Abstract
The purpose of this study was to determine whether plasma lactate and skeletal muscle glucose regulatory pathways, specifically PDH dephosphorylation, are impaired during hyperinsulinemic conditions in middle- to older-aged individuals and determine whether exercise training could improve key variables responsible for skeletal muscle PDH regulation. Eighteen young (19–29 yr; n = 9 males and 9 females) and 20 middle- to older-aged (57–82 yr; n = 10 males and 10 females) individuals underwent a 2-h euglycemic hyperinsulinemic clamp. Plasma samples were obtained at baseline and at 30, 50, 90, and 120 min for analysis of lactate, and skeletal muscle biopsies were performed at 60 min for analysis of protein associated with glucose metabolism. In response to insulin, plasma lactate was elevated in aged individuals when normalized to insulin action. Insulin-stimulated phosphorylation of skeletal muscle PDH on serine sites 232, 293, and 300 decreased in young individuals only. Changes in insulin-stimulated PDH phosphorylation were positively related to changes in plasma lactate. No age-related differences were observed in skeletal muscle phosphorylation of LDH, GSK-3α, or GSK-3β in response to insulin or PDP1, PDP2, PDK2, PDK4, or MPC1 total protein. Twelve weeks of endurance- or strength-oriented exercise training improved insulin-stimulated PDH dephosphorylation, which was related to a reduced lactate response. These findings suggest that impairments in insulin-induced PDH regulation in a sedentary aging population contribute to impaired glucose metabolism and that exercise training is an effective intervention for treating metabolic inflexibility.
Keywords: pyruvate dehydrogenase, age
aging is associated with a number of detrimental health issues, including insulin resistance and type 2 diabetes (T2D). Skeletal muscle dysfunction occurs during the aging process, involving impaired glucose uptake (10, 41, 46), increased intramuscular lipid (12), and reduced mitochondria oxidative capacity (21, 46), and is speculated to contribute to age-related whole body insulin resistance. Metabolic flexibility is defined as the capacity of an organism to efficiently adjust substrate oxidation to match fuel availability (26) and is thought to protect individuals from insulin resistance (25). Recent research suggests that aging is associated with the inability to switch from lipid to glucose oxidation during insulin stimulation (42), which could explain the increased incidence of type 2 diabetes in the elderly. Despite this knowledge, the role of skeletal muscle and the cellular mechanism(s) responsible for metabolic inflexibility, especially in the elderly, remain unclear and warrant further investigation.
A number of proteins are responsible for controlling the fate of glucose during insulin-stimulated uptake, which can be altered by age (41), disease (19), and energy demands (53). Insulin-stimulated glucose enters skeletal muscle and becomes phosphorylated to glucose 6-phosphate by the enzyme hexokinase and either is stored as intramuscular glycogen in response to increased GSK-3 phosphorylation and glycogen synthase activity or enters glycolysis (4, 13, 37, 47). The end product of glycolysis, pyruvate, enters the mitochondria via the mitochondrial pyruvate carrier (MPC) (18) and is converted to either acetyl-CoA by pyruvate dehydrogenase (PDH) for oxidation (oxidative metabolism) (45) or lactate via lactate dehydrogenase (LDH; anaerobic metabolism). It is thought that imbalances in any of these pivotal proteins may contribute to impairments in metabolic flexibility and the ultimate fate of glucose during insulin-stimulated glucose uptake.
Elevated plasma lactate is present in insulin-resistant conditions, including obesity and type 2 diabetes (2, 49), and has been shown to be a risk factor for the development of type 2 diabetes (23), suggesting that a diminished oxidative capacity may precede the onset of diabetes (47). Lactate has been reported to inhibit insulin-stimulated glucose uptake by inhibiting glycolysis and insulin signaling (7), indicating that the cellular mechanism(s) responsible for increasing lactate, especially during hyperinsulinemia, is unfavorable. Although not fully understood, one line of research suggests that lactate accumulation occurs as a result of pyruvate production exceeding pyruvate oxidation (2), possibly implicating the role of PDH.
During insulin-stimulated conditions, PDH activity has been reported to increase in healthy lean individuals (8, 27, 37, 39) but is impaired during lipid-induced insulin resistance (39), suggesting a link between insulin resistance and impaired PDH regulation. Exercise-induced PDH activity has been reported to be attenuated in elderly compared with young individuals (17), providing evidence that PDH activity may be compromised with aging, but it remains unclear whether other physiological conditions that favor glucose oxidation are impacted. The regulation of PDH activity is under the control of posttranslational modifications, including phosphorylation/dephosphorylation on at least three different serine residues (Ser232, Ser293, and Ser300). Previous research suggests that phosphorylation on any of these serine sites has the ability to inhibit PDH activity (29). An inverse linear relationship between PDH activity and PDH phosphorylation has been demonstrated (43), with phosphorylation (inactivation) being regulated by PDH kinases (PDK) 1–4 and dephosphorylation (activation) under the control of pyruvate dehydrogenase phosphatase (PDP) 1–2. In vitro research has demonstrated that PDK1, which is only modestly expressed in skeletal muscle, is capable of phosphorylating all three serine sites, whereas PDK2-4 may phosphorylate only Ser293 and Ser300 (30). PDP1 has been reported to be expressed in higher concentrations in skeletal muscle compared with PDP2, but both isoforms appear capable of dephosphorylating the three PDH serine sites (24). To date, it remains unclear how human aging may influence the regulation of PDH dephosphorylation during hyperinsulinemia.
Collectively, these findings suggest that with aging, metabolic flexibility is attenuated in response to physiological stimuli; however, the cellular mechanisms remain unknown. The purpose of the current study was to determine whether plasma lactate and skeletal muscle glucose regulatory pathways, including PDH dephosphorylation, are impaired during hyperinsulinemic conditions in middle- to older-aged individuals and whether exercise training could improve key variables responsible for skeletal muscle PDH regulation.
METHODOLOGY
Participants.
Eighteen young (19–29 yr; n = 9 males and 9 females) and 20 middle- to older-aged (57–82 yr; n = 10 males and 10 females) individuals were recruited from a larger study examining aging, exercise training, and insulin sensitivity (10). A detailed description of subject inclusion/exclusion criteria was published previously (10). Briefly, all participants were nonsmokers and participated in <1 h/wk of organized physical activity. Individuals with diabetes, heart disease, and metabolic disorders and those taking lipid-altering medications were excluded. In an attempt to study a representative population reflective of the “normal” aging process, BMI was between the 25th and 75th percentiles of the US population for the respective decade of age (38). Written, informed consent was obtained, and the protocol was in accordance with the Declaration of Helsinki and approved by the East Carolina University Policy and Review Committee on Human Research.
Euglycemic hyperinsulinemic clamp.
Subjects reported to the laboratory at 0700 after a 12-h overnight fast. A 2-h euglycemic hyperinsulinemic clamp was used to determine insulin sensitivity, as described previously (10, 20). Briefly, insulin was infused for 120 min at a rate of 100 mU·m2·min−1. Blood samples were obtained every 5 min and autoanalyzed for glucose (YSI 2300 STAT Plus Glucose and Lactate Analyzer; YSI, Yellow Springs, OH), with glucose infusion (20% dextrose) adjusted as needed to maintain euglycemia. A steady-state M value was determined from the final 30 min of the clamp (14). Blood plasma was obtained prior to insulin infusion and at 30, 50, 90, and 120 min and stored at −80°C for the subsequent analysis of lactate. A biopsy specimen was obtained from the vastus lateralis with the percutaneous muscle biopsy technique at baseline and at 60 min of the clamp. Tissue samples were immediately frozen in liquid nitrogen for subsequent analyses.
Exercise training.
A detailed description of exercise training has been reported previously (10). Briefly, subjects were randomized into a 12-wk endurance (n = 10 young and 10 aged) or strength (n = 8 young and 10 aged) training program. Endurance training consisted of exercising on a graded treadmill, stationary cycle, or elliptical trainer at 70–75% V̇o2 peak for a total of 180 min/wk (3–4 sessions/wk). Strength training consisted of five upper body exercises (chest press, latissimus pulldown, seated row, triceps pulldown, and biceps curl) and three lower body exercises (leg press, leg extension, and leg curl). Subjects performed two to three sets of 10–12 repetitions to failure three times/wk (∼45 min/session). All subjects performed preliminary cardiovascular and body composition measurements, which were repeated during the final week of training and were reported previously (10). V̇o2 peak was measured with an incremental maximal treadmill test (16), with expired gases analyzed continuously (TrueMax 2400; ParvoMedics, Sandy, UT). Body composition was measured by dual X-ray absorptiometry. The euglycemic hyperinsulinemic clamp with muscle biopsies was performed before exercise training and ∼40 h after the final exercise training session.
