Abstract
Nucleotide-binding domain, leucine-rich-repeat-containing family, pyrin domain-containing 3 (NLRP3) is a cytosolic protein that nucleates assembly of inflammasome signaling platforms, which facilitate caspase-1-mediated IL-1β release and other inflammatory responses in myeloid leukocytes. NLRP3 inflammasomes are assembled in response to multiple pathogen- or environmental stress-induced changes in basic cell physiology, including the destabilization of lysosome integrity and activation of K+-permeable channels/transporters in the plasma membrane (PM). However, the quantitative relationships between lysosome membrane permeabilization (LMP), induction of increased PM K+ permeability, and activation of NLRP3 signaling are incompletely characterized. We used Leu-Leu-O-methyl ester (LLME), a soluble lysosomotropic agent, to quantitatively track the kinetics and extent of LMP in relation to NLRP3 inflammasome signaling responses (ASC oligomerization, caspase-1 activation, IL-1β release) and PM cation fluxes in murine bone marrow-derived dendritic cells (BMDCs). Treatment of BMDCs with submillimolar (≤1 mM) LLME induced slower and partial increases in LMP that correlated with robust NLRP3 inflammasome activation and K+ efflux. In contrast, supramillimolar (≥2 mM) LLME elicited extremely rapid and complete collapse of lysosome integrity that was correlated with suppression of inflammasome signaling. Supramillimolar LLME also induced dominant negative effects on inflammasome activation by the canonical NLRP3 agonist nigericin; this inhibition correlated with an increase in NLRP3 ubiquitination. LMP elicited rapid BMDC death by both inflammasome-dependent pyroptosis and inflammasome-independent necrosis. LMP also triggered Ca2+ influx, which attenuated LLME-stimulated NLRP3 inflammasome signaling but potentiated LLME-induced necrosis. Taken together, these studies reveal a previously unappreciated signaling network that defines the coupling between LMP, changes in PM cation fluxes, cell death, and NLRP3 inflammasome activation.
Keywords: caspase-1, dendritic cell, IL-1, ion channels, cell death
il-1β is a proinflammatory cytokine involved in the induction of fever and mobilization of leukocytes to sites of infection (9). Active IL-1β is produced via proteolytic processing of pro-IL-1β by caspase-1 (49). Caspase-1 is activated via proximity-induced autocatalysis within macromolecular signaling platforms called inflammasomes (49), and a major inflammasome is based on the cytosolic pattern recognition receptor nucleotide-binding domain, leucine-rich-repeat-containing family, pyrin domain-containing 3 (NLRP3) (15, 49). Upon conformational activation, NLRP3 interacts with the ASC adapter protein to nucleate formation of large cytosolic aggregates known as ASC specks (7, 29). ASC specks form a platform for recruitment of pro-caspase-1 monomers and thereby activate caspase-1 with consequent proteolytic maturation of IL-1β for release by noncanonical secretion (15, 49). Caspase-1 also triggers pyroptosis, a regulated cell death pathway defined by activation of plasma membrane channels/pores permeable to osmotically active ions (Na+, K+, Cl−) and small metabolites; this induces osmotic swelling and eventual cell lysis (1). The various proteins required for NLRP inflammasome signaling are predominantly expressed in myeloid leukocytes including macrophages, dendritic cells (DCs), neutrophils, and microglia.
Although the critical biochemical signal that drives conformational activation of NLRP3 is unknown, NLRP3 inflammasomes are rapidly assembled in response to multiple pathogen- or environmental stress-induced changes in basic cell physiology, including ion channel-mediated decreases in cytosolic [K+] and the disruption of lysosome integrity (51). NLRP3 activation in response to lysosome destabilization correlates with lysosomal membrane permeabilization (LMP) and release of lysosomal hydrolases, including multiple cathepsins, into the cytosol (20). LMP is induced by a broad range of proinflammatory stimuli that include phagocytized particulates, such as alum, silica, monosodium urate (MSU), and crystalline cholesterol (10, 12, 19, 33), lipotoxic saturated fatty acids (54), and soluble lysosomotropic compounds, such as the dipeptide Leu-Leu-O-Me (LLME) (19). Pharmacologic inhibition of cathepsin activity or genomic deletion of various cathepsins markedly attenuates LMP-induced NLRP3 inflammasome signaling (6, 17, 41, 56). LMP-mediated activation of inflammasomes markedly contributes to inflammatory disease pathogenesis or progression elicited by some particulate stimuli, including MSU-induced gout (33, 47) and crystalline cholesterol-induced atherosclerosis (10, 34, 45).
The mechanism(s) by which LMP and accumulation of cytosolic cathepsins induce NLRP3 inflammasome complex assembly are incompletely defined. Although direct proteolytic modification of NLRP3 or other inflammasome regulatory proteins may be involved, multiple studies have demonstrated potent suppression of NLRP3 signaling when diverse LMP stimuli are presented to macrophages or DCs bathed in media containing elevated extracellular [K+] (23, 38, 56). This prevents the efflux of cytosolic K+ and consequent decrease in intracellular [K+] that is a necessary (but not sufficient) signal for NLRP3 inflammasome activation in normal cells that express nonmutated NLRP3 protein. Moreover, all LMP stimuli tested also trigger robust decreases in cytosolic K+ before, and independently from, NLRP3 inflammasome assembly and accumulation of active caspase-1 (38). This suggests a model wherein activation of plasma membrane cation channels or transporters by lysosome-derived signals (cathepsins, other hydrolases, or small metabolites) is a critical early signal for licensing NLRP3 inflammasome assembly by LMP stimuli. We previously reported that robust Ca2+ influx and K+ efflux are rapidly triggered by LLME treatment of murine DC (23).
Although the role of LMP in stimulating NLRP3 inflammasomes is well established, recent studies by Brojatsch and colleagues (4, 5, 21, 27) have demonstrated that the efficacy of LMP stimuli in activating inflammasome signaling can be very low under some experimental conditions. Those investigators observed that LLME or particulate alum failed to induce significant caspase-1 processing or IL-1β release in murine macrophages despite causing massive lysosome rupture. The low level of inflammasome signaling in response to LLME and alum correlated with proteolytic degradation of various cytosolic proteins, including pro-caspase-1 and pro-IL-1β, and extensive cytolysis (27). These findings indicate that lysosome destabilization can either activate or suppress NLRP3 inflammasome signaling in different cellular contexts and raise several questions: What quantitative parameters determine the threshold levels of lysosome destabilization to trigger the inflammasome signaling cascade? What percentage of lysosomes must become permeabilized to cause activation of NLRP3? Might LMP-induced suppression of inflammasome signaling involve mechanisms other than, or in addition to, direct proteolytic degradation of inflammasome proteins?
We addressed these questions using LLME, a soluble lysosomotropic agent, to quantitatively track the kinetics and extent of LMP in relation to the time courses of multiple NLRP3 inflammasome signaling responses and plasma membrane cation fluxes in murine bone marrow-derived DCs (BMDCs). We found that submillimolar LLME induced slowly developing and partial increases in LMP that correlated with robust NLRP3 inflammasome activation and K+ efflux. In contrast, supramillimolar LLME elicited extremely rapid and complete collapse of lysosome integrity accompanied by coordinated suppression or attenuation of inflammasome signaling that correlated with increased NLRP3 ubiquitination. LMP also induced a parallel Ca2+ influx, which contributed in part to the attenuated NLRP3 inflammasome activation. In contrast, this Ca2+ influx potentiated LMP-induced DC death. These results reveal a previously unappreciated signaling network between lysosome destabilization, changes in plasma membrane cation fluxes, entrainment of necrotic death, and NLRP3 inflammasome activation.1
MATERIALS AND METHODS
Reagents.
Key reagents and their sources were as follows: Escherichia coli LPS serotype O1101:B4 (List Biological Laboratories), nigericin (NG) (Sigma-Aldrich), PR-619 (Sigma-Aldrich), ionomycin (LC Laboratories), H-Leu-Leu-OMe·HBr (Bachem), CA-074-methyl ester (Bachem), Imject Alum (Pierce), monosodium urate (Invivogen), disuccinimidyl suberate (DSS; Sigma-Aldrich), anti-caspase-1 (p20) mouse mAb (Casper-1; Adipogen), anti-NLRP3 mouse mAb (Cryo-2; Adipogen), and anti-NLRP3 rat mAb (Clone 768319; R&D Systems). Anti-ASC rabbit polyclonal Ab (N-15), anti-β-actin goat polyclonal Ab (C-11), anti-ubiquitin mouse mAb (sc-8017), all horseradish peroxidase (HRP)-conjugated secondary Abs, G5 deubiquitinase (DUB) inhibitor, and protein A-agarose (sc-200) were from Santa Cruz Biotechnology. Murine IL-1β ELISA kit (Biolegend), fluo 4-AM (Life Technologies), probenecid (Sigma-Aldrich), propidium iodide (Life Technologies), FITC-conjugated 10-kDa dextran (Life Technologies), lactate dehydrogenase (LDH) cytotoxicity detection kit (Roche), and CellTiter-Glo ATP assay kit (Promega) were used. Anti-IL-1β mouse mAb was provided by the Biological Resources Branch, National Cancer Institute, Frederick Cancer Research and Development Center (Frederick, MD).
Murine models.
Wild-type (WT) C57BL/6 mice were purchased from Jackson Laboratories. Mice lacking both caspase-1 and caspase-11 on a C57BL/6 background (Casp1/11−/−) have been previously described (24). All experiments and procedures involving mice were approved by the Institutional Animal Care and Use Committee of Case Western Reserve University. BMDCs were isolated from 9- to 12-wk-old mice euthanized by CO2 inhalation. Bones from TRPM2 knockout mice were shipped in serum-free DMEM on ice from Penn State Hershey Children's Hospital to Case Western Reserve University by overnight courier service. Femurs and tibiae were removed, and the intact bones were briefly immersed in 70% ethanol and then washed in sterile PBS before exposing the marrow cavity for removal. PBS was used to wash out the marrow plugs, and the bone marrow cells were triturated, filtered through sterile mesh, and then resuspended in DMEM (Sigma-Aldrich) supplemented with 10% bovine calf serum (HyClone Laboratories), 100 U/ml penicillin, 100 μg/ml streptomycin (Invitrogen), 2 mM l-glutamine (Lonza), and 1.5% J558L cell-conditioned medium (which contains the granulocyte macrophage colony-stimulating factor necessary for BMDC differentiation). BMDCs were cultured and prepared for experiments exactly as described in our recent study (23). Loosely adherent BMDCs were collected, pelleted by centrifugation (300 g, 5 min), resuspended to 1 × 106 cells/ml in fresh growth medium, and plated in six-well (2 ml/well) tissue culture plates for Western blot assays, 12-well (1 ml/well) plates for K+ atomic absorbance analysis, and 24-well (0.5 ml/well) plates for IL-1β ELISA, fluo 4 assays of cytosolic [Ca2+] changes, propidium2+ influx assays, LDH release assays of cytotoxicity, or assays of lysosome integrity using FITC-dextran-loaded cells. All assays of BMDC function were performed between days 7 and 10 postisolation.
Priming and stimulation of BMDCs.
BMDCs were primed with 1 μg/ml LPS for 4 h at 37°C. The primed cells were processed for experiments as described previously (23). Briefly, LPS priming medium was aspirated after 4 h and replaced with either Ca2+-containing balanced salt solution (BSS) (130.0 mM NaCl, 4.0 mM KCl, 1.5 mM CaCl2, 1.0 mM MgCl2, 25.0 mM Na HEPES, 5.0 mM d-glucose, 0.1% BSA, pH 7.4) or Ca2+-free BSS (130.0 mM NaCl, 4.0 mM KCl, 300.0 μM EGTA, 1.0 mM MgCl2, 25.0 mM Na HEPES, 5.0 mM d-glucose, 0.1% BSA, pH 7.4). BMDCs in BSS were preincubated for 5 min at 37°C and then stimulated with 10 μM NG, 6 μM ionomycin, indicated concentrations of LLME, indicated concentrations of Imject Alum, or indicated concentrations of MSU for various times as indicated in specific experiments. Where indicated, BMDCs were treated with 100 μM CA-074-ME during the last 60 min of LPS priming.
