Abstract
β-Catenin (βcat) is a major downstream signaling node in canonical Wingless-related integration site (Wnt) signaling pathway, and its activity is crucial for canonical Wnt signal transduction. Wnt signaling has recently been implicated in the osteo-anabolic response to PTH, a potent calcium-regulating factor. We investigated whether βcat is essential for the anabolic action of intermittent PTH by generating male mice with adult-onset deletion of βcat in a subpopulation of bone cells (osteocytes and late-stage osteoblasts), treating them with an anabolic regimen of PTH, and measuring the skeletal responses. Male 10kbDmp1-CreERt2 transgenic mice that also harbored floxed loss-of-function βcat alleles (βcatf/f) were induced for Cre activity using tamoxifen, then injected daily with human PTH 1–34 (30 μg/kg) or vehicle for 5 weeks. Mice in which βcat was deleted showed either total lack of bone mineral density (BMD) gain, or BMD loss, and did not respond to PTH treatment. However, bone mass measurements in the trabecular compartment of the femur and spine revealed PTH-induced bone gain whether βcat was deleted or not. PTH-stimulated increases in periosteal and cancellous bone formation rates were not impaired by βcat deletion, but resorption markers and cortical porosity were significantly increased in induced mice, particularly induced mice treated with PTH. These results suggest that βcat is required for net-positive BMD effects of PTH therapy but that the anabolic effects per se of PTH treatment might not require osteocytic/osteoblastic βcat.
PTH, a hormone secreted by parathyroid glands, is a major regulator of plasma calcium levels (1). Daily injection of teriparatide, a truncated form of human PTH (amino acid residues 1–34), increases bone turnover but bone formation is preferentially stimulated to a greater extent than resorption, resulting in an anabolic effect on the skeleton. Although clearly efficacious in reducing fractures (2–5), teriparatide is expensive (6, 7), has inherent compliance issues due to its requirement for daily self-injection (8, 9), and is typically efficacious for only a short period of time, typically 18–24 months (10, 11). Those shortcomings have prompted the biomedical research community to better understand the signaling pathways activated by PTH, in order to identify more economical, longer-lasting, safer, and/or higher compliance therapies that harness the anabolic power of PTH.
PTH activates number of signaling pathways that could potentially be exploited for skeletal therapy. The most widely studied downstream cascade is the activation of adenylate cyclase, which stimulates cAMP production and subsequently activates protein kinase A. More recently, it was discovered that PTH signaling in bone cells activates components of the Wingless-related integration site (Wnt) signal transduction network (12, 13), a potent pathway that plays a significant role in bone metabolism (14). The mechanism of action for PTH-induced activation of intracellular Wnt signaling nodes was first discovered using Förster resonance energy transfer-based technology to reveal physical interaction between the PTH type 1 receptor (PTH1R) and the low-density lipoprotein-receptor related protein 6 (Lrp6) in the presence of PTH ligand (15). Lrp6 is a coreceptor for the large family of Wnt ligands that trigger the intracellular Wnt cascade. Further experiments revealed that the PTH1R-Lrp6 interaction has functional consequences; osteoblasts and osteocytes in mice (16) and from mice (17) with Lrp6 deletion fail to undergo PTH-induced activation of the downstream Wnt signaling mediator β-catenin (βcat). Perhaps more importantly, these mice fail to respond to an otherwise anabolic regimen of PTH (16). The PTH1R-Lrp6 interaction appears to be highly selective, because we (18) and others (19) have found that the closely related Wnt coreceptor Lrp5 is not required for the anabolic action of intermittent PTH.
Although the involvement of Lrp6 in PTH-induced bone anabolism has been established (16), the requirement of one of its major downstream Wnt targets, βcat, has not been elucidated. βcat plays a curious and paradoxical role in bone cells. Mice with βcat deletion from 2.3kbCol1a1-expressing cells (20) or from 10kbDmp1-expressing cells (21) exhibit very low bone mass but show no measurable reduction in bone formation rates, yet deletion of either canonical upstream Lrp coreceptor (Lrp5 or Lrp6) from the same cell populations induces a dramatic reduction in bone formation rates (16, 22–24). This disconnect between Lrp5/6 and βcat skeletal phenotypes raises the possibility that βcat might not be obligatory for PTH-induced anabolism in bone. To address this, we evaluated whether the anabolic effects of intermittent PTH stimulation would be maintained in mice with osteocytic/late-stage osteoblastic deletion of βcat. This cell population has previously been reported to be crucial for anabolic PTH action (25, 26). We used an inducible model of osteocyte-selective Cre activity (10kbDmp1-CreERt2) to recombine floxed loss-of-function βcat alleles in adult mice, in order to avoid the early lethality phenotype reported in mice with osteocyte-null βcat from conception (21).
Materials and Methods
Mice
All mice were homozygous for a floxed loss-of-function βcat (βcatf/f) allele that has been described previously (27). Briefly, these mice harbor loxP sites in introns 1 and 6 of the Ctnnb1 gene. 10kbDmp1-CreERt2 transgenic (TG) mice have been described previously (28). These mice harbor a cDNA for the Cre recombinase-mutant estrogen receptor fusion protein that results in Cre sequestration in the cytosol (away from the chromatin) until the selective ligand tamoxifen is encountered (29). The CreERt2 gene was driven by a 10-kb fragment of the dentin matrix protein-1 promoter, which provides osteocyte and late osteoblast selectivity of expression (30). Ai9 recombination reporter mice have been described previously (31). Briefly, these mice harbor a CAG promoter-driven floxed stop codon 5′ to a tdTomato sequence, knocked into Rosa26. βcatf/f mice were maintained on a C57BL6 background, 10kbDmp1-CreERt2 mice were maintained on a mixed 129/C57BL6 background, and Ai9 reporter mice were maintained on a C57BL6 background. βcatf/f mice were bred to 10kbDmp1-CreERt2 over several generations to generate βcatf/f mice that were either hemizygous (TG) for the 10kbDmp1-CreERt2 transgene or non-TG (NTG) for the 10kbDmp1-CreERt2 transgene. In separate crosses, mice homozygous for the Ai9 allele were bred to 10kbDmp1-CreERt2 TG mice to monitor recombination. Male mice were selected for the experiments. Offspring were same sex housed in cages of 3–5 (independent of genotype; NTG and TG littermates were used) and given standard mouse chow (Harlan Teklad 2018SX; 1% Ca; 0.65% P; vitamin D3 [2.1 IU/g]) and water ad libitum. All animal procedures were performed in accordance with Institutional Animal Care and Use Committee guidelines.
Dosing
To induce adult-onset recombination of the floxed βcat alleles, mice were treated with 20-mg/kg tamoxifen-free base (M&P Biomedicals). Tamoxifen powder was dissolved in dimethyl formamide (DMF) at a concentration of 100 mg/mL and then suspended in approximately 150 μL of corn oil for ip injection. Mice that received vehicle treatment (no Cre induction) were injected with an equivalent volume of DMF alone suspended in 150 μL of corn oil. Mice were treated with single injections of tamoxifen or vehicle at the following ages: 12, 12.4, 13, and 14 weeks.
Three days after beginning tamoxifen/vehicle injections, all mice began a 5-week course of daily (7 d/wk) sc injection of human PTH 1–34 at 30 μg/kg·d (Bachem, Inc), or vehicle control. PTH concentrations were adjusted weekly, based on weekly body mass measurements, to maintain the proper dose in a 100-μL injection volume. PTH was made fresh each day from frozen aliquots of 1-mg/mL stock solution.
