Abstract
The proton-coupled folate transporter (PCFT) mediates folate absorption across the brush-border membrane of the proximal small intestine and is required for folate transport across the choroid plexus into the cerebrospinal fluid. In this study, the functional role and accessibility of the seven PCFT Trp residues were assessed by the substituted-cysteine accessibility method. Six Trp residues at a lipid-aqueous interface tolerated Cys substitution in terms of protein stability and function. W85C, W202C, and W213C were accessible to N-biotinyl aminoethylmethanethiosulfonate; W48C and W299C were accessible only after treatment with dithiotreitol (DTT), consistent with modification of these residues by an endogenous thiol-reacting molecule and their extracellular location. Neither W107C nor W333C was accessible (even after DTT) consistent with their cytoplasmic orientation. Biotinylation was blocked by pemetrexed only for the W48C (after DTT), W85C, W202C residues. Function was impaired only for the W299C PCFT mutant located in the 4th external loop between the 7th and 8th transmembrane helices. Despite its aqueous location, function could only be fully preserved with Phe and, to a lesser extent, Ala substitutions. There was a 6.5-fold decrease in the pemetrexed influx Vmax and a 3.5- and 6-fold decrease in the influx Kt and Ki, respectively, for the W299S PCFT. The data indicate that the hydrophobicity of the W299 residue is important for function suggesting that during the transport cycle this residue interacts with the lipid membrane thereby impacting on the oscillation of the carrier and, indirectly, on the folate binding pocket.
Keywords: hereditary folate malabsorption, HFM, proton-coupled folate transporter, PCFT, solute transporter, substituted cysteine accessibility assay, folate, methotrexate, pemetrexed
folate transport across the brush-border membrane of the proximal small intestine, and across the epithelial cells of the choroid plexus, is mediated by the proton-coupled folate transporter (PCFT-SLC46A1), a member of the superfamily of solute transporters (15, 25). Mutations of this gene that cause loss-of-function of the PCFT protein result in the rare autosomal recessive disorder, hereditary folate malabsorption (HFM), indicative of the critical role PCFT plays in folate homeostasis and in the delivery of folates to the central nervous system (7, 15, 26). PCFT also plays an important role in the delivery of antifolates, such as pemetrexed, to cancer cells (6, 28). Because PCFT operates most efficiently at a pH far below that of normal systemic tissues, novel antifolates are being developed with high affinity and specificity for this transporter with the objective of achieving selective uptake into cancer cells within their acidic microenvironment (6). Hence, because of PCFT's role in cancer therapeutics and in the causation of HFM, understanding the structure-function of this transporter is of importance.
The structural and functional properties of PCFT have been characterized by the changes in the protein's stability, trafficking, and intrinsic function that occur when the gene is mutated in HFM, along with site-directed and random mutagenesis and the substituted cysteine accessibility method (SCAM). Based on these studies, the secondary structure of the carrier has been characterized along with residues and domains that play a key role in folate and proton binding and proton coupling and that line the aqueous translocation pathway (8, 9, 16–18, 20, 21, 23, 24, 27, 29, 32). These studies have been informed by, and correlated with, a homology model of PCFT based upon the structure of the glycerol-3-phosphate (GlpT) bacterial transporter (11, 17, 20, 27, 30).
Recently, this laboratory initiated evaluation of the role aromatic residues play in PCFT function. In an initial study focused on the 12 conserved Tyr residues, mutation of 4 residues to Cys resulted in a transport phenotype characterized by an increase in the influx Kt, Ki, and Vmax. (23). The data suggested that these Tyr residues maintain PCFT in a high-affinity configuration for its folate substrates while restricting the rate at which the carrier oscillates between its conformational states. The current study focuses on the role of all seven Trp residues, six of which are predicted to be located at a helix-loop interface, while one is located in the 4th external loop between the 7th and 8th transmembrane domains (TMDs). With the use of SCAM, the accessibility of the Cys-substituted residues to MTSEA-biotin, and the function of the Cys-mutants, were evaluated along with their role in substrate binding. The data identified the W299 residue in the 4th extracellular loop between the 7th and 8th TMDs as an important determinant of PCFT function.
MATERIALS AND METHODS
Chemicals.
[3′,5′,7-3H]Methotrexate (MTX) and [3H]pemetrexed were obtained from Moravek (Brea, CA). Unlabeled MTX was obtained from Sigma-Aldrich (St. Louis, MO) and unlabeled pemetrexed from LC Laboratories (Woburn, MA). EZ-Link Sulfo-NHS-LC-biotin[sulfosuccinimidyl-6-(biotinamido) hexanoate] (EZ-Link Sulfo-NHS-LC-biotin) was purchased from Pierce Biotechnology (Rockford, IL), streptavidin- agarose beads from Fisher Scientific (Pittsburgh, PA), and protease inhibitor cocktail from Roche Applied Science (Mannheim, Germany). The sulfhydryl reactive reagent N-biotinyl aminoethylmethanethiosulfonate (MTSEA-biotin) was obtained from Biotium (Hayward, CA).
