Abstract
Sphingomyelin synthase (SMS) catalyzes the conversion of phosphatidylcholine and ceramide to sphingomyelin and diacylglycerol. We previously showed that SMS1 deficiency leads to a reduction in expression of the K+ channel KCNQ1 in the inner ear (Lu MH, Takemoto M, Watanabe K, Luo H, Nishimura M, Yano M, Tomimoto H, Okazaki T, Oike Y, and Song WJ. J Physiol 590: 4029–4044, 2012), causing hearing loss. However, it remains unknown whether this change in expression is attributable to a cellular process or a systemic effect in the knockout animal. Here, we examined whether manipulation of SMS1 activity affects KCNQ1/KCNE1 currents in individual cells. To this end, we expressed the KCNQ1/KCNE1 channel in human embryonic kidney 293T cells and evaluated the effect of SMS1 manipulations on the channel using whole cell recording. Application of tricyclodecan-9-yl-xanthogenate, a nonspecific inhibitor of SMSs, significantly reduced current density and altered channel voltage dependence. Knockdown of SMS1 by a short hairpin RNA, however, reduced current density alone. Consistent with this, overexpression of SMS1 increased the current density without changing channel properties. Furthermore, application of protein kinase D inhibitors also suppressed current density without changing channel properties; this effect was nonadditive with that of SMS1 short hairpin RNA. These results suggest that SMS1 positively regulates KCNQ1/KCNE1 channel density in a protein kinase D-dependent manner.
Keywords: KCNQ1, KCNE1, sphingomyelin synthase, PKD
the slowly activating, delayed rectifier potassium channel encoded by KCNQ1 (α-subunit, also known as Kv7.1) and KCNE1 (β-subunit, also known as Isk or minK) plays important roles in a number of organs, including the inner ear, heart, pancreas, and brain (reviewed in Refs. 1, 15, 19). In the heart, the KCNQ1/KCNE1 channel [also known as the cardiac slowly activating K+ current (IKs) channel] is necessary for the termination of action potentials (5, 30, 42), and loss of function of the IKs channel causes prolongation of the QT interval in the ECG (26). In the inner ear, KCNQ1/KCNE1 channels are expressed exclusively on the apical surface of marginal cells of the stria vascularis (14, 29), maintaining the endocochlear potential and thereby the high sensitivity of the inner ear to sound. Dysfunction of KCNQ1/KCNE1 channels in the inner ear causes hearing impairment or congenital deafness (3, 15, 27). Understanding how KCNQ1/KCNE1 channels are regulated is, therefore, of profound biological significance.
We previously showed that KCNQ1 expression in the inner ear may be subject to regulation by sphingomyelin synthase 1 (SMS1) (21). SMS1 is one of the two isoforms of the enzyme that catalyzes the conversion of phosphatidylcholine and ceramide to sphingomyelin and diacylglycerol (DAG) (reviewed in Refs. 18, 38). We showed that SMS1 deficiency results in hearing impairment, which is attributable to a reduction in endocochlear potential caused by aberrant KCNQ1 protein expression in the marginal cells of the stria vascularis of the inner ear (21). Although these observations raise the possibility that SMS1 has a regulatory role in KCNQ1 expression, it is not known whether SMS1 affects KCNQ1 via a cellular mechanism, i.e., within the marginal cells, or indirectly via systemic pathways. Here, we examined whether manipulation of SMS1 activity affects KCNQ1/KCNE1 current at the cellular level. To this end, we expressed KCNQ1/KCNE1 channels in human embryonic kidney 293T (HEK-293T) cells, which express little endogenous potassium current, and evaluated the effect of SMS1 manipulations on the channel using whole cell recordings. Our results show that SMS1 positively regulates KCNQ1/KCNE1 channel density at the cellular level, without changing channel properties. Regulation of SMS1 activity, therefore, has therapeutic potential in hearing, cardiac, and metabolic disorders.
MATERIALS AND METHODS
Plasmids.
We constructed a plasmid for SMS1 overexpression by inserting human SMS1 cDNA, tagged with Myc at the COOH terminal, into the pLPCX plasmid vector (Takara, Shiga, Japan) at the EcoRI/NotI site. Plasmids for short hairpin RNA (shRNA) against SMS1, and control (scrambled) shRNA, were constructed previously (16). We constructed a FLAG-tagged human KCNE1 plasmid by inserting cDNA encoding human KCNE1, tagged with FLAG at the COOH terminal, into the pcDNA3 plasmid vector (Thermo Fisher Scientific, Waltham, MA) (24, 40). A green fluorescent protein-tagged human KCNQ1 cDNA plasmid (RG219869) was purchased from OriGene (Rockville, MD). All plasmid solutions for transfection were prepared using the PureLink HiPure Plasmid Maxiprep Kit (Thermo Fisher Scientific).
Cell culture and plasmid transfection.
HEK-293T cells were maintained in DMEM with 10% FBS in 35-mm dishes. One day after the cells were plated (1 × 105 cells per dish), KCNQ1 and KCNE1 plasmids (3 μg of each) were diluted in Opti-MEM I Reduced Serum Medium (Thermo Fisher Scientific) and transfected by mixing with either 5 μl Lipofectamine 3000 transfection reagent [Thermo Fisher Scientific; for CID-2011756 and phorbol 12-myristate 13-acetate (PMA) experiments] or 8 μl Lipofectamine 2000 transfection reagent (Thermo Fisher Scientific; for all other experiments). We added 2.2 μg of SMS1 plasmid for overexpression experiments, and 3 μg SMS1 shRNA plasmid for knockdown experiments. The empty vector (pLPCX) and scrambled shRNA plasmids were used as controls. Whole cell recordings were performed 48 h posttransfection. Approximately 5 h before recording, cells were transferred to poly-l-lysine-coated coverslips in DMEM containing 10% FBS.
Reverse transcription-polymerase chain reaction.
To examine whether SMS1 knockdown/overexpression had altered SMS1 mRNA level, we measured the SMS1/β-actin mRNA ratio by reverse transcription-polymerase chain reaction (RT-PCR) analysis. We also measured the SMS2-to-β-actin mRNA ratio to test the specificity of our shRNA. Total RNA was isolated from HEK-293T cells using the RNeasy Plus Mini kit (Qiagen, Hilden, Germany). Concentrations were normalized to the respective control groups. RT was carried out using the SuperScript III First-Strand Synthesis System for RT-PCR kit (Thermo Fisher Scientific), with oligo(dT) as the primer. All procedures followed the manufacturers' instructions.
PCR was carried out using KOD-FX DNA polymerase (Toyobo, Osaka, Japan), with the following primers: human β-actin (forward) 5′-GCA CAG AGC CTC GCC TTT GCC GAT-3′; (reverse) 5′-CTC CTT AAT GTC ACG CAC GAT TTC-3′; human SMS1 (forward) 5′-TGG CAG ACT GGC TGC TGG AGA AT-3′; (reverse) 5′-GTC ATT CAC CAG CCG GCT GTA TT-3′; human SMS2 (forward) 5′- CCA GTG ATC CTA CGA ACA CTT AT-3′; (reverse) 5′-CAT TGT CTT CAA TCT TCT GA-3′. PCR products were stained with ethidium bromide after agarose gel electrophoresis. Images were obtained with an ultraviolet transilluminator (CSF-20BF-S; Cosmo Bio, Tokyo, Japan) and a digital camera (DC120, Kodak); fluorescence intensity was measured using ImageJ 1.45 (National Institutes of Health, Bethesda, MD).
