Abstract
Vitamin C (ascorbic acid, AA) is indispensable for normal metabolism of all mammalian cells including pancreatic acinar cells (PACs). PACs obtain AA from their surroundings via transport across the cell membrane. Chronic alcohol exposure negatively affects body AA homeostasis; it also inhibits uptake of other micronutrients into PACs, but its effect on AA uptake is not clear. We examined this issue using both in vitro (266-6 cells) and in vivo (mice) models of chronic alcohol exposure. First, we determined the relative expression of the AA transporters 1 and 2 [i.e., sodium-dependent vitamin C transporter-1 (SVCT-1) and SVCT-2] in mouse and human PACs and found SVCT-2 to be the predominant transporter. Chronic exposure of 266-6 cells to alcohol significantly inhibited AA uptake and caused a marked reduction in SVCT-2 expression at the protein, mRNA, and heterogeneous nuclear RNA (hnRNA) levels. Similarly, chronic alcohol feeding of mice significantly inhibited AA uptake and caused a marked reduction in level of expression of the SVCT-2 protein, mRNA, and hnRNA. These findings suggest possible involvement of transcriptional mechanism(s) in mediating chronic alcohol effect on AA uptake by PACs. We also observed significant epigenetic changes (histone modifications) in the Slc23a2 gene (reduction in H3K4me3 level and an increase in H3K27me3 level) in the alcohol-exposed 266-6 cells. These findings show that chronic alcohol exposure inhibits PAC AA uptake and that the effect is mediated, in part, at the level of transcription of the Slc23a2 gene and may involve epigenetic mechanism(s).
Keywords: pancreas, acinar cells, vitamin C, uptake, epigenetics
ascorbic acid (AA), a member of the water-soluble family of vitamins, is essential for normal metabolism and function of all mammalian cells because of its role as a cofactor for several important enzymes and as an antioxidant (29). Systemic deficiency of the vitamin leads to a variety of clinical abnormalities that include scurvy, delayed wound healing, bone and connective tissue damage, and vasomotor instability (29). Optimization of body levels of vitamin C, on the other hand, has been reported to protect against gallbladder diseases, osteoporosis, cardiovascular disease, cancer, and cataract formation (7, 8, 33, 37). At the cellular level, deficiency of AA leads to an increase in oxidative stress (5). Therefore, studies that aim at improving our knowledge and understanding of the cellular and molecular mechanisms involved in maintaining and regulating vitamin C body and cellular homeostasis are of clear importance.
Human cells (including pancreatic acinar cells, PACs) cannot synthesize AA; rather they obtain the vitamin from their surroundings. Mice can synthesize AA endogenously, but their PACs still rely on the vitamin that exists in the circulation to meet their needs of this micronutrient, as shown by studies utilizing knockout mouse models (9, 38). In mammals (including human and mouse), two membrane transport systems have been identified for cellular uptake of AA, the sodium-dependent vitamin C transporter-1 and -2 (SVCT-1 and SVCT-2, products of the SLC23A1 and SLC23A2 genes, respectively) (7, 13, 31, 38, 47, 48). These membrane carriers transport l-ascorbic acid but not its oxidized form, i.e., dehydroascorbic acid. Also significant similarity exists between the human SVCT-1 and -2 and their mouse counterparts (36). The two AA transporters, however, show differential tissue distribution, with expression of SVCT-1 being confined to epithelia involved in bulk transport of the vitamin (like the intestine and the kidney), whereas expression of SVCT-2 is mainly in nonepithelial cell types (7).
Chronic alcohol use in humans is associated with lower plasma AA level (15, 17, 35). However, vitamin C appears to protect cells against alcohol-induced oxidative damage (27, 28). We have been interested in the effect of chronic alcohol exposure on the uptake of essential micronutrients by PACs (39, 40, 45), and in this study we examined its effect on physiological and molecular parameters of AA uptake. We utilized both an in vitro and an in vivo model of chronic alcohol exposure in our investigations. Our results showed that chronic exposure of PACs to alcohol leads to a significant inhibition in pancreatic acinar AA uptake. This inhibition is associated with a marked reduction in expression of the predominant AA transporter SVCT-2 in PACs at the protein, mRNA, and heterogeneous nuclear RNA (hnRNA) levels; it is also associated with a significant reduction in the level of the H3K4me3 (euchromatin) and an increase in the level of the H3K27me3 (heterochromatin) markers in the Slc23a2 predicted promoter region. These findings demonstrate that chronic alcohol exposure impairs AA uptake by PAC and that the effect is (in part) mediated via transcriptional mechanism(s) of the Slc23a2 gene and may involve epigenetic alterations.1
MATERIALS AND METHODS
Materials.
The mouse pancreatic acinar tumor cell line 266-6 was obtained from American Type Tissue Collection (ATCC, Rockville, MD). [14C]-AA (13 mCi/mmol, radiochemical purity <98%) was obtained from American Radiolabeled Chemicals (St. Louis, MO). Human pancreatic RNA was obtained from Origene (Rockville, MD). Human primary PAC cells used in this study were kindly provided by Dr. Balamurugan (Clinical Islet Cell Laboratory, Department of Surgery, University of Louisville, Louisville, Kentucky) (43). Anti-SVCT-2 and anti-GAPDH polyclonal primary antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-rabbit IRDye-800 and anti-rabbit IRDye-680 secondary antibodies were purchased from LI-COR Bioscience (Lincoln, NE). Oligonucleotide primers used in this study were synthesized by Sigma Genosys (Sigma, Woodland, TX).
Cell culture, alcohol exposure, and uptake studies.