Site-directed mutagenesis.
Trp residues were individually replaced with Cys or other amino acid residues using the Quickchange II XL site-directed mutagenesis kit (Stratagene, La Jolla, CA). The pcDNA3.1(+) expression vector that encodes a COOH-terminal-hemagglutinin (HA)-tagged wild-type (wt)-PCFT, or C66/C298 disulfide-less PCFT (PCFT-DSL), was the template (22, 27). The coding sequence of all mutant PCFTs was verified at the Albert Einstein Cancer Center Genomics Shared Resource.
Cell lines and culture conditions.
The HeLa-R1-11 cell line, which lacks PCFT and reduced folate carrier expression, was used for transient transfections. In this cell line, there is a genomic deletion of the reduced folate carrier and expression of PCFT is suppressed due to methylation of its promoter (27). These cells were maintained in Roswell Park Memorial Institute (RPMI) 1640 medium, under 5% CO2, with 10% fetal bovine serum (Gemini Bio-Products, Irvine, CA), 100 U/ml penicillin, 100 μg streptomycin. For transport studies, and subsequent flux determinations, 3 × 105 cells were seeded in 17-mm glass vials; for Western blot analyses, 5 × 105 cells/well were seeded in six-well plates. Two days later, cells were transfected with the PCFT constructs (0.8 μg/vial or 2 μg/well, respectively) using 2% lipofectamine 2000 (Invitrogen, Carlsbad, CA) in serum- and antibiotic free RPMI 1640.
Transport measurements.
Transport measurements were conducted as described in detail previously (27). The transport buffers include HBS (HEPES-buffered saline: 20 mM HEPES, 140 mM NaCl, 5 mM KCl, 2 mM MgCl2, and 5 mM dextrose, pH 7.4) and MBS (MES-buffered saline: 20 mM MES, 140 mM NaCl, 5 mM KCl, 2 mM MgCl2, and 5 mM dextrose, pH 5.5). Influx of tritiated MTX or pemetrexed was measured over 1 min at pH 5.5, 7.4 and a spectrum of intermediate pH levels and over a range of drug concentrations in which uptake was unidirectional. Pemetrexed influx kinetics (Kt and Vmax) was determined by nonlinear regression analysis of influx as a function of the extracellular pemetrexed concentration based on the Michaelis-Menten equation using prism software (version 6.07 for Windows; GraphPad Software, San Diego, CA). The pemetrexed and MTX influx Ki was determined based on inhibition of [3H]pemetrexed influx, at an extracellular [3H]pemetrexed concentration comparable to the influx Kt, and at concentrations of nonlabeled MTX or pemetrexed that resulted in 40–60% inhibition of influx. The Ki was calculated according to the Michaelis-Menten equation for competitive inhibition: V1/V2 = VmaxS/S + Kt [1 + (i/Ki)]/(VmaxS/S + Kt), where V1 is influx in the presence of inhibitor (i), V2 is influx in absence of inhibitor, and Kt and Vmax were derived from the measured [3H]pemetrexed (S) influx kinetics.
Biotinylation.
PCFT levels at the cell surface were determined by biotinylation of accessible Lys residues with EZ-Link Sulfo-NHS-LC-biotin, while MTSEA-biotin was used to evaluate the accessibility of the Cys residues in PCFT mutants as described in detail previously (27). For assessment of the effect of pemetrexed on MTSEA-biotin labeling, cells were first incubated with 1 mM pemetrexed in HBS (0.5 ml) at 0°C for 10 min before an equal volume of ice-cold MTSEA-biotin solution in HBS (0.5 ml) was added. The incubation was continued at 0°C for 30 min.
Gel electrophoresis and Western blot analysis.
Protein samples were resolved on 12% polyacrylamide Mini-Protean TGX gels (Bio-Rad, Hercules, CA) and then electroblotted onto polyvinylidene difluoride membranes (Merck, Darmstadt, Germany) and processed as described previously (27). The blots were probed with anti-HA antibody (no. H69080; Sigma) and horseradish peroxidase-conjugated secondary antibody (no. 7074S; Cell Signaling Technology, Danvers, MA) and developed with Western Lightning Plus-ECL (PerkinElmer Life Sciences, Waltham, MA). For the loading control, the blots were first probed with mouse monoclonal anti-actin antibody (no. A5441; Sigma) and then with anti-mouse HRP-linked secondary antibody (no. 7076; Cell Signaling). For some experiments, the same blots were probed for actin signals, washed in stripping buffer (2% SDS, 62.5 mM Tris·HCl, 0.5 M, and 100 mM β-mercaptoethanol, pH 6.7), and reprobed for HA signals.
