Abstract
The K+-Cl− cotransporters (KCC1-KCC4) encompass a branch of the SLC12 family of electroneutral cation-coupled chloride cotransporters that translocate ions out of the cell to regulate various factors, including cell volume and intracellular chloride concentration, among others. L-WNK1 is an ubiquitously expressed kinase that is activated in response to osmotic stress and intracellular chloride depletion, and it is implicated in two distinct hereditary syndromes: the renal disease pseudohypoaldosteronism type II (PHAII) and the neurological disease hereditary sensory neuropathy 2 (HSN2). The effect of L-WNK1 on KCC activity is unknown. Using Xenopus laevis oocytes and HEK-293 cells, we show that the activation of KCCs by cell swelling was prevented by L-WNK1 coexpression. In contrast, the activity of the Na+-K+-2Cl− cotransporter NKCC1 was remarkably increased with L-WNK1 coexpression. The negative effect of L-WNK1 on the KCCs is kinase dependent. Elimination of the STE20 proline-alanine rich kinase (SPAK)/oxidative stress-responsive kinase (OSR1) binding site or the HQ motif required for the WNK-WNK interaction prevented the effect of L-WNK1 on KCCs, suggesting a required interaction between L-WNK1 molecules and SPAK. Together, our data support that NKCC1 and KCCs are coordinately regulated by L-WNK1 isoforms.
Keywords: SPAK, WNK4, Xenopus oocytes, HEK-293 cells
the K+-CL− cotransporters (KCCs) constitute a branch of the electroneutral cation-coupled chloride cotransporter family SLC12 that is composed of four members: KCC1 to KCC4 (20, 22, 42, 45, 48). KCC1 is considered a housekeeping isoform due to its ubiquitous expression pattern (32), while KCC4 is expressed in several tissues with a strong presence in the heart and kidney (35, 42). KCC3 is expressed in multiple tissues with particularly abundant transcripts in heart, muscle, kidney, brain, and placenta (22, 42, 48). Although five different NH2-terminal isoforms of KCC3 have been identified at the molecular level (40) generated by alternative splicing of exons, here we will focus on the two most abundant isoforms in the organism, KCC3a and KCC3b. There are several potential phosphorylation sites for PKC (42) within the 51 amino acid residues present in KCC3a (exon 1a) that are not present in KCC3b (exon 1b) (22), suggesting different posttranslational regulation. Finally, KCC2, of which two different NH2-terminal isoforms, named KCC2a (62) and KCC2b (60) have been described, is only expressed in the central nervous system (CNS) (45). Both isoforms differ in the presence of a single sequence of 40 amino acid residues at the first exon of KCC2a, which contains a putative STE20 proline-alanine rich kinase (SPAK)/oxidative stress-responsive kinase (OSR1) binding site (62).
Following the potassium driving force developed and maintained by the Na+-K+-ATPase, the KCCs translocate ions to the outside of the cell, participating in various physiological roles including epithelial ion transport, cell volume, and intracellular chloride concentration ([Cl−]i) regulation (17). Erythroid function and differentiation (30), cancer cell growth and invasiveness (8, 57, 58), arterial blood pressure regulation (54), and neuronal excitability (23, 33, 53) are some of the important roles of these membrane proteins. At the renal level, KCC3 is located in the basolateral membranes of the proximal tubules (PT) (40), while KCC4 is expressed in proximal tubules, in the thick ascending limb of Henle's loop (TALH) (35), and in α-intercalated cells of the collecting duct (CD) (6, 35). In these zones of the nephron, KCC3 and KCC4 are involved in processes such as glucose and salt reabsorption and acid-base homeostasis (6, 35). It is known that KCCs are activated due to an increase in [Cl−]i or by cell swelling (39, 40, 60). This process is related to protein dephosphorylation, and its purpose is to reduce the [Cl−]i or mediate the regulatory volume decrease (RVD) (1, 14, 17).
The family of serine/threonine kinases proteins called WNK [with no lysine (K)] has been described for its ability to modulated the activity of members of the SLC12 family and other Cl− transport pathways (18, 24, 25). Up to date, four WNK isoforms have been identified but only WNK2, WNK3, and WNK4 have been extensively studied (3). In regard to full-length WNK1 (L-WNK1), this kinase has been related to renal ion transport and neuronal chloride concentration (5, 34, 66). Mutations in this gene cause two different diseases in humans: a form of salt-sensitive hypertension known as pseudohypoaldosteronism type II (PHAII) or familial hyperkalemic hypertension (65) and hereditary sensory neuropathy type 2 (HSN2), a recessive disorder in which peripheral sensory nerves and, therefore, sensitivity are lost (29, 55, 56, 59). Thus L-WNK1 kinase, which is ubiquitously expressed, is expected to be a modulator in the SLC12 family of cotransporters. Although previous studies have shown that other WNKs mediate the activity of all members of the SLC12 family (10, 12, 19, 44, 52), the precise effect of L-WNK1 on these cotransporters remains unknown. Rinehart et al. (51) observed that knocking down L-WNK1 with two distinct siRNAs reduced the phosphorylation of the key residues T991 and T1048 of the KCC3a isoform, a condition that is associated with its activation, suggesting that by promoting KCC3 phosphorylation, L-WNK1 could also be an inhibitor of this cotransporter. In the present study, we analyzed the effect of several human L-WNK1 isoforms on the activity of K+-Cl− cotransporters.
MATERIALS AND METHODS
cDNA constructs and mutations.
The full-length KCCs (39, 40, 60), as well as the wild-type and mutants of human L-WNK1 kinase cDNAs used in this work, were previously described (7, 46, 63). L-WNK1 gives rise to several distinct isoforms. In the present study, we used the full-length L-WNK1 and the variants lacking only exon 9 (L-WNK1-Δ9), only exon 11 (L-WNK1-Δ11), exons 11 and 12 (L-WNK1-Δ11–12), and exons 9, 11 and 12 (L-WNK1-Δ9–11–12). However, given that the most active variant against the renal thiazide-sensitive Na+-Cl− cotransporter (NCC) is L-WNK1-Δ11 (7), we focused our study on the effect of the full-length L-WNK1 and the variant lacking only exon 11. Mutant constructs were generated using custom-made primers (Sigma) and the Quick-Change mutagenesis system following the manufacturer's recommendations (Stratagene). All mutations were confirmed by sequencing and subcloned back into the appropriate expression constructs. To prepare cRNA, KCC1, KCC2a/b, and KCC4 cDNAs were linearized at their 3′-end with NheI and KCC3a/b cDNA with NotI, and the various isoforms of L-WNK1 kinase cDNAs were linearized with MluI and transcribed in vitro using a T7 RNA polymerase mMESSAGE kit (Ambion). The transcription product integrity was confirmed on agarose gels, and the concentration was determined by absorbance readings at 260 nm (SmartSpec Plus, Hercules, CA). cRNA was stored in aliquots at −80°C until used.
Assessment of the Na+-K+-2Cl− and K+-Cl− cotransporter function.
K+-Cl− cotransport activity of all four wild-type KCCs (KCC1-KCC4) and the endogenous Na+-K+-2Cl− (NKCC1) was assessed using the heterologous expression system of Xenopus laevis oocytes following our standard procedures (10, 36, 37, 39). Briefly, mature oocytes were injected with water alone or containing 0.2 μg/μl of KCCs cRNA alone or together with 0.1 μg/μl of the various isoforms and mutants of L-WNK1 kinase cRNA. Two days after injection, the activity of the KCCs was determined by measuring the Cl−-dependent 86Rb+ uptake under hypotonic conditions (110 mosmol/kgH2O). The activity of endogenous NKCC1 was determined by bumetanide-sensitive 86Rb+ uptake under hypotonic (155 mosmol/kgH2O) conditions, which is known to promote inhibition of the cotransporter (16, 27). All transport assays were performed with groups of 10–15 oocytes preincubated for 30 min at room temperature in a Cl−-free medium (in mM: 50 N-methyl-d-glucamine-gluconate, 10 K+-gluconate, 4.6 Ca2+-gluconate, 1 Mg+-gluconate, and 5 HEPES/Tris, pH 7.4) and then transferred to the Na+-free uptake medium (in mM: 40 N-methyl-d-glucamine-Cl−, 10 KCl, 1.8 CaCl2, 1 MgCl2, and 5 HEPES/Tris, pH 7.4) containing 1 μCi of 86Rb+/ml for 1 h at 32°C (10, 36, 37, 39). Tracer activity was determined for each oocyte by β-scintillation counting. Our experimental data are based on three to four independent experiments.
Antibodies.
The total and phospho-specific KCC3a antibodies for KCC3a and SPAK/OSR1 used in this study were previously described and validated (11, 36). Polyclonal anti-c-Myc antibody was used to identify L-WNK1 (Sigma). Secondary antibodies coupled to horseradish peroxidase that were used for immunoblotting were obtained from Sigma. Specificity of phosphoantibodies was assessed using protein extracts from oocytes or HEK-293 cells transfected with KCC3a cDNA.
Cell culture, transient cell lines, and stimulations.
The full-length human KCC3a and L-WNK1-Δ11 cDNAs were previously subcloned into the mammalian expression vector pCMV5 and pcDNA3.1, respectively. KCC3a was tagged with Flag and L-WNK1-Δ11 with c-Myc (7, 36). To generate the transient cell lines, HEK-293 cells underwent passages every 2–3 days to maintain the cells in their exponential growth phase. Cells were culture on 10-cm-diameter dishes in Dulbecco's modified Eagle medium (DMEM) supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, 10% FBS (vol/vol), and 2 mM l-glutamine in a humidified atmosphere containing 95% air-5% CO2 at 37°C (9, 36). Then, cells were transfected with 1–3 μg of full-length KCC3a and/or L-WNK1-Δ11 cDNA or empty vector using lipofectamine-2000 (Invitrogen) following the manufacturer's recommendations. After 36 h of transfection cells were stimulated with either isotonic or hypotonic Na+ and Cl−-free medium (in mM: 125 N-methyl-d-gluconate, 10 K-gluconate, 1 Mg-gluconate, 5 sucrose, and 5 HEPES/Tris, pH 7.4) for 30 min at 37°C and then transferred to a Na+-free medium (in mM: 125 N-methyl-d-glucamine-Cl−, 10 KCl, 1.8 CaCl2, 1 MgCl2, and 5 HEPES/Tris, pH 7.4) for 10 min at 37°C (10, 36). To reach the isotonic conditions for HEK-293 cells (∼300 mosmol/kgH2O), mediums were supplemented with 1.7 g/100 ml sucrose. Following the treatment, cells were harvested in ice-cold lysis buffer, 0.1 ml lysis buffer per well [in mM: 50 Tris·HCl (pH 7.5), 1 EGTA, 1 EDTA, 50 NaF, 5 Na4O7P2, 1 Na3VO4, 1% (wt/vol) Nonidet P40, 0.27 sucrose, and 0.1% 2-mercaptoethanol] supplemented with a protease inhibitor cocktail (1 tablet per 50 ml; Sigma), incubated for 10 min, and then scraped. Cell homogenates were centrifuged for 15 min at 26,000 g, and supernatants were collected and stored at −80°C until used. Protein concentrations were determined using the Bradford method.
