Abstract
Interdisciplinary studies in the research fields of endocrinology and immunology show that obesity-associated overnutrition leads to neuroinflammatory molecular changes, in particular in the hypothalamus, chronically causing various disorders known as elements of metabolic syndrome. In this process, neural or hypothalamic inflammation impairs the neuroendocrine and autonomic regulation of the brain over blood pressure and glucose homeostasis as well as insulin secretion, and elevated sympathetic activation has been appreciated as a critical mediator. This review describes the involved physiology and mechanisms, with a focus on glucose and blood pressure balance, and suggests that neuroinflammation employs the autonomic nervous system to mediate the development of diabetes and hypertension.
Keywords: autonomic nervous system, hypothalamus, inflammation
the hypothalamus, known as the neuroendocrine control center, functions to orchestrate the actions of both the neuroendocrine and autonomic nervous systems in the maintenance of organismwide internal homeostasis (18, 115). Neuroendocrine regulation of body weight, for example, involves multiple hypothalamic subregions, with ablation of ventral, dorsal, and/or lateral hypothalamic regions resulting in changes in food intake and body weight (9, 10). Among these hypothalamic subregions, the arcuate nucleus is of particular importance in the regulation of body weight because: 1) the arcuate nucleus is primarily responsive to a variety of regulatory hormonal signals from the circulation (4, 114, 144) and 2) this hypothalamic subregion houses a significant portion of the melanocortin system, including pro-opiomelanocortin (POMC) neurons and agouti-related peptide (AgRP) neurons, which act in concert to balance energy intake with expenditure (123). POMC and AgRP neurons in the arcuate nucleus further interact with neurons in other hypothalamic regions, such as the paraventricular nucleus (PVN), ventromedial hypothalamus, dorsomedial hypothalamus, and lateral hypothalamus to mediate downstream effects in metabolic regulation (27, 35, 138). These other hypothalamic regions are also responsive to many regulatory factors in the control of energy balance, for example, brain-derived neurotrophic factor in the PVN (85), orexin in the lateral hypothalamus (20, 95), and steroidogenic factor-1 in the ventral medial nucleus (139). In addition, it has been recently revealed that a population of GABA-producing AgRP neurons act to inhibit POMC neuronal signaling (54), indicating that the neuroendocrine roles of these neurons are integrated with synaptic neurotransmitter functions.
Regarding the influence on the autonomic nervous system, distinct populations of preautonomic neurons within the hypothalamus are known to innervate regions in the brain stem and spinal cord, which represent the control centers for sympathetic and parasympathetic outflows, such as the rostral ventrolateral medulla (RVLM), the dorsal vagal complex, and the intermediolateral spinal columns (87, 142, 149). Table 1 provides a brief summary on several hypothalamic subregions that can influence the autonomic regulation of blood glucose and blood pressure homeostasis. Concerning organismwide metabolic control, the autonomic nervous system innervates several metabolic organs, making it important in triggering various metabolic responses (Table 1). As an example, splanchnic sympathetic nerves and vagal parasympathetic nerves innervate the liver and the pancreas, and both the sympathetic and parasympathetic nervous systems innervate muscle (38, 67, 107, 142). The autonomic nervous system also innervates organs such as the kidney and arteries, which have crucial functions for blood pressure control (80, 99). In the hypothalamus, the PVN is a central site for integrating neuroendocrine information with autonomic nervous system functions (87, 142, 149). Recently, the mediobasal hypothalamic region, which contains the arcuate nucleus and the ventromedial hypothalamus, has further been appreciated for the effects of modulating the downstream autonomic nervous system to influence metabolic and cardiovascular functions (100). From the perspective of disease development, molecular inflammatory pathways in the mediobasal hypothalamus can cause autonomic nervous system dysfunctions, leading to the central mechanisms of metabolic and cardiovascular disorders (100, 102). Herein, this review will focus on summarizing recent findings on the hypothalamic and autonomic basis of glucose and blood pressure regulation as well as dysfunctions resulting from hypothalamic inflammation that contribute to glucose and blood pressure disorders.
Table 1.
Hypothalamic connections with autonomic control regions in regulating glucose and BP balance
| Hypothalamic Nucleus | Downstream Autonomic Control Regions | Target Organs | Physiological Effects | Reference No. |
|---|---|---|---|---|
| PVN | DMV, RVLM, NTS, IML | Liver, kidney, CVS | Glucose production, BP homeostasis, food intake | 129 |
| 37a | ||||
| 72a | ||||
| LHA | DMV, NTS, IML, VMH, ARC | Liver, BAT | Hepatic function, BAT activation, food intake | 129 |
| 89 | ||||
| 72a | ||||
| DMH | PVN, DMV, NTS | CVS | BP homeostasis | 122 |
| ARC, VMH (MBH) | PVN, LHA, IML | Liver, skeletal muscle, BAT, pancreas | Hepatic function, skeletal muscle glucose uptake, BAT activation, pancreatic insulin secretion, food intake | 129 |
| 119 | ||||
| 89 | ||||
| 72a |
ARC, arcuate nucleus, BAT, brown adipose tissue, BP, blood pressure, CVS, cardiovascular system, DMH, dorsal medial hypothalamus, DMV, dorsal motor nucleus of the vagus, IML, intermediolateral nucleus of the spinal cord, LHA, lateral hypothalamic area, MBH, mediobasal hypothalamus, NTS, nucleus of the solitary tract, PVN, paraventricular nucleus, RVLM, rostral ventrolateral medulla, VMH, ventromedial hypothalamus.
Autonomic and hypothalamic control of hepatic glucose production.
The liver is a critical organ in the regulation of glucose homeostasis, serving as the principal site for glycogen storage and acting to regulate levels of circulating glucose. For example, in response to increasing levels of blood glucose, the liver reduces hepatic glucose production; conversely, low levels of blood glucose lead to increases in hepatic glucose production (104). Regarding neuroendocrine regulation of hepatic glucose production, the arcuate nucleus and PVN of the hypothalamus respond to circulating levels of regulatory hormones to modulate glucose homeostasis. Experimental evidence demonstrates that restoration of leptin signaling in leptin receptor-deficient mice normalizes blood glucose concentrations (28, 56). These effects can also be achieved through restoring leptin receptor specifically in POMC neurons of mice that are genetically deficient in leptin receptor (40, 56). Other studies demonstrate the role of AgRP neurons in leptin's actions on glucose homeostasis (40, 44). Intracerebral ventricular administration of neuropeptide Y was shown to elevate hepatic glucose production due to an impairment in hepatic insulin sensitivity, and both of these effects were shown to be mediated by sympathetic activity in the autonomic nervous system (131). Additionally, glucocorticoid signaling in the arcuate nucleus was recently demonstrated to impair insulin sensitivity (141). Altogether, these findings signify the importance of the mediobasal hypothalamus in glucose homeostasis.
The central nervous system (CNS) innervates the liver through both the sympathetic and parasympathetic nervous systems (38, 142). Sympathetic efferent pathways from the intermediolateral nucleus of the spinal cord reach the liver to regulate hepatic functions, while parasympathetic efferent signals for the liver are generated significantly from the dorsal vagal complex (142). Several hypothalamic areas are also responsible in regulating autonomic signals to the liver, including the ventral medial nucleus, which participates in sympathetic function, the lateral hypothalamus in parasympathetic function, and the PVN, which integrates signals from several hypothalamic regions to affect the autonomic function (129). The PVN is anatomically connected to the arcuate nucleus and the ventral medial nucleus, functioning to convey information from these hypothalamic nuclei to the brain regions that employ the autonomic nervous system to regulate liver glucose production (131). Also, there is evidence suggesting that the PVN plays a role in regulating the biological clock, which is primarily controlled by the suprachiasmatic nucleus and thus contributes to circadian rhythms of blood glucose changes (62). This effect has been shown to be mediated through the actions of GABAergic and other neurons in the PVN (62). Furthermore, recent studies have linked the PVN to glucose intolerance induced by excess thyroid hormone. An administration of thyroid hormone in the PVN was shown to increase blood glucose levels and hepatic glucose production via hepatic sympathetic activation (38). Sympathetic regulation of hepatic glucose production is known to involve the ventral hypothalamus, since studies demonstrated that stimulation of the ventral hypothalamic region increases the activity of gluconeogenic enzyme phosphoenolpyruvate carboxykinase in the liver (118). Parasympathetic regulation of the liver is closely related to the lateral regions of the hypothalamus, with studies having shown that stimulation of these areas promotes glycogen synthesis in the liver, an effect that is associated with decreased levels in hepatic phosphoenolpyruvate carboxykinase gene expression (118). Also, it was reported that activation of orexin-expressing neurons, which are densely localized in the lateral hypothalamus, can increase the autonomic outflow to the liver (143). In summary, in addition to neuroendocrine pathways, the autonomic outflow regulated by the hypothalamus, involving both parasympathetic and sympathetic innervations of the liver, clearly plays an important role in controlling hepatic glucose output and therefore glucose homeostasis of the body.
