Abstract
Hepatocellular carcinoma (HCC) is the most common hepatic malignancy and the third leading cause of cancer related deaths. Previous studies have implicated bile acids in pathogenesis of HCC, but the mechanisms are not known. We investigated the mechanisms of HCC tumor promotion by bile acids the diethylnitrosamine (DEN)-initiation-cholic acid (CA)-induced tumor promotion protocol in mice. The data show that 0.2% CA treatment resulted in threefold increase in number and size of DEN-induced liver tumors. All tumors observed in DEN-treated mice were well-differentiated HCCs. The HCCs observed in DEN-treated CA-fed mice exhibited extensive CD3-, CD20-, and CD45-positive inflammatory cell aggregates. Microarray-based global gene expression studies combined with Ingenuity Pathway Analysis revealed significant activation of NF-κB and Nanog in the DEN-treated 0.2% CA-fed livers. Further studies showed significantly higher TNF-α and IL-1β mRNA, a marked increase in total and phosphorylated-p65 and phosphorylated IκBα (degradation form) in livers of DEN-treated 0.2% CA-fed mice. Treatment of primary mouse hepatocytes with various bile acids showed significant induction of stemness genes including Nanog, KLF4, Sox2, and Oct4. Quantification of total and 20 specific bile acids in liver, and serum revealed a tumor-associated bile acid signature. Finally, quantification of total serum bile acids in normal, cirrhotic, and HCC human samples revealed increased bile acids in serum of cirrhotic and HCC patients. Taken together, these data indicate that bile acids are mechanistically involved pathogenesis of HCC and may promote HCC formation via activation of inflammatory signaling.
Keywords: NF-κB, cholic acid, proliferation, Nanog
hepatocellular carcinoma (HCC) is the fifth most common cancer in men and seventh in women (9, 12). Each year an estimated 20,000 new cases of HCC are diagnosed in the US and the trend is rising. The treatment options for HCC remain limited partly because the mechanisms of pathogenesis of HCC are not completely known (27). Previous studies have implicated increased bile acids in pathogenesis of HCC but the mechanisms are not fully elucidated (3, 34, 35).
Bile acids, initially known for their role in lipid and vitamin metabolism, are now considered critical signaling molecules involved in cross tissue signaling, especially in the liver-gut axis (15). Both protective and pathogenic roles of bile acids in variety of diseases including drug-induced liver injury, steatohepatitis, and cancers of colon and liver have been identified (10). Whereas several studies have linked bile acids to hepatocellular carcinoma (HCC) (21, 22, 25, 34, 35), the exact mechanisms by which bile acids promote HCC are not known. Previous studies indicate that mouse models with increase in total circulating bile acids including FXR (NR1H4) knockout (KO), BSEP (Abcb11) KO, and MDR2 (Abcb4) KO mice exhibit spontaneous HCC formation. Decreasing bile acids in FXR KO mice results in reduction of spontaneous HCC (35). Although these studies implicate increase in bile acids to HCC promotion, the mechanisms remain elusive.
We studied the mechanisms of bile acid-mediated tumor promotion using a classic initiation-promotion protocol of diethylnitrosamine (DEN)-initiated liver tumors in C57BL/6 mice. Using a global gene expression analysis and traditional methods, we have identified NF-κB-mediated inflammatory signaling pathways as a mechanism behind the tumor promotion by bile acids. Furthermore, we show that increased bile acids may promote stemness-related genes in hepatocytes. Finally, our studies have also identified increase bile acid as a component of human HCC pathogenesis. These studies provide evidence that increased bile acids can promote liver cancer with diverse mechanisms.
METHODS
Animals, treatments, and tissue collection.
Animal studies were conducted as described before (32). The C57BL/6 mice were purchased from Jackson laboratories (Bar Harbor, Me) and housed in the University of Kansas Medical Center Laboratory Animal Resources Vivarium. All studied were performed in male mice only. The role of cholic acid in promoting DEN-induced hepatic tumors was evaluated using a classic initiation-promotion protocol (Fig. 1A). Male mice pups were injected with either vehicle or DEN (15 μg/kg dissolved in saline) at postnatal day 15. All mice (n = 5 to 8 per group) were weaned on normal rodent chow and maintained on rodent chow till 8 mo of age. At 8 mo, two groups of mice, one DEN treated and other vehicle treated were fed diet containing 0.2% cholic acid (CA) till 10 mo of age. All mice were killed at 10 mo of age. Livers and serum were collected and processed as described before (8, 32). All animal studies were approved by and performed in accordance with the Institutional Animal Care and Use Committee at University of Kansas Medical Center.
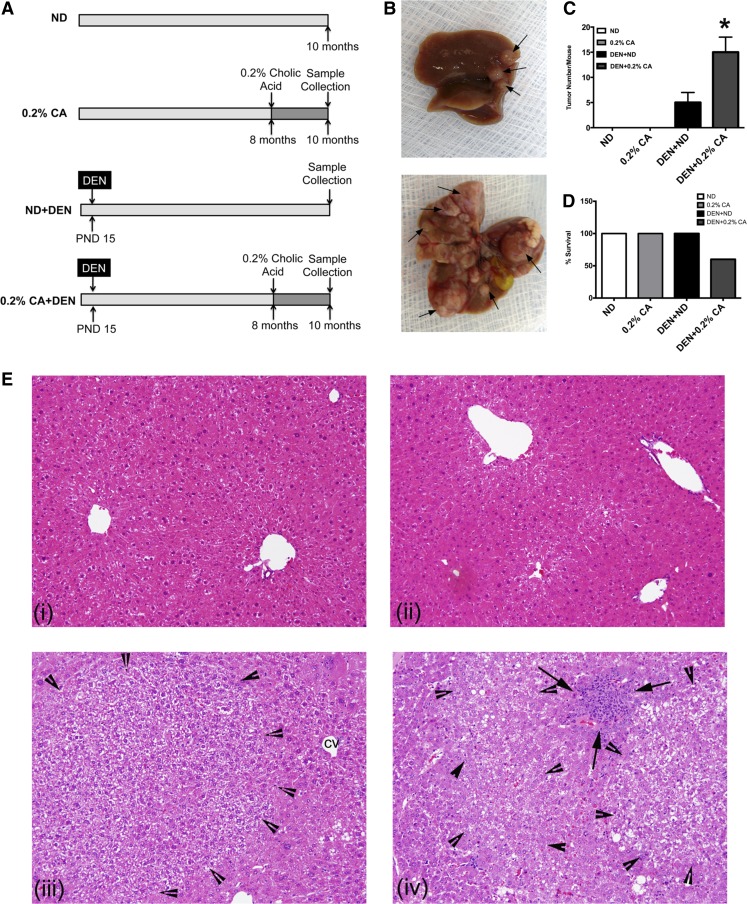
Fig. 1.
Cholic acid (CA) promotes diethylnitrosamine (DEN)-induced hepatic carcinogenesis. A: scheme showing the experimental design. B: representative photographs of mouse liver obtained from DEN + normal diet (ND) and DEN + 0.2% CA treated mice. Arrows point to liver tumors. C: bar graph showing number of liver tumors per mouse. D: percent survival in various treatment groups. Data are expressed as mean ± SE. *P < 0.05, significant difference from all other groups. E: representative photomicrographs of hemtoxylin-eosin (H&E)-stained liver sections from ND (i), 0.2% CA (ii), DEN + ND (iii), and DEN + 0.2% CA (iv) taken at ×400 magnification. Arrowheads demark the tumor boundary and arrows point to inflammatory cell infiltrate.
Mouse hepatocyte isolation and treatments.
Primary mouse hepatocytes were isolated from 2- to 3-mo-old male C57BL/6 mice using standard two-step collagenase perfusion method as previously described (1, 2). Hepatocytes were cultured on collagen-coated plates, allowed to adhere for 3 h, washed, and then treated with either vehicle or bile acids as described before (1). The bile acids used in these study include taurocholic acid (TCA), deoxycholic acid (DCA, also called desoxycholic), and chenodeoxycholic acid (CDCA) at either 100 or 200 μM. Cells were harvested 24 h after bile acid treatment, and mRNA was isolated and used for real-time PCR studies.
Histology and immunohistochemistry.
