Abstract
Overactive bladder (OAB) is a common debilitating bladder condition with unknown etiology and limited diagnostic modalities. Here, we explored a novel high-throughput and unbiased multiplex approach with cellular and molecular components in a well-characterized patient cohort to identify biomarkers that could be reliably used to distinguish OAB from controls or provide insights into underlying etiology. As a secondary analysis, we determined whether this method could discriminate between OAB and other chronic bladder conditions. We analyzed plasma samples from healthy volunteers (n = 19) and patients diagnosed with OAB, interstitial cystitis/bladder pain syndrome (IC/BPS), or urinary tract infections (UTI; n = 51) for proinflammatory, chemokine, cytokine, angiogenesis, and vascular injury factors using Meso Scale Discovery (MSD) analysis and urinary cytological analysis. Wilcoxon rank-sum tests were used to perform univariate and multivariate comparisons between patient groups (controls, OAB, IC/BPS, and UTI). Multivariate logistic regression models were fit for each MSD analyte on 1) OAB patients and controls, 2) OAB and IC/BPS patients, and 3) OAB and UTI patients. Age, race, and sex were included as independent variables in all multivariate analysis. Receiver operating characteristic (ROC) curves were generated to determine the diagnostic potential of a given analyte. Our findings demonstrate that five analytes, i.e., interleukin 4, TNF-α, macrophage inflammatory protein-1β, serum amyloid A, and Tie2 can reliably differentiate OAB relative to controls and can be used to distinguish OAB from the other conditions. Together, our pilot study suggests a molecular imbalance in inflammatory proteins may contribute to OAB pathogenesis.
Keywords: biomarkers, OAB, interstitial cystitis, bladder pain syndrome, UTI
oab syndrome is a debilitating disease that affects 9–35% of the population in Europe (EpiLUTS study) (7) and affects >17 million people in the United States (27). OAB is a symptom-complex condition, with multiple potential etiologies, but primarily characterized by patient-reported symptoms, which include symptoms of urgency, with or without urgency incontinence, usually with frequency, and nocturia (1). Although it is generally believed that OAB can be distinguished from other conditions affecting the lower urinary tract, there is notable overlap of symptoms and similarities between OAB and other urological conditions, particularly interstitial cystitis/bladder pain syndrome (IC/BPS) (8, 15). Tissue biopsies are typically not done in OAB as they are invasive, leading to a dearth of pathognomic findings on this condition. Several groups have previously associated OAB with an inflammatory disease of the lower urinary tract. Levels of serum C-reactive protein, nerve growth factor, and brain-derived neurotrophic factor, among others, have been found to be increased in OAB compared with control subjects (2, 4, 13, 16, 17, 21). A recent study of urinary chemokines in OAB patients showed increased monocyte chemotactic protein-1 and inflammatory proteins compared with controls, suggesting that chronic inflammation is a key feature in OAB (16). However, whether OAB is distinguishable by inflammatory markers or the presence of inflammatory cellular influx has not been elucidated. Thus urine or plasma biomarkers in conjunction with clinical data to diagnose OAB, distinguish it from other bladder diseases, and provide insights into its pathogenesis would be immensely valuable and could offer the potential for the discovery of novel biomarkers.
In this pilot study, using a highly sensitive, high-throughput, and unbiased multiplexed approach, we analyzed plasma samples from healthy volunteers and patients diagnosed with OAB, IC/BPS, or urinary tract infections (UTI) for proinflammatory, chemokine, cytokine, angiogenesis, and vascular injury factors with the goal of a broader classification of OAB biomarkers and potential insights into underlying mechanisms of OAB pathophysiology. Our studies have identified five plasma markers, including IL-4, TNF-α, macrophage inflammatory protein-1β (MIP-1β), serum amyloid A (SAA), and Tie2 that can distinguish between OAB and other bladder conditions. We propose that these findings could aid in the diagnosis of OAB from other urological conditions that share similar symptomatology and allow for new drug targets and treatment options for patients suffering from OAB.
MATERIALS AND METHODS
Subjects
Patients diagnosed with OAB, IC/BPS, UTI, and age-matched healthy volunteers who served as control subjects were recruited between October 2012 and July 2014. All participants signed an informed consent. The Washington University School of Medicine Institutional Review Board approved this study. Patient recruitment, consent, data and specimen collection, and processing were done using the resources and infrastructure of Washington University's Biorepository and Informatics Grid for Benign Urological Diseases (BIGBUD) and the Kidney Translational Research Core (KTRC).
One hundred and forty-three patients (56 OAB, 31 IC/BPS, 26 UTI, and 30 controls) consented to participate in the study, and data were collected from validated questionnaires completed by the patients. Dipstick analysis was performed on all patient urine to exclude active infectious components. Age-matched healthy volunteers (controls) were recruited by local advertisement and had no significant lower urinary tract symptoms or pain [American Urological Association (AUA) symptom index <7], no previous diagnosis of OAB or IC/BPS, and no evidence of infection based on a negative nitrite dipstick test and symptoms. Patients with an OAB diagnosis typically presented with urinary urgency, with or without urgency incontinence, usually with frequency and nocturia, in accordance with the 2002 International Continence Society (ICS) definitions (1) and published AUA OAB guidelines (10). Patients with a positive bacterial culture or a postvoid residual ≥150 ml were excluded. Patients diagnosed with IC/BPS presented with an unpleasant sensation (pain, pressure, or discomfort) perceived to be related to the bladder, which persisted for >6 wk in the absence of infection or another identifiable cause, in accordance to the AUA IC/BPS Guideline (11). For acute UTIs, symptomatic patients with positive nitrite on a urine dipstick and growth of a single organism were selected. For recurrent UTIs (rUTI), patients with history of at least two acute UTIs in the prior 12 mo were enrolled.