Autonomic and hypothalamic modulation of glucose uptake in skeletal muscle.
Skeletal muscle is a critical tissue responsible for insulin-stimulated glucose uptake in response to feeding. Under normal conditions, hyperglycemia triggers insulin secretion and increases glucose uptake in skeletal muscle; however, this ability is markedly impaired in the condition of insulin resistance, which is characteristically displayed in type 2 diabetes and also frequently seen in obesity (61, 76). The hypothalamus plays a significant role in affecting glucose update of skeletal muscle via the sympathetic nervous system and probably through insulin-independent pathways (86, 115). For instance, the intracerebral ventricular injection of leptin in the hypothalamic third ventricle leads to an increase in muscle glucose uptake, and this effect was shown to be dependent on β-adrenergic activation (81) and mediated through an insulin-independent mechanism (49). Furthermore, electrical stimulation of the ventromedial hypothalamus resulted in the increased uptake of glucose by skeletal muscle through sympathetic action, with this result also being independent of insulin signaling (119). In addition, injection of orexin-A in the ventral hypothalamic region was found to cause sympathetic excitation, leading to an increase in muscle glucose uptake (120). Finally, much of the sympathetic activation that regulates skeletal muscle glucose uptake appears to be regulated through norepinephrine signaling (86). To summarize, the hypothalamus has effects on glucose uptake and utilization in skeletal muscles through insulin-independent autonomic activation, and this effect might provide an appreciable contribution to the control of glucose homeostasis under certain physiological conditions.
Autonomic and hypothalamic control of glucose utilization in brown fat.
Thermogenic brown adipose tissue is known to be heavily innervated by sympathetic nerve fibers, although the role of the parasympathetic nervous system in brown fat is less well known (43). Brown adipose tissue is activated by the sympathetic nervous system through the stimulation of β-adrenergic receptors (6, 46, 93, 128, 136). For example, mice deficient in β1-adrenergic receptor showed a lower basal metabolic rate and defective cold tolerance (128). Consistently, blockade of β1- and β2-adrenergic receptors can reduce brown adipose tissue activity, as determined by imaging in mice as well as patients (93, 136). Interestingly, administration of a β3-adrenergic receptor agonist increased energy expenditure, mainly through white adipose tissue, rather than direct activation of brown adipose tissue (46). In recent years, specific neuronal types in the hypothalamus have been identified for the regulation on brown adipose tissue, for example, POMC neurons in the arcuate nucleus are present in the neural pathways that project to brown fat, whereas brown fat-innervating neurons in the lateral hypothalamus were found to express neuropeptides such as orexins and melanin-concentrating hormone (89). In addition, it has been demonstrated that brown adipose activation is associated with metabolism in relation to circadian rhythm or cold exposure (7, 127), and, in connection with this physiology, central administration of fibroblast growth factor-21 was shown to enhance the sympathetic mechanism of energy expenditure and weight loss (91). Treatment of type 2 diabetes with glucagon-like peptide-1 analog liraglutide has been shown to activate brown fat and to induce the browning of white adipose tissue through sympathetic activation (74). Additionally, glucagon-like peptide-1 activation through the use of dipeptdyl peptidase inhibitors has been demonstrated to activate brown fat, lower adiposity, and improve glucose metabolism (63, 117). Compared with brown adipose tissue, the neural control of white adipose tissue has been less well studied, despite white adipose tissue being the primary means of lipid energy storage. Of note, a recent study by Zeng et al. shows that induction of sympathetic activity induces lipolysis in white adipose tissue, suggesting that white adipose tissue is under similar neuronal control compared with brown adipose tissue (145). Moreover, the CNS has been recognized as an activator of the sympathetic innervation of white adipose tissue and is potentially a mediator in the control of peripheral lipid storage in this tissue (84). Despite this research progress, the role of the hypothalamus in the control of white adipose tissue has yet to be fully explored.
Autonomic and hypothalamic control of β-cell function.
The pancreas, including islets, is richly innervated by both the sympathetic and parasympathetic nervous systems (115), with sympathetic nerves innervating pancreatic α-cells, while the parasympathetic system innervates pancreatic α- and β-cells (108, 115). Sympathetic efferents to the pancreas are produced by neurons in the spinal cord that are connected to prevertebral ganglia neurons while the parasympathetic efferents are generated through preganglionic neurons in the brain stem and peripheral postganglionic neurons in the pancreas (17). In addition, several hypothalamic and extrahypothalamic (e.g., the dorsal vagal complex) sites have been identified that regulate the autonomic output to the pancreas (59, 60, 92, 97). The ventral hypothalamic region plays a critical role in the hypothalamic regulation of glucose response, in part because this region is important for glucose-induced pancreatic glucagon secretion (13, 14). The underlying mechanism of the ventral hypothalamus in modulating pancreatic function has been demonstrated by targeting propylendopeptidase, an enzyme that regulates neuropeptide production (65). Mice with propylendopeptidase gene knockdown developed pancreatic endocrine changes, including increased blood glucagon and decreased blood insulin in basal and glucose-stimulated conditions, and these changes were accompanied by increased sympathetic outflow to the pancreas (65). Stimulation of the dorsal motor nucleus of the vagus, either electrically or chemically, was demonstrated to activate not only endocrine but also exocrine functions, and vagotomy can block these effects (83, 134). Also, glucose-induced insulin secretion can be increased through the local injection of a chemical that antagonizes GABA to activate the dorsal motor nucleus of the vagus, and this effect can be suppressed by administration of a chemical that antagonizes muscarinic receptors (82). Furthermore, glucagon-like peptide-1 can depolarize neurons in the dorsal motor nucleus of the vagus that project to the pancreas, indicating that the effect of glucagon-like peptide-1 in modulating insulin secretion is mediated, at least in part, through the neural control of the pancreas (133, 134).
Autonomic and hypothalamic control of blood pressure.
Circumventricular organs, the hypothalamus, and the brain stem perform important functions in processing and integrating various stimuli from the periphery to maintain blood pressure and fluid homeostasis through influencing the sympathetic tone and the renin-angiotensin system (47, 51, 73, 75, 77, 96). Circumventricular organs, for example, the subfornical organ, organum vasculosum lamina terminalis, and median eminence, do not have a complete blood-brain barrier, and, of importance, angiotensin receptors are highly expressed in these brain areas (78). Hence, both circulating and locally produced angiotensin can activate these areas (57, 90), leading to the prediction that angiotensin in the CNS mediates the sympathetic upregulation and blood pressure elevation. In addition, these brain areas send projections to the paraventricular hypothalamus and the sympathetic center RVLM (57, 90). Activation of the circumventricular organs, the PVN and the RVLM, is important for angiotensin receptor activation, being one of the crucial neural mechanisms of hypertension (47, 57, 90, 96). Other brain sites, for example, the median preoptic nucleus, which receive neural signals from the subfornical organ or organum vasculosum lamina terminalis, are also responsible for angiotensin-induced blood pressure increase (90). Furthermore, angiotensin receptors and glutamatergic receptors in the RVLM have been related to hypertension in animals (58). In addition, leptin is also known to mediate increases in blood pressure via the sympathetic nervous system (2, 48, 121, 122). Furthermore, this effect of leptin on blood pressure was shown to be facilitated through neuronal circuits in the dorsal hypothalamus (48). To summarize, the brain renin-angiotensin system, as well as leptin, is known to play a substantial role in the central regulation of blood pressure through the mechanism of sympathetic activation, and the hypothalamus plays a significant role in this process.
Obesity-induced hypothalamic inflammation and autonomic dysfunction.
Obesity is associated with a set of chronic inflammatory changes in the hypothalamus (21, 100, 125, 148), with subsequent disease progression being promoted and sustained by dynamic effects of inflammatory signaling pathways. Molecules that interact with the IKKβ/NF-κB signaling cascade, such as MyD88 or JNK1, have been demonstrated to contribute to the development of hypothalamic inflammation (66). The cellular mediators for hypothalamic inflammation include microglia, which can spread inflammatory molecules to surrounding cells (33, 41, 88, 130, 146). Astrocytes have also been proposed to mediate the hypothalamus inflammation (55, 140). Cytokine receptors, such as TNF-α receptor, appeared to mediate obesity-related neural and hypothalamic inflammation (5, 109). In addition, intracellular endoplasmic reticulum stress has been associated with the induction of neural and hypothalamic inflammation (102). In general, while it remains to be determined how high-fat diet feeding causes endoplasmic reticulum stress and inflammation in the brain, the responsible mediators can include, at least, alterations in calcium homeostasis, production of toxic metabolites, upregulation of proinflammatory cytokines, and fatty acid-mediated reactive oxygen species (25, 64). More recently, hypothalamic RNA stress response was found to promote IκBα mRNA degradation and rapidly activate the NF-κB pathway, representing another mediator of neural inflammation in the prodiabetic high-fat diet condition (140).
