Abstract
Gastric hypersensitivity (GHS) and anxiety are prevalent in functional dyspepsia patients; their underlying mechanisms remain unknown largely because of lack of availability of live visceral tissues from human subjects. Recently, we demonstrated in a preclinical model that rats subjected to neonatal colon inflammation show increased basal plasma norepinephrine (NE), which contributes to GHS through the upregulation of nerve growth factor (NGF) expression in the gastric fundus. We tested the hypothesis that neonatal colon inflammation increases anxiety-like behavior and sympathetic nervous system activity, which upregulates the expression of NGF to induce GHS in adult life. Chemical sympathectomy, but not adrenalectomy, suppressed the elevated NGF expression in the fundus muscularis externa and GHS. The measurement of heart rate variability showed a significant increase in the low frequency-to-high frequency ratio in GHS vs. the control rats. Stimulus-evoked release of NE from the fundus muscularis externa strips was significantly greater in GHS than in the control rats. Tyrosine hydroxylase expression was increased in the celiac ganglia of the GHS vs. the control rats. We found an increase in trait but not stress-induced anxiety-like behavior in GHS rats in an elevated plus maze. We concluded that neonatal programming triggered by colon inflammation upregulates tyrosine hydroxylase in the celiac ganglia, which upregulates the release of NE in the gastric fundus muscularis externa. The increase of NE release from the sympathetic nerve terminals concentration dependently upregulates NGF, which proportionately increases the visceromotor response to gastric distention. Neonatal programming concurrently increases anxiety-like behavior in GHS rats.
Keywords: nerve growth factor, visceral hypersensitivity, norepinephrine, functional dyspepsia
functional dyspepsia (FD) is a complex, heterogeneous, biopsychosocial functional gastrointestinal disorder that is comprised of a cluster of symptoms, including postprandial epigastric pain/discomfort, impaired gastric emptying, and early satiety in the absence of any apparent structural, biochemical, or organic abnormality (14, 45, 49). Experimental clinical studies found that gastric sensitivity to distention is increased in a subset of FD patients (3, 9, 11, 12, 25, 34, 39, 44), and it contributes to the sensory symptoms, such as postprandial pain/discomfort, fullness, and early satiety. The cellular mechanisms of gastric hypersensitivity (GHS) in FD patients remain incompletely understood, primarily because of lack of availability of live visceral tissues from human subjects. In addition, ethical and safety considerations preclude the use of interventional experiments, such as induction of inflammation and severe chronic stress, in human subjects. However, epidemiological studies found that adverse early life experiences, including gastrointestinal infections, abuse, and psychological stress, are risk factors for the development of FD (2, 16, 24, 33, 41, 42).
With the use of clues from the epidemiological and experimental clinical findings, preclinical models of GHS show that either gastric (29, 30) or colon (50) inflammation during neonatal development induces GHS in adult life. We called these “gastric hypertensive rats” (GHS rats) (50). Cellular and neurohormonal findings revealed that neonatal colonic inflammation elevates basal plasma norepinephrine (NE), which makes a significant contribution to the induction of GHS; the blockade of α1/α2- and β1/β2-adrenergic receptors suppressed the visceromotor response (VMR) to gastric distention in GHS rats (50). The increase in plasma NE induced GHS by upregulating the expression of nerve growth factor (NGF) in the muscularis externa of the gastric fundus, which increased the expression of brain-derived growth factor (BDNF) in the thoracic dorsal root ganglia and spinal cord. The neutralization of NGF by its antibody suppressed the elevation of BDNF and GHS (50). There is evidence for increased sympathetic activity at baseline and in response to stress or a meal in FD patients (10). However, the source of increase in sympathetic activity in FD patients remains unknown.
Several, but not all, epidemiological studies found a greater prevalence of anxiety in a subset of FD patients than in healthy controls (48). However, the correlation between anxiety and the symptoms of FD has remained contentious. We tested the hypothesis that neonatal programming triggered by neonatal colon inflammation elevates the expression of tyrosine hydroxylase (TH) in the celiac ganglia to increase sympathetic nervous system activity in the gastric fundus as well as anxiety-like behavior.
METHODS
Animals.
Litters consisting of 10 5-day-old male Sprague Dawley pups with a nurturing primiparous mother were delivered from Harlan (Houston, TX). The litters were a combination of the mother's own pups and fostered pups. The pups were weaned at 22 days of age and were housed three to four per cage until they weighed 250 grams each at which time they were housed two per cage. The Institutional Animal Care and Use Committee at the University of Texas Medical Branch approved all procedures performed on these animals.
Neonatal colonic inflammation.
The rat pups received 0.2 ml of 130 mg/kg trinitrobenzenesulfonic (TNBS) acid in 10% ethanol in saline through a catheter inserted 2 cm in the distal colon on postnatal day (PND) 10 without anesthesia. The rats were placed on their backs with their four legs restrained by one hand of the investigator for about 2 min during the administration of TNBS. The control pups received saline only in a similar manner. Six-to-eight weeks later, when these rats were adults, they were evaluated for GHS and anxiety-like behavior, and tissues were collected for molecular experiments. Previous publications show that there is no residual colon or gastric inflammation 6–8 wk after the induction of neonatal colon inflammation (7, 50). We validated previously that the GHS in adult rats results when they were exposed to colon inflammation for the first time as neonates, but not when they were exposed to first-time colon inflammation as adults (50). One group of pups was treated daily with 16 μg/kg sc glucocorticoid receptor antagonist RU-486 from PND 9 to 17 or vehicle.
Implantation of gastric balloon and evaluation of gastric sensitivity to gastric distention.
The following procedures were performed under isoflurane anesthesia. Gastric balloon implantation: a 2-cm-long balloon was prepared from a condom and attached to PE-240 tubing. A 2-cm incision was made below the chest cavity to the right of the abdominal midline. The stomach was exteriorized and wrapped in gauze wetted with saline. The balloon was inserted through a small incision at the tip of the fundus; it was secured to the fundus wall with suture; the opening was closed. This placement left the lower esophageal sphincter and pylorus unobstructed. The stomach was returned to the abdominal cavity, and the muscle wall was closed with suture. The tubing tethering the balloon was pushed under the skin and externalized at the nape of the neck. The skin was closed. Electrodes implantation to record electromyographic (EMG) activity: a small incision was made at the nape of the neck to expose the acromeotrapezious muscle. Bipolar electrodes were sutured to the acromeotrapezious muscle, and the skin was closed.