Dual-energy x-ray absorptiometry (DEXA)
Whole-body DEXA scans were collected on isoflurane-anesthetized mice using a PIXImus II (GE Lunar) densitometer. All mice were scanned 3 days before beginning PTH/vehicle treatment, and again at 17 weeks of age, immediately before euthanasia. From the whole-body scans, areal bone mineral density (BMD) and bone mineral content were calculated for the entire postcranial skeleton and regionally for the lumbar spine (L3–L5, inclusive) using the Lunar ROI tools.
Microcomputed tomography (μCT)
Four days before beginning PTH/vehicle treatment, the distal 20% of the right femur was scanned on an in vivo μCT (vivaCT-40; Scanco Medical AG) while the mouse was under isoflurane-induced anesthesia. The μCT scan conditions were as follows: 60 kV, 114 mA, 100-ms integration time, and 21.5-μm voxel resolution. After euthanasia, the right femur was dissected from each mouse, fixed for 2 days in 10% neutral buffered formalin, and then transferred into 70% ethanol for μCT scanning on a high-throughput μCT specimen scanner (μCT-35; Scanco Medical AG). The middle 15% and distal 33% of each bone was scanned using the following conditions: 50 kV, 120 mA, 151-ms integration time, and 10-μm voxel resolution. Three-dimensional morphometric properties of the distal femur cancellous bone were measured as previously described (32). To facilitate accurate comparison between pre- and posttreatment μCT scans collected on different (in vivo vs specimen scanner) instruments, a set of 5 femurs was scanned on both machines on the same day to derive a calibration factor to adjust for differences between instruments. Identical scanning parameters were used in the calibration scans as were used in the actual experiments. A calibration factor was derived based on reduced major axis regression analysis of individual output parameters from the specimen scanner onto parameters from the in vivo scanner. Analyses of cortical bone parameters (cortical, total, and medullary area) were performed on posttreatment samples using a 20-slice stack that was centered around the midshaft slice of the femur, as previously described (32). Midshaft femur slices were imported into ImageJ (National Institutes of Health) to measure the total area within the periosteal border, between the periosteal and endocortical surfaces, and within the pores occupying the cortical volume.
Fluorochrome administration, bone quantitative histomorphometry, and histochemistry
Fluorochrome-derived histomorphometric indices of cortical and cancellous bone formation were measured in the midshaft femur and distal femur secondary spongiosa, respectively. Before killing, mice were injected with demeclocycline (75 mg/kg, sc) 1 day before the first PTH injection, calcein (12 mg/kg, ip) after 4 weeks of daily PTH treatment, and alizarin complexone (20 mg/kg, ip) 3 days before the euthanasia. After euthanasia and μCT scanning (see above), the right femur was dehydrated in graded ethanols and embedded in methylmethacrylate. Midshaft femur sections were cut in the transverse plane (∼200 μm thick) using a diamond-embedded wafering saw (Buehler, Inc) and ground to a final thickness of approximately 30 μm. Distal femur sections were cut in the longitudinal plane with a motorized microtome (Leica 2255; Leica Microsystems, Inc). Periosteal bone formation parameters were calculated from the demeclocycline and calcein labels by measuring the extent of unlabeled perimeter, single-labeled perimeter, double-labeled perimeter, and the area between the double labeling, using the Bioquant Osteo system (Bioquant Corp). Cancellous bone formation parameters were measured from the calcein and alizarin labels. Derived histomorphometric parameters, including mineralizing surface per unit bone surface (%), mineral apposition rate (μm/d), and bone formation rate per unit bone surface (BFR/BS) (μm3/μm2 per y), were calculated using standard equations.
The fifth lumbar vertebra was decalcified in 30% EDTA for 1 week, embedded in paraffin, sectioned at 5 μm, mounted on charged glass slides, stained using the Tartrate-resistant acid phosphatase reaction, and counterstained with methyl green, as previously described (33). The trabecular surface covered by Trap-positive (Trap+) cells containing 3 or more nuclei was measured in the central cancellous region, beginning 0.3 mm from the vertebral end plates. Osteoclast surface measurements were standardized to the total trabecular surface.
Serum collection and immunoassays
Whole blood samples were collected from 10kbDmp1-CreERt2 TG mice via cheek bleeding 1 day before beginning tamoxifen/oil treatment, and again 2 weeks after initiating PTH treatment. Approximately 150 μL of blood were collected into serum separator tubes (BD Microctainer), allowed to clot at room temperature for 40 minutes, then centrifuged at 10 000g for 1 minute. Serum was removed and stored at −80°C until all samples were collected and assayed together. Serum concentration of C-terminal telopeptide (CtX) and procollagen type 1 N-terminal propeptide (P1NP) were measured by commercially available ELISA kits (IDS, Inc) according to the manufacturer's instructions. Serum samples at each time point were measured in duplicate and averaged, and the percent change in each marker was calculated on a per mouse basis.
Cell sorting and RNA extraction
Whole tibiae and femora were removed from βcatf/f mice that also harbored the 10kbDmp1-CreERt2 and Ai9 transgenes. Eight-week-old mice were treated with 2 doses of tamoxifen, spaced 4 days apart, then killed 3 days later. At killing, both femora and tibiae were immediately removed, quickly cleaned of soft tissue, and the bone ends were removed to facilitate marrow removal via saline flush. The cortical tubes were subsequently fragmented to collect osteocytes using a published protocol (34). We retained and pooled digests 1–8, and resuspended the cells in PBS supplemented with 3% fetal calf serum, filtered using a 45-μm cell strainer. The cells were immediately loaded into an I-Cyt Reflection (Sony BioSciences) FACS unit. The fluidics operate using a sample pressure of approximately 20.0 ψ and a droplet frequency of 38 kHz. The sort phase was calibrated before sorting (on the same day) using Flow Check beads sorted onto a slide and counted. Cells were sorted using a 575/40-nm laser for detection of tdTomato (99% emission detection with 575/40), using a far red (675 nm) signal as control. Sorted cell populations (tdTomato-positive and tdTomato-negative) were collected in cold MEM with 10% FCS and washed in PBS. The cells were lysed and processed for RNA isolation using the TRIzol (Life Technologies, Inc) reagent, following the manufacturer's protocol for phenol-chloroform extraction. A total of 50 ng of RNA was reversed transcribed into cDNA using the ABI High Capacity cDNA
Reverse Transcription kit (Life Technologies, Inc), then amplified using custom designed primers and 6-carboxyfluorescein-labeled probes for βcat (Ctnnb1) and the housekeeper β-actin (Actb) on an Applied Biosciences 7900HT Fast Real Time PCR system (Life Technologies, Inc).
Statistical analysis
Statistical analyses were conducted with JMP (version 4.0; SAS Institute, Inc). The radiographic, histomorphometric, and biochemical endpoints were analyzed using one- or two-way (within transgene) ANOVA, with induction agent (oil/tamoxifen) and hormone therapy (veh/PTH) as main effects. Post hoc comparisons within ANOVAs that achieved overall significance were made using Fisher's protected least significant difference tests. Statistical significance was taken at P < .05. Two-tailed distributions were used for all analyses. Data are presented as means ± SEM.
