Gut is the first tissue affected in shock and is considered as the trigger for systemic response to blood loss. The accompanying barrier dysfunction is characterized by perturbation of the protein quality control mechanisms responsible for tissue adaptation and recovery. This study shows that resuscitation with artificially assembled liposome-encapsulated hemoglobin restores proteostasis and reduces oxidative stress in gut tissue. In comparison, saline resuscitation was not as effective, and it caused significant derangement in the constitutive protective machinery.
Keywords: oxidative stress, proteostasis, hemorrhagic shock, liposome-encapsulated hemoglobin
Abstract
Gut barrier dysfunction is the major trigger for multiorgan failure associated with hemorrhagic shock (HS). Although the molecular mediators responsible for this dysfunction are unclear, oxidative stress-induced disruption of proteostasis contributes to the gut pathology in HS. The objective of this study was to investigate whether resuscitation with nanoparticulate liposome-encapsulated hemoglobin (LEH) is able to restore the gut proteostatic mechanisms. Sprague-Dawley rats were recruited in four groups: control, HS, HS+LEH, and HS+saline. HS was induced by withdrawing 45% blood, and isovolemic LEH or saline was administered after 15 min of shock. The rats were euthanized at 6 h to collect plasma and ileum for measurement of the markers of oxidative stress, unfolded protein response (UPR), proteasome function, and autophagy. HS significantly increased the protein and lipid oxidation, trypsin-like proteasome activity, and plasma levels of IFNγ. These effects were prevented by LEH resuscitation. However, saline was not able to reduce protein oxidation and plasma IFNγ in hemorrhaged rats. Saline resuscitation also suppressed the markers of UPR and autophagy below the basal levels; the HS or LEH groups showed no effect on the UPR and autophagy. Histological analysis showed that LEH resuscitation significantly increased the villus height and thickness of the submucosal and muscularis layers compared with the HS and saline groups. Overall, the results showed that LEH resuscitation was effective in normalizing the indicators of proteostasis stress in ileal tissue. On the other hand, saline-resuscitated animals showed a decoupling of oxidative stress and cellular protective mechanisms.
NEW & NOTEWORTHY
Gut is the first tissue affected in shock and is considered as the trigger for systemic response to blood loss. The accompanying barrier dysfunction is characterized by perturbation of the protein quality control mechanisms responsible for tissue adaptation and recovery. This study shows that resuscitation with artificially assembled liposome-encapsulated hemoglobin restores proteostasis and reduces oxidative stress in gut tissue. In comparison, saline resuscitation was not as effective, and it caused significant derangement in the constitutive protective machinery.
hemorrhagic shock affects all organs, but a damage to the gut barrier function is recognized as the primary driver of shock pathology (19, 38, 49, 54). Hypoxia and hypoperfusion caused by hemorrhagic shock initiate the gut injury, but it is aggravated by the natural physiological response of splanchnic vasoconstriction and shifting of blood flow to the brain and heart. Despite recent studies advocating early resuscitation with blood products, the emergency condition of hemorrhagic shock is still initially managed by resuscitation with isotonic crystalloids such as saline and Ringer solution (34). These isotonic crystalloids primarily recover volume deficit but are unable to change the extent of tissue hypoxia. To correct volume as well as oxygen deficits, we are developing liposome-encapsulated hemoglobin (LEH). It is promoted as an oxygen-carrying substitute for red blood cells (RBCs), because the lack of safe and matched blood products in adequate supply at the place and time of need continues to threaten optimal treatment of trauma and shock victims (55). The objective of this work was to investigate the effects of LEH resuscitation on the intracellular protein quality control systems in the small intestine after hemorrhagic shock.