The 266-6 cells were cultured in DMEM growth medium containing 10% fetal bovine serum and penicillin/streptomycin. Cells were used between passages 3–20. Ethanol (50 mM) [a concentration similar to the blood alcohol level of chronic alcoholics (14)] containing DMEM medium was added to the 266-6 cells, and cells were grown in an ethanol-saturated 5% CO2 incubator maintained at 37°C for 96 h with media change every 12 h to minimize change in ethanol concentration in medium (30, 45). Uptake (3 min, initial rate) was measured at 37°C in cells suspended in Krebs-Ringer (K-R) buffer [containing (in mM) 133.00 NaCl, 4.93 KCl, 1.23 MgSO4, 0.85 CaCl2, 5.00 glucose, 5.00 glutamine, 10.00 HEPES, and 10.00 MES; pH 7.4], and the reaction was terminated by the addition of ice-cold K-R buffer, then digested and neutralized with 1 N NaOH and 10 N HCl, respectively. The radioactive content was measured using a scintillation counter (Beckman Coulter LS 6500 multipurpose counter; Miami, Florida). The protein content was determined using a protein assay kit (Bio-Rad, Hercules, CA).
Ethanol feeding of mice and isolation of mouse primary PACs.
The mice were fed the Lieber-DeCarli ethanol liquid diet (Dyets, Bethlehem, PA) [ethanol provided 25% of total ingested calories (25)] for 4 wk as described before (45). Control mice were pair fed with the same liquid diet but without ethanol (maltose-dextrin isocalorically replaced ethanol). PACs were isolated from the pancreas of chronically alcohol-fed and their pair-fed control mice using a collagenase-type IV (Lakewood, NJ) digestion method as described before (39, 42, 45). Isolated PAC viability was tested by Trypan blue exclusion method, and this preparation was shown to be greater than 90% viability. These cells were processed for 14C-AA uptake and for protein, RNA, and hnRNA expression studies. The use of mice in this study was approved by the Institutional Animal Care Use Committee (IACUC) of the Long Beach VA Medical Center.
RT-qPCR analysis.
One microgram of total RNA was treated with DNase I (Invitrogen, Carlsbad, CA) followed by cDNA synthesis using iScript cDNA synthesis kit (Bio-Rad). To amplify the coding region of mSVCT-1, mSVCT-2, hSVCT-1, hSVCT-2, hβ-actin, and acidic ribosomal phosphoprotein, we used the gene-specific primers (Table 1). RT-qPCR conditions and quantification were performed as described before (26, 42, 45).
Table 1.
Combination of primers used to amplify coding region of the respective genes by RT-qPCR
| Gene Name | Forward and Reverse Primers (5′-3′) |
|---|---|
| Real-Time PCR | |
| hSVCT-1 | TCATCCTCTTCTCCCAGTACCT; AGAGCAGCCACACGGTCAT |
| hSVCT-2 | TCTTTGTGCTTGGATTTTCGAT; ACGTTCAACACTTGATCGATTC |
| hβ-actin | CATCCTGCGTCTGGACCT; TAATGTCACGCACGATTTCC |
| mSVCT-1 | GAGCAGGGACTTCCACCA; CCACACAGGTGAAGATGGTA |
| mSVCT-2 | AACGGCAGAGCTGTTGGA; GAAAATCGTCAGCATGGCAA |
| mARP0 | GCTGAACATCTCCCCCTTCTC; ATATCCTCATCTGATTCCTCC |
| hnRNA Primers | |
| mSVCT-1 | GCTTCCAGGCTCTAGATGGT; GGGCAAAATCTTCGTTGGGT |
| mSVCT-2 | ACTCTTGTCCATGGCTCTGG; GGGCAAAATCTTCGTTGGGT |
| mARP0 | GGCATCTTCAGTTGTTCC; TTAGACACAGCCCCCAC |
| ChIP-qPCR | |
| Slc23a2 | |
| Promoter | GGTGTGTGGGGAAGGGTCTG; CAGCCGCCTGCAAAATGG |
| Bisulfite PCR Primers | |
| Slc23a2 | |
| Promoter | AAATTAAGTTTGGTGTGTTTTTTTT; AAACCTCCTTTCTACCCTACAAAAC; GGTAGAAAGGAGGTTTTG; CTCTCACCTACCTACAACT |
Western blot analysis.
Cell lysates were isolated from chronically alcohol-exposed 266-6 cells and control cells as well as from mice chronically fed alcohol and their pair-fed control primary PACs. The protein (60 μg) samples were electrophoretically separated in NuPAGE 4–12% Bis-Tris gradient minigels (Invitrogen), then transferred onto Immobilon polyvinylidene difluoride membrane (Fisher Scientific). The anti-SVCT-2 and GAPDH (1:200 dilutions) antibodies were used as primary antibodies. The anti-rabbit IRDye-800 and anti-rabbit IRDye-680 (both at 1:30,000 dilutions) were sequentially used as secondary antibodies. The immunoreactive bands were detected, and their densities were quantified using the Odyssey infrared imaging system and associated software, respectively (LI-COR Bioscience).
hnRNA analysis.
One microgram of RNA was isolated from the PACs (chronic alcohol-exposed 266-6 cells and their control cells; chronic alcohol-fed and their pair-fed control mice PACs) and treated with DNase I (Invitrogen). The treated RNA was then reverse transcribed and amplified by PCR using gene-specific hnRNA primers (Table 1) (1, 16, 19).
Chromatin immunoprecipitation assay and qPCR.
