Abstract
Myeloid-derived suppressor cells (MDSCs) are a CD11b+Gr1+ population in mice that can be separated into granulocytic (g-MDSC) and monocytic (m-MDSC) subtypes based on their expression of Ly6G and Ly6C. Both MDSC subtypes are potent suppressors of T cell immunity, and their contribution has been investigated in a plethora of diseases including renal cancer, renal transplant, and chronic kidney disease. Whether MDSCs contribute to the pathogenesis of acute kidney injury (AKI) remains unknown. Herein, using human C-reactive protein (CRP) transgenic (CRPtg) and CRP-deficient mice (CRP−/−) subjected to bilateral renal ischemia-reperfusion injury (IRI), we confirm our earlier finding that CRP exacerbates renal IRI and show for the first time that this effect is accompanied in CRPtg mice by a shift in the balance of kidney-infiltrating MDSCs toward a suppressive Ly6G+Ly6Clow g-MDSC subtype. In CRPtg mice, direct depletion of g-MDSCs (using an anti-Gr1 monoclonal antibody) reduced the albuminuria caused by renal IRI, confirming they play a deleterious role. Remarkably, treatment of CRPtg mice with an antisense oligonucleotide that specifically blocks the human CRP acute-phase response also led to a reduction in renal g-MDSC numbers and improved albuminuria after renal IRI. Our study in CRPtg mice provides new evidence that MDSCs participate in the pathogenesis of renal IRI and shows that their pharmacological depletion is beneficial. If ongoing investigations confirm that CRP is an endogenous regulator of MDSCs in CRPtg mice, and if this action is recapitulated in humans, then targeting CRP or/and MDSCs might offer a new approach for the treatment of AKI.
Keywords: innate immunity, acute phase proteins, pentraxin
ischemia-reperfusion injury (IRI) is injury caused by the restriction and subsequent restoration of blood flow to an organ. IRI can result from hypotension and cardiovascular surgery and is inevitable during organ transplantation, and it is thus a common cause of acute kidney injury (AKI) (6, 16, 30). There are few therapeutic options for AKI, so mortality is high and survivors have an increased likelihood of developing chronic kidney disease (23). The sequence of events leading to renal IRI/AKI are complex and have been the subject of much investigation (16, 30). There is now general agreement that cell death due to hypoxia triggers the release of proinflammatory cytokines (IL-6, IL-1, and TNF-α), thereby propelling a systemic inflammatory response that fosters the orchestrated recruitment of leukocytes to the injured kidney and the concomitant activation of resident ones, that together cause damage to the renal tubules (10, 16). C-reactive protein (CRP), an IL-1/IL-6-induced acute phase reactant in humans (3), marks the inflammatory response and its rise in the blood associates with worsened outcomes and increased mortality in patients with AKI (8, 17, 29). In a series of studies using CRP transgenic mice (CRPtg) (1) subjected to bilateral renal IRI (27), we found that CRP exacerbates AKI (21) and that this effect was linked to impairment of replication of renal tubular epithelial cells (26). Herein, we provide compelling new evidence that CRP-driven exacerbation of renal damage in CRPtg mice, and perhaps the impairment of renal tubular epithelial cell replication that accompanies it, is mediated by myeloid-derived suppressor cells (MDSCs).
As part of the body's response to systemic inflammation, immature myeloid cells are mobilized from the bone marrow and enter the circulation. These migrate to various tissues and organs, where they differentiate into effector cells pertinent to AKI (macrophages, dendritic cells, and neutrophils, etc.) (10, 13, 18, 19). Under certain conditions differentiation of these cells can be stalled, allowing the generation of myeloid cells that, despite their ability to replicate, retain an immature phenotype. As their name implies, these so-called MDSCs potently suppress the replication, and thereby the activation, of any cells in their proximity (4). This bystander-cell suppression by MDSCs is achieved in part by their upregulation of arginase, which thereby limits arginine availability to nearby cells (4). In mice, MDSCs are identified based on their coexpression of Gr1 and CD11b, their nuclear morphology, and their T cell-suppressive functions, and they can be further divided into granulocytic g-MDSCs (Ly6g+Ly6clow) or monocytic m-MDSCs (Ly6g−Ly6chigh) (2, 4, 5). While MDSCs have been shown to participate in cancer (5), autoimmunity (2, 7), and chronic kidney disease (9, 15), their potential contribution in AKI has only been hinted at (14). Herein, we show that 1) MDSCs recovered from the kidneys of CRPtg mice with renal IRI are strongly biased toward a suppressive g-MDSC subset; 2) prophylactic treatment of CRPtg mice with a human CRP-lowering drug is as effective as treatment with an anti-Gr1 antibody at lowering g-MDSC numbers in the injured kidneys; and 3) both therapeutic approaches improve AKI outcomes in CRPtg mice. Taken together, these findings provide strong initial evidence that g-MDSCS might be one of the CRP-responsive cell types driving exacerbation of renal IRI in CRPtg mice.
