Abstract
Kir4.1 is an inwardly rectifying potassium (K+) channel and is expressed in the brain, inner ear, and kidney. In the kidney, Kir4.1 is expressed in the basolateral membrane of the late thick ascending limb (TAL), the distal convoluted tubule (DCT), and the connecting tubule (CNT)/cortical collecting duct (CCD). It plays a role in K+ recycling across the basolateral membrane in corresponding nephron segments and in generating negative membrane potential. The renal phenotypes of the loss-function mutations of Kir4.1 include mild salt wasting, hypomagnesemia, hypokalemia, and metabolic alkalosis, suggesting that the disruption of Kir4.1 mainly impairs the transport in the DCT. Patch-clamp experiments and immunostaining demonstrate that Kir4.1 plays a predominant role in determining the basolateral K+ conductance in the DCT. However, the function of Kir4.1 in the TAL and CNT/CCD is not essential, because K+ channels other than Kir4.1 are also expressed. The downregulation of Kir4.1 in the DCT reduced basolateral chloride (Cl−) conductance, suppressed the expression of ste20 proline-alanine-rich kinase (SPAK), and decreased Na-Cl cotransporter (NCC) expression and activity. This suggests that Kir4.1 regulates NCC expression by the modulation of the Cl−-sensitive with-no-lysine kinase–SPAK pathway.
Keywords: Kcnj10, kidney
an inwardly rectifying potassium (K+) channel, Kir4.1 has been shown to be expressed in the brain, inner ear, eye, gastric parietal cells, and kidney (7, 14, 21, 28, 30). Loss-of-function mutations of Kir4.1 cause epilepsy, ataxia, sensorineural deafness, and renal tubulopathy/seizures, sensorineural deafness, ataxia, intellectual deficit, and electrolyte imbalance syndrome (1). In the brain, Kir4.1 is expressed in glial cells, particularly in astrocytes, and it is involved in the “K+ spatial buffering” process, which is essential for maintaining resting membrane potential of neurons (21). In the inner ear, Kir4.1 is required for generating the endocochlear potential of intermediate cells and for maintaining high K+ content of the endolymph (30). In the eye, Kir4.1 is expressed in retina Muller glia cells and in corneal epithelial cells, and it plays a role in the regulation of the extracellular K+ level and in regulating the healing process of cornea epithelial cells, respectively (14, 16). In the kidney, Kir4.1 interacts with Kir5.1 to form a 40-pS inwardly rectifying K+ channel in the basolateral membrane of the late thick ascending limb (TAL), distal convoluted tubule (DCT), connecting tubule (CNT), and initial collecting duct (CCD) (17, 41, 43). The importance of Kir4.1 in maintaining epithelial electrolyte transport in the DCT is convincingly documented by the report that patients with loss-of-function mutations of Kir4.1 in the kidney have metabolic alkalosis, hypomagnesemia, hypokalemia, and hypocalciuria (1, 28, 31). The aim of this short review is to provide an overview regarding the function and regulation of Kir4.1 in the TAL and distal nephron segments.
