Abstract
Mice deficient in the type 3 deiodinase (D3KO mice) manifest impaired clearance of thyroid hormone (TH), leading to elevated levels of TH action during development. This alteration causes reduced neonatal viability, growth retardation, and central hypothyroidism. Here we examined how these phenotypes are affected by a deficiency in the monocarboxylate transporter 8 (MCT8), which is a major contributor to the transport of the active thyroid hormone, T3, into the cell. MCT8 deficiency eliminated the neonatal lethality of type 3 deiodinase (D3)-deficient mice and significantly ameliorated their growth retardation. Double-mutant newborn mice exhibited similar peripheral thyrotoxicosis and increased brain expression of T3-dependent genes as mice with D3 deficiency only. Later in neonatal life and adulthood, double-mutant mice manifested central and peripheral TH status similar to mice with single MCT8 deficiency, with low serum T4, elevated serum TSH and T3, and decreased T3-dependent gene expression in the hypothalamus. In double-mutant adult mice, both thyroid gland size and the hypothyroidism-induced rise in TSH were greater than those in mice with single D3 deficiency but less than those in mice with MCT8 deficiency alone. Our results demonstrate that the marked phenotypic abnormalities observed in the D3-deficient mouse, including perinatal mortality, growth retardation, and central hypothyroidism in adult animals, require expression of MCT8, confirming the interdependent relationship between the TH transport into cells and the deiodination processes.
Adequate exposure to thyroid hormones (THs) is necessary for normal developmental and physiological function. THs play essential roles in the maturation of the central nervous system (CNS) and in the regulation of metabolism (1–3). To this end, a number of factors including TH secretion, cellular TH transporters, deiodinase enzymes, and TH receptors and their associated proteins work in a coordinate manner to ensure that local TH availability and signaling are confined within a range appropriate to the developmental and functional needs of particular cells and tissues (4, 5). Disruption of this homeostatic system leads to altered TH signaling and may have adverse consequences in both humans and in animal models (6, 7).
One of these determinants is the type 3 deiodinase (D3), an enzyme encoded by the Dio3 gene that metabolizes both the active hormone T3 and the major circulating hormone T4 into metabolites with no biological activity (4, 8). D3 thus limits TH biological actions by modulating the T3 level within cells and tissues and its availability systemically. The high expression of D3 in the pregnant uterus, placenta (9, 10), fetal tissues (11–13), and in the adult CNS (14, 15) suggests that preventing premature T3 action is important for developmental processes and for adult brain function. This is further underscored by our observation that transgenic mice devoid of D3 activity (D3KO mice) have excessive level of T3 during fetal and early neonatal life (16). The resulting enhancement in TH signaling during those critical periods leads to phenotypic abnormalities that include impaired neonatal viability, growth retardation, and adult central hypothyroidism due to aberrant programming of the hypothalamic-pituitary-thyroid (HPT) axis (17). Furthermore, despite low circulating levels of THs in adulthood, the CNS of D3KO mice manifests thyrotoxicosis and altered patterns of gene expression due to the lack of local TH clearance (18, 19).
The molecular effects of T3 require the entry of TH into cells, and thus, the deleterious effects of D3 deficiency are likely dependent on cellular transport mechanisms. THs can use multiple cellular transporters, which exhibit different tissue expression patterns and affinities for each of the THs (6, 20). Among them, the monocarboxylate transporter 8 (MCT8) features the highest affinity for the active hormone, T3 (21), and a relatively high abundance in the CNS and other tissues (22–24). In the human brain, the role of MCT8 in the CNS is prominent and unique because inactivating mutations result in the Allan-Herndon-Dudley syndrome, characterized by TH resistance and a hypothyroid state in the brain that is accompanied by severe neurological deficits including motor dysfunction (25–27). In mice, the genetic inactivation of the Mct8 gene does not cause such a recognizable neurological phenotype, probably due to the functional compensation by other TH transporters (28). However, the alterations in TH status and HPT axis physiology observed in the MCT8 knockout (KO) mice recapitulate those in Allan-Herndon-Dudley syndrome patients (29–32), indicating a unique role for MCT8 in HPT axis function also in mice.
Given the apparent primacy of the MCT8 in TH transport in the brain (14, 32), we hypothesized that the elimination of MCT8-mediated TH entry into this tissue, and specifically into the cells controlling the function of the HPT axis, will mitigate the enhanced TH signaling in the neonatal hypothalamus and the resultant adult central hypothyroidism characteristic of D3 deficiency. To address this hypothesis, we studied the phenotype of mice deficient in both D3 and MCT8 and compared it with that of mice with single deficiencies in either one of the proteins. We observed that the thyroid status and HPT axis phenotype of the D3KO mouse neonate is partially or totally reversed in the absence of MCT8, leading to an adult serum TH profile that resembles that of single MCT8 deficiency. Our results indicate that MCT8 and D3 functions are tightly associated in the neonatal ontogeny of the HPT axis and also provide new insights into the required role of MCT8 in abnormal viability and growth characteristic of D3 deficiency.
Materials and Methods
Experimental animals, genotyping, and tissue harvesting
D3KO and MCT8KO mice were in the inbred 129/Svj genetic background and the mutations they carry have been previously described (16, 29). Experimental animals were generated by mating males that were heterozygous for the Dio3 mutation (irrespective of their Mct8 genotype) with females that were double heterozygous for the Dio3 and Mct8 mutations. Only male mice were used in these studies. Given that Mct8 is an X-chromosome-linked gene, this mating strategy generates mice of all four experimental genotypes from the same parents. Mice were kept under a 12-hour light, 12-hour dark cycle and provided food and water ad libitum. Experimental animals of adult and weaning age were killed by asphyxiation with CO2, and blood was obtained postmortem from the vena cava. Trunk blood was collected from young neonates, which were killed by decapitation. Blood samples were stored at 4ºC for 4 hours, and serum was obtained by centrifugation and stored at −80ºC until analysis. Liver and brain tissues were harvested and frozen immediately on a dry ice-cold surface and then stored at −80ºC until being processed. Brain regions were identified according to the mouse atlas by Paxinos and Franklin (33). Dio3 and Mct8 genotyping was performed by PCR of genomic DNA obtained from tail snips as previously described (16, 29). The sequences of the primers used for this purpose are shown in Supplemental Table 1. To analyze the response of the HPT axis to induced hypothyroidism, adult mice were treated for 3 weeks with 0.4% potassium perchlorate and 0.05% methimazole in the drinking water. In another experiment designed to determine the thyroid gland response to TSH, mice were treated with T3 in the drinking water (0.25 μg/mL) for 10 days to induced thyrotoxicosis and reduce serum T4 and then injected ip with a single dose of 10 mU of TSH and killed 3 hours after the injection. In both experiments, serum and tissues were collected as described above. All animal procedures were approved by the Institutional Animal Care and Use Committees of Dartmouth College and/or Maine Medical Center Research Institute.
