antibodies overnight. Brain sections were stained for EGFP to visualize transduced brain regions and for ERα to evaluate the efficiency of ERα silencing. The sections were rinsed and incubated for 1 hour with secondary antibody Alexa Fluor 594 conjugate (1:1000, A-21207; Thermo Fisher Scientific). The sections were then rinsed 2 times with wash buffer TNT without Triton X-100 and mounted in mounting medium containing prolog gold antifade (P36930; Molecular Probes). Images were analyzed using a Carl Zeiss LSM 780 confocal microscope at the Center for Cellular Imaging at the Sahlgrenska Academy. Mice in which the injection had not been successful, as determined by postmortem immunofluorescence analysis of brain sections, were excluded. All surgical procedures were performed under isoflurane anesthesia (Forene; Abbot Scandinavia). The animal experiments were approved by the local Ethical Committee for Animal Research at the University of Gothenburg.
Statistical analysis
Values are given as mean ± SEM. Student's t test was used to compare 2 groups. To determine whether the estrogenic response differed between POMC-ERα−/− and control mice, the interaction term from a two-way ANOVA analyses was used. P < .05 was considered statistically significant.
Results
Increased cortical bone mass and mechanical strength in POMC-ERα−/− mice
We have previously demonstrated that POMC-ERα−/− mice have substantially reduced ERα expression in hypothalamic POMC neurons (26), and we now show that the ERα expression in cortical diaphyseal bone is unaffected in POMC-ERα−/− mice (POMC-ERα−/− 1.64 ± 0.32; control littermates 1.73 ± 0.21, arbitrary units, n = 4–6). The body weight, serum leptin levels (an indicator of body fat mass), femur length and vertebrae L5 height were unaffected in 6-month-old female POMC-ERα−/− mice compared with control mice (Supplemental Table 1). The mRNA levels of ERβ in hypothalamus were not affected in POMC-ERα−/− compared with control mice (Supplemental Table 1).
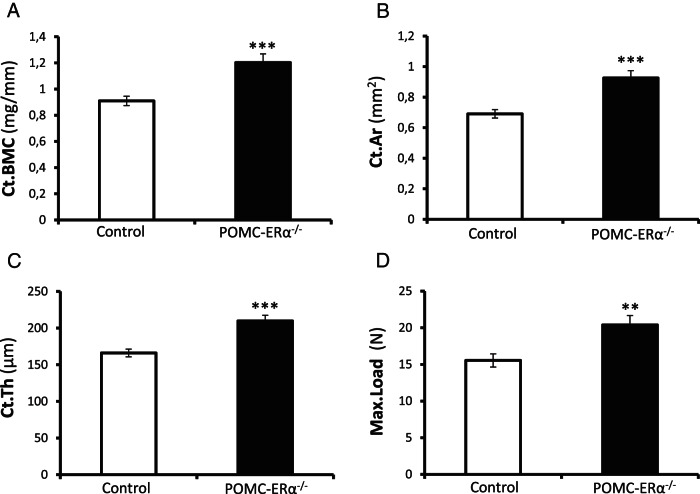
μCT analyses of the diaphyseal region of femur revealed that the cortical bone mineral content was significantly increased in the POMC-ERα−/− mice compared with control mice (Figure 1A). This was caused by an increased cortical bone area and thickness, whereas the cortical volumetric BMD (vBMD) was unaffected (Figure 1, B and C, and Table 1). These findings demonstrate that the amount of cortical bone is increased in the POMC-ERα−/− mice, whereas the degree of mineralization of the cortical bone seems to be unaffected. A normal composition of the cortical bone in POMC-ERα−/− mice is clearly supported by that the degree of mineralization, the collagen maturity, and the crystallinity reflecting the perfection of the hydroxylapatite crystals, as analyzed by FTIR spectroscopy in the cortical bone in the diaphyseal region of humerus were all unaffected in the POMC-ERα−/− mice compared with control mice (Table 1). Three-point bending analysis of the cortical diaphyseal bone in tibia revealed increased mechanical bone strength (maximal load at failure) in POMC-ERα−/− mice compared with control mice (Figure 1D).
Figure 1.
Increased cortical bone mass and mechanical strength in POMC-ERα−/− mice. Cortical bone mass and mechanical strength in 6-month-old female POMC-ERα−/− and control mice. A–C, μCT analysis of the diaphyseal region of femur (n = 8–12). A, Cortical bone mineral content (Ct.BMC), (B) cortical bone area (Ct.Ar), (C) cortical thickness (Ct.Th), and (D) 3-point bending test of the middiaphyseal region in tibia (n = 9–12). The maximal load (Max.Load) at failure is given. Data are presented as mean ± SEM. **, P < .01; ***, P < .001 POMC-ERα−/− vs control mice, Student's t test.
Table 1.
Cortical Bone Composition in POMC-ERα−/− Mice
| Control | POMC-ERα−/− | |
|---|---|---|
| μCT | ||
| Cortical vBMD (mg/cm3) | 1317 ± 5 | 1299 ± 16 |
| FTIR | ||
| Mineral to matrix ratio | 2.05 ± 0.53 | 1.35 ± 0.27 |
| Collagen maturity | 0.22 ± 0.02 | 0.25 ± 0.00 |
| Crystallinity | 0.083 ± 0.011 | 0.068 ± 0.010 |
μCT (femur; n = 8–12) and FTIR microspectroscopy (humerus; n = 4–6) analyses of cortical bone in 6-month-old female POMC-ERα−/− and control mice. Data are presented as mean ± SEM.