THICK ASCENDING LIMB
The TAL is responsible for absorption of 20–25% of the filtered NaCl, Ca2+, and Mg2+ and plays a key role in concentrating urine (9, 11). The basolateral K+ channels participate in generating the negative cell membrane potential, which provides the driving force for chloride (Cl−) exit through the basolateral Cl− channels. Since Cl− absorption in the TAL is completely through transcellular pathways, it is possible that the inhibition of basolateral K+ conductance should affect the transcellular Cl− absorption. Patch-clamp experiments have identified two types of K+ channels in the basolateral membrane of the TAL: a 40-pS inwardly rectifying K+ channel and an Na+- and Cl−-activated, 80- to 150-pS K+ channel (Kca4.1 or slo2.2) (24, 41). Because the 40-pS K+ channel was absent in the TAL of Kcnj10−/− mice, this suggests that the 40-pS K+ channel is a Kir4.1 and Kir5.1 heterotetramer (41). The expression of Kir4.1 is not uniformly distributed along the mouse TAL, and it is highly detected in the late part of the TAL (41). Although Kir4.1 is also detected in human TAL, loss-of-function mutations of Kir4.1 in humans did not show the phenotype of Bartter syndrome, suggesting that the disruption of Kir4.1 has no significant effect on transport function in the TAL (1, 31). Studies performed in postneonatal day 9 Kcnj10−/− mice demonstrated that disruption of Kir4.1 in mice leads to the stimulation of the 80- to 150-pS K+ channel expression, thereby compensating for the function of Kir4.1 (6). Therefore, the function of Kir4.1 in the TAL of mice is not indispensable. Because the disruption of Kir4.1 may lead to an increase in vasopressin release induced by volume contraction, it is suggested that an increase in vasopressin levels may be responsible for the stimulation of Na+- and Cl−-activated K+ channels (6). However, new experiments are needed in adult Kcnj10 knockout mice to confirm this observation.
DISTAL CONVOLUTED TUBULE
The DCT is responsible for the reabsorption of 5–9% of the filtered Na+ and plays a role in the reabsorption of Ca2+ and Mg2+ (5). The DCT is generally divided into the early part (DCT1) and the late portion (DCT2). Whereas the thiazide-sensitive Na-Cl cotransporter (NCC) is expressed in the apical membrane of both DCT1 and DCT2, the channel activity of renal outer medullary K (ROMK) and epithelial Na+ channel (ENaC) is only detected in the apical membrane of DCT2, although ROMK immunostaining is also detected in DCT1 (36). The reabsorption of NaCl in the DCT1 is a two-step process. Na+ and Cl− enter the cells across the apical membrane through the NCC, and Na+ is then pumped out of the cell through the basolateral Na-K-ATPase, whereas Cl− exits the cell along its electrochemical gradient by basolateral Cl− channels (ClC-kb) or the K/Cl cotransporter (KCC) (20, 22).
The basolateral K+ channels in the DCT participate in generating the cell membrane potential and are responsible for K+ recycling, which is important for sustaining the activity of the Na+-K+-ATPase (10). The patch-clamp experiments performed in the basolateral membrane of the DCT detected a 40-pS inwardly rectifying K+ channel (17). Because this 40-pS K+ channel is completely absent in Kcnj10−/− and Kcnj16−/− mice, it indicates that this 40-pS K+ channel is a heterotetramer of Kir4.1 and Kir5.1 (23, 43). Moreover, the disruption of Kir4.1 largely abolished the basolateral K+ conductance in the DCT, indicating that Kir4.1 plays a dominant role in determining the basolateral K+ conductance (43). The basolateral 40-pS K+ channel has been shown to be sensitive to cell pH, such that the K+ channel activity was inhibited by acidic pH (15). Moreover, Kir5.1 may play a role in the regulation of pH sensitivity of the Kir4.1/Kir5.1 heterotetramer (25, 33). This notion is also supported in experiments performed on Kcnj16−/− mice, in which the pH sensitivity of the basolateral 20-pS K+ channel, presumably a Kir4.1 homotetramer, in the DCT is blunted (23). In addition, the calcium-sensing receptor (CaSR) has been shown to be colocalized with Kir4.1 in the DCT. Because the expression of CaSR decreased the surface expression of Kir4.1 in human embryonic kidney cells, it is suggested that CaSR may play a role in modulating the activity of the basolateral 40-pS K+ channel in the DCT (2, 13). Kir4.1 is a substrate of src-family protein tyrosine kinase (SFK), and coexpression of c-Src phosphorylates Kir4.1 at Tyr9 (42). The SFK-induced tyrosine phosphorylation of Kir4.1 plays a role in the stimulation of the basolateral 40-pS K+ channel in the DCT, because the inhibition of SFK suppressed the 40-pS K+ channel activity. The stimulatory effect of SFK on the basolateral 40-pS K+ channel requires the participation of caveolin-1, which is highly expressed in the basolateral membrane of the DCT, CNT, and CCD (37). The disruption of caveolin-1 inhibited the Kcnj10/16 activity in the basolateral 40-pS K+ channel in the mouse DCT (37).