Paraffin-embedded liver sections (4-μm thick) were used for hemtoxylin-eosin (H&E) staining and immunohistochemical detection of proliferating cell nuclear antigen (PCNA), CD34, CD3, CD20, CD45, and reticulin as described before (8, 16, 32). H&E-stained slides were used for necrosis scoring and PCNA-stained slides were used for PCNA scoring as described before (7). Antibodies used include Dako rabbit polyclonal antibodies anti-α-1-antitrypsin, anti-α feto protein (AFP), and anti-CD3, CD20, and CD45 (Carpentaria, CA) as well as Cell Marque/Epitomics rabbit monoclonal anti-CD34 (Burlingame, CA).
Biochemical analysis of proteins and mRNA.
Western blotting was used to detect protein levels in RIPA extracts and real-time PCR was used to quantify mRNA as described before (32). Primary and secondary antibodies were purchased from Cell Signaling Technologies (Danvers, MA).
Bile acid analysis.
Serum and liver total bile acids were measured enzymatically using Total bile acid assay kit (BQ Kits, San Diego, CA), as per the manufacturer's protocol described previously (6). Specific serum and hepatic bile acids were analyzed by UPLC-MS (Waters, Milford, MA) using methods similar to described previously (6) at the NIH sponsored West Coast Metabolomics Center in University of California, Davis.
Microarray analysis.
Global gene expression analysis was performed using Affymetrix Mouse 430_2.0 Chip and pooled mRNA isolated from four to five separate mice livers per group. Microarrays and Ingenuity Pathway Analysis (IPA) analysis were performed as described before (33). The raw data have been submitted to GEO database (GSE75445).
Human serum bile acid analysis.
All human studies were approved by and were performed in accordance to Ethics Committee and the Clinical Trials office of the Third Affiliated Hospital of Sun Yat-sen University. Total serum bile acids were quantified in de-identified serum samples from normal, cirrhotic, and HCC patients using Automated Biochemical Analyzer Hitachi 7180 (Hitachi).
Statistical analysis.
All data presented as bar graphs are expressed as mean ± SE. Unpaired Student's t-test was used to evaluate statistically significant difference between two groups and differences between more than two groups were analyzed using ANOVA. P < 0.05 was considered statistically significant.
RESULTS
Cholic acid feeding results in increased DEN-induced hepatic tumors.
We used classic initiation-promotion protocol (Fig. 1A) where tumors were initiated by DEN and promoted by 0.2% CA feeding in the diet for 2 mo. The control groups including mice fed normal diet only (ND) and 0.2% CA diet only (0.2% CA) did not have any tumor development. Mice in the DEN + ND group had visible hepatic tumors as expected (Fig. 1B). However, the mice in the DEN + 0.2% CA group had threefold higher number of tumors compared with the DEN + ND group (Fig. 1C). The tumors observed in DEN + 0.2% CA were substantially larger. In addition to tumors, fluid filled hepatic cysts were also visible in the livers of the DEN + 0.2% CA treated group. No mortality was observed in the ND, 0.2% CA, or DEN + ND group but 40% mortality was observed in the DEN + 0.2% CA group (Fig. 1D). Autopsy of the mice that died indicated extensive liver tumors and cysts indicating the death was related to HCC formation.
Histological features of cholic acid-promoted liver tumors.
H&E-stained liver sections were reviewed by a board certified pathologist for detection of histological changes following cholic acid feeding and tumorigenesis. Apart from occasional inflammatory cell infiltration in the liver parenchyma and sinusoidal widening known to occur in cholestatic conditions, livers of 0.2% CA fed mice were histologically normal (Fig. 1, Ei and Eii). The livers of both DEN + ND and DEN + 0.2% CA treated mice had tumors. DEN + 0.2% CA treated mice had significantly higher number of tumors, which were also larger in size. Further histological analysis revealed that the DEN + ND treated mice had mainly hepatic adenoma and occasional well differentiated HCC (Fig. 1, Eiii and Eiv). However, majority of the tumors in the DEN + 0.2% CA group were well differentiated HCC with occasional hepatic adenoma and dysplastic nodules. The histopathological analysis was conducted using the criteria noted in Table 1. Tumors were identified as either hepatic adenoma, dysplastic nodules, or well-differentiated HCC. The tumors in the DEN + 0.2% CA group were accompanied with significant increase in inflammatory cell infiltration with visible inflammatory cell foci adjacent to and within the liver tumors (Fig. 1, Eiii and Eiv). Hepatic adenomas were characterized by liver cells resembling normal hepatocytes, which formed slightly thickened liver cell plates that do not exceed two cells thick. Adenomas were enclosed in a well-developed reticulin framework, lacked internal nodularity, portal tracts, or fibrosis. Dysplastic nodules showed a uniform population of hepatocytes, often with increased nuclear to cytoplasmic ratios. Focally, the hepatic plates were expanded greater than or equal to three cells thick, and there was focal disruption of reticulin framework. Few mitoses were visible. Well-differentiated hepatocellular carcinoma exhibited increased nuclear:cytoplasmic ratios, absence of portal tracts, expansion of hepatic plates greater than three cells thick, and disruption and loss of normal reticulin framework and focal pseudoglandular formation. Mitoses, including atypical mitoses, were frequent (Fig. 1, Eiii-Eiv).
Table 1.
Histopathological criteria used to differentiate various stages in progression of HCC
| Hepatic Adenoma (HA) | Dysplastic Nodule (DN) | Well-Differentiated Hepatocellular Carcinoma (HCC) | |
|---|---|---|---|
| Hepatocytes | Normal | Dysplastic | Dysplastic |
| N:C ratio | Normal | Increased | Increased |
| Nuclear hyperchromasia | Absent | Present | Present |
| Mitotic figures | Absent | Few | Frequent |
| Plate width | 1–2 Cells | 2–3 Cells thick | >3 Cells thick |
| Reticulin | Intact | Focally disrupted | Markedly disrupted or absent |
| Absence of portal tracts | + | ± | + |
| Sinusoidal capillarization (CD34) | ± | ± | + |
N:C ratio, nuclear-to-cytoplasmic ratio.
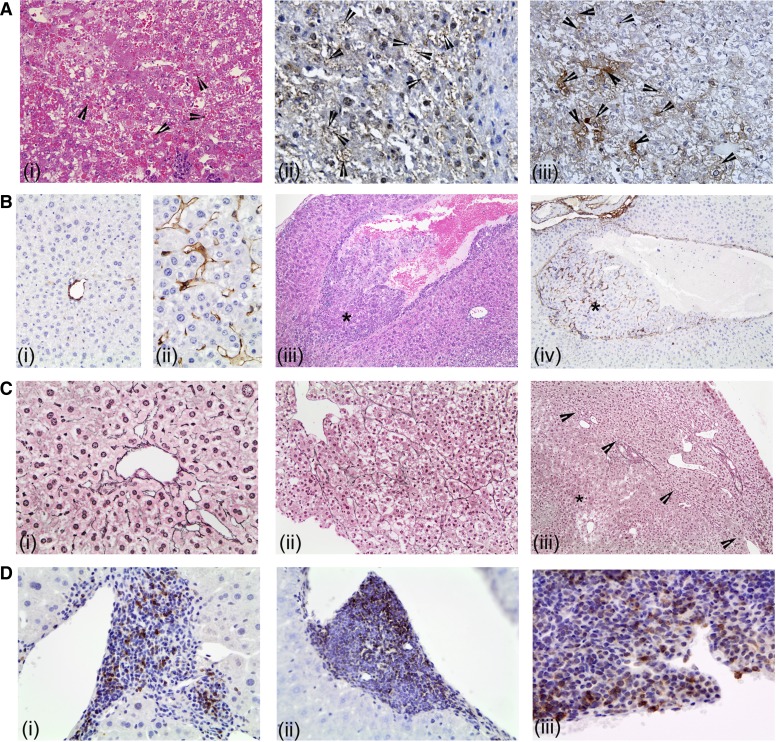
Some but not all tumors in the DEN + 0.2% CA group exhibited intracellular and intranuclear inclusions within the neoplastic hepatocytes (Fig. 2Ai). These globules were not observed in the background benign hepatocytes. These globules were brightly eosinophilic, varied in size and number per cell, and were concentrated within the tumor nodules. The globules were negative for both periodic acid-Schiff (PAS), PAS/diastase, α-1-antitrypsin, AFP, or lipids (Oil Red O staining). Further immunohistochemical analysis revealed that these granules were positive for ubiquitin and CK8 (Fig. 2, Aii-Aiii), which has been demonstrated in HCC associated with cholestasis (14).