The trabecular bone mass was augmented, as demonstrated by increased trabecular bone volume fraction (BV/TV) and trabecular number, in the metaphyseal region of femur but not in vertebrae L5 in the POMC-ERα−/− mice compared with control mice (Table 2). Neither serum markers of bone formation (OCN) nor bone resorption (CTX-I; type I collagen fragments) were significantly affected in the 6-month-old female POMC-ERα−/− mice (Supplemental Table 2).
Table 2.
Trabecular Bone in Femur and Vertebrae L5 of POMC-ERα−/− Mice
| Control | POMC-ERα−/− | |
|---|---|---|
| Femur | ||
| BV/TV (%) | 12.2 ± 1.1 | 22.8 ± 2.8a |
| Tb.N (L/mm) | 2.9 ± 0.2 | 5.2 ± 0.6a |
| Tb.Th (μm) | 41 ± 1 | 44 ± 2 |
| Tb.Sp (μm) | 130 ± 1 | 115 ± 5a |
| Vertebrae L5 | ||
| BV/TV (%) | 21.1 ± 1.4 | 25.6 ± 2.6 |
| Tb.N (L/mm) | 4.9 ± 0.3 | 6.0 ± 1.1 |
| Tb.Th (μm) | 44 ± 2 | 44 ± 3 |
| Tb.Sp (μm) | 70 ± 3 | 62 ± 7 |
High-resolution μCT analyses of BV/TV, trabecular number (Tb.N), trabecular thickness (Tb.Th), and trabecular separation (Tb.Sp) in femur (n = 8–11) and vertebrae L5 (n = 4–7) of 6-month-old female POMC-ERα−/− and control mice. Data are presented as mean ± SEM.
P < .01, POMC-ERα−/− vs control mice, Student's t test.
The negative feedback regulation of serum sex steroids is disturbed in female mice with global ERα inactivation, reflected by substantially increased serum levels of not only E2 but also of the 2 androgens testosterone and DHT (Supplemental Table 3) (38). Although serum testosterone and DHT were unaffected, serum levels of E2 were slightly elevated in the female POMC-ERα−/− mice compared with control mice (Supplemental Table 3). However, uterine weight, a sensitive bio-indicator of mean serum E2 levels, was not significantly altered in the POMC-ERα−/− mice compared with control mice. Furthermore, the POMC-ERα−/− had substantially lower serum E2 levels compared with female mice with global ERα inactivation (Supplemental Tables 1 and 3), suggesting that the disturbance in feedback regulation of serum sex steroids in POMC-ERα−/− mice is modest.
Enhanced estrogenic responses on cortical bone mass and mechanical strength in POMC-ERα−/− mice
To exclude the possibility that the slightly altered E2 levels could have confounded the interpretation of the bone phenotype in the gonadal intact POMC-ERα−/− mice, we next compared the estrogenic responses (E2; 0.5 μg/d for 6 wk) in OVX POMC-ERα−/− and OVX control mice.
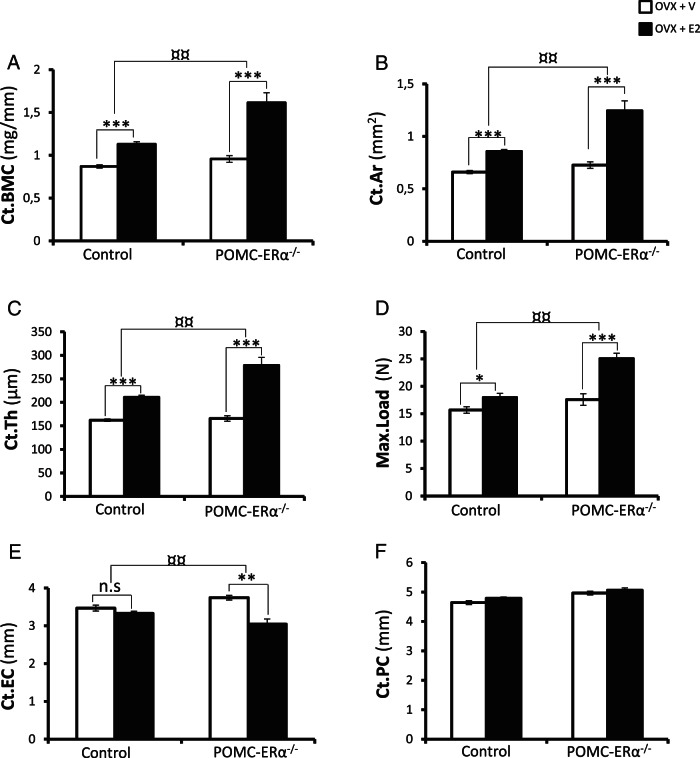
E2 treatment did neither in the POMC-ERα−/− nor in the control mice affect the body weight, the femur bone length or the height of vertebra L5 compared with vehicle-treated mice (data not shown). As expected, E2 treatment increased the cortical bone mineral content, cortical bone area and cortical bone thickness in the diaphyseal region of femur and the BV/TV in both the distal metaphyseal region of femur and in vertebrae L5 (Figure 2, A–C, and table 4 below). Importantly, for the cortical bone mineral content (+130 ± 41%, P < .01), the cortical bone area (+138 ± 44%, P < .01) and the cortical bone thickness (+126 ± 34%, P < .01) the estrogenic responses were substantially augmented in OVX POMC-ERα−/− mice compared with the estrogenic responses in OVX control mice (Figure 2, A–C). The cortical bone mineralization and composition parameters, analyzed by μCT in the diaphyseal region of femur (vBMD) and FTIR in the diaphyseal region of humerus (mineral/matrix ratio, collagen maturity and crystallinity), were not affected by E2 treatment in the control or POMC-ERα−/− mice (Table 3). Three-point bending analysis of the cortical diaphyseal bone in tibia revealed a substantially increased estrogenic response on mechanical bone strength (maximal load at failure) in POMC-ERα−/− mice compared with control mice (+193 ± 38%, P < .01) (Figure 2D).