Assessment of modification of the substituted Cys residues.
Modification of Cys residues by endogenous sulfhydryl reacting molecules was assessed by determining the impact of dithiothreitol (DTT) treatment on transport function and MTSEA-biotinylation. Two days after cells were transfected with the various PCFT constructs they were exposed to 10 mM DTT in HBS, pH 7.4, for 10 min at room temperature. After two washes with HBS, the cells were then assessed for PCFT function with a tritiated antifolate or for biotinylation with MTSEA-biotin as indicated above. The control was cells exposed to the HBS buffer alone.
Statistical analysis.
Statistical comparisons were performed with the Student's paired t-test using Graph Pad Prism (version 6.07 for Windows).
RESULTS
Analysis of the role of Trp residues in PCFT expression and function.
The human PCFT protein consists of 459 amino acids. There are seven Trp residues (48, 85, 107, 202, 213, 299, and 333); all are fully conserved in nine species (human, mouse, rat, monkey, dog, bovine, opossum, xenopus, zebra fish) except for bovine L202. Based on a hydropathy analysis and topological studies (32), six of the seven residues are predicted to be positioned at the lipid-aqueous interface of a transmembrane helix. W299 is located within the 4th extracellular loop between TMD 7 and 8 (Fig. 1). All these residues were replaced individually with Cys in the wt-PCFT scaffold and the properties of the mutants were assessed.
Fig. 1.
A topological model of human proton-coupled folate transporter (PCFT). Conserved Trp residues are indicated by the red closed circles. Conserved Tyr residues are indicated by the blue closed circles. The model is based, in part, on the data from the substituted cysteine accessibility analyses (8, 32).
As indicated in Fig. 2A, all the mutant proteins were stable and trafficked to the cell surface, although the level of expression at the cell membrane was somewhat reduced for the W85C, W107C, and W202C mutants (60 ± 6, 50 ± 2, and 45 ± 3%, of wt-PCFT; P < 0.02), respectively, based on densitometric analyses of three separate experiments. Transport function (unidirectional uptake) of the mutants was assessed at concentrations below (0.5 μM) and far above (50 μM) the MTX influx Kt, to approximate saturating (Vmax) conditions, at pH 5.5 (Fig. 2B). Influx was ≥50% of the wt-PCFT level at both the low and high concentrations for all these mutants except for W299C. For two of the mutants (W48C and W202C), transport at the high concentration exceeded the low and was comparable to the wt-PCFT level. Indeed, because expression of the W202C was decreased, the data suggested that the Vmax was higher than that of the wt-PCFT. Furthermore, the observation that transport was below that of wt-PCFT at the low concentration in the W202C mutant in the setting of an increased Vmax was consistent with an increase in the influx Kt, similar to what was observed with several Tyr mutants (23). Transport function of the W299C mutant was decreased by ∼90% irrespective of the substrate concentration. Hence, all the Trp residues tolerated the Cys-substitution except for W299C.
Fig. 2.

Cell surface expression and function of PCFT mutants. A, top: Western blot analysis of the level of Cys-substituted Trp PCFT mutants at the cell surface based on biotinylation of extracellular Lys residues. A, bottom: PCFT expression in the crude membrane fraction. A, middle: actin loading control. B: function of PCFT mutants assessed by [3′,5′,7-3H]methotrexate ([3H]MTX) influx (0.5 and 50 μM) at pH 5.5 for 1 min. The images in A are representative of 3 independent experiments; the data in B are the mean ± SE from 3 independent experiments. WT, wild type.
Analysis of the accessibility of the Cys mutants to the extracellular aqueous compartment; impact of DTT.
Accessibility of the various Cys mutants to the extracellular aqueous compartment was evaluated by the extent of biotinylation by MTSEA-biotin, a reagent that does not penetrate the cell membrane. Previous studies indicated that none of the endogenous Cys residues in wt-PCFT are accessible to biotinylation by this agent (17, 23, 32). In this study no reaction between MTSEA-biotin and an endogenous Cys residue, nor a nonspecific reaction with any other residue, was observed (Fig. 3A).
Fig. 3.
Accessibility of the Cys-substituted Trp residues to N-biotinyl aminoethylmethanethiosulfonate (MTSEA-biotin); impact of DTT and protection by pemetrexed. A: accessibility of the Cys-substituted Trp residues to MTSEA-biotin. B: impact of DTT treatment on MTSEA-biotinylation. C and D: impact of pemetrexed on accessibility of the Cys-substituted residues to MTSEA-biotin. The MTSEA-biotin concentration was 0.5 mM in A and B and 0.125 mM in C and D. The images in each panel are representative of 3 independent experiments; the MTSEA-biotin signals match the corresponding PCFT signals in the crude membrane fractions.