Fig. 2.
Histological characterization of hepatocellular carcinoma (HCC). A: representative photomicrographs of H&E-stained sections showing globular inclusions (i, arrowheads) and immunohistochemical detection of ubiquitin (ii) and CK8 (iii). B: representative photomicrographs of CD34 immunohistochemistry on liver sections from ND (i) and DEN + 0.2% CA (ii) treated mice. Liver section from DEN + 0.2% CA treated mouse showing vascular invasion of an HCC. iii: H&E staining (×200). iv: CD34 immunohistochemistry (×200). *Vascular invasion. C: representative photomicrographs of reticulin staining in ND (i) and DEN + 0.2% CA treated mice (ii: ×400. iii: ×200). *HCC within the section and arrowheads show tumor boundary. D: representative photomicrographs of CD3 (i), CD45 (ii), and CD20 (iii). All photographs at ×400 magnification unless mentioned otherwise.
We performed several additional special staining to further characterize the HCC in the DEN + 0.2% CA group. CD34 staining highlighted the capillarization of the sinusoids in neoplastic vs. nonneoplastic tissue (Fig. 2, Bi-Bii). The endothelial cells wrapping the nests and trabeculae phenotypically resembled capillary endothelium (CD34 positive) rather than normal hepatic sinusoidal endothelium (CD34 negative). Interestingly, we observed vascular invasion characterized by CD44-positive capillarization within the vessel in only the DEN + 0.2% CA treated mice (Fig. 2, Biii and Biv). Reticulin staining confirmed the presence of hepatocellular carcinoma and showed widened hepatic plates, disruption of normal hepatic plate architecture, and decreased number/amount of reticulin fibers compared with background nonlesional liver (Fig. 2, Ci-Ciii). The reticulin framework was found to be absent or markedly decreased or distorted, with irregular or absent staining of the borders of the hepatic plates in the HCC. Finally, we characterized the inflammatory infiltrates associated with HCC in the DEN + 0.2% CA group by staining for CD3, CD20, and CD45 (Fig. 2, Di-Diii). The data indicate that the inflammatory infiltrate in and around HCC observed in DEN + 0.2% CA group consisted mainly of CD45-positive lymphocytes with a mixed population of CD3-positive T cells and CD20-positive B cells (Fig. 2, Di and Dii).
Increased proliferation and decreased apoptosis in cholic acid-promoted liver tumors.
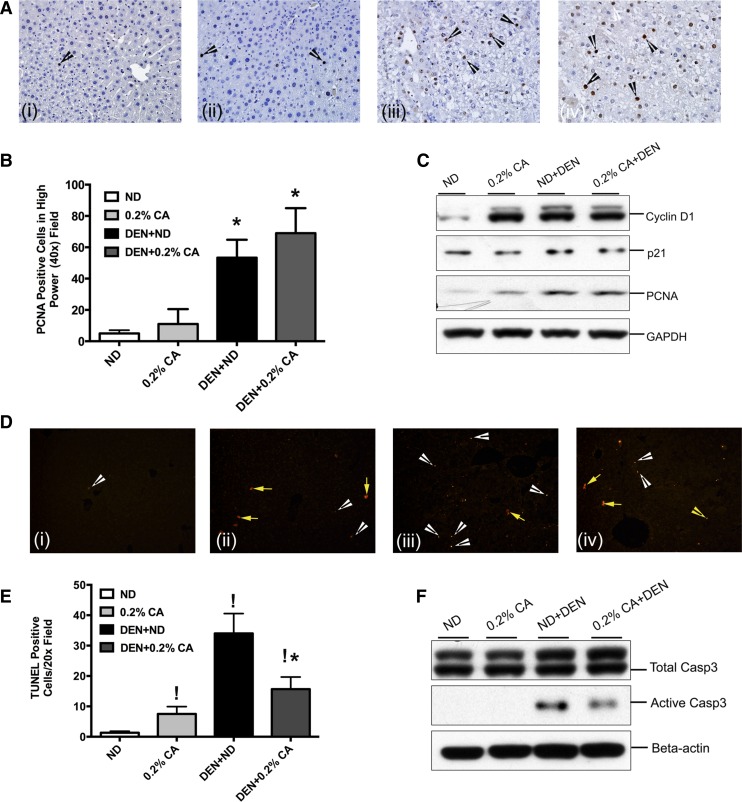
To determine changes in cell proliferation and cell death, we performed immunohistochemical analysis for PCNA and terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling (TUNEL) assay on paraffin embedded liver sections from all four groups (Fig. 3). PCNA staining indicated a moderate increase in hepatocyte proliferation after 0.2% CA feeding, which was not statistically significant (Fig. 3, A and B). A significant increase in cell proliferation was observed in both DEN + ND and DEN + 0.2% CA treated mice compared with ND and 0.2% CA treated mice. Although number of PCNA-positive cells were moderately higher in DEN + 0.2% CA treated mice, the values were not statistically significant than the DEN + ND group. Western blot analysis corroborated the PCNA immunohistochemistry. A significant increase in cyclin D1 and PCNA protein was observed in the 0.2% CA, DEN + ND, and DEN + 0.2% CA groups (Fig. 3C). Interestingly, levels of p21, the main cell cycle inhibitor, decreased in the 0.2% CA, DEN + ND, and DEN + 0.2% CA groups and were lowest in the DEN + 0.2% CA group.
Fig. 3.
Analysis of cell proliferation and apoptosis in CA-promoted DEN-induced HCC. A: representative photomicrographs of PCNA immunohistochemistry performed on paraffin embedded liver sections from various treatment groups. Arrows indicate cells in S phase of cell cycle. B: quantification of PCNA staining. Bar graph shows numbers of PCNA-positive cells in high power (×400) fields in various treatments groups. Data are expressed as mean ± SE. *P < 0.05, significant difference from ND. C: Western blot analysis of cyclin D1, PCNA, and p21 conducted using total liver cell extracts. D: representative photomicrographs of terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling (TUNEL) assay immunofluorescent staining on liver sections from ND (i), 0.2% CA (ii), DEN + ND (iii), and DEN + 0.2% CA (iv). White arrowheads point to cells in apoptosis and yellow arrows point to necrotic cells. E: bar graph showing quantification of TUNEL assay performed using paraffin embedded liver sections from various treatment groups. Data are expressed as mean ± SE. *P < 0.05, significant difference from ND. !P < 0.5, significantly different from DEN + ND group. F: Western blot analysis of total and activated caspase-3 conducted using total liver extracts from various treatment groups.
TUNEL assay results indicated a significant increase in apoptosis in the 0.2% CA, DEN + ND, and DEN + 0.2% CA groups compared with ND control mice (Fig. 3, D and E). However, DEN + 0.2% CA treated mice exhibited 50% lower apoptosis compared with the DEN + ND group. Interestingly, 0.2% CA treatment by itself and in combination with DEN induced significant scattered single cell necrosis as demonstrated by cytoplasmic TUNEL staining, a characteristic of necrotic cell death. TUNEL data were further corroborated by Western blot analysis of total and activated caspase 3 (Fig. 3F). A significant increase in activated caspase 3 was observed in both DEN + ND and DEN + 0.2% CA treated mouse livers but the levels of active caspase 3 protein were significantly lower in the DEN + 0.2% CA group compared with the DEN + ND treated group.
Global gene expression changes in cholic acid-promoted liver tumors.
To determine the mechanisms behind promotion of DEN-induced liver tumors by 0.2% CA, we studied global gene expression changes using Affymetrix microarrays. The extensive data generated in these studies have been submitted to GEO database. We used threefold change cut off for significance. With the use of this cut off, the 0.2% CA-induced expression of 20 genes and decreased expression of 20 genes compared with ND (Table 2). The DEN + ND group had 335 genes upregulated and 28 genes downregulated as compared ND. When gene expression changes in 0.2% CA were compared with those in the DEN + 0.2% CA group, 318 genes were significantly upregulated and 59 genes were downregulated. Finally, in the DEN + 0.2% CA group 18 genes were upregulated and 17 genes were downregulated compared with the DEN + ND group.
Table 2.
Global gene expression changes in promotion of DEN-induced liver tumors by 0.2% cholic acid
| Group Comparison | Upregulated genes | Downregulated Genes |
|---|---|---|
| 0.2% CA vs. ND | 20 | 20 |
| DEN + ND vs. ND | 335 | 28 |
| DEN + 0.2% CA vs. ND | 569 | 130 |
| DEN + 0.2% CA vs. 0.2% CA | 310 | 59 |
| DEN + 0.2% CA vs. DEN + ND | 18 | 17 |
Global gene expression changes are number of genes that changed (up or down) more than 3-fold.
