Abstract
Runt-related transcription factor 1 (Runx1), a master regulator of hematopoiesis, is expressed in preosteoclasts. Previously we evaluated the bone phenotype of CD11b-Cre Runx1fl/fl mice and demonstrated enhanced osteoclasts and decreased bone mass in males. However, an assessment of the effects of Runx1 deletion in female osteoclast precursors was impossible with this model. Moreover, the role of Runx1 in myeloid cell differentiation into other lineages is unknown. Therefore, we generated LysM-Cre Runx1fl/fl mice, which delete Runx1 equally (∼80% deletion) in myeloid precursor cells from both sexes and examined the capacity of these cells to differentiate into osteoclasts and phagocytic and antigen-presenting cells. Both female and male LysM-Cre Runx1fl/fl mice had decreased trabecular bone mass (72% decrease in bone volume fraction) and increased osteoclast number (2–3 times) (P < .05) without alteration of osteoblast histomorphometric indices. We also demonstrated that loss of Runx1 in pluripotential myeloid precursors with LysM-Cre did not alter the number of myeloid precursor cells in bone marrow or their ability to differentiate into phagocytizing or antigen-presenting cells. This study demonstrates that abrogation of Runx1 in multipotential myeloid precursor cells significantly and specifically enhanced the ability of receptor activator of nuclear factor-κB ligand to stimulate osteoclast formation and fusion in female and male mice without affecting other myeloid cell fates. In turn, increased osteoclast activity in LysM-Cre Runx1fl/fl mice likely contributed to a decrease in bone mass. These dramatic effects were not due to increased osteoclast precursors in the deleted mutants and argue that inhibition of Runx1 in multipotential myeloid precursor cells is important for osteoclast formation and function.
Hematopoietic stem cells can differentiate into a variety of lineages, including osteoclasts, which are essential for bone resorption (1). These cells have a key role in a number of musculoskeletal pathologies, including osteoporosis. After commitment to the myeloid precursor, Hematopoietic stem cells may differentiate into preosteoclasts under the influence of macrophage colony-stimulating factors (M-CSFs) (2). Early osteoclast precursors express the proteins lysozyme M (LysM) and cluster of differentiation 11b (CD11b) during their progression into mature osteoclasts, which occurs with exposure to both M-CSFs and receptor activator of nuclear factor-κB ligand (RANKL) (3).
Many signaling pathways, which regulate osteoclast differentiation, involve key transcription factors that play important roles in osteoclast formation and function (4–8). Among these is runt-related transcription factor 1 (Runx1) (9–11). This master regulator of hematopoiesis (12, 13) is widely expressed in hematopoietic progenitors within the bone marrow, and its expression is inhibited by RANKL in osteoclasts (9, 14). Runx1 has previously been shown to be involved in the differentiation of a variety of cell lineages. It is essential for hematopoietic commitment into the hemangioblast lineage (15). In addition, haploinsufficiency of Runx1 results in the acceleration of mesodermal development and hemangioblast specification after in vitro differentiation of embryonic stem cells (16). In the osteoclast nucleus, Runx1 participates with the scaffolding protein Four and a Half LIM domains 2 to sequester TNF receptor-associated factor 6, thereby preventing it from interacting with activator of nuclear factor-κB and subsequently mediating RANKL-induced activation of nuclear factor-κB (10, 11). Bai et al (11) showed that Runx1 transcriptionally activates Four and a Half LIM domains 2 in osteoclasts, and this is further enhanced by TNF receptor-associated factor 6, suggesting that Runx1 may be required for terminal osteoclast maturation.
Runx1 is also essential for the generation of a normal number of blast colonies (16). Our group previously published evidence that Runx1 negatively regulates osteoclast formation and function. We demonstrated that when Runx1 floxed mice (Runx1fl/fl) were crossed with CD11b-Cre mice to delete the expression of Runx1 in myeloid precursors (14), osteoclastogenesis was markedly enhanced and bone mass decreased. However, only male mice were examined in our previous study because the CD11b-Cre transgene used in these experiments was present on the Y chromosome (14, 17). In the current work, we sought to overcome this limitation by crossing Runx1 floxed mice with LysM-Cre transgenic mice, which deleted Runx1 in the myeloid precursors of both female and male mice. We hypothesized that female LysM-Cre Runx1fl/fl mice would display a similar phenotype to that of male CD11b-Cre Runx1fl/fl mice, including decreased trabecular bone mass and more osteoclasts both in vitro and in vivo. In addition, we evaluated the number and ability of myeloid progenitor cells to differentiate into other lineages after conditional deletion of Runx1 to determine the specificity of the role of Runx1 in regulating myeloid cell lineage commitment into a variety of cell fates.
Materials and Methods
Animals
LysM-Cre Runx1fl/fl mice were generated by breeding Runx1fl/fl mice (18) with LysM-Cre Runx1fl/+ transgenic mice (19). LysM-Cre Runx1fl/fl mutant mice were compared with control mice (Runx1fl/+). Both female and male mice were used for outcomes to evaluate gender differences. Mice were housed at the UConn Health Center for Comparative Medicine, in accordance with all applicable state and federal guidelines. The Institutional Animal Care and Use Committee of UConn Health approved the use of animals in all experiments.
Bone homeostasis experiments
Microcomputed tomography (CT)
Trabecular and cortical bone morphometry were measured using a cone-beam microfocus X-ray CT (Scanco Medical AG) as described previously (20, 21). A 9.6-mm segment of the trabecular bone was quantitatively analyzed at a 0.96-mm distance from the growth plate toward the femoral midshaft. This region encompassed the secondary spongiosa. Standard measurements were evaluated including bone volume fraction, trabecular number, trabecular thickness, trabecular spacing, connectivity density, and bone surface. Cortical thickness was measured within a 0.6-mm segment of cortical bone, located 4.5 mm from the growth plate. Standard measurements were evaluated including length, total cross-sectional area, bone cross-sectional area, medullary area, cortical thickness, and cortical porosity.