The metabolic perturbations, oxidative stress, and energy crisis associated with hypoxic and hypoperfused tissue cause an intracellular buildup of damaged or misfolded proteins in the lumen of the endoplasmic reticulum (ER). Consequently, an unfolded protein response (UPR) is induced that prevents the pathological accumulation of these proteins (24, 46). The ER stress in a normal cell is prevented by a constitutive level of UPR that addresses the accidental misfolding of proteins during the routine transcriptional turnover (37). The effector pathways of the UPR are determined by the protein kinase RNA-like ER kinase (PERK), the inositol-requiring enzyme (IRE)1, and the activating transcription factor (ATF)6. These three initiator proteins remain sequestered in the ER lumen by glucose-regulating peptide (GRP) 78, which also functions as a chaperone to properly refold the misfolded proteins. During a metabolic stress, there is an increased demand of GRP78 to carry out its chaperone activity, which results in its disengagement from PERK, IRE1, and ATF6. The free PERK, IRE1, and ATF6 initiate the distinct signaling pathways that characterize the UPR. The overall goals of the UPR are to 1) prevent accumulation of misfolded proteins by a translational block, 2) restore protein folding, and 3) permit synthesis of sufficient GRP78 to enable resequestering of PERK, IRE1, and ATF6. The activated IRE1 splices X box-binding protein 1 (XBP1) mRNA, and the spliced XBP1 mRNA encodes a transcription factor that induces the target genes, including GRP78, protein disulfide isomerases, and the components of the ER-associated protein degradation (ERAD). On the other hand, the activated PERK phosphorylates the α-subunit of the eukaryotic initiation factor (eIF2α) to suppress all new protein synthesis (14, 43).
In addition to the GRPs, other chaperones of the heat shock protein (HSP) family also participate in the detection and refolding of improperly folded proteins (41). The large-molecular-weight HSPs are the ATP-dependent foldases (HSP60/70/90), which are responsible for ensuring correct folding of newly synthesized proteins and refolding of misfolded or aggregated proteins. On the other hand, the small HSPs are the ATP-independent holdases (HSP25/27), which provide high-affinity binding platforms for unfolded proteins and prevent protein aggregation during stress (41). These HSPs also assist in degradation of irreversibly damaged proteins by the ERAD mechanisms. The ERAD is largely dependent on the ubiquitin-proteasome system (UPS) (60), and the molecular chaperones, such as HSP27, target damaged or mutated proteins to the UPS (41). The UPS function is mediated by a large multicatalytic complex of the 26S proteasome, which exhibits chymotrypsin-like, trypsin-like, and caspase-like activities localized in its 20S core (61). The process closely coordinates with another degradative pathway called macroautophagy, which serves to recycle the intracellular proteins for efficient utilization of assets during crisis (28, 53, 57).
The small intestine, particularly the mucin-secreting goblet and antimicrobial peptide-secreting Paneth cells lining the epithelium, is highly sensitive toward disturbances of the UPR (40). A prolonged or exaggerated activation of the UPS may also lead to a catastrophic loss of the structural and regulatory proteins critical for the integrity of the gut barrier. Moreover, when the demand on the homeostatic processes is excessive, the ER stress shifts the outcome from survival to cell death. For example, an excessive stimulation of the UPR was found to be detrimental to the cells populating gut epithelium in a rat model of ischemia-reperfusion injury (18). In this work, we examined the components and targets of the UPR in the small intestinal tissue of rats with hemorrhagic shock and LEH resuscitation.
MATERIALS AND METHODS
Small intestinal and plasma samples.
The small intestinal tissues and plasma samples employed in this work were from our previously reported work on a 45% hemorrhagic shock model in male Sprague-Dawley rats (250–300 g) obtained from Harlan (Indianapolis, IN) (47). All animal work was performed according to the NIH Animal Use and Care Guidelines and was approved by the institutional IACUC. Briefly, the rats with an indwelling left femoral artery catheter were anesthetized with 2.5% isoflurane in medical air stream supplied at 2 l/min. Hemorrhagic shock was induced by withdrawing ∼45% of blood from the catheter. An isovolemic resuscitation with LEH was administered after 15 min of the blood withdrawal. We used saline as a comparative fluid for resuscitation. The choice of saline over the other crystalloids, such as Ringer solution, was based on the data that normal saline is preferred over Ringer lactate for the prehospital intervention in a combat zone (31). Moreover, we employed isovolemic infusion based on a report showing that, compared with aggressive fluid overloading, mildly hypotensive resuscitation enhances survival rates in a rat model of hemorrhagic shock (33). It also eliminates the possibility of hyperchloremic metabolic acidosis, fluid overloading, and tissue edema, which are a cause of concern in clinical management of hemorrhagic shock (8). The fluids were warmed to ∼35°C and intravenously infused at 1 ml/min by using a syringe pump. As reported elsewhere (47), the blood pressure and blood gases were monitored through 6 h of the study. The rats were euthanized at 6 h, and immediately after euthanasia the small intestine was collected and washed in ice-cold saline. The intestine was segmented into small pieces, snap-frozen in liquid nitrogen, and stored at −80°C. On the assay day, the samples were thawed on ice and homogenized in a buffer appropriate for the assay. Similarly, the plasma samples obtained at baseline and 6 h after shock were stored at −80°C.
