Abstract
The process of entering the bloodstream, intravasation, is a necessary step in the development of distant metastases. The focus of this review is on the pathways and molecules that have been identified as being important based on current in vitro and in vivo assays for intravasation. Properties of the vasculature which are important for intravasation include microvessel density and also diameter of the vasculature, with increased intravasation correlating with increased vessel diameter in some tumors. TGFB signaling can enhance intravasation at least in part through induction of EMT, and we discuss other TGFB target genes that are important for intravasation. In addition to TGFB signaling, a number of studies have demonstrated that activation of EGF receptor family members stimulates intravasation, with downstream signaling through PI3K, N-WASP, RhoA, and WASP to induce invadopodia. With respect to proteases, there is strong evidence for contributions by uPA/uPAR, while the roles of MMPs in intravasation may be more tumor specific. Other cells including macrophages, fibroblasts, neutrophils, and platelets can also play a role in enhancing tumor cell intravasation. The technology is now available to interrogate the expression patterns of circulating tumor cells, which will provide an important reality check for the model systems being used. With a better understanding of the mechanisms underlying intravasation, the goal is to provide new opportunities for improving prognosis as well as potentially developing new treatments.
Keywords: intravasation, metastasis, TGFB, EGFR, uPA, angiogenesis
the metastatic cascade involves a number of steps including invasion, intravasation, and extravasation, leading to growth at a secondary site. Tumor cells invade into normal tissue, often towards lymphatic or blood vessels. Upon reaching the vessel, these cells must then cross the endothelial barrier and enter the circulation. In the circulation, tumor cells may be killed by shear stress and the immune system, or become lodged in a distant capillary bed, where they can undergo extravasation and form a secondary tumor. Although the metastatic process is very inefficient, with only about 0.01% of circulating tumor cells actually leading to the formation of tumors at secondary sites, it is important to study the process of intravasation, as the rate at which tumor cells gain access to the peripheral circulation can affect the efficiency of metastasis.
There are many factors that contribute to cancer cell intravasation, including other cells in the tumor microenvironment, proteases, signaling molecules, and environmental conditions at the tumor and associated vasculature. These cells and molecules play important roles in allowing the tumor cells to invade through the basement membrane, adhere, and pass through the endothelial cell junctions in order to enter the circulation. A number of in vivo and in vitro systems have been developed to study intravasation, and they have been useful in identifying the roles of specific cells and molecules in enhancing intravasation. A number of earlier reviews have discussed intravasation in cancer (10, 31, 32, 110, 121, 126). The focus of this review will be on what has been learned regarding the mechanisms contributing to intravasation.
A clearer understanding of intravasation may provide opportunities for developing new prognostic markers as well as therapeutic options for preventing the spread of metastases. One of the complexities of studying intravasation in cancer, however, is the wide variation in properties of different tumors. Thus different tumors are likely to utilize different mechanisms for intravasation. Successful development of new markers or treatments will need to go hand in hand with identifying the appropriate tumors for which they can be used.
Intravasation by Nontumor Cells
Although this review is on intravasation by cancer cells, intravasation is a naturally occurring process that is important during development, as well as for a normally functioning immune system. Signals such as growth factors or tissue injury can guide populations of cells through normal tissue, and can lead them into crossing the endothelial barrier and into vessels. The crossing of these cells maintains vessel structure and integrity by retaining cell junctions and keeping vessels from leaking, unlike tumor cell intravasation which may in some tumors be accompanied by loss of endothelial cells and disrupted cell junctions.
Entry into the blood from the bone marrow requires intravasation by developing immune cells. Depending on the circumstances, immune cells may cross via paracellular (through the cell) or transcellular (between cells) diapedesis (79, 103). During maturation in the thymus, T-cells go through a process of selection, eventually leading to egress from the thymus and entry into circulation. From there, they become activated by antigens in lymphoid organs, and again must enter the circulation to reach sites of infection (72). Egress from the thymus and lymphoid organs requires the S1P receptor S1P1, a factor also important for proper endothelial cell function (104).
Hematogenous vs. Lymphatic Intravasation
Intravasation of tumor cells into the circulation can occur through entry into blood vessels or lymphatic vessels, with the majority of entry occurring through hematogenous (blood vessel) routes. The process of intravasation is very inefficient, and the shear stress of blood flow alone may be enough to destroy many of the cells entering into the bloodstream. Once in the bloodstream, the cancer cells must also be able to evade the immune system and implant at a secondary site to form distant metastasis.
Hematogenous intravasation can be an active or passive event, depending on the tumor type, blood vessel structure, and conditions occurring in the tumor microenvironment. During intravasation, there are a number of changes that occur within the tumor cells themselves, as discussed in the sections below. It has been seen that tumor cells can undergo changes in cytoskeletal activity due to sensing a chemoattractant gradient. There is also evidence of the upregulation of integrins and other adhesion molecules in order to facilitate attachment of tumor cells to endothelial cells (31, 62).
Lymphatic intravasation (entry into lymphatics) is another pathway by which tumor cells can enter the circulation. After converging on the major thoracic duct, lymph vessels eventually drain into the blood, thereby indirectly allowing tumor cells to gain access to the venous blood supply. However, along the way, the tumor cells encounter a series of lymph nodes, which are often the first sites of metastasis observed for a number of cancers. These sentinel lymph nodes are the ones that are closest to the site of the primary tumor, and are resected and biopsied in order to check for early signs of metastasis.
Whether a tumor cell undergoes hematogenous or lymphatic intravasation is dependent on a number of factors (128). One obvious factor could be tumor cell accessibility to vessels. Tumor angiogenesis leads to a network of microvasculature that is then available for access to tumor cells, as discussed below. It has also been seen that tumors undergo lymphangiogenesis, but that these lymph vessels within the tumor may not function as well as those formed on the outside of the tumor tissue (83). The relative amount of hematogenous versus lymphatic intravasation may depend on the relative proportions of blood vessels and lymphatics induced by the tumor.
The differences in structure between blood vessels and lymphatics likely lead to differences in the mode of intravasation. Structurally, lymphatics do not have the tight endothelial junctions seen in blood vessels. Entry into lymphatics may be limited by the ability of tumor cells to invade through intervening connective tissue, with the actual entry into the lymphatics occurring with relative ease. Additionally, the flow in lymphatic vessels is far less than that in blood vessels, making survival in the lymphatics easier, due to decreased shear stress (2). Thus the presence of metastases in local lymph nodes may reflect the ability of tumor cells to penetrate connective tissue barriers. However, the step of penetrating the blood endothelial cell barrier may require capabilities in addition to simple invasion. Therefore, the importance of the lymphatic route for development of distant metastasis is open to debate. There is not a strict correlation between development of distant metastases and the presence of a chain of lymph nodes with tumor cells going to the major thoracic duct (107). It is likely that distant metastases are initiated by direct intravasation into the blood supply either in the primary tumor itself, or potentially in the lymph nodes. The focus of this review is on mechanisms of entry into blood vessels.
In Vivo Models
There are two in vivo common measures of intravasation. The direct measure of intravasation is the measurement of circulating tumor cells. This measurement assumes that there is fairly rapid filtration of tumor cells by capillary beds, especially the lungs for cells entering the venous system, and is supported by published data (34, 51). The intravasation efficiency is then inferred from the number of circulating tumor cells. Comparisons should ideally be done with tumors of similar size and vascular density in order to be focused on intravasation. Another direct method which is technically more challenging is to directly image cells protruding through the blood vessel wall (46, 112). An indirect but simpler method is to measure metastatic burden generated by a primary tumor. Because this “spontaneous metastasis” measurement can be influenced by the ability of cells to extravasate and survive in the target organ, an accompanying “experimental metastasis” measurement should be made in parallel, in which the metastatic efficiency of cells directly introduced into the vasculature by iv injection is also determined. Multiple in vivo models have been developed to study the intricate, multistep process of tumor metastasis, including chick embryo, fluorescent transgenic zebrafish, and murine models. Each model system provides unique advantages that have been utilized to investigate and visualize tumor cell intravasation as well as the interactions between tumor and stromal cells during this event.
The chick embryo is naturally immunodeficient, which allows human tumor cells to be transplanted onto the chorioallantoic membrane (CAM) without risk of immunological rejection. Furthermore, the CAM is extensively vascularized and located directly underneath the eggshell and is thus easily accessible for manipulation and visualization. A detailed review covers the various experimental approaches using the CAM model (28). Palmer et al. (84) provide methods for the spontaneous metastasis model, which involves exposure of the CAM and subsequent engraftment of the tumor cells onto the membrane. Quantitative analysis of what are termed intravasated cells is performed by a real-time PCR amplification of human-specific Alu sequences (144) that was first developed by Kim et al. (61). This is an indirect measurement of metastasis, as it does not distinguish between circulating tumor cells in the vasculature and those that have arrested and possibly proliferated in target organs.
The process of intravasation can also be visualized using fluorescent transgenic zebrafish(77). Zebrafish are cost-effective in maintenance and optically transparent, which allows noninvasive, high-resolution imaging of tumor progression. Using this model, it was shown that RhoC, a regulator of the actin cytoskeleton, induces cytoskeletal changes to mediate intravasation (112). In this study, tumor cells were injected into the zebrafish peritoneal cavity, allowing them to invade into the tissues, remodel the vasculature, and form microtumors. The intravasation events were then visualized by confocal microscopy. Tumor cells expressing RhoC formed large membrane extensions that protruded through VEGF-induced vascular openings into the vessel lumen.
Visualization and quantitation of intravasation can also be performed in mouse models of cancer. In a murine model of breast cancer, tumor cell intravasation was directly visualized using intravital multiphoton imaging of fluorescently labeled tumor cells (46, 130). Intravasated tumor cells can be quantified using whole blood drawn from the right ventricle before the blood perfuses the pulmonary vasculature, showing that the number of CTCs correlates with lung metastasis (129). Furthermore, two patterns of tumor cell migration were identified by multiphoton intravital imaging: single cells and multicellular streams, with the multicellular streams correlating with intravasation (86). Using a Mammary Imaging Window (MIW) combined with fluorescent photoconversion, trafficking of cells can be followed over a week (56). Using this method, it was shown that vascular microenvironments promote tumor cell intravasation (42). Spontaneous lymphogenic metastasis of metastatic breast tumor cells has also been investigated in vivo through high-resolution, noninvasive fluorescence imaging (25, 47). The presence of high interstitial fluid pressure (IFP) in the tumor and fast drainage rates of the FITC-dextran into the tumor-associated lymphatic vessels suggest that lymphatic intravasation can be induced by high IFP (92).
