Abstract
Engineered nanomaterials (ENMs) are increasingly entering the environment with uncertain consequences including potential ecological effects. Various research communities view differently whether ecotoxicological testing of ENMs should be conducted using environmentally relevant concentrations—where observing outcomes is difficult—versus higher ENM doses, where responses are observable. What exposure conditions are typically used in assessing ENM hazards to populations? What conditions are used to test ecosystem-scale hazards? What is known regarding actual ENMs in the environment, via measurements or modeling simulations? How should exposure conditions, ENM transformation, dose, and body burden be used in interpreting biological and computational findings for assessing risks? These questions were addressed in the context of this critical review. As a result, three main recommendations emerged. First, researchers should improve ecotoxicology of ENMs by choosing test endpoints, duration, and study conditions—including ENM test concentrations—that align with realistic exposure scenarios. Second, testing should proceed via tiers with iterative feedback that informs experiments at other levels of biological organization. Finally, environmental realism in ENM hazard assessments should involve greater coordination among ENM quantitative analysts, exposure modelers, and ecotoxicologists, across government, industry, and academia.
Introduction
As nanotechnology (the synthesis, manipulation, and measurement of matter at 10−9 meter scales)1 rapidly evolves,2 engineered nanomaterials (ENMs) are entering air, waters, soils,3 and sediments where they could adversely affect organisms to ecosystems.4-6 Actual environmental impacts of ENMs have not been documented, and there are uncertainties about the potential for, and how to evaluate, impacts.7
ENM ecotoxicology elucidates hazards and their mechanisms.8 The scope overlaps with conventional ecotoxicology, although ENMs are particulate and diverse,9 with varying cores, native or acquired surface chemistries,10 conditional agglomeration or dissolution,11 and size- plus composition-dependent electronic properties,12 affecting their reactivity and biological interactions.13
Focusing ENM ecotoxicology invokes exposure scenarios14, 15 relevant to ENM production,16-19 use,20-24 disposal,25, 26 and product release (Fig. 1).14, 27-31 Scenarios consider environmental fate and transport,15, 32-39 bioavailability,40, 41 and ENM uptake into ecological receptors.42-46 Scenarios also specify processes47, 48 and ecosystem services49 such as food production.50, 51
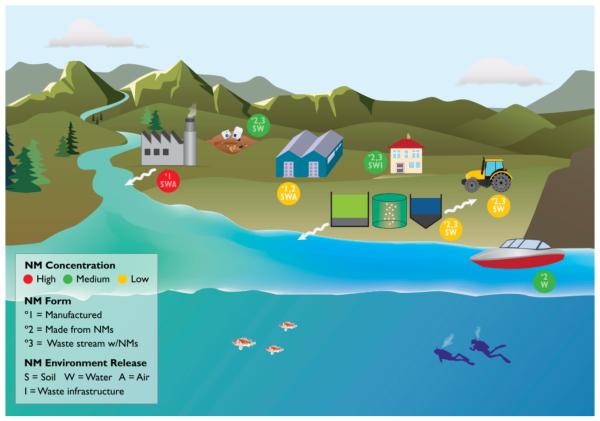
Figure 1.
Conceptual environmental release scenarios for engineered nanomaterials (ENMs) across their life cycles. Potential release sites, clockwise from upper left, include: Primary ENM manufacturing; Landfill with solid waste including nano-enabled electronics, consumer goods, and permitted industrial waste; Secondary processing or goods manufacturing sites using ENMs; Consumer (household) use of ENM-enabled products; Agricultural ENM-enabled product use; Marine or freshwater ENM-enabled product, including coatings, use; Waste treatment with aqueous effluent and solids residuals that may contain ENMs or transformation products thereof. According to the legend, colored circles adjacent to each location indicate the highest expected relative nanomaterial (NM) concentration; the NM forms are as-produced (°1) or in products (°2) or in mixed waste streams (°3); release destinations include waste infrastructure and major environmental compartments (soil, water, air).
In conventional chemical toxicology, observed and perceived exposures often diverge.52 In ENM ecotoxicology, water is emphasized, while soil and sediment impacts have received less attention.53 Where do exposures occur? What ENM forms54 and quantities are involved? Which ecological receptors are affected?55 Which local exposure conditions prevail? As with conventional chemical risk assessment, such questions unite hazard and exposure assessments.15, 56
Standardized test regimens do not derive from scenarios, since ENM test conditions are predefined (e.g., aqueous chemistry, temperature, pH, experiment duration, among others) for standardized endpoints.57 Ideally, ENM hazards are studied at realistic exposures for ecologically relevant receptors. An example would be studying real soils under controllable yet realistic conditions, i.e. in greenhouses58 or lysimeters.59 However, requiring absolute realism in all ENM ecotoxicology would pose scientific challenges associated with: measuring ENMs analytically in environmental media; measuring toxicity across a representative range of environmental conditions; characterizing environmental ENM forms and their transformations so that toxicity is measured for representative materials; ENMs altering physical or chemical exposure media conditions; and few efficient approaches for estimating hazards and exposures necessary to evaluate risks before ENM products develop. Another challenge is internal to the scientific community: multiple dissimilar working definitions of environmental relevance intruding on scholarship, including peer review.53 Environmental relevance remains undefined, leading to categorization of research around a few selected concepts.60
Previously, over 600 published studies were examined to compare modeled or measured environmental concentrations of ENMs versus concentrations administered in ENM ecotoxicity assessments.53 The study found nominal concentration disparities, but also infrequent testing at low ENM concentrations. The study noted uncertainties in ENM exposure modeling, and that other toxicity testing conditions beyond ENM concentration—including aqueous chemistry, biological receptor, system complexity, and ENM form—relate to real-world conditions. However, the study did not establish what constitutes environmental relevance in the ecotoxicology of ENMs.53
Issues regarding environmental detection, uncertain concentrations, and unknown toxicity are not unique to ENMs; they are also raised for other emerging contaminants.61 However, because the ENM industry is rapidly evolving and scientists seek to assist in advancing environmentally safe nanotechnology, environmental relevance in ENM hazard assessment should be prioritized. To accomplish this, representatives from academia, industry, and government regulation working in ecotoxicology, exposure modeling, and social science (Table S1) addressed four questions within this critical review: (i) What exposure conditions are used in assessing ENM ecotoxicity potential for model organisms? (ii) What exposure and design considerations drive mesocosm experiments for assessments of ENM environmental hazards? (iii) What is the state of knowledge regarding ENM environmental exposure conditions, via measurements or modeling simulations? (iv) How should concepts such as exposure conditions, ENM transformation, dose, and body burden be used in interpreting biological and computational findings for assessing risks? The main objective was to provide context and guidance to the meaning of environmental relevance (singular or multiple meanings) in ENM environmental hazard assessment. This critical review addresses the four motivating questions and expands on detailed topics that emerged during the project (Supporting Information). For each question, there are findings and recommendations. These serve to crystallize what is meant by environmental relevance in ENM ecotoxicology, and further coalesce ENM environmental exposure and hazard assessment endeavors.
What exposure conditions are used in assessing ENM ecological hazard potential for model organisms?
Findings
Many systems, approaches, and conditions (including standardized testing with model media, organisms, and endpoints)62 have been used to assess ENM ecotoxicity.63 The applicability and challenges of standardized testing protocols under aquatic conditions have been reviewed57, 64 and vetted in workshops.62 Also, the OECD reviewed and vetted its guidelines for testing ENMs65 in an expert meeting.66, 67 Readers are referred to those reports for deliberations of ENM standardized testing.
Research studies include laboratory-specific low-68, 69 and high-throughput dose-response evaluations using select media with ENM compositional and receptor variants70 for assessing uptake71-73 and effect mechanisms.69, 74, 75 Investigations include mechanistic gene transcriptional,76-79 DNA damage,80 metabolomics profiling,81 and transgenerational82, 83 experiments. Bottle-scale microcosms partially simulate limited levels of environmental complexity,84 e.g. in using natural soils or sediments with58 or without85, 86 plants and associated rhizosphere influences,87 or using seawater88 or marine sediments89, 90 and associated receptors based on expected ENM compartmentalization.91 Non-standard microcosms assess ecological endpoints, e.g. microbial community composition85, 92 and function92 related to C and N cycling.92, 93 Environmental factors are examined, including how ENMs interactively affect soil water availability94 and soil bacterial communities.95 Single-species experiments that assess ENM bio-association96, 97 or bioaccumulation71, 98 precede and motivate using microcosms for assessing dual species trophic transfer98-101 and potential biomagnification.102
Pristine ENMs, including those with surface functionalization, capping agents, or adsorbed species or coatings,103 are the most frequently assessed, although released and transformed versions are increasingly studied.28, 30, 104 Results for textiles, paints, and nanocomposites105 suggest that released particles significantly transform and age, and exhibit different environmental behavior and effects compared to pristine ENMs.106, 107 Assessing changes in form and associated behavior or activity across the material's life cycle are uniquely challenging.
Nominal test exposure concentrations vary widely53 (Fig. S1) and are sometimes related to scenarios such as repeated applications,108 accidental spills,109 or ranges over a spatial gradient.110 High exposure concentrations may be used:111 for assessing bioavailability in soil58 in comparison to simpler media, or to accommodate analytical instrument detection limits. High concentrations are also used to establish no-effect limits, using limit tests; if an effect is not observed, the ENM is assumed to be non-toxic at lower concentrations,62 although effects could occur at longer exposure times. However, this approach is problematic if agglomeration and sedimentation of ENMs are concentration dependent, or if effect mechanisms do not scale with concentration, which might occur if organisms adaptively respond to toxicants.
Some challenges to ENM ecotoxicology are familiar to conventional chemical ecotoxicology while others are unique. With conventional chemicals, toxicity is related to the effective dose of a toxicant molecule crossing the cell membrane and disrupting essential processes. However, ENMs can exert effects as particulates (e.g., physical effects) and in a molecular or ionic form depending on the dissolution extent in the medium. The effective ENM dose—which impacts the assay results112—may be unknown or changing because it varies with media conditions or with dynamic physicochemical interactions of receptors and toxicants.113 One effective dose metric may be insufficient to characterize an observed effect that could be related to both a physical interaction of the ENM particulate and the dissolved ionic form. Also, bioavailability and effective ENM doses114 change because ENMs can transform abiotically115 and biotically116, 117 during assessment. While such influences on stability exist in conventional ecotoxicology, stability in a particulate exposure must also consider the ENM size distribution, and potential physical changes to the ENM such as dissolution and agglomeration. Therefore, effective and nominal doses may or may not be related.
Although multiple ENMs may co-occur in product formulations, different ENMs are infrequently studied together 118 or with co-contaminants.119-121 ENMs can conditionally sorb and modulate the toxicity and uptake of other contaminants and vice versa.122-134 ENMs can acquire coatings135, 136 such as natural organic matter (NOM),137, 138 and age with varying pH and sunlight.139, 140 Assessment outcomes are affected by media chemistry, physical characteristics, and additives (e.g., soil pH,141 temperature,142 or organic matter content,108, 143 and dispersing agents in aqueous media144). The exposure concentration at the receptor and consequent effects145 depend on ENM dissolution146 perhaps assisted by biotic ligands,147 and speciation,103 shed ions,103 surface associations,148 and heteroaggregation with colloids and particulate matter,148-151 or agglomeration and settling.152 All of these vary with ENM types and their varying properties, and ambient conditions.148 Changes in ENM properties may change bioavailability94, 153 and toxicity.154
Various test durations and endpoints have been studied, from acute responses measured by standard test protocols141 to short term microbial biodiversity and community composition effects108 or plant genotoxicity111, 155 and nutrient composition changes.156, 157 Multiple endpoints, toxicant characterization methods, and experimental controls increase assessment comprehensiveness158 and reduce artifacts and misinterpretations.152 Experimental controls based on coatings and dispersants enable determining if the apparent toxicity is attributable to the ENM itself or to ligand or surface groups. Yet whether and how experimental controls are used varies widely, for example metal salts that allow interpreting biological responses relative to ENM dissolution products versus intact ENMs.159, 160
The appropriate analytical method for quantifying, locating and characterizing organism-associated ENMs depends on ENM chemistry and amounts or concentrations. For ENMs exhibiting fluorescence or for ENMs containing heavy elements, high resolution microscopy can assess biological uptake and compartmentalization.99, 102, 161-163 X-ray synchrotron methods can sensitively locate bioaccumulated metal oxide ENMs164 and their transformation products in biota.117, 165 No single analytical tool is suitable for all ENMs; however, the general lack of methodologies that can be routinely implemented to quantify ENM exposure in complex matrices continues to be a major challenge to the fundamental understanding of ecological effects.
Alternative testing strategies (e.g. miniaturized screening assessments using environmentally relevant bacteria)166 can simultaneously assess many material types, controls, and concentrations,70, 75 which is useful for ENMs not available in sufficient quantities for microcosms. However, the potential for interferences in screening assay performance, depending on the specific ENM properties and toxicity test conditions,167 are increasingly recognized, and therefore should be controlled or accounted for in study designs.152, 168
To date, ENM ecological hazard assessments have not adequately explored numerous well-conceived and plausible exposure scenarios that are founded in theory, hypothesis, mechanism and occurrence probability; yet scenarios increase certainty and predictability when addressing nanotechnology-related material safety. For instance, hazard assessments have been mainly skewed towards as-produced ENMs without full consideration of potential aging, since aging cannot be fully standardized in a realistic context. However, such studies can and should further be conducted. Biological uptake, and the compartmentalization and speciation of ENMs, are still infrequently studied, limiting possibilities for attributing ENM exposure to effects at biological receptors.9 Varying degrees of rigor have been applied in designing and incorporating controls that are relevant to experimental questions or hypotheses. Varying degrees of attention are paid to experimental artifacts. While great advancements have been made in ENM ecotoxicology, improvements are needed to increase the environmental relevance of future research.
Recommendations
To understand hazards for plausible exposure initiation scenarios, assessment conditions will need to depart from standardized testing protocols.62, 169, 170 It may be helpful to link or compare data obtained for ENMs under plausible scenarios to those obtained with standard methods using standardized media (e.g., standard soil mixtures) to facilitate interpreting complex multivariate experiments or comparing results among multiple laboratories. Herein, several recommendations regard exposure conditions that should be used in assessing ENM ecotoxicity (Table 1).
Table 1.
Summary recommendations regarding exposure conditions used in assessing ENM hazard potentials for model organisms
| Develop logically conceived, plausible exposure and receptor scenarios in designing experiments, addressing background chemical, physical, and biotic conditions, biological endpoints, and exposure periods. |
| Thoroughly characterize exposure media, including natural soils, waters, and sediments, and provide metadata to allow cross-comparisons and for mining influential factors across studies. |
| Select ENM forms—including ENM mixtures or other co-contaminants—and scale exposure concentrations to logically well-defined exposure scenarios, or to scenario-independent mechanistic and process-based modeling goals, or to best available analytical instrumentation limitations. |
| To the fullest extent possible, characterize ENMs under the exposure conditions and over the time frame of hazard assessments to allow homogeneous exposure or understanding bioavailability and relating endpoint measures to effective forms. |
| Examine and choose multiple endpoints for ENM physicochemical characterization, toxicity quantification, and toxicant characterization, subject to the exposure scenario or similar context. |
| Quantify body burden and determine compartmentalization of ENMs and transformation products in receptors, to allow comparing effective doses to measured biological responses. Carefully consider body burden assay approaches to avoid artifacts. |
| Adopt appropriate experimental control treatments for media additives such as dispersants, and for ENM transformation products that are expected during hazard assessment. |
| Incorporate appropriate rapid screening approaches, as prioritized tiers in an ecological hazard assessment hierarchy (Fig. 1). |
| Adapt these approaches in response to relevant knowledge generation. |
Adequately characterize exposure conditions
Soil ecotoxicity studies should specify the soil taxonomy171 and characteristics such as pH, clay content, and organic matter content, using standard methods.108, 172 Similarly, characterization of sediments should be provided. Natural soils and sediments are preferred, because artificial media do not harbor natural soil communities, and do not reflect chemical and physical characteristics (e.g. of specific surface area related to water holding, or of organic matter content) that influence ENM effects and bioavailability. Water characteristics determine ENM agglomeration, dissolution, and other behaviors affecting aquatic system compartmentalization (e.g., residing in the water column or depositing into sediments)91 and hence need definition. When adequately documented, media characteristics can be used retrospectively to interpret conditional ENM bioavailability or effect mechanisms.
Determine and report the form and concentrations of ENMs
ENMs in testing should represent the particulate material form relevant to the environmental scenario (i.e., as-produced materials near manufacturing sites, or as-released with associated product surface coatings or with relevant co-contaminants in waste streams, including industrial byproducts, Fig. 1). ENMs should be fully characterized and their history (including storage conditions and time, or other uses that may affect compositional or physical characteristics) should be adequately described to allow comparing between studies. For toxic coatings,136 coating identity and degree of coverage should be related to observed effects. Impurities in ENMs introduced during product synthesis and handling should be characterized, since they can sometimes account for apparent ENM ecotoxicity.152 Nominal concentrations should scale according to exposure scenarios, or to specific objectives such as mechanistic research or quantifying biotic uptake. Dose verification, including size distribution and ENM concentration, is also desirable, although heretofore challenging in soil and sediment exposures.
ENM physicochemical changes during release and in the environment should be studied to uncover properties of ENMs that reach biological receptors. All potential forms of ENMs, including transformation products and residual reagents used in synthesis, should be accounted for, such that all toxicants can be related to biological responses.152 Study designs should anticipate dispersing agent effects144 and the nature of transformed ENMs plus co-contaminants, by including controls to account for effects, fate, and kinetics of ENMs in the test medium.154
The ENM physicochemical states should be understood before conducting hazard assessments.173, 174 This is important because some ENMs agglomerate or dissolve or otherwise change in laboratory media, resulting in non-uniform exposures and uncertain bioavailability. Such unevenness precludes relating measured effects to the applied dose. Spatial bio-association, bioaccumulation,175 and intra-organism compartmentalization should be assessed (e.g., through imaging and mapping with sufficiently high resolution117) to locate ENMs and their components.
Relate biological receptors to the exposure scenario
Important advances have been made toward characterizing the physicochemical factors influencing ENM behavior in environmental and test media, and towards utilizing that information to develop standardized methods for conducting ENM ecotoxicity testing.62, 64, 67, 152 However, aquatic or terrestrial species8, 159, 166 —and even different species belonging to the same order176 —respond differently to ENMs using the same tests. Thus, test species and endpoints should be carefully chosen to enhance the relevance of ENM ecotoxicity testing. Some complex interrelationships and dependencies between species comprising ecosystems have been described.8, 159, 177 However, focused research could rationally identify species for routine evaluations; likewise, the scientific rationale behind test species should be reported. Ecosystems are more complex than conditions of routine ENM ecotoxicity evaluations. Thus, research should define an optimal suite of test species and endpoints to determine the ecosystem response to a given ENM.
In general, biological receptors should be chosen for expected exposures stemming from realistic exposure scenarios. For example, relatively insoluble ENMs may, depending on their density, size and agglomeration state, rapidly settle out of suspension and associate with aquatic sediments. In that case, initial hazard assessment could focus on benthic, rather than pelagic, receptor organisms.62 Conversely, for ENMs that rapidly dissolve under environmental exposure conditions, conventional ecotoxicological exposure scenarios may be applied and receptors chosen to assess dissolution product toxicity. However, ENM dissolution rates vary, and pelagic organisms can be more sensitive than benthic organisms.100, 101 Thus, both ENM compartmentalization and form must be accounted for when choosing receptors.