RNA isolation and real-time RT-PCR.
We used a Direct Zol RNA kit (Zymo Research, Irvine, CA) to extract total RNA from the gut tissues. Briefly, ∼100 mg of tissue was homogenized in the TRIzol reagent, ethanol precipitated, and separated on the columns provided with the kit. After the on-column DNase-treatment and washing, the RNA was eluted in 100 μl of RNase-free water. The average 260/280 ratio of the RNA preparations was 2.05 and the average concentration was 470 ng/μl by NanoDrop estimations. The RNA (2 μg) was used to synthesize cDNA using an iScript kit from Bio-Rad (Hercules, CA). These cDNA preparations were employed for the RT-PCR performed by SYBR Green chemistry using a Bio-Rad master mix. Each reaction was set up in triplicate wells of a 96-well plate in a total volume of ∼25 μl. The reaction mix contained ∼40 ng of total RNA (cDNA equivalents). A typical run consisted of denaturation (95°C 11.5 min), 40 repeats of amplification (95°C 15 s, 55°C 30 s, and 72°C 30 s), and 51 repeats of melting (55°C 30 s) on a Bio-Rad iCycler. The quantitative values of the genes of interest were normalized with β-actin as the endogenous reference, and the fold increase over control levels was calculated by the relative quantification method of 2−ΔΔCt. The primers for various rat genes were obtained from realtimeprimers.com or Integrated DNA Technologies (Coralville, IA).
Immunoblotting.
The protein samples were separated by denaturing gel electrophoresis and subjected to immunoblotting for the expression of phospho-HSP27, HSP27, HSP70, signal transducer and activator of transcription 1 (STAT1), phospho-STAT1, beclin-1, and light chain-3B (LC3B). Briefly, the frozen tissues were minced and incubated on ice for 30 min in ice-cold buffer consisting of 10% NP-40, 5 M NaCl, 1 M HEPES, 0.1 M ethyleneglycoltetraacetic acid, 0.5 M ethylenediaminetetraacetic acid (EDTA), 0.1 M phenylmethylsulfonyl fluoride (PMSF), 0.2 M sodium orthovanadate, 1 M NaF, 2 μg/ml aprotinin, and 2 μg/ml leupeptin. The protein was extracted by homogenization using a Dounce homogenizer and centrifugation at 18,000 g for 15 min at 4°C. The homogenates were boiled in a gel-loading dye (40% glycerol, 240 mM Tris·HCl, pH 6.8, 8% SDS, 0.04% bromophenol blue, 5% β-mercaptoethanol) and fractionated on a 10% polyacrylamide gel. The separated proteins were electrotransferred on to a nitrocellulose membrane, and the membrane was blocked with 5% fat-free milk for 1 h. The blocked membrane was probed overnight with the primary antibodies (dilution 1:500 to 1:1,000), followed by 1 h probing with horseradish peroxidase (HRP)-labeled anti-rabbit IgG secondary antibody (1:10,000). The antibodies for immunoblotting were purchased from Cell Signaling Technology (Danvers, MA). For protein loading control, the membranes were stripped and reprobed with an actin antibody (1:2,000; Santa Cruz Biotechnology, Dallas, TX).
Xanthine oxidase activity.
Xanthine oxidase activity was determined by using a Cayman fluorometric kit (Cayman Chemical, Ann Arbor, MI). Briefly, the gut tissues (∼100 mg) were homogenized in 0.2 ml of a Tris·HCl buffer (100 mM, pH 7.5) containing protease inhibitors. After centrifugation (10,000 g, 15 min at 4°C), ∼50 μl of the homogenate was used for the assay, which was performed as recommended by the kit manufacturer. Finally, the samples were read at an excitation λ of 520–550 nm and an emission λ of 585–595 nm.