In Vitro Models
In vitro systems such as microfluidic devices, artificial microvessels, and Transwell assays have been developed to mimic the pathophysiology of a tumor microenvironment. Microfluidic models provide a basis for studying complex cell-cell interactions within a 3D microenvironment (113). A single microfluidic chip that integrates intravasation and extravasation was used to study LOVO tumor cells and found that tumor cells degraded the ECM by the expression of MMPs. Thus, this system can be utilized to evaluate MMP inhibitors and drug candidates for intravasation (106). Zervantonakis et al. (139) designed a microfluidic-based assay that reconstructs the tumor-vascular interface in 3D and integrates it with real-time imaging and measurement of the endothelial barrier function. Specifically, this assay consists of two independent micro-channels that are interconnected via a 3D ECM hydrogel. Tumor and endothelial cells are seeded in each micro-channel and additional cell types may be added to model heterotypic cell-cell interactions during intravasation. Using this assay, the authors showed that macrophage-secreted TNFA increases endothelial permeability and intravasation rate.
Tumor-vascular interactions have also been investigated using an artificial microvessel system integrated with live-cell fluorescence microscopy (127). A perfusable artificial vessel is lined with an endothelial monolayer in a type I collagen matrix, and fluorescently labeled tumor cells are placed in the ECM around the vessel. Using this platform, it was found that during an intravasation event a single tumor cell forms membrane extensions into the lumen of the microvessel, disrupting the monolayer, and can leave a portion of its cytoplasm in the ECM as it enters the microvessel.
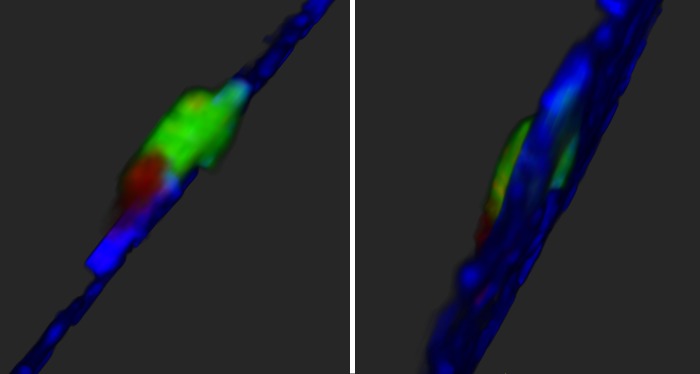
Transendothelial migration of tumor cells can also be quantified using a Transwell assay that measures movement of tumor cells from the basal to apical side of an endothelial monolayer (99). In this system, the endothelial monolayer is plated on the underside of a Transwell and establishes the appropriate polarity such that tumor cells and macrophages added to the interior of the Transwell face the basal side of the monolayer, mimicking intravasation. Imaging of the tumor cell transendothelial migration in this assay revealed that tumor cells preferentially cross the endothelial barrier via paracellular migration while in close association with macrophages (Fig. 1).
Fig. 1.
Two views of an MDA-MB-231 breast cancer cell (green) crossing an endothelial cell layer (blue) in association with a macrophage (red) in the Transwell in vitro intravasation assay (99). The basal side of the endothelium is on the right.
Angiogenesis
Intravasation is dependent on the access of tumor cells to vasculature, and thus tumors with more vasculature would be expected to have an increased intravasation rate, all other factors being equal. Consistent with this prediction, in colorectal cancer the density of intratumoral microvessels was found to be a predictive factor for the presence of circulating tumor cells (117, 133). Expression of the angiogenic factor VEGF correlated with the presence of liver metastases. Inhibition of VEGF using a functional blocking antibody was found to reduce microvessel density and intravasation of prostate cancer cells in the CAM model (22). Similarly, in the PyMT breast cancer model using intravital imaging, bursts in vascular permeability correlated with intravasation events, and both phenomena were inhibited by a VEGF blocking antibody (46). Fibroblast growth factors are also angiogenic factors (96), and expression of FGF1 in MCF7 breast cancer cells resulted in increased blood vessel density in parallel with increased intravasation frequency (141). However, these cells were unable to form macrometastases in the lung and seemed to be unable to extravasate and proliferate before eventually being cleared. On the other hand, a conflicting report concerning VEGF expression and microvessel density (120) in colorectal cancer showed metastasis associated with a lower VEGF-positive cell count and a decrease in microvessel density. However, metastasis did correlate with an increase in microvessel diameter, which is consistent with other reports supporting the importance of vessel diameter for intravasation.
Thus the specific characteristics of the blood vessels may influence the mechanism by which intravasation occurs. Yamamura et al. (133) compared the diameter of the microvessel lumens in the primary tumor tissues of patients with or without metastatic disease and found that patients with metastatic disease had significantly larger microvessels in the primary tumors. The presence of dilated sinusoidal vessels in the tumor may allow formation of tumor emboli in an invasion-independent manner (114, 115). In a renal cancer model, the majority of cells leaving the primary tumor were nonviable, consistent with a passive shedding mechanism (10, 11). Emboli surrounded by endothelium have been observed in the veins of renal cell carcinoma, hepatocellular carcinoma, and follicular thyroid carcinoma, suggesting a process where an endothelium-lined outpocketing of the tumor into the sinusoidal space could detach and enter the circulation through shearing. These emboli could have a higher efficiency of seeding distant sites (1, 34, 40, 68). Such tumor fragments have been found in the clear cell subtype of renal cell carcinoma to correlate with pulmonary metastases (55) and higher levels of VEGFA. Overexpression of VEGFA in a melanoma animal model was also shown to generate sinusoidal vessels and the presence of endothelium-coated tumor emboli (63). However, in the CAM model, expression of VEGF was found to enhance leakiness and intravasation in smaller diameter vessels (74).
Other perturbations of the vasculature can have complex consequences for intravasation. VEGFB expression can reduce blood perfusion efficiency, resulting in increased hypoxia, which can stimulate invasiveness. In parallel it can increase vessel leakiness, with the net result being increased intravasation and metastasis in tumors that grow less efficiently (135). Reduced expression of Shb, an adaptor protein downstream of VEGFR-2, can result in vascular leakiness and increased metastasis (138). Expression of the matrix metalloproteinase MMP-1 by tumor cells has also been shown to enhance vascular permeability and intravasation without affecting invasive properties of the tumor cells in the CAM assay, and a potential mediator of this effect is PAR1 (52). IL-6 and MMP-9 promote cancer metastasis by increasing resistance to apoptosis, enhancing angiogenesis, and accelerating degradation of the ECM (17, 29, 69). Conversely, activation of myosin light chain kinase in endothelial cells can be induced by intravasating tumor cells(60), leading to the activation of myosin II and inducing endothelial myosin contraction. Interestingly, inhibition of myosin regulatory light chain diphosphorylation led to a reduction in transcellular migration and an increase in paracellular migration. Thus, myosin light chain kinase expression in endothelial cells may play a key role in directing intravasation pathways.
An intriguing potential mechanism for intravasation involves vascular mimicry by tumor cells (105). Expression of the secreted proteins SERPINE2 or SLPI in mammary tumor cells increased their frequency of lining blood vessels in tumors without an identifiable endothelial surface layer, thus putting them in direct contact with blood. The expression of these proteins suppressed coagulation, likely enabling blood flow to be maintained and facilitating detachment of tumor cells from the main tumor body (122).
The vasculature has a major role in determining conditions within the tumor microenvironment including tumor hypoxia. The cell density at primary tumors, due to the limited blood supply, creates conditions where cells experience a shortage of nutrients and oxygen. These hypoxic conditions can induce the expression of VEGF, causing increased angiogenesis, lymphangiogenesis, and vessel permeability (12). Hypoxia can also increase tumor cell invasiveness by making tumor cells more sensitive to growth factors such as HGF (89, 116). This then allows tumor cells to invade through normal tissue, degrade the basement membrane, and eventually cross the endothelial barrier. Another factor regulated by hypoxia is the secreted protein angiopoietin-like 4 (ANGPTL4), which acts to disrupt endothelial cell junctions and therefore allows tumor cells to migrate into the vasculature. ANGPTL4 expression can enhance vascular permeability at the primary tumor and extravasation (49, 140); it would be interesting to test whether it plays a role in intravasation.
TGFB
A number of studies combine to make a strong case for TGFB signaling regulating intravasation in breast cancer and hepatocellular carcinoma. Muraoka et al. (80) utilized systemic long-term application of a soluble TGFB receptor fusion protein to sequester TGFB in the transgenic MMTV-PyMT breast cancer model and found suppression of circulating tumor cell numbers. Although the rate of tumor formation, vascularity, and tumor weight were not altered, treatment with the fusion protein resulted in more cystic morphology and increased apoptosis. Possible mediators of intravasation that were reduced included PI3K signaling, VIM, MMP-2, and MMP-9. A potential role for TGFBR2 in intravasation was shown by Keklikoglou et al. (58), who found that suppression of TGFBR2 expression inhibited intravasation of MDA-MD-231 breast cancer cells in the indirect CAM assay. The authors showed that expression of miR-373/520 family members also suppresses TGFBR2 expression as well as intravasation. Although miR-373 levels were too low to quantitate in patient tumors, a negative correlation was found between expression of miR-520c and TGFBR2 in ER-negative tumors, consistent with miR-520c regulating TGFBR2 levels in patients. TGFB target genes that were affected included ANGPTL4, PTHrP, and PAI-1.
Using a SMAD2-GFP expression construct to follow SMAD2 localization in the nucleus, and a CAGA12-CFP reporter for TGFB signaling, Giampieri et al. (38) visualized TGFB signaling in rat mammary adenocarcinoma MTLn3E cells undergoing single cell motility in vivo using intravital imaging. The authors also confirmed a role for SMAD signaling using suppression of SMAD4 expression. Entry into lymphatic vessels was not dependent on TGFB signaling, while overexpression of TGFB1 increased hematogenous intravasation. Intriguingly, overexpression of TGFB1 appeared to inhibit proliferation in the lung although it enhanced intravasation. Thus transient TGFB signaling at the intravasation step may be the most effective way for tumor cells to intravasate while not inhibiting their proliferation when arriving at a metastatic site. TGFB pathway activation in the primary tumor was found to be quite heterogeneous, suggesting the presence of transient signals being generated by specific local microenvironments within the tumor. Consistent with inhibition of metastatic growth by TGFB, overexpression of a downstream mediator of TGFB, p12CDK2-AP1, stimulated EMT and intravasation of hamster cheek pouch carcinoma cells, but did not enhance overall metastasis (119). Intriguingly, the presence of tumor cells which can intravasate can enhance the metastasis of other cells in the same tumor, suggesting that in a heterogeneous tumor, cells which have undergone EMT may enable the intravasation of other cells which have not undergone EMT but are better able to seed distant sites (16, 35, 118).
A number of downstream genes have been identified that could mediate TGFB's contribution to intravasation. Patsialou et al. (87) found that TGFB stimulation results in increased expression of CSF1R in basal breast cancer cells. Suppression of CSF1R expression in the tumor cells resulted in reduced in vivo motility and intravasation. Echoing the result found by Giampieri et al. (38), CSF1R activity appeared to inhibit proliferation in vivo, in parallel with stimulating invasion and intravasation. CSF1R activity was found to suppress the expression of keratins and claudins, and overexpression of claudins resulted in reduced invasion, suggesting that maintenance of the claudin low state is supported by TGFB driven expression of CSF1R.
