Abstract
Mitochondrial dysfunction is associated with many human diseases and results from mismatch of damage and repair over the life of the organelle. PARK2 is a ubiquitin E3 ligase that regulates mitophagy, a repair mechanism that selectively degrades damaged mitochondria. Deletion of PARK2 in multiple in vivo models results in susceptibility to stress-induced mitochondrial and cellular dysfunction. Surprisingly, Park2 knockout (KO) mice are protected from nutritional stress and do not develop obesity, hepatic steatosis or insulin resistance when fed a high-fat diet (HFD). However, these phenomena are casually related and the physiological basis for this phenotype is unknown. We therefore undertook a series of acute HFD studies to more completely understand the physiology of Park2 KO during nutritional stress. We find that intestinal lipid absorption is impaired in Park2 KO mice as evidenced by increased fecal lipids and reduced plasma triglycerides after intragastric fat challenge. Park2 KO mice developed hepatic steatosis in response to intravenous lipid infusion as well as during incubation of primary hepatocytes with fatty acids, suggesting that hepatic protection from nutritional stress was secondary to changes in energy balance due to altered intestinal triglyceride absorption. Park2 KO mice showed reduced adiposity after 1-wk HFD, as well as improved hepatic and peripheral insulin sensitivity. These studies suggest that changes in intestinal lipid absorption may play a primary role in protection from nutritional stress in Park2 KO mice by preventing HFD-induced weight gain and highlight the need for tissue-specific models to address the role of PARK2 during metabolic stress.
Keywords: lipid absorption, mitophagy, PARK2, insulin resistance, liver, small intestine, obesity
park2 is a ubiquitin e3 ligase that was first identified in patients with autosomal recessive juvenile Parkinsonism (ARJP) and has since emerged as one of the most frequently mutated genes in this disorder (25, 31). PARK2 is comprised of an NH2-terminal ubiquitin-like domain, a novel PARK2-specific domain, and two RING-finger domains separated by an in-between RING-finger (IBR) domain in the COOH terminus (25, 32). PARK2 mutations resulting in ARJP typically lead to loss of E3 ligase activity and commonly occur within the COOH-terminal RING-finger or IBR domains (9). PARK2 acts as a multifunctional E3 ligase in that it cooperates with several E2 conjugating enzymes, such as UBCH7, UBCH8, and UBCH13/UEV1, and catalyzes mono- and polyubiquitination with Lys48 and Lys63 linkages, indicating proteasome-dependent and -independent effects of PARK2-mediated ubiquitination (9, 11, 17, 41, 53). Multiple PARK2 substrates have been reported, but it still remains unclear how loss of PARK2 E3 ligase activity contributes to the pathogenesis of ARJP (10).
PARK2 is expressed in a number of metabolically active tissues, including liver, heart, skeletal muscle and kidney, suggesting a broad biological function outside the brain (4). A growing body of evidence suggests PARK2 plays a critical role in mitochondrial homeostasis by contributing to the generation of a phospho-ubiquitin signaling motif on the outer membrane of damaged mitochondria, marking them for selective removal through mitophagy (23, 27, 30, 33, 49). Pharmacological induction of mitochondrial stress results in the redistribution of PARK2 from the cytosol to the outer mitochondrial membrane, and mitochondria with PARK2 accumulation are selectively engulfed by autophagosomes for degradation (34). Further evidence of PARK2's role in mitochondrial maintenance comes from a growing list of in vivo models. Deletion of the PARK2 homolog in Drosophila results in severe mitochondrial morphological defects, muscular degeneration and reduced body weight (16, 50). Park2 knockout (KO) mice show an age-associated reduction in body weight, which remains incompletely understood, as well as loss of mitochondrial protein abundance and impaired mitochondrial respiration in the ventral midbrain (37). Interestingly, although multiple Park2 KO mouse lines were independently generated as models of ARJP, none exhibit robust dopaminergic or behavioral phenotypes associated with human Parkinsonism and are generally poor models of the disease (8, 10, 14, 15, 19, 38). However, Park2 KO mice are more prone to sepsis-induced mitochondrial and cardiac dysfunction, and fail to recover mitochondrial metabolic function and cardiac contractility following lipopolysaccharide treatment, highlighting the importance of PARK2 in peripheral tissues (39). A similar susceptibility to stress was demonstrated in liver of Park2 KO mice treated with alcohol; alcohol-induced mitochondrial dysfunction, oxidative stress, and steatosis were more pronounced in the absence of PARK2 (51).
Surprisingly, in contrast to their susceptibility to alcohol-induced liver disease, Park2 KO mice are protected from high-fat diet (HFD)-induced metabolic dysfunction in liver (24). Following 6 wk of HFD feeding, Park2 KO mice displayed reduced hepatic steatosis and plasma liver enzymes, as well as improved glucose tolerance and hepatic insulin signaling (24). Baseline studies in regular chow-fed mice detected no differences in body weight or adiposity between WT and Park2 KO mice, and measures of whole body energy balance including food intake, activity, and oxygen consumption were also not different (24). Interestingly, Park2 KO mice showed a modest reduction in plasma glucose levels compared with WT mice during glucose tolerance tests but no difference in plasma glucose levels during insulin tolerance tests (24). These studies also demonstrated a novel mitophagy-independent mechanism by which PARK2 regulates metabolism: ubiquitination and stabilization of the fatty acid transporter CD36 (24). Consistent with this model, CD36 levels were reduced in HFD-fed Park2 KO mouse liver (24). Strikingly, the Park2 KO mice were also protected from HFD-induced obesity, which could not be explained by reduced fatty acid transport in liver, and suggested a broader systemic role for PARK2 in lipid metabolism. Also, because obesity is strongly associated with the development of hepatic steatosis, the primary and secondary effects of PARK2 on liver lipid metabolism remain unclear. We therefore undertook a series of acute lipid challenge or short-term HFD feeding studies to more completely understand the response of Park2 KO mice to lipid oversupply. We found that intestinal lipid absorption was reduced in Park2 KO mice and propose that altered lipid absorption contributes to their resistance to HFD-induced obesity and subsequent protection from hepatic steatosis and insulin resistance.
MATERIALS AND METHODS
Animal use and care.
Mice were housed and studied at Yale University School of Medicine and the University of Pittsburgh according to guidelines established by the Institutional Animal Care and Use Committees at each institution. Mice were housed at 22 ± 2°C on a 12:12-h light-dark cycle with free access to food and water. Diets provided were Harlan Teklad 2018S (control rodent chow) and Research Diets D12492 (HFD, 60% kcal fat). Five- to six-week-old male WT (C57BL6/J) and Park2 KO mice on the C57BL6/J background (stock no. 006582) were purchased from the Jackson Laboratory and housed within the animal facilities for 7–8 wk before study. Mice were between 12 and 14 wk old at the time of study except where noted. The Comprehensive Lab Animal Monitoring System (Columbus Instruments) with Oxymax was used to evaluate energy expenditure, feeding, activity, and respiratory quotient. Mice were housed for a total of 72 h in the metabolic cages following 3–4 days of individual housing for acclimation. The first 24 h of metabolic cage data were considered acclimation and the remaining 48 h analyzed and presented as the 24-h average divided into dark (7PM–7AM) and light cycles (7AM–7PM). Studies were designed such that there was no difference in body weight between groups during metabolic cage studies, and data were normalized to body weight. Body composition was determined by 1H magnetic resonance spectroscopy (Bruker Minispec). Intestinal lipid absorption during regular chow studies was determined after feeding mice a custom diet consisting of base diet D12450 (Research Diets) supplemented with 5% sucrose polybehenate, a nonabsorbable additive, for 4 days. Fecal pellets collected on the fourth day were used to determine percent fat absorption as the ratio of behenic acid to dietary fatty acids in feces by GC-MS (20).
Biochemical analyses.
Plasma triglyceride levels were measured using Triglyceride SL detection reagent (Sekisui Diagnostics). Plasma glucose was measured using the YSI Glucose Analyzer. Plasma insulin was determined by radioimmunoassay (EMD Millipore), and plasma fatty acids were measured using a Cobas-Roche Analyzer and a spectrophotometric NEFA detection reagent (Wako Diagnostics). Fecal fatty acid levels and profiles were measured as methyl ester derivatives by GC-MS following extraction from feces with chloroform-methanol (1:1) plus an internal deuterated potassium palmitate standard (7,7,8,8-D4; Cambridge Isotopes) and treatment with methanolic borane (48). Liver and heart triglycerides were determined using the Triglyceride SL detection reagent following tissue homogenization and lipid extraction in chloroform-methanol (2:1), followed by acidification with H2SO4 and phase separation by centrifugation.
Histology.