Western blot analysis of wild-type flag-hKCC3a and cmyc-hL-WNK1-Δ11.
Total proteins were extracted either from Xenopus oocytes or HEK-293 cells. When extracted from oocytes, groups of 10 to 20 oocytes were previously injected with cRNA from various constructs, transferred to Eppendorf tubes, and homogenated in ice-cold lysis buffer, 10 μl/oocyte, supplemented with a protease inhibitor cocktail. The homogenates were centrifuged at 10,000 g for 10 min at 4°C, and the supernatant containing the total protein fraction was collected. Western blotting was performed using the total protein equivalent to one oocyte resolved in 7.5% polyacrylamide gels (SDS-PAGE) and transferred to polyvinylidine difluoride membranes (PVDF; Amersham).
The HEK-293 cells that were previously transfected with hKCC3a and hL-WNK1-Δ11 were cultured in T25 flasks, washed, and then scraped into ice-cold lysis buffer. Cell homogenates were sonicated and clarified by centrifugation (11,000 g for 5 min), and protein concentration was assessed in duplicate using the Bradford method. Then, 25 μg of protein were resolved in 7.5% SDS-PAGE and transferred to PVDF membranes. In both cases, membranes were blocked with TBST (Tris-buffered saline/Tween 20, in mM: 100 Tris·HCl, 150 NaCl, and 0.2% Tween 20, pH 7.5) containing 5% (wt/vol) of nonfat dry milk and incubated overnight at 4°C, with anti-KCC3a phospho-specific antibodies T991 and T1048 (2 μg/ml plus 2 μl/ml nonphosphopeptide) (11, 36), polyclonal anti-cMyc (1:1,000; Sigma), anti-β actin (1:2,500; Santa Cruz Biotechnology), or anti-GAPDH (1:10,000; Sigma). After further being washed, blots were incubated with (horseradish peroxidase) conjugated anti-sheep secondary antibody (1:5,000; Sigma) for 1 h at room temperature. Immunoreactive species were detected by chemiluminescence (Luminata Forte; Millipore), and bands were visualized using a C-DiGit Blot Scanner (Li-Cor, Lincoln, NE).
Statistical analysis.
Statistical significance was defined as two-tailed P < 0.05, and the results are presented as the mean ± SE. The significance of the differences between groups was tested by Student's t-test or nonparametric Mann-Whitney test. For the uptake experiments in oocytes, each condition was studied 3 to 4 times (frogs) with 10–15 oocytes per condition, while for the experiments using cell line HEK-293, each condition was tested three times; n represents the number of experiments using either frogs (oocytes) or HEK-293 cells (wells seeded).
RESULTS
Inhibition of the K+-Cl− cotransporters by full-length wild-type L-WNK1 (L-WNK1) is dependent on WNK1 catalytic activity.
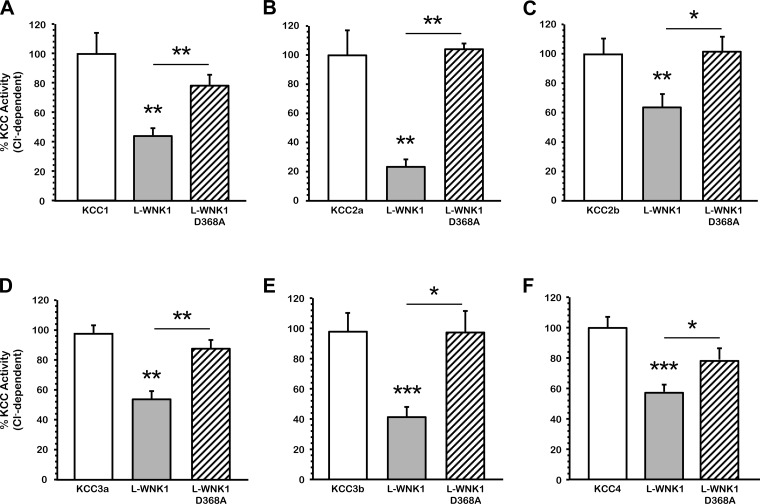
We have previously shown in Xenopus laevis oocytes that hypotonic conditions trigger the activity of the K+-Cl− cotransporters (36, 37, 39) and that wild-type WNK3 prevents their cell swelling-induced activation. Likewise catalytically inactive WNK3-D294A is able to bypass the tonicity requirements to regulate KCCs under isotonicity (10, 12, 16). Here, we analyzed the effect of L-WNK1 on the K+-Cl− cotransporters and whether its effect requires the kinase catalytic activity. Elimination of the catalytic activity of L-WNK1 was achieved by a single point mutation in which the aspartic acid residue 368 of the kinase domain was substituted by alanine (L-WNK1-D368A) (66). Figure 1, A-F, depicts a pool of three experiments in which oocytes were injected with KCC alone or together with the full-length wild-type L-WNK1 or the catalytically inactive L-WNK1-D368A kinase cRNA. The effect of the kinase was compared with its control 48 h later under hypotonic conditions in the absence or presence of extracellular chloride. As expected, all KCCs, taken as 100%, were activated by cell swelling (in pmol·oocyte−1·h−1: H2O-injected oocytes: 143 ± 323; KCC1: 2,587 ± 323; KCC2a: 5,395 ± 661; KCC2b: 5,830 ± 580; KCC3a: 10,546 ± 651; KCC3b: 7,705 ± 895; KCC4: 10,542 ± 718), and their activities were dramatically reduced in the absence of extracellular chloride. The overexpression of L-WNK1 significantly decreased the activity of all K+-Cl− cotransporters (in percentage: KCC1: 44.1 ± 5.9; KCC2a: 23.7 ± 3.4; KCC2b: 63.7 ± 9.1; KCC3a: 54.7 ± 5.8; KCC3b: 41.5 ± 7.6; KCC4: 57.9 ± 5.3; P < 0.001). Thus coexpression of all KCCs with L-WNK1 resulted in a significant reduction in their activity. In contrast, the catalytically inactive L-WNK1-D368A had no effect on the KCCs activity in hypotonic conditions (in percentage: KCC1: 78.6 ± 7.1; KCC2a: 101.4 ± 5.6; KCC2b: 101.3 ± 9.6; KCC3a: 88.7 ± 5.9; KCC3b: 99.3 ± 16; KCC4: 78.9 ± 7.3; P = NS). Comparison of KCCs activity in the presence of wild-type L-WNK1 or L-WNK1-D368A was statistically significant (*P < 0.01, **P <0.001). This observation suggests that catalytic activity of L-WNK1 is required for KCC inhibition by the kinase during cell swelling. To verify the integrity and processing of L-WNK1 constructs, we performed an immunoblot analysis and found that both kinases are well processed and similarly expressed in Xenopus oocytes, as shows in Fig. 2. Therefore, the lack of effect of L-WNK1-D368A upon the cotransporter's activity is not due to an abnormal protein processing.
Fig. 1.
Effect of the full-length wild-type WNK1 (L-WNK1) and catalytically inactive L-WNK1-D368A on the K+-Cl− cotransport (KCC) activity induced by cell swelling under hypotonic conditions. Xenopus laevis oocytes were injected with water or 0.2 μg/μl each of KCC1 (A), KCC2a (B), KCC2b (C), KCC3a (D), KCC3b (E), or KCC4 (F) cRNA alone (open bars) or together with 0.1 μg/μl of L-WNK1 (grey bars) or catalytically inactive L-WNK1 cRNA harboring the D368A mutation (hatched bars). 86Rb+ uptake was assessed 48 h later in hypotonic conditions (110 mosmol/kgH2O). The pooled data from 3 different experiments are shown as percentage of KCC activity, with means ± SE; n = 3 (10–15 oocytes per group, per experiment were tested). The Cl−-dependent 86Rb+ uptake in each KCC control group was taken as 100% and the groups coinjected with L-WNK1 or L-WNK1-D368A were normalized accordingly. Significantly different from the uptake observed in the corresponding control (absence of L-WNK1 construct, *P < 0.01, **P < 0.001, ***P < 0.0001, by Student's t-test).
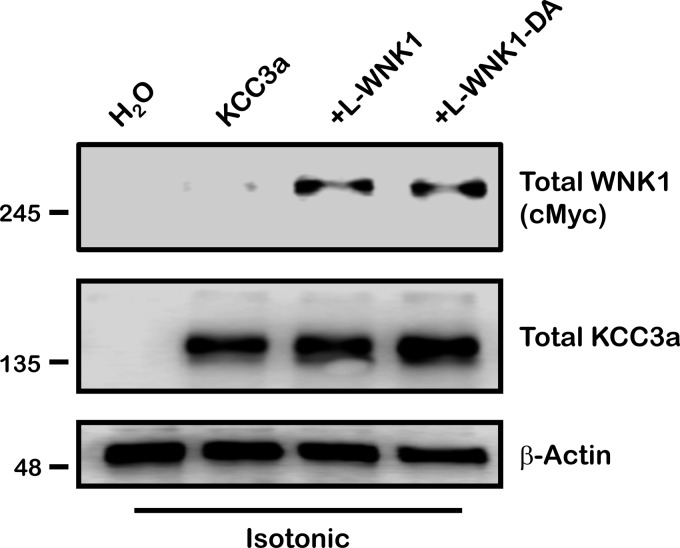
Fig. 2.
Protein expression of L-WNK1 and L-WNK1-D368A under isotonic conditions. Xenopus laevis oocytes were injected with water or 0.2 μg/μl of KCC3a cRNA alone or together with 0.1 μg/μl of L-WNK1 or the catalytically inactive L-WNK1-D368A mutation, as stated. Forty-eight hours later oocytes were homogenated in ice cold lysis buffer and immunoblotted to verify protein expression under isotonic conditions (210 mosmol/kgH2O). Blots were performed using specific antibodies against c-Myc-WNK1, total KCC3, and β-actin as loading control.
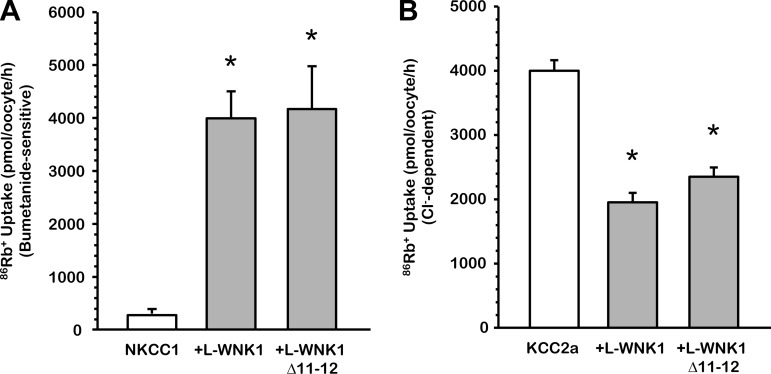
L-WNK1 and L-WNK1-Δ11–12 coordinate the activity of NKCC1 and KCC2.