Three of the Cys-substituted residues were accessible to MTSEA-biotin, W85C, W202C, and W213C; three were not, W107C, W299C and W333C; and W48C was only weakly labeled (Fig. 3A). Studies from this laboratory have shown that the lack of accessibility may be due to the modification of the Cys residue with an endogenous sulfhydryl-active molecule (27). If this is the case, the modification can be removed by pretreatment with a reducing agent (27). To explore this possibility, mutants were generated in a C66S and C298S PCFT background (identified as disulfide-less with the DSL suffice). These Cys residues form a disulfide bond in wt-PCFT that is broken by DTT making both residues accessible to biotinylation thereby confounding analyses of the impact of DTT on the accessibility of other residues. As indicated in Fig. 3B, after treatment with DTT the W48C and W299C residues in the disulfide-less scaffold (W48C-DSL, W299C-DSL) became accessible. The increase in W299C-DSL accessibility with DTT was accompanied by a small increase in function; however, influx remained <25% that of wt-PCFT (not shown). Hence, the loss of the major component of the W299C mutant function could not be attributed to the Cys modification. There was no change in accessibility of the W107C-DSL or W333C-DSL mutants with DTT, consistent with their intracellular location. While there was much lower expression of the W107C-DSL protein in the crude membrane fraction, nonetheless, this level of expression resulted in ∼50% of the influx activity of the PCFT-DSL (not shown).
The role of Trp residues in folate binding.
Studies then explored whether a PCFT substrate alters accessibility of the Cys-substituted residues either by directly interfering with the MTSEA-biotin or by altering the structure of the protein, which, in turn, limits accessibility to the labeling reagent. Pemetrexed was used for this analysis because these experiments are conducted at neutral pH and pemetrexed retains a high affinity for the carrier under these conditions (28). As indicated in Fig. 3, C and D, pemetrexed blocked MTSEA-biotin labeling of the W85C and W213C mutants but not W202C. After treatment with DTT, pemetrexed neither blocked MTSEA-biotin labeling of the W48C-DSL nor labeling of the W299C-DSL mutants.
Structural requirements for the W299 PCFT residue.
The Cys substitution at the W299 residue had a marked effect on function. To determine the structural requirements for function at this residue, a variety of other substitutions were made encompassing differences in size, charge and polarity, and transport assessed. These studies were performed with both MTX and pemetrexed to determine whether the mutations resulted in functional changes that had elements of substrate specificity. As indicated in Fig. 4, there were substantial losses of function for most of the mutants. However, function was fully preserved for W299F and there was substantial retention of function with the W299A mutant, particularly when pemetrexed was the transport substrate. Beyond this, there was a modest better retention of function with pemetrexed (B) compared with MTX (A), particularly at the low concentration for all the mutants except W299E. In contrast, the loss of function with MTX as substrate was generally comparable at low and high concentrations. As indicated in Fig. 5, the differences in function among the different W299 mutants cannot be attributed to differences in expression at the cell surface. Expression of all the mutants at the cell membrane was ≥50% of wt-PCFT (based upon densitometric measurements from 3 separate experiments). Hence, among the residues substituted, the two neutral residues preserved the most activity.
Fig. 4.
Impact of a spectrum of substitutions at the Trp 299 residue on MTX and pemetrexed influx. Influx of [3H]MTX (A) or [3H]pemetrexed (B) was measured over 1 min at pH 5.5 at 0.5- and 50-μM substrate concentrations. The data in A and B are the mean ± SE from 3 independent experiments.
Fig. 5.

Western blot analysis of the expression of W299 PCFT mutants. Top: expression at the cell surface of the W299 mutants based upon biotinylation of extracellular Lys residues. Middle: actin loading control. Bottom: expression in the crude membrane fraction. The images are representative of 3 independent experiments.
Influx kinetics and Ki determinations for the W299S mutant PCFT.