Static histomorphometric analyses
Femurs were fixed in 4% paraformaldehyde and decalcified in 10% EDTA prior to paraffin embedding. Seven-micrometer-thick serial sections were stained for tartrate-resistant acid phosphatase (TRAP) using a Kamiya Biomedical Co TRAP kit and counterstained with hematoxylin as previously described (14, 22). For histomorphometric evaluation we analyzed three sections, spaced 50 μm apart from eight mice per group. There were six fields analyzed for each section, located 20 μm distal to the growth plate. This region encompassed the secondary spongiosa, the same region of interest that trabecular bone was analyzed by micro-CT.
Bone marrow macrophage (BMM) osteoclast formation assays
Whole bone marrow from mutant and control mice was isolated from long bones (femurs and tibiae) and purified over a Ficoll-Paque Plus density gradient (GE Healthcare) as previously described (14). BMMs were seeded at a density of 5 × 103 cells/well of a 96-well tissue culture plate and induced to become osteoclasts in vitro by treatment with M-CSF (30 ng/mL) and various doses of RANKL (3, 10, or 30 ng/mL), as previously described (14, 22). Three wells per biological replicate were used for cells seeded in 96-well plates.
The ability of progenitor cells to form mature osteoclasts was evaluated via quantification of multinucleated (more than three nuclei) TRAP+ cells after culture for 4 days and the total number of osteoclasts per well was quantified.
Bone pit assay
BMMs were seeded onto UV-irradiated bovine cortical bone slices to determine whether the osteoclast function was affected in the LysM-Cre Runx1F/F mutant mice. Cells on the bone slices were initially stained for TRAP as above, photographed by microscopy, and then removed by sonication. The bone slices were then stained briefly with toluidine blue to visualize the pit area and osteoclast path. The area of pits formed by multinucleated TRAP+ cells were determined after 14 days of culture on the bone slices as previously described (14), using cellSens Dimension software (Olympus Life Science). The average pit area per osteoclast was determined by measuring the individual pit area of all osteoclasts on the bone slices of each group and then computing a mean. The total pit area per bone slice was determined by measuring the entire resorbed pit areas per bone slice for replicates of four slices per group.
Gene expression to verify loss of Runx1
Total RNA was extracted from cultured BMMs using TRI Reagent (Molecular Research Center) and converted to cDNA using a reverse transcription kit (Applied Biosystems) (20). cDNA for each sample was mixed with gene-specific PCR primers (Runx1) and SYBR Green Master Mix (Thermo Fischer) to assay for levels of gene expression by RT-PCR using an ABI Prism 7500 sequence detection system (Applied Biosystems). Relative expression of Runx1 was normalized to β-actin, and data were analyzed to determine the relative quantification values by the δδCT method, as previously described (14).
Western blotting to verify loss of Runx1
BMM cells were cultured for 3 days with M-CSF (30 ng/mL) and then extracted into sample lysis buffer (62.5 mM Tris-HCl, pH 6.8; 10% [vol/vol] glycerol; 2% sodium dodecyl sulfate) with HALT protease inhibitors (Thermo Fisher Sciences). Protein was determined by bicinchoninic assay (Thermo Fisher Sciences), and equal amounts of protein (20–40 μg) were run on 10% SDS-PAGE gels. Filters were blocked for 2 hours in a blocking buffer containing 5% powdered milk and TBS + 0.1% Tween 20. Filters were then incubated with a primary antibody (monoclonal rabbit antimurine Runx1) (Cell Signaling Technology) or polyclonal rabbit antimurine β-actin, (Cell Signaling Technology) at 4°C overnight. Samples were transferred onto nitrocellulose membranes by electroblotting (Bio-Rad Laboratories). After washing, membranes were incubated for 1 hour with the appropriate secondary antibodies that were complexed to horseradish peroxidase. Reactive bands were detected by enhanced chemiluminescence using Chemiluminescence LumiGLO (Cell Signaling Technology). Detection was by autoradiography.
Enzyme-linked immunosorbent assays
Eight-week-old male mice were fasted without food but with free access to water overnight prior to the mice being killed. Serum was obtained in the morning by cardiac puncture for measurement in ELISAs. Carboxy-terminal collagen cross-links (CTX) (RatLaps; ImmunoDiagnostic Systems) and N-terminal propeptide of type I procollagen (rat/mouse PINP; ImmunoDiagnostic Systems) were assayed in 5 μL of serum using ELISA kits according to the manufacturer's recommendations.
Flow cytometry to determine populations of progenitor cells in the bone marrow
Bone marrow cells were harvested for single-cell suspensions by flushing femurs and tibias with 10 mL of staining medium (1% BSA in PBS) into 15-mL tubes. Cells were spun down (1500 rpm for 5 min at 4°C), supernatants were removed, and the pellet containing leukocytes and red blood cells was resuspended in 1 mL of red blood cell lysing buffer (Sigma), incubated for 5 minutes, and washed with staining medium. The final pellet was resuspended in 10 mL of staining medium and filtered through a 100-mm Nytex mesh.
For analysis of progenitors, the cells were stained with a mix of antibodies in staining media and incubated on ice for 45 minutes, followed by washing in staining medium. As a second step, the cells were stained with streptavidin coupled to the fluorochromes of interest on ice for 45 minutes. Finally, cells were resuspended in staining medium containing 1 μg/mL of propidium iodide.
