Abstract
Ghrelin is a gastric hormone that stimulates hunger and worsens glucose metabolism. Circulating ghrelin is decreased after Roux-en-Y gastric bypass (RYGB) surgery; however, the mechanism(s) underlying this change is unknown. We tested the hypothesis that jejunal nutrient exposure plays a significant role in ghrelin suppression after RYGB. Feeding tubes were placed in the stomach or jejunum in 13 obese subjects to simulate pre-RYGB or post-RYGB glucose exposure to the gastrointestinal (GI) tract, respectively, without the confounding effects of caloric restriction, weight loss, and surgical stress. On separate study days, the plasma glucose curves obtained with either gastric or jejunal administration of glucose were replicated with intravenous (iv) infusions of glucose. These “isoglycemic clamps” enabled us to determine the contribution of the GI tract and postabsorptive plasma glucose to acyl ghrelin suppression. Plasma acyl ghrelin levels were suppressed to a greater degree with jejunal glucose administration compared with gastric glucose administration (P < 0.05). Jejunal administration of glucose also resulted in a greater suppression of acyl ghrelin than the corresponding isoglycemic glucose infusion (P ≤ 0.01). However, gastric and isoglycemic iv glucose infusions resulted in similar degrees of acyl ghrelin suppression (P > 0.05). Direct exposure of the proximal jejunum to glucose increases acyl ghrelin suppression independent of circulating glucose levels. The enhanced suppression of acyl ghrelin after RYGB may be due to a nutrient-initiated signal in the jejunum that regulates ghrelin secretion.
Keywords: ghrelin, gastric bypass, isoglycemic clamp, jejunal nutrient exposure, incretin
ghrelin was originally discovered as a gastric-derived hormone that stimulates growth hormone secretion (24) but has since emerged as a pleiotropic hormone with significant roles in the regulation of energy homeostasis (32). Ghrelin is commonly known as the “hunger hormone,” and more recent data indicate that it can also promote adiposity and insulin resistance (47, 49, 51). The biological functions of ghrelin are exerted primarily by acyl ghrelin, the active form of the hormone that has an n-octanoyl group at serine 3. It is important to note, however, that metabolic actions of des-acyl ghrelin have also been described (4). The ability of ghrelin to regulate metabolism and energy balance implicates it as a potential therapeutic target for regulation of body weight and glucose metabolism. Despite advances in understanding the physiological roles of ghrelin, the regulation of ghrelin secretion remains poorly defined (1).
The most notable aspect of ghrelin secretion is its response to fasting and feeding. Plasma ghrelin concentrations are highest before eating and subsequently suppressed after food intake, suggesting nutrient-mediated regulation of secretion (9, 10). The stomach is considered the primary source of circulating ghrelin (2). Gastric ghrelin-secreting cells are closed-type cells without luminal contact, suggesting that ghrelin-secreting cells are not regulated directly by ingested nutrients but rather indirectly through hormonal and/or neural pathways (41). Ghrelin-producing cells are also located in the intestine, albeit at lower levels than in the stomach (11). Interestingly, intestinal ghrelin cells are opened-type cells and thus can be regulated by luminal nutrients (41). Consistent with this histological difference, previous rodent and human studies support a postgastric, either preabsorptive and/or postabsorptive, regulation of ghrelin secretion (27, 36, 38, 45, 50).
Fasting and postprandial ghrelin levels are generally decreased after Roux-en-Y gastric bypass (RYGB) surgery (10, 14, 25, 26, 30, 52), although it should be noted that this is not a universal finding (4, 6, 15). The mechanisms underlying altered ghrelin secretion after RYGB remain elusive. Changes in postoperative nutrient flow are thought to be responsible for at least some of the weight-independent metabolic benefits of the operation. RYGB involves the creation of a small gastric pouch that is connected to the proximal jejunum such that nutrient flow bypasses the majority of the stomach and duodenum and is delivered directly to the proximal jejunum. We reported recently that the improvements in glucose homeostasis and insulin secretion and the incretin effect observed after RYGB could be recapitulated by acutely delivering nutrients directly to the jejunum via a nasally inserted feeding tube (3). In the current study, we extend these findings to determine whether the enhanced ghrelin suppression after RYGB can be attributed to jejunal nutrient exposure independent of changes in postabsorptive glucose levels.
MATERIALS AND METHODS
Subjects.
Obese subjects were recruited from the Vanderbilt Center for Surgical Weight Loss and through local advertisements. Exclusion criteria included a prior gastric, duodenal, or proximal jejunal operation, known malabsorptive disorders, a history of cancer within the past 5 yr, contraindication to nasal tube placement (e.g., deviated septum), and pregnancy. The study protocol was approved by the Vanderbilt University Institutional Review Board, and all subjects provided written, informed consent before participation in the study. This study was registered with ClinicalTrials.gov (NCT00568620).
Study protocol.
Partial data from this study were reported previously as an online observation letter (3). All studies were performed at the Clinical Research Center at Vanderbilt University Medical Center. Study subjects completed two study visits of 2 days each, with a minimum 3-day washout period between study visits. Five of the subjects had a diagnosis of type 2 diabetes treated only with metformin, which was discontinued 5 days prior to each study visit. Participants were admitted the morning of study day 1 after a 12-h fast. A history and physical examination were performed and anthropometric measures obtained. A peripheral iv was placed for blood draws, and a Dobhoff feeding tube (8 French, 43 in.; Viasys Medsystems, Wheeling, IL), was then inserted through the nose into the stomach or jejunum according to the randomization scheme. Gastric positioning of the feeding tube was achieved by advancing the tube according to the distance from nasal region to the xiphoid and by auscultating injected air; correct placement was confirmed by abdominal X-ray. Jejunal tube placement 15–20 cm distal to the ligament of Treitz was performed under fluoroscopic guidance. After tube placement, subjects rested for 30 min before commencing a frequently sampled enteral glucose tolerance test. Subjects were fed standardized meals and then fasted overnight. On the 2nd day of each visit, a second peripheral iv was placed in the contralateral arm, and the subjects underwent an isoglycemic iv glucose match. The same protocol was followed on the second study visit, except the tube was placed in the alternate location. Subjects were instructed to maintain their usual diet and physical activity between study visits.
