Abstract
Despite numerous reports of relationships between weight gain and butyrylcholinesterase (BChE), this enzyme's role in the genesis of obesity remains unclear, but recent research points to strong links with ghrelin, the “hunger hormone.” The availability of BChE knockout (KO) mice provides an opportunity to clarify the causal relationship between BChE and obesity onset. We now find that young KO mice have abnormally high plasma ghrelin levels that slowly decline during long-term high-fat feeding and ultimately drop below those in wild-type mice. On such a diet, the KO mice gained notably more weight, more white fat, and more hepatic fat than wild-type animals. In addition to a greater burden of hepatic triglycerides, the livers of these KO mice show distinctly higher levels of inflammatory markers. Finally, their energy expenditure proved to be lower than in wild-type mice despite similar activity levels and increased caloric intake. A gene transfer of mouse BChE with adeno-associated virus vector restored nearly all aspects of the normal phenotype. Our results indicate that BChE strongly affects fat metabolism, has an important impact on fat accumulation, and may be a promising tool for combating obesity.
Obesity has become a pandemic disorder with serious health concerns for affected individuals (1), raising the risks of insulin resistance, hypertension, atherosclerosis, type 2 diabetes, as well as breast and colon cancer. One predisposing factor may be a low circulating level of butyrylcholinesterase (BChE) (EC 1.1.1.8). Serum BChE activity shows a strong positive correlation with levels of serum triglyceride, total cholesterol, fasting insulin, and insulin resistance, but a negative correlation with high density lipoprotein-cholesterol (2–5). BChE is synthesized in liver and can serve as a biomarker of liver damage, cirrhosis, and metastatic cancer (3). Plasma BChE is often low in protein-energy malnutrition (6) but is elevated in populations that are obese or diabetic (7). Until now, few if any studies have thoroughly probed the causal links between BChE and obesity and its metabolic consequences, or explored how deliberate alterations of BChE levels might affect such disorders.
BChE's genetic variation clearly points out a link to obesity and obesity-related disorders. For example, studies show that individuals with a BChE phenotype expressing one extra protein band owing to a variant at the cholinesterase-2 locus (∼10% of the population) display up to 20% higher BChE activity in plasma, along with lower weight and body mass index (8, 9). In contrast, those with “K variant” BChE display 40% less plasma BChE activity and have a higher incidence of obesity, type 2 diabetes, and coronary artery disease (10–12). Overall, individuals with high BChE activity tend to be lean, whereas those with low BChE activity are prone to increased adiposity and weight gain. Paradoxically, BChE activity is positively correlated with body weight and body mass index in obese people (13–16). Moreover, a recent genome-wide association study found highly significant correlations between BChE activity and phenomena related to obesity, including insulin resistance and fatty liver (17). The study authors concluded that altered BChE expression was more likely to be a consequence rather than a cause of metabolic abnormalities. However, a full grasp of BChE's association with metabolic syndromes remains elusive and this topic deserves renewed attention in light of recent discoveries highlighting the enzyme's role in inactivating the peptide hormone, ghrelin (18).
Ghrelin is released primarily from the stomach to drive food intake and weight gain, and also regulates energy homeostasis (19, 20). This peptide has the unique feature of an n-octanoylated serine-3 residue that is essential for its pharmacokinetics and receptor activation (21). BChE cleaves the octanoyl group to transform ghrelin into “quasi-inactive” desacyl-ghrelin (22–25). Genotyping of single nucleotide polymorphisms of GHRELIN and BCHE genes in obese individuals indicates a close relationship between ghrelin and BChE expression (26).
In 2008, Lockridge's pioneering investigation of BChE knockout (KO) mice of the C57BL strain found that, on a high-fat diet (HFD), they gained body weight faster than wild-type (WT) mice (27). Very recently, Zhan and coworkers showed that weekly injections of mutated human BChE impeded weight gain in WT C57 mice (28). These outcomes show that plasma BChE can strongly influence the onset of obesity. However, no excess food consumption was observed in either study, and the mechanism behind the weight gain has remained obscure. Our own work with the same strain of mice (23) showed that a genetic deficiency of BChE leads to ghrelin levels 50% above normal. This finding suggests that BChE KO mice gain excess weight on rich diets because their superabundant ghrelin arouses appetite and promotes food consumption. Here, with methods that permit precise measures of food intake, we pursued the concept that a loss of BChE and a rise in ghrelin are strong drivers of food intake and weight gain. In addition we examined a wide range of metabolic phenomena relevant to fat deposition in the KO mice.
Materials and Methods
Animal subjects and ethics
Adult male WT and BChE KO mice in the same background C57BL/6 were obtained from The Jackson Laboratory under protocol A41212 approved by Mayo Clinic's Institutional Animal Care and Use Committee. These BChE KO mice were recovered from cryo-storage and bred as homozygotes at The Jackson Laboratory. Experiments were conducted in accord with the Guide for Care and Use of Laboratory Animals (29) in American Association for the Accreditation of Laboratory Animal Care accredited facility. Our high-fat diet contained 45% calories from fat, 20% from protein, and 35% from carbohydrate (Research Diet D12451). At first consumption was measured weekly in cages of 5 mice with wood shavings. For more accuracy, food intake was determined daily in singly housed mice with cage bedding replaced by iso-pads in order to account for every grain of residual food.
Viral gene transfer
cDNA encoding luciferase or mouse BChE was subcloned into an adeno-associated virus (AAV) backbone. The resulting transfer vectors were cotransfected into HEK293T cells with helper vectors pHELP (Applied Viromics) and pAAV 2/8 (University of Pennsylvania) as previously described (30). Viruses in cell lysates were isolated by ultracentrifugation and viral particles were quantitated by real-time PCR. Vector (200 μL, 9.9 × 1011 particles) was given via tail-vein in 4-week-old mice, followed by 200 μL of 0.9% sterile NaCl. BChE expression was monitored across the study as shown in Supplemental Figure 1.
Body composition and energy expenditure
Fat mass and lean mass were assessed every 2 months in an EchoMRI-100 body composition analyzer (Echo Medical System). Habitual physical activity, O2 consumption (VO2), and CO2 expiration (VCO2) were monitored over 24-hour periods (12-h light/12-h dark) using a Comprehensive Lab Animal Monitoring System equipped with an Oxymax Open Circuit Calorimeter System (Columbus Instruments). The resulting VO2 and VCO2 values were converted into respiratory exchange ratios (VCO2 to VO2).