Electrophysiology.
Whole cell recordings of K+ currents were performed according to our laboratory's previously published methods (12, 36). Green fluorescent protein-labeled (transfected) cells were randomly selected for recording. The recording chamber was perfused with a background solution containing (in mM) 140 NaCl, 2 KCl, 1 CaCl2, 2 MgCl2, 10 glucose, 15 HEPES; pH was adjusted to 7.4 with NaOH; osmolarity was 295–300 mOsm/l. The external solution consisted of (in mM) 140 NaCl, 2 KCl, 2 MgCl2, 10 glucose, 10 HEPES; pH = 7.4 with NaOH; 295–300 mOsm/l. Ca2+ was excluded from the external solution to eliminate Ca2+ currents. During recording, cells were bathed in extracellular solution applied via a gravity-fed capillary perfusion array positioned ∼2 mm away from the cell under study. The internal solution consisted of (in mM) 120 potassium-gluconate, 3 MgCl2, 10 HEPES, 10 EGTA, 2 ATP, 12 phosphocreatine, 0.2 GTP; pH = 7.2–7.3 with KOH; 275–285 mOsm/l. Room temperature was controlled at 25–27°C during recording.
Recordings were obtained with an Axon Instruments patch-clamp amplifier (Axopatch 200B, Molecular Devices, Foster City, CA) and were controlled and monitored with a computer running pCLAMP 7.0 with a 125-kHz interface (Molecular Devices). Electrode resistances were typically 3–6 MΩ in the bath. The tip potentials of the patch pipettes were zeroed before seal formation. After seal rupture, the series resistance (7–15 MΩ) was compensated (> 80%) and periodically monitored. The liquid junction potential between the pipette solution and the bath solution was not compensated. KCNQ1 and KCNQ1/KCNE1 currents were evoked by depolarization voltage steps. Unstable recordings were excluded from analysis.
Drugs.
To inhibit SMS activity, we added tricyclodecan-9-yl-xanthogenate (D609, Sigma, St. Louis, MO; 300 μM) to the culture dishes for 6 h (4, 37). Control cells received DMSO vehicle (0.05%) for the same period of time. To inhibit protein kinase D (PKD), we added either CID-755673 or CID-2011756 (Tocris, Bristol, UK; 40 μM) to the cultures for 6 h (9, 33, 34, 48), and control cells received DMSO (0.05%). The effect of PMA (Sigma-Aldrich; 50 nM) was also tested by adding the drug to the cultures for 6 h, and control cells received DMSO vehicle (0.05%).
Data analysis.
Recordings were analyzed using AxoGraph (AxoGraph, Berkeley, CA) and Matlab (Mathworks, Natick, MA). KaleidaGraph (Synergy Software, Reading, PA) was used for drawing box plots and for Boltzmann fitting. Conductance-voltage relationships were derived from tail currents. Sample statistics are given as the means ± SE, unless stated otherwise. The Wilcoxon signed-rank test was used to compare samples.
RESULTS
Expression of KCNQ1/KCNE1 currents in HEK-293T cells.
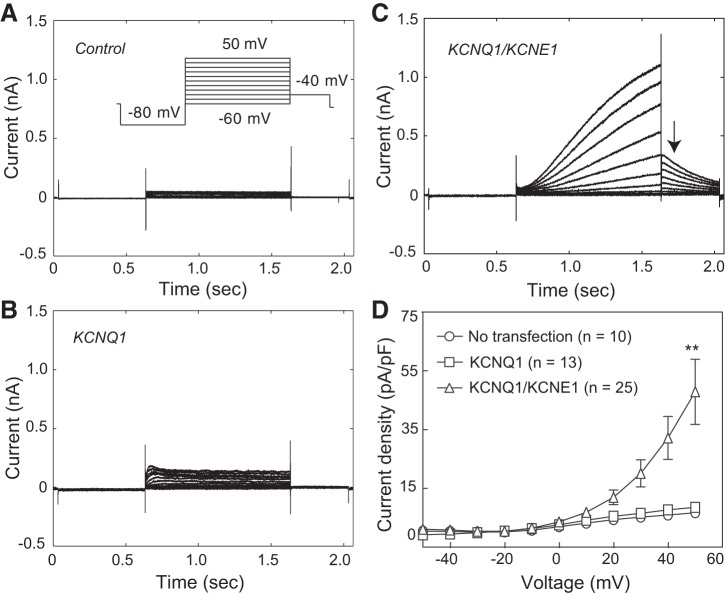
We used HEK-293T cells as models because they express low levels of depolarization-activated K+ currents (15) (Fig. 1A). The peak current density was 7.6 ± 0.8 pA/pF at 50 mV (n = 10). Endogenous K+ currents in HEK-293T cells activated with a short time constant of 25.7 ± 3.2 ms at 10 mV (n = 10). When KCNQ1 was expressed alone, peak current density showed no significant increase (8.6 ± 0.9 pA/pF at 50 mV; P > 0.05, n = 13; Fig. 1B), and channel activation, at 10 mV, could also be described by a single exponent, although with longer time constants than in control cells (37.4 ± 3.5 ms; P < 0.05, n = 13). In agreement with previous reports (31), coexpression of KCNQ1 and KCNE1 in HEK-293T cells resulted in much slower currents (Fig. 1C), which had a typical sigmoidal shape in response to depolarization voltage steps (15, 25); also in agreement with these previous studies, the channel deactivated slowly after termination of the depolarization pulse (Fig. 1C, arrow). The peak current density (47.9 ± 11.1 pA/pF at 50 mV; n = 25) was significantly greater than that of control cells (P < 0.01; Fig. 1D). Therefore, with KCNQ1/KCNE1 expression, 84% [(47.9 − 7.6)/47.9] of depolarization-activated K+ current in HEK-293T cells was KCNQ1/KCNE1 current, similar to that reported previously (15). We subsequently used this model system to study the mechanisms of KCNQ1/KCNE1 current regulation by SMS1.
Fig. 1.
Expression of KCNQ1/KCNE1 channels in HEK-293T cells. A: K+ currents evoked in a HEK-293T cell, with voltage steps from −60 mV to +50 mV at a 10-mV interval (inset: protocol). B: K+ currents evoked with the protocol shown in A, inset, in a cell transfected with KCNQ1 alone. C: K+ currents in a cell transfected with KCNQ1 and KCNE1. Arrow points to tail currents at −40 mV. D: current density in the three cell groups at different voltages. Values are means ± SE. **P < 0.01 vs. no transfection and vs. KCNQ1 transfection. Cell capacitances for the control, KCNQ1, and KCNQ1/KCNE1 groups were 17.9 ± 1.6, 14.0 ± 1.2, and 13.6 ± 1.3 pF, respectively. No statistical difference in cell capacitance was found between groups in this or any other figure.
Effects of D609 treatment on KCNQ1/KCNE1 currents.