Enteral glucose infusion.
After a baseline blood draw (−10 min), 50 g of glucose (50% dextrose) was delivered into the feeding tube over 10 min. A blood sample was obtained immediately after the glucose was administered (0 min), and samples were then collected at times 15, 30, 45, 60, 90, 120, and 180 min for the measurement of acyl ghrelin, insulin, glucagon-like peptide-1 (GLP-1), and glucose-dependent insulinotropic polypeptide (GIP). Glucose readings were taken every 5 min for the first 120 min and every 10 min thereafter.
Isoglycemic iv glucose match.
A baseline blood sample was drawn (−10 min) and an intravenous (iv) 20% dextrose infusion began. Glucose readings were taken every 5 min for the first 120 min and every 10 min thereafter. Adjustments were made to the 20% dextrose infusion rate throughout the 180 min to match the plasma glucose levels achieved the previous day during the enteral glucose infusion. Blood samples were collected at times 0, 15, 30, 45, 60, 90, 120, and 180 min for measurement of acyl ghrelin, insulin, GLP-1, and GIP.
Sample collection and analysis.
Blood was collected in chilled EDTA tubes containing AEBSF protease inhibitor (4 mM) and dipeptidyl peptidase IV inhibitor (50 μM) and immediately centrifuged to separate plasma, which was stored at −80°C until analysis. All samples were assayed in duplicate. Glucose was measured via the glucose oxidase method (Beckman Instruments, Fullerton, CA). Plasma insulin was measured by RIA (Millipore, Billerica, MA), and active GLP-1 (7–37 and 7–36 amide) and GIP (total) were measured using the Human Gut Hormone Panel LINCOplex immunoassays (Millipore) by the Vanderbilt Hormone Assay and Analytical Services Core. The intra- and interassay coefficients of variation for these assays were, respectively, 4.1 and 6.9% for insulin, 5.8 and 12% GLP-1, and 9.7 and 16% GIP. Acyl ghrelin was measured using the Milliplex MAP Human Metabolic Hormone Magnetic Bead Panel immunoassay (Millipore). The intra- and interassay coefficients of variation of the acyl ghrelin assay were 12 and 11%, respectively.
Data analyses.
The incremental (above or below baseline) area under the curve (AUC) was calculated according to the trapezoid rule and analyzed by repeated-measures ANOVA with Bonferroni adjustment for multiple comparisons using GraphPad Prism version 5. Differences in outcome measures over time among glucose infusion routes were analyzed by mixed-effects models with Bonferroni adjustment for pairwise comparisons in IBM SPSS Statistics version 22. All variables were distributed normally. The three planned comparisons among glucose infusion routes were gastric vs. jejunal, gastric vs. gastric iv match, and jejunal vs. jejunal iv match. Data are expressed as means ± SE.
RESULTS
Characteristics of the study population.
The study cohort consisted of 13 obese subjects, including nine females and four males, with an average age of 38 ± 3 yr. Five subjects had type 2 diabetes. The average weight and BMI on the first study visit were 120.5 ± 7.4 kg and 41.1 ± 1.9 kg/m2, respectively. There was no weight change between the two study visits (P = 0.323).
Glucose responses.
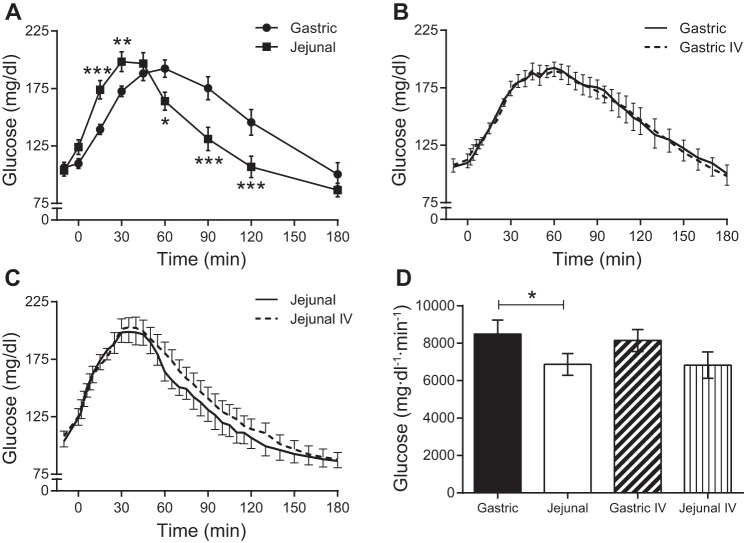
A 50-g glucose load was delivered directly to the stomach or jejunum via a nasally inserted feeding tube. Administration of glucose to the jejunum caused a left shift in the plasma glucose curve compared with gastric administration of glucose (Fig. 1A). Although peak concentrations of plasma glucose were similar between the two routes, glucose levels rose and fell more rapidly with the jejunal route. The glucose AUC was lower with jejunal compared with gastric administration of glucose (Fig. 1D). During the isoglycemic iv glucose matches, the plasma glucose curves (Fig. 1, B and C) and AUCs (Fig. 1D) did not differ from those obtained during enteral administration of glucose. The average amount of iv glucose required to match the gastric glucose curve was 43.3 ± 2.2 g, whereas only 32.7 ± 2.3 g of iv glucose was necessary to match the jejunal glucose curve (P = 0.003). The difference between the amount of glucose administered enterally (50 g) and that administered during the isoglycemic iv match (noted above) represents the contribution of the splanchnic bed to glucose disposal (20), the majority of which being the GI tract. The contribution of splanchnic glucose disposal was greater for jejunal compared with gastric delivery of glucose (17.3 ± 2.3 vs. 6.7 ± 2.2 g, P = 0.003).