MATERIALS AND METHODS
Animals and renal IRI.
Human CRPtg mice and CRP-deficient mice (CRP−/−; both backcrossed to C57BL/6) have been fully described elsewhere (1, 11, 25). Human CRP is present in the blood of CRPtg at concentrations mirroring humans (i.e., ∼1–10 μg/ml under steady-state conditions vs. 100–500 μg/ml during an acute phase response) (1, 25). Mouse CRP is expressed in CRPtg but is not a major acute phase protein (28). Bilateral renal IRI has been described previously (21). Briefly, following flank incisions the renal pedicles of both kidneys were exposed and clamped with an atraumatic microserrefine vascular clamp (catalog no. 18055-05; Fine Science Tools) for 30 min, and then the clamps were removed to allow kidney reperfusion for 24 h. Each separate renal IRI experiment included at least six mice (at least 3/genotype), and each experiment was repeated at least three times. Only males were used as they are more susceptible to renal IRI (20), and male CRPtg mice express more human CRP than females (25). All animal protocols were approved by the Institutional Animal Care and Use Committees at the University of Alabama at Birmingham, and all mouse experiments were consistent with the Guide for the Care and Use of Laboratory Animals (NIH publication 96-01, revised 1996).
Histology and biomarkers.
Thin sections (5 μm) of paraffin-embedded kidneys were stained with periodic acid-Schiff (PAS) reagent to quantitate organ damage by light microscopy. The number of degenerating/necrotic tubules, the number of tubules with brush border loss, and the number of tubules containing casts were counted as previously described (21) by observers blinded to the condition being scored. Human CRP in serum and albumin in urine were measured using ELISA, and creatinine in serum was determined by tandem mass spectrometry (LC-MS/MS) as described (21).
Flow cytometry, cell sorting, and cytospins.
Single-cell suspensions of the spleen and bone marrow were prepared, and erythrocytes were lysed using ACK buffer (Sigma). To collect renal cells, kidneys were minced and enzymatically digested in RPMI with 1.67 Wünsch U/ml liberase (Roche Diagnostics, Indianapolis, IN) for 30 min at 37°C, then filtered through a 70-μm cell strainer. Mononuclear cells were purified by centrifugation over a 30/70% Percoll gradient. The cell layer was removed, washed, and stained for Gr1 (Brilliant violet 605, clone RB6-8C5), CD11b (Alexa Fluor 700), Ly6g (Pacific blue), Ly6c (PE), F4/80 (FITC), and CD11c (APC); all from Biolegend. A viability dye (Life Technologies) was used to exclude dead cells. Samples were acquired using an LSRII flow cytometer (BD Biosciences) followed by data analysis with FlowJo (Tree Star). Renal MDSCs were sorted from wild-type (WT) kidneys 24 h post-IRI using fluorescence-activated cell sorting (FACS) ARIA (BD Biosciences) and used for T cell proliferation assays or to prepare cytospins. Nuclear morphology was analyzed by light microscopy after Wright-Giemsa staining (Sigma).
T cell proliferation.
Naive CD4+CD45RBhi T cells were sorted from healthy WT mice using a FACS ARIA cell sorter, and then labeled with carboxyfluorescein di-acetate succinimidyl ester (CFSE) (eBioscience). CFSE-labeled T cells were stimulated with anti-CD3/CD28 expander beads (Life Technologies) and cultured alone for 3 days or cocultured for 3 days with Gr1+CD11b+ MDSCs, Gr1+CD11b+Ly6g+Ly6clow g-MDSCs, or Gr1+CD11b+Ly6g−Ly6chigh m-MDSCs purified (by cell sorting) from WT post-IRI kidneys. Since far fewer m-MDSCs infiltrated the injured kidneys than g-MDSCs (see Fig. 1E) and the efficiency of m-MDSC cell sorting was lower, the MDSC:T cell ratios used in the T cell proliferation assays were lower for m-MDSCs (1:100) than for g-MDSCs (1:5).