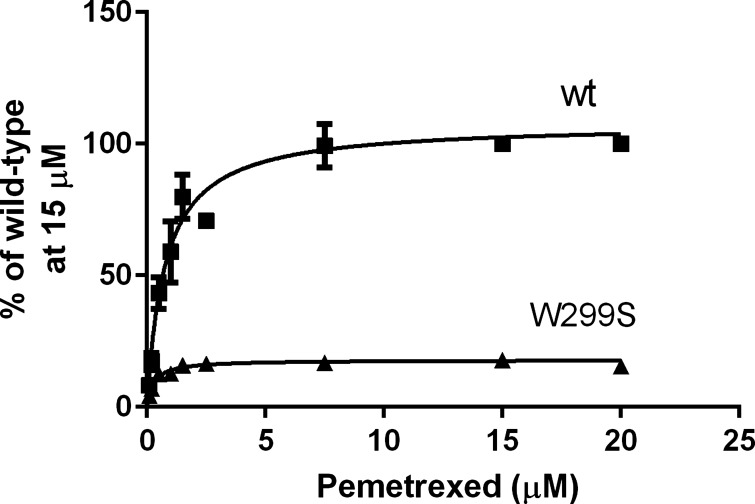
The kinetic basis for the decline in function with mutations at the W299 residue was assessed with the W299S mutant because it had sufficient residual activity to allow accurate transport measurements. Pemetrexed was chosen as the transport substrate because its activity was better retained than that of MTX, and its low Kt for wt-PCFT allows saturation even if the influx Kt for a mutant is substantially increased. Based on a nonlinear regression analysis of the relationship between pemetrexed influx and the extracellular concentration (Fig. 6), the loss of function for the W299S mutant was due to a sixfold decrease in the influx Vmax. However, the influx Kt was decreased by a factor of 3.5, from 0.99 to 0.28 μM (Table 1). This decrease in Kt was due to an increase in the affinity of the W299S mutant for pemetrexed as confirmed by the 5.9-fold decrease in Ki determined from the inhibition of 3H-pemetrexed influx by nonlabeled drug. Likewise, there was a small (∼50%), but significant, decrease in the influx Ki for MTX (Table 1). The data suggest that this change in pemetrexed influx kinetics may be relevant to most of the W299 mutants in which retention of function was greater at low than high pemetrexed concentrations relative to wt-PCFT (Fig. 4B).
Fig. 6.
Pemetrexed influx kinetics for the W299S PCFT mutant. Concentrations were chosen below and well above the pemetrexed influx Kt to ensure saturation of the carrier. Influx was assessed over 1 min, at pH 5.5. The data are the mean ± SE from 4 independent experiments plotted as percentage of the maximum transport rate for wt-PCFT.
Table 1.
Influx kinetics for pemetrexed and methotrexate for wt-PCFT and the W299S mutant
| wt-PCFT | W299S | W299S/wt-PCFT | |
|---|---|---|---|
| Pemetrexed Kt, μM | 0.99 ± 0.23 | 0.28 ± 0.01 | 0.28 |
| Pemetrexed Vmax, pmol/mg protein | 384 ± 83 | 58.3 ± 8.7 | 0.15 |
| Pemetrexed Ki, μM | 0.94 ± 0.07 | 0.16 ± 0.02 | 0.17 |
| MTX Ki, μM | 2.36 ± 0.06 | 1.05 ± 0.03 | 0.44 |
Values are the mean ± SE from 3 independent experiments. Influx Kt, Ki and Vmax were computed from the Michaelis-Menton equation. All differences between wild-type proton-coupled folate transporter (PCFT) (wt-PCFT) and the W299S mutant are significant to P ≤ 0.01. The difference between the fold decrease in the influx Ki for the W299S mutant relative to wt-PCFT for both [3′,5′,7-3H]methotrexate (MTX) and pemetrexed is significant (P < 0.03). The difference between MTX Ki and pemetrexed Ki for wt-PCFT as well as the difference between the W299S mutants is significant (P < 0.003).
Impact of pH on the function of the W299 mutants.
Proton coupling and proton binding are critical to PCFT function. To determine whether mutations at the W299 residue might cause a pH-specific loss of activity, transport was measured at both pH 5.5 and 7.4. As indicated in Fig. 7, A for MTX and B for pemetrexed, there was a substantial loss of activity at pH 5.5 for all the mutants with greater loss at pH 7.4 relative to wt-PCFT for both antifolates. The discrepancy between loss of activity at the low vs. high pH was greatest for W299A PCFT. MTX influx as a function of pH for the W299A and wt-PCFT is illustrated in Fig. 7C. It can be seen that there was a marked left shift in the relationship between MTX influx and pH. Hence, a decreasing pH tended to normalize transport of the mutant raising the possibility that this residue may play a role in proton binding as observed for the His281 residue (20).
Fig. 7.
Influx of MTX or pemetrexed mediated by W299 mutants as a function of pH. Influx of [3H]MTX (A) and [3H]pemetrexed (B) at pH 5.5 and 7.4, both at 1 μM. C: influx of [3H]MTX (1 μM) over a pH range of 4.5 to 7.0.
An analysis of tilting of PCFT transmembrane helices.
The orientation of PCFT helices relative to the membrane was obtained by the TMDET program that calculates the most likely position of the membrane bilayer for a given membrane protein structure (19). The largest momentum of inertia for each helix was compared with the normal vector of the membrane bilayer to obtain a tilt angle for each of the TMDs. Five Trp residues are associated with helices with a tilt angle >40°: W48-1st TMD; W85 and W107-2nd TMD; W202-5th TMD; W213-6th TMD. The 9th TMD with W333 at the cytoplasmic interface is not tilted. Neither the 7th nor the 8th helices that connect to the 4th external loop contain a Trp at the lipid-aqueous interface and only TMD 7 is tilted.