CA, cholic acid; ND, normal diet; DEN, diethylnitrosamine.
To further gain insights into specific signaling pathways changed during promotion of DEN-induced liver tumors by cholic acid, we analyzed the data using IPA. The upstream regulator analysis is used to identify possible activation of transcription factors and nuclear receptors predicted based on the gene expression data. Upstream regulator analysis on the gene array data indicated NF-κB and NANOG signaling pathways were predicted to be upregulated in the DEN + 0.2% CA group (Table 3). IPA also predicted downregulation of other transcription regulators including RBPJ (regulator in Notch signaling pathway), estrogen receptor, glucocorticoid receptor, and SREBF1. Furthermore, we performed upstream regulator analysis on cytokines and growth factors, which revealed several inflammatory cytokines including IL-1β and TNF-α to be activated (Table 3).
Table 3.
Upstream regulators activated and inhibited in DEN +0.2% CA group
| Upstream Regulator | Predicted Activation State | Activation z-Score | P Value of Overlap |
|---|---|---|---|
| Transcription Factors | |||
| NFκB | Activated | 2.792 | 1.38E-04 |
| NUPR1 | Activated | 2.558 | 7.10E-05 |
| NANOG | Activated | 2.236 | 4.73E-02 |
| RBPJ | Inhibited | −2.000 | 2.49E-02 |
| Estrogen receptor | Inhibited | −2.111 | 1.31E-01 |
| NR3C1 | Inhibited | −2.219 | 5.26E-02 |
| SREBF1 | Inhibited | −2.592 | 5.81E-06 |
| Cytokines and Growth Factors | |||
| IFNγ | Activated | 3.240 | 9.10E-05 |
| IL1β | Activated | 3.068 | 3.54E-06 |
| TNFα | Activated | 2.846 | 7.98E-06 |
| IFNα2 | Activated | 2.570 | 1.09E-05 |
| IL1α | Activated | 2.447 | 2.73E-03 |
| CSF2 | Activated | 2.236 | 1.94E-01 |
| IFNL1 | Activated | 2.138 | 8.11E-04 |
| IL1RN | Inhibited | −2.345 | 5.82E-04 |
Increased inflammatory signaling in cholic acid-promoted liver tumors.
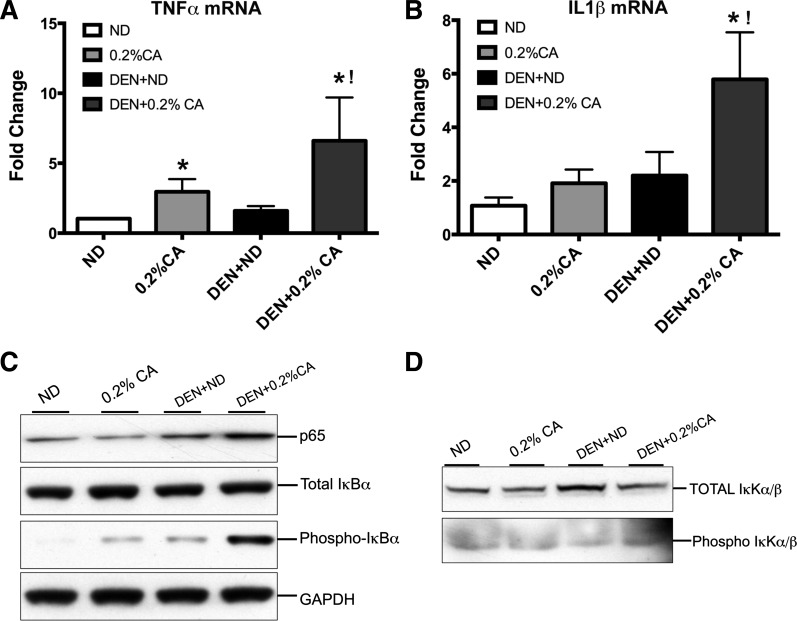
Histological analysis, gene array data and further upstream regulator analysis of gene expression data together indicated increase in inflammatory signaling in DEN + 0.2% CA treated livers compared with all other groups. The main difference between the DEN + ND and DEN + 0.2% CA groups was the increased inflammation in the DEN + 0.2% CA group. Therefore, we further investigated whether TNF-α-IL-1β-NF-κB activation is involved in increased inflammation and tumor promotion following cholic acid feeding. Real-time PCR analysis revealed a twofold increase in TNF-α and IL-1β transcripts following 0.2% CA treatment compared with ND (Fig. 4, A and B). The DEN alone did not cause increase in TNF-α mRNA but resulted in twofold increase in IL-1β mRNA. However, a sixfold increase in TNF-α and IL-1β mRNA in the DEN + 0.2% CA compared with the ND group. Western blot analysis indicated a significant increase in total p65 levels in the DEN + ND and DEN + 0.2% CA groups but the DEN + 0.2% CA group had substantially higher p65 protein compared with any other the group (Fig. 4C). The total IκBα protein was unchanged in any treatment, but the phosphorylated form of IκBα (targeted for degradation) was significantly higher in the DEN + 0.2% CA group consistent with increased p65 protein levels. Finally, Western blot analysis indicated a moderate increase in IKKα/β, the primary regulator of IκB/NF-κB interaction in DEN + ND treated mice. However, phosphorylated (active) IKKα/β protein was higher in the DEN + 0.2% CA group (Fig. 4D). Taken together, these data indicate higher activation of IKKα/β in the DEN + 0.2% CA group compared with all other groups.
Fig. 4.
Increased inflammation in CA-promoted DEN-induced HCC. Real-time PCR analysis of TNF-α (A) and IL-1β mRNA (B) in various groups. Data are expressed as mean ± SE. *P < 0.05, significant difference from the ND group. !P < 0.05, significant difference from all other groups. Western blot analysis of total p65, total and phosphorylated IκBα (C) and total and phosphorylated IκKα/β (D) conducted using total liver extracts from various groups.
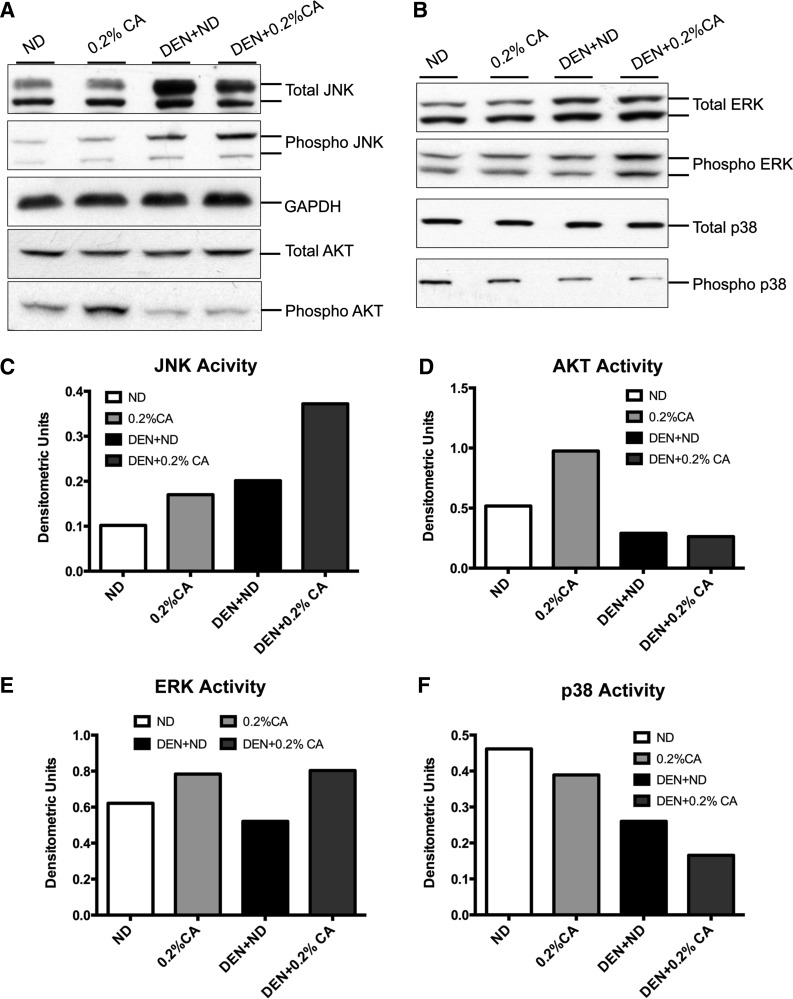
Changes in MAPK signaling in cholic acid-promoted liver tumors.