Preparation and staining of all histological samples were conducted at the Department of Pathology Histology Core at Yale University. Small intestines were removed during dissection of 6-wk-old mice following a 6-h fast and cut into three equal-length segments to approximate the duodenum, jejunum, and ileum. Segments were washed with saline and middle portions of each segment cut into ringlets or lengthwise and splayed on a histological cassette sponge and embedded in paraffin for sectioning. Sections were cut at 5 μm, dried, deparaffinized, and rehydrated with distilled water before being mounted on glass slides and stained with hematoxylin and eosin (H&E). A general histological evaluation of intestinal morphology was performed in a blinded fashion by a pathologist (D. J. Hartman) at magnifications ranging from ×40-×400. Because no gross differences in proximal, middle, or distal small-intestine morphology were observed between genotypes, representative images of middle (approximate jejunum) are shown. For determination of villi length and width and crypt length, three images per segment representing the entire length of the small intestine were taken randomly at ×100 magnification and analyzed using ImageJ in a blinded fashion by a technician. Data shown are the average of ∼50–60 villi and crypts per mouse.
Primary hepatocyte isolation.
Livers were perfused in situ with ∼50 ml of preheated 37°C perfusion buffer I (142 mM NaCl, 6.7 mM KCl, 10 mM HEPES, 2.23 μM EGTA, pH 7.4) and then with 50–100 ml of preheated 37°C perfusion buffer II [152 mM albumin, 67 mM NaCl, 6.7 mM KCl, 100 mM HEPES, 19 mM CaCl2, 0.02% type IV collagenase (Sigma), pH 7.6] at a flow rate of 8 ml/min. Liver was excised and placed in a 6-cm petri dish with ice-cold isolation media (RPMI, 10% heat-inactivated FBS, 1× nonessential amino acids, 1 mM sodium pyruvate, 100 IU/ml penicillin-streptomycin, 2 mM l-glutamine). Liver was then manually disrupted and filtered through a gauze pad followed by centrifugation at 4°C, 400 rpm, for 5 min. The cell pellet was resuspended in isolation medium, and 90% Percoll (GE Healthcare) was carefully added to the cell suspension followed by centrifugation at 4°C, 400 rpm, for 10 min. The supernatant was discarded and the pellet washed twice with isolation medium. The pellet was then resuspended in high-glucose medium (isolation medium plus 4.5 g/l glucose) and viability calculated using trypan blue exclusion. Cells were plated on collagen-coated 12-well tissue culture plates at a density of 5.0 × 105 live cells per well. After incubation for 1 h at 37°C and 5% CO2, media and any floating cells were removed and replaced with fresh media.
Primary hepatocyte studies.
For palmitate transport studies, cells were washed with prewarmed PBS and cultured for 1 h in RPMI plus 2% FBS medium prior to addition of 100, 250, or 500 μM palmitate conjugated to BSA plus 0.5 μCi [14C]palmitate per well in triplicate. Transport studies were conducted for 5 min at room temperature and terminated by rapid removal of media followed by 3× washing with ice-cold PBS on ice. Cells were collected in RIPA buffer plus protease inhibitors, and lysates were used to determine cellular 14C levels by liquid scintillation counting in Ultima Gold XR scintillation fluid (PerkinElmer) on a Beckman Coulter LS 6500 Multipurpose Scintillation Counter. Protein concentration was measured with a Pierce BCA protein assay (Thermo Scientific). For triglyceride accumulation studies, primary hepatocytes were incubated overnight with 0, 250, or 500 μM palmitate conjugated to BSA in duplicate followed by washing in PBS and collection of a cell pellet for lipid extraction. Pellets were resuspended in 2:1 chloroform-methanol and vortexed every 5 min for 30 min followed by addition of 1 M H2SO4 and centrifugation at 5,000 g for 10 min at room temperature. The lower organic phase was collected and a small portion dried for determination of triglyceride content using Infinity Triglyceride Reagent (Thermo Scientific). Protein levels were determined in quadruplicate from plates cultured in parallel under identical conditions. For respiration studies, primary hepatocytes were plated on collagen-coated Seahorse Biosciences XF-24 culture plates at a density of 1.25 × 104 cells per well. WT and Park2 KO hepatocytes were assayed on the same plate in sextuplicate, and respiration was measured in the presence of 200 μM palmitate conjugated to BSA or BSA alone.
RNA and protein isolation and analysis.
RNA was isolated from liver and small intestine using an RNeasy Kit (Qiagen) and then used for cDNA synthesis using a QuantiTect Reverse Transcriptase Kit (Qiagen), both according to the manufacturer's instructions. Quantitative PCR was performed using the Applied Biosystems 7300 RT-PCR System and PowerUp SYBR Green Master Mix (Thermo Fisher). Primer sequences were designed with Primer Express Software (Applied Biosystems) and were as follows (forward and reverse): Abcb11: F TCACATCTGTAGGGTTGTTGAGT, R GCGCCTTCCCCTTTCTTCTTA; Cyp7a1: F CTTGAGCCAGAGTCC AATGCT, R GGCAACCTCCTGCAATTCA; Cyp8b1: F GGTACGCTTCCTCTATCGCC, R GGATGGCGTCTTA TGGGGAG; Dgat2: F TGGGCCTTGGTGGTTTCTTAC, R GACTGCCCTTGCCCAGCTA; Fxr: F CAAGTGTAAGAACGGGGGCA, R CACTGGATTTCAGTTAACAAACCTG; Lxr: F GCGACAGTTTTGGTAGAGGGT, R GCTTCCCCACCAGACTAACT; Slc10a2: F ATGGGGTGCAATGTGGAAGT, R GAAGCCCACGAAGATACC CC; Mttp: F GCGTTGCATTTCTACCCACA, R TCGAGACCCGAGCTTACACA. A small subset of genes (Fatp4, Mgat2, Dgat1, Fabp2, Cd36, Actb) were measured using Quantitect Primer Assays (Qiagen), and the sequences are proprietary. The efficiency of each primer set was determined from a four-point standard curve and used to calculate relative expression using Actb as reference gene. Data are expressed as the fold change in relative expression relative to controls. For Western blotting, mucosal scrapings of the entire length of the small intestine were collected from overnight-fasted mice and lysed on ice for 30 min with intermittent vortexing in homogenization buffer plus protease inhibitors (1% CHAPS, 150 mM NaCl, 25 mM HEPES, pH 7.4, Halt Protease Inhibitor Cocktail, Thermo Fisher). Intestinal epithelial cell lysates were resolved on Bis-Tris polyacrylamide gels (Thermo Fisher) and transferred to nitrocellulose membranes for incubation with primary and HRP-conjugated secondary antibodies for visualization using enhanced chemiluminescence. Antibodies used: CD36 (Abcam ab133625), FABP2 (Abcam ab128860), DGAT1 (Abcam ab54037), MGAT2 (Thermo Fisher PA5-11263), APOA-IV (Santa Cruz sc-50376), MTTP (Abcam ab 75316), and GAPDH (Santa Cruz sc-25778).
Oral fat tolerance tests.
In one series of studies, mice were fasted overnight prior to receiving a 10 μl/kg oral gavage of canola oil. Plasma was isolated following centrifugation of whole blood collected by tail bleeding at set intervals for triglyceride measurements. In a second series of studies, overnight-fasted mice were placed in restrainers for basal blood collection followed by intraperitoneal injection of 500 mg/kg poloxamer 407. Blood was collected 30 min later, and mice were gavaged with 10 μl/kg canola oil plus 23.3 μCi/ml [14C]palmitate and [3H]triolein, and bled 120 min later. Hepatic lipid production was calculated as the change in plasma triglycerides from 0 to 30 min, and lipid absorption was calculated as the change in plasma triglycerides from 30 to 120 min minus the rate of hepatic lipid production. Plasma levels of the 14C and 3H tracers were determined by liquid scintillation counting, and the rate of appearance in plasma was calculated from 30 to 120 min.
Intralipid plus heparin infusions.
Mice recovered for 1 wk prior to infusion experiments following surgical implantation of an indwelling catheter in the right jugular vein. After a 6-h morning fast, mice received either a saline control (175 μl·kg−1·min−1) or Intralipid 20% lipid emulsion (175 μl·kg−1·min−1) plus heparin (0.4 U·kg−1·min−1) infusion to experimentally raise plasma fatty acids. After 180 min, mice were euthanized with an intravenous injection of 150 mg/kg pentobarbital sodium, and plasma and tissues were harvested for plasma fatty acid level and tissue triglyceride level measurements. Tissue triglyceride levels are expressed in absolute terms and as the fold change relative to the saline- infused group for that genotype.
Hyperinsulinemic euglycemic clamps.