Gene expression
Total RNA was isolated from mouse tissues using the RNeasy kit (QIAGEN). Total RNA (1 μg) was reverse transcribed for 1 hour at 42ºC with 1 μL of M-MLV reverse transcriptase (Life Technologies). The mix was heated at 75ºC for 15 minutes to inactivate the reverse transcriptase and diluted 1:20 with water. Aliquots of the mixes from a given experiment were pooled before dilution to establish the first point of an internal standard. Three consecutive 1:4 dilutions of this standard were done to generate three additional standard points. Ten microliters of each of the diluted samples were mixed with 12.5 μL of SYBR Select Master Mix (Life Technologies) and 2.5 μL of the appropriate gene specific primer mix (3.33 pmol/μL each). The mixture was subjected to PCR cycling using a MyiQ single-color real-time PCR detection system from Bio-Rad Laboratories. PCRs were performed in triplicate and data were read from the standard curve. Gapdh was used as a control gene, its expression level not varying significantly between experimental groups. Gene expression levels are reported in arbitrary units, after correction by Gapdh expression. The sequences of the primers used for Hr, Dio1, and Gapdh expression are shown in Supplemental Table 1. Cortex expression of Hr was determined by qPCR using Taqman probes.
Serum thyroid parameters
Serum total T4 and T3 in adult mice were determined using RIA kits (TKT4 and TKT3 from Siemens) following the manufacturer's directions. In neonatal mice, serum total T3 and/or T4 were determined by Xiao-Hui Laio (University of Chicago, Chicago, Illinois) using liquid chromatography (Shimadzu) followed by tandem mass spectrometry (API 4000; Applied Biosystems) as previously described (34). Serum TSH determinations were also performed at the University of Chicago using a sensitive, heterologous, disequilibrium, double-antibody precipitation RIA, as previously described (35).
Thyroid gland size and histology
After euthanasia and blood collection, the trachea with the thyroid gland still attached was removed from the mouse and fixed by overnight immersion in formalin at 4°C. The tissue was embedded in paraffin and 5-μm-thick transversal sections were cut, collecting every sixth section on slides. The thyroid sections were then stained with hematoxylin and eosin using standard protocols and photographed. The areas of the left and right thyroid gland were measured using Image J free software (http://imagej.nih.gov/ij/) and added up to determine the total thyroid gland tissue area per section. Then the three largest areas for each animal were averaged for each thyroid gland and used as total area of the thyroid gland per animal.
Statistical analysis
GraphPad Prism software was used for statistical analysis. Unless stated otherwise, differences between more than two experimental groups were determined by an ANOVA and a Tukey's post hoc test, whereas differences between two groups were determined by the Student's t test. The abundance of genotypes found in experimental mice was analyzed using the χ2 test. P < .05 is considered statistically significant, and higher levels of significance (P < .01, P <.001) are also noted.
Results
Viability of D3/MCT8 double-KO mice
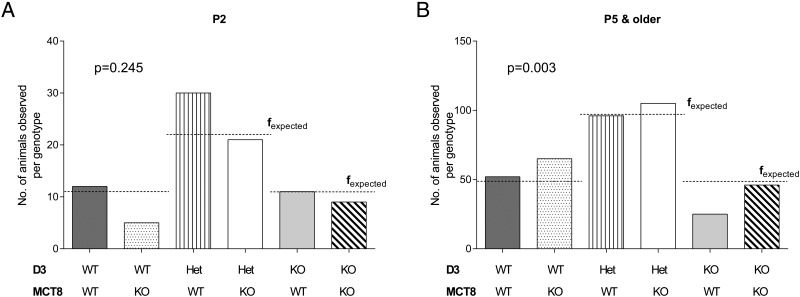
D3KO mice exhibit partial neonatal lethality. This is manifested by the fact that parents heterozygous for the D3 mutation produce a significantly lower number of D3KO offspring than that predicted by Mendelian inheritance (16). To determine how MCT8 deficiency impacts this phenotype, we examined the genotype of the male offspring generated by the mating of males heterozygous for the mutated Dio3 gene and females that were heterozygous for both the Dio3 and the Mct8 gene mutations. Because the Mct8 gene is located on the X-chromosome, six genotypes are possible in the male offspring as indicated in Figure 1, A and B. Mice were genotyped at postnatal day (P) 2, P5, or P15, and a χ2 test was performed to assess the distribution of genotypes (Figure 1 and Supplemental Table 2). At age P2, the distribution of pup genotypes between wild-type (WT/WT, 12 mice), D3-deficient (KO/WT, 11 mice), and D3/Mct8 double-knockout (KO/KO, 9 mice) mice corresponds with that expected. Although the number of MCT8-deficient pups (WT/KO, 5 mice) is smaller than predicted, this was not statistically significant (P = .245) (Figure 1A). However, at P5 and older, only 25 of the 389 total animals were D3KO mice. This number is significantly lower than the expected 48.625 animals (P = .003), whereas the number of MCT8KO mice (n = 65), is slightly higher. The number of animals that were D3/MCT8 double-KO mice at these ages conforms to the expected one (Figure 1B). This result indicates that MCT8 deficiency rescues the phenotype of partial neonatal lethality observed in D3KO mice.
Figure 1.
MCT8 deficiency rescues the neonatal lethality of D3KO mice. Genotype frequency distribution at age P2 (A) and ages P5 and P15 (B) of male offspring resulting from fathers heterozygous for the D3 mutation and mothers heterozygous for both D3 and MCT8 mutations. Bars represent the fobserved values and the dashed lines indicate the calculated fexpected values. The P values indicated were determined by the χ2 test. Genotypes are named WT, KO, or Het (heterozygous). Additional details can be found in Supplemental Table 1.