Select endpoints relevant to appropriate receptor and exposure scenarios
Multiple effects measurements152 should be applied to answer research questions. Rapid screening assessments should be prioritized within a testing strategy (Fig. 2). Mechanistically understanding overt toxicity is needed, which may require measuring more omics81 endpoints (i.e., gene transcripts, proteins, and pathway metabolites) and choosing variables for developing mathematical models to predict toxicity at untested concentrations or conditions.8 Omics technologies can also identify potential modes of action (MOAs) that are conserved among different species. However, different scientific communities will have varying preferences in defining needs for omics-level investigations.
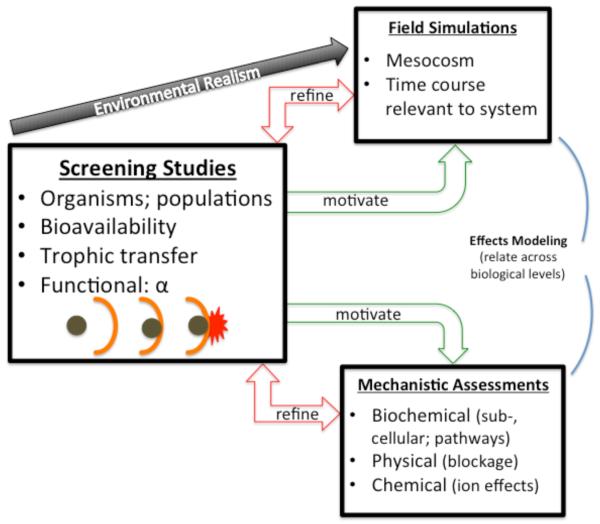
Figure 2.
Interactive “tiers” of ENM ecological hazard assessment. Screening studies (left) are recommended starting points for ENM assessments, and are typically conducted under laboratory conditions, using microcosms, batch reactors, or microplates, depending on the experiment. Examples include assessing organismal to population growth effects, bioavailability (including to communities), ENM physical behavior, and trophic transfer. If screening assay results warrant, mesocosms (upper right box) may be initiated to simulate actual environmental conditions in longer-term experiments, to determine the potential for ENMs to impinge on ecosystems. Screening assay results may also motivate determining mechanisms (lower right box) of observed effects on cells or macromolecules and to characterize biochemical, physical, and chemical interactions of ENMs with biological receptors. Knowledge gained within each tier is used to refine the approaches in the other tiers, thereby improving the relevance of each activity. Results inform development of dynamic process-based mathematical models (curved lines linking across tiers) of biological effects.
Perform adequate dosimetry to understand exposures
Effects interpretation requires understanding the effective toxicant dose or other (perhaps indirect) basis of impacts.9 For ENMs, the mass concentration basis of dosing may relate only partially to the effective applied dose, since biological effects often originate from surface interactions with receptors.178 Furthermore, ENMs are more complex than conventional chemicals because ENM shape, aggregation state and surface area may influence toxicity.67, 178-182 Thus, surface area applied has been suggested as a supplemental dosing metric.178 However, ENM surface area in suspension/solid media is not a straightforward assessment given that ENMs may aggregate with a size distribution that is affected by the medium in which they are dispersed. In addition, coatings, either on pristine ENMs or acquired in the test media or environment, may alter toxicity.104, 183
ENM amounts and forms effecting biological impacts should be understood and related to the administered dose to inform environmental risk assessment.9, 67, 184 This is the essence of dosimetry in ENM ecotoxicology. As with other exposure concerns related to hazard assessment, appropriate dose measurement depends on receptor and ENM characteristics, which are scenario-dependent. For example, mammalian cells are harmed by ENMs that become internalized, yet uptake pathways depend on ENM characteristics.185 Then again, bacterial receptors that affect ecosystem-level processes may be impacted by externally-associated ENMs at the cell membrane, or even in the surrounding environment. In those cases, dosimetry relies on understanding ENM behavior in the complex media in which bacteria reside (e.g., soil, water, sediments, and extracellular polymers186, 187), which is scenario-driven. Endpoint observations of ENM damage will also depend on ENM processing in cells. During hazard assessments, understanding the history of biological interactions with internalized, or otherwise associated ENMs may not be feasible. Yet efforts should be made to measure and spatially associate ENM bioburden (quantity and form) within biological receptors, and to examine the relationships of applied ENMs to apparent effective dose and to effects.
Summary
Overall, it is not recommended to categorically exclude select conditions, environmental compartments, protocols, receptors, or endpoints, since any may be environmentally relevant. Rather, careful experimental designs around well-conceived, plausible exposure scenarios should be emphasized; also, ENM characteristics that influence biological responses under the dynamic conditions that occur in the environment and in biota should be characterized and quantified. One could imagine identifying key material-environment system determinants that could be systematically varied to provide test results across relevant determinant ranges. Such ideas are not specific to ENM ecotoxicology, but could establish defensible practices for making progress in hazard assessment while the ENM industry rapidly advances.
What exposure and design considerations drive mesocosm assessment of ENM environmental hazards?
Findings
Mesocosms are “enclosed experimental systems [that] are intended to serve as miniaturized worlds for studying ecological processes.”84 While the distinctions between mesocosms and other experimental systems are not well delineated, mesocosms are generally larger experimental units and inherently more complex than bench-top microcosms or more simplified laboratory experiments.84, 188-191 Mesocosms for ENM ecotoxicology are intended to increase the complexity of experimental systems, such that more realistic ENM physical compartmentalization, speciation,117 and uptake into biota58, 192 can be achieved alongside biotic effects.89, 177 Also, the intent is to realistically characterize ENM fates and interactions with environmental system components, and to reveal fluxes among compartments of the ecosystems (e.g., the aqueous phase, sediments, and biota in aquatic systems) responsive to internal system influences that are unconstrained by investigator interventions.25, 177, 193
Mesocosms have been used for testing relative biotic effects of ENM variants (e.g., surface functionalization, capping agents, adsorbed surface species or coatings),177 and discerning ENM effects separately from effects of dissolution products (ions or complexes).194 Mesocosm testing may occur following individual organism and microcosm studies (Fig. 2). For example, to study how ENMs impact crops, one could first establish the potential for hydroponic plant population impacts,111 use soil microcosms to understand ENM bioavailability via observing soil microbial community shifts,85, 93 and then scale up to greenhouse mesocosms of soil-grown crops. This sequence could provide an understanding of plant-microbe interactions,58, 87 ENM transformation and uptake in plants,117 and effects on food nutritional quality.157 Still, there are relatively few published studies using mesocosms to assess ENM ecological hazards,53 and the design and operating variables (including size, media, biota, length of study, and ENMs tested) of existing mesocosm studies are wide ranging.60
By contrast, wastewater-associated ENMs,195, 196 and their transformations,197-199 effects, and fates200 in wastewater treatment plants (WWTPs),201, 202 along with the potential for ENMs to impact WWTP processes,48, 203, 204 have been more extensively studied. Since sewage contains ENMs, WWTPs are inherent forms of mesocosms.195, 205, 206 Studies at entire WWTP scales elucidate ENM fates during wastewater treatment, including significant association with biological treatment biomass201, 207 that becomes biosolids.76, 208 However, only 50% of biosolids produced in the U.S. are land-applied, and these biosolids are used on less than 1% of agricultural land in the U.S. (http://www.epa.gov/biosolids). Biosolids are land-applied even less in the European Union.209 Thus, knowledge of ENM fates in WWTPs and how final residues are disposed regionally are needed to develop plausible exposure scenarios.
Concerns with mesocosms include factors that can be difficult to control (e.g., pH or oxygenation) and that mesocosms may respond to artifacts including “wall” or “bottle” effects.84 Further, mesocosms can conflate direct and indirect toxicant effects, typically do not have a full complement of control conditions, and deliver inconclusive results (e.g., where bioaccumulation may be explicable by either direct toxicant uptake or by trophic transfer). Biological communities in mesocosms also lack realistic ecological interconnections, interactions, and energy flows. Nevertheless, outcomes can be improved by using carefully designed mesocosms and associated experiments.84 For example, combined with analyzing mesocosm samples, performing practical “functional assays”38 such as for heteroaggregation,210 allows for anticipating phenomena and later interpreting ENM transformation and compartmentalization in mesocosms.177 Similarly, batch physical association experiments—if conducted using realistic components, and over time frames that allow for quantifiable mass transfer—can assess ENM biomass association and readily suggest ENM fates in WWTPs.207 Still, hydrodynamic conditions are different in simplified tests versus mesocosms, which are different from those in the natural environment. Hydrodynamic conditions will impact ENM fate and transport and thus exposure concentrations at receptor boundaries. The inability to capture real environmental hydrodynamic conditions in any experimental scale is a general shortcoming for both ecotoxicology and transport studies.
Recommendations
Although mesocosms do not fully simulate real environments,84 mesocosms are useful and should be employed, albeit judiciously due to their resource intensity, within a strategy (Fig. 2). Recommendations regarding using mesocosms for assessing ENM environmental hazards are provided in Table 2.
Table 2.
Summary recommendations regarding exposure and design considerations in mesocosm assessment of ENM environmental hazards
| Establish mesocosms only to address clearly defined questions and hypotheses, including those motivated by testing results from lower complexity microcosms, and as related to expected exposure scenarios. |
| Design and operate mesocosms using established authoritative principles, and aim to minimize artifacts. |
| Follow the recommendations for assessing hazard potentials in using individualized or microcosm experiments (Table 1) |
| Make experiment and exposure durations sufficiently long to best represent the exposure scenario underpinning the mesocosm design. |
| Anticipate and address challenges in detecting and quantifying ENMs in complex media and myriad receptors in mesocosms, such that mass balances can be performed, and bioaccumulation and trophic transfer quantified. |
| Attend to issues of ENMs aging, or otherwise significantly transforming, over the duration of mesocosm operation, and control for, or otherwise assess, specific outcomes related to aged materials. |
| Consider assessing effects and ENM interactions with co-contaminants, since they are realistic constituents of most compartments into which ENMs are released. |
| Anticipate, recognize, and plan to assess secondary effects on non-target receptors that contribute to ecological processes in complex systems represented by mesocosms. |
| Increasingly use sensitive, e.g., omics type, endpoints as they are responsive to questions and hypotheses motivating mesocosm studies. |
Mesocosm studies must be designed and conducted around well-conceived questions related to plausible exposure scenarios; they should use select endpoints, potentially including sensitive omics measurements,211 to answer questions or test hypotheses.84 Internal process and constituent characterization should be thorough and equally responsive to well-conceived, realistic scenarios. Functional assays, i.e. “intermediary, semi-empirical measures of processes or functions within a specified system that bridge the gap between nanomaterial properties and potential outcomes in complex systems”,38 should precede mesocosm designs and experiments, and aid interpreting mesocosm results (Fig. 2).
Mesocosm artifacts are avoidable by following best practices for design and operation, although possible interferences of particulate material testing with assays must be evaluated.84 As for other hazard assessments, ENMs should be tested across the product life cycle, (i.e., ranging from as-produced to released from products) within a motivating exposure scenario. Similarly, suitable material controls should be used to test hypotheses regarding ENM-specific effects (Table 1).
The recommendations made regarding exposure conditions in assessing ENM hazard potentials for model organisms (including in microcosms) should be followed for mesocosm studies (Table 1). Additionally, mesocosm designs should incorporate exposure durations, which should be sufficiently long to address population growth, reproduction, bioaccumulation, trophic transfer, and possibly transgenerational effects. Sufficient measurements of ENM concentrations and time-dependent properties must be made for clear interpretations.
Key to successfully interpreting mesocosm studies is using validated methods for measuring ENMs in complex media. Measurements should include the size distribution, concentration and chemical composition of ENMs in the test system, including biological tissues,161, 212-214 over time.215 In some cases, transformation products are inventoried thoroughly during long term field-relevant exposures.193 Detection schemes require sample preparation to assess in situ exposures (e.g., aqueous or solvent extractions) before quantitative analyses, or drying and embedding before visual confirmation by electron microscopy. The potential for artifact introduction should be recognized. Recovery methods continually develop, such as cloud point extraction for concentrating ENMs from aqueous matrices.216, 217
Depending on the exposure scenario, in situ aging may be a study objective. However, it is important to define what ‘aging’ really means and the specific application domain, since ‘aging’ is a wide-ranging term and can be used in different contexts, making comparisons impossible. At least, studies should be undertaken over sufficiently long time frames (e.g., acknowledging time scales of soil processes141, 218), which may include repeated ENM applications,108 such that appropriate aging, i.e. time-dependent transformation under realistic conditions, could occur. Alternatively, pre-aged ENMs could be used. However pre-aging protocols (whether in the context of product use conditions or under environmental conditions) are not yet standardized and, while some convention could allow for comparing across studies, the appropriate aging protocol would depend on the envisioned exposure scenario.
Co-contaminants should be considered and potentially introduced into mesocosms, since some ENMs sorb, concentrate, and increase exposure to other contaminants.130 Select endpoints should account for ENMs as chemosensitizers.119, 120, 219, 220 Also, mesocosm study designs should anticipate and plan for measuring secondary effects (e.g., rhizosphere microbial shifts that affect plants87 and soil nutrient turnover).
In summary, while few mesocosms have been used in assessing ENM ecotoxicity and are also rare for conventional chemical testing, such systems potentially offer greater realism. Still, mesocosm exposure and design considerations should derive from immediate environmental applicability. The value of mesocosms to ENM ecotoxicology can increase by following recommendations including: addressing context-dependent questions while using relevant endpoints; considering and minimizing artifacts; using realistic exposure durations; quantifying ENMs and their products; and considering ENM aging, co-contaminants, and secondary biological effects (Table 2). Further, it should be acknowledged that mesocosms do not fully recreate natural environmental complexity. For example, aquatic mesocosms do not recreate actual environmental hydrodynamic, or temperature cycling, conditions. Hydrodynamics can significantly impact ENM aggregation or heteroaggregation, and fate and transport (e.g., sedimentation, dissolution, etc.). Therefore, potential impacts on the resulting concentrations at the receptor boundaries should be considered.
What is the state of knowledge regarding ENM environmental exposure conditions, via measurements or modeling simulations?
Findings
ENM environmental exposure conditions herein refer to where, how much, and in what forms ENMs may occur in the environment. These are central issues for ecotoxicology of ENMs because they suggest test exposure scenarios14, 15, 18 in which ENMs could impact biological receptors within environmental compartments (e.g., soil, sediments, air, and water) influenced by various factors (e.g., pH, redox potential, aeration, temperature, and ionic strength). These issues also influence outcomes of key regulatory concern: persistence, bioaccumulation, and toxicity.
In the parlance of exposure science,221, 222 three knowledge domains inform ecotoxicology, including the ecotoxicology of ENMs6:
-
1)
“far-field”223 “environmental science”222 regarding contaminant release, transport, fate, and transformation external to biological receptors;
-
2)
“near-field”223 exposures at the receptor that lead to biological outcomes, as affected by contaminant bioavailability, affinity, uptake, accumulation, and biological transformation; and
-
3)
“at point” responses and outcomes that occur at the biological receptor and include toxicity (Fig. 3).
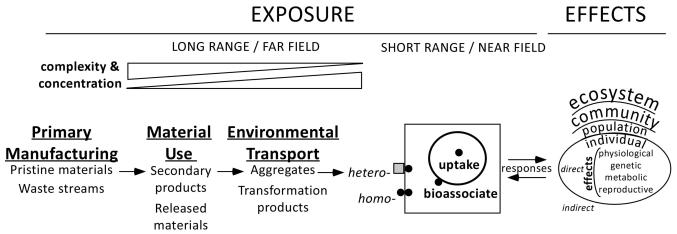
Figure 3.
Conceptual exposure and effects assessments in ENM environmental risk assessment. Exposure derives from far-field emissions, transport, and transformation processes leading to environmental accumulation (bottom “wedge” of ENM complexity and concentration) of either more complex mixtures, or of specific ENMs or transformation products. Top “wedges” depict that either ENM complexity or ENM concentration can increase or decrease along the path of far-field ENM transport. At biological receptors, adverse effects are predicated on near-field exposures. Homo- and heteroaggregation (of multiple particles, here depicted as two) are particle-specific phenomena that may prevent near-field exposure. Biotic responses can influence bioavailability, and thus near-field exposures. Direct effects to biota may manifest across all levels of biological organization (subcellular, to individual, population, community, and ecosystem); effects can also be indirect, e.g., from physical effects of ENMs on nutrient availability.
Discharges (locations, types, and amounts) underpin exposure scenarios,14, 15, 37, 224 are initiated by situational contaminant releases (Fig. 1), and are referred to as source terms. Mass balance-based multimedia simulations225 mathematically account for released contaminants as they are transported and exchanged between environmental media, where contaminants may be transformed and may ultimately concentrate, potentially with altered compositions and structures (Fig. 3).
Far-field exposure modeling approaches vary by question, the modeling purpose (e.g., regulatory compliance, priority setting, research, material design, or industrial use), the required spatial resolution (e.g., a site or region, and the need to resolve environmental heterogeneity), the temporal conditions (e.g., steady state, dynamic, or episodic), and the predictive accuracy required.56, 226 Material Flow Analysis (MFA), which is a type of life-cycle inventory analysis, has been advanced to track ENM flows through various use patterns into volumes released into broad environmental compartments,227 scaled to regional ENM concentrations that release via WWTPs to water, air, landfills, and soil.227 Such models estimate exposure concentrations in part via engineering assumptions and in part via heuristics (i.e. expert judgment regarding the potential amount released to various media).228 Also, such material flow analysis models depend on the underlying data (e.g. production and release estimates) which are not readily available, making it difficult to validate model results and potentially leading to inaccurate estimates.32, 228
Multimedia models for ENMs32, 37, 228-230 can predict environmental concentrations based on sources of continuous, time-dependent, or episodic releases32, 228 and are similar to multimedia models that predict environmental concentrations of organic chemicals231-233 and particle-associated organic chemicals.233, 234 For ENMs, predicting particle size distribution—as affected by particle dissolution, agglomeration, and settling—is desired for various spatial and temporal endpoints. For one integrated MFA and multimedia model (MendNano and LearNano), user-defined inputs are flexible around product use and ENM release throughout material life cycles.228 It is noted that although validation of multimedia models is a formidable task, various components of such models have been validated as well as model predictions with such models for particle-bound pollutants.
Most far-field223 models of ENMs have major challenges. First, the quantities and types of ENMs being manufactured are unknown to the general public due to issues surrounding confidential business information, leading to a reliance on market research.53 The resulting public uncertainty will persist while nanotechnology continues a course of rapid innovation, as is typical of new industries.235 The rates of product use and ENM releases at all life-cycle stages (i.e., manufacturing, waste handling, product use, and product disposal) are also not defined.225 There are challenges associated with modeling transport processes through specific media and across media (i.e., water, soil, air), highly divergent time scales of processes, lack of required input parameters, and the need for validation of results (e.g., through measurements of ENMs in the environment).226 Several multimedia models developed for conventional chemicals could be adapted around ENMs, but few account for fate processes specific to nanoparticles (e.g., never in thermodynamic equilibrium).37, 236 In addition, various transport models for a single medium and in the multimedia environment could be adapted for far-field analysis of ENMs, but few account for fate processes distinctive to ENMs (e.g., considering the complexity of ENMs aggregating and that their transport is governed by physical rather than thermodynamic driving forces).37, 236 Moreover, their validation, which would require ENM monitoring data, is a major challenge.