Histology and determination of fat cell size
White and brown fat samples were taken from characteristic depots identified by location and color. Flash-frozen adipose tissues were fixed in 4% buffered paraformaldehyde and sections were stained with hematoxylin and eosin (H&E) or Oil Red O. To measure adipocyte cross-sectional area, 4 microscope fields in H&E-stained adipose tissue from 8 mice per group were digitally recorded. Images were analyzed using NIS-Elements imaging software (Nikon Instruments, Inc).
Tissue triglyceride
Liver lipids were analyzed as previously described (31) after homogenization in 20mM Tris-Cl buffer (pH 7.4) with 5mM NaF (Sigma-Aldrich), and cocktails of proteinase and phosphatase inhibitors (Roche). Lipids were extracted in 2:1 (vol/vol) chloroform/methanol, solubilized in tertbutanol:methanol:Triton (3:1:1, vol/vol/vol), and dried under nitrogen. Lipid content was determined using L-type triglyceride M reagent (Wako Chemicals USA, Inc).
Gene expression
Total tissue RNAs were extracted with TRIzol (Invitrogen) and 1-μg aliquots were processed with a high-capacity cDNA reverse transcription kit (Applied Biosystems) per the manufacturer's instructions. Real-time PCR was performed with a CFX384 detection system (Bio-Rad). Primer sequences are in Supplemental Table 1.
Plasma analysis
Blood (0.2–0.3 mL) was taken by cheek puncture with a mouse-bleeding lancet, and sterile gauze was applied to stop bleeding. Random-state measurements were made between 8 and 10 am, and fasting samples were obtained after 16 hours of food deprivation. Samples were collected in cooled EDTA-treated tubes with 0.1 vol of 1N HCl, protected by proteinase inhibitors (1mM p-hydroxymercuribenzoic acid; Sigma-Aldrich and 1.5μM aprotinin; Roche), centrifuged 10 minutes at 8000g, and stored at −80°C. ELISA kits were used to measure levels of insulin (Alipco) and insulin-like growth factor 1 (IGF-1) (R&D Systems, Inc), as well as ghrelin and desacyl-ghrelin (Cayman Chemical). Total, low, and very low density cholesterol (LDL and VLDL, respectively) were assayed by a Sigma kit. Blood glucose concentrations were measured by glucometer (Bayer HealthCare). BChE activity was determined by Ellman assay as described previously (23).
Data analysis
Body weight and daily food intake data were analyzed by two-factor mixed ANOVA followed by Holm-Sidak multiple comparison tests. Two-group comparisons were conducted with a 2-tailed t test, and P < .05 was considered significant.
Results
BChE-deficient mice become obese on high-fat diet
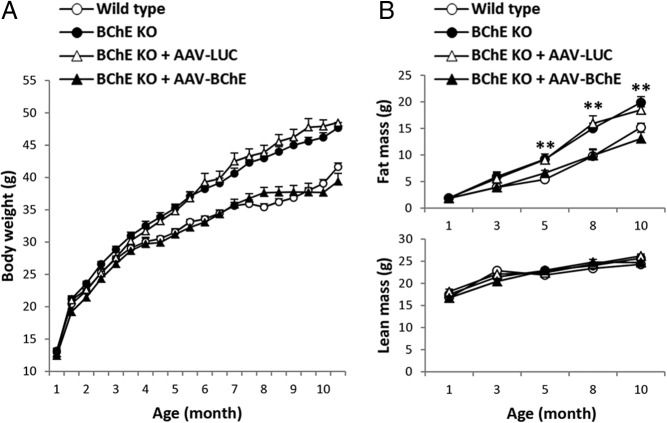
Mouse body weights were similar in all groups at baseline, but KO mice on high-fat diet slowly became heavier than their WT counterparts. Significant group differences emerged after 4 months of feeding and they grew further as time passed (Figure 1A). At final stages, whole-body fat mass was 35% higher in the KO mice than in WT (Figure 1B). The excess weight in KO mice was due to increased adipose tissue mass (Figure 1B, upper panel). Lean tissue gain was not different between groups (Figure 1B, lower panel). These dietary obesogenic effects were blunted in KO mice given viral transfer of the BChE gene at 4 weeks of age (Figure 1, A and B). Consistent with the weight gain difference, circulating leptin, total cholesterol, LDL/VLDL, insulin, and IGF-1 were respectively 62%, 88%, 62%, 80%, and 21% higher in BChE KO mice than in controls, with no change in blood glucose (Table 1). BChE vector in the KO mice led to stably high enzyme levels in plasma (>30 U/mL) (Supplemental Figure 1) as compared with approximately 1 U/mL in untreated WT animals. This treatment also normalized all the altered serum parameters, except for insulin and IGF-1 (Table 1).
Figure 1.
Effect of high-fat diet on body weight, fat mass, and lean mass in untreated wild-type and BChE-KO mice, as well as KO mice given AAV-luciferase or BChE vector. A, Body weights of male control (n = 27), BChE KO (n = 28), and BChE KO mice transduced with AAV encoding luciferase (LUC) (n = 10) or mouse BChE (n = 9). All mice were fed on 45% calorie high-fat diet. Two-way ANOVA showed significant differences in body weight: BChE KO VS wild type (P < .001), BChE KO VS LUC-vector-treated KO (not significant, n.s.); BChE KO VS BChE-vector-treated KO (P < .001), BChE-vector-treated KO VS wild type (n.s.). B, Fat and lean mass measured by Eco-MRI every 2 months. Results are mean ± SEM; **, BChE KO and LUC-vector treated-BChE KO vs wild-type group, P < .01. There were no significant group differences in lean mass.
Table 1.
Serum Parameters in Wild-Type, BChE KO, and AAV-BChE-treated BChE KO Mice
| Wild Type | BChE KO | BChE KO + AAV-BChE | |
|---|---|---|---|
| BChE (U/mL) | 1.16 ± 0.04 | 0c | 38.36 ± 5.0c |
| Leptin (ng/mL) | 45 ± 5 | 73 ± 8a | 45 ± 9 |
| Total cholesterol (mg/mL) | 0.78 ± 0.24 | 1.47 ± 0.16a | 0.66 ± 0.19 |
| LDL/VLDL (mg/mL) | 0.13 ± 0.01 | 0.21 ± 0.02c | 0.19 ± 0.03 |
| Fast insulin (ng/mL) | 0.54 ± 0.04 | 0.97 ± 0.15b | 0.88 ± 0.38a |
| Fast IGF-1 (ng/mL) | 541 ± 34 | 656 ± 34a | 639 ± 25a |
| Fast ghrelin (pg/mL) | 372 ± 38 | 222 ± 25b | 327 ± 71 |
| Fast desacyl-ghrelin (pg/mL) | 713 ± 94 | 349 ± 37b | 870 ± 186 |
| Blood glucose (mg/dL) | 167 ± 7 | 165 ± 10 | 174 ± 11 |
| Fast blood glucose (mg/dL) | 87 ± 11 | 91 ± 6 | 86 ± 10 |
Circulating concentrations were measured in 10-month-old mice after 9 months of high-fat feeding (9–10 animals per group). Results are mean ± SEM. Statistical significance was assessed by 2-tailed Student's t tests.