The rats were allowed to recover in their home cage. Each rat received twice daily subcutaneous injection of buprenorphine (0.03 mg/kg) for up to 3 or 4 days. Health was monitored by ensuring that the rats were maintaining or gaining weight and absence of lack of activity, such as lethargy and grooming. Seven-to-10 days later, gastric sensitivity to fundus distention was measured. The rats received a series of 20-s gastric balloon distention of increasing intensity: 30, 40, 50, 60, 80, 100, and 120 mmHg using a sphygmomanometer with a 2-min interval between successive distentions. The rats were awake and freely moving during gastric balloon dissentions. The pressures were applied in duplicate. A Biopac (Biopac, Goleta, CA) amplifier (sample rate 2,000/s; high-pass cut-off 1 Hz, low-pass cut-off 500 Hz) was used to record EMG activity. The traces were visualized and analyzed using Acknowledge (Biopac). EMG was rectified, and the area under the curve was calculated for each 20-s distention period. Baseline activity, 20 s before distention, was subtracted from the EMG induced by distention. Data were displayed as EMG activity in volts × seconds as a function of distention pressure. The area under the pressure-EMG curve for each rat was calculated.
Plasma NE levels.
Blood was drawn from the saphenous vein under isoflurane anesthesia into tubes containing EDTA and spun at 10,000 g for 10 min at 4°C. NE was measured by ELISA purchased from Rocky Mountain Diagnostics (Colorado Springs, CO).
Adrenalectomy and 6-hydroxydopamine treatment.
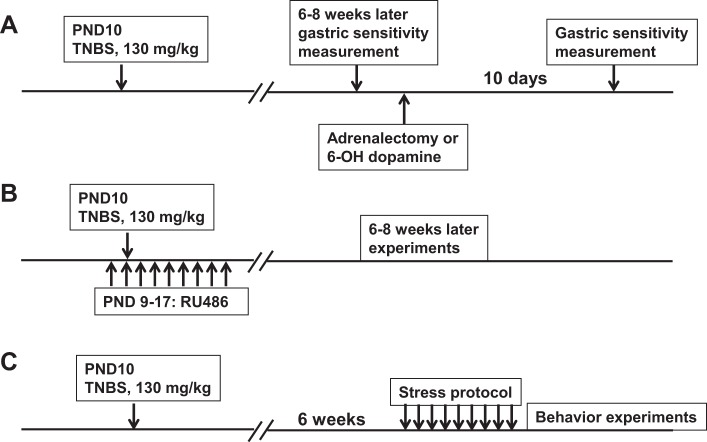
Gastric sensitivity was measured in 24 GHS rats that were then randomly assigned to one of three experimental groups (n = 8 each): adrenalectomy (ADx), sham surgery, and 6-hydroxydopamine (6-OHDA). A 1.5- to 2-cm dorsal incision was made over the center line behind the rib cage. One-centimeter incisions were made in the muscle layers to the left and the right of the rib cage. The adrenal glands were externalized from the abdominal cavity, and a ligature was placed below each adrenal gland. The adrenal glands were excised. Sham surgery consisted of making the incisions without removing the adrenal glands. ADx rats were given 0.9% saline in place of drinking water. 6-OHDA (150 mg/kg in 0.1% ascorbic acid) was administered intraperitoneally one time per day for three consecutive days. The rats were allowed to recover for 10 days; gastric sensitivity to fundus distention was measured again. Fundus muscularis externa tissue was saved for the measurement of NGF. Figure 1 illustrates the time line of the experiments.
Fig. 1.
Time line of experimental protocols. A: trinitrobenzenesulfonic (TNBS) was used to induce colonic inflammation on postnatal day (PND) 10. Later (6–8 wk), gastric sensitivity was measured, and the gastric hypersensitivity (GHS) rats were assigned to one of three groups: 6-hydroxydopamine (6-OHDA) treatment, sham surgery, and bilateral adrenalectomy (n = 8 each). Later (10 days) gastric sensitivity was measured again in each group of rats. B: TNBS inflammation was induced on PND 10, as above (n = 5). In addition, these rats were treated once a day with 16 mg/kg RU-486 from PND 9 to 17 to block the glucocorticoid receptor. Experiments were performed 6–8 wk later. C: adult GHS rats subjected previously to TNBS neonatal colon inflammation on PND 10, and age-matched controls (control, n = 6; GHS, n = 9; and GHS + RU-486, n = 10) were subjected to 9-day heterotypic intermittent chronic stress protocol; experiments were performed 24 h after the end of the stress protocol.
Heart rate variability.
Twelve GHS and 12 control rats were tested. A pair of electrodes was attached to the muscle layer under the skin overlying the chest and was externalized behind the nape of the neck. ECG activity was recorded for 30 min using a Biopac Systems data acquisition unit MP100A-CE, EG100C, universal interface module UIM100C, high-frequency (HF) and low-frequency (LF) components; the LF-to-HF ratio was calculated with Acknowledge software. The RR intervals were extracted from the ECG signal (modified Pan-Tompkins QRS detector). Frequency information was extracted from the RR intervals. A Welch periodogram was used to generate the Power Spectral Density (in units of s2). The efferent vagal activity is a major contributor to the HF component, and the LF component is considered a marker of sympathetic modulation. Data were expressed as the LF/HF ratio, which mirrors sympathetic-vagal balance.
Elevated plus maze test for anxiety-like behavior.
A total of 36 rats in 4 groups (control, control + stress, GHS, and GHS + stress) were tested. The rats were stressed by the application of a heterotypic intermittent chronic stress (HeICS) protocol that consisted of a random sequence of three types of stressors, 1 h water avoidance stress, 45 min cold restraint stress, or 20 min forced swim stress for 9 days (8, 51). One stressor was applied in the morning (9:00–11:00 AM) and the other in the afternoon (3:00–5:00 PM) in a random order, as described previously (51). The Elevated plus maze (EPM) tests were performed 1 day after the last stressor. Anxiety-like behavior was assessed on an EPM (San Diego Instruments) consisting of two open arms (38 × 5 cm) and two closed arms (38 × 5 × 15 cm) with a central open intersection (5 × 5 cm) elevated 75 cm above the ground. Each rat was tested for 5 min. At the beginning of the test, each rat was placed individually in the central platform facing an open arm. The rat's movement through the maze was followed with a video camera, and data were analyzed by AnyMaze flexible video tracking software (Stoelting, Wood Dale, IL). The time spent in the open and closed arms, the number of entries in open and closed arms, and the total distance traveled were measured.
