Abstract
Classical brown adipocytes such as those found in interscapular brown adipose tissue (iBAT) represent energy-burning cells, which have been postulated to play a pivotal role in energy metabolism. Brown adipocytes can also be found in white adipose tissue (WAT) depots [e.g., inguinal WAT (iWAT)] following adrenergic stimulation, and they have been referred to as “beige” adipocytes. Whether the presence of these adipocytes, which gives iWAT a beige appearance, can confer a white depot with some thermogenic activity remains to be seen. In consequence, we designed the present study to investigate the metabolic activity of iBAT, iWAT, and epididymal white depots in mice. Mice were either 1) kept at thermoneutrality (30°C), 2) kept at 30°C and treated daily for 14 days with an adrenergic agonist [CL-316,243 (CL)], or 3) housed at 10°C for 14 days. Metabolic activity was assessed using positron emission tomography imaging with fluoro-[18F]deoxyglucose (glucose uptake), fluoro-[18F]thiaheptadecanoic acid (fatty acid uptake), and [11C]acetate (oxidative activity). In each group, substrate uptakes and oxidative activity were measured in anesthetized mice in response to acute CL. Our results revealed iBAT as a major site of metabolic activity, which exhibited enhanced glucose and nonesterified fatty acid uptakes and oxidative activity in response to chronic cold and CL. On the other hand, beige adipose tissue failed to exhibit appreciable increase in oxidative activity in response to chronic cold and CL. Altogether, our results suggest that the contribution of beige fat to acute-CL-induced metabolic activity is low compared with that of iBAT, even after sustained adrenergic stimulation.
Keywords: beige adipose tissue, brown adipose tissue, energy metabolism, oxidative metabolism, positron emission tomography
in recent years, brown adipocyte thermogenesis has gained renewed attention. Brown adipocytes are thermogenic cells predominantly found in classical brown adipose tissue (BAT) depots such as interscapular BAT (iBAT). They are responsible for cold adaptation and survival during cold challenge (6). The thermogenic potential of classical BAT is indeed impressive; in cold-adapted rats, it can account for >60% of the increase in the metabolic rate induced by norepinephrine and is undoubtedly a prominent site of nonshivering thermogenesis (11). The brown adipocyte owes its thermogenic potential to the presence of uncoupling protein 1 (UCP1), which disconnects mitochondrial respiration from ATP synthesis (31, 32). Being uniquely found in brown adipocytes, UCP1 constitutes the ultimate marker of these cells (30).
Brown adipocytes can also develop in white adipose tissue (WAT) depots [e.g., inguinal WAT (iWAT)] under sustained adrenergic stimulation led to, for instance, by cold exposure (24–26, 54) or β3-adrenergic agonism (9, 17, 35, 53). Development of brown adipocytes in WAT (13, 47) led to the browning of white fat, giving it a “beige” appearance. Brown adipocytes in WAT have been designated as brite (brown-in-white) as well as recruitable and beige (16, 18, 23, 27, 37, 52). Brown adipocytes in WAT express UCP1 and are histologically similar to BAT adipocytes. However, they do not have, as the latter, a myogenic origin (1, 15, 37, 42, 48). Their mitochondria exhibit enhanced ex vivo oxygen consumption following chronic adrenergic stimulation (16, 43).
The browning of WAT has generated an unrelenting interest in recent years, in part due to the potential role that this process may have in elevating energy expenditure. However, there is still ambiguity regarding the metabolic activity of beige fat, and assuredly uncertainty regarding the contribution of such fat to energy homeostasis. In that respect, we conducted the present study, whose goal was to compare the metabolic activity of BAT (iBAT), “beige” (iWAT), and WAT [epididymal WAT (eWAT)] and in which we subjected mice to treatments aimed at inducing the development of brown adipocytes in WAT. Mice were chronically exposed to cold (10°C) or treated with the β3-agonist CL-316,243 (CL) for a period of two weeks. We investigated acute CL-induced metabolic activity in iBAT, iWAT, and eWAT (white fat exhibiting resistance to beiging) (51), with a triad of positron emission tomography (PET) tracers, namely fluoro-[18F]deoxyglucose (18FDG, glucose uptake), 14(R,S)-fluoro-[18F]-6-thiaheptadecanoic acid (18FTHA, nonesterified fatty acid uptake), and [11C]acetate (oxidative activity).
MATERIALS AND METHODS
Animals and treatments.
Male C57Bl/6 mice (10–12 wk old) weighing 20–25 g (Charles River, Quebec, Canada) were divided into three groups, which were 1) kept at thermoneutrality (30°C), 2) kept at 30°C and chronically (14 days) treated each morning at 0600 with CL (1 mg/kg, 10 ml/kg ip, no. C5976; Sigma), or 3) housed at 10°C, for 14 days. All animals were kept under a 12:12-h light-dark cycle (lights on at 0600) with ad libitum access to pelleted chow (Rodent Laboratory Chow 5001; Purina, St. Louis, MO) and tap water. Distinct groups of animals were used for the PET procedures and immunohistochemistry/biochemical measurements described below. All experimental protocols were approved by the Animal Ethics Committee of the Université de Sherbrooke and in accordance with the guidelines of the Canadian Council on Animal Care.
Body weight and food intake.
Body weight and food intake were measured weekly. Feed efficiency was determined as the ratio of total weight gained (mg) to total food consumed (g).
Blood and tissue sampling.
At the end of the 14 days of adaptation and before a 6-h fasting period (0600–1200), mice were killed under isoflurane while blood was sampled in EDTA-coated tubes through intracardiac puncture 1 h following the CL injection. Plasma glucose, nonesterified fatty acid (NEFA), and triglyceride levels were measured as described previously (19), and insulin was measured by an ultrasensitive mouse insulin ELISA kit (no. 90080; Crystal Chem, Downers Grove, IL). Adipose tissue depots [namely iBAT, iWAT (middle part of the dorsal region), and eWAT] were carefully excised, dissected on ice, and weighed. Collected adipose tissue samples were either immediately frozen in liquid nitrogen for RNA and DNA extractions or fixed in 4% (wt/vol) paraformaldehyde for immunohistochemical analyses.
Gene expression analysis.
Total mRNA was isolated from iBAT, iWAT, and eWAT using QIAzol and the RNeasy Lipid Tissue kit (Qiagen, Mississauga, ON, Canada). The RNA concentrations were estimated from absorbance at 260 nm. cDNA synthesis was performed using the expanded reverse transcriptase (50 U/μl; Roche, Laval, QC, Canada). mRNA extraction and cDNA synthesis were performed following the manufacturer's instructions. cDNA was diluted in DNase-free water (1:20) before the quantification by real-time PCR. Relative quantification of gene expression was performed on diluted cDNA (1:20) using mRNA; transcript levels were measured in duplicate samples using a CFX384 touch real-time PCR (Bio-Rad, Mississauga, ON, Canada). Chemical detection of the PCR products was achieved with SYBR Green (172–5271; Bio-Rad) on the CFX384 touch real-time PCR system (Bio-Rad, Mississauga, ON, Canada). Specificity of amplification was ensured using melt curve analyses followed by agarose gel electrophoresis of the amplified products, as described previously (19). Fold differences in target mRNA expression were calculated using the 2-delta cycle threshold method and β-microglobulin as the housekeeping gene.