P < .05 vs wild-type control.
P < .01 vs wild-type control.
P < .001 vs wild-type control.
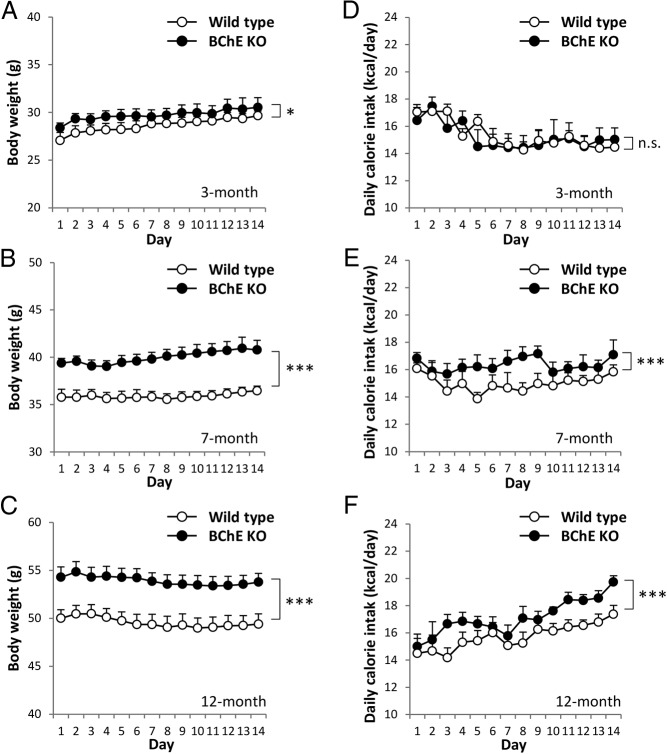
Initially, we saw no apparent difference in food intake with or without vector treatment, but food powder lost into bedding was not accounted for. More rigorous measures of food intake were then made on a daily basis with 2 cohorts of single-housed mice. One group was young (3 mo old) and had fed only on regular chow. Their average weight at this point was slightly higher than WT but not different by t test. However, a significant weight divergence emerged over the next 2 weeks on high-fat diet, P < .05 by two-way ANOVA (Figure 2A). This divergence grew further during 4 more months of HFD (Figure 2B), and it was sustained or enhanced at 9 months, the study endpoint (Figure 2C). Energy intake in the younger KO mice did not differ measurably from that in same-age wild-type controls (Figure 2D), but the older, obese KO mice (7- and 12-mo-old mice) consumed significantly more food than the age-matched controls (Figure 2, E and F). Although this excess was modest, the cumulative energy intake eventually surpassed the control group.
Figure 2.
Daily calorie intake in single-housed mice. Body weights (A–C) and daily calorie intake (D–F) of 3-, 7-, and 12-month-old mice were measured every day for 2 weeks. Data are mean ± SEM (n = 8). n.s., not significant; *, P < .05; ***, P < .001 vs wild-type control.
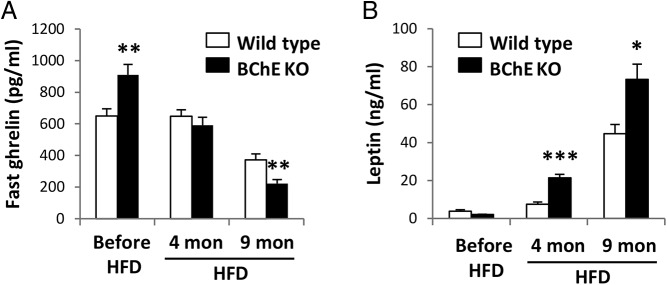
Plasma ghrelin decreased in all groups as body weights rose. In young mice lacking BChE, it was 40% greater than control, but after months of high-fat feeding, ghrelin levels dropped 40% below the lean WT (Figure 3A) or vector-treated KO mice (Table 1). Meanwhile, leptin levels rose in parallel with body fat (Figure 3B). The evidence that body weight/diet was affecting plasma ghrelin levels led us to examine the impact of BChE loss on energy expenditure.
Figure 3.
Plasma ghrelin and leptin levels. Fast plasma ghrelin (A) and random-fed leptin (B) levels determined at the experimental outset (before high-fat diet [HFD] feeding), and 4 or 9 months after HFD feeding. Results are mean ± SEM (n = 9–10); *, P < .05; **, P < .01; ***, P < .001 vs wild-type control.
Decreased energy expenditure in BChE KO mice
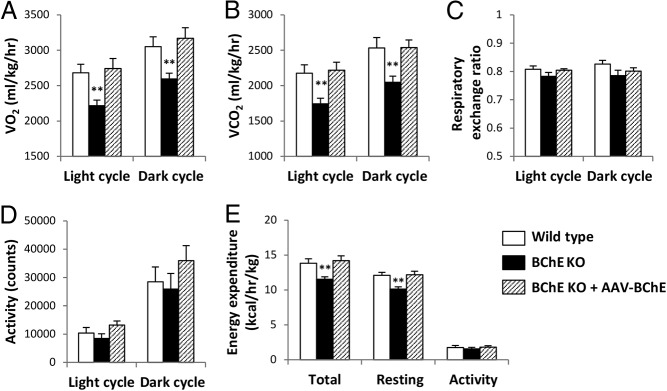
Because increases in body weight imply an excess of energy intake over energy expenditure, we examined the possibility that decreased energy expenditure contributed to excess fat gain in KO mice. At age 2.5 months after HFD for 1.5 months (before body weight differences arose), the KO mice showed slightly lower energy expenditure than controls, but the effect was not significant (Supplemental Figure 2). When the mice reached 7 months of age, after 6 months of HFD feeding, the body weight difference became obvious. At this point, during the light cycle, the KO mice consumed 17% less O2 per kilogram of total body weight than control mice, and 15% less than controls during the light cycle and 15% less during the dark cycle (P < .01). Likewise, O2 consumption in vector-treated KO mice was similar to WT controls (Figure 4A). Again, CO2 production was 19% lower in KO mice during both light and dark cycles but was normal in those given BChE vector (Figure 4B). There were no group differences in respiratory exchange ratio or spontaneous activity (Figure 4, C and D). Thus, we ascribed the altered energy expenditure to calories involved in maintaining body systems and normal temperature at the resting state (Figure 4E). However, it is worth noting that all mice had undergone long-term HFD feeding and their excess body weight reflected an expansion of adipose tissue, which has much lower metabolic activity than lean tissues. To account for such differences in body composition, we adjusted the data to lean body mass. After this normalization the differences in VO2,VCO2, and energy expenditure disappeared (Supplemental Figure 3). As the proper way to normalize energy expenditure remains a debated issue, we hesitate to draw strong conclusions from the above data. Nevertheless, reduced overall energy expenditure seemed the most likely cause of increased fat accumulation in the KO mice.