Cold stress-induced defecation and social interaction.
Twelve control and 12 GHS rats were tested. In cold stress defecation, the rats were placed in individual novel polycarbonate cages on ice, and the numbers of fecal pellets were counted over a 20-min period (19). For the social interaction test (13, 19), the test rat's behavior in its home cage with a novel rat of similar size was recorded for 10 min. The total time spent in active interaction, defined as sniffing, close following, and allo-grooming, was recorded. The observer was blinded to treatment when monitoring behaviors.
RT-PCR/western blot.
Celiac ganglia and fundus muscularis externa tissue were removed and frozen in liquid nitrogen. RNA was prepared from the frozen tissue with a MicroRNeasy kit (Qiagen, Valencia, CA). RT was performed using a Superscript III kit (Invitrogen). Quantitative PCR was performed on an Applied Biosystems Step One Plus Real-time PCR. TAQMAN primer/probe sets for each gene were purchased from Applied Biosystems. Western blots were performed as described (50).
NE release.
Freshly dissected fundus muscularis externa tissue strips were incubated in oxygenated Krebs [(in mmol/l) 126 NaCl, 5 KCl, 2.5 NaH2PO4, 1.2 MgCl2, 2.5 CaCl2, 11 glucose, 25 NaHCO3], bubbled with 95% O2-5% CO2 + 30 μM pargyline, a monoamine oxidase inhibitor, + 50 μCi [3H]NE (0.2 μmol/l, 42 Ci/mmol; Amersham, Piscataway, NJ) for 1 h at 37°C. The strips were washed three times and transferred either to Krebs to measure basal release or to Krebs with 70 mM KCl for stimulus-evoked release. The buffer was changed every 5 min over a period of 15 min. The buffer containing the released NE contained 30 μM pargyline and a NE transport inhibitor, 20 μM maprotiline. At the conclusion of NE release measurements, the tissues were blotted dry, weighed, and solubilized with 1 ml Soluene-350 tissue solubilizer (Perkin-Elmer, Waltham, MA) and neutralized with 50 μl glacial acetic acid. The solubilized tissue was then added to 3 ml scintillation fluid, and the samples were analyzed to determine the total residual tritium content in each tissue sample. Counts remaining in the tissue + counts released = total counts, which were normalized to wet tissue weight. The NE release was expressed as a percentage of total uptake.
Statistics.
Data were expressed as means ± SE. Paired or unpaired t-test or one- or two-way ANOVA were used where appropriate. Tukey test was used to compare individual means where a significant main effect was obtained by ANOVA; P < 0.05 was considered significant.
RESULTS
Source of NE to upregulate gastric fundus NGF and its correlation with visceral hypersensitivity.
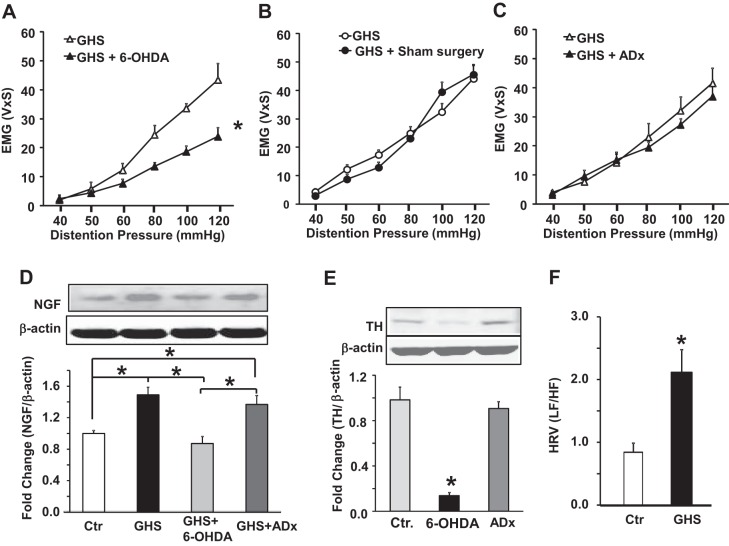
We reported previously that the basal plasma level of NE is significantly elevated in GHS rats and it underlies the upregulation of NGF in the fundus muscularis externa tissue, which contributes to the enhancement of sensitivity to gastric fundus distention (50). Here, we investigated the predominant source of NE to upregulate NGF and hence GHS, i.e., sympathetic neuronal overflow or adrenal gland. We performed chemical sympathectomy with 6-OHDA or bilateral ADx in separate groups of GHS rats (Fig. 1). 6-OHDA treatment significantly suppressed the gastric sensitivity to fundus distention in GHS rats (P < 0.05, n = 6; Fig. 2A). By contrast, ADx or sham surgery had no significant effect on the sensitivity to fundus distention (P > 0.05, n = 6 each; Fig. 2, B and C). 6-OHDA treatment also suppressed NGF expression in the fundus muscularis externa tissue vs. the baseline values in GHS rats, but ADx had no significant effect (Fig. 2D). To evaluate the effects of 6-OHDA treatment on fundus sympathetic innervation, we measured TH expression by Western blot. 6-OHDA treatment nearly ablated TH expression in the gastric fundus, but ADx had no significant effect (Fig. 2E). We found >90% decrease in TH expression in all 6-OHDA-treated rats.
Fig. 2.
Chemical sympathectomy suppressed GHS and nerve growth factor (NGF) expression in the gastric fundus. A: graphs showing the visceromotor response to gastric distention (electromyographic activity in volts × s) as a function of distention pressure in GHS rats, before and after 6-OHDA treatment (*P < 0.01). B: after adrenalectomy (ADx). C: after sham surgery (each comparison in A, B, and C used t-test, n = 8 for each group). D: panels and bar graph illustrating the results of Western blotting measuring NGF in the fundus muscularis externa normalized to β-actin. The significant increase in NGF in GHS rats was normalized by 6-OHDA treatment but not by ADx (*P < 0.05, n = 8; ANOVA). Ctr, control. E: bar graph showing that 6-OHDA treatment almost completely ablated the expression of tyrosine hydroxylase (TH) in the gastric fundus muscularis externa while ADx had no significant effect (*P < 0.05, n = 8; ANOVA). F: bar graph showing a significant increase in the ratio of sympathetic to vagal activity in GHS rats compared with controls rats (*P < 0.05, n = 12 each; t-test).