LHX2 has been identified as another downstream target of TGFB that can enhance intravasation and metastasis (64). High levels of LHX2 in the primary tumor correlated with metastasis and worse outcome in breast cancer patients. Increased expression of LHX2 as a transgene in the PyMT model resulted in increased intravasation and metastasis, with some increase in growth as well. Both invasion and vessel size are increased with expression of LHX2, and could result in the increased intravasation capability. A possible mediator of LHX2's effect is PDGFB.
A classic downstream mediator of TGFB signaling is the transcription factor Twist. Knockdown of Twist in the 4T1 breast cancer model did not affect primary tumor or anchorage independent growth, but did significantly reduce circulating tumor cells and metastasis (134). Twist expression was higher in more metastatic breast cancer cell lines, and also in lobular breast carcinoma, which is distinguished by its invasive character. A potential downstream mediator of Twist for intravasation in the 4T1 breast cancer model is miR-10b, which targets HoxD10, a suppressor of RHOC expression (70).
In hepatocellular carcinoma, the long noncoding mRNA lncRNA-ATB (lncRNA-AL589182.3, ENST00000493038) is upregulated by TGFB (137). LncRNA-ATB acts as a competing RNA for the miR-200 family, and by inhibiting miR-200 function, it stimulates the production of the miR-200 targets ZEB1 and ZEB2. Lnc-ATB also could enhance lung and liver colonization, but that was not through EMT and miR-200 regulation but rather through increased IL-11 production and STAT3 signaling, reiterating the separation between production of circulating tumor cells and enhanced growth capability at distant sites. Another TGFB target potentially important for intravasation in hepatocellular carcinoma is CTGF/CCN2. Suppression of CTGF expression results in reduced intravasation in the CAM assay in parallel with reduced fibrosis (73). It is possible that the paracrine stimulation of tumor-associated fibroblasts by CTGF mediates an enhancement of intravasation. The exact mechanism by which this occurs remains to be determined.
Receptor Tyrosine Kinases
The most detailed examination of receptor stimulation of intravasation has been based on the EGF receptor family. Although the EGFR family is associated with tumor growth, it also can have a significant contribution to invasion and intravasation. Overexpression of the EGFR was shown to affect in vivo invasiveness, intravasation, and metastasis without affecting primary tumor growth of MTLn3 cells in SCID mice (132). Intravasation was measured as the number of circulating tumor cells. Similar results were found for fibrosarcoma and head and neck cancer in the CAM assay (74). Since EGFR overexpression enhanced the ability of tumor cells to invade locally, the enhancement of intravasation could be due to enhanced ability to approach the blood vessel, or enhanced ability to cross the blood vessel barrier. Studies comparing EGFR inhibitors with ErbB2 inhibitors suggest that EGFR signaling may be more important for the approach to the vessel, while ErbB2 signaling may be more directly involved in the intravasation step (57). Similarly, overexpression of the EGFR ligand HBEGF was also able to stimulate in vivo invasion, intravasation and metastasis with little effect on primary tumor growth in MTLn3 cells and human MDA-MB-231 breast cancer cells (142). SPINK1 also appears to act as an autocrine EGFR ligand for prostate cancer cells independent of its protease inhibitor properties, and suppression of SPINK1 expression reduced intravasation in the CAM assay (4). The sensitivity of the EGFR to low levels of ligand can be enhanced by the actin-binding protein Mena/ENAH (100). Knockout of Mena expression results in reduced intravasation in the MMTV-PyMT breast cancer model (101), while overexpression of a particular isoform, termed MenaINV, can increase intravasation together with invasion in response to EGF (90, 100). Other members of the EGFR family include ErbB2 and ErbB3, and the ErbB2/ErbB3 heterodimer, which is activated by heregulin, has been shown to enhance intravasation in SCID mice of MTLn3 and MDA-MB-435 cells (131). Factors that regulate expression of EGFR family members could also direct intravasation; a possible example is the steroid receptor coactivator-1 SRC-1. Knockout of SRC-1 resulted in reduced ErbB2 expression in the MMTV-PyMT mammary tumor model in parallel with reduced intravasation and metastasis and little effect on primary tumor growth (124). However, the SRC-1 phenotype is more complex—the grade of the primary tumor may be altered as well as expression of important factors such as CSF1 discussed below.
Downstream of the receptor, PI3K activity has been shown to contribute to intravasation. Knockdown of the endogenous p110 alpha catalytic subunit in MDA-MB-231 breast cancer cells and replacement with two mutants commonly found in cancer resulted in increased intravasation, with the helical domain mutation E545 producing the strongest enhancement (85). Mutation of the PI3K-binding sites on ErbB3 led to reduced PI3K activation, and corresponding reductions in invasion in vivo and intravasation in an in vitro intravasation assay (108). A potential downstream target of PI3K is the actin regulator N-WASP. Either expression of a dominant-negative form of N-WASP or shRNA knockdown resulted in reduced intravasation of MTLn3 breast cancer cells without affecting primary tumor growth (43). N-WASP is important for formation of invadopodia, localized F-actin-containing structures which cause localized matrix degradation (5, 45, 50), and invadopodia could enhance penetration of the endothelial basement membrane during intravasation. Intravital imaging and multiparametric analysis shows a correlation between invadopodia and intravasation (41). Similarly, an in vitro intravasation assay shows that macrophages can stimulate intravasation and that in parallel macrophages stimulate invadopodium formation and RhoA activity in MDA-MB-231 breast cancer cells (99).
A number of other regulators of invadopodium function have been found to contribute to intravasation. Arg is a tyrosine kinase that regulates cortactin activity in invadopodia, and suppression of Arg in MDA-MB-231 cells dramatically reduced invasion and intravasation in vivo, while remarkably increasing primary tumor growth and the ability of tail vein injected tumor cells to grow in the lung (39). This experiment emphasizes how invasion and intravasation can be separated from tumor growth, and is consistent with the observations noted above that tumor cells can decide to “go or grow.” Invadopodia are also regulated by integrins, and the ERM protein talin integrates adhesion signals to regulate intracellular pH through the sodium/proton antiporter NHE-1. Like Arg, suppression of talin results in reduced intravasation although primary tumor growth is stimulated (6). Cofilin actin-severing activity is regulated by intracellular pH, and thus the talin regulation of NHE1 activity also contributes to cofilin activity. An alternative mechanism of regulation of cofilin is through phosphorylation by LIMK, which suppresses its activity, and perturbation of LIMK activity also affects intravasation (125).
The ERK pathway also can contribute to intravasation. Expression of the Raf inhibitor RKIP is reduced in a number of tumors, and increased expression of RKIP in MDA-MB-231 breast cancer cells resulted in reduced invasion and intravasation in vivo without affecting tumor growth (26). More detailed analysis indicated that through regulation of the ERK pathway, RKIP affects a pathway involving myc, LIN28, let-7, and HMGA2, resulting in changes in expression of Snail and other proteins involved in tumor cell invasion as well as secretion of the chemokine CCL5, which may recruit and program tumor-associated macrophages (36).
Proteases
There is significant evidence for a role of the uPA/uPAR system in intravasation. Studies with the inoculation of HEp3 cells into the CAM demonstrated that uPA blocking antibodies could inhibit invasiveness and metastasis but not extravasation, arguing for a role in intravasation (82). Quantitation of tumor cell intravasation in the CAM assay using uPA mRNA knockdown was consistent with this possibility (61). uPA was also identified as important for invasion and intravasation by comparing high and low metastatic derivatives of the HT1080 fibrosarcoma cell line using a protease activity-based profiling method (71). Treatment with the uPA inhibitor PAI-1/SERPINE1 or a uPA blocking antibody, mAB-112(9), could block intravasation while addition of uPA could stimulate intravasation with limited effects on primary tumor growth. Similar results were seen with a prostate cancer cell line (7, 22). The microRNA miR-182 has been shown to stimulate intravasation in a mouse sarcoma model, and part of its effect could be through suppression of the uPA inhibitor PAI-1 (102).
Reciprocal activation of uPA and plasminogen is strongly enhanced by binding of uPA to its receptor on the cell surface, uPAR. The importance of uPA binding to uPAR was demonstrated through the use of peptides that were developed to bind to uPAR and compete with uPA (94). Addition of the antagonist peptide significantly reduced intravasation of HEp3 cells in the CAM assay compared with a scrambled peptide control. Similarly, uPAR-binding peptide antagonists inhibited plasmin activation by uPA. Expression of uPAR can be increased by src through enhanced AP1 activity in colon cancer SW480 cells. Src expression stimulated invasion and intravasation, and the enhanced invasion was shown to be dependent on uPAR (66). Conversely, suppression of uPAR expression is a potential mechanism of action of the tumor suppressor PDCD4. PDCD4 expression was found to be inversely correlated with uPAR, and PDCD4 suppressed SP1-mediated activation of uPAR transcription. Overexpression of PDCD4 in HEp3 cells suppressed intravasation in the CAM assay (66). PDCD4 in turn can be suppressed by miR-21, and inhibition of miR-21 in RKO colon cancer cells reduced intravasation in the CAM assay (3).
The mechanism by which the uPA/uPAR system enhances intravasation may be multifactorial, given that plasmin can digest a wide range of proteins. One example of a downstream mediator for intravasation is CDCP1. A CDCP-1-specific antibody that blocks plasmin cleavage of CDCP1, mAB 10-D7, was able to block intravasation of prostate cancer cells in the CAM assay, as did treatment with aprotinin (14). mAB 10-D7 was also able to inhibit protease-stimulated intravasation using an in vitro transendothelial migration assay. Cleaved CDCP1 forms a complex with β1-integrins, resulting in FAK activation and PI3K activation. Inhibition of FAK or PI3K activity resulted in reduced intravasation.
Other proteases that may contribute to intravasation include the matrix metalloproteinases (MMPs). However, the literature is mixed regarding the role of MMPs in intravasation. The MMP inhibitor marimastat strongly inhibited intravasation of HEp3 cells in the CAM assay (61), while the MMP inhibitor GM6001 inhibited fibrosarcoma but not prostate cancer intravasation (7, 30). Both papers report that uPA/uPAR is important. GM6001 did inhibit block transendothelial migration of breast cancer cells in an in vitro intravasation assay, however (99).
