Abstract
In male rats, androgen supplements increase 20-hydroxyeicosatetraenoic acid (20-HETE) via cytochrome P-450 (CYP)4A ω-hydroxylase and cause an increase in blood pressure (BP). In the present study, we determined the roles of 20-HETE and CYP4A2 on the elevated BP in hyperandrogenemic female rats. Chronic dihydrotestosterone (DHT) increased mean arterial pressure (MAP) in female Sprague-Dawley rats (96 ± 2 vs. 108 ± 2 mmHg, P < 0.05) and was associated with increased renal microvascular CYP4A2 mRNA expression (15-fold), endogenous renal 20-HETE (5-fold), and ω-hydroxylase activity (3-fold). Chronic DHT also increased MAP in low salt-fed Dahl salt-resistant female rats (81 ± 4 vs. 95 ± 1 mmHg, P < 0.05) but had no effect on MAP in Dahl salt-sensitive female rats (154 ± 3 vs. 153 ± 3 mmHg), which are known to be 20-HETE deficient. To test the role of CYP4A2, female CYP4A2−/− and SS.5Bn (wild type) rats were treated with DHT. DHT increased MAP in SS.5Bn female rats (104 ± 1 vs. 128 ± 1 mmHg, P < 0.05) but had no effect in CYP4A2−/− female rats (118 ± 1 vs. 120 ± 1 mmHg). Renal microvascular 20-HETE was reduced in control CYP4A2−/− female rats and was increased with DHT in SS.5Bn female rats (6-fold) but not CYP4A2−/− female rats. ω-Hydroxylase activity was 40% lower in control CYP4A2−/− female rats than in SS.5Bn female rats, and DHT decreased ω-hydroxylase activity in SS.5Bn female rats (by 50%) but significantly increased ω-hydroxylase activity in CYP4A2−/− female rats (3-fold). These data suggest that 20-HETE via CYP4A2 contributes to the elevation in BP in hyperandrogenemic female rats. The data also suggest that 20-HETE synthesis inhibition may be effective in treating the elevated BP in women with hyperandrogenemia, such as women with polycystic ovary syndrome.
Keywords: polycystic ovary syndrome, dihydrotestosterone, Dahl salt-sensitive rats, CYP4A2−/− rats, cytochrome P-450, 20-hydroxyeicosatetraenoic acid
women who exhibit elevated levels of circulating androgens are at greater risk for hypertension and cardiovascular disease (19). Hyperandrogenemia is a major diagnostic characteristic of women with polycystic ovary syndrome (PCOS), the most common endocrine disorder affecting 6–10% of women during their reproductive life (3, 19). Furthermore, postmenopausal women often exhibit increases in circulating androgens as well. Shaw et al. (24) reported that postmenopausal women who had a history of irregular menses and elevated androgen levels were at greater risk for hypertension and coronary artery disease than women who did not have elevated androgens. Daan and colleagues (2) compared the free androgen index in cohorts of women with PCOS, premature ovarian insufficiency, natural menopause, and regular menstrual cycling and reported that women who had an increased free androgen index, irrespective of ovarian function or dysfunction, had a higher incidence of hypertension and metabolic disorders and an increased risk for developing cardiovascular disease. The mechanisms by which androgens modulate blood pressure (BP) control in women are unknown.
Several investigators have shown that renal cytochrome P-450 (CYP)4A ω-hydroxylase expression and 20-hydroxyeicosatetraenoic acid (20-HETE) synthesis are increased in male rats and mice given androgen supplements (9, 18, 25, 26, 27, 32). 20-HETE is a metabolite of arachidonic acid produced by ω-hydroxylases, mainly of the CYP4A and CYP4F families (8, 10, 21, 32), and plays a role in renal function, vascular tone, and BP regulation (5, 15, 17, 30). In the kidneys, 20-HETE can have a dual effect on BP regulation depending on the location of its synthesis: antihypertensive in renal tubules, where 20-HETE blocks Na+ reabsorption, and prohypertensive in the renal microvasculature, where 20-HETE is a vasoconstrictor (30, 32). Upregulation of 20-HETE synthesis by androgen supplements in males causes an increase in BP (25, 26, 32). Whether androgens increase 20-HETE synthesis in the kidneys of females and, if so, whether the increase in 20-HETE is pro- or antihypertensive are unknown.
We have recently characterized a rat model that mimics many of the characteristics of women with chronic hyperandrogenemia, including an increase in BP (34). Female Sprague-Dawley (SD) rats are treated with low doses of dihydrotestosterone (DHT), which increases circulating DHT levels by approximately threefold (34). These female rats develop hyperphagia, increases in body weight, insulin resistance, metabolism disorders, and inflammation and exhibit an increase of 10–15 mmHg in mean arterial pressure (MAP) (34), many characteristics that occur with elevated androgens in women with PCOS (3, 19).
In the present study, the hypothesis was tested that chronic androgen supplements in female rats cause an increase in BP in part by increasing synthesis of renal microvascular 20-HETE and that this is mediated by an increase in CYP4A ω-hydroxylase expression, in particular the CYP4A2 isoform. To test this hypothesis, we tested whether 20-HETE levels and ω-hydroxylase activity are increased in the renal microvasculature of SD female rats treated with DHT, our standard model of hyperandrogenemia (34). We also determined the effect of androgen supplements on renal microvascular mRNA expression of CYP4A ω-hydroxylase isoforms known to be present in the kidney (CYP4A1, CYP4A2, CYP4A3, and CYP4A8) (20, 21). Based on these data, which supported a role for 20-HETE mediated by microvascular CYP4A2 in the increase in BP with DHT supplements, we created the hyperandrogenemic model first in female rats with 20-HETE deficiency [Dahl salt-sensitive (SS) rats] and compared the response to hyperandrogenemia in Dahl salt-resistant (SR) control rats with normal 20-HETE synthesis (6, 14). Then, to determine the specific contribution of CYP4A2 ω-hydroxylase in the pressor response to hyperandrogenemia, we used female rats of the CYP4A2−/− rat strain that was developed at the Medical College of Wisconsin using zinc finger nuclease technology (7, 16, 16a) and compared the response to hyperandrogenemia in SS.5BN consomic strain female rats (wild type). SS.5Bn rats were produced at the Medical College of Wisconsin by transferring chromosome 5 carrying CYP4A genes from Brown Norway rats onto the Daht SS genetic background, increasing the renal expression of CYP4A and thus 20-HETE production by approximately fourfold compared with Dahl SS rats (5, 12, 19, 27, 29). The CYP4A2−/− strain was developed on the SS.5Bn background.
