Abstract
Loss of function mutations in the actin motor myosin Vb (Myo5b) lead to microvillus inclusion disease (MVID) and death in newborns and children. MVID results in secretory diarrhea, brush border (BB) defects, villus atrophy, and microvillus inclusions (MVIs) in enterocytes. How loss of Myo5b results in increased stool loss of chloride (Cl−) and sodium (Na+) is unknown. The present study used Myo5b loss-of-function human MVID intestine, polarized intestinal cell models of secretory crypt (T84) and villus resembling (CaCo2BBe, C2BBe) enterocytes lacking Myo5b in conjunction with immunofluorescence confocal stimulated emission depletion (gSTED) imaging, immunohistochemical staining, transmission electron microscopy, shRNA silencing, immunoblots, and electrophysiological approaches to examine the distribution, expression, and function of the major BB ion transporters NHE3 (Na+), CFTR (Cl−), and SLC26A3 (DRA) (Cl−/HCO3−) that control intestinal fluid transport. We hypothesized that enterocyte maturation defects lead villus atrophy with immature secretory cryptlike enterocytes in the MVID epithelium. We investigated the role of Myo5b in enterocyte maturation. NHE3 and DRA localization and function were markedly reduced on the BB membrane of human MVID enterocytes and Myo5bKD C2BBe cells, while CFTR localization was preserved. Forskolin-stimulated CFTR ion transport in Myo5bKD T84 cells resembled that of control. Loss of Myo5b led to YAP1 nuclear retention, retarded enterocyte maturation, and a cryptlike phenotype. We conclude that preservation of functional CFTR in immature enterocytes, reduced functional expression of NHE3, and DRA contribute to Cl− and Na+ stool loss in MVID diarrhea.
Keywords: CFTR, brush border, MVI, Myo5b, NHE3, MVID
microvillus inclusion disease (MVID) is a rare but life-threatening disease that affects newborns and children and leads to rapid death from severe secretory diarrhea. MVID clusters in the Middle East and Navajo Indian populations in the US and is associated with consanguinity (41, 42, 47, 51, 61). Stool volumes are greater than 125 ml·kg−1·day−1 with elevated levels of chloride (Cl−) and sodium (Na+) (47) resulting in diarrhea that is often worse than cholera. Death is inevitable unless patients receive continuous support with parenteral nutrition or undergo bowel transplantation (42). Secretory diarrhea is associated with specific pathological features most commonly observed in the small intestine including villus atrophy, disordered brush border (BB), and microvillus-containing inclusions (MVIs) that are characteristically found within the apical cytoplasm of mature enterocytes (51).
Loss of function mutations in the actin motor myosin Vb (Myo5b) is responsible for most cases of MVID (41). Recently, mutations in the SNARE fusion protein Syntaxin 3 (STX3) were reported in the milder MVID variant (61). Myo5b is present in epithelial cells, where it directs apical polarity together with Rab GTPases 8 and 11 (16, 31). Previous studies from our group demonstrated disruption of apical polarity as a central feature of MVID long before Myo5b and STX3 were implicated in the disease (5). However, why loss of Myo5b results in severe intestinal disease and secretory diarrhea is not understood (17).
Cystic fibrosis transmembrane conductance regulator (CFTR) chloride channels regulate Cl− and HCO3− secretion from the BBM of epithelial cells in a cell type-specific manner (2, 8, 52). In the intestine, CFTR is central to fluid secretion as lack of functional CFTR on the enterocyte BBM leads to complete intestinal obstruction as observed in the genetic disease cystic fibrosis (8). Under steady-state conditions in the intestine, CFTR is most abundant in subapical vesicles of crypt and villus enterocytes, with less expression on the BBM. In secretory diarrhea, second messengers stimulate exocytic insertion of CFTR from subapical vesicles to markedly increase its functional expression on the enterocyte BBM (4, 6, 18). A number of factors regulate CFTR function include apical recycling, cAMP, cGMP-regulated traffic, myosin motors including myosin VI (Myo6), myosin Ia (Myo1a), Rab GTPases, and STX3 (6, 11, 18, 34, 52, 56).
Two other brush border membrane (BBM) transporters, SLC26A3 (Downregulated in Adenoma, DRA) and sodium-hydrogen exchanger 3 (NHE3), contribute to intestinal fluid transport and electroneutral NaCl absorption in the intestine. However, the role of BBM ion transporters in MVID-associated diarrhea has not been investigated.
Na+ and Cl− transport abnormalities were documented in MVID long before gene defects were identified (13, 41), but the mechanisms and specific transporters underlying the intestinal ion transport defects were unknown. Here we sought to address the mechanisms underlying the secretory diarrhea by examining the distribution of the major BB ion transporters implicated in secretory diarrhea including CFTR, NHE3, and DRA, using human MVID intestine. Radioisotope and electrophysiological studies were employed to examine ion transporter functions in the crypt and villus enterocyte using two independent polarized intestinal cell models lacking Myo5b expression: CaCo2BBe (C2BBe) resembling villus enterocytes and T84 crypt model. Silencing or knockdown (KD) of Myo5b expression in CaCo2 or C2BBe cells has been used to model MVID (31, 48). Although KD of Myo5b yielded some morphological features of MVID enterocytes, no cellular model has replicated the complete array of ultrastructural features with complete absence of Myo5b, BB disorganization, and MVIs in the cytoplasm resembling human MVID enterocytes. In the present study, we used shRNA approaches to silence Myo5b in the C2BBe cell line (C2BBeKD) with the goal of replicating the key ultrastructural features of human villus MVID enterocytes including MVIs (a pathognomonic feature of MVID). C2BBe is a subclone of parental CaCo2 cells and an excellent model for studies of villus enterocyte BB development and assembly. This cell line expresses endogenous BB myosins, STX3, Rab11, absorptive apical hydrolases, and ion transporters such as sucrase isomaltase (SI) and CFTR that are found on the BBM of villus enterocytes in the native small intestine (11, 34, 43, 58). Finally, the observations that MVID villus enterocytes possess disordered BB and resemble crypt cells led us to test the hypothesis that loss of Myo5b leads to enterocyte maturation defects and a prosecretory cryptlike phenotype due to alterations in the Hippo pathway, an evolutionary conserved signaling pathway that regulates tissue growth in organs (62).
MATERIALS AND METHODS
Reagents and antibodies.
The following antibodies were used in this study: Myo5b (Novus Biologicals, Littleton, CO); Myo1a (KB1032) (55); Myo6 (22) and Myo1e (55) (a gift from Dr. Mooseker, Yale University); NHE3 (3H3) (gift from Dr. Orson Moe, University of Texas Southwestern Medical Center); CFTR 450 (CFF Therapeutics, University of North Carolina, Chapel Hill, NC); SLC26A3/DRA (gift from Dr. C. Schweinfest, Medical University South Carolina, Charleston, SC) (46); YAP1 (Abnova, Walnut, CA); pYAP1 (Cell Signaling, Danvers, MA); EBP-50 (Thermo-Fisher, Rockford, IL); NHERF2 (Santa Cruz, Dallas, TX); GAPDH (Millipore, Temekula, CA); Sec8 (Enzo Life Sciences, Farmingdale, NY); β-actin (Sigma-Aldrich, St. Louis, MO); and phalloidin-Alexa-555, Alexa-488, Alexa-568, and Alexa-647-conjugated anti-rabbit and anti-mouse secondary (Life Technologies, Grand Island, NY). Cell culture medium, antibiotics, and supplements were obtained from Life Technologies. All chemicals and other reagents were obtained from Sigma (St. Louis, MO) and J. T. Baker (Phillipsburg, NJ) unless stated otherwise.
Cell culture.
C2BBe and T84 cells were purchased from American Type Culture Collection (ATCC; Manassas, VA). C2BBe were grown at 37°C in 5% CO2-90% air atmosphere in high glucose with l-glutamine Dulbecco's modified Eagle's medium, supplemented with 5% FBS, 10 μg/ml apo-transferrin (Millipore, Billerica, MA), 1 mM sodium pyruvate, and 1× antibiotic-antimycotic. T84 required DMEM/F12 medium supplemented with 1× Antibiotic-Antimycotic and 5% FBS. C2BBe cells were seeded at 1 × 105 cells/cm2 onto 12.5-mm cell culture dishes (Corning, NY). At 70% confluence, cells were passed onto 12.5-mm Transwell permeable supports (Costar, Cambridge, MA) and resistance was measured every day up to the value of 200 Ω (day 0 or D0 time point) using EVOM voltohmmeter (World Precision Instruments, Sarasota, FL). Transepithelial resistance (TEER) was measured twice weekly for 21 days (D21). Confluent monolayers of C2BBe cells were used for Western blot analysis, immunofluorescence labeling, RT-PCR, and functional experiments.
Myo5b silencing in C2BBe (Myo5bKD C2BBe) cells.
MYO5B KD cells were generated with MYO5B mRNA targeted shRNA (NM_001080467) delivered by a pLKO.1-Puro-based lentiviral system and maintained with a selection marker puromycin (12 μg/ml; Cayman Chemical, Ann Arbor, MI). Cells were transduced to stably express either scrambled or MYO5B-targeting shRNA (32). C2BBe cells at passage number 15 or less were grown on 100-mm dishes (Corning, Corning, NY) to ∼70–90% confluency and then subjected to reverse transduction (cells were seeded in the titrated viral medium supplemented with 8 μg/ml Polybrene at 1:2 ratio; Millipore, Waltham, MA). shRNA-induced silencing of protein expression was assessed after one passage by immunoblot using Myo5b antibodies.
3D cyst culture and imaging of Myo5bKD C2BBe.
C2BBe cells were cultured at 37°C in 5% CO2-90% air atmosphere in high glucose l-glutamine Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 10 μg/ml human apotransferrin (Millipore), 1 mM sodium pyruvate, and 1× antibiotic-antimycotic. To produce cysts, C2BBe cells were plated on growth factor-reduced Matrigel (Corning, NY) and grown as described previously (39). In brief, cells were trypsinized and resuspended (104 cells/ml) in cell culture medium plus 2% Matrigel; then 400 μl of suspension was plated in each well of an eight-well chamber slide (Corning, NY) precoated with 30 μl of Matrigel and cultured for 12 days. Medium was changed at 4-day intervals. For imaging by confocal microcopy, cysts were fixed in 2% (wt/vol) paraformaldehyde-PBS (PFA) for 20 min at room temperature. After fixation cysts were washed with 1× PBS, permeabilized with 0.5% Triton-X in PBS for 10 min at 4°C, and stained with phalloidin-Alexa-546 diluted 1:100 in PBS for 30 min. Nuclei were stained for 20 s with 1% Hoechst and mounted in Prolong Gold anti-fade reagent (Molecular Probes). Mounting medium was allowed to dry at room temperature overnight. Slides were stored at 4°C and examined on Zeiss LSM 710-META laser scanning confocal microscope outfitted with a Mai-Tai laser.