Previous studies have indicated that bile acids are able to induce MAPKs, which further exacerbate inflammatory signals. To determine MAPK activation during promotion of DEN-induced HCC by cholic acid, we investigated levels of total and phosphorylated AKT, ERK, JNK, and p38 MAPK by Western blot (Fig. 5). The results indicate that 0.2% CA and DEN + ND treated mice showed a moderate increase in JNK activation (ratio of phosphorylated protein to total protein) but DEN + 0.2% CA mice had significant activation (4-fold higher than ND) of JNK (Fig. 5, A and C). AKT activation was induced twofold by 0.2% CA treatment but was surprisingly decreased in both the DEN + ND and DEN + 0.2% CA groups compared with ND treated mice (Fig. 5, A and D). A very mild increase in ERK activity was observed in both the 0.2% CA and DEN + 0.2% CA groups (Fig. 5, B and E). Finally, p38 activity decreased significantly in the DEN + ND and DEN + 0.2% CA treated groups with more than threefold decrease in the DEN + 0.2% CA group compared with the ND group (Fig. 5, B and F). Taken together, these data indicate a significant increase in JNK activity and significant decrease in p38 MAPK activity accompanied higher tumor incidence in DEN + 0.2% CA treated mice.
Fig. 5.
Changes in MAPK signaling in CA-promoted DEN-induced HCC. Western blot analysis of total and phosphorylated JNK and AKT (A) and total and phosphorylated ERK and p38 MAPK (B) conducted using total liver extracts from various groups. C–F: densitometric analysis of Western blots of JNK, AKT, ERK, and p38, respectively, conducted using blots shown in A and B.
Increased bile acids during HCC pathogenesis in mice.
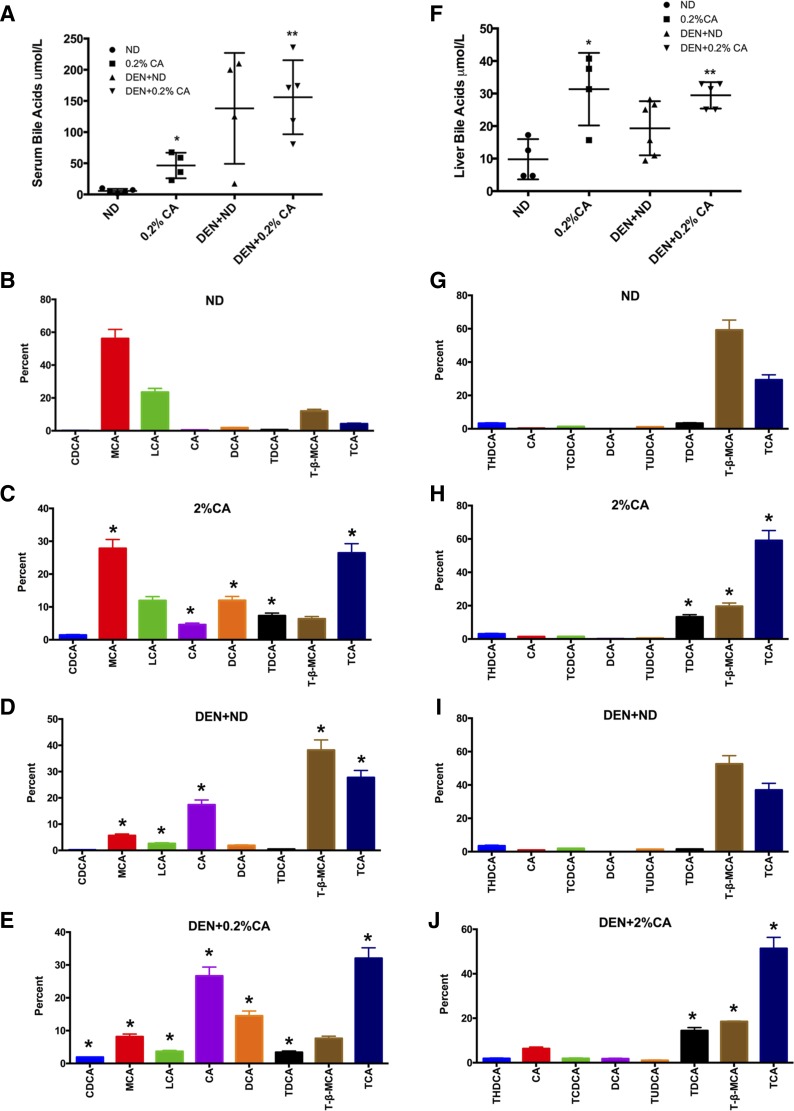
To determine whether development of HCC is accompanied with changes in bile acids, we quantified total and specific bile acids in serum and livers from all groups of mice. We analyzed a total of 20 specific bile acids in liver and serum out of which eight bile acids contributed to over 97% of the total bile acids. These data were further analyzed to determine the percentage each bile acid contributed to total bile acids measured. The data indicate significant increase in serum bile acids in 0.2% CA and DEN + 0.2% CA treated mice (Fig. 6A). Interestingly, the mice in the DEN + ND group also showed an overall increase in total serum bile acids but there was significant variation. Further analysis revealed substantial changes in the bile acid composition (Fig. 6, B–E). Cholic acid feeding alone substantially decreased amounts of muricholic acid and tauro-β-muricholic acid and increased levels of cholic acid, taurocholic acid, chenodeoxycholic acid, deoxycholic acid. Intriguingly, DEN treatment alone (in the DEN + ND treated group) increased levels of cholic acid, taurocholic acid, and tauro-β-muricholic acid and decreased the levels of muricholic acid. Similarly, a substantial increase in deoxycholic acid and cholic acid along with decrease in tauro-β-muricholic acid was observed in serum of DEN + 0.2% CA treated mice.
Fig. 6.
Changes in bile acids in serum and liver during CA-induced promotion of DEN-initiated tumors. A: quantification of total bile acids in serum in mice after various treatment groups. B–E: bar graphs showing contribution of each bile acid to total bile acids in serum of ND, 0.2% CA, DEN + ND, and DEN + 0.2% CA groups. F: quantification of total bile acids in livers of mice after various treatment groups. G–J: bar graphs showing contribution of each bile acid to total bile acids in serum of ND, 0.2% CA, DEN + ND, and DEN + 0.2% CA groups. Data are expressed as mean ± SE. In B–E, all comparisons are made to respective bile acid concentration in ND group. *P < 0.05, significant difference. **P < 0.01, significant difference.
Total liver bile acid analysis showed substantial increase in total hepatic bile acid content in the 0.2% CA and DEN + 0.2% CA treated groups (Fig. 6F). The DEN + ND treated mice also had increase in total liver bile acid with a lesser degree of variation compared with serum values but it was not statistically significant than the ND group. Specific bile acid analysis (Fig. 6, G–J) revealed decrease in tauro-β-muricholic acid and increase in taurocholic acid and taurodeoxycholic acid in mice fed 0.2% CA diet. Treatment with DEN alone (in DEN + ND treated group) did not show significant change in bile acid composition except moderate increase in taurocholic acid and slight decrease in tauro-β-muricholic acid. However, DEN + 0.2% CA treated mice showed significant decrease in tauro-β-muricholic acid and substantial increase in cholic acid, taurocholic acid, and deoxycholic acid. Taurodeoxycholic acid levels increased compared with the ND and DEN + ND group but decreased compared with DEN + 0.2% CA.
Bile acid treatment induces genes associated with stemness.