IL-1β release.
LPS-primed BMDCs in 24-well plates (5 × 105 cells, 0.5 ml BSS/well) were stimulated with NG, LLME, or a combination of NG + LLME at 37°C. After various times (routinely for 30 min), extracellular medium was removed from each well and centrifuged at 10,000 g for 15 s to pellet detached cells. In some experiments, the LPS-primed BMDCs were stimulated with increasing concentrations of Imject Alum (30- 3,000 μg/ml) or MSU (50-1,000 μg/ml) crystal suspension added to BSS additionally supplemented with nonessential and essential amino acids, 2 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin for 6 h. The cell-free supernatants were assayed for murine IL-1β by sandwich ELISA according to the manufacturer's protocol.
Western blot analyses.
LPS-primed BMDCs in six-well plates (2 × 106 cells, 1 ml BSS/well) were stimulated with NG, LLME, or combination (NG + LLME) at 37°C. After various times (routinely for 30 min), the cells and extracellular media samples were processed for SDS-PAGE and Western blot as described previously (23). Primary Abs were used at the following concentrations: 1.0 μg/ml for caspase-1, 5.0 μg/ml for IL-1β, 4.0 μg/ml for NLRP3, 0.4 μg/ml for ASC, and 0.2 μg/ml β-actin. HRP-conjugated secondary Abs were used at a concentration of 0.13 μg/ml. Chemiluminescent images of Western blots were developed and saved using a FluorChem E image processor (Cell Biosciences).
Assay of ASC oligomerization.
Isolation of detergent-insoluble ASC oligomeric complexes from the extracts of LLME- or NG-stimulated BMDC was performed as described previously (23). Briefly, this involved isolation of detergent-soluble and detergent-insoluble fractions from whole cell lysates by centrifugation at 15,000 g for 15 min at 4°C. The resuspended detergent-insoluble pellets were incubated with 2 mM DSS (in PBS) to cross link ASC multimers, repelleted, and processed for SDS-PAGE and anti-ASC Western blotting.
Assay of NLRP3 ubiquitination status.
LPS-primed BMDC in six-well plates (2 × 106 cells, 1 ml BSS/well) were pretreated with or without 15 μM PR-619 DUB inhibitor for 15–30 min before stimulation with NG, LLME, or combination of NG + LLME for 30 min at 37°C. After removal of the extracellular medium, the cells were lysed in 550 μl RIPA buffer (PBS supplemented with 0.5% sodium deoxycholate, 0.1% SDS, 1.0% Igepal, pH 7.4, plus protease inhibitors, and, where indicated, 2.0 μM G5 DUB inhibitor). After clearance by centrifugation (13,000 g, 15 min, 4°C), a 500-μl aliquot of each lysate was supplemented with 0.5 μg Cryo-2 anti-NLRP3 mAb. After overnight incubation at 4°C with continuous rotation, the lysates were supplemented with 20 μl of protein A agarose beads and incubated 60 min at 4°C to bind anti-NLRP3 immune complexes. The protein A beads were sedimented (13,000 g; 5 min), washed three times with 500-μl aliquots of RIPA buffer, and then extracted into 40 μl of SDS-PAGE sample buffer with boiling for 5 min. The extracted anti-NLRP3 immune complexes were resolved by SDS-PAGE (4–20% polyacrylamide minigels), transferred to PVDF, and analyzed by serial Western blotting with anti-ubiquitin (1 μg/ml) and anti-NLRP3 (1 μg/ml Cryo-2 mAb).
Analysis of cellular K+ content by atomic absorbance spectrophotometry.
LPS-primed BMDCs in 12-well plates (106 cells, 1 ml BSS/well) were stimulated for indicated times with NG or LLME at 37°C before removal of the extracellular medium. The adherent BMDCs were extracted into 10% nitric acid, and the K+ content in the acid extracts was quantified by atomic absorbance spectrophotometry (Agilent 50AA atomic absorption spectrophotometer) as described previously (23).
Propidium2+ influx assay.
LPS-primed BMDCs in 24-well plates (5 × 105 cells/well) were briefly washed with PBS before the addition of 0.5 ml BSS (Ca2+-containing or Ca2+-free BSS) supplemented with 2.0 μg/ml propidium iodide to each well. LLME- or NG-stimulated propidium2+ influx was fluorimetrically monitored (540em → 620ex at 30-s intervals) with a Synergy HT plate reader (BioTek) preheated to 37°C. The agonist-induced increases in fluorescence at each time point were normalized as a percentage of the maximum fluorescence measured after complete permeabilization of all BMDCs within the well with 1% Triton X-100.
Fluo 4 assay of cytosolic [Ca2+].
LPS-primed BMDCs in 24-well plates (5 × 105 cells/well) were loaded with fluo 4-AM and assayed for LLME-induced changes in cytosolic [Ca2+] as described previously (23) using the Synergy HT reader preheated to 37°C. Baseline fluorescence (485em → 528ex) was recorded at 30-s intervals for 10 min. Cells were then stimulated with 6 μM ionomycin or indicated concentrations of LLME for 30 min, and 485ex → 528em fluorescence was recorded at 30-s intervals. Assays were terminated by BMDC permeabilization of the cells with 1% Triton X-100. The changes in 485ex → 528em fluorescence were used to calculate cytosolic [Ca2+] by standard calibration methods (16).
Assay of cytotoxicity (LDH release).
LPS-primed BMDCs in 24-well plates (5 × 105 cells, 0.5 ml BSS/well) were stimulated for various times with the indicated concentrations of LLME or 10 μM NG at 37°C. Extracellular medium was removed from each well and centrifuged at 10,000 g for 15 s to pellet detached cells. The cell-free supernatants were then assayed for LDH enzyme activity using an LDH cytotoxicity detection kit (Roche) according to the manufacturer's protocol. The released LDH was normalized to total LDH content measured in 1% Triton X-100-permeabilized samples of BMDCs.
Assay of cellular ATP content.
Intracellular ATP content was assayed using the Cell Titer-Glo Viability kit (Promega) that uses a proprietary thermostable recombinant luciferase for quantification of cell viability based on ATP content. BMDCs (500,000 cells/well) in 24-well plates were incubated in 0.5 ml BSS and stimulated for 10 or 30 min with 10 μM NG or various concentrations of LLME. The BSS was removed, and each well was extracted into 100 μl of CellTiter-Glo permeabilization buffer. After permeabilization on ice, 10 μl of each extract was diluted 1:10 with 90 μl of BSS and mixed with 100 μl reconstituted Cell Titer-Glo assay reagent per well of a 96-well white plate. The ATP-dependent bioluminescence was recorded using the BioTek Synergy HT plate reader (1-s integration of emitted light) and quantified by comparison to ATP standards assayed under identical conditions.
Assay of lysosomal membrane permeabilization.
A previously described method (8) was adapted for online analysis of lysosomal membrane permeabilization during stimulation of BMDCs with LLME or NG. BMDCs in 24-well plates (5 × 105 cells/well) were incubated in complete DMEM supplemented with 100 μg/ml FITC-conjugated dextran (10 kDa). After overnight (∼15 h) loading, the FITC-dextran-supplemented DMEM was replaced, and the cell monolayers were washed once with 1 ml PBS. The FITC-dextran-loaded BMDCs were then primed with LPS (1.0 μg/ml in complete DMEM, 0.5 ml/well) for 4 h. The priming medium was replaced with 0.5 ml BSS (Ca2+-containing or Ca2+-free BSS) per well and the plate transferred to the Synergy HT reader preheated to 37°C. Baseline fluorescence (485em → 528ex) was recorded for 5 min at 30-s intervals. Cells were then stimulated with 10 μM NG or indicated concentrations of LLME for 30 min with 485ex → 528em fluorescence recorded at 30-s intervals. Immediately afterward, phase-contrast and epifluorescence images of the BMDCs in each well were recorded using a Zeiss Axiovert 25 Microscope equipped with a 485-nm/540-nm filter set, QCam1394 digital camera, and QCapturePro imaging software (QImaging).
Data processing and analysis.
All experiments were repeated two to five times with separate BMDC preparations. Figures illustrating Western blot results and changes in FITC-dextran subcellular localization are from representative experiments. Figures illustrating quantified changes in IL-1β secretion, intracellular K+ content, cytosolic [Ca2+], pyroptotic propidium2+ influx, or extracellular LDH activity represent the means (± SE) from two to five independent experiments. Quantified data were statistically evaluated by one-way ANOVA with Newman-Keuls posttest using Prism 3.0 software.
RESULTS
LLME induces concentration-dependent and synchronized increases in LMP in murine DCs.
We previously reported that treatment of LPS-primed murine BMDCs with 1 mM LLME for 30 min induces robust (∼10 ng/ml from 106 cells) release of IL-1β via a mechanism strictly dependent on NLRP3·ASC inflammasome assembly (23). The ability of LLME to elicit particularly rapid NLRP3 inflammasome assembly distinguishes it from the particulate stimuli typically used in studies of LMP-mediated inflammasome activation. Particulate activators require phagocytosis and fusion of the particulate-loaded phagosomes with lysosomes. This complex cell biology will limit the rate and increase the heterogeneity of LMP-dependent responses in DC or macrophage populations, such that lysosome destabilization and NLRP3 activation within the cells will be slower and less synchronized. We reasoned that synchronization of lysosomal destabilization within the cells comprising a BMDC test population would facilitate analysis of the temporal and quantitative relationships between LMP and the extent of NLRP3 activation. LLME is transported into the cytosol via plasma membrane amino acid transporters and then into the lysosome lumen via lysosomal amino acid transporters. Lysosomes in leukocytes function as effective sinks for LLME accumulation due to their particularly high content of cathepsin C, also known as dipeptidyl peptidase-I (DPP-I). Via its reverse reaction, DPP-I rapidly catalyzes the conjugation of Leu-Leu dipeptides into Leu4, Leu6, and Leu8 polymers that act as intraluminal destabilizers of the lysosomal membrane permeability barrier (53).