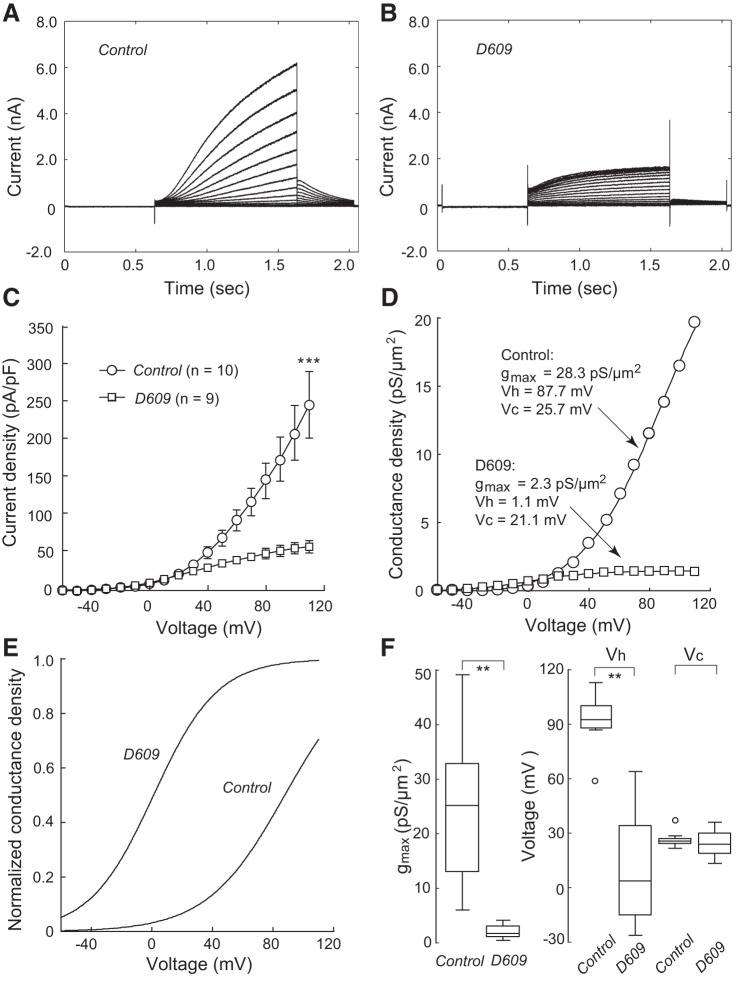
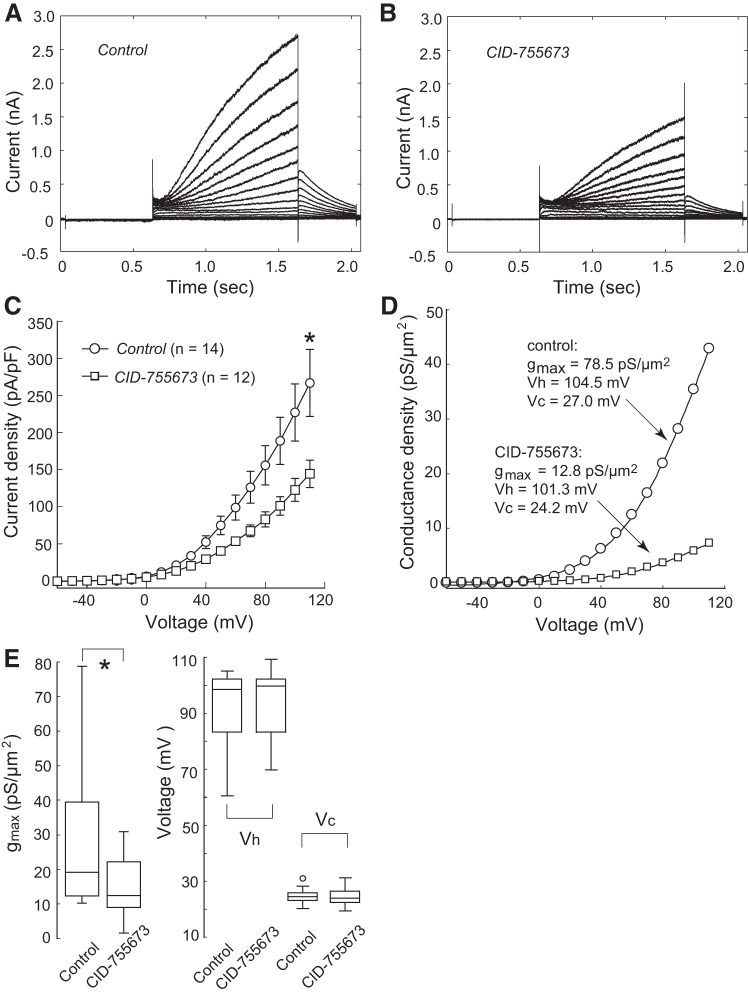
To determine whether SMS1 affects KCNQ1/KCNE1 currents, we first explored the effects of D609, a nonspecific inhibitor of SMS1 (reviewed in Ref. 2). Cells treated with D609 for 6 h had much lower current density (Fig. 2B) than control cells (Fig. 2A). At 110 mV, peak current density was 58.7 ± 8.1 pA/pF (n = 9) in the D609-treated cells, significantly lower than that in the control group (255.6 ± 43.8 pA/pF; P < 0.001, n = 10; Fig. 2C).
Fig. 2.
Effect of D609 on KCNQ1/KCNE1 currents. Currents shown here and in all subsequent figures were evoked with the voltage protocol shown in Fig. 1A, inset, except that the highest voltage was increased to 110 mV. A: currents from a cell cultured in control conditions. B: currents from a cell that had received D609 before recording. C: current densities of the control and D609-treated groups. Values are means ± SE. ***P < 0.001. Cell capacitances were 16.2 ± 1.6 and 12.0 ± 2.3 pF for the control and D609 groups, respectively. D: conductance density-voltage relationship in a control cell and a D609-treated cell. Boltzmann fit parameters are shown for each cell. E: Boltzmann fits for the two cells in D are shown with normalized maximum conductance density. D609 induced a marked shift in voltage dependence. F: box plots for maximum conductance density (gmax), half-activation voltage (Vh), and slope factor (Vc); open circles represent outliers. n = 10 (Control) or 9 (D609). **P < 0.01.
To examine how D609 alters channel voltage dependence, the conductance-voltage relationships in cells of both groups were fitted, for descriptive purposes, with a single Boltzmann function: g = gmax/{1 + exp[− (V − Vh)/Vc]}, where gmax is the maximum conductance density, Vh is the half-activation voltage, and Vc is the slope factor (35) (Fig. 2D). D609 markedly shifted the Vh to negative potential (control, 92.1 ± 4.4 mV, n = 10; D609, 12.3 ± 9.7 mV, n = 9; P < 0.01; Fig. 2, E and F) and reduced the gmax (control, 24.4 ± 4.0 pS/μm2, n = 10; D609, 1.9 ± 0.3 pS/μm2, n = 9; P < 0.01; Fig. 2F). No significant change was found for the Vc (control, 26.5 ± 1.2 mV, n = 10; D609, 24.6 ± 2.7 mV, n = 9; P > 0.05; Fig. 2F).
