Abstract
Cisplatin, a wildly used chemotherapy drug, induces nephrotoxicity that is characterized by renal tubular cell apoptosis. In response to toxicity, tubular cells can activate cytoprotective mechanisms, such as the heat shock response. However, the role and regulation of the heat shock response in cisplatin-induced nephrotoxicity remain largely unclear. In the present study, we demonstrated the induction of heat shock factor (Hsf)1 and the small heat shock protein crystallin-αB (CryAB) during cisplatin nephrotoxicity in mice. Consistently, cisplatin induced Hsf1 and CryAB in a cultured renal proximal tubular cells (RPTCs). RPTCs underwent apoptosis during cisplatin treatment, which was increased when Hsf1 was knocked down. Transfection or restoration of Hsf1 into Hsf1 knockdown cells suppressed cisplatin-induced apoptosis, further supporting a cytoprotective role of Hsf1 and its associated heat shock response. Moreover, Hsf1 knockdown increased Bax translocation to mitochondria and cytochrome c release into the cytosol. In RPTCs, Hsf1 knockdown led to a specific downregulation of CryAB. Transfection of CryAB into Hsf1 knockdown cells diminished their sensitivity to cisplatin-induced apoptosis, suggesting that CryAB may be a key mediator of the cytoprotective effect of Hsf1. Taken together, these results demonstrate a heat shock response in cisplatin nephrotoxicity that is mediated by Hsf1 and CryAB to protect tubular cells against apoptosis.
Keywords: cisplatin nephrotoxicity, crystallin-αB, heat shock factor 1, heat shock response, renal tubular cell
cisplatin has been widely used for cancer therapy (8, 32, 35). However, the side effects of cisplatin, particularly nephrotoxicity in the kidneys, remain a major factor that limits the use and efficacy of cisplatin in chemotherapy (4, 9, 18, 24, 26, 27, 30). Renal disorders, especially acute kidney injury, occur in 20–30% of cisplatin-treated patients. It is recognized that renal tubules are the major sites of cell injury and death during cisplatin nephrotoxicity. Tubular cell apoptosis is widespread in kidney tissues of cisplatin nephrotoxicity, and the intrinsic pathway, characterized by Bax accumulation in mitochondria resulting in cytochrome c release, plays a critical role in cisplatin-induced apoptosis in renal tubular cells (4, 9, 14, 18, 19, 24, 26, 27, 30).
In response to injury or stress, mammalian cells may activate a myriad of adaptive or cytoprotective mechanisms. For example, in response to hypoxia or ischemia, cells of various origins, including the kidneys, may induce hypoxia-inducible factors that lead to the transcription of a series of genes for cellular adaptations to the condition of oxygen deficiency (12, 31). More recently, autophagy has been demonstrated to be an early response of kidney cells to acute injury, including cisplatin nephrotoxicity (16, 29, 41); upon activation, autophagy may protect tubular cells and promote their survival (17, 21). The heat shock response is a classic pathway in mammalian cells that facilitates their adaptation to, and survival from, a variety of stressful or injurious conditions (2, 3, 11).
The heat shock response consists of two main layers of molecular players: heat shock factors (Hsfs) and heat shock proteins (Hsps) (2, 3, 11). Hsfs are transcription factors for Hsps. Upon cell stress, Hsfs are activated to bind specifically to heat shock sequence elements of Hsp genes, leading to the transcription and expression of Hsps. There are several Hsfs, among which Hsf1 is ubiquitously expressed in mammalian tissues and functions as the major transcription factor for Hsp induction (2, 3, 11). Hsps are a specific group of highly conserved molecular chaperones that promote the proper folding, transport, subcellular localization, and activity of client proteins. Named according to their molecular weight, common Hsps include Hsp90, Hsp70, and small Hsps such as Hsp20 and crystallin-αB (CryAB) (2, 3, 11). The heat shock response, including both Hsfs and Hsps, plays important roles in kidney development and physiology as well as the pathogenesis of a myriad of renal diseases (5, 7). Hsf1-dependent expression of selective Hsps was suggested to be required for normal renal homeostasis and to protect renal cells against oxidative stress in vivo under physiological conditions (40). In experimental models of renal ischemia-reperfusion, Hsf1 and Hsf2 were activated primarily by metabolic stresses associated with ATP depletion and might protect against renal tubular cell injury (10). However, Hsf1 mRNA was not induced during renal ischemia-reperfusion in rats in another study (1). Moreover, a recent study (33) showed that Hsf1 knockout mice were more resistant to ischemic renal injury probably due to the presence of more T regulatory cells and that tubular injury appeared similar in wild-type and Hsf1 knockout mice, negating the protective role of Hsf1 and the associated heat shock response. Until now, the role and regulation of Hsf1 in cisplatin nephrotoxicity remain unknown.
In the present study, we demonstrated the induction of Hsf1 and CryAB during cisplatin nephrotoxicity in mice and in cultured renal proximal tubular cells (RPTCs). Gene knockdown (KD) and expression experiments further verified the cytoprotective role of Hsf1 in renal tubular cells. We further demonstrated that CryAB induction by cisplatin depends on Hsf1 and that the expression of CryAB in Hsf1 KD cells may decrease cisplatin injury. Taken together, these results demonstrate a heat shock response in cisplatin nephrotoxicity that is mediated by Hsf1 and CryAB to protect tubular cells against apoptosis.
MATERIALS AND METHODS
Materials.
The rat kidney proximal tubular cell line (RPTCs) was originally obtained from Dr. Hopfer (Case Western Reserve University, Cleveland, OH) and maintained as previously described (6, 28, 39). Rabbit monoclonal antibodies, including anti-Hsf1, anti-poly(ADP-ribose) polymerase (PARP), anti-caspase3, anti-Hsp70, anti-Hsp90, and anti-hemagglutinin, were purchased from Cell Signaling Technology (Beverly, MA). Polyclonal rabbit anti-CryAB was from Santa Cruz Technology (Lexington, Kentucky). Mouse monoclonal anti-β-actin antibody was from Sigma (St. Louis, MO). Horseradish peroxidase-conjugated secondary antibodies against mouse or rabbit IgG were from Jackson ImmunoResearch (West Grove, PA). Cisplatin was purchased from Sigma. Carbobenzoxy-Asp-Glu-Val-Asp-7-amino-4-trifluoromethylcoumarin (DEVD.AFC) and 7-amino-4-trifluoromethylcoumarin (AFC) for the caspase assay were purchased from Enzyme Systems Products (Dublin, CA).
