Abstract
In the absence of ovarian estrogens, increased levels of estrogen receptor (ER)α in the hippocampus are associated with improvements in cognition. In vitro evidence indicates that under conditions of low estrogen, growth factors, including Insulin-Like Growth Factor 1 (IGF-1), can activate ERα and regulate ERα-mediated transcription through mechanisms that likely involve modification of phosphorylation sites on the receptor. The goal of the current work was to investigate a role for IGF-1 in ligand-independent activation of ERα in the hippocampus of female rats. Ovariectomized rats received a single intracerebroventricular infusion of IGF-1 and hippocampi were collected 1 or 24 hours later. After 1 h, IGF-1 increased hippocampal levels of phosphorylated ERα at serine 118 (S118) as revealed by Western blotting. Coimmunoprecipitation revealed that at 1 hour after infusion, IGF-1 increased association between ERα and steroid receptor coactivator 1, a histone acetyltransferase that increases transcriptional activity of phosphorylated ERα. IGF-1 infusion increased levels of the ERα-regulated proteins ERα, choline acetyltransferase, and brain-derived neurotrophic factor in the hippocampus 24 hours after infusion. Results indicate that IGF-1 activates ERα in ligand-independent manner in the hippocampus via phosphorylation at S118 resulting in increased association of ERα with steroid receptor coactivator 1 and elevation of ER-regulated proteins. To our knowledge, these data are the first in vivo evidence of ligand-independent actions of ERα and provide a mechanism by which ERα can impact memory in the absence of ovarian estrogens.
Estrogens exert effects in the hippocampus to impact memory (for reviews, see Refs. 1, 2). Estrogens act by binding 2 intracellular estrogen receptors (ERs), ERα and ERβ (3). In the brain, increasing levels of ERα are associated with improved cognition even in the absence of ovarian or exogenously administered estrogens as demonstrated in rodents (4, 5) and humans (6–8). For example, when levels ERα are elevated in the hippocampus via viral vector-mediated delivery, ovariectomized rodents show improved hippocampus-dependent spatial memory (4, 9). In humans, decreased levels of ERα are associated with increases in cognitive decline in Alzheimer's patients (6) and certain polymorphisms of ERα in women are indicative of increased cognitive impairment with age (7, 8). Taken together these findings underscore not only the role of estrogens in memory processes; they specifically find that ERα levels correspond to improvements in memory in the absence of circulating estrogens.
Under conditions of low levels of estrogens, ER function can be impacted by extracellular signals (10). These ligand-independent actions include the ability of growth factors, including IGF-1 to activate ER and impact ER-dependent transcription. IGF-1 exerts its effects by binding to its receptor, which is a transmembrane protein with tyrosine kinase activity (11). The mechanism by which IGF-1 receptor activation impacts ER function is unclear, but evidence from a series of studies employing in vitro models indicates it most likely involves modification of phosphorylation sites on ERα by cellular kinases (12). Activation of IGF-1 receptors leads to activation of 2 main downstream signaling cascades, the Ras-ERK/MAPK and Phosphatidylinositol-4,5-bisphosphate 3-kinase pathways (13). IGF-1 activation of ERα is regulated by phosphorylation of serine residues at position 118 (S118) through the Ras-ERK/MAPK pathway in COS-1 cells (14) and by the Phosphatidylinositol-4,5-bisphosphate 3-kinase pathway in MCF-7 cells (15). However, mutation of either S118 or serine residue at position 167 (S167) on the Accessory-Factor-1 domain resulted in complete loss of ERα activation by Akt (15). Finally, ligand-independent transcriptional activity of ERα in vitro is enhanced by its association with the histone acetyltransferase steroid receptor coactivator 1 (SRC-1) (16), and that association is regulated by phosphorylation of ERα at S118 (17).
Although the ability of IGF-1 to activate ERα has not been demonstrated in vivo, previous results from our lab employing a rat model support a role for IGF-1 in ligand-independent activation of ERα. Short-term administration of estradiol during a critical window after cessation of ovarian function in middle-aged rats has long-term benefits for hippocampus-dependent memory and results in corresponding lasting elevations of hippocampal levels ERα and the ERα-regulated protein choline acetyltransferase (ChAT) (5). These effects persist for months after the termination of estradiol treatment. Short-term administration of estradiol in midlife also results in lasting increases in levels of IGF-1 receptors in the hippocampus (18). Additionally, the long-term effects of short-term estradiol treatment on memory and on ERα-regulated proteins were blocked by chronic antagonism of brain IGF-1 receptors that was initiated after the estradiol treatment was terminated. ERα and IGF-1 receptors colocalize in neurons in the brain, including in the hippocampus, allowing for interactions in their signaling mechanisms (19). These results, in light of previous in vitro work documenting that IGF-1 can activate ERα in a ligand-independent manner (10, 12), are consistent with the hypothesis that IGF-1 can activate ERα in the hippocampus, increasing ERα-transcribed proteins in the absence of estradiol resulting in a benefit to cognition.
The goal of the current study was to provide a direct test of the ability of IGF-1 to activate ERα in the hippocampus of ovariectomized rats. Ovariectomized rats received an intracerebroventricular (i.c.v.) infusion of IGF-1 or vehicle and hippocampi were examined 1 and 24 hours after infusion. One hour after infusions, we determined the impact of IGF-1 on levels of phosphorylated ERα at S118 and S167 as well the association between ERα and SRC-1. Twenty-four hours after infusions, we determined the impact of IGF-1 on levels of proteins associated with ERα activation, including ERα itself, ChAT, brain-derived neurotrophic factor (BDNF), the synaptic protein postsynaptic density 95 (PSD-95) and the antiapoptotic protein B-cell lymphoma 2 (BCL-2). These proteins were selected as all are reportedly up-regulated as a result of ligand-bound activation by ER (20–24).
