to the editor: In their recent paper, Cornachione et al. (1) draw three major conclusions: 1) that cardiac myofibrils do not show static tension or residual force enhancement; 2) that static tension/residual force enhancement is directly related to titin isoforms; and 3) that static tension/residual force enhancement is not associated with titin-actin interactions.
Here, I would like to point out that much of the raw data displayed by Cornachione et al. (Fig. 2, A–C, in their manuscript) are inconsistent with accepted mechanical properties of skeletal muscle contraction in general, and long-known properties of sarcomeres and myofibrils specifically, thereby undermining confidence in the conclusions drawn from their results.
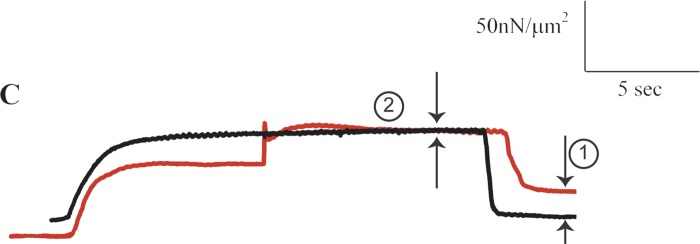
Fig. 2.
Isometric force-time trace of a rabbit cardiac myofibril at an average sarcomere length of 2.0 μm (black line), and corresponding experimental contraction of that same myofibril starting with an isometric contraction at an average sarcomere length of 1.8 μm, then stretched to 2.0 μm, and held isometrically at that final length (red line). Note the substantial passive force enhancement (arrow 1), indicating that cardiac myofibrils are predicted to exhibit residual force enhancement and static tension. The apparent lack of residual force enhancement (arrow 2), is inconsistent as the total force enhancement should be at least as large as the passive force enhancement (3, 4, 5). [From Cornachione et al. (1).]
In the following, I will only focus on the most obvious inconsistencies in the results shown by Cornachione et al. in their Fig. 2, A–C.
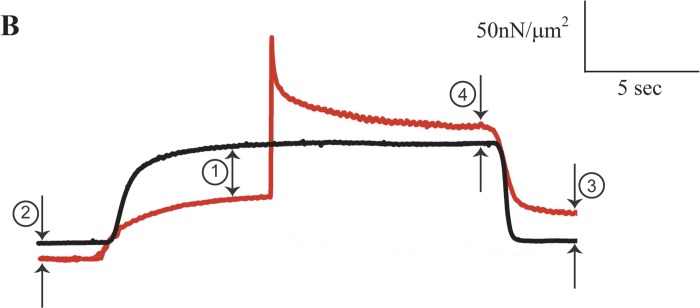
Below, I reproduced part of Fig. 2B from Cornachione et al. (Fig. 1, here labeled B). Specifically, I reproduced the isometric reference contraction at 3.0 μm (black trace) and the active stretch experiment from 2.8 to 3.0 μm (red trace) of the rabbit soleus myofibrils that were shown by Cornachione et al. as representative records of their results. I added arrows and numbers to the traces to illustrate selected inconsistencies. I also deleted the beginning and end of the traces, which are not relevant to the experiments. Otherwise, I have not changed the traces in any manner.
Fig. 1.
Isometric reference force-time trace from a rabbit soleus myofibril contracting at an average sarcomere length of 3.0 μm (black line) and corresponding experimental contraction of that same myofibril starting with an isometric contraction at an average sarcomere length of 2.8 μm, then stretched to 3.0 μm and held isometrically at 3.0 μm (red line). The active force for the red trace at arrow 1 should be ∼14% greater than that for the black trace but it is only about half of the expected value. The total residual force enhancement (arrow 4) should be greater than the passive force enhancement (arrow 3), whereas it is clearly smaller. Together, these results suggest that the active force in the experimental (red trace) is ∼50% lower than expected compared with the black trace. The reasons for this inconsistency are not known but are likely associated with damage to the myofibril, incomplete activation, or deviation from the experimental protocol described in the methods section. [From Cornachione et al. (1).]