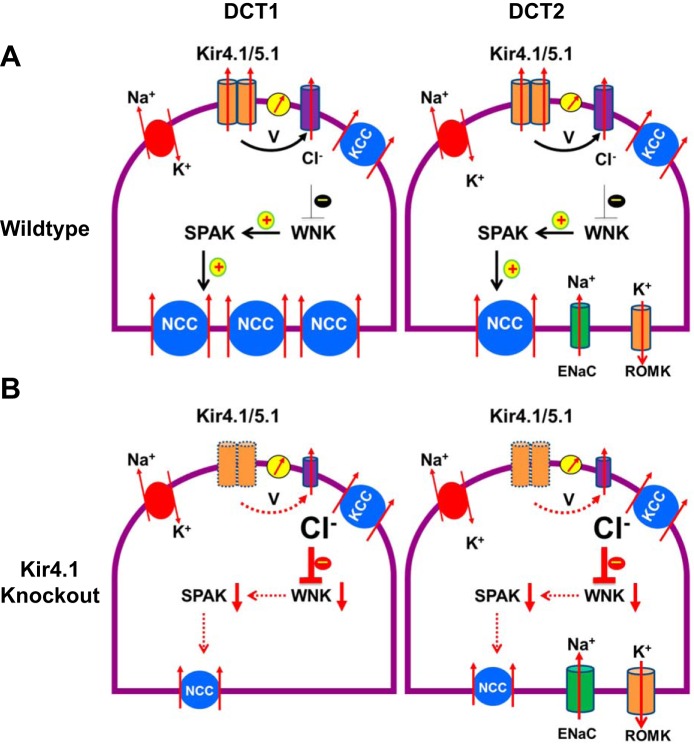
Figure 1 is a scheme illustrating the physiological role of Kir4.1 in the DCT. It is possible that the basolateral Kir4.1/5.1 may control intracellular Cl− concentrations (Cli) in the DCT. For instance, inhibition of Kir4.1/5.1 depolarizes the negative cell voltage (V), thereby decreasing Cl− exit across the basolateral membrane and increasing Cli in the DCT. This speculation is supported by the report that an increase in Cli, induced by depolarization, is responsible for the inhibition of NCC activity in the cells expressing loss-of-function Kcnj10 mutants (35). Because an increase in Cli inhibits with-no-lysine kinase (WNK) and ste20 proline-alanine-rich kinase (SPAK) activity (26, 34), a decrease in SPAK activity reduces the phosphorylation of NCC. Since SPAK-induced phosphorylation has been shown not only to activate NCC but also to inhibit NCC degradation (19, 27, 29, 39), a decrease in the basolateral K+ conductance in the DCT leads to the suppression of NCC expression. Our previous experiments also showed that the membrane potential in the DCT was less negative in Kcnj10−/− mice compared with their wild-type (WT) littermates (43). Thus it is conceivable that the activity of the basolateral K+ conductance in the DCT controls NCC activity through a Cl−-sensitive WNK pathway. In addition to Kir4.1, Cl channels and KCCs in the basolateral membrane could have an effect on the Cli concentration. Our previous study has demonstrated that Cl channel activity is tightly correlated with Kir4.1 activity, such that the inhibition of Kir4.1 decreased Cl channel activity (43). Thus the downregulation of Cl channels should amplify the effect of the Kir4.1 inhibition-induced increase in Cli. On the other hand, WNK-regulated SPAK/oxidative stress response kinases have been shown to phosphorylate KCC, thereby inhibiting the KCC (3). Thus the inhibition of SPAK should activate KCC, thereby facilitating Cl exit and reducing Cli. This may serve as a negative-feedback mechanism for controlling Cli. Further experiments are required to explore the role of both Cl channels and KCC in the regulation of Cli. Furthermore, it is essential to measure Cli in vivo or in vitro to validate this model.