Multiple studies have demonstrated that the [Cl−]i in the nervous system affects the type of response to neurotransmitters acting on chloride channels in the postsynaptic membranes, such as GABA (2, 5, 13, 28, 38, 53). [Cl−]i is determined by the balance between the activity of NKCC1, involved in chloride influx, and that of KCC2, which promotes chloride efflux. Several variants of L-WNK1 are generated by alternative splicing of 7 exons (8b, HSN2, 9, 11, 12, 26a, and 26b). The full-length L-WNK1 and L-WNK1-Δ11–12 are two isoforms with higher expression in the CNS, including the brain, cerebellum, dorsal root ganglia, and spinal cord (63). Therefore, to analyze whether these two isoforms can regulate, in a coordinated manner, the activity of these two players of the CNS, we coinjected oocytes with NKCC1 or KCC2a cRNA in the absence or presence of wild-type L-WNK1 or L-WNK1-Δ11–12 cRNA. As shown in Fig. 3A, under hypotonic conditions, NKCC1 was similarly activated in the presence of L-WNK1 (4,194 ± 765 pmol·oocyte−1·h−1 vs. NKCC1 alone, P < 0.001) and L-WNK1-Δ11–12 (4,029 ± 443 pmol·oocyte−1·h−1 vs. NKCC1 alone, P < 0.001) compared with the control NKCC1 alone (241 ± 16 pmol·oocyte−1·h−1). KCC2a under hypotonic conditions shows a swelling activation (4,143 ± 151 pmol·oocyte−1·h−1) that was partially prevented in the presence of L-WNK1 (2,033 ± 103 pmol·oocyte−1·h−1, P < 0.001, vs. KCC2a alone) or L-WNK1-Δ11–12 (2,447 ± 138 pmol·oocyte−1·h−1, P < 0.001, vs. KCC2a alone) (Fig. 3B). Overall, these data suggest that both isoforms, L-WNK1 and L-WNK1-Δ11–12, are kinases that have a potential role in coordinating the activity of NKCC1 and KCC2.
Fig. 3.
Effect of L-WNK1 and L-WNK1-Δ11–12 on NKCC1 and KCC2a activity under hypotonic conditions. Xenopus laevis oocytes were injected with 0.2 μg/μl of either NKCC1 (A) or KCC2a (B) cRNA alone or together with 0.1 μg/μl of L-WNK1 or L-WNK1-Δ11–12 cRNA, as stated. 86Rb+ uptake was assessed 48 h later in hypotonic conditions (155 and 110 mosmol/kgH2O, respectively, for NKCC1 and KCC2a). The pooled data from 3 different experiments are shown as means ± SE; n = 3 (10–15 oocytes per group, per experiment were tested). *Significantly different from the uptake observed in control alone (P < 0.001 by Student's t-test).
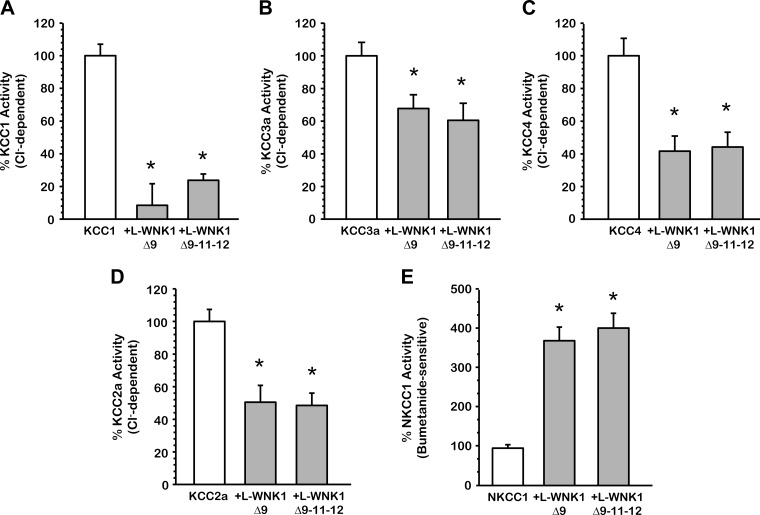
Similar effects of other L-WNK1 variants on the activity of the SLC12 family members.
To determine whether the variants of L-WNK1 similarly affect the activity of the SLC12 family members, we coinjected oocytes with KCC1, KCC2a, KCC3a, KCC4, and NKCC1 cRNA in the absence or presence of L-WNK1-Δ9 or L-WNK1-Δ9–11–12 cRNA. As shown in Fig. 4, A-E, all K+-Cl− cotransporters, as well as NKCC1, were similarly inhibited by these isoforms of L-WNK1. Coinjecting NKCC1 either with L-WNK1-Δ9 or L-WNK1-Δ9–11–12 cRNA resulted in a significant activation of the cotransporter (NKCC1 alone represented as 100%, with L-WNK1-Δ9 368.7 ± 31.1% and with L-WNK1-Δ9–11–12 404.6 ± 33.8%, P < 0.001) as is depicted in Fig. 4E. Chávez-Canales et al. (7) recently showed that the effect of these L-WNK1 variants was different on NCC, where L-WNK1-Δ9 has no impact on NCC's activation, while L-WNK1-Δ9–11–12 increases its activity ∼250%, (P < 0.001). The effect observed on NKCC1 in the presence of these isoforms could be related to a differential regulation of WNK kinases towards the cotransporters. Altogether, our observations indicate that all variants of L-WNK1 are potent activators of NKCC1 and inhibitors of KCCs.
Fig. 4.
Effect of various L-WNK1 isoforms on the activity of the SLC12 family members under hypotonic conditions. Xenopus laevis oocytes were injected with 0.2 μg/μl of KCC1 (A), KCC3a (B), KCC4 (C), KCC2a (D), or NKCC1 (E) cRNA alone or together with 0.1 μg/μl of L-WNK1-Δ9 or L-WNK1-Δ9–11–12 cRNA, as stated. 86Rb+ uptake was assessed 48 h later in hypotonic conditions (110 and 155 mosmol/kgH2O, respectively for KCCs and NKCC1). The pooled data from 3 different experiments are shown as percentage of cotransporter activity with means ± SE; n = 3 (10–15 oocytes per group, per experiment were tested). *Significantly different from the uptake observed in control alone (P < 0.001, by Student's t-test).
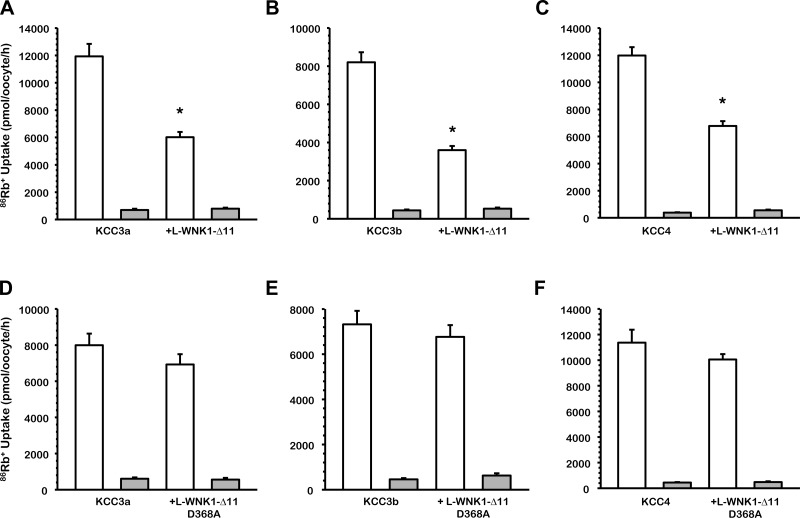
Inhibitory effect of L-WNK1-Δ11 on the renal K+-Cl− cotransporter activity induced by cell swelling in Xenopus oocytes.
Among L-WNK1 variants, the most abundant in the nephron is the variant that lacks exon 11 (63). We therefore analyzed the effect of this isoform on the renal K+-Cl− cotransporters KCC4 and KCC3a/b variants, which are the most abundant forms of KCC expressed in this organ (22, 40, 48). Figure 5, A-C, shows that the presence of L-WNK1-Δ11 reduces the swelling-induced 86Rb+ uptake through the KCCs: KCC3a activity was reduced by 50% (P < 0.001 vs. KCC3a alone), KCC3b activity was reduced by 44% (P < 0.001 vs. KCC3b alone), and KCC4 activity was reduced by 56% (P < 0.001 vs. KCC4 alone). Additionally, as shown in Fig. 5, D-F, the effect of L-WNK1-Δ11 on KCC3 and KCC4 is kinase dependent.
Fig. 5.
Effect of L-WNK1-Δ11 and L-WNK1-Δ11-D368A on the renal K+-Cl− cotransporters activity induced by cell swelling under hypotonic conditions. Xenopus laevis oocytes were injected with water or 0.2 μg/μl each of KCC3a (A), KCC3b (B) or KCC4 (C) cRNA alone or together with 0.1 μg/μl of L-WNK1-Δ11 cRNA, and KCC3a (D), KCC3b (E) or KCC4 (F) cRNA alone or together with 0.1 μg/μl of catalytically inactive L-WNK1-Δ11 harboring the D368A mutation cRNA, as stated. 86Rb+ uptake was assessed 48 h later in hypotonic conditions (110 mosmol/kgH2O) in the presence (white bars) or absence (grey bars) of Cl− in the uptake medium. The pooled data from 3 different experiments are shown, with means ± SE; n = 3 (10–15 oocytes per group, per experiment were tested). *Significantly different from the uptake observed in the corresponding control (absence of L-WNK1-Δ11, P < 0.001, by Student's t-test).
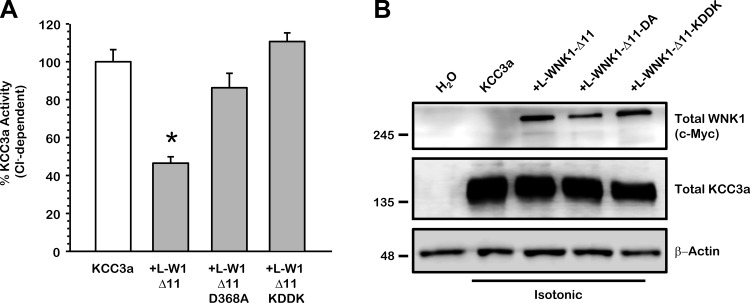
Effect of the double-charge K233D/D368K mutant of L-WNK1-Δ11 upon KCC3a: kinase dependency.