Animals.
C57BL/6 mice (∼8 wk old) were maintained in the animal facility of Charlie Norwood Veterans Affairs Medical Center (Augusta, GA). Animal experiments were conducted according to a protocol approved by the Institutional Animal Care and Use Committee of the Charlie Norwood Veterans Affairs Medical Center and Medical College of Georgia.
Cell culture.
RPTCs were plated at a density of 1 × 106 cells/dish in 35-mm dishes to reach confluence by the next day. After cells had reached confluence, they were treated with the indicated amounts of cisplatin. All RPTCs were maintained in Ham's F-12-DMEM supplemented with 10% FBS, 5 μg/ml transferrin, 5 μg/ml insulin, 1 ng/ml EGF, 4 μg/ml dexamethasone, and 1% antibiotics.
Examination of apoptotic cell morphology.
Apoptotic cells were identified by their morphology, as previously described (6, 28, 39). Briefly, after cisplatin treatment, cells were incubated in media containing 10 μg/ml Hoechst 33342 for 2∼5 min and then photographed under phase-contrast and fluorescence microscopy. Typical apoptotic cell morphology included a shrunken configuration, apoptotic blebs or bodies, and a condensed and fragmented nucleus. Four fields of ∼200 cells were randomly selected in each dish for apoptotic cell counting. Student's t-test was used to determine the significance of the differences between cisplatin-treated and control groups. Representative images were also recorded.
Caspase activity.
Caspase activity in the cell lysate was measured using a DEVD.AFC kit (6, 28, 39). Briefly, cells were lysed in buffer containing 10 mM Tris·HCl, 10 mM NaH2PO4/NaHPO4 (pH 7.5), 130 mM NaCl, and 1% Triton X-100. Protein (25 μg) was incubated with enzymatic reaction buffer containing 50 μM DEVD.AFC. After a 1-h incubation at 37°C, fluorescence intensity was measured at an excitation wavelength of 360 nm and an emission wavelength of 530 nm. To indicate caspase activity, the fluorescence reading from each sample was calculated into the nanomolar amount of liberated AFC per milligram protein, based on a standard curve constructed using free AFC.
Transfections.
RPTCs were transfected with Hsf1 small hairpin (sh)RNA according to previously published methods (42). Briefly, cells were plated in 35-mm dishes at a density of 5 × 105 cells/well and allowed to adhere for 20–24 h. The Hsf1 shRNA plasmids were purchased from Qiagen. The shRNA target sequence for rat Hsf1 was 5′-TGTCAACAAGCTCATCCAATT-3′. Scrambled shRNA (2 μg) or Hsf1-specific shRNA was mixed with 2 μl of plus regents and 6 μl of Lipofectamine LTX (Invitrogen) in 0.5 ml of Opti-MEM (Invitrogen) for 20 min. Cells were incubated for 4 h with the prepared shRNA medium. In the next day, cultures were washed once with PBS, and fresh RPTC medium was added. The transfected cells were selected in 400 μg/ml Hygromycin for 2 wk in the selection medium, which was replaced every 2 days. Well-separated antibiotic resistant clones were individually picked with cloning discs and transferred to 24-well plates in selection medium. Cells were then transferred to larger culture vessels before confluence, and aliquots of early passages of cells were frozen in liquid nitrogen.
Cellular fractionation for the analysis of protein distribution.
Cytosolic and membrane-bound organelle fractions were prepared using digitonin buffer, as previously described (28, 43). Briefly, after treatment, cells were exposed to 0.05% digitonin in isotonic buffer (250 mM sucrose, 10 mM HEPES, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, and 1 mM EGTA; pH 7.1) for 2 min at room temperature. The soluble fraction was collected as the cytosolic extract. The insoluble part was washed with isotonic buffer and then dissolved in 2% SDS buffer to collect the membrane-bound organelle fraction. Digitonin-soluble and -insoluble fractions were analyzed for immunoblot analysis of cytochrome c, Bax, CryAB, and the other indicated proteins.
Nuclear and cytoplasmic fractions were prepared using Nuclear and Cytoplasmic Extraction Reagents (Thermo Scientific) according to the manufacturer's instruction.
Immunoblot analysis.
Cells were plated on 35-mm dishes and allowed to adhere and grow for overnight for treatment. At the end of treatment, cells were rinsed once with ice-cold PBS and then lysed in lysis buffer containing 62.5 mmol/l Tris·HCl (pH 7.4), 2% (wt/vol) SDS, 10% glycerol, Protease cocktail (Roche), and Benzonase. The total protein concentration of supernatants was estimated by the bicinchoninic acid method. Aliquots containing 30 μg protein were separated by one-dimensional SDS-PAGE, transferred to polyvinylidene difluoride membrants, probed with antibodies, and visualized by enhanced chemiluminescence, as previously described (36).
Statistical analyses.
Results are presented as means ± SE. Data were analyzed by Student's t-test or two-way ANOVA. P values of <0.05 were considered statistically significant.
RESULTS
Activation of the heat shock response during cisplatin-induced kidney injury in mice.
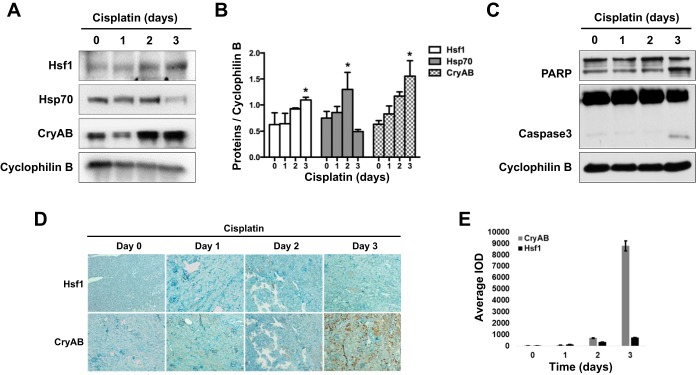
To determine whether the heat shock response is activated in cisplatin nephrotoxicity, mice were treated with cisplatin for 1, 2, or 3 days. Kidney cortical tissues mainly containing renal tubules were collected for analysis. In the immunoblot analysis (Fig. 1A), Hsf1 was induced at day 2 of cisplatin treatment, and the induction became more evident at day 3. Hsp70 showed a marginal induction at day 2 but then decreased at day 3 of cisplatin treatment. Renal expression of Hsp27 and Hsp90 was not affected by cisplatin (data not shown). In contrast, the small Hsp CryAB showed a continuous induction after 2 and 3 days of cisplatin treatment (Fig. 1, A and B). Consistent with previous work, cisplatin induced significant renal apoptosis at day 3 of cisplatin treatment, as shown by the increase of PARP cleavage and appearance of cleaved/active caspase 3 (Fig. 1C). We further verified Hsf1 and CryAB induction during cisplatin treatment by immunohistochemistry. As shown in Fig. 1, D and E, CryAB showed a remarkable increase at day 3 in cisplatin treatment tissues, whereas Hsf1 induction was moderate. Taken together, these results suggest that the heat shock response is activated during cisplatin nephrotoxicity.