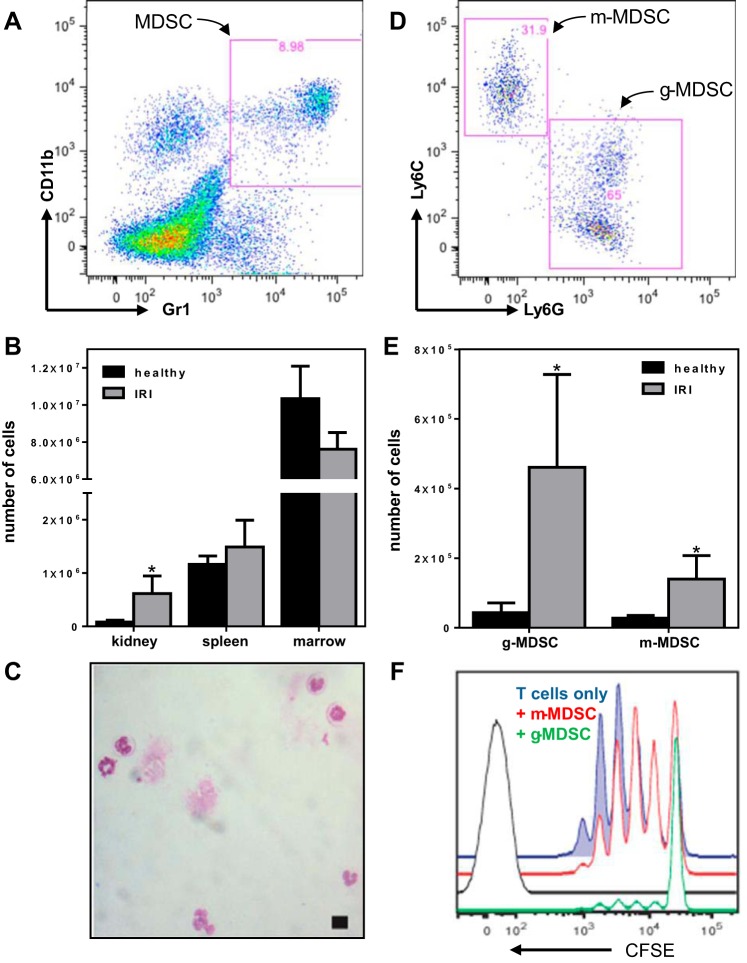
Fig. 1.
Myeloid-derived suppressor cells (MDSCs) isolated from healthy and renal ischemia-reperfusion injury (IRI) mice. A: representative scatter plot showing the gating strategy used to identify Gr1+CD11b+ renal MDSCs. B: the numbers of Gr1+CD11b+ MDSCs recovered from the kidneys, spleens, and bone marrow of healthy (black bars) vs. renal IRI mice (gray bars). Note the break in the y-axis. C: representative Wright-Giemsa-stained cytospin of fluorescence-activated cell sorting (FACS) renal Gr1+CD11b+ MDSCs, demonstrating their predominantly immature nuclear morphology (scale bar = 10 μm). D: representative scatter plot showing the gating strategy used to identify Gr1+CD11b+Ly6clowLy6g+ granulocytic (g)-MDSCs and Gr1+CD11b+Ly6chigh Ly6g− monocytic (m)-MDSCs subpopulations. E: the numbers of g-MDSCs and m-MDSCs recovered from healthy (black bars) vs. renal IRI kidneys (gray bars). F: representative flow cytometry histograms showing unimpeded proliferation of carboxyfluorescein di-acetate succinimidyl ester (CFSE)-labeled naive T cells after 3-day stimulation with CD3/CD28 (blue line) compared with partially suppressed proliferation in the presence of m-MDSCs (red line) and fully suppressed proliferation in the presence of g-MDSCs (green line). Unstained naive T cells are shown as a control (black line). In B and E, each bar and whisker represent the mean ± SE for n = 3–6 wild-type (WT) mice; the numbers of cells recovered (healthy vs. IRI) were compared using unpaired t-tests (1-tailed with Holm-Sidak correction), and when the difference was significant (P < 0.05) this is indicated with an asterisk.
Depletion of Gr1+ cells and CRP.
To deplete Gr1+ cells, mice were given 200 μg of purified anti-Gr1 monoclonal antibody (RB6-8C5, Biolegend) via intraperitoneal (ip) injection 24 h before IRI surgery, as described (14, 24). Pilot studies verified that within 24 h this regimen achieved a 93% reduction of circulating CD11b+Gr1+ cells but did not lower blood CRP levels in CRPtg mice. To deplete human CRP, mice received via an ip injection an antisense oligonucleotide that specifically targets human CRP mRNA (35 mg/kg twice a week for 2 wk before IRI surgery) (12). Blood was collected to confirm Gr1+ cell and CRP depletion. Controls received an equivalent volume of PBS.