The lack of understanding of many fundamental ENM behaviors under environmental conditions propagates into broad uncertainties, for example in predicting ENM removal to solids or aqueous fractions in WWTPs.225, 227 ENM surface chemistries fundamentally affect ENM agglomeration or dispersion237 and likely affect bioavailabilty.94 Some species on ENM surfaces may degrade in the environment,238 while other adsorbates can be acquired.239, 240 Carbonaceous ENMs may be transformed or degraded by environmental processes such as photo-,241, 242 enzymatic,243, 244 chemical,245 and biodegradation.246 Redox and other environmental conditions will affect nanomaterial surfaces, which for nano-Ag includes formation of sulfide that inhibits dissolution.39, 247 Surface chemistry also affects transformation rates of primary particles and aggregates (e.g., in WWTP biological processes).248 For many ENMs such as nano-ceria,249 reactivity is highly size-dependent. To accurately model material fates thus requires understanding how material surface properties affect integrity, how both change under varying environmental conditions such as pH, clay content,250 and organic matter content,143, 251 and how surface properties and particle reactivity affect physicochemical processes that are parameterized in far-field models. This is especially true for ENMs used as pesticide delivery mechanisms, including carbon nanotube composites with specifically-reactive surface monomers. Yet only recently has modeling attempted to address differing properties of a material's structural variants (e.g., photocatalytic versus photostable nano-TiO2).230
Evaluating computational model predictions is a challenge for ENMs, which presently are estimated to occur in the environment at low (ng/L, to mg/L) concentrations.32, 230, 252-254 Also, detection methods for ENMs in environmental media and distinguishing ENMs from natural chemical analogs are still under development,161, 255-257 with more evaluation strategies needed including a framework for validating new ENM analytical detection methods.215 Fullerenes from incidental sources (combustion products) were quantified in river sediments collected from locations across the globe258 and quantified in the atmosphere over the Mediterranean Sea.259 Perhaps related to a viable exposure scenario, fullerenes were quantified at relatively high concentrations in treated wastewater effluent260 and at ng/L to μg/L261 concentrations in river waters receiving effluent discharge. While not necessarily nanoscale, similarly high concentrations of TiO2 were reported for sediments sampled near a WWTP outfall.262
The greatest uncertainty in ENM exposures is near-field (Fig. 3), at the receptor where toxicant dose manifests as internal dose. Heteroaggregation is a dominant fate process for ENMs when they interact with natural colloids.37, 263-265 Given sufficient residence time for ENMs in environmental matrices, heteroaggregation (i.e., kinetically-controlled association with colloids) and to a lesser degree homoaggregation will affect localized compartmentalization, including stability in the water column and therefore, sedimentation.177 However, these processes do not preclude biological impacts under simulated environmental conditions, as has been shown for nano-ceria in a complex aquatic mesocosm.177 Exposure can be confirmed by quantifying receptor body burdens, thereby allowing for quantitatively relating near-field exposure to biological effects.177 Thus, in the absence of detailed, biologically complex, near-field models for local exposures to environmental receptors, the ability to trace ENMs to biological receptors sampled directly from the environment becomes the best available approach to relate far-field exposures to biological impacts.177
Overall, material flow models and multimedia modeling of ENMs have advanced to inform ENM ecotoxicology. Available far-field modeling frameworks are adaptable to changing inputs despite uncertainties in production volumes. Major uncertainties remain at the nexus of ENM surface and core chemistries as related to nanomaterial transport, aggregation, and degradation characteristics. However, fundamental research (e.g., of conditional hetero- and homoaggregation, and of dissolution) is needed to discover and parameterize complex fate processes. New approaches, such as assays that can be used to rapidly probe surface associations,38 demonstrate how to populate far-field models and how to determine near-field exposures associated with effects. Although existing models can simulate particle movement, deposition, and some transformations, the knowledge state regarding ENM environmental exposure conditions via measurements or modeling simulations cannot be assumed to accurately represent actual conditions at biological receptors.
Recommendations
Despite significant progress in modeling potential ENM environmental distributions, several key constraints exist. Models predict the average concentration within broad compartments such as soils, sediments, water, or wastewater, even when constrained to regional releases. Actual concentrations at biological exposure sites are typically unknown, but can be constrained within upper and lower bounds. Due to the potential for bioassociation, bioaccumulation, and hetero- or homoaggregation, predicted environmental ENM concentrations may inaccurately estimate bioactive concentrations at receptors. Following are recommendations for using measurements or modeling simulations to understand conditions of ENM environmental exposures.
The limitations of modeling approaches should be recognized more broadly, such that generic model predictions are not used to rigidly define or judge ecotoxicity testing conditions.
Modeling should be simplified where possible and coupled with evaluation and iteration while varying assumptions to develop an understanding of the sensitivity of modeling parameters.
Meteorological conditions play an important role in ENM transport, and thus should be carefully documented when assessing model predictions relative to monitoring.
ENM surface characteristics relevant to the receptor and the exposure context should be considered by modelers and experimentalists. Considerations should include: which ENM characteristics prevail near receptors, how this changes in the environment, and how particle stability, migration and bioavailability are affected.
Batch experimental approaches to rapidly assess relationships between environmental factors (e.g., pH, redox potential or aeration, ionic strength, and organic matter content) and ENM physicochemical characteristics that affect fate processes (e.g., homo- and heteroaggregation, sedimentation, and dissolution) could be more routinely incorporated into assessments (Fig. 2), to justify further testing and for interpreting other testing results.
Improvements in quantifying ENMs in complex media, including biological tissue, are needed to validate multimedia models and to determine near-field exposures to biological receptors.
Improved fundamental understanding of how ENM variants (e.g., in transformable coatings, shapes, sizes, or primary chemistry) comparatively migrate under varying environmental conditions can be advanced through research that intentionally develops and uses relatively stable ENMs as tracers.188, 189, 266
In summary, understanding exposure conditions should continue to improve from fundamental experimental research of ENM environmental behaviors. Modeling should continue to improve with such understanding, and avenues for model evaluation should continue to grow. Environmental relevance in ENM ecotoxicology need not be defined or constrained by model outputs, especially given recognized uncertainties and the need for continued model evolution. Lastly, echoing prior recommendations,15 the efforts of exposure modelers, analytical methods developers, and ecotoxicologists should continue to influence each other for maximum individual and mutual benefit.
How should concepts such as exposure conditions, ENM transformation, dose, and body burden be utilized in interpreting biological and computational findings for assessing risks?
Findings
Many of the outstanding research issues and recommendations for evolving ENM ecotoxicology (Tables 1 and 2) are echoed in the discourse for other chemicals of emerging concern (CECs).267 These include the need for systematically understanding ENM and decomposition product toxicity across various receptors within linked levels of biological organization,8, 268, 269 quantifying actual exposures270 and uptake271 into environmental receptors,57, 161 gaining mechanistic insights into and biological markers for acute and chronic low level exposures,272, 273 and understanding how environmental factors including co-contaminants affect ENM transformation274 and biological impacts. Still, how can the potential for exposure and impacts of ENMs be anticipated, prevented, managed, or mitigated? Further, what data and tools do decision makers need to inform their work? Innovation in nanotechnology hinges on having the science to evaluate ENM safety.
While no formalized process for incorporating all exposure conditions and concepts of ENM transformation, dose, and body burden into risk assessments currently exists, a proposed framework approach to risk characterization over the life cycle of ENMs has been published and is available.63 This framework advocates an initial decision cut-off in regards to exposure; in the absence of exposure, the need for further assessment is diminished or negated.26, 63 In this available framework, ENMs that are certain to rapidly dissolve into ionic components in a destined environmental compartment would be assessed for risk based on the released components rather than the original nanoparticles.63 Persistent ENMs are expected to accumulate in matrices such as sediments.63 The consequences of ENMs to successive generations, biodiversity, and ecosystem services49 are not addressed by model organism-specific assays of discrete growth and mortality.8, 63 Nonetheless, in this available framework, toxicity endpoints associated with standardized testing protocols for sediment, aquatic, and terrestrial standard population-level endpoints over short and long time frames are advocated for assessing hazards of simulated ENM concentrations in the environment.63 In this framework, sunlight is an environmental variable, bioaccumulation is measured, and ENM modifications during product and material life cycles that may change bioavailability are considered.63 While such a framework has broad organizational appeal, priority setting within the framework is required and thus could focus on tests that are relatively well aligned with likely exposure scenarios.
Even with a risk assessment framework that considers ENMs across product life cycles and considers sediments, water, and soil in testing endpoints,63 major hurdles hinder regulatory agencies, and research scientists, in using concepts such as exposure conditions, ENM transformation, dose, and body burden in interpreting biological and computational findings for assessing risks. Toxicity tests developed for dissolved chemicals typically require significant modification for use with ENMs.57 Tests may not apply to ENMs if they are not appropriate for solids.275 Additional scientifically-based hazard information from the peer-reviewed literature may or may not be available for consideration. ENMs used in ecotoxicity tests, which are sometimes laboratory-synthesized to overcome uncertainty regarding proprietary coating or other commercial formulations, may be insufficiently analogous to allow for extrapolating information or risk comparisons.
Issues include the need to know test material characteristics and how they relate to testing results and the ENM life cycle. Even if an initial risk assessment considers ENM solubility,63 ENM dissolution is not instantaneous; therefore, at what stage of dissolution does the contaminant no longer pose a hazard as an ENM? Also, where biological impacts stem from ENM surface characteristics, how can mass concentration be used to judge hazards? Environmental ENM effects in benchtop experiments can be indirect, stemming from physical nutrient depletion,152 or amplifying organism uptake of co-contaminants.276 Other indirect physical effects derive from ENMs adhering to the organism surface,277 light shading,278 or internal food displacement.99 Near-field exposures (Fig. 3) can result in biological hazards from specific ENMs based on their properties (e.g., electronic).75, 279
By definition, ecological risk assessment (ERA) is “the process for evaluating how likely the environment will be impacted as a result of exposure to one or more environmental stressors.”280 ERA involves predicting effects for individuals, populations, communities and ecosystems, and concerns itself with valuable49 ecosystem services such as nutrient cycling.280 Thus, conducting ERAs for ENMs could benefit from an ecological outlook. All levels of biological organization, and interactions between them, would be considered when assessing responses to ENM exposure (Fig. 3).
Release and exposure scenarios (Fig. 1), use of functional assays for assessing environmental compartmentalization (Fig. 2),38 and combined life-cycle and multi-media modeling228 have important roles in focusing ENM ecotoxicology. Less recognized is that mechanistically based models of dynamic biological effects are informed by hazard assessment research. Different types of process-based, dynamic models (e.g., Adverse Outcome Pathway or AOP,281, 282 and Dynamic Energy Budget or DEB283) allow for predicting effects from exposures stepwise, starting at subcellular levels, into individuals, through populations, and conceivably to communities and ecosystems. Developing process-based models requires researching key effects processes71, 284, 285 and ecological feedbacks.153 Models are formalized to describe interactive processes culminating in toxicity such as reactive oxygen species (ROS) generation and cellular damage. Process-based mathematical expressions evolve with empirically-based discoveries or through model reconciliation with experimental data. Parameters are independent of toxicity testing protocols, although models could be informed by standard test results. Thus, ENM ecotoxicity research could support predictive toxicology by informing and populating process-based, dynamic ecological effects models.
A comprehensive fate and effects research agenda is needed for addressing ENM quantification in complex media.286, 287 Such an agenda has allowed for assessing experimental compartmentalization,288 and sensitively assessing environmental persistence,289 toxicity, bioaccumulation,287, 290 trophic transfer,291 and indirect effects from the uptake of ENMs coated in other hazardous materials.292, 293 Such research could substantially inform ENM risk assessment for a relevant environmental exposure scenario. However, most ENMs have not been studied comprehensively along the entire exposure and effects continuum (Fig. 3). Further, the approach is not sustainable. Rather, the need is to develop efficient approaches applicable within an overall approach to rapidly evaluate the large number of ENMs under commercialization (Fig. 2). A research agenda that focuses on distilling key determinants of exposure and hazard for ENM-environment systems that can be measured experimentally would be most compelling.
Thus, while the science of ENM ecotoxicology and exposure characterization has advanced, there are disconnects between how regulators review ENM-based products for environmental safety and the research that is conducted to evaluate hazards. Except for results published in open source outlets or directly reported, research may be unknown to government bodies. Ongoing synthesis of published research results is challenging due to high variability across study conditions and ENMs tested, and due to effort needed to regularly update such comparisons. Moreover, there is a systematic resistance to publishing “no effect” studies in the peer-reviewed literature.294 As a result, relying only on published research to inform regulatory decisions can present challenges. A life cycle-based framework facilitates exposure modeling and hazard testing to support risk assessment. However, extrapolation of effects to untested concentrations, study, or environmental conditions, and across biological levels of organization, requires understanding dynamic biological process-based effects, which current standard tests neither deliver nor sufficiently inform. Ultimately, exposure scenarios are useful for framing and focusing ENM ecotoxicology, and some version of a tiered intelligent testing and risk assessment (Fig. 2) strategy is needed.
Such a conceptual tiered strategy considering the impact of the ENMs’ varying properties on ecological risks at different life cycle stages was proposed in the EU FP7 MARINA (Managing Risks of Nanoparticles) project295 and is being further developed in the EU NANoREG programme. This strategy considers several domains (exposure, fate, kinetics, and hazard) represented by specific tools ranging from relatively simple in the lower tiers to more complex and specific in the higher tiers. The framework aim is to structure information collection and generation for cost-efficient risk assessment, compliant with 3R animal-use testing principles (i.e., replacement, reduction, and refinement), which should also be pursued by means of grouping ENMs.
A strategy for grouping ENMs based on releases, uses, physicochemical properties, bioaccumulation, bioavailability, and effects for both human and ecological risk assessment is currently in development across a number of EU research projects such as MARINA, NANoREG, SUN, and GUIDEnano. These efforts have been challenged by the complexity of ENM identity and interactions, but this approach is necessary, as the costs for safety assessment on a case-by-case basis would be exorbitant.295 Therefore, a vision on ENM grouping is needed, which should apply in a regulatory context.296, 297 Applying grouping in regulatory risk assessments should enable read-across, i.e., filling a data gap by using information on one ENM, or a non-ENM, for another substance in the same group.
Recommendations
The abovementioned tools should be fitted into a risk assessment strategy for ENMs. This strategy should be flexible enough to address different assessment goals depending on the user's needs, considering all data already available as a starting point, contingent upon data quality evaluation298 and selecting the most appropriate tools to fill existing data gaps. Such a strategy should ideally be exposure-driven, starting with identifying the most relevant exposure scenarios in the ENM life cycle, and evaluating completeness and quality of the available data from a risk assessment perspective. This facilitates careful prioritization of ENMs to optimize testing efforts and can inform more realistic ecotoxicological investigations. Doing so can allow one to screen-out irrelevant exposure routes, eliminate unnecessary testing, and support prioritization of exposure scenarios. Exposure assessment should begin with an analysis of plausible exposure scenarios; where none is expected, further testing may be precluded for the applicable use patterns and volumes.26, 63 Researchers and regulators need to understand actual exposures at biological receptors. This exposure-driven approach can also provide important information on realistic environmental conditions to affect test designs for improved interpretation of laboratory toxicology studies. Such practices can ensue in the interim, while research continues to discover best hazard assessment practices.
Experimental ENM toxicity assessments, using ecologically relevant receptors and across linked biological levels of organization, should inform developing and parameterizing dynamic process-based models. Such models should respond to future scenarios and predict impacts. ENM characteristics, exposure conditions, and ENM transformation, dose, and body burden should be used in interpreting biological and computational findings for assessing ENM risks. ENM test results should be benchmarked to results for appropriate controls to establish relative hazard (e.g., comparing dissolving ENMs to their dissolution products or comparing persistent ENMs to non-nano analogs). This applies to pinnacle concerns in ecological fate assessment of bioaccumulation, biomagnification, and biopersistence.299
How to develop, interpret, and use pertinent information in ENM environmental risk assessment is a larger issue that should become part of an extended dialog among regulators, industry, civil society organizations, researchers, and other societal members so that the fundamental research will inform decision making. Collaborative decisions are recommended for focusing ENM ecotoxicology towards relevant scenarios, including testing the most relevant materials throughout ENM life cycles and employing appropriate hazard assessment approaches, towards meaningful ecological risk assessment.
Summary and Outcomes: Towards Defining “Environmental Relevance”
The overarching question motivating this critical review was: how can we ensure that hazard assessment in ENM ecotoxicology is as environmentally relevant as possible? The answer requires considering how ecotoxicity tests are performed, what constitutes pertinent concentration and test conditions for ENMs (Table 1), the main biotic and abiotic attributes of the environment, how ecologically oriented hazard assessment is undertaken (Table 2), and how the resulting information should be interpreted. Answering this question yielded three primary insights. First, environmental relevance is informed by a logical consideration of what exposures might occur, to which receptors, and to what outcomes. The consideration should begin with a plausible release and exposure scenario (Fig. 1), and use best available knowledge and technologies to develop the full assessment approach. Concerns regarding ENM concentrations used in hazard assessments are paramount, but are not the only concerns. ENM concentrations should be selected to assess potential effects, but overly high concentrations that fundamentally change media conditions should be avoided. Still, concentrations ranging above and below predicted ENM average concentrations must be assessed for understanding potential organismal effects, underlying mechanisms and their concentration dependencies, and for informing process-based dynamic biological effects models. In addition to the nanomaterial, the conventional material (e.g., dissolved metal) should be tested. ENM distributions and fates in broad environmental compartments do not equate to concentrations and forms near, or effective at, actual biological receptors.114 Therefore, research results on ENM effects should not be disregarded on the limited basis of environmentally relevant exposure concentrations when the study conditions were predicated on a broader hypothesis.
In addition to tethering ENM ecotoxicology to exposure initiation scenarios (Fig. 1), the concept of employing tiered approaches in hazard and risk assessment resonated (Fig. 2). Multi-stage approaches to ENM hazard assessment are advocated.114 A highly developed tiered approach for health and safety testing of nanotechnologies has been published26 and strategies for tiered risk assessment and grouping are underway.295, 297, 298 Staging ENM ecotoxicology efforts, such that potential interactive impacts at all levels of biological organization (Fig. 2 and 3) are evaluated, could simultaneously inform risk assessment and predictive process-based effects model development. As some ENMs can cause biological impacts from ENM properties or characteristics,300 ENM ecotoxicology should be oriented to logical exposure initiation scenarios (Fig. 1) based on ENM life cycles, via testing tiers (Fig. 2). Finally, coordination is recommended among multiple disciplines in ENM environmental analysis, fate and transport modeling, and hazard assessment, towards rapidly advancing research using tiered approaches around realistic exposure scenarios.
Supplementary Material
Acknowledgements
Funding was from the National Science Foundation and the U.S. Environmental Protection Agency under Cooperative Agreement DBI-1266377. Any opinions, findings, and conclusions are those of the authors and do not necessarily reflect the views of the National Science Foundation, the U.S. Environmental Protection Agency, or the National Institute of Standards and Technology. Although this document has been reviewed in accordance with U.S. Environmental Protection Agency policy and approved for publication, the views are those of the authors and may not necessarily reflect official Agency policy. Mention of trade names or commercial products should not be interpreted as endorsement or recommendation for use. The authors acknowledge the helpful comments of three anonymous reviewers.
Footnotes
Supporting Information Available
Supporting Information includes a description of the process and methods used to develop this critical review, one supporting table, and one supporting figure.