Immunohistochemistry.
Mounted sections of adipose tissue samples were deparaffinized using 0.1 M sodium citrate (pH 6.0), followed by inhibition of endogenous peroxidases using 3% (vol/vol) H2O2 in methanol for 20 min. Slides were washed four times in 50 mM potassium PBS (KPBS) (pH 7.2) for 15 min each, followed by incubation with a sheep antihamster UCP1 antibody (no. ab10983; Abcam, Cambridge, MA) at a dilution of 1:100 in 1% (wt/vol) BSA, 0.4% (vol/vol) Triton X-100, and 1% (vol/vol) rabbit serum in KPBS overnight at 4°C in a humid chamber. The next day, slides were washed two times with KPBS for 15 min, followed by incubation with a biotinylated rabbit antisheep antibody (Vector Laboratories, Burlingame, CA) at a dilution of 1:500 in 1% BSA, 0.4% Triton, and 1% rabbit serum in KPBS for 2 h at room temperature. After being washed, the samples were incubated with ABC complex (Vector Laboratories) and stained using diaminobenzidine. All images were taken on an Olympus BX60 microscope (Tokyo, Japan) at a magnification of ×40.
Small animal PET-computed tomography protocol.
All in vivo PET/computed tomography (CT) experiments were initiated immediately after the insertion of a cannula in the tail vein for injections of PET tracers. All imaging experiments were performed on the Avalanche photodiode-based small animal PET scanner (LabPET/Triumph; Gamma Medica, Northridge, CA) of the Sherbrooke Molecular Imaging Center, having a 7.5-cm axial field-of-view. The animals were anesthetized with isoflurane (1.75%) delivered through a nose cone and were placed in the prone position on the scanner bed with the heart centered within the field-of-view of the scanner to include the interscapular region and also the inguinal fat. Just before the injection of the first PET tracer (<1 min), an intravenous injection of CL (2 mg/kg, no. C5976; Sigma) was performed. Acute CL allows for appreciating the effects of chronic cold and CL on both substrate uptakes and oxidative activity, which are otherwise minimal under anesthesia, especially in mice kept at thermoneutrality. Boluses of each radiopharmaceutical compound (10 MBq, in 0.2 ml of 0.9% NaCl) were injected via the caudal vein over 30 s after starting PET data acquisition ([11C]acetate) and over 60 s for 18FDG and 18FTHA. In one set of experiments, a 20-min dynamic data acquisition with [11C]acetate was done to determine tissue blood flow and oxidative metabolism, followed 10 min later by a 30-min dynamic data acquisition with either 18FDG or 18FTHA to also determine glucose or NEFA utilization, respectively, as previously described (29). List-mode dynamic data acquisition allowed for flexible time framing of the data for kinetic modeling of all tracers. Low-dose CT scan imaging was performed using a Gamma Medica Triumph X-O small-animal CT scanner composed of a 40 W X-ray tube with a 75-μm focal spot diameter and a 2,240 × 2,368 CsI flat panel X-ray detector. The detector pixel size was 50 μm, and a 2 × 2 pixel binning scheme was used. Scans were performed at 60 kVp and 230 μA using 512 projections in fly mode to reduce exposure. Blood samples were taken at the end of experiments by heart punctures.
Imaging data analysis.
For [11C]acetate images, dynamic series of 28 frames (1 × 30, 12 × 10, 8 × 30, 6 × 90, and 1 × 300 s) were sorted out, whereas 30 frames (1 × 30, 12 × 10, 8 × 30, 6 × 90, and 3 × 300 s) were used for 18FDG and 18FTHA imaging, and three-dimensional images were reconstructed using 15 iterations of a maximum-likelihood expectation-maximization algorithm incorporating physical description of the detector response function. Regions of interest (ROIs) were drawn on short-axis images and confirmed with the μCT scan (19). Time-activity curves were quantified in matching ROIs of [11C]acetate with either 18FDG or 18FTHA. Input curves were extracted by means of a ROI drawn on the left ventricular cavity blood pool in summed last-frame images to seek better contrast. The sizes of these almost-circular ROIs were compared with images of eight cylinders of different diameters from which a recovery factor was extracted and applied to the ROIs for partial volume correction (29). For [11C]acetate, we used a three-compartment kinetic model that estimates the generation of CO2 from the tricarboxylic acid cycle using the k2 constant (50) and the tissue blood flow through the K1 constant (12, 49). The dynamic glucose and NEFA uptake was determined using the Patlak graphical analysis (36). Whole tissue glucose and NEFA uptake was corrected for the total mass of adipose tissue determined at the end of PET experiments.
Measurement of mitochondrial DNA content.
Tissues for assessing mitochondrial DNA (mtDNA) content were prepared as described previously (10, 41). Briefly, total DNA was isolated by proteinase K digestion followed by phenol/chloroform extraction and ethanol precipitation. Ten (10) nanograms of total DNA were then used to amplify mtDNA-encoded NADH dehydrogenase I (ND1) and nuclear DNA-encoded β-actin using real-time PCR in a 10-μl reaction mixture. mtDNA content was calculated from the ratio of ND1 to genomic β-actin quantity.
Statistical analyses.
Results are expressed as means ± SE. Comparisons were done on normally distributed data using ANOVA followed by Bonferroni post hoc tests to assess the differences between the various treatments with Graph Pad Prism Software version 6.0h for Mac (San Diego, CA). Differences of P < 0.05 were considered statistically significant.
RESULTS
After 14 days, cold-exposed (10°C) mice tended to exhibit lower weight than mice kept at thermoneutrality (30°C) or kept at 30°C and chronically injected with CL (Table 1). The trend for lower weight gain was accompanied by a significant increase in total food intake and by a tendency for a decrease in feed efficiency (Table 1). This cold-induced decrease in food/energetic efficiency supports previous observations (6, 11, 19).
Table 1.