Clamps were performed as previously described, with minor modifications (21). Mice recovered for 1 wk prior to clamp experiments following surgical implantation of an indwelling catheter in the right jugular vein. Mice were fasted 6 h prior to infusion with [3-3H]glucose at a rate of 0.05 μCi/min for 120 min to determine basal glucose turnover. A primed infusion of insulin and [3-3H]glucose was administered at 7.14 mU·kg−1·min−1 and 0.24 μCi/min, respectively, for 4 min, after which rates were reduced to 3 mU·kg−1·min−1 insulin and 0.1 μCi/min [3-3H]glucose. Blood was collected by tail massage for plasma measurements at set time points, and a variable infusion of 20% dextrose was given to maintain euglycemia. Glucose turnover was calculated as the ratio of the [3-3H]glucose infusion rate to the specific activity of plasma glucose at the end of the basal infusion and during the last 40 min of the hyperinsulinemic infusion. Endogenous or hepatic glucose output represents the difference between the glucose infusion rate and the rate of glucose appearance. After the final blood sample, mice were euthanized with an intravenous injection of 150 mg/kg pentobarbital sodium, and tissues were harvested and frozen with aluminum forceps in liquid nitrogen. All tissues were stored at −80°C until later use.
Statistical analyses.
Data are reported as means ± SE, and comparisons were made between groups using two-tailed, unpaired Student's t-test or by two-way ANOVA with Sidak's multiple comparisons test where indicated. A P value of <0.05 was considered significant. Analyses were performed using GraphPad Prism 6. Blinding during experiments and analysis was used only where indicated.
RESULTS
Fecal lipid content is increased in Park2 KO mice.
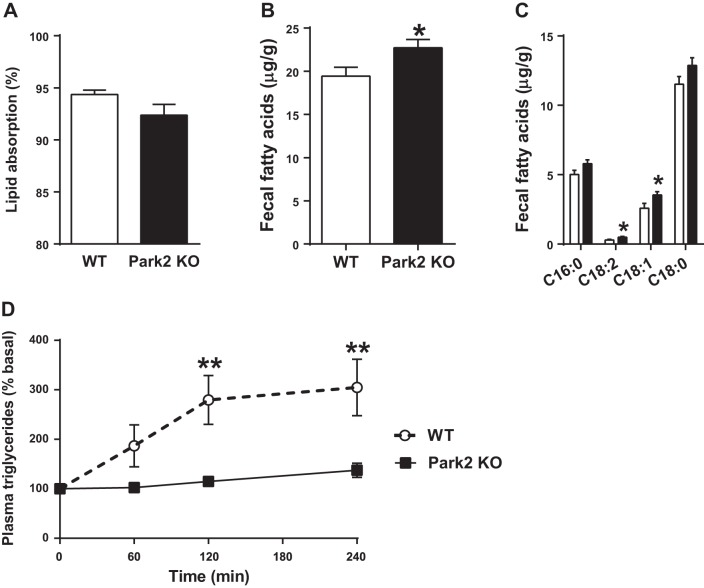
Park2 KO mice were previously shown to have slightly reduced body weight on regular chow after 6 mo of age (19, 37), as well as resistance to HFD-induced obesity (24). Interestingly, we observed a modest trend of ∼2% toward reduced intestinal lipid absorption in regular chow-fed Park2 KO mice, in which fat accounted for only 10% of the energy provided by the diet (P = 0.09; Fig. 1A). To determine the effect of Park2 deletion on intestinal lipid absorption during HFD feeding, WT and Park2 KO fecal samples were collected 4 days after initiating a HFD intervention. Total fecal fatty acid levels were ∼20% greater in Park2 KO mouse feces (P < 0.05, Fig. 1B). Palmitate (C16:0), linoleate (C18:2), oleate (C18:1), and stearate (C18:0), which account for ∼93% of the fatty acid species found in the HFD used, were measured to determine whether malabsorption of a particular fatty acid accounted for the overall effect. Fecal linoleate and oleate were significantly greater in Park2 KO feces (P < 0.05; Fig. 1C), and there were strong trends toward increased palmitate and stearate levels as well (P = 0.07 and P = 0.09, respectively; Fig. 1C), suggesting a generalized defect in dietary lipid absorption. Next, oral fat tolerance tests (OFTTs) were performed after an overnight fast to evaluate lipid absorption acutely in chow-fed mice. Mice were gavaged with 10 μl/kg body wt canola oil, and plasma triglyceride levels were determined over the course of 4 h. Plasma triglycerides increased ∼200% from baseline in WT mice and were significantly greater compared with Park2 KO mice, which displayed an ∼40% increase (Fig. 1D; P < 0.01). There was a significant effect of genotype on plasma triglyceride levels during the OFTT (P < 0.01; Fig. 1D), and the change in plasma triglycerides was significantly greater in WT mice at both the 120- and 240-min time points (P < 0.01; Fig. 1D).
Fig. 1.
Fecal dietary lipid levels are increased in Park2 knockout (KO) mice after short-term high-fat diet (HFD) feeding. A: lipid absorption in chow-fed mice measured as the ratio of behenic acid to dietary fatty acids in feces after 4 days of feeding a diet supplemented with sucrose polybehenate (n = 8 per group). B and C: feces were collected 4 days after start of HFD, and total (B) and specific (C) fatty acid content of lipids extracted from feces was determined by GC-MS (n = 15–16 per group). D: change in plasma triglyceride levels after overnight fast and oral gavage of 10 μl/kg canola oil at time 0 (n = 6 per group). Data are means ± SE. *P < 0.05, **P < 0.01 vs. WT. Data were analyzed by 2-way ANOVA (D), and Student's t-test (A–C).
Intestinal lipid absorption is reduced in Park2 KO mice.
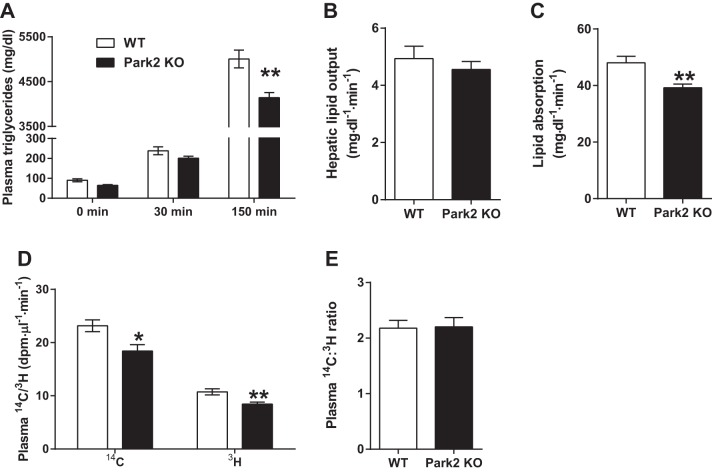
The OFTT suggested that intestinal lipid absorption was reduced in Park2 KO mice, but changes in hepatic lipid output or clearance of plasma triglycerides postabsorption during OFTT could also account for the effect. To address these alternative mechanisms, plasma triglyceride levels were measured after an overnight fast, followed by administration of poloxamer 407 to block triglyceride clearance. Plasma triglycerides were measured 30 min later, and an oral gavage of canola oil plus [14C]palmitate and [3H]triolein was administered. Consistent with the OFTT data, plasma triglycerides were reduced in the Park2 KO mice 120 min after lipid gavage (P < 0.01; Fig. 2A), suggesting that increased triglyceride clearance could not account for the change. There was no difference in plasma triglycerides at 30 min following poloxamer dosing prior to lipid gavage, and the rate of hepatic lipid output did not differ between groups (Fig. 2, A and B). However, the rate of lipid absorption over the 120-min interval was ∼20% less in Park2 KO compared with WT mice (P < 0.01; Fig. 2C), and, importantly, the rate of appearance of both the 14C and 3H tracers, which occur independently of hepatic lipid output, were similarly reduced by 20% in Park2 KO mice (P < 0.05 and P < 0.01, respectively; Fig. 2D). The increased rate of appearance of the 14C tracer compared with the 3H tracer in plasma of both groups likely reflected the fact that the palmitate tracer did not require hydrolysis prior to absorption, reesterification, and export as chylomicron triglyceride, whereas the [3H]triolein tracer did. The ratio of the rate of appearance of 14C to 3H in plasma can therefore serve as a surrogate for the rate of intestinal triglyceride hydrolysis, and, interestingly, there was no difference in this ratio between groups (Fig. 2E), suggesting that the deficit in lipid absorption in Park2 KO mice was not due to triglyceride hydrolysis.
Fig. 2.