Growth of D3/MCT8 double-KO mice
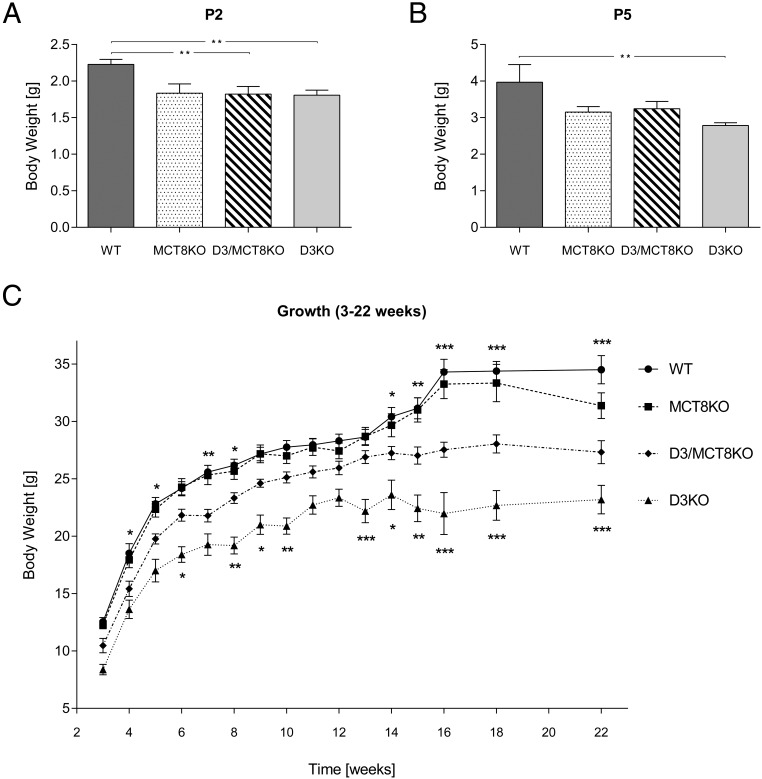
As previously described (16), D3KO mice show marked growth retardation that persists into adulthood. At P2, body weight (BW) is reduced in D3KO mice when compared with WT mice, but significant growth retardation is also observed in D3/MCT8 double-KO mice (Figure 2A). MCT8KO mice showed reduced BW at P2, but the reduction was not statistically significant. At P5, D3KO mice were the only group exhibiting significant growth retardation (Figure 2B). D3/MCT8 double-KO mice exhibited significantly reduced BW during neonatal life and into adult age, but BW reduction was not as severe as that observed in mice with single D3 deficiency (Figure 2C). MCT8KO mice did not exhibit any reduction in BW when compared with WT mice except later in adulthood at the age of 22 weeks. These results indicate that MCT8 deficiency partially ameliorates the growth retardation of D3KO mice.
Figure 2.
Growth of mice of all four genotypes. The BW of male WT, MCT8KO, D3KO, and D3/MCT8 double-KO mice is represented at P2 (A), P5 (B), and as a overall growth curve (C). Each data point represents the mean ± SEM of determinations in 5–22 mice. *, P < .05, **, P < .01, ***, P < .001, as determined by a one-way-ANOVA followed by Tukey's test (A and B) or a two-way-ANOVA with a random mouse effect.
Neonatal thyroid axis phenotype of D3/MCT8 double-KO mice
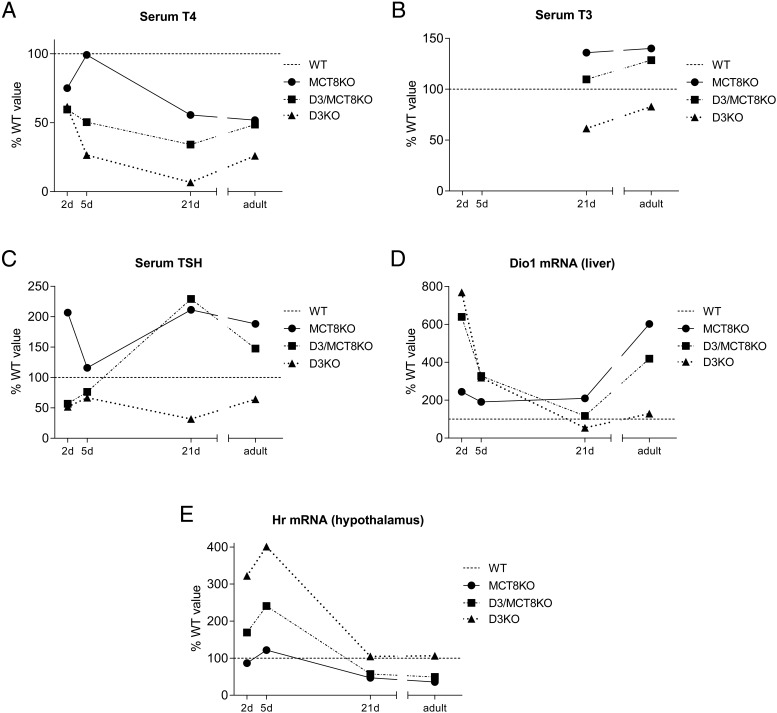
To analyze the peripheral and central thyroid status of mice of all four genotypes at different neonatal stages, we determined serum levels of TSH, T3, and T4 and the expression of selected T3-regulated genes in the liver, hypothalamus, and neocortex. The genes were hairless (Hr) in the hypothalamus and neocortex and type 1 deiodinase (Dio1) in the liver. Serum T3 was not determined at early neonatal ages due to the limitations in the serum sample size. (A general summary of these data is later shown in Figure 9.)
Figure 9.
Ontogeny of thyroid status parameters. Summary of data representing serum T4 (A), serum T3 (B), serum TSH (C), hepatic Dio1 mRNA (D), and hypothalamic Hr mRNA (E) across developmental stages. Data for each genotype is represented as a percentage of the values obtained for WT mice (dotted line).
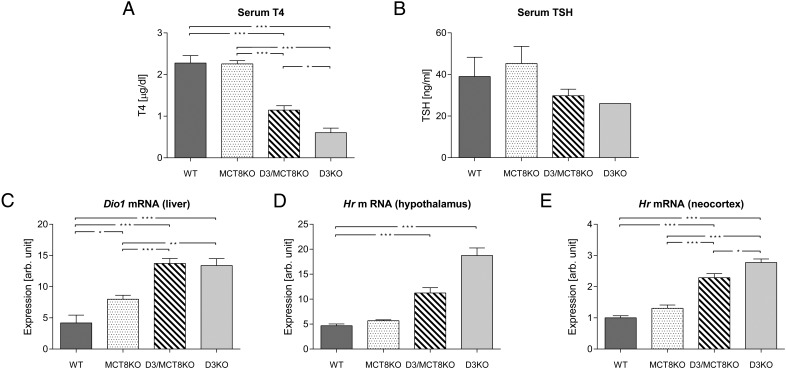
At P2 (Figure 3), serum T4 level was decreased in D3-, MCT8-, and D3/MCT8-deficient mice when compared with the wild type (Figure 3A), but this trend did not achieve statistical significance. The published serum TSH suppression in neonatal D3KO mice (16) did not achieve statistical significance in our experiment but was not improved by MCT8 deficiency (Figure 3B). Hepatic Dio1 mRNA expression was not significantly higher in MCT8-KO mice compared with WT mice, but it was markedly increased in both D3KO and D3/MCT8-KO animals (Figure 3C). The Hr mRNA expression level in the hypothalamus and in the neocortex (the latter a brain region not involved in the regulation of the HPT axis) was not significantly different in WT and MCT8KO mice but trended higher in the neocortex of the MCT8KO mice. However, Hr expression was increased 3-fold relative to WT mice in D3KO animals in both tissues. D3/MCT8 double-KO mice showed increases in neocortical and hypothalamic Hr mRNA expression, but they were significantly lower than those in D3KO mice (Figure 3, D and E).
