Abstract
Proteomics studies have identified Ste20-related proline/alanine-rich kinase (SPAK) and oxidative stress response 1 (OSR1) in exosomes isolated from body fluids such as blood, saliva, and urine. Because proteomics studies likely overestimate the number of exosome proteins, we sought to confirm and extend this observation using traditional biochemical and cell biology methods. We utilized HEK293 cells in culture to verify the packaging of these Ste20 kinases in exosomes. Using a series of centrifugation and filtration steps of conditioned culture medium isolated from HEK293 cells, we isolated nanovesicles in the range of 40–100 nm. We show that these small vesicles express the tetraspanin protein CD63 and lack endoplasmic reticulum and Golgi markers, consistent with these being exosomes. We show by Western blot and immunogold analyses that these exosomes express SPAK, OSR1, and Na-K-Cl cotransporter 1 (NKCC1). We show that exosomes are not only secreted by cells, but also accumulated by adjacent cells. Indeed, exposing cultured cells to exosomes produced by other cells expressing a fluorescently labeled kinase resulted in the kinase finding its way into the cytoplasm of these cells, consistent with the idea of exosomes serving as cell-to-cell communication vessels. Similarly, coculturing cells expressing different fluorescently tagged proteins resulted in the exchange of proteins between cells. In addition, we show that both SPAK and OSR1 kinases entering cells through exosomes are preferentially expressed at the plasma membrane and that the kinases in exosomes are functional and maintain NKCC1 in a phosphorylated state.
Keywords: Na-K-2Cl cotransport, NKCC1, HEK293 cells, HeLa cells, extracellular vesicles
ste20-related proline/alanine-rich kinase (SPAK, also known as STK39) and oxidative stress response 1 (OSR1, also known as OXSR1) belong to the 28-member family of mammalian Sterile20p-like Ser/Thr kinases (5). Through a protein-protein interaction that involves a highly conserved 90-residue domain located in the carboxyl terminus of the kinases (33, 43) and Arg-Phe-Xaa-Val/Iso-containing peptides located in target proteins (6, 33), SPAK and OSR1 anchor to their targets and phosphorylate downstream Ser/Thr residues. The major targets of SPAK and OSR1 are membrane proteins involved in ion transport: the two Na-K-2Cl cotransporters (32, 33), the Na-Cl cotransporter (30), K-Cl cotransporters (26, 37), Cl− channels (7), and Na+-bicarbonate cotransporter, NBCe1 (18, 48).
In Cl−-secreting epithelia (e.g., salivary, sweat, lacrimal gland, lung, intestine) Na-K-Cl cotransporter 1 (NKCC1) replenishes Cl− at the basolateral membrane, as it is transported by Cl− channels at the apical membrane (3, 9, 16, 29). In sensory neurons, NKCC1 accumulates Cl−, thereby facilitating GABA depolarization and presynaptic inhibition (1). The activity of the cotransporter in these cells is equally controlled by SPAK and OSR1 (12).
In the distal convoluted tubule (DCT) segment of the mouse kidney, SPAK controls the phosphorylation state and activity of Na+-Cl− cotransporter (NCC), resulting in the control of the amount of Na+ reabsorption that is mediated by this nephron segment (30). The activity of SPAK is itself under the control of two upstream With-No-K (lysine) kinases: WNK1 and WNK4 (11, 28, 44). Absence of SPAK upon genetically engineered disruption of the Stk39 gene results in low blood pressure, a propensity to waste sodium when challenged with a low sodium diet, and increased fractional excretion of K+ and Mg2+ while decreased fractional excretion of Ca2+ (15, 25, 36, 51), whereas increased activity of WNK4 due to pseudohypoaldosteronism type II (PHAII) mutations results in increased SPAK activity leading to increased NCC function, increased Na+ reabsorption, and increased blood pressure (50).
While the relationship between SPAK/OSR1 and membrane transporters is being under intense investigation, other aspects of SPAK/OSR1 biology are receiving less attention. An intriguing finding made in the SPAK knockout mouse was the evidence of OSR1-containing punctae in DCT cells (15, 25). The nature of these structures is still unknown and could be related to multivesicular bodies or large endosome-like structures that contain intraluminal vesicles. Upon fusion with the plasma membrane, these multivesicular bodies release in the extracellular environment these intraluminal vesicles, which are now called exosomes. Exosomes are known to be formed and released by all types of cells and to participate in cell-cell communication (10, 41, 52). Of interest, the number of intravesicular bodies and release of exosomes have been shown to increase in cardiomyocytes treated with hypotonic conditions (34). This indicates that the production of multivesicular bodies and release of exosomes might be increased by cellular stress, a feature that likely occurs in the distal convoluted cells of SPAK-deficient mice, as the segment undergoes atrophy.
Proteomics studies have indicated that only a fraction of proteins in a cell are packaged in exosomes (4, 35, 38, 39, 46). Proteomic profiling of human urine revealed that transporters and kinases relevant to Na+ reabsorption, e.g., NKCC2, NCC, SPAK, OSR1, and WNK kinases, are detected in urine exosomes (35). However, it has been argued that proteomics data likely overestimate the number of proteins found in exosomes, as a number of detected proteins might be included because of their mere presence at the side of budding (49). Thus, it is important to confirm the proteomics findings that SPAK and OSR1 are packaged in exosomes and extend this observation to a well-established cell cultured model.
In this study, we show that SPAK, OSR1, and NKCC1 are packaged by HEK293 cells in exosomes, that these microvesicles are released in the extracellular environment and internalized by neighboring cells. We show that the cotransporter in exosomes is highly phosphorylated and that the kinases extracted from exosomes are active and able to phosphorylate immunoprecipitated NKCC1.
MATERIALS AND METHODS
Reagents.
Rabbit polyclonal antibodies raised against mouse SPAK, OSR1, and NKCC1 were produced in the laboratory and previously described (22, 32, 33). Sheep polyclonal antibody against pNKCC1 (S763B, pThr203 + pThr207 + pThr212) was obtained from the Protein Phosphorylation Unit of the Medical Research Council (University of Dundee, Dundee, UK). Monoclonal antibodies to CD63 (clone E-12), and protein A-Agarose were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit monoclonal antibody to EGFR (clone D38B1) was obtained from Cell Signaling (Danvers, MA). Monoclonal anti-HA (clone HA-7) and anti-FLAG (clone M2) antibodies and gold-conjugated anti-rabbit IgG were obtained from Sigma-Aldrich (St. Louis, MO). Monoclonal antibodies directed against the ER marker protein disulfide isomerase (PDI, clone RL90) and the 58K Golgi marker (clone 58K-9) were obtained from Abcam (Cambridge, MA). Horseradish peroxidase (HRP)-conjugated secondary antibodies (anti-mouse, anti-rabbit, and anti-sheep) were purchased from Jackson Immunochemicals (West Grove, PA). The plasmid-encoding GFP-tagged CD63 was a kind gift from Dr. Josiah Ochieng (Meharry Medical College, Nashville, TN).
Cell culture and transfection.