Among all 143 subjects enrolled in the study, 17 IC/BPS, 17 OAB, 17 UTI (including acute, chronic, and recurrent UTIs), and 19 control subjects were selected for Meso Scale Discovery (MSD) analysis (Table 1). The 17 IC/BPS patients with high pain scores and low OAB/incontinence scores were selected using the following criteria: Genitourinary Pain Index (GUPI) (6) pain score ≥11, 0–10 numeric rating scale (NRS) pain score ≥5, International Consultation on Incontinence- Urinary Incontinence (ICIQ-UI) (3) total score ≤9, and International Consultation on Incontinence-Overactive Bladder (ICIQ-OAB) (12) total score ≤7. The 17 OAB patients with high OAB/incontinence scores and low pain scores were selected using the following criteria: GUPI pain score ≤10, 0–10 NRS pain score ≤4, ICIQ-UI ≥10, and ICIQ-OAB ≥8 (high incontinence and OAB symptom scores). UTI patients in the study presented with an acute, chronic, or recurrent UTI. Control patients were selected according to the following criteria: GUPI pain score <1, 0–10 NRS pain score <1, ICIQ-UI <1, and ICIQ-OAB <1.
Table 1.
Meso Scale Discovery cohort
| Control | IC/BPS | OAB | UTI | |
|---|---|---|---|---|
| n | 19 | 17 | 17 | 17 |
| Gender | ||||
| Female | 10 | 17 | 15 | 13 |
| Male | 9 | 0 | 2 | 4 |
| Race | ||||
| Black | 8 | 3 | 9 | 7 |
| White | 11 | 14 | 7 | 10 |
| Other | 0 | 0 | 1 | 0 |
| Age | ||||
| Median | 52 | 42 | 58 | 48 |
| Range | 27–81 | 20–78 | 27–72 | 21–78 |
IC/BPS, interstitial cystitis/bladder pain syndrome; OAB, overactive bladder; UTI, urinary tract infection; n, no. of animals.
Urine Inflammation Scores
Midstream clean catch voided urine was collected in a container with protease inhibitors and stored at −80°C until use. One hundred microliters of each sample was centrifuged onto poly-l-lysine-coated glass slides at 1,000 rpm for 5 min and heat-fixed under a flame. Urine sediments were stained using either the Hema3 or Papanicolaou staining protocol and examined by light microscopy for the presence of polymorphonuclear leukocytes (PMN), sloughed epithelial cells, bacteria, and crystals. Inflammation scores were assigned on a 0–4 scale by averaging the number of PMNs per ×200 magnification field calculated from counting five separate fields. A semiquantitative scoring system was established for analysis: 0, <5 PMN/high-power field (hpf); 1, 5–25 PMN/hpf; 2, 26–50 PMN/hpf; 3, 51–100 PMN/hpf; and 4, >100 PMN/hpf (21). Stained urine samples were scored in a blinded fashion by two investigators (E. Ma and L. Bliss) and averaged to obtain a final score.
Meso Scale Discovery Analysis
Plasma from patients was obtained from the KTRC, and specific markers for inflammation, chemokines, cytokines, angiogenesis factors, and vascular injury markers were assayed using a V-PLEX human biomarker 40-plex kit from Meso Scale Discovery (MSD; Rockville, MD) (Table 2). All 40 analytes were measured by an electrochemiluminescence immunoassay technique per the manufacturer's protocol and analyzed on a Meso Scale Discovery model 1250 Sector Imager 2400. TNF-α, MIP-1β, SAA, IL-4, and Tie2 concentrations were measured according to the manufacturer's instructions. Controls and plasma samples were diluted 2-fold, 4-fold, 1,000-fold, 2-fold, and 2-fold, respectively, in assay diluent for a final volume of 50 μl (TNF-α, MIP-1β, IL-4, Tie2), and a final volume of 25 μl (SAA). The units of concentration for all analytes shown are picograms per milliliter.
Table 2.
Complete analyte list of V-PLEX human biomarker 40-plex kit selected for MSD analysis
| Proinflammatory | Angiogenesis | Chemokine | Cytokine | Vascular Injury |
|---|---|---|---|---|
| IFN-y | bFGF | Eotaxin | GMCSF | CRP |
| IL-10 | PIGF | Eotaxin3 | IL-12_IL-23p40 | SAA |
| IL-12p70 | sFLT1 | IL-8HA | IL-15 | sICAM1 |
| IL-13 | Tie2 | IP-10 | IL-16 | sVCAM1 |
| IL-1β | VEGF_Angio | MCP-1 | IL-17A | |
| IL-2 | VEGFC | MCP-4 | IL-1α | |
| IL-4 | VEGFD | MDC | IL-5 | |
| IL-6 | MIP-1α | IL-7 | ||
| IL-8 | MIP-1β | TNF-β | ||
| TNF-α | TARC | VEGF_Cyto |
MSD, Meso Scale Discovery.