Fig. 1.
Activation of the heat shock response in cisplatin-induced nephrotoxicity in mice. B6 mice were given 30 mg/kg cisplatin via one intraperitoneal injection. Animals were then euthanized at days 1, 2, and 3 after the injection. A: immunoblot analysis of the heat shock response in kidney tissues during cisplatin treatment of mice. Kidney cortical tissue lysate was collected for immunoblot analysis of heat shock factor (Hsf)1, heat shock protein (Hsp)70, crystallin-αB (CryAB), and cyclophilin B (internal loading control). B: quantification and statistical analysis of Hsf1, Hsp70, and CryAB proteins were performed using ImageJ software and two-way ANOVA. Data are expressed as means ± SD; n = 3. *P < 0.05 vs. the other three time points of cisplatin treatment. C: immunoblot analysis of apoptotic poly(ADP-ribose) polymerase (PARP) and caspase 3 cleavage. Kidney tissue lysate was subjected to immunoblot analysis of PARP, caspase 3, and cyclophilin B. D: immunohistochemical staining of Hsf1 and CryAB in kidney tissues. Kidney tissue sections were subjected to immunohistochemical staining using a streptavidin-biotin system and the antibodies specific to anti-CryAB and anti-Hsf1 antibodies. E: quantification of the immunohistochemical staining of Hsf1 and CryAB in kidney tissues. The average integrated optical density (IOD) value of each slide was calculated using Image-Pro plus 6.0 software.
Hsf1 activation and CryAB induction during cisplatin treatment of RPTCs.
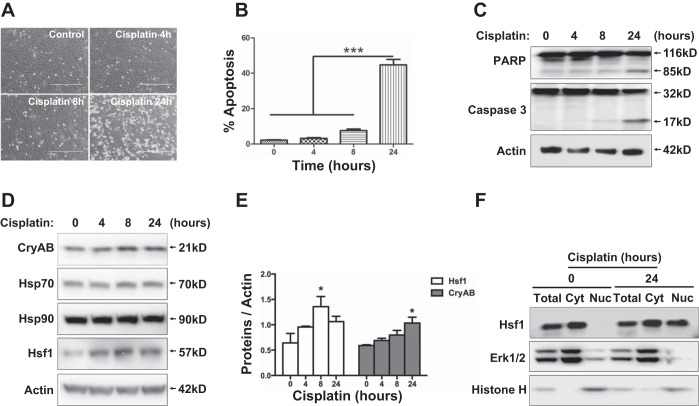
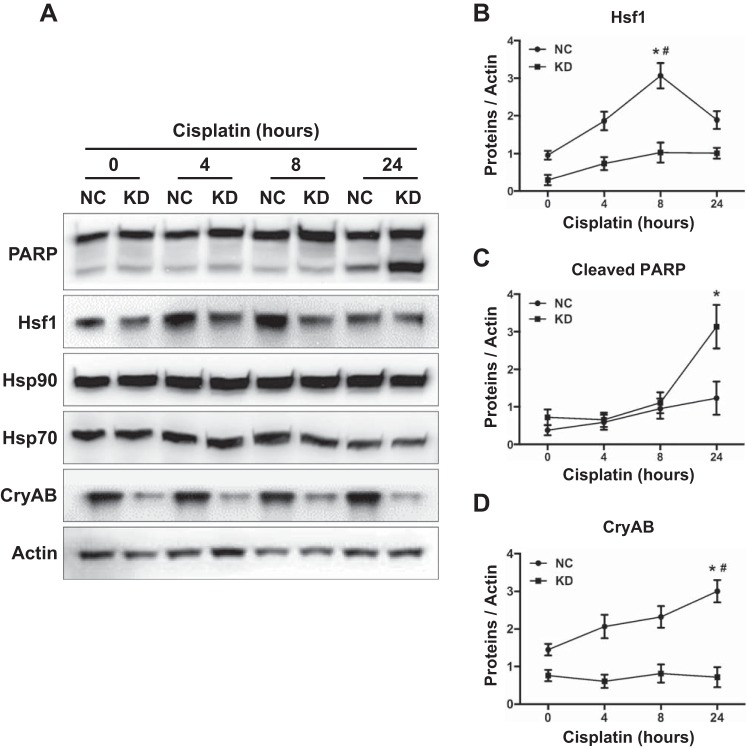
To further examine the role and regulation of the heat shock response in cisplatin nephrotoxicity, we used an in vitro model of RPTCs, a rat kidney proximal tubular cell line. RPTCs were treated with cisplatin for 0, 4, 8, and 24 h. Cisplatin induced apoptosis in RPTCs in a treatment time-dependent manner. Very little or limited apoptosis was induced by cisplatin during the first 8 h, but at 24 h of treatment, 45% of RPTCs underwent apoptosis (Fig. 2, A and B). The morphological observation was verified by immunoblot analysis of PARP and caspase 3 cleavage (Fig. 2C), further confirming cisplatin-induced apoptosis. Interestingly, Hsf1 was induced by cisplatin at the earliest time point of analysis, i.e., 4 h, and the induction continued to 24 h (Fig. 2D). Cisplatin also induced CryAB, which became notable at 8–24 h. In contrast, the induction of Hsp70 was minimal at 8–24 h of cisplatin treatment, and no induction was shown for Hsp90 (Fig. 2, D and E). A characteristic feature of Hsf1 activation is its translocation to the nucleus to bind to target gene promoter sequences (2, 3, 11). Thus, to further examine Hsf1 activation, we determined if Hsf1 accumulates in the nucleus during cisplatin treatment. As shown in Fig. 2F, very little Hsf1 was detected in the nuclear fraction of control (0 h of cisplatin exposure) cells, but after 24 h of cisplatin treatment, significant amounts of Hsf1 appeared in the nuclear fraction. These results, together with the in vivo data shown in Fig. 1, demonstrate the activation of the heat shock response in cisplatin nephrotoxicity, which is mainly characterized by Hsf1 activation and CryAB induction.