Figure 4.
Energy expenditure and spontaneous activity across light and dark cycles. VO2 (A) and CO2 production (B), respiratory exchange ratio (C), spontaneous locomotor activity (D), and energy expenditure (E) were measured in Comprehensive Lab Animal Monitoring System metabolic chambers. All mice were 7 months old and had been on HFD for 6 months. Data are mean ± SEM (n = 8); **, P < .01 vs wild-type control.
BChE KO mice have more white and brown adipose tissue
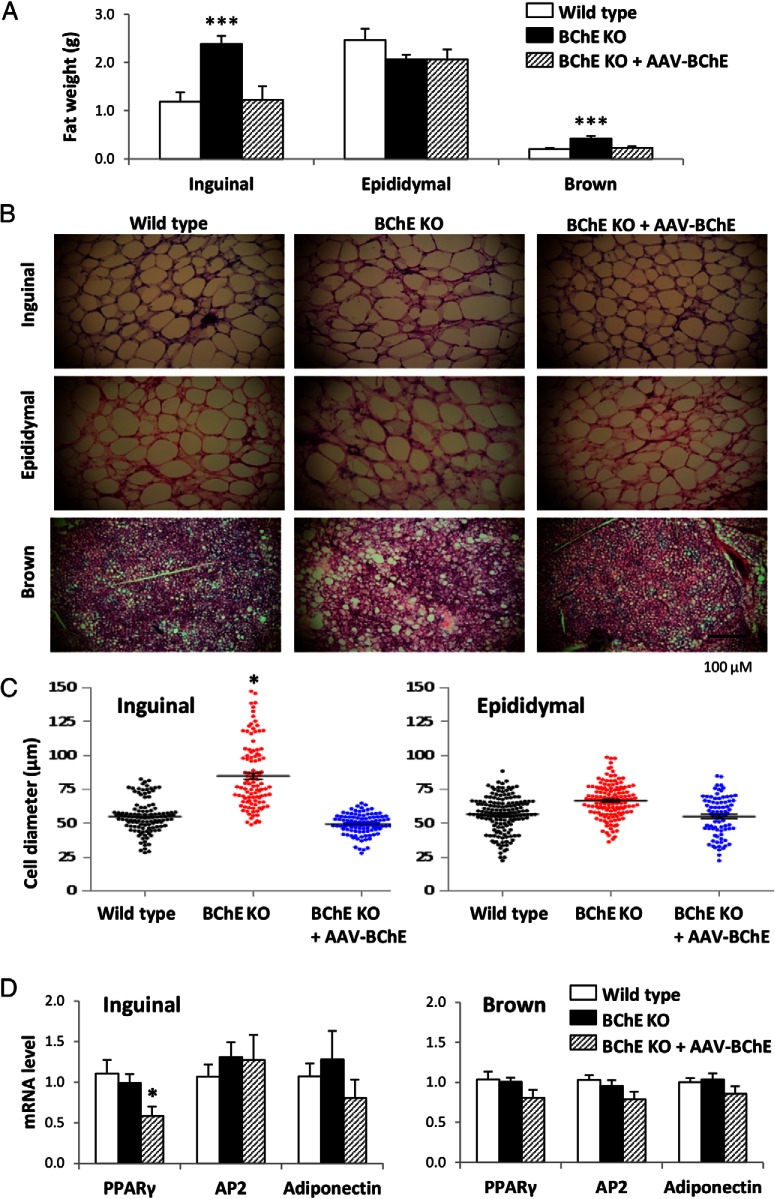
Terminal issue harvests revealed that inguinal fat pads and interscapular brown fat in untreated KO mice were heavier than in controls but not in KO mice given BChE vector (Figure 5A). Thus, at 10 months, inguinal fat weighed 119 and 123 mg in WT and vector-treated BChE KO mice vs 239 mg in untreated KO mice (P < .001). Brown fat weights followed the same pattern: 210 mg in WT mice, 230 mg in BChE-vector-treated KO mice, and 430 mg in untreated KO mice (P < .001). There were no differences in epididymal fat pad weights among the 3 groups. Tissue weights in the luciferase vector controls were similar to the untreated group (Supplemental Figure 4), suggesting that AAV by itself had no effect on diet-induced body weight gain.
Figure 5.
Adipose tissue weight, adipocyte cell size, and adiposity-related gene expression. A, Inguinal sc, epididymal, and brown adipose tissue depot weights in 10-month-old controls, BChE KO, and vector-rescued BChE KO mice on HFD. B, H&E-stained sections of adipose tissues. Scale bar, 100 μm. C, Cell diameters of isolated inguinal and epididymal fat pads. D, mRNA levels of adipogenesis markers (PPARγ, adipocyte protein 2 [AP2], and adiponectin). Results are mean ± SEM (n = 9–10); *, P < .05; ***, P < .001, relative to wild-type control.
White adipose and brown fat tissues were sectioned and stained with H&E for microscopic evaluation, which revealed a substantial increase in the size of inguinal but not epididymal adipocytes in BChE KO mice vs WT and vector-treated BChE KO (Figure 5B). Average inguinal fat cell diameters in KO mice were 50% greater than in WT animals (Figure 5C). If cells were perfectly spherical this would translate to a 2.5-fold increase in cell volume, roughly mirroring the relative fat pad weights. Brown fat tissue in KO mice also contained more lipid than in the other 2 groups (Figure 5B). Nonetheless, transcript levels of adipogenesis markers, including peroxisome proliferator-activated receptor (PPAR)γ, adipocyte protein 2, and adiponectin, were not altered in inguinal or brown fat (Figure 5D). We conclude that the fat excess was not due to enhanced adipogenesis (ie, proliferation of fat cells). Instead it reflected increased fat cell volume and lipid content.