Characteristics of mice following 14-day adaptation
| 30°C | 30°C-Chronic CL | 10°C | |
|---|---|---|---|
| Weight gain, g | 0.54 ± 0.24 | 0.52 ± 0.19 | 0.00 ± 0.28 |
| Total food intake, g | 49 ± 2 | 45 ± 2 | 98 ± 3***### |
| Energetic efficiency, mg weight gain/g food intake | 10 ± 5 | 11 ± 4 | −1 ± 3 |
| iBAT weight, mg | 66.5 ± 2.4 | 69.8 ± 2.6 | 92.2 ± 4.0***### |
| iWAT weight, mg | 162.9 ± 14.5 | 153.1 ± 13.1 | 70.8 ± 8.7***### |
| eWAT weight, mg | 389.6 ± 36.2 | 141.7 ± 14.2*** | 219.7 ± 18.2***## |
Data are expressed as means ± SE. CL, CL-316,243; iBAT, inguinal brown adipose tissue; iWAT, inguinal white adipose tissue; eWAT, epididymal white adipose tissue.
P < 0.001 vs. 30°C and
P < 0.01 and
P < 0.001 vs. 30°C-chronic CL, assessed by post hoc Bonferroni test following ANOVA.
Chronic adrenergic stimulation effects on adipose tissues.
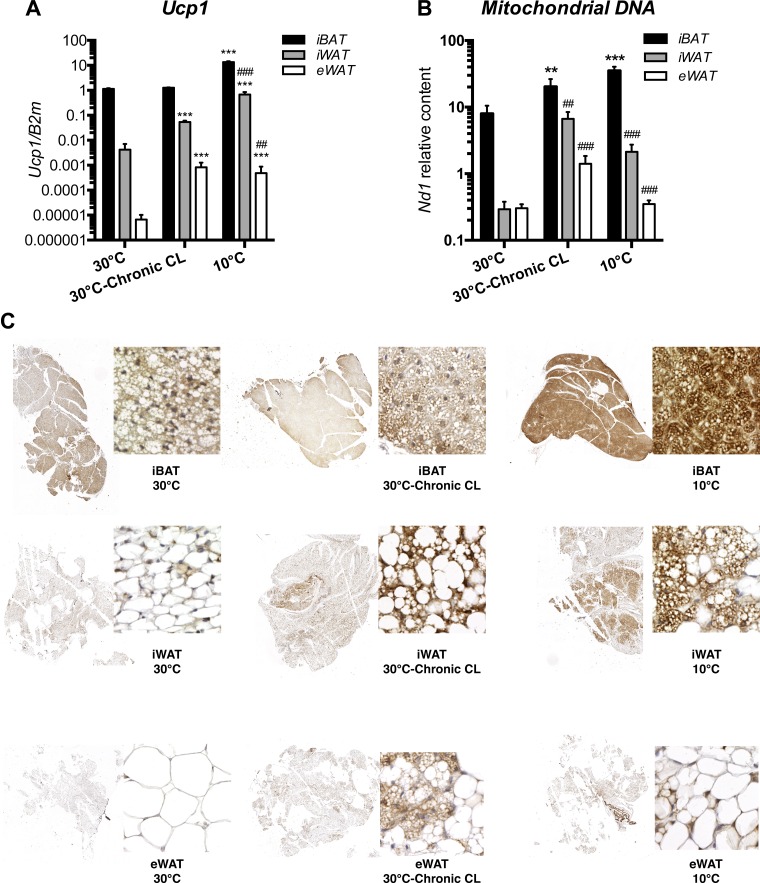
As shown in Fig. 1A, cold exposure (10°C) induced a 12-fold increase (over 30°C) in Ucp1 expression in iBAT, whereas chronic CL (30°C-chronic CL) had no effect. We also observed significant increases (over 30°C) in Ucp1 expression in iWAT and eWAT following chronic CL (13- and 124-fold) and chronic cold (168- and 72-fold).
Fig. 1.
Chronic adrenergic stimulation promotes the formation of multilocular and uncoupling protein 1 (UCP1)-expressing cells. Black bars represent interscapular brown adipose tissue (iBAT), gray bars represent interscapular white adipose tissue (iWAT), and open bars represent epididymal white adipose tissue (eWAT) (n = 6 mice for each experimental condition). A: Ucp1 gene expression. B: ratio of mitochondrial DNA to nuclear DNA was determined by quantitative PCR of nuclear (Actin) and mitochondrial (Nd1) genes. C: UCP1-immunostained brown adipose tissue (BAT), iWAT, and eWAT. Representative sections from indicated tissues and conditions are shown. Data are expressed as means ± SE. ***P < 0.001 vs. 30°C and ##P < 0.01 and ###P < 0.001 vs. iBAT, assessed by post hoc Bonferroni test following 2-way ANOVA.
As shown in Fig. 1C, UCP1-positive multilocular cells were indisputably detected in iWAT, following both chronic CL (30°C-chronic CL) and cold (10°C), with cold being the strongest inducer. As shown in Fig. 1C, brown adipocytes were heterogeneously grouped in multiple clusters across iWAT. At 10°C, multilocular UCP1-immunostained adipocytes of iWAT appeared morphologically similar to the brown adipocytes in iBAT at 30°C. However, following chronic CL and cold, brown adipocytes in iBAT looked more lipid depleted and more densely immunostained for UCP1 than brown adipocytes in iWAT. In eWAT, we also observed sparse UCP1-positive multilocular cells following chronic CL and cold.
As shown in Fig. 1B, mtDNA content was significantly increased in iBAT after chronic CL (2.5-fold, P < 0.01) and even more after chronic cold exposure (4.4-fold, P < 0.001). In iWAT, chronic CL increased mtDNA levels by 23-fold, and chronic cold exposure increased these levels by 7.3-fold. However, the ratio of mtDNA to nuclear DNA in iWAT following chronic CL was lower than that of iBAT at 30°C (6.06 vs. 7.98). In eWAT, this ratio was even lower than iWAT both after chronic CL (1.41) and chronic cold (0.35).
Glucose uptake in response to acute CL following chronic CL at 30°C and cold.