Statistical analysis.
Where appropriate, the data are presented as means with associated standard errors. Statistical analysis was performed using GraphPad Prism 6. One-way ANOVA, unpaired Student's t-tests with Holm-Sidak and Welch's corrections for multiple comparisons and unequal variance, and linear trends tests were used for comparisons among and between genotypes. A P value of <0.05 was considered significant.
RESULTS
MDSCs are recruited to the injured kidneys after IRI.
Since 1) renal IRI is exacerbated in CRPtg mice (21, 26), 2) CRP has been shown to influence the biology of monocytes/macrophages, dendritic cells, and neutrophils (reviewed in Ref. 3), and 3) each of these myeloid cell types has been linked to the pathogenesis of IRI (13, 18, 19), we sought to determine whether CRP-mediated exacerbation of renal IRI is associated with a change in the community of renal-infiltrating myeloid cells. Initial studies done using WT mice showed that after renal IRI, the number of Gr1+CD11b+ cells (gating strategy shown in Fig. 1A) was significantly increased in the kidneys, remained unchanged in the spleen, and decreased substantially in the bone marrow (Fig. 1B). This pattern is consistent with the expected mobilization of Gr1+CD11b+ myeloid cells from the bone marrow followed by their homing to the injured organ (2, 4, 14). Coexpression of Gr1 and CD11b and mobilization in response to injury is also a characteristic of mouse neutrophils, but surprisingly few of the Gr1+CD11b+ cells purified by FACS from the injured kidneys had the polymorphonuclear morphology that is characteristic of mature neutrophils (Fig. 1C) (22). This seemingly paradoxical finding prompted us to check whether the Gr1+CD11b+ population represented Ly6c+ or/and Ly6g+ MDSCs rather than mature neutrophils. We found that the numbers of Gr1+CD11b+Ly6g+Ly6clow cells (g-MDSCs) and Gr1+CD11b+Ly6g−Ly6chigh cells (m-MDSCs; gating strategy shown in Fig. 1D) increased significantly in the kidneys after IRI (Fig. 1E). Importantly, at an MDSC:T cell ratio of 1:5 the g-MDSCs recovered and purified from the injured kidneys completely blocked T cell replication induced by CD3/CD28 stimulation (Fig. 1F; compare the green and blue lines). The low yield of renal m-MDSCs precluded us from testing their suppressive activity at the same MDSC:T cell ratio, but at a ratio of 1:100 the m-MDSCs were still able to slightly suppress T cell proliferation (Fig. 1F; compare the red and blue lines). These data confirm that both populations of MDSCs have authentic suppressive action (22).
After renal IRI, CRP shifts the balance toward the g-MDSC subset.
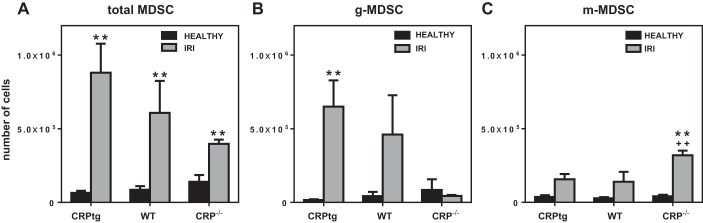
To determine whether CRP influenced the numbers or/and types of MDSCs infiltrating the injured kidneys, we compared renal MDSCs recovered from healthy CRPtg, WT, and CRP−/− mice vs. injured ones. The total number of MDSCs recovered from the kidneys of healthy mice was comparable among the genotypes (Fig. 2A, black bars), and following renal IRI the total number of renal MDSCs increased significantly for all three genotypes (Fig. 2A, gray bars). Notably, the total number of renal MDSCs recovered after renal IRI was lowest in CRP−/− and highest in CRPtg (Fig. 2A, compare gray bars). The paucity of total MDSCs in CRP−/− mice was attributable to a failure of g-MDSCs to increase in the injured kidneys of this genotype; thus kidneys from both of the CRP-sufficient genotypes (i.e., CRPtg and WT mice) showed a distinct increase in renal g-MDSCs following IRI (achieving significance in CRPtg), whereas the response was completely absent in CRP−/− mice (Fig. 2B). In stark contrast, after renal IRI the number of renal infiltrating m-MDSCs was highest in CRP−/− mice (Fig. 2C).
Fig. 2.