Proteasome activity.
Proteasome activity was measured in the gut tissue homogenates by adapting a method described elsewhere (29). The substrates for trypsin-like, chymotrypsin-like, and caspase-like enzyme activities were Boc-Leu-Arg-Arg-AMC, Suc-Leu-Leu-Val-Tyr-AMC, and Z-Leu-Leu-Glu-AMC, respectively. Briefly, the gut tissue (100 mg) was homogenized in 400 μl of an ice-cold homogenization buffer (50 mM Tris·HCl, pH 7.5, 250 mM sucrose, 5 mM MgCl2, 2 mM ATP, 1 mM DTT, and 0.5 mM EDTA) containing 0.025% digitonin. After centrifugation at 20,000 g for 15 min (4°C), the protein concentration of supernatants was measured by Bradford assay (Bio-Rad, Hercules, CA). Approximately 10 μg protein equivalent of the tissue homogenates was incubated with 100 μl of assay buffer (50 mM Tris·HCl, pH 7.5, 40 mM KCl, 5 mM MgCl2, 0.5 mM ATP, 1 mM dithiothreitol, 0.5 mg/ml bovine serum albumin) in a 96-well plate. The fluorogenic peptides were added at 1 mM working concentration in each well and the plate was incubated at 37°C for 30 min. Fluorescence reading was taken at 380–440 nm. As assay controls, boiled homogenate (to kill the enzyme activity) and bortezomib (a known proteasome inhibitor) were used.
Carbonyl content.
We used a Cayman colorimetric kit to determine the gut tissue carbonyl content. The kit utilizes a reaction of 2,4-dinitrophenylhydrazine (DNPH) with the protein carbonyl groups to produce a yellow-colored complex. Briefly, ∼100 mg of tissue was homogenized in 200 μl of an ice-cold phosphate buffer (pH 6.7) containing 1 mM EDTA. The homogenate was cleared by centrifugation (10,000 g, 15 min at 4°C). The supernatant was treated with streptomycin sulfate and centrifuged to remove the contaminating nucleic acids. The samples were allowed to react with the DNPH reagent for 1 h in darkness before precipitation with an equal volume of 20% trichloroacetic acid. After the suggested washing steps, the protein pellets were dissolved in guanidine HCl solution supplied with the kit and the solution was read at 360–385 nm.
TBARS.
Thiobarbituric acid reactive species (TBARS) in the small gut samples was determined by using a kit from Cayman Chemical and following the manufacturer's recommendations. We monitored absorbance at 532 as well as at 450 nm because it has been shown that alkanals and alk-2-enals favor TBARS(450) formation, whereas alka-2,4-dienals favor TBARS(532) generation (56).
IFNγ ELISA.
IFNγ was estimated in the plasma and small gut tissue samples by using a rat ELISA kit (BioLegend, San Diego, CA). We used 50 μl of plasma and tissue homogenates (∼161 μg of protein). The protein concentration in homogenates was determined by a standard bicinchoninic acid method (Thermo Fisher Scientific, Waltham, MA), and the tissue IFNγ values were normalized with the protein content.
LEH preparation and characterization.
The procedure for the manufacturing of LEH has been described elsewhere (1, 47, 62). The phospholipids were purchased from Lipoid (Ludwigshafen, Germany), Avanti Polar Lipids (Alabaster, AL), or NOF (Tokyo, Japan), whereas cholesterol was obtained from Calbiochem (Gibbstown, NJ). The anionic lipid N1-(2-aminoethyl)-N4-hexadecyl-2-tetradecylsuccinamide (HDAS) was synthesized by using the methods described elsewhere (39). The outdated RBC units were sourced from Oklahoma Blood Institute (Oklahoma City, OK) for the isolation of ultrapure stroma-free hemoglobin as described elsewhere (1).