Substitution of the conserved catalytic residue D368 by alanine (D368A) in the L-WNK1 kinase domain abolishes its ability to inhibit KCCs, as previously shown in Figs. 1 and 5. It has been suggested, however, that the single point mutation generated in the WNKs kinase domain could have indirect effects on the structure of the NH2 terminus. The crystal structure of WNK1 kinase domain indicates that residues K233 and D368 are in close vicinity perhaps generating ionic interactions critical for the kinase domain to exerts its effect upon target proteins (21). Recently, Bazúa-Valenti et al. (4) demonstrated that introduction of the double mutant KDDK in WNK4 lacking the Cl−-binding sites prevented the activation of NCC and eliminated most of its activity. Thus we generated the double-charge K233D/D368K mutant (KDDK) into L-WNK1-Δ11 to analyze the kinase dependency on the activity of KCC3a after cell swelling. Consistent with the idea that K233 and D368 are critical for the catalytic activity of L-WNK1, the double mutant KDDK, previously shown to be a kinase dead (21), did not modify the activity of KCC3a (Fig. 6A) and the absence of the effect was not related to an anomalous protein processing as shown in Fig. 6B. Overall, these results provide convincing evidence that WNK1 regulates KCC3a in a kinase-dependent manner.
Fig. 6.
Effect of the L-WNK1-Δ11 double-charge KDDK (K233D/D368K) mutant upon KCC3a. Xenopus laevis oocytes were injected with 0.2 μg/μl of KCC3a cRNA alone or together with 0.1 μg/μl of L-WNK1-Δ11, L-WNK1-Δ11 harboring the D368A (DA) mutation or L-WNK1-Δ11 harboring the K233D/D368K (KDDK) mutation cRNA, as stated. A: 86Rb+ uptake was assessed 48 h later under hypotonic conditions. The pooled data from 4 different experiments are shown as percentage of KCC3a activity, with means ± SE; n = 4 (10–15 oocytes per group, per experiment were tested). *Significantly different from the uptake observed in control alone (absence of L-WNK1-Δ11 mutant P < 0.001, by Student's t-test). B: representative Western blot assay of total protein extracted from oocytes injected with water or wild-type KCC3a with or without L-WNK1-Δ11, L-WNK1-Δ11-DA, or L-WNK1-Δ11-KDDK cRNA under isotonic conditions, as stated. Blots were performed using specific antibodies against c-Myc-WNK1, total KCC3a and, β-actin as loading control.
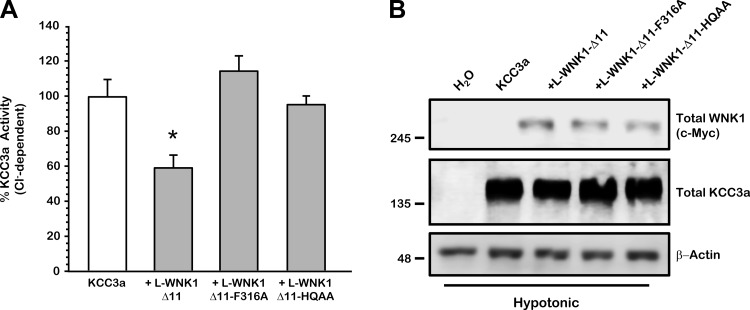
The SPAK/OSR1 binding site F316 of L-WNK1 is required for the regulation of KCCs.
The SPAK/OSR1 can directly or indirectly modulate the activity of members of the SLC12 family. The activity of the Na+-K+-2Cl− cotransporter NKCC1 is regulated by the interaction of WNK4 with SPAK (16). The activation of NKCC2 depends on the WNK3 interaction with SPAK (44). Finally, the activity of Na+-Cl− cotransporter NCC depends on the L-WNK1-SPAK interaction (7). All three cotransporters contain SPAK/OSR1 binding sites within their NH2-terminal domains. When these sites are eliminated, the SPAK-induced activation is dramatically reduced. In contrast, KCCs cotransporters do not contain SPAK/OSR1 binding sites, with the exception of the KCC3a and KCC2a isoforms that contains one in the NH2-terminal domain. This site, however, is not required for the regulation of KCC3a by WNK4 (19). Whereas the elimination of the SPAK/OSR1 binding site in NCC reduced the basal activity of the cotransporter, it did not preclude SPAK activation by WNK3 because the WNK3-SPAK-NCC complex can be formed if WNK3 contains the SPAK/OSR1 binding site. Thus, to determine whether the L-WNK1 effect on the KCCs also requires SPAK interaction, we eliminated the SPAK/OSR1 binding motif in L-WNK1-Δ11 (L-WNK1-Δ11-F316A). As shown in Fig. 7A, the presence of L-WNK1-Δ11 reduced KCC3a by 41% (KCC3a + L-WNK1-Δ11 5,665 ± 708 pmol·oocyte−1·h−1, P < 0.01 vs. KCC3a alone). In contrast, L-WNK1-Δ11-F316A failed to inhibit the K+-Cl− cotransporter (KCC3a + L-WNK1-Δ11-F316A 10,973 ± 838 pmol·oocyte−1·h−1, P = NS vs. KCC3a alone), suggesting that elimination of the SPAK/OSR1 binding site on L-WNK1-Δ11 prevents the inhibition induced by the kinase. To verify equal protein expression of L-WNK1-Δ11 and L-WNK1-Δ11-F316A constructs, Western blot analysis was done under the same experimental conditions. As is shown in Fig. 7B, both proteins are well processes when expressed in Xenopus oocytes, indicating that the absence of the effect on KCC3a is not due to an irregular protein processing. Our results suggest that the loss of L-WNK1-Δ11-F316A effect on KCCs activity is probably due to a reduced capacity of this mutant to interact with endogenous OSR1.
Fig. 7.
L-WNK1-Δ11-F316A and L-WNK1-Δ11-HQAA lose the inhibitory effect on KCC3a under hypotonic conditions. Xenopus laevis oocytes were injected with 0.2 μg/μl of KCC3a cRNA alone or together with 0.1 μg/μl of L-WNK1-Δ11, L-WNK1-Δ11-F316A, or L-WNK1-Δ11-HQAA cRNA, as stated. A: 86Rb+ uptake was assessed 48 h later under hypotonic conditions (110 mosmol/kgH2O). The chloride-dependent Rb+ uptake observed in KCC3a control was taken as 100%, and the effect of the WT kinase and mutants was normalized accordingly. The pooled data from 4 different experiments are shown in percentage of KCC3a activity with means ± SE; n = 4 (10–15 oocytes per group, per experiment were tested). *Significantly different from the uptake observed in KCC3a alone (P < 0.001, by Student's t-test). B: representative Western blot assay of total protein extracted from oocytes injected with water or wild-type KCC3a alone or with L-WNK1-Δ11, L-WNK1-Δ11-F316A, or L-WNK1-Δ11-HQAA mutants cRNA under hypotonic conditions, as stated. Blots were performed using specific antibodies against c-Myc-WNK1, total KCC3a, and β-actin as loading control.
It has been suggested that WNK kinases interact with each other physically by oligomerization (7, 31, 66). The formation of WNK oligomers is most likely due to a small HQ motif (HIQEVVSLQT) located at the COOH-terminal domain of the protein (61). Replacing the HIQEVVSLQT sequence with AIQEVVSLAT has been shown to prevent the interaction between WNK kinases, abrogating the formation of homo- and heteromers (61). Thus we investigated whether the mutation of the HQ motif in L-WNK1-Δ11 (L-WNK1-Δ11-HQAA) can impair the regulation of KCC3a. As depicted in Fig. 7A, L-WNK1-Δ11-HQAA mutant proved to be unable to modify KCC3a activity after cell swelling conditions. Same as L-WNK1-Δ11 and L-WNK1-Δ11-F136A, this protein was properly processed by the oocyte and showed similar expression levels under hypotonic conditions (Fig. 7B). Together, these data suggest that, expressed in Xenopus oocytes, L-WNK1-Δ11 mutants F316A and HQAA are well processed and unable to inhibit KCC3a activity after cell swelling. It also shows that L-WNK1-Δ11 needs to interact with SPAK/OSR1 and other WNKs to inhibit KCC3a activity.
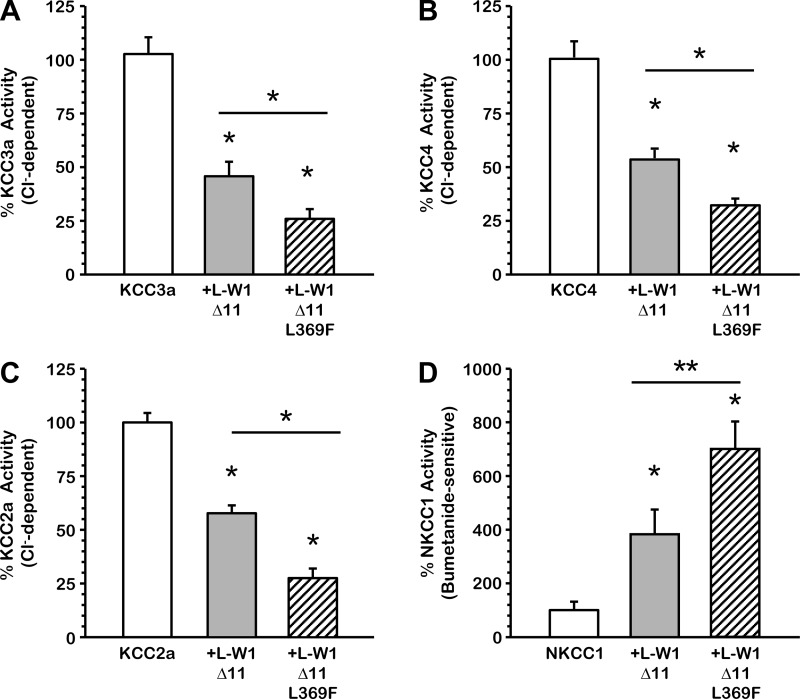
Effect of the elimination of the chloride binding site on L-WNK1-Δ11 upon the SLC12 members.
The responsiveness of the SLC12 family members to changes in extracellular chloride parallels their phosphorylation state, suggesting that these transporters are controlled by chloride-sensitive protein kinases (4, 43, 64). In a recent study, Piala et al. (46) demonstrated that chloride stabilizes the inactive conformation of L-WNK1, preventing kinase autophosphorylation and activation. The leucine residue at position 369 in L-WNK1 is critical for the formation of the Cl−-binding pocket within the catalytic domain (46). The substitution of the leucine for phenylalanine converts L-WNK1 into a constitutively phosphorylated kinase that is insensitive to changes in Cl− concentrations. This mutated kinase could be a more potent inhibitor of KCCs. Therefore, we tested this hypothesis in oocytes exposed to hypotonic conditions by injecting KCCs or NKCC1 cRNA with wild-type L-WNK1-Δ11 or mutant L-WNK1-Δ11-L369F. As shown in Fig. 8, A-D, the activity of each cotransporter alone under hypotonic conditions was represented as 100% and then compared with the activity in the presence of either wild type L-WNK1-Δ11 or mutant L-WNK1-Δ11-L369F. KCC3a activity was 44.4 ± 6.0% in the presence of L-WNK1-Δ11 and 25.6 ± 3.6% in the presence of L-WNK1-Δ11-L369F compared with KCC3a alone. KCC4 activity was 57.8 ± 4.5% in the presence of L-WNK1-Δ11 and 35.8 ± 3.1% with the L369F mutant. When coinjected with L-WNK1-Δ11, KCC2a activity was 57.6 ± 4.3%, and in the presence of the L369F mutant, its activity dropped to 26.9 ± 2.8%. In regard to NKCC1, under hypotonic conditions, L-WNK1-Δ11 increased its activity to 394.4 ± 89.1%, whereas the L369F mutant activity increased to 697.2 ± 97.9%. Therefore, elimination of the Cl−-binding site turned L-WNK1-Δ11-L369F more efficient in inhibiting KCCs (P < 0.001) or activating NKCC1 (P < 0.01) than wild-type L-WNK1-Δ11.