Plasma lactate and Western blot procedures.
Plasma lactate was analyzed in duplicate using the Sigma Lactate II colorimetric assay (Sigma, St. Louis, MO). Skeletal muscle was homogenized and protein content determined as described previously (9, 10). Twenty to 25 μg of cellular protein was separated by SDS-PAGE, electrotransferred onto polyvinylidene difluoride membranes (Millipore, Billerica, MA), and probed overnight with Cell Signaling Technology (Beverly, MA) antibodies for phosphorylated (p)-GSK-3α-Ser21, p-GSK-3β-Ser9, GSK-3α, GSK-3β, p-GS (glycogen synthase)-Ser641, GS, p-LDH-Tyr10, LDH-A, hexokinase II, MPC1, CalBiochem (Darmstadt, Germany) antibodies for p-PDH-E1α-Ser232, p-PDH-E1α-Ser293, and p-PDH-E1α-Ser300, Abcam (Cambridge, MA) antibodies for PDP1, PDP2, and PDK2, Sigma antibody for PDK4, and Santa Cruz Biotechnology (Santa Cruz, CA) antibody for PDH-E1a. Samples were normalized to a control sample on each gel, and phosphorylation levels were additionally normalized to their corresponding total protein after membranes were stripped, as reported previously (10). Nonphosphorylated total protein was normalized to actin (Santa Cruz Biotechnology).
PDH activity.
PDH activity (in the active form) was determined using the PDH Activity Colorimetric Assay Kit (Biovision, Mountain View, CA) according to the manufacturer's instructions, with minor modifications. Briefly, PDH activity was determined using 10 mg of skeletal muscle tissue homogenized in buffer containing NaF (phosphatase inhibitor) and dichloroacetic acid (kinase inhibitor). PDH activity was measured by the kinetic reduction of NAD+ to NADH, which resulted in a colorimetric (450 nm) product proportional to the enzymatic activity present.
Statistics.
Analyses were performed using SPSS version 21.0 software (SPSS, Chicago, IL). Pearson correlation coefficients and stepwise regression analysis were used to determine associations. Comparisons between young and aged individuals under basal and insulin-stimulated conditions as well as before and after exercise training were performed with repeated-measures ANOVA. Significant main effects and interactions were further analyzed using unpaired (age group) and paired (pre- vs. posttraining and baseline vs. insulin-stimulated) contrast-contrast comparisons. Data are presented as means ± SE. Statistical significance was defined as P < 0.05.
RESULTS
Subject characteristics and plasma lactate.
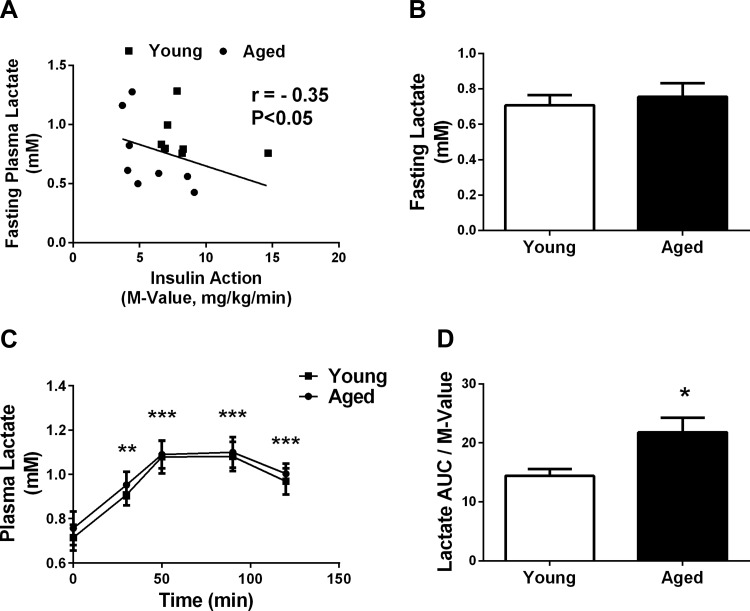
Aged individuals had lower whole body insulin action (M value), aerobic capacity, and higher percent body fat, as we published previously (10). There was a significant negative relationship between fasting plasma lactate and whole body insulin action (r = −0.35, P < 0.05; Fig. 1A); however, fasting plasma lactate did not differ between the two age groups (P = 0.62; Fig. 1B). In response to the hyperinsulinemic euglycemic clamp, plasma lactate levels were significantly increased by 30 min (∼25%, P < 0.0005) and further increased at 50 min (∼50%, P < 0.0001), irrespective of age (Fig. 1C). Plasma lactate area under the curve (AUC) during the hyperinsulinemic euglycemic clamp did not differ between the age groups; however, when expressed relative to insulin action, lactate AUC was greater (51%, P < 0.05; Fig. 1D) in the aged group.
Fig. 1.
Plasma lactate during fasting and hypersinsulinemic conditions. A: relationship of fasting plasma lactate and whole body insulin action in the whole group (n = 32). ■, Response from young individuals; ●, response from aged individuals. B: absolute fasting plasma lactate in young (n = 16) and aged (n = 16) individuals. C and D: plasma lactate (C) and plasma lactate relative to insulin action (D) in response to a hyperinsulinemic euglycemic clamp in young (n = 16) and aged individuals (n = 16). *P < 0.05 vs. young; **P < 0.0005; ***P < 0.0001 vs. baseline.
Skeletal muscle PDH activity in response to the hyperinsulinemic euglycemic clamp and relationship with PDH phosphorylation status.
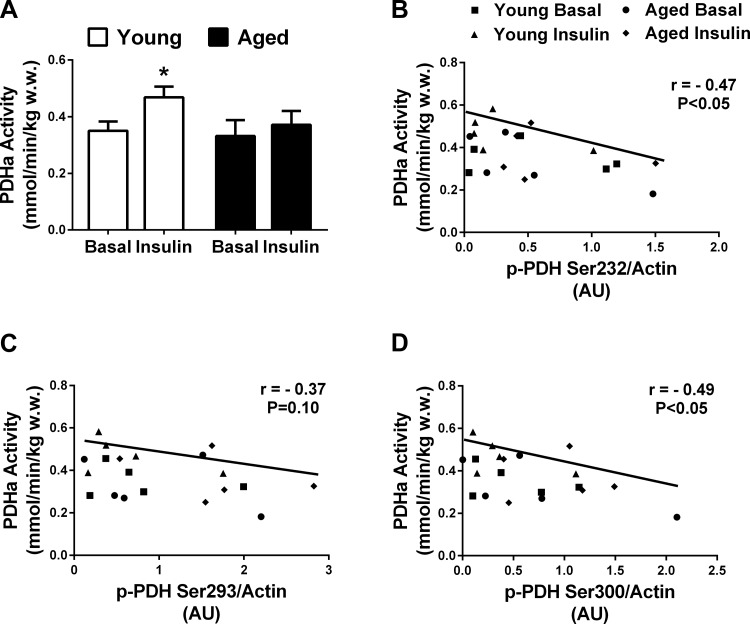
Due to limited skeletal muscle tissue, PDH activity was determined only during basal and insulin-stimulated conditions in five young (mean age: 24 yr) and five aged (mean age: 71 yr) individuals (Fig. 2A). In the fasted state, PDH activity did not differ between the two age groups. In response to insulin, PDH activity increased in the young (∼34%, P < 0.05; Fig. 2A) but not the aged individuals. Skeletal muscle PDH activity (all age and conditions pooled) was negatively associated with PDH phosphorylation on serine sites 232 (r = −0.47, P < 0.05; Fig. 2B), 293 (r = −0.37, P = 0.10; Fig. 2C), and 300 (r = −0.49, P < 0.05; Fig. 2D).
Fig. 2.
Skeletal muscle insulin-stimulated (1 h) pyruvate dehydrogenase (PDH) activity is impaired in aged individuals, and PDH activity is negatively associated with PDH phosphorylation. A: skeletal muscle PDH activity under basal and insulin-stimulated conditions in a subset of young (n = 5) and aged individuals (n = 5). B–D: skeletal muscle PDH activity and skeletal muscle PDH phosphorylation on serine site 232 (B), 293 (C), and 300 (D); ■, data points for young individuals in the basal state; ●, data points for aged individuals in the basal state; ▲, data points for young individuals in the insulin-stimulated state; ⧫, aged individuals in the insulin-stimulated state.