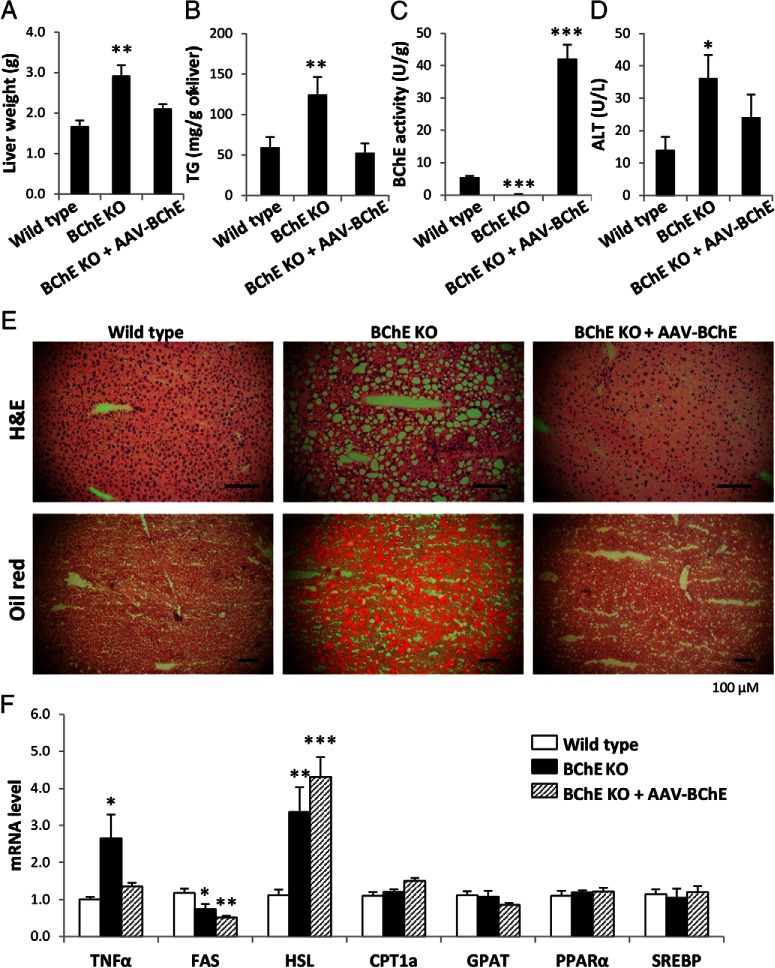
BChE KO mice develop fatty liver after long-term high-fat feeding
Clinical obesity is often associated with hepatic steatosis. Livers from BChE KO mice were 70% heavier and contained twice as much lipid as controls (Figure 6, A and B). The vector-treated KO mice had 8 times more BChE activity in liver than the WT controls (Figure 6C), and their liver weights and triglyceride levels were distinctly lower (Figure 6, A and B). Plasma from untreated KO mice showed substantially higher levels of serum alanine aminotransferase, a marker of hepatic damage (Figure 6D). In addition, H&E and Oil Red O staining of KO liver sections revealed abundant lipid droplets (micro- or macrovesicular fat), which were rare or absent in WT and vector-treated KO mice (Figure 6E). These observations were consistent with the approximately 3-fold increase in TNFα mRNA in KO livers, suggesting a persistent, low-level hepatic inflammation (Figure 6F). Hence, it is clear that BChE directly or indirectly affects fat metabolism in mice experiencing a high-fat challenge.
Figure 6.
Liver weight, histology, and fat metabolism-related gene expression. Liver weight (A), hepatic triglyceride content (B), hepatic BChE activity (C), plasma alanine aminotransferase (ALT) level (D), H&E and Oil Red O staining of liver sections (E), and mRNA levels of TNFα and hepatic enzymes and transcription factors involved in fatty acid synthesis (fatty acid synthase [FAS], sterol regulatory element binding protein [SREBP]), lipolysis (hormone-sensitive lipase [HSL]), oxidation (PPARα, carnitine palmitoyl-transferase 1a [CPT1a]), and TG synthesis (glycerol-3-phosphate acyltransferase [GPAT]) (F). Data expressed as mean ± SEM (n = 9–10); *, P < .05; **, P < .01; ***, P < .001.
Hepatic lipid content reflects a complex balance between fatty acid uptake, de novo lipogenesis, export as VLDL-triglyceride, and lipid oxidation. Real-time PCR data showed reduced expression of mRNA for fatty acid synthase and increased mRNA for hormone-sensitive lipase in KO mice (Figure 6F). These effects were not rescued by BChE vector treatment. There were no differences in mRNAs for enzymes that regulate fatty acid entry into the mitochondria (carnitine palmitoyl-transferase 1a) or fatty acid esterification (glycerol-3-phosphate acyltransferase). There was also no difference in expression of the transcription factors, proliferator-activated receptor-α and sterol regulatory element binding protein, which regulate lipid metabolism in liver. We conclude that the abnormal liver fat in BChE KO mice is not due to gene regulation in liver cells but to lipid relocation from adipose tissues elsewhere in the body.
Discussion
Several human studies have made connections between altered BChE activity and the incidence of obesity, perturbed lipid metabolism, type 2 diabetes, and cardiovascular risks (3, 4, 7), but none were able to determine whether abnormally high or low BChE levels were a cause or consequence. We have now taken advantage of available BChE KO mice to explore multiple aspects of this enzyme's role in genesis of obesity. As noted, Lockridge's group reported in 2008 that BChE−/− S129 mice gained excess weight on a rich diet (29% calories from fat) that did not lead to obesity in mice with normal BChE levels (27). We now confirm that key finding in another strain, C57BL/6, and we show for the first time that BChE gene transfer in enzyme-deleted mice restores resistance to obesity. Interestingly, our vector-treated KO mice carried much higher BChE activities in plasma and liver, but they were not leaner or smaller than the WT controls. We also found that mouse ghrelin levels depend at least partly on the degree of BChE expression, being somewhat higher when the enzyme is absent and substantially lower when it is greatly overexpressed. The higher fat burden in BChE KO mice clearly reflects excess of energy intake over outgo. Lower energy expenditure on basic body functions is a likely additional factor. Although fat is metabolically less active than lean tissue, it accounted for a large share of body mass in our high-fat-fed mice, making a substantial contribution to the total metabolic rate. In any event the large mass of sc adipose tissue and the accumulation of fat droplets in the KO mouse liver are striking demonstrations of BChE's importance in modulating weight gain and lipid processing.