To verify that LLME can induce rapid, synchronous, and concentration-dependent lysosome destabilization in our BMDC experimental model, we loaded the cells with 10-kDa FITC-dextran. Dextrans are internalized via endocytosis and trafficked to lysosomes. In healthy cells, FITC-dextran possesses low fluorescence within the acidic pH environment of the lysosomal lumen. If only the lysosomal pH gradient is dissipated, the resulting alkalinization increases fluorescence of FITC-dextran compartmentalized within lysosomes to produce brighter lysosomal puncta within individual cells and increased total fluorescence within the cell population. If lysosomal integrity is completely disrupted, the entrapped FITC-dextran is released into the neutral (pH 7.2) cytosol; this results in bright but nonpunctate fluorescence at the single cell level and increased total fluorescence within the cell population (Fig. 1A). We used a plate reader to continuously track changes in total fluorescence in FITC-dextran-loaded BMDC monolayers (Fig. 1, B and 1D) and fluorescence microscopy to image FITC-dextran subcellular localization (Fig. 1, E and F). In most LPS-primed but unstimulated BMDCs, FITC-dextran localizes to endosomes/lysosomes as perinuclear punctae with dim fluorescence (Fig. 1, E and F, i). However, in a minor fraction of nonstimulated cells, the FITC-dextran is released into the cytosol to produce a bright but diffuse pattern of fluorescence, which fills the entire cell volume (Fig. 1E, arrowhead), consistent with complete loss of lysosomal membrane integrity. As a positive control, we observed that NG induced a slowly developing (over 15 min) increase in total fluorescence in the plate reader assay (Fig. 1B) and the retention of punctate labeling but with brighter punctal intensity by cell imaging (Fig. 1F, ii). This is consistent with dissipation of the lysosomal transmembrane pH gradient via NG K+/H+ exchanger activity but retention of the 10-kDa dextran within the lysosomal lumen. In contrast, stimulation of BMDCs with LLME triggered concentration-dependent and time-dependent increases in total cell fluorescence (Fig. 1, B and C) that correlated with increases in cytosolic accumulation of 10-kDa dextran, indicative of collapsed lysosomal integrity (Fig. 1F, iii–vi). Notably, cells treated with 0.5 mM LLME were characterized by both low-level accumulation of cytosolic dextran and retention of bright punctae but at lower numbers (Fig. 1F, iii). This suggests complete membrane permeabilization in only a subset of lysosomes. The phase-contrast images showed large vacuoles consistent with osmotic swelling of lysosomes. LLME (1 mM) induced a more rapid, but still submaximal, increase in total fluorescence (Fig. 1B) and higher cytosolic dextran levels but with some retention of punctate labeling (Fig. 1F, iv). Stimulation of BMDCs with 2 or 3 mM LLME for 30 min resulted in complete collapse of most lysosomes, as evidenced by bright cytosolic staining (Fig. 1F, v and vi) in the majority of cells and very rapid (t½ < 2 min) increases in total fluorescence (Fig. 1, B and C). Notably, preincubation of FITC-dextran-loaded/LPS-primed BMDCs with 100 μM CA074-Me markedly attenuated the rates of LMP (as indicated by the increase in total fluorescence) induced by all tested concentrations of LLME (Fig. 1D). This verified that the ability of LLME to induce LMP requires cathepsin-C-mediated conversion of the LLME precursor to polyleucine peptides. Although CA074-Me has previously been considered a specific cathepsin-B inhibitor, Orlowski et al. (41) recently reported that this reagent broadly inhibits multiple cathepsins in both WT and cathepsin-B knockout murine macrophages. This results in strong suppression of LMP-mediated activation of IL-1β release in response to LLME or particulate stimuli (41). Brojatch and colleagues (4, 5) have determined that the ability of CA074-Me to suppress LLME-mediated LMP is due to inhibition of cathepsin-D-mediated processing of pro-cathepsin-C into active cathepsin-C.
Fig. 1.
Leu-Leu-O-methyl ester (LLME) induces concentration-dependent and synchronized increases in lysosomal membrane permeabilization (LMP) in murine dendritic cells. A: schematic of experiments utilizing FITC-dextran to measure lysosome membrane integrity. B: dissipation of lysosomal pH gradient and/or release of lysosomal FITC-dextran into the cytosol was assayed by measuring pH-dependent changes in the fluorescence of FITC-dextran-loaded bone marrow-derived dendritic cells (BMDCs) (485ex → 528em). Baseline recordings were taken for 5 min. Nigericin (NG) or indicated concentration of LLME was added at t = 5 min. Fluorescence was measured for 30 min subsequent to addition of stimulus. Data represent the average ± range of the fluorescence changes recorded in separate wells of BMDCs treated under identical conditions; the results are representative of n = 3 independent experiments with different BMDC preparations. C: slope of curves in B between t = 5 min and t = 10 min was calculated for each concentration of LLME. D: FITC-dextran-loaded BMDCs were treated with or without 100 μM CA074Me during the final 2 h of the 4-h LPS priming incubation before kinetic analysis of changes in FITC-dextran fluorescence during exposure to the indicated concentrations of LLME. Data points indicate the values from separate wells (n = 1 for each [LLME]) in 1 experiment representative of 2 similar experiments. E: loss of lysosome membrane integrity was assayed by fluorescence microscopy of nonstimulated, FITC-dextran-loaded BMDCs. Dashed arrow shows a cell with punctate fluorescence indicative of lysosome-restricted FITC-dextran compartmentation. Solid triangle shows a cell with cytosolic localization of FITC-dextran indicative of a complete loss of lysosome integrity. F: BMDCs were loaded with FITC-dextran and stimulated with NG or the indicated concentrations of LLME for 30 min. Cells were subsequently visualized by both phase contrast and fluorescence microscopy. Data are representative of images from 2 independent experiments; each illustrated image is from the same experiment using the same BMDC preparation. RFU, relative fluorescence units.
NLRP3 inflammasome signaling is activated by low-level LMP but inhibited by high-level LMP.
The Fig. 1 data indicate that LLME elicits rapid and synchronous increases in LMP, which are graded in magnitude depending on [LLME]. We used identical experimental conditions and the same range of LLME concentrations to characterize the kinetics and efficacy of LMP-triggered NLRP3 inflammasome signaling, as indicated by IL-1β release, in LPS-primed BMDCs (Fig. 2, B and C). Consistent with our previous study (23), 1 mM LLME elicited maximal IL-1β release within 30 min, and no additional release was observed with 60- or 120-min exposure times (Fig. 2B). Unexpectedly, stimulation of BMDCs with either lower (0.5 mM) or higher (2 or 3 mM) concentrations of LLME resulted in less IL-1β release at each time point; this indicated that the attenuated IL-1β release observed with supramillimolar LLME was not due to slower rates of inflammasome activation. Thus a bell-shaped (or inverted V) concentration-response relationship defines the effect of LLME on IL-1β release with a peak response at 1 mM. The ability of LLME to stimulate IL-1β release reflected inflammasome signaling and was absent in caspase-1-deficient BMDCs. Notably, the maximal IL-1β release (8–10 ng/ml) observed with 1 mM LLME was invariably twofold lower in magnitude than that (16–30 ng/ml) triggered by NG in parallel samples of the same BMDC preparations (Fig. 2C).
Fig. 2.
Nucleotide-binding domain, leucine-rich-repeat-containing family, pyrin domain-containing 3 (NLRP3) inflammasome signaling is activated by low-level LMP but inhibited by high-level LMP. A: schematic illustration of how LLME activates LMP induction of the NLRP3 inflammasome signaling pathway. B and C: kinetics and concentration-response relationships that define LLME-induced regulation of IL-1β release (measured by ELISA) in LPS-primed wild-type (WT) or caspase-1−/− BMDCs. Data points represent the means ± SE from 4 experiments; *P < 0.05; ns, not significant compared with 1 mM LLME. Parallel wells from each BMDC preparation were also stimulated with 10 μM NG for the indicated times (B) or 30 min (C). D–G: kinetics and concentration-response relationships that define LLME-induced activation of pyroptotic pores, as measured by influx of propidium2+ and accumulation of fluorescent propidium-DNA complexes, in LPS-primed WT or caspase-1−/− BMDCs. D and E: data points represent the average ± range of the mean values from 2 independent experiments, each based on 2 technical replicates. Parallel wells from each BMDC preparation were also stimulated with 10 μM NG for 30 min. F: rates of propidium2+ influx calculated from the time courses in D and E and 1 other experiment were plotted as a function of [LLME]. Data points for WT cells represent the means ± SE from 4 independent assays performed with 3 different BMDC preparations; *P < 0.05; ns compared with 0.5 mM LLME. Data points for Casp1−/− BMDCs are the average ± range from 2 experiments. G: BMDCs were treated with or without 100 μM CA074Me during the final 2 h of the 4-h LPS priming incubation before kinetic analysis of changes in propidium2+ accumulation during exposure to the indicated concentrations of LLME. Data points represent the average ± range of technical duplicates from a single experiment. H: LPS-primed WT BMDCs were stimulated with the indicated concentrations of Imject alum for 6 h. IL-1β release was measured by ELISA, and data points represent the means ± SE of triplicate wells from a single experiment representative of 2 similar experiments; *P < 0.05 compared with 1,000 μg/ml alum. I: LPS-primed WT BMDCs were stimulated with the indicated concentrations of monosodium urate (MSU) for 6 h. IL-1β release was measured by ELISA, and data points represent the means ± SE of values from 2 independent experiments, each based on 2 technical replicates.
Activation of NLRP3 and other inflammasome platforms also triggers pyroptotic cell death. A critical signal for initiation of the pyroptotic cascade is the caspase-1-mediated induction of plasma membrane channels or pores permeable to the major inorganic osmolytes (e.g., Na+, Cl−, K+) and small osmotically active metabolites (Fig. 2A). Opening of these pyroptotic channels leads to osmotic cell swelling, which, if sustained, results in cytolysis. We (2, 23) have shown that pyroptotic channel/pores are also permeable to cationic DNA-intercalating dyes, such as propidium2+ (Mr 416 Da), which can be used to kinetically track the caspase-1-dependent opening of the channel/pores in DCs or macrophages under experimental conditions identical to those in the assays of lysosome disruption (Fig. 1B) and IL-1β release (Fig. 2, B and C). Consistent with our previously reported findings, NG-stimulated propidium2+ influx was initiated after a 10–12-min lag phase in WT BMDCs (Fig. 2D) and was absent in caspase-1−/− BMDCs (Fig. 2E). Treatment of BMDCs with increasing concentrations of LLME also induced propidium2+ influx in both WT and caspase-1-deficient BMDCs after a 10-min lag time. However, LLME-induced propidium2+ influx was defined by a bell-shaped concentration-response relationship in WT cells but a near-hyperbolic curve in the cells lacking caspase-1 (Fig. 2F). Collapse of lysosome membrane integrity triggers a caspase-independent form of necrotic cell death involving permeabilization of the plasma membrane (3). Figure 2E shows that all tested concentrations of LLME induced a slowly developing increase in propidium2+ fluorescence in the caspase-1-deficient BMDCs. These results indicate that LLME-induced LMP can trigger propidium2+ uptake both via caspase-1-dependent pyroptotic channel/pores and a caspase-1-independent mechanism. Preincubation of LPS-primed BMDCs with CA074-Me markedly suppressed the induction of propidium2+ influx by all tested concentrations of LLME (Fig. 2G illustrates the effects on 1 and 3 mM LLME); this verified that the effects of LLME on plasma membrane permeability to propidium2+ required cathepsin-mediated conversion of LLME to lysosome-disrupting polyleucine peptides.
Eisenbarth et al. (12) previously reported a bell-shaped concentration-response relationship for alum-induced IL-1β release from murine macrophages. We observed a similar relationship in BMDCs stimulated with increasing concentrations of alum for 6 h (Fig. 2H). In contrast, a drop-off in IL-1β release was not apparent in BMDCs stimulated with increasing concentrations of MSU, another type of particulate inflammasome activator (Fig. 2I). Thus biphasic effects on NLRP3 inflammasome signaling can be elicited by some, but not all, stimuli that compromise lysosome integrity.
Thus two different readouts of inflammasome signaling (IL-1β release and pyroptotic pore activity) suggest that the slower, partial LMP induced by submillimolar LLME generates stimulatory signals for NLRP3 activation but that these signals are antagonized or reversed by the high-level LMP triggered by supramillimolar LMP.
The inhibitory effect of high-level LMP on NLRP3 inflammasome signaling occurs downstream of K+ efflux, upstream of ASC oligomerization, and independently of decreases in intracellular ATP and proteolytic degradation of inflammasome protein components.
We (23) and others (38) have established a critical role for decreased cytosolic [K+] as a proximal signal for initiation of NLRP3 inflammasome assembly by LMP stimuli (see signaling cascade in Fig. 2A). We tested whether the inhibitory effects of high-level LMP on IL-1β release were due to attenuated K+ efflux. Kinetic analysis indicated that 1 mM LLME triggered a rapid decrease (near maximal within 10 min) in the intracellular [K+] of WT BMDCs that was insensitive to pharmacological inhibition of caspase-1 (data not shown). In either WT (Fig. 3A) or caspase-1-deficient (Fig. 3B) cells, LLME elicited concentration-dependent decreases in cytosolic [K+] that were maximal at 1 mM LLME. Notably, the maximal decrease induced by LLME was less that that elicited by NG. Thus the lower efficacy of LLME relative to NG as an activator of IL-1β release correlates with its lower efficacy as an activator of K+ efflux. However, in contrast to LLME-induced IL-1β release or pyroptotic pore formation, LLME-stimulated K+ efflux was not characterized by a biphasic concentration-response relationship; this indicates that the suppressive effect of high-level LMP on NLRP3 inflammasome signaling occurs downstream of the initiation by decreased cytosolic [K+].