Fig. 1.
Plasma glucose concentrations following enteral or intravenous (iv) administration of glucose. A: gastric and jejunal administration of glucose. B: gastric and isoglycemic iv administrations of glucose. C: jejunal and isoglycemic iv administrations of glucose. D: areas under the curve (AUCs) for enteral administrations of glucose and the corresponding isoglycemic iv glucose matches. *P < 0.05; **P ≤ 0.01; ***P ≤ 0.001.
Insulin responses.
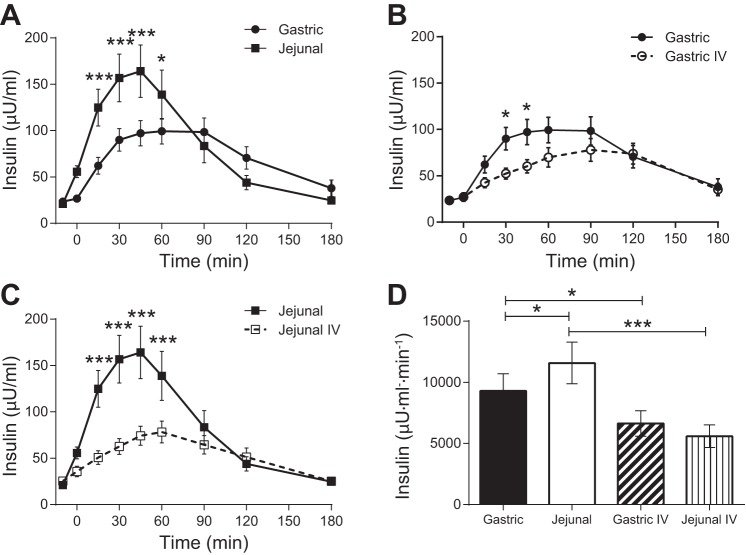
Plasma insulin reached higher concentrations with jejunal glucose administration compared with gastric glucose administration (Fig. 2A), which resulted in a higher AUC for jejunal glucose (Fig. 2D). The isoglycemic iv administrations of glucose produced lower plasma insulin concentrations (Fig. 2, B and C) and AUCs (Fig. 2D) compared with the corresponding enteral glucose administrations.
Fig. 2.
Plasma insulin concentrations following enteral or iv administration of glucose. A: gastric and jejunal administrations of glucose. B: gastric and isoglycemic iv administrations of glucose. C: jejunal and isoglycemic iv administrations of glucose. D: AUCs for enteral administrations of glucose and the corresponding isoglycemic iv glucose matches. *P < 0.05; ***P ≤ 0.001.
Incretin responses.
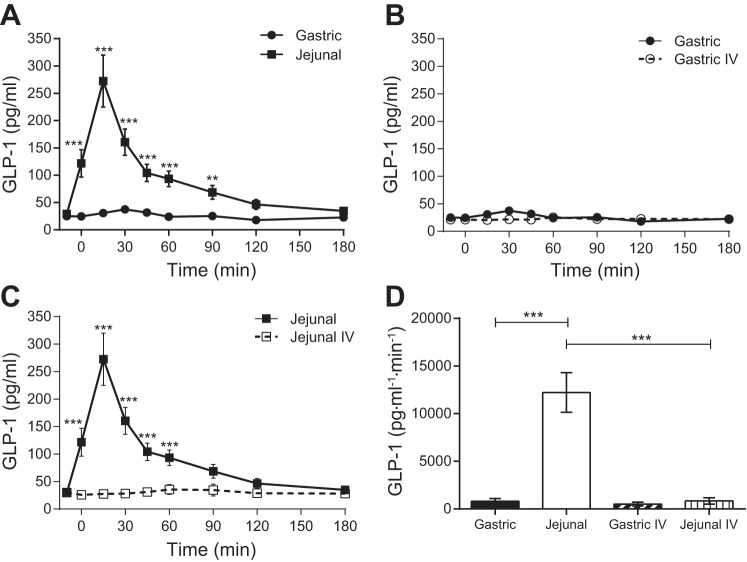
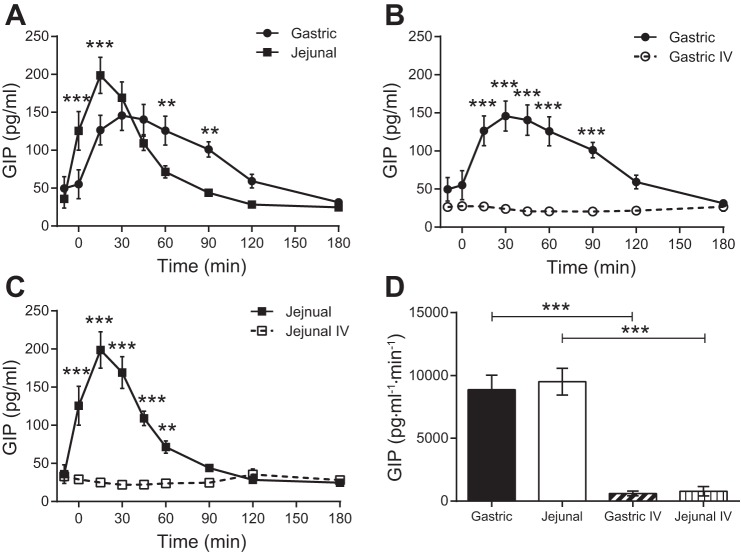
GLP-1 secretion (Fig. 3) was low with gastric administration of glucose but was significantly enhanced with jejunal administration of glucose. In contrast, GIP secretion (Fig. 4) occurred with both gastric and jejunal administration of glucose. Although the total amount of GIP secretion (AUC) was similar in response to gastric and jejunal delivery of glucose, GIP concentrations increased and declined more rapidly with a higher peak with jejunal glucose. Neither GLP-1 nor GIP secretion was stimulated with iv administration of glucose.