Lipid absorption is reduced during oral fat tolerance test in Park2 KO mice. Mice were treated with 500 mg/kg poloxamer 407 after overnight fast at time 0 min followed by an intragastric dose of 10 μl/kg canola oil plus 23.3 μCi/ml [14C]palmitate and [3H]triolein at time 30 min, and plasma triglycerides were determined 120 min later. A: plasma triglyceride levels at indicated time points where 0 min represents fasting levels, 30 min post-poloxamer 407 levels and 150 min post-poloxamer and canola oil plus tracer levels. B: hepatic lipid output calculated as change in plasma triglyceride from 0 to 30 min. C: lipid absorption calculated as change in plasma triglycerides from 30 to 150 min corrected for the rate of hepatic lipid production in B. D: rate of appearance of 14C/3H tracers in plasma at 150 min. E: ratio of rate of appearance of 14C/3H tracers in plasma at 150 min. Data are means ± SE; n = 5–6 per group. *P < 0.05 and **P < 0.01 vs. WT; data were analyzed by Student's t-test.
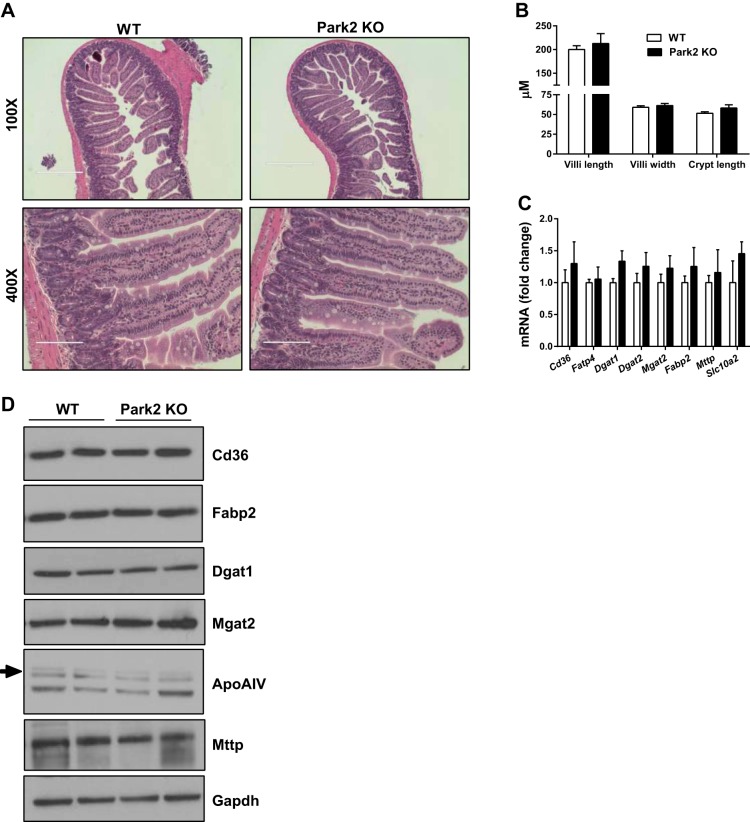
To determine whether the observed changes in intestinal lipid absorption were associated with morphological changes in the small intestine, representative sections of proximal, middle, and distal small intestine were collected from WT and Park2 KO mice and analyzed in a blinded fashion. Sections from both the WT and KO animals demonstrated normal histology findings, and no gross abnormalities in the architecture or inflammatory content within the specimens were identified (Fig. 3A; representative middle or jejunal small intestine). Furthermore, no epithelial abnormalities, including abnormal inflammatory cells, were identified in Park2 KO mice, and there were no differences in villi length or width, nor crypt length between groups (Fig. 3B). The expression of several genes involved in intestinal lipid transport and esterification, including the fatty acid transporters Cd36 and Fatp4, and the monoacylglycerol and diacylglycerol transferases Mgat2, Dgat1 and Dgat2 did not differ between groups (Fig. 3C). Likewise, expression of the intestinal fatty acid binding protein Fabp2, microsomal triglyceride transfer protein Mttp, or the sodium/bile acid transporter Slc10a2, did not differ (Fig. 3C). There was also no change in expression of several genes involved in bile acid metabolism within liver, including the nuclear receptors Fxr and Lxr, nor their transcriptional targets Shp, Cyp7a1, Cyp8b1, and Abcb11 (data not shown). Protein levels of many of these same genes did not differ in intestinal epithelial cell lysates when WT and Park2 KO mice were compared, including CD36, FABP2, DGAT1, MGAT2, MTTP, and APOA-IV (Fig. 3D), which is a critical protein component of chylomicrons that regulates intestinal triglyceride absorption (26).
Fig. 3.
Small intestine morphology appears normal in Park2 KO mice. A: representative images of middle small intestine (jejunum) stained with H&E. Bar, 400 μm at ×100 and 100 μm at ×400. B: villi length and width, and crypt length measurements determined at ×100 from H&E-stained small intestine shown in A (n = 3 per group). C: relative gene expression of factors involved in lipid absorption in small intestine expressed as fold change vs. WT (n = 6 per group). D: Western blots from intestinal epithelial cell lysates of factors involved in lipid absorption (representative images from n = 4 per group). Data are means ± SE and were analyzed by Student's t-test; no significant differences were detected.
Park2 KO mice are not protected from hepatic steatosis following intravenous lipid infusion.
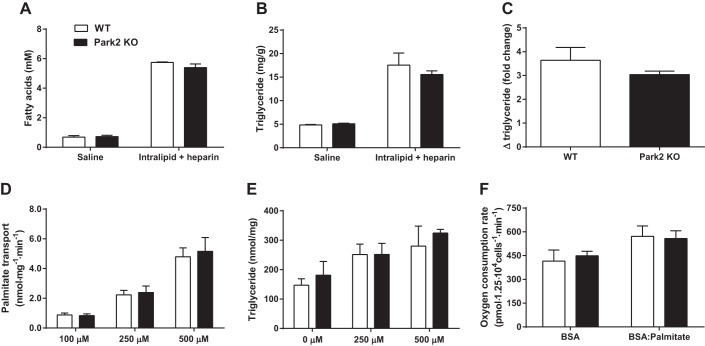
Previously, Park2 KO mice were shown to be protected from diet-induced obesity and hepatic steatosis (24), but whether the hepatic effect occurred independently of changes in body weight and adiposity was not determined. To address this question, WT and Park2 KO mice were given an intravenous infusion of saline control or Intralipid plus heparin to raise plasma fatty acids over the course of 180 min. There was no difference in plasma fatty acids between genotypes after either the saline or Intralipid plus heparin infusions, and both groups experienced an ∼5.5-fold increase in plasma fatty acid concentrations in response to the intravenous lipid challenge (Fig. 4A). Similarly, there was no difference in liver triglyceride levels between groups after either infusion; liver triglyceride levels increased 3.0- to 3.5-fold in response to lipid challenge in both groups (Fig. 4, B and C). These data suggest that protection from HFD-induced steatosis in the Park2 KO mice may be secondary to changes in body weight resulting from impaired intestinal lipid absorption. The change in triglyceride levels in heart after lipid infusion did not differ between groups, and the fold effect was notably lower than the fold effect in liver (∼1.2-fold vs. 3.25-fold increase; data not shown).
Fig. 4.
Park2 KO mice are not protected from hepatic steatosis during intravenous lipid challenge. Mice received either saline control (175 μl·kg−1·min−1) or Intralipid 20% lipid emulsion (175 μl·kg−1·min−1) plus heparin (0.4 U·kg−1·min−1) infusion after a 6-h fast to experimentally raise plasma fatty acid levels for 180 min. A: pPlasma nonesterified fatty acid levels following saline or Intralipid plus heparin infusion. B: liver triglyceride levels at the end of 180-min infusion. C: fold change in liver triglyceride levels after Intralipid plus heparin infusion for each genotype relative to saline-infused mice of the same genotype. D: palmitate transport rates in the presence of 100, 250, and 500 μM palmitate conjugated to BSA measured with [14C]palmitate in primary hepatocytes in triplicate. Data normalized to total protein levels. E: triglyceride levels measured from primary hepatocytes following overnight incubation with 0, 250, or 500 μM palmitate conjugated to BSA measured in duplicate. Data normalized to total protein levels. F: rates of primary hepatocyte respiration in response to 200 μM palmitate-BSA stimulation or BSA alone performed in sextupliate. Data are means ± SE; for A–C; n = 3 per group for saline; n = 5 per group for Intralipid plus heparin. For D, n = 4 per group; n = 3 per group in E and F. Data were analyzed by Student's t-test or 2-way ANOVA, and no significant differences between genotypes were detected.