Immunofluorescence labeling and confocal microscopy of tissues and cells.
Freshly frozen deidentified human Intestinal tissues were sectioned (6 μm) and fixed in 2% (wt/vol) PFA for 10 min at room temperature. Cells on Transwell permeable supports were fixed for 10 min in 2% PFA at room temperature. Following fixation, cells and tissues were immunolabeled as follows: all steps were carried out in a humidified chamber at room temperature, except for overnight incubation with primary antibodies, which was carried out at 4°C. Where necessary, antigen retrieval was performed by incubating sections with a solution of 1% NaBH4 for 30 min. The sections were rehydrated in PBS, and nonspecific proteins were blocked with 2% BSA in PBS and incubated with primary antibodies overnight. The sections were incubated with appropriate secondary antibodies for 1 h, stained with phalloidin-Alexa-555 diluted 1:100 in PBS for 30 min followed by 1% Hoechst nuclear stain and mounted in Prolong Gold mounting medium (Thermo Fisher, Waltham, MA). Control sections were labeled in the absence of primary antibodies or with nonspecific IgG. Immunolabeled sections were stored at 4°C and examined on Zeiss LSM 710-META laser scanning confocal microscope outfitted with a Mai-Tai laser and electronics capable of spectrally separating fluorescence emission profiles. Image acquisition and processing were done using ZEN (Zeiss Efficient Navigation) software. Some images were obtained using a Zeiss inverted fluorescent microscope equipped with a Hamamatsu Orca R2-C10600 digital camera. The acquisition parameters were standardized in relation to the highest-intensity regions to avoid oversaturation of pixel intensity. TUNEL labeling was done using In Situ Apoptosis Detection Set (MK500, Clontech/TaKaRa, Mountain View, CA).
Image processing.
Image stacks were taken with oversampling rate of 40 nm/pixel in the XY-dimension and 150 nm/pixel in the Z-dimension, followed by deconvolution in Volocity software (PerkinElmer, Waltham, MA). Analysis of confocal images was performed using Adobe Photoshop and Image J software. Unsaturated raw confocal images were standardized as was described previously (30), converted to a single-channel mode and quantified using mean gray value and integrated density algorithms. For actin quantifications, confocal sections in XY were adjusted by using such a combination of lenses and a pinhole value that the resulting images would encompass the entire depth of the BB [±1.5 μm from the center of microvillus (MV)]. Eight ×40 fields of view were quantified for each control and Myo5bKD.
Immunohistochemical staining of human intestine.
Sections from deidentified paraffin blocks of human MVID intestine were immunolabeled to detect CFTR and imaged as described before (12).
Immunoblots.
Cells were lysed in TGH buffer (1% Triton X-100, 25 mM HEPES, and 10% glycerol, pH 7.4) containing Complete protease inhibitors cocktail, 2 pills/10 ml, and 1 mM Pefablock (Roche, Branchburg, NJ). Lysates were centrifuged at 12,000 rpm for 15 min at 4°C. Protein concentrations were determined from supernatants, samples were eluted with 2× Laemmli sample buffer (Bio-Rad, Hercules, CA), and equivalent protein loads were analyzed by PAGE as before (11, 32).
Electron microscopy.
Filters of confluent C2BBe cells were fixed in 2% glutaraldehyde for 10 min at room temperature followed by 50 min on ice, washed, and postfixed in 1% osmium tetroxide, pH 6.0 for 1 h, prestained with 1% uranyl acetate overnight at 4°C, dehydrated, and embedded in EMBed 812 resin. After solidification, blocks were sectioned on the ultramicrotome at 60-nm setting (silver or silver-gold colored section appearance) and grids with sections were stained with 1% uranyl acetate for 20 min and Reynold's lead citrate for 1 min at room temperature. Grids were examined under the electron microscope equipped with Hamamatsu Orca HR camera and AMT camera system, HV = 80.0 kV.
Assessment of Cl−/HCO3− exchange activity.
Cl−/HCO3− exchange activity was measured as described previously (53). C2BBe cells were incubated with loading buffer, pH 8.5, for 30 min at room temperature. The medium was removed and the cells were rapidly washed with 1 ml tracer-free uptake mannitol buffer containing 260 mM mannitol, 20 mM Tris/2-(N-morpholino)ethanesulfonic acid (Tris/MES buffer) pH 7.0. The cells were then incubated with the uptake buffer containing 1 μCi of 125I− (3 mM) of sodium iodide for 5 min in the absence or presence of 600 μM DIDS. The 5-min time period was chosen because it falls within the linear range of 125I− uptake in this system. The uptake was stopped by removing the buffer and washing the cells rapidly twice with 1 ml of ice-cold PBS, pH 7.2. Finally, the cells were solubilized by incubation with 0.5 N NaOH for 4 h. The protein concentration was measured by the method of Bradford, and radioactivity was counted by a Packard Liquid Scintillation Analyzer, TRI-CARB 1600-TR (Packard Instruments; PerkinElmer, Boston, MA). The Cl−/HCO3− exchange activity was assessed as DIDS-sensitive 125I− uptake, and the values were expressed as nanomoles per milligram protein per 5 min.
Isolation of RNA and real-time PCR.
RNA was isolated from C2BBe intestinal epithelial cells using RNeasy Mini Kit (Qiagen, Valencia, CA) according to manufacturer's instructions. An equal amount of RNA for each sample was reverse-transcribed and amplified in a one-step reaction using Brilliant SYBR Green QRT-PCR master mix kit (Stratagene, La Jolla, CA) and using Mx 3000 (Stratagene). The gene-specific primers for human DRA and NHE3 were used for the RT-PCR reactions. The ΔCt-DRA/NHE3 and ΔCt-GAPDH represent the differences between the threshold cycle of amplification of DRA/NHE3 and GAPDH.
Measurement of Na+/H+ exchange activity.
The function of NHEs, measured as Na+/H+ exchange activity, was determined in acid-loaded postconfluent C2BBe monolayers as ethylisopropylamiloride (EIPA)-sensitive 22Na uptake, as previously described (15). Briefly, confluent cell monolayers were preincubated for 20 min at room temperature following incubation in a medium containing (in mM) 50 NH4Cl, 70 choline chloride, 5 KCl, 1 MgCl2, 2 CaCl2, 5 glucose, and 15 MOPS (pH 7.0). The cells were then washed with a solution containing (in mM) 120 choline chloride and 15 Tris·HEPES (pH 7.5) and were incubated in uptake buffer containing (in mM) 3 NaCl, 110 choline chloride, 1 MgCl2, 2 CaCl2, and 20 HEPES (pH 7.4) and 1 μCi/ml of 22Na with or without 50 μM EIPA or 50 μM HOE-694. After 5 min, the uptake of 22Na was terminated by washing the monolayers twice with ice-cold PBS. The cells were then solubilized by incubation with 0.5 N NaOH, and incorporated radioactivity was determined. The protein content of the cell lysate was estimated by the method of Bradford, and incorporated radioactivity was determined by Packard Tri-Carb 1600TR Liquid Scintillation. The values of 22Na uptake were expressed as nanomoles 22Na per milligram protein per 5 min.
The activity of NHE isoforms (NHE2 and NHE3) was measured in the presence of HOE-694 (NHE2-specific inhibitor at 50 μM). NHE2 activity was calculated as NHE activity sensitive to 50 μM HOE-694. NHE3 activity was calculated by subtracting 50 μM HOE-694-sensitive NHE activity from total NHE activity (50 μM EIPA-sensitive NHE activity).
Measurement of CFTR-mediated Cl− transport.
Cl− transport studies in the Ussing chambers were performed as before (32) with the following modifications: T84 cells were grown on Snapwell (Costar, Cambridge, MA) supports to confluency and mounted in Ussing chambers (Physiologic Instruments, San Diego, CA). CFTR-specific Cl− currents were recorded following the consecutive apical treatment with 5 mM Ba+, 100 μM amiloride in the Cl−-free buffer, 10 μM forskolin, and 20 μM CFTR(inh)-172. Cells were allowed to equilibrate for 10–15 min after each treatment, while the electrophysiological parameters were continuously recorded during the experiment under the clamped current (so-called “open circuit” conditions). Isc was calculated by Ohm's law and the resistance was corrected for the surface area.
Use of human tissues.
All localization studies on intestinal sections were conducted on deidentified samples under an approved protocol from Yale University School of Medicine.
RESULTS
Myo5bKD C2BBe cells as a model of villus MVID enterocytes.