Fig. 1.
A cell scheme illustrating the role of Kir4.1 in the regulation of Na-Cl cotransport (NCC) of the DCT by a Cl−-sensitive with-no-lysine kinase (WNK) signaling in WT (A) and Kir4.1 knockout mice (B). Dotted lines indicate deletion of Kir4.1 and decrease of the stimulation effect on downstream factors (B). SPAK, ste20 proline-alanine rich kinase; V, negative cell voltage.
CONNECTING TUBULE/CORTICAL COLLECTING DUCT
Immunostaining and electrophysiological experiments have shown that Kir4.1 is expressed in the basolateral membrane of the CNT and CCD (8, 15). Moreover, dopamine has been shown to inhibit Kir4.1/Kir5.1 directly in the CCD (40). Although Kir4.1 is expressed in the basolateral membrane of the CCD, the disruption of Kir4.1, however, causes a modest depolarization in the CNT/CCD, suggesting that Kir4.1 may not be the only type of K+ channel in the basolateral membrane of the CNT/CCD (32). Indeed, previous studies have identified three types of K+ channel activity in the basolateral membrane of the CCD (12, 38). We have also observed the activity of the 23-, 40-, and 60-pS K+ channels in the basolateral membrane of CNT and early CCD in WT mice (32). Since only 40 pS K+ channel was absent in the basolateral membrane of CNT/CCD in Kcnj10−/− mice, it strongly suggests that the 23- and 60-pS K+ channels are not related to Kir4.1. Therefore, it is conceivable that the electrophysiological gradient for Na+ entry across the apical membrane should be maintained. Immunostaining and Western blot have also revealed that the disruption of Kir4.1 increased the expression of ENaC-β and -γ subunits and also the cleaved form of ENaC-α in the kidney (32). However, because these experiments were performed in postneonatal day 9 Kcnj10−/− mice, further experiments in adult Kir4.1 knockout mice are required to validate the finding. Furthermore, we speculate that the increase in ENaC expression in Kcnj10−/− mice may be induced partially by the activation of the renin-ANG II-aldosterone system and vasopressin, which has been shown to stimulate ENaC activity (4). Thus the increase of Na+ absorption in the CCD compensates the Na+ wasting partially in the DCT, but it causes K+ wasting and hypokalemia.
SUMMARY
Studies performed in the global Kir4.1 knockout mice have firmly established the role of Kir4.1 in the regulation of NCC in the DCT. Moreover, since the disruption of Kir4.1 also inhibits the basolateral Cl− channel activity, this indicates that the Cli should be involved in mediating the effect of Kir4.1 inhibition on NCC expression.
GRANTS
Support for this work is provided by the National Institute of Diabetes and Digestive and Kidney Diseases (Grant DK 54983) and the National Heart, Lung, and Blood Institute (Grant HL34100).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
X-T.S. and W-H.W. conception and design of research; X-T.S. performed experiments; X-T.S. and W-H.W. analyzed data; X-T.S. and W-H.W. interpreted results of experiments; X-T.S. and W-H.W. prepared figures; X-T.S. and W-H.W. drafted manuscript; X-T.S. and W-H.W. edited and revised manuscript; X-T.S. and W-H.W. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Ms. Gail Anderson for editing the manuscript.