Fig. 3.
The inhibitory effect of high-level LMP on NLRP3 inflammasome signaling occurs downstream of K+ efflux, upstream of ASC oligomerization, and independently of proteolytic degradation of inflammasome protein components. A and B: concentration-response relationships for LLME-induced regulation of K+ efflux in WT (A) or caspase-1−/− (B) BMDCs. In A, parallel wells from the WT BMDC preparations were also stimulated with 10 μM NG for 30 min. Data points represent the means ± SE from 4 experiments for A and 3 experiments for B. C and D: LPS-primed WT BMDCs were stimulated with various combinations of NG (10 μM) and LLME (1 mM or 5 mM) for 30 min. C: IL-1β release was assayed by ELISA. Data points represent the means ± SE from 3 experiments; *P < 0.05, ***P < 0.001. D: expression levels of ASC, procaspase-1, and pro-IL-1β, as well as the processing and release of caspase-1 and IL-1β, were assayed by Western blot analysis of cell lysates or extracellular conditioned medium (ECM). The detergent-insoluble pellets from the whole cell lysates were isolated and treated with DSS cross linker to assay formation of ASC inflammasome filaments. E: kinetics of pyroptotic pore induction (as assayed by influx of propidium2+) was measured during single stimulation with 10 μM NG, 1 mM LLME, or 5 LMME, or during serial costimulation with LLME plus NG in LPS-primed WT BMDCs. Baseline propidium2+ uptake was recorded for 5 min; LLME was added to the indicated wells at t = 5 min, and NG was added to indicated wells at t = 15 min. Data represent the average ± range of 2 technical replicates from 1 experiment representative of 2 independent experiments with different BMDC preparations. F: intracellular ATP was measured in LPS-primed BMDCs stimulated for 10 or 30 min with 1 or 5 mM LLME or 10 μM NG. Values were normalized to the ATP content in untreated (Utx) cells and represent the average ± range of values from 2 experiments with the LLME stimuli and 1 experiment with NG.
We next tested whether the reduced efficacy of supramillimolar LLME in stimulating IL-1β release reflected deficient assembly of the NLRP3 inflammasome complex. If the robust LMP triggered by high [LLME] alters the expression levels or function of one or more of the members of this complex (NLRP3, ASC, caspase-1, or pro-IL-1β), this may suppress inflammasome activation by other NLRP3 agonists such as NG. Indeed, cotreatment of BMDCs with 5 mM LLME prevented the robust IL-1β release response to NG (Fig. 3C). Consistent with the bell-shaped [LLME]-IL-1β release response curve, 5 mM LLME by itself elicited minimal IL-1β secretion. Interestingly, cells cotreated with 1 mM LLME plus NG released the same amount of IL-1β as cells stimulated with only 1 mM LLME, rather than the twofold greater amount of cytokine elicited by NG alone (Fig. 3C). We also observed suppressed processing/release of caspase-1 and IL-1β by Western blot analysis of lysates and conditioned medium from BMDCs cotreated with 5 mM LLME vs. NG alone (Fig. 3D). The Western blots confirmed that 1 mM LLME alone triggers caspase-1 activation and IL-1β processing but prevents the even more robust responses induced by NG alone. LLME alone, at 1 but not 5 mM, induced strong ASC oligomerization, albeit less intense than observed in BMDCs stimulated with NG (Fig. 3D). As with the other indices of NLRP3 inflammasome signaling, 5 mM LLME completely prevented NG-induced ASC oligomerization in cotreated cells, whereas cotreatment with 1 mM LLME plus NG yielded ASC oligomerization intensity similar to that produced by 1 mM LLME alone.
Lima et al. (27) reported that weak NLRP3 inflammasome signaling responses in murine macrophages treated for 2 h with 2.5 mM LLME correlated with cathepsin-mediated degradation of procaspase-1 and pro-IL-1β. In contrast, treatment of BMDCs with 5 mM LLME for 30 min in our experimental model did not result in obvious decreases in intracellular levels of pro-caspase-1, pro-IL-1β, monomeric ASC, or actin despite the prevention of ASC oligomerization, pro-caspase-1 autocatalytic processing, IL-1β maturation, and the release of mature IL-1β and the p20 subunit of active caspase-1 (Fig. 3D). Consistent with the ability of supramillimolar LLME to suppress NG-induced inflammasome activation (Fig. 3, C and D), pretreatment of BMDCs with 5 mM, but not 1 mM, LLME also inhibited NG-activation of pyroptotic pore opening (Fig. 3E).
The dominant-negative action of supramillimolar LLME on NLRP3 inflammasome activation by NG was rapidly (within 10 min) induced (Fig. 3E) and upstream of ASC oligomerization (Fig. 3D) but independent of inflammasome component degradation (Fig. 3D). This suggested that high-level LMP triggers rapid changes in cytosolic cofactors/conditions required for optimal conformational activation of NLRP3 oligomers per se or their interaction with ASC. NLRP3 oligomerization requires adenine nucleotide binding and hydrolysis by the central NACHT domain (11, 30). We tested whether decreases in intracellular ATP correlate with NLRP3 inflammasome suppression by high-level LMP by assaying total ATP levels after treating LPS-primed BMDCs for 10 or 30 min with 1 mM LLME, 5 mM LLME, or 10 μM NG (Fig. 3F). Indeed, 5 mM LLME triggered more rapid and greater magnitude decreases in ATP (80% within 10 min) than 1 mM LLME. However, NG per se elicited an equally rapid and robust reduction in cellular ATP consistent with its action as both a mitochondrial uncoupler in myeloid leukocytes (18) and an inducer of pyroptotic pores permeable to 400–600-Da organic molecules. The similar effects of supramillimolar LLME and NG on cellular ATP levels contrast with their dissimilar actions on NLRP3 inflammasome assembly. This suggests that an acute decrease in intracellular ATP is unlikely to be the critical factor in mediating inflammasome suppression by high-level LMP.
Taken together, the Fig. 3 experiments indicate that the K+ efflux-initiated NLRP3/ASC inflammasome assembly process is rapidly activated by lysosome-derived factors released or produced during submaximal or low-level LMP. However, this assembly process is antagonized, downstream of the K+ efflux initiation signal but upstream of ASC oligomerization, by other lysosomal factors that rapidly change the cytosolic milieu during the high-level LMP induced by supramillimolar LLME.
The inhibitory effect of high-level LMP on NLRP3 inflammasome signaling correlates with perturbation of NLRP3 ubiquitination status.
Previous studies have demonstrated that NLRP3 is ubiquitinated (via conjugation to nondegradative K63-linked polyubiquitin chains) in the basal state and rapidly deubiquitinated by various DUBs in response to “signal 2” inflammasome agonists, such as NG (22, 28, 44). Because pharmacological inhibition of DUBs greatly reduces NLRP3 inflammasome activation by such agonists, we hypothesized that high-level LMP might similarly attenuate DUB activity (Fig. 4A). Pretreatment (10–30 min) of LPS-primed BMDCs with the PR-619 DUB inhibitor markedly suppressed NG-mediated induction of the propidium2+-permeable pyroptotic pores similar to the effects of 5 mM LLME (Fig. 4B, i) or caspase-1 knockout (Fig. 2E). PR-619 also decreased pryoptotic pore activation by 0.5 and 1.0 mM LLME (Fig. 4B, ii) to the magnitudes observed in caspase-1-deficient BMDCs (Fig. 2E). Activation of IL-1β release by NG was suppressed by PR-619 (Fig. 4C) similar to the action of 5 mM LLME (Fig. 3C); the stimulatory effects of 0.5 and 1.0 mM LLME on IL-1β production were also prevented in the presence of this DUB inhibitor (Fig. 4C).
Fig. 4.
The inhibitory effect of high-level LMP on NLRP3 inflammasome signaling correlates with perturbation of NLRP3 ubiquitination status. A: model for NLRP3 ubiquitination (Ubi) status as a regulator of inflammasome activation. DUBs, deubiquitinases; PR-619 and G5, small molecule DUB inhibitors. B: kinetics of pyroptotic pore induction was measured during single stimulation with 10.0 μM NG (i), 0.5 mM LLME (ii), 1.0 mM LLME (ii), 5.0 LMME (i), or costimulation with 5.0 mM LLME plus NG (i) in LPS-primed WT BMDCs that were pretreated with or without 15 μM PR-619 for 15–20 min before stimulation. Baseline propidium2+ uptake was recorded for 5 min; NG, LLME, or NG plus LLME was added at t = 5 min. Data represent the means ± SE of values from 2 independent experiments each based on 2 technical replicates. C: BMDCs were primed or not with LPS for 4 h and then treated without or with 15 μM PR-619 for 20 min before stimulation with 10 μM NG (i) or the indicated concentration of LLME (ii) for 30 min. IL-1β release was measured by ELISA; data represent the means ± SE of 4 wells from 2 independent experiments each performed in duplicate; ***P < 0.001. D: BMDCs were primed or not with LPS for 4 h before stimulation with 10 μM NG, the indicated concentration of LLME, or 2 μM G5 for 30 min. Cell lysates were incubated with anti-NLRP3 for immunoprecipitation (IP) overnight. Immune complexes were collected with protein A agarose, resolved by SDS-PAGE, and sequentially analyzed by Western blot (WB) with anti-NLRP3 or anti-ubiquitin. E: LPS-primed BMDCs were treated without or with 15 μM PR-619 for 20 min before stimulation with 10 μM NG or the indicated concentration of LLME for 30 min. Lysates were processed for NLRP3 IP and Western blot analysis as in D. Data are from 1 experiment representative of 2 independent experiments.
We assessed the ubiquitination status of NLRP3 immunoprecipitated from the lysates of BMDCs treated for 30 min with LPS, NG, various LLME concentrations, and DUB inhibitors, alone or in different combinations (Fig. 4, D and E). Consistent with the induction of NLRP3 expression by Toll-like receptor 4 priming, there was more monomeric 120-kDa NLRP3 protein in the immunoprecipitation from LPS-treated cells; the lower content of monomeric NLRP3 in the LPS plus NG-stimulated cells likely reflects loss of NLRP3 inflammasome complexes during robust pyroptosis plus a shift to more slowly migrating species, indicative of denaturation-resistant complexes (Fig. 4, D and E). Inclusion of PR-619 during the NG stimulus suppressed accumulation of these higher-order immunoreactive NLRP3 species (Fig. 4E). Some anti-NLRP3 reactive protein migrated as a higher molecular mass band in immunoprecipitation from cells treated with 0.5–3.0 mM LLME but not 5.0 mM LLME. Probing the NLRP3 immunoprecipitation from non-LPS-primed BMDCs with anti-ubiquitin revealed modest basal ubiquitination in the 120-kDa region corresponding to monomeric NLRP3 but also an ∼250-kDa band. The intensity of this latter band was reduced in LPS-primed cells but restored when LPS priming was performed in the presence of the G5 DUB inhibitor (Fig. 4D). Although only weak ubiquitin signals were apparent in the immunoprecipitation from LPS plus NG-treated BMDCs, stronger anti-ubiquitin signals characterized the immunoprecipitation from LPS-primed cells cotreated with LLME. Treatment with 0.5–3.0 mM LLME resulted in a ladder of diffuse ubiquitinated signals in the 120–175-kDa range; the ∼250-kDa band was enhanced in the immunoprecipitation from cells treated with 3.0 or 5.0 mM LLME (Fig. 4D). These results indicate that even submillimolar LLME results in increased ubiquitination of NLRP3 but that an increasing extent of polyubiquitination to higher molecular weight conjugates is induced with supramillimolar LLME. Cotreatment of BMDCs with either NG or increasing [LLME] plus PR-619 results in enhanced accumulation of the higher molecular mass ubiquitinated conjugates in the NLRP3 immunoprecipitation (Fig. 4E).