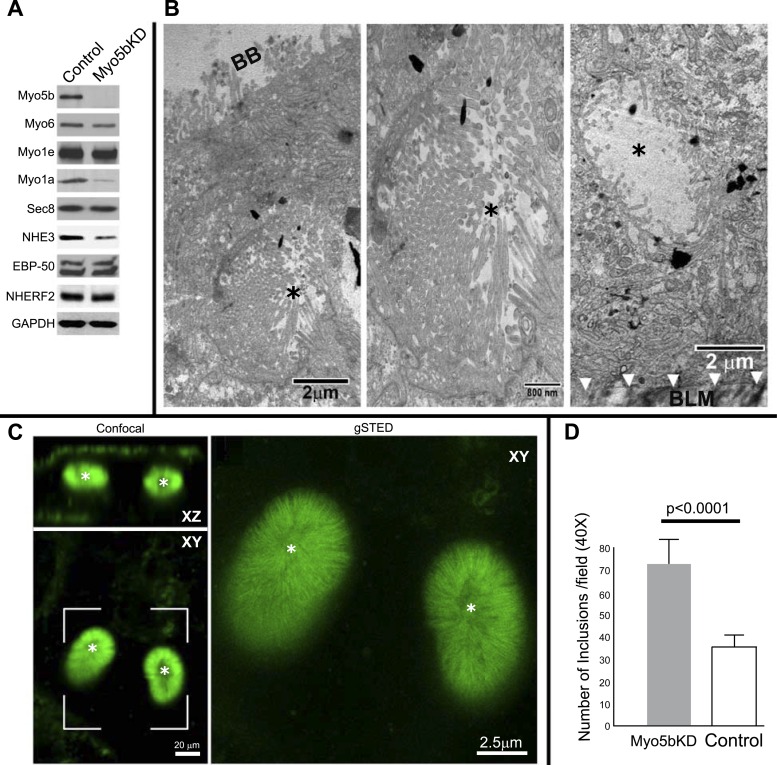
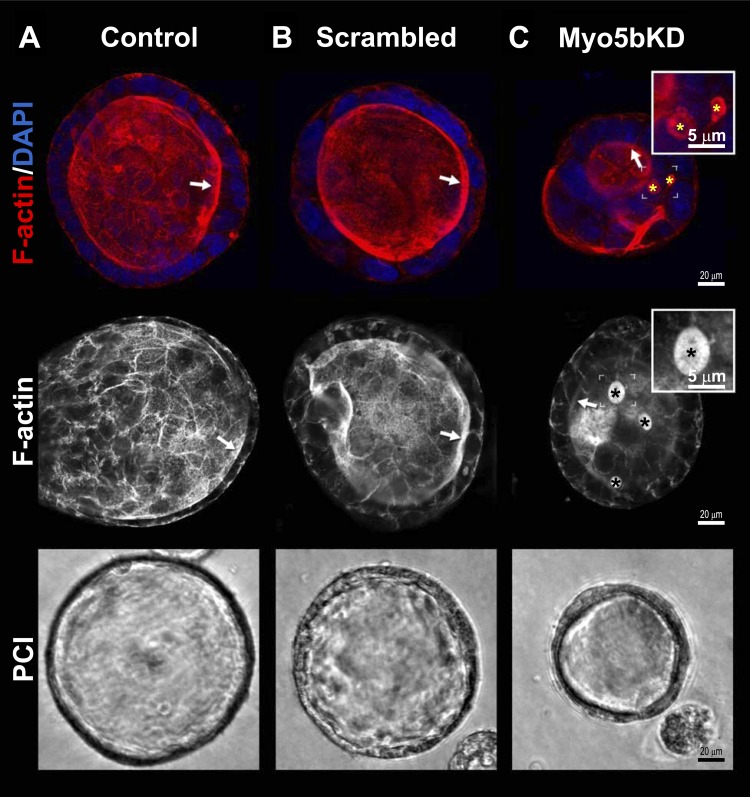
Immunoblot analysis of lysates from control and Myo5bKD C2BBe cells confirmed specific and efficient KD of Myo5b (Fig. 1A) with preservation of the endocytic motors myosin 6 (Myo6) and myosin 1e (Myo1e). Levels of myosin 1a (Myo1a) protein were markedly reduced in Myo5bKD cells, consistent with the observation that MVID enterocytes are immature and more cryptlike (Figs. 3, C and D, and 7). Protein levels of the exocyst component Sec-8 and PDZ proteins EBP50 and NHERF2 that regulate CFTR, DRA, and NHE3 were unaffected in Myo5b-deficient cells; however, levels of the absorptive BBM sodium hydrogen exchanger NHE3 were reduced (Fig. 1A). Transmission electron microscopy (TEM) examination of confluent Myo5bKD C2BBe cells revealed BB disorganization and MVIs containing fully formed BB in the apical and basal domains similar to human MVID (Figs. 1, B and C, and 3, B–D) (5, 31, 47). Reduced apical actin and F-actin-positive MVIs are characteristic features of MVID enterocytes (1, 31) (Figs. 1–3 and 7). In addition, polarity defects are also observed in MVID, although basolateral defects are not a consistent feature of MVID (49). Confocal images of F-actin stained 3D cysts (Fig. 2) generated from Myo5bKD C2BBe cells confirmed the presence of MVIs and redistribution of apical F-actin consistent with polarity defects.
Fig. 1.
Myo5bKD C2BBe cells model MVID villus enterocytes. A: immunoblots of Myo5b, Myo6, Myo1e, Myo1a, Sec 8, NHE3, EBP50, NHERF2, and GAPDH (for protein load) from equivalent protein loads of lysates prepared from fully polarized mature control (scrambled) and Myo5bKD C2BBe cells. B: transmission electron micrographs (TEM) of mature Myo5bKD C2BBe cells show apical microvillus inclusion (MVI) with brush border (black asterisk) at low-power (left, scale bar 2 μm) and high-power (middle, scale bar 800 nm) magnification and basolateral MVI with BB (black asterisk) at low-power (right, scale bar 2 μm) magnification. BB, brush border; BLM, basolateral membrane. C: confocal (XZ and XY, scale bar 20 μm) and gSTED images of a bracketed XY confocal area shows actin labeling in green. Asterisk marks MVIs. Scale bar, 2.5 μm. D: quantification in fully polarized Transwell-grown C2BBe cells reveals increased number of inclusions in Myo5bKD C2BBe cells (expressed as a total number per ×40 power field of view).
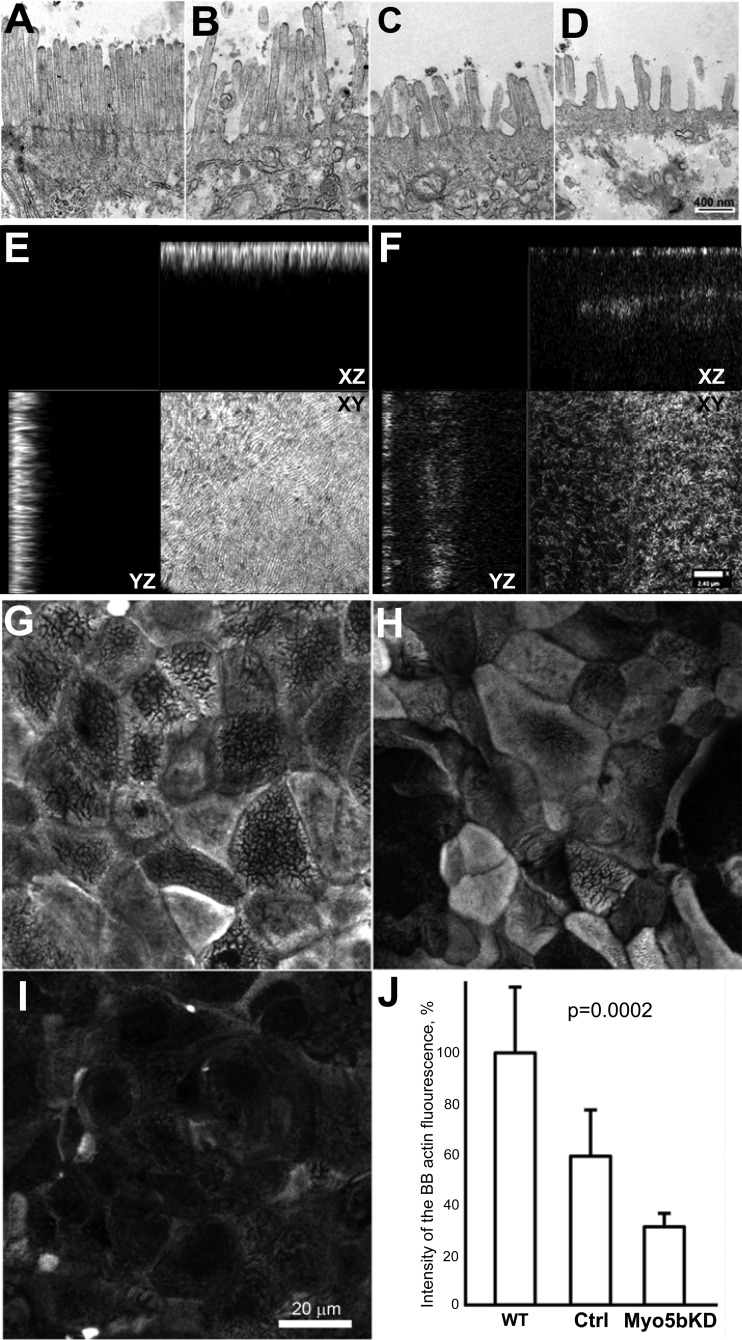
Fig. 3.
Brush border disorganization in Myo5bKD C2BBe cells. A–D: TEM demonstrates significant BB disorganization following Myo5bKD. Control C2BBe cells (A) and Myo5bKD C2BBe cells (B–D) with various degrees of BB disorganization. Scale bar, 400 nm. E–I: actin IFL in polarized C2BBe cells. gSTED Z-stack images demonstrate the dependence of fluorescence intensity on the degree of BB disorganization. XY planes are at the mid-BB level. E: control cells. F: Myo5bKD cells. G–I: representative confocal microscopy images of large areas of apical actin-stained scans used for quantification. Scale bar 20 μm. G: WT cells. H: scrambled control cells. I: Myo5bKD cells. J: quantification of apical actin immunofluorescence intensity.
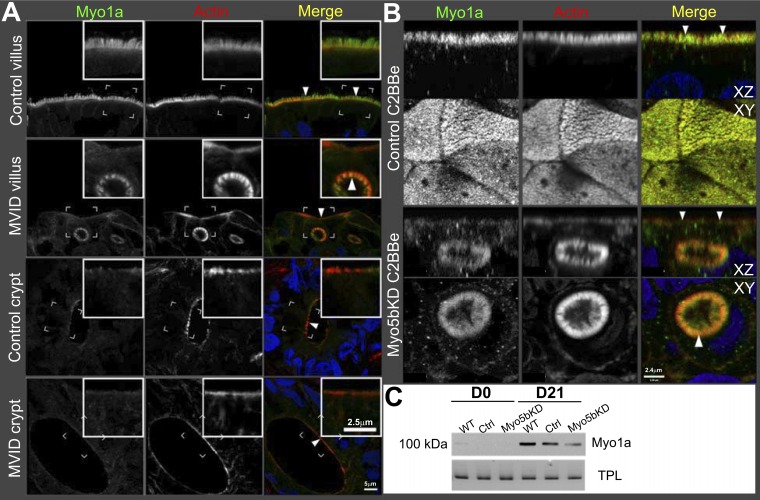
Fig. 7.
Myo1a localization in the human duodenum and C2BBe cells. Confocal microscopy images of Myo1a (green) IFL is shown in relation to F-actin (red); nuclei are stained in blue. A: control human and MVID duodenum cryosections. Insets, scale bar 2.5 μm; main panel, scale bar 5 μm. Brackets circumscribe the areas inserted under higher magnification. Arrowheads indicate Myo1a labeling in the microvilli of the normal brush border (control villus) and MVIs (MVID villus). No difference is visible in the Myo1a labeling pattern in crypts of control and MVID duodenum. B: confocal XY and XZ projections of Transwell-grown C2BBe monolayers. Arrowheads indicate Myo1a labeling in the microvilli of the normal brush border (scrambled C2BBe) and MVIs (Myo5bKD C2BBe). Scale bar, 2.4 μm. C: immunoblot analysis of Myo1a protein expression in immature (D0) mature (D21) control and Myo5bKD C2BBe cells. Total protein load (TPL) is shown.
Fig. 2.
Polarized 3D cysts of Myo5bKD C2BBe cells in culture display MVIs and F-actin redistribution. 3D cysts were cultured, immunolabeled for F-actin, and imaged by confocal microscopy as described in materials and methods. Control C2BBe cells (A), scrambled C2BBe cells (B), and Myo5bKD C2BBe cells (C) display varying degrees of BB disorganization. Top: images of F-actin (red) and nuclei (blue). Middle: images of F-actin-only-labeled cysts. Brackets circumscribe the areas inserted under higher magnification; asterisks and arrows indicate inclusions and brush borders, respectively. Inset, scale bar 5 μm; main panel, scale bar 20 μm. Bottom: representative phase contrast micrographs of polarized cysts. Images were taken on a phase-contrast inverted microscope at ×400 magnification.