References
- 1.Renn O, Roco MC. Nanotechnology and the need for risk governance. Journal of Nanoparticle Research. 2006;8(2):153–191. [Google Scholar]
- 2.Roco MC. The long view of nanotechnology development: the National Nanotechnology Initiative at 10 years. J. Nanopart. Res. 2011;13(2):427–445. [Google Scholar]
- 3.Schaumann GE, Baumann T, Lang F, Metreveli G, Vogel H-J. Engineered nanoparticles in soils and waters. Science of The Total Environment. 2015;535:1–2. doi: 10.1016/j.scitotenv.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 4.U. S. Environmental Protection Agency . In: Nanotechnology White Paper. Council, S. P., editor. U.S. Environmental Protection Agency; 2007. p. 120. [Google Scholar]
- 5.The Royal Society Nanoscience and nanotechnologies: opportunities and uncertainties. The Royal Society and The Royal Academy of Engineering; London: 2004. p. 116. [Google Scholar]
- 6.Klaine SJ, Alvarez PJJ, Batley GE, Fernandes TF, Handy RD, Lyon DY, Mahendra S, McLaughlin MJ, Lead JR. Nanomaterials in the environment: behavior, fate, bioavailability, and effects. Environmental Toxicology and Chemistry. 2008;27(9):1825–1851. doi: 10.1897/08-090.1. [DOI] [PubMed] [Google Scholar]
- 7.Stark WJ, Stoessel PR, Wohlleben W, Hafner A. Industrial applications of nanoparticles. Chemical Society Reviews. 2015;44(16):5793–5805. doi: 10.1039/c4cs00362d. [DOI] [PubMed] [Google Scholar]
- 8.Holden PA, Nisbet RM, Lenihan HS, Miller RJ, Cherr GN, Schimel JP, Gardea-Torresdey JL. Ecological nanotoxicology: integrating nanomaterial hazard considerations across the subcellular, population, community, and ecosystems levels. Accounts of Chemical Research. 2013;46(3):813–822. doi: 10.1021/ar300069t. [DOI] [PubMed] [Google Scholar]
- 9.Hinderliter P, Minard K, Orr G, Chrisler W, Thrall B, Pounds J, Teeguarden J. ISDD: A computational model of particle sedimentation, diffusion and target cell dosimetry for in vitro toxicity studies. Particle and Fibre Toxicology. 2010;7(1):36. doi: 10.1186/1743-8977-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mudunkotuwa IA, Pettibone JM, Grassian VH. Environmental implications of nanoparticle aging in the processing and fate of copper-based nanomaterials. Environmental Science & Technology. 2012;46(13):7001–7010. doi: 10.1021/es203851d. [DOI] [PubMed] [Google Scholar]
- 11.Borm P, Klaessig FC, Landry TD, Moudgil B, Pauluhn J, Thomas K, Trottier R, Wood S. Research strategies for safety evaluation of nanomaterials, Part V: Role of dissolution in biological fate and effects of nanoscale particles. Toxicological Sciences. 2006;90(1):23–32. doi: 10.1093/toxsci/kfj084. [DOI] [PubMed] [Google Scholar]
- 12.Burello E, Worth AP. A theoretical framework for predicting the oxidative stress potential of oxide nanoparticles. Nanotoxicology. 2010:1–8. doi: 10.3109/17435390.2010.502980. 0, (0) [DOI] [PubMed] [Google Scholar]
- 13.Nel AE, Madler L, Velegol D, Xia T, Hoek EMV, Somasundaran P, Klaessig F, Castranova V, Thompson M. Understanding biophysicochemical interactions at the nano-bio interface. Nature Materials. 2009;8(7):543–557. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- 14.Nowack B, Ranville JF, Diamond S, Gallego-Urrea JA, Metcalfe C, Rose J, Horne N, Koelmans AA, Klaine SJ. Potential scenarios for nanomaterial release and subsequent alteration in the environment. Environmental Toxicology and Chemistry. 2012;31(1):50–59. doi: 10.1002/etc.726. [DOI] [PubMed] [Google Scholar]
- 15.Praetorius A, Arvidsson R, Molander S, Scheringer M. Facing complexity through informed simplifications: a research agenda for aquatic exposure assessment of nanoparticles. Environmental Science: Processes & Impacts. 2013;15(1):161–168. doi: 10.1039/c2em30677h. [DOI] [PubMed] [Google Scholar]
- 16.Eckelman MJ, Mauter MS, Isaacs JA, Elimelech M. New perspectives on nanomaterial aquatic ecotoxicity: production impacts exceed direct exposure impacts for carbon nanotoubes. Environmental Science & Technology. 2012;46(5):2902–2910. doi: 10.1021/es203409a. [DOI] [PubMed] [Google Scholar]
- 17.Walser T, Demou E, Lang DJ, Hellweg S. Prospective environmental life cycle assessment of nanosilver t-shirts. Environmental Science & Technology. 2011;45(10):4570–4578. doi: 10.1021/es2001248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gottschalk F, Ort C, Scholz RW, Nowack B. Engineered nanomaterials in rivers - exposure scenarios for Switzerland at high spatial and temporal resolution. Environmental Pollution. 2011;159(12):3439–3445. doi: 10.1016/j.envpol.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 19.Aitken RJ, Chaudhry MQ, Boxall ABA, Hull M. Manufacture and use of nanomaterials: current status in the UK and global trends. Occupational Medicine-Oxford. 2006;56(5):300–306. doi: 10.1093/occmed/kql051. [DOI] [PubMed] [Google Scholar]
- 20.Hong J, Peralta-Videa JR, Gardea-Torresdey JL. Nanomaterials in agricultural production: benefits and possible threats? In: Shamin N, Sharma VK, editors. Sustainable Nanotechnology and the Environment: Advances and Achievements. Vol. 1124. American Chemical Society; 2013. pp. 73–90. [Google Scholar]
- 21.MacCuspie RI, Hyman H, Yakymyshyn C, Srinivasan SS, Dhau J, Drake C. A framework for identifying performance targets for sustainable nanomaterials. Sustainable Materials and Technologies. 2014;1-2:17–25. [Google Scholar]
- 22.Johnson AC, Park B. Predicting contamination by the fuel additive cerium oxide engineered nanoparticles within the United Kingdom and the associated risks. Environmental Toxicology and Chemistry. 2012;31(11):2582–2587. doi: 10.1002/etc.1983. [DOI] [PubMed] [Google Scholar]
- 23.Arvidsson R, Molander S, Sanden BA. Impacts of a silver-coated future. Journal of Industrial Ecology. 2011;15(6):844–854. [Google Scholar]
- 24.Wigger H, Hackmann S, Zimmermann T, Köser J, Thöming J, von Gleich A. Influences of use activities and waste management on environmental releases of engineered nanomaterials. Science of The Total Environment. 2015;535:160–171. doi: 10.1016/j.scitotenv.2015.02.042. [DOI] [PubMed] [Google Scholar]
- 25.Colman BP, Arnaout CL, Anciaux S, Gunsch CK, Hochella MF, Jr., Kim B, Lowry GV, McGill BM, Reinsch BC, Richardson CJ, Unrine JM, Wright JP, Yin L, Bernhardt ES. Low concentrations of silver nanoparticles in biosolids cause adverse ecosystem responses under realistic field scenario. PLoS ONE. 2013;8(2):e57189. doi: 10.1371/journal.pone.0057189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collier ZA, Kennedy AJ, Poda AR, Cuddy MF, Moser RD, MacCuspie RI, Harmon A, Plourde K, Haines CD, Steevens JA. Tiered guidance for risk-informed environmental health and safety testing of nanotechnologies. Journal of Nanoparticle Research. 2015;17(3):1–21. [Google Scholar]
- 27.Windler L, Lorenz C, von Goetz N, Hungerbuhler K, Amberg M, Heuberger M, Nowack B. Release of titanium dioxide from textiles during washing. Environmental Science & Technology. 2012;46(15):8181–8188. doi: 10.1021/es301633b. [DOI] [PubMed] [Google Scholar]
- 28.Stacey H, Wendel W, Maria D, Bernd N, Shaun C, Richard C, Andrew M. Measuring nanomaterial release from carbon nanotube composites: review of the state of the science. Journal of Physics: Conference Series. 2015;617(1):012026. [Google Scholar]
- 29.Nowack B, David RM, Fissan H, Morris H, Shatkin JA, Stintz M, Zepp R, Brouwer D. Potential release scenarios for carbon nanotubes used in composites. Environment International. 2013;59:1–11. doi: 10.1016/j.envint.2013.04.003. (0) [DOI] [PubMed] [Google Scholar]
- 30.Hirth S, Cena L, Cox G, Tomovic Z, Peters T, Wohlleben W. Scenarios and methods that induce protruding or released CNTs after degradation of nanocomposite materials. Journal of Nanoparticle Research. 2013;15(4):1–15. doi: 10.1007/s11051-013-1504-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petersen EJ, Lam T, Gorham JM, Scott KC, Long CJ, Stanley D, Sharma R, Liddle JA, Pellegrin B, Nguyen T. Methods to assess the impact of UV irradiation on the surface chemistry and structure of multiwall carbon nanotube epoxy nanocomposites. Carbon. 2014;69:194–205. [Google Scholar]
- 32.Liu HH, Cohen Y. Multimedia environmental distribution of engineered nanomaterials. Environmental Science & Technology. 2014;48(6):3281–3292. doi: 10.1021/es405132z. [DOI] [PubMed] [Google Scholar]
- 33.Goodwin DG, Marsh KM, Sosa IB, Payne JB, Gorham JM, Bouwer EJ, Fairbrother DH. Interactions of microorganisms with polymer nanocomposite surfaces containing oxidized carbon nanotubes. Environmental Science & Technology. 2015;49(9):5484–5492. doi: 10.1021/acs.est.5b00084. [DOI] [PubMed] [Google Scholar]
- 34.Khan FR, Laycock A, Dybowska A, Larner F, Smith BD, Rainbow PS, Luoma SN, Rehkaemper M, Valsami-Jones E. Stable isotope tracer to determine uptake and efflux dynamics of ZnO nano- and bulk particles and dissolved Zn to an estuarine snail. Environmental Science & Technology. 2013;47(15):8532–8539. doi: 10.1021/es4011465. [DOI] [PubMed] [Google Scholar]
- 35.Filser J, Arndt D, Baumann J, Geppert M, Hackmann S, Luther EM, Pade C, Prenzel K, Wigger H, Arning J, Hohnholt MC, Koser J, Kuck A, Lesnikov E, Neumann J, Schutrumpf S, Warrelmann J, Baumer M, Dringen R, von Gleich A, Swiderek P, Thoming J. Intrinsically green iron oxide nanoparticles? From synthesis via (eco-)toxicology to scenario modelling. Nanoscale. 2013;5(3):1034–1046. doi: 10.1039/c2nr31652h. [DOI] [PubMed] [Google Scholar]
- 36.Levard C, Hotze EM, Lowry GV, Brown GE. Environmental transformations of silver nanoparticles: impact on stability and toxicity. Environmental Science & Technology. 2012;46(13):6900–6914. doi: 10.1021/es2037405. [DOI] [PubMed] [Google Scholar]
- 37.Praetorius A, Scheringer M, Hungerbuhler K. Development of environmental fate models for engineered nanoparticles-a case study of TiO2 nanoparticles in the Rhine River. Environmental Science & Technology. 2012;46(12):6705–6713. doi: 10.1021/es204530n. [DOI] [PubMed] [Google Scholar]
- 38.Hendren CO, Lowry GV, Unrine JM, Wiesner MR. A functional assay-based strategy for nanomaterial risk forecasting. Science of the Total Environment. 2015;536:1029–1037. doi: 10.1016/j.scitotenv.2015.06.100. [DOI] [PubMed] [Google Scholar]
- 39.Kaegi R, Voegelin A, Sinnet B, Zuleeg S, Siegrist H, Burkhardt M. Transformation of AgCl nanoparticles in a sewer system — A field study. Science of The Total Environment. 2015;535:20–27. doi: 10.1016/j.scitotenv.2014.12.075. [DOI] [PubMed] [Google Scholar]
- 40.Liu XY, Vinson D, Abt D, Hurt RH, Rand DM. Differential toxicity of carbon nanomaterials in Drosophila: larval dietary uptake Is benign, but adult exposure causes locomotor impairment and mortality. Environmental Science & Technology. 2009;43(16):6357–6363. doi: 10.1021/es901079z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petersen EJ, Pinto RA, Zhang LW, Huang QG, Landrum PF, Weber WJ. Effects of polyethyleneimine-mediated functionalization of multi-walled carbon nanotubes on earthworm bioaccumulation and sorption by soils. Environmental Science & Technology. 2011;45(8):3718–3724. doi: 10.1021/es103004r. [DOI] [PubMed] [Google Scholar]
- 42.Johnson AC, Bowes MJ, Crossley A, Jarvie HP, Jurkschat K, Jurgens MD, Lawlor AJ, Park B, Rowland P, Spurgeon D, Svendsen C, Thompson IP, Barnes RJ, Williams RJ, Xu N. An assessment of the fate, behaviour and environmental risk associated with sunscreen TiO2 nanoparticles in UK field scenarios. Science of the Total Environment. 2011;409(13):2503–2510. doi: 10.1016/j.scitotenv.2011.03.040. [DOI] [PubMed] [Google Scholar]
- 43.Cong Y, Banta GT, Selck H, Berhanu D, Valsami-Jones E, Forbes VE. Toxic effects and bioaccumulation of nano-, micron- and ionic-Ag in the polychaete, Nereis diversicolor. Aquatic Toxicology. 2011;105(3-4):403–411. doi: 10.1016/j.aquatox.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 44.Al-Salim N, Barraclough E, Burgess E, Clothier B, Deurer M, Green S, Malone L, Weir G. Quantum dot transport in soil, plants, and insects. Sci. Total Environ. 2011;409(17):3237–48. doi: 10.1016/j.scitotenv.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 45.Hildebrand H, Kühnel D, Potthoff A, Mackenzie K, Springer A, Schirmer K. Evaluating the cytotoxicity of palladium/magnetite nano-catalysts intended for wastewater treatment. Environmental Pollution. 2010;158(1):65–73. doi: 10.1016/j.envpol.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 46.Gardea-Torresdey JL, Rico CM, White JC. Trophic transfer, transformation, and impact of engineered nanomaterials in terrestrial environments. Environmental Science & Technology. 2014;48(5):2526–2540. doi: 10.1021/es4050665. [DOI] [PubMed] [Google Scholar]
- 47.Musee N, Thwala M, Nota N. The antibacterial effects of engineered nanomaterials: implications for wastewater treatment plants. Journal of Environmental Monitoring. 2011;13(5):1164–1183. doi: 10.1039/c1em10023h. [DOI] [PubMed] [Google Scholar]
- 48.Nyberg L, Turco RF, Nies L. Assessing the impact of nanomaterials on anaerobic microbial communities. Environmental Science & Technology. 2008;42(6):1938–1943. doi: 10.1021/es072018g. [DOI] [PubMed] [Google Scholar]
- 49.Costanza R, d'Arge R, de Groot R, Farber S, Grasso M, Hannon B, Limburg K, Naeem S, O'neill RV, Paruelo J, Raskin RG, Sutton P, van den Belt M. The value of the world's ecosystem services and natural capital. Nature. 1997;387(6630):253–260. [Google Scholar]
- 50.Rico CM, Lee SC, Rubenecia R, Mukherjee A, Hong J, Peralta-Videa JR, Gardea-Torresdey JL. Cerium oxide nanoparticles impact yield and modify nutritional parameters in wheat (Triticum aestivum L.) Journal of Agricultural and Food Chemistry. 2014;62(40):9669–9675. doi: 10.1021/jf503526r. [DOI] [PubMed] [Google Scholar]
- 51.Rico CM, Barrios AC, Tan WJ, Rubenecia R, Lee SC, Varela-Ramirez A, Peralta-Videa JR, Gardea-Torresdey JL. Physiological and biochemical response of soil-grown barley (Hordeum vulgare L.) to cerium oxide nanoparticles. Environmental Science and Pollution Research. 2015;22(14):10551–10558. doi: 10.1007/s11356-015-4243-y. [DOI] [PubMed] [Google Scholar]
- 52.Kraus N, Malmfors T, Slovic P. Intuitive toxicology: expert and lay judgements of chemical risks. Risk Analysis. 1992;12(2):215–232. doi: 10.1177/019262339402200214. [DOI] [PubMed] [Google Scholar]
- 53.Holden PA, Klaessig F, Turco RF, Priester JH, Rico CM, Avila-Arias H, Mortimer M, Pacpaco K, Gardea-Torresdey JL. Evaluation of exposure concentrations used in assessing manufactured nanomaterial environmental hazards: are they relevant? Environmental Science & Technology. 2014;48(18):10541–10551. doi: 10.1021/es502440s. [DOI] [PubMed] [Google Scholar]
- 54.El Badawy AM, Silva RG, Morris B, Scheckel KG, Suidan MT, Tolaymat TM. Surface charge-sependent toxicity of silver nanoparticles. Environmental Science & Technology. 2011;45(1):283–287. doi: 10.1021/es1034188. [DOI] [PubMed] [Google Scholar]
- 55.Corsi I, Cherr GN, Lenihan HS, Labille J, Hassellov M, Canesi L, Dondero F, Frenzilli G, Hristozov D, Puntes V, Della Torre C, Pinsino A, Libralato G, Marcomini A, Sabbioni E, Matranga V. Common strategies and technologies for the ecosafety assessment and design of nanomaterials entering the marine environment. ACS Nano. 2014;8(10):9694–9709. doi: 10.1021/nn504684k. [DOI] [PubMed] [Google Scholar]
- 56.Hendren CO, Lowry M, Grieger KD, Money ES, Johnston JM, Wiesner MR, Beaulieu SM. Modeling approaches for characterizing and evaluating environmental exposure to engineered nanomaterials in support of risk-based decision making. Environmental Science & Technology. 2013;47(3):1190–1205. doi: 10.1021/es302749u. [DOI] [PubMed] [Google Scholar]
- 57.Handy RD, van den Brink N, Chappell M, Muhling M, Behra R, Dusinska M, Simpson P, Ahtiainen J, Jha AN, Seiter J, Bednar A, Kennedy A, Fernandes TF, Riediker M. Practical considerations for conducting ecotoxicity test methods with manufactured nanomaterials: what have we learnt so far? Ecotoxicology. 2012;21(4):933–972. doi: 10.1007/s10646-012-0862-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Priester JH, Ge Y, Mielke RE, Horst AM, Moritz SC, Espinosa K, Gelb J, Walker SL, Nisbet RM, An Y-J, Schimel JP, Palmer RG, Hernandez-Viezcas JA, Zhao L, Gardea-Torresdey JL, Holden PA. Soybean susceptibility to manufactured nanomaterials with evidence for food quality and soil fertility interruption. Proceedings of the National Academy of Sciences. 2012;109(37):E2451–E2456. doi: 10.1073/pnas.1205431109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Du WC, Gardea-Torresdey JL, Ji R, Yin Y, Zhu JG, Peralta-Videa JR, Guo HY. Physiological and biochemical changes imposed by CeO2 nanoparticles on wheat: a life cycle field study. Environmental Science & Technology. 2015;49(19):11884–11893. doi: 10.1021/acs.est.5b03055. [DOI] [PubMed] [Google Scholar]