These results suggest the possibility that SMS1 regulates the expression and voltage dependence of KCNQ1/KCNE1 channels.
Regulation of KCNQ1/KCNE1 currents by SMS1.
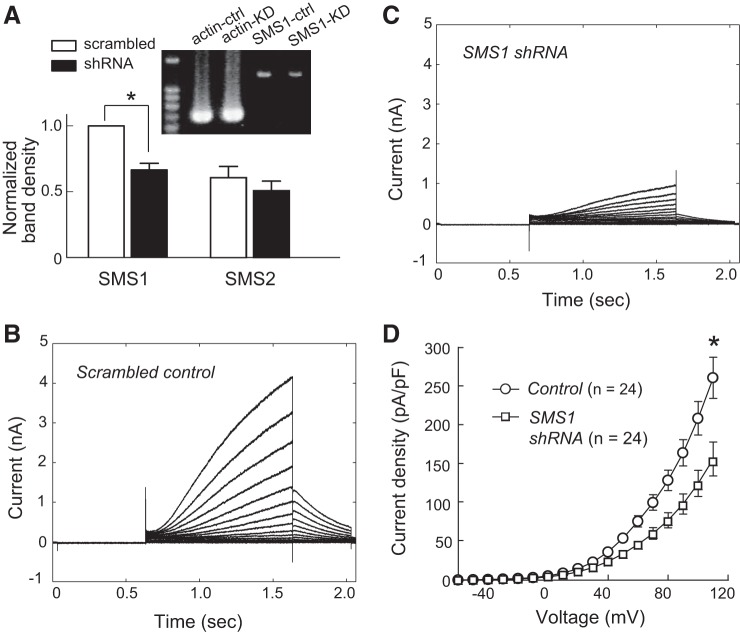
To manipulate SMS1 activity more specifically, we used an shRNA to knock down SMS1 expression. Transfection of the SMS1 shRNA plasmid resulted in significantly less SMS1 mRNA than in the scrambled control (P < 0.05, n = 7; Fig. 3A). The degree of mRNA reduction was similar to that reported by a real-time PCR study that used the same shRNA (16). The knockdown effect was specific to SMS1 because no change was observed in the amount of SMS2 mRNA in the same experiment (P > 0.05, n = 7; Fig. 3A).
Fig. 3.
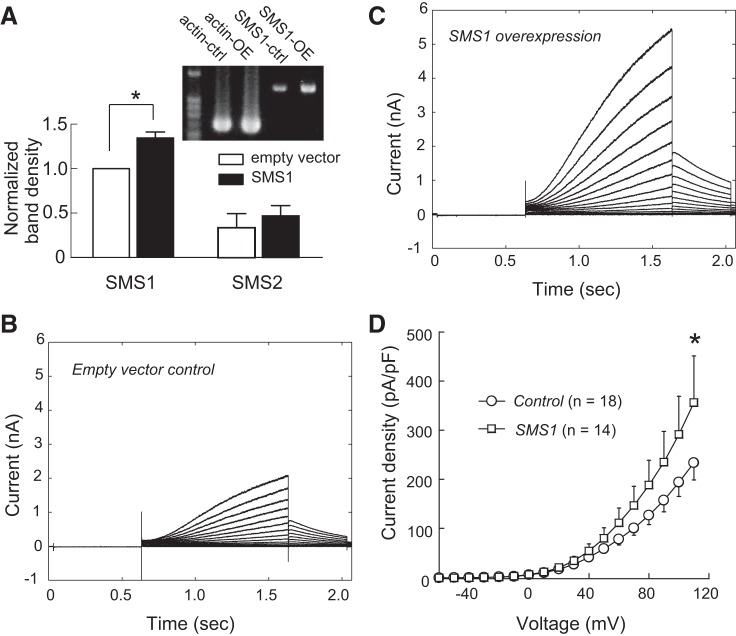
SMS1 downregulation reduced KCNQ1/KCNE1 current density. A: effect of SMS1 knockdown on mRNA levels of SMS1 and SMS2. Fluorescence ratios of SMS1 and SMS2 to β-actin cDNA in the control and shRNA groups were normalized to the control for SMS1. Values are means ± SE; n = 7 for SMS1 and SMS2. *P < 0.05. Inset: photograph showing SMS1 RT-PCR products in a control sample (SMS1-ctrl) and a SMS1 knockdown sample (SMS1-KD), together with products of β-actin. B: currents from a control cell. C: currents from an SMS1 knockdown cell. D: voltage-dependent current density in the control and knockdown groups. Values are means ± SE. *P < 0.05. Cell capacitances were 13.7 ± 1.3 and 13.5 ± 1.3 pF for the control and shRNA groups, respectively.
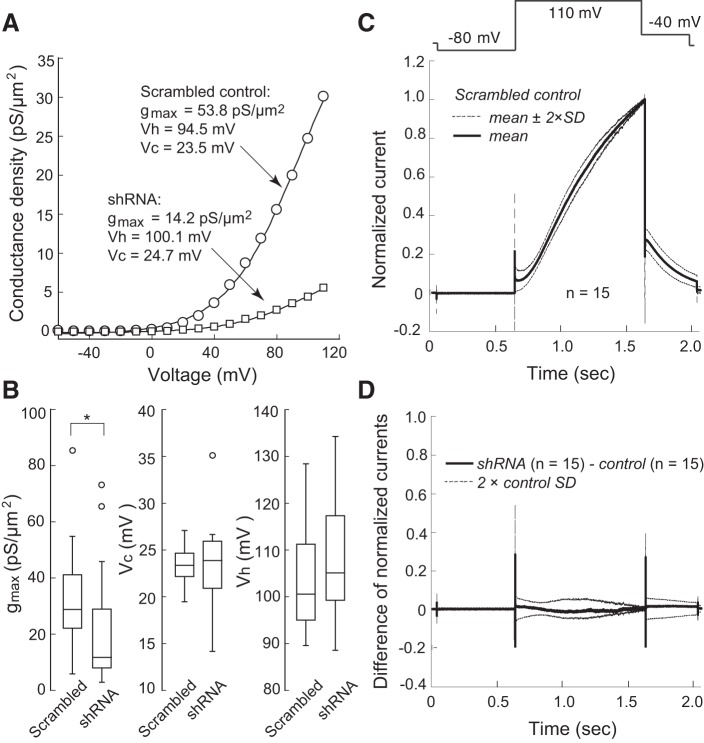
KCNQ1/KCNE1 current density was significantly lower after transfection of the SMS1 shRNA plasmid than after scrambled control plasmid transfection (at 110 mV: SMS1 shRNA, 155.2 ± 21.5 pA/pF, n = 24; control, 260.4 ± 26.5 pA/pF, n = 24; P < 0.05; Fig. 3, B–D). However, the effect of SMS1 knockdown was limited to channel density. Conductance density-voltage relationships were fitted with the Boltzmann function to reveal significantly lower gmax in the SMS1 shRNA group than in the scrambled control group (control: 32.2 ± 3.5 pS/μm2, n = 24; shRNA: 21.4 ± 4.0 pS/μm2, n = 24; P < 0.05), but no change in Vh or Vc was observed (Vh: shRNA, 108.0 ± 2.4 mV, n = 24; control, 104.3 ± 2.2 mV, n = 24; P > 0.05. Vc: shRNA, 23.0 ± 0.9 mV, n = 24; control, 23.3 ± 0.4 mV, n = 24; P > 0.05; Fig. 4, A and B). Furthermore, time-dependent current change during activation at 110 mV and during deactivation at −40 mV in knockdown cells was in close agreement with that in control cells (Fig. 4, C and D).