Taken together, the Fig. 4 data indicate that the inhibitory effect of high-level LMP on NLRP3 inflammasome signaling correlates with increased NLRP3 ubiquitination status.
Influx of extracellular Ca2+ elicited by lysosome destabilization attenuates LLME-induced NLRP3 inflammasome signaling.
We previously reported that increased cytosolic [Ca2+] is neither a sufficient nor necessary signal for inflammasome activation by either canonical NLRP3 agonists (NG and extracellular ATP) or LLME. Indeed, we found that removal of extracellular Ca2+ enhanced IL-1β release in response to 1 mM LLME (23). This suggested that influx of extracellular Ca2+ may have a selective inhibitory or restraining action on NLRP3 inflammasome activation by LMP stimuli. Experiments in Fig. 5 tested this by examining multiple readouts of inflammasome signaling in LPS-primed BMDCs treated with increasing [LLME] in Ca2+-free media vs. media with normal (1.5 mM) extracellular Ca2+. Ca2+-free conditions enhanced the magnitude of IL-1β release in response to all tested [LLME] (Fig. 5, A and E); thus, the bell-shaped concentration-response relationship observed in Ca2+-containing saline was retained but with enhanced efficacy of LLME. In contrast, the efficacy of NG in stimulating IL-1β release was similar in the presence or absence of extracellular Ca2+. Western blot analysis showed greater accumulation of ASC oligomers (Fig. 5D) over the entire range of [LLME] when tested in the absence of extracellular Ca2+. The presence or absence of Ca2+ did not markedly alter intracellular expression levels of pro-caspase-1 (Fig. 5D), pro-IL-1β (Fig. 5, D and E), ASC (Fig. 5D), or NLRP3 protein (Fig. 5E) during these 30-min treatments with LLME (in one of three similar experiments, 5 mM LLME did decrease NLRP3 protein by >50%, data not shown). Importantly, removal of extracellular Ca2+ did not attenuate, but modestly accelerated, the LLME-induced LMP responses observed in FITC-dextran BMDCs (Fig. 5, B and C).
Fig. 5.
Influx of extracellular Ca2+ elicited by lysosome destabilization attenuates LLME-induced NLRP3 inflammasome signaling. A–E: LPS-primed WT BMDCs were stimulated with the indicated concentrations of LLME or 10 μM NG for 30 min in the presence/absence of extracellular Ca2+. A: IL-1β release was quantified by ELISA. Data points represent the means ± SE from 6 experiments; *P < 0.05; **P < 0.01; ***P < 0.001. B: dissipation of lysosomal pH gradient and/or release of lysosomal FITC-dextran into the cytosol was assayed in BMDCs bathed in Ca2+-free medium as described for Fig 1B. After being loaded with FITC-dextran, cells were transferred into Ca2+-free extracellular medium, and baseline recordings were taken for 5 min. NG or the indicated concentration of LLME was added at t = 5 min. Fluorescence was measured for 30 min subsequent to addition of stimulus. Data are representative of n = 2 independent experiments. C: slopes of FITC-dextran fluorescence curves from Fig. 1B ([Ca2+] = 1.5 mM) and Fig. 5B ([Ca2+] = 0 mM) were calculated between t = 10 min and t = 5 min. D and E: expression levels of ASC, NLRP3, pro-caspase-1, and pro-IL-1β, as well as the processing and release of caspase-1 and IL-1β, were assayed by Western blot analysis of cell lysates or ECM. In D, the detergent-insoluble pellets from the whole cell lysates were isolated and treated with DSS cross linker to assay formation of ASC inflammasome filaments. In E, aliquots of the extracellular medium from the BMDC stimulation conditions indicated for each Western blot lane were analyzed for IL-1β release by ELISA. D and E are representative of results from 2 experiments.
Taken together, the experiments in Fig. 5 indicate that influx of extracellular Ca2+, which is a parallel response to LLME-induced LMP, attenuates the assembly of NLRP3 inflammasomes and consequent proteolytic maturation and release of IL-1β.
Influx of extracellular Ca2+ elicited by lysosome destabilization potentiates LLME-induced cell death.
Disruption of lysosome membrane integrity can trigger a caspase-independent mode of necrotic cell death, termed LMP-induced cell death, that is characterized by subsequent disruption of plasma membrane integrity (3). In inflammasome-competent cells, such as BMDCs, LMP-induced cell death will reflect both caspase-1-dependent pyroptosis and caspase-independent necrosis (Fig. 6A). LLME induced time-dependent (Fig. 6B) and concentration-dependent (Fig. 6C) increases in release of the cytosolic enzyme LDH from WT BMDCs as an indicator of lytic cell death. Maximal LDH release induced by each concentration of LLME (or 10 μM NG) required ≥60 min of stimulation (Fig. 6B). In contrast to the bell-shaped concentration-response curve for IL-1β release (Fig. 2C), LLME-induced LDH release from WT cells was characterized by a conventional hyperbolic dependence on [LLME] with a plateau at ≥1 mM (Fig. 6C).
Fig. 6.
Influx of extracellular Ca2+ elicited by lysosome destabilization potentiates LLME-induced cell death. A: model shows signaling network that integrates LMP with the activation of plasma membrane cation fluxes, NLRP3 inflammasome activation, and induction of inflammasome-dependent and inflammasome-independent modes of regulated myeloid cell death. B and C: LPS-primed WT or caspase-1−/− BMDCs were stimulated with the indicated concentrations of LLME for 30–120 min in the presence/absence of extracellular Ca2+. Cell death was quantified by assay of LDH released into the extracellular medium. Data points represent the means ± SE from 4 experiments; **P < 0.01; ***P < 0.001. D and E: kinetics of pyroptotic pore induction (as assayed by influx of propidium2+) was measured during stimulation of LPS-primed WT or caspase-1−/− BMDCs with 10 μM NG, 1 mM LLME, or 5 mM LLME for 30 min in the presence/absence of extracellular Ca2+. Baseline propidium2+ uptake was recorded for 5 min before addition of either LLME or NG. Data points represent the average ± range of the mean values from 2 independent experiments, each based on 2 technical replicates. F: plot of the differential propidium2+ accumulation rates measured between the 15- and 30-min time points from the time courses in D and E.
Notably, despite the inability of caspase-1-deficient BMDCs to undergo canonical pyroptosis, the concentration-response relationship describing LLME-induced LDH release from these cells was nearly identical to that in WT DCs (Fig. 6C). This is consistent with previous observations of Brojatsch and colleagues (21), who reported that LLME elicited death of murine macrophages independently of pyroptosis or necroptosis. However, possible mechanisms for this alternative LMP-induced necrosis were not defined. Given 1) the known roles for Ca2+ influx in other modes of nonapoptotic cell death (14, 35, 40, 52) and 2) the modulating action of Ca2+ influx on LMP-activated NLRP3 inflammasome signaling (Fig. 5), we tested whether Ca2+ influx might contribute to LMP-induced death signaling in BMDCs. We compared the concentration-response relationships in WT and caspase-1-deficient BMDCs treated for 1 h with increasing [LLME] in the presence or absence of extracellular Ca2+ (Fig. 6C). LLME-elicited LDH release from both DC genotypes was markedly attenuated in the absence of extracellular Ca2+. The cytoprotective effect of the low Ca2+ stimulus condition was particularly pronounced in caspase-1 knockout cells as indicated by the fivefold decrease in the magnitude of LDH release.
The absence of extracellular Ca2+ also attenuated the rate of propidium2+ accumulation in both cell genotypes treated with 1 or 3 mM LLME (Fig. 6, D–F). As with the LDH release response, the ability of low Ca2+ to sustain a normal plasma membrane permeability barrier to propidium2+ was most efficacious in caspase-1-deficient BMDCs during LMP triggered by 1 mM LLME (Fig. 6E). This is consistent with the data in Figs. 2F and 6F, which indicated that only 30% of the propidium2+ influx induced by 1 mM LLME is mediated by caspase-1-activated pyroptotic pores. The combination of caspase-1 deficiency and absence of extracellular Ca2+ synergistically reduced the rate of propidium2+ uptake elicited by 1 mM LLME. Because 3 mM LLME attenuates NLRP3 inflammasome assembly, propidium2+ accumulation under these test conditions is predominated by the Ca2+-sensitive nonpyroptotic pathway and is thus equivalent in WT and caspase-1-deficient BMDCs. Consistent with our previous findings (23), the absence of extracellular Ca2+ increased the rate of pyroptotic pore-mediated propidium2+ influx elicited by NG (Fig. 6, B and C). In contrast to LLME, NG induces no caspase-1-independent propidium2+ influx [even after 2 h, as we have reported (2)], in the presence or absence of extracellular Ca2+ (Fig. 6, E and F).
Thus the experiments in Fig. 6 indicate that influx of extracellular Ca2+ is a critical signaling event in the LMP-induced necrotic cell death that occurs in parallel with, but independently from, caspase-1-mediated pyroptosis.
LMP-induced increases in cytosolic [Ca2+]: kinetics and attenuation by high-level LMP.
Given the significant but opposite effects of extracellular Ca2+ depletion on LMP-induced NLRP3 inflammasome signaling vs. LMP-induced DC death, it was important to characterize the temporal regulation of intracellular Ca2+ homeostasis during LMP. Our previous studies demonstrated that 1 mM LLME induced rapid increases in cytosolic [Ca2+] in both WT and caspase-1-deficient BMDCs but did not test whether Ca2+ homeostasis might be differentially modulated by low-level vs. high-level LMP. Thus we compared the kinetics of cytosolic [Ca2+] changes in both WT (Fig. 7A) and caspase-1 knockout (Fig. 7B) BMDCs stimulated with the same range of LLME concentrations used in the assays of LMP, inflammasome signaling, K+ efflux, propidium2+ influx, and cell death. After an ∼3-min lag, all concentrations of LLME triggered increases in cytosolic [Ca2+] in both cell genotypes. By comparison, the Ca2+ ionophore ionomycin elicited near-immediate 50-fold increases in cytosolic [Ca2+] in these cells. Unexpectedly, LLME-induced increases in [Ca2+] in both WT and caspase-1-deficient BMDCs were characterized by bell-shaped concentration-response relationships (Fig. 7C) similar to that characterizing LLME-induced IL-1β release (Fig. 2C). As for IL-1β release, 1 mM LLME stimulated the greatest response, whereas 3 mM LLME elicited a markedly slower rate of [Ca2+] increase. The response to 1 mM LLME was characterized by increasing Ca2+ influx over 10 min to reach a steady-state cytosolic [Ca2+] of ∼750 nM that was 10-fold higher than the basal level. We previously demonstrated that removal of extracellular Ca2+ prevents the sustained [Ca2+] response to 1 mM LLME with a residual small and transient [Ca2+] elevation, likely reflecting the release of lysosomal Ca2+ stores (23). Importantly, pretreatment of the BMDCs with the CA074Me cathepsin inhibitor completely suppressed the Ca2+ increases triggered by 0.5 and 1.0 mM LLME and markedly attenuated the Ca2+ responses to 2.0 or 3.0 mM LLME (Fig. 7D). This indicates that the observed perturbation of Ca2+ homeostasis by LLME requires its enzymatic conversion to lysosome-disrupting products rather than direct effects of LLME on plasma membrane Ca2+ permeability.
Fig. 7.
LMP-induced increases in cytosolic [Ca2+]: kinetics and attenuation by high-level LMP. A, B, and D: changes in cytosolic [Ca2+] were measured in fluo 4-loaded BMDCs (WT or Casp1−/− as indicated) primed with LPS. Baseline fluo 4 fluorescence was recorded for 5 min before stimulation of the cells with the indicated concentrations of LLME or 6 μM ionomycin. D: parallel wells of BMDCs were treated with or without 100 μM CA074Me during the final 2 h of the 4-h LPS priming incubation. In A and B, cytosolic [Ca2+] was calculated based on calibration after Triton X-100 (Tx)-mediated release of intracellular fluo 4 into the 1.5 mM CaCl2-containing medium followed by chelation of Ca2+ with EGTA; data points represent the means ± SE from 4 experiments. In D, time-dependent changes in fluo 4 fluorescence for WT BMDCs are directly plotted; data represent the average ± range of technical duplicates from a single experiment. C: plot of the differential rates of [Ca2+] increase measured between the 10- and 20-min time points from the time courses in A and B; **P < 0.01; ***P < 0.001; ns compared with 1 mM LLME-treated WT cells (blue symbols) or 1 mM LLME-treated Casp1−/− cells (red symbols).