MATERIALS AND METHODS
Animal models.
SD, Dahl SS, and Dahl SR female rats were obtained from the vendor (Harlan Laboratories, Indianapolis, IN) at 3 wk of age. SS.5Bn rats were produced at the Medical College of Wisconsin by transferring chromosome 5 carrying CYP4A genes from Brown Norway rats onto the DS genetic background, increasing the renal expression of CYP4A and thus 20-HETE production in male rats by approximagely fourfold compared with Dahl SS rats (5, 12, 16, 16a, 19, 27, 29). The CYP4A2−/− strain was developed on the SS.5Bn background. SS.5BN consomic rats (wild type) and homozygous CYP4A2−/− rat breeder pairs were obtained from Dr. Richard Roman (University of Mississippi Medical Center). SD rats were maintained on normal salt rodent chow (0.8% NaCl, Teklad, Harlan Laboratories), and Dahl SS, Dahl SR, CYP4A2−/−, and SS.5Bn rats were maintained on a low-salt diet (0.3% NaCl, Teklad 7034) and tap water in a temperature-controlled room with 12:12-h light-dark cycle. Rats were used at ∼16 wk of age. All protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Mississippi Medical Center, and experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (8th ed.).
Experimental design.
In experiment 1, control and DHT-treated SD female rats were compared. In experiment 2, control and DHT-treated Dahl SR and SS female rats were compared. In experiment 3, control and DHT-treated SS.5BN and CYP4A2−/− female rats were compared.
DHT treatment.
At 4 wk of age, all animals were divided into groups that received either DHT pellets (7.5 mg/90 days, daily dose: 83 μg, Innovative Research, Sarasota, FL) or placebo pellets (Innovative Research) implanted subcutaneously on the back of the neck, as we have previously described (34).
Measurement of plasma steroids.
Plasma DHT and 17β-estradiol were measured in all rats using commercially available radioimmunoassay kits (DHT: DSL 9600 Active Free DHT RIA kit and DSL 4800 17β-estradiol: DSL 4800 Ultrasensitive Estradiol RIA kit, all from Diagnostic Systems Laboratories, now Beckman Coulter, Indianapolis, IN), as previously described (34). All measurements are presented as picograms per millilter.
Measurement of MAP.
At 14 wk of age, under gas anesthesia with isofluorane (Mallinckrodt Veterinary, Hazelwood, CA) and with aseptic techniques, control (n = 6–8 rats/group of each strain) and DHT-treated (n = 6–8 rats/group of each strain) female rats were implanted with radiotelemetry transmitters (TA11PA-C40, Data Sciences, St. Paul, MN) into the abdominal aorta below the renal arteries, as previously described (22, 33, 34). Rats were placed into individual cages above a receiver (RLA-3000) and allowed 2 wk of recovery. MAP was obtained during a 10-s sampling period (500 Hz) and recorded and averaged every 5 min for 24 h/day for 5 consecutive days. Data are presented as mmHg and were averaged over 5 days for each group of rats.
Measurement of endogenous 20-HETE in cerebral and renal microvessels.
Endogenous 20-HETE and ω-hydroxylase activity in cerebral and renal microvessels were measured in additional control and DHT-treated SD, SS.5Bn, and CYP4A2−/− female rats (n = 3–6 rats/group) using HPLC-mass spectroscopy in the University of Mississippi Medical Center LC/MS Core under the direction of Dr. Richard Roman, as we have previously described (33). Briefly, cerebral (used as control measurements) and renal microvessels were isolated using 1.5% Evans blue and standard sieving techniques. After isolation, vessels were homogenized, and samples were extracted twice with 3 ml ethyl acetate. After extraction, the organic layer was dried using nitrogen. Samples were reconstituted in acetonitrile and analyzed using LC/MS/MS using an AB Sciex 4000 Q TRAP system (Framingham, MA). Data were averaged for each group of rats and are presented as picomoles of endogenous 20-HETE per milligram of renal microvascular protein or ω-hydroxylase activity as picomoles of 20-HETE produced per minute per milligram of protein.
Real time RT-PCR for CYP4A isoforms in renal microvessels of SD female rats.
CYP4A1, CYP4A2, CYP4A3, and CYP4A8 isoform expressions from renal microvessels were measured in control or DHT-treated SD female rats (n = 6 rats/group) using primers and methods previously described by us (33). Renal microvessel mRNA was extracted with TRIzol reagent (Molecular Research Center, Cincinnati, OH), resuspended in diethylpyrocarbonate-H2O, DNase treated with a DNA-free kit (Ambion, Austin, TX), and quantified by spectrophotometry. Five micrograms of RNA were reverse transcribed with 0.5 μg T12VN primer and Superscript III (Invitrogen, Carlsbad, CA) in a final volume of 20 μl. The reaction was performed for 60 min at 50°C and terminated by an incubation at 75°C for 15 min. Elongation factor I (EF-1) primers were used as controls. Real-time RT-PCRs contained 1 μl reverse transcription product, 0.1 μM of each primer, 0.2 mM dNTPs, SYBR green I (1:20,000 final concentration, Molecular Probes, Eugene, OR), and 1 μl Titanium Taq DNA polymerase (Clontech, Palo Alto, CA). Amplifications were performed in an iCycler real-time thermal cycler (Bio-Rad). Cycling conditions were 1 min at 95°C followed by 50 cycles of 15 s at 95°C, 15 s at 60.0°C, and 60 s at 72°C. Fluorescence data were collected during the elongation step. After PCR amplification, the specificity of the PCR was confirmed by melting temperature determination of the PCR products and electrophoretic analysis in 2% agarose gels. Standard curves were made with serial dilutions of pooled reverse transcription samples. Results are expressed as arbitrary units and were standardized against EF-1 mRNA expression. Data were averaged for each group of rats and are presented as arbitrary units (CYP4A “X”/EF-1).