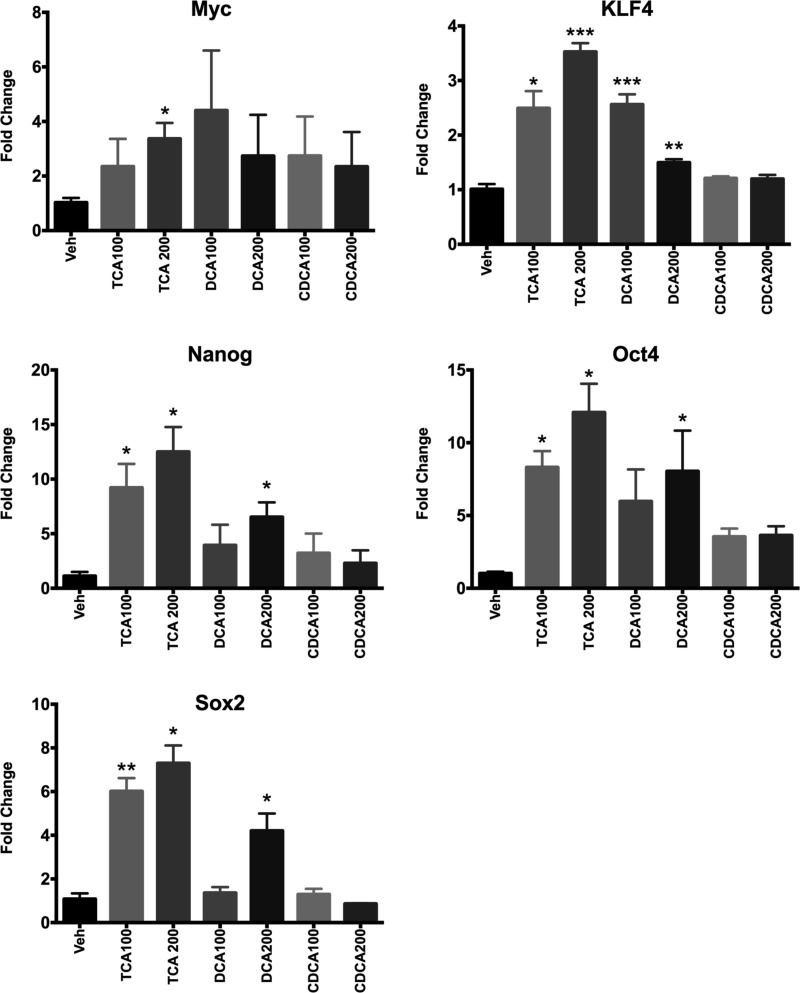
Our gene array studies indicate extensive activation of Nanog, a factor associated with stemness. To determine whether bile acids can regulate factors associated with stemness in hepatocytes, we treated primary mouse hepatocytes with bile acids including taurocholic acid, deoxycholic acid, and chenodeoxycholic acid. Our data indicate that taurocholic acid and deoxycholic acid, but not chenodeoxycholic acid, could induce stemness genes including Myc, KLF4, Nanog, Oct4, and Sox2 between 2- and 15-fold (Fig. 7). These data indicate that high concentrations of bile acids may induce factors associated with hepatic dedifferentiation and stemness.
Fig. 7.
Bile acids induce stemness genes in mouse hepatocytes. Bar graphs showing mRNA levels of Myc, KLF4, Nanog, Oct4, and Sox2 as measured by Rea Time PCR using total RNA extracted from primary mouse hepatocytes treated with various bile acids for 24 h. Data are expressed as mean ± SE. *P < 0.05, significant difference. **P < 0.01, significant difference. ***P < 0.001, significant difference.
Increased bile acids during HCC pathogenesis in humans.
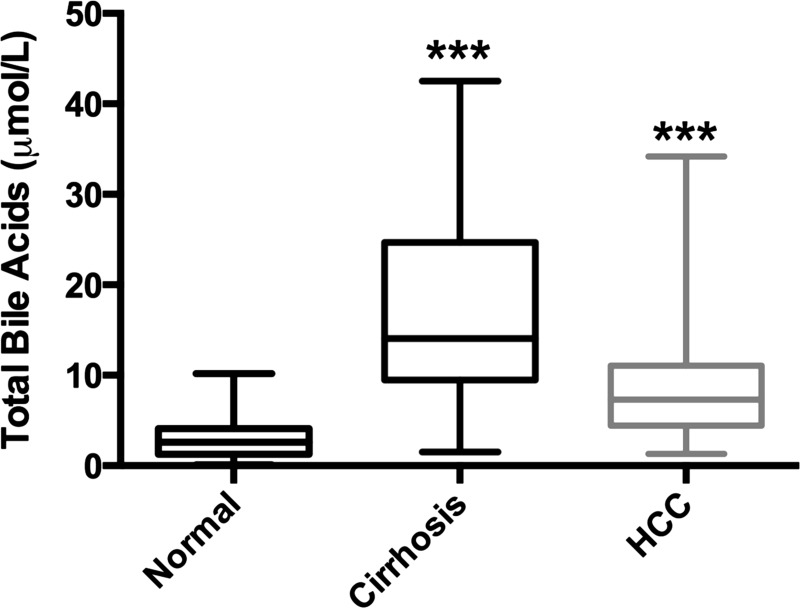
To determine whether increase in bile acid is associated with HCC pathogenesis in humans, we determined serum bile acids in a total of 32 normal, 30 cirrhotic without HCC, and 36 HCC patients (Fig. 8). The clinical characteristics of patients are provided in Table 4. The data indicate an overall increase in serum bile acids in both cirrhotic without HCC and with HCC compared with normal patients with no difference between the two cirrhotic groups.
Fig. 8.
Serum total bile acids in normal, cirrhotic and HCC patient samples. Data are expressed as mean ± SE. ***P < 0.001, significant difference.
Table 4.
Gender and age distribution of clinical cases
| Gender | Number of Cases | Median Age | Standard Deviation |
|---|---|---|---|
| Normal | |||
| Male | 16 | 40.5 | 14.93 |
| Female | 16 | 34 | 14.36 |
| HCC | |||
| Male | 27 | 48 | 10.8 |
| Female | 9 | 51 | 10.61 |
| Cirrhosis | |||
| Male | 24 | 42.5 | 8.72 |
| Female | 6 | 55.5 | 3.72 |
DISCUSSION
Role of bile acids in regulation of metabolism, cell growth, and cancer promotion apart from their classic role in digestion has been well recognized (10, 15). In the liver, bile acids play a critical role in liver homeostasis, liver regeneration, and development of liver cancers (4). Several animal models with increase in total bile acids exhibit spontaneous liver cancer development including the whole body FXR−/− mice and the BSEP-KO mice (21, 22, 35). Interestingly, sequestration of bile acids using cholestyramine can decrease liver cancer incidence in FXR−/− mice (35). Whereas these studies clearly indicate that increased bile acids are a risk factor in liver cancer pathogenesis, the mechanisms remain unknown. Furthermore, whether increased bile acids are associated with HCC development in humans is also not known. The data presented here demonstrate that the increase in total bile acids is associated with human HCC development and show that increased inflammation as the possible mechanisms of bile acid-mediated tumor promotion.
We used a classic initiation-promotion model of DEN-induced liver carcinogenesis to study the mechanisms of bile acid-induced HCC promotion. We choose cholic acid mainly because it is one of the major bile acids in humans and previous studies with cholic acid feeding in diet provided background information necessary for dose selection (6, 36). Our results obtained from a DEN-induced initiation-promotion model clearly demonstrate that bile acids can act as tumor promoters in liver carcinogenesis. Additionally, our data taken together with previous studies confirms that bile acids alone are not complete carcinogens, as bile acid feeding, albeit for short duration, did not result in liver cancers. Previous studies have demonstrated that bile acids can stimulate cell proliferation during liver regeneration (6, 19). We investigated whether the higher tumor promotion by bile acids is simply due to increases cell proliferation in the liver. However, our data indicate that cholic acid-promoted tumors exhibited only a moderate increase in cell proliferation but a much significant decrease in apoptosis as compared all other treatments. Further, cholic acid feeding resulted in scattered single cell necrotic cell death in the liver both in the 0.2% CA and DEN + 0.2% CA groups, which was absent in the ND and DEN + ND groups. It is plausible that the necrotic cell death observed may be a trigger for inflammation and in the DEN + 0.2% CA group; the inflammatory signaling further helps in selecting the mutated cells towards tumorigenesis. The molecular mechanisms by which increased bile acids induce cell death, increase inflammation, and promote cell proliferation have been investigated in the past. Bile acids can signal via a number of FXR-dependent and -independent mechanisms to induce cell death including activation of EGFR-dependent Egr1 activation, TGR5-mediated signaling in nonparenchymal cells in the liver, and S1P2 receptor-mediated signaling (1, 24, 29). These data suggest that bile acids may promote tumorigenesis by disturbing the natural balance between cell proliferation and compensatory cell death in the liver.