HEK293 cells were purchased from ATCC (Manassas, VA). HeLa cells were kindly donated by Dr. James Goldenring (Department of Surgery, Vanderbilt University). These cells were propagated in DMEM/F-12 or RPMI supplemented with 10% heat-inactivated fetal bovine serum (FBS), 200 U/ml penicillin and 200 μg/ml streptomycin, in a humidified 95% air and 5% CO2 incubator at 37°C. Where indicated, serum-free medium consisted of DMEM/F-12 in which FBS was replaced with 0.1% bovine serum albumin (BSA) or 0.5% FBS. Cells stably expressing HA-tagged SPAK and FLAG-tagged OSR1 were transfected with 2 μg of the plasmid DNA (pCDNA3 vector) using FuGene 6 (Roche Applied Science, Indianapolis, IN) and selected in complete DMEM/F-12 medium containing 500 μg/ml geneticin (G418) for 3 wk. In separated coexpression and coculture experiments, 2 μg of each plasmid—CD63-GFP, Venus-SPAK, Cerulean-OSR1, tdTomato-OSR1, and tdTomato-NKCC1—were transiently transfected in HEK293 or HeLa cells.
Exosome isolation.
Exosomes were purified as previously described (24). Briefly, control HEK293-, or HA-SPAK-, or FLAG-OSR1-transfected HEK293 cells were cultured in 15-cm dishes in complete medium until confluency, then transferred to serum-free medium overnight prior to collection of supernatant. The conditioned medium was then centrifuged at 3,000 g for 10 min to eliminate cells and large cellular debris, followed by a centrifugation at 20,000 g for 30 min to remove microvesicles and other cellular debris. The resultant supernatant was then carefully collected and filtered through a 0.22-μm filter (Millipore), and the exosomes were pelleted by ultracentrifugation at 120,000 g for 90 min at 4°C using a SW32 rotor. The exosome-containing pellet was washed by resuspension in 10 ml ice-cold PBS, and exosomes were again pelleted by ultracentrifugation at 120,000 g for 90 min at 4°C using a SW41Ti rotor. The exosome-containing final pellets were resuspended in 100 μl PBS and stored at −80°C until use. For characterization of exosomes on sucrose gradient, exosomes were mixed with 2 ml of 2.5 M sucrose in PBS and placed at the bottom of a SW41 centrifuge tube, overlaid with 6 ml of 2 M sucrose and 3 ml of 0.25 M sucrose, and ultracentrifuged at 120,000 g for 16 h. Twelve fractions (800 μl each) were then collected from the top of the gradient. These fractions were resuspended in PBS and ultracentrifuged at 100,000 g. Pellet were resuspended in 100 μl of PBS and 20 μl were then resolved on 10% polyacrylamide gel electrophoresis and analyzed by Western blotting.
Western blot analysis.
Cells were washed once in ice-cold PBS, harvested by scraping, and lysed in RIPA buffer (150 mM NaCl, 50 mM Tris·HCl, pH 7.4, 1% Nonidet P-40, 0.1% sodium deoxycholate, 1 mM EDTA) containing a cocktail of protease inhibitors (Roche Applied Science) and phosphatase inhibitors (20 mM sodium fluoride, 50 mM β-glycerophosphate, and 1 mM sodium orthovanadate). Cell lysates, exosomes pellets, or sucrose gradients fractions were separated in 10% SDS-PAGE and blotted on PVDF membranes (Thermo Fisher Scientific, Waltham, MA). Membranes were probed with the indicated primary antibodies followed by HRP-conjugated secondary antibodies and revealed using enhanced chemiluminescence (Perkin Elmer, Wellesley, MA).
Electron microscopy.
Exosomes (50 μl) in PBS were fixed by suspension in 2.5% gluteraldehyde in 0.1 M sodium cacodylate (50 μl). Fixed exosomes were dropped onto a sheet of Parafilm, and Formvar-carbon-coated grids were floated for 30 and 60 s at room temperature to adsorb the exosomes. The Formvar-carbon-coated grids were then washed in PBS and negatively stained with 2% uranyl acetate for 30 s and viewed using a JEOL transmission electron microscope at 80 kV. For immunogold labeling, grids with absorbed fixed exosomes were blocked with 0.5% bovine serum albumin, permeabilized with 0.02% Triton for 5 min, washed, and incubated with primary antibody (1:100) in blocking solution for 2 h. After extensive washing with blocking solution, the grids were incubated for 1 h with protein A-gold conjugates (1:100), washed with PBS, fixed with 2.5% glutaraldehyde for 30 s, washed with PBS and water, stained for 30 s with 2% uranyl acetate for 30 s, and observed under an electron microscope as described above.
Kinase assay.
Subconfluent HEK293 cells were lysed with 1 ml of RIPA, and 500 μl of cell lysate were mixed with 5 μl of NKCC1 or IgG antibodies and incubated in a 1.5-ml Eppendorff tube overnight at 4°C, under constant rotation using a Hematology/Chemistry mixer. The next day, 200 μl of protein-A Agarose beads/slurry were added and incubated at 4°C for 2 h with rotation. Immunoprecipitates were centrifuged at 10,000 rpm for 1 min and then washed 5 times with ice-cold PBS. Increasing exosome lysate amounts were added to the immunoprecipitates, supplemented with 10 μM ATP/MgCl2 and incubated at 37°C for 45 min. The reaction was stopped by adding 4% SDS sample buffer containing DTT and heated at 75°C for 15 min. Boiled sampled were then resolved on a 7.5% polyacrylamide gel and analyzed by Western blotting. For phosphorylation of exosome NKCC1, exosome lysates were directly incubated with 10 μM ATP/MgCl2 and incubated at 37°C for 45 min and analyzed by Western blotting.
Immunofluorescence and live cell imaging of exosomes secretion and internalization.
For fixed cells, HEK293 or HeLa cells transiently expressing GFP or tdTomato constructs were grown on microscope glass (Thermo Fisher Scientific). Cells were then fixed with 4% formaldehyde for 15 min at room temperature. Fixed cells were washed three times with ice-cold PBS for 5 min. Cells were then mounted on glass coverslips with ProLong Gold antifade containing DAPI (Invitrogen, Carlsbad, CA). Confocal Z-stack images were acquired on a Zeiss LSM 710 confocal microscope. For live cell imaging, HEK293 cells were plated on 35-mm imaging dishes (MatTek, Ashland, MA) and cultured overnight in complete DMEM/F-12 medium. For secretion or internalization of exosomes in real time, cells were maintained at 37°C. Time-lapse images were captured using a ×63 oil objective (numerical aperture, 1.4) in the GFP or tdTomato channel at 1-s intervals using a Zeiss laser scanning LSM 710 META inverted confocal microscope. Movie frames were saved as TIFF for presentation and printing.