Statistical analyses.
Statistical analyses were performed with SigmaPlot (version 11, Systat Software, San Jose, CA). Body weights in control and DHT-treated female rats as well as plasma steroid measurements were analyzed using one-Way ANOVA followed by a Tukey honestly significant different post hoc test. MAP, real time RT-PCR, 20-HETE, and ω-hydroylase activity changes in SD female rats were analyzed by a Student's t-test. 20-HETE and ω-hydroxylase activity in SS.5Bn and CYP4A2−/− female rats were compared by two-Way ANOVA followed by a Tukey honestly significant different post hoc test. MAP changes between the two factors (strain factor: Dahl SS vs. Dahl SR and SS.5BN vs. CYP4A2−/− and treatment factor: placebo vs. DHT) were analyzed using two-way ANOVA followed by a Tukey honestly significant different post hoc test.
RESULTS
Experiment 1: SD female rats.
As shown in Table 1, plasma DHT levels were increased by threefold in DHT-supplemented SD female rats and estradiol levels were not different in DHT-treated rats compared with control rats. DHT is not aromatizable, and thus the increase in estradiol is independent of the DHT supplements. DHT also increased body weight in SD female rats by 25% by 16 wk of age. As shown in Fig. 1, DHT increased MAP in 16-wk-old SD female rats by ∼12 mmHg compared with control rats.
Table 1.
Body weight as well as plasma DHT and estradiol levels in control and DHT-treated SD female rats
| Control | DHT | |
|---|---|---|
| Body weight, g | 215 ± 2 | 268 ± 3* |
| DHT, pg/ml | 32.13 ± 11.85 | 103.57 ± 23.22* |
| Estradiol, pg/ml | 4.35 ± 0.92 | 5.14 ± 0.58 |
n = 7–10 rats/group. DHT, dihydrotestosterone; SD, Sprague-Dawley.
P < 0.05 compared with control rats.
Fig. 1.
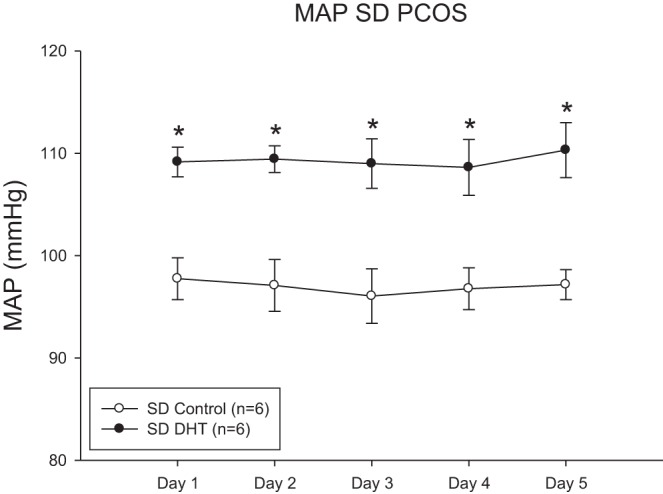
Dihydrotestosterone (DHT) increases mean arterial pressure (MAP) in Sprague-Dawley (SD) female rats. MAP was measured by telemetry for 5 consecutive days. Data were analyzed by t-test with significance defined as P < 0.05. *P < 0.05 DHT-treated rats compared with control rats. PCOS, polycystic ovary syndrome.
To determine if chronic DHT supplementation in SD female rats affected 20-HETE synthesis, endogenous 20-HETE and ω-hydroxylase activity were measured in cerebral microvessels (as a control) and renal microvessels, as shown in Table 2. DHT increased 20-HETE levels in renal microvessels but not in cerebral vessels. In contrast, hyperandrogenemic SD female rats had 20-HETE levels below detection in renal microsomal fraction, an indication of renal tubular 20-HETE levels. DHT also increased ω-hydroxylase activity in renal microvessels but not in cerebral microvessels. In renal microsomes, DHT reduced ω-hydroxylase activity.
Table 2.
20-HETE and ω-hydroxylase activities in cerebral and renal vessels and renal microsomes of control and hyperandrogenemic (DHT-treated) SD female rats
| 20-HETE Level, pmol/mg |
ω-Hydroxylase Activity, pmol 20-HETE·min−1·mg−1 |
|||||
|---|---|---|---|---|---|---|
| Cerebral Vessels | Renal Vessels | Renal Microsomes | Cerebral Vessels | Renal Vessels | Renal Microsomes | |
| Control rats | 0.51 ± 0.04 | 0.15 ± 0.02 | 1.25 ± 0.0 | 0.62 ± 0.03 | 0.20 ± 0.04 | 119.06 ± 4.9 |
| DHT-treated rats | 0.51 ± 0.06 | 0.81 ± 0.08 | Below detection | 0.09 ± 0.02 | 0.69 ± 0.14 | 101.15 ± 3.4 |
| P value | NS | <0.0001 | <0.0001 | <0.0001 | <0.009 | <0.01 |
n = 6 rats/group. 20-HETE, 20-hydroxyeicosatetraenoic acid; NS, not significant.