Chromatin immunoprecipitation (ChIP) analysis was done using the Simple ChIP enzymatic chromatin IP kit (Cell Signaling Technology, Danvers, MA). Briefly, chronic alcohol-exposed (50 mM, 96 h) and control 266-6 cells (4 × 106 cells) were used for ChIP assay. Chromatin was cross linked with 1% formaldehyde (final concentration), and the reaction was terminated by adding glycine stop solution. Two milliliters of ice-cold PBS containing protease inhibitor cocktail (PIC) was added to the cells, which were then scraped and centrifuged at 1,500 revolution/min for 5 min and resuspended in 1 ml ice-cold buffer A containing DTT and PIC. Then the nuclei were prepared, and the chromatin was digested with micrococcal nuclease; the samples were sonicated to shear DNA into fragments followed by centrifugation at 10,000 revolution/min for 10 min, following which the chromatin was analyzed for proper digestion and concentration. Five micrograms of the digested chromatin was incubated overnight with 2 μg of the specific antibody [H3, H3K4me3, H3K27me3 and IgG (Millipore, Billerica, MA)]. Finally, the immunoprecipitated samples were subjected to DNA purification and analyzed by qPCR using Slc23a2 promoter-specific primers (Table 1) to amplify the region −202 to −88 relative to transcriptional start site (TSS) (relative to TSS as +1) using the PCR conditions as described before (44).
Bisulfite sequencing analysis.
Bisulfite sequencing was performed to determine the methylation status of the mouse Slc23a2 promoter as described before (24, 39, 42). DNA was isolated using Wizard Genomic DNA Purification Kit (Promega, Madison, WI) from 266-6 cells exposed to alcohol and control conditions. Bisulfite reactions were performed by following the manufacturer protocol using EpiTect Bisulfite Kit (Qiagen, Valencia, CA), and the bisulfite-treated DNA was amplified by PCR using Slc23a2 bisulfite primers (Table 1), which span the −163 to +31 (relative to TSS as +1) region of CpG islands in the Slc23a2 promoter. The CpG islands were identified, and Slc23a2 bisulfite primers were designed using Methprimer (23). The PCR was performed as described before (39), and the resulting amplified products were ligated into pGEM-T easy vector (Promega). A minimum of 8–10 clones from alcohol-exposed and control samples was sequenced to determine the methylation status of Slc23a2 promoter by commercial vendor (Laragen, Los Angeles, CA). After DNA sequencing, the sequence was analyzed using QUMA methylation analysis tool as described previously (21, 39).
Data presentation and statistical analysis.
14C-AA uptake data represent the result of at least three separate experiments and are expressed as means ± SE in fmol/mg protein per 3 min. Western blot, PCR, histone modification, and DNA methylation studies were performed on at least three or more separate occasions. Data were analyzed by the Student's t-test with statistical significance set at P < 0.05.
RESULTS
Relative expression of SVCT-1 and -2 in mice and human pancreas.
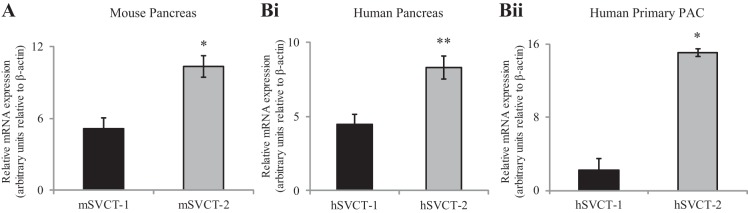
The relative expression of vitamin C transporters in PACs is not known and thus was determined in native mouse and human total pancreas as well as in isolated purified primary human PACs. Levels of expression of SVCT-1 and -2 mRNA were determined by RT-qPCR using total RNA. The results showed that both SVCT-1 and -2 are expressed in mouse and human pancreas as well as in human primary PACs, with expression of SVCT-2 being significantly higher than that of SVCT-1 (P < 0.01 for mouse pancreas; P < 0.02 for human pancreas; P < 0.01 for human primary PACs) (Fig. 1, A and B, i and ii, respectively).
Fig. 1.
Expression of sodium-dependent vitamin C transporter-1 (SVCT-1) and -2 mRNA in mouse and human pancreas. RT-qPCR was performed using mouse and human gene-specific primers and cDNA from mouse pancreas (A), human pancreas (B, i), and human primary pancreatic acinar cells (PACs) (B, ii). Data are means ± SE of at least 3 independent samples. *P < 0.01, **P < 0.02.
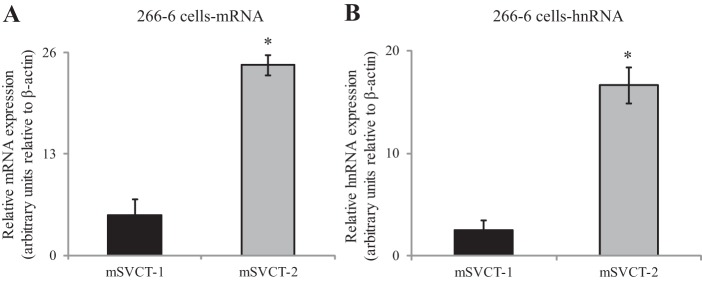
We also determined the relative expression of SVCT-1 and -2 mRNA in 266-6 cells, our in vitro model in these investigations. The results again showed that both transporters are expressed in these cells with expression of SVCT-2 being significantly (P < 0.01) higher than that of SVCT-1 (Fig. 2A). We further determined the level of expression of SVCT-1 and -2 hnRNA [hnRNA is the first product of gene transcription (1, 16, 19)] in 266-6 cells, with the results showing a significantly (P < 0.01) higher level of SVCT-2 hnRNA expression compared with SVCT-1 hnRNA in these cells (Fig. 2B).
Fig. 2.
Expression of SVCT-1 and -2 mRNA and heterogeneous nuclear RNA (hnRNA) in cultured mouse pancreatic acinar 266-6 cells. A: RT-qPCR was performed using mouse gene-specific primers to determine SVCT-1 and -2 mRNA expression levels. B: RT-qPCR was performed using mouse gene-specific primers to amplify SVCT-1 and -2 hnRNA in 266-6 cells. Data are means ± SE of at least 3 independent samples. *P < 0.01.