Skeletal muscle signaling response during hyperinsulinemic euglycemic clamp.
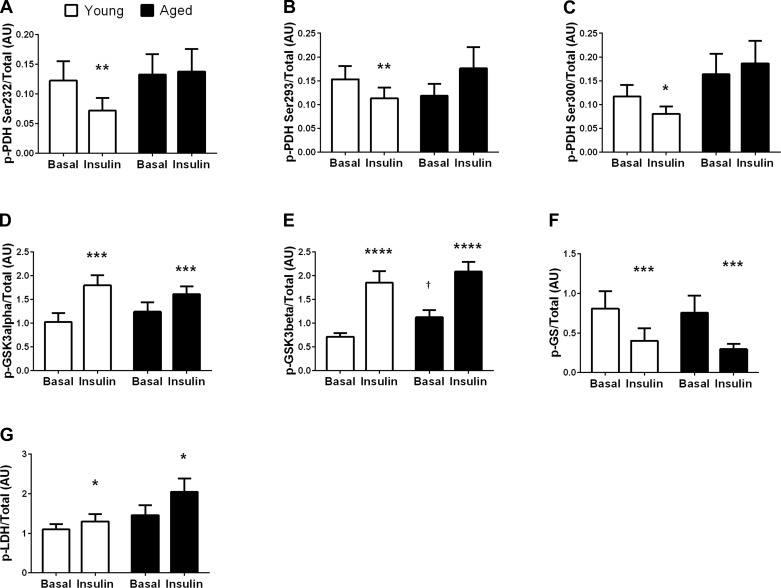
In the fasted state, skeletal muscle GSK-3β phosphorylation was greater in aged compared with young individuals (∼55%, P < 0.05; Fig. 3E), whereas fasting skeletal muscle phosphorylation of PDH (Fig. 3, A–C), GSK-3α (Fig. 3D), GS (Fig. 3F), and LDH (Fig. 3G) did not differ based on age or sex.
Fig. 3.
Phosphorylated protein responsible for skeletal muscle glucose metabolism under basal and insulin-stimulated (1 h) conditions. Phosphorylation levels of skeletal muscle PDH on sites Ser232 (n = 15 young and 16 aged; A), Ser293 (n = 16 young and 16 aged; B), Ser300 (n = 14 young and 15 aged; C), GSK-3α (n = 16 young and 16 aged; D), GSK-3β (n = 16 young and 16 aged; E), glycogen synthase (GS) (n = 6 young and 6 aged, due to limited tissue; F), and lactate dehydrogenase (LDH) (n = 16 young and 16 aged) in response to basal and hyperinsinsulinemic conditions Phosphorylation data are normalized to total protein. *P < 0.05, **P < 0.01, ***P < 0.005, and ****P < 0.0001 vs. basal condition; †P < 0.05 vs. young individuals under the same condition.
There was a significant interaction between age and the insulin response of PDH Ser232, Ser293, and Ser300, as the young individuals decreased phosphorylation by −43 (P < 0.01, Fig. 3A), −26 (P < 0.01, Fig. 3B), and −31% (P < 0.05; Fig. 3C), respectively, whereas PDH phosphorylation did not change in aged individuals. There was a tendency for PDH Ser293 phosphorylation to increase in aged individuals (49%, P = 0.09, Fig. 3B). Insulin infusion increased skeletal muscle phosphorylation of GSK-3α (∼50%, P < 0.005; Fig. 3D), GSK-3β (∼115%, P < 0.0001; Fig. 3E), and LDH-A (∼30%, P < 0.05; Fig. 3G) and decreased phosphorylation of GS (∼−55%, P < 0.005; Fig. 3F), regardless of age or sex. Insulin stimulation had no effect on total protein content. Significant changes with insulin stimulation were similar whether phosphorylation status was expressed relative to total protein (Fig. 3) or relative to actin.
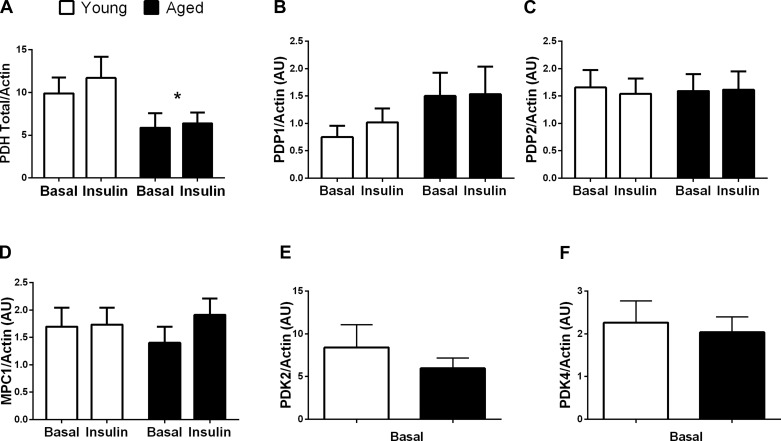
Skeletal muscle total PDH was significantly higher in young compared with aged individuals (∼70%, P < 0.05; Fig. 4A) but did not differ by sex. The increased PDH content in young individuals resulted in a trend for the overall phosphorylation of all three PDH sites to be increased under basal and insulin-stimulated conditions compared with aged individuals (but did not affect the age × insulin interaction; Fig. 3). Skeletal muscle total LDH (not shown), GSK-3α (not shown), GSK-3β (not shown), GS (not shown), hexokinase II (not shown), PDP1 (Fig. 4B), PDP2 (Fig. 4C), MPC1 (Fig. 4D), PDK2 (Fig. 4E), and PDK4 (Fig. 4F) protein content did not differ with age or sex. PDH phosphorylation in the fasted state was not related to skeletal muscle PDKs or PDPs.
Fig. 4.
Skeletal muscle total protein responsible for regulating PDH. Skeletal muscle PDH (n = 16 young and 16 aged; A), pyruvate dehydrogenase phosphatase (PDP)1 (n = 16 young and 16, aged; B), PDP2 (n = 16 young and 16, aged; C), mitochondrial pyruate carrier (MPC1) (n = 15 young and 15 aged; D), PDH kinase (PDK)2 (n = 12 young and 12 aged; E), and PDK4 (n = 15 young and 15 aged; F). Protein analyzed with fewer than n = 16 in each group or only under basal conditions (PDKs) is reflective of limited skeletal muscle tissue. Total protein data are presented relative to β-actin. *P < 0.05 vs. basal condition. AU, abritrary units.
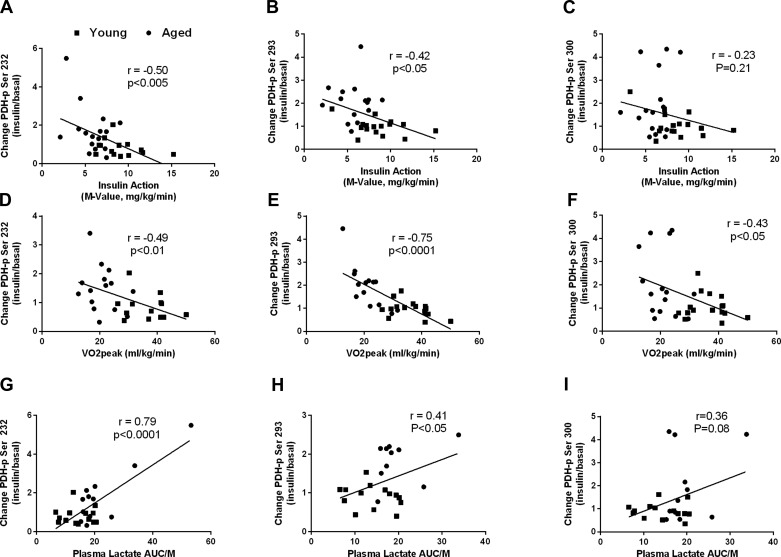
Univariate correlational analyses indicated that the changes in phosphorylation of PDH Ser and PDH Ser293 with insulin were inversely related to whole body insulin action (r = −0.50, P < 0.005, and r = −0.42, P < 0.05; Fig. 5, A and B, respectively) and V̇o2 peak (r = −0.49, P < 0.01, and r = −0.75, P < 0.0001; Fig. D and E, respectively). There was a positive relationship between the changes in phosphorylation of PDH Ser232 and PDH Ser293 with insulin and the change in plasma lactate AUC relative to insulin action (r = 0.79, P < 0.0001, and r = 0.41, P < 0.05; Fig. 5, G and H, respectively). Fasting plasma lactate was positively related to the change in PDH phosphorylation on Ser232 in response to insulin (r = 0.48, P < 0.05; not shown). Changes in the phosphorylation of PDH on serine site 300 during insulin were negatively related to V̇o2 peak (r = −0.43, P < 0.05; Fig. 5F) and skeletal muscle PDP2 content (r = −0.42, P < 0.05; not shown). There were no significant relationships between skeletal muscle PDK2 or PDK4 and insulin-induced changes in PDH phosphorylation.