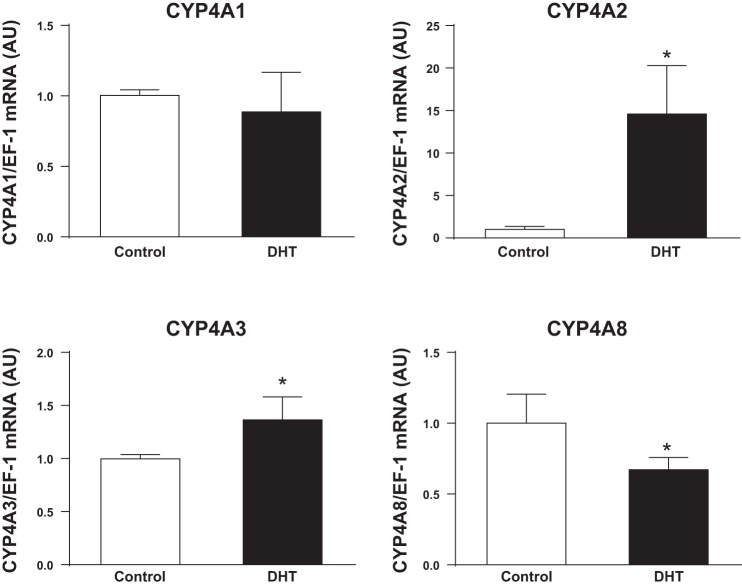
As shown in Fig. 2, real time RT-PCR showed that DHT had no effect on renal microvascular expression of CYP4A1, reduced expression of CYP4A8, slightly increased expression of CYP4A3, but significantly increased expression of CYP4A2 ω-hydroxylase by ∼15-fold.
Fig. 2.
mRNA expression of cytochrome P-450 (CYP)4A1, CYP4A2, CYP4A3, and CYP4A8 in the kidneys of control and DHT-treated SD female rats. n = 6 rats/group. Real-time RT-PCR was performed as described in materials and methods. Data were analyzed by t-test with significance defined as P < 0.05. *P < 0.05, DHT-treated female rats vs. control rats. EF-1, elongation factor I; AU, arbitrary units.
Experiment 2: Dahl SR versus Dahl SS female rats.
Based on the experiments in SD female rats, we determined the effect of DHT supplements in 20-HETE-sufficient Dahl SR rats and 20-HETE-deficient Dahl SS rats. As shown in Table 3, DHT levels were similar in control Dahl SR and SS female rats and were increased with DHT to similar levels (∼10-fold in both Dahl SS and SR rats). Plasma estradiol was ∼2.5-fold higher in Dahl SS compared with control Dahl SR female rats and was reduced with DHT treatment in both Dahl SR and SS female rats to similar levels. As also shown in Table 3, body weight was greater in control Dahl SS female rats than control Dahl SR female rats and was increased with DHT supplements by ∼25% in both groups.
Table 3.
Body weight as well as plasma DHT and estradiol levels in control and DHT-treated Dahl SR and Dahl SS female rats
| Dahl SR |
Dahl SS |
|||
|---|---|---|---|---|
| Control | DHT | Control | DHT | |
| Body weight, g | 230 ± 4 | 284 ± 6* | 256 ± 6†‡ | 319 ± 6*†‡ |
| DHT, pg/ml | 54.23 ± 5.33 | 578.06 ± 74.92* | 46.88 ± 2.68‡ | 579.68 ± 31.50* |
| Estradiol, pg/ml | 10.52 ± 1.77 | 7.46 ± 1.69* | 25.96 ± 5.86†‡ | 9.41 ± 0.76*† |
n = 6–7 rats/group. SR, salt resistant; SS, salt sensitive.
P < 0.05 compared with controls of the same strain;
P < 0.05 compared with control Dahl SR rats;
P < 0.05 compared with DHT- treated Dahl SR rats.
As shown in Fig. 3, despite being maintained on a low-salt diet, MAP was significantly higher in control Dahl SS female rats than control Dahl SR female rats. DHT supplements increased MAP in Dahl SR female rats by ∼20% but had no effect on MAP in Dahl SS female rats.
Fig. 3.
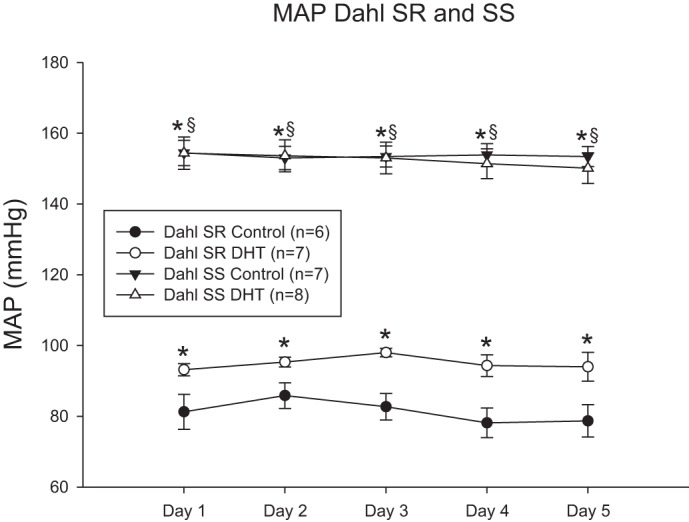
DHT increases MAP in Dahl salt-resistant (SR) female rats but not Dahl salt-sensitive (SS) female rats. MAP was measured by telemetry for 5 consecutive days. Data were analyzed by two-way ANOVA with significance defined as P < 0.05. *P < 0.05 vs. control Dahl SR rats; §P < 0.05 vs. DHT-treated Dahl SR rats.
Experiment 3: SS.5Bn and CYP4A2−/− female rats.
Because we found that there was a 15-fold increase in CYP4A2 expression in renal microvessels of female rats supplemented with DHT, we evaluated the effects of DHT supplements on CYP4A2−/− female rats compared with wild-type SS.5Bn female rats. As shown in Table 4, plasma DHT was similar in control SS.5Bn and CYP4A2−/− female rats and was increased with DHT supplements by approximately threefold in female rats of both strains compared with control rats. Plasma estradiol was slightly higher in control SS.5Bn rats than control CYP4A2−/− rats and was reduced to similar levels in rats of both strains with DHT supplements. Body weight was similar in control SS.5Bn and control CYP4A2−/− female rats, and DHT supplements increased body weights in SS.5Bn and CYP4A2−/− female rats by 30% and 50%, respectively.
Table 4.