DISCUSSION
PCFT is one of several members of the superfamily of proton-coupled solute transporters expressed in the brush-border membrane of the proximal small intestine where the microenvironment is acidic and the absorption of their substrates is initiated (1, 25). Unlike most of the other proton-coupled transporters of organic substrates, PCFT-mediated transport is a high-affinity process with Kt and Ki in the 10−7/10−6 molar range and is highly specific for its folate/antifolate monoglutamyl substrates (1, 15). A variety of studies have been undertaken to elucidate the structural determinants of PCFT activity employing a number of different strategies. The current report focuses on the role of Trp residues in PCFT function and their accessibility to the aqueous compartment.
The only Trp residue for which there was substantial loss of function when a Cys residue was substituted was Trp299 located within the 4th extracellular loop. The W299C mutant was modified by an endogenous thiol-reacting molecule but became accessible to MTSEA-biotin after treatment with DTT, although function remained impaired. While this residue was accessible to the extracellular compartment, consistent with its location in the aqueous portion of the loop (32), full function was preserved with a Phe or, to a lesser extent, a Ala substitution. Hence, despite its location, it would appear to be the hydrophobic nature of this residue that is required for function. The data suggest that at some point in the transport cycle the W299 residue interacts with the lipid membrane and this is important to the cycling of the carrier between its conformational states and impacts indirectly on the folate binding pocket. This could be related to the role that the 7th TMD plays in the formation of an extracellular gate involving a residue (L290) at the opposite end of this loop. It is of interest that L290C-DSL was also modified resulting in a loss of function and accessibility; however, both were reversed by DTT (27).
These finding are unlike what was observed when all 12 of the conserved Tyr residues, 6 located within a few residues of the lipid-aqueous interface, were substituted with Cys. Function was preserved particularly when assessed at saturating substrate concentrations (23). Four of the mutated Tyr residues had influx Vmax increases to levels far beyond those of wt-PCFT; however, the relative modest reduction, or unchanged, function relative to cells with wt-PCFT at low substrate concentrations for these mutants was consistent a marked a decrease in the affinity of the mutated carrier (increase in Kt and Ki) for its substrates, quantitated for the Y315A mutant. It is of interest that, save for a modest apparent increase in the maximum velocity for the W202C PCFT mutant, none of the Trp mutants in the current study had a transport phenotype similar to the Tyr mutants.
Trp residues are enriched at the lipid-aqueous interface of transmembrane proteins at the extracellular and cytoplasmic boundaries of the cell membrane (5, 10). The Trp indole moiety forms H-bonds with lipid carbonyl and phosphate groups and that, along with cation-π interactions involving the indole ring, draw the Trp side chain to the lipid-aqueous interface and anchors the transmembrane protein to the lipid bilayer. This localization plays an important role in stabilizing transmembrane proteins particularly when there is hydrophobic mismatch. This is due to a difference between the length of the protein helix and the thickness of the lipid bilayer resulting in helix tilting and membrane deformations that impact on the stability of the protein that is modulated by the presence of a Trp or Tyr residue at or near to the helix interface (4, 5, 10). While the majority of the Tyr residues in PCFT are located within four to five residues of the predicted water-lipid interface, all Trp residues except W299 are predicted to be located at the loop-helix interface. However, despite the location of five Trp residues at highly tilted helices, mutation of any single Trp residue to Cys was well tolerated.
Of the Cys-substituted PCFT Trps predicted to be located contiguous with the extracellular compartment, W85C and W202C were both accessible to MTSEA-biotin and not modified. Besides the W299C-DSL, the W48C-DSL mutant was modified and became accessible after DTT treatment. The W107C-DSL, W333C-DSL and PCFT-DSL mutants were not accessible before or after treatment with DTT consistent with their location at the cytosolic interface of the 2nd and 9th TMD, respectively, and previous studies that identified as intracellular the location of the domains in which these residues reside (3, 32). Another laboratory reported that Cys substituted residues in the 2nd intracellular loop are accessible to biotinylation and suggested that this is a reentrant loop intermittently moving sufficiently deep into the translocation pathway to be accessible to the extracellular compartment (24). In that study, the W107C mutant was generated in a Cys-less scaffold and the protein was found to be unstable, a frequent observation in membrane transporters (31), so that its accessibility could not be assessed (24). In the current study, the W107C mutant was not accessible in either the wild-type PCFT scaffold or a scaffold in which all Cys residues were retained except for C66 and C298. In the latter scaffold, the level of protein expressed was reduced in the crude membrane fraction, consistent with decreased stability of the mutant protein; however, while intrinsic function was preserved, no MTSEA-biotin labeling was detected. These observations are also consistent with recent findings in Xenopus oocytes that both A106C and V111C hPCFT mutants in a Cys-less scaffold, located in the 2nd intracellular loop, were not labeled by MTSEA-biotin (3). Taken together, the data affirm the intracellular location of this domain.