The numbers of MDSCs (A), g-MDSCs (B), and m-MDSCs (C) recovered from the kidneys of healthy (black bars) vs. renal IRI (gray bars) CRPtg, WT, and CRP−/− mice. Each bar and whisker represent the mean ± SE for n = 3–13 mice. The numbers of cells recovered (healthy vs. IRI) were compared using unpaired t-tests (2-tailed with Holm-Sidak correction), and when the difference was significant (P < 0.05) this is indicated with a double asterisk. The numbers of cells recovered from CRPtg and CRP−/− vs. treatment-matched WT mice were compared using unpaired t-tests (1-tailed with Welch's correction), and when P < 0.05 this is indicated by ++.
In CRPtg mice, g-MDSCs have a deleterious effect on renal IRI and their depletion is beneficial.
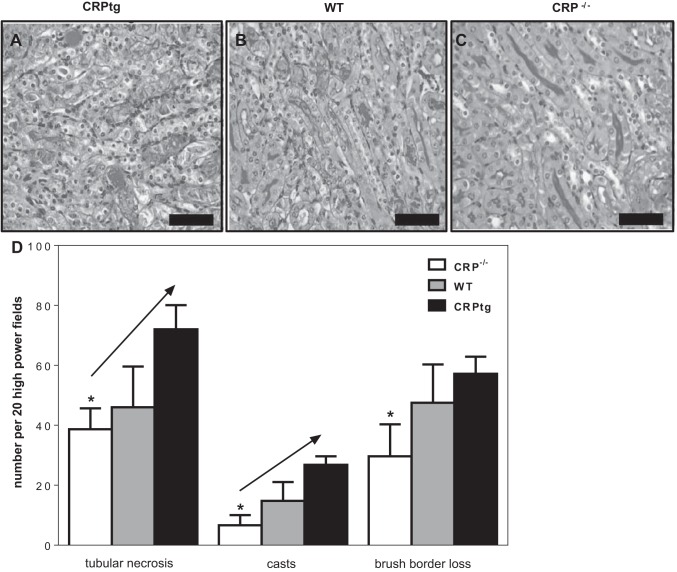
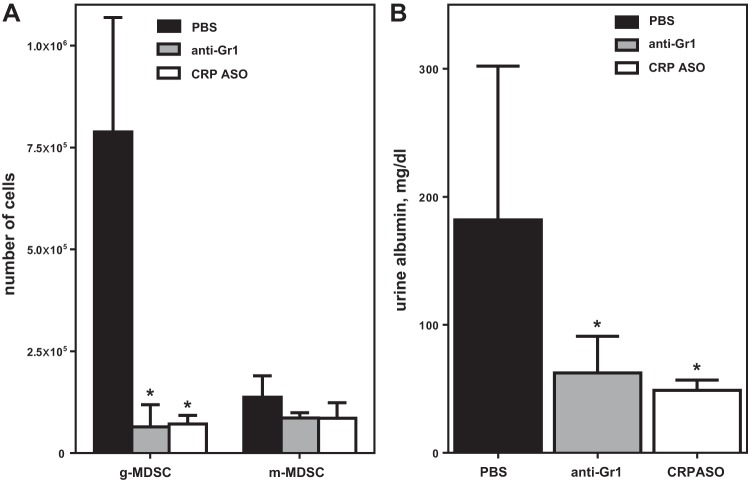
Each measure of tissue damage in the renal medulla (tubular necrosis, casts, brush border loss) was significantly less severe for CRP−/− than CRPtg mice (Fig. 3). Furthermore, all measures of tissue damage increased sequentially in the order CRP−/− → WT → CRPtg (Fig. 3), a trend that achieved statistical significance for tubular necrosis and cast formation (Fig. 3). Since these trends paralleled the genotype-associated differences in MDSCs (see Fig. 1), to determine more directly whether MDSCs contributed to the renal IRI process, we employed a method used by others to specifically eliminate g-MDSCs from mice, i.e., the administration of an anti-Gr1 monoclonal antibody (14, 24). CRPtg mice were used for these experiments because they reproducibly showed the greatest increase in renal MDSCs and g-MDSCs after renal IRI (Fig. 2, A and B, respectively) and because they reproducibly were the most severely affected genotype (Fig. 3). For these experiments, anti-Gr1 (200 μg) was administered 24 h before renal IRI surgery. Flow cytometry confirmed that in the kidneys of anti-Gr1-treated animals (Fig. 4A, gray bars) the renal g-MDSC population, but not the m-MDSC population, was significantly depleted compared with that observed in PBS-treated controls (Fig. 4A, black bars). Importantly, a pronounced elevation of urine albumin following renal IRI surgery was observed for PBS-treated CRPtg (Fig. 4B, black bars) but not for anti-Gr1 treated CRPtg mice (Fig. 4B, gray bars). Since albuminuria is an accepted hallmark of AKI, these results suggest that g-MDSC depletion had a renoprotective effect in CRPtg mice.