All these antibodies were obtained directly conjugated to fluorochromes or biotinylated (e-Biosciences): anti-B cell lineage monoclonal antibody (mAb), anti-CD45R/B220 (RA3-6B2); anti-T-cell lineage mAb, anti-CD3 (145-2C11); antimonocyte/macrophage lineage mAb, anti-CD11b/Mac-1 (M1/70), and anti-F4/80 (BM8); antidendritic cell mAb, anti-CD11c (N418); anti-ckit/CD117 (2B8); and anti-c-fms/CD115 (AFS98). Labeling of cells for flow cytometric analysis was performed by standard staining procedures wherein directly conjugated or biotinylated mAb plus second-step reagents were sequentially added to the cell preparation of interest.
Labeling of bone marrow cells for flow cytometric analysis was performed by standard staining procedures in 1× Hanks' balanced salt solution (Gibco, Invitrogen Corp) containing 0.01 M HEPES (pH 7.4) and supplemented with 2% heat-inactivated fetal bovine serum. Bone marrow was harvested by flushing long bones with medium using a 25-G needle. After washing, the red blood cells were lysed using ammonium chloride, and the cell preparation was filtered through a nylon mesh and counted. The cells were kept on ice at all times. Dead cells were excluded by their ability to incorporate propidium iodide. All antibodies were titrated for optimal dilutions. Unless indicated, all other antibodies and secondary step reagents were obtained directly conjugated to fluorochromes or biotinylated from either of two commercial sources (BD Biosciences or eBiosciences). Flow cytometric analysis was done on a FACSCalibur instrument (BD Biosciences), and data analysis was performed using FlowJo software from Tree Star, Inc as previously described (23).
We previously demonstrated a phenotyping scheme, which identifies three populations (IV, V, and VI) of OC precursor cells in murine bone marrow (3, 23). All three OC precursor cell populations are negative for expression of CD3 and CD45R and negative to low for expression of CD11b. All also express CD115 (the receptor for M-CSF) and high (population IV) intermediate (population V), or low (population VI) levels of CD117. The antibodies used for flow cytometric analysis are all commercially available. These include the following: antimouse CD45R (B220) for B-cell lineage cells; antimouse CD3 for T-cell lineage cells; antimouse CD11b (Mac-1) for macrophage lineage cells; antimouse CD117 (c-kit), and antimouse CD115 (c-fms).
Phagocytosis assay
The phagocytic function of enriched BMMs from LysM-Cre Runx1fl/fl and Runx1fl/+ mice was evaluated. BMMs were cultured in the presence of M-CSF for 3 days followed by 2 hours of culture with Escherichia coli particles at 37°C, according to the Vybrant (Thermo Fisher) phagocytosis assay kit protocol. Briefly, after fluorescent E coli particles were added to the wells, the cultures were returned to the incubator for 2 hours. The culture medium and excess fluorescent E coli were then aspirated, and 100 μL of a trypan blue suspension was added to each well and removed after 1 minute. The purpose of the trypan blue was to quench the fluorescence of any E coli particles that were adhering to the surface of the cells. Any remaining fluorescence in the wells resulted from E coli that were phagocytosed and therefore intracellular after the 2-hour incubation period. Fluorescence in the wells (a measure of cellular phagocytic activity) was read in a fluorescence plate reader (Synergy HT; BioTek) using a 480-nm excitation wavelength and a 520-nm emission wavelength.
Antigen presentation assay
The dendritic cell potential of BMMs isolated from LysM-Cre Runx1fl/fl and Runx1fl/+ mice was evaluated to determine whether the deletion of Runx1 affects the ability of cells to differentiate into antigen-presenting dendritic cells. Bone marrow macrophages from both groups were differentiated by culturing with granulocyte macrophage colony-stimulating factor (GM-CSF; 20 ng/mL) for 5 days. B3Z T-cell clones were added to the BMMs and exposed to either ovalbumin (1 mg/mL) (IBA) or SIINFECKL (10 μg/mL) (Endotoxin free from Biovendor LLC) overnight. Media were replaced with β-galactosidase substrate: chlorophenol red-D-galactopyranoside solution (Roche) in lysis solution (0.5% Nonidet P-40 in PBS), mixed, and transferred to a new plate. Plates were read for β-galactosidase activity produced by B3Z cells at 1.5 and 8 hours as previously described (23).
Statistical analyses
Data were analyzed using paired Student's t tests between two groups and a one-way ANOVA with a Bonferroni post hoc analysis between multiple groups with a 95% confidence interval.
Results
Runx1 gene is deleted by LysM-driven Cre recombinase in vivo
To evaluate and contrast the effect of Runx1 on the formation and activity of osteoclasts in female and male mice, we crossed LysM-Cre mice with Runx1fl/fl mice to generate LysM-Cre Runx1fl/fl mice, which had Runx1 deleted in myeloid precursor cells, including those that could differentiate into osteoclasts.
We first verified the conditional deletion of Runx1 in LysM-Cre Runx1fl/fl mice by culturing BMMs isolated from whole bone marrow in monolayer for 3 days in the presence of MCSF (to enrich the population of myeloid precursors). Western blotting for Runx1 protein expression, relative to the endogenous β-actin, showed conditional deletion of Runx1 protein in LysM-Cre Runx1fl/fl mice but not in Runx1fl/+ mice (Figure 1A). We also examined the ability of RANKL to inhibit Runx1 expression in BMMs isolated from Runx1fl/+ and LysM-Cre Runx1fl/fl mice in the M-CSF-treated monolayer culture. We found significantly lower Runx1 gene expression in LysM-Cre Runx1fl/fl mice compared with controls (Figure 1B) and that RANKL further decreased Runx1 mRNA levels in LysM-Cre Runx1fl/fl mice, as previously published (14) (data not shown).
Figure 1.
The Runx1 gene was deleted by Cre recombinase in vivo. Western blotting (A) and gene expression analyses by QRT-PCR (B) demonstrate the loss of Runx1 in LysM-Cre Runx1fl/fl mutant mice. The housekeeping gene for both experiments was β-actin. Data obtained for Western blotting (n = 2) were representative of two replicate experiments. Data obtained for quantitative RT-PCR were means ± SE (n = 5). *, P < .05 vs Runx1fl/+ mice.