Fig. 4.
SMS1 downregulation did not change channel voltage dependence or channel kinetics. A: conductance density-voltage relationship of a control cell transfected with scrambled RNA and a knockdown cell transfected with SMS1 shRNA. Boltzmann fit parameters are shown for each cell. B: box plots for maximum conductance density (gmax), half-activation voltage (Vh), and slope factor (Vc); open circles represent outliers. n = 24 (Control) or 24 (knockdown). *P < 0.05. C: currents evoked by a voltage step to 110 mV in 15 control cells, normalized to the maximum value. Thick line, mean; thin lines, mean ± 2 SD. D: difference current between the means of the control and knockdown groups (n = 15). Thick line, difference in mean currents; thin lines, ±2 SD for the control condition; the difference current during and after the voltage step never exceeded the 2 SD range, suggesting a lack of statistical difference between control and knockdown conditions.
Conversely, overexpression of SMS1 resulted in a greater amount of SMS1 mRNA compared with the empty vector control (P < 0.05, n = 6; Fig. 5A, left). This effect was specific to SMS1, because no change in SMS2 mRNA level was detected in the same experiment (P > 0.05, n = 6; Fig. 5A, right).
Fig. 5.
SMS1 overexpression increased KCNQ1/KCNE1 current density. A: effect of SMS1 overexpression on SMS1 and SMS2 mRNA levels. Fluorescence ratios of SMS1 and SMS2 to β-actin cDNA in the control and overexpression groups, normalized to the control for SMS1. Values are means ± SE; n = 6 (SMS1 and SMS2). *P < 0.05. Inset: photograph showing SMS1 and β-actin RT-PCR products in a control sample and an overexpression sample. B: currents from a control cell transfected with the empty vector. C: currents from a cell transfected with SMS1. D: voltage-dependent current density of control and overexpression groups. Values are means ± SE. *P < 0.05. Cell capacitances were 13.9 ± 5.2 and 12.0 ± 0.7 pF for the control and overexpression groups, respectively.
Cells overexpressing SMS1 had significantly higher KCNQ1/KCNE1 current density than empty vector cells (at 110 mV: overexpression, 356.1 ± 95.2 pA/pF, n = 14; control, 211.9 ± 28.4 pA/pF, n = 18; P < 0.05; Fig. 5, B–D). Similar to the knockdown experiments, we found no change in channel voltage dependence (Vh: overexpression, 95.9 ± 4.3 mV, n = 14; control, 110.1 ± 15.5 mV, n = 18; P > 0.05. Vc: overexpression, 24.4 ± 0.8 mV, n = 14; control, 27.0 ± 2.5 mV, n = 18; P > 0.05), or in kinetics in the overexpression experiments (data not shown). The gmax in cells overexpressing SMS1 was not statistically significantly different from that in empty vector cells (overexpression: 65.5 ± 19.7 pS/μm2, n = 14; control: 27.7 ± 5.0 pS/μm2, n = 18; P = 0.09), probably owing to the large variance of the overexpression group.
Together, the results of the knockdown and overexpression experiments suggest that SMS1 positively regulates KCNQ1/KCNE1 channel expression without affecting channel properties.
Suppression of KCNQ1/KCNE1 currents by PKD inhibitors.
We next investigated the cellular mechanism by which SMS1 regulates the expression of KCNQ1/KCNE1 channels. SMS1 is known to reside in the Golgi apparatus and catalyzes the conversion of phosphatidylcholine and ceramide to sphingomyelin and DAG (18, 38). Because SMS1 changed KCNQ1/KCNE1 current density only, without changing channel properties, it is likely that SMS1 affects the trafficking of channel proteins to the cytoplasmic membrane, rather than modifying the channel proteins themselves. We, therefore, focused on the reaction product DAG and speculated that DAG might lead to the regulation of KCNQ1/KCNE1 expression via the DAG-binding kinase, PKD (6). We found that the PKD inhibitor CID-755673 (9, 33) significantly reduced the current density of the KCNQ1/KCNE1 channel (Fig. 6, A–C). Similar to that observed after SMS1 downregulation, the effect of CID-755673 was limited to a reduction in channel density (at 110 mV: CID-755673, 144.2 ± 17.2 pA/pF, n = 12; control, 266.8 ± 43.6 pA/pF, n = 14; P < 0.05). Analysis of the channel conductance-voltage relationship revealed that CID-755673 significantly reduced the maximum conductance (control, 28.7 ± 5.0 pS/μm2, n = 14; CID-755673, 15.9 ± 3.0 pS/μm2, n = 12; P < 0.05), but had no effect on Vh or Vc (Vh: control, 92.6 ± 3.4 mV, n = 14; CID-755673, 94.4 ± 3.6 mV, n = 12; P > 0.05. Vc: control, 24.6 ± 0.7 mV, n = 14; CID-755673, 24.1 ± 0.9 mV, n = 12; P > 0.05; Fig. 6, D and E).
Fig. 6.
PKD inhibition reduced KCNQ1/KCNE1 current density. A: control cell currents. B: currents from a cell treated with the PKD inhibitor CID-755673 for 6 h before recording. C: current density in the control and CID-755673-treated groups. Values are means ± SE. *P < 0.05. Cell capacitances were 16.0 ± 1.7 and 17.8 ± 1.5 pF for the control and CID-755673 groups, respectively. D: conductance density-voltage relationship in a control cell and a CID-755673-treated cell. Boltzmann fit parameters are shown for each cell. E: box plots of maximum conductance density (gmax), half activation voltage (Vh), and slope factor (Vc); open circles represent outliers. n = 14 (Control) or 12 (CID-755673). *P < 0.05.