Fig. 2.
Hsf1 activation and CryAB induction during cisplatin treatment of renal proximal tubular cells (RPTCs). RPTCs were incubated with 20 μM cisplatin for 0, 4, 8, and 24 h. A: representative images of cell morphology. B: percentage of apoptosis evaluated by counting cells with typical apoptotic morphology (***P < 0.001). C and D: whole cell lysates were collected for immunoblot analysis of PARP and caspase 3 (C) or Hsf1, CryAB, Hsp70, and Hsp90 (D). The same blots were reprobed for β-actin to control sample loading and transferring. E: quantification and statistical analysis of Hsf1 and CryAB proteins were performed using ImageJ software and two-way ANOVA. Data are expressed as means ± SD; n = 3. *P < 0.05 vs. the other three time points of cisplatin treatment. F: nuclear accumulation of Hsf1 during cisplatin treatment of RPTCs. RPTCs were untreated or treated with cisplatin for 24 h. Whole cell lysates or cytosolic (Cyt) and nuclear fractions of the cells were collected for immunoblot analysis of Hsf1, Erk1/2 (cytosolic control), and histone H (nuclear control).
Hsf1 is protective during cisplatin treatment of RPTCs.
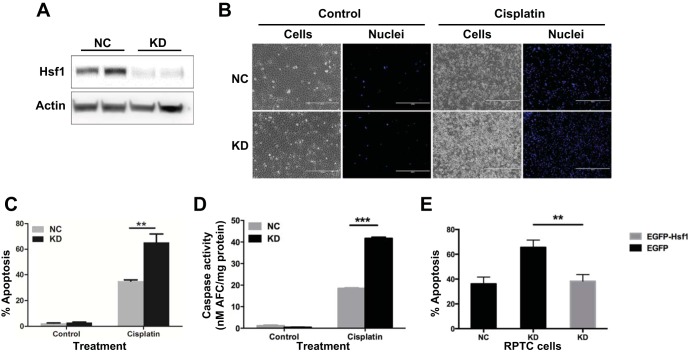
Whether Hsf1 and its associated heat shock response protects against ischemia kidney injury remains controversial, and their role in cisplatin nephrotoxicity has not been examined. To this end, we first determined the effect of Hsf1 KD on cisplatin-induced apoptosis in RPTCs. Hsf1 shRNA or the scrambled sequence was stably transfected and expressed in RPTCs to generate cell lines for comparison. The expression level of Hsf1 in Hsf1 shRNA-transfected (i.e., KD) cells was decreased by ∼85% compared with that in scrambled sequence-transfected [negative control (NC)] cells (Fig. 3A). These cells were treated with 20 μM cisplatin for 24 h to initially analyze apoptosis by morphology. Obviously, Hsf1 KD cells developed significantly more apoptosis during cisplatin treatment than NC cells (Fig. 3B). Quantification of apoptotic cells further verified this conclusion: cisplatin treatment induced 58% apoptosis in KD cells but only 33% in NC cells (Fig. 3C). We further analyzed caspase activity and showed that significantly higher caspase activation was induced by cisplatin in KD cells versus NC cells (Fig. 3D). Finally, we tested the effect of Hsf1 overexpression. As shown in Fig. 3E, cisplatin induced ∼65% apoptosis in Hsf1 KD cells, which was significantly higher than that of NC cells. Notably, transient transfection of enhanced green fluorescent protein-Hsf1 could significantly reduce the apoptosis of Hsf1 KD cells to ∼40%. Taken together, both Hsf1 KD and overexpression experiments supported a cytoprotective role of Hsf1 and its associated heat shock response in cisplatin nephrotoxicity.
Fig. 3.
Hsf1 knockdown (KD) increases apoptosis during cisplatin treatment. A: verification of Hsf1 knockdown via short hairpin (sh)RNA stable transfection. RPTCs were transfected with a Hsf1 shRNA plasmid or scrambled sequence plasmid containing a hygromycin-resistance cassette. After hygromycin selection, resistant colonies were identified and expanded. The selected cells were analyzed for the expression of Hsf1 and β-actin by immunoblot analysis. NC, negative control cells transfected with scrambled sequence. B: higher apoptosis in Hsf1 KD cells induced by cisplatin: cell and nuclear morphology. NC or KD cells were incubated with or without 20 μM cisplatin. Cells were then fixed with 4% paraformaldehyde and stained with Hoechst 33342. Representative images of cell morphology and nuclear staining of the same fields of cells were recorded. C: higher apoptosis in Hsf1 KD cells induced by cisplatin: counting of cells with typical apoptotic morphology. **P < 0.01. D: higher apoptosis in Hsf1 KD cells induced by cisplatin: caspase activity measured by enzymatic assays. Data are expressed as means ± SD; n = 3. ***Statistically significantly different between NC and KD cells treated with cisplatin (P < 0.001). E: suppression of apoptosis in Hsf1 KD cells by reconstitution of Hsf1. KD cells were transfected with pEGFP-Hsf1 or with pEGFP and then incubated with 20 μM cisplatin for 24 h. The percentage of apoptosis was evaluated by counting cells with typical apoptotic morphology. **P < 0.01.
Hsf1 protects against the intrinsic pathway of apoptosis during cisplatin treatment of RPTCs.