One of the intriguing findings of this study is that some of the HCC observed in the DEN + 0.2% CA treated mice but not in DEN + ND treated mice showed characteristic eosinophillic intracellular and intranuclear inclusions in tumor cells. Similar inclusions have been observed in livers of cholic acid fed cholestatic mice and were found to be CK8 and ubiquitin-positive Mallory bodies (14). Our studies revealed that the intracellular and intranuclear inclusions found in HCC of DEN + 0.2% CA treated mice were also positive for CK8 and ubiquitin. These data suggests that these CK8/ubiquitin-positive intracellular inclusions are associated with increased bile acids and could be potentially used diagnostically in clinical settings to identify HCC with bile acid abnormalities.
Global gene expression analysis, especially the upstream regulator analysis (23), revealed three transcription factors including NF-κB, NUPR1 (also called p8), and NANOG as major upstream regulators of gene expression changes observed. NUPR1 is a stress-activated transcription factor linked to endoplasmic reticulum stress and chemoresistance and deletion of NUPR1 results in higher CCl4-induced liver injury and PPAR-mediated liver cancer formation in the liver (11, 18). Whereas we did not further pursue NUPR1 in this study, the role of this stress-activated protein in tumor promotion by bile acids is intriguing and warrants further scrutiny. Nanog, a pluripotency factor, well known for its role in conferring pluripotency to iPS cells, is also implicated in cancer pathogenesis including that of liver cancers (5, 20, 28, 31). Our observation of increased Nanog expression in cholic acid-promoted DEN-induced HCC in mice is consistent with the putative role of Nanog in liver cancer and indicate that bile acids may promote survival of tumor initiating stem cells in the cancer. This is supported by our studies where primary mouse hepatocytes treated with high concentrations of bile acids exhibited increased expression of stemness factors including Myc, Nanog, KLF4, Oct4, and Sox2 (all Yamanaka factors). The mechanism by which bile acids induce these factors is currently not known. Recent studies have indicated role of yes associated protein (Yap), the downstream effector of Hippo kinase signaling pathway in bile acid-mediated tumor promotion (3). Yap has also been shown to stimulate cellular reprogramming inducing stemness in the liver. although our detailed analysis of the Hippo kinase pathway (data not shown) revealed that Yap was equally activated in both the DEN + ND and DEN + 0.2% CA groups, it is possible that an underlying Nanog-Yap signaling axis may be involved in bile acid-induced promotion of liver cancers, which require additional investigations.
Our upstream analysis on transcription factors and cytokines revealed NF-κB, TNF-α, and IL-1β to be highly upregulated in the DEN + 0.2% CA group, which was corroborated by detailed analysis of these factors in the liver tissues. These data suggest that enhanced inflammatory signaling is involved in tumor promotion by bile acids. These data are consistent with previous observation that bile acids are proinflammatory molecules (1, 2, 10, 15, 26). Previous studies have also shown increase in IL-1β in whole body FXR-KO mice, which have spontaneous liver tumors (21). These data also raise a question regarding the primary receptor involved in this proinflammatory and protumorigenic effect of bile acids. Previous observations indicate that FXR and TGR5 activation by bile acids is anti-inflammatory whereas EGFR activation by bile acids leads to proinflammatory signaling (2, 26, 29). Further studies are needed to determine the role of these various receptors, which are expressed in different cell types in the liver, in bile acid-induced tumor promotion.
One of the important contributions of this study is the determination of changes in bile acid composition during HCC pathogenesis. Although our data, not surprisingly, revealed increased bile acids in cholic acid fed groups, they also indicated an increase in total serum and liver bile acids in the DEN + ND treated group. These mice were only treated with DEN and experienced a slow progressive tumor growth. The significant increase in total and specific bile acids in DEN treated mice indicates that loss of bile acid regulation is an integral part of HCC pathogenesis. These data were further supported by highest increase in bile acids in the DEN + 0.2% CA treated mice above all other groups, indicating an additive effect of DEN and cholic acid treatment. The common theme in bile acids changes associated with HCC formation was a decrease in tauro-β-muricholic acid and an increase in cholic acid, muricholic acid, and deoxycholic acid. Out of these, the significant increase in deoxycholic acid (DCA, also called desoxycholic acid) known to induce inflammatory signaling in the liver (17, 30) is consistent with our other observations. It is plausible that increased DCA may trigger proinflammatory signaling resulting in tumor promotion in the DEN + 0.2% CA group. Finally, our analysis of serum bile acids in normal, cirrhotic, and HCC patients indicated an increase in circulating bile acids in cirrhotic and HCC patients further supporting the role of bile acids in tumor promotion.
Previous observations indicate that HCC development is relative rare in adult cholestatic disease but is more common in children with cholestasis, as seen in children with mutations in BSEP (22). Given the fact that HCC is an adult disease, formation of HCC in children with cholestasis further indicates that increased bile acids may result in faster promotion of an adult disease in the children. HCC may be rare in adult cholestatic patients but deregulation of bile acid homeostasis secondary to loss of FXR has been observed in both liver cirrhosis and nonalcoholic steatohepatitis (NASH), two conditions closely associated with HCC development (13, 34). Thus our findings have revealed a previously unrecognized role of bile acids in promotion of HCC of multiple etiologies.
In summary, our data indicate that bile acids can promote liver tumors via increasing inflammatory signaling, endoplasmic reticulum stress, and possibly selective survival of tumor-initiating stem cells. Whereas additional studies are needed to fully understand the mechanisms behind bile acid-mediated tumor growth, our data suggest that reducing bile acids may be a plausible treatment option in inhibiting tumor formation in early cirrhotic patients without HCC and reducing tumor growth in HCC patients.
GRANTS
These studies were supported by National Institutes of Health Grants P20-RR-021940, 5T32-E-8007079, and R01-DK-098414 and an American Association for the Study of Liver Diseases/Acute Liver Failure Liver Scholar Award (to U. Apte). The gene array studies were performed by the Kansas University Medical Center-Microarray Facility (KUMC-MF), which is supported by the Kansas University-School of Medicine, KUMC Biotechnology Support Facility, the Smith Intellectual and Developmental Disabilities Research Center (HD-02528), and the Kansas IDeA Network of Biomedical Research Excellence (RR-016475). Studies in human bile acid analysis were supported by National Natural Science Foundation of China Grant 30772135 (to H. Li). The mouse specific bile acid analysis was conducted in West Coast Metabolomics Center at University of California, Davis funded by National Institute of Diabetes and Digestive and Kidney Diseases Grant 1U24-DK-097154.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
L.S., K.B., P.B., G.E., B.B., C.W., N.R., M.W.M.J., S.G., M.O., H.L., and U.A. performed experiments; L.S., K.B., P.B., B.B., C.W., N.R., S.G., M.O., H.L., and U.A. analyzed data; L.S., P.B., G.E., B.B., C.W., M.W.M.J., S.G., H.L., and U.A. approved final version of manuscript; P.B. and U.A. edited and revised manuscript; S.G., M.O., and U.A. interpreted results of experiments; M.O. and U.A. prepared figures; U.A. conception and design of research; U.A. drafted manuscript.
ACKNOWLEDGMENTS
Present address: Department of Pediatrics, Third Hospital of Hebei Medical University, Shijiazhuang 050051, Hebei, China.