Taken together, these findings suggest that LLME-induced LMP triggers rapid increases in the Ca2+ permeability of the plasma membrane that occurs parallel to, but independently from, the NLRP3 inflammasome activation response. However, as for inflammasome activation, increases in cytosolic [Ca2+] are optimally activated by signals generated during low-level LMP but attenuated by signals or cellular conditions produced during high-level LMP.
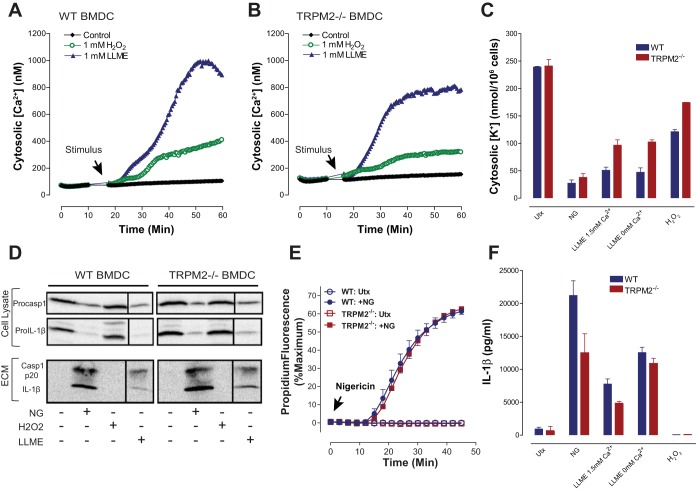
TRPM2 cation channels are not major regulators of LMP-induced NLRP3 inflammasome signaling in BMDCs but partially contribute to LMP-activated K+ efflux.
The ability of LLME to rapidly stimulate both Ca2+ influx and K+ efflux suggests that LMP may trigger gating of both Ca2+ channels and K+ channels or activation of nonselective cation channels/transporters in the plasma membrane. TRPM2 channels are expressed in many cell types including myeloid leukocytes (25). Zhong et al. (56) reported that LMP stimuli (e.g., phagocytosis of alum and silica crystals) induce mitochondrial reactive oxygen species (ROS) production in macrophages with consequent gating of ROS-sensitive TRPM2 nonselective cation channels measured by Ca2+ influx (56). Moreover, inhibition or knockout of TRPM2 channels caused an ∼50% reduction in NLRP3 inflammasome signaling. Although K+ efflux was not measured in that study, TRPM2 is known to function as a nonselective cation channel with significant K+ permeability (43, 55). Thus we tested whether TRPM2 contributes to LLME-induced Ca2+ influx, K+ efflux, and NLRP3 inflammasome signaling by comparing the responses in WT and Trpm2−/− BMDCs. The latter DCs were isolated from a TRPM2-null mouse strain that has been extensively characterized in various in vivo models of metabolic stress-induced tissue dysfunction, including cardiac and renal ischemia-reperfusion injury (13, 36). Comparative measurements of Ca2+ influx activated by 1 mM LLME in WT (Fig. 8A) and TRPM2-deficient (Fig. 8B) cells indicated that TRPM2 mediates only a minor component of this response. Likewise, Fig. 8C shows that TRPM2 deletion attenuated the magnitude of the 1 mM LLME-induced [K+] decrease by ∼25% in the either presence or absence of the normal 1.5 extracellular CaCl2. By comparison, NG-stimulated K+ efflux was similar in both BMDC genotypes. We also measured K+ efflux and Ca2+ influx in BMDCs treated with 1 mM H2O2, a commonly used extrinsic redox stress stimulus for TRPM2 channel activation. H2O2 induced a decrease in cytosolic [K+] in the WT DCs but with much lower efficacy compared with NG or LLME; this response to H2O2 was attenuated by ∼40% in the TRPM2-deficient cells (Fig. 8C). The H2O2-dependent sustained increases in BMDC cytosolic [Ca2+] were similarly reduced by ∼50% in the absence of TRPM2 (Fig. 8, A and B). Control experiments indicated that ∼200 μM H2O2 was the threshold concentration for inducing Ca2+ influx, and 1 mM H2O2 produced the maximal rate of Ca2+ influx in BMDCs (data not shown). Taken together, these direct assays of K+ efflux and Ca2+ influx indicate that plasma membrane channels or transporters other than TRPM2 are the major targets for LMP-dependent perturbation of cation homeostasis in BMDCs. Accordingly, the TRPM2-deficient DCs were characterized by a trend to a decrease in LLME-induced processing and release of IL-1β, as measured by Western blot (Fig. 8D) or ELISA (Fig. 8F). Trpm2−/− BMDCs also exhibited robust NG-activated caspase-1 cleavage (Fig. 8D), IL-1β processing (Fig. 8D), and induction of pyroptotic pores (Fig. 8E). Interestingly, H2O2 treatment failed to stimulate significant inflammasome signaling responses in either WT or TRPM2-deficient BMDCs (Fig. 8, D and F).
Fig. 8.
TRPM2 cation channels are not major regulators of LMP-induced NLRP3 inflammasome signaling in BMDCs but partially contribute to LMP-activated K+ efflux. A and B: changes in cytosolic [Ca2+] were measured in LPS-primed, fluo 4-loaded BMDCs from WT or TRPM2 knockout mice. Baseline fluo 4 fluorescence was recorded for 5 min before stimulation of the cells with 1 mM LLME or 1 mM H2O2. Cytosolic [Ca2+] was calculated based on calibration after Tx-mediated release of intracellular fluo 4 into the 1.5 mM CaCl2-containing medium followed by chelation of Ca2+ with EGTA; data points represent the average ± ranges of technical duplicates from 1 experiment representative of 2 similar experiments. C and F: LPS-primed WT or TRPM2−/− BMDCs were stimulated with 10 μM NG for 30 min, 1 mM LLME for 30 min, or 1 mM hydrogen peroxide (H2O2) for 3 h. Where indicated, BMDCs in Ca2+-free saline were also stimulated with 1 mM LLME. In C, the BMDCs were extracted with 10% nitric acid, and K content in the extracts was measured by atomic absorption spectroscopy. Data points represent the average ± range of the mean values from 2 independent experiments, each based on 2 technical replicates. In F, the extracellular medium samples were analyzed for IL-1β release by ELISA. Data points represent the average ± range of the mean values from 2 independent experiments, each based on 2 technical replicates. D: LPS-primed WT or TRPM2−/− BMDCs were stimulated with 10 μM NG, 1 mM LLME, or 1 mM H2O2 for 30 min. Intracellular pro-caspase-1 and pro-IL-1β and extracellular p20 caspase-1 subunit and mature IL-1β were assayed by Western blot. E: activation of pyroptotic pores in response to 10 μM NG was assayed by propidium2+ influx in LPS-primed WT or TRPM2−/− BMDCs. NG was added at t = 0 min, and fluorescence was recorded for 45 min. Data represent the average of duplicates from a single experiment.
Thus cation channels or transporters other than TRPM2 likely mediate the predominant component of the LMP-induced K+ efflux required to initiate NLRP3 inflammasome signaling in murine DCs. Likewise, the robust increases in cytosolic [Ca2+] triggered by LMP mainly reflect the perturbation of Ca2+-homeostatic channels or transporters other than TRPM2.
DISCUSSION
This study provides several new insights regarding the proximal signaling mechanisms that couple LMP to the assembly of NLRP3 inflammasomes and induction of regulated cell death in myeloid leukocytes. First, the study extends previous findings that indicate that changes in plasma membrane cation fluxes comprise one of the earliest (within minutes) responses to LMP. These cation fluxes include both K+ efflux, which is critically permissive for NLRP3 activation, and Ca2+ influx, which attenuates NLRP3 activation in the context of LMP. Second, we identified a biphasic relationship between the extent of LMP and the efficacy of NLRP3 activation such that inflammasome signaling is stimulated by low-level LMP but rapidly and efficiently inhibited by high-level lysosome disruption. Costimulation experiments indicated that the inhibitory signals generated by maximal LMP act in a dominant-negative manner to prevent ASC oligomerization by the canonical NLRP3 agonist NG. Third, the LMP-induced transinhibition of NG-stimulated NLRP3 inflammasome signaling occurred in the absence of, or before, any major decreases in the protein levels of NLRP3, ASC, pro-caspase-1, or pro-IL-1β. This inhibitory action correlated with an increased ubiquitination of NLRP3 and mimicked the functional effects of known DUB inhibitors on efficient NLRP3·ASC complex formation. Fourth, the study extends recent studies indicating that lysosome disruption can trigger either caspase-1-dependent or caspase-1-independent regulated cell death by showing that LMP-induced Ca2+ influx is a critical signal for the caspase-1-independent death pathway in myeloid leukocytes. Taken together, these new observations suggest a model (Fig. 6A) whereby a nonlinear signaling network defines the coupling between LMP, gating of PM cation channels, regulated cell death, and NLRP3 inflammasome activation.
LMP-generated signals for the activation of plasma membrane ion channels/transporters.
Decreased cytosolic [K+] is an obligatory signal for activation of NLRP3 inflammasome signaling by all tested LMP stimuli, including crystalline particulates and LLME (38). However, the mechanisms by which perturbation of lysosome membrane integrity is coupled to changes in plasma membrane function are largely undefined. Observations from this study and our previous report (23) indicate that LLME-induced LMP causes maximal increases in both Ca2+ influx and K+ efflux within 5–10 min independently of NLRP3 and caspase-1 activation. However, K+ efflux was defined by a simple hyperbolic dependence on [LLME] (Fig. 3, A and B), whereas a bell-shaped dose response characterized LLME-activated Ca2+ influx (Fig. 7C). These distinct concentration-response relationships strongly suggest that Ca2+ influx and K+ efflux are mediated in part by different and/or differentially regulated plasma membrane ion channels or transporters. The marked suppression of Ca2+ influx with increased LMP also argues against nonselective plasma membrane permeabilization or cell lysis as an underlying mechanism at least at early (<15 min) time points following massive LMP. An important area for future studies is to define the molecular identities of plasma membrane ion channels/transporters activated by LMP. Several TRP family cation channels are highly expressed in myeloid leukocytes; these include TRPM2, TRPV2, and TRPM7, which are characterized both by differential Na+:K+:Ca2+ permeability ratios and differential gating by various metabolic or cellular stress signals. We found that TRPM2 channels mediate only a fraction of the LLME-induced K+ efflux and Ca2+ influx responses in BMDCs (Fig. 8, A–C). In contrast to a previous study in bone marrow macrophages, TRPM2 channels in DCs only modestly contributed to LMP-induced NLRP3 inflammasome signaling (Fig. 8, D–F). This indicates that multiple species of plasma membrane ion channels (TRP family or non-TRP family) and/or transporters that are variably expressed in different subsets of myeloid leukocytes may be targeted by lysosome-derived signals as part of the LMP/NLRP3 signaling pathway. Our complementary kinetic characterization of LLME-induced lysosome disruption and Ca2+ influx indicated only a brief lag time (<5 min) between cytosolic accumulation of lysosomal molecules and increased plasma membrane ion fluxes. This may be consistent with direct gating of plasma membrane-resident cation channels by lysosomal proteases or lysosome-derived second messengers.
LMP-generated signals for the activation of NLRP3 inflammasome signaling.