Among various outcomes of hypothalamic inflammation, one important one seems to be upregulation in sympathetic excitation. In 2011, Purkayastha et al. demonstrated that the acute induction of hypothalamic endoplasmic reticulum stress led to increased spontaneous renal sympathetic activity and hemodynamic changes, indicating cardiovascular sympathetic activation (102). In parallel, inhibition of inflammation in the mediobasal hypothalamus was shown to reverse the sympathetic effects caused by hypothalamic endoplasmic reticulum stress (102). Further research demonstrated that intracerebral ventricular injection of TNF-α could lead to sympathetic upregulation and sympathetic hemodynamic changes (101), with these actions being reversible through anti-inflammatory treatment in the hypothalamus (116). Despite these understandings, therapeutic options of directly inhibiting NF-κB might have pitfalls such as altered response to bacterial infections (8, 45), calling for alternative approaches of suppressing hypothalamic inflammation. Interestingly, while excess hypothalamic TGF-β was recently found to affect hepatic glucose production potentially through the sympathetic nervous system (140), it should be noted that suppression of TGF-β already shows clinical promise in the treatment of cancers (50, 72, 105), and these medications were considered generally safe (3, 106). Because it is still early to therapeutically predict, it holds promise that practical and safe anti-inflammatory approaches of targeting the brain should exist and will be identified through future research.
Glucose intolerance by hypothalamic inflammation and autonomic dysfunction.
Recent studies using experimental mouse models of hypothalamic inflammation have revealed that these animals manifest diabetic or prediabetic symptoms (102, 125). Purkayastha et al. demonstrated that short-term endoplasmic reticulum stress in hypothalamus upregulates the proinflammatory NF-κB pathway to cause hepatic glucose disorder, a metabolic effect that was associated with increased levels of gluconeogenic enzymes in the liver (102). In agreement, site-specific NF-κB inhibition in the hypothalamic arcuate nucleus was revealed to attenuate the induction of glucose intolerance by high-fat diet feeding or brain endoplasmic reticulum stress (102). Yan et al. found that hypothalamic TGF-β production under conditions of aging or obesity is higher than normal and that administration of TGF-β to the hypothalamus led to glucose intolerance, which was independent of feeding or body weight (140). Mechanistically, this effect of hypothalamic TGF-β excess was induced through hypothalamic NF-κB (140). Consistent with this conceptual model, oxidative stress has been demonstrated to be related with inflammation in the hypothalamus. Researchers found that the intracerebral ventricular injection of apelin (an endogenous ligand for G protein-coupled apelin receptor that affects diverse biological functions, including fluid homeostasis, cardiovascular function, and insulin secretion) induced hypothalamic overproduction of reactive oxygen species and increased expression of inflammatory factors leading to hyperglycemia in mice. This impairment in systemic glucose homeostasis caused by hypothalamic apelin was attributed to the enhanced sympathetic activity that increased glucose production from the liver (32). Interestingly, an intracerebral ventricular injection of an antioxidant before apelin injection was found to prevent the establishment of hyperglycemia in a body weight-independent manner (32). Furthermore, Burgos-Ramos et al. demonstrated that diabetic insulin receptor substrate 2 (IRS2)-deficient mice displayed greater amounts of hypothalamic inflammation than prediabetic IRS2-deficient mice, being the result of NF-κB and JNK activation (19). In summation, numerous studies have demonstrated a strong association between hypothalamic inflammation and glucose intolerance, supporting the concept of the hypothalamus as being a crucial central regulator for glucose homeostasis.
Insulin resistance and β-cell dysfunction by hypothalamic inflammation.
As described previously, studies have demonstrated that hypothalamic inflammation via endoplasmic reticulum stress activates the proinflammatory NF-κB pathway leading to glucose intolerance, with this metabolic disorder being partly attributed to insulin resistance (102). Indeed, injection of endoplasmic reticulum stress suppressor in the hypothalamus improved systemic glucose tolerance and peripheral insulin signaling (102). Despite these observations, it should be noted that endoplasmic reticulum stress is not the only cause of hypothalamic inflammation in affecting insulin resistance. For instance, increased redox signaling and reactive oxygen species in the hypothalamus were also observed to be associated with NF-κB activation, leading to hyperglycemia, hyperinsulinemia, and insulin resistance in mice (32). Furthermore, an intracerebral ventricular injection of antioxidant chemical trolox (a water-soluble analog of vitamin E) before the induction of reactive oxygen species in the hypothalamus inhibits the development of diabetic characteristics (32). Besides, long-term overnutrition can impair hypothalamic autophagic function, also contributing to chronic hypothalamic inflammation, which causes not only obesity but also systemic insulin resistance (22, 79). Excess lipids through chemical injection or feeding were shown to activate inflammatory Toll-like receptor 4 signaling in the hypothalamus, whereas the inhibition of this receptor signaling in the brain prevents overnutrition from causing metabolic disorders, including systemic insulin resistance (66). The proinflammatory TNF-α pathway was also studied, showing that obesity and insulin resistance can both be abrogated in mice that lack either TNF-α or TNF-α receptor (52, 132). Resistin, another proinflammatory cytokine that is produced mainly from the fat, was found to induce hepatic insulin resistance via neural actions (1). Recently, it was revealed that central TGF-β excess employs an RNA metabolism-dependent mechanism to activate proinflammatory NF-κB in the hypothalamus, leading to a rapid manifestation of systemic and hepatic insulin resistance (140). In addition, and in line with the fact that pancreatic β-cell function is, at least in part, under the control of the autonomic system, it was shown that hypothalamic inflammation led to the loss of first-phase insulin secretion, with this loss being restored following sympathectomy (115). Altogether, hypothalamic inflammation and associated autonomic changes have a significant role in impairing insulin sensitivity, as well as insulin secretion, contributing to the development of type 2 diabetes.
Hypothalamic inflammation links aging to diabetes development.
Obesity, type 2 diabetes, and their related complications increase in prevalence with aging and with aging-related diseases such as Alzheimer's disease and Parkinson's disease (11, 70). In addition, therapeutic interventions for metabolic diseases (e.g., diabetes) are known to counteract symptoms related to neurodegeneration-associated disorders, suggesting that metabolic and age-related diseases share several mechanistic pathways (31, 112). Indeed, studies with the use of animal models have frequently demonstrated the causal relationships between diabetes and neurodegenerative diseases. For instance, degeneration of dopaminergic neurons in a mouse model of Parkinson's disease can be significantly promoted by overnutrition (15). Also, while diabetes-associated brain insulin-signaling changes can cause neuronal oxidative stress and mitochondrial dysfunction, these changes in the brain were also shown to mediate Huntington's disease (111). Furthermore, obesity and diabetes are known to alter the functioning of phosphatase and tensin homolog-induced putative kinase-1, which mediates neuronal apoptosis and is causally related to familial Parkinson's disease (113). IL-6 is a classical NF-κB product and is overproduced in obesity and diabetic conditions. This cytokine has been reported to mediate GABAergic neuronal degeneration in the forebrain, with this neurodegenerative outcome induced through neuronal NF-κB activation (34). Conceptually, it has been suggested that metabolic overload and neuronal insulin resistance in obesity and diabetes render neurons more susceptible to neural inflammation and neuronal death (16, 30). Recent studies have also implicated that glial cells in the brain play roles in the etiology of diabetes symptoms. For instance, hyperglycemia is associated with a reduction in the expression level of glucose transporter-1 in hypothalamic glial cells (26), and reduced levels of glucose transporter-1 were shown to impair the hypothalamic regulation of hepatic glucose production, while this impairment can be prevented by restoring glucose transporter-1 in hypothalamic glial cells (26). In addition, glucose transporter-2 in astrocytes and tanycytes has been suggested to contribute to glucose sensing of the brain (42, 71).
Hypertensive effect by hypothalamic inflammation and autonomic dysfunction.
Evidence from both neuroendocrinology and neuroimmunology studies indicates that chronic overnutrition is an independent environmental factor in the pathogenesis of obesity-related hypertension (100, 125). As discussed earlier, overnutrition is known to lead to hypothalamic inflammation, and several studies have eluded to the chronic activation of proinflammatory pathways within both central and peripheral tissues as being associated with obesity-induced hypertension (135). Within the CNS, NF-κB-driven hypothalamic inflammation employs an obesity-dissociable mechanism to induce the loss of blood pressure homeostasis, likely in parallel to producing glucose intolerance and insulin resistance, acting as an uncoupling point between hypertension and obesity-associated metabolic diseases (5, 98, 101, 102). Increased proinflammatory cytokines in the hypothalamus are closely related to the condition of hypertension, and recent literature highlighted the relationship between the renin-angiotensin system and inflammation in understanding the neural mechanism of hypertension (103). For example, blocking NF-κB activation in the brain can significantly attenuate angiotensin-induced hypertension (23). Within the hypothalamus, TNF-α and IL-1β stimulation in the PVN was shown to augment adrenocorticotropic hormone release, enhance cardiac sympathetic afferent reflex, and increase sympathetic outflow, all of which belong to important factors for the development of hypertension (36, 37). Furthermore, angiotensin-induced prohypertensive mechanisms may be significantly attenuated by PVN-specific blockade of TNF-α (23, 36). The key role of TNF-α was also recognized for angiotensin-stimulated inflammation, which may involve cross talk between this inflammatory cytokine and reactive oxygen species signals, given that central administration of reactive oxygen species scavenger tempol is able to decrease angiotensin-induced IL-1β in the PVN, further decreasing sympathetic activity and blood pressure (124). Angiotensin and prorenin both increase proinflammatory cytokine expression, such as TNF-α and IL-β, in the nucleus tractus solitarius via the NF-κB complex (150). Purkayastha et al. further showed that TNF-α stimulates the IKKβ and NF-κB pathway in POMC neurons, leading to elevated blood pressure via a central mechanism involving sympathetic nervous system activation (101). Although it is clear that inflammatory cytokines are directly or indirectly induced by angiotensin stimulation, studies are needed to address which molecules and processes work to initiate and mediate this inflammatory response.