Fig. 8.
Elimination of putative Cl−-binding site L369 on L-WNK1-Δ11 increased the inhibitory effect on KCCs and the activating effect on NKCC1. Xenopus laevis oocytes were injected with 0.2 μg/μl of either KCC3a (A), KCC4 (B), KCC2a (C), or NKCC1 (D) cRNA alone or together with 0.1 μg/μl of L-WNK1-Δ11 or L-WNK1-Δ11 harboring the L369F mutation cRNA, as stated. 86Rb+ uptake was assessed 48 h later in hypotonic conditions (110 and 155 mosmol/kgH2O, respectively for KCCs and NKCC1). The pooled data from 4 different experiments are shown as percentage of the cotransporter activity, with means ± SE; n = 4 (10–15 oocytes per group, per experiment were tested). Significantly different from the uptake observed in control alone (*P < 0.001, **P < 0.01, by Student's t-test).
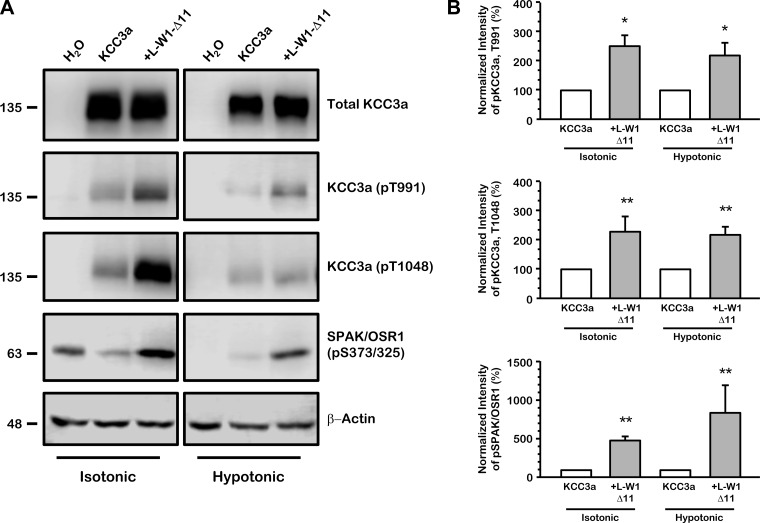
L-WNK1-Δ11 increases phosphorylation of the K+-Cl− cotransporters under isotonic and hypotonic conditions.
As mentioned above, the phosphorylation of KCC3 inhibits its activity. Therefore, L-WNK1 could inhibit the cotransporter by inducing its phosphorylation. To test this hypothesis, we used previously generated and validated phospho-specific antibodies that recognize KCC3a at the phospho-residues Thr991 and Thr1048 (11, 35, 36) and SPAK/OSR1 at the phospho-residue Ser373/Ser325, respectively (11). These residues, as well as the corresponding to the endogenous SPAK/OSR1 kinase, are phosphorylated in Xenopus oocytes maintained in isotonic conditions, in which KCC3 is inactive. As it was expected, under isotonic conditions, the coinjection of L-WNK1-Δ11 significantly increased the phosphorylation status of KCC3a and the endogenous SPAK/OSR1 through their phospho-residues (KCC3a: T991, 255 ± 43%, P < 0.01; KCC3a: T1048, 231 ± 53%, P < 0.001 and SPAK/OSR1: S373/S325, 465 ± 51%, P < 0.01 vs. KCC3a alone taken as 100%) as is depicted in Fig. 9A and in the densitometric analysis on Fig. 9B. When oocytes are exposed to hypotonic conditions, these residues become considerably dephosphorylated, activating KCC3a. Thus, in the presence of L-WNK1-Δ11 kinase, which precludes the activation of KCC3a in hypotonic conditions, the level of phosphorylation increases (Fig. 9, A and B).
Fig. 9.
L-WNK1-Δ11 induces phosphorylation of KCC3a and inhibits its activation under hypotonic conditions. A: representative Western blot assay of total protein extracted from oocytes injected with water or wild-type KCC3a with or without L-WNK1-Δ11 cRNA under isotonic or hypotonic conditions, as stated. Blots were performed using specific antibodies against total KCC3a and phospho-antibodies directed to Thr991 and Thr1048 of KCC3a and Ser373/325 of STE20 proline-alanine rich kinase (SPAK)/oxidative stress-responsive kinase (OSR1), respectively. β-Actin was used as loading control. B: densitometric analysis of data compiled from 4 different experiments. KCC3a alone (white bars) under isotonic or hypotonic conditions was arbitrarily set as 100% and the corresponding groups coinjected with L-WNK1-Δ11 (grey bars) were normalized accordingly. Significantly different from KCC3a alone; n = 4, *P < 0.01, **P < 0.001 by Mann-Whitney test.
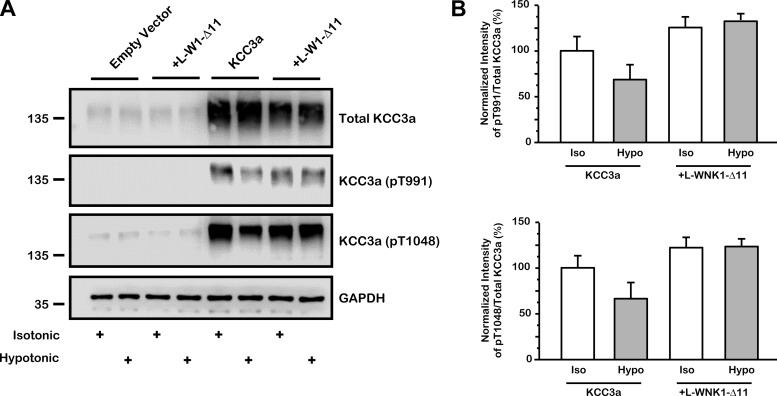
The effect of L-WNK1-Δ11 on the KCCs was analyzed using mammalian HEK-293 cells transiently transfected with KCC3a alone or cotransfected with L-WNK1-Δ11, stimulated under isotonic or hypotonic conditions (Fig. 10A). Cells were immunoblotted using a specific antibody against total KCC3a and phospho-antibodies directed against Thr991 and Thr1048 of KCC3a. GAPDH was used as the loading control. Figure 10A shows that in HEK-293 cells after hypotonic conditions the activation of KCC3a is associated with partial dephosphorylation of residues Thr991 and Thr1048. However, when coexpressed with L-WNK1-Δ11 dephosphorylation of the cotransporter in hypotonic conditions was not observed. Figure 10B corresponds to the densitometric analysis of pT991 and pT1048 ratios towards total KCC3a immunoblots. All groups were compared with phosphorylation of KCC3a under isotonic conditions, which was taken as 100%. KCC3a pT991 phosphorylation cotransfected with L-WNK1-Δ11 was 126 ± 7 and 133 ± 8% in isotonic and hypotonic conditions, respectively. KCC3a pT1048 was 120 ± 11 and 124 ± 19% compared with isotonic or hypotonic conditions in presence of L-WNK1-Δ11, respectively. These results suggest that phosphorylation of T991 and T1048 is increased in presence of L-WNK1-Δ11 either in isotonic or hypotonic conditions. We assume that only partial dephosphorylation of KCC3a was achieved given the short time of hypotonic stimulation (see Fig. 11).
Fig. 10.
Inhibitory effect of L-WNK1-Δ11 correlates with KCC3a phosphorylation status in HEK-293 cells A: HEK-293 cells were transiently transfected with full-length wild-type Flag-KCC3a with or without c-Myc-L-WNK1-Δ11. Thirty-six hours after transfection, cells were preincubated in either isotonic or hypotonic Na+ and Cl−-free medium during 30 min and later transferred to either isotonic or hypotonic Na+-free medium for another 10 min. Cells were lysed, and total cell extracts were immunoblotted using specific antibodies against total KCC3a, pT991 and pT1048 of KCC3a and GAPDH as loading control. B: densitometric analysis of data compiled from 3 different experiments. KCC3a basal isotonic phosphorylation (white bar) was arbitrarily set as 100% and the corresponding groups were normalized accordingly. The result of densitometric analysis is expressed as the percentage of pT991 (iso or hypo) over total KCC3a (iso or hypo) and the percentage of pT1048 (iso or hypo) over total KCC3a (iso or hypo). P = NS by Mann-Whitney test; n = 3.
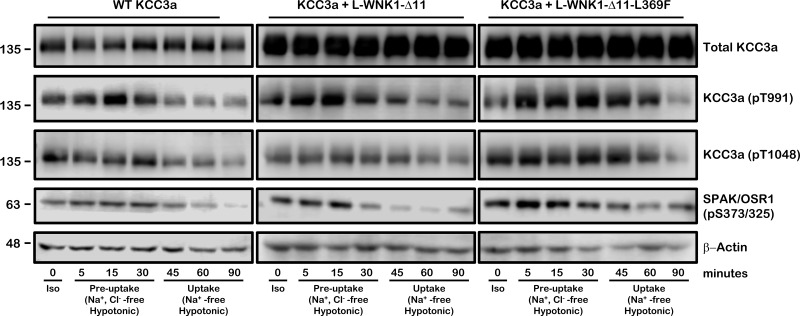
Fig. 11.
Time course of phosphorylation status of KCC3a induced by cell swelling. Representative Western blot assay of total protein extracted from oocytes injected with 0.2 μg/μl KCC3a cRNA alone (right) or together with 0.1 μg/μl of L-WNK1-Δ11 (middle) or L-WNK1-Δ11-L369F cRNA (left) assessed 48 h later in hypotonic conditions. Phosphorylation status of KCC3a and SPAK/OSR1 was monitored over a 90-min time period, as stated. Oocytes were immediately lysed and extracts were analyzed by immunoblot using specific antibodies against total KCC3a, phosphoantibodies directed to Thr991 and Thr1048 of KCC3a and Ser373/Ser325 of SPAK/OSR1, respectively. β-Actin was used as loading control. Similar results were obtained in three independent experiments. Iso, isotonic (210 mosmol/kgH2O); Preuptake, Na+, Cl−-free hypotonic (110 mosmol/kgH2O); Uptake, Na+-free hypotonic (110 mosmol/kgH2O).
SPAK/OSR1 kinase activity is associated with KCC3a phosphorylation status.