REFERENCES
- 1.Bockenhauer D, Feather S, Stanescu HC, Bandulik S, Zdebik AA, Reichold M, Tobin J, Lieberer E, Sterner C, Landoure G, Arora R, Sirimanna T, Thompson D, Cross JH, van't Hoff W, Al Masri O, Tullus K, Yeung S, Anikster Y, Klootwijk E, Hubank M, Dillon MJ, Heitzmann D, Arcos-Burgos M, Knepper MA, Dobbie A, Gahl WA, Warth R, Sheridan E, Kleta R. Epilepsy, ataxia, sensorineural deafness, tubulopathy, and KCNJ10 mutations. N Engl J Med 360: 1960–1970, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cha SK, Huang C, Ding Y, Qi X, Huang CL, Miller RT. Calcium-sensing receptor decreases cell surface expression of the inwardly rectifying K+ channel Kir4.1. J Biol Chem 286: 1828–1835, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de los Heros P, Alessi D, Gourlay R, Campbell D, Deak M, Macartney TJ, Kahle KT, Zhang J. The WNK-regulated SPAK/OSR1 kinases directly phosphorylate and inhibit the K+Cl− co-transporters. Biochem J 458: 559–573, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.Ecelbarger CA, Kim GH, Terris J, Masilamani S, Mitchell C, Reyes I, Verbalis JG, Knepper MA. Vasopressin-mediated regulation of epithelial sodium channel abundance in rat kidney. Am J Physiol Renal Physiol 279: F46–F53, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Ellison DH. Divalent cation transport by the distal nephron: insights from Bartter's and Gitelman's syndromes. Am J Physiol Renal Physiol 279: F616–F625, 2000. [DOI] [PubMed] [Google Scholar]
- 6.Fan L, Wang X, Zhang D, Duan X, Zhao C, Zu M, Meng X, Zhang C, Su XT, Wang MX, Wang WH, Gu R. Vasopressin-induced stimulation of the Na+-activated K+ channels is responsible for maintaining the basolateral K+ conductance of the thick ascending limb (TAL) in EAST/SeSAME syndrome. Biochim Biophys Acta 1852: 2554–2562, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujita A, Horio Y, Higashi K, Mouri T, Hata F, Takeguchi N, Kurachi Y. Specific localization of an inwardly rectifying K+ channel, Kir4.1, at the apical membrane of rat gastric parietal cells; its possible involvement in K+ recycling for the H+-K+-pump. J Physiol 540: 85–92, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gray DA, Frindt G, Zhang YY, Palmer LG. Basolateral K+ conductance in principal cells of rat CCD. Am J Physiol Renal Physiol 288: F493–F504, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Greger R. Ion transport mechanisms in thick ascending limb of Henle's loop of mammalian nephron. Physiol Rev 65: 760–797, 1985. [DOI] [PubMed] [Google Scholar]
- 10.Hebert SC, Desir G, Giebisch G, Wang W. Molecular diversity and regulation of renal potassium channels. Physiol Rev 85: 319–371, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hebert SC, Friedman PA, Culpepper RM, Andreoli TE. Salt absorption in the thick ascending limb of Henle's loop: NaCl cotransport mechanism. Sem Nephrol 2: 316–327, 1982. [Google Scholar]
- 12.Hirsch J, Schlatter E. K channels in the basolateral membrane of rat cortical colleting duct. Pflugers Arch 424: 470–477, 1993. [DOI] [PubMed] [Google Scholar]
- 13.Huang C, Sindic A, Hill CE, Hujer KM, Chan KW, Sassen M, Wu Z, Kurachi Y, Nielsen S, Romero MF, Miller RT. Interaction of the Ca2+-sensing receptor with the inwardly rectifying potassium channels Kir4.1 and Kir42 results in inhibition of channel function. Am J Physiol Renal Physiol 292: F1073–F1081, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Kofuji P, Ceelen P, Zahs KR, Surbeck LW, Lester HA, Newman EA. Genetic inactivation of an inwardly rectifying potassium channel (Kir4.1 subunit) in mice: phenotypic impact in retina. J Neurosci 20: 5733–5740, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lachheb S, Cluzeaud F, Bens M, Genete M, Hibino H, Lourdel S, Kurachi Y, Vandewalle A, Teulon J, Paulais M. Kir4.1/Kir5.1 channel forms the major K+ channel in the basolateral membrane of mouse renal collecting duct principal cells. Am J Physiol Renal Physiol 294: F1398–F1407, 2008. [DOI] [PubMed] [Google Scholar]