Figure 3.
Thyroid status parameters of mice of all four genotypes at age P2. Serum T4 (A), serum TSH (B), hepatic Dio1 mRNA (C), hypothalamic Hr mRNA (D), and cerebrocortical Hr mRNA (E) in P2 WT, MCT8KO, D3/MCT8 double-KO, and D3KO male mice. Data represent the mean ± SEM of determinations in three to eight mice. *, P < .05, **, P < .01, ***, P < .001, as determined by a one-way-ANOVA followed by Tukey's test.
At P5 (Figure 4), the serum T4 level in MCT8KO mice was not different from those in the WT mice, whereas it was significantly lower in the D3/MCT8 double-KO and D3KO mice, with the latter manifesting the lowest level (Figure 4A). No significant differences were noted in serum TSH across the four experimental groups (Figure 4B). Hepatic Dio1 mRNA expression level was significantly higher in all three mutants compared with that in WT mice, although D3KO and D3/MCT8 double-KO mice exhibited the highest level of expression (Figure 4C). Hr mRNA expression levels were comparable in WT and MCT8KO mice in both the hypothalamus and the neocortex, whereas the levels were significantly elevated in D3/MCT8 double-KO mice and even more elevated in D3KO mice (Figure 4, D and E).
Figure 4.
Thyroid status parameters of mice of all four genotypes at age P5. Serum T4 (A), serum TSH (B), hepatic Dio1 mRNA (C), hypothalamic Hr mRNA (D), and cerebrocortical Hr mRNA (E) in P5 WT, MCT8KO, D3/MCT8 double-KO, and D3KO male mice. Data represent the mean ± SEM of determinations in five to eight mice. *, P < .05, **, P < .01, ***, P < .001, as determined by a one-way-ANOVA followed by Tukey's test.
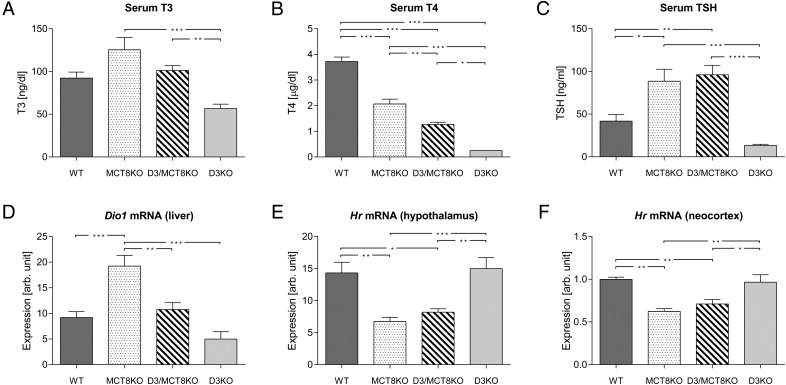
At P21 (Figure 5), compared with WT mice, serum T3 trended higher in MCT8KO mice, trended lower in D3KO mice, and was not changed in D3/MCT8 double-KO mice. At this age, serum T3 was significantly different between D3KO and D3/MCT8 double-KO mice, although other comparisons were not statistically significant (Figure 5A). Serum T4 was significantly decreased in all three groups of mutant mice, the degree of decrease being greatest in D3KO mice, modest in MCT8KO mice, and intermediate in D3/MCT8 double-KO mice (Figure 5B). The serum TSH level was significantly elevated in both MCT8KO and D3/MCT8 double-KO mice compared with those in WT mice, but they were decreased (not statistically significant) in D3KO mice (Figure 5C). Compared with WT mice, the pattern of hepatic Dio1 mRNA expression across genotypes mimicked that of serum T3: increased in MCT8KO mice, decreased in D3KO mice, and unchanged in double-D3/MCT8 KO mice, although not all comparisons were statistically significant (Figure 5D). Expression levels of Hr mRNA in both the hypothalamus and neocortex of WT and D3KO mice were not significantly different at this age, whereas it was reduced to a comparable extent in both MCT8KO and D3/MCT8 double-KO mice (Figure 5, D and E).
Figure 5.
Thyroid status parameters of mice of all four genotypes at age P21. Serum T3 (A), serum T4 (B), serum TSH (C), hepatic Dio1 mRNA (D), hypothalamic Hr mRNA (E), and cerebrocortical Hr mRNA (F) in P21 WT, MCT8KO, D3/MCT8 double-KO, and D3KO male mice. Data represent the mean ± SEM of determinations in six to eight mice. *, P < .05, **, P < .01, ***, P < .001, as determined by a one-way-ANOVA followed by Tukey's test.