This mixed record is also reflected in studies of individual MMPs. In the CAM assay, suppression of MMP-1 expression in HEp3 cells resulted in reduced intravasation, possibly due to perturbed angiogenesis and endothelial permeability via regulation of endothelial PAR1 (52). Conversely, although GM6001 inhibited intravasation of fibrosarcoma in the CAM assay, MT1MMP suppression had no effect while MMP-1, MMP-2, or MMP-9 suppression was found to actually increase intravasation (30). In the fibrosarcoma case, one possibility is that tumor cell and host MMPs have contrasting contributions to intravasation in this system. Host MMPs may contribute to angiogenesis, with increased angiogenesis enhancing the likelihood of intravasation (8, 29, 74). Overexpression of membrane type MMPs in breast cancer cells can enhance intravasation, potentially through stimulating invasion or perturbing angiogenesis and generating larger, more permeable blood vessels (15, 82).
Antitumor treatments may induce increased intravasation in parallel with cell killing. In a mouse model using lung cancer cells, irradiation resulted in increased intravasation and MMP-9 expression. Suppression of MMP-9 inhibited the radiation-induced increase in intravasation (19). In wild-type p53 lung cancer cells, irradiation led to the induction of SULF2 which in turn upregulated IL6 expression, invasion, and intravasation (53).
Other Contributors
Other receptors that can contribute to intravasation include G protein-coupled receptors. CXCR1 and CXCR2 are upregulated by CYR61/CCN1 expression in gastric cancer cells, and neutralizing antibodies for CXCR1 and CXCR2 inhibit intravasation induced by CCN1 expression in the CAM assay (67). Expression of the SDF1/CXCL12 receptor CXCR4 in MTLn3 breast cancer cells could enhance intravasation in a mouse model, while CXCR7 could not (48), although CXCR7 enhanced primary tumor growth through angiogenesis. A role for Notch signaling in colon cancer cells has been shown, where loss of an inhibitor of Notch, Aes, resulted in increased invasion of tumor glands into blood vessels (109).
A number of other potential contributors to intravasation have been identified. Oxidative stress and reactive oxygen species generated by UV radiation were shown to enhance basal to apical transendothelial migration of melanoma cells using an in vitro assay (18). Tissue factor expression on osteosarcoma cells enhanced intravasation in the CAM assay (23).
Regulation of tumor cell adhesion interactions can also perturb intravasation. Increased cell-cell adhesion through ICAM1 in a T-cell leukemia model resulted in reduced intravasation (33). Increased osteopontin expression can enhance intravasation in the CAM assay (59). An antibody to the tetraspanin CD151 inhibited tumor cell motility and intravasation without affecting proliferation (143). α2β1-integrin expression inhibits intravasation of the MMTV-Neu mouse model of breast cancer (97). Conversely, β3-integrin expression was important for metastasis of MDA-MB-231 breast cancer cells (13).
Other Participating Cells
Tumor cell intravasation can be facilitated by other cells in the tumor stroma. Macrophages, fibroblasts, neutrophils, and platelets can all aid in migration across the endothelial barrier.
While macrophages normally play a role in tumor suppression by eliminating damaged and mutated cells, it has been shown that macrophages can be reprogrammed by tumor cells to support tumor growth and help in the metastatic spread of cancer (21, 95). One such relationship is the EGF/CSF1 paracrine interaction between breast cancer cells and macrophages. Tumor cells secrete CSF1 to recruit macrophages, and in turn macrophages secrete EGF in order to stimulate tumor cell invasion towards blood vessels (44). Once at the endothelial barrier, both the tumor cell and macrophage produce proteases at the site of tumor cell entry (130), showing a direct interaction between tumor cells and macrophages involved in hematogenous intravasation. This interaction is contact dependent as shown by Roh et al. (99), where contact between tumor cells and macrophages induces the formation of tumor cell invadopodia, allowing the cell to penetrate the endothelial tight junctions and migrate into the bloodstream. One population of tumor-associated macrophages, perivascular macrophages, is of particular interest. It has been shown that tumor cells tend to intravasate in areas enriched for these perivascular macrophages, which express VEGFA (46, 130). Quantifying the association of a Mena-expressing tumor cell with a perivascular macrophage and an endothelial cell, termed TMEM (tumor microenvironment of metastasis), may have prognostic value (98).
Macrophage-secreted TNFA may also play a role in mediating tumor cell intravasation. Studies by Zervantonakis et al. (139) show that treating an endothelial cell monolayer with TNFA from macrophages can increase endothelial layer permeability. This has been further supported in a recent study by Wang et al. (123) in zebrafish, showing that tumor-associated macrophages bind to tumor cells to aid in intravasation. In their findings, the authors show that macrophage TNFA and IL6 are important signaling molecules for this step (123).
Cancer-associated fibroblasts (CAFs) can interact with tumor cells and mediate migration through a CXCL12/CXCR4 paracrine loop. Much like the paracrine interaction between tumor cells and macrophages, this allows for migration through tissue and towards blood vessels (37, 75). Pena et al. (88) have found that STC1, a protein expressed by PDGF-activated fibroblasts, may be important for colorectal cancer cell intravasation. Tumors formed in mice with STC1-deficient CAFs showed decreased numbers of tumor cell emboli in the tumor vasculature (88).
Neutrophils play a role in assisting tumor angiogenesis and intravasation by being a source of MMP-9, a metalloproteinase that stimulates the production of new blood vessels within the tumor. In a study using aggressive fibrosarcoma and prostate carcinoma cells, it was found that the ability of these cells to cross the endothelial barrier was dependent on how well they were able to recruit MMP-9-positive neutrophils (8).
Platelets, like many of the other interacting cells in the tumor microenvironment, are a source of several growth factors that are important for cancer cell intravasation. Platelet-derived growth factor, PDGF, has been shown to increase tumor cell proliferation, as well as activate other cells within the tumor stroma, such as fibroblasts. TGFB, a protein released by the alpha granules of platelets, can promote cancer cell intravasation as described above.
Circulating Tumor Cells
A number of studies have shown that the presence of circulating tumor cells (CTCs) correlates with disease progression and metastasis in different human cancers (20, 24, 27, 76, 78). For example, in a study of metastatic breast cancer, patients with 5 or more CTCs per 7.5 ml of whole blood had a significantly shorter median progression-free survival and shorter overall survival as compared with patients with less than 5 CTCs (24). Rapidly advancing technologies to measure CTCs are allowing many studies to utilize the measurement of CTCs in model systems to validate analyses performed in human cancers and provide novel insights into the mechanisms of intravasation.
In a study using the 4T1 breast cancer model, the gene expression patterns of CTCs were compared with primary tumor cells (65). There were significant increases in TWIST1 and SNAI1, consistent with increased EMT in intravasating cells and studies indicating that TWIST1 expression can enhance metastasis (134). In addition, there was a significant increase in the mitochondrial inducer, PGC-1, and intravasation correlated with PGC-1 expression in both animal studies and patient CTCs. In another study of CTCs during treatment, changes in gene expression were followed and EMT patterns were detected (136). However, in this study, direct comparisons with the tumors giving rise to the CTCs were not made, and thus it is unclear in this case whether the CTC signatures simply reflected the expression in the source tumor. Genomic signatures of CTCs were characterized in a study of 40 breast cancer patients (54). CTCs from the peripheral blood of patients were isolated using single-cell laser microdissection and analyzed for copy number aberrations (CNA) by whole genome amplification. A signature of recurrent copy number gains was identified containing genes and miRNAs which have known roles in motility, invasion, and intravasation. Examples that could contribute to enhanced intravasation as discussed above include PAI-1, CD151, ErbB2, and ANGPTL4. Furthermore, the analysis clustered the CTCs into two broad signatures: dormancy-related versus tumor-aggressiveness related. However, there are a number of potential complications in interpreting the results, including whether the CTCs are from metastases or the primary tumor, possible contamination with lymphoid cells, and how the CTCs have avoided being trapped in capillaries (93).
Aside from tumor expression of key proteins and miRNA, extracellular elements in the blood may also regulate CTCs. The role of platelets in CTC survival and colonization has been reviewed (111). Briefly, once in the circulation, CTCs activate and cause aggregation of platelets, leading to the formation of tumor cell-induced platelet aggregations (TCIPA). As aggregates, platelets enhance survival and extravasation of CTCs into new metastatic sites by facilitating the dissemination and embolization in the microvasculature as well as protection from immunological attacks.
Conclusions and Future Prospects
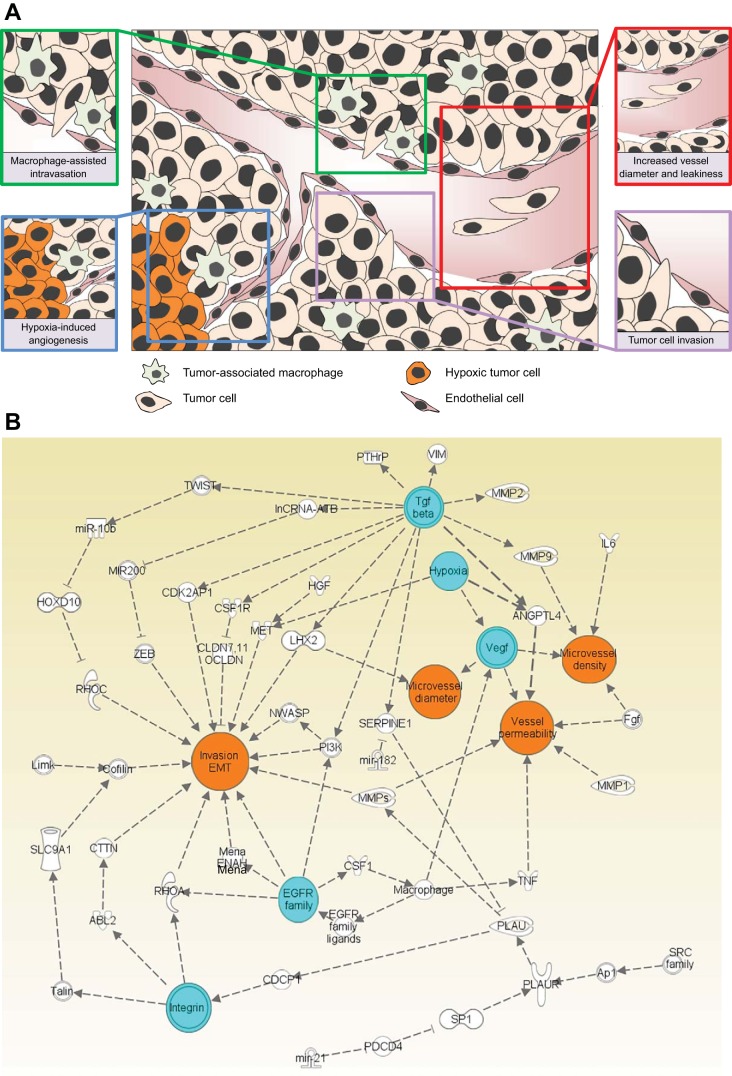
A number of molecular pathways for regulation of intravasation have been identified (Fig. 2). The development of sophisticated in vivo and in vitro assays for the analysis of intravasation has been important for our current advances and will enable more detailed understanding of the events that take place during intravasation. It is possible that different tumor types (or subtypes) will utilize different mechanisms of intravasation, and this will be important to clarify development of clinical applications. The expression patterns of CTCs may be helpful in confirming the pathways that are important for intravasation.