Fig. 3.
Plasma glucagon-like peptide-1 (GLP-1) concentrations following enteral or iv administration of glucose. A: gastric and jejunal administrations of glucose. B: gastric and isoglycemic iv administrations of glucose. C: jejunal and isoglycemic iv administrations of glucose. D: AUCs for enteral administrations of glucose and the corresponding isoglycemic iv glucose matches. **P ≤ 0.01; ***P ≤ 0.001.
Fig. 4.
Plasma glucose-dependent insulinotropic polypeptide (GIP) concentrations following enteral or iv administration of glucose. A: gastric and jejunal administrations of glucose. B: gastric and isoglycemic iv administrations of glucose. C: jejunal and isoglycemic iv administrations of glucose. D: AUCs for enteral administrations of glucose and the corresponding isoglycemic iv glucose matches. **P ≤ 0.01; ***P ≤ 0.001.
Acyl ghrelin responses.
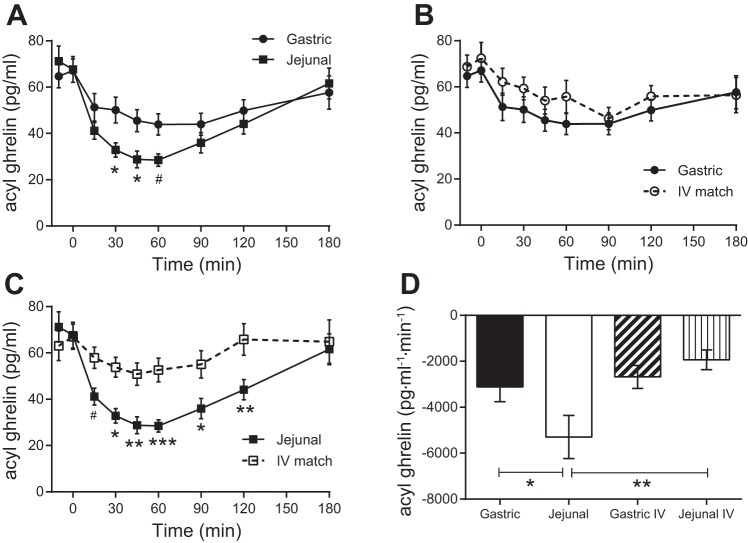
Jejunal administration of glucose resulted in a greater suppression of plasma acyl ghrelin concentrations (∼60%) compared with gastric administration of glucose (∼30%) (Fig. 5, A and D). The isoglycemic iv glucose match to the gastric glucose curves produced a similar suppression of ghrelin observed with the gastric administration of glucose (Fig. 5, B and D). Conversely, the isoglycemic iv glucose match to the jejunal glucose curves produced less ghrelin suppression compared with the jejunal administration of glucose (Fig. 5, C and D).
Fig. 5.
Plasma acyl ghrelin concentrations following enteral or iv administration of glucose. A: gastric and jejunal administrations of glucose. B: gastric and isoglycemic iv administrations of glucose. C: jejunal and isoglycemic iv administrations of glucose. D: AUCs for enteral administrations of glucose and the corresponding isoglycemic iv glucose matches. #P = 0.05; *P < 0.05; **P ≤ 0.01; ***P ≤ 0.001.
DISCUSSION
RYGB produces profound metabolic improvements that can be attributed to the combined effects of altered enteral nutrient flow, caloric restriction, and weight loss. In this study, we examined the contribution of jejunal nutrient exposure and postabsorptive glucose levels to ghrelin suppression after RYGB. To avoid the confounding metabolic effects of caloric restriction, weight loss, and the stress of the surgical procedure, we used gastric and jejunal feeding tubes in obese subjects to simulate pre-RYGB and post-RYGB nutrient flow, respectively. We found that direct delivery of glucose to the jejunum produced a more robust reduction in plasma ghrelin levels compared with either gastric or isoglycemic iv delivery of glucose. These data suggest that the enhanced ghrelin suppression after RYGB results from a nutrient-initiated signal in the jejunum that is independent of circulating glucose levels.
Our data in this obese cohort of subjects demonstrate that administration of glucose to the jejunum is more effective in suppressing ghrelin levels than an identical glucose load to the stomach. This is consistent with the fact that closed-type cells predominate in the stomach and potentially more nutrient-sensitive opened-type cells predominate in the intestine (41). Given these results, we propose that the enhanced ghrelin suppression observed with jejunal administration of glucose (simulating post-RYGB nutrient flow) is initiated by nutrient contact with the jejunal mucosa and subsequently communicated to the stomach, the primary site of ghrelin secretion, via neuronal and/or hormonal signals. This apparent postgastric regulation of ghrelin secretion is consistent with a model previously introduced by Overduin et al. (36) and Williams et al. (50), who demonstrated in rodents that gastric emptying was required for nutrient-stimulated ghrelin suppression. Similar observations were noted in lean, healthy humans in which continuous infusions of the same glucose load into the duodenum or the stomach resulted in identical suppression of plasma ghrelin concentrations (38, 45). An elegant study by Little et al. (27) demonstrated that the intestinal signal for ghrelin suppression in lean, healthy humans is located distal to the duodenum and proximal jejunum. Our study extends these findings to demonstrate that the jejunal signal to suppress ghrelin can be activated in obese humans to overcome the blunted postprandial ghrelin suppression observed in obesity (13, 31, 48). It should be noted that hyperosmolarity in the small intestine, rather than nutrients themselves, has been shown to induce ghrelin suppression in rodents (37) and could contribute to the enhanced ghrelin suppression observed in our current studies and after RYGB. After RYGB, gastric emptying is more rapid and has been reported recently to be ∼100 kcal/min for a glucose drink (35), much faster than a normal rate of 1–4 kcal/min (21). Although in our study the rate of glucose delivery to the jejunum, 17 kcal/min, was slower than after RYGB to minimize GI discomfort, we cannot rule out the possibility that more rapid nutrient exposure produces an osmotic effect that contributed to acyl ghrelin suppression. Our current data support the concept that a signal to suppress ghrelin is located distal to the stomach and further suggest that it is enhanced by direct jejunal nutrient contact, as would occur after RYGB.