Deletion of Runx1 gene in LysM+ cells reduced bone mass in both female and male mice and enhanced resorption in vivo
Micro-CT imaging demonstrated that the femoral trabecular bone of both female and male LysM-Cre Runx1fl/fl mice was microstructurally more disconnected with a high degree of spacing between adjacent trabeculae, compared with the femora of Runx1fl/+ mice (Figure 2). Micro-CT analysis quantitatively revealed that female mice conditionally lacking Runx1 in myeloid progenitor cells exhibited a 72% decrease in femoral trabecular bone volume fraction, a 28% decrease in trabecular number, and a 24% increase in trabecular spacing, all of which were statistically significant differences (P < .05) (Figure 2 and Supplemental Table 1). We observed a similar significant loss of trabecular bone mass in male LysM-Cre Runx1fl/fl mice (Figure 2 and Supplemental Table 1). However, unlike our results with the CD11b-Cre Runx1fl/fl conditionally deleted mice (14), micro-CT analyses did not show any significant differences in cortical bone parameters between male or female LysM-Cre Runx1fl/fl and Runx1fl/+ mice (Supplemental Table 1).
Figure 2.
Conditional deletion of Runx1 in preosteoclasts decreased bone mass for both female and male mice (16 wk of age). A, The left panels are representative three-dimensional trabecular bone microstructure for male Runx1fl/+ and LysM-Cre Runx1fl/fl mice. B, A graphical representation of the micro-CT analysis of femora from female and male Runx1fl/+ and LysM-Cre Runx1fl/fl mice. Data obtained for micro-CT were means ± SE (n = 8). *, P < .05 vs Runx1F/+ mice.
When trabecular bone histomorphometry was analyzed, osteoclast perimeter and osteoclast surface per bone perimeter were significantly increased in female LysM-Cre Runx1fl/fl mice, compared with female Runx1fl/+ controls with no difference in osteoblast parameters. Furthermore, the trabecular bone volume fraction of LysM-Cre Runx1fl/fl was decreased (Figure 3 and Supplemental Table 2). We observed similar differences in these histomorphometric parameters in male mice, with no changes in osteoblast parameters (Figure 3 and Supplemental Table 2). We also found that the levels of serum N-terminal propeptide of type I procollagen, a measure of bone formation, were not different between male LysM-Cre Runx1fl/fl and Runxfl/+ mice. However, the serum levels of CTX, a measure of bone formation, were significantly greater (by 44%) in LysM-Cre Runx1fl/fl mice compared with Runx1fl/+ mice (Supplemental Figure 1).
Figure 3.
Loss of Runx1 in preosteoclasts decreased bone mass and increased the number of osteoclasts in vivo. Femurs from 16-week-old Runx1fl/+ and LysM-Cre Runx1fl/fl female and male mice were embedded, sectioned, TRAP stained, and used for histomorphometric analyses. A, A representative TRAP-stained histological sections (×20 magnification), indicating increased osteoclast size for male LysM-Cre Runx1fl/fl mutant mice, compared with Runx1fl/+ controls. B, A summary of several histomorphometric parameters for osteoclasts. Data obtained for histomorphometry were means ± SE (n = 8). *, P < .05 vs Runx1F/+ mice. BV/TV, bone volume fraction; Oc Pm, osteoclast perimeter; Oc Bone Pm, osteoclast bone perimeter.
Preosteoclast-specific loss of Runx1 increases osteoclastogenesis in vitro
Equal numbers of BMM cells from female and male mice were cultured with M-CSF and with or without RANKL (3, 10, or 30 ng/mL) for 4 days to determine whether there were differences in osteoclastogenesis in vitro between LysM-Cre Runx1fl/fl and Runx1fl/+ mice (Figure 4). For both genotypes, female BMMs formed more osteoclasts than did male cells for all concentrations of RANKL. In cells from female mice, osteoclast formation was greater in LysM-Cre Runx1fl/fl cultures only at the lowest dose of RANKL (3 ng/mL) (P < .05; Figure 4). In contrast, in male cultures osteoclast number at 4 days was significantly increased in the cultures of the LysM-Cre Runx1fl/fl BMMs for all concentrations of RANKL (P < .05; Figure 4).
Figure 4.
Top panels, Loss of Runx1 accelerated in vitro osteoclastogenesis. Bone marrow cells were isolated from the long bones of either 8-week-old Runx1fl/+ or LysM-Cre Runx1fl/fl female and male mice. Cells were cultured in the presence of 30 ng/mL M-CSF and 3, 10, or 30 ng/mL RANKL for 4 days to induce osteoclast formation. Bottom panels, Representative images of 4-day cultures from male Runx1fl/+ or LysM-Cre Runx1fl/fl mice treated with M-CSF and RANKL (30 ng/mL for each). Image are ×10 magnifications of TRAP-stained cells. Data are means ± SE (n = 3). *, P < .05 vs Runx1fl/+ mice.
In a separate experiment with BMMs from male mice, we examined the time course for the development and loss of osteoclasts in cultures that were treated with M-CSF and RANKL (30 ng/mL for each) for up to 10 days (Supplemental Figure 2). In this experiment we found that the peak of osteoclastogenesis was somewhat delayed relative to the studies of Figure 4, with maximum osteoclast number occurring at day 6 for cells from both LysM-Cre Runx1fl/fl and Runx1fl/+ mice. In this study the number of osteoclasts in the LysM-Cre Runx1fl/fl cultures was significantly greater than in cultures of cells from Runx1fl/+ mice at days 6 and 8. In addition, the rate of osteoclast formation between days 4 and 6 was greater in LysM-Cre Runx1fl/fl cells. In contrast, the rate that osteoclast number decreased in the cultures for both genotypes between days 6 and 8 was similar. Few osteoclasts remained in the cultures on day 10.