Materials and Methods
Subjects
Twenty young adult (∼70 d) female Long-Evans rats were obtained from Harlan, Inc and group housed in a temperature-controlled Assessment and Accreditation of Laboratory Animal Care-accredited vivarium under a 12-hour light, 12-hour dark cycle with ad libitum access to food and water. Animal care was in accordance with guidelines set by the National Institutes of Health Guide for the Care and Use of Laboratory Animals (1996). All procedures were approved by the Institutional Animal Care and Use Committee of Tulane University.
Ovariectomy
Rats were ovariectomized under anesthesia induced by injection of ketamine (100 mg/kg, ip; Fort Dodge Animal Health) and xylazine (7 mg/kg, ip; MWI). One rat was lost due to complications from anesthetic.
Infusion and tissue dissection
Ten days after ovariectomy, rats were randomly assigned to receive a single i.c.v. infusion of aCSF vehicle (n = 9) or IGF-1 (n = 10). Rats were anesthetized with ketamine and xylazine and placed into a stereotaxic frame. An incision was made in the scalp and fascia that overlie the skull. A hole was drilled in the skull, and a 10-μL Hamilton syringe was lowered to the appropriate depth (coordinates: −0.3 mm anterior-posterior, +1.2 mm medial-lateral, and −4.5 mm dorsal-ventral relative to bregma) (18). IGF-1 (GroPep), 2 μg/μL (25) in a total volume of 1 μL or aCSF vehicle (Tocris Bioscience) was delivered over a period of 2 minutes. Rats were killed by decapitation either 1 hour (4 vehicle and 5 IGF-1) or 24 hours (5 vehicle and 5 IGF-1) after infusions. Hippocampi were dissected from both hemispheres, quick frozen on dry ice, and stored at −80°C until processing.
Western blotting sample preparation
Tissue was homogenized by sonication in 15 μL/mg lysis buffer containing 1mM EGTA, 1mM EDTA, 20mM Tris, 1mM sodium pyrophosphate tetrabasic decahydrate, 4mM 4-nitrophenyl phosphate disodium salt hexahydrate, 0.1μM microcystin, and 1% protease inhibitor cocktail (Sigma-Aldrich). Samples were centrifuged for 15 minutes at 1000g at 4°C, protein concentration of supernatants was determined (Bradford protein assay kit; Pierce), and each sample was diluted 1:1 with Laemmli sample buffer (Bio-Rad Laboratories) mixed with 350mM D, L-dithiothreitol, boiled for 5 minutes, and stored at −80°C.
Coimmunopreciptiation (IP) sample preparation
Tissue was homogenized in 10-μL/mg IP lysis/wash buffer included in the Pierce Classic Immunoprecipitation kit (Pierce) then centrifuged at 13 000g for 10 minutes. Protein concentration of supernatant was determined using the Bradford Protein Assay kit (Pierce). Supernatant containing 1 mg of protein was incubated in either the primary antibody against SRC-1 (mouse monocolonal, 1:2000; Millipore) or ERα (H-184 rabbit polyclonal, 1:750; Santa Cruz Biotechnology, Inc) overnight at 4°C. Samples were then incubated for 1 hour with Protein A/G beads. After incubation, samples were eluted by nonreducing electrophoresis buffer and 20mM dithiothreitol and boiled for 5 minutes followed by centrifugation at 1000g. Elution was collected for Western blotting.
Electrophoresis and immunostaining
For total protein samples, 25 μg (ChAT, BDNF, PSD-95, and BCL-2) or 35 μg (ERα, phospho-ERα S118 and S167) of total protein were loaded and separated at 250 V on 10% Tris-Glycine-Extended Polyacrylamide gels (Bio-Rad Laboratories) for 40 minutes. For samples in which we IP either ERα or SRC-1, 15 μL of each sample were loaded and separated as described above in order to probe for ERα-associated SRC-1 protein levels or SRC-1-associated ERα protein levels. We conducted probes for ERα-associated SRC-1 as well as the reverse, SRC-1-associated ERα, to provide verification of the association between these proteins. Molecular weight markers (Kaleidoscope; Bio-Rad Laboratories) were included with each run. Uterus samples were included as positive controls for ERα and phospho-ERα (S118, S167). Proteins were transferred to nitrocellulose membranes at 100 V for 20 minutes. Membranes were blocked with 5% nonfat dry milk in 1% Tween 20/1× Tris-buffered saline (TTBS) at room temperature for 1 hour. After