Disruption of lysosome integrity induced by internalized crystalline particles, insoluble protein aggregates, or lysosomotropic small molecules is recognized as a major cellular stress stimulus for initiation of NLRP3 inflammasome-mediated proinflammatory response (20, 50). However, the reported efficacy of different lysosome destabilizing agents as inflammasome stimuli varies widely. Hornung et al. (19) first characterized LLME as an NLRP3 activator by demonstrating that 0.5–1.0 mM LLME elicited release of nanogram per milliliter levels of IL-1β in BMDCs. In contrast, Brojatsch and colleagues (27) reported only minor IL-1β production in BMDCs treated with LLME in the 2.5–20.0 mM range. Our kinetic analyses in a BMDC model resolve these disparate observations by demonstrating that bell-shaped concentration-response relationships describe the effects of LLME on multiple readouts of NLRP3 inflammasome signaling, including ASC oligomerization, caspase-1 autocatalytic processing and release, IL-1β maturation and release, and induction of pyroptotic channels/pores.
Although the signaling pathway linking lysosome permeabilization to activation of NLRP3 remains an area of active investigation, multiple studies have indicated that the proteolytic activity of lysosomal cathepsins is required for inflammasome assembly in response to particulates and LLME (10, 19). Cathepsins are released into the cytosol by lysosome destabilizing agents, and pharmacological cathepsin inhibitors such as CA-074 prevent inflammasome complex assembly downstream of LMP. Orlowski et al. (41) have recently reported that the proteolytic activity of multiple redundant cathepsins is required for NLRP3 inflammasome activation in response to LMP-inducing particulates or LLME.
LMP-generated signals for the suppression of NLRP3 inflammasome signaling.
Our findings suggest that an inhibitory factor or change in cytosolic milieu is released or produced upon extensive lysosomal disruption to attenuate NLRP3 inflammasome activation or assembly. This factor or change in milieu does not cause degradation of inflammasome components (NLRP3, ASC, pro-caspase-1, pro-IL-1β) (Fig. 5, D and E). Rather, it exerts a dominant negative inhibitory effect on the inflammasome machinery and prevents inflammasome assembly in response to the K+ efflux agonist NG (Fig. 3, D and E). Inflammasome components are regulated at the posttranslational level, and the lysosomal inhibitory factor may interfere with one or several of these posttranslational modifications. Several studies have reported that NLRP3 is ubiquitinated in the basal state and must be deubiquitinated by BRCC3 to facilitate regulation by signal 2 activators (44). Another report indicated that ASC must be linearly ubiquitinated by linear ubiquitin assembly complex to mediate efficacious inflammasome signaling (48). We observed that LLME enhanced the accumulation of ubiquitinated NLRP3 consistent with the possibility that lysosome disruption results in inhibition of DUBs (Fig. 5, D and E). Moreover, the inhibitory effect of supramillimolar LLME on NG-induced inflammasome signaling mimicked the action of PR-619, a known DUB inhibitor and suppressor of NLRP3 activation (23). However, only a minor fraction of the total NLRP3 pool was differentially ubiquitinated, consistent with observations in the BRCC3 study. In LPS-primed myeloid cells, ASC is localized to the perinuclear space and associates with IKK-α (32). Dephosphorylation of ASC by phosphatase 2A (PP2A) and dissociation from IKK-α has been linked to active inflammasome complex assembly (ASC specks) (32). Thus the inhibitory effect of high-level LMP may also modulate ASC dissociation from IKK-α and/or dephosphorylation by PP2A.
LLME also induced robust Ca2+ influx within 3–4 min (Fig. 7) that was a parallel response to, and independent of, inflammasome assembly and caspase-1 activation. In contrast to some earlier studies (39), we recently determined that increased cytosolic [Ca2+] is neither a necessary nor sufficient signal for NLRP3 inflammasome activation in different experimental models (23). This was an unexpected result given the canonical role of Ca2+ as a stimulatory second messenger in many signaling cascades. The results in this study now suggest that LMP-induced Ca2+ influx acts to attenuate the NLRP3-dependent ASC filament formation initiated by LMP-induced or NG-induced K+ efflux. This inhibitory action of elevated [Ca2+] at a very proximal step in the inflammasome signaling cascade suggests effects at 1) conformational activation of NLRP3 per se, 2) the association of NLRP3 with ASC, or 3) the process of ASC filament assembly. Given that the attenuation of NLRP3 inflammasome signaling was induced by high-level LMP and exacerbated by LMP-induced Ca2+ influx, it seemed paradoxical that high-level LMP resulted in attenuated rates of increase in cytosolic [Ca2+] (Fig. 7). This might indicate a highly nonlinear relationship between the extent of LMP and the perturbation of plasma membrane Ca2+ homeostatic mechanisms, such that Ca2+-permeable channels/transporters are initially activated by cytosolic factors or biophysical conditions generated during submaximal lysosomal disruption but suppressed by other factors/conditions that increasingly accumulate with more extensive lysosomal disruption. Given that increasing LMP resulted in inhibition of DUBs that target NLRP3, it is possible that these DUBS also modulate the ubiquitination status of the Ca2+-permeable channels or channel regulatory proteins.
Lima et al. (27) found that treatment of BMDCs with 2.5 mM LLME for 2 h resulted in proteolytic degradation of many cytosolic proteins, including pro-IL-1β and pro-caspase-1 (27). However, we observed that a 30-min incubation of BMDCs with [LLME] >1 mM strongly suppressed NLRP3 inflammasome assembly and signaling but did not decrease levels of the key inflammasome proteins (NLRP3, ASC, pro-caspase-1, pro-IL-1β) (Fig. 5, D and E). Thus, although proteolytic degradation of inflammasome components may occur at later times following LMP, this cannot explain the rapid suppression of inflammasome assembly induced within several minutes after exposure to supramillimolar LLME.
The ability of supramillimolar LLME to strongly suppress NLRP3 inflammasome activation correlated with rapid, synchronous, and complete disruption of lysosome integrity. Particulate lysosomal disrupting agents elicited only modest (by alum in Fig. 2H) or no (by MSU in Fig. 2I) declines in inflammasome signaling (as indicated by IL-1β release), as the concentrations of these stimuli were elevated beyond maximally efficacious levels in vitro. Thus the slower and incomplete LMP induced by such stimuli (data not shown) is less likely to drive the inflammasome-restrictive signals, such as increased NLRP3 ubiquitination. This raises the question whether suppression of NLRP3 inflammasome signaling by high-level LMP might occur in vivo during the inflammation of tissues that accumulate either environmental or endogenous particulate stimuli. In this regard, it is relevant to note that in vivo tissue inflammation additionally involves local perturbation of blood flow, oxygenation, pH homeostasis, and metabolite clearance. Changes in these physiological parameters may act additively or synergistically with the particulate stimuli per se to drive more extensive lysosomal destabilization. Future in vitro studies with DCs or macrophages could test this by combining the particulate stimuli with conditions, such as acidosis (46), that also regulate NLRP3 inflammasome signaling.
LMP-generated signals for activation of caspase-1-independent necrotic cell death.
Lysosome-dependent necrosis is induced by extensive disruption of the lysosomal membrane and cytosolic accumulation of lysosomal cathepsins and other hydrolases (3). Cathepsin activity is required for LMP-induced cell death, as cell viability is restored upon treatment with the pharmacological cathepsin inhibitor CA-074 or overexpression of cystatins (endogenous inhibitors of cathepsins) (3). However, the downstream signaling pathways leading to cell death subsequent to LMP remain uncharacterized. Our findings identified Ca2+ influx as one mechanism linking lysosome destabilization and necrotic cell death. We found that withdrawal of extracellular Ca2+ markedly suppressed LMP-induced necrotic cell death, as indicated by reduced LDH release in BMDCs treated with LLME in 0 mM Ca2+ medium (Fig. 6C). As schematically modeled in Fig. 6A, our findings suggest a dual role for influx of extracellular Ca2+ in the response of BMDCs to lysosome destabilization; increased cellular [Ca2+] inhibits NLRP3 inflammasome activation (and the subsequent processing/release of IL-1β) while promoting necrotic cell death in response to LMP. Notably, this Ca2+-dependent pathway for LMP-induced cell death operates in parallel with, and independently from, the caspase-1-dependent pyroptotic cell death pathway triggered by LMP-induced NLRP3 inflammasome assembly.
A pathophysiologically relevant LMP-induced, but caspase-independent, cell death is suggested by the multiple modes of death triggered by Salmonella infection of macrophages. Although NLRC4-mediated activation of caspase-1-dependent pyroptosis is a major mechanism for host control of Salmonella typhimurium (S. typhimurium) infection (31), Lage et al. (26) recently demonstrated that accumulation of bacterial flagellin in macrophage cytosol, which drives NLRC4 signaling, additionally engages a caspase-independent necrotic cell death pathway that contributes to clearance of S. typhimurium infection. That study used caspase-1/11−/− macrophages to show that bacterial flagellin causes lysosome destabilization, release of lysosome cathepsins into the cytosol, and necrotic cell death with export of IL-1α into the extracellular space. Cathepsin-mediated necrosis may be the predominant mechanism for clearing S. typhimurium infection in B cells, where the bacterium downregulates NLRC4 gene expression via phosphorylation of the Yap1 transcription factor (42).
In summary, our observations support a nonlinear signaling network (Fig. 6A) that integrates LMP with the activation of multiple PM cation channels/transporters, NLRP3 inflammasome activation, and induction of inflammasome-dependent and inflammasome-independent modes of regulated myeloid cell death. This network may be operative in multiple models of bacterial infection or sterile inflammation linked to lysosomal dysfunction. For example, both inflammasome signaling and necrotic macrophage death contribute to the development of atheromatous lesions in cardiovascular disease (10, 37). Early atheroma are characterized by viable macrophages, which internalize crystalline cholesterol and activate NLRP3 inflammasome signaling, whereas advanced atheroma are defined by accumulation of macrophages undergoing necrotic cell death. Lysosome destabilization in response to accumulation of cholesterol crystals and/or destabilizing fatty acids may drive two distinct cellular responses, with low-level LMP promoting inflammasome activation but high-level LMP driving inflammasome-independent necrotic cell death.
GRANTS
This work was supported in part by NIH-R01-GM36387 (G. Dubyak), NIH-R01-EY014362 (G. Dubyak), National Multiple Sclerosis Society grant NMSS RG5130A2/1 (G. Dubyak), and the Four Diamonds Fund of the Pennsylvania State University (B. Miller). M. Katsnelson was supported in part by 13PRE16860052 Predoctoral Fellowship from the American Heart Association and NIH training grants T32-HL105338 and T32-GM007250. K. Lozada-Soto is a Case Post-Baccalaureate Research Education Program (PREP) Scholar supported by NIH-R25-GM075207. H. Russo was supported in part by NIH training grants T32-AI089474 and T32-GM007250.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.A.K., K.M.L.-S., and G.R.D. conception and design of research; M.A.K., K.M.L.-S., H.M.R., and G.R.D. performed experiments; M.A.K., K.M.L.-S., H.M.R., and G.R.D. analyzed data; M.A.K., K.M.L.-S., H.M.R., B.A.M., and G.R.D. interpreted results of experiments; M.A.K., K.M.L.-S., and G.R.D. prepared figures; M.A.K. and G.R.D. drafted manuscript; M.A.K., K.M.L.-S., H.M.R., B.A.M., and G.R.D. edited and revised manuscript; M.A.K., K.M.L.-S., H.M.R., B.A.M., and G.R.D. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Caroline El-Sanadi for technical assistance with animal husbandry and BMDC isolation and cell culture.
Footnotes
This article is the topic of an Editorial Focus by Joel D. Schilling (48a).