RESULTS
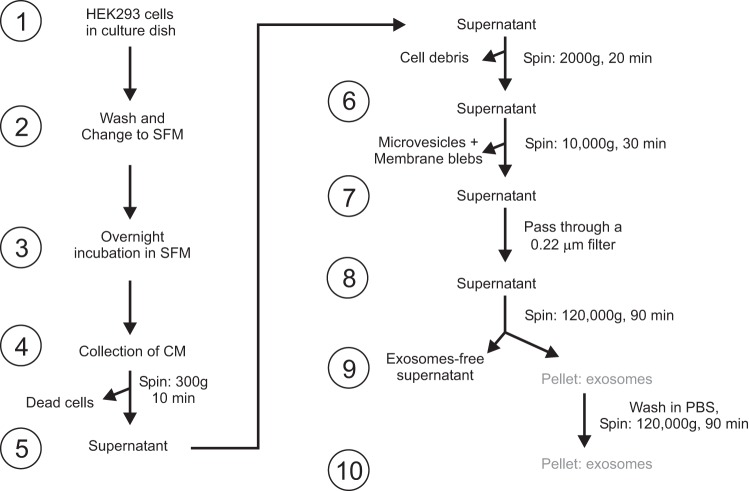
All cells, in vivo or in culture, generate multivesicular bodies which, when fused to the plasma membrane, release in the extracellular space their content in the form of exosomes (10, 41). As only a fraction of proteins expressed by a cell end up within the exosomal compartment (4), we sought to determine whether the Ste20 kinases SPAK and OSR1 are packaged in these exosomes. As fetal bovine serum contains their own exosomes, confluent HEK293 cells were first washed and exposed to serum-free medium overnight to isolate newly secreted exosomes. As these vesicles are very small is size (30–100 nm), a multistep and extensive centrifugation and filtration process, designed to eliminate cellular debris, dead cell material, and microvesicles of larger size (250-1,000 nm), was required (Fig. 1). This process led to a final high-speed pellet that contains the exosomal fraction (Fig. 2A).
Fig. 1.
Isolation of exosomes. Schematic representation is shown of the differential centrifugation protocol used to purify exosomes from the conditioned medium of HEK293 cells.
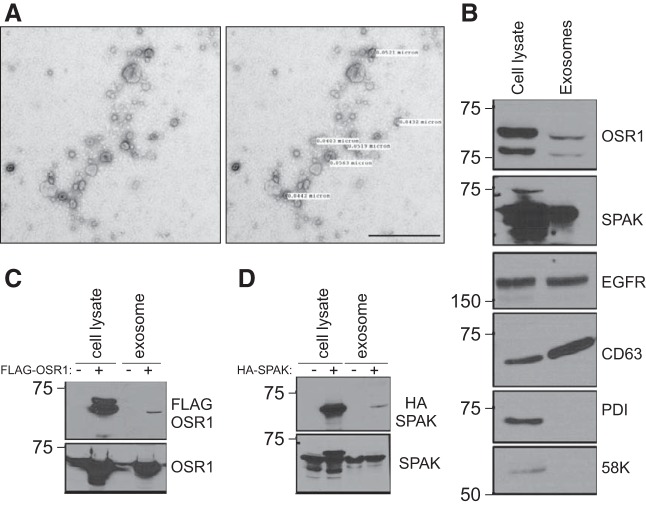
To facilitate the identification of the kinases, HEK293 cells were transfected with epitope-tagged kinases: the FLAG tag was added to the N-terminus of OSR1, whereas an HA-tag was added to the N-terminal tail of SPAK. Exosomes (∼40–100 nm) were isolated from HEK293 cells transfected with either FLAG-OSR1 or HA-SPAK (Fig. 2A). Western blot analysis of cell lysates and exosomal fractions identifies both kinases in exosomes. Exosomes were characterized by their expression of the exosomal membrane markers, EGFR and CD63, and the absence of ER and Golgi markers, PDI and 58K protein, respectively (Fig. 2B). As expected, exosomes contain more native kinases than tagged kinases, as only a fraction of the cells get transfected (Fig. 2, C and D). Additional evidence that the final high-speed fraction contains mostly exosomes is provided in Fig. 3, which shows that protein signal is mostly restricted to fractions 3–5 of a sucrose gradient, which is where the exosomes typically lie (24, 40). Of interest, we also identified NKCC1 as a resident protein of HEK293 cell exosomes.
Fig. 2.
SPAK and OSR1 are found in exosomes. A: representative transmission electron microscopy image of HEK293 exosomes. Bar, 500 nm. B: exosomes were purified by ultracentrifugation from HEK293 cell-conditioned media. Ten micrograms were analyzed by Western blotting. OSR1 and SPAK as well as the exosome markers EGFR and CD63 were detected in exosome fraction, while the Golgi (58K) and ER (PDI) markers were detected only in the cell lysate fraction. OSR1 and SPAK blots are probing lysate and exosomes from HEK293. C: HEK293 cells transfected with HA-SPAK and FLAG-OSR1 package exogenous SPAK and OSR1 inside exosomes. Exosomes were purified from the conditioned media of HEK293 cells expressing HA-SPAK and FLAG-OSR1 and analyzed by Western blotting. OSR1 and SPAK blots are probing lysate and exosomes from HEK293 expressing both native and recombinant tagged FLAG-OSR1 and HA-SPAK.
Fig. 3.
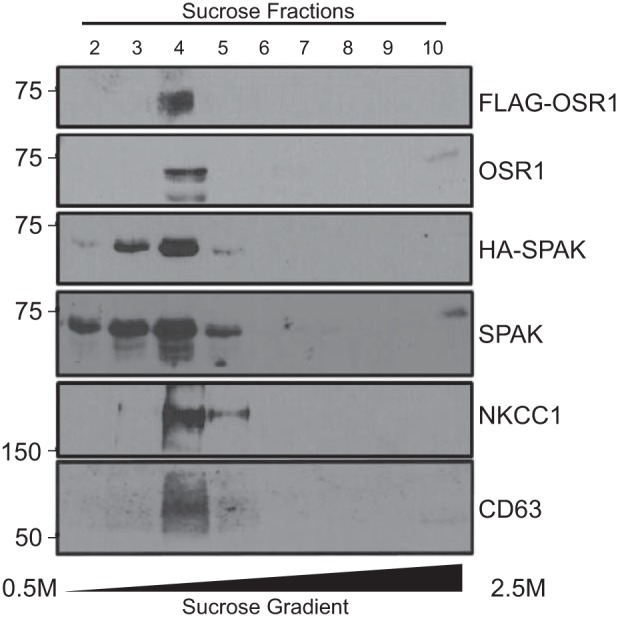
Confirmation of SPAK and OSR1 in exosomal fraction. Exosomes from conditioned media of HEK293 cells stably transfected with FLAG-OSR1 and HA-SPAK were fractionated on a discontinuous (0.25M/2.0M/2.5M) sucrose gradient. Fractions collected from the top of the sucrose gradient (50 μl) were separated by SDS-PAGE and analyzed by Western blotting for the presence of OSR1, SPAK, NKCC1, and the exosome marker, CD63.
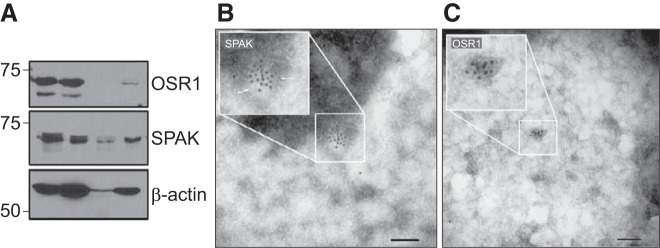
To further demonstrate that the structures we isolated are exosomes or cell-derived vesicles, we performed an experiment in which we treated some exosomes with trypsin and others with Triton X-100, prior to trypsin treatment. As seen in Fig. 4A, the exosome membrane protects the content from trypsin digestion because the exosomes maintain a certain amount of OSR1 and SPAK after lysis in sample buffer, in opposition to exosomes that were first permeabilized with Triton X-100 and then treated with trypsin where we observe an absence of signal. Using gold-labeling and electron microscopy to visualize purified exosomes, we verified that endogenous SPAK and OSR1 are detected inside the lumen of exosomes (Fig. 4B). Note, however, that due to the extensive washes that are related with the immunogold staining, the membrane is often not seen in the electron micrograph (Fig. 4C). The size of the structure in Fig. 4, B and C, is consistent with the size of exosomes.