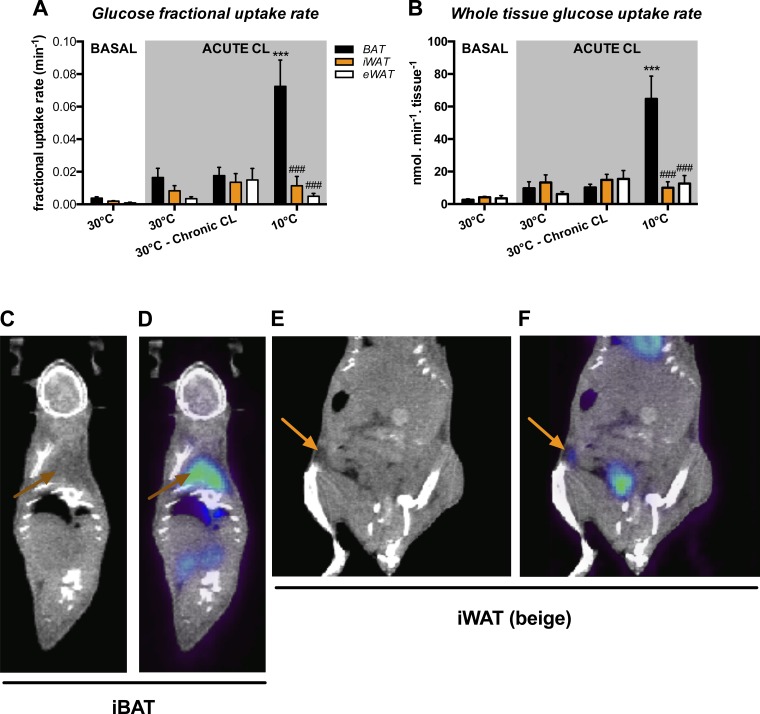
Figure 2 shows the effects of chronic CL and cold on glucose uptake in iBAT, iWAT, and eWAT. Table 2 presents the blood glucose levels, which were similar under each condition (average value around 9.0 mmol/l). Figure 2A presents the glucose fractional uptake rate, whereas Fig. 2B shows the rate of glucose uptake by the whole tissue. We first measured basal glucose uptake at 30°C without any stimulation. As shown in Fig. 2, A and B, there was no statistical difference in uptake in either iBAT or WAT depots. However, uptake was statistically different in iBAT and iWAT when animals were kept at 30°C and acutely injected with CL. Although there was no difference between the 30°C and 30°C-chronic CL groups in their glucose uptake, chronic cold exposure significantly increased CL-induced glucose uptake in iBAT. This increase was accounted for by elevations in both the ability of iBAT to extract glucose from blood (fractional uptake rate, 4-fold increase) and its mass (1.4-fold). The iWAT and eWAT glucose uptakes were much lower than that of iBAT after cold. Figure 2, C–F, shows representative examples of 18FDG uptake in iBAT (Fig. 2D) and iWAT in mice adapted to 10°C (Fig. 2F).
Fig. 2.
Glucose metabolism in various adipose depots. Black bars represent iBAT, orange bars represent iWAT, and open bars represent eWAT [n = 5 mice for 30°C at basal state, n = 4 mice for 30°C and 30°C-chronic CL-316,243 (CL), and n = 5 mice for 10°C following acute CL]. A: fractional glucose uptake determined using the Patlak graphic approach following a 30-min dynamic scan. B: total dynamic glucose uptake corrected for tissue weight. C: representative coronal computed tomography (CT) images. Brown arrows show iBAT region. D: representative positron emission tomography (PET)-CT coregistration following chronic cold exposure. Brown arrows show positive fluoro-[18F]deoxyglucose (18FDG) uptake in iBAT. E: representative coronal CT images. Orange arrows show iWAT region. F: representative PET-CT coregistration following chronic cold exposure. Orange arrows show positive 18FDG uptake in iWAT. Data are expressed as means ± SE. ***P < 0.001 vs. 30°C + acute CL and ###P < 0.001 vs. iBAT, assessed by post hoc Bonferroni test following 2-way ANOVA.
Table 2.
Plasma profile of mice following acute CL injection
| 30°C | 30°C-Chronic CL | 10°C | |
|---|---|---|---|
| Glucose, mmol/l | 8.58 ± 0.46 | 8.89 ± 0.80 | 9.59 ± 0.52 |
| Insulin, pmol/l | 3,812 ± 61 | 20 ± 9*** | 27 ± 8*** |
| NEFA, μmol/l | 1,448 ± 114 | 298 ± 26*** | 231 ± 32*** |
| TG, μmol/l | 697 ± 35 | 397 ± 78** | 332 ± 54*** |
Data are expressed as means ± SE. NEFA, nonesterified fatty acid; TG, triglyceride.
P < 0.01 and
P < 0.001 vs. 30°C, assessed by post hoc Bonferroni test following ANOVA.
NEFA uptake in response to acute CL following chronic CL at 30°C and cold.
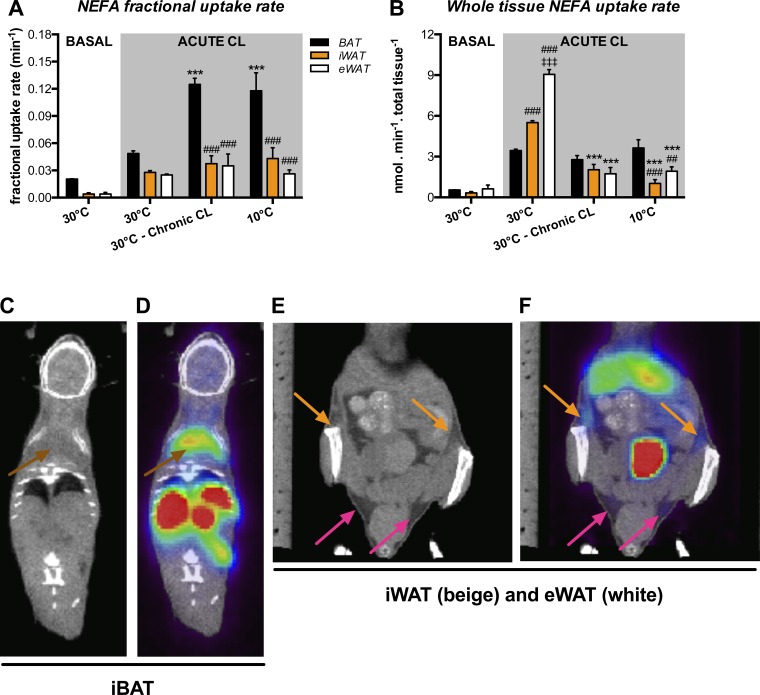
Figure 3 shows the effects of chronic CL and cold exposure on NEFA uptake in the adipose depots. As shown in Table 2, we observed that NEFA levels were lower in chronic CL and cold mice than in animals kept at 30°C. At baseline, NEFA fractional uptake was larger in iBAT than in iWAT and eWAT (Fig. 3A). However, the net uptake contribution was similar in all depots (Fig. 3B). Similar to glucose, acute injection of CL following 30°C adaptation was associated with a significant increase in NEFA uptake. Both chronic CL and cold were associated with an elevation (2.5-fold above thermoneutrality) of NEFA fractional uptake rate in iBAT (Fig. 3A). However, NEFA fractional uptake rate in iWAT and eWAT was not significantly increased (above 30°C) following either chronic CL or cold, despite being significantly lower than that of iBAT (Fig. 3A). Nonetheless, whole iBAT NEFA uptake rate was not significantly increased following chronic CL and cold (Fig. 3B). The absence of effects in chronic CL and cold mice on whole iBAT NEFA uptake rate was likely accounted for by the reduced levels (below acute CL at 30°C) of circulating NEFA (Table 2). NEFA uptake rate in a tissue in part depends on the circulating NEFA levels. In iWAT (1.6-fold) and eWAT (2.6-fold), acute CL at 30°C induced an increase in whole NEFA uptake rate compared with iBAT. The responses for chronic CL and cold were, however, different; iWAT and eWAT exhibited whole NEFA uptake rate comparable to and lower than that of iBAT after chronic CL and cold, respectively. Similar to iBAT, the absence of effects in chronic CL and cold mice on whole iWAT and eWAT NEFA uptake rate was dependent on the reduced levels (below acute CL at 30°C) of circulating NEFA, as well as on the reduction of whole tissue mass observed in both chronic CL and cold-exposed mice (Table 1). Figure 3, C–F, shows representative examples of 18FTHA uptakes in iBAT (Fig. 3D) and iWAT or eWAT in mice adapted to cold (Fig. 3F).