The measurement of heart rate variability is often used as a noninvasive test to evaluate the balance between the sympathetic and vagal tones. We investigated whether the increase of sympathetic tone in GHS rats reflects changes in heart rate variability. The measurement of heart rate variability showed a significant increase in the LF/HF ratio in GHS vs. the control rats (Fig. 2F). This increase reflected both an increase in the LF component (0.57 ± 0.04 vs. 0.36 ± 0.04, GHS vs. control) and a decrease in the HF component (0.43 ± 0.03 vs. 0.64 ± 0.05, GHS vs. control). These data were consistent with increased sympathetic tone and a decrease in vagal tone.
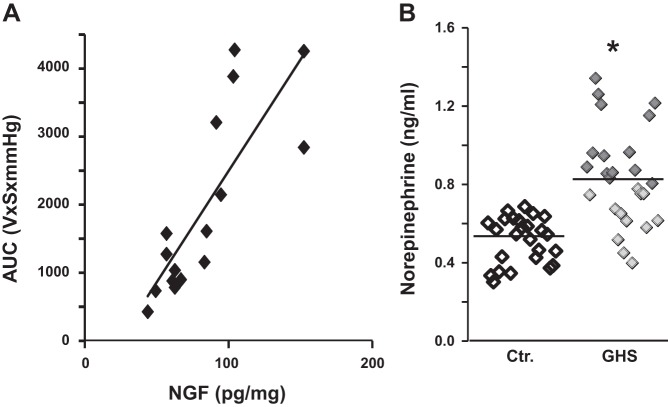
FD is a heterogeneous disorder where the intensity of symptoms varies among patients. We investigated whether biological variables, such as plasma NE and NGF expression in the gastric fundus, may relate to the intensity of gastric sensitivity and therefore with the symptoms related to it, such as epigastric pain and discomfort. We reported previously that, even though all neonate rats were subjected to the same TNBS inflammatory insult, only about one-half of them developed GHS (50). Here, we investigated whether the level of NGF expression in the fundus correlates with the intensity of GHS. We found that the intensity of VMR to gastric distention was linearly correlated with the expression of NGF in the fundus muscularis externa (r2 = 0.66; F = 27.1, P = 0.0001, n = 5 control and n = 11 neonatal inflammation; Fig. 3A). Consistent with this finding, we found that the plasma NE levels in 14/26 adult rats (Fig. 3B) subjected previously to neonatal colon insult were >2 SDs above the mean of controls (1.03 ± 0.05, n = 14 responder rats vs. 0.64 ± 0.04, n = 12 nonresponder rats and 0.53 ± 0.03, n = 25 control rats). We also found a wide scatter in the plasma concentration of NE among the GHS rats subjected to the same inflammatory insult as neonates. The scatter and mean concentration of plasma NE were significantly greater in GHS than in vehicle-treated control rats (P < 0.05, rank-sum test; Fig. 3B).
Fig. 3.
Plots representing gastric fundus NGF levels vs. sensitivity to gastric distention and individual plasma NE levels in control and GHS rats. A: gastric sensitivity, calculated as area under the distention pressure curve in volts-seconds × mmHg (EMG), was significantly correlated with NGF expression in the muscularis externa of the gastric fundus of the control and GHS rats (r2 = 0.66; F = 27.1, P = 0.0001, n = 16). B: mean values of NE were significantly different between the entire group of GHS and control rats (P < 0.05, rank-sum test, control, n = 25; GHS, n = 26). However, the plasma levels of NE showed a wide variation in GHS rats. In 14 out of 26 GHS rats (shown by dark diamonds, designated responder rats), each individual plasma level of NE was >2 SDs of the mean of control rats. The mean value of plasma NE in nonresponder rats (shown by lightly shaded diamonds) was not different from that in control rats (n = 25).
Alterations in the release and uptake of NE from the fundus muscularis externa sympathetic nerve endings in GHS rats.
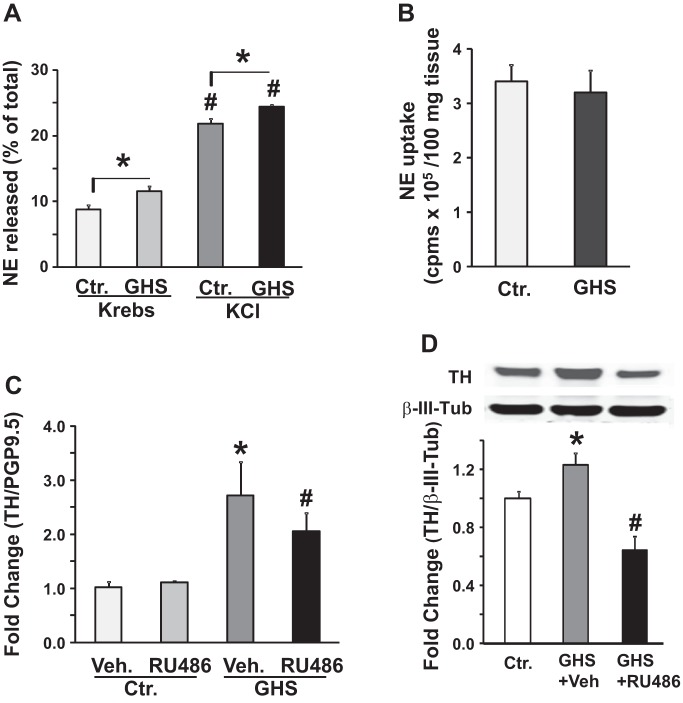
We found a significantly greater basal and KCl-evoked release of NE from the fundus muscularis externa strips of GHS rats than those from the control rats (Fig. 4A). By contrast, the uptake of NE from the fundus strips of the GHS and control rats did not differ (Fig. 4B). To investigate the molecular mechanisms of increase of fundus NE release in GHS rats, we measured TH, the rate-limiting enzyme for the synthesis of NE, expression levels in the celiac ganglia, which supplies sympathetic innervation to the stomach. The Th mRNA in the celiac ganglia was significantly greater (2.5-fold; P < 0.05) in GHS than in control rats (Fig. 4C).
Fig. 4.