Our previous work has shown that BChE regulates ghrelin by hydrolyzing the peptide's octanoyl group required for its main biological actions, as loss of this enzyme in mice results in a 50% rise of plasma ghrelin (23). It is logical that ghrelin elevation in BChE KO mice should drive excessive food intake. However, many factors influence ghrelin's impact, one being diet-induced weight change (32). We suggest that the slow decline in plasma ghrelin as KO mice grew heavier was an adaptation to the positive energy balance associated with obesity. This is consistent with the decreased circulating ghrelin levels in human obesity (33).
A plausible interpretation is that ghrelin reduction enhances the sensitivity or abundance of its main target, the GH secretagogue receptor 1a. Continued excess of food consumption in obese mice is consistent with human studies showing that low-dose ghrelin infusion has little effect in lean individuals but increases energy intake in obese subjects (34). Animal research confirms that ghrelin is a hunger-stimulating hormone, but ghrelin-induced overeating typically requires nonphysiological doses (35, 36) and neither ghrelin KO nor ghrelin receptor KO cause decreased food intake and body weight on normal diets (37, 38). However, in contrast with WT animals, such mice do gain less body weight and fat mass on HFD (39, 40). Furthermore, ghrelin is an important physiological regulator of blood glucose levels during periods of caloric restriction. It serves this role by driving GH secretion (41). Thus, the intertwined roles of BChE and ghrelin in obesity progression are far more complex than we originally envisioned.
Body weight is regulated by complex mechanisms involving cooperation between peripheral and central systems. Leptin and ghrelin are 2 hormones that strongly impact energy balance (42). Both originate primarily in the periphery and signal to the central nervous system (CNS) through different pathways leading primarily to the hypothalamus (35, 43, 44). Leptin mediates long-term energy balance, slowly suppressing food intake, and preventing weight gain (45). By contrast, ghrelin is a fasting-acting hormone that arouses appetite and promotes fat storage (46). In a seeming paradox, the circulating anorectic hormone leptin rises in obese people (47), whereas orexigenic ghrelin falls (33). Our obese mice maintained constitutively high leptin concentrations, probably secreted from fat cells.
As a rule, unburned energy is conserved as fat. Nutrient overload induces both sc and visceral fat enlargement, but as obesity develops, visceral fat mass can increase disproportionally (48), and health risks grow. Visceral fat portends greater risk for diabetes, cardiovascular disease, hypertension, and certain cancers (48–50). Subcutaneous fat is not linked to most of the classic obesity-related pathologies, and some evidence suggests it may even be protective (51). In our study, after high-fat feeding for 9 months, the major contributor to excess body weight in KO mice was sc inguinal fat rather than epididymal fat. In other words, it appears that intraabdominal adipose stores saturated quickly, whereas sc fat did not.
A dramatic new finding in high-fat-fed KO mice was the major lipid accumulation in liver. Liver is a surrogate reservoir for fat when adipose tissue nears saturation (52). A buildup of fatty acids in the liver promotes triglyceride synthesis and VLDL production (53). Discordance between triglyceride synthesis and VLDL secretion may lead to cytotoxicity and dysfunction from accumulated intrahepatic triglycerides. Our data show that when fat calories were superabundant, BChE deficiency led to an overload of liver fat and triggered gene expression of inflammatory cytokines. Hence, we posit a key role for BChE in lipid regulation, either directly or through synergistic actions with cholesterol esterase.
In humans, activation of the ghrelin/GH endocrine axis promotes the synthesis and secretion of IGF-1 (21), whereas plasma ghrelin levels are negatively regulated by circulating IGF-1 (54, 55). Here, we saw a negative correlation between plasma ghrelin and IGF-1 in older, fat BChE KO mice, supporting the idea of a negative feedback loop between ghrelin and the GH/IGF-1 system. In line with previous reports (56), the elevated circulating IGF-1 was associated with chronic hyperinsulinemia in BChE KO mice, which involved increased abundance of GH receptors in liver, leading to elevated production of IGF-1.
Despite a full rescue of body weight and other variables in KO mice given BChE vector, plasma insulin and IGF-1 in these animals remained higher than in WT littermates. A likely cause of the persisting elevation despite healthy body weight was lack of BChE in KO brains. Systemic injection of BChE vector drives enzyme transduction in liver and release into plasma, generating supernormal levels in all tissues outside the CNS, with no sign of toxicity (57). However, this transduced BChE cannot cross the blood-brain barrier and remains absent in the CNS of KO mice. Because the systemic gene transfer did normalize weight gain and other functions, we attribute the persistent physiological abnormality to the continued BChE deficit in brain and spinal cord. Therefore the continued hyperinsulinemia points to unexpected functions for BChE in a CNS locus unreached by gene transfer vector. Acetylcholinesterase evidently cannot serve these functions although its levels remain normal in BChE KO mice. Data to define BChE's role are still lacking but we propose it involves modulating ghrelin in the hypothalamus to regulate vagal stimulation of pancreatic insulin release (58).
Finally, we are well aware that the role of sex is a fundamental issue in the incidence and evolution of obesity, diabetes, and metabolic syndrome (59). Plasma BChE is reportedly higher in female rats than in males (60), and female rats generally have more body fat than males. Unfortunately, the present study was unable include female mice, but this is a high priority for future research on BChE interactions with sex hormones in regulating fat metabolism and storage. All in all, our findings strongly support a role for BChE in mitigating diet-induced weight gain by regulating energy expenditure, adipose tissue growth, and fat metabolism in liver. Efforts to clarify that role may lead to new therapeutic approaches for treating obesity and related disorders.
Acknowledgments
This work was supported by the Minnesota Partnership for Biotechnology and Medical Genomics Grant MNP13.05, the Mayo Foundation for Medical Research, and National Institute on Drug Addiction Grant DP1 DA31340–01.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AAV
- adeno-associated virus
- BChE
- butyrylcholinesterase
- CNS
- central nervous system
- H&E
- hematoxylin and eosin
- HFD
- high-fat diet
- KO
- knockout
- PPAR
- peroxisome proliferator-activated receptor
- VCO2
- CO2 expiration
- VO2
- O2 consumption
- WT
- wild-type.