To further explore the effect of Park2 deletion on hepatic fatty acid metabolism in a system not influenced by the observed changes in intestinal lipid absorption, primary hepatocytes were isolated from WT and Park2 KO mice. Rates of palmitate transport were measured using a [14C]palmitate tracer across a range of palmitate concentrations. There was a significant dose-dependent increase in palmitate transport rates with increasing palmitate concentrations in both groups (2-way ANOVA, main row effect P < 0.001); however, there was no difference between groups at any concentration (Fig. 4D). Next, primary hepatocytes were incubated overnight in the presence or absence of palmitate at two different concentrations to determine triglyceride accumulation in the presence of fatty acids. There was a significant effect of palmitate on hepatocyte triglyceride levels, specifically when we compared the 0 to 500 μM conditions (2-way ANOVA, main row effect P < 0.05; Sidak's multiple comparison test 0 vs. 500 P < 0.05), but there was no difference in triglyceride levels between WT and Park2 KO mice at any dose (Fig. 4E). Finally, hepatocyte respiration was measured in the presence and absence of 200 μM palmitate. Palmitate stimulation resulted in a significant increase in respiration in both groups that was not different (Fig. 4F; 2-way ANOVA, main row effect P = 0.05).
Park2 KO mice gain less fat mass during short-term HFD feeding.
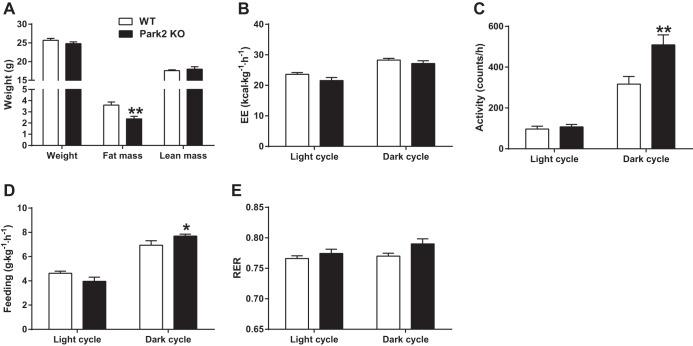
To further characterize the physiological effects of a HFD challenge prior to changes in body weight, WT and Park2 KO mice were studied in metabolic cages 1 wk after start of HFD. Following 1 wk of HFD feeding, there was no difference in body weight between WT and Park2 KO mice (25.6 ± 0.5 vs. 24.8 ± 0.5 g, P = 0.22; Fig. 5A) nor lean mass determined by proton-NMR (17.6 ± 0.2 vs. 18.0 ± 0.6 g, P = 0.54; Fig. 5A). Interestingly, fat mass was modestly, but significantly, reduced at this early time point (3.6 ± 0.3 vs. 2.4 ± 0.2 g, P < 0.01; Fig. 5A), consistent with the previously reported protection from diet-induced obesity after 6 wk HFD (24). There was no detectable difference in energy expenditure when we compared WT with Park2 KO mice during either the light or dark cycle (Fig. 5B), and accordingly, the 24-h average energy expenditure was similar between groups. Park2 KO mice were more active during the dark cycle than WT mice, but not during the light cycle, and dark cycle activity was increased by 60% (P < 0.01; Fig. 5C). A similar pattern of feeding behavior was observed; Park2 KO mice consumed significantly more during the dark cycle than WT mice (P < 0.05; Fig. 5D), whereas there was no detectable difference in feeding in Park2 KO mice during the light cycle. There was a modest trend toward reduced feeding in the light cycle in Park2 KO mice (P = 0.11; Fig. 5D), such that the 24-h average food intake was not different between groups. Interestingly, there was a modest trend toward an increased respiratory quotient during the dark cycle in the Park2 KO mice (Fig. 5E; P = 0.056).
Fig. 5.
Short-term HFD feeding alters energy balance in Park2 KO mice. After 1 wk of HFD, body weight-matched mice were housed in metabolic cage units and maintained on HFD. A: body weight and composition determined by 1H magnetic resonance spectroscopy. B: energy expenditure (EE) determined by indirect calorimetry. C: activity or X-ambulatory counts. D: feeding activity. E: respiratory exchange ratio (RER). Data are means ± SE; n = 7 per group. *P < 0.05 and **P < 0.01 vs. WT. Data were analyzed by Student's t-test.
Park2 KO mice are protected from HFD-induced insulin resistance independently of body weight differences.
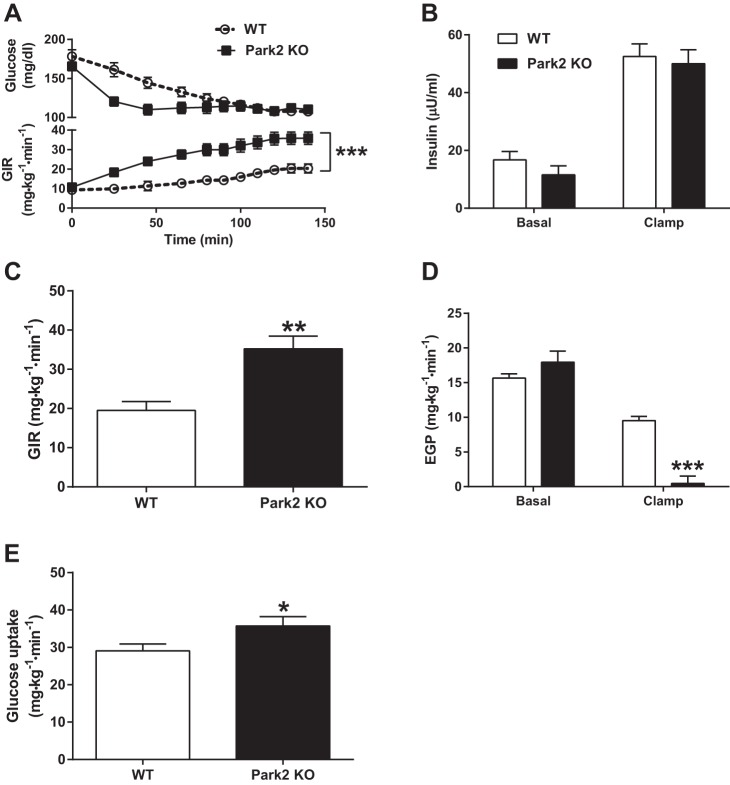
Next, hyperinsulinemic euglycemic clamps were performed after 1 wk of HFD to determine the effect of Park2 deletion on insulin sensitivity independently of differences in body weight. There was no difference in 6-h-fasted plasma glucose or insulin levels between groups, and plasma glucose and insulin levels were matched during the clamp at ∼120 mg/dl and 50 μU/ml, respectively (Fig. 6, A and B). The glucose infusion rate (GIR) required to maintain euglycemia was significantly greater in Park2 KO mice over the course of the 140-min infusion (Fig. 6A), and the average GIR during steady state or the last 40 min of the clamp was 1.8-fold greater in the Park2 KO mice, demonstrating improved whole body insulin sensitivity (Fig. 6C; P < 0.01). Consistent with the lack of difference in fasting plasma glucose, there was no difference in basal rates of endogenous glucose production (EGP) between groups (Fig. 6D). However, insulin-stimulated rates of EGP were markedly reduced in Park2 KO mice, demonstrating improved hepatic insulin sensitivity (Fig. 6D; P < 0.001). Peripheral insulin sensitivity was also improved in the Park2 KO mice, demonstrated by an ∼20% increase in insulin-stimulated glucose uptake (GU) during the clamp (Fig. 6E; P < 0.05). Overall, the improvement in hepatic insulin sensitivity accounted for ∼60% of the difference in whole body insulin sensitivity, while changes in peripheral glucose uptake accounted for 40% (ΔGIR = 15.7 mg·kg−1·min−1; ΔEGP = 9.1 mg·kg−1·min−1; ΔGU = 6.6 mg·kg−1·min−1).
Fig. 6.
Park2 KO mice are protected from HFD-induced insulin resistance independently of changes in body weight. Hyperinsulinemic euglycemic clamps were performed after 1-wk HFD and a morning 6-h fast. A: plasma glucose levels (top) and glucose infusion rate (GIR; bottom) during 140-min infusion. B: Basal (fasting) and clamped plasma insulin during 3 mU·kg−1·min−1 infusion. C: GIR during steady state or the last 40 min (100–140 min) of the clamp. D: endogenous glucose production (EGP) prior to clamp (basal or fasted) and during steady state. E: whole body glucose uptake during steady state. Data are means ± SE; n = 7–8 per group. *P < 0.05, **P < 0.01, ***P < 0.001 vs. WT. Data were analyzed by 2-way ANOVA (A) and Student's t-test (B–E).