Fig. 5.
Relationships among insulin-stimulated phosphorylation levels of skeletal muscle PDH and whole body and plasma lactate variables. Changes in skeletal muscle PDH Ser232 and whole body insulin sensitivity (A), V̇o2 peak (D), and plasma lactate AUC/insulin action (AUC/M; G). Changes in skeletal muscle PDH Ser293 and whole body insulin sensitivity (B), V̇o2 peak (E), and plasma lactate AUC/M (H). Changes in skeletal muscle PDH Ser300 and whole body insulin sensitivity (C), V̇o2 peak (F), and plasma lactate AUC/M (H). ■, Data points for young individuals; ●, data points for aged individuals.
Stepwise linear regression was performed using variables linked to metabolic flexibility (age, sex, insulin sensitivity, aerobic capacity, and %body fat) and pyruvate transport into the mitochondria (skeletal muscle MPC1), as well as the dephosphorylation/phosphorylation of PDH (skeletal muscle PDP1, PDP2, PDK2, and PDK4), to determine variables that independently predicted the site-specific phosphorylation response of PDH to insulin (Table 1). Chronological age and skeletal muscle PDP1 content were determined to be independent predictors of the insulin-induced change in PDH phosphorylation on serine site 232 (r2 = 0.41, P < 0.005). Aerobic capacity was determined to be the sole predictor of insulin-induced changes in the phosphorylation of PDH Ser293 (r2 = 0.50, P < 0.001), whereas chronological age and skeletal muscle PDP2 content were determined to be the best predictors of insulin-induced changes in PDH Ser300 (r2 = 0.36, P < 0.005).
Table 1.
Stepwise linear regression analysis for predictors of insulin-stimulated changes in PDH phosphorylation
| Dependent Variable | Significant Predictor(s) | β-Coefficient | Adjusted r2 | P Value |
|---|---|---|---|---|
| Change in PDH-Ser232 | Age, skeletal muscle PDP1 | 0.588, −0.478 | 0.41 | P < 0.005 |
| Change in PDH-Ser293 | V̇o2 peak | −0.723 | 0.50 | P < 0.001 |
| Change in PDH-Ser300 | Skeletal muscle PDP2, age | −0.501, 0.405 | 0.36 | P < 0.005 |
PDH, pyruvate dehydrogenase; PDP1 and -2, pyruvate dehydrogenase phosphatase 1 and 2, respectively. Age was coded (0, 1), with the higher number indicating aged individuals.
Exercise training.
Differences in whole body insulin action (M value) (young: 8.6 ± 0.7 mg·kg−1·min−1; aged: 5.8 ± 0.5 mg·kg−1·min−1; P < 0.005), total percent body fat (young: 24 ± 3%, aged: 38 ± 2%, P < 0.0005), and V̇o2 peak (young: 37 ± 2 mg·kg−1·min−1; aged: 21 ± 1 mg·kg−1·min−1; P < 0.0001) were evident between the young and aged groups before 12 wk of endurance or strength training was initiated, as published previously (10). In response to exercise training, whole body insulin action and aerobic capacity increased (28 and 11%, respectively, P < 0.01), regardless of age or mode of training. Percent body fat decreased (−5%, P < 0.01) with endurance training only.
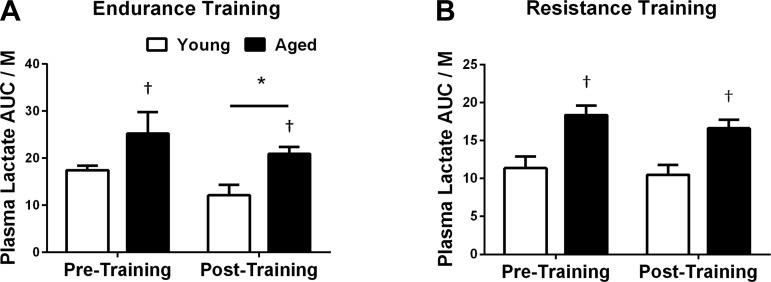
In response to the hyperinsulinemic euglycemic clamp, plasma lactate AUC relative to insulin action decreased after endurance (−23%, P < 0.05; Fig. 6A) but not strength training (−10%, P = 0.17; Fig. 6B), regardless of age. After 12 wk of training, the plasma lactate response remained higher in aged individuals (P < 0.05), regardless of the type of exercise training (Fig. 6).
Fig. 6.
Plasma lactate in response to exercise training. Plasma lactate AUC relative to insulin action in response to endurance (n = 8 young and 8 aged; A) and strength training (n = 8, young and 8 aged; B). Plasma lactate AUC was normalized to insulin action. *P < 0.05 vs. pretraining; †P < 0.05 vs. young at the same time point.
Exercise training and skeletal muscle.
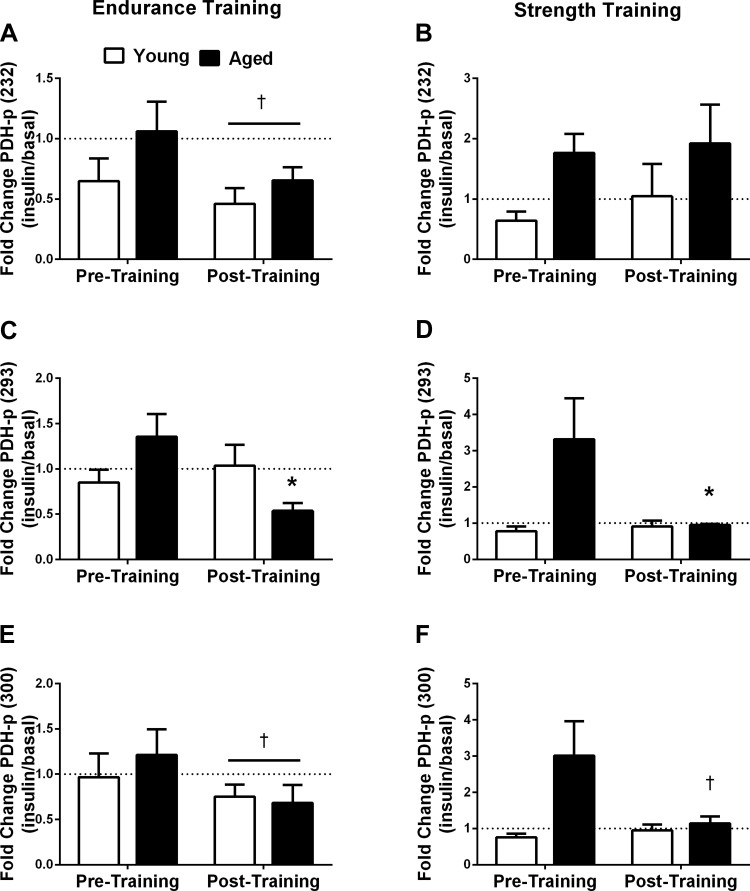
There was a significant interaction (P < 0.05) between age and exercise training for the change in phosphorylation of skeletal muscle PDH on serine site 293. In response to insulin, the change in the phosphorylation of PDH Ser293 decreased in the aged group only after endurance (−60%, P < 0.05; Fig. 7C) and resistance (−71%, P < 0.05; Fig. 7D) training. Similarly, there was a tendency (age × training interaction, P = 0.07) for only aged individuals to suppress PDH serine 300 phosphorylation in response to strength training (−62%, interaction P = 0.07; Fig. 7F) during insulin infusion. There was a trend for endurance training to decrease the phosphorylation of PDH on sites 232 (−35%, P = 0.06; Fig. 7A) and 300 (−23% P = 0.09; Fig. 7E) during insulin infusion, regardless of age. Exercise training had no effects on the insulin-induced phosphorylation of GSK-3α, GSK-3β, or LDH (not shown).