- 16.Lin D, Halilovic A, Yue P, Bellner L, Wang K, Wang L, Zhang C. Inhibition of miR-205 impairs the wound-healing process in human corneal epithelial cells by targeting KIR4.1 (KCNJ10)miR-205 inhibition and the wound-healing process. Invest Ophthalmol Vis Sci 54: 6167–6178, 2013. [DOI] [PubMed] [Google Scholar]
- 17.Lourdel S, Paulais M, Cluzeaud F, Bens M, Tanemoto M, Kurachi Y, Vandewalle A, Teulon J. An inward rectifier K+ channel at the basolateral membrane of the mouse distal convoluted tubles: similarites with Kir4-Kir5.1 heteromeric channels. J Physiol 538: 391–404, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCormick JA, Mutig K, Nelson JH, Saritas T, Hoorn EJ, Yang CL, Rogers S, Curry J, Delpire E, Bachmann S, Ellison DH. A SPAK isoform switch modulates renal salt transport and blood pressure. Cell Metab 14: 352–364, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melo Z, Cruz-Rangel S, Bautista R, Vázquez N, Castañeda-Bueno M, Mount DB, Pasantes-Morales H, Mercado A, Gamba G. Molecular evidence for a role for K(+)-Cl(−) cotransporters in the kidney. Am J Physiol Renal Physiol 305: F1402–F1411, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neusch C, Papadopoulos N, Müller M, Maletzki I, Winter SM, Hirrlinger J, Handschuh M, Bähr M, Richter DW, Kirchhoff F, Hülsmann S. Lack of the Kir4.1 channel subunit abolishes K+ buffering properties of astrocytes in the ventral respiratory group: impact on extracellular K+ regulation. J Neurophysiol 95: 1843–1852, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Nissant A, Lourdel S, Baillet S, Paulais M, Marvao P, Teulon J, Imbert-Teboul M. Heterogeneous distribution of chloride channels along the distal convoluted tubule probed by single-cell RT-PCR and patch clamp. Am J Physiol Renal Physiol 287: F1233–F1243, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Paulais M, Bloch-Faure M, Picard N, Jacques T, Ramakrishnan SK, Keck M, Sohet F, Eladari D, Houillier P, Lourdel S, Teulon J, Tucker SJ. Renal phenotype in mice lacking the Kir5.1 (Kcnj16) K+ channel subunit contrasts with that observed in SeSAME/EAST syndrome. Proc Natl Acad Sci USA 108: 10361–10366, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paulais M, Lachheb S, Teulon J. A Na+ and Cl-activated K+ channel in the thick ascending limb of mouse kidney. J Gen Physiol 127: 205–215, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pessia M, Imbrici P, D'Adamo MC, Salvatore L, Tucker SJ. Differential pH sensitivity of Kir4.1 and Kir42 potassium channels and their modulation by heteropolymerisation with Kir51. J Physiol 532: 359–367, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piala AT, Moon TM, Akella R, He HX, Cobb MH, Goldsmith EJ. Chloride sensing by WNK1 involves inhibition of autophosphosphorylation. Sci Signal 7: ra41, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piechotta K, Lu J, Delpire E. Cation chloride cotransporters interact with the stress-related kinases Ste20-related proline-alanine-rich kinase (SPAK) and oxidative stress response 1 (OSR1). J Biol Chem 277: 50812–50819, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Reichold M, Zdebik AA, Lieberer E, Rapedius M, Schmidt K, Bandulik S, Sterner C, Tegtmeier I, Penton D, Baukrowitz T, Hulton SA, Witzgall R, Ben Zeev B, Howie AJ, Kleta R, Bockenhauer D, Warth R. KCNJ10 gene mutations causing EAST syndrome (epilepsy, ataxia, sensorineural deafness, and tubulopathy) disrupt channel function. Proc Natl Acad Sci USA 107: 14490–14495, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenbaek LL, Kortenoeven ML, Aroankins TS, Fenton RA. Phosphorylation decreases ubiquitylation