Collectively, the above findings clearly show that the SVCT-2 system is the predominant vitamin C transporter expressed in mouse and human PACs, and thus we focused on this transporter in our subsequent investigations.
Effect of in vitro and in vivo exposure of PACs to alcohol on physiological and molecular parameters of AA uptake process.
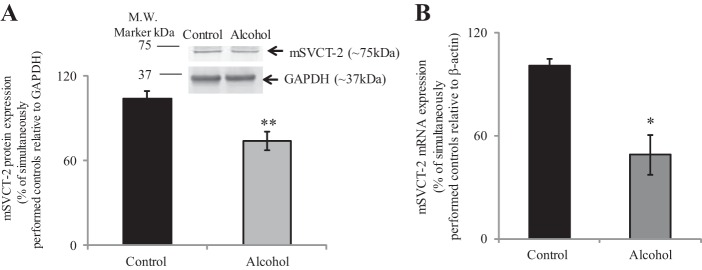
We investigated the effect of chronic exposure of the 266-6 cells to alcohol [50 mM, 96 h (14, 30, 45)] on carrier-mediated 14C-AA (32 μM) uptake. As mentioned earlier, although the mouse synthesize AA endogenously, PACs require extracellular AA, as shown in studies utilizing knockout mouse models for both vitamin C transporters (9, 38). Our results showed a significant (P < 0.01) inhibition in AA uptake by chronic alcohol-exposed cells compared with controls (Fig. 3). This inhibition was associated with a significant decrease in SVCT-2 protein (P < 0.02) (Fig. 4A) and mRNA (P < 0.01) (Fig. 4B) expression compared with controls.
Fig. 3.
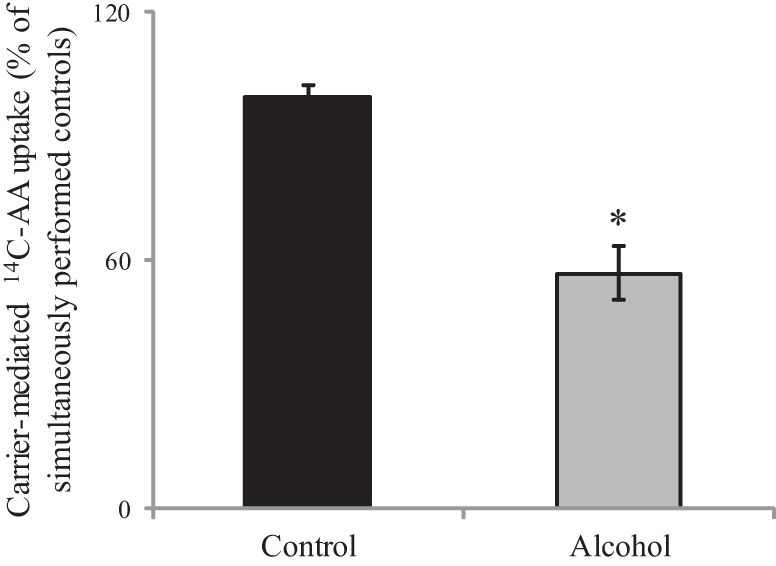
Effect of chronic alcohol exposure of pancreatic acinar 266-6 cells on 14C-ascorbic acid (AA) uptake. The 266-6 cells were exposed chronically to alcohol (50 mM, 96 h), and carrier-mediated 14C-AA uptake was performed. Data are means ± SE of at least 3 separate uptake determinations. *P < 0.01.
Fig. 4.
Effect of chronic alcohol exposure of pancreatic acinar 266-6 cells on level of expression of SVCT-2 protein and mRNA. 266-6 cells were exposed chronically to alcohol (50 mM, 96 h), and SVCT-2 protein (A) and mRNA (B) expression levels were determined on isolated protein and mRNA by Western blot and RT-qPCR analysis, respectively. Data are means ± SE of at least 3 separate determinations. **P < 0.02, *P < 0.01.
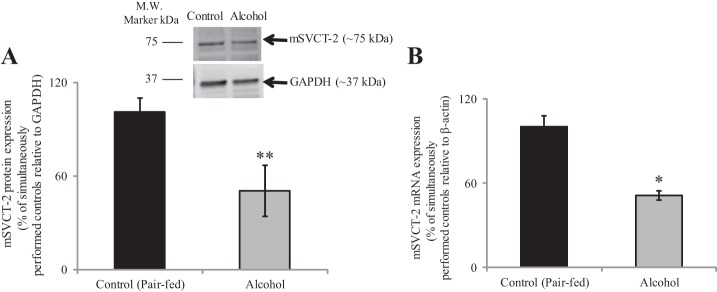
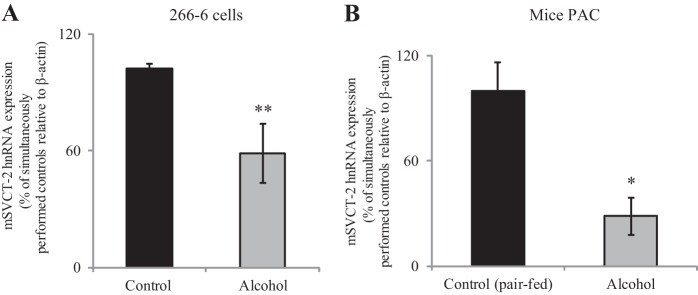
To confirm the above-mentioned findings on the effect of the in vitro model of chronic exposure of PACs to alcohol on molecular parameters of AA uptake process in an in vivo model, we investigated the effect of chronic alcohol feeding of mice on carrier-mediated 14C-AA (32 μM) uptake and molecular parameters of the predominant transporter (SVCT-2) by primary PACs. In these studies, mice were fed an ethanol-liquid diet, while their control mice were pair fed the same diet but without alcohol (see materials and methods). Our results show a significant (P < 0.02) inhibition in AA uptake in alcohol-fed mice compared with their pair-fed controls (Fig. 5). This inhibition was associated with a marked decrease in expression of SVCT-2 protein (P < 0.05) (Fig. 6A) and mRNA (P < 0.01) (Fig. 6B) in alcohol-fed mice compared with pair-fed controls.