Previous studies have shown that, often, pemetrexed activity is better preserved than that of MTX when PCFT is mutated (13, 18). In one case, while the mutation resulted in a decrease in the affinity of the carrier for MTX the affinity for pemetrexed actually increased (18). Hence, changes in the protein that adversely affect MTX binding can have a lesser, or even opposite, effect on pemetrexed association with the carrier. Likewise, in this study there was a much greater decrease in the influx Kt and Ki of the W299S mutant for pemetrexed than MTX consistent with substantial differences in the way these antifolates bind to the carrier. The observation that pemetrexed blocked MTSEA-biotinylation of the W85C and W213C mutants at 0°C suggests that these residues may be directly involved in substrate binding. At this temperature, an allosteric conformational change upon substrate binding is unlikely (2, 12, 14). The lack of an inhibitory effect of pemetrexed on the biotinylation of the W299C residue is consistent with the lack of a direct role for this residue in folate substrate binding.
GRANTS
This study was supported by National Cancer Institute Grant CA-82621.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.N., R.Z., and I.D.G. conception and design of research; M.N. and R.Z. performed experiments; M.N., R.Z., A.F., and I.D.G. analyzed data; M.N., R.Z., A.F., and I.D.G. interpreted results of experiments; M.N. and R.Z. prepared figures; M.N. and I.D.G. drafted manuscript; M.N., R.Z., A.F., and I.D.G. edited and revised manuscript; M.N., R.Z., A.F., and I.D.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Michele Visentin for construction of several Trp mutants.
REFERENCES
- 1.Anderson CM, Thwaites DT. Hijacking solute carriers for proton-coupled drug transport. Physiology (Bethesda) 25: 364–377, 2010. [DOI] [PubMed] [Google Scholar]
- 2.Androutsellis-Theotokis A, Ghassemi F, Rudnick G. A conformationally sensitive residue on the cytoplasmic surface of serotonin transporter. J Biol Chem 276: 45933–45938, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Date SS, Chen CS, Chen Y, Jansen M. Experimentally optimized threading structure of the proton-coupled folate transporter. FEBS Open Bio 6: 216–230, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Jesus AJ, Allen TW. The determinants of hydrophobic mismatch response for transmembrane helices. Biochim Biophys Acta 1828: 851–863, 2013. [DOI] [PubMed] [Google Scholar]
- 5.de Jesus AJ, Allen TW. The role of tryptophan side chains in membrane protein anchoring and hydrophobic mismatch. Biochim Biophys Acta 1828: 864–876, 2013. [DOI] [PubMed] [Google Scholar]
- 6.Desmoulin SK, Hou Z, Gangjee A, Matherly LH. The human proton-coupled folate transporter: Biology and therapeutic applications to cancer. Cancer Biol Ther 13: 1355–1373, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diop-Bove N, Kronn D, Goldman ID. Hereditary folate malabsorption. In: GeneReviews (Internet), edited by Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJ, Bird TD, Dolan CR, Fong CT, Smith RJ, Stephens K. Seattle, WA: University of Washington, 2014. [PubMed] [Google Scholar]
- 8.Duddempudi PK, Goyal R, Date SS, Jansen M. Delineating the extracellular water-accessible surface of the proton-coupled folate transporter. PLoS One 8: e78301, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hou Z, Desmoulin SK, Etnyre E, Olive M, Hsiung B, Cherian C, Wloszczynski PA, Moin K, Matherly LH. Identification and functional impact of homo-oligomers of the human proton-coupled folate transporter. J Biol Chem 287: 4982–4995, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Killian JA, von HG. How proteins adapt to a membrane-water interface. Trends Biochem Sci 25: 429–434, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Lasry I, Berman B, Straussberg R, Sofer Y, Bessler H, Sharkia M, Glaser F, Jansen G, Drori S, Assaraf YG. A novel loss of function mutation in the proton-coupled folate transporter from a patient with hereditary folate malabsorption reveals that Arg 113 is crucial for function. Blood 112: 2055–2061, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Lopez-Corcuera B, Nunez E, Martinez-Maza R, Geerlings A, Aragon C. Substrate-induced conformational changes of extracellular loop 1 in the glycine transporter GLYT2. J Biol Chem 276: 43463–43470, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Mahadeo K, Diop-Bove N, Shin D, Unal E, Teo J, Zhao R, Chang MH, Fulterer A, Romero MF, Goldman ID. Properties of the Arg376 residue of the proton-coupled folate transporter (PCFT-SLC46A1) and a glutamine mutant causing hereditary folate malabsorption. Am J Physiol Cell Physiol 299: C1153–C1161, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pajor AM, Randolph KM. Conformationally sensitive residues in extracellular loop 5 of the Na+/dicarboxylate co-transporter. J Biol Chem 280: 18728–18735, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qiu A, Jansen M, Sakaris A, Min SH, Chattopadhyay S, Tsai E, Sandoval C, Zhao R, Akabas MH, Goldman ID. Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell 127: 917–928, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Shin DS, Min SH, Russell L, Zhao R, Fiser A, Goldman ID. Functional roles of aspartate residues of the proton-coupled folate transporter (PCFT; SLC46A1); a D156Y mutation causing hereditary folate malabsorption. Blood 116: 5162–5169, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shin DS, Zhao R, Fiser A, Goldman ID. Role of the fourth transmembrane domain in proton-coupled folate transporter function as assessed by the substituted cysteine accessibility method. Am J Physiol Cell Physiol 304: C1159–C1167, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shin DS, Zhao R, Yap EH, Fiser A, Goldman ID. A P425R mutation of the proton-coupled folate transporter causing hereditary folate malabsorption produces a highly selective alteration in folate binding. Am J Physiol Cell Physiol 302: C1405–C1412, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tusnady GE, Dosztanyi Z, Simon I. TMDET: web server for detecting transmembrane regions of proteins by using their 3D coordinates. Bioinformatics 21: 1276–1277, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Unal ES, Zhao R, Chang MH, Fiser A, Romero MF, Goldman ID. The functional roles of the His247 and His281 residues in folate and proton translocation mediated by the human proton-coupled folate transporter SLC46A1. J Biol Chem 284: 17846–17857, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Unal ES, Zhao R, Goldman ID. Role of the glutamate 185 residue in proton translocation mediated by the proton-coupled folate transporter SLC46A1. Am J Physiol Cell Physiol 297: C66–C74, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Unal ES, Zhao R, Qiu A, Goldman ID. N-linked glycosylation and its impact on the electrophoretic mobility and function of the human proton-coupled folate transporter (HsPCFT). Biochim Biophys Acta 1178: 1407–1414, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Visentin M, Unal ES, Najmi M, Fiser A, Zhao R, Goldman ID. Identification of Tyr residues that enhance folate substrate binding and constrain oscillation of the proton-coupled folate transporter (PCFT-SLC46A1). Am J Physiol Cell Physiol 308: C631–C641, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson MR, Hou Z, Matherly LH. Substituted cysteine accessibility reveals a novel transmembrane 2–3 reentrant loop and functional role for transmembrane domain 2 in the human proton-coupled folate transporter. J Biol Chem 289: 25287–25295, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao R, Goldman ID. Folate and Thiamine Transporters mediated by Facilitative Carriers (SLC19A1-3 and SLC46A1) and Folate Receptors. Mol Aspects Med 34: 373–385, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao R, Min SH, Qiu A, Sakaris A, Goldberg GL, Sandoval C, Malatack JJ, Rosenblatt DS, Goldman ID. The spectrum of mutations in the PCFT gene, coding for an intestinal folate transporter, that are the basis for hereditary folate malabsorption. Blood 110: 1147–1152, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao R, Najmi M, Fiser A, Goldman ID. Identification of an extracellular gate for the proton-coupled folate transporter (SLC46A1) by cysteine cross-linking. J Biol Chem 291: 8162–8172, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao R, Qiu A, Tsai E, Jansen M, Akabas MH, Goldman ID. The proton-coupled folate transporter (PCFT): impact on pemetrexed transport and on antifolate activities as compared to the reduced folate carrier. Mol Pharmacol 74: 854–862, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao R, Shin DS, Diop-Bove N, Ovits CG, Goldman ID. Random mutagenesis of the proton-coupled folate transporter (PCFT, SLC46A1), clustering of mutations and the bases for associated losses of function. J Biol Chem 286: 24150–24158, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao R, Shin DS, Fiser A, Goldman ID. Identification of a functionally critical GXXG motif and its relationship to the folate binding site of the proton-coupled folate transporter (PCFT-SLC46A1). Am J Physiol Cell Physiol 303: C673–C681, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao R, Shin DS, Goldman ID. Vulnerability of the cysteine-less proton-coupled folate transporter (PCFT-SLC46A1) to mutational stress associated with the substituted cysteine accessibility method. Biochim Biophys Acta 1808: 1140–1145, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao R, Unal ES, Shin DS, Goldman ID. Membrane topological analysis of the proton-coupled folate transporter (PCFT-SLC46A1) by the substituted cysteine accessibility method. Biochemistry 49: 2925–2931, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]