Results
Induced Dmp1-CreERt2 activity localizes to cortical and trabecular osteocytes and osteoblasts and selectively recombines floxed βcat alleles
To confirm that the inducible CreERt2 allele had selective activity in osteocytes and late-stage osteoblasts upon induction via tamoxifen injection, we evaluated cryosections from the femur and spine of 10kbDmp1-CreERt2-positive mice that also carried the fluorescent recombination reporter allele Ai9. Consistent with previous reports (30), mice treated with vehicle (DMF/corn oil) exhibited observable but minimal recombination in osteocytes and surface cells (Figure 1, A and B, and Supplemental Figure 1). Mice treated with tamoxifen twice during the 9-day period before killing exhibited a strong tdTomato signal in most of the cortical and cancellous osteocytes, and also in some surface cells (presumably osteoblasts) (Figure 1B). Recombination was not detected in skeletal muscle or marrow (Supplemental Figure 1).
Figure 1.

A, Undecalcified cryosection through the femur of a 14-week-old Ai9 TG Cre reporter mouse that also carried the 10kbDmp1-CreErt2 transgene. The mouse was given tamoxifen twice in 1 week, then killed 7 days after the first injection. Red fluorescent cells indicate recombination of the Ai9 allele (ie, Cre activity). Sections were stained with DAPI to reveal cell nuclei (fluorescent blue staining). The white ROI box in A is shown at higher magnification in B. Note the recombination (red fluorescence) in osteocytes and surface cells (presumably late-stage osteoblasts) but not in marrow. C, Double TG (10kbDmp1-CreERt2 × Ai9) mice that also harbored homozygous loss-of-function floxed βcat alleles (βcatf/f) were injected with tamoxifen, and the cells were flow sorted for the 575-nm (tdTomato) signal. The tdTomato-positive fraction and a portion of the tdTomato-negative fraction were identified using the indicated gates and were subsequently lysed and assayed for βcat expression using quantitative polymerase chain reaction (D). Note the significant reduction in βcat expression among the tdTomato-positive cells, indicating that the inducible transgene was selectively cutting βcat loxP sites in bone cells; *, P < .05.
To verify that the 10kbDmp1-CreERt2 transgene was capable of recombining the floxed βcat alleles per se (and not just Ai9), we bred the βcat-flox alleles onto the 10kbDmp1-CreERt2 × Ai9 double TG background. The mice were treated with tamoxifen according to the previously described schedule, and the femora and tibia were collected, flushed to remove marrow, and the cortical tubes were fragmented to collect osteocytes. We flow sorted the cells for the tdTomato signal (Figure 1C) and performed quantitative polymerase chain reaction for βcat using the lysates from the tdTomato-positive (osteocyte enriched) and tdTomato-negative (marrow, vessels, nerves) fractions (Figure 1D). βcat expression was significantly reduced in the osteocyte-enriched (Ai9-positive) fraction, compared with the tdTomato-negative pool, indicating that the inducible Cre model had high fidelity for recombining and inactivating βcat in osteocytes in vivo.
DEXA-derived bone mass measurements reveal that βcat deletion from 10kbDmp1-expressing cells results in compromised PTH-induced bone gain
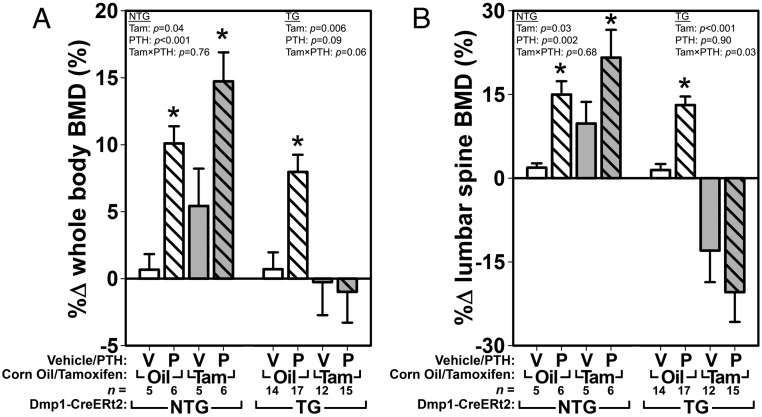
To assess the effects of intermittent PTH in mice with βcat-deficient osteocytes, βcatf/f mice were treated for 5 weeks with 30 μg/kg·d of PTH or vehicle. Comparison of the 12-week pretreatment DEXA scan with the 17 weeks after treatment among NTG (10kbDmp1-CreERt2 negative) mice revealed that both tamoxifen and PTH had significant effects on whole-body and spine BMD as individual treatments (ie, PTH alone in oil-treated mice and tamoxifen alone in vehicle-treated mice) and in combination (Figure 2). Among TG (10kbDmp1-CreERt2 positive) mice, PTH was efficacious in increasing BMD in noninduced (oil-treated) mice but not in Cre-induced (tamoxifen-treated) mice. Spinal BMD was reduced to below baseline values by βcat deletion, and PTH was not effective at rescuing bone mass in those mice. Changes in whole-body and spinal bone mineral content, and other skeletal properties, were similar to those found for BMD (Supplemental Figures 2 and 3).
Figure 2.
DEXA-derived measures of BMD using whole-body (A) and lumber spine (B) ROIs, calculated as the percent difference from 12 weeks of age (beginning of Cre induction and PTH treatment) to 17 weeks of age (end of PTH treatment), reveal that PTH treatment failed to increase BMD when βcat was deleted from 10kbDmp1-expressing cells. The left side of each graph indicates the effect of tamoxifen (Tam) and PTH (P) in essentially wild-type mice (no Cre transgene present [NTG] to recombine the floxed βcat alleles that are present in all groups). The right side of each graph indicates the effect of PTH in TG mice with (Tam) or without (Oil) βcat deletion. Among the TG mice, whole-body BMD and spine BMD ANOVAs (upper corners) yielded near significant (P = .06) and significant (P = .03) interaction terms, respectively, suggesting that βcat deletion significantly affects the efficacy of PTH (at least in the spine). Asterisks indicate significant (P < .05) PTH-induced difference from the tam/oil-matched vehicle control, using PLSD post hoc pairwise tests.