Fig. 2.
Summary of intravasation mechanisms. A: diagram summarizing basic contributors to intravasation. B: interaction network of the major mechanisms described in this review. Major stimulators of intravasation are noted in blue and key properties regulating intravasation are indicated in orange.
Possible clinical applications of our understanding of intravasation broadly fall into two general categories: prognosis and therapy. With respect to prognosis, measurements of histological grading, staging, and hormone receptor status are used by clinicians to determine the most appropriate treatments. However, these clinical criteria only roughly assess intravasation capability (and the resulting metastatic capability). Mechanism-based tests have the potential to provide additional prognostic value. For example, the observation that macrophages can enhance tumor cell intravasation capability through increases in the expression of Mena/ENAH has been used to develop a new test based on the tumor microenvironment of metastasis (TMEM). TMEM has been shown to independently predict the risk of metastasis (81, 91). The ability to more accurately identify which patients are unlikely to have metastases could significantly reduce the complications due to unnecessary chemotherapy and/or radiation therapy.
Development of new therapies based on anti-intravasation treatments is complex. If an anti-intravasation therapy does not affect tumor cell viability, then it may simply constrain the tumor cells to the primary tumor. This can potentially be useful for reducing tumor cell dissemination induced by other treatments (such as radiation as noted above) and may also enhance killing efficiency of the combined therapy. This may be more effective in treating tumors where complete removal of the primary tumor with adequate margins is often difficult (such as prostate or head and neck cancer). For tumors such as breast, in which the primary tumor often can be completely removed, anti-intravasation therapy may be useful to treat metastases once they recur. In this context, it will be important to determine whether the mechanism of intravasation from a metastasis is the same as intravasation from the primary tumor.
GRANTS
This work was supported by CA100324.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
S.P.H.C., R.M.C., and J.E.S. drafted manuscript; S.P.H.C., R.M.C., and J.E.S. edited and revised manuscript; S.P.H.C., R.M.C., and J.E.S. approved final version of manuscript.
Glossary
- ANGPTL4
Angiopoietin-like 4
- AP1
Activator protein 1
- CAF
Cancer-associated fibroblast
- CAM
Chorioallantoic membrane
- CDCP1
CUB domain-containing protein 1
- CSF1R
Colony-stimulating factor 1 receptor
- CTCs
Circulating tumor cells
- CTGF
Connective tissue growth factor
- CXCR1,2
Chemokine (C-X-C motif) receptor 1, 2
- CYR61
Cysteine-rich angiogenic inducer 61
- ECM
Extracellular matrix
- EGF
Epidermal growth factor
- EGFR
Epidermal growth factor receptor
- EMT
Epithelial-mesenchymal transition
- FAK
Protein tyrosine kinase 2 (PTK2) Focal adhesion protein
- FGF1
Fibroblast growth factor 1
- HBEGF
Heparin-binding EGF
- HGF
Hepatocyte growth factor
- HIF
Hypoxia-inducible factor
- HMGA2
High mobility group AT-hook 2
- HOXD10
Homeobox D10
- ICAM1
Intercellular adhesion molecule 1
- IFP
Interstitial fluid pressure
- IL-6
Interleukin 6
- IL-11
Interleukin 11
- LHX2
LIM homeobox 2
- LIMK
LIM domain kinase
- MIW
Mammary imaging window
- MMP
Matrix metalloproteinase
- N-WASP
Wiskott-Aldrich syndrome-like (WASL)
- PAR1
Protease-activated receptor 1
- PAI-1
Plasminogen activator inhibitor-1
- PCR
Polymerase chain reaction
- PDCD4
Programmed cell death 4
- PI3K
Phosphoinositide 3-kinase
- PTHrP
Parathyroid hormone-related protein
- PTK2
Protein tyrosine kinase 2
- PVT
Prevascularized tumor
- RHOA
ras homolog family member A
- S1P
Sphingosine 1 phosphate
- SERPINE
Serpin peptidase inhibitor, Clade E
- SLPI
Secretory leukocyte peptidase inhibitor
- SNAI1
Snail family zinc finger 1
- SPINK1
Serine peptidase inhibitor, Kazal type 1
- SRC-1
Steroid receptor coactivator-1
- STAT3
Signal transducer and activator of transcription 3
- STC1
Stanniocalcin 1
- SULF2
Sulfatase 2
- TGFB
Transforming growth factor-β
- TMEM
Tumor microenvironment of metastasis
- TNFA
Tumor necrosis factor-α
- uPA
Urokinase type plasminogen activator
- VEGF
Vascular endothelial growth factor
- VIM
Vimentin
- ZEB1,2
Zinc finger E-box binding homeobox 1,2
REFERENCES
- 1.Aceto N, Bardia A, Miyamoto DT, Donaldson MC, Wittner BS, Spencer JA, Yu M, Pely A, Engstrom A, Zhu H, Brannigan BW, Kapur R, Stott SL, Shioda T, Ramaswamy S, Ting DT, Lin CP, Toner M, Haber DA, Maheswaran S. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 158: 1110–1122, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alitalo K, Carmeliet P. Molecular mechanisms of lymphangiogenesis in health and disease. Cancer Cell 1: 219–227, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Asangani IA, Rasheed SA, Nikolova DA, Leupold JH, Colburn NH, Post S, Allgayer H. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene 27: 2128–2136, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Ateeq B, Tomlins SA, Laxman B, Asangani IA, Cao Q, Cao X, Li Y, Wang X, Feng FY, Pienta KJ, Varambally S, Chinnaiyan AM. Therapeutic targeting of SPINK1-positive prostate cancer. Sci Transl Med 3: 72ra17, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beaty BT, Condeelis J. Digging a little deeper: the stages of invadopodium formation and maturation. Eur J Cell Biol 93: 438–444, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beaty BT, Wang Y, Bravo-Cordero JJ, Sharma VP, Miskolci V, Hodgson L, Condeelis J. Talin regulates moesin-NHE-1 recruitment to invadopodia and promotes mammary tumor metastasis. J Cell Biol 205: 737–751, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bekes EM, Deryugina EI, Kupriyanova TA, Zajac E, Botkjaer KA, Andreasen PA, Quigley JP. Activation of pro-uPA is critical for initial escape from the primary tumor and hematogenous dissemination of human carcinoma cells. Neoplasia 13: 806–821, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bekes EM, Schweighofer B, Kupriyanova TA, Zajac E, Ardi VC, Quigley JP, Deryugina EI. Tumor-recruited neutrophils and neutrophil TIMP-free MMP-9 regulate coordinately the levels of tumor angiogenesis and efficiency of malignant cell intravasation. Am J Pathol 179: 1455–1470, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blouse GE, Botkjaer KA, Deryugina E, Byszuk AA, Jensen JM, Mortensen KK, Quigley JP, Andreasen PA. A novel mode of intervention with serine protease activity: targeting zymogen activation. J Biol Chem 284: 4647–4657, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bockhorn M, Jain RK, Munn LL. Active versus passive mechanisms in metastasis: do cancer cells crawl into vessels, or are they pushed? Lancet Oncol 8: 444–448, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bockhorn M, Roberge S, Sousa C, Jain RK, Munn LL. Differential gene expression in metastasizing cells shed from kidney tumors. Cancer Res 64: 2469–2473, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R, Maxwell P, Koch CJ, Ratcliffe P, Moons L, Jain RK, Collen D, Keshert E. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 394: 485–490, 1998. [DOI] [PubMed] [Google Scholar]
- 13.Carter RZ, Micocci KC, Natoli A, Redvers RP, Paquet-Fifield S, Martin AC, Denoyer D, Ling X, Kim SH, Tomasin R, Selistre-de-Araujo H, Anderson RL, Pouliot N. Tumour but not stromal expression of beta3 integrin is essential, and is required early, for spontaneous dissemination of bone-metastatic breast cancer. J Pathol 235: 760–772, 2015. [DOI] [PubMed] [Google Scholar]
- 14.Casar B, Rimann I, Kato H, Shattil SJ, Quigley JP, Deryugina EI. In vivo cleaved CDCP1 promotes early tumor dissemination via complexing with activated beta1 integrin and induction of FAK/PI3K/Akt motility signaling. Oncogene 33: 255–268, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chabottaux V, Ricaud S, Host L, Blacher S, Paye A, Thiry M, Garofalakis A, Pestourie C, Gombert K, Bruyere F, Lewandowsky D, Tavitian B, Foidart JM, Duconge F, Noel A. Membrane-type 4 matrix metalloproteinase (MT4-MMP) induces lung metastasis by alteration of primary breast tumour vascular architecture. J Cell Mol Med 13: 4002–4013, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaffer CL, Brennan JP, Slavin JL, Blick T, Thompson EW, Williams ED. Mesenchymal-to-epithelial transition facilitates bladder cancer metastasis: role of fibroblast growth factor receptor-2. Cancer Res 66: 11271–11278, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Chang Q, Bournazou E, Sansone P, Berishaj M, Gao SP, Daly L, Wels J, Theilen T, Granitto S, Zhang X, Cotari J, Alpaugh ML, de Stanchina E, Manova K, Li M, Bonafe M, Ceccarelli C, Taffurelli M, Santini D, Altan-Bonnet G, Kaplan R, Norton L, Nishimoto N, Huszar D, Lyden D, Bromberg J. The IL-6/JAK/Stat3 feed-forward loop drives tumorigenesis and metastasis. Neoplasia 15: 848–862, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng GC, Schulze PC, Lee RT, Sylvan J, Zetter BR, Huang H. Oxidative stress and thioredoxin-interacting protein promote intravasation of melanoma cells. Exp Cell Res 300: 297–307, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Chou CH, Teng CM, Tzen KY, Chang YC, Chen JH, Cheng JC. MMP-9 from sublethally irradiated tumor promotes Lewis lung carcinoma cell invasiveness and pulmonary metastasis. Oncogene 31: 458–468, 2012. [DOI] [PubMed] [Google Scholar]