Body weight as well as plasma DHT and estradiol levels in control and DHT-treated SS.5Bn and CYP4A2−/− female rats
| SS.5Bn |
CYP4A2−/− |
|||
|---|---|---|---|---|
| Control | DHT | Control | DHT | |
| Body weight, g | 269 ± 3 | 358 ± 11* | 254 ± 9‡ | 384 ± 13*†‡ |
| DHT, pg/ml | 27.29 ± 2.50 | 110.88 ± 5.67* | 27.30 ± 2.68†‡ | 101.82 ± 10.74*† |
| Estradiol, pg/ml | 9.54 ± 1.64 | 3.91 ± 0.85* | 6.92 ± 0.73‡ | 4.40 ± 0.50*† |
n = 6–8 rats/group. CYP, cytochrome P-450.
P < 0.05 compared with controls of the same strain;
P < 0.05 compared with control SS.5Bn rats;
P < 0.05 compared with DHT-treated SS.5Bn rats.
As shown in Fig. 4, control CYP4A2−/− female rats maintained on a low-salt diet had significantly higher MAP compared with SS.5Bn female rats. DHT increased MAP in SS.5Bn female rats by 20% but had no effect on MAP in CYP4A2−/− female rats.
Fig. 4.
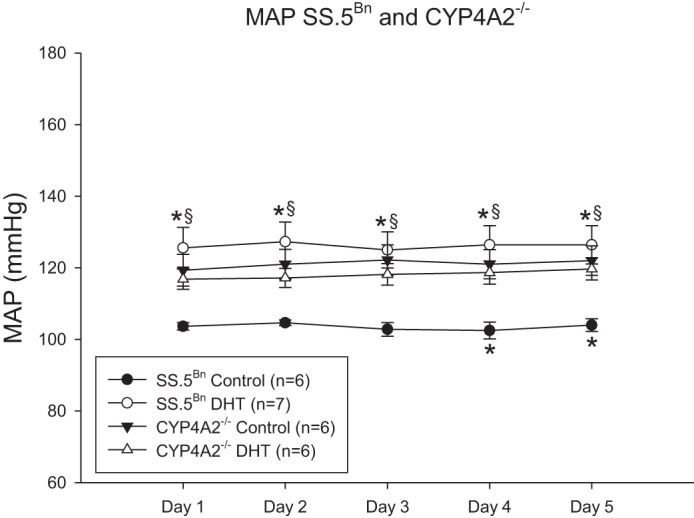
DHT increases MAP in SS.5Bn female rats but not in CYP4A2−/− female rats. MAP was measured by telemetry for 5 consecutive days. Data were analyzed by two-way ANOVA with significance defined as P < 0.05. *P < 0.05, control SS.5Bn vs. DHT-treated SS.5Bn rats; §P < 0.05, control CYP4A2−/− and DHT-treated rats vs. control SS.5Bn rats.
As shown in Table 5, cerebral and renal microvascular endogenous 20-HETE was lower in untreated control CYP4A2−/− than SS.5Bn female rats (P < 0.05). DHT supplements increased endogenous 20-HETE in cerebral microvessels of SS.5Bn female rats by almost fourfold and by twofold in CYP4A2−/− female rats (P < 0.01, CYP4A2−/− vs. SS.5Bn rats). DHT supplements increased renal microvascular 20-HETE by sevenfold in SS.5Bn female rats but had no effect in CYP4A2−/− female rats. Cerebral microvascular ω-hydroxylase activity was slightly higher in SS.5Bn female rats (P < 0.05 vs. CYP4A2−/− female rats) and was not affected by DHT supplements, whereas cerebral ω-hydroxylase activity was increased with DHT in CYP4A2−/− female rats. Renal microvascular ω-hydroxylase activity was 40% lower in CYP4A2−/− than control SS.5Bn female rats (P < 0.05). DHT supplements reduced ω-hydroxylase activity by ∼50% in SS.5Bn female rats but increased activity by approximately threefold in CYP4A2−/− female rats (P < 0.01 vs. SS.5Bn female rats).
Table 5.
Endogenous 20-HETE and ω-hydroxylase activity in control and hyperandrogenemic female (DHT-treated) SS.5Bn and CYP4A2−/− rats
| Endogenous 20-HETE, pmol/mg |
ω-Hydroxylase Activity pmol 20-HETE·min−1·mg−1 |
|||
|---|---|---|---|---|
| Cerebral vessels | Renal vessels | Cerebral vessels | Renal vessels | |
| SS.5Bn female rats | ||||
| Control | 0.047 ± 0.010 (n = 3) | 0.059 ± 0.006 (n = 3) | 0.034 ± 0.004 (n = 6) | 7.346 ± 0.588 (n = 5) |
| DHT | 0.082 ± 0.019 (n = 3) | 0.118 ± 0.009 (n = 3) | 0.033 ± 0.004 (n = 8) | 3.715 ± 0.330 (n = 7) |
| P value | NS | <0.005 | NS | <0.01 |
| CYP4A−/− female rats | ||||
| Control | 0.029 ± 0.004 (n = 5) | 0.035 ± 0.008 (n = 5) | 0.023 ± 0.004 (n = 8) | 4.299 ± 0.8760 (n = 8) |
| DHT | 0.056 ± 0.006 (n = 4) | 0.075 ± 0.026 (n = 3) | 0.068 ± 0.016 (n = 7) | 13.789 ± 0.9841 (n = 7) |
| P value | <0.01 | NS | <0.001 | <0.0001 |
Statistics are from two-way ANOVA comparison of DHT- versus placebo-treated control rats. Statistics for intergroup comparison are in text.
DISCUSSION
In the present study, we followed up on our previous observations that chronic DHT increased MAP in SD female rats to evaluate the role of 20-HETE as a potential mechanism responsible for the DHT-mediated increase in BP. In this study, we found that 1) DHT increased renal microvascular mRNA expression of CYP4A2 and CYP4A3 and reduced CYP4A8 in SD female rats, and these data are supported by the increases in endogenous renal microvascular 20-HETE in DHT-treated SD female rats; 2) despite a similar significant increase in body weight with DHT in Dahl SS and Dahl SR female rats, DHT did not increase MAP in 20-HETE-deficient Dahl SS female rats, whereas DHT did increase MAP in Dahl SR female rats, suggesting that an intact 20-HETE system capable of upregulation contributes to the elevated BP with DHT in female rats; and 3) despite a greater increase in body weight and increases in endogenous renal 20-HETE levels (albeit modest compared with SS.5Bn female rats) with DHT supplements, lack of CYP4A2 ω-hydroxylase prevented the DHT-mediated pressor response in CYP4A2−/− female rats. Taken together, these data suggest that not only does 20-HETE contribute to the DHT-mediated elevated BP in female rats but that the CYP4A2 isoform may at least in part be responsible for the increase in BP. In support of our findings for a role for CYP4A2 in the DHT-mediated increases in BP in female rats, Schwartzman and colleagues (27) showed that lentiviral vectors that increase intrarenal CYP4A2 expression in male rats caused an increase in their BP. Holla and colleagues (9) made similar observations regarding CYP4A isoforms.