βcat deletion from 10kbDmp1-expressing cells does not reduce PTH-induced cancellous bone gain
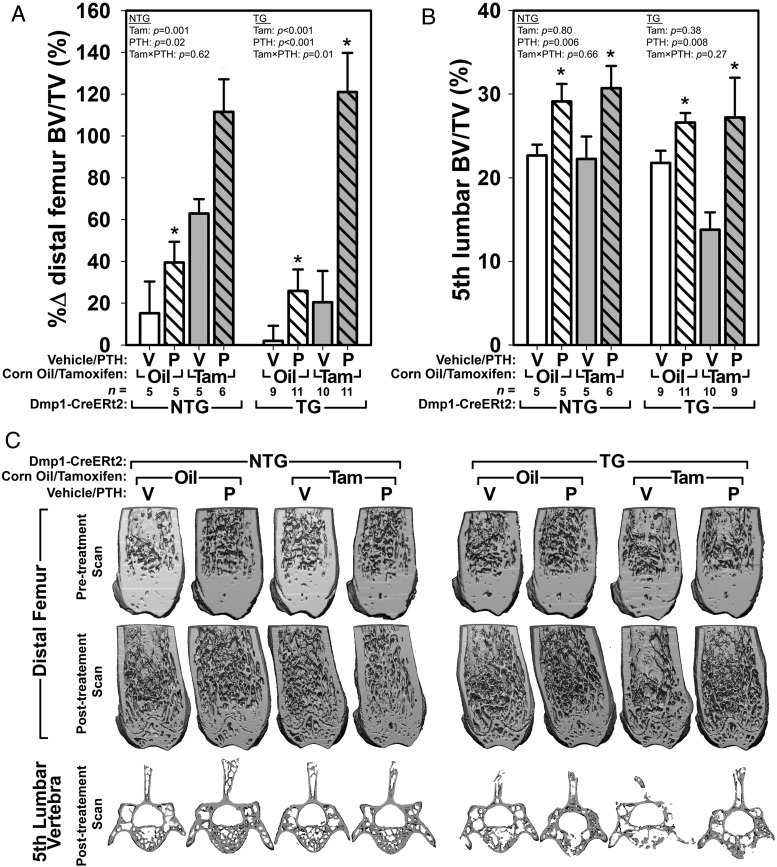
Analysis of the cancellous bone compartment yielded a different result than that obtained by DEXA. For evaluation of the distal femur trabecular envelope, we were able to increase our effect size sensitivity by collecting a pretreatment distal femur μCT scan using an in vivo μCT, for comparison with a posttreatment scan (after euthanasia) using our standard specimen scanner. Among NTG mice, both tamoxifen and PTH increased femoral bone volume fraction (BV/TV) significantly as individual treatments and in combination (Figure 3, A and C). PTH had similar effects in TG mice to those observed in NTG mice, where PTH treatment in the presence of corn oil improved BV/TV modestly, but PTH treatment in the presence of tamoxifen (ie, in mice with recombined βcat alleles) increased BV/TV to the same degree as was observed in NTG mice (Figure 3, A and C). Tamoxifen alone in TG mice did not significantly improve BV/TV compared with control (oil/veh-treated) mice. Additional μCT outcome measures (trabecular number, thickness, and spacing) followed similar trends as those described for BV/TV (Supplemental Table 1).
Figure 3.
BV/TV in the distal femoral metaphysis and the body of the fifth lumbar vertebra was enhanced by PTH treatment in βcat mutant and replete mice. A, In the femur, PTH and tamoxifen had significant independent effects on the change over time in femoral BV/TV. Only in TG mice was a significant interaction term encountered, indicating that βcat deletion significantly affects the way PTH acts on femoral BV/TV. The significant interaction term was likely driven by the reduction in BT/TV among tamoxifen-treated mice. B, In the spine, BV/TV measurements collected at the completion of the experiment indicated that PTH but not tamoxifen significantly increased trabecular bone mass, regardless of transgene presence or tamoxifen status. Asterisks indicate significant (P < .05) PTH-induced difference from the tam/oil-matched vehicle control, using PLSD post hoc pairwise tests. C, μCT reconstructions through the distal femur (anterior portion digitally removed) and L5 vertebrae (cranial and caudal portions digitally removed). Note that the pretreatment (in vivo) scans were collected on a different instrument than the posttreatment scans, and the difference in resolution is apparent.
Vertebral cancellous bone architecture was evaluated posttreatment; pretreatment in vivo scans were not collected due to trunk radiation concerns and to poor image quality stemming from trunk movement during respiration. Among NTG mice, PTH increased fifth lumbar BV/TV to the same degree in the presence of tamoxifen and corn oil (Figure 3, B and C). Tamoxifen alone had no detectable effect on vertebral BV/TV. In TG mice that were not induced (oil-treated), PTH treatment was associated with a significant increase in fifth lumbar BV/TV, similar to that found in NTG mice. In tamoxifen-induced TG mice, BV/TV was reduced significantly in vehicle-treated mice, but PTH treatment in this group resulted in a similar degree of PTH-induced bone gain as was observed in oil-treated TG mice. Additional L5 μCT outcome measures (trabecular number, thickness, and spacing) followed similar trends as those described for BV/TV (Supplemental Table 1).
Cortical and cancellous bone formation are stimulated by PTH in mice with βcat deletion from 10kbDmp1-expressing cells
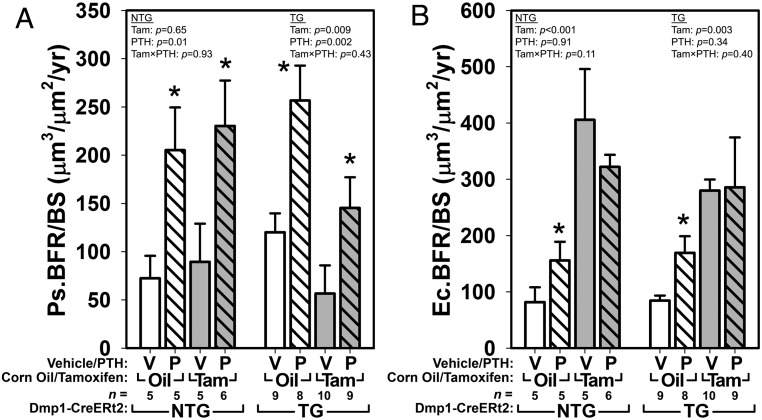
Having found that changes in bone mass derived from DEXA (a measure dominated by cortical bone) were inconsistent with those derived from changes derived from μCT of the trabecular network, we next looked at fluorochrome-derived bone formation parameters in these envelopes to gain insight into the discrepancy. Periosteal and endocortical bone formation parameters were measured at the midshaft femur, using the pretreatment (12 wk of age) demeclocycline labels and the 4 weeks after treatment (16 wk of age) calcein labels. Cancellous parameters were measured at the distal femur metaphysis using the 4 weeks after treatment calcein labels and the final alizarin labels. In NTG control mice, periosteal bone formation rates were increased significantly by PTH, but no measureable tamoxifen effect was observed (Figure 4A and Supplemental Figure 5). In oil-treated TG mice, periosteal BFR/BS was similarly increased by PTH treatment. In tamoxifen-treated TG mice, bone formation rates were suppressed in mice receiving vehicle, but PTH was able to significantly increase BFR/BS even in the presence of recombined βcat alleles. The changes in periosteal BFR/BS were driven by changes in both mineralizing surface and apposition rates (Supplemental Table 1). Endocortical BFR/BS was not consistently increased by PTH, but tamoxifen had significant effects at that surface in both TG and NTG groups (Figure 4B). The combination of tamoxifen and PTH had similar effects to tamoxifen alone, regardless of transgene status. Cancellous BFR/BS was enhanced by PTH in TG and NTG groups, regardless of tamoxifen treatment (Supplemental Figure 4 and Supplemental Table 1).
Figure 4.
A, Midshaft femur fluorochrome-derived bone formation rates on the periosteal surface (Ps.BFR/BS) were significantly increased by PTH treatment. In NTG mice, PTH increased BFR/BS significantly in both corn oil- and tamoxifen-treated mice, but no tamoxifen effect was detected. In TG mice, tamoxifen significantly lowered BFR/BS overall, but the PTH effect on bone formation remained in induced mice. B, Measurements from the endocortical surface of the same sections yielded a significant effect only for tamoxifen. Asterisks indicate significant (P < .05) PTH-induced difference from the tam/oil-matched vehicle control, using PLSD post hoc pairwise tests.