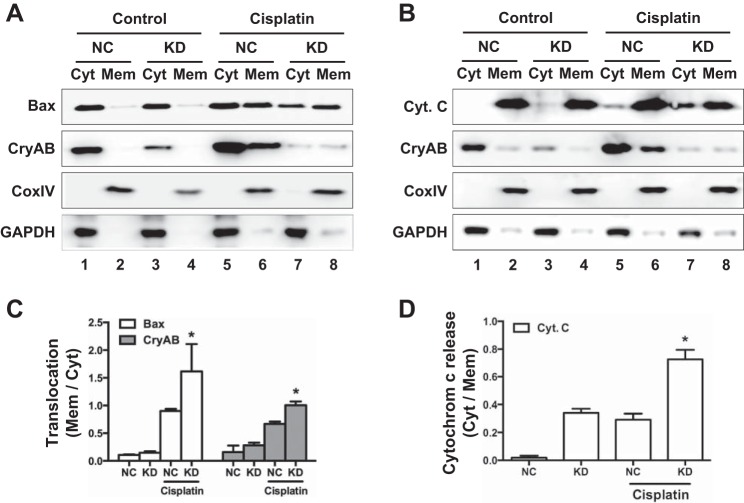
The intrinsic pathway of apoptosis is characterized by the accumulation and insertion of Bax in mitochondria, leading to the release of apoptogenic factors such as cytochrome c. This pathway plays a critical role in tubular cell apoptosis during cisplatin nephrotoxicity (19, 38). Thus, to further delineate the cytoprotective role of Hsf1, we compared Hsf1 KD cells and NC cells for changes of Bax and cytochrome c. These two types of cells were treated with cisplatin for 24 h to isolate the membrane-bound mitochondrial fraction and cytosolic fraction for immunoblot analysis of Bax and cytochrome c. Cytochrome c oxidase IV and GAPDH were analyzed as internal controls of mitochondrial and cytosolic samples, respectively. We also analyzed CryAB in these samples to determine if CryAB redistributes during cisplatin-induced injury. As shown in Fig. 4A, Bax was detected almost entirely in the cytosolic fractions (lanes 1 and 3) with very weak signals in the membrane-bound mitochondrial fraction (lanes 2 and 4) in both NC and KD cells. After 24 h of cisplatin treatment, NC cells showed a good amount of Bax in both cytosolic and membrane fractions (Fig. 4A, lane 5 vs. lane 6), indicative of Bax accumulation in mitochondria. In cisplatin-treated KD cells, more Bax appeared in the membrane fraction than in the cytosolic fraction (Fig. 4A, lane 8 vs. lane 7), suggesting that cisplatin induced higher Bax accumulation to mitochondria in Hsf1 KD cells. Cytochrome c was detected almost completely in the membrane fraction in control KD and NC cells without cisplatin injury (Fig. 4B, lanes 1–4). After cisplatin treatment, some cytochrome c appeared in the cytosolic fraction of NC cells (Fig. 4B, lane 5), whereas significantly more cytochrome c was detected in the cytosolic fraction of KD cells (Fig. 4B, lane 7), suggesting that Hsf1 KD increased cytochrome c release from mitochondria during cisplatin treatment. Interestingly, in both KD and NC cells, a portion of CryAB accumulated in the membrane-bound mitochondrial fraction during cisplatin treatment (Fig. 4, lanes 6 and 8 vs. lanes 2 and 4). In addition, the fractions of KD cells appeared to have much lower CryAB than NC cells, an observation consistent with the results described below (see Fig. 5). The ratio of Bax and CryAB translocation from the mitochondria to cytosol and cytochrome c released in the cytosol were quantified and are shown in Fig. 4, C and D. Collectively, these data indicate that Hsf1 KD sensitized RPTCs to the intrinsic pathway of apoptosis. Conversely, it is suggested that Hsf1 may protect renal tubular cells during cisplatin nephrotoxicity by attenuating mitochondrial damage and the intrinsic pathway of apoptosis.
Fig. 4.
Hsf1 inhibits Bax translocation and cytochrome c (Cyt. c) release during cisplatin treatment of RPTCs. Hsf1 KD and NC cells were incubated with cisplatin. Cells were harvested for fractionation into cytosolic and membrane-bound mitochondrial (Mem) fractions for immunoblot analysis of Bax (A) and cytochrome c (B). Cytochrome c oxidase (Cox)IV and GAPDH were analyzed in the same samples as mitochondrial and cytosolic protein controls, respectively. CryAB was analyzed to verify its accumulation in membrane-bound mitochondrial fractions during cisplatin treatment. C and D: the ratio of Bax and CryAB translocation from mitochondria to the cytosol (C) as well as cytochrome c released in the cytosol (D) were quantified and analyzed by Student's t-test. *P < 0.05 vs. NC cells treated with cisplatin.
Fig. 5.
Hsf1 KD lead to a downregulation of CryAB in RPTCs. Hsf1 KD cells and NC cells were incubated with 20 μM cisplatin for 0, 4, 8, and 24 h. A: whole cell lysates were collected for immunoblot analysis of Hsf1, PARP, CryAB, Hsp90, and Hsp70. The same blots were reprobed for β-actin. B–D: quantification of Hsf1 (B), cleaved PARP (C), and CryAB (D) proteins was performed using ImageJ software. Data are expressed as means ± SD; n = 3. *P < 0.05 vs. KD cells treated with cisplatin at the same time point (Student's t-test); #P < 0.05 vs. NC at the other three time points of cisplatin treatment (two-way ANOVA).
Hsf1 regulates CryAB in RPTCs.
We went on to identify the main Hsps transactivated by Hsf1 during cisplatin nephrotoxicity. To this end, we first analyzed the effect of Hsf1 KD on the expression of Hsps. Hsf1 KD cells and NC cells were treated cisplatin for 0–24 h to collect cell lysates for immunoblot analysis. The analysis first verified that Hsf1 expression was lower in KD cells at every time point of cisplatin treatment (Fig. 5, A and B). Moreover, Hsf1 KD significantly increased PARP cleavage at 24 h of cisplatin treatment, confirming the higher apoptosis in these cells (Fig. 5, A and C). Importantly, CryAB expression was markedly diminished in Hsf1 KD cells compared with NC cells (Fig. 5, A and D), whereas expression of Hsp70 and Hsp90 was similar in these two types of cells. The results suggest that Hsf1 may specifically activate the transcription and expression of CryAB, a small Hsp.
CryAB mediates the cytoprotective effect of Hsf1 during cisplatin treatment of RPTCs.