It is also worth noting that circulating angiotensin can send signals to the nucleus tractus solitarius neuronal networks leading to an increase in the blood-brain barrier permeability (94, 147). Because of this change, circulating factors including angiotensin have better access to brain areas such as the PVN, the RVLM, and the nucleus tractus solitaries, which are important for the autonomic control of blood pressure (12). Inflammatory cytokines IL-1β, TNF-α, and IL-6 can dysregulate adherens and tight junctions, further increasing the blood-brain barrier permeability (68). In addition, inflammatory cytokines are also produced by astroglia and microglia, which might also contribute to the development of hypertension (29, 33, 41, 88, 110, 130, 137), given that these glial cells play a particular role for the induction of hypothalamic inflammation in diet-induced obesity (24, 55, 69, 126, 135). Anti-inflammatory cytokines, such as IL-10, inhibit microglia activation and lead to reduced blood pressure levels in animals (116), but it remains to be studied if this effect on microglia was mechanistically involved in the decrease in blood pressure. Taken together, angiotensin and its associated inflammatory pathways interact closely in the hypothalamus and the rest of the brain to mediate the development of hypertension and, in particular, obesity-related hypertension.
Concluding remarks.
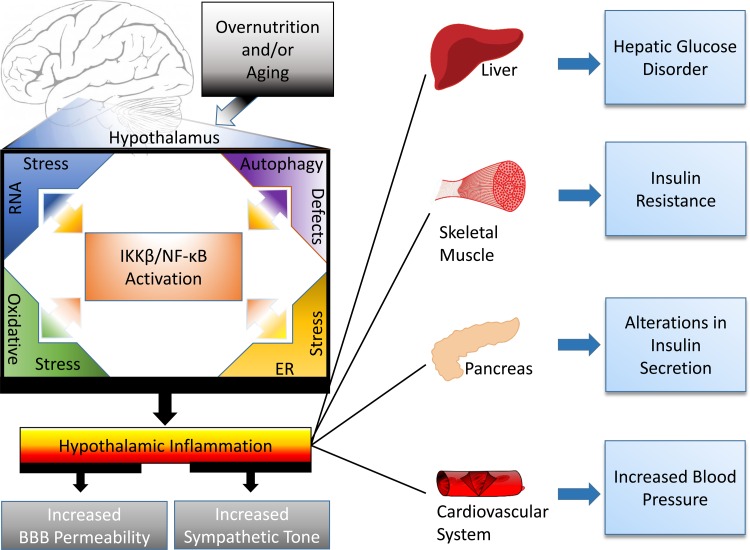
Multiple lines of evidence indicate that the CNS is a mediating factor in the pathogenesis of diabetes and hypertension, rather than these ailments simply being disorders of peripheral tissues. Within the CNS, the hypothalamus lies at the intersection of the neuroendocrine and autonomic systems, and is a central component in the regulation of glucose and blood pressure homeostasis. As summarized in Fig. 1, provocative evidence has recently emerged to indicate that hypothalamic inflammation is a major contributor to the central dysregulation of glucose and blood pressure homeostasis. Although the cause of hypothalamic inflammation is not fully understood, this condition is closely associated with endoplasmic reticulum stress, RNA stress, oxidative damage, and autophagy defects, all of which can trigger or promote proinflammatory NF-κB activation. The amelioration of hypertensive and diabetic symptoms following administration of anti-inflammatory agents (39, 53) or targeting inflammatory molecular pathways (50, 72, 105) indicates therapeutic possibilities of reducing hypothalamic inflammation to control these epidemic diseases and related complications. While the pathogenic basis of neural and hypothalamic inflammation is formed dynamically through multiple processes, many of them still need further investigation. Regardless, recent findings have made it safe to conclude that neural inflammation, especially in the hypothalamus, alters the autonomic system to contribute to the development of diabetes and hypertension.
Fig. 1.
Hypothalamic inflammation in the pathogenesis of diabetes and hypertension. Overnutrition and aging trigger induction of hypothalamic inflammation. This inflammation can stem from, in part, the activation of IKKβ/NF-κB cascade in association with functional changes of intracellular organelles such as RNA stress response, endoplasmic reticulum and oxidative stresses, and, more chronically, autophagic defect. Such hypothalamic inflammation affects not only neuroendocrine signaling but also the connections between the hypothalamus and the autonomic nervous system leading to increased sympathetic outflow. Consequently, the autonomic control over peripheral organs, including the liver, skeletal muscle, pancreas, and cardiovascular system, is perturbed, resulting in glucose disorder, insulin resistance, insulin secretion impairment, and blood pressure increase, which are predicted to chronically contribute to the development of diabetes and hypertension.
GRANTS
These projects were supported by National Institutes of Health Grants R01-DK-078750, R01-AG-031774, R01-HL-113180, and R01-DK-099136 (all awarded to D. Cai).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.H. drafted manuscript; M.W.R. prepared figures; M.W.R. and D.C. edited and revised manuscript; D.C. conception and design of research; D.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the members of the laboratory of D. Cai for contributions to projects that were related to this review.
REFERENCES
- 1.Ahima RS, Lazar MA. Adipokines and the peripheral and neural control of energy balance. Mol Endocrinol 22: 1023–1031, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aizawa-Abe M, Ogawa Y, Masuzaki H, Ebihara K, Satoh N, Iwai H, Matsuoka N, Hayashi T, Hosoda K, Inoue G, Yoshimasa Y, Nakao K. Pathophysiological role of leptin in obesity-related hypertension. J Clin Invest 105: 1243–1252, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akhurst RJ, Hata A. Targeting the TGFbeta signalling pathway in disease. Nat Rev Drug Discov 11: 790–811, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aronsson M, Fuxe K, Dong Y, Agnati LF, Okret S, Gustafsson JA. Localization of glucocorticoid receptor mRNA in the male rat brain by in situ hybridization. Proc Natl Acad Sci USA 85: 9331–9335, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arruda AP, Milanski M, Coope A, Torsoni AS, Ropelle E, Carvalho DP, Carvalheira JB, Velloso LA. Low-grade hypothalamic inflammation leads to defective thermogenesis, insulin resistance, and impaired insulin secretion. Endocrinology 152: 1314–1326, 2011. [DOI] [PubMed] [Google Scholar]
- 6.Bachman ES, Dhillon H, Zhang CY, Cinti S, Bianco AC, Kobilka BK, Lowell BB. betaAR signaling required for diet-induced thermogenesis and obesity resistance. Science 297: 843–845, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Bamshad M, Song CK, Bartness TJ. CNS origins of the sympathetic nervous system outflow to brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 276: R1569–R1578, 1999. [DOI] [PubMed] [Google Scholar]
- 8.Baud V, Karin M. Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discov 8: 33–40, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bellinger LL, Bernardis LL. The dorsomedial hypothalamic nucleus and its role in ingestive behavior and body weight regulation: lessons learned from lesioning studies. Physiol Behav 76: 431–442, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Bernardis LL, Bellinger LL. The lateral hypothalamic area revisited: neuroanatomy, body weight regulation, neuroendocrinology and metabolism. Neurosci Biobehav Rev 17: 141–193, 1993. [DOI] [PubMed] [Google Scholar]
- 11.Beydoun MA, Beydoun HA, Wang Y. Obesity and central obesity as risk factors for incident dementia and its subtypes: a systematic review and meta-analysis. Obes Rev 9: 204–218, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biancardi VC, Son SJ, Ahmadi S, Filosa JA, Stern JE. Circulating angiotensin II gains access to the hypothalamus and brain stem during hypertension via breakdown of the blood-brain barrier. Hypertension 63: 572–579, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borg MA, Sherwin RS, Borg WP, Tamborlane WV, Shulman GI. Local ventromedial hypothalamus glucose perfusion blocks counterregulation during systemic hypoglycemia in awake rats. J Clin Invest 99: 361–365, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borg WP, Sherwin RS, During MJ, Borg MA, Shulman GI. Local ventromedial hypothalamus glucopenia triggers counterregulatory hormone release. Diabetes 44: 180–184, 1995. [DOI] [PubMed] [Google Scholar]
- 15.Bousquet M, St-Amour I, Vandal M, Julien P, Cicchetti F, Calon F. High-fat diet exacerbates MPTP-induced dopaminergic degeneration in mice. Neurobiol Dis 45: 529–538, 2012. [DOI] [PubMed] [Google Scholar]