this process, membranes were incubated with primary antibody overnight at 4°C in 1% nonfat dry milk-TTBS. Primary antibodies used were: ERα (H-184 rabbit polyclonal, 1:750; Santa Cruz Biotechnology, Inc), phospho-ERα S118 (rabbit polyclonal, 1:1000; Millipore), phospho-ERα S167 (rabbit polyclonal, 1:750; Millipore), SRC-1 (mouse monocolonal, 1:2000; Millipore), BCL-2 (rabbit polyclonal, 1:100; Santa Cruz Biotechnology, Inc), BDNF (rabbit polyclonal, 1:600; Santa Cruz Biotechnology, Inc), ChAT (mouse monoclonal, 1:1500; Millipore), and PSD-95 (rabbit polyclonal, 1:1500; Millipore). Blots were washed 3 times for 15 minutes each with TTBS and incubated with 5% nonfat dry milk containing secondary antibody conjugated to horseradish peroxidase for 1.5 hours at room temperature. Secondary antibodies used were goat antirabbit IgG (ERα, pERα S118, S167 1:40 000: BCL-2, 1:22 000: BDNF, 1:4000: PSD-95, 1:2000; Santa Cruz Biotechnology, Inc) or goat antimouse IgG (ChAT, 1:70 000; Santa Cruz Biotechnology, Inc) and for co-IP samples Clean-Blot IP Detection reagent was used (1:2000; Pierce). Blots were washed again 3 times for 15 minutes each and incubated with the chemiluminescent substrate Pierce ECL (BDNF, BCL-2; Fisher Scientific) for 1 minute or SuperSignal West Femto (ERα, pERα S118, pERα S167, SRC-1, ChAT, PSD-95; Fisher Scientific) for 5 minutes and exposed to film (Kodak Biomax MR; Kodak) for varying durations to capture optimal signal intensity. To stain for loading control β-actin, blots were washed and stripped with stripping buffer (RestorePlus Western Blot; Fisher Scientific) for 15 minutes at 37°C. Blots were then blocked and incubated with primary antibody for β-actin (mouse monoclonal, 1:15 000; Santa Cruz Biotechnology, Inc) overnight at 4°C in TTBS. Blots were washed 3 times for 15 minutes each with TTBS and incubated in 5% nonfat dry milk containing goat antimouse IgG (1:10 000; Santa Cruz Biotechnology, Inc) conjugated to horseradish peroxidase for 1.5 hours at room temperature, washed, and detected by chemiluminescence. To determine levels of phosphorylated ERα relative to total ERα, phospho-ERα S118 and S167 blots were washed and stripped with stripping buffer, blocked, and immunostained for total ERα as described above. Films were imaged using MCID Core imaging software (InterFocus Imaging Ltd), and optical density by area was measured for bands of interest. Levels of phosphorylated ERα were calculated as density × area relative to total ERα. For all other proteins measured, mean values were calculated for the aCSF control samples and values were represented as a percentage relative to the average control value.
Statistical analysis
Data were analyzed by one-way ANOVAs with infusion (aCSF or IGF-1) as the factor.
Results
One hour after infusion
Phosphorylated and total ERα
Western blottings revealed bands of phosphorylated and total ERα-like immunoreactivity at approximately 66 kDa. In addition, a single band at approximately 43 kDa was detected on immunostaining for the loading control, β-actin. There was a significant effect of infusion, F1,7 = 5.987, P = .044, for levels of phosphorylated ERα at S118 calculated as a percent of total ERα (Figure 1A), indicating the IGF-1 infusion increases levels of phosphorylated ERα at S118 in the hippocampus. There was no effect of infusion on phosphorylated ERα at S167 (Figure 1B) or on total levels of ERα or β-actin (Figure 1C).
Figure 1.
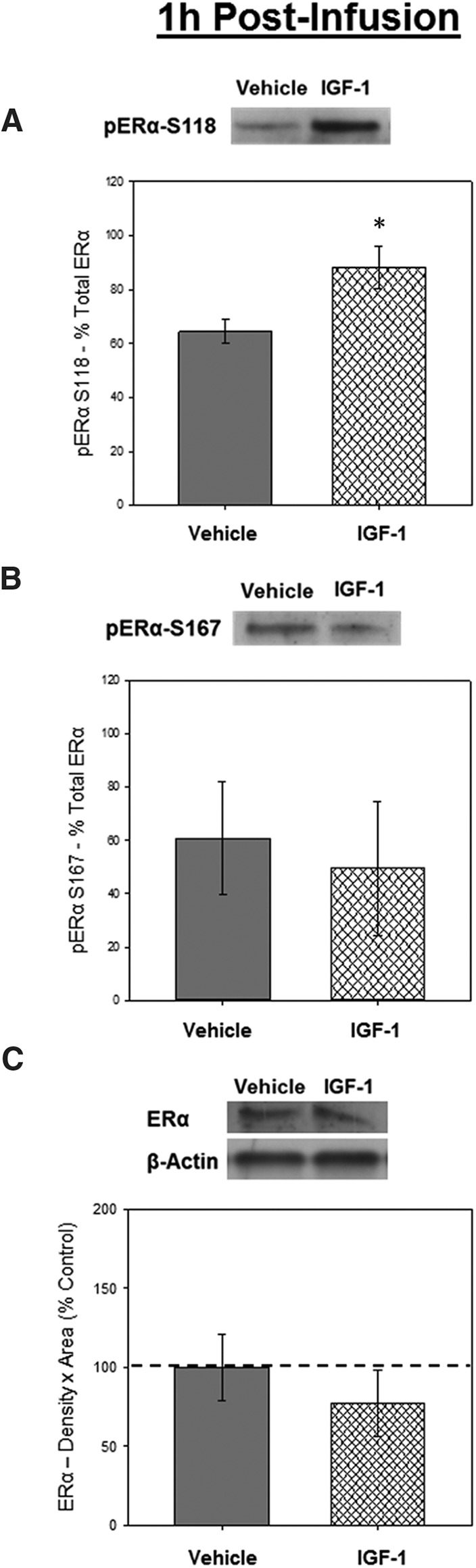
Effects of IGF-1 infusion on levels of phosphorylated ERα in the hippocampus 1 hour after infusion. Hippocampi were collected from ovariectomized rats 1 hour after an i.c.v. infusion of aCSF vehicle or IGF-1. Western blotting data showing effects of treatments on levels of phosphorylated ERα at site S118 (A), phosphorylated ERα at site S167 (B), and total ERα (C). Mean density × area (±SEM) relative to total ERα (A and B) or relative to vehicle control group (C); *, P < .05. Representative blot images for phosphorylated ERα at S118, phosphorylated ERα at S167, total ERα, and the loading control β-actin are shown in insets above respective graphs.