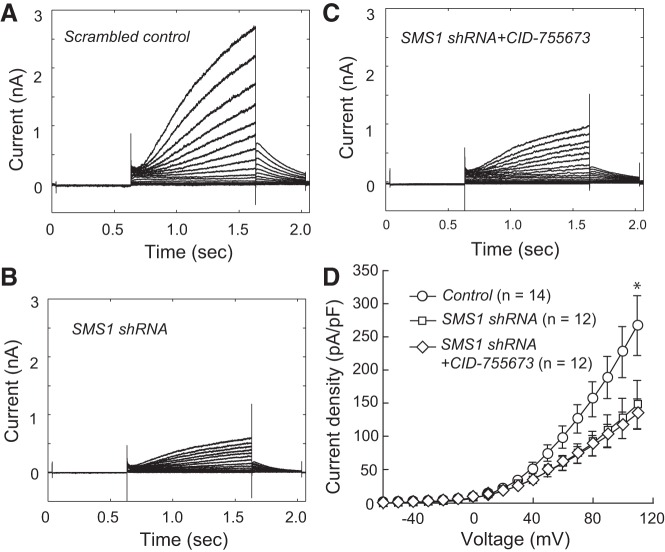
To determine whether SMS1 and PKD regulate KCNQ1/KCNE1 channel density via a common signaling pathway, we tested whether the effects of SMS1 knockdown and PKD inhibition were additive (Fig. 7). Knockdown by SMS1 shRNA plus CID-755673 suppressed current density to a similar level as SMS1 shRNA knockdown alone (at 110 mV: control, 266.8 ± 43.6 pA/pF, n = 14; shRNA, 147.3 ± 36.9 pA/pF, n = 12; shRNA + CID-755673, 133.8 ± 22.5 pA/pF, n = 12; P < 0.05 for control vs. shRNA and for control vs. shRNA + CID-755673; P > 0.05 for shRNA and shRNA + CID-755673). Similar changes were found for gmax derived from Boltzmann fits (control, 28.7 ± 5.0 pS/μm2, n = 14; shRNA, 14.1 ± 5.1 pS/μm2, n = 12; shRNA + CID-755673, 12.7 ± 2.2 pS/μm2, n = 12; P < 0.05 for control vs. shRNA and for control vs. shRNA + CID-755673; P > 0.05 for shRNA vs. shRNA + CID-755673).
Fig. 7.
Nonadditive effects of SMS1 knockdown and PKD inhibition. A: currents in a control cell transfected with scrambled RNA. B: currents in a cell transfected with SMS1 shRNA. C: currents in a cell transfected with SMS1 shRNA and treated with CID-755673. D: current density-voltage relationships in the three groups. Values are means ± SE. *P < 0.05 for control vs. SMS1 shRNA and for control vs. SMS1 shRNA + CID-755673. Cell capacitances were 16.0 ± 1.7, 15.4 ± 1.2, and 18.4 ± 1.9 pF for the control, shRNA, and shRNA + CID-755673 groups, respectively.
To further test the involvement of PKD in the regulation of KCNQ1/KCNE1 channels by SMS1, we studied the effect of another antagonist of PKD, CID-2011756 (34, 48). Similarly to CID-755673, CID-2011756 significantly reduced current density at 110 mV (CID-2011756, 140.7 ± 24.6 pA/pF, n = 33; control, 321.2 ± 22.8 pA/pF, n = 56; P < 0.05; data not shown). Furthermore, the suppressive effect of CID-2011756 was also found to be nonadditive with the effect of SMS1 knockdown (at 110 mV: control, 340.1 ± 37.4 pA/pF, n = 41; shRNA, 253.1 ± 29.1 pA/pF, n = 56; shRNA + CID-2011756, 220.3 ± 32.0 pA/pF, n = 41; P < 0.05 for control vs. shRNA and for control vs. shRNA + CID-2011756; P > 0.05 for shRNA vs. shRNA + CID-2011756).
These results suggest that PKD works downstream of SMS1 in the regulation of KCNQ1/KCNE1 channels. An immediate question is whether DAG analogs mimic the effects of SMS1. To address this, we tested the effect of a phorbol ester, PMA (50 nM) (17, 43). However, no significant effect of PMA was found on current density (at 110 mV: control, 302.1 ± 49.3 pA/pF, n = 32; PMA, 258.0 ± 39.2 pA/pF, n = 34; P > 0.05; data not shown).
DISCUSSION
We have shown here that knockdown of SMS1 in HEK-293T cells downregulates KCNQ1/KCNE1 channel density without changing channel voltage dependence. Consistent with this, overexpression of SMS1 increased current density only. Furthermore, we have demonstrated that inhibition of PKD reduces KCNQ1/KCNE1 channel density, and that this effect is not additive with that of SMS1 knockdown. Our results suggest that SMS1 positively regulates KCNQ1/KCNE1 channel density via a PKD-dependent pathway.
SMS1 positively regulates KCNQ1/KCNE1 current expression.
All lines of evidence obtained here (D609 treatment, SMS1 knockdown, SMS1 overexpression) support the notion that SMS1 positively regulates the expression of KCNQ1/KCNE1 channels.
The alteration in channel voltage dependence by D609 was not observed after overexpression or knockdown of SMS1, and D609 suppressed the current more drastically than SMS1 knockdown (compare Fig. 2C to Fig. 3D). These may be attributable to the effect of D609 on other enzymes, including SMS2 and phosphatidylcholine-specific phospholipase C (2); it is also possible that D609 acts directly on the channel. These possibilities need to be explored in future studies.
Because KCNQ1/KCNE1 channels in this study appeared not fully activated under most conditions (e.g., Fig. 2E), consistent with previous studies (20, 23, 32), the Boltzmann fits obtained here may have been biased toward the hyperpolarized direction. Furthermore, because the gmax values reported here were the results of extrapolation, they should be interpreted with caution. We have presented statistical analyses for current density at 110 mV in parallel with gmax and prefer to put more weight on the current density than gmax when interpreting our results.
Together, our results establish for the first time the role of SMS1 in the regulation of KCNQ1/KCNE1 channels. These results are of broad biological significance because of the important roles played by KCNQ1/KCNE1 channels in a number of organs, including the inner ear (14, 21), heart (5, 30, 42), pancreas (13, 45), and brain (10).
Mechanism underlying the regulation of KCNQ1/KCNE1 channels by SMS1.
SMS catalyzes the conversion of phosphatidylcholine and ceramide to sphingomyelin and DAG. Two isoforms of SMS (SMS1 and SMS2) have been identified; SMS2 is found in the cytoplasmic membrane, whereas SMS1 is primarily localized in the Golgi apparatus (18, 38). Both isoforms are shown here to be expressed in HEK-293T cells, but the focus here is on SMS1. Manipulation of SMS1 activity is expected to change the level of all four molecules in the reaction it catalyzes, as well as other phospholipids in related networks (11, 20). The question is, therefore, which of these molecules mediates the effect of SMS1 manipulation on KCNQ1/KCNE1?
Sphingomyelin may appear as the immediate candidate. Indeed, a growing body of evidence suggests that membrane lipids regulate voltage-dependent ion channels. Sphingomyelin, in particular its phospho-head group, is essential for the proper gating of certain voltage-dependent K+ channels (28, 44). Another phospholipid, phosphatidylinositol 4,5-bisphosphate, is critical for KCNQ channel opening (reviewed in Ref. 47). Furthermore, recent studies investigating the mechanism underlying the effect of lipids on channels have revealed that polyunsaturated fatty acid analogs modulate cardiac IKs channel functions via an electrostatic mechanism (20); the negative or positive charges of the fatty acids change channel voltage dependence by acting electrostatically on the channel voltage sensor (7). In all of these studies, a common effect of lipids is to affect channel gating. In the present study, however, in response to manipulation of SMS1 expression, we observed only changes in channel density, but not in voltage dependence or channel kinetics. The mechanism for the regulation of KCNQ1/KCNE1 channels by SMS1 must be one that controls membrane protein level.