Our data show that CYP4A2 and CYP4A3 isoform mRNA was increased and that CYP4A8 isoform mRNA was reduced in the renal microvasculature of DHT-treated SD female rats. These data were somewhat surprising since Schwartzman and colleagues reported that androgens cause an upregulation of renal vascular CYP4A8 in male rats (25–27, 32). One caveat is that the androgen levels produced by testosterone supplements in male rats in the Schwartzman et al. studies were significantly higher than in the female rats in our study. However, taken together, these data are exciting, because they suggest that different isoforms of CYP4A are androgen sensitive in male and female rats and yet contribute to an androgen-mediated increase in BP in both sexes.
The role of 20-HETE to modulate BP is well known (20, 21), and 20-HETE has differential effects on the kidney depending on the location of synthesis. In the renal microvasculature, 20-HETE is prohypertensive, acting as a vasoconstrictor, whereas in the renal tubules, 20-HETE attenuates Na+ reabsorption and thus is antihypertensive. Since we found that there was no increase in endogenous renal microsomal 20-HETE with DHT in SD female rats, the data suggest that it was the increase in renal microvascular 20-HETE with DHT that affected their BP. Simiarly, the increase in MAP in SS.5Bn female rats was also accompanied with a sevenfold increase in endogenous 20-HETE in the renal microvessels, whereas there was no increase in renal microvascular endogenous 20-HETE or MAP with DHT in CYP4A2−/− female rats, also suggesting that the increase in endogenous 20-HETE mediated via CYP4A2 played a role in the increased BP in SS.5Bn female rats.
However, the differences in ω-hydroxylase activity in response to DHT in SS.5Bn and CYP4A2−/− female rats are not clear. In SD female rats, the increase in endogenous 20-HETE was accompanied by an increase in ω-hydroxylase activity (Table 2). However, the significant increase in renal microvascular endogenous 20-HETE in SS.5Bn female rats was accompanied by a reduction in ω-hydroxylase activity (Table 5). It is possible that this could be a compensatory negative feedback effect since endogenous 20-HETE levels in SS.5Bn female rats increased by sevenfold. However, the reason why ω-hydroxylase activity increased significantly in the renal microvasculature of CYP4A2−/− female rats with DHT is not clear since there was no effect of DHT on endogenous 20-HETE levels or on MAP. Perhaps the increased ω-hydroxylase activity reflected a compensatory increase in the synthesis of other CYP4A isoforms; however, since there was no pressor effect in response to DHT in CYP4A2−/− female rats, it remains unclear as to the physiological relevance of the DHT-mediated increase in ω-hydroxylase activity.
Although we performed these experiments separately comparing control and DHT supplements in SD female rats, control and DHT supplements in Dahl SR versus Dahl SS female rats, and control and DHT supplements in SS.5Bn and CYP4A2−/− female rats, and thus cannot statistically compare the data among the strains, it was surprising that DHT levels increased to much higher levels with DHT supplements in Dahl SS and Dahl SR rats than in the other strains, despite similar DHT treatment, similar DHT-mediated increases in body weight, and similar estradiol levels among all strains. It was also surprising to us that there was such a significant difference in MAP between control Dahl SS and Dahl SR female rats at 16 wk of age. We used Dahl SS female rats in a study (22) published in 2011 and found that MAP, also measured by telemetry, was ∼110 ± 2 mmHg at 10–11 wk of age when rats were maintained on a low-salt diet (0.3%) that was free of phytoestrogens. In this previous study (22), when Dahl SS female rats were given a 4% salt diet at 12 wk of age, their MAP increased to ∼125 ± 4 mmHg. In the present study, control Dahl SS female rats were maintained on a similar low-salt diet that was not phytoestrogen free (this would in fact tend to reduce BP), and MAP was ∼155 ± 4 mmHg (Fig. 3). We have not previously measured telemetry BP in Dahl SR females, but we used 6–8 rats/group and the SEs in the measurements were ≤1–2 mmHg. In support of our data, Gillis and colleagues (8) also measured telemetry BP in Dahl SS female rats and found similar BPs as we did in the present study. In addition, in control CYP4A2−/− female rats, which are on a Dahl SS background and in which only one CYP4A isoform was missing, MAP (120 ± 3 mmHg) was intermediate between control SS.5Bn (with intact CYP4A isoforms) and Dahl SS (with deficient CYP4A isoforms) strains, as might be expected.
One caveat for our study is that it is possible that in Dahl SS and CYP4A2−/− female rats, endogenous CYP4A ω-hydroxylase and 20-HETE levels may be lower in renal tubules than in controls, leading to significant increases in tubular Na+ reabsorption and higher BP. In the presence of reduced 20-HETE in the tubules of control Dalh SS and CYP4A2−/− female rats, it is possible then that an increase in renal microvascular 20-HETE mediated by DHT would not be able to overcome this reduction in tubular 20-HETE, thus preventing a further increase in BP in these strains compared with controls. Furthermore, since reductions in nitric oxide are also a feature of hypertension in Dahl SS rats (1), it is also possible that DHT-mediated increases in renal microvascular 20-HETE in Dahl SS rats were not capable of overcoming the reduced nitric oxide to increase BP. Furthermore, since we did not measure the mRNA expression of other CYP4A isoforms in DHT-treated CYP4A2−/− rats, it is possible that DHT-mediated upregulation of other CYP4A isoforms in the tubules of CYP4A2−/− rats could have contributed to the lack of a pressor response to DHT.