Fig. 7.
Change in insulin-induced PDH phosphorylation (PDH-p) with exercise training. Fold change in the phosphorylation levels of skeletal muscle PDH Ser232 in response to insulin before and after 12 wk of endurance (n = 7 young and 8 aged; A) and strength training (n = 8 young and 8 aged; B). Fold change in the phosphorylation levels of skeletal muscle PDH Ser293 in response to insulin before and after endurance (n = 7 young and 8 aged; C) and strength training (n = 8 young and 8 aged; D). Fold change in the phosphorylation levels of skeletal muscle PDH Ser300 in response to insulin before and after endurance (n = 7 young and 7 aged; E) and strength training (n = 8 young and 8 aged; F). *P < 0.05 vs. pretraining for aged only; †P < 0.10 vs. pretraining for aged only; †tendency for a main effect for training (P < 0.10).
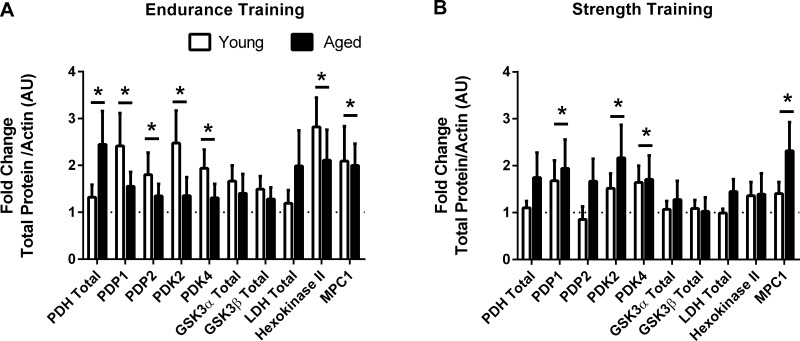
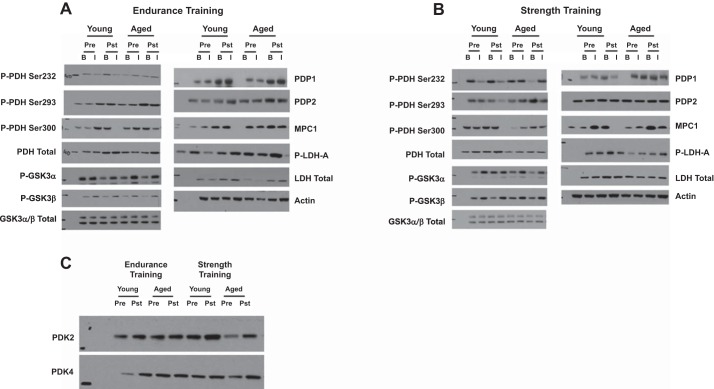
Endurance training increased PDH (89%, P < 0.05), PDP1 (98%, P < 0.05), PDP2 (57%, P < 0.05), PDK2 (92%, P = 0.05), PDK4 (62%, P < 0.05), hexokinase II (147%, P < 0.01), and MPC1 (105%, P < 0.05) protein content, with a tendency to increase GSK-3α (54%, P = 0.08) and GSK-3β (39%, P = 0.07) protein content, regardless of age or sex (Fig. 8A). Twelve weeks of strength training increased PDK2 (84%, P < 0.05), PDK4 (68%, P < 0.05), MPC1 (86%, P < 0.05), and PDP1 (82%, P < 0.05), regardless of age or sex (Fig. 8B). Representative blots for skeletal muscle proteins are presented in Fig. 9.
Fig. 8.
Fold change in skeletal muscle total protein in response to exercise. Fold change (posttraining relative to pretraining) in total protein in response to endurance (A) and resistance (B) training in young and aged individuals (n = 6–8 young and aged each). *P ≤ 0.05, main effect for training.
Fig. 9.
Representative Western blots for Figs. 3, 4, 7, and 8. A and B: representative blots for young and aged individuals under basal and insulin-stimulated conditions in response to endurance (A) and resistance training (B). C: because of limited skeletal muscle availability, the effects of endurance and resistance training were measured only under basal conditions for PDK2 and PDK4.
When endurance and strength training data were pooled, changes in insulin-induced PDH phosphorylation on sites Ser232 and Ser300 were positively related with changes in plasma lactate AUC relative to insulin action (r = 0.39, P < 0.05, and r = 0.40, P < 0.05, respectively; not shown). Training-induced changes in whole body insulin action were negatively related to changes in insulin-stimulated PDH Ser300 phosphorylation (r = −0.36, P < 0.05; not shown). Exercise-induced changes in skeletal muscle PDP2 content were negatively related to insulin-stimulated changes in phosphorylation of PDH on site Ser293 (r = −0.39, P < 0.05; not shown).
DISCUSSION
In the current study, we demonstrate for the first time that the insulin-stimulated dephosphorylation of skeletal muscle PDH, which is indicative of PDH activity, is impaired with the aging process (Fig. 3, A–C) and associated with an enhanced plasma lactate response (Fig. 5, G–I). Increased PDH activity during hyperinsulinemic conditions has been observed in healthy lean individuals (37, 40, 52), providing evidence of metabolic flexibility, whereas PDH activity has been reported to be impaired during lipid infusion (37). Collectively, the current findings in aged individuals and the previous findings with insulin resistance provide evidence of a link between conditions of insulin resistance and the desensitization of PDH during physiological conditions that is indicative of metabolic inflexibility.
To our knowledge, this is the first study to demonstrate an augmented plasma lactate response relative to insulin action during hyperinsulinemia in aged individuals (Fig. 1D), which is suggestive of impaired oxidative capacity or inefficient substrate utilization in insulin-responsive tissue. Despite having a lower glucose uptake, our aged individuals had similar absolute lactate concentrations during the hyperinsulinemic clamp (Fig. 1C), suggesting an exaggerated anaerobic response relative to glucose uptake. We acknowledge that our measured plasma lactate levels were the net effect of lactate production and uptake and that a number of tissue are responsible for lactate production; however, skeletal muscle is a primary site for insulin-stimulated glucose uptake and lactate production during fasting (44) and hyperinsulinemic conditions (11) and could contribute to the elevated lactate response. Recent findings by Vigelsø et al. (54) suggest the augmented lactate response with aging is not limited to insulin stimulation. Compared with their younger counterparts, aged individuals had an increased lactate response during acute exercise, and similar to the present study (Fig. 3, G–I), this increased lactate response was associated with an attenuated PDH response. These findings suggest that when modest amounts of glucose are taken into the skeletal muscle of aged individuals, inefficiencies in glucose regulatory pathways within the tissue are accentuated likely as a result of impaired PDH activity, resulting in the preferential shuttling of glucose toward lactate.
Of particular interest is that the insulin-induced phosphorylation of PDH on serine sites 232, 293, and 300 decreased in our young but not aged individuals (Fig. 3, A–C), suggesting that the capacity for glucose oxidation during hyperinsulinemia is impaired in the skeletal muscle of aged individuals. This finding was further supported by our data demonstrating that insulin increased PDH activity in our subgroup of young individuals but not our aged individuals (Fig. 2A). The current study adds to previous studies (5, 17, 42) by suggesting that a specific cellular impairment, attenuated PDH dephosphorylation, contributes to the metabolic inflexibility with aging. Despite our previous research documenting reduced insulin-stimulated glucose uptake with aging, which is likely the result of diminished skeletal muscle AS160 phosphorylation and reduced GLUT4 protein, we do not feel the reduced PDH responsiveness was the sole result of diminished glucose uptake. Although insulin action was related to some site-specific changes in PDH phosphorylation (Fig. 5, A and B), regression analysis determined that it was not an independent predictor of any of these PDH sites (Table 1). Our exercise training data provide further support since 12 wk of either endurance or strength training did not abolish age-related discrepancies in insulin action but did normalize PDH responsiveness between the two age groups. Furthermore, it has been reported that aged individuals have diminished PDH activity in response to acute exercise despite having a glucose uptake during exercise similar to their younger counterparts (54). Taken together, these findings provide evidence that a mechanism besides diminished glucose uptake contributed to the PDH desensitization in our aged individuals.