References
- 1. Malik VS, Willett WC, Hu FB. Global obesity: trends, risk factors and policy implications. Nat Rev Endocrinol. 2013;9:13–27. [DOI] [PubMed] [Google Scholar]
- 2. Iwasaki T, Yoneda M, Nakajima A, Terauchi Y. Serum butyrylcholinesterase is strongly associated with adiposity, the serum lipid profile and insulin resistance. Intern Med. 2007;46:1633–1639. [DOI] [PubMed] [Google Scholar]
- 3. Randell EW, Mathews MS, Zhang H, Seraj JS, Sun G. Relationship between serum butyrylcholinesterase and the metabolic syndrome. Clin Biochem. 2005;38:799–805. [DOI] [PubMed] [Google Scholar]
- 4. Santarpia L, Grandone I, Contaldo F, Pasanisi F. Butyrylcholinesterase as a prognostic marker: a review of the literature. J Cachexia Sarcopenia Muscle. 2013;4:31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alcantara VM, Chautard-Freire-Maia EA, Scartezini M, Cerci MSJ, Braun-Prado K, Picheth G. Butyrylcholinesterase activity and risk factors for coronary artery disease. Scand J Clin Lab Inv. 2002;62:399–404. [DOI] [PubMed] [Google Scholar]
- 6. Lampón N, Hermida-Cadahia EF, Riveiro A, Tutor JC. Association between butyrylcholinesterase activity and low-grade systemic inflammation. Ann Hepatol. 2012;11:356–363. [PubMed] [Google Scholar]
- 7. Abbott CA, Mackness MI, Kumar S, et al. Relationship between serum butyrylcholinesterase activity, hypertriglyceridaemia and insulin sensitivity in diabetes mellitus. Clin Sci (Lond). 1993;85:77–81. [DOI] [PubMed] [Google Scholar]
- 8. Alcântara VM, Rodrigues LC, Oliveira LC, Chautard-Freire-Maia EA. Association of the CHE2 locus with body mass index and butyrylcholinesterase activity. Hum Biol. 2001;73:587–595. [DOI] [PubMed] [Google Scholar]
- 9. Chautard-Freire-Maia EA, Primo-Parmo SL, Picheth G, Lourenço MA, Vieira MM. The C5 isozyme of serum cholinesterase and adult weight. Hum Hered. 1991;41:330–339. [DOI] [PubMed] [Google Scholar]
- 10. Hashim Y, Shepherd D, Wiltshire S, et al. Butyrylcholinesterase K variant on chromosome 3 q is associated with type II diabetes in white Caucasian subjects. Diabetologia. 2001;44:2227–2230. [DOI] [PubMed] [Google Scholar]
- 11. Vaisi-Raygani A, Rahimi Z, Entezami H, et al. Butyrylcholinesterase K variants increase the risk of coronary artery disease in the population of western Iran. Scand J Clin Lab Invest. 2008;68:123–129. [DOI] [PubMed] [Google Scholar]
- 12. Lima JK, Leite N, Turek LV, et al. 1914G variant of BCHE gene associated with enzyme activity, obesity and triglyceride levels. Gene. 2013;532:24–26. [DOI] [PubMed] [Google Scholar]
- 13. Alcântara VM, Oliveira LC, Réa RR, Suplicy HL, Chautard-Freire-Maia EA. Butyrylcholinesterase and obesity in individuals with the CHE2 C5+ and CHE2 C5- phenotypes. Int J Obes Relat Metab Disord. 2003;27:1557–1564. [DOI] [PubMed] [Google Scholar]
- 14. Souza RL, Fadel-Picheth C, Allebrandt KV, Furtado L, Chautard-Freire-Maia EA. Possible influence of BCHE locus of butyrylcholinesterase on stature and body mass index. Am J Phys Anthropol. 2005;126:329–334. [DOI] [PubMed] [Google Scholar]
- 15. Vallianou NG, Evangelopoulos AA, Bountziouka V, et al. Association of butyrylcholinesterase with cardiometabolic risk factors among apparently healthy adults. J Cardiovasc Med (Hagerstown). 2014;15:377–383. [DOI] [PubMed] [Google Scholar]
- 16. Milano GE, Leite N, Chaves TJ, Souza RL, Alle LF. [Butyrylcholinesterase activity and cardiovascular risk factors in obese adolescents submitted to an exercise program]. Arq Bras Endocrinol Metabol. 2013;57:533–537. [DOI] [PubMed] [Google Scholar]
- 17. Benyamin B, Middelberg RP, Lind PA, et al. GWAS of butyrylcholinesterase activity identifies four novel loci, independent effects within BCHE and secondary associations with metabolic risk factors. Hum Mol Genet. 2011;20:4504–4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brimijoin S, Chen VP, Pang Y-P, Geng L, Gao Y. Physiological roles for butyrylcholinesterase: a BChE-ghrelin axis [published online ahead of print February 23, 2016]. Chem-Biol Interact. 10.1016/j.cbi.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. De Vriese C, Delporte C. Influence of ghrelin on food intake and energy homeostasis. Curr Opin Clin Nutr Metab Care. 2007;10:615–619. [DOI] [PubMed] [Google Scholar]
- 20. Gil-Campos M, Aguilera CM, Cañete R, Gil A. Ghrelin: a hormone regulating food intake and energy homeostasis. Br J Nutr. 2006;96:201–226. [DOI] [PubMed] [Google Scholar]
- 21. Kojima M, Kangawa K. Ghrelin: structure and function. Physiol Rev. 2005;85:495–522. [DOI] [PubMed] [Google Scholar]
- 22. De Vriese C, Gregoire F, Lema-Kisoka R, Waelbroeck M, Robberecht P, Delporte C. Ghrelin degradation by serum and tissue homogenates: identification of the cleavage sites. Endocrinology. 2004;145:4997–5005. [DOI] [PubMed] [Google Scholar]
- 23. Chen VP, Gao Y, Geng L, Parks RJ, Pang YP, Brimijoin S. Plasma butyrylcholinesterase regulates ghrelin to control aggression. Proc Natl Acad Sci USA. 2015;112:2251–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schopfer LM, Lockridge O, Brimijoin S. Pure human butyrylcholinesterase hydrolyzes octanoyl ghrelin to desacyl ghrelin. Gen Comp Endocrinol. 2015;224:61–68. [DOI] [PubMed] [Google Scholar]
- 25. Chen VP, Gao Y, Geng L, Brimijoin S. Radiometric assay of ghrelin hydrolase activity and 3H-ghrelin distribution into mouse tissues. Biochem Pharmacol. 2015;98:732–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dantas VG, Furtado-Alle L, Souza RL, Chautard-Freire-Maia EA. Obesity and variants of the GHRL (ghrelin) and BCHE (butyrylcholinesterase) genes. Genet Mol Biol. 2011;34:205–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li B, Duysen EG, Lockridge O. The butyrylcholinesterase knockout mouse is obese on a high-fat diet. Chem Biol Interact. 2008;175:88–91. [DOI] [PubMed] [Google Scholar]