DISCUSSION
Herein, we report the first detailed characterization of impaired intestinal lipid absorption in Park2 KO mice. We also provide a comprehensive analysis of the acute response to lipid oversupply in Park2 KO mice, before the potentially confounding development of divergence in body weight. Importantly, we provide evidence that the previously reported protection from HFD-induced liver steatosis in Park2 KO mice is likely secondary to altered triglyceride absorption, in that intravenous infusion of mice with a lipid emulsion or incubation of primary hepatocytes with palmitate results in similar changes in hepatic fatty acid uptake and triglyceride accumulation compared with WT mice. We detected a modest trend toward reduced lipid absorption in Park2 KO mice fed control chow, in which fat comprised only 10% of the kilocalories provided. Furthermore, the defect in intestinal lipid absorption was readily detected following short-term HFD feeding; fecal lipid levels were significantly increased in Park2 KO mice. Reduced plasma triglycerides following intragastric lipid gavage provided additional evidence that lipid absorption was impaired in Park2 KO mice. Importantly, we observed this effect in the absence and presence of a detergent to block triglyceride clearance. The finding of reduced appearance of isotopic triglyceride and fatty acid tracers in plasma after oral gavage in Park2 KO mice is also consistent with altered absorption. Metabolic cage studies in body weight-matched, 1-wk HFD-fed mice detected increased activity during the dark cycle and no difference in energy expenditure or feeding over the course of 24-h, although dark cycle feeding was significantly increased. Despite this, fat mass was reduced in Park2 KO mice during the short-term HFD challenge. Consistent with the apparent negative energy balance after 1-wk HFD, Park2 KO mice displayed improved whole body insulin sensitivity that was accounted for by both improved insulin-stimulated suppression of hepatic glucose production and whole body glucose uptake. We conclude that impaired intestinal lipid absorption contributes to the apparent negative energy balance phenotype of fat-fed Park2 KO mice.
Previously, Park2 KO mice were shown to be protected from HFD-induced obesity, hepatic steatosis, and insulin resistance after 6 wk of HFD feeding (24). However, the primary and secondary effects of the HFD on energy balance and insulin sensitivity in this model were unclear due to significant differences in body weight at the time of study, which can confound the interpretation of both metabolic cage studies and glucose/insulin tolerance tests (2, 22, 46). Specifically, when mice with significantly different body weights due to changes in adiposity are compared by metabolic cage, the data are often inappropriately corrected to body weight that in the heavier mice is due to changes in adipose and not lean mass. In the same vein, glucose dosing during GTT is typically made on the basis of body weight and does not take differences in body composition into account. A mouse with increased body weight due to changes in adiposity will therefore be inadvertently dosed with more glucose per gram lean mass, biasing the result toward impaired glucose tolerance in the heavier animal. We therefore evaluated Park2 KO mice using a series of acute lipid challenge studies under body weight-matched conditions. In contrast to the previous study, we detected no difference in energy expenditure during HFD feeding. It is possible that a small change in energy expenditure in response to short-term HFD feeding was below the level of detection of the metabolic cage units in our studies. Given the robust increase in activity during the dark cycle, it seems likely that energy expenditure would be increased in Park2 KO mice during this time, and we cannot rule out that this contributes to their protection from diet-induced obesity. However, proportionately equal changes in body weight and energy expenditure in the previous study suggest that the increased energy expenditure in HFD-fed Park2 mice may be confounded by normalization to decreased body weight (22, 24). In support of this interpretation, feeding behavior was disproportionately increased relative to the change in body weight in the previous study, suggesting a true increase in feeding behavior that we also observed during the dark cycle, but not over the course of 24 h, under body weight matched conditions. Importantly, activity measures, which are body weight independent, were similarly increased in both studies. Kim et al. (24) also observed improved insulin tolerance in HFD-fed Park2 KO mice, consistent with our observation of improved whole body insulin sensitivity.
However, we find that Park2 KO mice are as susceptible to lipid-induced hepatic steatosis as WT mice when lipids are delivered intravenously to bypass intestinal absorption, in contrast to the previous study (24). Furthermore, using primary hepatocytes from Park2 KO mice, we find no differences in palmitate transport, accumulation of triglyceride in the presence of exogenous palmitate, or changes in the rate of hepatocyte respiration in response to palmitate stimulation. In the model of Kim et al., PARK2 is upregulated in liver in response to HFD and ubiquitinates and stabilizes the fatty acid transporter CD36, such that in Park2 KO mice liver CD36 protein levels and hepatic steatosis are reduced during HFD feeding. The lack of difference in liver triglyceride levels after intravenous lipid challenge in our model could therefore result from similar levels of hepatic CD36 protein levels in chow-fed WT and Park2 KO mice (24). However, as noted by Kim et al., it seems unlikely that differences in CD36 protein levels in Park2 KO mice account for the entire whole body phenotype during HFD feeding. Indeed, CD36 KO mice do not precisely phenocopy Park2 KO mice and show no differences in liver fatty acid transport or fecal lipid levels, as previously reported (7, 12, 24). It therefore is likely that other systemic changes in lipid metabolism in the Park2 KO mice, such as intestinal malabsorption, mediate their resistance to HFD-induced obesity and hepatic steatosis. And although the Park2 KO mice used in these studies do not present with a robust Parkinson's-like phenotype (10, 15), we cannot rule out the possibility that central deletion of Park2 confers a change in systemic metabolism that contributes to their protection from nutritional stress.
The mechanism for reduced intestinal lipid absorption in the Park2 KO mice remains unclear. We found no gross differences in small intestine morphology between groups that could account for the effect. However, these observations were made in chow-fed mice. We therefore cannot rule out the possibility that changes in morphology in response to HFD contributed to the effect, although the observed trend toward reduced lipid absorption even on chow diet suggests this may not be the case. We also found no differences in gene or protein expression of a number of factors known to regulate key aspects of lipid absorption, including emulsification/hydrolysis, fatty acid transport, binding and reesterification, or chylomicron production, in the small intestine or liver. Of these factors, DGAT1 and MGAT2 are intriguing candidates. Although changes in DGAT1 and MGAT2 could not account for the impaired lipid absorption in the Park2 KO mice, these models provide some insight into how altering lipid absorption can impact whole body physiology and provide protection from HFD-induced obesity. Dgat1 and Mgat2 KO mice both appear normal on regular chow but are protected from HFD-induced obesity similarly to Park2 KO mice, and their plasma triglyceride levels are also reduced during OFTT (3, 43, 52). However, dietary fat is completely absorbed in these models, and their phenotypes seem to result from delayed triglyceride absorption, which partitions dietary fat toward energy use instead of storage, and increased energy expenditure with no change in activity or feeding (3, 5, 43, 52). In contrast, Park2 KO mice in our studies displayed no difference in energy expenditure despite increased dark cycle activity. There was also no difference in total energy intake, but Park2 KO mice consumed more during the dark cycle and less during the light cycle compared with controls and gained less fat mass within 1 wk of initiating the HFD. Interestingly, there is a growing body of evidence demonstrating that meal timing influences body weight gain, and mice restricted to dark cycle HFD feeding gain less weight than mice fed HFD ad libitum, despite matched caloric intake (1, 18, 40). Future studies to determine whether changes in gut motility or the kinetics of triglyceride absorption occur in the Park2 KO mice and contributed to their increased activity and apparent shift in time of day feeding behavior and protection from HFD-induced weight gain are of great interest.
The observations described above highlight the different phenotypic outcomes that result from delayed compared with reduced triglyceride absorption, namely increased energy expenditure in the case of the former and negative energy balance due to absorptive inefficiency in the latter. Importantly, both result in protection from HFD-induced obesity. Dgat1 and Mgat2 KO mice are also protected from HFD-induced insulin resistance (6, 35, 52) similarly to Park2 KO mice; this was associated with reduced skeletal muscle triglyceride and liver diacylglycerol levels in the case of Dgat1 KO mice (6). Although we did not determine ectopic lipid levels in our studies, the apparent negative energy balance in our model is consistent with the idea that Park2 KO mice are protected from HFD-induced insulin resistance in a body weight-independent fashion due to reduced accumulation of intracellular lipids in liver and skeletal muscle, which are associated with insulin resistance (42). Because there was a modest but significant reduction in fat mass in the Park2 KO mice during the clamp, we cannot rule out the possibility that a reduction(s) in proinflammatory cytokines, such as leptin, tumor necrosis factor-α or interleukin-6, which are positively associated with insulin resistance (29), contributed to the effect. The observation here that improvements in liver insulin sensitivity accounted for the majority of the whole body improvement in insulin action after short-term HFD feeding is consistent with the time course for the onset of insulin resistance in specific tissues in response to nutritional stress in rodents: liver develops insulin resistance first, followed by skeletal muscle (47).