Adult thyroid axis phenotype of D3/MCT8 double-KO mice
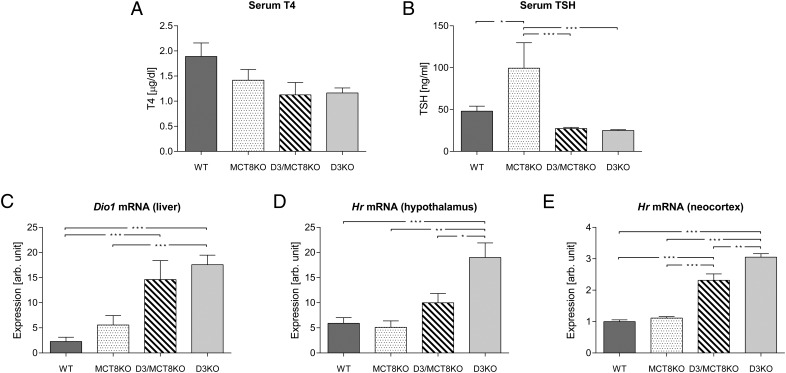
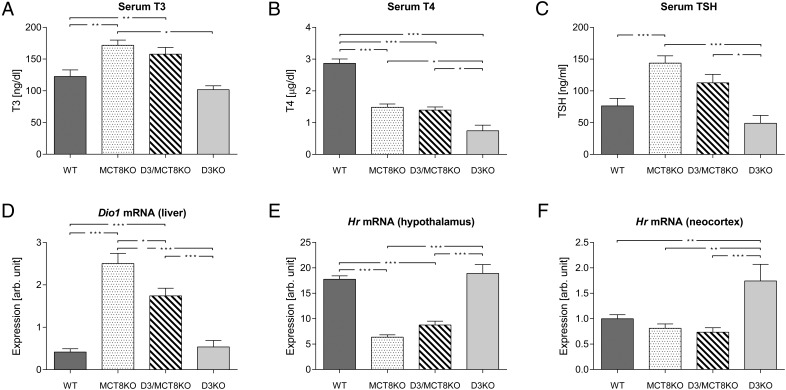
At P90 (Figure 6), serum T3 was elevated in adult MCT8KO and D3/MCT8 double-KO mice, but it was not significantly altered in D3KO mice (Figure 6A). Serum T4 levels were markedly decreased in all mutant animals, with D3KO mice exhibiting the largest decrease, whereas MCT8KO and D3/MCT8 double-KO mice demonstrated comparable but lesser decreases (Figure 6B). Serum TSH in MCT8KO mice was significantly higher than in WT mice and also trended higher in D3/MCT8 double-KO mice, although in this case the difference was not statistically significant (Figure 6C). Serum TSH was not altered in D3KO mice. Hepatic Dio1 mRNA expression was markedly elevated in MCT8KO mice, and this elevation was more moderate in D3/MCT8 double-KO mice. Hepatic Dio1 mRNA expression (Figure 6D) was markedly elevated in MCT8KO mice, no different from WT in D3KO mice, and elevated to an intermediate degree in the D3/MCT8 double-KO mice. These results, as those at P21, reflect the pattern of serum T3 in the four groups. Hypothalamic levels of Hr mRNA expression were significantly reduced to a similar extent in both MCT8KO and D3/MCT8 double-KO mice when compared with WT values. Levels in D3KO mice (Figure 6E) were no different from WT controls. In the neocortex, however, the Hr mRNA expression level was significantly elevated in D3KO mice when compared with that in all other groups (Figure 6F), in which Hr mRNA expression was not significantly different (Figure 6F). Taken together, the results in Figure 6 demonstrate that by adult age, MCT8 deficiency reverts the abnormal D3KO thyroid status to one that resembles most closely that of the single MCT8-deficient model.
Figure 6.
Thyroid status parameters of mice of all four genotypes at age P90. Serum T3 (A), serum T4 (B), serum TSH (C), hepatic Dio1 mRNA (D), hypothalamic Hr mRNA (E), and cerebrocortical Hr mRNA (F) in P21 WT, MCT8KO, D3/MCT8 double-KO, and D3KO male mice. Data represent the mean ± SEM of determinations in six to eight mice. *, P < .05, **, P < .01, ***, P < .001, as determined by a one-way-ANOVA followed by Tukey's test.
Responsiveness of the HPT axis
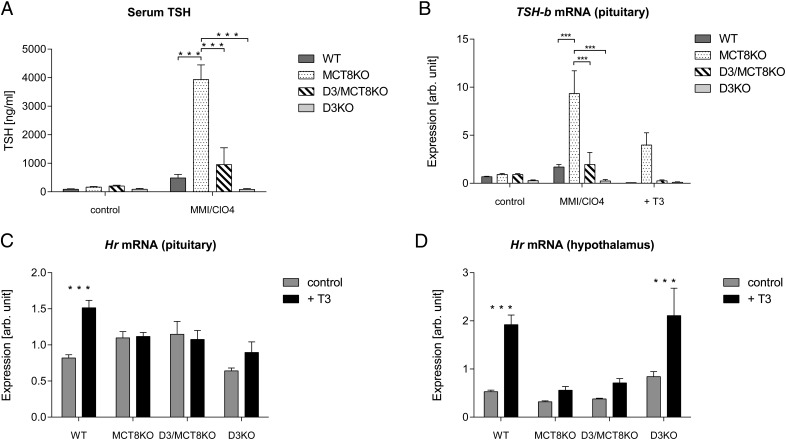
To assess the responsiveness of the HPT axis to alterations in TH levels (Figure 7), we first analyzed the TSH response to induced hypothyroidism in adult mice. After a 3-week treatment with methimazole and perchlorate, serum TSH was elevated 10-fold in WT mice. Serum TSH in hypothyroid MCT8KO mice increased an additional 7-fold and was completely blunted in the D3KO animals, consistent with previously reported data (17) (Figure 7A). In hypothyroid D3/MCT8 double-KO mice TSH values were similar to that in WT mice, suggesting an improved response, but were not statistically different from those in D3KO mice (Figure 7A). In the pituitary, the pattern of Tshb mRNA expression across groups was very similar to that of serum TSH (Figure 7B). A 10-day treatment with T3 in the drinking water suppressed Tshb mRNA expression in mice of all genotypes except in MCT8KO mice, in which Tshb mRNa expression was still elevated (Figure 7B), as previously reported (36). These findings indicate that concurrent MCT8 deficiency restores to normal the diminished TSH response to induced hypothyroidism that is characteristic of D3KO mice.
Figure 7.
Effects of an induced change in TH status on the pituitary and hypothalamus of P120 male mice of all four genotypes. A, Effect of induced hypothyroidism on serum TSH. B, Effect of hypothyroidism and T3 treatment on pituitary expression of Tshb mRNA. C and D, Effect of T3 treatment on Hr mRNA expression in the pituitary and hypothalamus, respectively. Data represent the mean ± SEM of determinations in four to seven mice. *, P < .05, **, P < .01, ***, P < .001, as determined by a one-way-ANOVA followed by Tukey's test (A and B) or by the Student's t test (C and D).
T3 treatment significantly increased the pituitary expression of Hr mRNA in WT mice but not in mice of the other three genotypes (Figure 7C). In the hypothalamus, Hr mRNA expression was induced in WT and D3KO mice after T3 treatment but not in MCT8KO or D3/MCT8 double-KO mice (Figure 7D). These results confirm the insensitivity of the MCT8KO brain to systemic T3 administration (37, 38) and further shows that concurrent MCT8 deficiency abrogates the T3 response of the D3KO brain.