Gastric NE release and tyrosine hydroxylase expression in the celiac ganglia of control (Ctr.) and gastric hypersensitive (GHS) rats. A: bar graph shows NE release in the presence of reuptake and monooxygenase inhibitors from the fundus muscularis externa isolated from GHS and Ctr. rats in Krebs or high KCl Krebs. KCl Krebs significantly increased NE release in the fundus tissues from both Ctr. and GHS rats (n = 5, #P < 0.05). NE release was significantly greater in tissues from GHS vs. Ctr. rats in Krebs as well as in KCl Krebs (n = 5, *P < 0.05). B: bar graphs show no significant difference in NE uptake, expressed as cpms/100 mg tissue, by fundus muscularis externa strips between Ctr. and GHS rats (n = 5, P > 0.05). C: bar graph shows the levels of Th mRNA expression normalized to protein gene product (PGP9.5) in the celiac ganglia of adult Ctr. and GHS rats that were treated with RU-486 during the neonatal period. RU-486 treatment did not significantly alter Th mRNA expression in Ctr. rats (n = 6, P > 0.05). Th mRNA expression was significantly greater in GHS rats vs. vehicle treated Ctr. rats (n = 6, *P < 0.05). Neonatal treatment with RU486 significantly decreased Th mRNA expression in GHS rats (n = 6, #P < 0.05). D: Western blot panel and bar graph show tyrosine hydroxylase expression in the celiac ganglia extracts from Ctr. (n = 6), GHS (n = 9) and GHS+RU486 (n = 10) rats. TH expression in the celiac ganglia was significantly greater in GHS + vehicle rats vs. Ctr. rats (*P < 0.05). Neonatal RU-486 treatment significantly suppressed TH expression in the celiac ganglia of GHS rats (#P < 0.05).
Neonatal colon inflammation upregulates Th gene transcription.
We reported previously that colon inflammation on PND 10 prematurely upregulates the plasma corticosterone by PND 15, which triggers neonatal programming to induce GHS in adult life (50). In control rats, the increase in plasma corticosterone occurs by PND 17 (50). We found that the blockade of glucocorticoid receptors by RU-486 in neonates prevented the induction of GHS in adult life (50). Here, we investigated whether neonatal programming targets the Th gene to upregulate its transcription in GHS rats (experimental protocol, Fig. 1B). Treatment of neonates with RU-486 significantly reduced the upregulation of Th mRNA in the GHS rats; it had no effect in vehicle-treated control rats (Fig. 4C). TH protein was significantly increased 1.3-fold (P < 0.05) in the celiac ganglia of GHS vs. control rats; this increase was significantly reduced in rats treated with RU-486 as neonates (Fig. 4D).
Increase in trait but not stress-induced anxiety-like behavior in GHS rats.
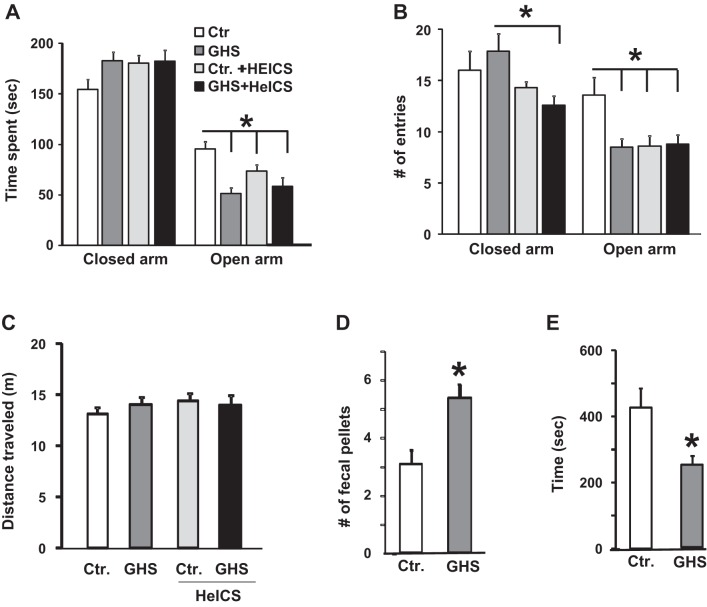
Because anxiety is often a comorbid condition in FD patients, we used EPM, cold stress-induced defecation, and social interaction tests to investigate whether GHS rats displayed anxiety-like behavior. In the EPM test, we subjected the GHS and control rats to sham stress or a 9-day HeICS protocol (51) (Fig. 1C). Sham-stressed GHS rats spent significantly less time in the open arm of the EPM compared with that by the control rats (P < 0.05) (Fig. 5A). HeICS significantly reduced the time spent in the open arm by the control rats, but it was without additional significant effect on the time spent in the closed arm by the GHS rats. Control + HeICS, GHS, and GHS + HeICS rats made significantly fewer open arm entries compared with the control rats (Fig. 5B). HeICS significantly reduced the number of closed arm entries by GHS rats (Fig. 5B), indicating an increased unwillingness to cross the open center space in the maze. There were no significant differences in the total distance traveled in the open and closed arms (Fig. 5C), suggesting that neonatal inflammatory insult did not alter locomotor activity in GHS rats. Cold stress significantly increased the number of fecal pellets in GHS than in control rats (Fig. 5D). Social interaction between GHS rats was significantly lower than that between control rats (Fig. 5E).
Fig. 5.
GHS rats exhibited anxiety-like behavior in the elevated plus maze, social interaction, and cold stress tests relative to controls. A: bar graph showing the average times spent on the closed and open arms of the elevated plus maze by control, control + HeICS, GHS, and GHS + HeICS rats. GHS rats spent significantly less time in the open arm of the elevated plus maze compared with controls. HeICS significantly reduced the open arm time of control rats but was without significant effect on the open arm time in GHS rats (*P < 0.05; 2-way ANOVA, n = 12 each). B: bar graph showing the average number of entries in the open and closed arms. GHS, control + HeICS, and GHS + HeICS rats made significantly fewer open arm entries compared with controls (*P < 0.05; 2-way ANOVA, n = 12 each). C: bar graph showing no significant differences in the average total distance traveled in meters (m) on the elevated plus maze (P > 0.05; 2-way ANOVA, n = 12 each). D: bar graphs showing a significant increase in the number of fecal pellets defecated in response to cold stress in GHS rats compared with controls (*P < 0.05, n = 12 each; t-test). E: bar graphs showing a significant decline in time spent in social interaction by GHS rats compared with controls (*P < 0.05, n = 12 each; t-test).