High-resolution stimulated emission depletion (gSTED) confocal imaging of Myo5bKD C2BBe cells also confirmed alterations in apical actin (Fig. 3, E–J) and the presence of MVIs (Figs. 1C, 2C, and 7B) that are usually detected in human biopsies by the more expensive and labor-intensive technique of TEM. Control cells expressing Myo5b are enriched with apical F-actin (Figs. 2, A and B; 3, E, G, H; and 7B), as it constitutes the structural core of microvilli. En face views of Myo5b-expressing cells reveal highly developed BBs (Fig. 3, E, G, and H) in contrast to cells lacking Myo5b (Fig. 3, F and I). As reported before, inclusions are also observed in control cells; however, MVIs were more abundant in Myo5bKD cells (48) (Figs. 1D and 2C).
Loss of Myo5b downregulates and delocalizes NHE3 and DRA.
Functional expression of NHE3 at the BBM of mature enterocytes is the main mechanism for Na+ absorption in the small intestine. DRA is an apical Cl−/HCO3− exchanger on the BBM of villus enterocytes that regulates chloride absorption and bicarbonate secretion. Since NHE3 activity is coupled to DRA to mediate electroneutral NaCl absorption in the intestine (35, 54), we also examined DRA expression in Myo5bKD C2BBe cells. To determine whether the distribution and function of NHE3 and DRA are altered in MVID, we examined intestine from a child with MVID who carried a homozygous mutation c.4366C>T in exon 33 of Myo5b (59) that results in a premature stop codon at position Gln 1456X of Myo5b.
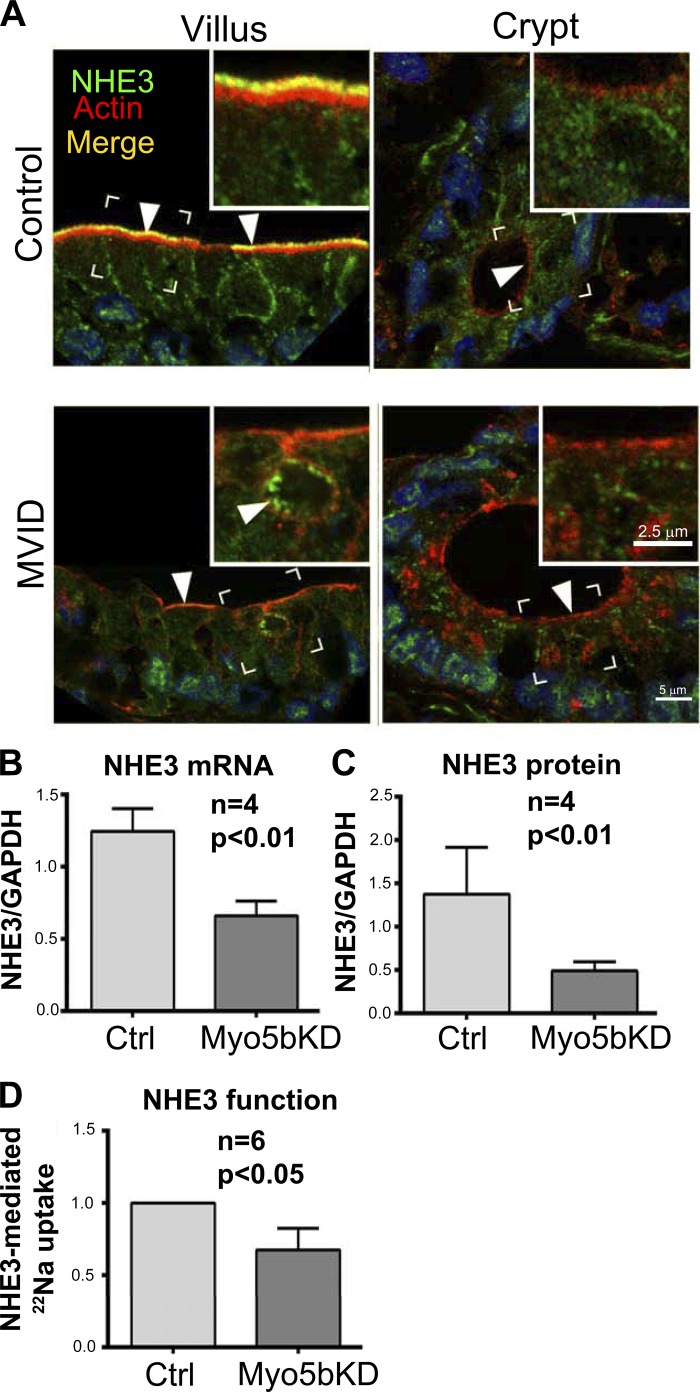
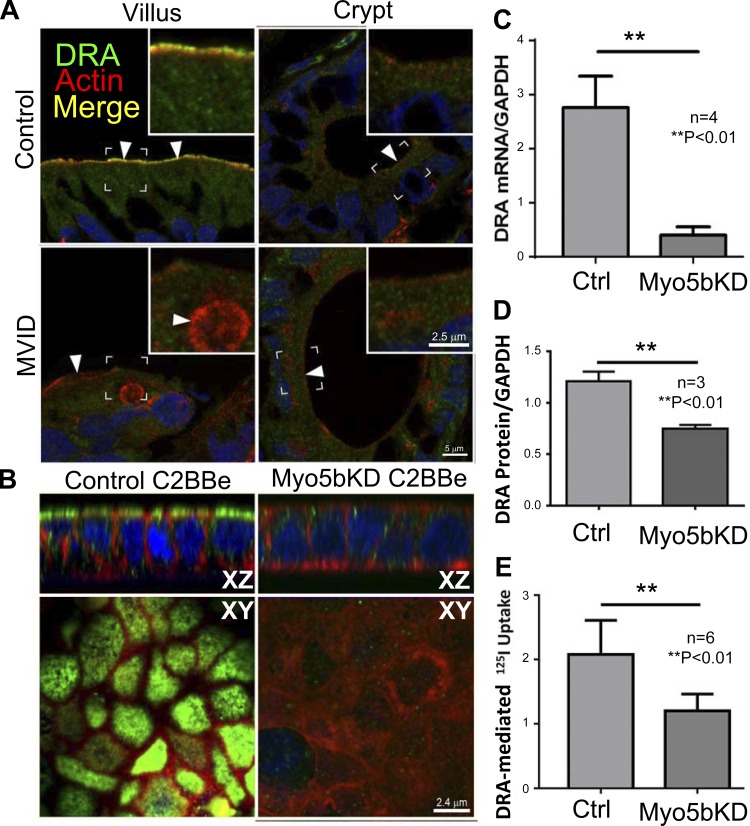
Immunofluorescence labeling (IFL) of normal human villus sections revealed higher apical BB label for NHE3 (Fig. 4) and DRA (Fig. 5) and lower diffuse intracellular labeling consistent with previous reports. As predicted, apical NHE3 (Fig. 4) and DRA (Fig. 5) were absent in the crypt. In MVID tissues described above, BB label for both proteins was markedly reduced in the villus (Figs. 4 and 5). In contrast to the normal BB staining, NHE3 decorated the BB of MVIs in the apical cytoplasm. Interestingly, no DRA staining was detected in MVIs (Fig. 5). IFL in the crypt MVID sections resembled control (Fig. 4 and 5). The distribution of DRA in control C2BBe cells closely resembled human villus enterocytes, revealing a distinct apical band with very low intracellular label (Fig. 5B). In the Myo5bKD C2BBe cells, DRA fluorescence was markedly reduced, and no distinct apical DRA label was detected (Fig. 5B). The NHE3 and DRA IFL patterns in human MVID villus tissues and Myo5bKD C2BBe cells closely resembled that of the crypt, rather than villus enterocytes.
Fig. 4.
NHE3 localization, expression, and function in the human duodenum and C2BBe cells. A: confocal microscopy images of NHE3 (green) IFL in the human duodenum are shown in relation to F-actin (red); nuclei are stained in blue. Brackets circumscribe the areas inserted under higher magnification. Inset, scale bar 2.5 μm; main panel, scale bar 5 μm. B: bar graph shows NHE3 mRNA levels expressed relative to GAPDH in the mature polarized C2BBe cells. C: quantification of NHE3 protein relative to GAPDH in lysates of mature polarized C2BBe cells. D: NHE3 exchanger function assayed by 22Na uptake by mature polarized C2BBe cells represents EIPA-sensitive but HOE 694-insensitive 22Na uptake.
Fig. 5.
DRA localization, expression, and function in the human duodenum, control, and Myo5bKD C2BBe cells. A: confocal microscopy images of DRA (green) IFL in cryosections from normal control and MVID human duodenum are shown in relation to F-actin (red); nuclei are stained in blue. Brackets circumscribe the areas inserted under higher magnification, arrowheads indicate location of DRA label in merge images. Inset, scale bar 2.5 μm; main panel, scale bar 5 μm. B: confocal Z-stack XY and XZ projections of Transwell-grown C2BBe monolayer cells. DRA (green) staining is shown in relation to F-actin (red); nuclei are stained in blue. Scale bar, 2.4 μm. C: DRA mRNA levels relative to GAPDH in mature polarized C2BBe cells. D: quantification of DRA protein relative to GAPDH in lysates of mature polarized C2BBe cells. E: Cl−/HCO3− exchange using DIDS-sensitive 125I uptake in mature polarized C2BBe cells.
DRA and NHE3 expression are reduced in Myo5bKD C2BBe cells.
NHE3 mRNA levels were reduced almost twofold in Myo5bKD C2BBe cells, resulting in the reduction of total NHE3 protein (Fig. 4, B and C). DRA mRNA and protein levels decreased compared with control cells (Fig. 5, C and D). These data indicate that Myo5b is important for maintaining NHE3 and DRA gene expression in the intestine.
Loss of Myo5b downregulates Na+/H+ and Cl−/HCO3− activity in C2BBe cells.
Since loss of Myo5b leads to decreased NHE3 and DRA expression, we examined whether this decrease altered apical ion exchange activity. NHE3 activity was measured as EIPA-sensitive 22Na uptake after subtracting HOE 694-sensitive NHE2-mediated uptake; DIDS-sensitive 125I− uptake was measured to examine DRA activity. Consistent with the mRNA expression, immunoblot, and IFL data, both NHE3 and DIDS-sensitive Cl−/HCO3− exchange activities were significantly decreased by (35 and 45%, respectively) in Myo5bKD cells (Figs. 4D and 5E). Altogether, the results suggest that Myo5b is required for proper functioning of apical Na+/H+ and Cl−/HCO3− exchangers NHE3 and DRA. Moreover, functional impairment resulting from their loss appears to promote a diarrheal phenotype resulting from luminal accumulation of Na+ and Cl−.
CFTR localizes to the BB in villus and crypt enterocytes in MVID intestine.