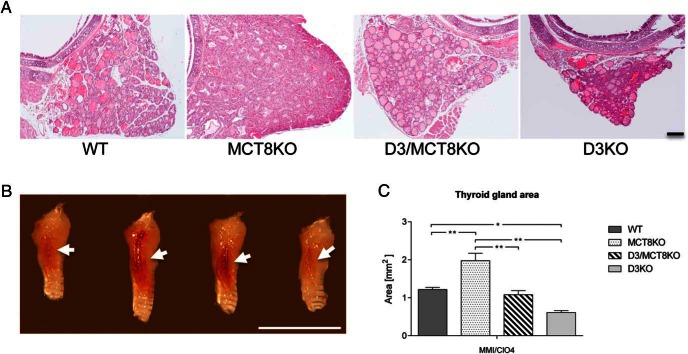
Thyroid gland size was estimated in mice with induced hypothyroidism. Representative photographs of thyroid gland sections and whole thyroid glands are shown in Figure 8A (also Supplemental Figure 1) and Figure 8B, respectively. After 3 weeks of methimazole and perchlorate treatment, relative thyroid gland size in MCT8KO mice was larger than in WT mice and other groups (Figure 8C). Consistent with previous observations (17), thyroid gland size in D3KO mice was reduced, but no significant difference in thyroid size was noticed between D3KO and D3/MCT8 double-KO mice (Figure 8C). To assess the response of the thyroid gland to TSH, a function that is impaired in both MCT8KO and D3KO mice (17, 39), we treated adult mice with T3 in the drinking water for 10 days (see Materials and Methods) to suppress serum T4. Then a single TSH dose was administered and serum T4 was determined 3 hours later. There was no significant difference between D3/MCT8 double-KO and D3KO mice in the increment in serum T4 after TSH injection (0.404 ± 0.096 μg/dL vs 0.654 ± 0.299 μg/dL, n = 4 and n = 3, respectively). Before the TSH injection, serum T4 was equally suppressed in both groups (0.025 ± 0.025 vs 0.02μg/dL ± 0.011 μg/dL, n = 4, and n = 3, respectively). This observation demonstrates that the impaired thyroid responsiveness to TSH of D3KO mice is not significantly altered by MCT8 deficiency.
Figure 8.
Thyroid gland size in short-term induced hypothyroidism. A, Representative transversal midsections of paraffin-embedded thyroid glands of mice with induced hypothyroidism. Scale bar, 100 μm. B, Representative photographs of the thyroid gland of P120 mice with induced hypothyroidism. Scale bar, 5 mm. Pictures were taken laterally and only one thyroid lobe is shown, as indicated by the arrow. C, Quantification of midsection areas (see Materials and Methods) of thyroid glands after short-term induced hypothyroidism. *, P < .05, **, P < .01, ***, P < .001, as determined by a one-way-ANOVA followed by Tukey's test.
Summary of ontogeny of TH status
Examining the TH status along the developmental process, we observed that at P2 and P5, MCT8 deficiency does not significantly change the abnormally low levels of serum T4 and TSH or the elevated hepatic Dio1 expression, which are all characteristics of D3KO mice at this age (Figure 9, A–C). Also, MCT8 deficiency only partially reduces the elevated hypothalamic expression of Hr in neonatal D3KO mice (Figure 9D). In contrast, at P21 and adult age, the thyroid status profile of D3KO mice is largely mitigated by a concurrent MCT8 deficiency. Thus, D3/MCT8 double-KO mice manifest serum thyroid hormone and gene expression patterns in the hypothalamus and neocortex that are not different from those in MCT8 deficiency alone (Figure 9, A–D). The results reveal a clear shift in the TH status of mice with double-D3/MCT8 deficiency: it is very similar to that in D3KO mice at the perinatal age and then changes to one similar to that in MCT8KO mice when the HPT axis is established and the animals move toward an independent, adult life.
Discussion
D3 deficiency in rodents leads to impaired T3 clearance and enhanced TH action in tissues in the perinatal period. Some consequences of this insult include reduced neonatal viability, retarded growth, and functional deficits in the HPT axis (16, 17). On the other hand, MCT8 is an important protein to transport TH through the blood-brain barrier into neural cells, including neurons (6, 23), the cell type expressing D3 (14). Thus, the abnormally elevated T3 signaling that occurs in the brain tissue of D3KO mice (18) may require the permissive role of MCT8 to transport T3 into neural cells. To determine the extent to which MCT8-mediated T3 transport is required for the pathophysiological consequences of D3 deficiency, we studied mice with combined D3/MCT8 deficiency to determine to what extent, if any, the D3KO phenotype is ameliorated. Reduced neonatal viability was corrected by concurrent MCT8 deficiency; D3/MCT8 double-KO mice survived in the proportions anticipated by Mendelian laws, whereas only about 50% of expected D3KO littermates survived to P5. Although the specific cause of death of D3KO mice in the neonatal period is not known, alterations in gene expression affecting the Dlk1-Dio3 imprinted domain lead to perinatal lethality (40). The cause of this phenotype notwithstanding, our finding indicates that MCT8-mediated transport of T3 contributes to the development of the cellular thyrotoxicosis and its associated morbidity in D3-deficient cells and tissues.
The pattern of growth retardation observed in D3KO mice is also abrogated, although only partially, by concurrent MCT8 deficiency. Because THs are typically involved in the cell differentiation processes of many tissues (41), we speculate that the stunted growth of D3KO mice is the result of a broad increase in the proliferation arrest and premature differentiation of stem cells across multiple tissues, ultimately limiting tissue growth. In this scenario, the reduction in T3 transport in multiple tissues as a result of MCT8 deficiency may ameliorate those cellular effects of excessive and untimely TH action. That the growth rate of D3KO mice is not fully recovered in concurrent MCT8 deficiency may reflect the fact that transporters in addition to MCT8 contribute significantly to T3 transport in some tissues and cell types (42). It is also possible that D3 deficiency alters the regulation of the GH-IGF-1 axis and that the improvement in growth observed in double-mutant mice reflects a compensatory effect of MCT8 deficiency in that physiological system.
Regarding the consequences of MCT8 deficiency for the TH status and HPT axis function of D3KO mice, our results reveal that those consequences vary, depending on the developmental stage. At P2 and P5, D3/MCT8 double-KO mice exhibit a serum and peripheral tissue TH status that appears similar to that of D3KO mice, including decreased serum T4 and TSH, and elevated hepatic expression of Dio1. These data indicate that in the early neonatal period, the degree of hepatic thyrotoxicosis in D3KO and D3/MCT8 double-KO mice, presumably mediated by elevated serum T3 level, are very similar. In contrast, hypothalamic and neocortical expression of Hr at these stages is less in D3/MCT8 double-KO mice compared with single-D3KO mice. These results suggest that MCT8 is significantly contributing to the hyperthyroid state of the D3KO neonatal brain by facilitating T3 transport into neural cells, probably both at the blood-brain-barrier and at the level of individual neurons.
We observed that he reduced response of the D3KO thyroid gland to TSH administration is not improved by concurrent MCT8 deficiency. This is not surprising because this response is also abnormally low in MCT8KO mice (39), probably due in part to the involvement of MCT8 in TH secretion from the thyroid gland (39). It is possible that the deficient response to TSH in both MCT8KO and D3KO mice results partially from an abnormal set point of the thyroid gland due to elevated levels of TH during perinatal life. This developmental hyperthyroid state is well documented in D3KO mice (16), but it has also been described in MCT8 mice (34).