Fig. 4.
SPAK and OSR1 are located inside membrane-containing vesicles. A: sucrose gradient-purified exosomes were left untreated (Exo; lane 2), incubated with trypsin for 30 min (Exo + trypsin; lane 3), or incubated with 0.1% Triton X-100 for 5 min followed by trypsin treatment (Exo + TX + trypsin, lane 4). Lane 1 was loaded with 30 μg of HEK293 cell lysate as a control. B and C: sucrose gradient-purified exosomes were fixed on carbon grids, permeabilized with Triton, labeled with rabbit anti-SPAK and anti-OSR1, respectively, and detected with protein A-gold conjugates. Exosomes were then stained with uranyl acetate and observed under an electron microscope. Bars, 100 nm.
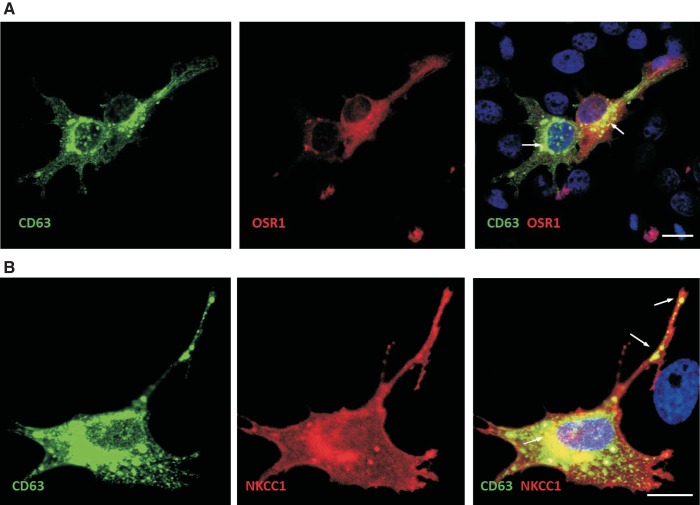
To visualize OSR1- and NKCC1-containing exosomes in cells, HeLa cells were cotransfected with an EGFP-CD63 cDNA and tdTomato-OSR1 or tdTomato-NKCC1 cDNAs. Live cell microscopy was done using a confocal microscope. As seen in Fig. 5, intense fluorescence signal is observed for all transfected protein and colocalization is established by intense perinuclear yellow signal as well as signal of trafficking vesicles punctae (presumably endosomes) traveling along cell processes (arrows). Note that abundant NKCC1 (red) signal is observed at the plasma membrane.
Fig. 5.
Colocalization of OSR1 and NKCC1 with exosome CD63 marker. HeLa cells were transiently cotransfected with GFP-CD63 and tdTomato-NKCC1 (A) or GFP-CD63 and tdTomato-OSR1 (B). After 48 h, cell were fixed with 4% paraformaldehyde and mounted on glass coverslips with ProLong Gold Antifade reagent with DAPI. Z-stack images were taken on a Zeiss LSM710 confocal microscope. Images with both merged images of green and red filters (colocalization) are displayed on the right. Bars, 10 μm.
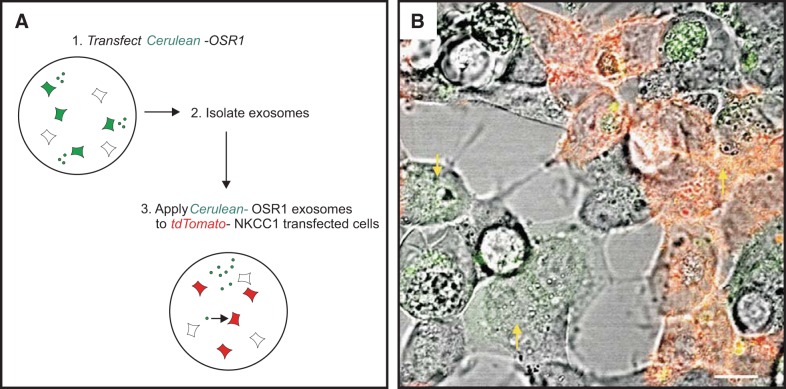
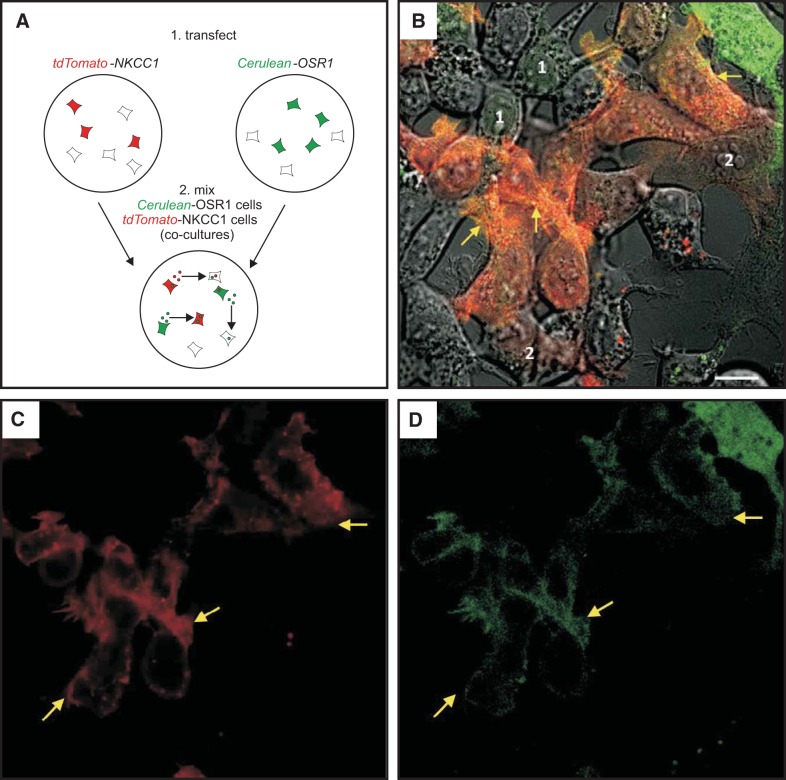
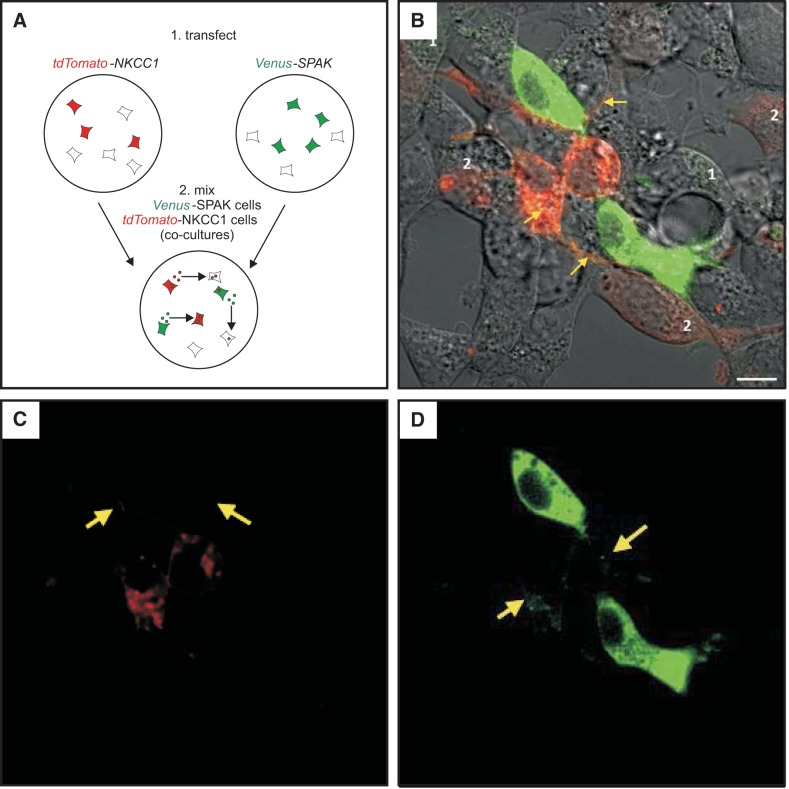
To demonstrate that exosomes are not only released in the extracellular space, but also taken up by cells, we designed experiments to establish the transfer of fluorescently labeled kinases from cell to cell. First, we exposed cells transfected with tdTomato-NKCC1 with exosomes purified from conditioned medium of cells transfected with Cerulean-OSR1 (protocol depicted in Fig. 6A). As seen in Fig. 6B, many cells demonstrate green fluorescence when not expressing tdTomato-NKCC1 and yellow fluorescent signal when expressing the red tagged-NKCC1 protein. Exosomes may have transferred both mRNA and proteins. However, since Cerulean-OSR1-containing exosomes were incubated on tdTomato-NKCC1 expressing cells only for 1 h prior to live cell imaging, the majority of the green signal in this case is likely due to internalization of labeled proteins. In a variation of this experiment, dishes of HEK293 cells were first transfected with either Cerulean-OSR1, or Venus-SPAK, or tdTomato-NKCC1, and after 2 days in culture, cells were washed, detached from the dishes, and mixed to generate cocultures of cells expressing either kinases or cotransporter. To demonstrate secretion and internalization of Cerulean-, Venus-, and dtTomato-tagged exosomes, cocultured dishes were visualized by live cell microscopy. When observed under green channel (excitation = 488 nm) and imaged for 2 min, the movement of Cerulean-OSR1 (Fig. 7) and Venus-SPAK (Fig. 8) green particles could be seen within transfected cells. Green signal was also observed in neighboring cells, indicating that these cells have internalized exosomes containing Cerulean-tagged OSR1 or Venus-tagged SPAK, respectively. On the other hand, when cells were excited at 568 nm, red tdTomato-NKCC1 punctae and particles (Figs. 7 and 8) could be seen moving within the cytoplasm of transfected cells. After fusion with the plasma membrane, these punctae disappeared, indicating that large multivesicular bodies presumably had fused to the plasma membrane and released their intraluminal vesicles or exosomes in the extracellular space. Red fluorescent signal was also observed within surrounding cells, including Cerulean-OSR1 and Venus-SPAK transfected cells, indicating that exosome containing tdTomato-NKCC1 have entered surrounding cells. Note that the NKCC1 signal observed in surrounding cells is strongly associated with the plasma membrane and intracytoplasmic endosomes. When the kinases are not overexpressed in cells but observed in adjacent cells, their expression is also mainly localized at the plasma membrane with NKCC1.