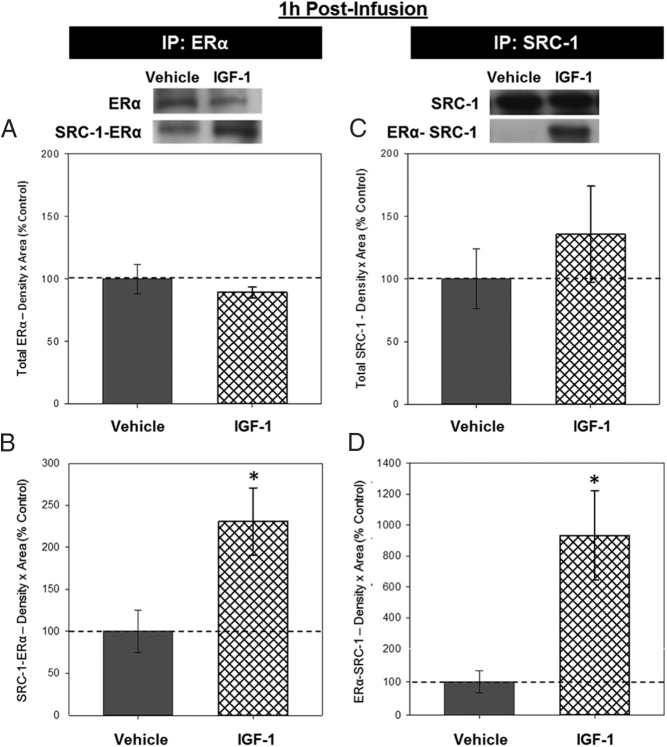
Association of ERα and SRC-1
Insufficient levels of protein were collected from one of the samples (IGF-1 group) and thus was excluded from the co-IP experiments. Western blottings from both co-IP experiments revealed bands of ERα-like immunoreactivity at approximately 66 kDa and bands of SRC-1-like immunoreactivity at approximately 160 kDa. In lysate from which ERα was IP, there was no effect of infusion on levels of ERα (Figure 2A), but there was a significant effect of infusion, F1,6 = 6.530, P = .038, on levels of SRC-1 (Figure 2B), indicating that IGF-1 infusion increased levels of SCR-1 that was associated with ERα in the hippocampus. In lysate from which SRC-1 was IP, there was no effect of infusion on levels of SRC-1 (Figure 2C), but there was a significant effect of infusion, F1,6 = 7.648, P = .033, on levels of ERα (Figure 2D) indicating that IGF-1 infusion increased levels of ERα that was associated with SCR-1 in the hippocampus.
Figure 2.
Effects of IGF-1 infusion on the association between ERα and SRC-1 in the hippocampus 1 hour after infusion. Hippocampi were collected from ovariectomized rats 1 hour after an i.c.v. infusion of aCSF vehicle or IGF-1 and processed for co-IP. Western blotting data showing effects of treatments on levels of ERα (A) and SRC-1 associated with ERα (B) in lysate in which ERα was IP for ERα and on levels of SRC-1 (C) and ERα that was associated with SRC-1 (D) in lysate that was IP for SRC-1. Mean density × area (±SEM) relative to vehicle control group; *, P < .05. Representative blot images for ERα, SRC-1-ERα, SRC-1, and ERα-SRC-1 are shown in insets above graphs.