In weanlings and adults, the serum, peripheral, and central TH status of the D3/MCT8 double-KO mouse is not like that in D3KO mice but is rather similar to that in MCT8KO mice. At these later stages, D3/MCT8 double-KO mice manifest elevated levels of serum T3, TSH, and hepatic Dio1 expression and low levels of serum T4 and hypothalamic Hr expression. These results indicate that in adulthood, the phenotypic features of MCT8 deficiency predominate in the D3/MCT8 double-KO mutant.
The shift in the TH status of D3/MCT8 double-KO mice occurs between P5 and weaning, precisely when the final establishment of the HPT axis takes place (43). This concurrence in timing suggests that MCT8 transport is critical for allowing the abnormal programming of the axis characteristic of D3KO mice.
It is conceivable that the abnormal expression of other TH transporters in MCT8 deficiency may modify the extent of phenotypic rescue observed. An increase in the perinatal expression of TH transporters linker for activation of Solute carrier family 7 member 5, linker for activation of Solute carrier family 7 member 8, organic anion-transporting polypeptide 3A1, and organic anion-transporting polypeptide 1C1 has been described in MCT8KO mice (34). This suggest that the up-regulation of other TH transporters may impair the full rescue of the D3KO phenotype by MCT8 deficiency. However, the contributions of alternative transporters are difficult to assess, given their differences in affinities for TH and in their developmental and cellular patterns of expression.
In summary, our results indicate that MCT8-mediated T3 transport is required for the full expression of the phenotypic abnormalities that result from a D3 deficiency. Thus, in D3-expressing cells, a loss of D3 function likely results in a thyrotoxic cellular environment due to the failure of MCT8-transported T3 and T4 to be metabolized and thus inactivated. In those cells that normally fail to express D3, any enhanced MCT8-mediated uptake resulting from elevated circulating T3 level, such as occurs in the perinatal period of the D3KO, will also lead to enhanced TH action. Thus, these studies confirm the hypothesis that the MCT8 transporter plays a critical permissive role in the development of the thyrotoxic state and the phenotypic abnormalities resulting from a D3 deficiency.
Acknowledgments
We are grateful to Xiao-Hui Liao for the TSH determinations and to Samuel Refetoff for providing us with the MCT8-deficient mice. Our studies used the Histopathology and the Molecular Phenotyping Core Facilities at the Maine Medical Center Research Institute.
This work was partially supported by Grants MH083220, DK095908, and MH096050 from the National Institutes of Health; Grant CIVP16A1805 from Fundación Ramón Areces; and Grant SAF2014-54919-R from Spain's Plan Nacional de I+D. The Core Facilities at Maine Medical Center Research Institute are supported by National Institutes of Health COBRE Grants P30GM103392 and P30GM106391 (to R. Friesel and D.Wojchowski, principal investigators).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BW
- body weight
- CNS
- central nervous system
- D3
- type 3 deiodinase
- D3KO mice
- devoid of D3 activity
- HPT
- hypothalamic-pituitary-thyroid
- KO
- knockout
- MCT8
- monocarboxylate transporter 8
- P
- postnatal day
- TH
- thyroid hormone
- WT
- wild type.
References
- 1. Bernal J, Guadano-Ferraz A, Morte B. Perspectives in the study of thyroid hormone action on brain development and function. Thyroid. 2003;13:1005–1012. [DOI] [PubMed] [Google Scholar]
- 2. Morreale de Escobar G, Obregon MJ, Escobar del Rey F. Role of thyroid hormone during early brain development. Eur J Endocrinol. 2004;151(suppl 3):U25–U37. [DOI] [PubMed] [Google Scholar]
- 3. Patel J, Landers K, Li H, Mortimer RH, Richard K. Thyroid hormones and fetal neurological development. J Endocrinol. 2011;209:1–8. [DOI] [PubMed] [Google Scholar]
- 4. Bianco AC. Minireview: cracking the metabolic code for thyroid hormone signaling. Endocrinology. 2011;152:3306–3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hennemann G, Docter R, Friesema EC, de Jong M, Krenning EP, Visser TJ. Plasma membrane transport of thyroid hormones and its role in thyroid hormone metabolism and bioavailability. Endocr Rev. 2001;22:451–476. [DOI] [PubMed] [Google Scholar]
- 6. Bernal J, Guadano-Ferraz A, Morte B. Thyroid hormone transporters—functions and clinical implications. Nat Rev Endocrinol. 2015;11:506. [DOI] [PubMed] [Google Scholar]
- 7. Fu J, Refetoff S, Dumitrescu AM. Inherited defects of thyroid hormone-cell-membrane transport: review of recent findings. Curr Opin Endocrinol Diabetes Obes. 2013;20:434–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hernandez A. Structure and function of the type 3 deiodinase gene. Thyroid. 2005;15:865–874. [DOI] [PubMed] [Google Scholar]
- 9. Galton VA, Martinez E, Hernandez A, St Germain EA, Bates JM, St Germain DL. Pregnant rat uterus expresses high levels of the type 3 iodothyronine deiodinase. J Clin Invest. 1999;103:979–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang SA, Dorfman DM, Genest DR, Salvatore D, Larsen PR. Type 3 iodothyronine deiodinase is highly expressed in the human uteroplacental unit and in fetal epithelium. J Clin Endocrinol Metab. 2003;88:1384–1388. [DOI] [PubMed] [Google Scholar]
- 11. Huang T, Chopra IJ, Boado R, Solomon DH, Chua Teco GN. Thyroxine inner ring monodeiodinating activity in fetal tissues of the rat. Pediatr Res. 1988;23:196–199. [DOI] [PubMed] [Google Scholar]
- 12. Chan S, Kachilele S, McCabe CJ, et al. Early expression of thyroid hormone deiodinases and receptors in human fetal cerebral cortex. Brain Res Dev Brain Res. 2002;138:109–116. [DOI] [PubMed] [Google Scholar]
- 13. Darras VM, Hume R, Visser TJ. Regulation of thyroid hormone metabolism during fetal development. Mol Cell Endocrinol. 1999;151:37–47. [DOI] [PubMed] [Google Scholar]
- 14. Tu HM, Legradi G, Bartha T, Salvatore D, Lechan R, Larsen PR. Regional expression of the type 3 iodothyronine deiodinase messenger ribonucleic acid in the rat central nervous system and its regulation by thyroid hormone. Endocrinology. 1999;140:784–790. [DOI] [PubMed] [Google Scholar]