Circulating ghrelin concentrations are highest before a meal and are suppressed in the postprandial period (9, 10). This meal-related pattern of secretion suggests that nutrients regulate ghrelin suppression. As such, the postprandial increase in plasma glucose has been investigated as a mediator of ghrelin suppression. Studies exploring the contribution of circulating glucose to ghrelin suppression in humans have yielded conflicting results. Two studies demonstrated that an iv glucose bolus in healthy subjects caused decreases in plasma ghrelin levels similar to oral glucose; however, the iv glucose resulted in supraphysiological plasma glucose concentrations that were much higher than those from an oral glucose load (34, 44). Another study using stepwise increases in iv glucose to reach postprandial glucose concentrations only observed ghrelin suppression with a coinfusion of supraphysiological insulin levels (43). In a slightly different study design, an iv bolus of glucose followed by an iv bolus of insulin also had no effect on ghrelin (7). In contrast to these previous studies, we explored the role of postprandial hyperglycemia by using an isoglycemic iv glucose infusion that replicated exactly the circulating glucose concentrations observed after the enteral administrations of glucose. We demonstrate that the ghrelin suppression observed after gastric glucose administration can be replicated with iv glucose, suggesting that elevations in plasma glucose are responsible for ghrelin suppression when glucose is given into the stomach. In vitro studies in primary cultures of mouse gastric mucosal cells demonstrate that glucose can directly inhibit ghrelin secretion (42). However, jejunal administration of glucose, as would occur after gastric bypass, causes greater suppression of ghrelin than a matched iv administration of glucose, suggesting that factors beyond plasma glucose can regulate plasma ghrelin levels.
We observed that an acute delivery of glucose to the jejunum resulted in an increased extraction of glucose by the splanchnic tissues (GI tract, liver, visceral adipose tissue, and spleen). An increase in intestinal glucose uptake after RYGB has been described in rodent models (40) and in humans (8, 29). After RYGB, chronic nutrient delivery to the jejunum causes adaptive morphological and molecular changes that may contribute to increased intestinal glucose uptake (8, 40). Our data suggest that increased splanchnic glucose uptake can occur by acutely delivering glucose more distal without invoking intestinal adaption. It is unclear whether or not splanchnic glucose retention contributes to the enhanced ghrelin suppression observed with jejunal administration of glucose. It would be interesting to determine whether limiting glucose absorption from the GI tract would increase glucose concentrations and osmolarity (37) in the intestinal lumen and perhaps provide a stronger stimulus for ghrelin suppression, as we observed with direct jejunal administration of glucose.
The inverse relationship between insulin secretion and ghrelin suppression after a meal suggests that insulin may inhibit ghrelin secretion. Most human studies indicate that hyperinsulinemia produced by exogenous insulin infusion can suppress ghrelin secretion (16, 23, 28, 33, 39) and that the effect occurs independently of circulating glucose levels (16). Insulin has also been shown to directly inhibit ghrelin secretion from rat primary stomach cell culture (17). There have been human studies, however, that did not find an effect of insulin infusion to lower plasma ghrelin levels (7, 43). In our study, jejunal administration of glucose caused an exaggerated insulin response compared with gastric and iv infusions of glucose that paralleled the degree of ghrelin suppression. Although we did not find a correlation between insulin and ghrelin responses (data not shown), we cannot rule out the possibility that the greater ghrelin suppression after jejunal administration of glucose is due to intestinal factors acting indirectly through insulin. The involvement of an intestinal signal regulating ghrelin suppression is supported by the different ghrelin responses between the jejunal and iv glucose infusions. The nature of this signal is presently unclear, but the rapidity of the response suggests a hormonal and/or neural mechanism. Our data also show that the GLP-1 response to jejunal delivery of glucose is increased compared with iv (and gastric) delivery of glucose (3). GLP-1 at supraphysiological levels has been shown to suppress ghrelin levels in humans, most likely indirectly through its effect to increase insulin secretion (19). Additionally, both human and rodent studies suggest that the autonomic nervous system can regulate ghrelin secretion (5, 17, 22, 54). Further studies are necessary to determine the principle pathways that regulate ghrelin secretion.
As the role of ghrelin evolves from a hunger hormone to a pleiotropic metabolic hormone, it will be necessary to better define the metabolic consequences of altered ghrelin secretion. Exogenous infusion of acyl ghrelin has been shown to inhibit insulin secretion (46). This raises the possibility that the postprandial decrease in ghrelin is permissive for insulin secretion. Recent data on ghrelin regulating GLP-1 secretion have emerged. Gagnon et al. (18) demonstrated in mice that ghrelin administration before oral glucose enhances GLP-1 release, thereby improving glucose tolerance, and suggested that the preprandial rise in ghrelin acts directly on L cells to “prime” the cell for nutrient-stimulated GLP-1 secretion. On the other hand, intraportal infusion of ghrelin in rats decreased glucose-stimulated GLP-1 secretion (53), consistent with a postprandial suppression of ghrelin secretion being permissive for a GLP-1 response. Several studies have demonstrated that ghrelin worsens glucose metabolism. Thus, an enhanced postprandial suppression of ghrelin with jejunal delivery of glucose would be consistent with the improved glucose metabolism observed in our studies and after RYGB. Our study cannot exclude the possibility that the greater decrease in ghrelin levels with jejunal administration of glucose contributes to the enhanced insulin and GLP-1 secretion and glucose metabolism observed with jejunal glucose.