of the thiazide-sensitive cotransporter NCC and subsequent clathrin-mediated endocytosis. J Biol Chem 289: 13347–13361, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rozengurt N, Lopez I, Chiu CS, Kofuji P, Lester HA, Neusch C. Time course of inner ear degeneration and deafness in mice lacking the Kir4.1 potassium channel subunit. Hear Res 177: 71–80, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Scholl UI, Choi M, Liu T, Ramaekers VT, Hausler MG, Grimmer J, Tobe SW, Farhi A, Nelson-Williams C, Lifton RP. Seizures, sensorineural deafness, ataxia, mental retardation, and electrolyte imbalance (SeSAME syndrome) caused by mutations in KCNJ10. Proc Natl Acad Sci USA 106: 5842–5847, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Su XT, Zhang C, Wang L, Gu R, Lin DH, Wang WH. Disruption of KCNJ10 (Kir4.1) stimulates the expression of ENaC in the collecting duct. Am J Physiol Renal Physiol. 310: F985–F993, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanemoto M, Kittaka N, Inanobe A, Kurachi Y. In vivo formation of a proton-sensitive K+ channel by heteromeric subunit assembly of Kir5.1 with Kir41. J Physiol 525: 587–592, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Terker AS, Zhang C, Erspamer KJ, Gamba G, Yang CL, Ellison DH. Unique chloride-sensing properties of WNK4 permit the distal nephron to modulate potassium homeostasis. Kidney Int 89: 127–134, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Terker AS, Zhang C, McCormick JA, Lazelle RA, Zhang C, Meermeier NP, Siler DA, Park HJ, Fu Y, Cohen DM, Weinstein AM, Wang WH, Yang CL, Ellison DH. Potassium modulates electrolyte balance and blood pressure through effects on distal cell voltage and chloride. Cell Metab 21: 39–50, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wade JB, Fang L, Coleman RA, Liu J, Grimm PR, Wang T, Welling PA. Differential regulation of ROMK (Kir1.1) in distal nephron segments by dietary potassium. Am J Physiol Renal Physiol 300: F1385–F1393, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang L, Zhang C, Su X, Lin DH, Wang W. Caveolin-1 deficiency inhibits the basolateral K+ channels in the distal convoluted tubule and impairs renal K+ and Mg2+ transport. J Am Soc Nephrol 26: 2678–2690, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang WH, McNicholas CM, Segal AS, Giebisch G. A novel approach allows identification of K channels in the lateral membrane of rat CCD. Am J Physiol 266: F813–F822, 1994. [DOI] [PubMed] [Google Scholar]
- 39.Yang SS, Lo YF, Wu CC, Lin SW, Yeh CJ, Chu P, Sytwu HK, Uchida S, Sasaki S, Lin SH. SPAK-knockout mice manifest Gitelman syndrome and impaired vasoconstriction. J Am Soc Nephrol 21: 1868–1877, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zaika OL, Mamenko M, Palygin O, Boukelmoune N, Staruschenko A, Pochynyuk O. Direct inhibition of basolateral Kir4.1/51 and Kir41 channels in the cortical collecting duct by dopamine. Am J Physiol Renal Physiol 305: F1277–F1287, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang C, Wang L, Su XT, Lin DH, Wang WH. KCNJ10 (Kir4.1) is expressed in the basolateral membrane of the cortical thick ascending limb. Am J Physiol Renal Physiol 308: F1288–F1296, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang C, Wang L, Thomas S, Wang K, Lin DH, Rinehart J, Wang WH. Src-family protein tyrosine kinase regulates the basolateral K channel in the distal convoluted tubule (DCT) by phosphorylation of KCNJ10. J Biol Chem 288: 26135–26146, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang C, Wang L, Zhang J, Su XT, Lin DH, Scholl UI, Giebisch G, Lifton RP, Wang WH. KCNJ10 determines the expression of the apical Na-Cl cotransporter (NCC) in the early distal convoluted tubule (DCT1). Proc Natl Acad Sci USA 111: 11864–11869, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]