Fig. 3.
Nonesterified fatty acid (NEFA) metabolism in various adipose depots. Black bars represent iBAT, orange bars represent iWAT, and open bars represent eWAT (n = 5 mice for 30°C at basal state, n = 4 mice for 30°C and 30°C-chronic CL, and n = 6 mice for 10°C following acute CL). A: fractional NEFA uptake determined using the Patlak graphic approach following a 30-min dynamic scan. B: total dynamic NEFA uptake corrected for tissue weight. C: representative coronal CT images. Brown arrows show iBAT region. D: representative PET-CT coregistration following chronic cold exposure. Brown arrows show positive 14(R,S)-fluoro-[18F]-6-thiaheptadecanoic acid (18FTHA) uptake in iBAT. E: representative coronal CT images. Orange arrows show iWAT region, and pink arrows show eWAT region. F: representative PET-CT coregistration following chronic cold exposure. Orange arrows show positive 18FTHA uptake in iWAT, and pink arrows show positive 18FTHA uptake in eWAT. Data are expressed as means ± SE. ***P < 0.001 vs. 30°C + acute CL, ##P < 0.01 and ### P < 0.001 vs. iBAT, and ‡P < 0.001 vs. iWAT assessed by post hoc Bonferroni test following 2-way ANOVA.
Oxidative activity in response to acute CL following chronic CL at 30°C and cold.
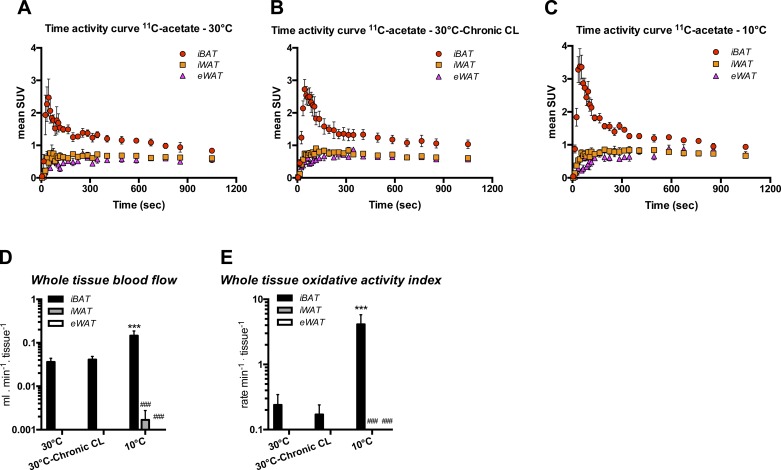
[11C]acetate was used to assess the oxidative activity of each adipose depot. The time-activity curves in Fig. 4, A–C, revealed that only iBAT exhibited significant uptake of [11C]acetate and disappearance rate of [11C]CO2 in response to acute CL. The blood flow response in iBAT was higher in cold mice than in animals kept at 30°C (Fig. 4D). As shown in Fig. 4E, chronic CL did not affect oxidative activity in iBAT, which contrasted with the 17.2-fold increase (above 30°C) seen following chronic cold. Importantly, we were not able to detect any CL-induced oxidative activity in iWAT (beige) and eWAT (white) following chronic CL and cold.
Fig. 4.
Effects of chronic adrenergic stimulation on blood flow and total oxidative activity index of various adipose depots. Mean standard uptake value (SUV) time-activity curves for 30°C mice (A), 30°C-chronic CL mice (B), and 10°C mice (C). Red dots show iBAT, orange squares show iWAT, and pink triangles show eWAT. Black bars represent iBAT, gray bars represent iWAT, and open bars represent eWAT (n = 4 mice for 30°C, n = 5 mice for 30°C-chronic CL, and n = 6 mice for 10°C). Total tissue blood flow (D) and total oxidative activity index (E) were calculated based on a 3-compartment kinetic model following a 20-min dynamic scan and corrected for respective tissue weight. Data are expressed as means ± SE. ***P < 0.001 vs. 30°C and ###P < 0.001 vs. iBAT, assessed by post hoc Bonferroni test following 2-way ANOVA.
DISCUSSION
We have investigated the metabolic activity of three adipose depots using 18FDG (glucose uptake), 18FTHA (NEFA uptake), and [11C]acetate (oxidative activity). The use of this triad of PET tracers has proven to be invaluable to assess brown fat metabolism in laboratory rodents (19) and humans (3, 4, 33). Specifically, our study aimed at comparing the metabolic activity of iBAT, the most investigated classical BAT depot, with that of iWAT and eWAT, which are known to, respectively, undergo or resist browning following adrenergic stimulation (cold exposure or β-adrenergic agonism) (51). We used groups of mice that were kept at either 30°C, 30°C treated chronic CL, or 10°C for 14 days. The metabolic activity was measured acutely after an injection of CL. Our results confirm the ability of iBAT to strongly respond to the chronic adrenergic treatments (CL and cold). Furthermore, our observations emphasize the limited ability of iWAT and eWAT to significantly enhance their metabolic activity in response to chronic CL and cold.
Classical BAT is a thermogenic tissue (6) that strongly responds to chronic adrenergic stimulation by enhancing its thermogenic capacity and activity. In the present study, chronic cold expectedly boosted the stimulating effect of acute CL on the iBAT oxidative activity (measured with [11C]acetate) and iBAT glucose uptake (measured with 18FDG). Cold constitutes a potent stimulator of classical BAT (19) via enhancing BAT sympathetic activity (6, 7, 20). In this study, chronic CL at 30°C was less efficient than cold in enhancing the metabolic activity of iBAT. It did not indeed enrich acute CL-induced oxidative activity in iBAT. It is noteworthy that acute CL per se resulted in an increased oxidative activity of iBAT following the three chronic conditions (30°C, chronic CL at 30°C, chronic 10°C). Chronic CL at 30°C nonetheless heightened iBAT NEFA fractional uptake rate following acute CL and increased (above 30°C) iBAT mitochondrial DNA content. It is worth insisting on the fact that chronic CL mice were housed at thermoneutrality (30°C), a condition that per se reduces the thermogenic need and hence likely might have potentially opposed stimulating effects of chronic CL in inducing the thermogenic capacity/activity of iBAT, which is further supported by the lack of iBAT mass expansion (Table 1).