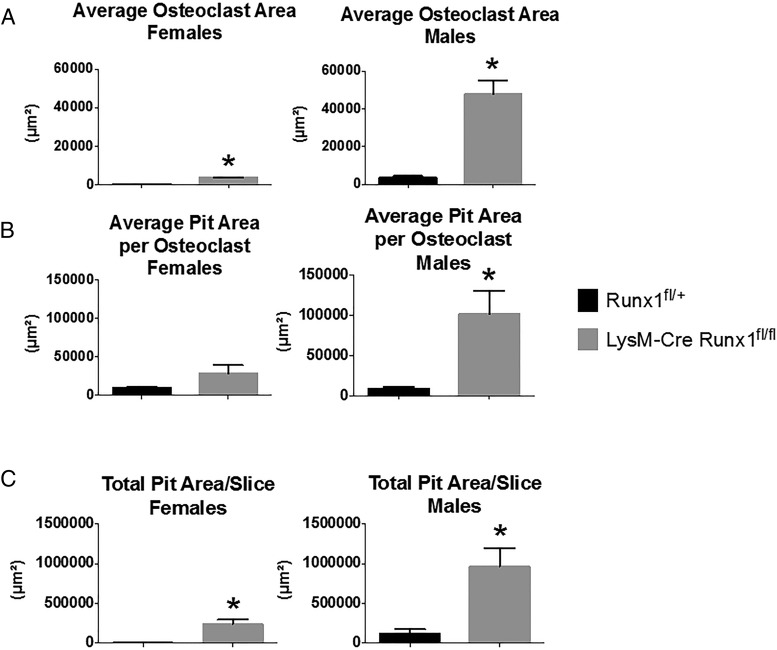
We found that osteoclasts from female mice were noticeably smaller than those from male mice both when cultured on plastic (data not shown) and on bovine cortical bone slices (Figure 5A). Male LysM-Cre Runx1fl/fl osteoclasts were also functionally more active and produced a larger average pit area on cortical bone slices than did osteoclasts from Runx1fl/+ male mice (Figure 5). For both female and male BMM cultures, the total pit area resorbed per bone slice was greater in cultures from the LysM-Cre Runx1fl/fl mice than from Runx1fl/+ mice.
Figure 5.
Conditional deletion of Runx1 increases RANKL-stimulated pit formation on bovine bone slices. Bone marrow cells from either Runx1fl/+ or LysM-Cre Runx1fl/fl female and male mice were cultured on bovine cortical bone chips with M-CSF and RANKL (30 ng/mL for each) for 14 days. The average osteoclast area (A), the average pit area per osteoclast (B), and the total pit area per slice (C) were measured for both Runx1fl/+ and LysM-Cre Runx1fl/fl groups. Data obtained were means ± SE (n = 4). *, P < .05 vs Runx1F/+ mice.
Percentages of osteoclast precursor populations were not altered by conditional deletion of Runx1 in myeloid cells from female and male mice
Because we found significantly more osteoclasts in cultures of LysM-Cre Runx1fl/fl bone marrow cells, we examined whether conditional loss of Runx1 altered a variety of populations of osteoclast precursor cells in bone marrow (Figures 6 and 7), using the phenotyping scheme that we previously described (23). This identifies multiple populations of osteoclast precursor cells (2, 7). These include cells in bone marrow that are CD3 negative, CD45R negative, CD11b negative or low, and CD115 (or c-fms) positive. The latter population can be further subdivided according to expression of CD117 as population IV (CD117 high), population V (CD117 intermediate), and population VI (CD117 low). Analysis of these osteoclast precursor populations in LysM-Cre Runx1fl/fl and Runx1fl/+ mice did not demonstrate any differences for either gender (Figure 6, A and B).
Figure 6.
Loss of Runx1 does not affect the distribution of osteoclast precursor cells in bone marrow of female (A) or male (B) mice. Cells isolated from murine long bones of either 8-week-old Runx1fl/+ or LysM-Cre Runx1fl/fl mice were analyzed using flow cytometry for the percentage of osteoclast precursors (populations IV, V, and VI) and total osteoclast precursors (populations IV + V + VI). All three osteoclast precursor cell populations are negative for expression of CD3 and CD45R and negative to low for expression of CD11b. All also express CD115 (the receptor for M-CSF) and high (population IV), intermediate (population V), or low (population VI) levels of CD117. Data are means ± SE (n = 3 mice per group).
Figure 7.
A, Cartoon demonstrating that multipotential myeloid precursor cells can differentiate into antigen-presenting dendritic cells, phagocytic macrophages, or mature osteoclasts. B, Cells isolated from murine long bones of either 8-week-old Runx1fl/+ or LysM-Cre Runx1fl/fl mice were analyzed using flow cytometry for the percentage of dendritic precursors (CD11c+), macrophage precursors (F4/80+), and more committed osteoclast precursors (CD11b+, CD115+). Data are means ± SE (n = 3 mice per group).
The myeloid precursor cells that differentiate into osteoclasts are multipotential (23) and can also differentiate into antigen-presenting dendritic cells and phagocytic macrophages (Figure 7A). After verifying the effective deletion of Runx1 in LysM-Cre Runx1fl/fl cells, we used flow cytometry to examine the percentage of cells expressing the cell surface antigen F4/80, which is found on phagocytic macrophages, and the cell surface antigen CD11c, which is found on antigen presenting dendritic cells, in the bone marrow of LysM-Cre Runx1fl/fl and Runx1fl/+ mice. We found no differences in the percentage of cells expressing these antigens between male or female LysM-Cre Runx1fl/fl and Runx1fl/+ mice (Figure 7B). In addition, treatment of osteoclast precursor populations IV, V, or VI with M-CSF and RANKL induces the transient high-level expression of CD11b in CD115-expressing osteoclast precursors (2, 7). Analysis of the percentage of cells positive for both CD11b and CD115 in the bone marrow of LysM-Cre Runx1fl/fl and Runx1fl/+ mice of both genders also failed to find differences among any of the groups (Figure 7B).