Figure 2.
Enhanced estrogenic responses on cortical bone mass and mechanical strength in POMC-ERα−/− mice. Cortical bone mass and mechanical strength in OVX POMC-ERα−/− and OVX control mice treated with either E2 (0.5 μg/d) or vehicle. A–C, High-resolution μCT analysis of the diaphyseal region of femur (n = 7–10). A, Cortical bone mineral content (Ct.BMC), (B) cortical bone area (Ct.Ar), (C) cortical thickness (Ct.Th), and (D) 3-point bending test of the middiaphyseal region in tibia. The maximal load (Max.Load) at failure is given, (E) cortical endosteal circumference (Ct.EC), and (F) cortical periosteal circumference (Ct.PC) (n = 9–10). Data are presented as mean ± SEM. *, P < .05; **, P < .01; and ***, P < .001 E2 treated vs vehicle treated, Student's t test; ¤¤, P < .01 E2 effect in POMC-ERα−/− mice vs E2-effect in control mice (interaction term from two-way ANOVA).
Table 3.
Effect of E2 on Cortical Bone Composition in POMC-ERα−/− Mice
| Control |
POMC-ERα−/− |
|||
|---|---|---|---|---|
| OVX | OVX + E2 | OVX | OVX + E2 | |
| μCT | ||||
| Cortical vBMD (mg/cm3) | 1319 ± 12 | 1317 ± 11 | 1318 ± 13 | 1300 ± 12 |
| FTIR | ||||
| Mineral to matrix ratio | 1.36 ± 0.09 | 1.66 ± 0.14 | 1.46 ± 0.13 | 1.66 ± 0.22 |
| Collagen maturity | 0.21 ± 0.02 | 0.19 ± 0.01 | 0.24 ± 0.07 | 0.20 ± 0.02 |
| Crystallinity | 0.078 ± 0.004 | 0.081 ± 0.004 | 0.080 ± 0.006 | 0.082 ± 0.003 |
μCT (femur; n = 7–10) and FTIR microspectroscopy (humerus; n = 4–7) analyses of cortical bone from OVX POMC-ERα−/− and OVX control mice treated with either E2 (0.5 μg/d) or vehicle. Data are presented as mean ± SEM.
The augmented estrogenic response on cortical bone thickness was mainly caused by increased amount of bone on the endosteal side in the E2-treated POMC-ERα−/− mice (Figure 2, E and F). However, when evaluated with cortical dynamic histomorphometry during the last week of the 6-week-long E2 treatment, no robust difference between the estrogenic response on endosteal or periosteal bone formation rate was observed for POMC-ERα−/− compared with control mice (Supplemental Table 4).
The estrogenic responses on trabecular bone parameters were unchanged in vertebrae L5 and only modestly augmented in the distal metaphyseal region of femur in POMC-ERα−/− compared with control mice (Table 4). Thus the estrogenic response on cortical bone mass was substantially increased, whereas no robust effect on the estrogenic response on trabecular bone mass was observed in POMC-ERα−/− compared with control mice (Figure 2 and Table 4).
Table 4.
Effect of E2 on Trabecular Bone Mass in POMC-ERα−/− Mice
| Control |
POMC-ERα−/− |
|||
|---|---|---|---|---|
| OVX | OVX + E2 | OVX | OVX + E2 | |
| Femur | ||||
| BV/TV (%) | 9.5 ± 1.0 | 39.8 ± 1.9c | 9.2 ± 1.0 | 47.4 ± 2.0c,d |
| Tb.N (L/mm) | 2.4 ± 0.2 | 9.6 ± 0.4c | 2.3 ± 0.2 | 10.6 ± 0.6c |
| Tb.Th (μm) | 39 ± 1 | 42 ± 1 | 40 ± 1 | 45 ± 2 |
| Tb.Sp (μm) | 132 ± 1 | 74 ± 5c | 132 ± 1 | 60 ± 5c |
| Vertebrae L5 | ||||
| BV/TV (%) | 15.7 ± 1.1 | 26.8 ± 1.6c | 16.4 ± 1.7 | 34.2 ± 2.8b |
| Tb.N (L/mm) | 4.1 ± 0.3 | 6.3 ± 0.4b | 3.9 ± 0.5 | 8.1 ± 1.0a |
| Tb.Th (μm) | 39 ± 1 | 43 ± 1a | 42 ± 1 | 43 ± 3 |
| Tb.Sp (μm) | 71 ± 3 | 59 ± 2a | 74 ± 5 | 50 ± 4b |
High-resolution μCT analysis of BV/TV, trabecular number (Tb. N), trabecular thickness (Tb.Th), and trabecular separation (Tb.Sp) in femur (n = 7–10) and vertebrae L5 (n = 4–6) of OVX POMC-ERα−/− and OVX control mice treated with either E2 (0.5 μg/d) or vehicle. Data are presented as mean ± SEM.