The current research adds to previous research demonstrating that PDH activity is attenuated in response to exercise in the elderly (17) and highlights the severity of this impairment based on its presence in more than one physiological condition (exercise and hyperinsulinemia). Based on our findings (Fig. 2A) and others (54), PDH activity in the resting state does not appear to be compromised with age, but instead the capacity for PDH to become activated under conditions favoring glucose oxidation is diminished with aging. It remains unclear whether the cellular mechanisms that contribute to the age-related reduction in PDH activity under hyperinsulinemia and exercise are similar since the two conditions stimulate independent and dependent signaling pathways. The use of hyperinsulinemia in the current study highlights the specific role that PDPs may have in age-related PDH responsiveness since insulin stimulation is thought to increase PDP activity but not PDK activity (6, 51), whereas exercise is expected to impact both PDK and PDP activity.
In contrast to the age-dependent response of PDH, the insulin-stimulated responses of nonoxidative pathways (GSK3, GS, and LDH) were similar between the two age groups (Fig. 3, D–G). Although we did not measure glycogen content or glycogen synthase (GS) activity, we do not expect that this pathway was increased to compensate for potential decrements in pyruvate oxidation since we did not observe age-related differences in insulin-stimulated GSK-3 or GS phosphorylation (Fig. 3, D–F). A paucity of research exists regarding the effects of aging and nonoxidative pathways in response to insulin. Insulin-induced GSK-3 phosphorylation and GS activity have been reported to be reduced with type 2 diabetes (19, 55), suggesting that this pathway is impaired with other etiologies of insulin resistance. A novel finding in the current study was that skeletal muscle LDH-A, the enzyme responsible for the preferential conversion of pyruvate to lactate, increased phosphorylation in response to insulin, and there was a tendency for increased LDH phosphorylation (∼60%; Fig. 3G) in the aged individuals.
In an attempt to gain an understanding of factors that regulate site-specific dephosphorylation of PDH during insulin, we performed regression analyses using factors linked directly to skeletal muscle pyruvate transport into the mitochondria and PDH phosphorylation/dephosphorylation as well as factors known to influence metabolic flexibility. In addition to being young (under the age of 35 yr), increased skeletal muscle PDP1 and PDP2 levels were independent predictors of insulin-stimulated dephosphorylation of PDH on serine sites 232 and 300, respectively (Table 1). To date, PDP1 and PDP2 are the only identified PDPs known to regulate PDH dephosphorylation. Therefore, it was not totally unexpected that these would be predictors of PDH dephosphorylation; however, the fact that each was predictive of different serine sites is of significance since it suggests that each phosphatase could have a preferential site of action. In contrast to PDPs, neither PDK2 nor PDK4 were predictors of PDH phosphorylation changes, which supports previous research indicating that insulin has an effect on PDPs and not PDKs (6, 51). Aerobic capacity was determined to be the lone independent predictor of PDH 293 dephosphorylation. This relationship was also not surprising since aerobic capacity was strongly correlated with PDH dephosphorylation during insulin stimulation (Fig. 5, D–F), and previously, Love et al. (35) determined that skeletal muscle citrate synthase activity (a measure of skeletal muscle aerobic capacity) was related to PDP activity and speculated that individuals with a greater aerobic capacity would also have a greater capacity for PDH dephosphorylation, PDH activity, and glucose oxidation, depending on cellular needs.
Differences in nutrient and metabolite ratios during hyperinsulinemia may have contributed to the age-related PDH response. Previously, during insulin stimulation, we have observed a blunted decline in plasma free fatty acid (FFA) levels with the aging process (Consitt LA and Houmard JA, unpublished data), suggesting that lipolysis is elevated and desensitized to insulin in older individuals. Based on previous research demonstrating that elevated FFA levels were responsible for the inhibition of PDH activity (43), it is conceivable that elevated FFA levels during hyperinsulinemia in the aged individuals played a role in the impaired ability to dephosphorylate PDH. Increased reliance on β-oxidation in the aged individuals would also increase the ratios of NADH to NAD and acetyl-CoA to CoA, which have been shown to inhibit PDH activity (48). Although not measured in the present study, it is also possible that age-related differences in skeletal muscle fiber type composition played a role in the phosphorylation status of PDH. It was reported recently in humans that insulin-stimulated PDH phosphorylation was decreased in type II fibers only (1), and in a rodent study it was determined that PDP activity was higher in fast-twitch oxidative muscle compared with slow-twitch oxidative muscle (31). Despite no differences in PDP content between the age groups (Fig. 4, B and C), the well-recognized age-associated decline in cross-sectional area of type II but not type I fibers (28, 34) could have played a role in decreasing PDP activity, resulting in the loss of PDH responsiveness. Insulin has been shown to increase mitochondrial calcium (15), a known activator of PDP1, and conditions of insulin resistance have been associated with decreased mitochondria retention of calcium (50). Therefore, it is plausible that during insulin stimulation, less calcium was available in the mitochondria of aged individuals to activate PDP1, and this contributed to the inability to dephosphorylate PDH. Taken together, it is evident that a number of age-related mechanisms have the potential to contribute to the desensitization of PDH to insulin and warrant further investigation.
Exercise has long been recognized as a method to improve insulin sensitivity and substrate utilization in individuals, including the elderly (36). In general, there was a tendency for enhanced dephosphorylation of PDH with insulin in both young and aged individuals in response to exercise training (Fig. 7), which was associated with a depressed lactate response, providing evidence that improving PDH responsiveness may reduce the flux of glucose toward anaerobic metabolism. Of particular importance is that only aged individuals experienced exercise-induced improvements in insulin-stimulated dephosphorylation of PDH on site 293 (Fig. 7, C and D), suggesting that either endurance or strength training can offset initial age-related deficits on this site. For the most part, changes in phosphorylation were due to increased PDH phosphorylation in the fasted state, which was likely a result of exercise-induced increases in PDKs; however, there was an inclination for greater dephosphorylation during insulin stimulation, particularly for aged individuals on serine site 300 after endurance and strength training. Of interest, we determined that PDP2 content, which is known to be activated by insulin (22), was the best predictor of PDH 300 phosphorylation. These findings suggest that this site may be more receptive to insulin after exercise training in aged individuals in an effort to compensate for the initial age-related deficit. It could also explain differences between our findings and the study by Biensø et al. (3) that reported that PDH phosphorylation in response to an oral glucose tolerance test did not change after 8 wk of endurance training in the elderly. Since insulin levels during an oral glucose tolerance test are variable and are generally lower after exercise training, it is difficult to measure the specific responsiveness of PDH relative to insulin levels during this procedure.
Our finding that exercise training increased both PDK2 and PDK4 expression has been reported previously with respect to PDK2 but not PDK4 (33). To our knowledge, this is the first study to investigate the response of PDK expression to exercise training in older adults and provides evidence of continued muscle plasticity with aging. To date, no known data have been reported in humans regarding the effect of exercise training on PDP content. Our finding that both endurance and resistance training increase PDP1 content, regardless of age, supports previous research reporting that PDP1 content and activity were increased in obese rats in response to 8 wk of endurance training (32), suggesting that this isoform is responsive to exercise training even in conditions of metabolic inflexibility and insulin resistance. Since PDP2 is believed to be activated by insulin (22), the increase in PDP2 content that occurred in the aged individuals in response to endurance training (and trend toward increasing with resistance training) may have contributed to the increased PDH dephosphorylation that occurred in response to insulin after training. Collectively, our data in sedentary and exercise-trained individuals highlight the importance of PDP content in the ability to dephosphorylate PDH and suggest that interventions to increase skeletal muscle PDP content may be important in decreasing variables that inhibit insulin signaling, including lactate (7).
In response to exercise training, especially endurance training, there was an upregulation of glucose (10) and pyruvate transport proteins (Fig. 8). To our knowledge, this is the first study to examine the effects of age and exercise training on skeletal muscle MPC1, one of two proteins responsible for the transport of pyruvate into the mitochondria. Whereas, MPC1 protein content did not appear to be affected by age, it was interesting to note that protein content increased in response to both endurance and strength training, which could increase the import of pyruvate into the mitochondria during physiological conditions requiring increased glucose oxidation.
In summary, the findings from the current study indicate for the first time that insulin-stimulated dephosphorylation of PDH is impaired on multiple serine sites with the aging process, which is indicative of metabolic inflexibility. The impaired PDH response was associated with an augmented lactate response, suggesting that under insulin-stimulated conditions, pyruvate is shuttled toward anaerobic metabolism with aging. With respect to 12 wk of exercise training, impairments in PDH phosphorylation were rescued and associated with a blunted lactate response, providing evidence that exercise training is an effective option to counteract metabolic inflexibility with aging.