Fig. 6.
HEK293 cells internalize exosomes provided to their extracellular environment. A: schematic representation of exosomes isolated from cells and internalized by other cells. B: exosomes collected from HEK293 cells overexpressing Venus-OSR1 were transferred to three separate MatTek culture dishes containing HEK293 cells transiently expressing tdTomato-NKCC1, and three fields of view per plate were imaged. Internalization of Venus-exosome was visualized by live cell confocal microscopy. Note the number of green cells and orange cells establishing the internalization of the Venus-OSR1-containing exosomes. Bar, 10 μm.
Fig. 7.
Transfer of Cerulean-OSR1 from cell to cell in HEK293 cells. A: HEK293 cells were transfected with tdTomato-NKCC1 or Cerulean-OSR1, detached, mixed, and cocultured in new dishes. B: live microscopy photograph of HEK293 cells showing both red and green signals. Intense green cells (top right corner) were successfully transfected with Cerulean-OSR1, whereas cells that show paler green signal (labeled “1”) are recipient cells of exosomes generated by Cerulean-OSR1-transfected cells. Cells showing pale red signal (labeled “2”) are recipient cells of exosomes generated by tdTomato-NKCC1-transfected cells. Cells showing orange signal, including yellow signal at the membrane (arrows), are tdTomato-transfected cells that have received Cerulean-OSR1 exosomes. C and D: same photograph showing red and green signals, separately. Each transfection was plated on 3 MatTek dishes, and 3 fields of view per plate were imaged. The experiment was repeated once. Bar, 10 μm.
Fig. 8.
Transfer of Venus-SPAK from cell to cell in HEK-293 cells. A: HEK293 cells were transfected with tdTomato-NKCC1 or Venus-SPAK, detached, mixed, and cocultured in new dishes. B: live microscopy photograph of HEK293 cells showing both red and green signals. Intense green cells were successfully transfected with Venus-SPAK, whereas cells that show paler green signal (labeled “1”) are recipient cells of exosomes generated by Venus-SPAK-transfected cells. Cells showing pale red signal (labeled “2”) are recipient cells of exosomes generated by tdTomato-NKCC1-transfected cells. Cells showing orange/yellow signal are tdTomato-transfected cells that have received Venus-SPAK exosomes. C and D: same photograph showing red and green signals, separately. Arrows point to plasma membrane. Each transfection was plated on 3 MatTek dishes, and 3 fields of view per plate were imaged. The experiment was repeated once. Bar, 10 μm.
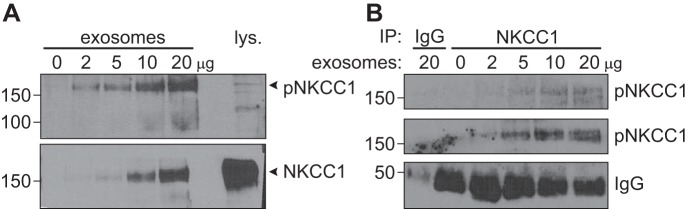
Finally, we sought to demonstrate that the kinases that are packaged in exosomes are functional. Using a phospho-specific antibody directed against the key residues in the N-terminal tail of NKCC1 that are targeted by SPAK/OSR1 kinases, we demonstrate that the kinases are able to phosphorylate the transporter. First, we show that the level of native NKCC1 phosphorylation is high in the exosome, compared with cotransporter isolated from cell lysate (Fig. 9A), indicating that exosomes contain functional kinases. Whether phosphorylation of NKCC1 occurs within the lumen of exosomes remains to be determined. Second, we show that exosome lysate is able to de novo phosphorylate immunoprecipitated NKCC1 from HEK293 cells. In this experiment, NKCC1 immobilized on beads was incubated with increasing amount of exosome lysate in the presence of Mg2+-ATP, washed to eliminate exosome material, denatured in sample buffer, subjected to SDS-PAGE, transferred, and exposed to phospho-specific or total NKCC1 antibody. We can see increasing NKCC1 phosphorylation with increasing amount of exosome material (Fig. 9B).
Fig. 9.