- 20.Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, Picus J, Morse M, Mitchell E, Miller MC, Doyle GV, Tissing H, Terstappen LW, Meropol NJ. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol 26: 3213–3221, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell 124: 263–266, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Conn EM, Botkjaer KA, Kupriyanova TA, Andreasen PA, Deryugina EI, Quigley JP. Comparative analysis of metastasis variants derived from human prostate carcinoma cells: roles in intravasation of VEGF-mediated angiogenesis and uPA-mediated invasion. Am J Pathol 175: 1638–1652, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conn EM, Madsen MA, Cravatt BF, Ruf W, Deryugina EI, Quigley JP. Cell surface proteomics identifies molecules functionally linked to tumor cell intravasation. J Biol Chem 283: 26518–26527, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LW, Hayes DF. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 351: 781–791, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Dadiani M, Kalchenko V, Yosepovich A, Margalit R, Hassid Y, Degani H, Seger D. Real-time imaging of lymphogenic metastasis in orthotopic human breast cancer. Cancer Res 66: 8037–8041, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Dangi-Garimella S, Yun J, Eves EM, Newman M, Erkeland SJ, Hammond SM, Minn AJ, Rosner MR. Raf kinase inhibitory protein suppresses a metastasis signalling cascade involving LIN28 and let-7. EMBO J 28: 347–358, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Bono JS, Scher HI, Montgomery RB, Parker C, Miller MC, Tissing H, Doyle GV, Terstappen LW, Pienta KJ, Raghavan D. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res 14: 6302–6309, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Deryugina EI, Quigley JP. Chick embryo chorioallantoic membrane model systems to study and visualize human tumor cell metastasis. Histochem Cell Biol 130: 1119–1130, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deryugina EI, Quigley JP. Tumor angiogenesis: MMP-mediated induction of intravasation- and metastasis-sustaining neovasculature. Matrix Biol 44–46: 94–112, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deryugina EI, Zijlstra A, Partridge JJ, Kupriyanova TA, Madsen MA, Papagiannakopoulos T, Quigley JP. Unexpected effect of matrix metalloproteinase down-regulation on vascular intravasation and metastasis of human fibrosarcoma cells selected in vivo for high rates of dissemination. Cancer Res 65: 10959–10969, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Dua RS, Gui GP, Isacke CM. Endothelial adhesion molecules in breast cancer invasion into the vascular and lymphatic systems. Eur J Surg Oncol 31: 824–832, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Fein MR, Egeblad M. Caught in the act: revealing the metastatic process by live imaging. Dis Model Mech 6: 580–593, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feng H, Stachura DL, White RM, Gutierrez A, Zhang L, Sanda T, Jette CA, Testa JR, Neuberg DS, Langenau DM, Kutok JL, Zon LI, Traver D, Fleming MD, Kanki JP, Look AT. T-lymphoblastic lymphoma cells express high levels of BCL2, S1P1, and ICAM1, leading to a blockade of tumor cell intravasation. Cancer Cell 18: 353–366, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fidler IJ. The relationship of embolic homogeneity, number, size and viability to the incidence of experimental metastasis. Eur J Cancer 9: 223–227, 1973. [DOI] [PubMed] [Google Scholar]
- 35.Fischer KR, Durrans A, Lee S, Sheng J, Li F, Wong ST, Choi H, El Rayes T, Ryu S, Troeger J, Schwabe RF, Vahdat LT, Altorki NK, Mittal V, Gao D. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature 527: 472–476, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frankenberger C, Rabe D, Bainer R, Sankarasharma D, Chada K, Krausz T, Gilad Y, Becker L, Rosner MR. Metastasis suppressors regulate the tumor microenvironment by blocking recruitment of prometastatic tumor-associated macrophages. Cancer Res 75: 4063–4073, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gaggioli C, Hooper S, Hidalgo-Carcedo C, Grosse R, Marshall JF, Harrington K, Sahai E. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat Cell Biol 9: 1392–1400, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Giampieri S, Manning C, Hooper S, Jones L, Hill CS, Sahai E. Localized and reversible TGFbeta signalling switches breast cancer cells from cohesive to single cell motility. Nat Cell Biol 11: 1287–1296, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gil-Henn H, Patsialou A, Wang Y, Warren MS, Condeelis JS, Koleske AJ. Arg/Abl2 promotes invasion and attenuates proliferation of breast cancer in vivo. Oncogene 32: 2622–2630, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Glaves D. Correlation between circulating cancer cells and incidence of metastases. Br J Cancer 48: 665–673, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gligorijevic B, Bergman A, Condeelis J. Multiparametric classification links tumor microenvironments with tumor cell phenotype. PLoS Biol 12: e1001995, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gligorijevic B, Entenberg D, Kedrin D, Segall J, van Rheenen J, Condeelis J. Intravital imaging and photoswitching in tumor invasion and intravasation microenvironments. Micros Today 18: 34–37, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gligorijevic B, Wyckoff J, Yamaguchi H, Wang Y, Roussos ET, Condeelis J. N-WASP-mediated invadopodium formation is involved in intravasation and lung metastasis of mammary tumors. J Cell Sci 125: 724–734, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goswami S, Sahai E, Wyckoff JB, Cammer M, Cox D, Pixley FJ, Stanley ER, Segall JE, Condeelis JS. Macrophages promote the invasion of breast carcinoma cells via a colony-stimulating factor-1/epidermal growth factor paracrine loop. Cancer Res 65: 5278–5283, 2005. [DOI] [PubMed] [Google Scholar]
- 45.Gould CM, Courtneidge SA. Regulation of invadopodia by the tumor microenvironment. Cell Adh Migr 8: 226–235, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harney AS, Arwert EN, Entenberg D, Wang Y, Guo P, Qian BZ, Oktay MH, Pollard JW, Jones JG, Condeelis JS. Real-time imaging reveals local, transient vascular permeability, and tumor cell intravasation stimulated by TIE2hi macrophage-derived VEGFA. Cancer Discov 5: 932–943, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hayashi K, Jiang P, Yamauchi K, Yamamoto N, Tsuchiya H, Tomita K, Moossa AR, Bouvet M, Hoffman RM. Real-time imaging of tumor-cell shedding and trafficking in lymphatic channels. Cancer Res 67: 8223–8228, 2007. [DOI] [PubMed] [Google Scholar]
- 48.Hernandez L, Magalhaes MA, Coniglio SJ, Condeelis JS, Segall JE. Opposing roles of CXCR4 and CXCR7 in breast cancer metastasis. Breast Cancer Res 13: R128, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang RL, Teo Z, Chong HC, Zhu P, Tan MJ, Tan CK, Lam CR, Sng MK, Leong DT, Tan SM, Kersten S, Ding JL, Li HY, Tan NS. ANGPTL4 modulates vascular junction integrity by integrin signaling and disruption of intercellular VE-cadherin and claudin-5 clusters. Blood 118: 3990–4002, 2011. [DOI] [PubMed] [Google Scholar]
- 50.Jacob A, Prekeris R. The regulation of MMP targeting to invadopodia during cancer metastasis. Front Cell Dev Biol 3: 4, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Juacaba SF, Horak E, Price JE, Tarin D. Tumor cell dissemination patterns and metastasis of murine mammary carcinoma. Cancer Res 49: 570–575, 1989. [PubMed] [Google Scholar]
- 52.Juncker-Jensen A, Deryugina EI, Rimann I, Zajac E, Kupriyanova TA, Engelholm LH, Quigley JP. Tumor MMP-1 activates endothelial PAR1 to facilitate vascular intravasation and metastatic dissemination. Cancer Res 73: 4196–4211, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jung CH, Ho JN, Park JK, Kim EM, Hwang SG, Um HD. Involvement of SULF2 in -irradiation-induced invasion and resistance of cancer cells by inducing IL-6 expression. Oncotarget. [Epub ahead of print]. doi: 10.18632/oncotarget.7449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kanwar N, Hu P, Bedard P, Clemons M, McCready D, Done SJ. Identification of genomic signatures in circulating tumor cells from breast cancer. Int J Cancer 137: 332–344, 2015. [DOI] [PubMed] [Google Scholar]
- 55.Kats-Ugurlu G, Roodink I, de Weijert M, Tiemessen D, Maass C, Verrijp K, van der Laak J, de Waal R, Mulders P, Oosterwijk E, Leenders W. Circulating tumour tissue fragments in patients with pulmonary metastasis of clear cell renal cell carcinoma. J Pathol 219: 287–293, 2009. [DOI] [PubMed] [Google Scholar]
- 56.Kedrin D, Gligorijevic B, Wyckoff J, Verkhusha VV, Condeelis J, Segall JE, van Rheenen J. Intravital imaging of metastatic behavior through a mammary imaging window. Nat Methods 5: 1019–1021, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kedrin D, Wyckoff J, Boimel PJ, Coniglio SJ, Hynes NE, Arteaga CL, Segall JE. ERBB1 and ERBB2 have distinct functions in tumor cell invasion and intravasation. Clin Cancer Res 15: 3733–3739, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Keklikoglou I, Koerner C, Schmidt C, Zhang JD, Heckmann D, Shavinskaya A, Allgayer H, Guckel B, Fehm T, Schneeweiss A, Sahin O, Wiemann S, Tschulena U. MicroRNA-520/373 family functions as a tumor suppressor in estrogen receptor negative breast cancer by targeting NF-kappaB and TGF-beta signaling pathways. Oncogene 31: 4150–4163, 2012. [DOI] [PubMed] [Google Scholar]
- 59.Khodavirdi AC, Song Z, Yang S, Zhong C, Wang S, Wu H, Pritchard C, Nelson PS, Roy-Burman P. Increased expression of osteopontin contributes to the progression of prostate cancer. Cancer Res 66: 883–888, 2006. [DOI] [PubMed] [Google Scholar]
- 60.Khuon S, Liang L, Dettman RW, Sporn PH, Wysolmerski RB, Chew TL. Myosin light chain kinase mediates transcellular intravasation of breast cancer cells through the underlying endothelial cells: a three-dimensional FRET study. J Cell Sci 123: 431–440, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim J, Yu W, Kovalski K, Ossowski L. Requirement for specific proteases in cancer cell intravasation as revealed by a novel semiquantitative PCR-based assay. Cell 94: 353–362, 1998. [DOI] [PubMed] [Google Scholar]
- 62.Konstantopoulos K, Thomas SN. Cancer cells in transit: the vascular interactions of tumor cells. Annu Rev Biomed Eng 11: 177–202, 2009. [DOI] [PubMed] [Google Scholar]
- 63.Kusters B, Kats G, Roodink I, Verrijp K, Wesseling P, Ruiter DJ, de Waal RM, Leenders WP. Micronodular transformation as a novel mechanism of VEGF-A-induced metastasis. Oncogene 26: 5808–5815, 2007. [DOI] [PubMed] [Google Scholar]