P < .05 E2 treated vs vehicle treated, Student's t test.
P < .01 E2 treated vs vehicle treated, Student's t test.
P < .001 E2 treated vs vehicle treated, Student's t test.
P < .05 E2 effect in POMC-ERα−/− mice vs E2 effect in control mice (interaction term from two-way ANOVA).
E2 treatment reduced serum OCN to a similar extent in POMC-ERα−/− and control mice, whereas the serum levels of CTX-1 (type I collagen fragments mice) were not significantly altered by E2 treatment in POMC-ERα−/− or control mice when analyzed after 6 weeks of E2 treatment (Supplemental Table 5). Serum leptin, as an indicator of body fat mass, was reduced to a similar extent by E2 treatment in POMC-ERα−/− and control mice (Supplemental Table 5). Serum levels of corticosterone were not affected by E2 treatment in OVX POMC-ERα−/− or OVX control mice (Supplemental Table 5).
To identify a possible mechanism for the augmented cortical E2-response in POMC-ERα−/− mice, mRNA levels of bone-related transcripts were evaluated in the cortical bone. At termination of the experiment, no significant effect of E2 treatment on mRNA levels of osteoprotegering, receptor activator of nuclear κB ligand, or OCN was observed in POMC-ERα−/− or control mice (Supplemental Table 6). As we have previously observed that central ERα action regulates mRNA levels of the CIITA (a regulator of MHC II, which is involved in antigen presentation by macrophages and T-cell activation in cortical bone) (5), CIITA mRNA levels were analyzed in cortical bone. Interestingly, although CIITA mRNA levels were reduced by E2 treatment in both POMC-ERα−/− and control mice, the estrogenic response was significantly more pronounced in POMC-ERα−/− compared with the estrogenic response in control mice (Supplemental Table 6).
We also characterized the mRNA levels of a number of central hypothalamic genes previously described to be involved in the regulation of bone and/or fat mass. The mRNA levels of NPY, AgRP, αMSH, MC4R, PTP1B, and SOCS3 were not significantly affected by E2 treatment in POMC-ERα−/− or control mice (Supplemental Table 7). Hypothalamic leptin receptor mRNA was significantly reduced by E2 treatment in control mice, whereas only a tendency of reduction was observed in POMC-ERα−/− mice but the estrogenic response was not significantly different between the POMC-ERα−/− and control mice (Supplemental Table 7).
Silencing of ERα expression in hypothalamic VMN does not affect bone mass
To evaluate the role of ERα in hypothalamic VMN for bone mass, ERα expression was silenced in VMN using stereotactic injection of AAV-shRNA-ERα. First, to test the efficacy of the shRNA viral vector for ERα silencing, mice were unilaterally injected with AAV-shRNA-ERα in the hypothalamic VMN area. ERα immunostaining was clearly visible in the VMN of the noninjected side, whereas almost no ERα immunostaining was observed in the VMN of the AAV-shRNA-ERα injected side (Supplemental Figure 1B).
Next, to determine the role of ERα in VMN for bone mass, mice were injected bilaterally with AAV-shRNA-ERα or AAV-shRNA-scramble. To avoid possible confounding effects of disturbed feedback regulation of serum sex steroids, all mice were OVX and treated with a constant dose of E2 (E2-pellet, 111 ng/mouse per d). As it is previously described that specific inactivation of ERα in hypothalamic VMN neurons results in obesity (28), we followed the change in body weight of the AAV-shRNA-ERα and AAV-shRNA-scramble-treated mice. Supporting a successful functional inactivation of ERα in VMN, the body weights of the AAV-shRNA-ERα-injected mice increased significantly more than the body weights of the AAV-shRNA-scramble-treated mice (Supplemental Figure 1C). In contrast, after 6 weeks, there was no difference in cortical or trabecular bone mass between the AAV-shRNA-ERα and AAV-shRNA-scramble-treated mice (Supplemental Figure 1, D–F), suggesting that ERα in hypothalamic VMN neurons is involved in the regulation of body weight but not bone mass.
Discussion
Elegant studies using several different conditional gene targeted mouse models or local injection of E2 in bone have clearly established that estrogens exert crucial direct peripheral effects on osteoclasts and osteoblasts in bone, resulting in augmented trabecular and cortical bone mass in female mice (8, 11, 12). We, here, demonstrate that the stimulatory peripheral effects of E2 on cortical bone in female mice are modulated by central inhibitory ERα-mediated actions in hypothalamic POMC neurons in ARC.
To evaluate the effect of ERα in ARC for energy metabolism and body composition, we recently developed a mouse model with specific inactivation of ERα in POMC neurons (26). In our previous study, we demonstrated that the ERα expression is reduced by more than 90% in POMC neurons in ARC of POMC-ERα−/− mice, and we here show that these mice have normal ERα expression in bone, demonstrating that the POMC-ERα−/− mouse model is useful to evaluate the specific role of ERα in hypothalamic POMC neurons for bone metabolism. We have earlier reported that female POMC-ERα−/− mice display hyperphagia but normal energy expenditure (26), and in the present study, we observed normal body weight, fat mass, and axial as well as appendicular bone growth, suggesting that the POMC-ERα−/− mice are in general healthy.