One important aspect of the present study was the investigation of the metabolic activity of iWAT after its transformation into beige fat following chronic adrenergic stimulation. It has been known for years that cold exposure (24–26, 54) and β3-adrenergic agonism (9, 17, 35, 53) both lead to the browning of iWAT. Our results confirm the ability to iWAT to undergo browning after chronic CL and cold and to exhibit increased mtDNA content. However, our results do not demonstrate that the browning of iWAT was paralleled by a major increase in its metabolic activity as assessed with a triad of PET tracers that have been proven to readily detect any metabolic changes in BAT (3, 4, 19, 33) as well as in other metabolically active tissues such as heart or liver (2, 8, 21, 29, 46). The oxidative activity ([11C]acetate) of iWAT following chronic CL and cold was not only trivial compared with that of iBAT, but it was also not higher than that of eWAT, which resisted browning. This finding was somewhat unexpected considering the simultaneous observations of increased oxidative capacity (mitochondria and multilocular UCP1-positive cells) of iWAT following chronic adrenergic stimulation. At the same time, our findings do not really contradict the existing literature, since there is not much evidence that beige adipose tissue can markedly contribute to energy metabolism when activated, despite the evidence that beige fat mitochondria exhibit enhanced ex vivo oxygen consumption following chronic adrenergic stimulation (43). There is also evidence that its thermogenic potential is much less than that of iBAT (16, 30). Recently, Park et al. (35) reported that browning of iWAT following chronic CL treatment (10 days) was associated with an increase in 18FDG fractional uptake in response to acute CL, a finding that tends to be corroborated by the 18FDG uptake results of the present study. However, one has to consider that 18FDG uptake, in contrast to [11C]acetate, does not represent a direct marker of tissue oxidative/thermogenic activity. Moreover, 18FDG uptake following acute CL can be modulated by insulin, whose levels significantly raised following CL, and served for de novo lipogenesis rather than for oxidative activity (22). It is noteworthy that acute CL led to an increase in NEFA uptake in iWAT and eWAT compared with iBAT in mice kept at 30°C. This was likely due to the increase in insulin levels seen as a result of acute CL injection.
The reasons as to why our PET/CT imaging protocol with [11C]acetate did not reveal any enhanced activity in beige adipose certainly deserve attention. One can argue that the PET/CT imaging protocol is not sensitive enough to detect oxidative activity in every circumstance. However, this argument lacks strength, since PET/CT imaging with [11C]acetate has proven to be a very reliable tool for detecting metabolic activity changes in the heart (2, 29), liver (8, 46), skeletal muscle (21), cancer cells (28, 34), and, more recently, in brown fat (3, 4, 19, 33), even in humans, where the density of brown fat adipocytes appears not higher than that of beige fat in laboratory mice (55). One can also argue that either the mouse strain that we used was not a good responder to cold or the cold stimulus that we used did not sufficiently enhance iWAT thermogenic capacity. This reason cannot be excluded, since there are mouse strains that respond more to cold than C57Bl/6 mice (43), and there could be longer and stronger cold stimuli than the one that we used. However, it is clear from our data that we induced a noticeable “beiging” of iWAT and yet no detectable enhanced oxidative activity (with [11C]acetate), even in response to an acute CL injection. It is noteworthy that the amount of UCP1 (protein) following chronic adrenergic activation remains much lower in iWAT than in iBAT (16, 30), whatever the model or species used (43). Further studies need to be conducted to elucidate the beige fat incapacity to demonstrate oxidative activity, and one cannot even exclude at this point the possibility that there could be metabolic particularities of iWAT preventing brown fat cells from increasing their oxidative activity under stimulation.
The inability of beige iWAT to show enhanced 11C disappearance rate may question the physiological relevance of this depot in contributing to enhanced oxidative metabolism and hence in enhanced energy expenditure. Several investigators have reported strong correlations between the development of beige fat and enhanced energy expenditure with resistance to diet-induced obesity, at least in animal models (5, 14, 38–40, 44, 14a). However, the causality link between beige fat and enhanced energy expenditure remains to be firmly established. It is noteworthy that every beige fat inducer also stimulates classical iBAT, whose oxidative activity exceeds by far that of beige fat, as clearly and further demonstrated here. Finally, one cannot deny a potential indirect role of beige fat in influencing metabolic activity of classical BAT or other metabolic tissues such liver, skeletal muscle, or heart (45) to improve the overall metabolic profile.
In conclusion, our results demonstrate the ability of iBAT to increase its metabolic activity following chronic adrenergic stimulation induced by chronic CL or cold. They also demonstrate the limited ability of beige fat to show a meaningful enhanced substrate uptake and oxidative activity following chronic adrenergic stimulation.
GRANTS
This work was supported by a grant from the Natural Sciences and Engineering Research Council of Canada (2014-06721).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.M.L. and D.R. conception and design of research; S.M.L. and A.C. performed experiments; S.M.L. analyzed data; S.M.L., K.C., M.L., R.L., and D.R. interpreted results of experiments; S.M.L. prepared figures; S.M.L. drafted manuscript; S.M.L., A.C., K.C., M.L., R.L., and D.R. edited and revised manuscript; S.M.L., A.C., K.C., M.L., R.L., and D.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Jean-François Beaudoin and Maxime Paillé for helpful PET scan assistance and Serge Phoenix and Sébastien Tremblay for radiotracer synthesis.