Statistical Analysis
Wilcoxon rank-sum tests were used to perform univariate comparisons between patient groups (controls, OAB, IC/BPS, and UTI) to determine whether the MSD analytes could differentiate OAB patients from the others. Multivariate logistic regression models were fit for each analyte, and odds ratios were calculated on a subset of the data: one that included only OAB patients and controls, one that included only OAB patients and IC/BPS patients, and one that included only OAB patients and UTI patients. To control for confounding effects of age, race, and sex, these were included as independent variables in all multivariate analysis (Table 3). The MSD analyte expression level in plasma was correlated with comorbid inflammatory diseases including joint disease, inflammatory bowel disease, rheumatoid arthritis, ulcerative colitis, lupus, and connective tissue disorder. Receiver operating characteristic (ROC) curves were fit to the analytes that had the strongest associations in univariate and multivariate analysis comparing OAB patients to controls. All statistical analysis was performed using R v3.2.0.
Table 3.
Multivariate models (controlling for age, sex, and race)
| P Value | Odds Ratio | Lower 95% CI | Upper 95% CI | Odds Ratio Units | |
|---|---|---|---|---|---|
| OAB vs. Controls | |||||
| IL-4 | 0.014 | 0.51 | 0.26 | 0.77 | 0.01 pg/ml |
| TNF-α | 0.072 | 3.44 | 0.90 | 13.20 | 1 pg/ml |
| MIP-1β | 0.035 | 1.07 | 1.02 | 1.18 | 1 pg/ml |
| Tie2 | 0.004 | 0.90 | 0.83 | 0.96 | 100 pg/ml |
| SAA | 0.069 | 1.61 | 0.96 | 2.70 | 1,000,000 pg/ml |
| OAB vs. IC/BPS | |||||
| IL-4 | 0.970 | 1.00 | 0.77 | 1.27 | 0.01 pg/ml |
| TNF-α | 0.226 | 1.90 | 0.67 | 5.31 | 1 pg/ml |
| MIP-1β | 0.719 | 1.00 | 0.97 | 1.02 | 1 pg/ml |
| Tie2 | 0.267 | 1.04 | 0.97 | 1.13 | 100 pg/ml |
| SAA | 0.387 | 1.01 | 0.99 | 1.03 | 1,000,000 pg/ml |
| OAB vs. UTI | |||||
| IL-4 | 0.256 | 0.89 | 0.70 | 1.09 | 0.01 pg/ml |
| TNF-α | 0.159 | 1.98 | 0.76 | 5.14 | 1 pg/ml |
| MIP-1β | 0.849 | 1.00 | 0.97 | 1.03 | 1 pg/ml |
| Tie2 | 0.451 | 0.98 | 0.94 | 1.02 | 100 pg/ml |
| SAA | 0.650 | 1.03 | 0.91 | 1.16 | 1,000,000 pg/ml |
SAA, serum amyloid A.
RESULTS
Urine Inflammatory Cell Scores Reveal That OAB is not Characterized by Acute Inflammatory PMN Influx
It has been unclear whether benign bladder diseases have an underlying inflammation that can be distinguished by urine cytological analysis. To determine this, we performed cytological examination of urine from patients with OAB, IC/BPS, UTIs, and controls for the presence of PMNs. We found that OAB patients had low inflammation scores (≤2), suggesting that OAB is not characterized by acute inflammatory cell influx (Fig. 1). Furthermore, IC/BPS was also not associated with an influx of PMNs (P = 0.80). IC/BPS and OAB patients could not be distinguished based on a urine inflammation score (P = 0.314, Fisher's Exact test). However, as expected, patients diagnosed with an acute UTI had high inflammatory scores. Incidentally, patients with a history of recurrent UTIs but with no active infection at the time of diagnosis also had low inflammation scores (data not shown).
Fig. 1.
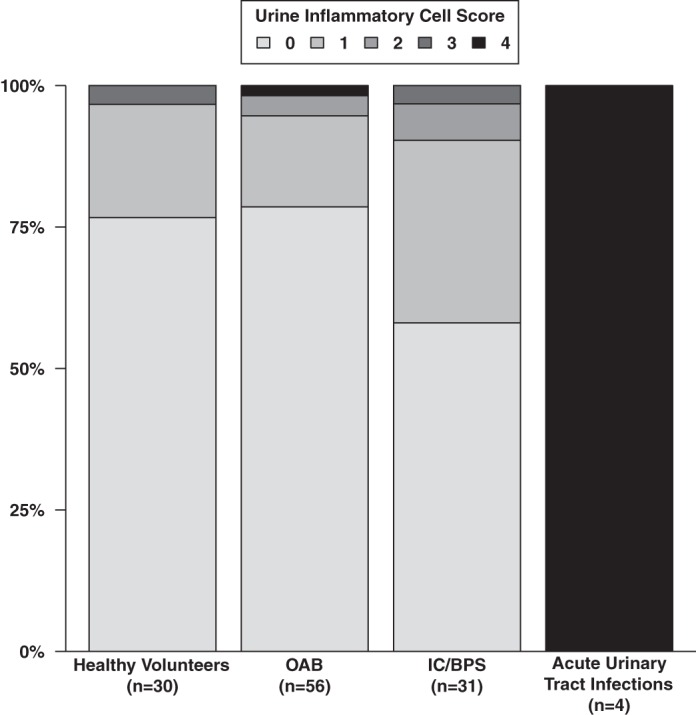
Urine Inflammatory cell scores do not distinguish overactive bladder (OAB) from other bladder diseases. Urine sediments from OAB, interstitial cystitis/bladder pain syndrome (IC/BPS), acute urinary tract infcetion (UTI), and controls were spun onto slides and analyzed under a microscope. A semiquantitative polymorphonuclear cell (PMN) score ranging from 0 to 4 was assigned to each sample, represented by the bar graph.