- 16.Bruce-Keller AJ, Keller JN, Morrison CD. Obesity and vulnerability of the CNS. Biochim Biophys Acta 1792: 395–400, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buijs RM, Chun SJ, Niijima A, Romijn HJ, Nagai K. Parasympathetic and sympathetic control of the pancreas: a role for the suprachiasmatic nucleus and other hypothalamic centers that are involved in the regulation of food intake. J Comp Neurol 431: 405–423, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Burbridge S, Stewart I, Placzek M. Development of the Neuroendocrine Hypothalamus. Compr Physiol 6: 623–643, 2016. [DOI] [PubMed] [Google Scholar]
- 19.Burgos-Ramos E, Gonzalez-Rodriguez A, Canelles S, Baquedano E, Frago LM, Revuelta-Cervantes J, Gomez-Ambrosi J, Fruhbeck G, Chowen JA, Argente J, Valverde AM, Barrios V. Differential insulin receptor substrate-1 (IRS1)-related modulation of neuropeptide Y and proopiomelanocortin expression in nondiabetic and diabetic IRS2-/- mice. Endocrinology 153: 1129–1140, 2012. [DOI] [PubMed] [Google Scholar]
- 20.Butterick TA, Billington CJ, Kotz CM, Nixon JP. Orexin: pathways to obesity resistance? Rev Endocr Metab Disord 14: 357–364, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cai D. Neuroinflammation and neurodegeneration in overnutrition-induced diseases. Trends Endocrinol Metab 24: 40–47, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cai D, Liu T. Hypothalamic inflammation: a double-edged sword to nutritional diseases. Ann NY Acad Sci 1243: E1–E39, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cardinale JP, Sriramula S, Mariappan N, Agarwal D, Francis J. Angiotensin II-induced hypertension is modulated by nuclear factor-kappaBin the paraventricular nucleus. Hypertension 59: 113–121, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carmichael CY, Wainford RD. Hypothalamic signaling mechanisms in hypertension. Curr Hypertens Rep 17: 39, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castro G, MFCA, Weissmann L, Quaresma PG, Katashima CK, Saad MJ, Prada PO. Diet-induced obesity induces endoplasmic reticulum stress and insulin resistance in the amygdala of rats. FEBS Open Bio 3: 443–449, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chari M, Yang CS, Lam CK, Lee K, Mighiu P, Kokorovic A, Cheung GW, Lai TY, Wang PY, Lam TK. Glucose transporter-1 in the hypothalamic glial cells mediates glucose sensing to regulate glucose production in vivo. Diabetes 60: 1901–1906, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci 8: 571–578, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Coppari R, Ichinose M, Lee CE, Pullen AE, Kenny CD, McGovern RA, Tang V, Liu SM, Ludwig T, Chua SC Jr, Lowell BB, Elmquist JK. The hypothalamic arcuate nucleus: a key site for mediating leptin's effects on glucose homeostasis and locomotor activity. Cell Metab 1: 63–72, 2005. [DOI] [PubMed] [Google Scholar]
- 29.de Kloet AD, Krause EG, Shi PD, Zubcevic J, Raizada MK, Sumners C. Neuroimmune communication in hypertension and obesity: a new therapeutic angle? Pharmacol Ther 138: 428–440, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de la Monte SM, Longato L, Tong M, Wands JR. Insulin resistance and neurodegeneration: roles of obesity, type 2 diabetes mellitus and non-alcoholic steatohepatitis. Curr Opin Investig Drugs 10: 1049–1060, 2009. [PMC free article] [PubMed] [Google Scholar]
- 31.Dinarello CA. Anti-inflammatory agents: present and future. Cell 140: 935–950, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drougard A, Duparc T, Brenachot X, Carneiro L, Gouaze A, Fournel A, Geurts L, Cadoudal T, Prats AC, Penicaud L, Vieau D, Lesage J, Leloup C, Benani A, Cani PD, Valet P, Knauf C. Hypothalamic apelin/reactive oxygen species signaling controls hepatic glucose metabolism in the onset of diabetes. Antioxid Redox Signal 20: 557–573, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duffy CM, Yuan C, Wisdorf LE, Billington CJ, Kotz CM, Nixon JP, Butterick TA. Role of orexin A signaling in dietary palmitic acid-activated microglial cells. Neurosci Lett 606: 140–144, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dugan LL, Ali SS, Shekhtman G, Roberts AJ, Lucero J, Quick KL, Behrens MM. IL-6 mediated degeneration of forebrain GABAergic interneurons and cognitive impairment in aged mice through activation of neuronal NADPH oxidase. PLoS One 4: e5518, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fekete C, Sarkar S, Rand WM, Harney JW, Emerson CH, Bianco AC, Lechan RM. Agouti-related protein (AGRP) has a central inhibitory action on the hypothalamic-pituitary-thyroid (HPT) axis; comparisons between the effect of AGRP and neuropeptide Y on energy homeostasis and the HPT axis. Endocrinology 143: 3846–3853, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Felder RB. Mineralocorticoid receptors, inflammation and sympathetic drive in a rat model of systolic heart failure. Exp Physiol 95: 19–25, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Felder RB, Yu Y, Zhang ZH, Wei SG. Pharmacological treatment for heart failure: a view from the brain. Clin Pharmacol Ther 86: 216–220, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37a.Ferguson AV, Latchford KJ, Samson WK. The paraventricular nucleus of the hypothalamus: a potential target for integrative treatment of autonomic dysfunction. Expert Opin Ther Target 12: 717–727, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fliers E, Klieverik LP, Kalsbeek A. Novel neural pathways for metabolic effects of thyroid hormone. Trends Endocrinol Metab 21: 230–236, 2010. [DOI] [PubMed] [Google Scholar]
- 39.Fraillon D, Wynne KN, Funder JW. Further studies on neomycin and experimental hypertension. Clin Exp Pharmacol Physiol 11: 339–341, 1984. [DOI] [PubMed] [Google Scholar]
- 40.Fujikawa T, Coppari R. Living without insulin: the role of leptin signaling in the hypothalamus. Front Neurosci 9: 108, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao Y, Ottaway N, Schriever SC, Legutko B, Garcia-Caceres C, de la Fuente E, Mergen C, Bour S, Thaler JP, Seeley RJ, Filosa J, Stern JE, Perez-Tilve D, Schwartz MW, Tschop MH, Yi CX. Hormones and diet, but not body weight, control hypothalamic microglial activity. Glia 62: 17–25, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garcia M, Millan C, Balmaceda-Aguilera C, Castro T, Pastor P, Montecinos H, Reinicke K, Zuniga F, Vera JC, Onate SA, Nualart F. Hypothalamic ependymal-glial cells express the glucose transporter GLUT2, a protein involved in glucose sensing. J Neurochem 86: 709–724, 2003. [DOI] [PubMed] [Google Scholar]
- 43.Giordano A, Frontini A, Castellucci M, Cinti S. Presence and distribution of cholinergic nerves in rat mediastinal brown adipose tissue. J Histochem Cytochem 52: 923–930, 2004. [DOI] [PubMed] [Google Scholar]
- 44.Goncalves GH, Li W, Garcia AV, Figueiredo MS, Bjorbaek C. Hypothalamic agouti-related peptide neurons and the central melanocortin system are crucial mediators of leptin's antidiabetic actions. Cell Rep 7: 1093–1103, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Greten FR, Arkan MC, Bollrath J, Hsu LC, Goode J, Miething C, Goktuna SI, Neuenhahn M, Fierer J, Paxian S, Van Rooijen N, Xu Y, O'Cain T, Jaffee BB, Busch DH, Duyster J, Schmid RM, Eckmann L, Karin M. NF-kappaB is a negative regulator of IL-1beta secretion as revealed by genetic and pharmacological inhibition of IKKbeta. Cell 130: 918–931, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grujic D, Susulic VS, Harper ME, Himms-Hagen J, Cunningham BA, Corkey BE, Lowell BB. Beta3-adrenergic receptors on white and brown adipocytes mediate beta3-selective agonist-induced effects on energy expenditure, insulin secretion, and food intake. A study using transgenic and gene knockout mice. J Biol Chem 272: 17686–17693, 1997. [DOI] [PubMed] [Google Scholar]
- 47.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci 7: 335–346, 2006. [DOI] [PubMed] [Google Scholar]
- 48.Hall JE, da Silva AA, do Carmo JM, Dubinion J, Hamza S, Munusamy S, Smith G, Stec DE. Obesity-induced hypertension: role of sympathetic nervous system, leptin, and melanocortins. J Biol Chem 285: 17271–17276, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haque MS, Minokoshi Y, Hamai M, Iwai M, Horiuchi M, Shimazu T. Role of the sympathetic nervous system and insulin in enhancing glucose uptake in peripheral tissues after intrahypothalamic injection of leptin in rats. Diabetes 48: 1706–1712, 1999. [DOI] [PubMed] [Google Scholar]