A time course of KCC3a and SPAK/OSR1 phosphorylation was assessed in oocytes exposed to hypotonicity in similar conditions as those used for uptake experiments. Oocytes were injected with KCC3a alone or coinjected with L-WNK1-Δ11 or L-WNK1-Δ11-L369F. KCC3a T991 and T1048 phosphorylation and SPAK/OSR1 S373/325 phosphorylation was assessed over a 90-min time period. As shown in Fig. 11, oocytes injected only with KCC3a cRNA revealed that within 5 min of incubation in hypotonic conditions, there was an increase in the phosphorylation of SPAK/OSR1, which was maximal within 30 min. When KCC3a was coinjected with L-WNK1-Δ11, SPAK/OSR1 phosphorylation was higher than in absence of the L-WNK1-Δ11, reaching its maximal within 15 min. As expected, coinjection of KCC3a with the active L-WNK1-Δ11-L369F mutant showed an even higher increase in the phosphorylation of SPAK/OSR1, which was maximal within 5 min and sustained for longer than in the other two situations, KCC3a alone and KCC3a + L-WNK1-Δ11. In all cases, and consistent with SPAK/OSR1 mediating the phosphorylation of KCC3a at the COOH-terminal threonine residues, there was an increased phosphorylation of T991 and T1048. As shown in Fig. 11, dephosphorylation of KCC3a, which is known to be associated with its activation (36, 51), takes more than 30 min. In addition, in the presence of L-WNK1-Δ11 or L-WNK1-Δ11-L369F dephosphorylation of KCC3a takes even longer times. Overall, our results suggest that WNK1 kinase regulate the activity of the K+-Cl− cotransporters through SPAK/OSR1 phosphorylation.
DISCUSSION
Data from several laboratories including ours have implicated a complex pathway of protein phosphorylation and dephosphorylation in the regulation of the members of the SLC12 family. It is known that phosphorylation promotes the activation of the Na-coupled members of the family (NKCC1/2 and NCC), and it inhibits the activity of the K-coupled members, KCCs. Rinehart et al. (51) identified two phosphorylation sites in the COOH-terminal domain of KCC3a. Recently, our group demonstrated functionally and biochemically that the full activation of KCC3a by cell swelling is only achieved after dephosphorylation of three distinct residues (Ser96 in the NH2-terminal domain and Thr991 and Thr1048 in the COOH-terminal domain) (35, 51). de los Heros et al. (11) confirmed that SPAK/OSR1 phosphorylates KCCs, thus preventing efflux of Cl− and K+. Phosphorylation of threonine residues T991 and T1048 of KCC3a greatly contributes to the regulation of the KCC activity. In their study, however, de los Heros et al. (11) indicated that SPAK/OSR1 phosphorylates T1048 but may not itself phosphorylate the T991 directly. Regarding S96, which is only present in KCC3a, Melo et al. (36) showed that phosphorylation of this site plays a role in regulating KCC3a activity. It is currently accepted that an increase in [Cl−]i or cell swelling promotes dephosphorylation of the SLC12 cotransporters by inhibiting protein kinases and/or activating protein phosphatases, inhibiting the NKCCs and activating the KCCs. Decreased [Cl−]i or cell shrinkage promotes the opposite effect. In this regard, it has been established that WNK autophosphorylation and activity are modulated by [Cl−]i (4, 46), and they are able to bypass tonicity requirements for the activation or inhibition of the SLC12 cotransporters (12, 19). Thus WNK kinases are good candidates to translate the changes of [Cl−]i or cell volume into activation/deactivation of the SLC12 transporters.
L-WNK1 is a ubiquitous kinase and its activity is regulated by hypertonicity or hypotonicity. It has been involved in cell volume regulation during changes in osmolality (31, 49, 68). L-WNK1 is an activator of the downstream kinase SPAK/OSR1 that is known to be the kinase that actually promotes phosphorylation of the SLC12 cotransporters (11, 64). However, the effect of L-WNK1 on any of the SLC12 cotransporters remained unknown for many years. Initial studies suggested that the rat L-WNK1 had no effect on NCC activity (67). In our studies, we were not able to demonstrate any effect of rat L-WNK1 on any of the SLC12 cotransporters (unpublished data). More recently, however, we analyzed the effect of human L-WNK1 on the activity of NCC and observed that it is a powerful activator (7). In searching for the difference between rat and human L-WNK1, we found an unexpected mutation in rat L-WNK1 cDNA (G2120S) that abrogated the effect of the kinase on NCC. Reversing this mutation turned rat L-WNK1 into the expected activator of NCC.
In the present study, we analyzed the effect of the human L-WNK1 on the functional expression of all K+-Cl− cotransporters isoforms that are expressed in the kidney and CNS. We observed that the activity of all four KCCs is reduced when these cotransporters were expressed with the full length L-WNK1 that contains all exons or with the variants that lack exon 9; exon 11; exons 11 and 12; and exons 9, 11, and 12. Thus the K+-Cl− cotransporters are another Cl− transport pathway that is regulated by L-WNK1. Chávez-Canales et al. (7) showed that L-WNK1 and WNK3 mediate the activity of NCC through SPAK/OSR1, and WNK4 lies upstream of L-WNK1 and WNK3, modulating their effects towards NCC through WNK-WNK interactions. In the present study, we observed that L-WNK1 is a negative regulator of all K+-Cl− cotransporters (Figs. 1, 3–5, 8, and 11) and that this effect is SPAK/OSR1 dependent because the elimination of the SPAK/OSR1 binding site in L-WNK1 completely prevented the effect on KCCs (Fig. 7). Piechotta et al. (47) previously demonstrated that SPAK directly interacts with some, but not all, members of the SLC12 family. Among the KCCs, only the NH2-terminal sequence of KCC3a and KCC2a isoforms contains a true SPAK/OSR1 binding site. However, we previously showed that, although the unique SPAK/OSR1 binding site on NCC and NKCC2 seems to be required for basal activity of the cotransporters, it is not necessary for WNK-induced activation (44). We indeed observed that the WNK3-SPAK-NCC complex could be formed as long as WNK3 is able to interact with SPAK, which in turn activates NCC, even in the absence of the SPAK binding site on the cotransporter (44). As shown in the present work, L-WNK1 can inhibit the activity of all KCCs, even though only KCC3a and KCC2a isoforms contain a SPAK/OSR1 binding site. We have also previously observed that other WNKs inhibit the KCCs cotransporter that contains no SPAK/OSR1 binding sites (12, 19, 52). Our data thus suggest that L-WNK1 activates SPAK/OSR1, which in turn phosphorylates KCCs, decreasing their activity.
The inhibitory effect of L-WNK1 also depends on its ability to form dimers through the HQ motif with other kinases. Particularly, if the osmolarity and/or intracellular chloride concentration decreases, KCCs become phosphorylated and inhibited, whereas the NKCCs become active. Under this condition, L-WNK1 requires the presence of a binding site for SPAK kinase and the integrity of the HQ motif to allow its interaction with other WNKs.
Many cells express NKCC1 and KCC1 and/or other KCCs. Thus a balance between the activity of the influx (NKCC1) and efflux (KCCs) pathways is critical to modulate the [Cl−]i or the response to changes in cell volume. Because L-WNK1 is an inhibitor of KCCs, it is expected to also be an activator of NKCC1. Therefore, activation of L-WNK1 by a decrease of [Cl−]i or cell shrinkage will simultaneously increase the influx while decreasing the efflux of ions from the cell. Supporting this role for L-WNK1, we observed in the present study that in the same assay, L-WNK1 is a powerful activator of NKCC1 and inhibitor of KCC2 (Fig. 3). Additionally, it has been recently shown that L-WNK1 is a chloride-sensitive kinase. Here, we show that eliminating one leucine residue (L369) of the chloride-binding pocket made L-WNK1 more efficient in affecting the function of NKCC1 and KCCs (Fig. 8), most likely because chloride no longer inhibits the autophosphorylation of L-WNK1, as described by Piala et al. (46).
Our data support the proposal that L-WNK1 is a potential modulator of [Cl−]i in cells expressing both NKCC1 and some of the KCCs, which is the case in many epithelial and nonepithelial cells and in neurons. In this regards, recently, Friedel et al. (15) showed that in immature neurons WNK1, SPAK, and KCC2 form a physical complex that triggers a shift in GABA activity (from excitatory to inhibitory) by promoting the dephosphorylation of two COOH-terminal threonines in KCC2 (T906 and T1007) and thus enhancing KCC2-mediated Cl−-extrusion. The authors suggest that WNK1 is a specific kinase that contributes to the developmental control of KCC2 activity and due to its relevance in several neurodevelopmental disorders such as neonatal seizures, autism among others, this interaction has an important neuropsychiatric value, since KCC2 activity is suppressed and GABAergic disinhibition promotes the hyperexcitability of neurons and circuits (15). We have recently report the case of a patient with an activating mutation of NKCC1 that is associated with the development of schizophrenia (41).
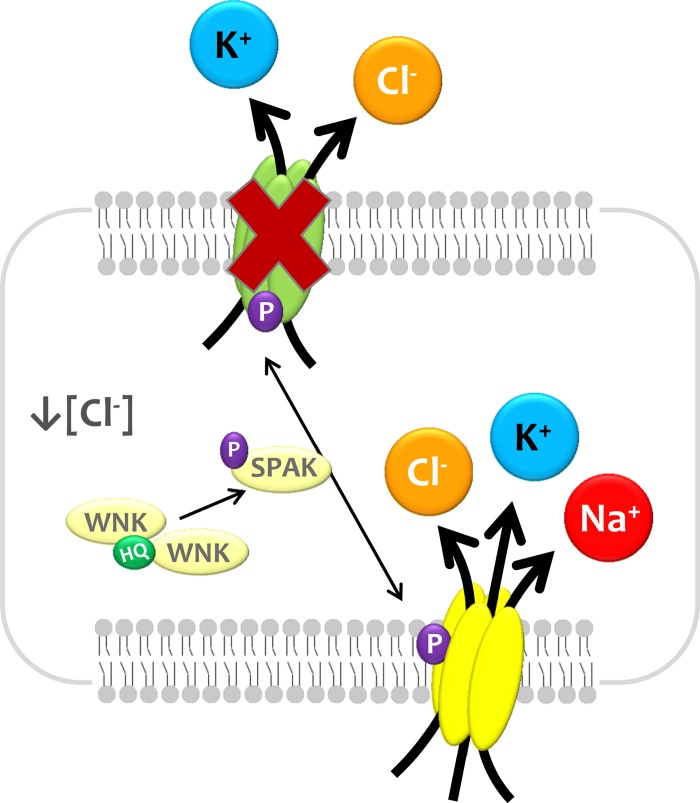
As shown in Fig. 12, we propose that changes in [Cl−]i or cell volume are sensed by L-WNK1, which becomes active and thus able to phosphorylate NKCC1 and KCCs in key residues by an SPAK/OSR1-dependent mechanism. The phosphorylation results in the activation of NKCC1 and inhibition of KCCs, promoting the increase of [Cl−]i or cell volume. The opposite occurs if there is an increase in [Cl−]i or cell volume. Whereas the recent study by Piala et al. (46) clarified how L-WNK1 senses intracellular chloride, how the kinase senses changes in cell volume remains to be understood.