Loss of Runx1 in myeloid precursor cells does not affect their ability to differentiate into antigen-presenting or phagocytic cells
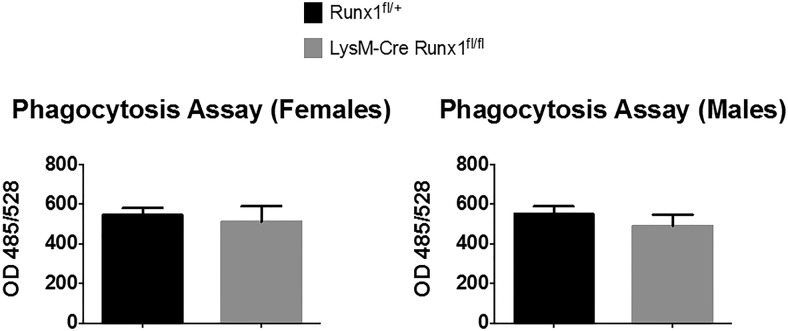
We also evaluated the antigen-presenting potential of BMMs isolated from LysM-Cre Runx1fl/fl and Runx1fl/+ mice, which were differentiated by treatment with GM-CSF. These studies were designed to determine the effects that Runx1 deletion had on the functional capacity of differentiated dendritic cells. The antigen-presenting assay relies on the ability of dendritic cells to process ovalbumin and present a specific fragment, SIINFECKL, to engineered B3Z T-lymphocytes, which express only T-cell receptors for SIINFECKL. Once activated, B3Z cells express β-galactosidase activity, which is used to quantitate the antigen-presenting activity of the BMMs. Figures 8 and Supplemental Figure 1 show that deletion of Runx1 in myeloid precursors did not affect the antigen-presenting cell activity of differentiated BMMs for either gender. Similarly, we evaluated the phagocytic activity of BMMs from LysM-Cre Runx1fl/fl and Runx1fl/+ mice that were cultured with M-CSF to drive their differentiation toward phagocytic cells. This assay uses fluorescent bacteria and relies on the ability of phagocytic cells to engulf the bacteria and become fluorescent. Figure 9 shows that deletion of Runx1 in myeloid precursor cells did not affect the phagocytic activity of BMMs that were driven toward the macrophage lineage.
Figure 8.
The dendritic cell potential of BMMs isolated from female (A) or male (B) LysM-Cre Runx1fl/fl and Runx1fl/+ mice was evaluated. BMMs from both groups were differentiated to antigen-presenting cells by culture with GM-CSF, plated with B3Z T-cell clones, and exposed to either ovalbumin (1 mg/mL) or SIINFECKL (30 μg/mL) for 1.5 or 8 hours. Data are means ± SE (n = 3 mice per group).
Figure 9.
The phagocytic function of enriched BMMs from LysM-Cre Runx1fl/fl and Runx1fl/+ mice was evaluated. BMMs were cultured in the presence of M-CSF for 3 days followed by 2 hours of culture with E coli particles at 37°C, according to the Vybrant phagocytosis assay kit (V-6694) protocol. Data are means ± SE (n = 3 mice per group).
Discussion
Although many signaling pathways may be associated with osteoclastogenic processes, the critical role of the transcription factor Runx1 in regulating these processes remains largely unexplored. In this study we examined the effects that conditional loss of Runx1 using LysM-Cre in both female and male myeloid precursor cells had on a variety of parameters. These included osteoclast formation and function and the capacity of the myeloid precursor cells to differentiate toward other lineages. A recent study identified Runx1 as one of nine genes with potentially functional single-nucleotide polymorphisms, which were associated with a loss of trabecular bone mineral density in men aged 65 years and older. This result demonstrates that Runx1 may be critical for bone homeostasis in humans (24). Our previously published work with CD11b-Cre Runx1fl/fl male mice demonstrated a decreased trabecular bone mass and more osteoclasts (14). These osteoclasts were larger than those in Runx1fl/+ controls, and their osteoclastogenic activity was increased. Similarly, in both LysM-Cre Runx1fl/fl female and male mice, we observed decreased trabecular bone mass, more osteoclasts, and increased osteoclastogenic activity. These results imply that loss of Runx1 in both female and male myeloid precursor cells produced a similar bone phenotype.
Table 1.
Antibody Table
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog Number, and/or Name of Individual Providing the Antibody | Species Raised (Monoclonal or Polyclonal) | Dilution Used |
|---|---|---|---|---|---|
| Runx1 | N/A | Anti-Runx1 | Cell Signaling | Murine, monoclonal | 1:200 |
| β-Actin | N/A | Anti-β-actin | Cell Signaling | Murine, polyclonal | 1:200 |
| CD45R/B220 | N/A | Anti-CD45R/B220 | e-Biosciences, RA3-6B2 | Murine, monoclonal | 1:400 |
| CD3 | N/A | Anti-CD3 | e-Biosciences, 145-2C11 | Murine, monoclonal | 1:200 |
| CD11b/Mac-1 | N/A | Anti-CD11b/Mac-1 | e-Biosciences, M1/70 | Murine, monoclonal | 1:800 |
| F4/80 | N/A | Anti-F4/80 | e-Biosciences, BM8 | Murine, monoclonal | 1:200 |
| CD11c | N/A | Anti-CD11c | e-Biosciences, N418 | Murine, monoclonal | 1:200 |
| ckit/CD117 | N/A | Anti-ckit/CD117 | e-Biosciences, 2B8 | Murine, monoclonal | 1:800 |
| c-fms/CD115 | N/A | Anti-c-fms/CD115 | e-Biosciences, AFS98 | Murine, monoclonal | 1:400 |
Abbreviation: N/A, not available.