- 60.Bour A, Mouchet F, Silvestre J, Gauthier L, Pinelli E. Environmentally relevant approaches to assess nanoparticles ecotoxicity: A review. Journal of Hazardous Materials. 2015;283:764–777. doi: 10.1016/j.jhazmat.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 61.Murray KE, Thomas SM, Bodour AA. Prioritizing research for trace pollutants and emerging contaminants in the freshwater environment. Environmental Pollution. 2010;158(12):3462–3471. doi: 10.1016/j.envpol.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 62.Petersen EJ, Diamond SA, Kennedy AJ, Goss GG, Ho K, Lead J, Hanna SK, Hartmann NB, Hund-Rinke K, Mader B, Manier N, Pandard P, Salinas ER, Sayre P. Adapting OECD aquatic toxicity tests for use with manufactured nanomaterials: key issues and consensus recommendations. Environmental Science & Technology. 2015;49(16):9532–9547. doi: 10.1021/acs.est.5b00997. [DOI] [PubMed] [Google Scholar]
- 63.Hund-Rinke K, Herrchen M, Schlich K. Integrative test strategy for the environmental assessment of nanomaterials; (UBA-FB) 002028/E. Environmental Research of the Federal Ministry for the Environment, Nature Conservation, Building and Nuclear Safety; Dessau-RoBlau, Germany: Mar, 2015. p. 143. [Google Scholar]
- 64.Handy RD, Cornelis G, Fernandes T, Tsyusko O, Decho A, Sabo-Attwood T, Metcalfe C, Steevens JA, Klaine SJ, Koelmans AA, Horne N. Ecotoxicity test methods for engineered nanomaterials: practical experiences and recommendations from the bench. Environmental Toxicology and Chemistry. 2012;31(1):15–31. doi: 10.1002/etc.706. [DOI] [PubMed] [Google Scholar]
- 65.OECD . Preliminary Review of OECD Test Guidelines for Their Applicability to Manufactured Nanomaterials. Vol. 15. OECD Publishing; Paris: 2009. p. 71. [Google Scholar]
- 66.OECD . Ecotoxicology and Environmental Fate of Manufactured Nanomaterials: Test Guidelines. Vol. 40. OECD Publishing; Paris: 2014. p. 84. [Google Scholar]
- 67.Kuhnel D, Nickel C. The OECD expert meeting on ecotoxicology and environmental fate - Towards the development of improved OECD guidelines for the testing of nanomaterials. Science of the Total Environment. 2014;472:347–353. doi: 10.1016/j.scitotenv.2013.11.055. [DOI] [PubMed] [Google Scholar]
- 68.Priester JH, Singhal A, Wu B, Stucky GD, Holden PA. Integrated approach to evaluating the toxicity of novel cysteine-capped silver nanoparticles to Escherichia coli and Pseudomonas aeruginosa. Analyst. 2014;139(5):954–963. doi: 10.1039/c3an01648j. [DOI] [PubMed] [Google Scholar]
- 69.Miller RJ, Bennett S, Keller AA, Pease S, Lenihan HS. TiO2 nanoparticles are phototoxic to marine phytoplankton. PLoS ONE. 2012;7(1):e30321. doi: 10.1371/journal.pone.0030321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kaweeteerawat C, Chang CH, Roy KR, Liu R, Li R, Toso D, Fischer H, Ivask A, Ji Z, Zink JI, Zhou ZH, Chanfreau GF, Telesca D, Cohen Y, Holden PA, Nel AE, Godwin HA. Cu nanoparticles have different impacts in Escherichia coli and Lactobacillus brevis than their microsized and ionic analogues. ACS Nano. 2015;9(7):7215–7225. doi: 10.1021/acsnano.5b02021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Priester JH, Stoimenov PK, Mielke RE, Webb SM, Ehrhardt C, Zhang JP, Stucky GD, Holden PA. Effects of soluble cadmium salts versus CdSe quantum dots on the growth of planktonic Pseudomonas aeruginosa. Environmental Science & Technology. 2009;43(7):2589–2594. doi: 10.1021/es802806n. [DOI] [PubMed] [Google Scholar]
- 72.Guo XK, Dong SP, Petersen EJ, Gao SX, Huang QG, Mao L. Biological uptake and depuration of radio-labeled graphene by Daphnia magna. Environmental Science & Technology. 2013;47(21):12524–12531. doi: 10.1021/es403230u. [DOI] [PubMed] [Google Scholar]
- 73.Tervonen K, Waissi G, Petersen EJ, Akkanen J, Kukkonen JVK. Analysis of fullerene-C-60 and kinetic measurements for its accumulation and depuration in Daphnia magna. Environmental Toxicology and Chemistry. 2010;29(5):1072–1078. doi: 10.1002/etc.124. [DOI] [PubMed] [Google Scholar]
- 74.Ivask A, Suarez E, Patel T, Boren D, Ji ZX, Holden P, Telesca D, Damoiseaux R, Bradley KA, Godwin H. Genome-wide bacterial toxicity screening uncovers the mechanisms of toxicity of a cationic polystyrene nanomaterial. Environmental Science & Technology. 2012;46(4):2398–2405. doi: 10.1021/es203087m. [DOI] [PubMed] [Google Scholar]
- 75.Kaweeteerawat C, Ivask A, Liu R, Zhang H, Chang CH, Low-Kam C, Fischer H, Ji Z, Pokhrel S, Cohen Y, Telesca D, Zink J, Mädler L, Holden PA, Nel A, Godwin H. Toxicity of metal oxide nanoparticles in Escherichia coli correlates with conduction band and hydration energies. Environmental Science & Technology. 2015;49(2):1105–1112. doi: 10.1021/es504259s. [DOI] [PubMed] [Google Scholar]
- 76.Chen C, Unrine JM, Judy JD, Lewis RW, Guo J, McNear DH, Tsyusko OV. Toxicogenomic responses of the model legume Medicago truncatula to aged biosolids containing a mixture of nanomaterials (TiO2, Ag, and ZnO) from a pilot wastewater treatment plant. Environmental Science & Technology. 2015;49(14):8759–8768. doi: 10.1021/acs.est.5b01211. [DOI] [PubMed] [Google Scholar]
- 77.Gou N, Onnis-Hayden A, Gu AZ. Mechanistic toxicity assessment of nanomaterials by whole-cell-array stress genes expression analysis. Environmental Science & Technology. 2010;44(15):5964–5970. doi: 10.1021/es100679f. [DOI] [PubMed] [Google Scholar]
- 78.Ma C, Chhikara S, Xing B, Musante C, White JC, Dhankher OP. Physiological and molecular response of Arabidopsis thaliana (L.) to nanoparticle cerium and indium oxide exposure. ACS Sustainable Chem. Eng. 2013;1:768–778. [Google Scholar]
- 79.Marmiroli M, Pagano L, Sardaro MLS, Villani M, Marmiroli N. Genome-wide approach in Arabidopsis thaliana to assess the toxicity of cadmium sulfide quantum dots. Environmental Science & Technology. 2014;48(10):5902–5909. doi: 10.1021/es404958r. [DOI] [PubMed] [Google Scholar]
- 80.Lan J, Gou N, Gao C, He M, Gu AZ. Comparative and mechanistic genotoxicity assessment of nanomaterials via a quantitative toxicogenomics approach across multiple species. Environmental Science & Technology. 2014;48(21):12937–12945. doi: 10.1021/es503065q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chang XJ, Henderson WM, Bouchard DC. Multiwalled carbon nanotube dispersion methods affect their aggregation, deposition, and biomarker response. Environmental Science & Technology. 2015;49(11):6645–6653. doi: 10.1021/acs.est.5b00654. [DOI] [PubMed] [Google Scholar]
- 82.Wang Q, Ebbs SD, Chen YS, Ma XM. Trans-generational impact of cerium oxide nanoparticles on tomato plants. Metallomics. 2013;5(6):753–759. doi: 10.1039/c3mt00033h. [DOI] [PubMed] [Google Scholar]
- 83.Arndt DA, Chen J, Moua M, Klaper RD. Multigeneration impacts on Daphnia magna of carbon nanomaterials with differing core structures and functionalizations. Environmental Toxicology and Chemistry. 2014;33(3):541–547. doi: 10.1002/etc.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Petersen JE, Kemp WM. Mesocosms: enclosed experimental ecosystems in ocean science. In: Steele JH, Turekian KK, Thorpe SA, editors. Encyclopedia of Ocean Sciences. second ed Academic Press; 2009. pp. 732–747. [Google Scholar]
- 85.Ge Y, Schimel JP, Holden PA. Evidence for negative effects of TiO2 and ZnO nanoparticles on soil bacterial communities. Environmental Science & Technology. 2011;45(4):1659–1664. doi: 10.1021/es103040t. [DOI] [PubMed] [Google Scholar]
- 86.Pakarinen K, Petersen EJ, Leppanen MT, Akkanen J, Kukkonen JVK. Adverse effects of fullerenes (nC60) spiked to sediments on Lumbriculus variegatus (Oligochaeta) Environmental Pollution. 2011;159(12):3750–3756. doi: 10.1016/j.envpol.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 87.Ge Y, Priester JH, Van De Werfhorst LC, Walker SL, Nisbet RM, An Y-J, Schimel JP, Gardea-Torresdey JL, Holden PA. Soybean plants modify metal oxide nanoparticle effects on soil bacterial communities. Environmental Science & Technology. 2014;48(22):13489–13496. doi: 10.1021/es5031646. [DOI] [PubMed] [Google Scholar]
- 88.Hanna SK, Miller RJ, Muller EB, Nisbet RM, Lenihan HS. Impact of engineered zinc oxide nanoparticles on the individual performance of Mytilus galloprovincialis. Plos One. 2013;8(4):1–7. doi: 10.1371/journal.pone.0061800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hanna SK, Miller RJ, Zhou DX, Keller AA, Lenihan HS. Accumulation and toxicity of metal oxide nanoparticles in a soft-sediment estuarine amphipod. Aquatic Toxicology. 2013;142:441–446. doi: 10.1016/j.aquatox.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 90.Dai L, Syberg K, Banta GT, Selck H, Forbes VE. Effects, uptake, and depuration kinetics of silver oxide and copper oxide nanoparticles in a marine deposit feeder, Macoma balthica. ACS Sustainable Chemistry & Engineering. 2013;1(7):760–767. [Google Scholar]
- 91.Keller AA, Wang HT, Zhou DX, Lenihan HS, Cherr G, Cardinale BJ, Miller R, Ji ZX. Stability and aggregation of metal oxide nanoparticles in natural aqueous matrices. Environmental Science & Technology. 2010;44(6):1962–1967. doi: 10.1021/es902987d. [DOI] [PubMed] [Google Scholar]
- 92.Echavarri-Bravo V, Paterson L, Aspray TJ, Porter JS, Winson MK, Thornton B, Hartl MGJ. Shifts in the metabolic function of a benthic estuarine microbial community following a single pulse exposure to silver nanoparticles. Environmental Pollution. 2015;201:91–99. doi: 10.1016/j.envpol.2015.02.033. [DOI] [PubMed] [Google Scholar]
- 93.Ge Y, Schimel JP, Holden PA. Identification of soil bacteria susceptible to TiO2 and ZnO nanoparticles. Applied & Environmental Microbiology. 2012;78(18):6749–6758. doi: 10.1128/AEM.00941-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Priester JH, Ge Y, Chang V, Stoimenov PK, Schimel JP, Stucky GD, Holden PA. Assessing interactions of hydrophilic nanoscale TiO2 with soil water. Journal of Nanoparticle Research. 2013;15(9):1–13. [Google Scholar]
- 95.Ge Y, Priester JH, De Werfhorst LCV, Schimel JP, Holden PA. Potential mechanisms and environmental controls of TiO2 nanoparticle effects on soil bacterial communities. Environmental Science & Technology. 2013;47(24):14411–14417. doi: 10.1021/es403385c. [DOI] [PubMed] [Google Scholar]
- 96.Horst AM, Neal AC, Mielke RE, Sislian PR, Suh WH, Mädler L, Stucky GD, Holden PA. Dispersion of TiO2 nanoparticle agglomerates by Pseudomonas aeruginosa. Appl. Environ. Microbiol. 2010;76(21):7292–7298. doi: 10.1128/AEM.00324-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rhiem S, Riding MJ, Baumgartner W, Martin FL, Semple KT, Jones KC, Schaffer A, Maes HM. Interactions of multiwalled carbon nanotubes with algal cells: Quantification of association, visualization of uptake, and measurement of alterations in the composition of cells. Environmental Pollution. 2015;196:431–439. doi: 10.1016/j.envpol.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 98.Jarvis TA, Miller RJ, Lenihan HS, Bielmyer GK. Toxicity of ZnO nanoparticles to the copepod Acartia tonsa, exposed through a phytoplankton diet. Environmental Toxicology and Chemistry. 2013;32(6):1264–1269. doi: 10.1002/etc.2180. [DOI] [PubMed] [Google Scholar]
- 99.Mielke RE, Priester JH, Werlin RA, Gelb J, Horst AM, Orias E, Holden PA. Differential growth of and nanoscale TiO2 accumulation in Tetrahymena thermophila by direct feeding versus trophic transfer from Pseudomonas aeruginosa. Applied and Environmental Microbiology. 2013;79(18):5616–5624. doi: 10.1128/AEM.01680-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Khan FR, Paul KB, Dybowska AD, Valsami-Jones E, Lead JR, Stone V, Fernandes TF. Accumulation dynamics and acute toxicity of silver nanoparticles to Daphnia magna and Lumbriculus variegatus: implications for metal modeling approaches. Environmental Science & Technology. 2015;49(7):4389–4397. doi: 10.1021/es506124x. [DOI] [PubMed] [Google Scholar]
- 101.Kalman J, Paul KB, Khan FR, Stone V, Fernandes TF. Characterisation of bioaccumulation dynamics of three differently coated silver nanoparticles and aqueous silver in a simple freshwater food chain. Environmental Chemistry. 2015;12(6):662–672. [Google Scholar]
- 102.Werlin R, Priester JH, Mielke RE, Krämer S, Jackson S, Stoimenov PK, Stucky GD, Cherr GN, Orias E, Holden PA. Biomagnification of cadmium selenide quantum dots in a simple experimental microbial food chain. Nature Nanotechnology. 2011;6(1):65–71. doi: 10.1038/nnano.2010.251. [DOI] [PubMed] [Google Scholar]
- 103.Yue Y, Behra R, Sigg L, Freire PF, Pillai S, Schirmer K. Toxicity of silver nanoparticles to a fish gill cell line: Role of medium composition. Nanotoxicology. 2015;9(1):54–63. doi: 10.3109/17435390.2014.889236. [DOI] [PubMed] [Google Scholar]
- 104.Smulders S, Luyts K, Brabants G, Golanski L, Martens J, Vanoirbeek J, Hoet PHM. Toxicity of nanoparticles embedded in paints compared to pristine nanoparticles, in vitro study. Toxicology Letters. 2015;232(2):333–339. doi: 10.1016/j.toxlet.2014.11.030. [DOI] [PubMed] [Google Scholar]
- 105.Gottschalk F, Nowack B. The release of engineered nanomaterials to the environment. Journal of Environmental Monitoring. 2011;13(5):1145–1155. doi: 10.1039/c0em00547a. [DOI] [PubMed] [Google Scholar]
- 106.Solovitch N, Labille J. r. m., Rose J. r. m., Chaurand P, Borschneck D, Wiesner MR, Bottero J-Y. Concurrent aggregation and deposition of TiO2 nanoparticles in a sandy porous media. Environmental Science & Technology. 2010;44(13):4897–4902. doi: 10.1021/es1000819. [DOI] [PubMed] [Google Scholar]
- 107.Auffan M, Bottero JY, Chaneac C, Rose J. Inorganic manufactured nanoparticles: how their physicochemical properties influence their biological effects in aqueous environments. Nanomedicine. 2010;5(6):999–1007. doi: 10.2217/nnm.10.61. [DOI] [PubMed] [Google Scholar]
- 108.Tong ZH, Bischoff M, Nies LF, Myer P, Applegate B, Turco RF. Response of soil microorganisms to as-produced and functionalized single-wall carbon nanotubes (SWNTs) Environmental Science & Technology. 2012;46(24):13471–13479. doi: 10.1021/es303251r. [DOI] [PubMed] [Google Scholar]
- 109.Simonin M, Guyonnet JP, Martins JMF, Ginot M, Richaume A. Influence of soil properties on the toxicity of TiO2 nanoparticles on carbon mineralization and bacterial abundance. Journal of Hazardous Materials. 2015;283:529–535. doi: 10.1016/j.jhazmat.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 110.Schlich K, Klawonn T, Terytze K, Hund-Rinke K. Effects of silver nanoparticles and silver nitrate in the earthworm reproduction test. Environmental Toxicology and Chemistry. 2013;32(1):181–188. doi: 10.1002/etc.2030. [DOI] [PubMed] [Google Scholar]
- 111.Lopez-Moreno ML, de la Rosa G, Hernandez-Viezcas JA, Castillo-Michel H, Botez CE, Peralta-Videa JR, Gardea-Torresdey JL. Evidence of the differential biotransformation and genotoxicity of ZnO and CeO2 nanoparticles on soybean (Glycine max) plants. Environmental Science & Technology. 2010;44(19):7315–7320. doi: 10.1021/es903891g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mackay D, McCarty LS, Arnot JA. Relationships between exposure and dose in aquatic toxicity tests for organic chemicals. Environmental Toxicology and Chemistry. 2014;33(9):2038–2046. doi: 10.1002/etc.2649. [DOI] [PubMed] [Google Scholar]
- 113.Gordon AK, Palmer CG. Defining an exposure-response relationship for suspended kaolin clay particulates and aquatic organisms: work toward defining a water quality guideline for suspended solids. Environmental Toxicology and Chemistry. 2015;34(4):907–912. doi: 10.1002/etc.2872. [DOI] [PubMed] [Google Scholar]
- 114.Koelmans AA, Diepens NJ, Velzeboer I, Besseling E, Quik JTK, van de Meent D. Guidance for the prognostic risk assessment of nanomaterials in aquatic ecosystems. Science of The Total Environment. 2015;535:141–249. doi: 10.1016/j.scitotenv.2015.02.032. [DOI] [PubMed] [Google Scholar]
- 115.Sekine R, Brunetti G, Donner E, Khaksar M, Vasilev K, Jamting AK, Scheckel KG, Kappen P, Zhang H, Lombi E. Speciation and lability of Ag−, AgCl−, and Ag2S-nanoparticles in soil determined by X-ray absorption spectroscopy and diffusive gradients in thin films. Environmental Science & Technology. 2015;49(2):897–905. doi: 10.1021/es504229h. [DOI] [PubMed] [Google Scholar]
- 116.Cui D, Zhang P, Ma YH, He X, Li YY, Zhang J, Zhao YC, Zhang ZY. Effect of cerium oxide nanoparticles on asparagus lettuce cultured in an agar medium. Environmental Science-Nano. 2014;1(5):459–465. [Google Scholar]
- 117.Hernandez-Viezcas JA, Castillo-Michel H, Andrews JC, Cotte M, Rico C, Peralta-Videa JR, Ge Y, Priester JH, Holden PA, Gardea-Torresdey JL. In situ synchrotron X-ray fluorescence mapping and speciation of CeO2 and ZnO nanoparticles in soil cultivated soybean (Glycine max) ACS Nano. 2013;7(2):1415–1423. doi: 10.1021/nn305196q. [DOI] [PubMed] [Google Scholar]
- 118.Zou XY, Shi JP, Zhang HW. Coexistence of silver and titanium dioxide nanoparticles: enhancing or reducing environmental risks? Aquatic Toxicology. 2014;154:168–175. doi: 10.1016/j.aquatox.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 119.Canesi L, Frenzilli G, Balbi T, Bernardeschi M, Ciacci C, Corsolini S, Della Torre C, Fabbri R, Faleri C, Focardi S, Guidi P, Kocan A, Marcomini A, Mariottini M, Nigro M, Pozo-Gallardo K, Rocco L, Scarcelli V, Smerilli A, Corsi I. Interactive effects of n-TiO2 and 2,3,7,8-TCDD on the marine bivalve Mytilus galloprovincialis. Aquatic Toxicology. 2014;153:53–65. doi: 10.1016/j.aquatox.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 120.Della Torre C, Balbi T, Grassi G, Frenzilli G, Bernardeschi M, Smerilli A, Guidi P, Canesi L, Nigro M, Monaci F, Scarcelli V, Rocco L, Focardi S, Monopoli M, Corsi I. Titanium dioxide nanoparticles modulate the toxicological response to cadmium in the gills of Mytilus galloprovincialis. Journal of Hazardous Materials. 2015;297:92–100. doi: 10.1016/j.jhazmat.2015.04.072. [DOI] [PubMed] [Google Scholar]