Fig. 3.
Organ histology after renal IRI. Representative periodic acid-Schiff (PAS)-stained thin sections of the outer medulla of kidneys collected 24 h after renal IRI from CRPtg (A), WT (B), and CRP−/− mice (C; ×40, scale bars = 25 μm). D: tubular necrosis, casts, and brush border loss were quantitated in kidney sections from CRP−/− (white bars), WT (gray bars), and CRPtg (black bars). Each bar represents the average ± SE of 20 microscopic fields examined for n = 3–6 mice of each genotype. Each measure of kidney damage was tested for a linear increase among genotypes (in left-to-right order) using ANOVA coupled with a linear trends test, and when P < 0.05 this is indicated by a diagonal arrow. The asterisks indicate P < 0.05 for unpaired t-tests (2-tailed) of CRP−/− vs. CRPtg.
Fig. 4.
Depletion of g-MDSCs improves renal IRI outcome in CRPtg mice. A: the numbers of renal g-MDSCs and m-MDSC determined 24 h after renal IRI for mice treated with anti-Gr1-depleting antibody (gray bars), CRP-lowering antisense oligonucleotide (ASO; open bars), or PBS (black bars). B: urine albumin was measured 24 h after IRI surgery. Each bar and whisker represent the mean ± SE for n = 3–8 mice/group. The asterisks indicate P < 0.05 for unpaired t-tests of the indicated groups with its matched PBS control.
In CRPtg, CRP lowering prevents renal infiltration by g-MDSCs and protects the kidney.
To connect human CRP expression in CRPtg mice to exacerbation of renal IRI and increased renal g-MDSC infiltration, we tested whether therapeutic lowering of human CRP would also lower renal g-MDSC numbers and thereby protect the kidney. To lower human CRP, we used an antisense oligonucleotide (ASO) that we previously showed specifically reduces human CRP blood levels in CRPtg mice and humans (12). CRPtg were treated with the human CRP-specific ASO twice weekly for 2 wk before undergoing renal IRI. This regimen completely blocked the human CRP acute phase response induced by renal IRI (121.8 ± 38.49 μg human CRP/ml serum in PBS-treated/IRI mice vs. 21.89 ± 12.44 μg/ml in ASO-treated/IRI mice). Importantly, compared with PBS-treated CRPtg mice (Fig. 4, black bars), in the ASO-treated CRPtg mice significantly fewer g-MDSCs were recovered from the injured kidneys (Fig. 4A, open bars) and elevation of urine albumin after renal IRI was prevented (Fig. 4B, open bars). To our surprise, the benefit of human CRP lowering on both measures of renal IRI severity were comparable to that seen with anti-Gr1 treatment (Fig. 4, compare the white and gray bars). In the same experiment, serum creatinine in ASO-treated CRPtg mice (0.18 ± 0.03 mg/dl) was also lower than in PBS-treated ones (0.75 ± 0.3 mg/dl).
DISCUSSION
In sum, our new findings demonstrate for the first time that 1) a substantial population of suppressive MDSCs are recruited to the kidneys of mice subjected to renal IRI, 2) human CRP expression in CRPtg mice skews their balance toward the g-MDSC subset, and 3) reduction of g-MDSC numbers before renal IRI lessens injury to the kidneys in CRPtg mice. Furthermore, our data show that for CRPtg mice, targeted lowering of human CRP is as effective as an anti-Gr1 antibody at lessening infiltration of g-MDSCs into the kidney and lessening renal damage after renal IRI. The mechanism(s) in CRPtg mice by which human CRP promotes selective recruitment of g-MDSCs to the injured kidney, and how MDSCs exacerbate organ damage once they arrive there, are both currently under active investigation. Based on previous experiments showing that both Fc receptors and inducible nitric oxide synthase are upregulated in the injured kidneys of CRPtg mice (21), we are exploring the possibility that human CRP binding to Fc receptors on immature myeloid cells might selectively promote the generation of g-MDSCs in CRPtg, perhaps amplifying tissue damage via increased generation of reactive oxygen species. We are also exploring whether the suppressive action of MDSCs contributes to the reduced proliferation of tubular epithelial cells seen in CRPtg mice undergoing renal IRI (26). Our preliminary results do suggest that in CRPtg mice treated with the CRP-lowering ASO there is an ∼60% reduction in renal MDSCs expressing arginase (FACS data not shown) and an approximate doubling of the proliferation of CD133+ epithelial cells (Ki-67 immunostaining data not shown). Regardless of the exact mechanisms involved, our findings suggest that (at least in CRPtg mice) human CRP possibly acts as an endogenous regulator of MDSC mobilization and differentiation. If further study confirms this is indeed the case in CRPtg mice, and if this action is recapitulated in humans, then targeting CRP or/and g-MDSCs might offer a new approach for the clinical treatment of renal IRI. Since CRP is associated with cancer and autoimmunity (3, 11, 12) and MDSCs are known to participate in these diseases also (2, 5, 7), the relevance of our findings could extend far beyond renal IRI.