Kinases from exosomes are active and phosphorylate NKCC1. A: increasing amount of exosomes and a cell lysate control were separated by SDS-PAGE and analyzed by Western blotting for phospho-NKCC1 and total NKCC1 signals. NKCC1 packaged inside the exosomes is phosphorylated by OSR1/SPAK inside the exosomes. B: OSR1 and SPAK in exosomes phosphorylate cellular NKCC1. HEK293 exosome lysate was incubated with immunoprecipitated NKCC1 from HEK293 cell lysate, incubated at 37°C for 45 min, then subjected to Western blot analysis for phospho-NKCC1 and total NKCC1 signals.
DISCUSSION
In this study we tested the hypothesis that OSR1 and SPAK are secreted from HEK293 cells in exosomes and transferred from cell to cell. The kinases transported by these extracellular nanovesicles are active kinases and able to phosphorylate NKCC1, one of their specific targets.
Multiple proteomics studies have identified members of the WNK-OSR1/SPAK-N(K)CC pathway in exosomes from human saliva (14), urine (13, 45), as well as in mast cells and erythrocyte-conditioned media (2). Other data from proteomic studies are consolidated within ExoCarta, an online tool (23). As exosomes are found in the urine, they can serve as biomarkers for diseases. For example, pNCC in urinary exosomes can be possibly used as a biomarker for Gitelman's syndrome (21) and pseudohypoaldosteronism type II (PHAII) (19, 42), as the Na+-reabsorbing transporter is mutated in the first disorder, while hyperphosphorylated in the other. While exosomes in urine, saliva, and other fluids can provide useful tools for disease diagnosis, they are certainly not secreted for that purpose and neither likely, as originally postulated, to constitute a mechanism of elimination of unwanted proteins (17, 20, 31). Instead, consensus exists today to consider exosomes as a mechanism of cell-cell communication (4).
Our study confirmed that SPAK, OSR1, and NKCC1 are proteins packaged in exosomes. We show that both OSR1 and NKCC1 in HeLa cells colocalize with CD63, a well-recognized exosomal marker. While CD63 is also found in perinuclear endosomes (bright yellow signal around nucleus), it is found in large size bodies, i.e., multivesicular bodies, within the cytoplasm (Fig. 5). A multistep purification process of HEK293 cell-conditioned medium was used to isolate exosomes (Fig. 2). Isolated exosomes were further characterized by Western blots to confirm the expression of the exosomal marker CD63 and the absence of endoplasmic reticulum and Golgi markers. In addition, we showed by electron microscopy analysis that the isolated particles are consistent with their being exosomes as they are within the right size range (40–100 nm) and are surrounded by a membrane. Immunogold staining demonstrated that they contain both SPAK and OSR1 kinases. The membrane of the exosome protects the content from degradation by extracellular proteases.
Our study also confirms that exosomes are not only secreted by living cells, but also internalized or accumulated by adjacent cells, supporting the notion that they constitute communication vehicles between neighboring cells. The mechanism of entry of exosomes into cells is still controversial, with many different mechanisms having been described (for review, see Ref. 8). Our data cannot distinguish between the fusion of exosomal membrane with the plasma membrane of a cell leading to the release of exosomal content into the cytosol, versus phagocytosis/endocytosis of whole exosomes inside the cell. Indeed, the fact that no large particles are observed in the receiving cell might be related to the fact that exosomes, if internalized by cells through an endocytotic mechanism, might be too small in size to visualize by light microscopy, in opposition to multivesicular bodies (in donor cells) that contain a larger number of exosomes. In addition, the fact that NKCC1 is mostly observed in the membrane of receiving cells is consistent with exosomal membrane fusion to the plasma membrane, but could as well be due from insertion from the inside (i.e., recycling) after internalization of the intact exosomes. Note that we cannot exclude the possibility that some of the fluorescently labeled proteins found in receiving cells might come from mRNA transported by exosomes, especially in coculture experiments. However, live imaging of cells in Fig. 6 was done 1 h after application of exosomes, making it unlikely that translation was a significant contributor to the signal.
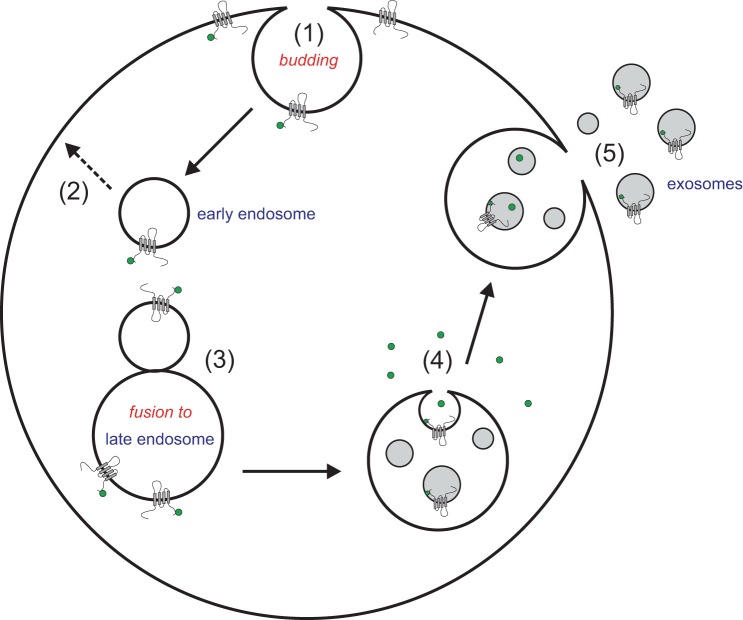
Interestingly, the fluorescently labeled kinases in receiving cells are also preferentially associated with the plasma membrane when NKCC1 is overexpressed (Figs. 7, B and D, and 8, B and D). This observation is consistent with the kinases binding to their transporter target, as we previously observed with native tissues such as choroid plexus where NKCC1 and SPAK signals are colocalized on the apical membrane or in salivary gland, where NKCC1 and SPAK signals are observed on the basolateral membrane (33). It has been argued that proteins found in exosomes are preferentially associated with higher-order oligomeric complexes that also exist in the plasma membrane (49) and these complexes possibly include their interacting proteins. This is consistent with the origin of the exosomes, which form from early endosomes budded from the plasma membrane (Fig. 10). Note that the process of exosome formation conserves the polarity of membrane receptors, channels, and transporters, with extracellular domains remaining on the outside of exosomes. It is therefore not surprising that SPAK and OSR1, the function of which requires binding to the N-terminal tail of NKCC1, would also be detected in exosomes.
Fig. 10.
Polarity of membrane proteins in exosomes is explained by exosome formation. Process starts from the budding of the plasma membrane into early endosomes (1), which in some cases can recycle back to the membrane (2). In other cases, the early endosomes fuse with late endosomes (3). Budding of the late endosome membrane creates multivesicular bodies (4). Fusion of these multivesicular bodies with the plasma membrane releases the exosomes to the extracellular space (5). SPAK/OSR1 kinases (pictured as small green dots) can be found in the cell, bound to plasma membrane proteins (transporter drawn at the membrane with a green dot), as well as in the cytosol. Note that cytosolic proteins can be trapped in exosomes merely by diffusion during the budding process.