Another caveat for the present study is the lack of an effect on endogenous 20-HETE by DHT in the cerebral microvessels of SD female rats, whereas the levels of ω-hydroxylase were significantly reduced. ω-Hydroxylase activity is the capacity of the tissue to produce 20-HETE when nonrate-limiting levels of arachidonic acid are present. Endogenous 20-HETE is the level that is present in the tissue with no added substrate or cofactors. The fact that DHT had no effect on endogenous 20-HETE levels in the cerebral vessels suggests that DHT did not increase either substrate or enzyme levels to increase synthesis of 20-HETE in SD rats. Since ω-hydroxylase activity was reduced with DHT in cerebral vessels, the data suggest that DHT may actually reduce enzyme synthesis. We have not followed up on this possibility.
Whether 20-HETE is elevated in the renal microvasculature of women with elevated circulating androgens is unknown. 20-HETE has been shown to be associated with increases in cardiovascular disease, renal disease, and endothelial dysfunction in humans. Schuck and colleagues (23) reported that 20-HETE caused reduced flow-mediated dilation, but the data were not analyzed for men and women separately. Similarly, Dreisbach and colleagues (4) reported that 20-HETE was elevated in urine from African-American men and women who had chronic kidney disease, but, again, the data were not analyzed separately by sex. Thus, future studies will need to be performed to determine if androgens increase 20-HETE in men and/or women with PCOS or postmenopausal hypertension. More importantly, in future studies, clinical investigators need to make certain that their data are analyzed separately for men and women to fully understand the effect of sex on their data.
Perspectives
Young women with hyperandrogenemia such as PCOS that have modest elevations in BP are typically not treated since the BP levels do not meet the recommendations prescribed in Joint National Committee Guidelines (12). However, women who have elevated androgens may be protected from early cardiovascular disease risk and the development of essential hypertension associated with aging using modest BP-lowering therapies early on. Furthermore, if androgens increase 20-HETE synthesis in the kidneys of women, they may benefit from 20-HETE synthesis antagonists. Studies will be necessary to evaluate how androgens affect 20-HETE synthesis in women and where, in the human female kidney, androgen-mediated 20-HETE synthesis occurs. Furthermore, in postmenopausal women who receive androgens to improve their libido, care should be taken to monitor their BP carefully to evaluate and treat any increases.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants R01-HL66072 and P01 HL51971 (to J. F. Reckelhoff) and by American Heart Association Postdoctoral Fellowships 14POST18640015 (to R. Maranon) and 11POST7450006 to (to M. Moulana).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
C.D., R.O.M., C.N.P., M.M., and D.G.R. performed experiments; C.D., R.O.M., C.N.P., M.M., D.G.R., and J.F.R. analyzed data; C.D., R.O.M., C.N.P., M.M., D.G.R., and J.F.R. interpreted results of experiments; C.D., R.O.M., C.N.P., and J.F.R. prepared figures; C.D., R.O.M., C.N.P., and J.F.R. drafted manuscript; C.D., R.O.M., C.N.P., M.M., D.G.R., and J.F.R. edited and revised manuscript; C.D., R.O.M., C.N.P., M.M., D.G.R., and J.F.R. approved final version of manuscript; J.F.R. conception and design of research.
ACKNOWLEDGEMENTS
The authors thank Dr. Richard Roman (University of Mississippi Medical Center) for giving the authors the breeding pairs for the SS.5Bn and CYP4A2−/− rats, and the LC/MS Core Facility for 20-HETE and ω-hydroxylase activity measurements.
REFERENCES
- 1.Chen PY, Sanders PW. Role of nitric oxide synthesis in salt-sensitive hypertension in Dahl/Rapp rats. Hypertension 22: 812–818, 1993. [DOI] [PubMed] [Google Scholar]
- 2.Daan NM, Jaspers L, Koster MP, Broekmans FJ, de Rijke YB, Franco OH, Laven JS, Kavousi M, Fauser BC. Androgen levels in women with various forms of ovarian dysfunction: associations with cardiometabolic features. Hum Reprod 30: 2376–2386, 2015. [DOI] [PubMed] [Google Scholar]
- 3.Diamanti-Kandarakis E. Polycystic ovarian syndrome; pathophysiology, molecular aspects and clinical implications. Expert Rev Mol Med 10: 1–21, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Dreisbach AW, Smith SV, Kyle PB, Ramaiah M, Amenuke M, Garrett MR, Lirette ST, Griswold ME, Roman RJ. Urinary CYP eicosanoid excretion correlates with glomerular filtration in African-Americans with chronic kidney disease. Prostaglandins Other Lipid Mediat 113–115: 45–51, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fleming I. Cytochrome p450 and vascular homeostasis. Circ Res 89: 753–762, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Ge Y, Murphy SR, Fan F, Williams JM, Falck JR, Liu R, Roman RJ. Role of 20-HETE in the impaired myogenic and TGF responses of the Af-Art of Dahl salt-sensitive rats. Am J Physiol Renal Physiol 307: F509–F515, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geurts AM, Moreno C. Zinc-finger nucleases: new strategies to target the rat genome. Clin Sci (Lond) 119: 303–311, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gillis EE, Williams JM, Garrett MR, Mooney JN, Sasser JM. The Dahl salt-sensitive rat is a spontaneous model of superimposed preeclampsia. Am J Physiol Regul Integr Comp Physiol 309: R62–R70, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hasler JA. Pharmacogenetics of cytochromes P450. Mol Aspects Med 20: 12–137, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Holla VR, Adas F, Imig JD, Hao X, Price E Jr, Olsen N, Kovacs WJ, Magnuson MA, Keeney DS, Breyer MD, Falck JR, Waterman MR, Capdevila JH. Alterations in the regulation of androgen sensitive Cyp 4a monooxygenases cause hypertension. Proc Natl Acad Sci USA 98: 5211–5216, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu MH, Savas U, Griffin KJ, Johnson EF. Human cytochrome p450 family 4 enzymes: function, genetic variation and regulation. Drug Metab Rev 39: 515–538, 2007. [DOI] [PubMed] [Google Scholar]