GRANTS
This work was supported by NIH grant 1R01DK099092 to A. J. Szalai, American Heart Association Grant 13PRE14490057 to M. A. Pegues, and the UAB-UCSD O'Brien Core Center for AKI Research (supported by NIH Grant P30DK079337).
DISCLOSURES
A. J. Szalai has in the past received research funding from Ionis Pharmaceuticals. No other authors have any relationships with any companies that may have a financial interest in the information contained in this manuscript.
AUTHOR CONTRIBUTIONS
M.A.P. and A.J.S. provided conception and design of research; M.A.P. performed experiments; M.A.P. and A.J.S. analyzed data; M.A.P., I.L.M., and A.J.S. interpreted results of experiments; M.A.P. and A.J.S. prepared figures; M.A.P. and A.J.S. drafted manuscript; M.A.P. and A.J.S. edited and revised manuscript; M.A.P. and A.J.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank the members of the UAB comprehensive flow cytometry core facility [supported by National Institutes of Health (NIH) Grants P30 AR048311 and P30 AI27667] for assistance with FACS, and Ionis Pharmaceuticals for supplying the CRP-lowering ASO.
REFERENCES
- 1.Ciliberto G, Arcone R, Wagner EF, Ruther U. Inducible and tissue-specific expression of human C-reactive protein in transgenic mice. EMBO J 6: 4017–4022, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cripps JG, Gorham JD. MDSC in autoimmunity. Int Immunopharmacol 11: 789–793, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Du Clos TW. Pentraxins: structure, function, and role in inflammation. ISRN Inflamm 2013: 379040, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 9: 162–174, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol 12: 253–268, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haase M, Shaw A. Acute kidney injury and cardiopulmonary bypass: special situation or same old problem? Contrib Nephrol 165: 33–38, 2010. [DOI] [PubMed] [Google Scholar]
- 7.Haile LA, von Wasielewski R, Gamrekelashvili J, Krüger C, Bachmann O, Westendorf AM, Buer J, Liblau R, Manns MP, Korangy F, Greten TF. Myeloid-derived suppressor cells in inflammatory bowel disease: a new immunoregulatory pathway. Gastroenterology 135: 871–881, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Harrison NA, Masterton RG, Bateman JM, Rainford DJ. C-reactive protein in acute renal failure. Nephrol Dial Transplant 4: 864–869, 1989. [DOI] [PubMed] [Google Scholar]
- 9.Höchst B, Mikulec J, Baccega T, Metzger C, Welz M, Peusquens J, Tacke F, Knolle P, Kurts C, Diehl L, Ludwig-Portugall I. Differential induction of Ly6G and Ly6C positive myeloid derived suppressor cells in chronic kidney and liver inflammation and fibrosis. PLoS One 10: e0119662, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jang HR, Rabb H. Immune cells in experimental acute kidney injury. Nat Rev Nephrol 11: 88–101, 2015. [DOI] [PubMed] [Google Scholar]
- 11.Jones NR, Pegues MA, McCrory MA, Kerr SW, Jiang H, Sellati R, Berger V, Villalona J, Parikh R, McFarland M, Pantages L, Madwed JB, Szalai AJ. Collagen-induced arthritis is exacerbated in C-reactive protein-deficient mice. Arthritis Rheum 63: 2641–2650, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones NR, Pegues MA, McCrory MA, Singleton W, Bethune C, Baker BF, Norris DA, Crooke RM, Graham MJ, Szalai AJ. A selective inhibitor of human C-reactive protein translation is efficacious in vitro and in C-reactive protein transgenic mice and humans. Mol Ther Nucleic Acids 1: e52, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ko GJ, Boo CS, Jo SK, Cho WY, Kim HK. Macrophages contribute to the development of renal fibrosis following ischaemia/reperfusion-induced acute kidney injury. Nephrol Dial Transplant 23: 842–852, 2008. [DOI] [PubMed] [Google Scholar]