- 28. Zheng X, Deng J, Zhang T, Yao J, Zheng F, Zhan C-G. Potential anti-obesity effects of a long-acting cocaine hydrolase [published online ahead of print May 6, 2016]. Chem-Biol Interact. 10.1016/j.cbi.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. National Research Council. National Research Council 2011 Guide for the Care and Use of Laboratory Animals. 8th ed Washington, DC: National Academies Press; 2011. [Google Scholar]
- 30. Geng L, Gao Y, Chen X, et al. Gene transfer of mutant mouse cholinesterase provides high lifetime expression and reduced cocaine responses with no evident toxicity. PLoS One. 2013;8:e67446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stout MB, Tchkonia T, Pirtskhalava T, et al. Growth hormone action predicts age-related white adipose tissue dysfunction and senescent cell burden in mice. Aging (Albany NY). 2014;6:575–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cummings DE, Weigle DS, Frayo RS, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346:1623–1630. [DOI] [PubMed] [Google Scholar]
- 33. Tschöp M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML. Circulating ghrelin levels are decreased in human obesity. Diabetes. 2001;50:707–709. [DOI] [PubMed] [Google Scholar]
- 34. Druce MR, Wren AM, Park AJ, et al. Ghrelin increases food intake in obese as well as lean subjects. Int J Obes Relat Metab Disord. 2005;29:1130–1136. [DOI] [PubMed] [Google Scholar]
- 35. Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. [DOI] [PubMed] [Google Scholar]
- 36. Asakawa A, Inui A, Kaga T, et al. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology. 2001;120:337–345. [DOI] [PubMed] [Google Scholar]
- 37. Sun Y, Ahmed S, Smith RG. Deletion of ghrelin impairs neither growth nor appetite. Mol Cell Biol. 2003;23:7973–7981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sun Y, Wang P, Zheng H, Smith RG. Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proc Natl Acad Sci USA. 2004;101:4679–4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wortley KE, del Rincon JP, Murray JD, et al. Absence of ghrelin protects against early-onset obesity. J Clin Invest. 2005;115:3573–3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zigman JM, Nakano Y, Coppari R, et al. Mice lacking ghrelin receptors resist the development of diet-induced obesity. J Clin Invest. 2005;115:3564–3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhao TJ, Liang G, Li RL, et al. Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proc Natl Acad Sci USA. 2010;107:7467–7472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Klok MD, Jakobsdottir S, Drent ML. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review. Obes Rev. 2007;8:21–34. [DOI] [PubMed] [Google Scholar]
- 43. Sahu A. Leptin signaling in the hypothalamus: emphasis on energy homeostasis and leptin resistance. Front Neuroendocrinol. 2003;24:225–253. [DOI] [PubMed] [Google Scholar]
- 44. Kageyama H, Takenoya F, Shiba K, Shioda S. Neuronal circuits involving ghrelin in the hypothalamus-mediated regulation of feeding. Neuropeptides. 2010;44:133–138. [DOI] [PubMed] [Google Scholar]
- 45. Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. [DOI] [PubMed] [Google Scholar]
- 46. Tschöp M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. [DOI] [PubMed] [Google Scholar]
- 47. Lönnqvist F, Arner P, Nordfors L, Schalling M. Overexpression of the obese (ob) gene in adipose tissue of human obese subjects. Nat Med. 1995;1:950–953. [DOI] [PubMed] [Google Scholar]
- 48. Bergman RN, Kim SP, Catalano KJ, et al. Why visceral fat is bad: mechanisms of the metabolic syndrome. Obesity. 2006;14:16S–19S. [DOI] [PubMed] [Google Scholar]
- 49. Després JP. Body fat distribution and risk of cardiovascular disease: an update. Circulation. 2012;126:1301–1313. [DOI] [PubMed] [Google Scholar]
- 50. Stout M, Tchkonia T, Kirkland J. The aging adipose organ: lipid redistribution, inflammation, and cellular senescence. In: Fantuzzi G, Braunschweig C, eds. Adipose Tissue and Adipokines in Health and Disease. New York, NY: Humana Press; 2014:69–80. [Google Scholar]
- 51. Porter SA, Massaro JM, Hoffmann U, Vasan RS, O'Donnel CJ, Fox CS. Abdominal subcutaneous adipose tissue: a protective fat depot? Diabetes Care. 2009;32:1068–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Evans RM, Barish GD, Wang YX. PPARs and the complex journey to obesity. Nat Med. 2004;10:355–361. [DOI] [PubMed] [Google Scholar]
- 53. Fernandez ML, West KL. Mechanisms by which dietary fatty acids modulate plasma lipids1. J Nutr. 2005;135:2075–2078. [DOI] [PubMed] [Google Scholar]
- 54. Jarkovská Z, Rosická M, Marek J, et al. Plasma levels of total and active ghrelin in acromegaly and growth hormone deficiency. Physiol Res. 2006;55:175–181. [DOI] [PubMed] [Google Scholar]
- 55. Camurdan MO, Bideci A, Demirel F, Cinaz P. Serum ghrelin, IGF-I and IGFBP-3 levels in children with normal variant short stature. Endocr J. 2006;53:479–484. [DOI] [PubMed] [Google Scholar]
- 56. Cohen DH, LeRoith D. Obesity, type 2 diabetes, and cancer: the insulin and IGF connection. Endocr Relat Cancer. 2012;19:F27–F45. [DOI] [PubMed] [Google Scholar]
- 57. Murthy V, Gao Y, Geng L, LeBrasseur N, White T, Brimijoin S. Preclinical studies on neurobehavioral and neuromuscular effects of cocaine hydrolase gene therapy in mice. J Mol Neurosci. 2014;53:409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ferrini F, Salio C, Lossi L, Merighi A. Ghrelin in central neurons. Curr Neuropharmacol. 2009;7:37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mauvais-Jarvis F. Sex differences in metabolic homeostasis, diabetes, and obesity. Biol Sex Differ. 2015;6:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Edwards JA, Brimijoin S. Effects of hypophysectomy on acetylcholinesterase and butyrylcholinesterase in the rat. Biochem Pharmacol. 1983;32:1183–1189. [DOI] [PubMed] [Google Scholar]