DISCUSSION
Several, but not all, clinical studies found an increase in the ratio of sympathetic tone to vagal tone measured by heart rate variability in FD patients (5, 20–22, 37). Our findings in a preclinical model show that neonatal programming triggered by colon inflammation increases the ratio of sympathetic to vagal tone in later life. We show for the first time that neonatal programming triggered by colon inflammation increases activity of the sympathetic nerves innervating the gastric fundus. The upregulation of TH in the celiac ganglia contributed to an increase in the activity of the sympathetic neurons innervating the gastric fundus. The increase in the expression of TH was due to premature upregulation of plasma corticosterone by neonatal colon inflammation; RU-486 treatment of the neonates blocked the upregulation of TH in the celiac ganglia. Therefore, it appears that neonatal programming triggered by colon inflammation concurrently upregulates the activity of the sympathetic neurons innervating the gastric fundus and the heart. It remains unknown whether neonatal programming triggered by colon inflammation alters the sympathetic activity of the neurons originating from the other sympathetic ganglia, such as the inferior mesenteric ganglia.
We reported previously that neonatal colon inflammation elevates plasma NE and the expression of NGF in the fundus muscularis externa, which underlies the induction of GHS (50). The blockade of α1-, α2-, β1-, and β2-adrenergic receptors prevented the upregulation of NGF in the gastric fundus muscularis externa of the GHS rats. Our present findings show that the upregulation of NGF and the induction of GHS were due to an increase in the release of NE from the sympathetic neurons innervating the fundus muscularis externa, rather than due to contribution of the adrenal medulla to the plasma NE. Chemical sympathectomy, but not ADx, blocked the upregulation of NGF and GHS in GHS rats.
In vitro experiments showed that the increased release of NE in the gastric fundus of the GHS rats was due to its greater release from the nerve endings rather than due to impaired uptake. The biological effects of catecholamines are concentration dependent. The concentration of NE at the neuroeffector junctions and neural synapses is much greater than that in the plasma, which may be the reason that ADx, which partly decreases the plasma NE, did not affect the upregulation of NGF in the gastric fundus or the induction of GHS. An increase in the concentration of NGF is known to cause sprouting at the sympathetic nerve terminal (6, 23), which may set up a cycle to further enhance the release of NE in the fundus muscularis externa.
We found that, even though the plasma NE increased on average by ∼30% in the entire group of GHS rats, each one of which was subjected previously to the same neonatal inflammatory insults, there was a wide variation in the plasma NE concentration among the individual rats in the group. Our findings also show that the expression of NGF in the gastric fundus muscularis externa in the GHS rats correlates with the VMR to gastric distention. We reported previously that NE concentration dependently upregulates the expression of NGF in the fundus muscularis externa (50).
FD is a heterogeneous disorder; the intensity of symptoms differs among patients, and they show temporal variations in the same patient, probably due to environmental and psychosocial factors. Stress stimulates sympathetic neurons to release NE (8). A clinical study found that mental stress aggravates postprandial symptom severity in FD patients (10). In addition, a single subcutaneous dose of clonidine given just before a meal significantly suppressed the cumulative meal-related symptoms in FD patients with GHS (43). Clonidine, an α2-adrenergic receptor agonist (31), inhibits the release of NE from the sympathetic neurons. These clinical findings together with our findings in a preclinical model of FD that plasma NE concentration was significantly elevated in rats with GHS suggest that above normal plasma NE levels may contribute to FD sensation-related symptoms. However, the relationships between plasma NE levels and gastric sensitivity and severity of FD symptoms in patients remain to be investigated. Of course, in patients, the psychosocial factors may further modify the intensity of symptoms.
Anxiety and depression are prevalent in subsets of patients with chronic diseases, including chronic obstructive pulmonary disease (35, 38), inflammatory bowel disease (18, 36, 40), and functional bowel disorders (1). Psychological stress induced by the morbidity and chronicity of these diseases/disorders, including FD, is an obvious factor in the induction of mood disorders. Our findings and those of others (29) show that adverse early life experiences may induce anxiety-like behavior independent of psychosocial factors. Other types of prenatal and neonatal stress also induce anxiety-like behaviors in rodents (15, 47). However, the underlying mechanisms of the development of anxiety-like behaviors remain unknown. In this study, we did not obtain direct evidence that the increase in sympathetic nerve activity contributes to the development of anxiety-like behavior in GHS rats. However, experimental clinical evidence from patients with posttraumatic stress disorder (PTSD) suggests that an increase in the sympathetic nervous system activity contributes to anxiety. The sympathetic nervous system activity is chronically upregulated in PTSD patients (15, 26, 27, 32), resulting in elevated urine NE (32). In addition, the NE concentrations in the cerebrospinal fluid correlate with the severity of PTSD symptoms (17). The blockade of the stellate ganglion or the upper thoracic ganglion that innervate the brain or treatment with prazosin, an α1-adrenergic receptor antagonist, reduced anxiety in PTSD patients (4, 28, 46). The role of elevated sympathetic neuronal activity in inducing anxiety in FD patients remains to be investigated.
In conclusion, our findings show that neonatal programming triggered by colon inflammation upregulates TH expression in the celiac ganglia, which increases the release of NE in the fundus muscularis externa tissue. The increase of NE release at the sympathetic nerve terminals upregulates NGF in the muscularis externa, which proportionately increases the VMR to gastric distention. In our preclinical model, both the magnitude of NGF expression and plasma NE may serve as biological markers of the severity of GHS. This correlation remains to be investigated in FD patients. Concurrently, neonatal programming induces anxiety-like behavior in gastric hypersensitive rats. The targets of neonatal programming in the brain to induce anxiety-like behavior remain to be investigated.
GRANTS
This work was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases Grants 5R01-DK-088796 (S. K. Sarna) and DK-32346 (S. K. Sarna).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.H.W. and S.K.S. conception and design of research; J.H.W. performed experiments; J.H.W. analyzed data; J.H.W. and S.K.S. interpreted results of experiments; J.H.W. prepared figures; J.H.W. drafted manuscript; S.K.S. edited and revised manuscript; S.K.S. approved final version of manuscript.