In conclusion, this study demonstrates that simulating post-RYGB GI nutrient exposure in obese subjects by delivering glucose directly to the jejunum causes a more pronounced suppression of plasma ghrelin. This effect is independent of circulating glucose levels and requires intestinal nutrient exposure. It should be noted that this study examined only the effect of carbohydrates on ghrelin suppression, and further studies would be needed to determine whether responses differ based on type of macronutrient. A limitation of this study is that the acute nature of the jejunal nutrient exposure in this study does not take into account the contribution of intestinal adaptations that occur after RYGB. Also, due to the number of subjects in the study, we are unable to determine whether jejunal glucose administration elicited differential degrees of ghrelin suppression in subjects with and without type 2 diabetes or males and females. Future investigations are necessary to delineate the relative contributions of direct contact of nutrients with the GI mucosa, elevated concentrations of circulating nutrients, and/or neuroendocrine signals to ghrelin suppression in various states of energy balance. Interestingly, patients with a poor weight loss response to RYGB had a less pronounced postprandial suppression of ghrelin (12), underscoring the clinical importance in understanding the regulation of ghrelin secretion. Another important question that remains to be addressed is the contribution of decreased ghrelin levels to the metabolic improvements after RYGB.
GRANTS
This study was funded by grants from the National Institutes of Health: UL1-TR000445 (Vanderbilt Clincial and Translational Science Award) from National Center for Advancing Translational Sciences and DK-020593 (Vanderbilt Diabetes Research and Training Center), F32-DK-103474 (V. L. Albaugh), R01-DK-091748 (N. N. Abumrad), and R01 DK070860 (N. N. Abumrad) from the National Institute of Diabetes and Digestive and Kidney Diseases.
DISCLOSURES
The authors have nothing to disclose.
AUTHOR CONTRIBUTIONS
R.A.T., R.M.S., and J.A. performed experiments; R.A.T. and V.L.A. analyzed data; R.A.T., V.L.A., and N.N.A. interpreted results of experiments; R.A.T. prepared figures; V.L.A. and N.N.A. edited and revised manuscript; R.A.T., R.M.S., A.E.G., J.A., J.M.I., V.L.A., and N.N.A. approved final version of manuscript; R.A.T. and A.E.G. drafted manuscript; J.M.I. and N.N.A. conception and design of research.
ACKNOWLEDGMENTS
We like to thank our Vanderbilt colleagues Pam Marks-Shulman, Kareem Jabbour, Jabbar Saliba, Igal Breitman, and Charles Robb Flynn for their contributions to these studies as well as the study participants.
REFERENCES
- 1.Al Massadi O, Lear PV, Muller TD, Lopez M, Dieguez C, Tschop MH, Nogueiras R. Review of novel aspects of the regulation of ghrelin secretion. Curr Drug Metab 15: 398–413, 2014. [DOI] [PubMed] [Google Scholar]
- 2.Ariyasu H, Takaya K, Tagami T, Ogawa Y, Hosoda K, Akamizu T, Suda M, Koh T, Natsui K, Toyooka S, Shirakami G, Usui T, Shimatsu A, Doi K, Hosoda H, Kojima M, Kangawa K, Nakao K. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab 86: 4753–4758, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Breitman I, Isbell JM, Saliba J, Jabbour K, Flynn CR, Marks-Shulman PA, Laferrere B, Abumrad NN, Tamboli RA. Effects of proximal gut bypass on glucose tolerance and insulin sensitivity in humans. Diabetes Care 36: e57, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Broglio F, Gottero C, Prodam F, Gauna C, Muccioli G, Papotti M, Abribat T, Van Der Lely AJ, Ghigo E. Non-acylated ghrelin counteracts the metabolic but not the neuroendocrine response to acylated ghrelin in humans. J Clin Endocrinol Metab 89: 3062–3065, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Broglio F, Gottero C, Van Koetsveld P, Prodam F, Destefanis S, Benso A, Gauna C, Hofland L, Arvat E, van der Lely AJ, Ghigo E. Acetylcholine regulates ghrelin secretion in humans. J Clin Endocrinol Metab 89: 2429–2433, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ, Bantle JP, Sledge I. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med 122: 248.e5–256.e5, 2009. [DOI] [PubMed] [Google Scholar]
- 7.Caixás A, Bashore C, Nash W, Pi-Sunyer F, Laferrère B. Insulin, unlike food intake, does not suppress ghrelin in human subjects. J Clin Endocrinol Metab 87: 1902, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Cavin JB, Couvelard A, Lebtahi R, Ducroc R, Arapis K, Voitellier E, Cluzeaud F, Gillard L, Hourseau M, Mikail N, Ribeiro-Parenti L, Kapel N, Marmuse JP, Bado A, Le Gall M. Differences in Alimentary Glucose Absorption and Intestinal Disposal of Blood Glucose After Roux-en-Y Gastric Bypass vs Sleeve Gastrectomy. Gastroenterology 150: 454.e9–464.e9, 2016. [DOI] [PubMed] [Google Scholar]
- 9.Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 50: 1714–1719, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, Purnell JQ. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med 346: 1623–1630, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, Matsukura S, Kangawa K, Nakazato M. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology 141: 4255–4261, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Dirksen C, Jørgensen NB, Bojsen-Møller KN, Kielgast U, Jacobsen SH, Clausen TR, Worm D, Hartmann B, Rehfeld JF, Damgaard M, Madsen JL, Madsbad S, Holst JJ, Hansen DL. Gut hormones, early dumping and resting energy expenditure in patients with good and poor weight loss response after Roux-en-Y gastric bypass. Int J Obes (Lond) 37: 1452–1459, 2013. [DOI] [PubMed] [Google Scholar]