βcat deletion from 10kbDmp1-expressing cells does not affect PTH-induced changes in serum markers of bone formation, but serum and tissue indices of bone resorption and cortical porosity are exacerbated by PTH
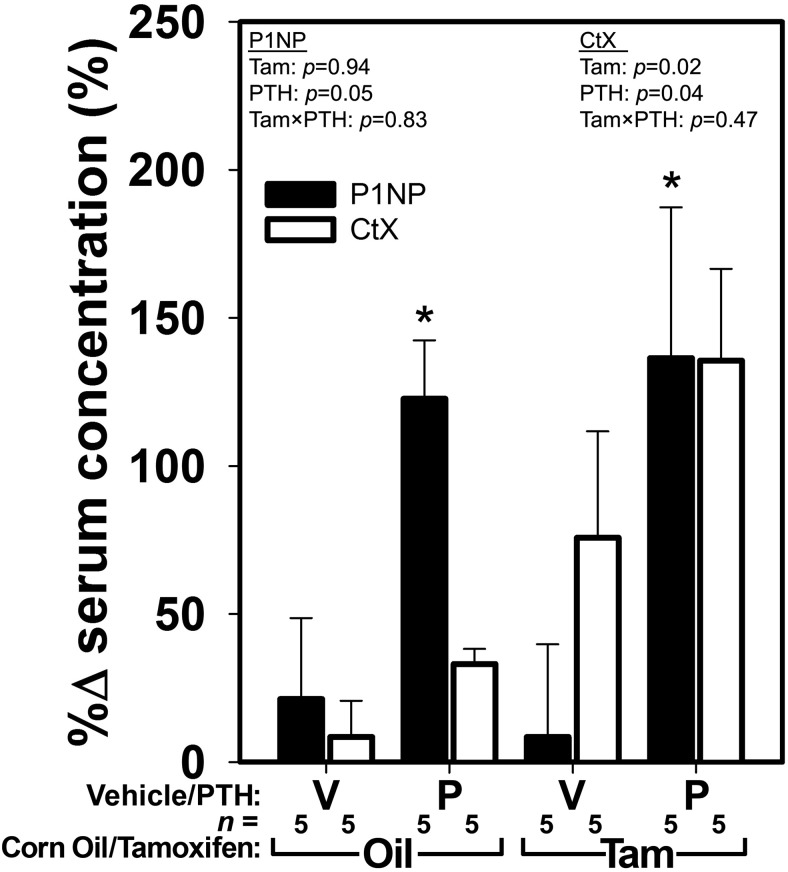
To evaluate global changes in bone formation and bone resorption induced by PTH in the presence or absence of osteocytic βcat, we collected serum from the TG mice just before initiating PTH treatment, and again after 2 weeks of treatment. Serum levels of the bone formation marker P1NP were increased significantly after 2 weeks of PTH treatment, regardless of βcat inactivation (Figure 5). Surprisingly, tamoxifen administration had no measureable effect on P1NP levels. Serum levels of the bone resorption marker CtX were significantly increased by tamoxifen and by PTH treatment after 2 weeks of PTH treatment in βcat replete mice (Figure 5), although the measurement error associated with this assay precluded a significant PTH effect when comparing PTH with vehicle treatment within induction (tamoxifen/oil) groups. Quantification of osteoclast surface in the fifth lumbar vertebra yielded similar results to those reported for serum CtX (Supplemental Figure 6). In NTG mice, PTH significantly increased osteoclast surface regardless of tamoxifen treatment. Among TG mice, a significant PTH effect was detected for osteoclast surface per unit bone surface, but post hoc tests indicated that the change was significant only for induced mice.
Figure 5.
Serum levels of P1NP (filled bars), a marker of bone formation, and type I collagen CtX (open bars), a marker of bone resorption, were increased significantly after 2 weeks of PTH treatment in TG mice. Cre induction via tamoxifen had no effect on P1NP levels, but CtX levels were significantly increased by βcat deletion. Asterisks indicate significant (P < .05) PTH-induced difference from the tam/oil-matched vehicle control, using PLSD post hoc pairwise tests.
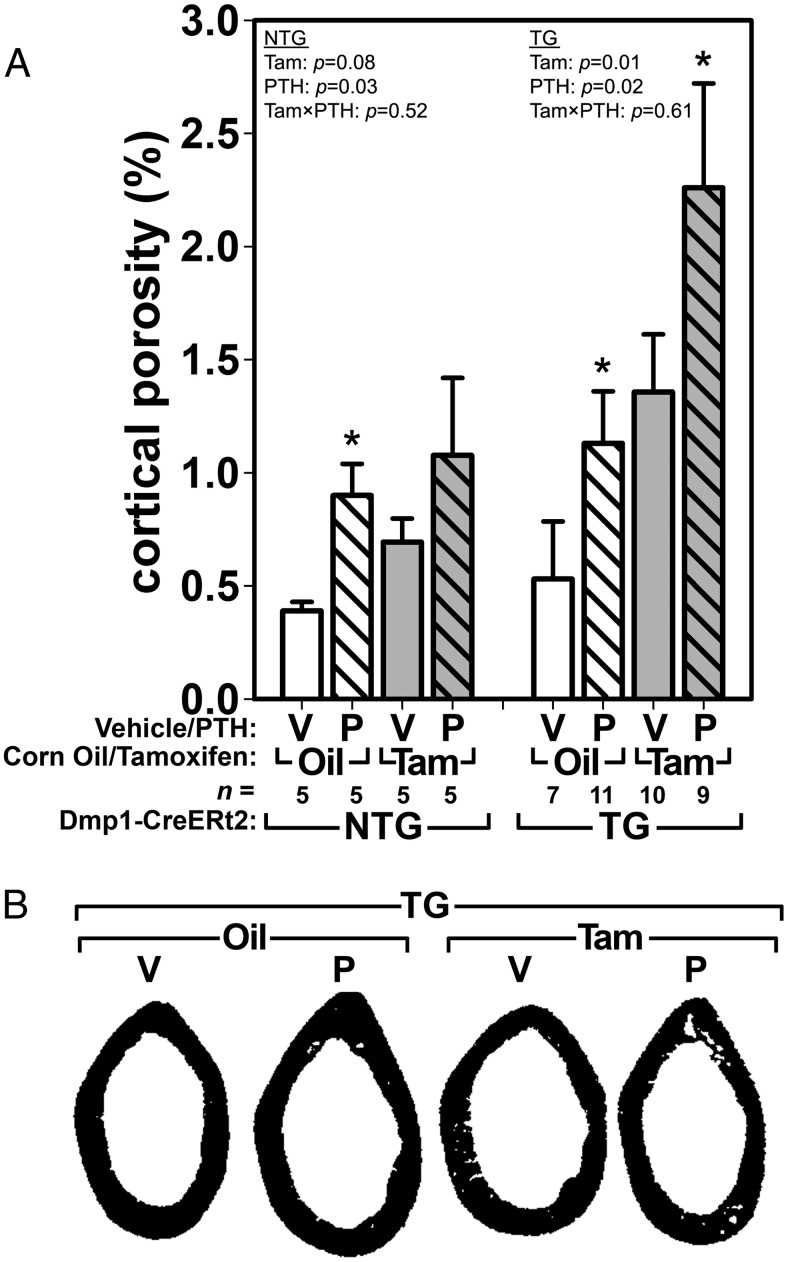
The relatively normal PTH-induced bone formation rates yet overall reduced bone mass in mice with recombined βcat alleles prompted us to investigate whether cortical porosity might explain these disparate observations. We measured cortical bone porosity (Ct.Po) within the femoral cortex from the same sections used to derive bone formation rates. In NTG mice, Ct.Po was increased by PTH but not by tamoxifen alone (Figure 6). In TG mice, PTH increased Ct.Po significantly among oil-treated mice. Recombination of βcat alleles with tamoxifen resulted in significantly increased Ct.Po compared with TG mice that were not given tamoxifen. Additional treatment of those mice with PTH further increased Ct.Po significantly. Midshaft femur bone tissue area, total area, and cortical thickness were increased by PTH treatment in all groups, whereas tamoxifen did not have a significant effect on those parameters (Supplemental Table 1).