Here, we focused on DAG, because binding of DAG is known to recruit PKD to the trans-Golgi network, thereby promoting protein transport to the cell membrane (6, 22). Although the modulation of SMS1 activity does not change the total cellular level of DAG (39, 41), DAG expression in the Golgi is reduced in SMS-knockdown cells and increased in SMS-overexpressing cells (41). Villani et al. (41) also demonstrated a reduction in localization of the DAG-binding kinase PKD to the Golgi. Furthermore, in HeLa cells, the vesicular stomatitis virus G protein was used as a reporter protein to reveal that SMS regulates trans-Golgi network-mediated protein trafficking (37). In line with this evidence, our results show that PKD inhibition suppresses KCNQ1/KCNE1 channel density, but not after knockdown of SMS1.
However, the DAG analog PMA appeared to have no effect on current density. DAG activates not only PKD but also PKC (reviewed in Ref. 46), and there is evidence that PKC activity is required for PKD activation (8, 49). Because PKC has also been reported to affect KCNQ1/KCNE1 channels (17, 43), PMA appears not to simply mimic the effect of SMS1 on KCNQ1/KCNE1, but may affect the channels through multiple mechanisms. In fact, previous studies on the action of phorbol esters on KCNQ1/KCNE1 channels in cells other than HEK-293T have reported either enhancement (43) or suppression (17). Compared with DAG, the effect of PKD can be addressed more specifically using selective inhibitors. The PKD inhibitors used here have been shown to inhibit PKD selectively, with little effect on PKC (9, 33).
To conclude, the present results suggest that SMS1 positively regulates KCNQ1/KCNE1 channel expression without changing channel properties. Our data provide evidence in support of a working hypothesis that SMS1 upregulates KCNQ1/KCNE1 channel density via DAG-binding PKD.
GRANTS
This work was supported by grants from the Japan Society for the Promotion of Science (nos. 25290006, 25670719, and 15H01442).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.W., M. Takemoto, M. Taniguchi, T.T., and T.O. performed experiments; M.W. and W.-J.S. analyzed data; M.W. drafted manuscript; M.W., M. Takemoto, M. Taniguchi, T.T., T.O., and W.-J.S. approved final version of manuscript; M. Takemoto and W.-J.S. edited and revised manuscript; W.-J.S. conception and design of research; W.-J.S. interpreted results of experiments.
REFERENCES
- 1.Abbott GW. Biology of the KCNQ1 potassium channel. New J Sci 2014: 237431, 2014. [Google Scholar]
- 2.Adibhatla RM, Hatcher JF, Gusain A. Tricyclodecan-9-yl-xanthogenate (D609) mechanism of actions: a mini-review of literature. Neurochem Res 37: 671–679, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anantharam A, Markowitz SM, Abbott GW. Pharmacogenetic considerations in diseases of cardiac ion channels. J Pharmacol Exp Ther 307: 831–838, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Barceló-Coblijn G, Martin ML, de Almeida RF, Noguera-Salvà MA, Marcilla-Etxenike A, Guardiola-Serrano F, Lüth A, Kleuser B, Halver JE, Escribá PV. Sphingomyelin and sphingomyelin synthase (SMS) in the malignant transformation of glioma cells and in 2-hydroxyoleic acid therapy. Proc Natl Acad Sci U S A 108: 19569–19574, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barhanin J, Lesage F, Guillemare E, Fink M, Lazdunski M, Romey G. KVLQT1 and IsK (minK) proteins associate to form the IKs cardiac potassium current. Nature 384: 78–80, 1996. [DOI] [PubMed] [Google Scholar]
- 6.Baron CL, Malhotra V. Role of diacylglycerol in PKD recruitment to the TGN and protein transport to the plasma membrane. Science 295: 325–328, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Börjesson SI, Parkkari T, Hammarström S, Elinder F. Electrostatic tuning of cellular excitability. Biophys J 98: 396–403, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Díaz Añel AM, Malhotra V. PKCeta is required for beta1gamma2/beta3gamma2- and PKD-mediated transport to the cell surface and the organization of the Golgi apparatus. J Cell Biol 169: 83–91, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.George KM, Frantz MC, Bravo-Altamirano K, Lavalle CR, Tandon M, Leimgruber S, Sharlow ER, Lazo JS, Wang QJ, Wipf P. Design, synthesis, and biological evaluation of PKD inhibitors. Pharmaceutics 3: 186–228, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldman AM1, Glasscock E, Yoo J, Chen TT, Klassen TL, Noebels J. Arrhythmia in heart and brain: KCNQ1 mutations link epilepsy and sudden unexplained death. Sci Transl Med 1: 2–6, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hannun YA, Obeid LM. The ceramide-centric universe of lipid-mediated cell regulation: stress encounters of the lipid kind. J Biol Chem 277: 25847–25850, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Hattori S, Murakami F, Song WJ. Quantitative relationship between between Kv4.2 mRNA and A-type potassium current in rat striatal cholinergic interneurons during postnatal development. J Neurophysiol 90: 175–183, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Hayashi M, Novak I. Molecular basis of potassium channels in pancreatic duct epithelial cells. Channels (Austin) 7: 432–441, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hibino H, Nin F, Tsuzuki C, Kurachi Y. How is the highly positive endocochlear potential formed? The specific architecture of the stria vascularis and the roles of the ion-transport apparatus. Pflügers Arch 459: 521–533, 2010. [DOI] [PubMed] [Google Scholar]
- 15.Jespersen T, Grunnet M, Olesen SP. The KCNQ1 potassium channel: from gene to physiological function. Physiology (Bethesda) 20: 408–416, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Jin ZX, Huang CR, Dong L, Goda S, Kawanami T, Sawaki T, Sakai T, Tong XP, Masaki Y, Fukushima T, Tanaka M, Mimori T, Tojo H, Bloom ET, Okazaki T, Umehara H. Impaired TCR signaling through dysfunction of lipid rafts in sphingomyelin synthase 1 (SMS1)-knockdown T cells. Int Immunol 20: 1427–1437, 2008. [DOI] [PubMed] [Google Scholar]