- 64.Kuzmanov A, Hopfer U, Marti P, Meyer-Schaller N, Yilmaz M, Christofori G. LIM-homeobox gene 2 promotes tumor growth and metastasis by inducing autocrine and paracrine PDGF-B signaling. Mol Oncol 8: 401–416, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.LeBleu VS, O'Connell JT, Gonzalez Herrera KN, Wikman H, Pantel K, Haigis MC, de Carvalho FM, Damascena A, Domingos Chinen LT, Rocha RM, Asara JM, Kalluri R. PGC-1alpha mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat Cell Biol 16: 992–1003, 1001–1015, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Leupold JH, Yang HS, Colburn NH, Asangani I, Post S, Allgayer H. Tumor suppressor Pdcd4 inhibits invasion/intravasation and regulates urokinase receptor (u-PAR) gene expression via Sp-transcription factors. Oncogene 26: 4550–4562, 2007. [DOI] [PubMed] [Google Scholar]
- 67.Lin BR, Chang CC, Chen LR, Wu MH, Wang MY, Kuo IH, Chu CY, Chang KJ, Lee PH, Chen WJ, Kuo ML, Lin MT. Cysteine-rich 61 (CCN1) enhances chemotactic migration, transendothelial cell migration, and intravasation by concomitantly up-regulating chemokine receptor 1 and 2. Mol Cancer Res 5: 1111–1123, 2007. [DOI] [PubMed] [Google Scholar]
- 68.Liotta LA, Saidel MG, Kleinerman J. The significance of hematogenous tumor cell clumps in the metastatic process. Cancer Res 36: 889–894, 1976. [PubMed] [Google Scholar]
- 69.Lissat A, Joerschke M, Shinde DA, Braunschweig T, Meier A, Makowska A, Bortnick R, Henneke P, Herget G, Gorr TA, Kontny U. IL6 secreted by Ewing sarcoma tumor microenvironment confers anti-apoptotic and cell-disseminating paracrine responses in Ewing sarcoma cells. BMC Cancer 15: 552, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ma L, Reinhardt F, Pan E, Soutschek J, Bhat B, Marcusson EG, Teruya-Feldstein J, Bell GW, Weinberg RA. Therapeutic silencing of miR-10b inhibits metastasis in a mouse mammary tumor model. Nat Biotechnol 28: 341–347, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Madsen MA, Deryugina EI, Niessen S, Cravatt BF, Quigley JP. Activity-based protein profiling implicates urokinase activation as a key step in human fibrosarcoma intravasation. J Biol Chem 281: 15997–16005, 2006. [DOI] [PubMed] [Google Scholar]
- 72.Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 427: 355–360, 2004. [DOI] [PubMed] [Google Scholar]
- 73.Mazzocca A, Fransvea E, Dituri F, Lupo L, Antonaci S, Giannelli G. Down-regulation of connective tissue growth factor by inhibition of transforming growth factor beta blocks the tumor-stroma cross-talk and tumor progression in hepatocellular carcinoma. Hepatology 51: 523–534, 2010. [DOI] [PubMed] [Google Scholar]
- 74.Minder P, Zajac E, Quigley JP, Deryugina EI. EGFR regulates the development and microarchitecture of intratumoral angiogenic vasculature capable of sustaining cancer cell intravasation. Neoplasia 17: 634–649, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mishra P, Banerjee D, Ben-Baruch A. Chemokines at the crossroads of tumor-fibroblast interactions that promote malignancy. J Leukoc Biol 89: 31–39, 2011. [DOI] [PubMed] [Google Scholar]
- 76.Mocellin S, Del Fiore P, Guarnieri L, Scalerta R, Foletto M, Chiarion V, Pilati P, Nitti D, Lise M, Rossi CR. Molecular detection of circulating tumor cells is an independent prognostic factor in patients with high-risk cutaneous melanoma. Int J Cancer 111: 741–745, 2004. [DOI] [PubMed] [Google Scholar]
- 77.Moore FE, Langenau DM. Through the looking glass: visualizing leukemia growth, migration, and engraftment using fluorescent transgenic zebrafish. Adv Hematol 2012: 478164, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moreno JG, O'Hara SM, Gross S, Doyle G, Fritsche H, Gomella LG, Terstappen LW. Changes in circulating carcinoma cells in patients with metastatic prostate cancer correlate with disease status. Urology 58: 386–392, 2001. [DOI] [PubMed] [Google Scholar]
- 79.Muller WA. The regulation of transendothelial migration: new knowledge and new questions. Cardiovasc Res 107: 310–320, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Muraoka RS, Dumont N, Ritter CA, Dugger TC, Brantley DM, Chen J, Easterly E, Roebuck LR, Ryan S, Gotwals PJ, Koteliansky V, Arteaga CL. Blockade of TGF-beta inhibits mammary tumor cell viability, migration, and metastases. J Clin Invest 109: 1551–1559, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Oktay MH, Gertler FB, Liu YF, Rohan TE, Condeelis JS, Jones JG. Correlated immunohistochemical and cytological assays for the prediction of hematogenous dissemination of breast cancer. J Histochem Cytochem 60: 168–173, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ossowski L. Plasminogen activator dependent pathways in the dissemination of human tumor cells in the chick embryo. Cell 52: 321–328, 1988. [DOI] [PubMed] [Google Scholar]
- 83.Padera TP, Kadambi A, di Tomaso E, Carreira CM, Brown EB, Boucher Y, Choi NC, Mathisen D, Wain J, Mark EJ, Munn LL, Jain RK. Lymphatic metastasis in the absence of functional intratumor lymphatics. Science 296: 1883–1886, 2002. [DOI] [PubMed] [Google Scholar]
- 84.Palmer TD, Lewis J, Zijlstra A. Quantitative analysis of cancer metastasis using an avian embryo model. J Vis Exp 30: pii: 2815, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pang H, Flinn R, Patsialou A, Wyckoff J, Roussos ET, Wu H, Pozzuto M, Goswami S, Condeelis JS, Bresnick AR, Segall JE, Backer JM. Differential enhancement of breast cancer cell motility and metastasis by helical and kinase domain mutations of class IA phosphoinositide 3-kinase. Cancer Res 69: 8868–8876, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Patsialou A, Bravo-Cordero JJ, Wang Y, Entenberg D, Liu H, Clarke M, Condeelis JS. Intravital multiphoton imaging reveals multicellular streaming as a crucial component of in vivo cell migration in human breast tumors. Intravital 2: e25294, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Patsialou A, Wang Y, Pignatelli J, Chen X, Entenberg D, Oktay M, Condeelis JS. Autocrine CSF1R signaling mediates switching between invasion and proliferation downstream of TGFbeta in claudin-low breast tumor cells. Oncogene 34: 2721–2731, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pena C, Cespedes MV, Lindh MB, Kiflemariam S, Mezheyeuski A, Edqvist PH, Hagglof C, Birgisson H, Bojmar L, Jirstrom K, Sandstrom P, Olsson E, Veerla S, Gallardo A, Sjoblom T, Chang AC, Reddel RR, Mangues R, Augsten M, Ostman A. STC1 expression by cancer-associated fibroblasts drives metastasis of colorectal cancer. Cancer Res 73: 1287–1297, 2013. [DOI] [PubMed] [Google Scholar]
- 89.Pennacchietti S, Michieli P, Galluzzo M, Mazzone M, Giordano S, Comoglio PM. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell 3: 347–361, 2003. [DOI] [PubMed] [Google Scholar]
- 90.Philippar U, Roussos ET, Oser M, Yamaguchi H, Kim HD, Giampieri S, Wang Y, Goswami S, Wyckoff JB, Lauffenburger DA, Sahai E, Condeelis JS, Gertler FB. A Mena invasion isoform potentiates EGF-induced carcinoma cell invasion and metastasis. Dev Cell 15: 813–828, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pignatelli J, Goswami S, Jones JG, Rohan TE, Pieri E, Chen X, Adler E, Cox D, Maleki S, Bresnick A, Gertler FB, Condeelis JS, Oktay MH. Invasive breast carcinoma cells from patients exhibit MenaINV- and macrophage-dependent transendothelial migration. Sci Signal 7: ra112, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pisano M, Triacca V, Barbee KA, Swartz MA. An in vitro model of the tumor-lymphatic microenvironment with simultaneous transendothelial and luminal flows reveals mechanisms of flow enhanced invasion. Integr Biol (Camb) 7: 525–533, 2015. [DOI] [PubMed] [Google Scholar]
- 93.Plaks V, Koopman CD, Werb Z. Cancer. Circulating tumor cells. Science 341: 1186–1188, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ploug M, Ostergaard S, Gardsvoll H, Kovalski K, Holst-Hansen C, Holm A, Ossowski L, Dano K. Peptide-derived antagonists of the urokinase receptor. Affinity maturation by combinatorial chemistry, identification of functional epitopes, and inhibitory effect on cancer cell intravasation. Biochemistry 40: 12157–12168, 2001. [DOI] [PubMed] [Google Scholar]
- 95.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer 4: 71–78, 2004. [DOI] [PubMed] [Google Scholar]
- 96.Presta M, Dell'Era P, Mitola S, Moroni E, Ronca R, Rusnati M. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Rev 16: 159–178, 2005. [DOI] [PubMed] [Google Scholar]
- 97.Ramirez NE, Zhang Z, Madamanchi A, Boyd KL, O'Rear LD, Nashabi A, Li Z, Dupont WD, Zijlstra A, Zutter MM. The alpha(2)beta(1) integrin is a metastasis suppressor in mouse models and human cancer. J Clin Invest 121: 226–237, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Robinson BD, Sica GL, Liu YF, Rohan TE, Gertler FB, Condeelis JS, Jones JG. Tumor microenvironment of metastasis in human breast carcinoma: a potential prognostic marker linked to hematogenous dissemination. Clin Cancer Res 15: 2433–2441, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Roh-Johnson M, Bravo-Cordero JJ, Patsialou A, Sharma VP, Guo P, Liu H, Hodgson L, Condeelis J. Macrophage contact induces RhoA GTPase signaling to trigger tumor cell intravasation. Oncogene 33: 4203–4212, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Roussos ET, Balsamo M, Alford SK, Wyckoff JB, Gligorijevic B, Wang Y, Pozzuto M, Stobezki R, Goswami S, Segall JE, Lauffenburger DA, Bresnick AR, Gertler FB, Condeelis JS. Mena invasive (MenaINV) promotes multicellular streaming motility and transendothelial migration in a mouse model of breast cancer. J Cell Sci 124: 2120–2131, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Roussos ET, Wang Y, Wyckoff JB, Sellers RS, Wang W, Li J, Pollard JW, Gertler FB, Condeelis JS. Mena deficiency delays tumor progression and decreases metastasis in polyoma middle-T transgenic mouse mammary tumors. Breast Cancer Res 12: R101, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sachdeva M, Mito JK, Lee CL, Zhang M, Li Z, Dodd RD, Cason D, Luo L, Ma Y, Van Mater D, Gladdy R, Lev DC, Cardona DM, Kirsch DG. MicroRNA-182 drives metastasis of primary sarcomas by targeting multiple genes. J Clin Invest 124: 4305–4319, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sage PT, Carman CV. Settings and mechanisms for trans-cellular diapedesis. Front Biosci 14: 5066–5083, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schwab SR, Cyster JG. Finding a way out: lymphocyte egress from lymphoid organs. Nat Immunol 8: 1295–1301, 2007. [DOI] [PubMed] [Google Scholar]