The fact that transporters and kinases not only colocalize at the plasma membrane of cells, but are also found in exosomes raises the possibility of functionally active transporters in exosomes, either inside multivesicular bodies within cells, or as isolated particles in the extracellular environment. One aspect in favor of transport function is the observation in both proteomic studies and in our data (Fig. 9), that NKCC1 is phosphorylated in the exosomes. In fact, our data indicate that NKCC1 phosphorylation is very high, when compared with NKCC1 from whole cell lysate, suggesting the possibility of functional transporters in the exosomal membrane. To date, there are no data assigning any membrane transport function across the membrane of exosomes. Obviously, secondary active transport through Na-K-2Cl cotransporter would require that an ionic gradient is maintained across the exosomal membrane, which we speculate could be generated by expression of the Na+-K+-ATPase in these vesicles.
As mentioned earlier, multivesicular bodies and exosome production are increased in condition of cell stress, e.g., hypotonic swelling (34). This observation can be related to the significant increase in cell blebs that was observed many years ago with cells exposed to hypotonic media (27, 47). In fact, blebs can pinch off the cell surface and then be counted as extracellular microvesicles as well, although of much larger sizes than exosomes (in the μm range). To be detectable as intracellular punctae, vesicles need to be of a certain size, and multivesicular bodies are certainly big enough to be visualized by light microscopy (15). Thus, it is possible that the OSR1-containing punctae observed in the remaining DCT cells of SPAK knockout animals (15, 25) are multivesicular bodies that accumulate in the absence of SPAK-mediated NCC transport in these cells. The fate of these accumulated structures (punctae) and of possible exosomes released from these cells is unknown, but likely to affect cells downstream of this nephron segment.
GRANTS
This work was supported by NIH Grant DK093501 to E. Delpire. Confocal imaging and transmission electron microscopy imaging were performed in part through the use of the Vanderbilt University Medical Center Cell Imaging Shared Resource (supported by NIH Grants CA68485, DK20593, DK58404, DK59637, and EY08126).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
R.K. and E.D. conception and design of research; R.K. performed experiments; R.K. and E.D. analyzed data; R.K. and E.D. interpreted results of experiments; R.K. and E.D. prepared figures; R.K. and E.D. drafted manuscript; R.K. and E.D. edited and revised manuscript; R.K. and E.D. approved final version of manuscript.
REFERENCES
- 1.Alvarez-Leefmans FJ. Chloride transporters in presynaptic inhibition, pain and neurogenic inflammation. In: Physiology and Pathology of Chloride Transporter and Channels in the nervous System: From Molecules TO Diseases, edited by Alvarez-Leefmans FJ, Delpire E. London: Academic, 2009, p. 439–470. [Google Scholar]
- 2.Carayon K, Chaoui K, Ronzier E, Lazar I, Bertrand-Michel J, Roques V, Balor S, Terce F, Lopez A, Salomé L, Joly E. Proteolipidic composition of exosomes changes during reticulocyte maturation. J Biol Chem 286: 34426–34439, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collaco AM, Jakab RL, Hoekstra NE, Mitchell KA, Brooks A, Ameen NA. Regulated traffic of anion transporters in mammalian Brunner's glands: a role for water and fluid transport. Am J Physiol Gastrointest Liver Physiol 305: G258–G275, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 30: 255–289, 2014. [DOI] [PubMed] [Google Scholar]
- 5.Delpire E. The Mammalian family of Sterile20p-like protein kinases. Pflügers Arch 458: 953–967, 2009. [DOI] [PubMed] [Google Scholar]
- 6.Delpire E, Gagnon KB. Genome-wide analysis of SPAK/OSR1 binding motifs. Physiol Genomics 28: 223–231, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Denton J, Nehrke K, Yin X, Morrison R, Strange K. GCK-3, a newly identified Ste20 kinase, binds to and regulates the activity of a cell cycle-dependent ClC anion channel. J Gen Physiol 125: 113–125, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erdbrügger U, Le TH. Extracellular vesicles in renal diseases: more than novel biomarkers? J Am Soc Nephrol 27: 12–26, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans RL, Park K, turner RJ, Watson GE, Nguyen HV, Dennett MR, Hand AR, Flagella M, Shull GE, Melvin JE. Severe impairment of salivation in Na+/K+/2Cl− cotransporter (NKCC1)-deficient mice. J Biol Chem 275: 26720–26726, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Février B, Raposo G. Exosomes: endosomal-derived vesicles shipping extracellular messages. Curr Opin Cell Biol 16: 415–421, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Gagnon KB, England R, Delpire E. Volume sensitivity of cation-chloride cotransporters is modulated by the interaction of two kinases: Ste20-related proline-alanine-rich kinase and WNK4. Am J Physiol Cell Physiol 290: C134–C142, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Geng Y, Hoke A, Delpire E. The Ste20 kinases Ste20-related proline-alanine-rich kinase and oxidative-stress response 1 regulate NKCC1 function in sensory neurons. J Biol Chem 284: 14020–14028, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzales PA, Pisitkun T, Hoffert JD, Tchapyjnikov D, Star RA, Kleta R, Wang NS, Knepper MA. Large-scale proteomics and phosphoproteomics of urinary exosomes. J Am Soc Nephrol 20: 363–379, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez-Begne M, Lu B, Han X, Hagen FK, Hand AR, Melvin JE, Yates JR. Proteomic analysis of human parotid gland exosomes by multidimensional protein identification technology (MudPIT). J Proteome Res 8: 1304–1314, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grimm PR, Taneja TK, Liu J, Coleman R, Chen YY, Delpire E, Wade JB, Welling PA. SPAK isoforms and OSR1 regulate sodium-chloride co-transporters in a nephron-specific manner. J Biol Chem 287: 37673–37690, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grubb BR, Pace AJ, Lee E, Koller BH, Boucher RC. Alterations in airway ion transport in NKCC1-deficient mice. Am J Physiol Cell Physiol 281: C615–C623, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Harding C, Heuser J, Stahl P. Endocytosis and intracellular processing of transferrin and colloidal gold-transferrin in rat reticulocytes: demonstration of a pathway for receptor shedding. Eur J Cell Biol 35: 256–263, 1994. [PubMed] [Google Scholar]
- 18.Hong JH, Yang D, Shcheynikov N, Ohana E, Shin DM, Muallem S. Convergence of IRBIT, phosphatidylinositol (4,5) bisphosphate, and WNK/SPAK kinases in regulation of the Na+-HCO3− cotransporters family. Proc Natl Acad Sci USA 110: 4105–4110, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Isobe K, Mori T, Asano T, Kawaguchi H, Nonoyama S, Kumagai N, Kamada F, Morimoto T, Hayashi M, Sohara E, Rai T, Sasaki S, Uchida S. Development of enzyme-linked immunosorbent assays for urinary thiazide-sensitive Na-Cl cotransporter measurement. Am J Physiol Renal Physiol 305: F1374–F1381, 2013. [DOI] [PubMed] [Google Scholar]
- 20.Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem 262: 9412–9420, 1987. [PubMed] [Google Scholar]