Significant gender differences were not observed in the bone phenotype of LysM-Cre Runx1fl/fl mice. Interestingly, osteoclasts from the marrow of both male Runx1-deleted and control mice were larger than those of their female littermate counterparts when cultured on bone, and, for male-derived cells, the pits they formed were also larger. This finding agrees with a recent publication by Herbert and Valerio (25), which evaluated sexual dimorphisms in mice after RANKL treatment of bone marrow macrophages for 3 and 5 days. The authors found that in vitro cultures of cells from female mice generally had a higher number of smaller osteoclasts than did equivalent cultures from male mice (25). These differences were particularly evident with shorter periods of exposure to RANKL.
The decrease in trabecular bone mass, increase in osteoclast number on trabecular bone, and increase in serum level of CTX in LysM-Cre Runx1fl/fl mice relative to Runx1fl/+ mice strongly argues that Runx1 represses osteoclastic bone resorption. In terms of cortical bone homeostasis, the data for the LysM-Cre Runx1fl/fl mice more closely reflect the results for the single-nucleotide polymorphism human study (24) than our previous work using CD11b-Cre Runx1fl/fl mice (14). Contrary to what we demonstrated for the CD11b-Cre Runx1fl/fl mice, neither male nor female LysM-Cre Runx1fl/fl mice exhibited a decrease in cortical bone mass relative to Runx1fl/fl mice. This suggests that there may be differences in the cells targeted in the LysM and CD11b Cre mice.
When bone marrow macrophages were cultured with different concentrations of RANKL, we saw significant increases in osteoclast formation and function with LysM-Cre Runx1fl/fl BMMs. This was true for the lowest concentration of RANKL (3 ng/mL) that we studied in female cells and all concentrations of RANKL (3, 10, and 30 ng/mL) in male cells. This result in male LysM-Cre Runx1fl/fl cells was similar to that observed with male CD11b-Cre Runx1fl/fl BMMs (14). We also observed increases in the resorptive activity of osteoclasts for the LysM-Cre Runx1fl/fl group compared with the Runx1fl/+ control group. This was measured by the total area of resorbed bone in a bone pit assay for both genders, which was previously demonstrated for the CD11b-Cre Runx1fl/fl group (14). The size of the osteoclasts in the LysM-Cre Runx1fl/fl BMM cultures was also significantly larger for both female and male cultures compared with cultures of Runx1fl/+ cells, which is similar to what we observed in male CD11b-Cre Runx1fl/fl mice (14). Osteoclast size correlates with resorption activity (26), so it was expected that the total area resorbed by LysM-Cre Runx1fl/fl-derived osteoclasts would be greater than that of control cultures.
The time-course study for in vitro osteoclast formation and loss (Supplemental Figure 2) demonstrated a more rapid increase in osteoclast number in LysM-Cre Runx1fl/fl cells compared with control cultures. In addition, we found little difference between the LysM-Cre Runx1fl/fl group and the Runx1fl/+ group in the rate that osteoclast number decreased between the peak levels achieved for both genotypes on day 6 and the values on day 8. These results argue that increased osteoclastogenesis and not decreased osteoclast loss is responsible for the increased number of osteoclasts that are seen in LysM-Cre Runx1fl/fl mice.
We found no effect of Runx1 deletion in myeloid precursor abundance as measured by flow cytometry. Runx1 regulates essential genes for hematopoiesis, which are also known to regulate osteoclast differentiation. The most prominent example is that of PU.1, which is necessary for hematopoietic commitment to the myeloid lineage (27). The effects of Runx1 on PU.1 transcriptional activity are complex and can be either stimulatory or inhibitory, depending on which response element in the PU.1 promoter is mutated (28). We have previously reported that discrete populations of precursor cells have different osteoclastogenic potentials (23). The fact that none of these precursor populations was altered upon Runx1 conditional deletion suggests either that Runx1 is not involved in regulating the abundance of these cells or, if it is involved, its regulation occurs at a stage in lineage commitment that is prior to expression of LysM.
Our data demonstrated that deleting Runx1 in an established pool of myeloid precursors affected only their capacity to differentiate into osteoclasts and not antigen-presenting dendritic cells or phagocytic macrophages. This strongly argues that the down-regulation of Runx1 by RANKL is a critical and specific step in the lineage commitment of multipotential myeloid precursor cells into mature osteoclasts.
Acknowledgments
We thank Dr Ernesto Canalis and David Bridgewater for their assistance with the microcomputed tomography scanning.
This work was supported by the National Institutes of Health Grant R01AR060867 (to H.D. and J.L.). Support for D.N.P. comes from National Institute of Dental and Craniofacial Research T90 funding.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMM
- bone marrow macrophage
- CD
- cluster of differentiation
- CT
- computed tomography
- CTX
- carboxy-terminal collagen cross-links
- GM-CSF
- granulocyte macrophage colony-stimulating factor
- LysM
- lysozyme M
- mAb
- monoclonal antibody
- M-CSF
- macrophage colony-stimulating factor
- RANKL
- receptor activator of nuclear factor-κB ligand
- Runx1
- runt-related transcription factor 1
- TRAP
- tartrate-resistant acid phosphatase.