The female POMC-ERα−/− mice had, in the present study, clearly increased cortical bone mass and mechanical strength. This was the result of increased cortical bone thickness, whereas the cortical vBMD, as analyses by μCT and the cortical bone properties, as analyzed by detailed FTIR, were not affected in the POMC-ERα−/−mice. Thus, the amount but not the quality of cortical bone was altered, resulting in increased bone strength in female POMC-ERα−/− mice. Bone formation and resorption indices by serum assays were unchanged in the 6-month-old female POMC-ERα−/− mice, suggesting that alterations in bone turnover may have occurred at an earlier stage of development.
The female POMC-ERα−/− mice had slightly elevated serum levels of E2 but unchanged uterine weight, indicating that they have a modest disturbance in feedback regulation of serum sex steroids. To avoid the possibility that the slightly altered serum E2 levels could have confounded the interpretation of the bone phenotype in the gonadal intact POMC-ERα−/− mice, the main conclusions from the present study are based on the subsequent study comparing the estrogenic responses in OVX POMC-ERα−/− and OVX control littermate mice.
The most important finding in the present study was that the estrogenic responses for cortical bone thickness and mechanical strength were substantially augmented in OVX POMC-ERα−/− mice compared with the estrogenic responses in OVX control mice. This was mainly caused by a significantly reduced endosteal circumference in the E2-treated OVX POMC-ERα−/− mice. In contrast, cortical bone composition as analyzed by μCT and detailed FTIR were not affected by E2 treatment in POMC-ERα−/− mice or control mice and the estrogenic response on trabecular bone mass was mainly similar between POMC-ERα−/− mice and control mice. In addition, serum leptin levels, as an indicator of body fat mass, was reduced to a similar extent by E2 treatment in POMC-ERα−/− and control mice. Thus, estrogenic effects mediated via ERα in POMC neurons of ARC seem to rather specifically inhibit the peripheral stimulatory effects of estrogens on cortical bone mass and mechanical strength.
Our findings that the estrogenic response on the amount of trabecular bone was modestly augmented in femur and unchanged in vertebrae L5 of POMC-ERα−/− mice, suggest that the role of ERα in hypothalamic POMC neurons for trabecular bone mass is modest. Interestingly, Khosla et al have proposed that the main physiological target for estrogens in bone is cortical and not trabecular bone (42). This statement is based on a number of observations. First, detailed clinical investigations using CT revealed that trabecular bone loss begins in sex hormone replete young adults of both sexes and might be the result of cell autonomous age-related factors as suggested by Manolagas et al (12) and Manolagas (43). Second, the same studies, using CT, demonstrated that the onset of cortical bone loss in humans is closely tied to estrogen deficiency. Thus, for cortical bone, which comprises more than 80% of the skeleton and is likely the major contributor to overall fracture risk, there are data supporting the view that estrogen deficiency is the major cause of bone loss (42, 43). Our finding that the stimulatory effect of E2 on cortical bone mass is modulated by inhibitory actions of ERα in POMC neurons of ARC might therefore be useful for the development of selective ERα agonists with reduced penetrance to ARC, resulting in stimulatory peripheral ERα-mediated effects without inhibitory central ERα-mediated effects on cortical bone mass. Other estrogen-related targets separating the estrogenic effects on cortical and trabecular bone mass includes 1) activation function 1 in ERα, as ERα activation function 1 inactivated mice display an estrogenic response in cortical but not trabecular bone (44); 2) the steroid receptor coactivator 1, as steroid receptor coactivator 1 knock-out mice also display an estrogenic response in cortical but not trabecular bone (45); and 3) ERα in osteoclasts, as mice with osteoclast-specific ERα inactivation display reduced trabecular but not cortical bone mass (46, 47). Collectively, we believe that the cortical and trabecular bone compartments should be considered as separate functional entities when designing tissue specific ERα modulators.
Central ERα has in a previous study been reported to exert an inhibitory role on bone mass, partly counteracting the peripheral stimulatory effect of E2 (5). The present study extends these previous findings by identifying ERα in hypothalamic POMC neurons to mediate the central inhibitory effect of estrogens on cortical bone mass.
The hypothalamus is a central regulator of energy metabolism, and it also contributes to bone homeostasis (20–24, 48–51). The present result that ERα in hypothalamic POMC neurons regulates bone mass together with the recent finding that the transcription factor AP1 in POMC neuron is involved in the regulation of bone mass (24), strongly suggest that hypothalamic POMC neurons are crucial regulators of bone homeostasis. In addition, a role of POMC neurons for the regulation of bone mass is supported by that mice devoid of MC4R, the receptor for the POMC-neuron-derived ligand αMSH, have elevated bone mass (52) and that humans with MC4R mutations have high bone mass (53).
To identify possible downstream mediators of the modulatory effect of ERα in hypothalamic POMC neurons on cortical bone mass, the mRNA levels of several central hypothalamic genes (NPY, AgRP, αMSH, MC4R, leptin receptor, PTP1B, and SOCS3) were analyzed but no clear underlying pathway was identified. In addition, circulating leptin was not elevated in the POMC-ERα−/− mice, arguing against decreased central leptin sensitivity as an underlying mechanism (5). The immune system is involved in the regulation of bone homeostasis. Interestingly, although CIITA mRNA levels in cortical bone were reduced by E2 treatment in both POMC-ERα−/− and control mice, the estrogenic response was significantly more pronounced in POMC-ERα−/− compared with the estrogenic response in control mice. CIITA is a regulator of MHC II, which is involved in antigen presentation by macrophages and T-cell activation, and it is earlier reported to be regulated by central ERα signaling (5). These findings suggest that immune-mediated mechanisms might contribute to the modulatory effect of ERα in hypothalamic POMC neurons on cortical bone mass. However, further functional studies are clearly warranted to in detail characterize the downstream pathways of ERα action in hypothalamic POMC neurons for cortical bone mass.