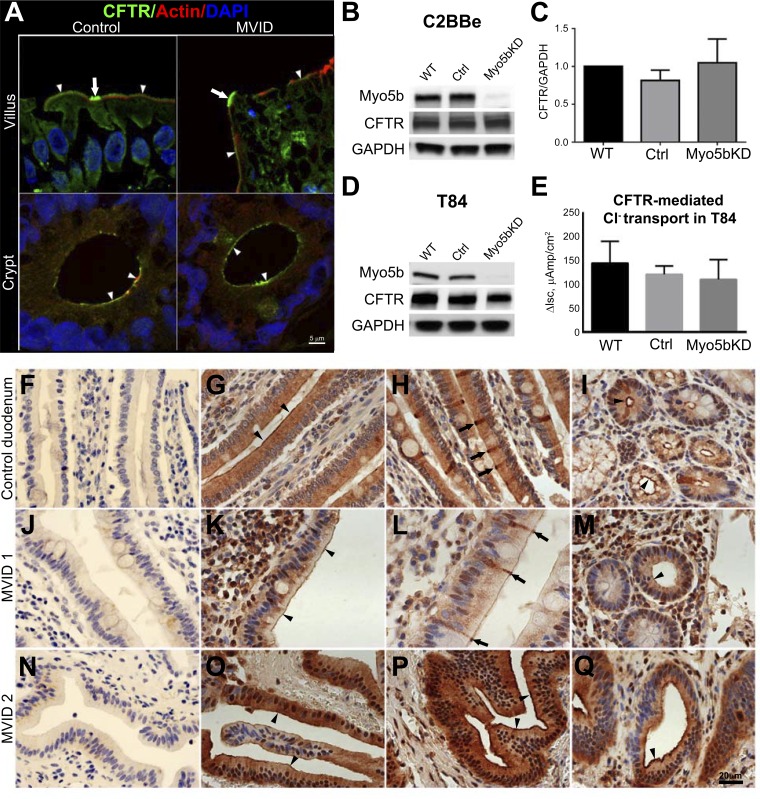
The distribution of CFTR IFL in cryosections from control and Myo5b loss-of-function MVID human jejunum [homozygous mutation c.4366C>T in exon 33 of Myo5b (59)] is shown in Fig. 6A. In the normal human, CFTR decorates the BBM of villus and crypt enterocytes, and very high levels are observed in a subpopulation of predominantly villus CFTR High Expresser (CHE) cells (3) (Fig. 6A). Interestingly, in the Myo5b loss-of-function MVID intestine very high levels of apical IFL for CFTR are observed in CHE cells similar to the normal (Fig. 6A, top, arrows). BB label for CFTR in villus enterocytes was also present although slightly reduced compared with control, consistent with shorter and disorganized BB in MVID villus enterocytes (Fig. 6A, top, arrowheads). Apical IFL for CFTR was present in the crypt of MVID similar to control (Fig. 6A, bottom, arrowheads). Immunohistochemical (IHC) staining for CFTR in paraffin sections of intestine from two independent nongenotyped MVID patients (Fig. 6, J–Q) replicated the CFTR localization patterns observed in the intestine of the Myo5b loss-of-function MVID tissue. In the nongenotyped MVID tissues (MVID 1 and 2), IHC staining confirmed the presence of very high levels of apical CFTR in CHE cells on the villi (Fig. 6L, arrows) and apical CFTR on the BB of the villi (Fig. 6, K and O) and the crypt (Fig. 6, M and Q), similar to control (Fig. 6, G–I). Consistent with its distribution in MVID intestine, immunoblots confirmed that CFTR levels were comparable in Myo5bKD C2BBe, Myo5bKD T84, wild-type (WT), or scrambled cells (Fig. 6, B and D).
Fig. 6.
CFTR localization, expression, and ion transport in the human duodenum, C2BBe, and T84 cells. A: confocal microscopy images of CFTR (green) IFL in cryosections of normal and MVID human duodenum are shown in relation to F-actin (red); nuclei are stained in blue. Arrowheads indicate BB IFL; arrows indicate CFTR High Expressor (CHE) cells. Scale bar, 5 μm. B: immunoblot analysis of Myo5b and CFTR in lysates of mature polarized C2BBe cells relative to GAPDH. C: comparison of GAPDH-corrected CFTR expression in mature polarized C2BBe cells. D: immunoblot analysis of Myo5b and CFTR in lysates of mature polarized T84 cells relative to GAPDH. Cells were analyzed following Isc measurements. E: CFTR short-circuit delta current (ΔIsc) measurements in the polarized T84 cells. Cl− secretion was stimulated with forskolin and inhibited with CFTR(inh)-172 inhibitor. F–Q: immunohistochemical localization of CFTR in normal and MVID human intestine. Horseradish peroxidase staining detects CFTR in small intestine sections from paraffin-embedded blocks of normal control (G–I) and two independent nongenetically characterized but confirmed MVID patients, MVID 1 (J–M), and MVID 2 (N–Q). F, J, and N are controls for negative staining. Nuclei are stained blue. Scale bar 25 μm. G: staining of CFTR in the BB (arrowheads) and supranuclear cytoplasm of villus enterocytes. H: intense BB and subapical CFTR staining in villus CHE cells (arrows). I: apical CFTR staining in crypt (black arrowheads). K: BB CFTR stain (arrowheads). L: intense BB and subapical CFTR staining in villus CHE cells (arrows). M: apical CFTR stain in crypt. O–Q: IHC stain for CFTR in MVID 2. O–P: BB (arrowheads) and subapical stain for CFTR. Q: apical CFTR stain in crypt (arrowhead).
CFTR ion transport is not affected by loss of Myo5b.
The presence of an apical distribution for CFTR in the crypt of MVID tissues led us to investigate CFTR ion transport in the secretory compartment. T84 cells resemble native crypt enterocytes, express endogenous CFTR, and are ideally suited for electrophysiology studies of CFTR anion secretion (52). To examine CFTR ion transport in MVID crypt, we silenced Myo5b in T84 cells. Immunoblots confirmed efficient silencing (Fig. 6D) of Myo5b expression but preserved CFTR protein. In WT or scrambled cells, the cAMP agonist forskolin activates robust CFTR currents that were abrogated by the specific CFTR inhibitor CFTR(inh)-172. In Myo5bKD cells, cAMP-elicited CFTR currents (Isc) were comparable to WT or scrambled control (n = 18, in three independent sets of experiments) (Fig. 6E), indicating that CFTR anion transport function is preserved in the absence of Myo5b.
Myosin 1a is mislocalized in human MVID intestine and Myo5bKD C2BBe cells.
Consistent with its role in BB assembly and development, Myo1a localizes to the BB of villus enterocytes in normal human duodenum similar to mouse small intestine and low levels of diffuse cytosolic labeling are in the crypts (Fig. 7A) (34). In human MVID duodenum, Myo1a IFL was drastically reduced in the BB and cytosolic labeling intensity was increased compared with control villus enterocytes. Myo1a IFL was consistently found in the BBs of the MVIs (Fig. 7A). In the MVID crypts, the labeling pattern is very similar to the control (Fig. 7A).
IFL of fully mature C2BBe cells at 21 days in culture (D21) reveal a pattern for Myo1a that is indistinguishable from normal human villus enterocytes (Fig. 7B). Following Myo5bKD, the cells lose the apical band of Myo1a IFL and display a slight increase in the cytosolic labeling and recruitment of Myo1a into MVIs (Fig. 7B). With the exception of MVI labeling, the Myo1a distribution in villus MVID enterocytes and Myo5bKD C2BBe cells is remarkably similar to that in the crypt cells. Immunoblot analysis revealed an increase in the Myo1a signal from D0 to D21 in control cells (Fig. 7C), consistent with enterocyte BB maturation. Myo5bKD cells displayed markedly reduced Myo1a protein at D21 compared with control (Fig. 7C, Fig. 1A), consistent with the IFL observations. The changes in Myo1a distribution and expression are consistent with defects in Myo5b-dependent enterocyte maturation and BB development in MVID.
Myo5bKD C2BBe cells exhibit YAP1-dependent maturation defects.
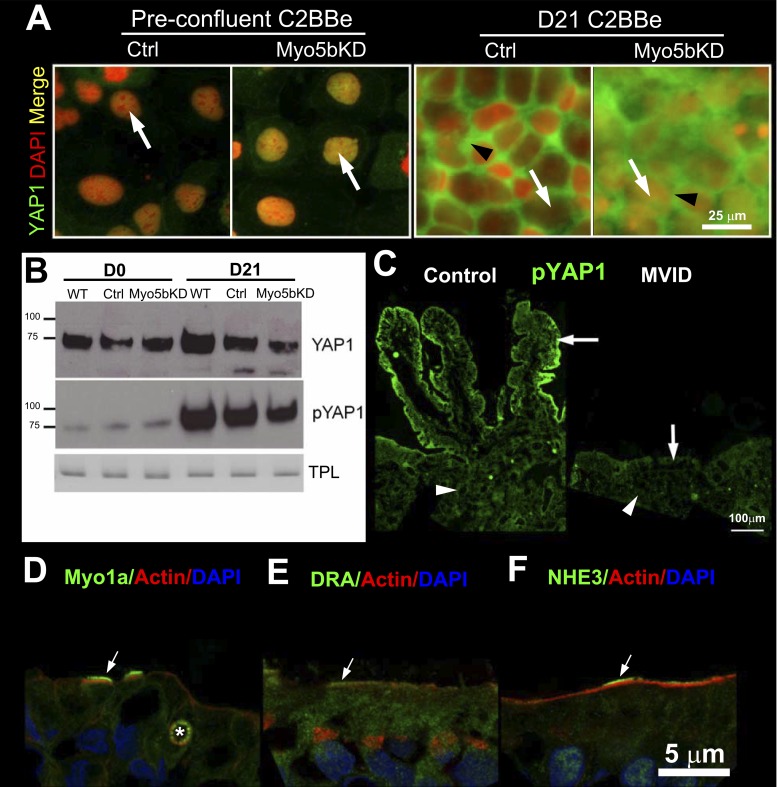
YAP1/2 complex acts downstream of the Hippo phosphorylation cascade that controls cell proliferation and maturation (62). In the nonphosphorylated state, YAP1 is in the nucleus and associates with TEAD transcription factors to promote division and cell proliferation. Upon confluency, YAP1 is phosphorylated by LATS1/2 kinases and transported from the nucleus into the cytosol, allowing transition to differentiation. Our observations that “mature” MVID villus enterocytes possess morphological and functional properties of immature crypt cells prompted evaluation of an enterocyte maturation defect in MVID. We labeled preconfluent (D0) and mature (D21) C2BBe cells for YAP1 and found that it localized to the nuclei of dividing cells (Fig. 8A). In the mature D21 cell monolayers, YAP1 was largely observed in the cytosol of control cells (Fig. 8A). The images shown were taken at the nuclear level, and, owing to the relatively thin band of cytosol, the labeling appears almost basolateral. However, in the cells with wider band of cytosol (Fig. 8A), YAP1 labeling is diffusely cytosolic. In Myo5bKD cells at the same time point, YAP1 labeling was more abundant in the nuclei than control (Fig. 8A).