- 14.Lee DH, Wolstein JM, Pudasaini Basu Plotkin M. INK4a deletion results in improved regeneration and decreased capillary rarefaction after ischemia-reperfusion injury. Am J Physiol Renal Physiol 302: F183–F191, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li L, Zhang T, Diao W, Jin F, Shi L, Meng J, Liu H, Zhang J, Zeng CH, Zhang MC, Liang S, Liu Y, Zhang CY, Liu Z, Zen K. Role of myeloid-derived suppressor cells in glucocorticoid-mediated amelioration of FSGS. J Am Soc Nephrol 26: 2183–2197, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malek M, Nematbakhsh M. Renal ischemia/reperfusion injury; from pathophysiology to treatment. J Renal Inj Prev 4: 20–27, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mittalhenkle A, Stehman-Breen CO, Shlipak MG, Fried LF, Katz R, Young BA, Seliger S, Gillen D, Newman AB, Psaty BM, Siscovick D. Cardiovascular risk factors and incident acute renal failure in older adults: the cardiovascular health study. Clin J Am Soc Nephrol 3: 450–456, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyazawa S, Watanabe H, Miyaji C, Hotta O, Abo T. Leukocyte accumulation and changes in extra-renal organs during renal ischemia reperfusion in mice. J Lab Clin Med 139: 269–278, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Okusa MD, Li L. Dendritic cells in acute kidney injury: cues from the microenvironment. Trans Am Clin Climatol Assoc 123: 54–62, 2012. [PMC free article] [PubMed] [Google Scholar]
- 20.Park KM, Kim JI, Ahn Y, Bonventre AJ, Bonventre JV. Testosterone is responsible for enhanced susceptibility of males to ischemic renal injury. J Biol Chem 279: 52282–52292, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Pegues MA, McCrory MA, Zarjou A, Szalai AJ. C-reactive protein exacerbates renal ischemia-reperfusion injury. Am J Physiol Renal Physiol 304: F1358–F1365, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pillay J, Tak T, Kamp VM, Koenderman L. Immune suppression by neutrophils and granulocytic myeloid-derived suppressor cells: similarities and differences. Cell Mol Life Sci 70: 3813–3827, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rewa O, Bagshaw SM. Acute kidney injury-epidemiology, outcomes and economics. Nat Rev Nephrol 10: 193–207, 2014. [DOI] [PubMed] [Google Scholar]
- 24.Stromnes IM, Brockenbrough JS, Izeradjene K, Carlson MA, Cuevas C, Simmons RM, Greenberg PD, Hingorani SR. Targeted depletion of an MDSC subset unmasks pancreatic ductal adenocarcinoma to adaptive immunity. Gut 63: 1769–1781, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szalai AJ, van Ginkel FW, Dalrymple SA, Murray R, McGhee JR, Volanakis JE. Testosterone and IL-6 requirements for human C-reactive protein gene expression in transgenic mice. J Immunol 160: 5294–5299, 1998. [PubMed] [Google Scholar]
- 26.Tang Y, Huang XR, Lv J, Chung AC, Zhang Y, Chen JZ, Szalai AJ, Xu A, Lan HY. C-reactive protein promotes acute kidney injury by impairing G1/S-dependent tubular epithelium cell regeneration. Clin Sci (Lond) 126: 645–659, 2014. [DOI] [PubMed] [Google Scholar]
- 27.Wei Q, Dong Z. Mouse model of ischemic acute kidney injury: technical notes and tricks. Am J Physiol Renal Physiol 303: F1487–F1494, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whitehead AS, Zahedi K, Rits M, Mortensen RF, Lelias JM. Mouse C-reactive protein. Generation of cDNA clones, structural analysis, and induction of mRNA during inflammation. Biochem J 266: 283–290, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yeun JY, Levine RA, Mantadilok V, Kaysen GA. C-Reactive protein predicts all-cause and cardiovascular mortality in hemodialysis patients. Am J Kidney Dis 35: 469–476, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Zager RA, Johnson AC, Lund S, Hanson S. Acute renal failure: determinants and characteristics of the injury-induced hyperinflammatory response. Am J Physiol Renal Physiol 291: F546–F556, 2006. [DOI] [PubMed] [Google Scholar]