MSD Analysis Revealed Increased Plasma TNF-α, MIP-1β, and SAA levels and Decreased IL-4 and Tie2 Levels in Patients With OAB Compared With Healthy Controls
Among the 40 plasma analytes (Table 2) assayed with MSD technology, 5 biomarkers demonstrated a significant difference between OAB and control subjects: IL-4, TNF-α, MIP-1β, SAA, and Tie2 (Fig. 2) (Table 4). In univariate analysis, levels of IL-4, an anti-inflammatory marker, were significantly lower in the patients with OAB than in control subjects (0.07 ± 0.01 vs. 0.11 ± 0.01 pg/ml, P = 0.02). Levels of the proinflammatory cytokine/chemokine TNF-α (3.4 ± 0.23 vs. 2.8 ± 0.14 pg/ml, P = 0.01), SAA, an acute phase apolipoprotein important for recruitment of immune cells to inflammatory sites (P < 0.01), and MIP-1β, a PMN-specific chemokine (91.37 ± 9.53 vs. 66.48 ± 15.25 pg/ml, P = 0.05), were significantly higher in OAB patients vs. control subjects. Additionally, levels of Tie2, a receptor involved with angiogenesis, were significantly lower in OAB patients than controls (5,940 ± 326 vs. 7,816 ± 512 pg/ml, P = 0.01). Controlling for age, sex, and race in the multivariate analysis, IL-4 (P = 0.01), MIP-1β (P = 0.04), and Tie2 (P < 0.01) all remained statistically significant in differentiating OAB patients from controls. TNF-α (P = 0.07) and SAA (P = 0.07) both showed a clear trend toward higher values in OAB patients compared with controls. Together, our findings indicate that these markers are indicative of OAB.
Fig. 2.
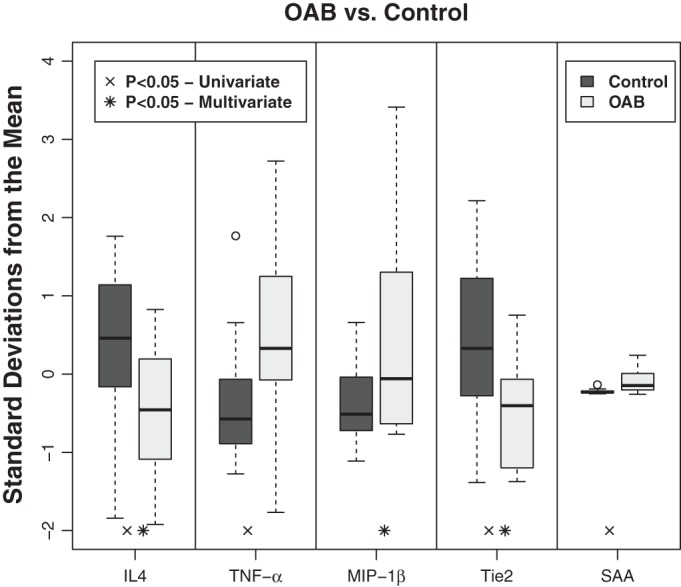
Selective pro- and anti-inflammatory markers can differentiate OAB from healthy controls. Box plots are shown of the normalized expressions of the 5 analytes [IL-4, TNF-α, macrophage inflammatory protein (MIP-1β), Tie2, and serum amyloid A (SAA)] measured in pg/ml using Meso Scale Discovery technology comparing overactive bladder patients (n = 17) with healthy controls (n = 19). Using Wilcoxon rank-sum analysis, levels of IL4, TNF-α, Tie2, and SAA in OAB patients were statistically different from healthy controls, with P < 0.05. Using multivariate linear models controlling age, race, and sex, IL4, MIP-1β, and Tie2 in OAB patients were statistically different from healthy controls, with P < 0.05.
Table 4.
MSD data (pg/ml) on the 5 analytes differentially expressed in OAB vs. control patients
| Control (19) |
OAB (17) |
|||||
|---|---|---|---|---|---|---|
| Analyte | Median | Mean | St. Dev. | Median | Mean | St. Dev. |
| IL-4 | 0.12 | 0.11 | 0.05 | 0.07 | 0.07 | 0.04 |
| TNF-α | 2.60 | 2.78 | 0.63 | 3.36 | 3.42 | 0.94 |
| MIP-1β | 61.86 | 66.48 | 17.43 | 76.33 | 91.37 | 39.31 |
| Tie2 | 7,611.30 | 7,816.21 | 2,229.97 | 6,095.11 | 5,940.50 | 1,345.48 |
| SAA | 1,623,543 | 2,148,642 | 1,392,385 | 5,945,401 | 25,911,793 | 72,916,492 |
Patients with OAB are more Likely to Exhibit Increased Proinflammatory Markers TNF-α and MIP-1β Relative to Patients With IC/BPS and rUTIs.