- 17.Kanda VA, Purtell K, Abbott GW. Protein kinase C downregulates I(Ks) by stimulating KCNQ1-KCNE1 potassium channel endocytosis. Heart Rhythm 8: 1641–1647, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitatani K, Taniguchi M, Okazaki T. Role of sphingolipids and metabolizing enzymes in hematological malignancies. Mol Cells 38: 482–495, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liin SI, Barro-Soria R, Larsson HP. The KCNQ1 channel–remarkable flexibility in gating allows for functional versatility. J Physiol 593: 2605–2615, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liin SI, Silverå Ejneby M, Barro-Soria R, Skarsfeldt MA, Larsson JE, Starck Härlin F, Parkkari T, Bentzen BH, Schmitt N, Larsson HP, Elinder F. Polyunsaturated fatty acid analogs act antiarrhythmically on the cardiac IKs channel. Proc Natl Acad Sci U S A 112: 5714–5719, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu MH, Takemoto M, Watanabe K, Luo H, Nishimura M, Yano M, Tomimoto H, Okazaki T, Oike Y, Song WJ. Deficiency of sphingomyelin synthase-1 but not sphingomyelin synthase-2 causes hearing impairments in mice. J Physiol 590: 4029–4044, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malhotra V, Campelo F. PKD regulates membrane fission to generate TGN to cell surface transport carriers. Cold Spring Harb Perspect Biol 3: a005280, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moreno C, de la Cruz A, Oliveras A, Kharche SR, Guizy M, Comes N, Starý T, Ronchi C, Rocchetti M, Baró I, Loussouarn G, Zaza A, Severi S, Felipe A, Valenzuela C. Marine n-3 PUFAs modulate IKs gating, channel expression, and location in membrane microdomains. Cardiovasc Res 105: 223–232, 2015. [DOI] [PubMed] [Google Scholar]
- 24.Murai T, Kakizuka A, Takumi T, Ohkubo H, Nakanishi S. Molecular cloning and sequence analysis of human genomic DNA encoding a novel membrane protein which exhibits a slowly activating potassium channel activity. Biochem Biophys Res Commun 161: 176–181, 1989. [DOI] [PubMed] [Google Scholar]
- 25.Nakajo K, Kubo Y. KCNQ1 channel modulation by KCNE proteins via the voltage-sensing domain. J Physiol 593: 2617–2625, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nerbonne JM, Kass RS. Molecular physiology of cardiac repolarization. Physiol Rev 85: 1205–1253, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Neyroud N, Tesson F, Denjoy I, LeiboviciM, Donger C, Barhanin J, Faure S, Gary F, Coumel P, Petit C, Schwartz K, Guicheney P. A novel mutation in the potassium channel gene KVLQT1 causes the Jervell and Lange-Nielsen cardioauditory syndrome. Nat Genet 15: 186–189, 1997. [DOI] [PubMed] [Google Scholar]
- 28.Ramu Y, Xu Y, Lu Z. Enzymatic activation of voltage-gated potassium channels. Nature 442: 696–699, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Sakagami M, Fukazawa K, Matsunaga T, Fujita H, Mori N, Takumi T, Ohkubo H, Nakanishi S. Cellular localization of rat Isk protein in the stria vascularis by immunohistochemical observation. Hear Res 56: 168–172, 1991. [DOI] [PubMed] [Google Scholar]
- 30.Sanguinetti MC, Curran ME, Zou A, Shen J, Spector PS, Atkinson DL, Keating MT. Coassembly of KVLQT1 and minK (IsK) proteins to form cardiac IKs potassium channel. Nature 384: 80–83, 1996. [DOI] [PubMed] [Google Scholar]
- 31.Schroeder BC, Waldegger S, Fehr S, Bleich M, Warth R, Greger R, Jentsch TJ. A constitutively open potassium channel formed by KCNQ1 and KCNE3. Nature 403: 196–199, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Seebohm G, Strutz-Seebohm N, Birkin R, Dell G, Bucci C, Spinosa MR, Baltaev R, Mack AF, Korniychuk G, Choudhury A, Marks D, Pagano RE, Attali B, Pfeufer A, Kass RS, Sanguinetti MC, Tavare JM, Lang F. Regulation of endocytic recycling of KCNQ1/KCNE1 potassium channels. Circ Res 100: 686–692, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Sharlow ER, Giridhar KV, LaValle CR, Chen J, Leimgruber S, Barrett R, Bravo-Altamirano K, Wipf P, Lazo JS, Wang QJ. Potent and selective disruption of protein kinase D functionality by a benzoxoloazepinolone. J Biol Chem 283: 33516–33526, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharlow ER, Mustata Wilson G, Close D, Leimgruber S, Tandon M, Reed RB, Shun TY, Wang QJ, Wipf P, Lazo JS. Discovery of diverse small molecule chemotypes with cell-based PKD1 inhibitory activity. PLoS One 6: e25134, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song WJ, Baba Y, Otsuka T, Murakami F. Characterization of Ca2+ channels in rat subthalamic nucleus neurons. J Neurophysiol 84: 2630–2637, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Song WJ, Tkatch T, Baranauskas G, Ichinohe N, Kitai S, Surmeier DJ. Somatodendritic depolarization-activated potassium currents in rat neostriatal cholinergic interneurons are predominantly of the A-type and attributable to co-expression of Kv4.2 and Kv41 subunits. J Neurosci 18: 3124–3137, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Subathra M, Qureshi A, Luberto C. Sphingomyelin synthases regulate protein trafficking and secretion. PLoS One 6: e23644, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tafesse FG, Ternes P, Holthuis JC. The multigenic sphingomyelin synthase family. J Biol Chem 281: 29421–29425, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Tafesse F, Huitema K, Hermansson M, van der Poel S, van den Dikkenberg J, Uphoff A, Somerharju P, Holthuis J. Both sphingomyelin synthases SMS1 and SMS2 are required for sphingomyelin homeostasis and growth in human HeLa cells. J Biol Chem 282: 17537–17547, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Takumi T, Ohkubo H, Nakanishi S. Cloning of a membrane protein that induces a slow voltage-gated potassium current. Science 242: 1042–1045, 1988. [DOI] [PubMed] [Google Scholar]
- 41.Villani M, Subathra M, Im YB, Choi Y, Signorelli P, Del Poeta M, Luberto C. Sphingomyelin synthases regulate production of diacylglycerol at the Golgi. Biochem J 414: 31–41, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang KW, Tai KK, Goldstein SA. MinK residues line a potassium channel pore. Neuron 16: 571–577, 1996. [DOI] [PubMed] [Google Scholar]
- 43.Xiao GQ, Mochly-Rosen D, Boutjdir M. PKC isozyme selective regulation of cloned human cardiac delayed slow rectifier K current. Biochem Biophys Res Commun 306: 1019–1025, 2003. [DOI] [PubMed] [Google Scholar]
- 44.Xu Y, Ramu Y, Lu Z. Removal of phospho-head groups of membrane lipids immobilizes voltage sensors of K+ channels. Nature 451: 826–830, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamagata K, Senoguchi T, Lu MH, Takemoto M, Karim F, Go C, Sato Y, Hatta M, Yoshizawa T, Araki E, Miyazaki J, Song WJ. Voltage-gated K+ channel KCNQ1 regulates insulin secretion in MIN6 beta-cell line. Biochem Biophys Res Commun 407: 620–625, 2011. [DOI] [PubMed] [Google Scholar]
- 46.Yang C, Kazanietz MG. Divergence and complexities in DAG signaling: looking beyond PKC. Trends Pharmacol Sci 24: 602–608, 2003. [DOI] [PubMed] [Google Scholar]
- 47.Zaydman MA, Cui J. PIP2 regulation of KCNQ channels: biophysical and molecular mechanisms for lipid modulation of voltage-dependent gating. Front Physiol 5: 195, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao S, Zhou L, Niu G, Li Y, Zhao D, Zeng H. Differential regulation of orphan nuclear receptor TR3 transcript variants by novel vascular growth factor signaling pathways. FASEB J 28: 4524–4533, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zugaza JL, Sinnett-Smith J, Van Lint J, Rozengurt E. Protein kinase D (PKD) activation in intact cells through a protein kinase C-dependent signal transduction pathway. EMBO J 15: 6220–6230, 1996. [PMC free article] [PubMed] [Google Scholar]