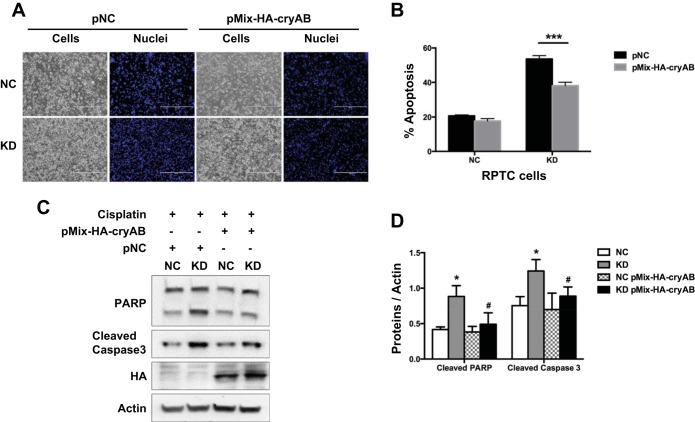
We hypothesized that Hsf1 protected against cisplatin-induced renal tubular cell apoptosis mainly through the induction of CryAB. This hypothesis was based on two observations: 1) CryAB was the main Hsp that was induced along with Hsf1 in both in vitro and in vivo models of cisplatin nephrotoxicity (Figs. 1 and 2) and 2) in RPTCs, KD of Hsf1 led to the attenuation of CryAB in both control and cisplatin-treated cells. To further test this hypothesis, we examined the effect of CryAB overexpression in Hsf1 KD cells. The rationale was that if CryAB was the key mediator of the cytoprotective effect of Hsf1, then CryAB expression would reverse the phenotype of injury sensitivity in Hsf1 KD cells. As shown in Fig. 6A, expression of HA-tagged CryAB reduced cisplatin-induced apoptosis in Hsf1 KD cells, whereas it only had marginal effects in NC cells. Quantification of apoptotic cells further verified the results (Fig. 6B). In addition, HA-CryAB transfection suppressed PARP and caspase 3 cleavage during cisplatin treatment of Hsf1 KD cells, whereas the effect in NC cells was minimal (Fig. 6, C and D). HA-CryAB expression in transfected cells was verified by immunoblot analysis using anti-HA antibody in the same samples (Fig. 6C). These results suggest that Hsf1 protects RPTCs from cisplatin-induced apoptosis at least in part by inducing CryAB.
Fig. 6.
CryAB contributes the cytoprotective effect of Hsf1 during cisplatin treatment of RPTCs. Hsf1 KD cells and NC cells were transfected with pMix-HA-CryAB or negative control plasmid (pNC). Cells were then incubated with 20 μM cisplatin for 24 h. A: representative images of cell morphology. B: percentage of apoptosis evaluated by counting cells with typical apoptotic morphology (P < 0.001). C: whole cell lysate was collected for immunoblot analysis of PARP and active/cleaved caspase 3 to further verify apoptosis. Blots were reprobed for hemagglutinin (HA) to verify HA-CryAB expression and β-actin to control protein loading. D: quantification and statistical analysis of cleaved PARP and cleaved caspase 3 proteins were performed using ImageJ software and Student's t-test. Data are expressed as means ± SD; n = 3. *P < 0.05 vs. NC cells; #P < 0.05 vs. KD cells with cisplatin treatment.
DISCUSSION
Cisplatin-induced nephrotoxicity is the main side effect that limits the clinical use of the otherwise highly potent chemotherapy drug; however, the underlying mechanism remains poorly understood (4, 9, 18, 24, 26, 27, 30). In response to stress or injury, kidney cells may activate multiple cytoprotective mechanisms for adaptation and cell survival. The heat shock response is one of the cellular mechanisms of protection or adaption under cell stress. The heat shock response has been studied in experimental models of ischemia kidney injury (1, 10, 33). However, somewhat surprisingly, there have been no reports about the heat shock response in cisplatin nephrotoxicity. In the present study, we demonstrated the activation of the heat shock response during cisplatin nephrotoxicity using mouse and renal tubular cell culture models. The heat shock response is characterized by Hsf1 activation accompanied by the induction of the small Hsp CryAB. We further verified that Hsf1 has a cytoprotective role during cisplatin treatment of renal tubular cells. In addition, we have shown that the cytoprotective effect of Hsf1 is partly mediated by CryAB.
Hsfs are the transcription factors that lead to the expression of Hsps under conditions of cell stress. It is noteworthy that, although initially discovered in heat shock, these factors respond to a variety of cellular stress far beyond heat shock (2, 3, 11). In addition to Hsf1, there are several other Hsfs, such as Hsf1, Hsf2, and Hsf4, which may have tissue- or cell type-specific distributions. Hsf1 is known to be the major transcription Hsf that is ubiquitously expressed and responsible for the transactivation of Hsps in response to stress (2, 3, 11). Consistently, in the present study, Hsf1 was activated in both in vivo and in vitro models of cisplatin nephrotoxicity and responsible for at least the induction of the small Hsp CryAB. Hsf1 activation was also shown in models of renal ischemia-reperfusion (10). Taken together, these results demonstrated the activation of the Hsf1-mediated heat shock response in both ischemia and nephrotoxic kidney injury. Whether other Hsf isoforms contribute to the heat shock response remains unclear, but KD of Hsf1 was associated with a marked downregulation of CryAB in both control and cisplatin-treated cells (Fig. 5), an observation that supports a critical role of Hsf1 in the heat shock response during cisplatin nephrotoxicity.
However, it is unclear how Hsf1 is activated for the heat shock response in cisplatin nephrotoxicity. We showed moderate but consistent increases of Hsf1 expression in mouse kidneys and RPTC cells during cisplatin treatment (Figs. 1, 2). Nonetheless, key Hsf1 activation events include the dissociation from sequestering chaperone proteins, translocation to the nucleus, and binding to promoters of target genes (2, 3, 11). In this regard, we detected the accumulation or translocation of Hsf1 to the nucleus during cisplatin treatment of RPTCs (Fig. 2E), suggesting that Hsf1 is activated in cisplatin nephrotoxicity via the classic pathway. What triggers this pathway? One interesting possibility is the endoplasmic reticulum stress-associated accumulation of misfolded proteins or proteostatic stress. Endoplasmic reticulum stress occurs in models of cisplatin nephrotoxicity and has been reported to contribute to tubular cell death (20). We speculate that, upon cisplatin exposure, endoplasmic reticulum stress leads to the accumulation of unfolded or misfolded proteins that may activate Hsf1 and the associated heat shock response for cell protection and survival, but severe and persistent endoplasmic reticulum stress results in a complete loss of cellular proteostasis with the ensuing activation of the cell death program.