Table 5.
Antibody Table
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog Number, and/or Name of Individual Providing the Antibody | Species Raised in; Monoclonal or Polyclonal | Dilution Used |
|---|---|---|---|---|---|
| ERα | Anti-ERα antibody | 06-935 | Polyclonal | 1:500 | |
| GFP | MSKGEELFTGVVPILVELDGD VNGHKFSVSGEGEGDATYG KLTLKFICTT GKLPVPWPTL VTTFSYGVQCFSRYPDHMKQ HDFFKSAMPEGYVQERTIFF KDDGNYKTRA EVKFEGDTLV NRIELKGIDFKEDGNILGHKLE YNYNSHNVYIMADKQKNGIK VNFKIRHN IEDGSVQLAD HYQQNTPIGDGPVLLPDNHY LSTQSALSKDPNEKRDHMVL LEFVTAAGIT HGMDELYK |
Anti-GFP antibody (FITC) | ab6662 | Polyclonal | 1:500 |
| Antirabbit IgG | Donkey antirabbit IgG (H + L) secondary antibody | Alexa Fluor 594 | Polyclonal | 1:1000 |
In addition to POMC cells in the hypothalamus, POMC (ACTH-secreting) cells also exist in the pituitary and we have previously shown that ERα mRNA levels were significantly reduced in the POMC (ACTH) cells in the pituitary of POMC-ERα−/− mice (26). However, neither ACTH expression in pituitary nor plasma levels of corticosterone at either basal or stressed conditions were affected in the POMC-ERα−/− mice. In addition, the T3/T4 levels were normal (26). Therefore, it is unlikely that the cortical bone phenotype in POMC-ERα−/− mice is due to loss of ERα from the pituitary POMC (ACTH) cells, although we cannot fully rule out this possibility.
Because ERα is abundantly expressed in hypothalamic VMN neurons, we also evaluated the possible role of ERα in VMN for bone mass. ERα expression in VMN was silenced using an AAV vector. Supporting our previous report regarding the metabolic function of ERα in VMN (28) and confirming a successful functional inactivation of ERα in VMN, the body weights of the AAV-shRNA-ERα-injected mice increased significantly more than the body weights of the AAV-shRNA-scramble-treated mice. In contrast, no bone phenotype was observed, indicating that ERα in hypothalamic VMN neurons is involved in the regulation of body weight but not bone mass.
In conclusion, mice lacking ERα in POMC neurons display substantially enhanced estrogenic response on cortical bone mass and modestly increased estrogenic response on the amount of trabecular bone in femur. We propose that the balance between inhibitory effects of central ERα activity in hypothalamic POMC neurons in ARC and stimulatory peripheral ERα-mediated effects in bone determines cortical bone mass in female mice.
Acknowledgments
We thank A. Hansevi, B. Aleksic, and C. Uggla for technical assistance. We also thank the Centre for Cellular Imaging at the Sahlgrenska Academy, University of Gothenburg, for the use of imaging equipment and for the support from the staff and the MAX-IV laboratory, Lund, Sweden, for providing the beamtime at the D7 beamline.
This work was supported by the Swedish Research Council, the Swedish Foundation for Strategic Research, an Agreement for Medical Education and Research from the University of Gothenburg and Västra Götaland region, the Lundberg Foundation, the Torsten and Ragnar Söderberg's Foundations, and the Novo Nordisk Foundation.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AAV
- adeno-associated viral
- AgRP
- aguti-related protein
- ARC
- arcuate nucleus
- BMD
- bone mineral density
- BV/TV
- trabecular bone volume fraction
- CIITA
- class II MHC transactivator
- CTX
- C-terminal type I collagen fragments
- μCT
- microcomputed tomography
- DHT
- dihydrotestosterone
- E2
- estradiol
- ER
- estrogen receptor
- FTIR
- Fourier transform infrared
- MC4R
- melanocortin 4 receptor
- MHC
- major histocompatibility complex
- αMSH
- α-melanocyte-stimulating hormone
- NPY
- neuropeptide Y
- OCN
- osteocalcin
- OVX
- ovariectomy
- POMC
- proopiomelanocortin
- PTP1B
- protein-tyrosin phosphatase 1b
- shRNA
- short hairpin RNA
- SOCS3
- supressor of cytokine signaling 3
- vBMD
- volumetric BMD
- VMN
- ventromedial nucleus.