Since OAB, IC/BPS, and UTI share some overlapping symptoms (e.g., they all present with varying degree of urinary frequency and urgency) (8, 15), we examined whether each of the 40 analytes could distinguish OAB from rUTI and IC/BPS patients. In a multivariate analysis which took into account the age, gender, and race of the patients, we were not able to identify statistically significant associations with the five analytes compared with patients with OAB relative to those with IC/BPS or UTI. However, by univariate analysis, we found that plasma TNF-α and MIP-1β levels were significantly higher in OAB patients compared with IC/BPS patients (P = 0.02 and P = 0.02, respectively). TNF-α was also significantly different in OAB patients (higher levels in OAB, P = 0.01) relative to patients with rUTI. The anti-inflammatory cytokine IL-4 as well as SAA and Tie2 levels in OAB patients were not significantly different from IC/BPS patients. In addition, we examined comorbid inflammatory disorders as a whole as well as separately in our patient cohort and found no detectable relationship for the five analytes.
ROC Curves of MSD Analytes can Differentiate Patients With OAB From Controls
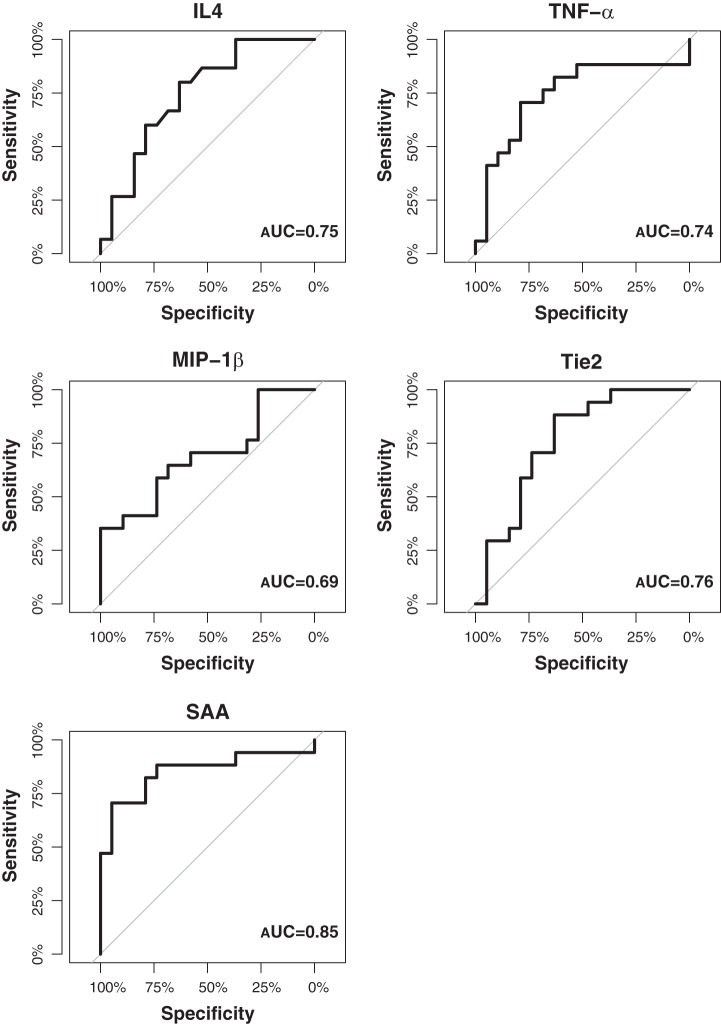
Next, we sought to determine whether any or all of the five analytes could be used as reliable predictors of OAB. Thus we performed ROC curves, which are a highly reliable way to compare diagnostic tests (19), on the analytes that had the strongest associations in univariate and multivariate analysis comparing OAB patients with controls. We found that the ROC curves show all five analytes to be able to differentiate OAB patients from controls (Fig. 3). SAA has the highest area-under the curve (AUC) of 0.85, indicating a reasonable degree of accuracy as a predictive and/or discriminating factor. Similarly, Tie2, TNF-α, and IL-4 all have AUC of 0.76, 0.75, and 0.74, respectively, suggesting that these markers have a 75% chance of correctly discriminating between patient conditions. MIP-1β has an AUC of 0.69, which only makes it marginally less accurate. These results identify potential analytes for supporting an OAB diagnosis.
Fig. 3.
Receiver operating characteristic (ROC) curves can distinguish OAB patients from healthy controls. ROC curves for TNF-α, MIP-1β, Tie2, and SAA can differentiate OAB patients (n = 17) from healthy controls (n = 19).
DISCUSSION
In this pilot study, we applied a multiplex, highly sensitive approach of 40 analytes simultaneously to identify potential plasma markers that can distinguish OAB from controls and extended it to further test the possibility of leads into markers that can distinguish three major benign urological diseases (OAB, IC/BPS, and UTIs; acute, chronic, and recurrent) as there is often symptomatic overlap between these conditions that can lead to diagnostic conundrums. An important finding of our study is that OAB patients showed increased levels of several plasma proinflammatory cytokines and chemokines, including TNF-α, MIP-1β, and SAA. Furthermore, we noted decreased levels of an anti-inflammatory cytokine, IL-4, and an angiogenesis marker, Tie2, compared with age-matched controls without OAB.