References
- 1. Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. [DOI] [PubMed] [Google Scholar]
- 2. Arai F, Miyamoto T, Ohneda O, et al. Commitment and differentiation of osteoclast precursor cells by the sequential expression of c-Fms and receptor activator of nuclear factor κB (RANK) receptors. J Exp Med. 1999;190:1741–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jacquin C, Gran DE, Lee SK, Lorenzo JA, Aguila HL. Identification of multiple osteoclast precursor populations in murine bone marrow. J Bone Miner Res. 2006;21:67–77. [DOI] [PubMed] [Google Scholar]
- 4. Lee J, Kim K, Kim JH, et al. Id helix-loop-helix proteins negatively regulate TRANCE-mediated osteoclast differentiation. Blood. 2006;107:2686–2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Smink JJ, Begay V, Schoenmaker T, Sterneck E, de Vries TJ, Leutz A. Transcription factor C/EBPβ isoform ratio regulates osteoclastogenesis through MafB. EMBO J. 2009;28:1769–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhao B, Takami M, Yamada A, et al. Interferon regulatory factor-8 regulates bone metabolism by suppressing osteoclastogenesis. Nat Med. 2009;15:1066–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miyauchi Y, Ninomiya K, Miyamoto H, et al. The Blimp1-Bcl6 axis is critical to regulate osteoclast differentiation and bone homeostasis. J Exp Med. 2010;207:751–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhao B, Grimes SN, Li S, Hu X, Ivashkiv LB. TNF-induced osteoclastogenesis and inflammatory bone resorption are inhibited by transcription factor RBP-J. J Exp Med. 2012;209:319–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Saltman LH, Javed A, Ribadeneyra J, et al. Organization of transcriptional regulatory machinery in osteoclast nuclei: compartmentalization of Runx1. J Cell Physiol. 2005;204:871–880. [DOI] [PubMed] [Google Scholar]
- 10. Bai S, Kitaura H, Zhao H, et al. FHL2 inhibits the activated osteoclast in a TRAF6-dependent manner. J Clin Invest. 2005;115:2742–2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bai S, Zha J, Zhao H, Ross FP, Teitelbaum SL. Tumor necrosis factor receptor-associated factor 6 is an intranuclear transcriptional coactivator in osteoclasts. J Biol Chem. 2008;283:30861–30867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Niki M, Okada H, Takano H, et al. Hematopoiesis in the fetal liver is impaired by targeted mutagenesis of a gene encoding a non-DNA binding subunit of the transcription factor, polyomavirus enhancer binding protein 2/core binding factor. Proc Natl Acad Sci USA. 1997;94:5697–5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang Q, Stacy T, Binder M, Marin-Padilla M, Sharpe AH, Speck NA. Disruption of the Cbfa2 gene causes necrosis and hemorrhaging in the central nervous system and blocks definitive hematopoiesis. Proc Natl Acad Sci USA. 1996;93:3444–3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Soung do Y, Kalinowski J, Baniwal SK, et al. Runx1-mediated regulation of osteoclast differentiation and function. Mol Endocrinol. 2014;28:546–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lacaud G, Gore L, Kennedy M, et al. Runx1 is essential for hematopoietic commitment at the hemangioblast stage of development in vitro. Blood. 2002;100:458–466. [DOI] [PubMed] [Google Scholar]
- 16. Lacaud G, Kouskoff V, Trumble A, Schwantz S, Keller G. Haploinsufficiency of Runx1 results in the acceleration of mesodermal development and hemangioblast specification upon in vitro differentiation of ES cells. Blood. 2004;103:886–889. [DOI] [PubMed] [Google Scholar]
- 17. Ferron M, Vacher J. Targeted expression of Cre recombinase in macrophages and osteoclasts in transgenic mice. Genesis. 2005;41:138–145. [DOI] [PubMed] [Google Scholar]
- 18. Growney JD, Shigematsu H, Li Z, et al. Loss of Runx1 perturbs adult hematopoiesis and is associated with a myeloproliferative phenotype. Blood. 2005;106:494–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8:265–277. [DOI] [PubMed] [Google Scholar]
- 20. Jastrzebski S, Kalinowski J, Stolina M, et al. Changes in bone sclerostin levels in mice after ovariectomy vary independently of changes in serum sclerostin levels. J Bone Miner Res. 2013;28:618–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Soung DY, Devareddy L, Khalil DA, et al. Soy affects trabecular microarchitecture and favorably alters select bone-specific gene expressions in a male rat model of osteoporosis. Calcif Tissue Int. 2006;78:385–391. [DOI] [PubMed] [Google Scholar]
- 22. Aguila HL, Mun SH, Kalinowski J, Adams DJ, Lorenzo JA, Lee SK. Osteoblast-specific overexpression of human interleukin-7 rescues the bone mass phenotype of interleukin-7-deficient female mice. J Bone Miner Res. 2012;27:1030–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jacome-Galarza CE, Lee SK, Lorenzo JA, Aguila HL. Identification, characterization, and isolation of a common progenitor for osteoclasts, macrophages, and dendritic cells from murine bone marrow and periphery. J Bone Miner Res. 2013;28:1203–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yerges LM, Klei L, Cauley JA, et al. Candidate gene analysis of femoral neck trabecular and cortical volumetric bone mineral density in older men. Osteoporotic Fractures in Men Study Group. J Bone Miner Res. 2010;25:330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Herbert BA, Valerio MS, Gaestel M, Kirkwood KL. Sexual dimorphism in MAPK-activated protein kinase-2 (MK2) regulation of RANKL-induced osteoclastogenesis in osteoclast progenitor subpopulations. PloS One. 2015;10:e0125387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lees RL, Heersche JN. Macrophage colony stimulating factor increases bone resorption in dispersed osteoclast cultures by increasing osteoclast size. J Bone Miner Res. 1999;14:937–945. [DOI] [PubMed] [Google Scholar]
- 27. Tondravi MM, McKercher SR, Anderson K, et al. Osteopetrosis in mice lacking haematopoietic transcription factor PU.1. Nature. 1997;386:81–84. [DOI] [PubMed] [Google Scholar]
- 28. Huang G, Zhang P, Hirai H, et al. PU.1 is a major downstream target of AML1 (Runx1) in adult mouse hematopoiesis. Nat Genet. 2008;40:51–60. [DOI] [PubMed] [Google Scholar]