References
- 1. Riggs BL, Khosla S, Melton LJ., 3rd Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002;23(3):279–302. [DOI] [PubMed] [Google Scholar]
- 2. Vandenput L, Ohlsson C. Estrogens as regulators of bone health in men. Nat Rev Endocrinol. 2009;5(8):437–443. [DOI] [PubMed] [Google Scholar]
- 3. Vanderschueren D, Vandenput L, Boonen S, Lindberg MK, Bouillon R, Ohlsson C. Androgens and bone. Endocr Rev. 2004;25(3):389–425. [DOI] [PubMed] [Google Scholar]
- 4. Lindberg MK, Weihua Z, Andersson N, et al. Estrogen receptor specificity for the effects of estrogen in ovariectomized mice. J Endocrinol. 2002;174(2):167–178. [DOI] [PubMed] [Google Scholar]
- 5. Ohlsson C, Engdahl C, Börjesson AE, et al. Estrogen receptor-α expression in neuronal cells affects bone mass. Proc Natl Acad Sci USA. 2012;109(3):983–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Turner RT. Mice, estrogen, and postmenopausal osteoporosis. J Bone Miner Res. 1999;14(2):187–191. [DOI] [PubMed] [Google Scholar]
- 7. Nicks KM, Fujita K, Fraser D, et al. Deletion of estrogen receptor β in osteoprogenitor cells increases trabecular but not cortical bone mass in female mice. J Bone Miner Res. 2016;31(3):606–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vanderschueren D, Laurent MR, Claessens F, et al. Sex steroid actions in male bone. Endocr Rev. 2014;35(6):906–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Windahl SH, Hollberg K, Vidal O, Gustafsson JA, Ohlsson C, Andersson G. Female estrogen receptor β−/− mice are partially protected against age-related trabecular bone loss. J Bone Miner Res. 2001;16(8):1388–1398. [DOI] [PubMed] [Google Scholar]
- 10. Windahl SH, Vidal O, Andersson G, Gustafsson JA, Ohlsson C. Increased cortical bone mineral content but unchanged trabecular bone mineral density in female ERβ (−/−) mice. J Clin Invest. 1999;104(7):895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Takano-Yamamoto T, Rodan GA. Direct effects of 17 β-estradiol on trabecular bone in ovariectomized rats. Proc Natl Acad Sci USA. 1990;87(6):2172–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Manolagas SC, O'Brien CA, Almeida M. The role of estrogen and androgen receptors in bone health and disease. Nat Rev Endocrinol. 2013;9(12):699–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gajda M, Litwin JA, Cichocki T, Timmermans JP, Adriaensen D. Development of sensory innervation in rat tibia: co-localization of CGRP and substance P with growth-associated protein 43 (GAP-43). J Anat. 2005;207(2):135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ducy P, Amling M, Takeda S, et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100(2):197–207. [DOI] [PubMed] [Google Scholar]
- 15. Cirmanova V, Bayer M, Starka L, Zajickova K. The effect of leptin on bone: an evolving concept of action. Physiol Res. 2008;57(1):13. [DOI] [PubMed] [Google Scholar]
- 16. Sienkiewicz E, Magkos F, Aronis KN, et al. Long-term metreleptin treatment increases bone mineral density and content at the lumbar spine of lean hypoleptinemic women. Metabolism. 2011;60(9):1211–1221. [DOI] [PubMed] [Google Scholar]
- 17. Yadav VK, Oury F, Suda N, et al. A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell. 2009;138(5):976–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cui Y, Niziolek PJ, MacDonald BT, et al. Lrp5 functions in bone to regulate bone mass. Nat Med. 2011;17(6):684–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yadav VK, Ryu JH, Suda N, et al. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell. 2008;135(5):825–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Driessler F, Baldock PA. Hypothalamic regulation of bone. J Mol Endocrinol. 2010;45(4):175–181. [DOI] [PubMed] [Google Scholar]
- 21. Idelevich A, Sato K, Rowe G, Gori F, Baron R. Inhibition of AP-1 in specific hypothalamic neurons increases both energy expenditure and bone density in mice. Am Soc Bone Miner Res. 2013; abstract 1043. [Google Scholar]
- 22. Rowe GC, Vialou V, Sato K, et al. Energy expenditure and bone formation share a common sensitivity to AP-1 transcription in the hypothalamus. J Bone Miner Res. 2012;27(8):1649–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sato K, Idelevich A, Rowe G, Gori F, Baron R. Leptin independent regulation of bone homeostasis by ΔFosB in the ventral hypothalamus contrasts with its leptin-dependent effects on energy and glucose metabolism. Am Soc Bone Miner Res. 2013; abstract MO0125. [Google Scholar]
- 24. Idelevich A, Sato K, Rowe G, Gori F, Baron R. Critical role of galanin in the hypothalamic neuronal regulation of bone density and energy expenditure by AP-1 antagonists. Am Soc Bone Miner Res. 2015; abstract 1083. [Google Scholar]