- 50.Hawinkels LJ, Ten Dijke P. Exploring anti-TGF-beta therapies in cancer and fibrosis. Growth Factors 29: 140–152, 2011. [DOI] [PubMed] [Google Scholar]
- 51.Hirooka Y, Kishi T, Sakai K, Takeshita A, Sunagawa K. Imbalance of central nitric oxide and reactive oxygen species in the regulation of sympathetic activity and neural mechanisms of hypertension. Am J Physiol Regul Integr Comp Physiol 300: R818–R826, 2011. [DOI] [PubMed] [Google Scholar]
- 52.Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature 420: 333–336, 2002. [DOI] [PubMed] [Google Scholar]
- 53.Honour JW, Borriello SP, Ganten U, Honour P. Antibiotics attenuate experimental hypertension in rats. J Endocrinol 105: 347–350, 1985. [DOI] [PubMed] [Google Scholar]
- 54.Horvath TL, Bechmann I, Naftolin F, Kalra SP, Leranth C. Heterogeneity in the neuropeptide Y-containing neurons of the rat arcuate nucleus: GABAergic and non-GABAergic subpopulations. Brain Res 756: 283–286, 1997. [DOI] [PubMed] [Google Scholar]
- 55.Horvath TL, Sarman B, Garcia-Caceres C, Enriori PJ, Sotonyi P, Shanabrough M, Borok E, Argente J, Chowen JA, Perez-Tilve D, Pfluger PT, Bronneke HS, Levin BE, Diano S, Cowley MA, Tschop MH. Synaptic input organization of the melanocortin system predicts diet-induced hypothalamic reactive gliosis and obesity. Proc Natl Acad Sci USA 107: 14875–14880, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huo L, Gamber K, Greeley S, Silva J, Huntoon N, Leng XH, Bjorbaek C. Leptin-dependent control of glucose balance and locomotor activity by POMC neurons. Cell Metab 9: 537–547, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ito S, Komatsu K, Tsukamoto K, Kanmatsuse K, Sved AF. Ventrolateral medulla AT1 receptors support blood pressure in hypertensive rats. Hypertension 40: 552–559, 2002. [DOI] [PubMed] [Google Scholar]
- 58.Ito S, Komatsu K, Tsukamoto K, Sved AF. Tonic excitatory input to the rostral ventrolateral medulla in Dahl salt-sensitive rats. Hypertension 37: 687–691, 2001. [PubMed] [Google Scholar]
- 59.Jansen AS, Hoffman JL, Loewy AD. CNS sites involved in sympathetic and parasympathetic control of the pancreas: a viral tracing study. Brain Res 766: 29–38, 1997. [DOI] [PubMed] [Google Scholar]
- 60.Joly-Amado A, Denis RG, Castel J, Lacombe A, Cansell C, Rouch C, Kassis N, Dairou J, Cani PD, Ventura-Clapier R, Prola A, Flamment M, Foufelle F, Magnan C, Luquet S. Hypothalamic AgRP-neurons control peripheral substrate utilization and nutrient partitioning. EMBO J 31: 4276–4288, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest 106: 473–481, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kalsbeek A, Foppen E, Schalij I, Van Heijningen C, van der Vliet J, Fliers E, Buijs RM. Circadian control of the daily plasma glucose rhythm: an interplay of GABA and glutamate. PLoS One 3: e3194, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kato H, Nagai Y, Ohta A, Tenjin A, Nakamura Y, Tsukiyama H, Sasaki Y, Fukuda H, Ohshige T, Terashima Y, Sada Y, Kondo A, Sasaoka T, Tanaka Y. Effect of sitagliptin on intrahepatic lipid content and body fat in patients with type 2 diabetes. Diabetes Res Clin Pract 109: 199–205, 2015. [DOI] [PubMed] [Google Scholar]
- 64.Kawasaki N, Asada R, Saito A, Kanemoto S, Imaizumi K. Obesity-induced endoplasmic reticulum stress causes chronic inflammation in adipose tissue. Sci Rep 2: 799, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim JD, Toda C, D'Agostino G, Zeiss CJ, DiLeone RJ, Elsworth JD, Kibbey RG, Chan O, Harvey BK, Richie CT, Savolainen M, Myohanen T, Jeong JK, Diano S. Hypothalamic prolyl endopeptidase (PREP) regulates pancreatic insulin and glucagon secretion in mice. Proc Natl Acad Sci USA 111: 11876–11881, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kleinridders A, Schenten D, Konner AC, Belgardt BF, Mauer J, Okamura T, Wunderlich FT, Medzhitov R, Bruning JC. MyD88 signaling in the CNS is required for development of fatty acid-induced leptin resistance and diet-induced obesity. Cell Metab 10: 249–259, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Klieverik LP, Janssen SF, van Riel A, Foppen E, Bisschop PH, Serlie MJ, Boelen A, Ackermans MT, Sauerwein HP, Fliers E, Kalsbeek A. Thyroid hormone modulates glucose production via a sympathetic pathway from the hypothalamic paraventricular nucleus to the liver. Proc Natl Acad Sci USA 106: 5966–5971, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Labus J, Hackel S, Lucka L, Danker K. Interleukin-1beta induces an inflammatory response and the breakdown of the endothelial cell layer in an improved human THBMEC-based in vitro blood-brain barrier model. J Neurosci Methods 228: 35–45, 2014. [DOI] [PubMed] [Google Scholar]
- 69.Lee EB, Ahima RS. Alteration of hypothalamic cellular dynamics in obesity. J Clin Invest 122: 22–25, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lees AJ, Hardy J, Revesz T. Parkinson's disease. Lancet 373: 2055–2066, 2009. [DOI] [PubMed] [Google Scholar]
- 71.Leloup C, Arluison M, Lepetit N, Cartier N, Marfaing-Jallat P, Ferre P, Penicaud L. Glucose transporter 2 (GLUT 2): expression in specific brain nuclei. Brain Res 638: 221–226, 1994. [DOI] [PubMed] [Google Scholar]
- 72.Levy L, Hill CS. Alterations in components of the TGF-beta superfamily signaling pathways in human cancer. Cytokine Growth Factor Rev 17: 41–58, 2006. [DOI] [PubMed] [Google Scholar]
- 72a.Llewellyn-Smith IJ, Verberne AJM. Central Regulation of Autonomic Functions. Oxford, UK: Oxford University Press, 2011. [Google Scholar]
- 73.Lohmeier TE, Iliescu R. Chronic lowering of blood pressure by carotid baroreflex activation: mechanisms and potential for hypertension therapy. Hypertension 57: 880–886, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lopez M, Dieguez C, Nogueiras R. Hypothalamic GLP-1: the control of BAT thermogenesis and browning of white fat. Adipocyte 4: 141–145, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Malpas SC. Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiol Rev 90: 513–557, 2010. [DOI] [PubMed] [Google Scholar]
- 76.Marino JS, Xu Y, Hill JW. Central insulin and leptin-mediated autonomic control of glucose homeostasis. Trends Endocrinol Metab 22: 275–285, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Marvar PJ, Lob H, Vinh A, Zarreen F, Harrison DG. The central nervous system and inflammation in hypertension. Curr Opin Pharmacol 11: 156–161, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McKinley MJ, Albiston AL, Allen AM, Mathai ML, May CN, McAllen RM, Oldfield BJ, Mendelsohn FA, Chai SY. The brain renin-angiotensin system: location and physiological roles. Int J Biochem Cell Biol 35: 901–918, 2003. [DOI] [PubMed] [Google Scholar]
- 79.Meng Q, Cai D. Defective hypothalamic autophagy directs the central pathogenesis of obesity via the IkappaB kinase beta (IKKbeta)/NF-kappaB pathway. J Biol Chem 286: 32324–32332, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Milosavljevic Z, Zelen I, Sazdanovic M. Autonomic innervation of the periglomerular arteries. Anal Quant Cytopathol Histpathol 36: 161–166, 2014. [PubMed] [Google Scholar]
- 81.Minokoshi Y, Haque MS, Shimazu T. Microinjection of leptin into the ventromedial hypothalamus increases glucose uptake in peripheral tissues in rats. Diabetes 48: 287–291, 1999. [DOI] [PubMed] [Google Scholar]
- 82.Mussa BM, Sartor DM, Rantzau C, Verberne AJ. Effects of nitric oxide synthase blockade on dorsal vagal stimulation-induced pancreatic insulin secretion. Brain Res 1394: 62–70, 2011. [DOI] [PubMed] [Google Scholar]
- 83.Mussa BM, Verberne AJ. The dorsal motor nucleus of the vagus and regulation of pancreatic secretory function. Exp Physiol 98: 25–37, 2013. [DOI] [PubMed] [Google Scholar]