REFERENCES
- 1.Ballenger JC, Davidson JR, Lecrubier Y, Nutt DJ, Lydiard RB, Mayer EA, International Consensus Group on Depression and Anxiety. Consensus statement on depression, anxiety, and functional gastrointestinal disorders. J Clin Psychiatry 62, Suppl 8: 48–51, 2001. [PubMed] [Google Scholar]
- 2.Bonilla S, Saps M. Early life events predispose the onset of childhood functional gastrointestinal disorders. Rev Gastroenterol Mex 78: 82–91, 2013. [DOI] [PubMed] [Google Scholar]
- 3.Bouin M, Lupien F, Riberdy M, Boivin M, Plourde V, Poitras P. Intolerance to visceral distention in functional dyspepsia or irritable bowel syndrome: an organ specific defect or a pan intestinal dysregulation? Neurogastroenterol Motil 16: 311–314, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Brady K, Pearlstein T, Asnis GM, Baker D, Rothbaum B, Sikes CR, Farfel GM. Efficacy and safety of sertraline treatment of posttraumatic stress disorder: a randomized controlled trial. J Am Med Assoc 283: 1837–1844, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Camilleri M, Ford MJ. Functional gastrointestinal disease and the autonomic nervous system: a way ahead? Gastroenterology 106: 1114–1118, 1994. [DOI] [PubMed] [Google Scholar]
- 6.Chen PS, Chen LS, Cao JM, Sharifi B, Karagueuzian HS, Fishbein MC. Sympathetic nerve sprouting, electrical remodeling and the mechanisms of sudden cardiac death. Cardiovasc Res 50: 409–416, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Choudhury BK, Shi XZ, Sarna SK. Gene plasticity in colonic circular smooth muscle cells underlies motility dysfunction in a model of postinfective IBS. Am J Physiol Gastrointest Liver Physiol 296: G632–G642, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choudhury BK, Shi XZ, Sarna SK. Norepinephrine mediates the transcriptional effects of heterotypic chronic stress on colonic motor function. Am J Physiol Gastrointest Liver Physiol 296: G1238–G1247, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corsetti M, Caenepeel P, Fischler B, Janssens J, Tack J. Impact of coexisting irritable bowel syndrome on symptoms and pathophysiological mechanisms in functional dyspepsia. Am J Gastroenterol 99: 1152–1159, 2004. [DOI] [PubMed] [Google Scholar]
- 10.De Giorgi F, Sarnelli G, Cirillo C, Savino IG, Turco F, Nardone G, Rocco A, Cuomo R. Increased severity of dyspeptic symptoms related to mental stress is associated with sympathetic hyperactivity and enhanced endocrine response in patients with postprandial distress syndrome. Neurogastroenterol Motil 25: 31–38 e32–e33, 2013. [DOI] [PubMed] [Google Scholar]
- 11.Di Stefano M, Miceli E, Tana P, Mengoli C, Bergonzi M, Pagani E, Corazza GR. Fasting and postprandial gastric sensorimotor activity in functional dyspepsia: postprandial distress vs. epigastric pain syndrome. Am J Gastroenterol 109: 1631–1639, 2014. [DOI] [PubMed] [Google Scholar]
- 12.Farre R, Vanheel H, Vanuytsel T, Masaoka T, Tornblom H, Simren M, Van Oudenhove L, Tack JF. In functional dyspepsia, hypersensitivity to postprandial distention correlates with meal-related symptom severity. Gastroenterology 145: 566–573, 2013. [DOI] [PubMed] [Google Scholar]
- 13.File SE, Seth P. A review of 25 years of the social interaction test. Eur J Pharmacol 463: 35–53, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Fischler B, Tack J, De Gucht V, Shkedy ZI, Persoons P, Broekaert D, Molenberghs G, Janssens J. Heterogeneity of symptom pattern, psychosocial factors, and pathophysiological mechanisms in severe functional dyspepsia. Gastroenterology 124: 903–910, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Fride E, Weinstock M. Prenatal stress increases anxiety related behavior and alters cerebral lateralization of dopamine activity. Life Sci 42: 1059–1065, 1988. [DOI] [PubMed] [Google Scholar]
- 16.Geeraerts B, Van Oudenhove L, Fischler B, Vandenberghe J, Caenepeel P, Janssens J, Tack J. Influence of abuse history on gastric sensorimotor function in functional dyspepsia. Neurogastroenterol Motil 21: 33–41, 2009. [DOI] [PubMed] [Google Scholar]
- 17.Geracioti TD Jr, Baker DG, Ekhator NN, West SA, Hill KK, Bruce AB, Schmidt D, Rounds-Kugler B, Yehuda R, Keck PE Jr, Kasckow JW. CSF norepinephrine concentrations in posttraumatic stress disorder. Am J Psychiatry 158: 1227–1230, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Goodhand JR, Wahed M, Mawdsley JE, Farmer AD, Aziz Q, Rampton DS. Mood disorders in inflammatory bowel disease: relation to diagnosis, disease activity, perceived stress, and other factors. Inflamm Bowel Dis 18: 2301–2309, 2012. [DOI] [PubMed] [Google Scholar]
- 19.Green TA, Alibhai IN, Roybal CN, Winstanley CA, Theobald DE, Birnbaum SG, Graham AR, Unterberg S, Graham DL, Vialou V, Bass CE, Terwilliger EF, Bardo MT, Nestler EJ. Environmental enrichment produces a behavioral phenotype mediated by low cyclic adenosine monophosphate response element binding (CREB) activity in the nucleus accumbens. Biol Psychiatry 67: 28–35, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haug TT, Svebak S, Hausken T, Wilhelmsen I, Berstad A, Ursin H. Low vagal activity as mediating mechanism for the relationship between personality factors and gastric symptoms in functional dyspepsia. Psychosomatic Med 56: 181–186, 1994. [DOI] [PubMed] [Google Scholar]
- 21.Hausken T, Svebak S, Wilhelmsen I, Haug TT, Olafsen K, Pettersson E, Hveem K, Berstad A. Low vagal tone and antral dysmotility in patients with functional dyspepsia. Psychosomatic Med 55: 12–22, 1993. [DOI] [PubMed] [Google Scholar]
- 22.Hveem K, Svebak S, Hausken T, Berstad A. Effect of mental stress and cisapride on autonomic nerve functions in functional dyspepsia. Scand J Gastroenterol 33: 123–127, 1998. [DOI] [PubMed] [Google Scholar]
- 23.Isaacson LG, Billieu SC. Increased perivascular norepinephrine following intracerebroventricular infusion of NGF into adult rats. Exp Neurol 139: 54–60, 1996. [DOI] [PubMed] [Google Scholar]