- 12.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT Jr, Narva AS, Ortiz E. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 311: 507–520, 2014. [DOI] [PubMed] [Google Scholar]
- 13.Lukaszewicz KM, Lombard JH. Role of the CYP4A/20-HETE pathway in vascular dysfunction of the Dahl salt-sensitive rat. Clin Sci (Lond) 124: 695–700, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma YH, Schwartzman ML, Roman RJ. Altered renal P-450 metabolism of arachidonic acid in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 267: R579–R589, 1994. [DOI] [PubMed] [Google Scholar]
- 15.Maier KG, Roman RJ. Cytochrome P450 metabolites of arachidonic acid in the control of renal function. Curr Opin Nephrol Hypertens 10: 81–87, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Medical College of Wisconsin Transgenic Rat Facility. Gene: Cyp4a2em1Mcwi (Cytochrome P450, Family 4, Subfamily a, Polypeptide 2; Zinc Finger Nuclease Induced Mutant 1, Medical College of Wisconsin) Rattus norvegicus (online). http://rgd.mcw.edu/rgdweb/report/gene/main.html?id=5687724&view=1 [1 June 2016]. [Google Scholar]
- 16a.Medical College of Wisconsin Transgenic Rat Facility. Strain: SS-Chr 5BN-Cyp4a2em1Mcwi (online). http://rgd.mcw.edu/rgdweb/report/strain/main.html?id=5687974 [1 June 2016]. [Google Scholar]
- 17.Miyata N, Roman RJ. Role of 20-hydroxyeicosatetraenoic acid (20-HETE) in vascular system. J Smooth Muscle Res 41: 175–193, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Nakagawa K, Marji JS, Schwartzman ML, Waterman MR, Capdevila JH. Androgen-mediated induction of the kidney arachidonate hydroxylases is associated with the development of hypertension. Am J Physiol Regul Integr Comp Physiol 284: R1055–R1062, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Reckelhoff JF. Polycystic ovary syndrome: androgens and hypertension. Hypertension 49: 1220–1221, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Roman RJ, Maier KG, Sun CW, Harder DR, Alonso-Galicia M. Renal and cardiovascular actions of 20-hydroxyeicosatetraenoic acid and epoxyeicosatrienoic acids. Clin Exp Pharmacol Physiol 27: 855–865, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev 82: 131–185, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Sartori-Valinotti JC, Venegas-Pont MR, Lamarca BB, Romero DG, Yanes LL, Racusen LC, Jones AV, Ryan MJ, Reckelhoff JF. Rosiglitazone reduces blood pressure in female Dahl salt-sensitive rats. Steroids 75: 794–799, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schuck RN, Theken KN, Edin ML, Caughey M, Bass A, Ellis K, Tran B, Steele S, Simmons BP, Lih FB, Tomer KB, Wu MC, Hinderliter AL, Stouffer GA, Zeldin DC, Lee CR. Cytochrome P450-derived eicosanoids and vascular dysfunction in coronary artery disease patients. Atherosclerosis 227: 442–448, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaw LJ, Bairey Merz CN, Azziz R, Stanczyk FZ, Sopko G, Braunstein GD, Kelsey SF, Kip KE, Cooper-Dehoff RM, Johnson BD, Vaccarino V, Reis SE, Bittner V, Hodgson TK, Rogers W, Pepine CJ. Postmenopausal women with a history of irregular menses and elevated androgen measurements at high risk for worsening cardiovascular event-free survival: results from the National Institutes of Health-National Heart, Lung, and Blood Institute sponsored Women's Ischemia Syndrome Evaluation. J Clin Endocrinol Metab 93: 1276–1284, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Singh H, Cheng J, Deng H, Kemp R, Ishizuka T, Nasjletti A, Schwartzman ML. Vascular cytochrome P450 4A expression and 20-hydroxyeicosatetraenoic acid synthesis contribute to endothelial dysfunction in androgen-induced hypertension. Hypertension 50: 123–129, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Singh H, Schwartzman ML. Renal vascular cytochrome P450-derived eicosanoids in androgen induced hypertension. Pharmacol Rep 60: 29–37, 2010. [PubMed] [Google Scholar]
- 27.Sodhi K, Wu CC, Cheng J, Gotlinger K, Inoue K, Goli M, Flack JR, Abraham NG, Schwartzman ML. CYP4A2-induced hypertension is 20-HETE and angiotensin II dependent. Hypertension 56: 871–878, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stec DE, Deng AY, Rapp JP, Roman RJ. Cytochrome P4504A genotype cosegregates with hypertension in Dahl S rats. Hypertension 27: 564–568, 1996. [DOI] [PubMed] [Google Scholar]
- 29.Weiner CP, Lizasoain I, Baylis SA, Knowles RG, Charles IG, Moncada S. Induction of calcium dependent NO synthase by sex hormones. Proc Natl Acad Sci USA 91: 5212–5216, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams JM, Murphy S, Burke M, Roman RJ. 20-Hydroxyeicosatetraeonic acid: a new target for the treatment of hypertension. J Cardiovasc Pharmacol 56: 336–344, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams JM, Fan F, Murphy S, Schreck C, Lazar J, Jacob HJ, Roman RJ. Role of 20-HETE in the antihypertensive effect of transfer of chromosome 5 from Brown Norway to Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 302: R1209–R1218, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu CC, Schwartzman ML. The role of 20-HETE in androgen-mediated hypertension. Prostaglandins Other Lipid Mediat 96: 45–53, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yanes LL, Lima R, Moulana M, Romero DG, Yuan K, Ryan MJ, Baker R, Zhang H, Fan F, Davis DD, Roman RJ, Reckelhoff JF. Postmenopausal hypertension: role of 20-HETE. Am J Physiol Regul Integr Comp Physiol 300: R1543–R1548, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yanes LL, Romero DG, Moulana M, Lima R, Davis DD, Zhang H, Lockhart R, Racusen LC, Reckelhoff JF. Cardiovascular-renal and metabolic characterization of a rat model of polycystic ovary syndrome. Gend Med 8: 103–115, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]