Figure 6.
A, Cortical porosity at the midshaft femur was significantly increased by PTH in NTG mice, although post hoc tests yielded a significant increase only among oil-treated mice. Among TG mice, PTH increased cortical porosity significantly in both noninduced (oil-treated) and Cre-induced (tamoxifen-treated) mice. B, μCT reconstructions of the midshaft femur in TG mice, illustrating the changes in intracortical porosity resulting from tamoxifen and PTH treatments.
Discussion
Our main objective in the current study was to understand whether loss of βcat in the osteocyte/late-stage osteoblast population would prevent the anabolic effects of intermittent PTH treatment in adult mice. Our rationale for conducting these studies was based on published results showing that 1) βcat is activated by PTH (12, 13, 17, 35, 36); 2) βcat is a major downstream target of Lrp6 in the canonical Wnt cascade (37–39); and 3) Lrp6 is crucial for PTH-induced anabolism (16). We found that mice with recombined βcat alleles in 10kbDmp1-expressing cells failed to exhibit PTH-induced net bone gain as assessed by DEXA, but μCT measurements of cancellous properties revealed significant positive of PTH in induced mutant mice. The anabolic effect per se of intermittent PTH was not inhibited in induced mutant mice. This latter observation is supported by histomorphometric measurements of cortical and cancellous bone formation rates from tissue sections, and from serum measurements of a formation biomarker. Both of these lines of evidence indicate relatively normal bone formation in the absence of βcat, and enhanced bone formation in response to PTH, regardless of osteocyte/late osteoblast βcat status.
Among induced mice, the DEXA measurements (which showed a failure to gain bone with PTH treatment) appear inconsistent with those derived from cortical and cancellous histomorphometry (which showed normal PTH-induced bone formation) and trabecular μCT (which showed normal PTH-induced increases in trabecular bone properties). However, some explanation for the apparent discrepancy is provided by the resorption-related data (serum CtX, cortical porosity, and osteoclast surface), which indicate that resorption is significantly increased in induced mice, and further increased in induced mice treated with PTH. Thus, when the data are interpreted in total, they suggest that PTH treatment increases bone formation rates whether βcat is present in Dmp1-expressing cells or not. However, loss of βcat in those cells significantly increases resorption (eg, cortical porosity), which nullifies any DEXA-related gains in bone mass that derive from the intact anabolic effect. We have encountered a similar phenomenon with another protein, sclerostin, in the Wnt pathway, where sclerostin-deficient mice have an intact anabolic response to PTH but also have a concomitant exacerbated resorption response to PTH (40). These factors combine to yield a net-neutral response to PTH as measured by DEXA (despite normal PTH-induced increases in bone formation rates).
Because βcat−/− mice are embryonic lethal (41) and the noninducible 10kbDmp1-Cre × βcatf/f are peripubertal lethal (21), we used the tamoxifen-inducible version of the Dmp1-Cre transgene (CreERt2) to achieve delayed recombination of the βcat flox alleles so that we had enough lifespan to study PTH signaling in a 5-week hormone treatment course, and to do so in the adult skeleton. Although this approach offers several advantages over traditional noninducible Cre lines, there are limitations to the inducible model which are primarily related to tamoxifen's side effects on bone metabolism. It is well known that women treated with tamoxifen for breast cancer therapy experience increased BMD and a reduction in fracture risk (42), and analogous effects on bone metabolism have been observed in rodents. The tamoxifen-inducible CreER fusion protein was initially brought into mouse in vivo transgenesis experiments for targeting recombination in ectoderm (skin) (43), a tissue where tamoxifen has negligible side effects. Adoption of the CreERt2 system into skeletal studies using various bone promoters, eg, 2.3kbCol1a1-CreERt2 (44), 3.2kbCol1a1-CreERt (45), Col1a1+/Frt-CreERt2-Frt (46), BACOsx-CreERt2 (45), and 10kbDmp1-CreERt2 (28) soon followed, but the independent effects of tamoxifen on bone properties has required additional controls to understand the effects of tamoxifen-induced gene deletion vs tamoxifen's direct effects on bone properties.
In our experiments, we were compelled to include NTG (10kbDmp1-CreERt2 negative) mice in the study design, which allowed us to probe the effects of tamoxifen alone, PTH alone, and the interaction of tamoxifen and PTH on catabolic and anabolic responses in a physiologically normal skeleton (where tamoxifen is incapable of inducing βcat recombination due to the lack of Cre). Tamoxifen and PTH elicit different effects in bone; tamoxifen increases the amount of mineral, whereas PTH increases the amount of bone that is inadequately mineralized. Our DEXA measurements are incapable of distinguishing between these 2 mechanisms, but the individual components (ie, contribution of formation, contribution of resorption) that drove the whole skeletal net changes measured using DEXA can be parsed out with specific tissue/serum measurements. Despite these analyses, it still remains impossible to unequivocally separate the effects of tamoxifen and genetic recombination in induced TG mice because the former is required for the latter. We used a lower tamoxifen dose than in previous studies (28) but we were unable to avoid the bone-active side effects of tamoxifen when used as an induction agent. In reporter mice experiments, we observed red fluorescence in some osteocytes in the absence of tamoxifen. However, it is unlikely that βcat was ablated in those mice because 1) the tdTomato reporter is exceptionally sensitive, perhaps much more so than other floxed loci (30); 2) basal skeletal phenotypes of NTG (oil/veh-treated) and TG (oil/veh-treated) mice were similar (Figures 2–4); and 3) the peripubertal lethality phenotype caused by early-onset βcat deletion from Dmp1-expressing cells (21) was not encountered in our study. Next generation inducible Cre models that use antibiotic ligands are being developed that might circumvent the tamoxifen issue regarding bone metabolism (47).
PTH1R deletion from 10kbDmp1-expressing cells prevents anabolic action of intermittent PTH in vivo (26). Thus, cell signaling downstream of PTHR1 in this population is crucial for the osteoanabolic action of PTH. We found that the anabolic action of PTH (hormone-stimulated new bone formation) was intact in mice with induced loss-of-function mutations in βcat, suggesting that the PTH-induced bone formation might occur through collateral mechanisms that either normally bypass βcat or that are able to activate accessory pathways when βcat is blocked. Our data are consistent with previous reports indicating unaltered bone formation rates in (noninducible) 10kbDmp1-Cre × βcatf/f mice (21), and also in mice that exhibit deletions slightly earlier in the differentiation program (2.3kbCol1a1-Cre × βcatf/f) (20). Moreover, mice with βcat exon 3 deletion (reduced degradation) in 2.3kbCol1a1-expressing cells show no change in bone formation. Yet in all 3 models, resorption was significantly affected (increased in loss-of-function models and reduced in gain-of-function models). We similarly found a significant perturbation in bone resorption when βcat was deleted from 10kbDmp1-expressing cells, which was significantly exacerbated by intermittent PTH.