GRANTS
This work was supported by National Institutes of Health Grants DK-102115 to L. A. Consitt and AG-025205 and DK-56112 to J. A. Houmard.
DISCLOSURES
No potential conflicts of interest relevant to this article, financial or otherwise, are reported.
AUTHOR CONTRIBUTIONS
L.A.C. conception and design of research; L.A.C., G.S., and A.S. performed experiments; L.A.C., G.S., and A.S. analyzed data; L.A.C. and J.A.H. interpreted results of experiments; L.A.C. prepared figures; L.A.C. drafted manuscript; L.A.C. edited and revised manuscript; L.A.C. and J.A.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Jessica Van Meter at East Carolina University for assistance with subject recruitment, screening, and exercise training.
REFERENCES
- 1.Albers PH, Pedersen AJ, Birk JB, Kristensen DE, Vind BF, Baba O, Nohr J, Hojlund K, Wojtaszewski JF. Human muscle fiber type-specific insulin signaling: impact of obesity and type 2 diabetes. Diabetes 64: 485–497, 2015. [DOI] [PubMed] [Google Scholar]
- 2.Avogaro A, Toffolo G, Miola M, Valerio A, Tiengo A, Cobelli C, Del Prato S. Intracellular lactate- and pyruvate-interconversion rates are increased in muscle tissue of non-insulin-dependent diabetic individuals. J Clin Invest 98: 108–115, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biensø RS, Olesen J, Gliemann L, Schmidt JF, Matzen MS, Wojtaszewski JF, Hellsten Y, Pilegaard H. Effects of Exercise Training on Regulation of Skeletal Muscle Glucose Metabolism in Elderly Men. J Gerontol A Biol Sci Med Sci 70: 866–872, 2015. [DOI] [PubMed] [Google Scholar]
- 4.Bogardus C, Lillioja S, Stone K, Mott D. Correlation between muscle glycogen synthase activity and in vivo insulin action in man. J Clin Invest 73: 1185–1190, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonadonna RC, Groop LC, Simonson DC, DeFronzo RA. Free fatty acid and glucose metabolism in human aging: evidence for operation of the Randle cycle. Am J Physiol Endocrinol Metab 266: E501–E509, 1994. [DOI] [PubMed] [Google Scholar]
- 6.Caruso M, Maitan MA, Bifulco G, Miele C, Vigliotta G, Oriente F, Formisano P, Beguinot F. Activation and mitochondrial translocation of protein kinase Cdelta are necessary for insulin stimulation of pyruvate dehydrogenase complex activity in muscle and liver cells. J Biol Chem 276: 45088–45097, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Choi CS, Kim YB, Lee FN, Zabolotny JM, Kahn BB, Youn JH. Lactate induces insulin resistance in skeletal muscle by suppressing glycolysis and impairing insulin signaling. Am J Physiol Endocrinol Metab 283: E233–E240, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Chokkalingam K, Jewell K, Norton L, Littlewood J, van Loon LJ, Mansell P, Macdonald IA, Tsintzas K. High-fat/low-carbohydrate diet reduces insulin-stimulated carbohydrate oxidation but stimulates nonoxidative glucose disposal in humans: An important role for skeletal muscle pyruvate dehydrogenase kinase 4. J Clin Endocrinol Metab 92: 284–292, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Consitt LA, Bell JA, Koves TR, Muoio DM, Hulver MW, Haynie KR, Dohm GL, Houmard JA. Peroxisome proliferator-activated receptor-gamma coactivator-1alpha overexpression increases lipid oxidation in myocytes from extremely obese individuals. Diabetes 59: 1407–1415, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Consitt LA, Van Meter J, Newton CA, Collier DN, Dar MS, Wojtaszewski JF, Treebak JT, Tanner CJ, Houmard JA. Impairments in site-specific AS160 phosphorylation and effects of exercise training. Diabetes 62: 3437–3447, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Consoli A, Nurjahan N, Gerich JE, Mandarino LJ. Skeletal muscle is a major site of lactate uptake and release during hyperinsulinemia. Metabolism 41: 176–179, 1992. [DOI] [PubMed] [Google Scholar]
- 12.Cree MG, Newcomer BR, Katsanos CS, Sheffield-Moore M, Chinkes D, Aarsland A, Urban R, Wolfe RR. Intramuscular and liver triglycerides are increased in the elderly. J Clin Endocrinol Metab 89: 3864–3871, 2004. [DOI] [PubMed] [Google Scholar]
- 13.DeFronzo RA, Jacot E, Jequier E, Maeder E, Wahren J, Felber JP. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes 30: 1000–1007, 1981. [DOI] [PubMed] [Google Scholar]
- 14.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol Endocrinol Metab Gastrointest Physiol 237: E214–E223, 1979. [DOI] [PubMed] [Google Scholar]
- 15.Dorman DM, Barritt GJ, Bygrave FL. Stimulation of hepatic mitochondrial calcium transport by elevated plasma insulin concentrations. Biochem J 150: 389–395, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duscha BD, Slentz CA, Johnson JL, Houmard JA, Bensimhon DR, Knetzger KJ, Kraus WE. Effects of exercise training amount and intensity on peak oxygen consumption in middle-age men and women at risk for cardiovascular disease. Chest 128: 2788–2793, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Gurd BJ, Peters SJ, Heigenhauser GJ, LeBlanc PJ, Doherty TJ, Paterson DH, Kowalchuk JM. O2 uptake kinetics, pyruvate dehydrogenase activity, and muscle deoxygenation in young and older adults during the transition to moderate-intensity exercise. Am J Physiol Regul Integr Comp Physiol 294: R577–R584, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Herzig S, Raemy E, Montessuit S, Veuthey JL, Zamboni N, Westermann B, Kunji ER, Martinou JC. Identification and functional expression of the mitochondrial pyruvate carrier. Science 337: 93–96, 2012. [DOI] [PubMed] [Google Scholar]
- 19.Hojlund K, Staehr P, Hansen BF, Green KA, Hardie DG, Richter EA, Beck-Nielsen H, Wojtaszewski JF. Increased phosphorylation of skeletal muscle glycogen synthase at NH2-terminal sites during physiological hyperinsulinemia in type 2 diabetes. Diabetes 52: 1393–1402, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Houmard JA, Shaw CD, Hickey MS, Tanner CJ. Effect of short-term exercise training on insulin-stimulated PI 3-kinase activity in human skeletal muscle. Am J Physiol Endocrinol Metab 277: E1055–E1060, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Houmard JA, Weidner ML, Gavigan KE, Tyndall GL, Hickey MS, Alshami A. Fiber type and citrate synthase activity in the human gastrocnemius and vastus lateralis with aging. J Appl Physiol (1985) 85: 1337–1341, 1998. [DOI] [PubMed] [Google Scholar]
- 22.Huang B, Gudi R, Wu P, Harris RA, Hamilton J, Popov KM. Isoenzymes of pyruvate dehydrogenase phosphatase. DNA-derived amino acid sequences, expression, and regulation. J Biol Chem 273: 17680–17688, 1998. [DOI] [PubMed] [Google Scholar]
- 23.Juraschek SP, Shantha GP, Chu AY, Miller ER 3rd, Guallar E, Hoogeveen RC, Ballantyne CM, Brancati FL, Schmidt MI, Pankow JS, Young JH. Lactate and risk of incident diabetes in a case-cohort of the atherosclerosis risk in communities (ARIC) study. PLoS One 8: e55113, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karpova T, Danchuk S, Kolobova E, Popov KM. Characterization of the isozymes of pyruvate dehydrogenase phosphatase: implications for the regulation of pyruvate dehydrogenase activity. Biochim Biophys Acta 1652: 126–135, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Kelley DE, Goodpaster B, Wing RR, Simoneau JA. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol Endocrinol Metab 277: E1130–E1141, 1999. [DOI] [PubMed] [Google Scholar]
- 26.Kelley DE, Mandarino LJ. Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes 49: 677–683, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Kelley DE, Mokan M, Simoneau JA, Mandarino LJ. Interaction between glucose and free fatty acid metabolism in human skeletal muscle. J Clin Invest 92: 91–98, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Korhonen MT, Cristea A, Alen M, Hakkinen K, Sipila S, Mero A, Viitasalo JT, Larsson L, Suominen H. Aging, muscle fiber type, and contractile function in sprint-trained athletes. J Appl Physiol 101: 906–917, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Korotchkina LG, Patel MS. Mutagenesis studies of the phosphorylation sites of recombinant human pyruvate dehydrogenase. Site-specific regulation. J Biol Chem 270: 14297–14304, 1995. [DOI] [PubMed] [Google Scholar]