ChAT, BDNF, PSD-95, and BCL-2
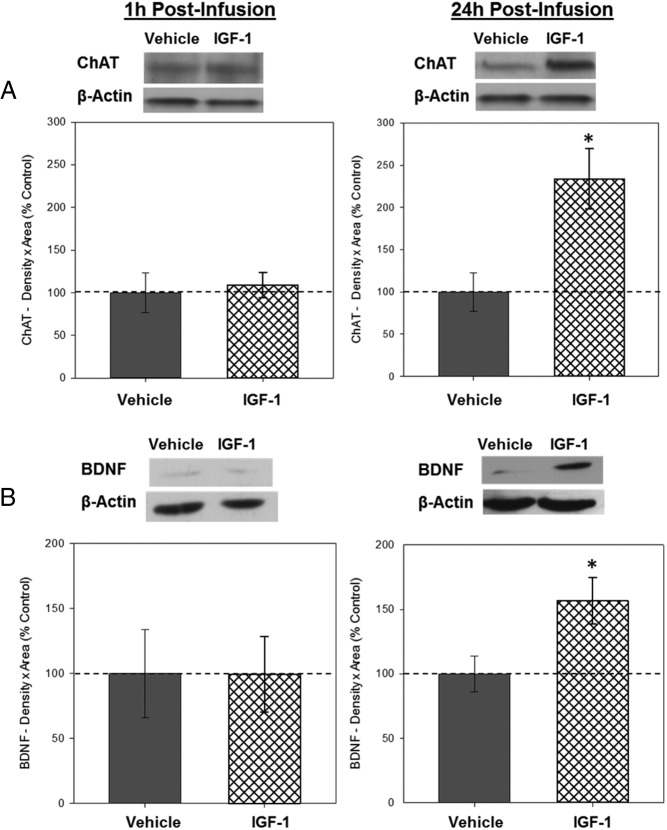
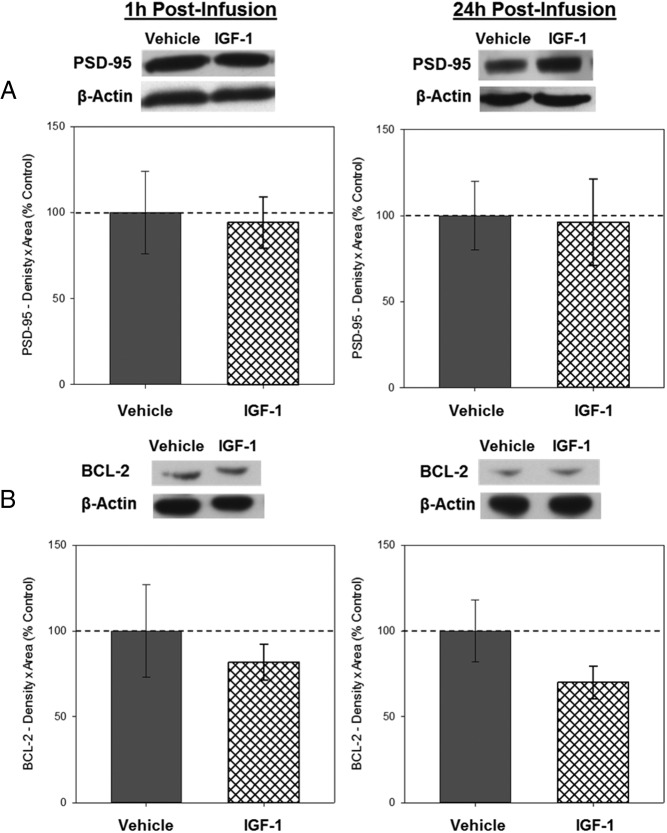
Western blottings revealed bands of ChAT-like immunoreactivity at approximately 67 kDa, BDNF-like immunoreactivity at approximately 14 kDa, PSD-95-like immunoreactivity at approximately 95 kDa, and BCL-2-like immunoreactivity at approximately 26 kDa. There was no effect of infusion on hippocampal levels of ChAT (see figure 4 below), BDNF (see figure 4 below), PSD-95 (see figure 5 below), or BCL-2 (see figure 5 below) in tissue collected 1 hour after infusion.
Twenty-four hours after infusion
ERα
Western blottings revealed bands of ERα-like immunoreactivity at approximately 66 kDa. There was a significant effect of infusion, F1,8 = 5.957, P = .041, indicating the IGF-1 infusion increases levels of ERα in the hippocampus 24 hours after infusion (Figure 3). There was no effect of infusion on β-actin.
Figure 3.
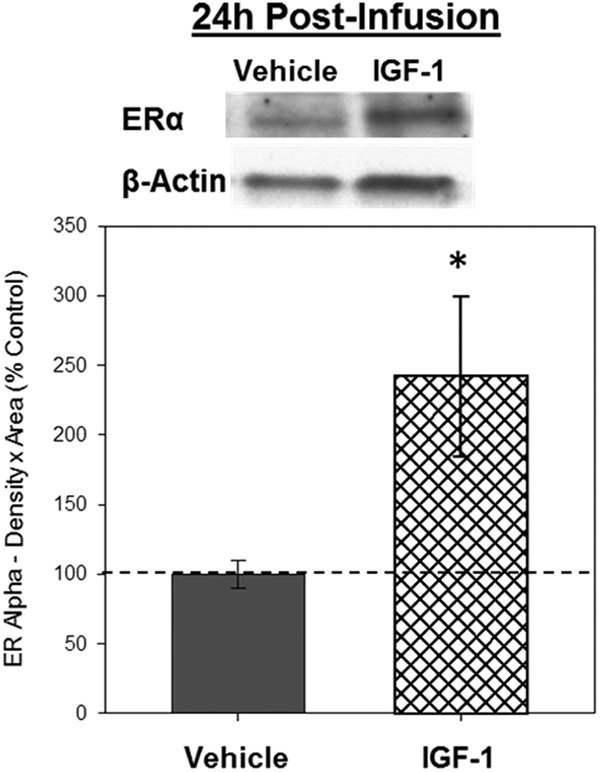
Effects of IGF-1 infusion on total levels of ERα in the hippocampus 24 hours after infusion. Hippocampi were collected from ovariectomized rats 24 hours after an i.c.v. infusion of aCSF vehicle or IGF-1. Western blotting data showing effects of treatments on levels of ERα. Mean density × area (±SEM) relative to vehicle control group; *, P < .05. Representative blot images for ERα and the loading control β-actin are shown in insets above the graph.