- 84.Nguyen NL, Randall J, Banfield BW, Bartness TJ. Central sympathetic innervations to visceral and subcutaneous white adipose tissue. Am J Physiol Regul Integr Comp Physiol 306: R375–R386, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Noble EE, Billington CJ, Kotz CM, Wang C. The lighter side of BDNF. Am J Physiol Regul Integr Comp Physiol 300: R1053–R1069, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nonogaki K. New insights into sympathetic regulation of glucose and fat metabolism. Diabetologia 43: 533–549, 2000. [DOI] [PubMed] [Google Scholar]
- 87.O'Hare JD, Zsombok A. Brain-liver connections: role of the pre-autonomic PVN neurons. Am J Physiol Endocrinol Metab 310: E183–E189, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Oh IS, Thaler JP, Ogimoto K, Wisse BE, Morton GJ, Schwartz MW. Central administration of interleukin-4 exacerbates hypothalamic inflammation and weight gain during high-fat feeding. Am J Physiol Endocrinol Metab 299: E47–E53, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Oldfield BJ, Giles ME, Watson A, Anderson C, Colvill LM, McKinley MJ. The neurochemical characterisation of hypothalamic pathways projecting polysynaptically to brown adipose tissue in the rat. Neuroscience 110: 515–526, 2002. [DOI] [PubMed] [Google Scholar]
- 90.Osborn JW, Hendel MD, Collister JP, Ariza-Guzman PA, Fink GD. The role of the subfornical organ in angiotensin II-salt hypertension in the rat. Exp Physiol 97: 80–88, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Owen BM, Ding X, Morgan DA, Coate KC, Bookout AL, Rahmouni K, Kliewer SA, Mangelsdorf DJ. FGF21 acts centrally to induce sympathetic nerve activity, energy expenditure, and weight loss. Cell Metab 20: 670–677, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Paris M, Bernard-Kargar C, Berthault MF, Bouwens L, Ktorza A. Specific and combined effects of insulin and glucose on functional pancreatic beta-cell mass in vivo in adult rats. Endocrinology 144: 2717–2727, 2003. [DOI] [PubMed] [Google Scholar]
- 93.Parysow O, Mollerach AM, Jager V, Racioppi S, San Roman J, Gerbaudo VH. Low-dose oral propranolol could reduce brown adipose tissue F-18 FDG uptake in patients undergoing PET scans. Clin Nucl Med 32: 351–357, 2007. [DOI] [PubMed] [Google Scholar]
- 94.Paton JF, Wang S, Polson JW, Kasparov S. Signalling across the blood brain barrier by angiotensin II: novel implications for neurogenic hypertension. J Mol Med (Berl) 86: 705–710, 2008. [DOI] [PubMed] [Google Scholar]
- 95.Perez-Leighton CE, Butterick-Peterson TA, Billington CJ, Kotz CM. Role of orexin receptors in obesity: from cellular to behavioral evidence. Int J Obes (Lond) 37: 167–174, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Peterson JR, Sharma RV, Davisson RL. Reactive oxygen species in the neuropathogenesis of hypertension. Curr Hypertens Rep 8: 232–241, 2006. [DOI] [PubMed] [Google Scholar]
- 97.Pick A, Clark J, Kubstrup C, Levisetti M, Pugh W, Bonner-Weir S, Polonsky KS. Role of apoptosis in failure of beta-cell mass compensation for insulin resistance and beta-cell defects in the male Zucker diabetic fatty rat. Diabetes 47: 358–364, 1998. [DOI] [PubMed] [Google Scholar]
- 98.Posey KA, Clegg DJ, Printz RL, Byun J, Morton GJ, Vivekanandan-Giri A, Pennathur S, Baskin DG, Heinecke JW, Woods SC, Schwartz MW, Niswender KD. Hypothalamic proinflammatory lipid accumulation, inflammation, and insulin resistance in rats fed a high-fat diet. Am J Physiol Endocrinol Metab 296: E1003–E1012, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Prieto I, Villarejo AB, Segarra AB, Banegas I, Wangensteen R, Martinez-Canamero M, de Gasparo M, Vives F, Ramirez-Sanchez M. Brain, heart and kidney correlate for the control of blood pressure and water balance: role of angiotensinases. Neuroendocrinology 100: 198–208, 2014. [DOI] [PubMed] [Google Scholar]
- 100.Purkayastha S, Cai D. Neuroinflammatory basis of metabolic syndrome. Mol Metab 2: 356–363, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Purkayastha S, Zhang G, Cai D. Uncoupling the mechanisms of obesity and hypertension by targeting hypothalamic IKK-beta and NF-kappaB. Nat Med 17: 883–887, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Purkayastha S, Zhang H, Zhang G, Ahmed Z, Wang Y, Cai D. Neural dysregulation of peripheral insulin action and blood pressure by brain endoplasmic reticulum stress. Proc Natl Acad Sci USA 108: 2939–2944, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Qi J, Zhang DM, Suo YP, Song XA, Yu XJ, Elks C, Lin YX, Xu YY, Zang WJ, Zhu Z, Kang YM. Renin-angiotensin system modulates neurotransmitters in the paraventricular nucleus and contributes to angiotensin II-induced hypertensive response. Cardiovasc Toxicol 13: 48–54, 2013. [DOI] [PubMed] [Google Scholar]
- 104.Ramnanan CJ, Edgerton DS, Kraft G, Cherrington AD. Physiologic action of glucagon on liver glucose metabolism. Diabetes Obes Metab 13, Suppl 1: 118–125, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Roberts AB, Wakefield LM. The two faces of transforming growth factor beta in carcinogenesis. Proc Natl Acad Sci USA 100: 8621–8623, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rodon J, Carducci MA, Sepulveda-Sanchez JM, Azaro A, Calvo E, Seoane J, Brana I, Sicart E, Gueorguieva I, Cleverly AL, Pillay NS, Desaiah D, Estrem ST, Paz-Ares L, Holdhoff M, Blakeley J, Lahn MM, Baselga J. First-in-human dose study of the novel transforming growth factor-beta receptor I kinase inhibitor LY2157299 monohydrate in patients with advanced cancer and glioma. Clin Cancer Res 21: 553–560, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rodriguez-Diaz R, Caicedo A. Neural control of the endocrine pancreas. Best Pract Res Clin Endocrinol Metab 28: 745–756, 2014. [DOI] [PubMed] [Google Scholar]
- 108.Rodriguez-Diaz R, Speier S, Molano RD, Formoso A, Gans I, Abdulreda MH, Cabrera O, Molina J, Fachado A, Ricordi C, Leibiger I, Pileggi A, Berggren PO, Caicedo A. Noninvasive in vivo model demonstrating the effects of autonomic innervation on pancreatic islet function. Proc Natl Acad Sci USA 109: 21456–21461, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Romanatto T, Roman EA, Arruda AP, Denis RG, Solon C, Milanski M, Moraes JC, Bonfleur ML, Degasperi GR, Picardi PK, Hirabara S, Boschero AC, Curi R, Velloso LA. Deletion of tumor necrosis factor-alpha receptor 1 (TNFR1) protects against diet-induced obesity by means of increased thermogenesis. J Biol Chem 284: 36213–36222, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 110.Saavedra JM. Angiotensin II AT(1) receptor blockers as treatments for inflammatory brain disorders. Clin Sci (Lond) 123: 567–590, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sadagurski M, Cheng Z, Rozzo A, Palazzolo I, Kelley GR, Dong X, Krainc D, White MF. IRS2 increases mitochondrial dysfunction and oxidative stress in a mouse model of Huntington disease. J Clin Invest 121: 4070–4081, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schapira AH. Mitochondrial diseases. Lancet 379: 1825–1834, 2012. [DOI] [PubMed] [Google Scholar]
- 113.Scheele C, Nielsen AR, Walden TB, Sewell DA, Fischer CP, Brogan RJ, Petrovic N, Larsson O, Tesch PA, Wennmalm K, Hutchinson DS, Cannon B, Wahlestedt C, Pedersen BK, Timmons JA. Altered regulation of the PINK1 locus: a link between type 2 diabetes and neurodegeneration? FASEB J 21: 3653–3665, 2007. [DOI] [PubMed] [Google Scholar]
- 114.Schwartz MW, Seeley RJ, Campfield LA, Burn P, Baskin DG. Identification of targets of leptin action in rat hypothalamus. J Clin Invest 98: 1101–1106, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Seoane-Collazo P, Ferno J, Gonzalez F, Dieguez C, Leis R, Nogueiras R, Lopez M. Hypothalamic-autonomic control of energy homeostasis. Endocrine 50: 276–291, 2015. [DOI] [PubMed] [Google Scholar]