Given PARK2's role in mitophagy and mitochondrial quality control (16, 34, 39), it is tempting to speculate that changes in mitochondrial function contribute to the impaired intestinal lipid absorption. Precedent for such a relationship was described in humans where mitochondrial DNA mutations resulting in respiratory chain defects were associated with reduced proliferation and increased apoptosis of colonic crypt cells taken from elderly subjects, who frequently present with intestinal malabsorption (36, 44). Mutation of the proofreading function of the mitochondrial DNA polymerase gamma in mice, which results in progressive mitochondrial DNA mutation and dysfunction, is also associated with reduced dietary fat absorption (13, 28, 45). Although there is strong evidence of reduced mitochondrial respiratory chain protein abundance and function in the ventral midbrain of Park2 KO mice, as well as susceptibility to mitochondrial dysfunction in cardiac myocytes (37, 39), no such evidence exists for Park2 KO intestinal epithelial cells, and this merits further study.
In summary, we provide direct evidence that intestinal lipid absorption is altered in Park2 KO mice and that changes in liver fatty acid transport do not contribute to the previously observed protection from HFD-induced hepatic steatosis in this model. Overall, these observations are consistent with the hypothesis that Park2 KO mice are protected from metabolic stress during HFD feeding secondarily, in part, to impaired intestinal lipid absorption, which reduces the metabolic load to which organs such as liver and skeletal muscle are exposed. These data highlight the limitations of the whole body Park2 KO model for addressing the role of PARK2 in specific organs in response to HFD-induced metabolic stress, as well as the need for tissue-specific models of PARK2 deletion to appropriately address such questions.
GRANTS
These studies were supported by funding from the National Institute of Diabetes and Digestive and Kidney Diseases (DK-099402 M. to J. Jurczak; DK-40936 to G. I. Shulman), and the Yale Diabetes Research Center (DK-45735).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
D.K.C., B.R.H., L.R.E., M.C.P., A.N., G.M.B., A.A., D.B.H., C.L., B.S.M., D.J.H., J.-P.G.C., G.W.C., and M.J.J. performed experiments; D.K.C., B.R.H., L.R.E., M.C.P., A.N., J.-P.G.C., G.W.C., and M.J.J. analyzed data; D.K.C., B.R.H., L.R.E., M.C.P., A.N., G.M.B., A.A., D.B.H., C.L., B.S.M., D.J.H., J.-P.G.C., R.M.O., G.W.C., G.I.S., and M.J.J. approved final version of manuscript; M.C.P., R.M.O., G.I.S., and M.J.J. edited and revised manuscript; R.M.O., G.I.S., and M.J.J. conception and design of research; R.M.O., G.I.S., and M.J.J. interpreted results of experiments; M.J.J. prepared figures; M.J.J. drafted manuscript.
ACKNOWLEDGMENTS
We thank Yanna Kosover and John Stack for providing technical expertise, specifically with GC-MS and Roche COBAS instrumentation, as well as the Yale MMPC for performing metabolic cage studies (DK-059635) and University of Cincinnati MMPC for lipid absorption measurements (DK-059630).
REFERENCES
- 1.Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. Circadian timing of food intake contributes to weight gain. Obes Silver Spring Md 17: 2100–2102, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayala JE, Samuel VT, Morton GJ, Obici S, Croniger CM, Shulman GI, Wasserman DH, McGuinness OP, NIH Mouse Metabolic Phenotyping Center Consortium . Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Dis Model Mech 3: 525–534, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buhman KK, Smith SJ, Stone SJ, Repa JJ, Wong JS, Knapp FF, Burri BJ, Hamilton RL, Abumrad NA, Farese RV. DGAT1 is not essential for intestinal triacylglycerol absorption or chylomicron synthesis. J Biol Chem 277: 25474–25479, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Cesari R, Martin ES, Calin GA, Pentimalli F, Bichi R, McAdams H, Trapasso F, Drusco A, Shimizu M, Masciullo V, D'Andrilli G, Scambia G, Picchio MC, Alder H, Godwin AK, Croce CM. Parkin, a gene implicated in autosomal recessive juvenile parkinsonism, is a candidate tumor suppressor gene on chromosome 6q25-q27. Proc Natl Acad Sci USA 100: 5956–5961, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen HC, Ladha Z, Smith SJ, Farese RV. Analysis of energy expenditure at different ambient temperatures in mice lacking DGAT1. Am J Physiol Endocrinol Metab 284: E213–E218, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Chen HC, Smith SJ, Ladha Z, Jensen DR, Ferreira LD, Pulawa LK, McGuire JG, Pitas RE, Eckel RH, Farese RV. Increased insulin and leptin sensitivity in mice lacking acyl CoA:diacylglycerol acyltransferase 1. J Clin Invest 109: 1049–1055, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coburn CT, Knapp FF, Febbraio M, Beets AL, Silverstein RL, Abumrad NA. Defective uptake and utilization of long chain fatty acids in muscle and adipose tissues of CD36 knockout mice. J Biol Chem 275: 32523–32529, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Coelln von R, Thomas B, Savitt JM, Lim KL, Sasaki M, Hess EJ, Dawson VL, Dawson TM. Loss of locus coeruleus neurons and reduced startle in parkin null mice. Proc Natl Acad Sci USA 101: 10744–10749, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dawson TM, Dawson VL. The role of parkin in familial and sporadic Parkinson's disease. Mov Disord Off J Mov Disord Soc 25, Suppl 1: S32–S39, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dawson TM, Ko HS, Dawson VL. Genetic animal models of Parkinson's disease. Neuron 66: 646–661, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doss-Pepe EW, Chen L, Madura K. Alpha-synuclein and parkin contribute to the assembly of ubiquitin lysine 63-linked multiubiquitin chains. J Biol Chem 280: 16619–16624, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Drover VA, Ajmal M, Nassir F, Davidson NO, Nauli AM, Sahoo D, Tso P, Abumrad NA. CD36 deficiency impairs intestinal lipid secretion and clearance of chylomicrons from the blood. J Clin Invest 115: 1290–1297, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fox RG, Magness S, Kujoth GC, Prolla TA, Maeda N. Mitochondrial DNA polymerase editing mutation, PolgD257A, disturbs stem-progenitor cell cycling in the small intestine and restricts excess fat absorption. Am J Physiol Gastrointest Liver Physiol 302: G914–G924, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujiwara M, Marusawa H, Wang HQ, Iwai A, Ikeuchi K, Imai Y, Kataoka A, Nukina N, Takahashi R, Chiba T. Parkin as a tumor suppressor gene for hepatocellular carcinoma. Oncogene 27: 6002–6011, 2008. [DOI] [PubMed] [Google Scholar]
- 15.Goldberg MS, Fleming SM, Palacino JJ, Cepeda C, Lam HA, Bhatnagar A, Meloni EG, Wu N, Ackerson LC, Klapstein GJ, Gajendiran M, Roth BL, Chesselet MF, Maidment NT, Levine MS, Shen J. Parkin-deficient mice exhibit nigrostriatal deficits but not loss of dopaminergic neurons. J Biol Chem 278: 43628–43635, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Greene JC, Whitworth AJ, Kuo I, Andrews LA, Feany MB, Pallanck LJ. Mitochondrial pathology and apoptotic muscle degeneration in Drosophila parkin mutants. Proc Natl Acad Sci USA 100: 4078–4083, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hampe C, Ardila-Osorio H, Fournier M, Brice A, Corti O. Biochemical analysis of Parkinson's disease-causing variants of Parkin, an E3 ubiquitin-protein ligase with monoubiquitylation capacity. Hum Mol Genet 15: 2059–2075, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, Leblanc M, Chaix A, Joens M, Fitzpatrick JAJ, Ellisman MH, Panda S. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab 15: 848–860, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Itier JM, Ibanez P, Mena MA, Abbas N, Cohen-Salmon C, Bohme GA, Laville M, Pratt J, Corti O, Pradier L, Ret G, Joubert C, Periquet M, Araujo F, Negroni J, Casarejos MJ, Canals S, Solano R, Serrano A, Gallego E, Sanchez M, Denefle P, Benavides J, Tremp G, Rooney TA, Brice A, Garcia de Yebenes J. Parkin gene inactivation alters behaviour and dopamine neurotransmission in the mouse. Hum Mol Genet 12: 2277–2291, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Jandacek RJ, Heubi JE, Tso P. A novel, noninvasive method for the measurement of intestinal fat absorption. Gastroenterology 127: 139–144, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Jurczak MJ, Lee AH, Jornayvaz FR, Lee HY, Birkenfeld AL, Guigni BA, Kahn M, Samuel VT, Glimcher LH, Shulman GI. Dissociation of inositol-requiring enzyme (IRE1α)-mediated c-Jun N-terminal kinase activation from hepatic insulin resistance in conditional X-box-binding protein-1 (XBP1) knock-out mice. J Biol Chem 287: 2558–2567, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaiyala KJ, Schwartz MW. Toward a more complete (and less controversial) understanding of energy expenditure and its role in obesity pathogenesis. Diabetes 60: 17–23, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kane LA, Lazarou M, Fogel AI, Li Y, Yamano K, Sarraf SA, Banerjee S, Youle RJ. PINK1 phosphorylates ubiquitin to activate Parkin E3 ubiquitin ligase activity. J Cell Biol 205: 143–153, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim KY, Stevens MV, Akter MH, Rusk SE, Huang RJ, Cohen A, Noguchi A, Springer D, Bocharov AV, Eggerman TL, Suen DF, Youle RJ, Amar M, Remaley AT, Sack MN. Parkin is a lipid-responsive regulator of fat uptake in mice and mutant human cells. J Clin Invest 121: 3701–3712, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, Mizuno Y, Shimizu N. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 392: 605–608, 1998. [DOI] [PubMed] [Google Scholar]
- 26.Kohan AB, Wang F, Lo CM, Liu M, Tso P. ApoA-IV: current and emerging roles in intestinal lipid metabolism, glucose homeostasis, and satiety. Am J Physiol Gastrointest Liver Physiol 308: G472–G481, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koyano F, Okatsu K, Kosako H, Tamura Y, Go E, Kimura M, Kimura Y, Tsuchiya H, Yoshihara H, Hirokawa T, Endo T, Fon EA, Trempe JF, Saeki Y, Tanaka K, Matsuda N. Ubiquitin is phosphorylated by PINK1 to activate parkin. Nature 510: 162–166, 2014. [DOI] [PubMed] [Google Scholar]
- 28.Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY, Sullivan R, Jobling WA, Morrow JD, Van Remmen H, Sedivy JM, Yamasoba T, Tanokura M, Weindruch R, Leeuwenburgh C, Prolla TA. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 309: 481–484, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Kwon H, Pessin JE. Adipokines mediate inflammation and insulin resistance. Front Endocrinol (Lausanne) 4: 71, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lazarou M, Sliter DA, Kane LA, Sarraf SA, Wang C, Burman JL, Sideris DP, Fogel AI, Youle RJ. The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature 524: 309–314, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lücking CB, Dürr A, Bonifati V, Vaughan J, De Michele G, Gasser T, Harhangi BS, Meco G, Denèfle P, Wood NW, Agid Y, Brice A, French Parkinson's Disease Genetics Study Group, European Consortium on Genetic Susceptibility in Parkinson's Disease. Association between early-onset Parkinson's disease and mutations in the parkin gene. N Engl J Med 342: 1560–1567, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Morett E, Bork P. A novel transactivation domain in parkin. Trends Biochem Sci 24: 229–231, 1999. [DOI] [PubMed] [Google Scholar]
- 33.Narendra DP, Youle RJ. Targeting mitochondrial dysfunction: role for PINK1 and Parkin in mitochondrial quality control. Antioxid Redox Signal 14: 1929–1938, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol 183: 795–803, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nelson DW, Gao Y, Yen MI, Yen CLE. Intestine-specific deletion of acyl-CoA:monoacylglycerol acyltransferase (MGAT) 2 protects mice from diet-induced obesity and glucose intolerance. J Biol Chem 289: 17338–17349, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nooteboom M, Johnson R, Taylor RW, Wright NA, Lightowlers RN, Kirkwood TBL, Mathers JC, Turnbull DM, Greaves LC. Age-associated mitochondrial DNA mutations lead to small but significant changes in cell proliferation and apoptosis in human colonic crypts. Aging Cell 9: 96–99, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palacino JJ, Sagi D, Goldberg MS, Krauss S, Motz C, Wacker M, Klose J, Shen J. Mitochondrial dysfunction and oxidative damage in parkin-deficient mice. J Biol Chem 279: 18614–18622, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Perez FA, Palmiter RD. Parkin-deficient mice are not a robust model of parkinsonism. Proc Natl Acad Sci USA 102: 2174–2179, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Piquereau J, Godin R, Deschênes S, Bessi VL, Mofarrahi M, Hussain SN, Burelle Y. Protective role of PARK2/Parkin in sepsis-induced cardiac contractile and mitochondrial dysfunction. Autophagy 9: 1837–1851, 2013. [DOI] [PubMed] [Google Scholar]
- 40.Sherman H, Genzer Y, Cohen R, Chapnik N, Madar Z, Froy O. Timed high-fat diet resets circadian metabolism and prevents obesity. FASEB J 26: 3493–3502, 2012. [DOI] [PubMed] [Google Scholar]
- 41.Shimura H, Hattori N, Kubo i S, Mizuno Y, Asakawa S, Minoshima S, Shimizu N, Iwai K, Chiba T, Tanaka K, Suzuki T. Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nat Genet 25: 302–305, 2000. [DOI] [PubMed] [Google Scholar]
- 42.Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med 371: 2237–2238, 2014. [DOI] [PubMed] [Google Scholar]
- 43.Smith SJ, Cases S, Jensen DR, Chen HC, Sande E, Tow B, Sanan DA, Raber J, Eckel RH, Farese RV. Obesity resistance and multiple mechanisms of triglyceride synthesis in mice lacking Dgat. Nat Genet 25: 87–90, 2000. [DOI] [PubMed] [Google Scholar]
- 44.Thomson ABR. Small intestinal disorders in the elderly. Best Pract Res Clin Gastroenterol 23: 861–874, 2009. [DOI] [PubMed] [Google Scholar]
- 45.Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, Bohlooly -YM, Gidlöf S, Oldfors A, Wibom R, Törnell J, Jacobs HT, Larsson NG. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 429: 417–423, 2004. [DOI] [PubMed] [Google Scholar]
- 46.Tschöp MH, Speakman JR, Arch JRS, Auwerx J, Brüning JC, Chan L, Eckel RH, Farese RV, Galgani JE, Hambly C, Herman MA, Horvath TL, Kahn BB, Kozma SC, Maratos-Flier E, Müller TD, Münzberg H, Pfluger PT, Plum L, Reitman ML, Rahmouni K, Shulman GI, Thomas G, Kahn CR, Ravussin E. A guide to analysis of mouse energy metabolism. Nat Methods 9: 57–63, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Turner N, Kowalski GM, Leslie SJ, Risis S, Yang C, Lee-Young RS, Babb JR, Meikle PJ, Lancaster GI, Henstridge DC, White PJ, Kraegen EW, Marette A, Cooney GJ, Febbraio MA, Bruce CR. Distinct patterns of tissue-specific lipid accumulation during the induction of insulin resistance in mice by high-fat feeding. Diabetologia 56: 1638–1648, 2013. [DOI] [PubMed] [Google Scholar]
- 48.Vatner DF, Majumdar SK, Kumashiro N, Petersen MC, Rahimi Y, Gattu AK, Bears M, Camporez JPG, Cline GW, Jurczak MJ, Samuel VT, Shulman GI. Insulin-independent regulation of hepatic triglyceride synthesis by fatty acids. Proc Natl Acad Sci USA 112: 1143–1148, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wauer T, Simicek M, Schubert A, Komander D. Mechanism of phospho-ubiquitin-induced PARKIN activation. Nature 524: 370–374, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Whitworth AJ, Theodore DA, Greene JC, Benes H, Wes PD, Pallanck LJ. Increased glutathione S-transferase activity rescues dopaminergic neuron loss in a Drosophila model of Parkinson's disease. Proc Natl Acad Sci USA 102: 8024–8029, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Williams JA, Ni HM, Ding Y, Ding WX. Parkin regulates mitophagy and mitochondrial function to protect against alcohol-induced liver injury and steatosis in mice. Am J Physiol Gastrointest Liver Physiol 309: G324–G340, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yen CLE, Cheong ML, Grueter C, Zhou P, Moriwaki J, Wong JS, Hubbard B, Marmor S, Farese RV. Deficiency of the intestinal enzyme acyl CoA:monoacylglycerol acyltransferase-2 protects mice from metabolic disorders induced by high-fat feeding. Nat Med 15: 442–446, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Y, Gao J, Chung KK, Huang H, Dawson VL, Dawson TM. Parkin functions as an E2-dependent ubiquitin- protein ligase and promotes the degradation of the synaptic vesicle-associated protein, CDCrel-1. Proc Natl Acad Sci USA 97: 13354–13359, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]