- 121.Hartmann NB, Von der Kammer F, Hofmann T, Baalousha M, Ottofuelling S, Baun A. Algal testing of titanium dioxide nanoparticles-testing considerations, inhibitory effects and modification of cadmium bioavailability. Toxicology. 2010;269(2-3):190–197. doi: 10.1016/j.tox.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 122.de La Torre-Roche R, Hawthorne J, Deng YQ, Xing BS, Cai WJ, Newman LA, Wang C, Ma XM, White JC. Fullerene-enhanced accumulation of p,p '-DDE in agricultural crop species. Environ. Sci. Technol. 2012;46(17):9315–9323. doi: 10.1021/es301982w. [DOI] [PubMed] [Google Scholar]
- 123.Hu XL, Liu JF, Mayer P, Jiang G. Impacts of some environmentally relevant parameters on the sorption of polycyclic aromatic hydrocarbons to aqueous suspensions of fullerene. Environmental Toxicology and Chemistry. 2008;27(9):1868–1874. doi: 10.1897/08-009.1. [DOI] [PubMed] [Google Scholar]
- 124.Gai K, Shi BY, Yan XM, Wang DS. Effect of dispersion on adsorption of atrazine by aqueous suspensions of fullerenes. Environmental Science & Technology. 2011;45(14):5959–5965. doi: 10.1021/es103595g. [DOI] [PubMed] [Google Scholar]
- 125.Sun K, Zhang ZY, Gao B, Wang ZY, Xu DY, Jin J, Liu XT. Adsorption of diuron, fluridone and norflurazon on single-walled and multi-walled carbon nanotubes. Science of the Total Environment. 2012;439:1–7. doi: 10.1016/j.scitotenv.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 126.Chen QQ, Yin DQ, Hu XL, Wang R, Zhang C. The effect of nC60 on tissue distribution of ibuprofen in Cyprinus carpio. Science of the Total Environment. 2014;496:453–460. doi: 10.1016/j.scitotenv.2014.07.074. [DOI] [PubMed] [Google Scholar]
- 127.Kelsey JW, White JC. Effect of C60 fullerenes on the accumulation of weathered p,p '-DDE by plant and earthworm species under single and multispecies conditions. Environmental Toxicology and Chemistry. 2013;32(5):1117–1123. doi: 10.1002/etc.2158. [DOI] [PubMed] [Google Scholar]
- 128.Guo HY, Zhang ZY, Xing BS, Mukherjee A, Musante C, White JC, He LL. Analysis of silver nanoparticles in antimicrobial products using surface-enhanced Raman spectroscopy (SERS) Environmental Science & Technology. 2015;49(7):4317–4324. doi: 10.1021/acs.est.5b00370. [DOI] [PubMed] [Google Scholar]
- 129.Zhao J, Wang ZY, Mashayekhi H, Mayer P, Chefetz B, Xing BS. Pulmonary surfactant suppressed phenanthrene adsorption on carbon nanotubes through solubilization and competition as examined by passive dosing technique. Environmental Science & Technology. 2012;46(10):5369–5377. doi: 10.1021/es2044773. [DOI] [PubMed] [Google Scholar]
- 130.Wang F, Yao J, Liu HJ, Liu RP, Chen HL, Yi ZJ, Yu Q, Ma L, Xing BS. Cu and Cr enhanced the effect of various carbon nanotubes on microbial communities in an aquatic environment. Journal of Hazardous Materials. 2015;292:137–145. doi: 10.1016/j.jhazmat.2015.03.032. [DOI] [PubMed] [Google Scholar]
- 131.Tian SY, Zhang YD, Song CZ, Zhu XS, Xing BS. Titanium dioxide nanoparticles as carrier facilitate bioaccumulation of phenanthrene in marine bivalve, ark shell (Scapharca subcrenata) Environmental Pollution. 2014;192:59–64. doi: 10.1016/j.envpol.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 132.Tian SY, Zhang YD, Song CZ, Zhu XS, Xing BS. Bioaccumulation and biotransformation of polybrominated diphenyl ethers in the marine bivalve (Scapharca subcrenata): Influence of titanium dioxide nanoparticles. Marine Pollution Bulletin. 2015;90(1-2):48–53. doi: 10.1016/j.marpolbul.2014.11.031. [DOI] [PubMed] [Google Scholar]
- 133.Liu FF, Zhao J, Wang SG, Du P, Xing BS. Effects of solution chemistry on adsorption of selected pharmaceuticals and personal care products (PPCPs) by graphenes and carbon nanotubes. Environmental Science & Technology. 2014;48(22):13197–13206. doi: 10.1021/es5034684. [DOI] [PubMed] [Google Scholar]
- 134.De La Torre-Roche R, Hawthorne J, Deng YQ, Xing BS, Cai WJ, Newman LA, Wang Q, Ma XM, Hamdi H, White JC. Multiwalled carbon nanotubes and C-60 fullerenes differentially impact the accumulation of weathered pesticides in four agricultural plants. Environmental Science & Technology. 2013;47(21):12539–12547. doi: 10.1021/es4034809. [DOI] [PubMed] [Google Scholar]
- 135.Zhang D, Pan B, Cook RL, Xing BS. Multi-walled carbon nanotube dispersion by the adsorbed humic acids with different chemical structures. Environmental Pollution. 2015;196:292–299. doi: 10.1016/j.envpol.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 136.Pan B, Zhang D, Li H, Wu M, Wang ZY, Xing BS. Increased adsorption of sulfamethoxazole on suspended carbon nanotubes by dissolved humic acid. Environmental Science & Technology. 2013;47(14):7722–7728. doi: 10.1021/es4008933. [DOI] [PubMed] [Google Scholar]
- 137.Wu FC, Bai YC, Mu YS, Pan B, Xing BS, Lin Y. Fluorescence quenching of fulvic acids by fullerene in water. Environmental Pollution. 2013;172:100–107. doi: 10.1016/j.envpol.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 138.Pakarinen K, Petersen EJ, Alvila L, Waissi-Leinonen GC, Akkanen J, Leppanen MT, Kukkonen JVK. A screening study on the fate of fullerenes (nC60) and their toxic implications in natural freshwaters. Environmental Toxicology and Chemistry. 2013;32(6):1224–1232. doi: 10.1002/etc.2175. [DOI] [PubMed] [Google Scholar]
- 139.Hüffer T, Kah M, Hofmann T, Schmidt TC. How redox conditions and irradiation affect sorption of PAHs by dispersed fullerenes nC60. Environmental Science & Technology. 2013;47(13):6935–6942. doi: 10.1021/es303620c. [DOI] [PubMed] [Google Scholar]
- 140.Hou WC, Chowdhury I, Goodwin DG, Henderson WM, Fairbrother DH, Bouchard D, Zepp RG. Photochemical transformation of graphene oxide in sunlight. Environmental Science & Technology. 2015;49(6):3435–3443. doi: 10.1021/es5047155. [DOI] [PubMed] [Google Scholar]
- 141.Schlich K, Hund-Rinke K. Influence of soil properties on the effect of silver nanomaterials on microbial activity in five soils. Environmental Pollution. 2015;196:321–330. doi: 10.1016/j.envpol.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 142.Adeleye AS, Keller AA. Long-term colloidal stability and metal leaching of single wall carbon nanotubes: Effect of temperature and extracellular polymeric substances. Water Research. 2014;49:236–250. doi: 10.1016/j.watres.2013.11.032. [DOI] [PubMed] [Google Scholar]
- 143.Farkas J, Peter H, Ciesielski TM, Thomas KV, Sommaruga R, Salvenmoser W, Weyhenmeyer GA, Tranvik LJ, Jenssen BM. Impact of TiO2 nanoparticles on freshwater bacteria from three Swedish lakes. Science of The Total Environment. 2015;535:85–93. doi: 10.1016/j.scitotenv.2015.03.043. [DOI] [PubMed] [Google Scholar]
- 144.Sauer UG, Aumann A, Ma-Hock L, Landsiedel R, Wohlleben W. Influence of dispersive agent on nanomaterial agglomeration and implications for biological effects in vivo or in vitro. Toxicology in Vitro. 2015;29(1):182–186. doi: 10.1016/j.tiv.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 145.Huynh KA, McCaffery JM, Chen KL. Heteroaggregation reduces antimicrobial activity of silver nanoparticles: evidence for nanoparticle-cell proximity effects. Environmental Science & Technology Letters. 2014;1(9):361–366. [Google Scholar]
- 146.Navarro E, Wagner B, Odzak N, Sigg L, Behra R. Effects of differently coated silver nanoparticles on the photosynthesis of Chlamydomonas reinhardtii. Environmental Science & Technology. 2015;49(13):8041–8047. doi: 10.1021/acs.est.5b01089. [DOI] [PubMed] [Google Scholar]
- 147.Juganson K, Mortimer M, Ivask A, Kasemets K, Kahru A. Extracellular conversion of silver ions into silver nanoparticles by protozoan Tetrahymena thermophila. Environmental Science-Processes & Impacts. 2013;15(1):244–250. doi: 10.1039/c2em30731f. [DOI] [PubMed] [Google Scholar]
- 148.Ghosh S, Pradhan NR, Mashayekhi H, Dickert S, Thantirige R, Tuominen MT, Tao S, Xing BS. Binary short-range colloidal assembly of magnetic iron oxides nanoparticles and fullerene (nC60) in environmental media. Environmental Science & Technology. 2014;48(20):12285–12291. doi: 10.1021/es501846s. [DOI] [PubMed] [Google Scholar]
- 149.Praetorius A, Tufenkji N, Goss K-U, Scheringer M, von der Kammer F, Elimelech M. The road to nowhere: equilibrium partition coefficients for nanoparticles. Environmental Science: Nano. 2014;1(4):317–323. [Google Scholar]
- 150.Zhao J, Liu FF, Wang ZY, Cao XS, Xing BS. Heteroaggregation of graphene oxide with minerals in aqueous phase. Environmental Science & Technology. 2015;49(5):2849–2857. doi: 10.1021/es505605w. [DOI] [PubMed] [Google Scholar]
- 151.Zhang LW, Petersen EJ, Zhang W, Chen YS, Cabrera M, Huang QG. Interactions of C-14-labeled multi-walled carbon nanotubes with soil minerals in water. Environmental Pollution. 2012;166:75–81. doi: 10.1016/j.envpol.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 152.Petersen EJ, Henry TB, Zhao J, MacCuspie RI, Kirschling TL, Dobrovolskaia MA, Hackley V, Xing B, White JC. Identification and avoidance of potential artifacts and misinterpretations in nanomaterial ecotoxicity measurements. Environmental Science & Technology. 2014;48(8):4226–4246. doi: 10.1021/es4052999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Stevenson LM, Dickson H, Klanjscek T, Keller AA, McCauley E, Nisbet RM. Environmental feedbacks and engineered nanoparticles: mitigation of silver nanoparticle toxicity to Chlamydomonas reinhardtii by algal-produced organic compounds. Plos One. 2013;8(9):1–7. doi: 10.1371/journal.pone.0074456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Nature Nanotechnology. 2012;7(9):545–545. doi: 10.1038/nnano.2012.150. Editor, Join the dialogue. [DOI] [PubMed] [Google Scholar]
- 155.Atha DH, Wang HH, Petersen EJ, Cleveland D, Holbrook RD, Jaruga P, Dizdaroglu M, Xing BS, Nelson BC. Copper oxide nanoparticle mediated DNA damage in terrestrial plant models. Environmental Science & Technology. 2012;46(3):1819–1827. doi: 10.1021/es202660k. [DOI] [PubMed] [Google Scholar]
- 156.Hong J, Rico CM, Zhao LJ, Adeleye AS, Keller AA, Peralta-Videa JR, Gardea-Torresdey JL. Toxic effects of copper-based nanoparticles or compounds to lettuce (Lactuca sativa) and alfalfa (Medicago sativa) Environmental Science-Processes & Impacts. 2015;17(1):177–185. doi: 10.1039/c4em00551a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Peralta-Videa JR, Hernandez-Viezcas JA, Zhao L, Diaz BC, Ge Y, Priester JH, Holden PA, Gardea-Torresdey JL. Cerium dioxide and zinc oxide nanoparticles alter the nutritional value of soil cultivated soybean plants. Plant Physiology and Biochemistry. 2014;80:128–135. doi: 10.1016/j.plaphy.2014.03.028. [DOI] [PubMed] [Google Scholar]
- 158.Tong Z, Bischoff M, Nies L, Applegate B, Turco RF. Impact of fullerene (C60) on a soil microbial community. Environmental Science & Technology. 2007;41(8):2985–2991. doi: 10.1021/es061953l. [DOI] [PubMed] [Google Scholar]
- 159.Garner KL, Suh S, Lenihan HS, Keller AA. Species sensitivity distributions for engineered nanomaterials. Environmental Science & Technology. 2015;49(9):5753–5759. doi: 10.1021/acs.est.5b00081. [DOI] [PubMed] [Google Scholar]
- 160.Juganson K, Ivask A, Blinova I, Mortimer M, Kahru A. NanoE-Tox: New and in-depth database concerning ecotoxicity of nanomaterials. Beilstein Journal of Nanotechnology. 2015;6:1788–1804. doi: 10.3762/bjnano.6.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.von der Kammer F, Ferguson PL, Holden PA, Masion A, Rogers KR, Klaine SJ, Koelmans AA, Horne N, Unrine JM. Analysis of engineered nanomaterials in complex matrices (environment and biota): general considerations and conceptual case studies. Environmental Toxicology and Chemistry. 2012;31(1):32–49. doi: 10.1002/etc.723. [DOI] [PubMed] [Google Scholar]
- 162.Edgington AJ, Petersen EJ, Herzing AA, Podila R, Rao A, Klaine SJ. Microscopic investigation of single-wall carbon nanotube uptake by Daphnia magna. Nanotoxicology. 2014;8:2–10. doi: 10.3109/17435390.2013.847504. [DOI] [PubMed] [Google Scholar]
- 163.Edgington AJ, Roberts AP, Taylor LM, Alloy MM, Reppert J, Rao AM, Mao JD, Klaine SJ. The influence of natural organic matter on the toxicity of multiwalled carbon nanotubes. Environmental Toxicology and Chemistry. 2010;29(11):2511–2518. doi: 10.1002/etc.309. [DOI] [PubMed] [Google Scholar]
- 164.Servin AD, Morales MI, Castillo-Michel H, Hemandez-Viezcas JA, Munoz B, Zhao LJ, Nunez JE, Peralta-Videa JR, Gardea-Torresdey JL. Synchrotron verification of TiO2 accumulation in cucumber fruit: a possible pathway of TiO2 nanoparticle transfer from soil into the food chain. Environmental Science & Technology. 2013;47(20):11592–11598. doi: 10.1021/es403368j. [DOI] [PubMed] [Google Scholar]
- 165.Zhao LJ, Sun YP, Hernandez-Viezcas JA, Hong J, Majumdar S, Niu GH, Duarte-Gardea M, Peralta-Videa JR, Gardea-Torresdey JL. Monitoring the environmental effects of CeO2 and ZnO nanoparticles through the life cycle of corn (Zea mays) plants and in situ μ-XRF mapping of nutrients in kernels. Environmental Science & Technology. 2015;49(5):2921–2928. doi: 10.1021/es5060226. [DOI] [PubMed] [Google Scholar]
- 166.Holden PA, Schimel JP, Godwin HA. Five reasons to use bacteria when assessing manufactured nanomaterial environmental hazards and fates. Current Opinion in Biotechnology. 2014;27:73–78. doi: 10.1016/j.copbio.2013.11.008. (0) [DOI] [PubMed] [Google Scholar]
- 167.Ong KJ, MacCormack TJ, Clark RJ, Ede JD, Ortega VA, Felix LC, Dang MKM, Ma GB, Fenniri H, Veinot JGC, Goss GG. Widespread nanoparticle-assay interference: implications for nanotoxicity testing. Plos One. 2014;9(3):1–9. doi: 10.1371/journal.pone.0090650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Horst AM, Vukanti R, Priester JH, Holden PA. An assessment of fluorescence-and absorbance-based assays to study metal-oxide nanoparticle ROS production and effects on bacterial membranes. Small. 2013;9(9-10):1753–1764. doi: 10.1002/smll.201201455. [DOI] [PubMed] [Google Scholar]
- 169.Park S, Woodhall J, Ma GB, Veinot JGC, Cresser MS, Boxall ABA. Regulatory ecotoxicity testing of engineered nanoparticles: are the results relevant to the natural environment? Nanotoxicology. 2014;8(5):583–592. doi: 10.3109/17435390.2013.818173. [DOI] [PubMed] [Google Scholar]
- 170.Turco RF, Bischoff M, Tong ZH, Nies L. Environmental implications of nanomaterials: are we studying the right thing? Current Opinion in Biotechnology. 2011;22(4):527–532. doi: 10.1016/j.copbio.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 171.Schimel J, Chadwick O. What's in a name? The importance of soil taxonomy for ecology and biogeochemistry. Frontiers in Ecology and the Environment. 2013;11(8):405–406. [Google Scholar]
- 172.Sparks DL, Page AL, Helmke PA, Loeppert RH. Soil Science Society of America. American Society of Agronomy; Madison, WI: 1996. Methods of Soil Analysis Part 3—Chemical Methods. [Google Scholar]
- 173.Ji Z, Jin X, George S, Xia T, Meng H, Wang X, Suarez E, Zhang H, Hoek EMV, Godwin H, Nel AE, Zink JI. Dispersion and stability optimization of TiO2 nanoparticles in cell culture media. Environmental Science & Technology. 2010;44(19):7309–7314. doi: 10.1021/es100417s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Horst AM, Ji ZX, Holden PA. Nanoparticle dispersion in environmentally relevant culture media: a TiO2 case study and considerations for a general approach. Journal of Nanoparticle Research. 2012;14(8):1–14. [Google Scholar]
- 175.Maes HM, Stibany F, Giefers S, Daniels B, Deutschmann B, Baumgartner W, Schaffer A. Accumulation and distribution of multiwalled carbon nanotubes in zebrafish (Danio rerio) Environmental Science & Technology. 2014;48(20):12256–12264. doi: 10.1021/es503006v. [DOI] [PubMed] [Google Scholar]
- 176.Song L, Vijver MG, de Snoo GR, Peijnenburg W. Assessing toxicity of copper nanoparticles across five cladoceran species. Environmental Toxicology and Chemistry. 2015;34(8):1863–1869. doi: 10.1002/etc.3000. [DOI] [PubMed] [Google Scholar]
- 177.Tella M, Auffan M, Brousset L, Issartel J, Kieffer I, Pailles C, Morel E, Santaella C, Angeletti B, Artells E, Rose J, Thiery A, Bottero JY. Transfer, transformation, and impacts of ceria nanomaterials in aquatic mesocosms simulating a pond ecosystem. Environmental Science & Technology. 2014;48(16):9004–9013. doi: 10.1021/es501641b. [DOI] [PubMed] [Google Scholar]
- 178.Handy RD, von der Kammer F, Lead JR, Hassellov M, Owen R, Crane M. The ecotoxicology and chemistry of manufactured nanoparticles. Ecotoxicology. 2008;17(4):287–314. doi: 10.1007/s10646-008-0199-8. [DOI] [PubMed] [Google Scholar]
- 179.Pal S, Tak YK, Song JM. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Applied and Environmental Microbiology. 2007;73(6):1712–1720. doi: 10.1128/AEM.02218-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hjorth R, Sorensen SN, Olsson ME, Baun A, Hartmann NB. A certain shade of green: can algal pigments reveal shading effects of nanoparticles? Integrated Environmental Assessment and Management. 2016;12(1):200–202. doi: 10.1002/ieam.1728. [DOI] [PubMed] [Google Scholar]