- 13.English PJ, Ghatei MA, Malik IA, Bloom SR, Wilding JP. Food fails to suppress ghrelin levels in obese humans. J Clin Endocrinol Metab 87: 2984, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Engstrom BE, Ohrvall M, Sundbom M, Lind L, Karlsson FA. Meal suppression of circulating ghrelin is normalized in obese individuals following gastric bypass surgery. Int J Obes (Lond) 31: 476–480, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Falkén Y, Hellström PM, Holst JJ, Näslund E. Changes in glucose homeostasis AFTER Roux-en-Y gastric bypass surgery for obesity at day three, two months, and one year after surgery: role of gut peptides. J Clin Endocrinol Metab 96: 2227–2235, 2011. [DOI] [PubMed] [Google Scholar]
- 16.Flanagan DE, Evans ML, Monsod TP, Rife F, Heptulla RA, Tamborlane WV, Sherwin RS. The influence of insulin on circulating ghrelin. Am J Physiol Endocrinol Metab 284: E313–E316, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Gagnon J, Anini Y. Insulin and norepinephrine regulate ghrelin secretion from a rat primary stomach cell culture. Endocrinology 153: 3646–3656, 2012. [DOI] [PubMed] [Google Scholar]
- 18.Gagnon J, Baggio LL, Drucker DJ, Brubaker PL. Ghrelin Is a Novel Regulator of GLP-1 Secretion. Diabetes 64: 1513–1521, 2015. [DOI] [PubMed] [Google Scholar]
- 19.Hagemann D, Holst JJ, Gethmann A, Banasch M, Schmidt WE, Meier JJ. Glucagon-like peptide 1 (GLP-1) suppresses ghrelin levels in humans via increased insulin secretion. Regul Pept 143: 64–68, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Hare KJ, Vilsboll T, Holst JJ, Knop FK. Inappropriate glucagon response after oral compared with isoglycemic intravenous glucose administration in patients with type 1 diabetes. Am J Physiol Endocrinol Metab 298: E832–E837, 2010. [DOI] [PubMed] [Google Scholar]
- 21.Hellström PM, Grybäck P, Jacobsson H. The physiology of gastric emptying. Best Pract Res Clin Anaesthesiol 20: 397–407, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Hosoda H, Kangawa K. The autonomic nervous system regulates gastric ghrelin secretion in rats. Regul Pept 146: 12–18, 2008. [DOI] [PubMed] [Google Scholar]
- 23.Karczewska-Kupczewska M, Straczkowski M, Adamska A, Nikolajuk A, Otziomek E, Gorska M, Kowalska I. Increased suppression of serum ghrelin concentration by hyperinsulinemia in women with anorexia nervosa. Eur J Endocrinol 162: 235–239, 2010. [DOI] [PubMed] [Google Scholar]
- 24.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402: 656–660, 1999. [DOI] [PubMed] [Google Scholar]
- 25.Korner J, Bessler M, Cirilo LJ, Conwell IM, Daud A, Restuccia NL, Wardlaw SL. Effects of Roux-en-Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY, and insulin. J Clin Endocrinol Metab 90: 359–365, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Korner J, Inabnet W, Conwell IM, Taveras C, Daud A, Olivero-Rivera L, Restuccia NL, Bessler M. Differential effects of gastric bypass and banding on circulating gut hormone and leptin levels. Obesity (Silver Spring) 14: 1553–1561, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Little TJ, Doran S, Meyer JH, Smout AJ, O'Donovan DG, Wu KL, Jones KL, Wishart J, Rayner CK, Horowitz M, Feinle-Bisset C. The release of GLP-1 and ghrelin, but not GIP and CCK, by glucose is dependent upon the length of small intestine exposed. Am J Physiol Endocrinol Metab 291: E647–E655, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Lucidi P, Murdolo G, Di Loreto C, De Cicco A, Parlanti N, Fanelli C, Santeusanio F, Bolli GB, De Feo P. Ghrelin is not necessary for adequate hormonal counterregulation of insulin-induced hypoglycemia. Diabetes 51: 2911–2914, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Magkos F, Bradley D, Eagon JC, Patterson BW, Klein S. Effect of Roux-en-Y gastric bypass and laparoscopic adjustable gastric banding on gastrointestinal metabolism of ingested glucose. Am J Clin Nutr 103: 61–65, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malin SK, Samat A, Wolski K, Abood B, Pothier CE, Bhatt DL, Nissen S, Brethauer SA, Schauer PR, Kirwan JP, Kashyap SR. Improved acylated ghrelin suppression at 2 years in obese patients with type 2 diabetes: effects of bariatric surgery vs standard medical therapy. Int J Obes (Lond) 38: 364–370, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mittelman SD, Klier K, Braun S, Azen C, Geffner ME, Buchanan TA. Obese adolescents show impaired meal responses of the appetite-regulating hormones ghrelin and PYY. Obesity (Silver Spring) 18: 918–925, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muller TD, Nogueiras R, Andermann ML, Andrews ZB, Anker SD, Argente J, Batterham RL, Benoit SC, Bowers CY, Broglio F, Casanueva FF, D'Alessio D, Depoortere I, Geliebter A, Ghigo E, Cole PA, Cowley M, Cummings DE, Dagher A, Diano S, Dickson SL, Diéguez C, Granata R, Grill HJ, Grove K, Habegger KM, Heppner K, Heiman ML, Holsen L, Holst B, Inui A, Jansson JO, Kirchner H, Korbonits M, Laferrère B, LeRoux CW, Lopez M, Morin S, Nakazato M, Nass R, Perez-Tilve D, Pfluger PT, Schwartz TW, Seeley RJ, Sleeman M, Sun Y, Sussel L, Tong J, Thorner MO, van der Lely AJ, van der Ploeg LH, Zigman JM, Kojima M, Kangawa K, Smith RG, Horvath T, Tschöp MH. Ghrelin. Mol Metab 4: 437–460, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murdolo G, Lucidi P, Di Loreto C, Parlanti N, De Cicco A, Fatone C, Fanelli CG, Bolli GB, Santeusanio F, De Feo P. Insulin is required for prandial ghrelin suppression in humans. Diabetes 52: 2923–2927, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Nakagawa E, Nagaya N, Okumura H, Enomoto M, Oya H, Ono F, Hosoda H, Kojima M, Kangawa K. Hyperglycaemia suppresses the secretion of ghrelin, a novel growth-hormone-releasing peptide: responses to the intravenous and oral administration of glucose. Clin Sci (Lond) 103: 325–328, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Nguyen NQ, Debreceni TL, Bambrick JE, Bellon M, Wishart J, Standfield S, Rayner CK, Horowitz M. Rapid gastric and intestinal transit is a major determinant of changes in blood glucose, intestinal hormones, glucose absorption and postprandial symptoms after gastric bypass. Obesity (Silver Spring) 22: 2003–2009, 2014. [DOI] [PubMed] [Google Scholar]