In our study, Hsf1 KD increased cisplatin-induced apoptosis, whereas reexpression of Hsf1 in Hsf1 KD cells led to a significant reduction of apoptosis (Fig. 3). These results demonstrate a cytoprotective role of Hsf1 in cisplatin nephrotoxicity. Such a conclusion is not surprising, in view of the well-documented cell biological function of Hsf1 and the heat shock response. However, further investigation may establish the role of Hsf1 in cisplatin nephrotoxicity conclusively by in vivo work preferably using gene knockout mouse models. Among the Hsps analyzed, we demonstrated a close association between Hsf1 activation and CryAB expression or induction. This conclusion is supported by both in vivo mouse tissue and in vitro cell culture results (Figs. 1 and 2). Although previous studies focused on Hsp70/Hsp72, a Hsp that plays an important role in renoprotection (23, 37), we only detected marginal Hsp70 induction by cisplatin in both kidney tissues and cells. Importantly, and somewhat surprisingly, KD of Hsf1 did not result in significant changes in Hsp70 expression in RPTCs (Fig. 5). In addition, Hsp90 expression was not affected by cisplatin or Hsf1 KD, either alone or in combination.
Our present data support a role of CryAB in mediating the cytoprotective effect of Hsf1 during cisplatin treatment of renal tubular cells. CryAB was shown to be one of the major Hsps downstream of Hsf1 under this condition. Moreover, overexpression of CryAB could partially yet significantly reduce the sensitivity of Hsf1 KD cells to cisplatin injury (Fig. 6). This observation is consistent with the antiapoptotic function of CryAB previously reported by others (13, 15, 22). A recent study by Volkmann et al. (34) further showed that overexpression of CryAB protected against cisplatin-induced apoptosis in human ovarian cancer cells. CryAB has been shown to bind and sequester proapoptotic proteins, such as Bax (13, 15). In our study, a portion of CryAB translocated to the membrane-bound mitochondrial fraction during cisplatin treatment (Fig. 4), but a further coimmunoprecipitation assay did not detect consistent results of the interaction between CryAB and Bax (data not shown). Thus, we speculate that upon induction during cisplatin treatment, CryAB may accumulate in the mitochondrial membrane to interact with other apoptosis regulatory proteins. In support of this possibility, Mitra and colleagues (25) recently reported that CryAB may interact with voltage-dependent anion-selective channel protein 1, a mitochondrial outer membrane protein that has been implicated in the control of the intrinsic pathway of apoptosis.
GRANTS
This work was supported in part by funds from the Henan University School of Medicine and grants from the National Institutes of Health and Department of Veterans Administration.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Q.L., Y.H., Y.M., and Z.D. conception and design of research; Q.L. performed experiments; Q.L., Y.H., and Z.D. analyzed data; Q.L., Y.H., Y.M., and Z.D. interpreted results of experiments; Q.L. and Z.D. prepared figures; Q.L. drafted manuscript; Q.L., Y.H., Y.M., and Z.D. edited and revised manuscript; Q.L., Y.H., Y.M., and Z.D. approved final version of manuscript.
REFERENCES
- 1.Akcetin Z, Pregla R, Darmer D, Bromme HJ, Holtz J. During ischemia-reperfusion in rat kidneys, heat shock response is not regulated by expressional changes of heat shock factor 1. Transpl Int 13: 297–302, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Akerfelt M, Morimoto RI, Sistonen L. Heat shock factors: integrators of cell stress, development and lifespan. Nat Rev Mol Cell Biol 11: 545–555, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anckar J, Sistonen L. Regulation of HSF1 function in the heat stress response: implications in aging and disease. Annu Rev Biochem 80: 1089–1115, 2011. [DOI] [PubMed] [Google Scholar]
- 4.Arany I, Safirstein RL. Cisplatin nephrotoxicity. Semin Nephrol 23: 460–464, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Beck FX, Neuhofer W, Muller E. Molecular chaperones in the kidney: distribution, putative roles, and regulation. Am J Physiol Renal Physiol 279: F203–F215, 2000. [DOI] [PubMed] [Google Scholar]
- 6.Bhatt K, Wei Q, Pabla N, Dong G, Mi QS, Liang M, Mei C, Dong Z. MicroRNA-687 induced by hypoxia-inducible factor-1 targets phosphatase and tensin homolog in renal ischemia-reperfusion injury. J Am Soc Nephrol 26: 1588–1596, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borkan SC, Gullans SR. Molecular chaperones in the kidney. Annu Rev Physiol 64: 503–527, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Cepeda V, Fuertes MA, Castilla J, Alonso C, Quevedo C, Perez JM. Biochemical mechanisms of cisplatin cytotoxicity. Anticancer Agents Med Chem 7: 3–18, 2007. [DOI] [PubMed] [Google Scholar]
- 9.dos Santos NA, Carvalho Rodrigues MA, Martins NM, dos Santos AC. Cisplatin-induced nephrotoxicity and targets of nephroprotection: an update. Arch Toxicol 86: 1233–1250, 2012. [DOI] [PubMed] [Google Scholar]
- 10.Eickelberg O, Seebach F, Riordan M, Thulin G, Mann A, Reidy KH, Van Why SK, Kashgarian M, Siegel N. Functional activation of heat shock factor and hypoxia-inducible factor in the kidney. J Am Soc Nephrol 13: 2094–2101, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Fujimoto M, Nakai A. The heat shock factor family and adaptation to proteotoxic stress. FEBS J 277: 4112–4125, 2010. [DOI] [PubMed] [Google Scholar]
- 12.Haase VH. Hypoxia-inducible factors in the kidney. Am J Physiol Renal Physiol 291: F271–F281, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamann S, Metrailler S, Schorderet DF, Cottet S. Analysis of the cytoprotective role of α-crystallins in cell survival and implication of the αA-crystallin C-terminal extension domain in preventing Bax-induced apoptosis. PLos One 8: e55372, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Havasi A, Borkan SC. Apoptosis and acute kidney injury. Kidney Int 80: 29–40, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu WF, Gong L, Cao Z, Ma H, Ji W, Deng M, Liu M, Hu XH, Chen P, Yan Q, Chen HG, Liu J, Sun S, Zhang L, Liu JP, Wawrousek E, Li DW. αA- and αB-crystallins interact with caspase-3 and Bax to guard mouse lens development. Curr Mol Med 12: 177–187, 2012. [DOI] [PubMed] [Google Scholar]