- 181.Rasmussen K, González M, Kearns P, Sintes JR, Rossi F, Sayre P. Review of achievements of the OECD Working Party on Manufactured Nanomaterials' Testing and Assessment Programme. From exploratory testing to test guidelines. Regulatory Toxicology and Pharmacology. 2016;74:147–160. doi: 10.1016/j.yrtph.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 182.Sorensen SN, Hjorth R, Delgado CG, Hartmann NB, Baun A. Nanoparticle ecotoxicity - physical and/or chemical effects? Integrated Environmental Assessment and Management. 2015;11(4):722–724. [Google Scholar]
- 183.Calder AJ, Dimkpa CO, McLean JE, Britt DW, Johnson W, Anderson AJ. Soil components mitigate the antimicrobial effects of silver nanoparticles towards a beneficial soil bacterium, Pseudomonas chlororaphis O6. Science of the Total Environment. 2012;429:215–222. doi: 10.1016/j.scitotenv.2012.04.049. [DOI] [PubMed] [Google Scholar]
- 184.Kennedy AJ, Chappell MA, Bednar AJ, Ryan AC, Laird JG, Stanley JK, Steevens JA. Impact of organic carbon on the stability and toxicity of fresh and stored silver nanoparticles. Environmental Science & Technology. 2012;46(19):10772–10780. doi: 10.1021/es302322y. [DOI] [PubMed] [Google Scholar]
- 185.Zhu M, Nie G, Meng H, Xia T, Nel A, Zhao Y. Physicochemical properties determine nanomaterial cellular uptake, transport, and fate. Accounts of Chemical Research. 2012;46(3):622–631. doi: 10.1021/ar300031y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Rodrigues DF, Elimelech M. Toxic effects of single-walled carbon nanotubes in the development of E. coli biofilm. Environmental Science & Technology. 2010;44(12):4583–4589. doi: 10.1021/es1005785. [DOI] [PubMed] [Google Scholar]
- 187.Saleh NB, Chambers B, Aich N, Plazas-Tuttle J, Phung-Ngoc HN, Kirisits MJ. Mechanistic lessons learned from studies of planktonic bacteria with metallic nanomaterials: implications for interactions between nanomaterials and biofilm bacteria. Frontiers in Microbiology. 2015;6:1–8. doi: 10.3389/fmicb.2015.00677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Burns JM, Pennington PL, Sisco PN, Frey R, Kashiwada S, Fulton MH, Scott GI, Decho AW, Murphy CJ, Shaw TJ, Ferry JL. Surface charge controls the fate of Au nanorods in saline estuaries. Environmental Science & Technology. 2013;47(22):12844–12851. doi: 10.1021/es402880u. [DOI] [PubMed] [Google Scholar]
- 189.Ferry JL, Craig P, Hexel C, Sisco P, Frey R, Pennington PL, Fulton MH, Scott IG, Decho AW, Kashiwada S, Murphy CJ, Shaw TJ. Transfer of gold nanoparticles from the water column to the estuarine food web. Nature Nanotechnology. 2009;4(7):441–444. doi: 10.1038/nnano.2009.157. [DOI] [PubMed] [Google Scholar]
- 190.Furtado LM, Norman BC, Xenopoulos MA, Frost PC, Metcalfe CD, Hintelmann H. Environmental fate of silver nanoparticles in boreal lake ecosystems. Environmental Science & Technology. 2015;49(14):8441–8450. doi: 10.1021/acs.est.5b01116. [DOI] [PubMed] [Google Scholar]
- 191.Furtado LM, Hoque ME, Mitrano DF, Ranville JF, Cheever B, Frost PC, Xenopoulos MA, Hintelmann H, Metcalfe CD. The persistence and transformation of silver nanoparticles in littoral lake mesocosms monitored using various analytical techniques. Environmental Chemistry. 2014;11(4):419–430. [Google Scholar]
- 192.Cleveland D, Long SE, Pennington PL, Cooper E, Fulton MH, Scott GI, Brewer T, Davis J, Petersen EJ, Wood L. Pilot estuarine mesocosm study on the environmental fate of silver nanomaterials leached from consumer products. Science of the Total Environment. 2012;421:267–272. doi: 10.1016/j.scitotenv.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 193.Lowry GV, Espinasse BP, Badireddy AR, Richardson CJ, Reinsch BC, Bryant LD, Bone AJ, Deonarine A, Chae S, Therezien M, Colman BP, Hsu-Kim H, Bernhardt ES, Matson CW, Wiesner MR. Long-term transformation and fate of manufactured Ag nanoparticles in a simulated large scale freshwater emergent wetland. Environmental Science & Technology. 2012;46(13):7027–7036. doi: 10.1021/es204608d. [DOI] [PubMed] [Google Scholar]
- 194.Buffet PE, Richard M, Caupos F, Vergnoux A, Perrein-Ettajani H, Luna-Acosta A, Akcha F, Amiard JC, Amiard-Triquet C, Guibbolini M, Risso-De Faverney C, Thomas-Guyon H, Reip P, Dybowska A, Berhanu D, Valsami-Jones E, Mouneyrac C. A mesocosm study of fate and effects of CuO nanoparticles on endobenthic species (Scrobicularia plana, Hediste diversicolor) Environmental Science & Technology. 2013;47(3):1620–1628. doi: 10.1021/es303513r. [DOI] [PubMed] [Google Scholar]
- 195.Benn TM, Westerhoff P. Nanoparticle silver released into water from commercially available sock fabrics. Environmental Science & Technology. 2008;42(11):4133–4139. doi: 10.1021/es7032718. [DOI] [PubMed] [Google Scholar]
- 196.Kiser MA, Westerhoff P, Benn T, Wang Y, Perez-Rivera J, Hristovski K. Titanium nanomaterial removal and release from wastewater treatment plants. Environmental Science & Technology. 2009;43(17):6757–6763. doi: 10.1021/es901102n. [DOI] [PubMed] [Google Scholar]
- 197.Kiser MA, Ladner DA, Hristovski KD, Westerhoff PK. Nanomaterial transformation and association with fresh and freeze-dried wastewater activated sludge: implications for testing protocol and environmental fate. Environmental Science & Technology. 2012;46(13):7046–7053. doi: 10.1021/es300339x. [DOI] [PubMed] [Google Scholar]
- 198.Kaegi R, Voegelin A, Ort C, Sinnet B, Thalmann B, Krismer J, Hagendorfer H, Elumelu M, Mueller E. Fate and transformation of silver nanoparticles in urban wastewater systems. Water Research. 2013;47(12):3866–3877. doi: 10.1016/j.watres.2012.11.060. [DOI] [PubMed] [Google Scholar]
- 199.Kaegi R, Voegelin A, Sinnet B, Zuleeg S, Hagendorfer H, Burkhardt M, Siegrist H. Behavior of metallic silver nanoparticles in a pilot wastewater treatment plant. Environmental Science & Technology. 2011;45(9):3902–3908. doi: 10.1021/es1041892. [DOI] [PubMed] [Google Scholar]
- 200.Wang YF, Westerhoff P, Hristovski KD. Fate and biological effects of silver, titanium dioxide, and C-60 (fullerene) nanomaterials during simulated wastewater treatment processes. Journal of Hazardous Materials. 2012;201:16–22. doi: 10.1016/j.jhazmat.2011.10.086. [DOI] [PubMed] [Google Scholar]
- 201.Westerhoff PK, Kiser A, Hristovski K. Nanomaterial removal and transformation during biological wastewater treatment. Environmental Engineering Science. 2013;30(3):109–117. [Google Scholar]
- 202.Westerhoff P, Song GX, Hristovski K, Kiser MA. Occurrence and removal of titanium at full scale wastewater treatment plants: implications for TiO2 nanomaterials. Journal of Environmental Monitoring. 2011;13(5):1195–1203. doi: 10.1039/c1em10017c. [DOI] [PubMed] [Google Scholar]
- 203.Priester JH, Van De Werfhorst LC, Ge Y, Adeleye AS, Tomar S, Tom LM, Piceno YM, Andersen GL, Holden PA. Effects of TiO2 and Ag nanoparticles on polyhydroxybutyrate biosynthesis by activated sludge bacteria. Environmental Science & Technology. 2014;48(24):14712–14720. doi: 10.1021/es504117x. [DOI] [PubMed] [Google Scholar]
- 204.Yang Y, Zhang CQ, Hu ZQ. Impact of metallic and metal oxide nanoparticles on wastewater treatment and anaerobic digestion. Environmental Science-Processes & Impacts. 2013;15(1):39–48. doi: 10.1039/c2em30655g. [DOI] [PubMed] [Google Scholar]
- 205.Benn T, Cavanagh B, Hristovski K, Posner JD, Westerhoff P. The release of nanosilver from consumer products used in the home. Journal of Environmental Quality. 2010;39(6):1875–1882. doi: 10.2134/jeq2009.0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Weir A, Westerhoff P, Fabricius L, Hristovski K, von Goetz N. Titanium dioxide nanoparticles in food and personal care products. Environmental Science & Technology. 2012;46(4):2242–2250. doi: 10.1021/es204168d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kiser MA, Ryu H, Jang HY, Hristovski K, Westerhoff P. Biosorption of nanoparticles to heterotrophic wastewater biomass. Water Research. 2010;44(14):4105–4114. doi: 10.1016/j.watres.2010.05.036. [DOI] [PubMed] [Google Scholar]
- 208.Judy JD, McNear DH, Chen C, Lewis RW, Tsyusko OV, Bertsch PM, Rao W, Stegemeier J, Lowry GV, McGrath SP, Durenkamp M, Unrine JM. Nanomaterials in biosolids inhibit nodulation, shift microbial community composition, and result in increased metal uptake relative to bulk/dissolved metals. Environmental Science & Technology. 2015;49(14):8751–8758. doi: 10.1021/acs.est.5b01208. [DOI] [PubMed] [Google Scholar]
- 209.Evans TD. Biosolids in Europe. 26th WEF Residuals & Biosolids Conference; Raleigh, North Carolina, USA: Water Environment Federation; 2012. [Google Scholar]
- 210.Barton LE, Auffan M, Olivi L, Bottero JY, Wiesner MR. Heteroaggregation, transformation and fate of CeO2 nanoparticles in wastewater treatment. Environmental Pollution. 2015;203:122–129. doi: 10.1016/j.envpol.2015.03.035. [DOI] [PubMed] [Google Scholar]
- 211.Mosier AC, Li Z, Thomas BC, Hettich RL, Pan CL, Banfield JF. Elevated temperature alters proteomic responses of individual organisms within a biofilm community. ISME Journal. 2015;9(1):180–194. doi: 10.1038/ismej.2014.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Speed D, Westerhoff P, Sierra-Alvarez R, Draper R, Pantano P, Aravamudhan S, Chen KL, Hristovski K, Herckes P, Bi X, Yang Y, Zeng C, Otero-Gonzalez L, Mikoryak C, Wilson BA, Kosaraju K, Tarannum M, Crawford S, Yi P, Liu X, Babu SV, Moinpour M, Ranville J, Montano M, Corredor C, Posner J, Shadman F. Physical, chemical, and in vitro toxicological characterization of nanoparticles in chemical mechanical planarization suspensions used in the semiconductor industry: towards environmental health and safety assessments. Environmental Science-Nano. 2015;2(3):227–244. [Google Scholar]
- 213.Bandyopadhyay S, Peralta-Videa JR, Gardea-Torresdey JL. Advanced analytical techniques for the measurement of nanomaterials in food and agricultural samples: a review. Environmental Engineering Science. 2013;30(3):118–125. doi: 10.1089/ees.2012.0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Petersen EJ, Zhang LW, Mattison NT, O'Carroll DM, Whelton AJ, Uddin N, Nguyen T, Huang QG, Henry TB, Holbrook RD, Chen KL. Potential release pathways, environmental fate, and ecological risks of carbon nanotubes. Environmental Science & Technology. 2011;45(23):9837–9856. doi: 10.1021/es201579y. [DOI] [PubMed] [Google Scholar]
- 215.Mader BT, Ellefson ME, Wolf ST. Measurements of nanomaterials in environmentally relevant water matrices using liquid nebulization/differential mobility analysis. Environmental Toxicology and Chemistry. 2015;34(4):833–842. doi: 10.1002/etc.2865. [DOI] [PubMed] [Google Scholar]
- 216.Hartmann G, Baumgartner T, Schuster M. Influence of particle coating and matrix constituents on the cloud point extraction efficiency of silver nanoparticles (Ag-NPs) and application for monitoring the formation of Ag-NPs from Ag+ Analytical Chemistry. 2014;86(1):790–796. doi: 10.1021/ac403289d. [DOI] [PubMed] [Google Scholar]
- 217.Ojeda CB, Rojas FS. Separation and preconcentration by cloud point extraction procedures for determination of ions: recent trends and applications. Microchimica Acta. 2012;177(1-2):1–21. [Google Scholar]
- 218.McShane HVA, Sunahara GI, Whalen JK, Hendershot WH. Differences in soil solution chemistry between soils amended with nanosized CuO or Cu reference materials: implications for nanotoxicity tests. Environmental Science & Technology. 2014;48(14):8135–8142. doi: 10.1021/es500141h. [DOI] [PubMed] [Google Scholar]
- 219.Epel D, Luckenbach T, Stevenson CN, Macmanus-Spencer LA, Hamdoun A, Smital T. Efflux transporters: newly appreciated roles in protection against pollutants. Environmental Science & Technology. 2008;42(11):3914–3920. doi: 10.1021/es087187v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Vannuccini ML, Grassi G, Leaver MJ, Corsi I. Combination effects of nano-TiO2 and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on biotransformation gene expression in the liver of European sea bass Dicentrarchus labrax. Comparative Biochemistry and Physiology C-Toxicology & Pharmacology. 2015;176:71–78. doi: 10.1016/j.cbpc.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 221.NRC . Exposure Science in the 21st Century: A Vision and a Strategy. The National Academies Press; Washington, DC: 2012. p. 196. [PubMed] [Google Scholar]
- 222.Lioy P, Weisel C. Exposure Science: Basic Principles and Applications. Academic Press; Oxford: 2014. pp. 1–16. [Google Scholar]
- 223.U.S. Environmental Protection Agency . In: High-throughput exposure forecasting. Development, O. o. R. a., editor. U. S. Environmental Protection Agency; 2014. p. 2. [Google Scholar]
- 224.Blaser SA, Scheringer M, MacLeod M, Hungerbuhler K. Estimation of cumulative aquatic exposure and risk due to silver: Contribution of nano-functionalized plastics and textiles. Science of the Total Environment. 2008;390(2-3):396–409. doi: 10.1016/j.scitotenv.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 225.Scheringer M. Nanoecotoxicology - environmental risks of nanomaterials. Nature Nanotechnology. 2008;3(6):322–323. doi: 10.1038/nnano.2008.145. [DOI] [PubMed] [Google Scholar]
- 226.Johnston JM, Lowry M, Beaulieu S, Bowles E. In: State-of-the-science report on predictive models and modeling approaches for characterizing and evaluating exposure to nanomaterials. Ecosystems Research Division, N. E. R. L., editor. U. S. Environmental Protection Agency; Athens, GA: 2010. [Google Scholar]
- 227.Lazareva A, Keller AA. Estimating potential life cycle releases of engineered nanomaterials from wastewater treatment plants. ACS Sustainable Chemistry & Engineering. 2014;2(7):1656–1665. [Google Scholar]
- 228.Liu HH, Bilal M, Lazareva A, Keller A, Cohen Y. Simulation tool for assessing the release and environmental distribution of nanomaterials. Beilstein Journal of Nanotechnology. 2015;6:938–951. doi: 10.3762/bjnano.6.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Meesters JAJ, Koelmans AA, Quik JTK, Hendriks AJ, van de Meentt D. Multimedia modeling of engineered nanoparticles with SimpleBox4nano: model definition and evaluation. Environmental Science & Technology. 2014;48(10):5726–5736. doi: 10.1021/es500548h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Gottschalk F, Lassen C, Kjoelholt J, Christensen F, Nowack B. Modeling flows and concentrations of nine engineered nanomaterials in the Danish environment. Int. J. Environ. Res. Public Health. 2015;12(5):5581–602. doi: 10.3390/ijerph120505581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.MacLeod M, Scheringer M, McKone TE, Hungerbuhler K. The state of multimedia mass-balance modeling in environmental science and decision-making. Environmental Science & Technology. 2010;44(22):8360–8364. doi: 10.1021/es103297w. [DOI] [PubMed] [Google Scholar]
- 232.Ryan PA, Cohen Y. Multimedia transport of particle-bound organics - benzoapyrene test case. Chemosphere. 1986;15(1):21–47. [Google Scholar]
- 233.Cohen Y, Ryan PA. Multi-media modeling of environmental transport - trichloroethylene test case. Environmental Science & Technology. 1985;19(5):412–417. doi: 10.1021/es00135a004. [DOI] [PubMed] [Google Scholar]
- 234.Cohen Y. Organic pollutant transport. Environmental Science & Technology. 1986;20(6):538–544. doi: 10.1021/es00148a001. [DOI] [PubMed] [Google Scholar]
- 235.Utterback T. Innovation and industrial evolution in manufacturing industries. In: Guile BR, Brooks H, editors. Technology and Global Industry: Companies and Nations in the World Economy. The National Academies Press; Washington, DC: 1987. pp. 16–48. [Google Scholar]
- 236.Schnoor JL, Sato C, McKechnie D, Sahoo D. In: Processes, Coefficients, and Models for Simulating Toxic Organics and Heavy Metals in Surface Waters. Environmental Research Laboratory, O. o. R. a. D., editor. U.S. Environmental Protection Agency; Athens, GA: 1987. p. 303. [Google Scholar]
- 237.Sehgal A, Lalatonne Y, Berret JF, Morvan M. Precipitation-redispersion of cerium oxide nanoparticles with poly(acrylic acid): toward stable dispersions. Langmuir. 2005;21(20):9359–9364. doi: 10.1021/la0513757. [DOI] [PubMed] [Google Scholar]
- 238.Auffan M, Masion A, Labille J, Diot MA, Liu W, Olivi L, Proux O, Ziarelli F, Chaurand P, Geantet C, Bottero JY, Rose J. Long-term aging of a CeO2 based nanocomposite used for wood protection. Environmental Pollution. 2014;188:1–7. doi: 10.1016/j.envpol.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 239.Siriwardane IW. Adsorption of citric acid on cerium oxide nanoparticles (nanoceria) : effects of pH, surface charge and aggregation. University of Iowa; 2012. [Google Scholar]
- 240.Mudunkotuwa IA, Grassian VH. Citric acid adsorption on TiO2 nanoparticles in aqueous suspensions at acidic and circumneutral pH: surface coverage, surface speciation, and Its impact on nanoparticle-nanoparticle interactions. Journal of the American Chemical Society. 2010;132(42):14986–14994. doi: 10.1021/ja106091q. [DOI] [PubMed] [Google Scholar]