It is unclear why femoral cancellous bone formation rates were equally responsive to PTH in βcat replete and deficient mice, yet μCT measurements in the same region indicated a significantly greater degree of bone gain in PTH-treated βcat-deficient mice. Unlike the labeling schedule used to measure bone periosteal formation rates in the femoral cortex, which flanked almost all of the treatment period, we used a double label administered toward the end of the experiment to quantify cancellous formation parameters in the femoral metaphysis. It is possible that most of the trabecular bone mass gains were made toward the beginning of the experiment, which would be captured by the pre- and posttreatment μCT measurements, whereas the histomorphometric measurements in the same region reflected trabecular dynamics at the very end of the study. Alternatively, because PTH changes the rate of preosteoblast proliferation/differentiation, and because the tamoxifen regimen that we used might not have been sufficient to ablate βcat in some newly formed osteocytes, it is possible that we might have generated more osteocytes (and osteoblasts) in the induced PTH-treated mice that do express βcat.
A number of signaling pathways converge on βcat, and Wnt/PTH/Lrp6 is not the only axis that can alter or require βcat signaling. For example, PTH activates several Map kinases (48) and jun N-terminal kinase-1 can phosphorylate Lrp6 and alter βcat-dependent transcription (49). Alternatively, in chondrocytes, βcat binds directly to the PTHR1 C terminus and causes downstream transduction switching (suppression of Gs and enhancement of Gq signaling) (50). Although this mechanism does not appear to affect canonical βcat signaling endpoints, it nonetheless might alter upstream PTH responses. Thus, the involvement of βcat in bone cell transduction of PTH signals is likely to occur at multiple nodes within one pathway, and at the same time, in multiple pathways (eg, phosphatidylinositol-3-kinase/protein kinase B, and protein kinase A).
In summary, we found that mice with induced βcat deletion from late-stage osteoblasts and osteocytes respond poorly to PTH treatment as measured by DEXA. However, the anabolic response to PTH in cancellous compartments (femur and spine) was not suppressed by βcat deletion. βcat deletion induced a significant increase in bone resorption and cortical porosity, which was enhanced by PTH; those effects might account for the lack of PTH-induced gains in BMD, despite normal PTH-induced bone formation. Our results are consistent with reports indicating that βcat has preferential effects on the resorption axis of bone cells, and further, that the formation axis might be less affected when βcat is deleted.
Acknowledgments
This work was supported by National Institutes of Health Grant AR53237 and Veterans Administration Grant BX001478 (to A.G.R.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BFR/BS
- bone formation rate per unit bone surface
- BMD
- bone mineral density
- BV/TV
- bone volume fraction
- βcat
- β-catenin
- μCT
- microcomputed tomography
- Ct.Po
- cortical bone porosity
- CtX
- C-terminal telopeptide
- DEXA
- dual-energy x-ray absorptiometry
- DMF
- dimethyl formamide
- Lrp6
- LDL-receptor related protein 6
- NTG
- non-TG
- P1NP
- procollagen type 1 N-terminal propeptide
- PTH1R
- PTH type 1 receptor
- TG
- transgenic
- veh
- vehicle
- Wnt
- Wingless-related integration site.
References
- 1. Silva BC, Costa AG, Cusano NE, et al. Catabolic and anabolic actions of parathyroid hormone on the skeleton. J Endocrinol Invest. 2011;34(10):801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ettinger B, San Martin J, Crans G, et al. Differential effects of teriparatide on BMD after treatment with raloxifene or alendronate. J Bone Miner Res. 2004;19(5):745–751. [DOI] [PubMed] [Google Scholar]
- 3. Stroup JS, Rivers SM, Abu-Baker AM, et al. Two-year changes in bone mineral density and T scores in patients treated at a pharmacist-run teriparatide clinic. Pharmacotherapy. 2007;27(6):779–788. [DOI] [PubMed] [Google Scholar]
- 4. Body JJ, Gaich GA, Scheele WH, et al. A randomized double-blind trial to compare the efficacy of teriparatide [recombinant human parathyroid hormone (1–34)] with alendronate in postmenopausal women with osteoporosis. J Clin Endocrinol Metab. 2002;87(10):4528–4535. [DOI] [PubMed] [Google Scholar]
- 5. Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344(19):1434–1441. [DOI] [PubMed] [Google Scholar]
- 6. Murphy DR, Smolen LJ, Klein TM, et al. The cost effectiveness of teriparatide as a first-line treatment for glucocorticoid-induced and postmenopausal osteoporosis patients in Sweden. BMC Musculoskelet Disord. 2012:13:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Conwell LJ, Esposito D, Garavaglia S, et al. Out-of-pocket drug costs and drug utilization patterns of postmenopausal Medicare beneficiaries with osteoporosis. Am J Geriatr Pharmacother. 2011;9(4):241–249. [DOI] [PubMed] [Google Scholar]
- 8. Briot K, Ravaud P, Dargent-Molina P, et al. Persistence with teriparatide in postmenopausal osteoporosis; impact of a patient education and follow-up program: the French experience. Osteoporos Int. 2009;20(4):625–630. [DOI] [PubMed] [Google Scholar]
- 9. Mulgund M, Beattie KA, Wong AK, et al. Assessing adherence to teriparatide therapy, causes of nonadherence and effect of adherence on bone mineral density measurements in osteoporotic patients at high risk for fracture. Ther Adv Musculoskelet Dis. 2009;1(1):5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vescini F, Grimaldi F. PTH 1–84: bone rebuilding as a target for the therapy of severe osteoporosis. Clin Cases Miner Bone Metab. 2012;9(1):31–36. [PMC free article] [PubMed] [Google Scholar]
- 11. Muschitz C, Kocijan R, Fahrleitner-Pammer A, et al. Antiresorptives overlapping ongoing teriparatide treatment result in additional increases in bone mineral density. J Bone Miner Res. 2013;28(1):196–205. [DOI] [PubMed] [Google Scholar]
- 12. Kulkarni NH, Halladay DL, Miles RR, et al. Effects of parathyroid hormone on Wnt signaling pathway in bone. J Cell Biochem. 2005;95(6):1178–1190. [DOI] [PubMed] [Google Scholar]
- 13. Tobimatsu T, Kaji H, Sowa H, et al. Parathyroid hormone increases β-catenin levels through Smad3 in mouse osteoblastic cells. Endocrinology. 2006;147(5):2583–2590. [DOI] [PubMed] [Google Scholar]
- 14. Williams BO, Insogna KL. Where Wnts went: the exploding field of Lrp5 and Lrp6 signaling in bone. J Bone Miner Res. 2009;24(2):171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wan M, Yang C, Li J, et al. Parathyroid hormone signaling through low-density lipoprotein-related protein 6. Genes Dev. 2008;22(21):2968–2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li C, Xing Q, Yu B, et al. Disruption of LRP6 in osteoblasts blunts the bone anabolic activity of PTH. J Bone Miner Res. 2013;28(10):2094–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Revollo L, Kading J, Jeong SY, et al. N-cadherin restrains PTH activation of Lrp6/β-catenin signaling and osteoanabolic action. J Bone Miner Res. 2015;30(2):274–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sawakami K, Robling AG, Ai M, et al. The Wnt co-receptor LRP5 is essential for skeletal mechanotransduction but not for the anabolic bone response to parathyroid hormone treatment. J Biol Chem. 2006;281(33):23698–23711. [DOI] [PubMed] [Google Scholar]