We employed Emulsiflex-C3 homogenizer (Avestin, Ottawa, ON, Canada) to homogenize a mixture of lipids and stroma-free hemoglobin. First, we made empty proliposomes of lipid composition dipalmitoylphosphatidylcholine (∼38 mol%), cholesterol (∼38 mol%), HDAS (∼20 mol%), HDAS-PEG2K (0.3 mol%), and α-tocopherol (∼2.4 mol%). The proliposomes were lyophilized to obtain a free-flowing powder. The proliposomes were gently mixed with the stroma-free hemoglobin and homogenized at 20,000 psi for four cycles at ∼20°C. The unencapsulated hemoglobin was separated from the LEH particles by tangential-flow filtration (TFF) through a 50-nm hollow fiber filter using PBS (pH 7.4) as a wash fluid. The LEH was surface modified with polyethylene glycol (PEG)-conjugated HDAS (HDAS-PEG2K) by a postinsertion method. The postinsertion method and the scheme for the synthesis of HDAS-PEG2K have been described elsewhere (1, 5, 39). Finally, the LEH was concentrated by TFF and added with human serum albumin (5% wt/vol). The final LEH preparation was stored at 4°C. All the stock and working solutions were prepared in endotoxin-free water or reagents, and the procedures were performed under aseptic conditions.
For characterization of the LEH preparation, we determined the hemoglobin content, methemoglobin %, oxygen affinity (p50), and lipid concentration by employing methods described in our previous articles (1, 47). The particle size was determined by dynamic light scattering (DLS) using a ZetaPALS particle size analyzer (Brookhaven Instruments, Holtsville, NY).
Histopathology.
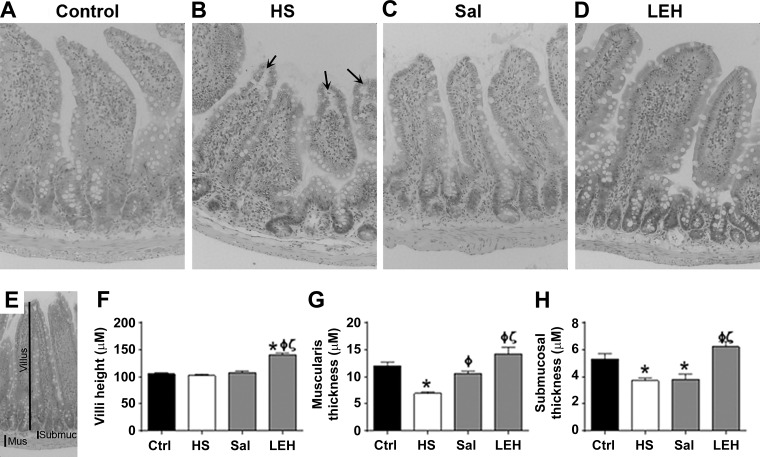
The intestinal tissues were sliced and stained with hematoxylin and eosin (H&E) by the imaging core facility of the Oklahoma Medical Research Foundation (Oklahoma City, OK). The stained slices were observed at ×200 magnification through a light microscope that was fitted with an image-capturing color camera. The captured images were evaluated by quantitative morphometry using Amira 6.1 software (FEI, Hillsboro, OR) which was cross-calibrated for spatial distance. We measured average villus height, thickness of the submucosal layer, and thickness of the muscularis layer as the morphometric parameters (25).
Data analysis.
The data are reported as means ± SE. For a comparison among four groups [control (Ctrl), HS, saline (Sal), and LEH] at the same time point, an ordinary one-way ANOVA with Bonferroni correction (single-pooled variance) was employed using Prism 6 software (GraphPad, San Diego, CA). A P value < 0.05 was considered statistically significant.
RESULTS
We studied the effect of hemorrhagic shock and subsequent isovolemic LEH or saline resuscitation on ER stress in gut tissue from a rat model of hemorrhagic shock. The gut tissues were obtained as a part of a larger study on preclinical efficacy of LEH (47). Hence, the characteristics of LEH employed in the present study and the hematological consequences of 45% blood volume loss and resuscitation are provided elsewhere (47).
LEH resuscitation reduces the oxidative stress associated with hemorrhagic shock.