Fig. 8.
YAP1 localization, expression, and phosphorylation in preconfluent and mature polarized day 21 C2BBe cells. IFL of pYAP1 distribution in normal and MVID human intestine and BB enterocyte compensatory responses in MVID. A: confocal microscopy images of YAP1 (green) and nuclear DAPI (red, arrow) IFL in control and Myo5bKD preconfluent (D0) and mature polarized (D21) C2BBe cells. Black arrowhead indicates cytosolic label. Scale bar, 25 μm. B: immunoblots of YAP1 and phospho-YAP1 (pYAP1) in lysates of prepolarized day 0 (D0) and polarized day 21 (D21) C2BBe cells is shown relative to the total protein load (TPL). C: IFL of pYAP1 (green) in crypt (arrowhead) and villus (arrow) of normal (left) and MVID (right) human intestine. Scale bar, 100 μm. D–F: confocal images of IFL of BB proteins in villus sections of human MVID duodenum. F-actin is shown in red and nuclei in blue in all panels. D: Myosin1a (green) labels the BB in two MVID enterocytes (arrows) and decorates an MVI marked with an asterisk. E: DRA (green) IFL in the BB of cells marked (arrow) is higher than in its neighbors. F: NHE3 (green) IFL in the BB of some enterocytes is significantly higher (arrow) than neighboring cells. Scale bar, 5 μm.
Immunoblot of YAP1 demonstrated that, in control cells, levels of total YAP1 increase slightly with maturation but remain unchanged in the Myo5bKD cells (Fig. 8B). Immunoblot revealed that although pYAP1 increased significantly in the WT and control D21 cells, levels were reduced in Myo5bKD cells (Fig. 8B). These findings were further supported by the IFL patterns of pYAP1 (Fig. 8C) in human intestine. In normal intestine, pYAP1 displays a crypt-villus gradient with the highest levels in villus enterocytes. In MVID intestine, pYAP1 staining is reduced and largely confined to the crypt. Since changes in YAP1 synthesis and phosphorylation could reflect increased rate of cell death and turnover, we performed TUNEL assays on mature monolayers. No difference was seen in the apoptotic rate between control and Myo5bKD cells (TUNEL fluorescence intensity 42.62 ± 0.8144, n = 11 vs. 41.08 ± 0.7323, n = 11).
DISCUSSION
This is the first study to specifically examine the role of BBM ion transport defects in the pathogenesis of MVID diarrhea that is central to the mortality in affected infants and children.
Until recently, no animal models of MVID were reported with features resembling human MVID. Targeted inactivation of Myo5b (10) in the first reported MVID mouse model resulted in diarrhea and early death, but no ion transport defects were described. The first intestine-specific conditional mouse model of MVID was recently reported (49). These Myo5b-deficient mice possess characteristic features of human MVID in the intestine and develop severe diarrhea, suggesting that this model closely mirrors human disease. In addition, two intestinal epithelial cellular models, CaCo2 and the subclone C2BBe, have been invaluable in contributing to our understanding of enterocyte defects in MVID as they resemble villus enterocytes and are amenable to Myo5b silencing approaches. C2BBe cells are superior to CaCo2 as a cellular model for MVID because they possess a highly developed BB upon confluency and express apical proteins and transporters similar to native villus enterocytes of the small intestine (11, 34), where the major morphological changes in MVID are observed. Similar to the observations supporting polarity defects in humans (47), mouse (49), and cellular models of MVID (39), we confirmed the presence of basal MVIs in Myo5bKD C2BBe cells, reduced apical F-actin with basal redistribution in Myo5bKD 3D cysts generated from C2BBe cells in culture. Although the loss of Myo5b can explain apical polarity defects in MVID, why basal defects are observed in the absence of functional Myo5b remains unknown.
Several reports silenced Myo5b with varied efficiency in C2BBe and demonstrated BB defects similar to MVID. However, since MVID disease results from complete loss of function of Myo5b, incomplete silencing of Myo5b replicates some, but not all, features of human MVID enterocytes. Efficient silencing of Myo5b in our C2BBe cells reproduced all the morphological features of human villus MVID enterocytes, BB ion transporter redistribution, and functional defects in NHE3 and DRA-dependent uptake of Na+ and Cl− that can contribute to sodium and chloride loss in human MVID. The data here also support our characterization of ion transporters and apical trafficking machinery in C2BBe cells (11). Except for the presence of CHE cells (that lack NHE3) (27), IFL of BB transporters in Myo5b loss-of-function human MVID intestine corroborated our findings in C2BBe cells. The observed downregulation of functional NHE3 expression in MVID intestine and Myo5bKD cells is consistent with previous reports and explains the increased stool Na+ observed in MVID (47, 51). NHE3 and DRA were both reduced on the BBM in human MVID intestine and Myo5bKD cells. Myo5b is important for targeting and delivery of selective proteins into the BBM (60). Myo5b is important for apical delivery of NHE3 (60) but its role in apical delivery of DRA is unknown. Reduced mRNA and protein levels of both transporters in the absence of Myo5b may reflect degradation and resulting decrease in BBM absorptive transport activities. Our finding that DRA is functionally downregulated from the BBM in MVID enterocytes is also consistent with reduced Cl− absorption and provides the first evidence of its involvement in MVID. Mutations in the DRA gene lead to congenital chloride diarrhea (24) and defective Cl−/HCO− exchange functions on the BBM of villus enterocytes (37). Impaired Na+ absorption due to the reduced functional activity of NHE3 is the underlying cause of congenital sodium diarrhea (28). Reduction in the surface area of the small intestine due to the villus atrophy and loss of MVs can further account for the absorptive loss (45) in MVID.
Like villus enterocytes of the small intestine (except for CHE cells), low levels of CFTR are on the BB of C2BBe cells (11). Villus enterocytes also possess secretory functions; however, the major site of CFTR expression and anion secretion that accounts for its central role in secretory diarrhea is its functional expression in the crypt (7). The role of villus CHE cells in secretory diarrhea has not been elucidated because isolation of this subpopulation has been a challenge. However, the presence of very high levels of BBM CFTR in CHE cells in MVID human intestine is intriguing. If CFTR is indeed functionally active in these cells, they could contribute significantly to the secretory diarrhea in MVID. Morphological studies of villus CHE cells support a secretory function as they express higher levels of basolateral NKCC1, lack apical NHE3, but possess very high levels of CFTR that undergo robust trafficking into the BBM upon cyclic nucleotide stimulation (3, 26).
T84 cells were used to examine the role of CFTR in MVID diarrhea since it is an excellent cellular model to study ion transport in human crypt (52). We previously reported CFTR redistribution in a nongenotyped patient but no functional evaluation was conducted in that study (5). Efficient silencing of Myo5b in T84 cells ensured a phenotype consistent with human MVID crypt. Forskolin-stimulated CFTR current (Isc), confirmed by using the CFTR-specific inhibitor CFTR(inh)-172, was elicited albeit slightly lower (∼120 μAmp/cm2) in Myo5bKD T84 cells compared with WT (∼150 μAmp/cm2) and scrambled control (∼125 μAmp/cm2), indicating that CFTR is delivered into the BBM and is indeed functional in the absence of Myo5b. These data in T84 cells model CFTR secretion in the crypt and do not take into account any contribution of villus enterocyte-mediated anion secretion or anion secretion originating from villus CHE cells in human MVID. Nevertheless, our functional studies in Myo5bKD T84 cells align with recent data reported in studies of the intestine-specific Myo5b-deficient mice. While all the key features of human MVID including severe diarrhea are observed in this mouse model, the failure to display increased forskolin-elicited fluid secretion in intestinal organoids suggests that factors other than cAMP-activated CFTR-mediated fluid secretion may contribute to the severity of the secretory diarrhea in human MVID (49). However, the combination of absorptive defects in NHE3-dependent sodium, DRA-dependent chloride, SGLT1 regulated glucose as reported by others, and villus atrophy in the setting of functional CFTR-mediated secretion can contribute to the diarrhea and metabolic acidosis in MVID (50, 51).
The observation that CFTR is present and functional on the BBM in Myo5b loss-of-function MVID human tissues, Myo5b-deficient cells, and mouse intestine begs the question of how CFTR is delivered to the BBM in the absence of Myo5b. As shown before, regulation of CFTR is tissue and cell type specific, and different transport pathways regulate CFTR trafficking in airway epithelial cells, in the absorptive enterocytes of intestinal villi and secretory crypt enterocytes (33). Apical CFTR trafficking in T84 cells and crypt has been shown to be dependent on microtubular transport (MT) (14, 40, 57) but not actin motors (34, 57). Thus it is possible that, in the absence of Myo5b, CFTR delivery into the crypt BBM is facilitated by MT motors. Alternatively, as we recently demonstrated in a Myo6/Myo1a double-knockout mouse model, loss of endocytic (Myo6) and exocytic (Myo1a) myosin motors in the enterocyte BB leads to rapid upregulation of compensatory myosin members (21). A similar scenario could explain CFTR delivery to the BBM in MVID in the absence of Myo5b.
Our Myo5bKD C2BBe cells demonstrate significant levels of BB disorganization as observed in human MVID enterocytes (Figs. 1 and 3). In the small intestine, the development of MVs begins after polarization, with the most mature enterocytes displaying the highest organization of BBs (23, 38). The microvilli on the apical surface of undifferentiated cells in the crypt are less abundant and considerably shorter (9). Density and length of microvilli correlate with the degree of maturation of C2BBe cells (44). Short, sparse, and disordered MVs in our D21 Myo5bKD C2BBe cells appear strikingly similar to the developing MVs of the early (D2–D5) stages of C2BBe maturation (44). Myo1a is one of the most abundant myosins in the enterocyte BB and critical for BB assembly. Only myosin II (Myo2) is expressed at higher levels in the BB (22). Unlike most microvillar (MV) core components (actin, villin, fimbrin) that localize to the BB, Myo1a associates with the cytoplasm and the cell perimeter in differentiating enterocytes. Its expression increases as enterocytes differentiate, and at the crypt-villus transition zone Myo1a assumes its characteristic distribution in the BB (55). Myo1a is thus an ideal marker of enterocyte differentiation. Expression and localization of Myo1a, NHE3, and DRA in MVID villus enterocytes and Myo5bKD C2BBe model closely resemble the patterns of normal crypt cells. Our findings are further supported by reports of increased expression of the intestinal proliferative crypt marker Ki-67 in human and intestine-specific mouse models of MVID (19, 49). Combined, these observations suggest that, in the absence of Myo5b, immature cryptlike secretory cells prevail throughout the intestinal epithelium and contribute to diarrhea.