Fig. 12.
Proposed model for the L-WNK1/SPAK phosphorylation effect upon electroneutral cation-coupled chloride cotransporters. See text for explanation.
We previously reported that eliminating the catalytic activity of WNK3 and WNK4 by the D294A and D318A substitution, respectively (4, 10, 12, 19, 36, 50), switched the way in which these kinases affect the SLC12 family members. Wild-type WNK3 activates NCC, NKCC1, and NKCC2, whereas the catalytically inactive version WNK3-D294A completely prevents the activity of these Na+-driven cotransporters, even in the presence of cell shrinkage (26, 50). A similar situation occurs with the K+-Cl− cotransporters. Wild-type WNK3 completely inhibits the activity of all four KCC cotransporters, even if oocytes were exposed to hypotonicity in which KCCs are maximally active. Conversely, WNK3-D294A activates the KCC cotransporters, even if oocytes are incubated in isotonic medium during the uptake where it is known that KCCs are inactive (10, 12, 19). In the present study, we explored whether a similar result is obtained by eliminating the catalytic activity of L-WNK1. Our data show, however, that although the catalytic activity of L-WNK1 is required for the inhibition of KCCs in hypotonic conditions (Fig. 1), the inactive kinase cannot activate KCCs in isotonic conditions as WNK3 does and, this lack of effect is not due to an abnormal protein processing in the cell (Figs. 2 and 6).
In summary, we show that K+-Cl− cotransporters are all inhibited by L-WNK1, elucidating another Cl− transport mechanism that is modulated by this kinase. Thus, all WNK kinases modulate the activity of the SLC12 cotransporters by activating the Na+-coupled, and inhibiting the K+-coupled, members of the family in a SPAK-dependent mechanism.
GRANTS
This work was supported by the Mexican Council of Science and Technology (CONACYT) Grants 132503, 222928, and 165815 (to A. Mercado, E. Moreno, and G. Gamba, respectively) and the Collaborative International Grant No. 188712 from CONACYT and ANR-12-ISVS1-0001-01 from the Agence Nationale pour la Recherche (ANR, France; to G. Gamba and J. Hadchouel).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
A.M., P.d.l.H., Z.M., E.M., J.H., and G.G. conception and design of research; A.M., P.d.l.H., Z.M., M.C.-C., A.R.M.-dO., E.M., S.B.-V., and N.V. performed experiments; A.M., P.d.l.H., Z.M., E.M., and G.G. analyzed data; A.M., P.d.l.H., Z.M., J.H., and G.G. interpreted results of experiments; A.M., P.d.l.H., and Z.M. prepared figures; A.M., P.d.l.H., and Z.M. drafted manuscript; A.M., P.d.l.H., Z.M., J.H., and G.G. edited and revised manuscript; A.M., P.d.l.H., Z.M., M.C.-C., A.R.M.-dO., E.M., S.B.-V., N.V., J.H., and G.G. approved final version of manuscript.
ACKNOWLEDGMENTS
This work was presented in part at the Experimental Biology meeting in San Diego, CA 2014.
REFERENCES
- 1.Adragna NC, Di Fulvio M, Lauf PK. Regulation of K-Cl cotransport: from function to genes. J Membr Biol 201: 109–137, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Alessi DR, Zhang J, Khanna A, Hochdorfer T, Shang Y, Kahle KT. The WNK-SPAK/OSR1 pathway: Master regulator of cation-chloride cotransporters. Sci Signal 7: re3, 2013. [DOI] [PubMed] [Google Scholar]
- 3.Arroyo JP, Kahle KT, Gamba G. The SLC12 family of electroneutral cation-coupled chloride cotransporters. Mol Asp Med 34: 288–298, 2013. [DOI] [PubMed] [Google Scholar]
- 4.Bazúa-Valenti S, Chávez-Canales M, Rojas-Vega L, González-Rodríguez X, Vázquez N, Rodríguez-Gama A, Argaiz ER, Melo Z, Plata C, Ellison DH, García-Valdés J, Hadchouel J, Gamba G. The effect of WNK4 on the Na+-Cl− cotransporter is modulated by intracellular chloride. J Am Soc Nephrol 26: 1781–1786, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blaesse P, Airaksinen MS, Rivera C, Kaila K. Cation-chloride cotransporters and neuronal function. Neuron 61: 820–838, 2009. [DOI] [PubMed] [Google Scholar]
- 6.Boettger T, Hubner CA, Maier H, Rust MB, Beck FX, Jentsch TJ. Deafness and renal tubular acidosis in mice lacking the K-Cl co-transporter Kcc4. Nature 416: 874–878, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Chávez-Canales M, Zhang C, Soukaseum C, Moreno E, Pacheco-Alvarez D, Vidal-Petiot E, Castañeda-Bueno M, Vázquez N, Rojas-Vega L, Meermeier NP, Rogers S, Jeunemaitre X, Yang CL, Ellison DH, Gamba G, Hadchouel J. WNK-SPAK-NCC cascade revisited WNK1 stimulates the activity of the Na-Cl cotransporter via SPAK, an effect antagonized by WNK4. Hypertension 64: 1047–1053, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen YF, Chou CY, Ellory JC, Shen MR. The emerging role of KCl cotransport in tumor biology. Am J Transl Res 2: 345–355, 2009. [PMC free article] [PubMed] [Google Scholar]
- 9.Cruz-Rangel S, Gamba G, Ramos-Mandujano G, Pasantes-Morales H. Influence of WNK3 on intracellular chloride concentration and volume regulation in HEK293 cells. Pflügers Arch 464: 317–330, 2012. [DOI] [PubMed] [Google Scholar]
- 10.Cruz-Rangel S, Melo Z, Vázquez N, Meade P, Bobadilla NA, Pasantes-Morales H, Gamba G, Mercado A. Similar effects of all WNK3 variants on SLC12 cotransporters. Am J Physiol Cell Physiol 301: C601–C608, 2011. [DOI] [PubMed] [Google Scholar]
- 11.de los Heros P, Alessi DR, Gourlay R, Campbell DG, Deak M, Macartney TJ, Kahle KT, Zhang J. The WNK-regulated SPAK/OSR1 kinases directly phosphorylate and inhibit the K+-Cl− co-transporters. Biochem J 458: 559–573, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.de los Heros P, Kahle KT, Rinehart J, Bobadilla NA, Vázquez N, San Cristobal P, Mount DB, Lifton RP, Hebert SC, Gamba G. WNK3 bypasses the tonicity requirement for K-Cl cotransporter activation via a phosphatase-dependent pathway. Proc Natl Acad Sci USA 103: 1976–1981, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farrant M, Kaila K. The cellular, molecular and ionic basis of GABA(A) receptor signalling. Prog Brain Res 160: 59–87, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Flatman PW. Cotransporters, WNKs and hypertension: important leads from the study of monogenetic disorders of blood pressure regulation. Clin Sci 112: 203–216, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Friedel P, Kahle KT, Zhang J, Hertz N, Pisella LI, Buhler E, Schaller F, Duan J, Khanna AR, Bishop PN, Shokat KM, Medina I. WNK1-regulated inhibitory phosphorylation of the KCC2 cotransporter maintains the depolarizing action of GABA in immature neurons. Sci Signal 8: ra65, 2015. [DOI] [PubMed] [Google Scholar]
- 16.Gagnon KB, England R, Delpire E. Volume sensitivity of cation-Cl− cotransporters is modulated by the interaction of two kinases: Ste20-related proline-alanine-rich kinase and WNK4. Am J Physiol Cell Physiol 290: C134–C142, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Gamba G. Molecular physiology and pathophysiology of electroneutral cation-chloride cotransporters. Physiol Rev 85: 423–493, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Gamba G. Role of WNK kinases in regulating tubular salt and potassium transport and in the development of hypertension. Am J Physiol Renal Physiol 288: F245–F252, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Garzón-Muvdi T, Pacheco-Alvarez D, Gagnon KB, Vázquez N, Ponce-Coria J, Moreno E, Delpire E, Gamba G. WNK4 kinase is a negative regulator of K+-Cl− cotransporters. Am J Physiol Renal Physiol 292: F1197–F1207, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Gillen CM, Brill S, Payne JA, Forbush B. Molecular cloning and functional expression of the K-Cl cotransporter from rabbit, rat, and human. A new member of the cation-chloride cotransporter family. J Biol Chem 271: 16237–16244, 1996. [DOI] [PubMed] [Google Scholar]
- 21.He G, Wang HR, Huang SK, Huang CL. Intersectin links WNK kinases to endocytosis of ROMK1. J Clin Invest 117: 1078–1087, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hiki K, D'Andrea RJ, Furze J, Crawford J, Woollatt E, Sutherland GR, Vadas MA, Gamble JR. Cloning, characterization, and chromosomal location of a novel human K+-Cl− cotransporter. J Biol Chem 274: 10661–10667, 1999. [DOI] [PubMed] [Google Scholar]
- 23.Jarolimek W, Lewen A, Misgeld U. A furosemide-sensitive K+-Cl− cotransporter counteracts intracellular Cl−accumulation and depletion in cultured rat midbrain neurons. J Neurosci 19: 4695–4704, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kahle KT, Gimenez I, Hassan H, Wilson FH, Wong RD, Forbush B, Aronson PS, Lifton RP. WNK4 regulates apical and basolateral Cl− flux in extrarenal epithelia. Proc Natl Acad Sci USA 101: 2064–2069, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kahle KT, MacGregor GG, Wilson FH, Van Hoek AN, Brown D, Ardito T, Kashgarian M, Giebisch G, Hebert SC, Boulpaep EL, Lifton RP. Paracellular Cl− permeability is regulated by WNK4 kinase: insight into normal physiology and hypertension. Proc Natl Acad Sci USA 101: 14877–14882, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kahle KT, Rinehart J, de Los Heros P, Louvi A, Meade P, Vazquez N, Hebert SC, Gamba G, Gimenez I, Lifton RP. WNK3 modulates transport of Cl− in and out of cells: implications for control of cell volume and neuronal excitability. Proc Natl Acad Sci USA 102: 16783–16788, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kahle KT, Rinehart J, Lifton RP. Phosphoregulation of the Na-K-2Cl and K-Cl cotransporters by the WNK kinases. Biochim Biophys Acta 1802: 1150–1158, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kahle KT, Staley KJ, Nahed BV, Gamba G, Hebert SC, Lifton RP, Mount DB. Roles of the cation-chloride cotransporters in neurological disease. Nat Clin Pract Neurol 4: 490–503, 2008. [DOI] [PubMed] [Google Scholar]