- 25. Gillies GE, McArthur S. Estrogen actions in the brain and the basis for differential action in men and women: a case for sex-specific medicines. Pharmacol Rev. 2010;62(2):155–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xu Y, Nedungadi TP, Zhu L, et al. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 2011;14(4):453–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Frank A, Brown LM, Clegg DJ. The role of hypothalamic estrogen receptors in metabolic regulation. Front Neuroendocrinol. 2014;35(4):550–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Musatov S, Chen W, Pfaff DW, et al. Silencing of estrogen receptor α in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc Natl Acad Sci USA. 2007;104(7):2501–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Movérare-Skrtic S, Henning P, Liu X, et al. Osteoblast-derived WNT16 represses osteoclastogenesis and prevents cortical bone fragility fractures. Nat Med. 2014;20(11):1279–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boskey A, Pleshko Camacho N. FT-IR imaging of native and tissue-engineered bone and cartilage. Biomaterials. 2007;28(15):2465–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Paschalis EP, Mendelsohn R, Boskey AL. Infrared assessment of bone quality: a review. Clin Orthop Relat Res. 2011;469(8):2170–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Isaksson H, Turunen MJ, Rieppo L, et al. Infrared spectroscopy indicates altered bone turnover and remodeling activity in renal osteodystrophy. J Bone Miner Res. 2010;25(6):1360–1366. [DOI] [PubMed] [Google Scholar]
- 33. Rieppo J, Hyttinen MM, Jurvelin JS, Helminen HJ. Reference sample method reduces the error caused by variable cryosection thickness in Fourier transform infrared imaging. Appl Spectrosc. 2004;58(1):137–140. [DOI] [PubMed] [Google Scholar]
- 34. Boskey A, Mendelsohn R. Infrared analysis of bone in health and disease. J Biomed Opt. 2005;10(3):031102. [DOI] [PubMed] [Google Scholar]
- 35. Paschalis EP, Verdelis K, Doty SB, Boskey AL, Mendelsohn R, Yamauchi M. Spectroscopic characterization of collagen cross-links in bone. J Bone Miner Res. 2001;16(10):1821–1828. [DOI] [PubMed] [Google Scholar]
- 36. Paschalis EP, DiCarlo E, Betts F, Sherman P, Mendelsohn R, Boskey AL. FTIR microspectroscopic analysis of human osteonal bone. Calcif Tissue Int. 1996;59(6):480–487. [DOI] [PubMed] [Google Scholar]
- 37. Pleshko N, Boskey A, Mendelsohn R. Novel infrared spectroscopic method for the determination of crystallinity of hydroxyapatite minerals. Biophys J. 1991;60(4):786–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nilsson ME, Vandenput L, Tivesten Å, et al. Measurement of a comprehensive sex steroid profile in rodent serum by high-sensitive gas chromatography-tandem mass spectrometry. Endocrinology. 2015;156(7):2492–2502. [DOI] [PubMed] [Google Scholar]
- 39. Alves SE, McEwen BS, Hayashi S, Korach KS, Pfaff DW, Ogawa S. Estrogen-regulated progestin receptors are found in the midbrain raphe but not hippocampus of estrogen receptor α (ER α) gene-disrupted mice. J Comp Neurol. 2000;427(2):185–195. [DOI] [PubMed] [Google Scholar]
- 40. Nomura M, Akama KT, Alves SE, et al. Differential distribution of estrogen receptor (ER)-α and ER-β in the midbrain raphe nuclei and periaqueductal gray in male mouse: predominant role of ER-β in midbrain serotonergic systems. Neuroscience. 2005;130(2):445–456. [DOI] [PubMed] [Google Scholar]
- 41. Schéle E, Benrick A, Grahnemo L, et al. Inter-relation between interleukin (IL)-1, IL-6 and body fat regulating circuits of the hypothalamic arcuate nucleus. J Neuroendocrinol. 2013;25(6):580–589. [DOI] [PubMed] [Google Scholar]
- 42. Khosla S, Melton LJ, 3rd, Riggs BL. The unitary model for estrogen deficiency and the pathogenesis of osteoporosis: is a revision needed? J Bone Miner Res. 2011;26(3):441–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Manolagas SC. From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis. Endocr Rev. 2010;31(3):266–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Börjesson AE, Windahl SH, Lagerquist MK, et al. Roles of transactivating functions 1 and 2 of estrogen receptor-α in bone. Proc Natl Acad Sci USA. 2011;108(15):6288–6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mödder UI, Sanyal A, Kearns AE, et al. Effects of loss of steroid receptor coactivator-1 on the skeletal response to estrogen in mice. Endocrinology. 2004;145(2):913–921. [DOI] [PubMed] [Google Scholar]
- 46. Martin-Millan M, Almeida M, Ambrogini E, et al. The estrogen receptor-α in osteoclasts mediates the protective effects of estrogens on cancellous but not cortical bone. Mol Endocrinol. 2010;24(2):323–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nakamura T, Imai Y, Matsumoto T, et al. Estrogen prevents bone loss via estrogen receptor α and induction of Fas ligand in osteoclasts. Cell. 2007;130(5):811–823. [DOI] [PubMed] [Google Scholar]
- 48. Elefteriou F, Karsenty G. [Bone mass regulation by leptin: a hypothalamic control of bone formation]. Pathol Biol. 2004;52(3):148–153. [DOI] [PubMed] [Google Scholar]
- 49. Karsenty G. Leptin controls bone formation through a hypothalamic relay. Recent Prog Horm Res. 2001;56:401–415. [DOI] [PubMed] [Google Scholar]
- 50. Karsenty G, Ducy P. The hypothalamic control of bone mass, implication for the treatment of osteoporosis. Ann Endocrinol. 2006;67(2):123. [DOI] [PubMed] [Google Scholar]
- 51. Oury F, Yadav VK, Wang Y, et al. CREB mediates brain serotonin regulation of bone mass through its expression in ventromedial hypothalamic neurons. Genes Dev. 2010;24(20):2330–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Braun TP, Orwoll B, Zhu X, et al. Regulation of lean mass, bone mass, and exercise tolerance by the central melanocortin system. PLoS One. 2012;7(7):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Farooqi IS, Keogh JM, Yeo GS, Lank EJ, Cheetham T, O'Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med. 2003;348(12):1085–1095. [DOI] [PubMed] [Google Scholar]