Several unrelated proteins are affected in MVID enterocytes, suggesting a defect in differentiation early in the life of the enterocyte. From the multitude of pathways regulating cellular maturation, we focused on the Hippo pathway since deficiency of the key kinase, WTS-1, leads to the appearance of the MVIs in Caenorhabditis elegans (29). LATS-1, the human homolog of WTS-1, regulates the effector protein YAP1/2 by phosphorylation (62), thus controlling the switch from proliferation to differentiation. This process is initiated and controlled by polarization of cells (20). Phosphorylation of YAP1 results in its uncoupling from TEAD transcription factors, extrusion from the nucleus, and sequestration in the cytosol (62). Retention of the nonphosphorylated YAP1 in the nucleus, as demonstrated here, prevents or delays the switch to differentiation, resulting in the cryptlike phenotype of Myo5bKD/MVID enterocytes. Furthermore, Myo5b is a nuclear motor (36) that may translocate YAP1/pYAP1, resulting in the nuclear accumulation of YAP1 in its absence. Previously, higher rates of enterocyte proliferation and apoptosis were reported in patients with MVID (19). Characterization of the precise mechanisms of interaction between Myo5b and Hippo pathway members will require further study. In view of the observed partial Yap1 defect in our cell models, the cryptlike phenotype is expected to be incomplete, with cells demonstrating various stages of maturation in the epithelium. Indeed, we observed this in the MVID human intestine, where cells displaying relatively normal BB localization of Myo1a, NHE3, and DRA were found randomly scattered throughout the epithelium (Fig. 8, D–F). Similar trends were noted for BB development (45), where variations in the structure and density of apical MV in several MVID patients were noted. We also observed similar changes in our Myo5bKD C2BBe model (Fig. 3, B–D).
The Hippo pathway may be linked to defects in polarity in MVID (25, 63). Defective polarity arising from the absence of Myo5b may fail to provide the necessary signaling for “switching off” the proliferative function of Hippo cascade. STX3 mutations in variant MVID (61) also affect the polarization process in enterocytes and may lead to diarrhea via similar polarity-related errors in Hippo signaling. Both mechanisms appear to be of importance in the disease pathogenesis and will require further investigations to understand whether they are linked.
GRANTS
This work was supported by NIH grant DK R01 DK077065 to N. Ameen; NIH grant P30 DK34989 to the Yale Liver Center; and DK 54016, DK 81858, DK 92441, Dept. of Veterans Affairs BX 002011 to P. K. Dudeja.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
D.V.K., M.K.A., P.K.D., and N.A.A. conception and design of research; D.V.K., M.K.A., V.K., A.K., and T.G. performed experiments; D.V.K., M.K.A., A.K., P.K.D., and N.A.A. analyzed data; D.V.K., M.K.A., A.K., P.K.D., and N.A.A. interpreted results of experiments; D.V.K. and M.K.A. prepared figures; D.V.K., M.K.A., and N.A.A. drafted manuscript; D.V.K., M.K.A., V.K., S.C.v.I., M.R.-M., A.K., T.G., P.K.D., and N.A.A. approved final version of manuscript; M.K.A. and N.A.A. edited and revised manuscript.
ACKNOWLEDGMENTS
Present address for D. Kravtsov: Vanessa Research, 925 Sherman Ave., Hamden, CT 06514.
Present address for T. Gujral: Second Genome Inc., 349 Allerton Ave., South San Francisco, CA 94080.
REFERENCES
- 1.Ameen N, Alexis J, Salas P. Cellular localization of the cystic fibrosis transmembrane conductance regulator in mouse intestinal tract. Histochem Cell Biol 114: 69–75, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Ameen N, Silvis M, Bradbury NA. Endocytic trafficking of CFTR in health and disease. J Cyst Fibros 6: 1–14, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ameen NA, Ardito T, Kashgarian M, Marino CR. A unique subset of rat and human intestinal villus cells express the cystic fibrosis transmembrane conductance regulator. Gastroenterology 108: 1016–1023, 1995. [DOI] [PubMed] [Google Scholar]
- 4.Ameen NA, Marino C, Salas PJ. cAMP-dependent exocytosis and vesicle traffic regulate CFTR and fluid transport in rat jejunum in vivo. Am J Physiol Cell Physiol 284: C429–C438, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Ameen NA, Salas PJ. Microvillus inclusion disease: a genetic defect affecting apical membrane protein traffic in intestinal epithelium. Traffic 1: 76–83, 2000. [DOI] [PubMed] [Google Scholar]
- 6.Ameen NA, van Donselaar E, Posthuma G, de Jonge H, McLaughlin G, Geuze HJ, Marino C, Peters PJ. Subcellular distribution of CFTR in rat intestine supports a physiologic role for CFTR regulation by vesicle traffic. Histochem Cell Biol 114: 219–228, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Barrett KE, Keely SJ. Chloride secretion by the intestinal epithelium: molecular basis and regulatory aspects. Annu Rev Physiol 62: 535–572, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Bertrand CA, Frizzell RA. The role of regulated CFTR trafficking in epithelial secretion. Am J Physiol Cell Physiol 285: C1–C18, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Brown AL., Jr Microvilli of the human jejunal epithelial cell. J Cell Biol 12: 623–627, 1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carton-Garcia F, Overeem AW, Nieto R, Bazzocco S, Dopeso H, Macaya I, Bilic J, Landolfi S, Hernandez-Losa J, Schwartz S, Ramon YCS, van Ijzendoorn SC, Arango D. Myo5b knockout mice as a model of microvillus inclusion disease. Sci Rep 5: 12312, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collaco A, Marathe J, Kohnke H, Kravstov D, Ameen N. Syntaxin 3 is necessary for cAMP- and cGMP-regulated exocytosis of CFTR: implications for enterotoxigenic diarrhea. Am J Physiol Cell Physiol 299: C1450–C1460, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collaco AM, Jakab RL, Hoekstra NE, Mitchell KA, Brooks A, Ameen NA. Regulated traffic of anion transporters in mammalian Brunner's glands: a role for water and fluid transport. Am J Physiol Gastrointest Liver Physiol 305: G258–G275, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davidson GP, Cutz E, Hamilton JR, Gall DG. Familial enteropathy: a syndrome of protracted diarrhea from birth, failure to thrive, and hypoplastic villus atrophy. Gastroenterology 75: 783–790, 1978. [PubMed] [Google Scholar]
- 14.Fuller CM, Bridges RJ, Benos DJ. Forskolin-but not ionomycin-evoked Cl− secretion in colonic epithelia depends on intact microtubules. Am J Physiol Cell Physiol 266: C661–C668, 1994. [DOI] [PubMed] [Google Scholar]
- 15.Gill RK, Saksena S, Tyagi S, Alrefai WA, Malakooti J, Sarwar Z, Turner JR, Ramaswamy K, Dudeja PK. Serotonin inhibits Na+/H+ exchange activity via 5-HT4 receptors and activation of PKC alpha in human intestinal epithelial cells. Gastroenterology 128: 962–974, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Golachowska MR, Hoekstra D, van Ijzendoorn SC. Recycling endosomes in apical plasma membrane domain formation and epithelial cell polarity. Trends Cell Biol 20: 618–626, 2010. [DOI] [PubMed] [Google Scholar]
- 17.Golachowska MR, van Dael CM, Keuning H, Karrenbeld A, Hoekstra D, Gijsbers CF, Benninga MA, Rings EH, van Ijzendoorn SC. MYO5B mutations in patients with microvillus inclusion disease presenting with transient renal Fanconi syndrome. J Pediatr Gastroenterol Nutr 54: 491–498, 2012. [DOI] [PubMed] [Google Scholar]
- 18.Golin-Bisello F, Bradbury NA, Ameen NA. Heat Stable Enterotoxin (STa) and cGMP stimulate CFTR translocation to the surface of villus enterocytes in rat jejunum and is regulated by protein kinase G. Am J Physiol Cell Physiol 289: C708–C716, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Groisman GM, Sabo E, Meir A, Polak-Charcon S. Enterocyte apoptosis and proliferation are increased in microvillous inclusion disease (familial microvillous atrophy). Hum Pathol 31: 1404–1410, 2000. [PubMed] [Google Scholar]
- 20.Gumbiner BM, Kim NG. The Hippo-YAP signaling pathway and contact inhibition of growth. J Cell Sci 127: 709–717, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hegan PS, Kravtsov DV, Caputo C, Egan ME, Ameen NA, Mooseker MS. Restoration of cytoskeletal and membrane tethering defects but not defects in membrane trafficking in the intestinal brush border of mice lacking both myosin Ia and myosin VI. Cytoskeleton (Hoboken) 72: 455–476, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heintzelman MB, Hasson T, Mooseker MS. Multiple unconventional myosin domains of the intestinal brush border cytoskeleton. J Cell Sci 107: 3535–3543, 1994. [DOI] [PubMed] [Google Scholar]
- 23.Heintzelman MB, Mooseker MS. Assembly of the brush border cytoskeleton: changes in the distribution of microvillar core proteins during enterocyte differentiation in adult chicken intestine. Cell Motil Cytoskeleton 15: 12–22, 1990. [DOI] [PubMed] [Google Scholar]
- 24.Hoglund P, Haila S, Socha J, Tomaszewski L, Saarialho-Kere U, Karjalainen-Lindsberg ML, Airola K, Holmberg C, de la Chapelle A, Kere J. Mutations of the Down-regulated in adenoma (DRA) gene cause congenital chloride diarrhoea. Nat Genet 14: 316–319, 1996. [DOI] [PubMed] [Google Scholar]
- 25.Imajo M, Ebisuya M, Nishida E. Dual role of YAP and TAZ in renewal of the intestinal epithelium. Nat Cell Biol 17: 7–19, 2015. [DOI] [PubMed] [Google Scholar]