- 36.Overduin J, Frayo RS, Grill HJ, Kaplan JM, Cummings DE. Role of the duodenum and macronutrient type in ghrelin regulation. Endocrinology 146: 845–850, 2005. [DOI] [PubMed] [Google Scholar]
- 37.Overduin J, Tylee TS, Frayo RS, Cummings DE. Hyperosmolarity in the small intestine contributes to postprandial ghrelin suppression. Am J Physiol Gastrointest Liver Physiol 306: G1108–G1116, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parker BA, Doran S, Wishart J, Horowitz M, Chapman IM. Effects of small intestinal and gastric glucose administration on the suppression of plasma ghrelin concentrations in healthy older men and women. Clin Endocrinol (Oxf) 62: 539–546, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Saad MF, Bernaba B, Hwu CM, Jinagouda S, Fahmi S, Kogosov E, Boyadjian R. Insulin regulates plasma ghrelin concentration. J Clin Endocrinol Metab 87: 3997–4000, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Saeidi N, Meoli L, Nestoridi E, Gupta NK, Kvas S, Kucharczyk J, Bonab AA, Fischman AJ, Yarmush ML, Stylopoulos N. Reprogramming of intestinal glucose metabolism and glycemic control in rats after gastric bypass. Science 341: 406–410, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakata I, Nakamura K, Yamazaki M, Matsubara M, Hayashi Y, Kangawa K, Sakai T. Ghrelin-producing cells exist as two types of cells, closed- and opened-type cells, in the rat gastrointestinal tract. Peptides 23: 531–536, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Sakata I, Park WM, Walker AK, Piper PK, Chuang JC, Osborne-Lawrence S, Zigman JM. Glucose-mediated control of ghrelin release from primary cultures of gastric mucosal cells. Am J Physiol Endocrinol Metab 302: E1300–E1310, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schaller G, Schmidt A, Pleiner J, Woloszczuk W, Wolzt M, Luger A. Plasma ghrelin concentrations are not regulated by glucose or insulin: a double-blind, placebo-controlled crossover clamp study. Diabetes 52: 16–20, 2003. [DOI] [PubMed] [Google Scholar]
- 44.Shiiya T, Nakazato M, Mizuta M, Date Y, Mondal MS, Tanaka M, Nozoe S, Hosoda H, Kangawa K, Matsukura S. Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretion. J Clin Endocrinol Metab 87: 240–244, 2002. [DOI] [PubMed] [Google Scholar]
- 45.Steinert RE, Meyer-Gerspach AC, Beglinger C. The role of the stomach in the control of appetite and the secretion of satiation peptides. Am J Physiol Endocrinol Metab 302: E666–E673, 2012. [DOI] [PubMed] [Google Scholar]
- 46.Tong J, Prigeon RL, Davis HW, Bidlingmaier M, Kahn SE, Cummings DE, Tschop MH, D'Alessio D. Ghrelin suppresses glucose-stimulated insulin secretion and deteriorates glucose tolerance in healthy humans. Diabetes 59: 2145–2151, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature 407: 908–913, 2000. [DOI] [PubMed] [Google Scholar]
- 48.Van Name M, Giannini C, Santoro N, Jastreboff AM, Kubat J, Li F, Kursawe R, Savoye M, Duran E, Dziura J, Sinha R, Sherwin RS, Cline G, Caprio S. Blunted suppression of acyl-ghrelin in response to fructose ingestion in obese adolescents: the role of insulin resistance. Obesity (Silver Spring) 23: 653–661, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vestergaard ET, Gormsen LC, Jessen N, Lund S, Hansen TK, Moller N, Jorgensen JO. Ghrelin infusion in humans induces acute insulin resistance and lipolysis independent of growth hormone signaling. Diabetes 57: 3205–3210, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Williams DL, Cummings DE, Grill HJ, Kaplan JM. Meal-related ghrelin suppression requires postgastric feedback. Endocrinology 144: 2765–2767, 2003. [DOI] [PubMed] [Google Scholar]
- 51.Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, Dhillo WS, Ghatei MA, Bloom SR. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab 86: 5992, 2001. [DOI] [PubMed] [Google Scholar]
- 52.Yousseif A, Emmanuel J, Karra E, Millet Q, Elkalaawy M, Jenkinson AD, Hashemi M, Adamo M, Finer N, Fiennes AG, Withers DJ, Batterham RL. Differential effects of laparoscopic sleeve gastrectomy and laparoscopic gastric bypass on appetite, circulating acyl-ghrelin, peptide YY3-36 and active GLP-1 levels in non-diabetic humans. Obes Surg 24: 241–252, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang X, Li W, Li P, Chang M, Huang X, Li Q, Cui C. Intraportal infusion of ghrelin could inhibit glucose-stimulated GLP-1 secretion by enteric neural net in Wistar rat. Biomed Res Int 2014: 923564, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao TJ, Sakata I, Li RL, Liang G, Richardson JA, Brown MS, Goldstein JL, Zigman JM. Ghrelin secretion stimulated by {beta}1-adrenergic receptors in cultured ghrelinoma cells and in fasted mice. Proc Natl Acad Sci U S A 107: 15868–15873, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]