Fig. 5.
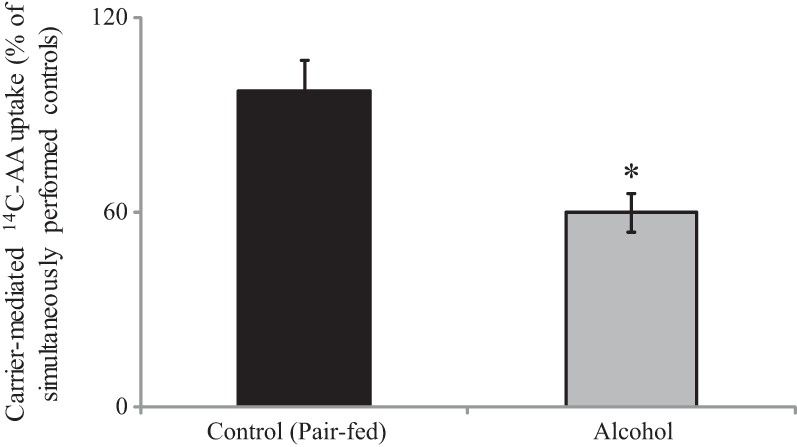
Effect of chronic alcohol feeding of mice on 14C-AA uptake by mice primary PACs. Primary PACs were isolated from the mice fed alcohol (25% total calories) for 4 wk and their pair-fed controls. The carrier-mediated 14C-AA uptake was performed as described before. Data are means ± SE of at least 3 independent experiments from 4 sets of mice. *P < 0.02.
Fig. 6.
Effect of chronic alcohol feeding of mice on the level of expression of SVCT-2 protein and mRNA in mice primary PACs. Primary PACs were isolated from the mice fed alcohol (25% of total calories) for 4 wk and their pair-fed controls. Protein (A) and mRNA (B) expression levels were determined by Western blot and RT-qPCR analysis, respectively, using isolated primary PACs. Data are means ± SE of at least 3 independent experiments from multiple sets of mice. *P < 0.01, **P < 0.05.
Molecular mechanism(s) involved in the inhibitory effect of chronic alcohol exposure on PAC AA uptake process.
The chronic exposure of 266-6 cells and mice to alcohol showed a significant inhibition in AA uptake, and this inhibition is associated with changes in SVCT-2 mRNA, which suggests possible involvement of transcriptional mechanism(s) affecting the Slc23a2 gene. To address this possibility, we examined the effect of chronic exposure of 266-6 cells to alcohol and of chronic alcohol feeding of mice on PAC level of expression of SVCT-2 hnRNA and obtained results showing a significant (P < 0.05 and P < 0.01, respectively) reduction in hnRNA expression compared with controls (Fig. 7, A and B). These findings support the suggestion that the effect of chronic alcohol exposure/feeding on AA uptake by PACs is in part mediated via transcriptional mechanism(s) affecting the Slc23a2 gene.
Fig. 7.
Effect of chronic alcohol exposure of 266-6 and mice on the level of expression of SVCT-2 hnRNA in PACs. hnRNA was quantified by RT-qPCR in 266-6 cells exposed to alcohol (A) and in primary PACs of mice fed alcohol chronically (B). Data are means ± SE at least 3 separate determinations. **P < 0.05. *P < 0.01.
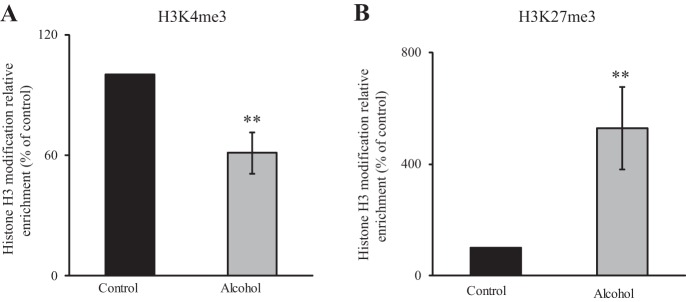
Chronic alcohol exposure affects transcriptional activity of a given gene via different mechanisms, including direct effect on activity of the involved promoter, e.g., inhibition in the level of expression of a transcription factor needed for driving the activity of the promoter and/or via epigenetic modifications (2, 4, 10, 11, 34, 39, 41). Previous studies have shown that KLF-4 and Sp-1 nuclear factors are important for transcriptional activity of the human SLC23A2 promoter (31); in addition, these cis-regulatory elements appear to be conserved in the predicted mouse Slc23a2 promoter (muscle and Mafft; mouse Slc23a2 promoter shares ∼75% homology to the human SLC23A2 promoter). Because we have shown previously that the levels of both of these nuclear factors (KLF-4 and Sp-1) are suppressed by chronic alcohol exposure (and thus may contribute to the observed inhibition in Slc23a2 transcription), we focused our effort here on examining whether epigenetic mechanisms (i.e., histone modifications and DNA methylation) are also involved in mediating the chronic alcohol effect on Slc23a2 transcription. Histone modifications were assessed by determining possible changes in H3K4me3 (an activation mark) and H3K27me3 (a repressive mark) in 266-6 cells chronically exposed to alcohol using ChIP-qPCR of the Slc23a2 promoter. The results showed a significant (P < 0.05) reduction in the level of H3K4me3 and a significant (P < 0.05) increase in the level of H3K27me3 in cells exposed to alcohol chronically compared with control cells (Fig. 8, A and B). Changes in methylation status of the Slc23a2 gene as a result of chronic alcohol exposure were also investigated using the Methprimer program (22) to identify CpG islands in the Slc23a2 promoter (−163 to +31 relative to TSS) followed by bisulfite sequencing (see materials and methods). The results, however, showed no significant changes in DNA methylation at the CpG islands of the specified Slc23a2 promoter region as a consequence of chronic alcohol exposure.