- 105.Seftor RE, Hess AR, Seftor EA, Kirschmann DA, Hardy KM, Margaryan NV, Hendrix MJ. Tumor cell vasculogenic mimicry: from controversy to therapeutic promise. Am J Pathol 181: 1115–1125, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shin MK, Kim SK, Jung H. Integration of intra- and extravasation in one cell-based microfluidic chip for the study of cancer metastasis. Lab Chip 11: 3880–3887, 2011. [DOI] [PubMed] [Google Scholar]
- 107.Sleeman JP, Nazarenko I, Thiele W. Do all roads lead to Rome? Routes to metastasis development. Int J Cancer 128: 2511–2526, 2011. [DOI] [PubMed] [Google Scholar]
- 108.Smirnova T, Zhou ZN, Flinn RJ, Wyckoff J, Boimel PJ, Pozzuto M, Coniglio SJ, Backer JM, Bresnick AR, Condeelis JS, Hynes NE, Segall JE. Phosphoinositide 3-kinase signaling is critical for ErbB3-driven breast cancer cell motility and metastasis. Oncogene 31: 706–715, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sonoshita M, Aoki M, Fuwa H, Aoki K, Hosogi H, Sakai Y, Hashida H, Takabayashi A, Sasaki M, Robine S, Itoh K, Yoshioka K, Kakizaki F, Kitamura T, Oshima M, Taketo MM. Suppression of colon cancer metastasis by Aes through inhibition of Notch signaling. Cancer Cell 19: 125–137, 2011. [DOI] [PubMed] [Google Scholar]
- 110.Stamenkovic I. Matrix metalloproteinases in tumor invasion and metastasis. Semin Cancer Biol 10: 415–433, 2000. [DOI] [PubMed] [Google Scholar]
- 111.Stegner D, Dutting S, Nieswandt B. Mechanistic explanation for platelet contribution to cancer metastasis. Thromb Res 133, Suppl 2: S149–S157, 2014. [DOI] [PubMed] [Google Scholar]
- 112.Stoletov K, Montel V, Lester RD, Gonias SL, Klemke R. High-resolution imaging of the dynamic tumor cell vascular interface in transparent zebrafish. Proc Natl Acad Sci USA 104: 17406–17411, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Stroka KM, Konstantopoulos K. Physical Biology in Cancer. 4. Physical cues guide tumor cell adhesion and migration. Am J Physiol Cell Physiol 306: C98–C109, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sugino T, Kawaguchi T, Suzuki T. Sequential process of blood-borne lung metastases of spontaneous mammary carcinoma in C3H mice. Int J Cancer 55: 141–147, 1993. [DOI] [PubMed] [Google Scholar]
- 115.Sugino T, Kusakabe T, Hoshi N, Yamaguchi T, Kawaguchi T, Goodison S, Sekimata M, Homma Y, Suzuki T. An invasion-independent pathway of blood-borne metastasis: a new murine mammary tumor model. Am J Pathol 160: 1973–1980, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sullivan R, Graham CH. Hypoxia-driven selection of the metastatic phenotype. Cancer Metastasis Rev 26: 319–331, 2007. [DOI] [PubMed] [Google Scholar]
- 117.Tien YW, Chang KJ, Jeng YM, Lee PH, Wu MS, Lin JT, Hsu SM. Tumor angiogenesis and its possible role in intravasation of colorectal epithelial cells. Clin Cancer Res 7: 1627–1632, 2001. [PubMed] [Google Scholar]
- 118.Tsuji T, Ibaragi S, Hu GF. Epithelial-mesenchymal transition and cell cooperativity in metastasis. Cancer Res 69: 7135–7139, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tsuji T, Ibaragi S, Shima K, Hu MG, Katsurano M, Sasaki A, Hu GF. Epithelial-mesenchymal transition induced by growth suppressor p12CDK2-AP1 promotes tumor cell local invasion but suppresses distant colony growth. Cancer Res 68: 10377–10386, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tsuji T, Sasaki Y, Tanaka M, Hanabata N, Hada R, Munakata A. Microvessel morphology and vascular endothelial growth factor expression in human colonic carcinoma with or without metastasis. Lab Invest 82: 555–562, 2002. [DOI] [PubMed] [Google Scholar]
- 121.van Zijl F, Krupitza G, Mikulits W. Initial steps of metastasis: cell invasion and endothelial transmigration. Mutat Res 728: 23–34, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wagenblast E, Soto M, Gutierrez-Angel S, Hartl CA, Gable AL, Maceli AR, Erard N, Williams AM, Kim SY, Dickopf S, Harrell JC, Smith AD, Perou CM, Wilkinson JE, Hannon GJ, Knott SR. A model of breast cancer heterogeneity reveals vascular mimicry as a driver of metastasis. Nature 520: 358–362, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang J, Cao Z, Zhang XM, Nakamura M, Sun M, Hartman J, Harris RA, Sun Y, Cao Y. Novel mechanism of macrophage-mediated metastasis revealed in a zebrafish model of tumor development. Cancer Res 75: 306–315, 2015. [DOI] [PubMed] [Google Scholar]
- 124.Wang S, Yuan Y, Liao L, Kuang SQ, Tien JC, O'Malley BW, Xu J. Disruption of the SRC-1 gene in mice suppresses breast cancer metastasis without affecting primary tumor formation. Proc Natl Acad Sci USA 106: 151–156, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang W, Mouneimne G, Sidani M, Wyckoff J, Chen X, Makris A, Goswami S, Bresnick AR, Condeelis JS. The activity status of cofilin is directly related to invasion, intravasation, and metastasis of mammary tumors. J Cell Biol 173: 395–404, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Weiss L, Orr FW, Honn KV. Interactions between cancer cells and the microvasculature: a rate-regulator for metastasis. Clin Exp Metastasis 7: 127–167, 1989. [DOI] [PubMed] [Google Scholar]
- 127.Wong AD, Searson PC. Live-cell imaging of invasion and intravasation in an artificial microvessel platform. Cancer Res 74: 4937–4945, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wong SY, Hynes RO. Lymphatic or hematogenous dissemination: how does a metastatic tumor cell decide? Cell Cycle 5: 812–817, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wyckoff JB, Jones JG, Condeelis JS, Segall JE. A critical step in metastasis: in vivo analysis of intravasation at the primary tumor. Cancer Res 60: 2504–2511, 2000. [PubMed] [Google Scholar]
- 130.Wyckoff JB, Wang Y, Lin EY, Li JF, Goswami S, Stanley ER, Segall JE, Pollard JW, Condeelis J. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res 67: 2649–2656, 2007. [DOI] [PubMed] [Google Scholar]
- 131.Xue C, Liang F, Mahmood R, Vuolo M, Wyckoff J, Qian H, Tsai KL, Kim M, Locker J, Zhang ZY, Segall JE. ErbB3-dependent motility and intravasation in breast cancer metastasis. Cancer Res 66: 1418–1426, 2006. [DOI] [PubMed] [Google Scholar]
- 132.Xue C, Wyckoff J, Liang F, Sidani M, Violini S, Tsai KL, Zhang ZY, Sahai E, Condeelis J, Segall JE. Epidermal growth factor receptor overexpression results in increased tumor cell motility in vivo coordinately with enhanced intravasation and metastasis. Cancer Res 66: 192–197, 2006. [DOI] [PubMed] [Google Scholar]
- 133.Yamamura T, Tsukikawa S, Yamada K, Yamaguchi S. Morphologic analysis of microvessels in colorectal tumors with respect to the formation of liver metastases. J Surg Oncol 78: 259–264, 2001. [DOI] [PubMed] [Google Scholar]
- 134.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 117: 927–939, 2004. [DOI] [PubMed] [Google Scholar]
- 135.Yang X, Zhang Y, Hosaka K, Andersson P, Wang J, Tholander F, Cao Z, Morikawa H, Tegner J, Yang Y, Iwamoto H, Lim S, Cao Y. VEGF-B promotes cancer metastasis through a VEGF-A-independent mechanism and serves as a marker of poor prognosis for cancer patients. Proc Natl Acad Sci USA 112: E2900–2909, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yu M, Bardia A, Wittner BS, Stott SL, Smas ME, Ting DT, Isakoff SJ, Ciciliano JC, Wells MN, Shah AM, Concannon KF, Donaldson MC, Sequist LV, Brachtel E, Sgroi D, Baselga J, Ramaswamy S, Toner M, Haber DA, Maheswaran S. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science 339: 580–584, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yuan JH, Yang F, Wang F, Ma JZ, Guo YJ, Tao QF, Liu F, Pan W, Wang TT, Zhou CC, Wang SB, Wang YZ, Yang Y, Yang N, Zhou WP, Yang GS, Sun SH. A long noncoding RNA activated by TGF-beta promotes the invasion-metastasis cascade in hepatocellular carcinoma. Cancer Cell 25: 666–681, 2014. [DOI] [PubMed] [Google Scholar]
- 138.Zang G, Gustafsson K, Jamalpour M, Hong J, Genove G, Welsh M. Vascular dysfunction and increased metastasis of B16F10 melanomas in Shb deficient mice as compared with their wild type counterparts. BMC Cancer 15: 234, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zervantonakis IK, Hughes-Alford SK, Charest JL, Condeelis JS, Gertler FB, Kamm RD. Three-dimensional microfluidic model for tumor cell intravasation and endothelial barrier function. Proc Natl Acad Sci USA 109: 13515–13520, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang H, Wong CC, Wei H, Gilkes DM, Korangath P, Chaturvedi P, Schito L, Chen J, Krishnamachary B, Winnard PT Jr, Raman V, Zhen L, Mitzner WA, Sukumar S, Semenza GL. HIF-1-dependent expression of angiopoietin-like 4 and L1CAM mediates vascular metastasis of hypoxic breast cancer cells to the lungs. Oncogene 31: 1757–1770, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 141.Zhang L, Kharbanda S, McLeskey SW, Kern FG. Overexpression of fibroblast growth factor 1 in MCF-7 breast cancer cells facilitates tumor cell dissemination but does not support the development of macrometastases in the lungs or lymph nodes. Cancer Res 59: 5023–5029, 1999. [PubMed] [Google Scholar]
- 142.Zhou ZN, Sharma VP, Beaty BT, Roh-Johnson M, Peterson EA, Van Rooijen N, Kenny PA, Wiley HS, Condeelis JS, Segall JE. Autocrine HBEGF expression promotes breast cancer intravasation, metastasis and macrophage-independent invasion in vivo. Oncogene 33: 3784–3793, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zijlstra A, Lewis J, Degryse B, Stuhlmann H, Quigley JP. The inhibition of tumor cell intravasation and subsequent metastasis via regulation of in vivo tumor cell motility by the tetraspanin CD151. Cancer Cell 13: 221–234, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zijlstra A, Mellor R, Panzarella G, Aimes RT, Hooper JD, Marchenko ND, Quigley JP. A quantitative analysis of rate-limiting steps in the metastatic cascade using human-specific real-time polymerase chain reaction. Cancer Res 62: 7083–7092, 2002. [PubMed] [Google Scholar]