REFERENCES
- 1.Allen K, Jaeschke H, Copple BL. Bile acids induce inflammatory genes in hepatocytes: a novel mechanism of inflammation during obstructive cholestasis. Am J Pathol 178: 175–186, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen K, Kim ND, Moon JO, Copple BL. Upregulation of early growth response factor-1 by bile acids requires mitogen-activated protein kinase signaling. Toxicol Appl Pharmacol 243: 63–67, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anakk S, Bhosale M, Schmidt VA, Johnson RL, Finegold MJ, Moore DD. Bile acids activate YAP to promote liver carcinogenesis. Cell Rep 5: 1060–1069, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baptissart M, Vega A, Maqdasy S, Caira F, Baron S, Lobaccaro JM, Volle DH. Bile acids: from digestion to cancers. Biochimie 95: 504–517, 2013. [DOI] [PubMed] [Google Scholar]
- 5.Ben-Porath I, Thomson MW, Carey VJ, Ge R, Bell GW, Regev A, Weinberg RA. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet 40: 499–507, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhushan B, Borude P, Edwards G, Walesky C, Cleveland J, Li F, Ma X, Apte U. Role of bile acids in liver injury and regeneration following acetaminophen overdose. Am J Pathol 183: 1518–1526, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhushan B, Walesky C, Manley M, Gallagher T, Borude P, Edwards G, Monga SP, Apte U. Pro-regenerative signaling after acetaminophen-induced acute liver injury in mice identified using a novel incremental dose model. Am J Pathol 184: 3013–3025, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borude P, Edwards G, Walesky C, Li F, Ma X, Kong B, Guo GL, Apte U. Hepatocyte-specific deletion of farnesoid X receptor delays but does not inhibit liver regeneration after partial hepatectomy in mice. Hepatology 56: 2344–2352, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention Hepatocellular carcinoma–United States CDC, 2001–2006. MMWR Morb Mortal Wkly Rep 59: 517–520, 2010. [PubMed] [Google Scholar]
- 10.Chiang JY. Bile acid metabolism and signaling. Compr Physiol 3: 1191–1212, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chowdhury UR, Samant RS, Fodstad O, Shevde LA. Emerging role of nuclear protein 1 (NUPR1) in cancer biology. Cancer Metastasis Rev 28: 225–232, 2009. [DOI] [PubMed] [Google Scholar]
- 12.El-Serag HB. Hepatocellular carcinoma. N Engl J Med 365: 1118–1127, 2011. [DOI] [PubMed] [Google Scholar]
- 13.Ferslew BC, Xie G, Johnston CK, Su M, Stewart PW, Jia W, Brouwer KL, Sidney Barritt A 4th. Altered bile acid metabolome in patients with nonalcoholic steatohepatitis. Dig Dis Sci 60: 3318–3328, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fickert P, Trauner M, Fuchsbichler A, Stumptner C, Zatloukal K, Denk H. Bile acid-induced Mallory body formation in drug-primed mouse liver. Am J Pathol 161: 2019–2026, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gadaleta RM, van Mil SW, Oldenburg B, Siersema PD, Klomp LW, van Erpecum KJ. Bile acids and their nuclear receptor FXR: Relevance for hepatobiliary and gastrointestinal disease. Biochim Biophys Acta 1801: 683–692, 2010. [DOI] [PubMed] [Google Scholar]
- 16.Gkretsi V, Apte U, Mars WM, Bowen WC, Luo JH, Yang Y, Yu YP, Orr A, St-Arnaud R, Dedhar S, Kaestner KH, Wu C, Michalopoulos GK. Liver-specific ablation of integrin-linked kinase in mice results in abnormal histology, enhanced cell proliferation, and hepatomegaly. Hepatology 48: 1932–1941, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta S, Natarajan R, Payne SG, Studer EJ, Spiegel S, Dent P, Hylemon PB. Deoxycholic acid activates the c-Jun N-terminal kinase pathway via FAS receptor activation in primary hepatocytes. Role of acidic sphingomyelinase-mediated ceramide generation in FAS receptor activation. J Biol Chem 279: 5821–5828, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Huang J, Viswakarma N, Yu S, Jia Y, Bai L, Vluggens A, Cherkaoui-Malki M, Khan M, Singh I, Yang G, Rao MS, Borensztajn J, Reddy JK. Progressive endoplasmic reticulum stress contributes to hepatocarcinogenesis in fatty acyl-CoA oxidase 1-deficient mice. Am J Pathol 179: 703–713, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang W, Ma K, Zhang J, Qatanani M, Cuvillier J, Liu J, Dong B, Huang X, Moore DD. Nuclear receptor-dependent bile acid signaling is required for normal liver regeneration. Science 312: 233–236, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Iv Santaliz-Ruiz LE, Xie X, Old M, Teknos TN, Pan Q. Emerging role of nanog in tumorigenesis and cancer stem cells. Int J Cancer 135: 2741–2748, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim I, Morimura K, Shah Y, Yang Q, Ward JM, Gonzalez FJ. Spontaneous hepatocarcinogenesis in farnesoid X receptor-null mice. Carcinogenesis 28: 940–946, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knisely AS, Strautnieks SS, Meier Y, Stieger B, Byrne JA, Portmann BC, Bull LN, Pawlikowska L, Bilezikci B, Ozcay F, Laszlo A, Tiszlavicz L, Moore L, Raftos J, Arnell H, Fischler B, Nemeth A, Papadogiannakis N, Cielecka-Kuszyk J, Jankowska I, Pawlowska J, Melin-Aldana H, Emerick KM, Whitington PF, Mieli-Vergani G, Thompson RJ. Hepatocellular carcinoma in ten children under five years of age with bile salt export pump deficiency. Hepatology 44: 478–486, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Kramer A, Green J, Pollard J Jr, Tugendreich S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 30: 523–530, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwong E, Li Y, Hylemon PB, Zhou H. Bile acids and sphingosine-1-phosphate receptor 2 in hepatic lipid metabolism. Acta Pharmacol Sin 5: 151–157, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li G, Kong B, Zhu Y, Zhan L, Williams JA, Tawfik O, Kassel KM, Luyendyk JP, Wang L, Guo GL. Small heterodimer partner overexpression partially protects against liver tumor development in farnesoid X receptor knockout mice. Toxicol Appl Pharmacol 272: 299–305, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li G, Zhu Y, Tawfik O, Kong B, Williams JA, Zhan L, Kassel KM, Luyendyk JP, Wang L, Guo GL. Mechanisms of STAT3 activation in the liver of FXR knockout mice. Am J Physiol Gastrointest Liver Physiol 305: G829–G837, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Llovet JM, Bruix J. Molecular targeted therapies in hepatocellular carcinoma. Hepatology 48: 1312–1327, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Machida K, Chen CL, Liu JC, Kashiwabara C, Feldman D, French SW, Sher L, Hyeongnam JJ, Tsukamoto H. Cancer stem cells generated by alcohol, diabetes, and hepatitis C virus. J Gastroenterol Hepatol 27, Suppl 2: 19–22, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pols TW, Noriega LG, Nomura M, Auwerx J, Schoonjans K. The bile acid membrane receptor TGR5 as an emerging target in metabolism and inflammation. J Hepatol 54: 1263–1272, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qiao L, Studer E, Leach K, McKinstry R, Gupta S, Decker R, Kukreja R, Valerie K, Nagarkatti P, El Deiry W, Molkentin J, Schmidt-Ullrich R, Fisher PB, Grant S, Hylemon PB, Dent P. Deoxycholic acid (DCA) causes ligand-independent activation of epidermal growth factor receptor (EGFR) and FAS receptor in primary hepatocytes: inhibition of EGFR/mitogen-activated protein kinase-signaling module enhances DCA-induced apoptosis. Mol Biol Cell 12: 2629–2645, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shan J, Shen J, Liu L, Xia F, Xu C, Duan G, Xu Y, Ma Q, Yang Z, Zhang Q, Ma L, Liu J, Xu S, Yan X, Bie P, Cui Y, Bian XW, Qian C. Nanog regulates self-renewal of cancer stem cells through the insulin-like growth factor pathway in human hepatocellular carcinoma. Hepatology 56: 1004–1014, 2012. [DOI] [PubMed] [Google Scholar]
- 32.Walesky C, Edwards G, Borude P, Gunewardena S, O'Neil M, Yoo B, Apte U. Hepatocyte nuclear factor 4 alpha deletion promotes diethylnitrosamine-induced hepatocellular carcinoma in rodents. Hepatology 57: 2480–2490, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walesky C, Gunewardena S, Terwilliger EF, Edwards G, Borude P, Apte U. Hepatocyte-specific deletion of hepatocyte nuclear factor 4α in adult mice results in increased hepatocyte proliferation. Am J Physiol Gastrointest Liver Physiol 304: G26–G37, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolfe A, Thomas A, Edwards G, Jaseja R, Guo GL, Apte U. Increased activation of Wnt/[beta]-catenin pathway in spontaneous hepatocellular carcinoma observed in farnesoid x receptor knockout mice. J Pharmacol Exp Ther 338: 12–21, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang F, Huang X, Yi T, Yen Y, Moore DD, Huang W. Spontaneous development of liver tumors in the absence of the bile acid receptor farnesoid X receptor. Cancer Res 67: 863–867, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Klaassen CD. Effects of feeding bile acids and a bile acid sequestrant on hepatic bile acid composition in mice. J Lipid Res 51: 3230–3242, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]