- 116.Shi P, Diez-Freire C, Jun JY, Qi Y, Katovich MJ, Li Q, Sriramula S, Francis J, Sumners C, Raizada MK. Brain microglial cytokines in neurogenic hypertension. Hypertension 56: 297–303, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shimasaki T, Masaki T, Mitsutomi K, Ueno D, Gotoh K, Chiba S, Kakuma T, Yoshimatsu H. The dipeptidyl peptidase-4 inhibitor des-fluoro-sitagliptin regulates brown adipose tissue uncoupling protein levels in mice with diet-induced obesity. PLoS One 8: e63626, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shimazu T, Ogasawara S. Effects of hypothalamic stimulation on gluconeogenesis and glycolysis in rat liver. Am J Physiol 228: 1787–1793, 1975. [DOI] [PubMed] [Google Scholar]
- 119.Shimazu T, Sudo M, Minokoshi Y, Takahashi A. Role of the hypothalamus in insulin-independent glucose uptake in peripheral tissues. Brain Res Bull 27: 501–504, 1991. [DOI] [PubMed] [Google Scholar]
- 120.Shiuchi T, Haque MS, Okamoto S, Inoue T, Kageyama H, Lee S, Toda C, Suzuki A, Bachman ES, Kim YB, Sakurai T, Yanagisawa M, Shioda S, Imoto K, Minokoshi Y. Hypothalamic orexin stimulates feeding-associated glucose utilization in skeletal muscle via sympathetic nervous system. Cell Metab 10: 466–480, 2009. [DOI] [PubMed] [Google Scholar]
- 121.Simonds SE, Cowley MA. Hypertension in obesity: is leptin the culprit? Trends Neurosci 36: 121–132, 2013. [DOI] [PubMed] [Google Scholar]
- 122.Simonds SE, Pryor JT, Ravussin E, Greenway FL, Dileone R, Allen AM, Bassi J, Elmquist JK, Keogh JM, Henning E, Myers MG Jr, Licinio J, Brown RD, Enriori PJ, O'Rahilly S, Sternson SM, Grove KL, Spanswick DC, Farooqi IS, Cowley MA. Leptin mediates the increase in blood pressure associated with obesity. Cell 159: 1404–1416, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sohn JW. Network of hypothalamic neurons that control appetite. BMB Rep 48: 229–233, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Su Q, Qin DN, Wang FX, Ren J, Li HB, Zhang M, Yang Q, Miao YW, Yu XJ, Qi J, Zhu Z, Zhu GQ, Kang YM. Inhibition of reactive oxygen species in hypothalamic paraventricular nucleus attenuates the renin-angiotensin system and proinflammatory cytokines in hypertension. Toxicol Appl Pharmacol 276: 115–120, 2014. [DOI] [PubMed] [Google Scholar]
- 125.Tang Y, Purkayastha S, Cai D. Hypothalamic microinflammation: a common basis of metabolic syndrome and aging. Trends Neurosci 38: 36–44, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, Zhao X, Sarruf DA, Izgur V, Maravilla KR, Nguyen HT, Fischer JD, Matsen ME, Wisse BE, Morton GJ, Horvath TL, Baskin DG, Tschop MH, Schwartz MW. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest 122: 153–162, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Uchida Y, Tokizawa K, Nagashima K. Characteristics of activated neurons in the suprachiasmatic nucleus when mice become hypothermic during fasting and cold exposure. Neurosci Lett 579: 177–182, 2014. [DOI] [PubMed] [Google Scholar]
- 128.Ueta CB, Fernandes GW, Capelo LP, Fonseca TL, Maculan FD, Gouveia CH, Brum PC, Christoffolete MA, Aoki MS, Lancellotti CL, Kim B, Bianco AC, Ribeiro MO. beta(1) Adrenergic receptor is key to cold- and diet-induced thermogenesis in mice. J Endocrinol 214: 359–365, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Uyama N, Geerts A, Reynaert H. Neural connections between the hypothalamus and the liver. Anat Rec A Discov Mol Cell Evol Biol 280: 808–820, 2004. [DOI] [PubMed] [Google Scholar]
- 130.Valdearcos M, Robblee MM, Benjamin DI, Nomura DK, Xu AW, Koliwad SK. Microglia dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function. Cell Rep 9: 2124–2138, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.van den Hoek AM, van Heijningen C, Schroder-van der Elst JP, Ouwens DM, Havekes LM, Romijn JA, Kalsbeek A, Pijl H. Intracerebroventricular administration of neuropeptide Y induces hepatic insulin resistance via sympathetic innervation. Diabetes 57: 2304–2310, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ventre J, Doebber T, Wu M, MacNaul K, Stevens K, Pasparakis M, Kollias G, Moller DE. Targeted disruption of the tumor necrosis factor-alpha gene: metabolic consequences in obese and nonobese mice. Diabetes 46: 1526–1531, 1997. [DOI] [PubMed] [Google Scholar]
- 133.Wan S, Browning KN, Travagli RA. Glucagon-like peptide-1 modulates synaptic transmission to identified pancreas-projecting vagal motoneurons. Peptides 28: 2184–2191, 2007. [DOI] [PubMed] [Google Scholar]
- 134.Wan S, Coleman FH, Travagli RA. Glucagon-like peptide-1 excites pancreas-projecting preganglionic vagal motoneurons. Am J Physiol Gastrointest Liver Physiol 292: G1474–G1482, 2007. [DOI] [PubMed] [Google Scholar]
- 135.Winklewski PJ, Radkowski M, Wszedybyl-Winklewska M, Demkow U. Brain inflammation and hypertension: the chicken or the egg? J Neuroinflammation 12: 85, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wu C, Cheng W, Xing H, Dang Y, Li F, Zhu Z. Brown adipose tissue can be activated or inhibited within an hour before 18F-FDG injection: a preliminary study with microPET. J Biomed Biotechnol 2011: 159834, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wu KL, Chan SH, Chan JY. Neuroinflammation and oxidative stress in rostral ventrolateral medulla contribute to neurogenic hypertension induced by systemic inflammation. J Neuroinflammation 9: 212, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wynne K, Stanley S, McGowan B, Bloom S. Appetite control. J Endocrinol 184: 291–318, 2005. [DOI] [PubMed] [Google Scholar]
- 139.Xu Y, Faulkner LD, Hill JW. Cross-talk between metabolism and reproduction: the role of POMC and SF1 neurons. Front Endocrinol 2: 98, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yan J, Zhang H, Yin Y, Li J, Tang Y, Purkayastha S, Li L, Cai D. Obesity- and aging-induced excess of central transforming growth factor-beta potentiates diabetic development via an RNA stress response. Nat Med 20: 1001–1008, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yi CX, Foppen E, Abplanalp W, Gao Y, Alkemade A, la Fleur SE, Serlie MJ, Fliers E, Buijs RM, Tschop MH, Kalsbeek A. Glucocorticoid signaling in the arcuate nucleus modulates hepatic insulin sensitivity. Diabetes 61: 339–345, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yi CX, la Fleur SE, Fliers E, Kalsbeek A. The role of the autonomic nervous liver innervation in the control of energy metabolism. Biochim Biophys Acta 1802: 416–431, 2010. [DOI] [PubMed] [Google Scholar]
- 143.Yi CX, Serlie MJ, Ackermans MT, Foppen E, Buijs RM, Sauerwein HP, Fliers E, Kalsbeek A. A major role for perifornical orexin neurons in the control of glucose metabolism in rats. Diabetes 58: 1998–2005, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yi CX, Tschop MH. Brain-gut-adipose-tissue communication pathways at a glance. Dis Model Mech 5: 583–587, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zeng W, Pirzgalska RM, Pereira MM, Kubasova N, Barateiro A, Seixas E, Lu YH, Kozlova A, Voss H, Martins GG, Friedman JM, Domingos AI. Sympathetic neuro-adipose connections mediate leptin-driven lipolysis. Cell 163: 84–94, 2015. [DOI] [PubMed] [Google Scholar]
- 146.Zhang G, Li J, Purkayastha S, Tang Y, Zhang H, Yin Y, Li B, Liu G, Cai D. Hypothalamic programming of systemic ageing involving IKK-beta, NF-kappaB and GnRH. Nature 497: 211–216, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhang M, Mao Y, Ramirez SH, Tuma RF, Chabrashvili T. Angiotensin II induced cerebral microvascular inflammation and increased blood-brain barrier permeability via oxidative stress. Neuroscience 171: 852–858, 2010. [DOI] [PubMed] [Google Scholar]
- 148.Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell 135: 61–73, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zsombok A, Smith BN. Plasticity of central autonomic neural circuits in diabetes. Biochim Biophys Acta 1792: 423–431, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zubcevic J, Jun JY, Lamont G, Murca TM, Shi P, Yuan W, Lin F, Carvajal JM, Li Q, Sumners C, Raizada MK, Shan Z. Nucleus of the solitary tract (pro)renin receptor-mediated antihypertensive effect involves nuclear factor-kappaB-cytokine signaling in the spontaneously hypertensive rat. Hypertension 61: 622–627, 2013. [DOI] [PubMed] [Google Scholar]