- 19. Iwaniec UT, Wronski TJ, Liu J, et al. PTH stimulates bone formation in mice deficient in Lrp5. J Bone Miner Res. 2007;22(3):394–402. [DOI] [PubMed] [Google Scholar]
- 20. Glass DA, 2nd, Bialek P, Ahn JD, et al. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell. 2005;8(5):751–764. [DOI] [PubMed] [Google Scholar]
- 21. Kramer I, Halleux C, Keller H, et al. Osteocyte Wnt/β-catenin signaling is required for normal bone homeostasis. Mol Cell Biol. 2010;30(12):3071–3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cui Y, Niziolek PJ, MacDonald BT, et al. Reply to Lrp5 regulation of bone mass and gut serotonin synthesis. Nat Med. 2014;20(11):1229–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Riddle RC, Diegel CR, Leslie JM, et al. Lrp5 and Lrp6 exert overlapping functions in osteoblasts during postnatal bone acquisition. PLoS One. 2013;8(5):e63323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhao L, Shim JW, Dodge TR, et al. Inactivation of Lrp5 in osteocytes reduces young's modulus and responsiveness to the mechanical loading. Bone. 2013;54(1):35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tu X, McAndrews K, Delgado-Calle J, et al. Osteocytic PTH receptor is required for bone anabolism induced by intermittent PTH administration, but is dispensable for bone resorption and the loss of bone induced by chronic PTH elevation. J Bone Miner Res. 2013;28(suppl 1):SU0125. [Google Scholar]
- 26. Saini V, Marengi DA, Barry KJ, et al. Parathyroid hormone (PTH)/PTH-related peptide type 1 receptor (PPR) signaling in osteocytes regulates anabolic and catabolic skeletal responses to PTH. J Biol Chem. 2013;288(28):20122–20134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brault V, Moore R, Kutsch S, et al. Inactivation of the β-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128(8):1253–1264. [DOI] [PubMed] [Google Scholar]
- 28. Powell WF, Jr, Barry KJ, Tulum I, et al. Targeted ablation of the PTH/PTHrP receptor in osteocytes impairs bone structure and homeostatic calcemic responses. J Endocrinol. 2011;209(1):21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Indra AK, Warot X, Brocard J, et al. Temporally-controlled site-specific mutagenesis in the basal layer of the epidermis: comparison of the recombinase activity of the tamoxifen-inducible Cre-ER(T) and Cre-ER(T2) recombinases. Nucleic Acids Res. 1999;27(22):4324–4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kalajzic I, Matthews BG, Torreggiani E, et al. In vitro and in vivo approaches to study osteocyte biology. Bone. 2013;54(2):296–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Madisen L, Zwingman TA, Sunkin SM, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13(1):133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Niziolek PJ, Farmer TL, Cui Y, et al. High-bone-mass-producing mutations in the Wnt signaling pathway result in distinct skeletal phenotypes. Bone. 2011;49(5):1010–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Munugalavadla V, Vemula S, Sims EC, et al. The p85α subunit of class IA phosphatidylinositol 3-kinase regulates the expression of multiple genes involved in osteoclast maturation and migration. Mol Cell Biol. 2008;28(23):7182–7198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stern AR, Stern MM, Van Dyke ME, et al. Isolation and culture of primary osteocytes from the long bones of skeletally mature and aged mice. Biotechniques. 2012;52(6):361–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tian Y, Xu Y, Fu Q, et al. Parathyroid hormone regulates osteoblast differentiation in a Wnt/β-catenin-dependent manner. Mol Cell Biochem. 2011;355(1–2):211–216. [DOI] [PubMed] [Google Scholar]
- 36. Yano M, Inoue Y, Tobimatsu T, et al. Smad7 inhibits differentiation and mineralization of mouse osteoblastic cells. Endocr J. 2012;59(8):653–662. [DOI] [PubMed] [Google Scholar]
- 37. Tamai K, Semenov M, Kato Y, et al. LDL-receptor-related proteins in Wnt signal transduction. Nature. 2000;407(6803):530–535. [DOI] [PubMed] [Google Scholar]
- 38. He X, Semenov M, Tamai K, et al. LDL receptor-related proteins 5 and 6 in Wnt/β-catenin signaling: arrows point the way. Development. 2004;131(8):1663–1677. [DOI] [PubMed] [Google Scholar]
- 39. Mi K. J ohnson GV. Role of the intracellular domains of LRP5 and LRP6 in activating the Wnt canonical pathway. J Cell Biochem. 2005;95(2):328–338. [DOI] [PubMed] [Google Scholar]
- 40. Robling AG, Kedlaya R, Ellis SN, et al. Anabolic and catabolic regimens of human parathyroid hormone 1–34 elicit bone- and envelope-specific attenuation of skeletal effects in Sost-deficient mice. Endocrinology. 2011;152(8):2963–2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Haegel H, Larue L, Ohsugi M, et al. Lack of β-catenin affects mouse development at gastrulation. Development. 1995;121(11):3529–3537. [DOI] [PubMed] [Google Scholar]
- 42. Tzeng HE, Muo CH, Chen HT, et al. Tamoxifen use reduces the risk of osteoporotic fractures in women with breast cancer in Asia: a nationwide population-based cohort study. BMC Musculoskelet Disord. 2015;16:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vasioukhin V, Degenstein L, Wise B, et al. The magical touch: genome targeting in epidermal stem cells induced by tamoxifen application to mouse skin. Proc Natl Acad Sci USA. 1999;96(15):8551–8556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kim JE, Nakashima K, de Crombrugghe B. Transgenic mice expressing a ligand-inducible cre recombinase in osteoblasts and odontoblasts: a new tool to examine physiology and disease of postnatal bone and tooth. Am J Pathol. 2004;165(6):1875–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Maes C, Kobayashi T, Selig MK, et al. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev Cell. 2010;19(2):329–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang M, Kirsch DG. The generation and characterization of novel Col1a1FRT-Cre-ER-T2-FRT and Col1a1FRT-STOP-FRT-Cre-ER-T2 mice for sequential mutagenesis. Dis Model Mech. 2015;8(9):1155–1166. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47. Sando R, 3rd, Baumgaertel K, Pieraut S, et al. Inducible control of gene expression with destabilized Cre. Nat Methods. 2013;10(11):1085–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Syme CA, Friedman PA, Bisello A. Parathyroid hormone receptor trafficking contributes to the activation of extracellular signal-regulated kinases but is not required for regulation of cAMP signaling. J Biol Chem. 2005;280(12):11281–11288. [DOI] [PubMed] [Google Scholar]
- 49. Ĉervenka I, Wolf J, Mašek J, et al. Mitogen-activated protein kinases promote WNT/β-catenin signaling via phosphorylation of LRP6. Mol Cell Biol. 2011;31(1):179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yano F, Saito T, Ogata N, et al. β-Catenin regulates parathyroid hormone/parathyroid hormone-related protein receptor signals and chondrocyte hypertrophy through binding to the intracellular C-terminal region of the receptor. Arthritis Rheum. 2013;65(2):429–435. [DOI] [PubMed] [Google Scholar]