ChAT, BDNF, PSD-95, and BCL-2
Western blottings revealed bands of ChAT-like immunoreactivity at approximately 67 kDa, BDNF-like immunoreactivity at approximately 14 kDa, PSD-95-like immunoreactivity at approximately 95 kDa, and BCL-2-like immunoreactivity at approximately 26 kDa. There was a significant effect of infusion on levels of ChAT, F1,8 = 5.485; P = .047 (Figure 4A) and on BDNF, F1,8 = 6.212, P = .037 (Figure 4B), indicating that IGF-1 infusion increases levels of ChAT and BDNF in the hippocampus 24 hours after infusion. There was no effect of infusion on levels of PSD-95 (Figure 5A) or BCL-2 (Figure 5B), indicating that IGF-1 infusion does not impact levels of these proteins in the hippocampus 24 hours after infusion. There were no effects of infusion on β-actin for any of the measures.
Figure 4.
Effects of IGF-1 on levels of ChAT and BDNF in the hippocampus 1 and 24 hours after infusion. Hippocampi were collected from ovariectomized rats 1 and 24 hours after an i.c.v. infusion of aCSF vehicle or IGF-1. Western blotting data showing effects of treatments on levels of ChAT (A) and BDNF (B) 1 and 24 hours after infusion. Mean density × area (±SEM) relative to vehicle control group; *, P < .05. Representative blot images for ChAT, BDNF, and the loading control β-actin are shown in insets above the respective graphs.
Figure 5.
Effects of IGF-1 on levels of PSD-95 and BCL-2 1 and 24 hours in the hippocampus 1 and 24 hours after infusion. Hippocampi were collected from ovariectomized rats 1 and 24 hours after an i.c.v. infusion of aCSF vehicle or IGF-1. Western blotting data showing effects of treatments on levels of PSD-95 (A) and BCL-2 (B) 1 and 24 hours after infusion. Mean density × area (±SEM) relative to vehicle control group. Representative blot images for PSD-95, BCl-2, and the loading control β-actin are shown in insets above the respective graphs.
Discussion
The current study examined the ability of IGF-1 to activate ERα in the hippocampus of ovariectomized rats in the absence of circulating estrogens. We found that a single i.c.v. infusion of IGF-1 resulted in increased phosphorylation of hippocampal ERα at S118, as well as increases in the association between hippocampal ERα and the coactivator SRC-1 at 1 hour after infusion. Furthermore, protein levels of ERα, ChAT, and BDNF were increased in the hippocampus 24 hours after IGF-1 infusions. Taken together, these findings indicate that in the absence of circulating estrogens, IGF-1 is capable of activating ERα and impacting levels of ERα-regulated proteins in the hippocampus. These data provide to our knowledge, the first evidence in the brain of ligand-independent activation of ERα by extracellular signaling.
Results of previous in vitro work indicate that IGF-1, through activation of downstream signaling pathways, is able to activate ERα via phosphorylation (26). Therefore, in the current study, we examined whether similar mechanisms were evident in vivo. Our results indicate that the ability of IGF-1 to activate ERα in the hippocampus in a ligand-independent manner is associated with increased phosphorylation of the receptor at S118 but not S167. Importantly, ligand dependent and independent phosphorylation of ERα at S118 in vitro is associated with the recruitment of the coactivator SRC-1 (17), which facilitates ER-dependent transcription. Current results show a similar relationship when brain ERα is activated by IGF-1. One hour after IGF-1 infusion, along with increased hippocampal levels of phosphorylated ERα at S118, there was increased association of ERα with SRC-1, consistent with the hypothesis that IGF-1 is able to activate hippocampal ERα in the absence of estradiol and to potentially induce ERα-dependent gene transcription.
To better understand impact of IGF-1 infusion on ERα-regulated proteins, we examined the levels of 5 estradiol-sensitive proteins, ERα, ChAT, BDNF, PSD-95, and BCL-2, after IGF-1infusion. We hypothesized that if IGF-1 is capable of activating ERα, levels of these proteins would be elevated by IGF-1 in a similar manner as they are by estradiol. As expected, no proteins were elevated in the hippocampus 1 hour after IGF-1 infusion. However, at 24 hours after IGF-1 infusion, levels of 3 of the 5 proteins, ERα, ChAT, and BDNF, were elevated. Although there are a wide range of proteins that are elevated when estradiol binds ERα, the mechanisms by which they are regulated by ERα differs. ERα-mediated regulation of ERα, ChAT, and BDNF occurs at the transcriptional level and the identification of a putative estrogen-response element (ERE) on the ERα (27), ChAT (3), and BDNF (28) genes provides a site for direct modulation of each of these by ERα. In contrast, those proteins that were not elevated by IGF-1, PSD-95, and BCL-2, are transcribed via other mechanisms after activation of ERα by estrogens. PSD-95 is proposed to be elevated by estradiol through nongenomic mechanisms at the membrane; increased PSD-95 after binding of estradiol does not happen through the ER specifically, rather it follows after alleviating repression of the initiation factor 4E binding protein 1 that results in increased PSD-95 translation (29). Similarly, elevation of BCL-2 in response to estradiol occurs via mechanisms other than direct interaction of ER with the ERE (30). When estradiol is introduced in vitro, BCL-2 is increased via tethering and activation of the cAMP-response element rather than the ERE (30). The finding in the current study that some, but not all estradiol-sensitive proteins were increased by IGF-1 infusion raises the possibility that though IGF-1 can activate ERα and increase ERα-regulated proteins in vivo, the impact of ligand-independent activation of ERα by IGF-1 on ERα-regulated proteins may vary depending upon the mechanism by which ERα activity regulates its expression.