- 24.Koloski NA, Talley NJ, Boyce PM. A history of abuse in community subjects with irritable bowel syndrome and functional dyspepsia: the role of other psychosocial variables. Digestion 72: 86–96, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Lemann M, Dederding JP, Flourie B, Franchisseur C, Rambaud JC, Jian R. Abnormal perception of visceral pain in response to gastric distention in chronic idiopathic dyspepsia. The irritable stomach syndrome. Dig Dis Sci 36: 1249–1254, 1991. [DOI] [PubMed] [Google Scholar]
- 26.Lemieux AM, Coe CL. Abuse-related posttraumatic stress disorder: evidence for chronic neuroendocrine activation in women. Psychosom Med 57: 105–115, 1995. [DOI] [PubMed] [Google Scholar]
- 27.Lipov E, Kelzenberg B. Sympathetic system modulation to treat post-traumatic stress disorder (PTSD): a review of clinical evidence and neurobiology. J Affect Disord 142: 1–5, 2012. [DOI] [PubMed] [Google Scholar]
- 28.Lipov EG, Joshi JR, Lipov S, Sanders SE, Siroko MK. Cervical sympathetic blockade in a patient with post-traumatic stress disorder: a case report. Ann Clin Psychiatry 20: 227–228, 2008. [DOI] [PubMed] [Google Scholar]
- 29.Liu L, Li Q, Sapolsky R, Liao M, Mehta K, Bhargava A, Pasricha PJ. Transient gastric irritation in the neonatal rats leads to changes in hypothalamic CRF expression, depression- and anxiety-like behavior as adults. PLoS One 6: e19498, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu LS, Winston JH, Shenoy MM, Song GQ, Chen JD, Pasricha PJ. A rat model of chronic gastric sensorimotor dysfunction resulting from transient neonatal gastric irritation. Gastroenterology 134: 2070–2079, 2008. [DOI] [PubMed] [Google Scholar]
- 31.Ma D, Rajakumaraswamy N, Maze M. alpha2-Adrenoceptor agonists: shedding light on neuroprotection? Br Med Bull 71: 77–92, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Mason JW, Giller EL, Kosten TR, Harkness L. Elevation of urinary norepinephrine/cortisol ratio in posttraumatic stress disorder. J Nerv Ment Dis 176: 498–502, 1988. [DOI] [PubMed] [Google Scholar]
- 33.Mearin F, Perez-Oliveras M, Perello A, Vinyet J, Ibanez A, Coderch J, Perona M. Dyspepsia and irritable bowel syndrome after a Salmonella gastroenteritis outbreak: one-year follow-up cohort study. Gastroenterology 129: 98–104, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Mertz H, Fullerton S, Naliboff B, Mayer EA. Symptoms and visceral perception in severe functional and organic dyspepsia. Gut 42: 814–822, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mikkelsen RL, Middelboe T, Pisinger C, Stage KB. Anxiety and depression in patients with chronic obstructive pulmonary disease (COPD). A review. Nord J Psychiatry 58: 65–70, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Mikocka-Walus AA, Turnbull DA, Moulding NT, Wilson IG, Andrews JM, Holtmann GJ. Controversies surrounding the comorbidity of depression and anxiety in inflammatory bowel disease patients: a literature review. Inflamm Bowel Dis 13: 225–234, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Muth ER, Koch KL, Stern RM. Significance of autonomic nervous system activity in functional dyspepsia. Dig Dis Sci 45: 854–863, 2000. [DOI] [PubMed] [Google Scholar]
- 38.Pumar MI, Gray CR, Walsh JR, Yang IA, Rolls TA, Ward DL. Anxiety and depression-Important psychological comorbidities of COPD. J Thorac Dis 6: 1615–1631, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosen JM, Cocjin JT, Schurman JV, Colombo JM, Friesen CA. Visceral hypersensitivity and electromechanical dysfunction as therapeutic targets in pediatric functional dyspepsia. World J Gastrointest Pharmacol Ther 5: 122–138, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sajadinejad MS, Asgari K, Molavi H, Kalantari M, Adibi P. Psychological issues in inflammatory bowel disease: an overview. Gastroenterol Res Pract 2012: 106502, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saps M, Lu P, Bonilla S. Cow's-milk allergy is a risk factor for the development of FGIDs in children. J Pediatr Gastroenterol Nutr 52: 166–169, 2011. [DOI] [PubMed] [Google Scholar]
- 42.Saps M, Pensabene L, Di Martino L, Staiano A, Wechsler J, Zheng X, Di Lorenzo C. Post-infectious functional gastrointestinal disorders in children. J Pediatr 152: 812–816, 2008. [DOI] [PubMed] [Google Scholar]
- 43.Tack J, Caenepeel P, Corsetti M, Janssens J. Role of tension receptors in dyspeptic patients with hypersensitivity to gastric distention. Gastroenterology 127: 1058–1066, 2004. [DOI] [PubMed] [Google Scholar]
- 44.Tack J, Caenepeel P, Fischler B, Piessevaux H, Janssens J. Symptoms associated with hypersensitivity to gastric distention in functional dyspepsia. Gastroenterology 121: 526–535, 2001. [DOI] [PubMed] [Google Scholar]
- 45.Tack J, Talley NJ. Functional dyspepsia–symptoms, definitions and validity of the Rome III criteria. Nat Rev Gastroenterol Hepatol 10: 134–141, 2013. [DOI] [PubMed] [Google Scholar]
- 46.Telaranta T. Treatment of social phobia by endoscopic thoracic sympathicotomy. Eur J Surg Suppl 27–32, 1998. [DOI] [PubMed]
- 47.Vallee M, Mayo W, Dellu F, Le Moal M, Simon H, Maccari S. Prenatal stress induces high anxiety and postnatal handling induces low anxiety in adult offspring: correlation with stress-induced corticosterone secretion. J Neurosci 17: 2626–2636, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van Oudenhove L, Aziz Q. The role of psychosocial factors and psychiatric disorders in functional dyspepsia. Nat Rev Gastroenterol Hepatol 10: 158–167, 2013. [DOI] [PubMed] [Google Scholar]
- 49.Vanheel H, Farre R. Changes in gastrointestinal tract function and structure in functional dyspepsia. Nat Rev Gastroenterol Hepatol 10: 142–149, 2013. [DOI] [PubMed] [Google Scholar]
- 50.Winston JH, Sarna SK. Developmental origins of functional dyspepsia-like gastric hypersensitivity in rats. Gastroenterology 144: 570–579, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Winston JH, Xu GY, Sarna SK. Adrenergic stimulation mediates visceral hypersensitivity to colorectal distention following heterotypic chronic stress. Gastroenterology 138: 294–304, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]