- 26.Jakab RL, Collaco AM, Ameen NA. Characterization of CFTR High Expresser cells in the intestine. Am J Physiol Gastrointest Liver Physiol 305: G453–G465, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jakab RL, Collaco AM, Ameen NA. Physiological relevance of cell-specific distribution patterns of CFTR, NKCC1, NBCe1, and NHE3 along the crypt-villus axis in the intestine. Am J Physiol Gastrointest Liver Physiol 300: G82–G98, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janecke AR, Heinz-Erian P, Yin J, Petersen BS, Franke A, Lechner S, Fuchs I, Melancon S, Uhlig HH, Travis S, Marinier E, Perisic V, Ristic N, Gerner P, Booth IW, Wedenoja S, Baumgartner N, Vodopiutz J, Frechette-Duval MC, De Lafollie J, Persad R, Warner N, Tse CM, Sud K, Zachos NC, Sarker R, Zhu X, Muise AM, Zimmer KP, Witt H, Zoller H, Donowitz M, Muller T. Reduced sodium/proton exchanger NHE3 activity causes congenital sodium diarrhea. Hum Mol Genet 24: 6614–6623, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang J, Shin D, Yu JR, Lee J. Lats kinase is involved in the intestinal apical membrane integrity in the nematode Caenorhabditis elegans. Development 136: 2705–2715, 2009. [DOI] [PubMed] [Google Scholar]
- 30.Kirkeby S, Thomsen CE. Quantitative immunohistochemistry of fluorescence labelled probes using low-cost software. J Immunol Methods 301: 102–113, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Knowles BC, Roland JT, Krishnan M, Tyska MJ, Lapierre LA, Dickman PS, Goldenring JR, Shub MD. Myosin Vb uncoupling from RAB8A and RAB11A elicits microvillus inclusion disease. J Clin Invest 124: 2947–2962, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kravtsov D, Mashukova A, Forteza R, Rodriguez MM, Ameen NA, Salas PJ. Myosin 5b loss of function leads to defects in polarized signaling: implication for microvillus inclusion disease pathogenesis and treatment. Am J Physiol Gastrointest Liver Physiol 307: G992–G1001, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kravtsov DV, Ameen NA. Molecular motors and apical CFTR traffic in epithelia. Int J Mol Sci 14: 9628–9642, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kravtsov DV, Caputo C, Collaco A, Hoekstra N, Egan ME, Mooseker MS, Ameen NA. Myosin Ia is required for CFTR brush border membrane trafficking and ion transport in the mouse small intestine. Traffic 13: 1072–1082, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lamprecht G, Heil A, Baisch S, Lin-Wu E, Yun CC, Kalbacher H, Gregor M, Seidler U. The down regulated in adenoma (dra) gene product binds to the second PDZ domain of the NHE3 kinase A regulatory protein (E3KARP), potentially linking intestinal Cl−/HCO3− exchange to Na+/H+ exchange. Biochemistry 41: 12336–12342, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Lindsay AJ, McCaffrey MW. Myosin Vb localises to nucleoli and associates with the RNA polymerase I transcription complex. Cell Motil Cytoskeleton 66: 1057–1072, 2009. [DOI] [PubMed] [Google Scholar]
- 37.Makela S, Kere J, Holmberg C, Hoglund P. SLC26A 3 mutations in congenital chloride diarrhea. Hum Mutat 20: 425–438, 2002. [DOI] [PubMed] [Google Scholar]
- 38.Mamajiwalla SN, Fath KR, Burgess DR. Development of the chicken intestinal epithelium. Curr Top Dev Biol 26: 123–143, 1992. [DOI] [PubMed] [Google Scholar]
- 39.Michaux G, Massey-Harroche D, Nicolle O, Rabant M, Brousse N, Goulet O, Le Bivic A, Ruemmele FM. The localisation of the apical Par/Cdc42 polarity module is specifically affected in microvillus inclusion disease. Biol Cell 108: 19–28, 2016. [DOI] [PubMed] [Google Scholar]
- 40.Morris RG, Tousson A, Benos DJ, Schafer JA. Microtubule disruption inhibits AVT-stimulated Cl− secretion but not Na+ reabsorption in A6 cells. Am J Physiol Renal Physiol 274: F300–F314, 1998. [DOI] [PubMed] [Google Scholar]
- 41.Muller T, Hess MW, Schiefermeier N, Pfaller K, Ebner HL, Heinz-Erian P, Ponstingl H, Partsch J, Rollinghoff B, Kohler H, Berger T, Lenhartz H, Schlenck B, Houwen RJ, Taylor CJ, Zoller H, Lechner S, Goulet O, Utermann G, Ruemmele FM, Huber LA, Janecke AR. MYO5B mutations cause microvillus inclusion disease and disrupt epithelial cell polarity. Nat Genet 40: 1163–1165, 2008. [DOI] [PubMed] [Google Scholar]
- 42.Oliva MM, Perman JA, Saavedra JM, Young-Ramsaran J, Schwarz KB. Successful intestinal transplantation for microvillus inclusion disease. Gastroenterology 106: 771–774, 1994. [DOI] [PubMed] [Google Scholar]
- 43.Peterson MD, Mooseker MS. Characterization of the enterocyte-like brush border cytoskeleton of the C2BBe clones of the human intestinal cell line, Caco-2. J Cell Sci 102: 581–600, 1992. [DOI] [PubMed] [Google Scholar]
- 44.Peterson MD, Mooseker MS. An in vitro model for the analysis of intestinal brush border assembly. I. Ultrastructural analysis of cell contact-induced brush border assembly in Caco-2BBe cells. J Cell Sci 105: 445–460, 1993. [DOI] [PubMed] [Google Scholar]
- 45.Phillips AD, Schmitz J. Familial microvillous atrophy: a clinicopathological survey of 23 cases. J Pediatr Gastroenterol Nutr 14: 380–396, 1992. [PubMed] [Google Scholar]
- 46.Priyamvada S, Anbazhagan AN, Gujral T, Borthakur A, Saksena S, Gill RK, Alrefai WA, Dudeja PK. All-trans-retinoic acid increases SLC26A3 DRA (Down-regulated in Adenoma) expression in intestinal epithelial cells via HNF-1beta. J Biol Chem 290: 15066–15077, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rhoads JM, Vogler RC, Lacey SR, Reddick RL, Keku EO, Azizkhan RG, Berschneider HM. Microvillus inclusion disease. In vitro jejunal electrolyte transport. Gastroenterology 100: 811–817, 1991. [DOI] [PubMed] [Google Scholar]
- 48.Ruemmele FM, Muller T, Schiefermeier N, Ebner HL, Lechner S, Pfaller K, Thoni CE, Goulet O, Lacaille F, Schmitz J, Colomb V, Sauvat F, Revillon Y, Canioni D, Brousse N, de Saint-Basile G, Lefebvre J, Heinz-Erian P, Enninger A, Utermann G, Hess MW, Janecke AR, Huber LA. Loss-of-function of MYO5B is the main cause of microvillus inclusion disease: 15 novel mutations and a CaCo-2 RNAi cell model. Hum Mutat 31: 544–551, 2010. [DOI] [PubMed] [Google Scholar]
- 49.Schneeberger K, Vogel GF, Teunissen H, van Ommen DD, Begthel H, El Bouazzaoui L, van Vugt AH, Beekman JM, Klumperman J, Muller T, Janecke A, Gerner P, Huber LA, Hess MW, Clevers H, van Es JH, Nieuwenhuis EE, Middendorp S. An inducible mouse model for microvillus inclusion disease reveals a role for myosin Vb in apical and basolateral trafficking. Proc Natl Acad Sci USA 112: 12408–12413, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shahid S, Fraser DD, Driman DK, Bax KC. Severe hypernatremic dehydration and metabolic acidosis due to neonatal intestinal microvillus inclusion disease. Neonatology 101: 154–158, 2012. [DOI] [PubMed] [Google Scholar]
- 51.Sherman PM, Mitchell DJ, Cutz E. Neonatal enteropathies: defining the causes of protracted diarrhea of infancy. J Pediatr Gastroenterol Nutr 38: 16–26, 2004. [DOI] [PubMed] [Google Scholar]
- 52.Silvis MR, Bertrand CA, Ameen N, Golin-Bisello F, Butterworth MB, Frizzell RA, Bradbury NA. Rab11b regulates the apical recycling of the cystic fibrosis transmembrane conductance regulator in polarized intestinal epithelial cells. Mol Biol Cell 20: 2337–2350, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Singh V, Kumar A, Raheja G, Anbazhagan AN, Priyamvada S, Saksena S, Jhandier MN, Gill RK, Alrefai WA, Borthakur A, Dudeja PK. Lactobacillus acidophilus attenuates downregulation of DRA function and expression in inflammatory models. Am J Physiol Gastrointest Liver Physiol 307: G623–G631, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Singh V, Yang J, Chen TE, Zachos NC, Kovbasnjuk O, Verkman AS, Donowitz M. Translating molecular physiology of intestinal transport into pharmacologic treatment of diarrhea: stimulation of Na+ absorption. Clin Gastroenterol Hepatol 12: 27–31, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Skowron JF, Bement WM, Mooseker MS. Human brush border myosin-I and myosin-Ic expression in human intestine and Caco-2BBe cells. Cell Motil Cytoskeleton 41: 308–324, 1998. [DOI] [PubMed] [Google Scholar]
- 56.Swiatecka-Urban A, Talebian L, Kanno E, Moreau-Marquis S, Coutermarsh B, Hansen K, Karlson KH, Barnaby R, Cheney RE, Langford GM, Fukuda M, Stanton BA. Myosin Vb is required for trafficking of the cystic fibrosis transmembrane conductance regulator in Rab11a-specific apical recycling endosomes in polarized human airway epithelial cells. J Biol Chem 282: 23725–23736, 2007. [DOI] [PubMed] [Google Scholar]
- 57.Tousson A, Fuller CM, Benos DJ. Apical recruitment of CFTR in T84 cells is dependent on cAMP and microtubules but not calcium or microfilaments. J Cell Sci 109: 1325–1334, 1996. [DOI] [PubMed] [Google Scholar]
- 58.Tyska MJ, Mooseker MS. A role for myosin-1A in the localization of a brush border disaccharidase. J Cell Biol 165: 395–405, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van der Velde KJ, Dhekne HS, Swertz MA, Sirigu S, Ropars V, Vinke PC, Rengaw T, van den Akker PC, Rings EH, Houdusse A, van Ijzendoorn SC. An overview and online registry of microvillus inclusion disease patients and their MYO5B mutations. Hum Mutat 34: 1597–1605, 2013. [DOI] [PubMed] [Google Scholar]
- 60.Vogel GF, Klee KM, Janecke AR, Muller T, Hess MW, Huber LA. Cargo-selective apical exocytosis in epithelial cells is conducted by Myo5B, Slp4a, Vamp7, and Syntaxin 3. J Cell Biol 211: 587–604, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wiegerinck CL, Janecke AR, Schneeberger K, Vogel GF, van Haaften-Visser DY, Escher JC, Adam R, Thoni CE, Pfaller K, Jordan AJ, Weis CA, Nijman IJ, Monroe GR, van Hasselt PM, Cutz E, Klumperman J, Clevers H, Nieuwenhuis EE, Houwen RH, van Haaften G, Hess MW, Huber LA, Stapelbroek JM, Muller T, Middendorp S. Loss of syntaxin 3 causes variant microvillus inclusion disease. Gastroenterology 147: 65–68, 2014. [DOI] [PubMed] [Google Scholar]
- 62.Yu FX, Guan KL. The Hippo pathway: regulators and regulations. Genes Dev 27: 355–371, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu FX, Meng Z, Plouffe SW, Guan KL. Hippo pathway regulation of gastrointestinal tissues. Annu Rev Physiol 77: 201–227, 2015. [DOI] [PubMed] [Google Scholar]