- 21.Joo KW, Lee JW, Jang HR, Heo NJ, Jeon US, Oh YK, Lim CS, Na KY, Kim J, Cheong HI, Han JS. Reduced urinary excretion of thiazide-sensitive Na-Cl cotransporter in Gitelman syndrome: preliminary data. Am J Kidney Dis 50: 765–773, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan MR, Plotkin MD, Brown D, Hebert SC, Delpire E. Expression of the mouse Na-K-2Cl cotransporter, mBSC2, in the terminal inner medullary collecting duct, the glomerular and extraglomerular mesangium, and the glomerular afferent arteriole. J Clin Invest 98: 723–730, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keerthikumar S, Chisanga D, Ariyaratne D, Al Saffar H, Anand S, Zhao K, Samuel M, Pathan M, Jois M, Chilamkurti N, Gangoda L, Mathivanan S. ExoCarta: a web-based compendium of exosomal cargo. J Mol Biol 428: 688–692, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khatua AK, Taylor HE, Hildreth JE, Popik W. Exosomes packaging APOBEC3G confer human immunodeficiency virus resistance to recipient cells. J Virol 83: 512–521, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCormick JA, Mutig K, Nelson JH, Saritas T, Hoorn EJ, Yang CL, Rogers S, Curry J, Delpire E, Bachmann S, Ellison DH. A SPAK isoform switch modulates renal salt transport and blood pressure. Cell Metab 14: 352–364, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melo Z, de los Heros P, Cruz-Rangel S, Vázquez N, Bobadilla NA, Pasantes-Morales H, Alessi DR, Mercado A, Gamba G. N-terminal serine dephosphorylation is required for KCC3 cotransporter full activation by cell swelling. J Biol Chem 288: 31468–31476, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mills JW, Falsig Pedersen S, Walmod PS, Hoffmann EK. Effect of cytochalasins on F-actin and morphology of Ehrlich ascites tumor cells. Exp Cell Res 161: 209–219, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Moriguchi T, Urushiyama S, Hisamoto N, Iemura SI, Uchida S, Natsume T, Matsumoto K, Shibuya H. WNK1 regulates phosphorylation of cation-chloride-coupled cotransporters via the STE20-related kinases, SPAK and OSR1. J Biol Chem 280: 42685–42693, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Nejsum LN, Praetorius J, Nielsen S. NKCC1 and NHE1 are abundantly expressed in the basolateral plasma membrane of secretory coil cells in rat, mouse, and human sweat glands. Am J Physiol Cell Physiol 289: C333–C340, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Pacheco-Alvarez D, Cristóbal PS, Meade P, Moreno E, Vazquez N, Muñoz E, Díaz A, Juárez ME, Giménez I, Gamba G. The Na+:Cl− cotransporter is activated and phosphorylated at the amino-terminal domain upon intracellular chloride depletion. J Biol Chem 281: 28755–28763, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Pan BT, Teng K, Wu C, Adam M, Johnstone RM. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol 101: 924–948, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piechotta K, Garbarini NJ, England R, Delpire E. Characterization of the interaction of the stress kinase SPAK with the Na+-K+-2Cl− cotransporter in the nervous system: evidence for a scaffolding role of the kinase. J Biol Chem 278: 52848–52856, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Piechotta K, Lu J, Delpire E. Cation chloride cotransporters interact with the stress-related kinases Ste20-related proline-alanine-rich kinase (SPAK) and oxidative stress response 1 (OSR1). J Biol Chem 277: 50812–50819, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Pironti G, Strachan RT, Abraham D, Mon-Wei Yu S, Chen M, Chen W, Hanada K, Mao L, Watson LJ, Rockman HA. Circulating exosomes induced by cardiac pressure overload contain functional angiotensin II type 1 receptors. Circulation 131: 2120–2130, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci USA 101: 13368–13373, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richardson C, Alessi DR. The regulation of salt transport and blood pressure by the WNK-SPAK/OSR1 signalling pathway. J Cell Sci 121: 3293–3304, 2008. [DOI] [PubMed] [Google Scholar]
- 37.Rinehart J, Maksimova YD, Tanis JE, Stone KL, Hodson CA, Zhang J, Risinger M, Pan W, Wu D, Colangelo CM, Forbush B, Joiner CH, Gulcicek EE, Gallagher PG, Lifton RP. Sites of regulated phosphorylation that control K-Cl cotransporter activity. Cell 138: 525–536, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simpson RJ, Jensen SS, Lim JW. Proteomic profiling of exosomes: current perspectives. Proteomics 8: 4083–4099, 2008. [DOI] [PubMed] [Google Scholar]
- 39.Staubach S, Razawi H, Hanisch FG. Proteomics of MUC1-containing lipid rafts from plasma membranes and exosomes of human breast carcinoma cells MCF-7. Proteomics 9: 2820–2835, 2009. [DOI] [PubMed] [Google Scholar]
- 40.Théry C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol Ch. 3, Unit 3.22, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol 2: 569–579, 2002. [DOI] [PubMed] [Google Scholar]
- 42.van der Lubbe N, Jansen PM, Salih M, Fenton RA, van den Meiracker AH, Danser AH, Zietse R, Hoorn EJ. The phosphorylated sodium chloride cotransporter in urinary exosomes is superior to prostasin as a marker for aldosteronism. Hypertension 60: 741–748, 2012. [DOI] [PubMed] [Google Scholar]
- 43.Villa F, Goebel J, Rafiqi FH, Deak M, Thastrup J, Alessi DR, van Aalten DM. Structural insights into the recognition of substrates and activators by the OSR1 kinase. EMBO Rep 8: 839–845, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vitari AC, Deak M, Morrice NA, Alessi DR. The WNK1 and WNK4 protein kinases that are mutated in Gordon's hypertension syndrome, phosphorylate and active SPAK and OSR1 protein kinases. Biochem J 391: 17–24, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Z, Hill S, Luther JM, Hachey DL, Schey KL. Proteomic analysis of urine exosomes by multidimensional protein identification technology (MudPIT). Proteomics 12: 329–338, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Welton JL, Khanna S, Giles PJ, Brennan P, Brewis IA, Staffurth J, Mason MD, Clayton A. Proteomics analysis of bladder cancer exosomes. Mol Cell Proteomics 9: 1324–1338, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilkerson EH, DiBona DR, Schafer JA. Analysis of structural changes during hypotonic swelling in Ehrlich ascites tumor cells. Am J Physiol Cell Physiol 251: C104–C114, 1986. [DOI] [PubMed] [Google Scholar]
- 48.Yang D, Li Q, So I, Huang CL, Ando H, Mizutani A, Seki G, Mikoshiba K, Thomas PJ, Muallem S. IRBIT governs epithelial secretion in mice by antagonizing the WNK/SPAK kinase pathway. J Clin Invest 121: 956–965, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang JM, Gould SJ. The cis-acting signals that target proteins to exosomes and microvesicles. Biochem Soc Trans 41: 277–282, 2013. [DOI] [PubMed] [Google Scholar]
- 50.Yang SS, Hsu YJ, Chiga M, Rai T, Sasaki S, Uchida S, Lin SH. Mechanisms for hypercalciuria in pseudohypoaldosteronism type II-causing WNK4 knock-in mice. Endocrinology 151: 1829–1836, 2010. [DOI] [PubMed] [Google Scholar]
- 51.Yang SS, Lo YF, Wu CC, Lin SW, Yeh CJ, Chu P, Sytwu HK, Uchida S, Sasaki S, Lin SH. SPAK-knockout mice manifest Gitelman syndrome and impaired vasoconstriction. J Am Soc Nephrol 21: 1868–1877, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zöller M. Tetraspanins: push and pull in suppressing and promoting metastasis. Nat Rev Cancer 9: 40–55, 2009. [DOI] [PubMed] [Google Scholar]