Fig. 8.
Effect of chronic alcohol exposure of pancreatic acinar 266-6 cells on epigenetic mechanisms. The qPCR data show the ratio of H3K4me3 (A) or H3K27me3 (B) relative to total H3 levels in chronic alcohol-exposed 266-6 cells and controls for 96 h (50 mM) in Slc23a2 promoter. Data are normalized to total input DNA and expressed as means ± SE of at least 3 independent samples. **P < 0.05.
DISCUSSION
The pancreas contains high levels of vitamin C (6, 18), where the vitamin assumes a variety of metabolic roles relevant to its normal cellular function and health. Little, however, is known about the factors (e.g., chronic alcohol exposure) that affect vitamin C uptake by PACs and about the mechanisms involved. We have begun to address these issues, and in this study we examined the effect of chronic alcohol exposure on PAC uptake of the reduced form of vitamin C, i.e., AA. We employed in vitro and in vivo chronic exposure approaches of PACs to alcohol in our investigations. First, we established the relative expression of the two known mammalian vitamin C transporters, i.e., SVCT-1 and -2 in mice and human pancreas and showed that both SVCT-1 and -2 are expressed in this tissue, with expression of the latter being significantly higher than that of the former in both species. The latter finding suggests that the SVCT-2 system assumes a more important role in AA uptake by these cells, and thus we focused on this transporter in our subsequent investigations.
Results of our investigations using the in vitro and in vivo models of chronic alcohol exposure showed a significant inhibition in carrier-mediated AA uptake by PACs exposed to alcohol compared with controls. This inhibition was associated with a significant reduction in the level of expression of the predominant SVCT-2 at the protein, mRNA, and hnRNA levels. These findings suggest that chronic alcohol exposure of PACs leads to inhibition in AA uptake and that the effect is exerted (at least in part) at the level of transcription of the Slc23a2 gene.
It is well known that a given factor can affect transcriptional activity of a particular gene via a variety of mechanisms, including an effect on the level of expression of an important nuclear factor needed to drive expression of that gene and/or via epigenetic modifications (2, 4, 11, 39, 41). Previous studies have shown that the nuclear factors KLF-4 and Sp-1 play important roles in driving the activity of the human SLC23A2 promoter (32) (cis-elements of these nuclear factors are conserved in mouse Slc23a2 promoter). We have also shown previously that chronic alcohol exposure leads to a decrease in the expression of these factors in PACs (39, 41). Thus it was not unreasonable to expect that part of the chronic alcohol inhibitory effect on SLC23A2 transcription in PACs was mediated via this mechanism. Whether other mechanisms, especially epigenetic modifications like histone modifications and DNA methylation (which mediate the effect of chronic alcohol exposure on expression of other genes; 2, 3, 4, 11, 12) are also involved in mediating the chronic alcohol exposure effect on the Slc23a2 transcription is not known and thus were also tested. Our findings showed that chronic exposure of 266-6 cells to alcohol is associated with a significant reduction in the level of expression of H3K4me3 (an activation mark) and a significant increase in the level of the H3K27me3 (a repressive mark) of the Slc23a2. No change, however, was seen in the methylation status of the Slc23a2 gene as a consequence of chronic alcohol exposure. These findings suggest that chronic alcohol exposure of PAC cells affects Slc23a2 transcription via mechanisms that include inhibition in the level of expression of relevant nuclear factors as well as via epigenetic modifications.
The observed inhibitory effect of chronic alcohol exposure on vitamin C transport by PACs is in contrast to its stimulatory effect on other transport genes (20, 22, 49). It is, however, similar to its inhibitory effects on uptake of other micronutrients (39, 41). These findings clearly show that alcohol exerts differential effects of alcohol on cellular physiological events. In summary, our results show that chronic alcohol exposure/feeding inhibits AA uptake by PACs and that the effect is, at least in part, mediated via transcriptional mechanism(s) of the Slc23a2 gene.
GRANTS
The study was supported by grants from the Dept. of Veterans Affairs and the NIH (DK-58057, DK-56061, and AA018071 to H. M. Said and DK-107474 to V. S. Subramanian).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
V.S.S., P.S., and H.M.S. conception and design of research; V.S.S. and P.S. performed experiments; V.S.S., P.S., and H.M.S. analyzed data; V.S.S., P.S., and H.M.S. interpreted results of experiments; V.S.S. and P.S. prepared figures; V.S.S., P.S., and H.M.S. drafted manuscript; V.S.S., P.S., and H.M.S. edited and revised manuscript; V.S.S., P.S., and H.M.S. approved final version of manuscript.
Footnotes
This article is the topic of an Editorial Focus by Alexander L. Ticho and Waddah A. Alrefai (46).