- 16.Jiang M, Wei Q, Dong G, Komatsu M, Su Y, Dong Z. Autophagy in proximal tubules protects against acute kidney injury. Kidney Int 82: 1271–1283, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaushal GP, Shah SV. Autophagy in acute kidney injury. Kidney Int 89: 779–791, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lebwohl D, Canetta R. Clinical development of platinum complexes in cancer therapy: an historical perspective and an update. Eur J Cancer 34: 1522–1534, 1998. [DOI] [PubMed] [Google Scholar]
- 19.Linkermann A, Chen G, Dong G, Kunzendorf U, Krautwald S, Dong Z. Regulated cell death in AKI. J Am Soc Nephrol 25: 2689–2701, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu H, Baliga R. Endoplasmic reticulum stress-associated caspase 12 mediates cisplatin-induced LLC-PK1 cell apoptosis. J Am Soc Nephrol 16: 1985–1992, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Livingston MJ, Dong Z. Autophagy in acute kidney injury. Semin Nephrol 34: 17–26, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malin D, Petrovic V, Strekalova E, Sharma B, Cryns VL. αB-crystallin: Portrait of a malignant chaperone as a cancer therapeutic target. Pharmacol Ther 160: 1–10, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mao H, Li Z, Zhou Y, Li Z, Zhuang S, An X, Zhang B, Chen W, Nie J, Wang Z, Borkan SC, Wang Y, Yu X. HSP72 attenuates renal tubular cell apoptosis and interstitial fibrosis in obstructive nephropathy. Am J Physiol Renal Physiol 295: F202–F214, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller RP, Tadagavadi RK, Ramesh G, Reeves PG. Mechanisms of cisplatin nephrotoxicity. Toxins 2: 2490–2518, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitra A, Basak T, Datta K, Naskar S, Sengupta S, Sarkar S. Role of α-crystallin B as a regulatory switch in modulating cardiomyocyte apoptosis by mitochondria or endoplasmic reticulum during cardiac hypertrophy and myocardial infarction. Cell Death Dis 4: e582, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ozkok A, Edelstein CL. Pathophysiology of cisplatin-induced acute kidney injury. Biomed Res Int 2014: 967826, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int 73: 994–1007, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Peng J, Li X, Zhang D, Chen JK, Su Y, Smith SB, Dong Z. Hyperglycemia, p53, and mitochondrial pathway of apoptosis are involved in the susceptibility of diabetic models to ischemic acute kidney injury. Kidney Int 87: 137–150, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Periyasamy-Thandavan S, Jiang M, Wei Q, Smith R, Yin XM, Dong Z. Autophagy is cytoprotective during cisplatin injury of renal proximal tubular cells. Kidney Int 74: 631–640, 2008. [DOI] [PubMed] [Google Scholar]
- 30.Sanchez-Gonzalez PD, Lopez-Hernandez FJ, Lopez-Novoa JM, Morales AI. An integrative view of the pathophysiological events leading to cisplatin nephrotoxicity. Crit Rev Toxicol 41: 803–821, 2011. [DOI] [PubMed] [Google Scholar]
- 31.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell 148: 399–408, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene 22: 7265–7279, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Sreedharan R, Chen S, Miller M, Haribhai D, Williams CB, Van Why SK. Mice with an absent stress response are protected against ischemic renal injury. Kidney Int 86: 515–524, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Volkmann J, Reuning U, Rudelius M, Hafner N, Schuster T, Becker VRA, Weimer J, Hilpert F, Kiechle M, Durst M, Arnold N, Schmalfeldt B, Meindl A, Ramser J. High expression of crystallin αB represents an independent molecular marker for unfavourable ovarian cancer patient outcome and impairs TRAIL- and cisplatin-induced apoptosis in human ovarian cancer cells. Int J Cancer 132: 2820–2832, 2013. [DOI] [PubMed] [Google Scholar]
- 35.Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov 4: 307–320, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Wang J, Biju MP, Wang MH, Haase VH, Dong Z. Cytoprotective effects of hypoxia against cisplatin-induced tubular cell apoptosis: involvement of mitochondrial inhibition and p53 suppression. J Am Soc Nephrol 17: 1875–1885, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Wang Z, Gall JM, Bonegio RG, Havasi A, Hunt CR, Sherman MY, Schwartz JH, Borkan SC. Induction of heat shock protein 70 inhibits ischemic renal injury. Kidney Int 79: 861–870, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wei Q, Dong G, Chen JK, Ramesh G, Dong Z. Bax and Bak have critical roles in ischemic acute kidney injury in global and proximal tubule-specific knockout mouse models. Kidney Int 84: 138–148, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei Q, Liu Y, Liu P, Hao J, Liang M, Mi QS, Chen JK, Dong Z. MicroRNA-489 induction by hypoxia-inducible factor-1 protects against ischemic kidney injury. J Am Soc Nephrol; doi: 10.1681/ASN.2015080870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yan LJ, Rajasekaran NS, Sathyanarayanan S, Benjamin IJ. Mouse HSF1 disruption perturbs redox state and increases mitochondrial oxidative stress in kidney. Antioxid Redox Signal 7: 465–471, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Yang C, Kaushal V, Shah SV, Kaushal GP. Autophagy is associated with apoptosis in cisplatin injury to renal tubular epithelial cells. Am J Physiol Renal Physiol 294: F777–F787, 2008. [DOI] [PubMed] [Google Scholar]
- 42.Yi X, Wang J, Seol DW, Dong Z. Characterization of cell clones stably transfected with short form caspase-9: apoptotic resistance and Bcl-XL expression. Mol Cell Biochem 282: 1–12, 2006. [DOI] [PubMed] [Google Scholar]
- 43.Zhang D, Liu Y, Wei Q, Huo Y, Liu K, Liu F, Dong Z. Tubular p53 regulates multiple genes to mediate AKI. J Am Soc Nephrol 25: 2278–2289, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]