- 29.Lafrenière RG, MacDonald ML, Dube MP, MacFarlane J, O'Driscoll M, Brais B, Meilleur S, Brinkman RR, Dadivas O, Pape T, Platon C, Radomski C, Risler J, Thompson J, Guerra-Escobio AM, Davar G, Breakefield XO, Pimstone SN, Green R, Pryse-Phillips W, Goldberg YP, Younghusband HB, Hayden MR, Sherrington R, Rouleau GA, Samuels ME. Identification of a novel gene (HSN2) causing hereditary sensory and autonomic neuropathy type II through the Study of Canadian Genetic Isolates. Am J Hum Genet 74: 1064–1073, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lauf PK, Bauer J, Adragna NC, Fujise H, Zade-Oppen AM, Ryu KH, Delpire E. Erythrocyte K-Cl cotransport: properties and regulation. Am J Physiol Cell Physiol 263: C917–C932, 1992. [DOI] [PubMed] [Google Scholar]
- 31.Lenertz LY, Lee BH, Min X, Xu B, Wedin K, Earnest S, Goldsmith EJ, Cobb MH. Properties of WNK1 and implications for other family members. J Biol Chem 280: 26653–26658, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Liapis H, Nag M, Kaji DM. K-Cl cotransporter expression in the human kidney. Am J Physiol Cell Physiol 275: C1432–C1437, 1998. [DOI] [PubMed] [Google Scholar]
- 33.Lu J, Karadsheh M, Delpire E. Developmental regulation of the neuronal-specific isoform of K-Cl cotransporter KCC2 in postnatal rat brains. J Neurobiol 39: 558–568, 1999. [PubMed] [Google Scholar]
- 34.McCormick JA, Ellison DH. The WNKs: atypical protein kinases with pleiotropic actions. Physiol Rev 91: 177–219, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melo Z, Cruz-Rangel S, Bautista R, Vázquez N, Castañeda-Bueno M, Mount DB, Pasantes-Morales H, Mercado A, Gamba G. Molecular evidence for a role for K+-Cl− cotransporters in the kidney. Am J Physiol Renal Physiol 305: F1402–F1411, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Melo Z, de Los Heros P, Cruz-Rangel S, Vázquez N, Bobadilla NA, Pasantes-Morales H, Alessi DR, Mercado A, Gamba G. N-terminal serine dephosphorylation is required for KCC3 cotransporter full activation by cell swelling. J Biol Chem 288: 31468–31476, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mercado A, Broumand V, Zandi-Nejad K, Enck AH, Mount DB. A C-terminal domain in KCC2 confers constitutive K+-Cl− cotransport. J Biol Chem 281: 1016–1026, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Mercado A, Mount DB, Gamba G. Electroneutral cation-chloride cotransporters in the central nervous system. Neurochem Res 29: 17–25, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Mercado A, Song L, Vázquez N, Mount DB, Gamba G. Functional comparison of the K+-Cl− cotransporters KCC1 and KCC4. J Biol Chem 275: 30326–30334, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Mercado A, Vázquez N, Song L, Cortés R, Enck AH, Welch R, Delpire E, Gamba G, Mount DB. NH2-terminal heterogeneity in the KCC3 K+-Cl− cotransporter. Am J Physiol Renal Physiol 289: F1246–F1261, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Merner ND, Mercado A, Khanna AR, Hodgkinson A, Bruat V, Awadalla P, Gamba G, Rouleau GA, Kahle KT. Gain-of-function missense variant in SLC12A2, encoding the bumetanide-sensitive NKCC1 cotransporter, identified in human schizophrenia. J Psychiatr Res 77: 22–24, 2016. [DOI] [PubMed] [Google Scholar]
- 42.Mount DB, Mercado A, Song L, Xu J, George AL, Delpire E, Gamba G. Cloning and characterization of KCC3 and KCC4, new members of the cation-chloride cotransporter gene family. J Biol Chem 274: 16355–16362, 1999. [DOI] [PubMed] [Google Scholar]
- 43.Pacheco-Alvarez D, San Cristobal P, Meade P, Moreno E, Vazquez N, Muñoz E, Diaz A, Juarez ME, Gimenez I, Gamba G. The Na+-Cl− cotransporter is activated and phosphorylated at the amino-terminal domain upon intracellular chloride depletion. J Biol Chem 281: 28755–28763, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Pacheco-Alvarez D, Vázquez N, Castañeda-Bueno M, de Los Heros P, Cortes-González C, Moreno E, Meade P, Bobadilla NA, Gamba G. WNK3-SPAK interaction is required for the modulation of NCC and other members of the SLC12 family. Cell Physiol Biochem 29: 291–302, 2012. [DOI] [PubMed] [Google Scholar]
- 45.Payne JA. Functional characterization of the neuronal-specific K-Cl cotransporter: implications for [K+]o regulation. Am J Physiol Cell Physiol 273: C1516–C1525, 1997. [DOI] [PubMed] [Google Scholar]
- 46.Piala AT, Moon TM, Akella R, He H, Cobb MH, Goldsmith EJ. Chloride sensing by WNK1 involves inhibition of autophosphorylation. Sci Signal 7: 1–9, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Piechotta K, Lu J, Delpire E. Cation chloride cotransporters interact with the stress-related kinases Ste20-related proline-alanine-rich kinase (SPAK) and oxidative stress response 1 (OSR1). J Biol Chem 277: 50812–50819, 2002. [DOI] [PubMed] [Google Scholar]
- 48.Race JE, Makhlouf FN, Logue PJ, Wilson FH, Dunham PB, Holtzman EJ. Molecular cloning and functional characterization of KCC3, a new K-Cl cotransporter. Am J Physiol Cell Physiol 277: C1210–C1219, 1999. [DOI] [PubMed] [Google Scholar]
- 49.Richardson C, Alessi DR. The regulation of salt transport and blood pressure by the WNK-SPAK/OSR1 signalling pathway. J Cell Sci 121: 3293–3304, 2008. [DOI] [PubMed] [Google Scholar]
- 50.Rinehart J, Kahle KT, de Los Heros P, Vázquez N, Meade P, Wilson FH, Hebert SC, Gimenez I, Gamba G, Lifton RP. WNK3 kinase is a positive regulator of NKCC2 and NCC, renal cation-Cl− cotransporters required for normal blood pressure homeostasis. Proc Natl Acad Sci USA 102: 16777–16782, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rinehart J, Maksimova YD, Tanis JE, Stone KL, Hodson CA, Zhang J, Risinger M, Pan W, Wu D, Colangelo CM, Forbush B, Joiner CH, Gulcicek EE, Gallagher PG, Lifton RP. Sites of regulated phosphorylation that control K-Cl cotransporter activity. Cell 138: 525–536, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rinehart J, Vázquez N, Kahle KT, Hodson CA, Ring AM, Gulcicek EE, Louvi A, Bobadilla NA, Gamba G, Lifton RP. WNK2 kinase is a novel regulator of essential neuronal cation-chloride cotransporters. J Biol Chem 286: 30171–30180, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature 397: 251–255, 1999. [DOI] [PubMed] [Google Scholar]
- 54.Rust MB, Faulhaber J, Budack MK, Pfeffer C, Maritzen T, Didié M, Beck FX, Boettger T, Schubert R, Ehmke H, Jentsch T, Hubner CA. Neurogenic mechanisms contribute to hypertension in mice with disruption of the K-Cl cotransporter KCC3. Cir Res 98: 549–556, 2006. [DOI] [PubMed] [Google Scholar]
- 55.Shekarabi M, Girard N, Rivière JB, Dion P, Houle M, Toulouse A, Lafrenière RG, Vercauteren F, Hince P, Laganière J, Rochefort D, Faivre L, Samuels M, Rouleau GA. Mutations in the nervous system–specific HSN2 exon of WNK1 cause hereditary sensory neuropathy type II. J Clin Invest 118: 2496–2505, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shekarabi M, Lafrenière RG, Gaudet R, Laganière J, Marcinkiewicz MM, Dion PA, Rouleau GA. Comparative analysis of the expression profile of Wnk1 and Wnk1/Hsn2 splice variants in developing and adult mouse tissues. PLoS One 8: e57807, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shen MR, Chou CY, Hsu KF, Hsu YM, Chiu WT, Tang MJ, Alper SL, Ellory JC. KCl cotransport is an important modulator of human cervical cancer growth and invasion. J Biol Chem 278: 39941–39950, 2003. [DOI] [PubMed] [Google Scholar]
- 58.Shen MR, Chou CY, Hsu KF, Liu HS, Dunham PB, Holtzman EJ, Ellory JC. The KCl cotransporter isoform KCC3 can play an important role in cell growth regulation. Proc Natl Acad Sci USA 98: 14714–14719, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Siew K, O'Shaughnessy KM. Extrarenal roles of the with-no-lysine[K] kinases (WNKs). Clin Exp Pharm Physiol 40: 885–894, 2013. [DOI] [PubMed] [Google Scholar]
- 60.Song L, Mercado A, Vázquez N, Xie Q, Desai R, George AL, Gamba G, Mount DB. Molecular, functional, and genomic characterization of human KCC2, the neuronal K-Cl cotransporter. Mol Brain Res 103: 91–105, 2002. [DOI] [PubMed] [Google Scholar]
- 61.Thastrup JO, Rafiqi FH, Vitari AC, Pozo-Guisado E, Deak M, Mehellou Y, Alessi DR. SPAK/OSR1 regulate NKCC1 and WNK activity: analysis of WNK isoform interactions and activation by T-loop trans-autophosphorylation. Biochem J 441: 325–337, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Uvarov P, Ludwig A, Markkanen M, Pruunsild P, Kaila K, Delpire E, Timmusk T, Rivera C, Airaksinen MS. A novel N-terminal isoform of the neuron-specific K-Cl cotransporter KCC2. J Biol Chem 282: 30570–30576, 2007. [DOI] [PubMed] [Google Scholar]
- 63.Vidal-Petiot E, Cheval L, Faugeroux J, Malard T, Doucet A, Jeunemaitre X, Hadchouel J. A new methodology for quantification of alternatively spliced exons reveals a highly tissue-specific expression pattern of WNK1 isoforms. PLoS One 7: e37751, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vitari AC, Deak M, Morrice NA, Alessi DR. The WNK1 and WNK4 protein kinases that are mutated in Gordon's hypertension syndrome phosphorylate and activate SPAK and OSR1 protein kinases. Biochem J 391: 17–24, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wilson FH, Disse-Nicodeme S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP. Human hypertension caused by mutations in WNK kinases. Science 293: 1107–1112, 2001. [DOI] [PubMed] [Google Scholar]
- 66.Xu BE, Enlishgh JM, Wilsbacher JL, Stippec S, Goldsmith EJ, Cobb MH. WNK1, a novel mammalian serine/threonine protein kinase lacking the catalytic lysine in subdomain II. J Biol Chem 275: 16795–16801, 2000. [DOI] [PubMed] [Google Scholar]
- 67.Yang CL, Angell J, Mitchell R, Ellison DH. WNK kinases regulate thiazide-sensitive Na-Cl cotransport. J Clin Invest 111: 1039–1045, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zagórska A, Pozo-Guisado E, Boudeau J, Vitari AC, Rafiqi FH, Tastrup JO, Deak M, Campbell DG, Morrice NA, Prescott AR. Regulation of activity and localization of the WNK1 protein kinase by hyperosmotic stress. J Cell Biol 176: 89–100, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]