In addition to ligand-independent mechanisms, brain ER may be activated by other mechanisms in the absence of circulating estrogens. For example, neuroestradiol can be synthesized in hippocampal neurons and impact neuroplasticity (31). Cross talk between ERα and IGF-1 receptors has been established, including evidence that IGF-1 interacts with estradiol to impact ERα-dependent transcription (for review, see Ref. 32). Therefore, we cannot discount the possibility that the current results may be due, at least in part, to a neuroestradiol-IGF-1 interaction. Nevertheless, results show that in the absence of circulating estrogens, administration of IGF-1 results in activation of ERα in vivo with consequences for ERα-regulated proteins.
There is growing evidence in humans (6–8) and in animal models (4, 9) that brain ERα can impact cognition in the absence of circulating estrogens. The current findings indicating that IGF-1 can activate ERα and increase levels of ERα-regulated proteins in the hippocampus provide a potential mechanism by which ERα can be activated and impact cognition in the absence of circulating estrogens. The functional implications for cognition of activation of IGF-1 receptors in the absence of circulating estrogens are evident from previous data from our lab in which antagonism of brain IGF-1 receptors blocked the lasting enhancing effects on cognition in aging ovariectomized rats that had previous estradiol exposure (18). In addition, this chronic antagonism of hippocampal IGF-1 receptors reversed the lasting elevation in hippocampal levels of ERα and ChAT that was evident in aging females that had previous estradiol treatment. These findings, along with the current data, indicate that activation of IGF-1 receptors impacts ERα function after cessation of ovarian function. Administration of IGF-1 to the aging brain can improve learning and memory (33) and enhance synaptic plasticity (34). Thus, ligand-independent actions by which IGF-1 can increase ERα activity may provide beneficial effects to the aging female brain and to cognition.
Acknowledgments
This work was supported by the National Institute on Aging Grant R01AG041374 (to J.M.D.) and the National Institutes of Health Office for Research in Women's Health Building Interdisciplinary Careers for Women's Health Program Grant K12HD043451 (to E.M.G.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- aCSF
- artificial cerebrospinal fluid
- Akt
- Protein Kinase B
- BCL-2
- B-cell lymphoma 2
- BDNF
- brain-derived neurotrophic factor
- ChAT
- choline acetyltransferase
- ER
- estrogen receptor
- ERE
- estrogen-response element
- i.c.v.
- intracerebroventricular
- IP
- immunoprecipitation
- pER
- phosphorylated estrogen receptor
- PSD-95
- postsynaptic density 95
- SRC-1
- steroid receptor coactivator 1
- TTBS
- 1% Tween 20/1× Tris-buffered saline.
References
- 1. Frick KM. Molecular mechanisms underlying the memory-enhancing effects of estradiol. Horm Behav. 2015;74:4–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Luine VN. Estradiol and cognitive function: past, present and future. Horm Behav. 2014;66:602–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hyder SM, Chiappetta C, Stancel GM. Interaction of human estrogen receptors α and β with the same naturally occurring estrogen response elements. Biochem Pharmacol. 1999;57:597–601. [DOI] [PubMed] [Google Scholar]
- 4. Foster TC, Rani A, Kumar A, Cui L, Semple-Rowland SL. Viral vector-mediated delivery of estrogen receptor-α to the hippocampus improves spatial learning in estrogen receptor-α knockout mice. Mol Ther. 2008;16:1587–1593. [DOI] [PubMed] [Google Scholar]
- 5. Rodgers SP, Bohacek J, Daniel JM. Transient estradiol exposure during middle age in ovariectomized rats exerts lasting effects on cognitive function and the hippocampus. Endocrinology. 2010;151:1194–1203. [DOI] [PubMed] [Google Scholar]
- 6. Kelly JF, Bienias JL, Shah A, et al. Levels of estrogen receptors α and β in frontal cortex of patients with Alzheimer's disease: relationship to Mini-Mental State Examination scores. Curr Alzheimer Res. 2008;5:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yaffe K, Lui LY, Grady D, Stone K, Morin P. Estrogen receptor 1 polymorphisms and risk of cognitive impairment in older women. Biol Psychiatry. 2002;51:677–682. [DOI] [PubMed] [Google Scholar]
- 8. Yaffe K, Lindquist K, Sen S, et al. Estrogen receptor genotype and risk of cognitive impairment in elders: findings from the Health ABC study. Neurobiol Aging. 2009;30:607–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Witty CF, Foster TC, Semple-Rowland SL, Daniel JM. Increasing hippocampal estrogen receptor α levels via viral vectors increases MAP kinase activation and enhances memory in aging rats in the absence of ovarian estrogens. PLoS One. 2012;7:e51385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Smith CL. Cross-talk between peptide growth factor and estrogen receptor signaling pathways. Biol Reprod. 1998;58:627–632. [DOI] [PubMed] [Google Scholar]
- 11. LeRoith D. Clinical relevance of systemic and local IGF-I: lessons from animal models. Pediatr Endocrinol Rev. 2008;5(suppl 2):739–743. [PubMed] [Google Scholar]