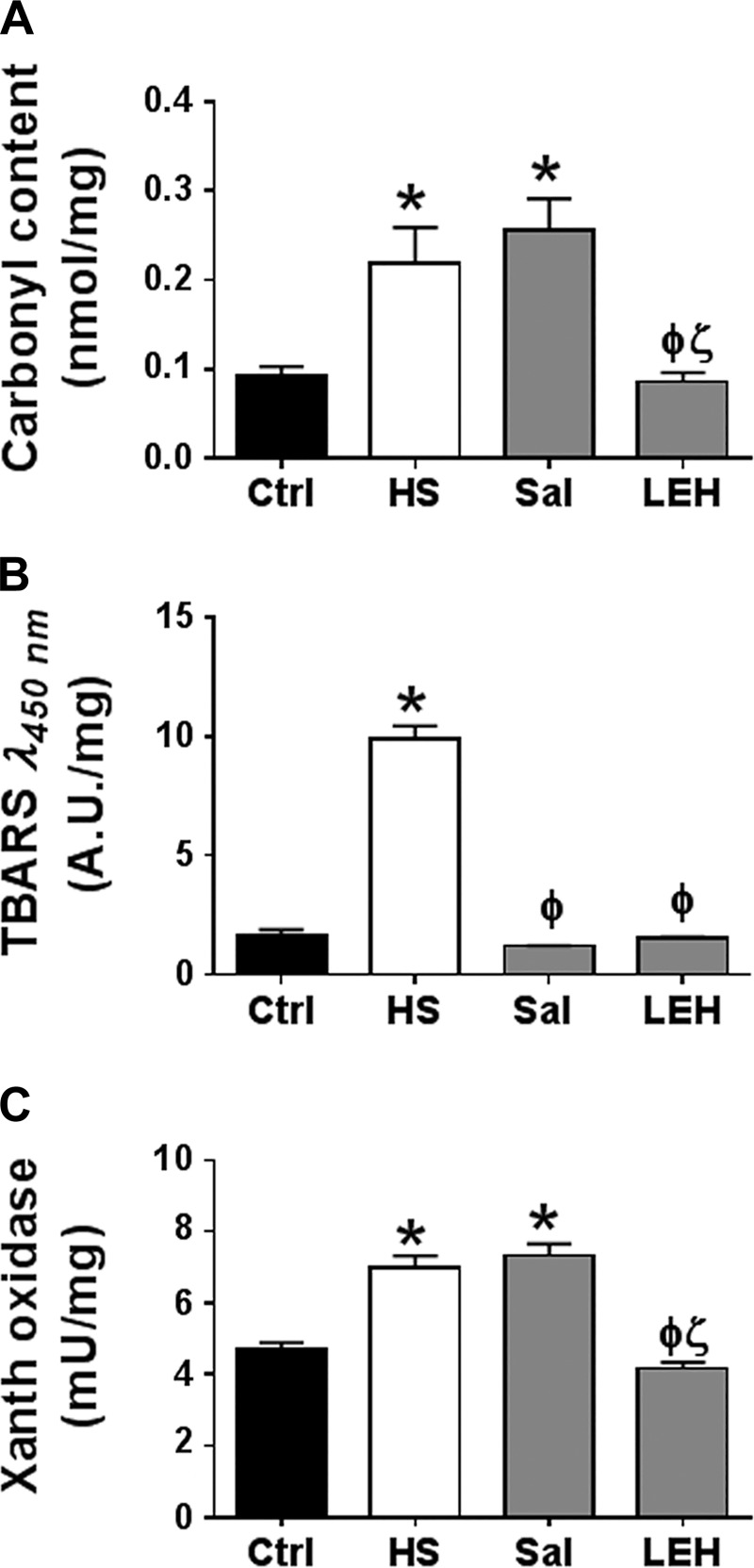
To assess the oxidative stress caused by hemorrhagic shock, we measured the carbonyl content (Fig. 1A) and TBARS (Fig. 1B) in the tissue homogenates. We found that hemorrhagic shock significantly increased the carbonyl content by ∼2.5-fold. Saline resuscitation was not able to reduce the intracellular carbonyl content, but LEH resuscitation was highly effective in reducing it to the normal levels (Fig. 1A). Hemorrhagic shock also increased the yellow TBARS absorbing at 450 nm (Fig. 1B). However, contrary to the effect on carbonyl content, both LEH and saline resuscitations were highly effective in reducing the TBARS. The typical absorbance of pink TBARS at 532 nm in our samples was found to be very weak (not shown), although it also followed the same pattern.