REFERENCES
- 1.Atit R, Sgaier SK, Mohamed OA, Taketo MM, Dufort D, Joyner AL, Niswander L, Conlon RA. Beta-catenin activation is necessary and sufficient to specify the dorsal dermal fate in the mouse. Dev Biol 296: 164–176, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Bessi VL, Labbé SM, Huynh DN, Ménard L, Jossart C, Febbraio M, Guérin B, Bentourkia M, Lecomte R, Carpentier AC, Ong H, Marleau S. EP 80317, a selective CD36 ligand, shows cardioprotective effects against post-ischaemic myocardial damage in mice. Cardiovasc Res 96: 99–108, 2012. [DOI] [PubMed] [Google Scholar]
- 3.Blondin DP, Labbé SM, Noll C, Kunach M, Phoenix S, Guérin B, Turcotte EE, Haman F, Richard D, Carpentier AC. Selective impairment of glucose but not fatty acid or oxidative metabolism in brown adipose tissue of subjects with type 2 diabetes. Diabetes 64: 2388–2397, 2015. [DOI] [PubMed] [Google Scholar]
- 4.Blondin DP, Labbé SM, Tingelstad HC, Noll C, Kunach M, Phoenix S, Guérin B, Turcotte EE, Carpentier AC, Richard D, Haman F. Increased brown adipose tissue oxidative capacity in cold-acclimated humans. J Clin Endocrinol Metab 99: E438–E446, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brestoff JR, Kim BS, Saenz SA, Stine RR, Monticelli LA, Sonnenberg GF, Thome JJ, Farber DL, Lutfy K, Seale P, Artis D. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature 519: 242–246, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 84: 277–359, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Chechi K, Carpentier AC, Richard D. Understanding the brown adipocyte as a contributor to energy homeostasis. Trends Endocrinol Metab 24: 408–420, 2013. [DOI] [PubMed] [Google Scholar]
- 8.Chen S, Ho C, Feng D, Chi Z. Tracer kinetic modeling of 11c-acetate applied in the liver with positron emission tomography. IEEE Trans Med Imaging 23: 426–432, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Cousin B, Cinti S, Morroni M, Raimbault S, Ricquier D, Penicaud L, Casteilla L. Occurrence of brown adipocytes in rat white adipose tissue: molecular and morphological characterization. J Cell Sci 103: 931–942, 1992. [DOI] [PubMed] [Google Scholar]
- 10.Festuccia WT, Blanchard PG, Turcotte V, Laplante M, Sariahmetoglu M, Brindley DN, Deshaies Y. Depot-specific effects of the PPARgamma agonist rosiglitazone on adipose tissue glucose uptake and metabolism. J Lipid Res 50: 1185–1194, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foster DO, Frydman ML. Brown adipose tissue: the dominant site of nonshivering thermogenesis in the rat. Experientia Suppl 32: 147–151, 1978. [DOI] [PubMed] [Google Scholar]
- 12.Gropler RJ, Siegel BA, Geltman EM. Myocardial uptake of carbon-11-acetate as an indirect estimate of regional myocardial blood flow. J Nucl Med 32: 245–251, 1991. [PubMed] [Google Scholar]
- 13.Himms-Hagen J, Cui J, Danforth E, Taatjes DJ, Lang SS, Waters BL, Claus TH. Effect of CL-316,243, a thermogenic β 3-agonist, on energy balance and brown and white adipose tissues in rats. Am J Physiol Regul Integr Comp Physiol 266: R1371–R1382, 1994. [DOI] [PubMed] [Google Scholar]
- 14.Jimenéz-Aranda A, Fernández-Vázquez G, Campos D, Tassi M, Velasco-Perez L, Tan DX, Reiter RJ, Agil A. Melatonin induces browning of inguinal white adipose tissue in Zucker diabetic fatty rats. J Pineal Res 55: 416–423, 2013. [DOI] [PubMed] [Google Scholar]
- 14a.Jimenéz-Aranda A, Fernández-Vázquez G, Mohammad A, Serrano M, Reiter RJ, Agil A. Melatonin improves mitochondrial function in inguinal white adipose tissue of Zücker diabetic fatty rats. J Pineal Res 57: 103–109, 2014. [DOI] [PubMed] [Google Scholar]
- 15.Kajimura S, Seale P, Spiegelman BM. Transcriptional control of brown fat development. Cell Metab 11: 257–262, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keipert S, Jastroch M. Brite/beige fat and UCP1: is it thermogenesis? Biochim Biophys Acta 1837: 1075–1082, 2014. [DOI] [PubMed] [Google Scholar]
- 17.Klaus S, Seivert A, Boeuf S. Effect of the beta(3)-adrenergic agonist Cl316,243 on functional differentiation of white and brown adipocytes in primary cell culture. Biochim Biophys Acta 1539: 85–92, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Kuhn E, Binart N, Lombès M. Brown, white, beige: the color of fat and new therapeutic perspectives for obesity. . . . Ann Endocrinol (Paris) 73, Suppl 1: S2–S8, 2012. [DOI] [PubMed] [Google Scholar]
- 19.Labbé SM, Caron A, Bakan I, Laplante M, Carpentier AC, Lecomte R, Richard D. In vivo measurement of energy substrate contribution to cold-induced brown adipose tissue thermogenesis. FASEB J 29: 2046–2058, 2015. [DOI] [PubMed] [Google Scholar]
- 20.Labbé SM, Caron A, Lanfray D, Monge-Roffarello B, Bartness TJ, Richard D. Hypothalamic control of brown adipose tissue thermogenesis. Front Syst Neurosci 9: 150–162, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Labbé SM, Croteau E, Grenier-Larouche T, Frisch F, Ouellet R, Langlois R, Guérin B, Turcotte EE, Carpentier AC. Normal postprandial nonesterified fatty acid uptake in muscles despite increased circulating fatty acids in type 2 diabetes. Diabetes 60: 408–415, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis GF, Carpentier A, Adeli K, Giacca A. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr Rev 23: 201–229, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Liu W, Shan T, Yang X, Liang S, Zhang P, Liu Y, Liu X, Kuang S. A heterogeneous lineage origin underlies the phenotypic and molecular differences of white and beige adipocytes. J Cell Sci 126: 3527–3532, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loncar D, Afzelius BA, Cannon B. Epididymal white adipose tissue after cold stress in rats. II. Mitochondrial changes. J Ultrastruct Mol Struct Res 101: 199–209, 1988. [DOI] [PubMed] [Google Scholar]
- 25.Loncar D, Afzelius BA, Cannon B. Epididymal white adipose tissue after cold stress in rats. I. Nonmitochondrial changes. J Ultrastruct Mol Struct Res 101: 109–122, 1988. [DOI] [PubMed] [Google Scholar]
- 26.Loncar D, Bedrica L, Mayer J, Cannon B, Nedergaard J, Afzelius BA, Svajger A. The effect of intermittent cold treatment on the adipose tissue of the cat. Apparent transformation from white to brown adipose tissue. J Ultrastruct Mol Struct Res 97: 119–129, 1986. [DOI] [PubMed] [Google Scholar]