- 12. Hall JM, Couse JF, Korach KS. The multifaceted mechanisms of estradiol and estrogen receptor signaling. J Biol Chem. 2001;276:36869–36872. [DOI] [PubMed] [Google Scholar]
- 13. Russo VC, Gluckman PD, Feldman EL, Werther GA. The insulin-like growth factor system and its pleiotropic functions in brain. Endocr Rev. 2005;26:916–943. [DOI] [PubMed] [Google Scholar]
- 14. Kato S, Endoh H, Masuhiro Y, et al. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science. 1995;270:1491–1494. [DOI] [PubMed] [Google Scholar]
- 15. Martin MB, Franke TF, Stoica GE, et al. A role for Akt in mediating the estrogenic functions of epidermal growth factor and insulin-like growth factor I. Endocrinology. 2000;141:4503–4511. [DOI] [PubMed] [Google Scholar]
- 16. Smith CL, Nawaz Z, O'Malley BW. Coactivator and corepressor regulation of the agonist/antagonist activity of the mixed antiestrogen, 4-hydroxytamoxifen. Mol Endocrinol. 1997;11:657–666. [DOI] [PubMed] [Google Scholar]
- 17. Dutertre M, Smith CL. Ligand-independent interactions of p160/steroid receptor coactivators and CREB-binding protein (CBP) with estrogen receptor-α: regulation by phosphorylation sites in the A/B region depends on other receptor domains. Mol Endocrinol. 2003;17:1296–1314. [DOI] [PubMed] [Google Scholar]
- 18. Witty CF, Gardella LP, Perez MC, Daniel JM. Short-term estradiol administration in aging ovariectomized rats provides lasting benefits for memory and the hippocampus: a role for insulin-like growth factor-I. Endocrinology. 2013;154:842–852. [DOI] [PubMed] [Google Scholar]
- 19. Cardona-Gómez GP, DonCarlos L, Garcia-Segura LM. Insulin-like growth factor I receptors and estrogen receptors colocalize in female rat brain. Neuroscience. 2000;99:751–760. [DOI] [PubMed] [Google Scholar]
- 20. Bohacek J, Daniel JM. The ability of oestradiol administration to regulate protein levels of oestrogen receptor α in the hippocampus and prefrontal cortex of middle-aged rats is altered following long-term ovarian hormone deprivation. J Neuroendocrinol. 2009;21:640–647. [DOI] [PubMed] [Google Scholar]
- 21. Bohacek J, Bearl AM, Daniel JM. Long-term ovarian hormone deprivation alters the ability of subsequent oestradiol replacement to regulate choline acetyltransferase protein levels in the hippocampus and prefrontal cortex of middle-aged rats. J Neuroendocrinol. 2008;20:1023–1027. [DOI] [PubMed] [Google Scholar]
- 22. Bora SH, Liu Z, Kecojevic A, Merchenthaler I, Koliatsos VE. Direct, complex effects of estrogens on basal forebrain cholinergic neurons. Exp Neurol. 2005;194:506–522. [DOI] [PubMed] [Google Scholar]
- 23. Zhao L, Wu TW, Brinton RD. Estrogen receptor subtypes α and β contribute to neuroprotection and increased Bcl-2 expression in primary hippocampal neurons. Brain Res. 2004;1010:22–34. [DOI] [PubMed] [Google Scholar]
- 24. Waters EM, Mitterling K, Spencer JL, Mazid S, McEwen BS, Milner TA. Estrogen receptor α and β specific agonists regulate expression of synaptic proteins in rat hippocampus. Brain Res. 2009;1290:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sun Y, Todd BJ, Thornton K, Etgen AM, Neal-Perry G. Differential effects of hypothalamic IGF-I on gonadotropin releasing hormone neuronal activation during steroid-induced LH surges in young and middle-aged female rats. Endocrinology. 2011;152:4276–4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Becker MA, Ibrahim YH, Cui X, Lee AV, Yee D. The IGF pathway regulates ERα through a S6K1-dependent mechanism in breast cancer cells. Mol Endocrinol. 2011;25:516–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Klinge CM, Jernigan SC, Mattingly KA, Risinger KE, Zhang J. Estrogen response element-dependent regulation of transcriptional activation of estrogen receptors α and β by coactivators and corepressors. J Mol Endocrinol. 2004;33:387–410. [DOI] [PubMed] [Google Scholar]
- 28. Sohrabji F, Miranda RC, Toran-Allerand CD. Identification of a putative estrogen response element in the gene encoding brain-derived neurotrophic factor. Proc Natl Acad Sci USA. 1995;92:11110–11114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Akama KT, McEwen BS. Estrogen stimulates postsynaptic density-95 rapid protein synthesis via the Akt/protein kinase B pathway. J Neurosci. 2003;23:2333–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dong L, Wang W, Wang F, et al. Mechanisms of transcriptional activation of bcl-2 gene expression by 17β-estradiol in breast cancer cells. J Biol Chem. 1999;274:32099–32107. [DOI] [PubMed] [Google Scholar]
- 31. Rune GM, Frotscher M. Neurosteroid synthesis in the hippocampus: role in synaptic plasticity. Neuroscience. 2005;136:833–842. [DOI] [PubMed] [Google Scholar]
- 32. Garcia-Segura LM, Arévalo MA, Azcoitia I. Interactions of estradiol and insulin-like growth factor-I signalling in the nervous system: new advances. Prog Brain Res. 2010;181:251–272. [DOI] [PubMed] [Google Scholar]
- 33. Markowska AL, Mooney M, Sonntag WE. Insulin-like growth factor-1 ameliorates age-related behavioral deficits. Neuroscience. 1998;87:559–569. [DOI] [PubMed] [Google Scholar]
- 34. Sonntag WE, Bennett SA, Khan AS, et al. Age and insulin-like growth factor-1 modulate N-methyl-D-aspartate receptor subtype expression in rats. Brain Res Bull. 2000;51:331–338. [DOI] [PubMed] [Google Scholar]