Cytokines and chemokines play an essential role in the pathogenesis of several chronic inflammatory diseases. Although the underlying pathophysiology of OAB remains unclear, our results suggest that OAB may have an underlying systemic inflammatory component, with upregulation of proinflammatory markers and downregulation of anti-inflammatory markers. One possibility contributing to this inflammation could be the newly described urinary microbiome (30). Interestingly, however, we noted limited acute inflammatory cellular influx or bacteriuria in OAB patient urine. Our findings are consistent with previous work where Antunes-Lopes et al. (2) suggest that chronic but not acute inflammation potentially plays a role in the development of OAB.
Uceyler et al. (28) had shown that plasma levels of IL-4, TNF-α, and MIP-1β were associated with systemic inflammatory diseases including arthritis, although only IL-4 was found to be significant, and their cohort had high pain scores and only one patient had bladder pain. Our OAB cohort had low pain scores, and the markers identified in the OAB vs. control group did not associate with comorbid inflammatory status nor affect the results in multivariate analysis. In this regard, our results support the notion that reduction in anti-inflammatory molecules such as IL-4 may have a broader role in conditions such as OAB in the absence of underlying chronic inflammatory disease.
Interestingly, we found that expression levels of a proinflammatory cytokine, TNF-α, differentiates OAB from other inflammatory conditions: IC/BPS and rUTI. TNF-α have been examined in the context of conditions affecting bladder morbidities, including OAB and diabetic bladder dysfunction (9, 29). For example, knocking out the hepatic-specific insulin receptor knockout exhibits detrusor overactivity (29). Treatment of these animals with soluble TNF receptor 1 reversed the bladder dysfunction (29). Similarly, the use of TNF-α receptor blockade gene therapy in the bladder has been shown to decrease overactivity induced by nociceptive bladder stimuli in rats (9). Our TNF-α findings in human OAB patients lend support to these observations in preclinical models. Thus we postulate that the high levels of plasma TNF-α observed in OAB patients likely affect bladder contractility and may have both nociceptive and inflammatory etiologies.
Our findings that MIP-1β is highly upregulated in OAB patients is supported by recent studies indicating that MIP-1β may be involved in the pathogenesis of OAB (26). Tyagi et al. (26) found a fivefold elevation of this molecule in the urine of OAB patients compared with controls, and they also did not see elevated PMN influx in urines from OAB patients. A study conducted by Suttmann et al. (23) showed that PMNs isolated from the urine of bacillus Calmette-Guérin (BCG)-treated patients are a source of MIP-1α and MIP-1β, involved in inflammation (23). Interestingly, MIP-1β was induced in peripheral blood PMNs but absent in urinary PMNs (23). The results of this study suggest that MIP-1β may be involved in a systemic inflammatory response which is not evident in situ in the bladder mucosa.
SAA is an acute-phase reactant that is involved in cholesterol transport and in immune cell recruitment. Studies suggest that acute-phase SAA is part of a systemic inflammatory response in which cholesterol from damaged cells is recycled and reused (14) (22), and SAA levels frequently increase as a consequence of acute and chronic inflammatory states, including chronic infection and rheumatoid arthritis. An SAA subtype, SAA4, has been identified as a possible biomarker for bladder cancer; however, there has been little to no mention of its effects on OAB (5). Our findings suggest that SAA is a biomarker that has high reliability in distinguishing patients with OAB from controls.
We found that OAB patients also exhibit a decrease in both the anti-inflammatory cytokine IL-4 and the angiogenesis marker and tyrosine kinase receptor Tie2. Recent investigation of IL-4 in mice showed its protective effects on the bladder wall in the setting of inflammation and overactivity (20). Oguchi et al. (20) demonstrated that vector-mediated expression of IL-4 in mice with irritated bladder walls had decreased bladder overactivity, as well as decreased inflammatory response and nociceptive behavior compared with untreated bladders. Thus IL-4 appears to have an anti-inflammatory, protective effect on the bladder wall, and IL-4 gene therapy could even potentially be a strategy for treating overactive bladder and bladder pain (20). This could be a compensatory response in the setting of OAB as well but may be overcome by the burden of proinflammatory cytokines, leading to symptomatic disease. Recent findings suggest that IL-4 expression in females is sensitive to the stage of menstrual cycle and sexual activity (18). We believe that the results of our data are less likely due to the menstrual cycle phase since our OAB control male and female cohorts are similarly represented, and included women who were pre- and postmenopausal. In support of this, comparative analysis between the sexes did not reveal any significant difference in IL-4 levels.
Little is known about the effects of Tie2 on OAB. Although the role for Tie2 in OAB pathogenesis remains unclear, the literature suggests that this angiogenesis marker plays a critical role in tumor growth related to bladder cancer (24). In a study conducted by Szarvas et al. (24), serum samples from 117 bladder cancer patients and 64 healthy volunteers were analyzed for Tie2 expression and their results showed that Tie2 levels were significantly lower than those of controls. Additionally, a higher Tie2 serum level was a risk factor for metastasis (24). Further studies are necessary to elucidate the role of Tie2 in OAB pathophysiology.
Strengths and Limitations
The strengths of our study include the use of two new approaches for biomarker identification, specifically that of urine inflammatory scores and MSD analysis. To our knowledge, this is the first multiplex analyte survey to distinguish these conditions in a well-matched cohort using univariate and multivariate analysis. Given the accuracy of urine inflammatory scores in predicting acute inflammatory conditions as well as the sensitivity of MSD in detecting low levels of analytes, both serve as methods for disease stratification, or biomarker identification that have previously not been explored in the context of OAB. The upregulated profile of plasma TNF-α, MIP-1β, and SAA and downregulation of Tie2 and plasma IL-4, despite low urine inflammation scores, suggest that OAB is a chronic systemic, rather than an acute or focal, inflammatory disease, which may elucidate some of the confusion behind the controversial inflammation-related OAB literature.