- 27.Long JZ, Svensson KJ, Tsai L, Zeng X, Roh HC, Kong X, Rao RR, Lou J, Lokurkar I, Baur W, Castellot JJ, Rosen ED, Spiegelman BM. A smooth muscle-like origin for beige adipocytes. Cell Metab 19: 810–820, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mena E, Turkbey B, Mani H, Adler S, Valera VA, Bernardo M, Shah V, Pohida T, McKinney Y, Kwarteng G, Daar D, Lindenberg ML, Eclarinal P, Wade R, Linehan WM, Merino MJ, Pinto PA, Choyke PL, Kurdziel KA. 11C-Acetate PET/CT in localized prostate cancer: a study with MRI and histopathologic correlation. J Nucl Med 53: 538–545, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menard SL, Croteau E, Sarrhini O, Gelinas R, Brassard P, Ouellet R, Bentourkia M, van Lier JE, Rosiers CD, Lecomte R, Carpentier AC. Abnormal in vivo myocardial energy substrate uptake in diet-induced type 2 diabetic cardiomyopathy in rats. Am J Physiol Endocrinol Metab 298: E1049–E1057, 2010. [DOI] [PubMed] [Google Scholar]
- 30.Nedergaard J, Cannon B. UCP1 mRNA does not produce heat. Biochim Biophys Acta 1831: 943–949, 2013. [DOI] [PubMed] [Google Scholar]
- 31.Nicholls DG, Bernson VS, Heaton GM. The identification of the component in the inner membrane of brown adipose tissue mitochondria responsible for regulating energy dissipation. Experientia Suppl 32: 89–93, 1978. [DOI] [PubMed] [Google Scholar]
- 32.Nicholls DG, Locke RM. Thermogenic mechanisms in brown fat. Physiol Rev 64: 1–64, 1984. [DOI] [PubMed] [Google Scholar]
- 33.Ouellet V, Labbé SM, Blondin DP, Phoenix S, Guérin B, Haman F, Turcotte EE, Richard D, Carpentier AC. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J Clin Invest 122: 545–552, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oyama N, Akino H, Kanamaru H, Suzuki Y, Muramoto S, Yonekura Y, Sadato N, Yamamoto K, Okada K. 11C-acetate PET imaging of prostate cancer. J Nucl Med 43: 181–186, 2002. [PubMed] [Google Scholar]
- 35.Park JW, Jung KH, Lee JH, Quach CHT, Moon SH, Cho YS, Lee KH. 18F-FDG PET/CT monitoring of β3 agonist-stimulated brown adipocyte recruitment in white adipose tissue. J Nucl Med 56: 153–158, 2015. [DOI] [PubMed] [Google Scholar]
- 36.Patlak CS, Blasberg RG. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Generalizations. J Cereb Blood Flow Metab 5: 584–590, 1985. [DOI] [PubMed] [Google Scholar]
- 37.Petrovic N, Waldén TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem 285: 7153–7164, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qian SW, Tang Y, Li X, Liu Y, Zhang YY, Huang HY, Xue RD, Yu HY, Guo L, Gao HD, Liu Y, Sun X, Li YM, Jia WP, Tang QQ. BMP4-mediated brown fat-like changes in white adipose tissue alter glucose and energy homeostasis. Proc Natl Acad Sci USA 110: E798–E807, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qiu Y, Nguyen KD, Odegaard JI, Cui X, Tian X, Locksley RM, Palmiter RD, Chawla A. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell 157: 1292–1308, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rao RR, Long JZ, White JP, Svensson KJ, Lou J, Lokurkar I, Jedrychowski MP, Ruas JL, Wrann CD, Lo JC, Camera DM, Lachey J, Gygi S, Seehra J, Hawley JA, Spiegelman BM. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell 157: 1279–1291, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ro SH, Nam M, Jang I, Park HW, Park H, Semple IA, Kim M, Kim JS, Park H, Einat P, Damari G, Golikov M, Feinstein E, Lee JH. Sestrin2 inhibits uncoupling protein 1 expression through suppressing reactive oxygen species. Proc Natl Acad Sci USA 111: 7849–7854, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seale P. Transcriptional control of brown adipocyte development and thermogenesis. Int J Obes Relat Metab Disord 34, Suppl 1: S17–S22, 2010. [DOI] [PubMed] [Google Scholar]
- 43.Shabalina IG, Petrovic N, de Jong JMA, Kalinovich AV, Cannon B, Nedergaard J. UCP1 in brite/beige adipose tissue mitochondria is functionally thermogenic. Cell Rep 5: 1196–1203, 2013. [DOI] [PubMed] [Google Scholar]
- 44.Shan T, Liang X, Bi P, Kuang S. Myostatin knockout drives browning of white adipose tissue through activating the AMPK-PGC1α-Fndc5 pathway in muscle. FASEB J 27: 1981–1989, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stanford KI, Middelbeek RJW, Townsend KL, Lee MY, Takahashi H, So K, Hitchcox KM, Markan KR, Hellbach K, Hirshman MF, Tseng YH, Goodyear LJ. A novel role for subcutaneous adipose tissue in exercise-induced improvements in glucose homeostasis. Diabetes 64: 2002–2014, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Territo PR, Maluccio M, Riley AA, McCarthy BP, Fletcher J, Tann M, Saxena R, Skill NJ. Evaluation of 11C-acetate and 18F-FDG PET/CT in mouse multidrug resistance gene-2 deficient mouse model of hepatocellular carcinoma. BMC Med Imaging 15: 15, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thurlby PL, Wilson S, Arch JR. Ciglitazone is not itself thermogenic but increases the potential for thermogenesis in lean mice. Biosci Rep 7: 573–577, 1987. [DOI] [PubMed] [Google Scholar]
- 48.Timmons JA, Wennmalm K, Larsson O, Waldén TB, Lassmann T, Petrovic N, Hamilton DL, Gimeno RE, Wahlestedt C, Baar K, Nedergaard J, Cannon B. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc Natl Acad Sci USA 104: 4401–4406, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van den Hoff J, Burchert W, Börner AR, Fricke H, Kühnel G, Meyer GJ, Otto D, Weckesser E, Wolpers HG, Knapp WH. [1-(11)C]Acetate as a quantitative perfusion tracer in myocardial PET. J Nucl Med 42: 1174–1182, 2001. [PubMed] [Google Scholar]
- 50.van den Hoff J, Burchert W, Wolpers HG, Meyer GJ, Hundeshagen H. A kinetic model for cardiac PET with [1-carbon-11]-acetate. J Nucl Med 37: 521–529, 1996. [PubMed] [Google Scholar]
- 51.Wang QA, Tao C, Gupta RK, Scherer PE. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med 19: 1338–1344, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu J, Boström P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, Huang K, Tu H, van Marken Lichtenbelt WD, Hoeks J, Enerbäck S, Schrauwen P, Spiegelman BM. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150: 366–376, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xiao C, Goldgof M, Gavrilova O, Reitman ML. Anti-obesity and metabolic efficacy of the β3-adrenergic agonist, CL316243, in mice at thermoneutrality compared to 22°C. Obesity In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Young P, Arch JR, Ashwell M. Brown adipose tissue in the parametrial fat pad of the mouse. FEBS Lett 167: 10–14, 1984. [DOI] [PubMed] [Google Scholar]
- 55.Zingaretti MC, Crosta F, Vitali A, Guerrieri M, Frontini A, CANNONB, Nedergaard J, Cinti S. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J 23: 3113–3120, 2009. [DOI] [PubMed] [Google Scholar]