In Fig. 1 (arrow 1), note that the force prior to stretch of the red trace (at a sarcomere length of 2.8 μm) is about half that of the isometric force of the black trace (at a sarcomere length of 3.0 μm). According to the force-length relationship (2), the isometric force of the red trace prior to stretch should be significantly greater (by ∼14%) than that of the black trace (at 3.0 μm) because for rabbit myofibrils, sarcomere lengths of 2.8 to 3.0 μm are on the descending limb of the force-length relationship (as correctly pointed out by the authors themselves). Even accounting for the passive force difference at sarcomere lengths of 2.8 and 3.0 μm (Fig. 1, arrow 2) does not come close to explaining why the red trace representing the experimental stretch contraction has a much lower active force than it should have relative to the black isometric reference force. The ∼50% deficit in force appears to indicate that the experimental stretch contraction (red trace) was executed with a damaged or incompletely activated myofibril. This conclusion is further supported by the passive force enhancement shown in this example (Fig. 1, arrow 3), which is approximately twice as large as the total residual force enhancement (Fig. 1, arrow 4), while it should be equal to (at best) or smaller than the total residual force enhancement (3, 4, 5). Together, these observations on the original force traces displayed by Cornachione et al. suggest an approximately 50% loss of active force in the experimental stretch contraction (red trace) compared with the isometric reference contraction (black trace).
Note that in the original Fig. 2B by Cornachione et al., the traces for the purely passive stretch and the active stretch in the presence of cross-bridge inhibition at a sarcomere length of 3.0 μm never reach the passive force of the isometric reference trace at 3.0 μm, suggesting that either stretch (sarcomere) lengths were not well controlled or that there was a loss of passive force caused by damage to structural components of the myofibrils. Similar inconsistencies were also present in Cornachione et al.'s raw data for rabbit psoas myofibrils (their Fig. 2A). Specifically, the active force prior to stretch (red trace in the original Fig. 2A by Cornachione et al.) relative to the isometric reference contraction (black trace in their original Fig. 2A) is also too small, suggesting damage or incomplete activation of the myofibril in the experimental stretch (red) compared with the isometric reference (black) contraction.
Below, I also reproduced the isometric reference contraction (black trace) and the experimental stretch contraction (red trace) for a rabbit cardiac myofibril shown by Cornachione et al. in their original Fig. 2C (Fig. 2 here labeled C). Again, the raw data traces for the experimentally relevant times are faithfully reproduced, but labeling was added for clarity. From these data, Cornachione et al. concluded that cardiac myofibrils showed no static tension or residual force enhancement in their experiments. The inconsistency here is that, while their exemplar graph shows an apparent lack of residual force enhancement, it also shows a large amount of passive force enhancement (Fig. 2, arrow 1). However, the total residual force enhancement should be at least as large as the passive force enhancement (3, 4, 5). Yet, no total residual force enhancement is apparent in this figure (Fig. 2, arrow 2). As above, this suggests that the active force following stretch in the experimental trace (red) was compromised relative to the isometric reference force (black trace). The large passive force enhancement shown in this graph indicates that cardiac myofibrils in fact exhibit total residual force enhancement, but that a compromised preparation, or an inappropriate stretch protocol (e.g., cross-bridge slippage during stretch) prevented the total residual force enhancement from being observed.
In summary, the raw data displayed in Fig. 2, A–C, of the original manuscript by Cornachione et al. are internally inconsistent, and basic properties of skeletal muscle, such as the decrease in isometric force with increasing length on the descending limb of the force-length relationship, are violated. The results shown in these figures suggest substantial deterioration of the active and passive forces in the experimental preparations (or other unidentified methodological errors), as well as misinterpretation of some results. Since the conclusions drawn by the authors crucially depend on accurate force enhancement and passive property measurements, the numerous inconsistencies and obvious inaccuracies in the raw data render the conclusions drawn by the authors questionable, and for some of the results, e.g., the cardiac myofibrils, they are demonstrably incorrect.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
W.H. drafted manuscript; W.H. edited and revised manuscript; W.H. approved final version of manuscript.
ACKNOWLEDGMENTS
The author's work is supported by NSERC (Natural Sciences and Engineering Research Council) of Canada, CIHR (the Canadian Institutes of Health Research), CRC (the Canada Research Chair Programme) and the Killam Memorial Chair.
REFERENCES
- 1.Cornachione AS, Leite F, Bagni MA, Rassier DE. The increase in non-cross-bridge forces after stretch of activated striated muscle is related to titin isoforms. Am J Physiol Cell Physiol 310: C19–C26, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gordon AM, Huxley AF, Julian FJ. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol 184: 170–192, 1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herzog W, Leonard TR. Force enhancement following stretching of skeletal muscle: a new mechanism. J Exp Biol 205: 1275–1283, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Rassier DE, Herzog W. Effects of shortening on the stretch-induced force enhancement in single skeletal muscle fibers. J Biomech 37: 1305–1312, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Rassier DE, Herzog W, Wakeling JM, Syme D. Stretch-induced, steady-state force enhancement in single skeletal muscle fibers exceeds the isometric force at optimal fibre length. J Biomech 36: 1309–1316, 2003. [DOI] [PubMed] [Google Scholar]