The limitation of this pilot study was the small patient cohort of control subjects and OAB patients. Nevertheless, several analytes showed significant association with OAB relative to controls and could differentiate OAB from other chronic bladder conditions. Additional investigations with larger OAB, IC/BPS, and control cohorts will be needed to power further discovery of analytes to discriminate between these conditions and provide additional clues to underlying etiologies.
Overall, our findings may serve as a premise to study the pathogenesis of OAB in the context of chronic systemic inflammation and the markers identified as potential tools to study the progression of, severity, and prognosis for OAB in future longitudinal and validation studies. Animal studies from Takagi-Matsumoto et al. (25) suggest that NSAIDs improve bladder capacity in a cystitis model of OAB. Thus we propose that anti-inflammatory drugs might be considered for future therapeutic trials to manage OAB. The pilot data identifying putative markers that can differentiate IC/BPS and OAB offer much promise since it has been challenging to differentiate these two syndromes clinically.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant P20-DK097798 (to H. H. Lai, I. U. Mysorekar, and S. Jain).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
E.M. and L.B. performed experiments; E.M., J.V., L.B., I.U.M., and S.J. analyzed data; E.M., J.V., and S.J. prepared figures; E.M., I.U.M., and S.J. drafted manuscript; E.M., J.V., H.H.L., I.U.M., and S.J. edited and revised manuscript; E.M., J.V., L.B., H.H.L., I.U.M., and S.J. approved final version of manuscript; J.V., H.H.L., I.U.M., and S.J. interpreted results of experiments; H.H.L., I.U.M., and S.J. provided conception and design of research.
ACKNOWLEDGMENTS
Present address of I. Mysorekar: Depts. of Obstetrics and Gynecology and Pathology and Immunology, Washington University School of Medicine, 660 South Euclid Ave., Box 8064, St. Louis, MO 63110 (e-mail: Indira@wustl.edu).
We thank Aleksandra Klim and Vivien Gardner for recruiting study participants, Mary Hoffman for regulatory approval, and Aleathea Paradis for managing the data. We also thank Dr. Joseph Gaut and Nancy Brada for use of the MSD Sector Imager and Dr. Jill Clampit for helping with the initial MSD data extraction. We thank Division of Nephrology for the use of KTRC resources (DK079333).
REFERENCES
- 1.Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, van Kerrebroeck P, Victor A, Wein A. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Am J Obstet Gynecol 187: 116–126, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Antunes-Lopes T, Pinto R, Barros SC, Botelho F, Silva CM, Cruz CD, Cruz F. Urinary neurotrophic factors in healthy individuals and patients with overactive bladder. J Urol 189: 359–365, 2013. [DOI] [PubMed] [Google Scholar]
- 3.Avery K, Donovan J, Peters TJ, Shaw C, Gotoh M, Abrams P. ICIQ: a brief and robust measure for evaluating the symptoms and impact of urinary incontinence. Neurourol Urodyn 23: 322–330, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Bhide AA, Cartwright R, Khullar V, Digesu GA. Biomarkers in overactive bladder. Int Urogynecol J 24: 1065–1072, 2013. [DOI] [PubMed] [Google Scholar]
- 5.Chen CL, Lin TS, Tsai CH, Wu CC, Chung T, Chien KY, Wu M, Chang YS, Yu JS, Chen YT. Identification of potential bladder cancer markers in urine by abundant-protein depletion coupled with quantitative proteomics. J Proteomics 85: 28–43, 2013. [DOI] [PubMed] [Google Scholar]
- 6.Clemens JQ, Calhoun EA, Litwin MS, McNaughton-Collins M, Kusek JW, Crowley EM, Landis JR. Validation of a modified National Institutes of Health chronic prostatitis symptom index to assess genitourinary pain in both men and women. Urology 74: 983–987, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coyne KS, Sexton CC, Thompson CL, Milsom I, Irwin D, Kopp ZS, Chapple CR, Kaplan S, Tubaro A, Aiyer LP, Wein AJ. The prevalence of lower urinary tract symptoms (LUTS) in the USA, the UK and Sweden: results from the Epidemiology of LUTS (EpiLUTS) study. BJU Int 104: 352–360, 2009. [DOI] [PubMed] [Google Scholar]
- 8.Elliott CS, Payne CK. Interstitial cystitis and the overlap with overactive bladder. Curr Urol Rep 13: 319–326, 2012. [DOI] [PubMed] [Google Scholar]
- 9.Funahashi Y, Oguchi T, Goins WF, Gotoh M, Tyagi P, Goss JR, Glorioso JC, Yoshimura N. Herpes simplex virus vector mediated gene therapy of tumor necrosis factor-alpha blockade for bladder overactivity and nociception in rats. J Urol 189: 366–373, 2013. [DOI] [PubMed] [Google Scholar]
- 10.Gormley EA, Lightner DJ, Burgio KL, Chai TC, Clemens JQ, Culkin DJ, Das AK, Foster HE Jr, Scarpero HM, Tessier CD, Vasavada SP. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline. J Urol 188: 2455–2463, 2012. [DOI] [PubMed] [Google Scholar]