- 30.Korotchkina LG, Patel MS. Site specificity of four pyruvate dehydrogenase kinase isoenzymes toward the three phosphorylation sites of human pyruvate dehydrogenase. J Biol Chem 276: 37223–37229, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Leblanc PJ, Harris RA, Peters SJ. Skeletal muscle fiber type comparison of pyruvate dehydrogenase phosphatase activity and isoform expression in fed and food-deprived rats. Am J Physiol Endocrinol Metab 292: E571–E576, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Leblanc PJ, Mulligan M, Antolic A, Macpherson L, Inglis JG, Martin D, Roy BD, Peters SJ. Skeletal muscle type comparison of pyruvate dehydrogenase phosphatase activity and isoform expression: effects of obesity and endurance training. Am J Physiol Regul Integr Comp Physiol 295: R1224–R1230, 2008. [DOI] [PubMed] [Google Scholar]
- 33.LeBlanc PJ, Peters SJ, Tunstall RJ, Cameron-Smith D, Heigenhauser GJ. Effects of aerobic training on pyruvate dehydrogenase and pyruvate dehydrogenase kinase in human skeletal muscle. J Physiol 557: 559–570, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci 50 Spec No: 11–16, 1995. [DOI] [PubMed] [Google Scholar]
- 35.Love LK, LeBlanc PJ, Inglis JG, Bradley NS, Choptiany J, Heigenhauser GJ, Peters SJ. The relationship between human skeletal muscle pyruvate dehydrogenase phosphatase activity and muscle aerobic capacity. J Appl Physiol 111: 427–434, 2011. [DOI] [PubMed] [Google Scholar]
- 36.Malin SK, Haus JM, Solomon TP, Blaszczak A, Kashyap SR, Kirwan JP. Insulin sensitivity and metabolic flexibility following exercise training among different obese insulin-resistant phenotypes. Am J Physiol Endocrinol Metab 305: E1292–E1298, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mandarino LJ, Wright KS, Verity LS, Nichols J, Bell JM, Kolterman OG, Beck-Nielsen H. Effects of insulin infusion on human skeletal muscle pyruvate dehydrogenase, phosphofructokinase, and glycogen synthase. Evidence for their role in oxidative and nonoxidative glucose metabolism. J Clin Invest 80: 655–663, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McDowell MA, Ogden CL, Flegal KM. Anthropometric Reference Data for Children and Adults: United States, 2003–2006. Hyattsville, MD: National Center for Health Statistics, 2008. [PubMed] [Google Scholar]
- 39.Pehleman TL, Peters SJ, Heigenhauser GJ, Spriet LL. Enzymatic regulation of glucose disposal in human skeletal muscle after a high-fat, low-carbohydrate diet. J Appl Physiol (1985) 98: 100–107, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Pehleman TL, Peters SJ, Heigenhauser GJ, Spriet LL. Enzymatic regulation of glucose disposal in human skeletal muscle after a high-fat, low-carbohydrate diet. J Appl Physiol 98: 100–107, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Petersen KF, Morino K, Alves TC, Kibbey RG, Dufour S, Sono S, Yoo PS, Cline GW, Shulman GI. Effect of aging on muscle mitochondrial substrate utilization in humans. Proc Natl Acad Sci USA 112: 11330–11334, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Petersen KF, Morino K, Alves TC, Kibbey RG, Dufour S, Sono S, Yoo PS, Cline GW, Shulman GI. Effect of aging on muscle mitochondrial substrate utilization in humans. Proc Natl Acad Sci USA 112: 11330–11334, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pilegaard H, Birk JB, Sacchetti M, Mourtzakis M, Hardie DG, Stewart G, Neufer PD, Saltin B, van Hall G, Wojtaszewski JF. PDH-E1alpha dephosphorylation and activation in human skeletal muscle during exercise: effect of intralipid infusion. Diabetes 55: 3020–3027, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Qvisth V, Hagström-Toft E, Moberg E, Sjöberg S, Bolinder J. Lactate release from adipose tissue and skeletal muscle in vivo: defective insulin regulation in insulin-resistant obese women. Am J Physiol Endocrinol Metab 292: E709–E714, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Randle PJ, Garland PB, Newsholme EA, Hales CN. The glucose fatty acid cycle in obesity and maturity onset diabetes mellitus. Ann NY Acad Sci 131: 324–333, 1965. [DOI] [PubMed] [Google Scholar]
- 46.Short KR, Vittone JL, Bigelow ML, Proctor DN, Rizza RA, Coenen-Schimke JM, Nair KS. Impact of aerobic exercise training on age-related changes in insulin sensitivity and muscle oxidative capacity. Diabetes 52: 1888–1896, 2003. [DOI] [PubMed] [Google Scholar]
- 47.Skurat AV, Dietrich AD, Roach PJ. Glycogen synthase sensitivity to insulin and glucose-6-phosphate is mediated by both NH2- and COOH-terminal phosphorylation sites. Diabetes 49: 1096–1100, 2000. [DOI] [PubMed] [Google Scholar]
- 48.Sugden MC, Holness MJ. Recent advances in mechanisms regulating glucose oxidation at the level of the pyruvate dehydrogenase complex by PDKs. Am J Physiol Endocrinol Metab 284: E855–E862, 2003. [DOI] [PubMed] [Google Scholar]
- 49.Szendroedi J, Schmid AI, Chmelik M, Toth C, Brehm A, Krssak M, Nowotny P, Wolzt M, Waldhausl W, Roden M. Muscle mitochondrial ATP synthesis and glucose transport/phosphorylation in type 2 diabetes. PLoS Med 4: e154, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taddeo EP, Laker RC, Breen DS, Akhtar YN, Kenwood BM, Liao JA, Zhang M, Fazakerley DJ, Tomsig JL, Harris TE, Keller SR, Chow JD, Lynch KR, Chokki M, Molkentin JD, Turner N, James DE, Yan Z, Hoehn KL. Opening of the mitochondrial permeability transition pore links mitochondrial dysfunction to insulin resistance in skeletal muscle. Mol Metab 3: 124–134, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thomas AP, Denton RM. Use of toluene-permeabilized mitochondria to study the regulation of adipose tissue pyruvate dehydrogenase in situ. Further evidence that insulin acts through stimulation of pyruvate dehydrogenase phosphate phosphatase. Biochem J 238: 93–101, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsintzas K, Chokkalingam K, Jewell K, Norton L, Macdonald IA, Constantin-Teodosiu D. Elevated free fatty acids attenuate the insulin-induced suppression of PDK4 gene expression in human skeletal muscle: potential role of intramuscular long-chain acyl-coenzyme A. J Clin Endocrinol Metab 92: 3967–3972, 2007. [DOI] [PubMed] [Google Scholar]
- 53.Vendelbo MH, Clasen BF, Treebak JT, Møller L, Krusenstjerna-Hafstrøm T, Madsen M, Nielsen TS, Stødkilde-Jørgensen H, Pedersen SB, Jørgensen JO, Goodyear LJ, Wojtaszewski JF, Møller N, Jessen N. Insulin resistance after a 72-h fast is associated with impaired AS160 phosphorylation and accumulation of lipid and glycogen in human skeletal muscle. Am J Physiol Endocrinol Metab 302: E190–E200, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vigelsø A, Gram M, Dybboe R, Kuhlman AB, Prats C, Greenhaff PL, Constantin-Teodosiu D, Birk JB, Wojtaszewski J, Dela F, Helge JW. The effect of age and unilateral leg immobilization for 2 weeks on substrate utilization during moderate-intensity exercise in human skeletal muscle. J Physiol 594: 2339–2358, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vind BF, Pehmoller C, Treebak JT, Birk JB, Hey-Mogensen M, Beck-Nielsen H, Zierath JR, Wojtaszewski JF, Hojlund K. Impaired insulin-induced site-specific phosphorylation of TBC1 domain family, member 4 (TBC1D4) in skeletal muscle of type 2 diabetes patients is restored by endurance exercise-training. Diabetologia 54: 157–167, 2011. [DOI] [PubMed] [Google Scholar]