- 15. Kaplan MM, Yaskoski KA. Phenolic and tyrosyl ring deiodination of iodothyronines in rat brain homogenates. J Clin Invest. 1980;66:551–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hernandez A, Martinez ME, Fiering S, Galton VA, St Germain D. Type 3 deiodinase is critical for the maturation and function of the thyroid axis. J Clin Invest. 2006;116:476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hernandez A, Martinez E, Liao X, et al. Type 3 deiodinase deficiency results in functional abnormalities at multiple levels of the thyroid axis. Endocrinology. 2007;148:5680–5687. [DOI] [PubMed] [Google Scholar]
- 18. Hernandez A, Quignodon L, Martinez ME, Flamant F, St Germain DL. Type 3 deiodinase deficiency causes spatial an temporal alterations in brain T3 signaling that are dissociated from serum thyroid hormone levels. Endocrinology. 2010;151:5550–5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hernandez A, Morte B, Belinchon MM, Ceballos A, Bernal J. Critical role of type 2 and 3 deiodinases in negative regulation of gene expression by T3 in the brain. Endocrinology. 2012;153(6):2919–2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Friesema EC, Jansen J, Milici C, Visser TJ. Thyroid hormone transporters. Vitam Horm. 2005;70:137–167. [DOI] [PubMed] [Google Scholar]
- 21. Friesema EC, Ganguly S, Abdalla A, Manning Fox JE, Halestrap AP, Visser TJ. Identification of monocarboxylate transporter 8 as a specific thyroid hormone transporter. J Biol Chem. 2003;278:40128–40135. [DOI] [PubMed] [Google Scholar]
- 22. Grijota-Martinez C, Diez D, Morreale de Escobar G, Bernal J, Morte B. Lack of action of exogenously administered T3 on the fetal rat brain despite expression of the monocarboxylate transporter 8. Endocrinology. 2011;152:1713–1721. [DOI] [PubMed] [Google Scholar]
- 23. Muller J, Heuer H. Expression pattern of thyroid hormone transporters in the postnatal mouse brain. Front Endocrinol (Lausanne). 2014;5:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Roberts LM, Woodford K, Zhou M, et al. Expression of the thyroid hormone transporters monocarboxylate transporter-8 (SLC16A2) and organic ion transporter-14 (SLCO1C1) at the blood-brain barrier. Endocrinology. 2008;149:6251–6261. [DOI] [PubMed] [Google Scholar]
- 25. Dumitrescu AM, Liao XH, Best TB, Brockmann K, Refetoff S. A novel syndrome combining thyroid and neurological abnormalities is associated with mutations in a monocarboxylate transporter gene. Am J Hum Genet. 2004;74:168–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Friesema EC, Grueters A, Biebermann H, et al. Association between mutations in a thyroid hormone transporter and severe X-linked psychomotor retardation. Lancet. 2004;364:1435–1437. [DOI] [PubMed] [Google Scholar]
- 27. Schwartz CE, May MM, Carpenter NJ, et al. Allan-Herndon-Dudley syndrome and the monocarboxylate transporter 8 (MCT8) gene. Am J Hum Genet. 2005;77:41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mayerl S, Muller J, Bauer R, et al. Transporters MCT8 and OATP1C1 maintain murine brain thyroid hormone homeostasis. J Clin Invest. 2014;124:1987–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dumitrescu AM, Liao XH, Weiss RE, Millen K, Refetoff S. Tissue-specific thyroid hormone deprivation and excess in monocarboxylate transporter (mct) 8-deficient mice. Endocrinology. 2006;147:4036–4043. [DOI] [PubMed] [Google Scholar]
- 30. Trajkovic M, Visser TJ, Mittag J, et al. Abnormal thyroid hormone metabolism in mice lacking the monocarboxylate transporter 8. J Clin Invest. 2007;117:627–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Trajkovic-Arsic M, Muller J, Darras VM, et al. Impact of monocarboxylate transporter-8 deficiency on the hypothalamus-pituitary-thyroid axis in mice. Endocrinology. 2010;151:5053–5062. [DOI] [PubMed] [Google Scholar]
- 32. Wirth EK, Roth S, Blechschmidt C, et al. Neuronal 3′,3,5-triiodothyronine (T3) uptake and behavioral phenotype of mice deficient in Mct8, the neuronal T3 transporter mutated in Allan-Herndon-Dudley syndrome. J Neurosci. 2009;29:9439–9449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. San Diego: Academic Press; 1997. [Google Scholar]
- 34. Ferrara AM, Liao XH, Gil-Ibanez P, et al. Changes in thyroid status during perinatal development of MCT8-deficient male mice. Endocrinology. 2013;154:2533–2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pohlenz J, Maqueem A, Cua K, Weiss RE, Van Sande J, Refetoff S. Improved radioimmunoassay for measurement of mouse thyrotropin in serum: strain differences in thyrotropin concentration and thyrotroph sensitivity to thyroid hormone. Thyroid. 1999;9:1265–1271. [DOI] [PubMed] [Google Scholar]
- 36. Di Cosmo C, Liao XH, Dumitrescu AM, Weiss RE, Refetoff S. A thyroid hormone analog with reduced dependence on the monocarboxylate transporter 8 for tissue transport. Endocrinology. 2009;150:4450–4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ceballos A, Belinchon MM, Sanchez-Mendoza E, et al. Importance of monocarboxylate transporter 8 for the blood-brain barrier-dependent availability of 3,5,3′-triiodo-L-thyronine. Endocrinology. 2009;150:2491–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Morte B, Ceballos A, Diez D, et al. Thyroid hormone-regulated mouse cerebral cortex genes are differentially dependent on the source of the hormone: a study in monocarboxylate transporter-8- and deiodinase-2-deficient mice. Endocrinology. 2010;151:2381–2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Di Cosmo C, Liao XH, Dumitrescu AM, Philp NJ, Weiss RE, Refetoff S. Mice deficient in MCT8 reveal a mechanism regulating thyroid hormone secretion. J Clin Invest. 2010;120:3377–3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lin SP, Coan P, da Rocha ST, et al. Differential regulation of imprinting in the murine embryo and placenta by the Dlk1-Dio3 imprinting control region. Development. 2007;134:417–426. [DOI] [PubMed] [Google Scholar]
- 41. Dentice M, Marsili A, Zavacki A, Larsen PR, Salvatore D. The deiodinases and the control of intracellular thyroid hormone signaling during cellular differentiation. Biochim Biophys Acta. 2013;1830:3937–3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Heuer H, Visser TJ. The pathophysiological consequences of thyroid hormone transporter deficiencies: insights from mouse models. Biochim Biophys Acta. 2013;1830:3974–3978. [DOI] [PubMed] [Google Scholar]
- 43. Dussault JH, Labrie F. Development of the hypothalamic-pituitary-thyroid axis in the neonatal rat. Endocrinology. 1975;97:1321–1324. [DOI] [PubMed] [Google Scholar]