- 11.Hanno PM, Burks DA, Clemens JQ, Dmochowski RR, Erickson D, Fitzgerald MP, Forrest JB, Gordon B, Gray M, Mayer RD, Newman D, Nyberg L Jr, Payne CK, Wesselmann U, Faraday MM. AUA guideline for the diagnosis and treatment of interstitial cystitis/bladder pain syndrome. J Urol 185: 2162–2170, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson S, Donovan J, Brookes S, Eckford S, Swithinbank L, Abrams P. The Bristol Female Lower Urinary Tract Symptoms questionnaire: development and psychometric testing. Br J Urol 77: 805–812, 1996. [DOI] [PubMed] [Google Scholar]
- 13.Kim JC, Park EY, Seo SI, Park YH, Hwang TK. Nerve growth factor and prostaglandins in the urine of female patients with overactive bladder. J Urol 175: 1773–1776, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Kisilevsky R, Manley PN. Acute-phase serum amyloid A: perspectives on its physiological and pathological roles. Amyloid 19: 5–14, 2012. [DOI] [PubMed] [Google Scholar]
- 15.Lai HH, Vetter J, Jain S, Gereau RWt, Andriole GL. The overlap and distinction of self-reported symptoms between interstitial cystitis/bladder pain syndrome and overactive bladder: a questionnaire based analysis. J Urol 192: 1679–1685, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu HT, Jiang YH, Kuo HC. Increased serum adipokines implicate chronic inflammation in the pathogenesis of overactive bladder syndrome refractory to antimuscarinic therapy. PloS One 8: e76706, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu HT, Lin H, Kuo HC. Increased serum nerve growth factor levels in patients with overactive bladder syndrome refractory to antimuscarinic therapy. Neurourol Urodyn 30: 1525–1529, 2011. [DOI] [PubMed] [Google Scholar]
- 18.Lorenz TK, Heiman JR, Demas GE. Sexual activity modulates shifts in TH1/TH2 cytokine profile across the menstrual cycle: an observational study. Fertil Steril 104: 1513–1521 e1514, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McNeil BJ, Hanley JA. Statistical approaches to the analysis of receiver operating characteristic (ROC) curves. Med Decis Making 4: 137–150, 1984. [DOI] [PubMed] [Google Scholar]
- 20.Oguchi T, Funahashi Y, Yokoyama H, Nishizawa O, Goins WF, Goss JR, Glorioso JC, Yoshimura N. Effect of herpes simplex virus vector-mediated interleukin-4 gene therapy on bladder overactivity and nociception. Gene Ther 20: 194–200, 2013. [DOI] [PubMed] [Google Scholar]
- 21.Pinto R, Lopes T, Frias B, Silva A, Silva JA, Silva CM, Cruz C, Cruz F, Dinis P. Trigonal injection of botulinum toxin A in patients with refractory bladder pain syndrome/interstitial cystitis. Eur Urol 58: 360–365, 2010. [DOI] [PubMed] [Google Scholar]
- 22.Rocken C, Menard R, Buhling F, Vockler S, Raynes J, Stix B, Kruger S, Roessner A, Kahne T. Proteolysis of serum amyloid A and AA amyloid proteins by cysteine proteases: cathepsin B generates AA amyloid proteins and cathepsin L may prevent their formation. Ann Rheum Dis 64: 808–815, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suttmann H, Riemensberger J, Bentien G, Schmaltz D, Stockle M, Jocham D, Bohle A, Brandau S. Neutrophil granulocytes are required for effective Bacillus Calmette-Guerin immunotherapy of bladder cancer and orchestrate local immune responses. Cancer Res 66: 8250–8257, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Szarvas T, Jager T, Droste F, Becker M, Kovalszky I, Romics I, Ergun S, Rubben H. Serum levels of angiogenic factors and their prognostic relevance in bladder cancer. Pathol Oncol Res 15: 193–201, 2009. [DOI] [PubMed] [Google Scholar]
- 25.Takagi-Matsumoto H, Ng B, Tsukimi Y, Tajimi M. Effects of NSAIDs on bladder function in normal and cystitis rats: a comparison study of aspirin, indomethacin, and ketoprofen. J Pharmacol Sci 95: 458–465, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Tyagi P, Barclay D, Zamora R, Yoshimura N, Peters K, Vodovotz Y, Chancellor M. Urine cytokines suggest an inflammatory response in the overactive bladder: a pilot study. Int Urol Nephrol 42: 629–635, 2010. [DOI] [PubMed] [Google Scholar]
- 27.Tyagi S, Thomas CA, Hayashi Y, Chancellor MB. The overactive bladder: Epidemiology and morbidity. Urol Clin North Am 33: 433–438, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Uceyler N, Valenza R, Stock M, Schedel R, Sprotte G, Sommer C. Reduced levels of antiinflammatory cytokines in patients with chronic widespread pain. Arthritis Rheum 54: 2656–2664, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Wang Z, Cheng Z, Cristofaro V, Li J, Xiao X, Gomez P, Ge R, Gong E, Strle K, Sullivan MP, Adam RM, White MF, Olumi AF. Inhibition of TNF-alpha improves the bladder dysfunction that is associated with type 2 diabetes. Diabetes 61: 2134–2145, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whiteside SA, Razvi H, Dave S, Reid G, Burton JP. The microbiome of the urinary tract–a role beyond infection. Nat Rev Urol 12: 81–90, 2015. [DOI] [PubMed] [Google Scholar]