REFERENCES
- 1.Aachoui Y, Leaf IA, Hagar JA, Fontana MF, Campos CG, Zak DE, Tan MH, Cotter PA, Vance RE, Aderem A, Miao EA. Caspase-11 protects against bacteria that escape the vacuole. Science 339: 975–978, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antonopoulos C, Russo HM, El Sanadi C, Martin BN, Li X, Kaiser WJ, Mocarski ES, Dubyak GR. Caspase-8 as an effector and regulator of NLRP3 inflammasome signaling. J Biol Chem 290: 20167–20184, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boya P, Kroemer G. Lysosomal membrane permeabilization in cell death. Oncogene 27: 6434–6451, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Brojatsch J, Lima H Jr, Palliser D, Jacobson LS, Muehlbauer SM, Furtado R, Goldman DL, Lisanti MP, Chandran K. Distinct cathepsins control necrotic cell death mediated by pyroptosis inducers and lysosome-destabilizing agents. Cell Cycle 14: 964–972, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brojatsch J, Lima H, Kar AK, Jacobson LS, Muehlbauer SM, Chandran K, Diaz-Griffero F. A proteolytic cascade controls lysosome rupture and necrotic cell death mediated by lysosome-destabilizing adjuvants. PLoS One 9: e95032, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruchard M, Mignot G, Derangere V, Chalmin F, Chevriaux A, Vegran F, Boireau W, Simon B, Ryffel B, Connat JL, Kanellopoulos J, Martin F, Rebe C, Apetoh L, Ghiringhelli F. Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nat Med 19: 57–64, 2013. [DOI] [PubMed] [Google Scholar]
- 7.Cai X, Chen J, Xu H, Liu S, Jiang QX, Halfmann R, Chen Zhijian J. Prion-like polymerization underlies signal transduction in antiviral immune defense and inflammasome activation. Cell 156: 1207–1222, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis MJ, Swanson JA. Technical advance: Caspase-1 activation and IL-1beta release correlate with the degree of lysosome damage, as illustrated by a novel imaging method to quantify phagolysosome damage. J Leukoc Biol 88: 813–822, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 117: 3720–3732, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464: 1357–1361, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duncan JA, Bergstralh DT, Wang Y, Willingham SB, Ye Z, Zimmermann AG, Ting JP. Cryopyrin/NALP3 binds ATP/dATP, is an ATPase, and requires ATP binding to mediate inflammatory signaling. Proc Natl Acad Sci USA 104: 8041–8046, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisenbarth SC, Colegio OR, O'Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature 453: 1122–1126, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao G, Wang W, Tadagavadi RK, Briley NE, Love MI, Miller BA, Reeves WB. TRPM2 mediates ischemic kidney injury and oxidant stress through RAC1. J Clin Invest 124: 4989–5001, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Dorado D, Ruiz-Meana M, Inserte J, Rodriguez-Sinovas A, Piper HM. Calcium-mediated cell death during myocardial reperfusion. Cardiovasc Res 94: 168–180, 2012. [DOI] [PubMed] [Google Scholar]
- 15.Groß O, Yazdi Amir S, Thomas Christina J, Masin M, Heinz Leonhard X, Guarda G, Quadroni M, Drexler Stefan K, Tschopp J. Inflammasome activators induce interleukin-1α secretion via distinct pathways with differential requirement for the protease function of caspase-1. Immunity 36: 388–400, 2012. [DOI] [PubMed] [Google Scholar]
- 16.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260: 3440–3450, 1985. [PubMed] [Google Scholar]
- 17.Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, Fitzgerald KA, Latz E, Moore KJ, Golenbock DT. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol 9: 857–865, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heid ME, Keyel PA, Kamga C, Shiva S, Watkins SC, Salter RD. Mitochondrial reactive oxygen species induces NLRP3-dependent lysosomal damage and inflammasome activation. J Immunol 191: 5230–5238, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol 9: 847–856, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hornung V, Latz E. Critical functions of priming and lysosomal damage for NLRP3 activation. Eur J Immunol 40: 620–623, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobson LS, Lima H Jr, Goldberg MF, Gocheva V, Tsiperson V, Sutterwala FS, Joyce JA, Gapp BV, Blomen VA, Chandran K, Brummelkamp TR, Diaz-Griffero F, Brojatsch J. Cathepsin-mediated necrosis controls the adaptive immune response by Th2 (T helper type 2)-associated adjuvants. J Biol Chem 288: 7481–7491, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Juliana C, Fernandes-Alnemri T, Kang S, Farias A, Qin F, Alnemri ES. Non-transcriptional priming and deubiquitination regulate NLRP3 inflammasome activation. J Biol Chem 287: 36617–36622, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katsnelson MA, Rucker LG, Russo HM, Dubyak GR. K+ efflux agonists induce NLRP3 inflammasome activation independently of Ca2+ signaling. J Immunol 194: 3937–3952, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kayagaki N, Warming S, Lamkanfi M, Walle LV, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, Zhang J, Lee WP, Roose-Girma M, Dixit VM. Non-canonical inflammasome activation targets caspase-11. Nature 479: 117–121, 2011. [DOI] [PubMed] [Google Scholar]
- 25.Knowles H, Li Y, Perraud AL. The TRPM2 ion channel, an oxidative stress and metabolic sensor regulating innate immunity and inflammation. Immunol Res 55: 241–248, 2013. [DOI] [PubMed] [Google Scholar]
- 26.Lage SL, Buzzo CL, Amaral EP, Matteucci KC, Massis LM, Icimoto MY, Carmona AK, D'Império Lima MR, Rodrigues MM, Ferreira LCS, Amarante-Mendes GP, Bortoluci KR. Cytosolic flagellin-induced lysosomal pathway regulates inflammasome-dependent and -independent macrophage responses. Proc Natl Acad Sci USA 110: E3321–E3330, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lima H Jr, Jacobson LS, Goldberg MF, Chandran K, Diaz-Griffero F, Lisanti MP, Brojatsch J. Role of lysosome rupture in controlling Nlrp3 signaling and necrotic cell death. Cell Cycle 12: 1868–1878, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopez-Castejon G, Luheshi NM, Compan V, High S, Whitehead RC, Flitsch S, Kirov A, Prudovsky I, Swanton E, Brough D. Deubiquitinases regulate the activity of caspase-1 and interleukin-1beta secretion via assembly of the inflammasome. J Biol Chem 288: 2721–2733, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu A, Magupalli VG, Ruan J, Yin Q, Atianand MK, Vos MR, Schröder GF, Fitzgerald KA, Wu H, Egelman EH. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell 156: 1193–1206, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacDonald JA, Wijekoon CP, Liao KC, Muruve DA. Biochemical and structural aspects of the ATP-binding domain in inflammasome-forming human NLRP proteins. IUBMB Life 65: 851–862, 2013. [DOI] [PubMed] [Google Scholar]
- 31.Man SM, Kanneganti TD. Regulation of inflammasome activation. Immunol Rev 265: 6–21, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin BN, Wang C, Willette-Brown J, Herjan T, Gulen MF, Zhou H, Bulek K, Franchi L, Sato T, Alnemri ES, Narla G, Zhong XP, Thomas J, Klinman D, Fitzgerald KA, Karin M, Nuñez G, Dubyak G, Hu Y, Li X. IKKα negatively regulates ASC-dependent inflammasome activation. Nat Commun 5: 4977, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440: 237–241, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Masters SL, Latz E, O'Neill LA. The inflammasome in atherosclerosis and type 2 diabetes. Sci Transl Med 3: 81ps17, 2011. [DOI] [PubMed] [Google Scholar]
- 35.Mattson MP, Chan SL. Neuronal and glial calcium signaling in Alzheimer's disease. Cell Calcium 34: 385–397, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Miller BA, Hoffman NE, Merali S, Zhang XQ, Wang J, Rajan S, Shanmughapriya S, Gao E, Barrero CA, Mallilankaraman K, Song J, Gu T, Hirschler-Laszkiewicz I, Koch WJ, Feldman AM, Madesh M, Cheung JY. TRPM2 channels protect against cardiac ischemia-reperfusion injury: role of mitochondria. J Biol Chem 289: 7615–7629, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell 145: 341–355, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muñoz-Planillo R, Kuffa P, Martínez-Colón G, Smith Brenna L, Rajendiran TM, Núñez G. K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 38: 1142–1153, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murakami T, Ockinger J, Yu J, Byles V, McColl A, Hofer AM, Horng T. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc Natl Acad Sci USA 109: 11282–11287, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neuhof C, Neuhof H. Calpain system and its involvement in myocardial ischemia and reperfusion injury. World J Cardiol 6: 638–652, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Orlowski GM, Colbert JD, Sharma S, Bogyo M, Robertson SA, Rock KL. Multiple cathepsins promote pro-IL-1beta synthesis and NLRP3-mediated IL-1beta activation. J Immunol 195: 1685–1697, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pedraza-Alva G, Pérez-Martínez L, Valdez-Hernández L, Meza-Sosa KF, Ando-Kuri M. Negative regulation of the inflammasome: keeping inflammation under control. Immunol Rev 265: 231–257, 2015. [DOI] [PubMed] [Google Scholar]
- 43.Perraud AL, Schmitz C, Scharenberg AM. TRPM2 Ca2+ permeable cation channels: from gene to biological function. Cell Calcium 33: 519–531, 2003. [DOI] [PubMed] [Google Scholar]
- 44.Py Bénédicte F, Kim MS, Vakifahmetoglu-Norberg H, Yuan J. Deubiquitination of NLRP3 by BRCC3 critically regulates inflammasome activity. Mol Cell 49: 331–338, 2013. [DOI] [PubMed] [Google Scholar]
- 45.Rajamaki K, Lappalainen J, Oorni K, Valimaki E, Matikainen S, Kovanen PT, Eklund KK. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS One 5: e11765, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rajamaki K, Nordstrom T, Nurmi K, Akerman KE, Kovanen PT, Oorni K, Eklund KK. Extracellular acidosis is a novel danger signal alerting innate immunity via the NLRP3 inflammasome. J Biol Chem 288: 13410–13419, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rock KL, Kataoka H, Lai JJ. Uric acid as a danger signal in gout and its comorbidities. Nat Rev Rheumatol 9: 13–23, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rodgers MA, Bowman JW, Fujita H, Orazio N, Shi M, Liang Q, Amatya R, Kelly TJ, Iwai K, Ting J, Jung JU. The linear ubiquitin assembly complex (LUBAC) is essential for NLRP3 inflammasome activation. J Exp Med 211: 1333–1347, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48a.Schilling JD. Dousing fire with gasoline: interplay between lysosome damage and the NLRP3 inflammasome. Focus on “NLRP3 inflammasome signaling is activated by low-level lysosome disruption but inhibited by extensive lysosome disruption: roles for K+ efflux and Ca2+ influx.” Am J Physiol Cell Physiol. doi: 10.1152/ajpcell.00174.2016. [DOI] [PubMed] [Google Scholar]
- 49.Schroder K, Tschopp J. The inflammasomes. Cell 140: 821–832, 2010. [DOI] [PubMed] [Google Scholar]
- 50.Sheedy FJ, Grebe A, Rayner KJ, Kalantari P, Ramkhelawon B, Carpenter SB, Becker CE, Ediriweera HN, Mullick AE, Golenbock DT, Stuart LM, Latz E, Fitzgerald KA, Moore KJ. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat Immunol 14: 812–820, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Storek KM, Monack DM. Bacterial recognition pathways that lead to inflammasome activation. Immunol Rev 265: 112–129, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Szydlowska K, Tymianski M. Calcium, ischemia and excitotoxicity. Cell Calcium 47: 122–129, 2010. [DOI] [PubMed] [Google Scholar]
- 53.Thiele DL, Lipsky PE. The action of leucyl-leucine methyl ester on cytotoxic lymphocytes requires uptake by a novel dipeptide-specific facilitated transport system and dipeptidyl peptidase I-mediated conversion to membranolytic products. J Exp Med 172: 183–194, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weber K, Schilling JD. Lysosomes integrate metabolic-inflammatory cross-talk in primary macrophage inflammasome activation. J Biol Chem 289: 9158–9171, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu LJ, Sweet TB, Clapham DE. International Union of Basic and Clinical Pharmacology. LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacol Rev 62: 381–404, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhong Z, Zhai Y, Liang S, Mori Y, Han R, Sutterwala FS, Qiao L. TRPM2 links oxidative stress to NLRP3 inflammasome activation. Nat Commun 4: 1611, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]