- 241.Bitter JL, Yang J, Beigzadeh Milani S, Jafvert CT, Fairbrother DH. Transformations of oxidized multiwalled carbon nanotubes exposed to UVC (254 nm) irradiation. Environmental Science: Nano. 2014;1(4):324–337. [Google Scholar]
- 242.Hou WC, Jafvert CT. Photochemical transformation of aqueous C-60 clusters in sunlight. Environmental Science & Technology. 2009;43(2):362–367. doi: 10.1021/es802465z. [DOI] [PubMed] [Google Scholar]
- 243.Flores-Cervantes DX, Maes HM, Schäffer A, Hollender J, Kohler H-PE. Slow biotransformation of carbon nanotubes by horseradish peroxidase. Environmental Science & Technology. 2014;48(9):4826–34. doi: 10.1021/es4053279. [DOI] [PubMed] [Google Scholar]
- 244.Lu K, Huang QG, Wang P, Mao L. Physicochemical changes of few-layer graphene in peroxidase-catalyzed reactions: characterization and potential ecological effects. Environmental Science & Technology. 2015;49(14):8558–8565. doi: 10.1021/acs.est.5b02261. [DOI] [PubMed] [Google Scholar]
- 245.Feng YP, Lu K, Mao L, Guo XK, Gao SX, Petersen EJ. Degradation of 14C-labeled few layer graphene via Fenton reaction: Reaction rates, characterization of reaction products, and potential ecological effects. Water Research. 2015;84:49–57. doi: 10.1016/j.watres.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 246.Zhang L, Petersen EJ, Habteselassie MY, Mao L, Huang Q. Degradation of multiwall carbon nanotubes by bacteria. Environmental Pollution. 2013;181(0):335–339. doi: 10.1016/j.envpol.2013.05.058. [DOI] [PubMed] [Google Scholar]
- 247.Levard C. m., Reinsch BC, Michel FM, Oumahi C, Lowry GV, Brown GE. Sulfidation processes of PVP-coated silver nanoparticles in aqueous solution: impact on dissolution rate. Environmental Science & Technology. 2011;45(12):5260–5266. doi: 10.1021/es2007758. [DOI] [PubMed] [Google Scholar]
- 248.Barton LE, Auffan M, Bertrand M, Barakat M, Santaella C, Masion A, Borschneck D, Olivi L, Roche N, Wiesner MR, Bottero JY. Transformation of pristine and citrate-functionalized CeO2 nanoparticles in a laboratory-scale activated sludge reactor. Environmental Science & Technology. 2014;48(13):7289–7296. doi: 10.1021/es404946y. [DOI] [PubMed] [Google Scholar]
- 249.Reed K, Cormack A, Kulkarni A, Mayton M, Sayle D, Klaessig F, Stadler B. Exploring the properties and applications of nanoceria: is there still plenty of room at the bottom? Environmental Science-Nano. 2014;1(5):390–405. [Google Scholar]
- 250.Avanasi R, Jackson WA, Sherwin B, Mudge JF, Anderson TA. C60 fullerene soil sorption, biodegradation, and plant uptake. Environmental Science & Technology. 2014;48(5):2792–2797. doi: 10.1021/es405306w. [DOI] [PubMed] [Google Scholar]
- 251.Ottofuelling S, von der Kammer F, Hofmann T. Commercial titanium dioxide nanoparticles in both natural and synthetic water: comprehensive multidimensional testing and prediction of aggregation behavior. Environmental Science & Technology. 2011;45(23):10045–10052. doi: 10.1021/es2023225. [DOI] [PubMed] [Google Scholar]
- 252.Nowack B, Baalousha M, Bornhoft N, Chaudhry Q, Cornelis G, Cotterill J, Gondikas A, Hassellov M, Lead J, Mitrano DM, von der Kammer F, Wontner-Smith T. Progress towards the validation of modeled environmental concentrations of engineered nanomaterials by analytical measurements. Environmental Science-Nano. 2015;2(5):421–428. [Google Scholar]
- 253.Keller AA, Lazareva A. Predicted releases of engineered nanomaterials: from global to regional to local. Environmental Science & Technology Letters. 2014;1(1):65–70. [Google Scholar]
- 254.Gottschalk F, Sun TY, Nowack B. Environmental concentrations of engineered nanomaterials: review of modeling and analytical studies. Environmental Pollution. 2013;181:287–300. doi: 10.1016/j.envpol.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 255.Lee S, Bi XY, Reed RB, Ranville JF, Herckes P, Westerhoff P. Nanoparticle size detection limits by single particle ICP-MS for 40 elements. Environmental Science & Technology. 2014;48(17):10291–10300. doi: 10.1021/es502422v. [DOI] [PubMed] [Google Scholar]
- 256.Montano MD, Lowry GV, von der Kammer F, Blue J, Ranville JF. Current status and future direction for examining engineered nanoparticles in natural systems. Environmental Chemistry. 2014;11(4):351–366. [Google Scholar]
- 257.Bustos ARM, Petersen EJ, Possolo A, Winchester MR. Post hoc interlaboratory comparison of single particle ICP-MS size measurements of NIST gold nanoparticle reference materials. Analytical Chemistry. 2015;87(17):8809–8817. doi: 10.1021/acs.analchem.5b01741. [DOI] [PubMed] [Google Scholar]
- 258.Sanchís J, Božović D, Al-Harbi NA, Silva LF, Farré M, Barceló D. Quantitative trace analysis of fullerenes in river sediment from Spain and soils from Saudi Arabia. Analytical and Bioanalytical Chemistry. 2013;405(18):5915–5923. doi: 10.1007/s00216-013-6924-z. [DOI] [PubMed] [Google Scholar]
- 259.Sanchís J, Berrojalbiz N, Caballero G, Dachs J, Farré M, Barceló D. Occurrence of aerosol-bound fullerenes in the Mediterranean sea atmosphere. Environmental Science & Technology. 2012;46(3):1335–1343. doi: 10.1021/es200758m. [DOI] [PubMed] [Google Scholar]
- 260.Farré M, Pérez S, Gajda-Schrantz K, Osorio V, Kantiani L, Ginebreda A, Barceló D. First determination of C60 and C70 fullerenes and N-methylfulleropyrrolidine C60 on the suspended material of wastewater effluents by liquid chromatography hybrid quadrupole linear ion trap tandem mass spectrometry. Journal of Hydrology. 2010;383(1-2):44–51. [Google Scholar]
- 261.Sanchís J, Oliveira LFS, de Leão FB, Farré M, Barceló D. Liquid chromatography-atmospheric pressure photoionization-Orbitrap analysis of fullerene aggregates on surface soils and river sediments from Santa Catarina (Brazil) Science of the Total Environment. 2015;505:172–179. doi: 10.1016/j.scitotenv.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 262.Luo ZX, Wang ZH, Li QZ, Pan QK, Yan CZ, Liu F. Spatial distribution, electron microscopy analysis of titanium and its correlation to heavy metals: occurrence and sources of titanium nanomaterials in surface sediments from Xiamen Bay, China. Journal of Environmental Monitoring. 2011;13(4):1046–1052. doi: 10.1039/c0em00199f. [DOI] [PubMed] [Google Scholar]
- 263.Barton LE, Therezien M, Auffan M, Bottero JY, Wiesner MR. Theory and methodology for determining nanoparticle affinity for heteroaggregation in environmental matrices using batch measurements. Environmental Engineering Science. 2014;31(7):421–427. [Google Scholar]
- 264.Praetorius A, Labille J, Scheringer M, Thill A, Hungerbuhler K, Bottero JY. Heteroaggregation of titanium dioxide nanoparticles with model natural colloids under environmentally relevant conditions. Environmental Science & Technology. 2014;48(18):10690–10698. doi: 10.1021/es501655v. [DOI] [PubMed] [Google Scholar]
- 265.Bouchard D, Chang X, Chowdhury I. Heteroaggregation of multiwalled carbon nanotubes with sediments. Environmental Nanotechnology, Monitoring & Management. 2015;4:42–50. [Google Scholar]
- 266.Sabo-Attwood T, Unrine JM, Stone JW, Murphy CJ, Ghoshroy S, Blom D, Bertsch PM, Newman LA. Uptake, distribution and toxicity of gold nanoparticles in tobacco (Nicotiana xanthi) seedlings. Nanotoxicolgy. 2012;6:353–360. doi: 10.3109/17435390.2011.579631. [DOI] [PubMed] [Google Scholar]
- 267.Novak PJ, Arnold WA, Blazer VS, Halden RU, Klaper RD, Kolpin DW, Kriebel D, Love NG, Martinovic-Weigelt D, Patisaul HB, Snyder SA, vom Saal FS, Weisbrod AV, Swackhamer DL. On the need for a national (US) research program to elucidate the potential risks to human health and the environment posed by contaminants of emerging concern. Environmental Science & Technology. 2011;45(9):3829–3830. doi: 10.1021/es200744f. [DOI] [PubMed] [Google Scholar]
- 268.Sturla SJ, Boobis AR, FitzGerald RE, Hoeng J, Kavlock RJ, Schirmer K, Whelan M, Wilks MF, Peitsch MC. Systems toxicology: from basic research to risk assessment. Chemical Research in Toxicology. 2014;27(3):314–329. doi: 10.1021/tx400410s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Nel A, Xia T, Meng H, Wang X, Lin SJ, Ji ZX, Zhang HY. Nanomaterial toxicity testing in the 21st century: use of a predictive toxicological approach and high-throughput screening. Accounts of Chemical Research. 2013;46(3):607–621. doi: 10.1021/ar300022h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Kashiwada S. Distribution of nanoparticles in the see-through medaka (Oryzias latipes) Environmental Health Perspectives. 2006;114(11):1697–1702. doi: 10.1289/ehp.9209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Doudrick K, Corson N, Oberdorster G, Eder AC, Herckes P, Halden RU, Westerhoff P. Extraction and quantification of carbon nanotubes in biological matrices with application to rat lung tissue. ACS Nano. 2013;7(10):8849–8856. doi: 10.1021/nn403302s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Arndt DA, Moua M, Chen J, Klaper RD. Core structure and surface functionalization of carbon nanomaterials alter impacts to daphnid mortality, reproduction, and growth: acute assays do not predict chronic exposure impacts. Environmental Science & Technology. 2013;47(16):9444–9452. doi: 10.1021/es4030595. [DOI] [PubMed] [Google Scholar]
- 273.Ivask A, ElBadawy A, Kaweeteerawat C, Boren D, Fischer H, Ji ZX, Chang CH, Liu R, Tolaymat T, Telesca D, Zink JI, Cohen Y, Holden PA, Godwin HA. Toxicity mechanisms in Escherichia coli vary for silver nanoparticles and differ from ionic silver. ACS Nano. 2014;8(1):374–386. doi: 10.1021/nn4044047. [DOI] [PubMed] [Google Scholar]
- 274.Maurer-Jones MA, Gunsolus IL, Meyer BM, Christenson CJ, Haynes CL. Impact of TiO2 nanoparticles on growth, biofilm formation, and flavin secretion in Shewanella oneidensis. Analytical Chemistry. 2013;85(12):5810–5818. doi: 10.1021/ac400486u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.OECD . Guidance Document on Aquatic Toxicity Testing of Difficult Substances and Mixtures. OECD Publishing; Paris: 2002. p. 53. [Google Scholar]
- 276.Boncel S, Kyziol-Komosinska J, Krzyewska I, Czupiol J. Interactions of carbon nanotubes with aqueous/aquatic media containing organic/inorganic contaminants and selected organisms of aquatic ecosystems - A review. Chemosphere. 2015;136:211–221. doi: 10.1016/j.chemosphere.2015.04.095. [DOI] [PubMed] [Google Scholar]
- 277.Auffan M, Bertin D, Chaurand P, Pailles C, Dominici C, Rose J, Bottero J-Y, Thiery A. Role of molting on the biodistribution of CeO2 nanoparticles within Daphnia pulex. Water Research. 2013;47(12):3921–3930. doi: 10.1016/j.watres.2012.11.063. [DOI] [PubMed] [Google Scholar]
- 278.Long Z, Ji J, Yang K, Lin D, Wu F. Systematic and quantitative investigation of the mechanism of carbon nanotubes' toxicity toward algae. Environmental Science & Technology. 2012;46(15):8458–8466. doi: 10.1021/es301802g. [DOI] [PubMed] [Google Scholar]
- 279.Zhang H, Ji Z, Xia T, Meng H, Low-Kam C, Liu R, Pokhrel S, Lin S, Wang X, Liao Y-P, Wang M, Li L, Rallo R, Damoiseaux R, Telesca D, Mädler L, Cohen Y, Zink JI, Nel AE. Use of metal oxide nanoparticle band gap to develop a predictive paradigm for oxidative stress and acute pulmonary inflammation. ACS Nano. 2012;6(5):4349–4368. doi: 10.1021/nn3010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.U.S. Environmental Protection Agency Ecological Risk Assessment [September 6, 2015]; http://www.epa.gov/risk/ecological-risk.htm.
- 281.Ankley GT, Bennett RS, Erickson RJ, Hoff DJ, Hornung MW, Johnson RD, Mount DR, Nichols JW, Russom CL, Schmieder PK, Serrrano JA, Tietge JE, Villeneuve DL. Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environmental Toxicology and Chemistry. 2010;29(3):730–741. doi: 10.1002/etc.34. [DOI] [PubMed] [Google Scholar]
- 282.Kramer VJ, Etterson MA, Hecker M, Murphy CA, Roesijadi G, Spade DJ, Spromberg JA, Wang M, Ankley GT. Adverse outcome pathways and ecological risk assessment bridging to population-level effects. Environmental Toxicology and Chemistry. 2011;30(1):64–76. doi: 10.1002/etc.375. [DOI] [PubMed] [Google Scholar]
- 283.Jager T, Zimmer EI. Simplified dynamic energy budget model for analysing ecotoxicity data. Ecological Modelling. 2012;225:74–81. [Google Scholar]
- 284.Klanjscek T, Nisbet RM, Priester JH, Holden PA. Modeling physiological processes that relate toxicant exposure and bacterial population dynamics. PLoS ONE. 2012;7(2):1–12. doi: 10.1371/journal.pone.0026955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Klanjscek T, Nisbet RM, Priester JH, Holden PA. Dynamic energy budget approach to modeling mechanisms of CdSe quantum dot toxicity. Ecotoxicology. 2013;22(2):319–330. doi: 10.1007/s10646-012-1028-7. [DOI] [PubMed] [Google Scholar]
- 286.Schierz A, Parks AN, Washburn KM, Chandler GT, Ferguson PL. Characterization and quantitative analysis of single-walled carbon nanotubes in the aquatic environment using near-infrared fluorescence Spectroscopy. Environmental Science & Technology. 2012;46(22):12262–12271. doi: 10.1021/es301856a. [DOI] [PubMed] [Google Scholar]
- 287.Bisesi JH, Merten J, Liu K, Parks AN, Afrooz A, Glenn JB, Klaine SJ, Kane AS, Saleh NB, Ferguson PL, Sabo-Attwood T. Tracking and quantification of single-walled carbon nanotubes in fish using near infrared fluorescence. Environmental Science & Technology. 2014;48(3):1973–1983. doi: 10.1021/es4046023. [DOI] [PubMed] [Google Scholar]
- 288.Schierz A, Espinasse B, Wiesner MR, Bisesi JH, Sabo-Attwood T, Ferguson PL. Fate of single walled carbon nanotubes in wetland ecosystems. Environmental Science-Nano. 2014;1(6):574–583. [Google Scholar]
- 289.Parks AN, Chandler GT, Ho KT, Burgess RM, Ferguson PL. Environmental biodegradability of 14C single-walled carbon nanotubes by Trametes veriicolor and natural microbial cultures found in New Bedford Harbor sediment and aerated wastewater treatment plant sludge. Environmental Toxicology and Chemistry. 2015;34(2):247–251. doi: 10.1002/etc.2791. [DOI] [PubMed] [Google Scholar]
- 290.Parks AN, Portis LM, Schierz PA, Washburn KM, Perron MM, Burgess RM, Ho KT, Chandler GT, Ferguson PL. Bioaccumulation and toxicity of single-walled carbon nanotubes to benthic organisms at the base of the marine food chain. Environmental Toxicology and Chemistry. 2013;32(6):1270–1277. doi: 10.1002/etc.2174. [DOI] [PubMed] [Google Scholar]
- 291.Parks AN, Burgess RM, Ho KT, Ferguson PL. On the likelihood of single-walled carbon nanotubes causing adverse marine ecological effects. Integrated Environmental Assessment and Management. 2014;10(3):472–474. [Google Scholar]
- 292.Ferguson PL, Chandler GT, Templeton RC, Demarco A, Scrivens WA, Englehart BA. Influence of sediment-amendment with single-walled carbon nanotubes and diesel soot on bioaccumulation of hydrophobic organic contaminants by benthic invertebrates. Environmental Science & Technology. 2008;42(10):3879–3885. doi: 10.1021/es702830b. [DOI] [PubMed] [Google Scholar]
- 293.Parks AN, Chandler GT, Portis LM, Sullivan JC, Perron MM, Cantwell MG, Burgess RM, Ho KT, Ferguson PL. Effects of single-walled carbon nanotubes on the bioavailability of PCBs in field-contaminated sediments. Nanotoxicology. 2014;8:111–117. doi: 10.3109/17435390.2013.858794. [DOI] [PubMed] [Google Scholar]
- 294.Warheit DB, Donner EM. How meaningful are risk determinations in the absence of a complete dataset? Making the case for publishing standardized test guideline and 'no effect' studies for evaluating the safety of nanoparticulates versus spurious 'high effect' results from single investigative studies. Science and Technology of Advanced Materials. 2015;16(3):1–5. doi: 10.1088/1468-6996/16/3/034603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Oomen AG, Bleeker EAJ, Bos PMJ, van Broekhuizen F, Gottardo S, Groenewold M, Hristozov D, Hund-Rinke K, Irfan MA, Marcomini A, Peijnenburg W, Rasmussen K, Jimenez AS, Scott-Fordsmand JJ, van Tongeren M, Wiench K, Wohlleben W, Landsiedel R. Grouping and read-across approaches for risk assessment of nanomaterials. Int. J. Environ. Res. Public Health. 2015;12(10):13415–13434. doi: 10.3390/ijerph121013415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Walser T, Studer C. Sameness: The regulatory crux with nanomaterial identity and grouping schemes for hazard assessment. Regulatory Toxicology and Pharmacology. 2015;72(3):569–571. doi: 10.1016/j.yrtph.2015.05.031. [DOI] [PubMed] [Google Scholar]
- 297.OECD . Series on the Safety of Manufactured Nanomaterials. OECD; Paris: 2015. Developments in Delegations on the Safety of Manufactured Nanomaterials - Tour De Table. [Google Scholar]
- 298.Hristozov DR, Zabeo A, Foran C, Isigonis P, Critto A, Marcomini A, Linkov I. A weight of evidence approach for hazard screening of engineered nanomaterials. Nanotoxicology. 2014;8(1):72–87. doi: 10.3109/17435390.2012.750695. [DOI] [PubMed] [Google Scholar]
- 299.Environment Canada . In: Screening Assessment for the Challenge: Carbon Black. Canada H, editor. Government of Canada; 2013. p. 88. [Google Scholar]
- 300.Nel AE, Parak WJ, Chan WCW, Xia T, Hersam MC, Brinker CJ, Zink JI, Pinkerton KE, Baer DR, Weiss PS. Where are we heading in nanotechnology environmental health and safety and materials characterization? ACS Nano. 2015;9(6):5627–5630. doi: 10.1021/acsnano.5b03496. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.