REFERENCES
- 1.Aydemir F, Jenkitkasemwong S, Gulec S, Knutson MD. Iron loading increases ferroportin heterogeneous nuclear RNA and mRNA levels in murine J774 macrophages. J Nutr 139: 434–438, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bleich S, Lenz B, Ziegenbein M, Beutler S, Frieling H, Kornhuber J, Bonsch D. Epigenetic DNA hypermethylation of the HERP gene promoter induces down-regulation of its mRNA expression in patients with alcohol dependence. Alcohol Clin Exp Res 30: 587–591, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Bonsch D, Lenz B, Fiszer R, Frieling H, Kornhuber J, Bleich S. Lowered DNA methyltransferase (DNMT-3b) mRNA expression is associated with genomic DNA hypermethylation in patients with chronic alcoholism. J Neural Transm 113: 1299–1304, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Bonsch D, Lenz B, Reulbach U, Kornhuber J, Bleich S. Homocysteine associated genomic DNA hypermethylation in patients with chronic alcoholism. J Neural Transm 111: 1611–1616, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Brown LA, Harris FL, Jones DP. Ascorbate deficiency and oxidative stress in the alveolar type II cell. Am J Physiol Lung Cell Mol Physiol 273: L782–L788, 1997. [DOI] [PubMed] [Google Scholar]
- 6.Brown S, Georgatos M, Reifel C, Song JH, Shin SH, Hong M. Recycling processes of cellular ascorbate generate oxidative stress in pancreatic tissues in in vitro system. Endocrine 18: 91–96, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Bürzle M, Suzuki Y, Ackermann D, Miyazaki H, Maeda N, Clémençon B, Burrier R, Hediger MA. The sodium-dependent ascorbic acid transporter family SLC23. Mol Aspects Med 34: 436–454, 2013. [DOI] [PubMed] [Google Scholar]
- 8.Carr AC, Frei B. Toward a new recommended dietary allowance for Vitamin C based on antioxidant and health effects in humans. Am J Clin Nutr 69: 1086–1107, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Corpe CP, Tu H, Eck P, Wang J, Faulhaber-Walter R, Schnermann J, Margolis S, Padayatty S, Sun H, Wang Y, Nussbaum RL, Espey MG, Levine M. Vitamin C transporter Slc23a1 links renal reabsorption, vitamin C tissue accumulation, and perinatal survival in mice. J Clin Invest 120: 1069–1083, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curry-McCoy TV, Guidot DM, Joshi PC. Chronic alcohol ingestion in rats decrease Kruppel-like factor 4 expression and intracellular zinc in the lung. Alcohol Clin Exp Res 37: 361–371, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Addario C, Caputin FF, Ekstrom TJ, Di Benedetto M, Maccarrone M, Romualdi P, Candeletti S. Ethanol induces epigenetic modulation of prodynorphin and pronociception gene expression in the rat amygdala Complex. J Mol Neurosci 49: 312–319, 2013. [DOI] [PubMed] [Google Scholar]
- 12.D'Addario C, Johansson S, Candeletti S, Romualdi P, Ogren SO, Terenius L, Ekstrom TJ. Ethanol and acetaldehyde exposure induces specific epigenetic modifications in the prodynorphin gene promoter in a human neuroblastoma cell line. FASEB J 25: 1069–1075, 2011. [DOI] [PubMed] [Google Scholar]
- 13.Daruwala R, Song J, Koh WS, Rumsey SC, Levine M. Cloning and functional characterization of the human sodium-dependent vitamin C transporters hSVCT1 and hSVCT2. FEBS Lett 460: 480–484, 1999. [DOI] [PubMed] [Google Scholar]
- 14.Devenyi R, Robinson GM, Kapur BM, Roncari DA. High-density lipoprotein cholesterol in male alcoholics with and without severe liver disease. Am J Med 71: 589–594, 1981. [DOI] [PubMed] [Google Scholar]
- 15.Devgun MS, Fiabane A, Paterson CR, Zarembski P, Guthrie A. Vitamin and mineral nutrition in chronic alcoholics including patients with Korsakoff's psychosis. Br J Nutr 45: 469–473, 1981. [DOI] [PubMed] [Google Scholar]
- 16.Elferink CJ, Reiners JJ Jr. Quantitative RT-PCR on CYP1A1 heterogeneous nuclear RNA: a surrogate for the in vitro transcription run-on assay. Biotechniques 20: 470–477, 1996. [DOI] [PubMed] [Google Scholar]
- 17.Fazio V, Flint DM, Wahlqvist ML. Acute effects of alcohol on plasma ascorbic acid in healthy subjects. Am J Clin Nutr 34: 2394–2396, 1981. [DOI] [PubMed] [Google Scholar]
- 18.Hornig D. Distribution of ascorbic acid, metabolites and analogues in man and animals. Ann NY Acad Sci 258: 103–118, 1975. [DOI] [PubMed] [Google Scholar]
- 19.Kohler CU, Roos PH. Focus on the intermediate state: immature mRNA of cytochromes P450-methods and insights. Anal Bioanal Chem 392: 1109–1122, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Kubisch CH, Gukovsky I, Lugea A, Pandol SJ, Kuick R, Misek R, Hanash SM, Logsdon CD. Long-term ethanol consumption alters pancreatic gene expression in rats: a possible connection to pancreatic injury. Pancreas 33: 68–76, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Kumaki Y, Oda M, Okano M. QUMA: quantification tool for methylation analysis. Nucleic Acids Res 36: 170–175, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li HS, Zhang JY, Thompson BS, Deng XY, Ford ME, Wood PG, Stolz DB, Eagon PK, Whitcomb DC. Rat mitochondrial ATP synthase ATP5G3:cloning and upregulation in pancreas after chronic ethanol feeding. Physiol Genomics 6: 91–98, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Li LC, Dahiya R. MethPrimer: designing primers for methylation PCRs. Bioinformatics 18: 1427–1431, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Li Y, Tollefsbol TO. DNA methylation detection: bisulfite genomic sequencing analysis. Methods Mol Biol 791: 11–21, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lieber CS, DeCarli LM. The feeding of ethanol in liquid diets. Alcohol Clin Exp Res 10: 550–553, 1986. [DOI] [PubMed] [Google Scholar]
- 26.Livek KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta DeltaC (T)) method. Methods 25: 402–408, 2001. [DOI] [PubMed] [Google Scholar]
- 27.McDonough KH. Antioxidant nutrients and alcohol. Toxicology 189: 89–97, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Navasumit P, Ward TH, Dodd NJ, O'Connor PJ. Ethanol-induced free radicles and hepatic DNA strand breaks are prevented in vivo by antioxidants: effects of acute and chronic ethanol exposure. Carcinogenesis 1: 93–99, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Packer L, Fuchs J. Vitamin C in Health and Disease. New York, NY: Marcel Dekker, 1997. [Google Scholar]
- 30.Pochareddy S, Edenberg HJ. Chronic alcohol exposure alters gene expression in HepG2 cells. Alcohol Clin Exp Res 36: 1021–1033, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rajan DP, Huang W, Dutta B, Devoe LD, Leibach FH, Ganapathy V, Prasad PD. Human placental sodium-dependent vitamin C transporter (SVCT2): molecular cloning and transport function. Biochem Biophys Res Commun 262: 762–768, 1999. [DOI] [PubMed] [Google Scholar]
- 32.Reidling JC, Rubin SA. Promoter analysis of the human ascorbic acid transporters SVCT1 and 2: mechanisms of adaptive regulation in liver epithelial cells. J Nutr Biochem 22: 344–350, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rondanelli M, Opizzi A, Perna S, Faliva MA. Update on nutrients involved in maintaining healthy bone. Endocrinol Nutr 60: 197–210, 2013. [DOI] [PubMed] [Google Scholar]
- 34.Rulten SL, Ripley TL, Hunt CL, Stephens DN, Mayne LV. Sp1 and NFkappaB pathways are regulated in brain in response to acute and chronic ethanol. Genes Brain Behav 5: 257–273, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Sajewicz W, Milnerowicz S, Nabzdyk S. Blood plasma antioxidant defense in patients with pancreatitis. Pancreas 32: 139–144, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Savini I, Rossi A, Pierro C, Avigliano L, Catani MV. SVCT1 and SVCT2: key proteins for vitamin C uptake. Amino Acids 34: 347–355, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Simon JA, Hudes ES. Serum ascorbic acid and gallbladder disease prevalence among US adults. Arch Intern Med 160: 931–936, 2000. [DOI] [PubMed] [Google Scholar]
- 38.Sotiriou S, Gispert S, Cheng J, Wang Y, Chen A, Hoogstraten-Miller S, Miller GF, Knon O, Levine M, Guttentag SH, Nussbaum RL. Ascorbic-acid transporter Slc23a2 is essential for vitamin C transport into the brain and for perinatal survival. Nat Med 8: 514–517, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Srinivasan P, Kapadia R, Biswas A, Said HM. Chronic alcohol exposure inhibits biotin uptake by pancreatic acinar cells: possible involvement of epigenetic mechanisms. Am J Physiol Gastrointest Liver Physiol 307: G941–G949, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Srinivasan P, Nabokina SM, Said HM. Chronic alcohol exposure affects pancreatic acinar mitochondrial thiamin pyrophosphate uptake: Studies with mouse 266-6 cell line and primary cells. Am J Physiol Gastrointest Liver Physiol 309: G750–G758, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Srinivasan P, Subramanian VS, Said HM. Mechanisms involved in the inhibitory effect of chronic alcohol exposure on pancreatic acinar thiamin uptake. Am J Physiol Gastrointest Liver Physiol 306: G631–G639, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Srinivasan P, Subramanian VS, Said HM. Effect of the cigarette smoke component, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), on physiological and molecular parameters of thiamin uptake by pancreatic acinar cells. PLoS One 8: e78853, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Srinivasan P, Thrower EC, Loganathan G, Balamurugan AN, Subramaian VS, Gorelick FS, Said HM. Chronic nicotine exposure in vivo, and in vitro inhibits vitamin B.1 (thiamin) uptake by pancreatic acinar cells. PLoS One 10: e0143575, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Subramanian VS, Ghosal A, Kapadia R, Nabokina SM, Said HM. Molecular mechanisms mediating the adaptive regulation of intestinal riboflavin uptake process. PLoS One 10: e0131698, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Subramanya SB, Subramanian VS, Sekar VT, Said HM. Thiamin uptake by pancreatic acinar cells: Effect of chronic alcohol feeding/exposure. Am J Physiol Gastrointest Liver Physiol 301: G896–G904, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ticho AL, Alrefai WA. Chronic ethanol exposure closes the door to vitamin C in pancreatic acinar cells. Focus on “Uptake of ascorbic acid by pancreatic acinar cells is negatively impacted by chronic alcohol exposure.” Am J Physiol Cell Physiol. doi: 10.1152/ajpcell.00149.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang H, Dutta B, Huang W, Devoe LD, Leibach FH, Ganapathy V, Prasad PD. Human Na(+)-dependent vitamin C transporter 1 (hSVCT1): Primary structure, functional characteristics and evidence for a non-functional splice variant. Biochim Biophys Acta 1461: 1–9, 1999. [DOI] [PubMed] [Google Scholar]
- 48.Wang Y, Mackenzie B, Tsukaguchi H, Weremowicz S, Morton CC, Hediger MA. Human vitamin C (L-ascorbic acid) transporter SVCT1. Biochem Biophys Res Commun 267: 488–494, 2000. [DOI] [PubMed] [Google Scholar]
- 49.Zinchuk V, Zinchuk O, Akimaru K, Moriya F, Okada T. Ethanol consumption alters expression and colocalization of bile salt export pump and multidrug resistance protein 2 in the rat. Histochem Cell Biol 127: 503–512, 2007. [DOI] [PubMed] [Google Scholar]