Fig. 1.
Effect of saline and LEH resuscitation on oxidative stress in the gut tissue of rats with hemorrhagic shock. Protein carbonyl content (A), TBARS (A.U. = arbitrary units) measured at 450 nm (B), and xanthine oxidase activity (C). The data are from at least 6 tissues from different animals per group. P < 0.05 vs. Ctrl*, HSϕ, and Salζ.
The reactive oxygen metabolites generated from a reaction catalyzed by xanthine oxidase play an important role in the pathogenesis of ischemia-induced oxidative tissue injury (58). We determined the enzyme activity of xanthine oxidase and found that hemorrhagic shock significantly increased it in the gut tissues (Fig. 1C, P < 0.05 Ctrl vs. HS). Infusion of LEH significantly suppressed the shock-induced activation of xanthine oxidase. However, saline resuscitation was not able to reduce the activity to its basal levels. The difference in the enzyme activity shown by the saline and LEH groups was statistically significant (P < 0.05 LEH vs. Sal).
LEH has no impact, but saline resuscitation drastically downregulates the markers of ER stress.
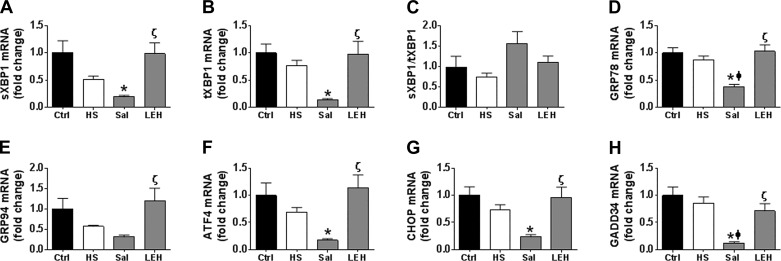
The phenomenon of ER stress is best monitored by determining the transcript levels of various players involved in the IRE1 and PERK pathways (14, 42). We examined XBP1 splicing, GRP78, and GRP94 for the IRE1 signaling, and ATF4, C/EBP homology protein (CHOP), and growth arrest and DNA-damage-inducible protein (GADD)34 for the PERK pathway. The RT-PCR data showed that sXBP1, tXBP1, GRP78, and GRP94 transcripts were reduced in HS group, but the reductions were not statistically significant (Fig. 2, A–E). However, saline resuscitation further reduced sXBP1, tXBP1, GRP78, and GRP94 levels to an extent that they were significantly lower in the saline group compared with the control group. Only GRP78 mRNA was significantly reduced in the saline group compared with the HS group. In contrast, LEH resuscitation maintained these elements at the normal levels. Since the sXBP1/tXBP1s ratio more closely correlates with IRE1 activity, we also calculated this ratio and found that there was no change in sXBP1/tXBP1s ratio among various groups (Fig. 2C). As with the elements of IRE1 signaling, we also did not observe any significant change in the transcripts of PERK-related elements ATF4, CHOP, and GADD34 in the HS and LEH groups (Fig. 2, F–H, P > 0.05, HS vs. Ctrl), but saline resuscitation resulted in further reduction in their levels (Fig. 2, F–H, P < 0.05, Sal vs. Ctrl). Importantly, the difference between the saline and LEH groups was significant for both IRE1- as well as PERK-related transcripts (P < 0.05).
Fig. 2.
Effect of saline and LEH resuscitation on the ER stress in the gut tissue of rats with hemorrhagic shock. Messenger RNA levels of spliced (s)XBP1 (A), total (t)XBP1 (B), spliced XBP1/total XBP1 ratio (C), GRP78 (D), GRP94 (E), ATF4 (F), CHOP (G), and GADD34 (H). The data are from at least 4 tissues from different animals per group. P < 0.05 vs. Ctrl*, HSϕ, and Salζ.
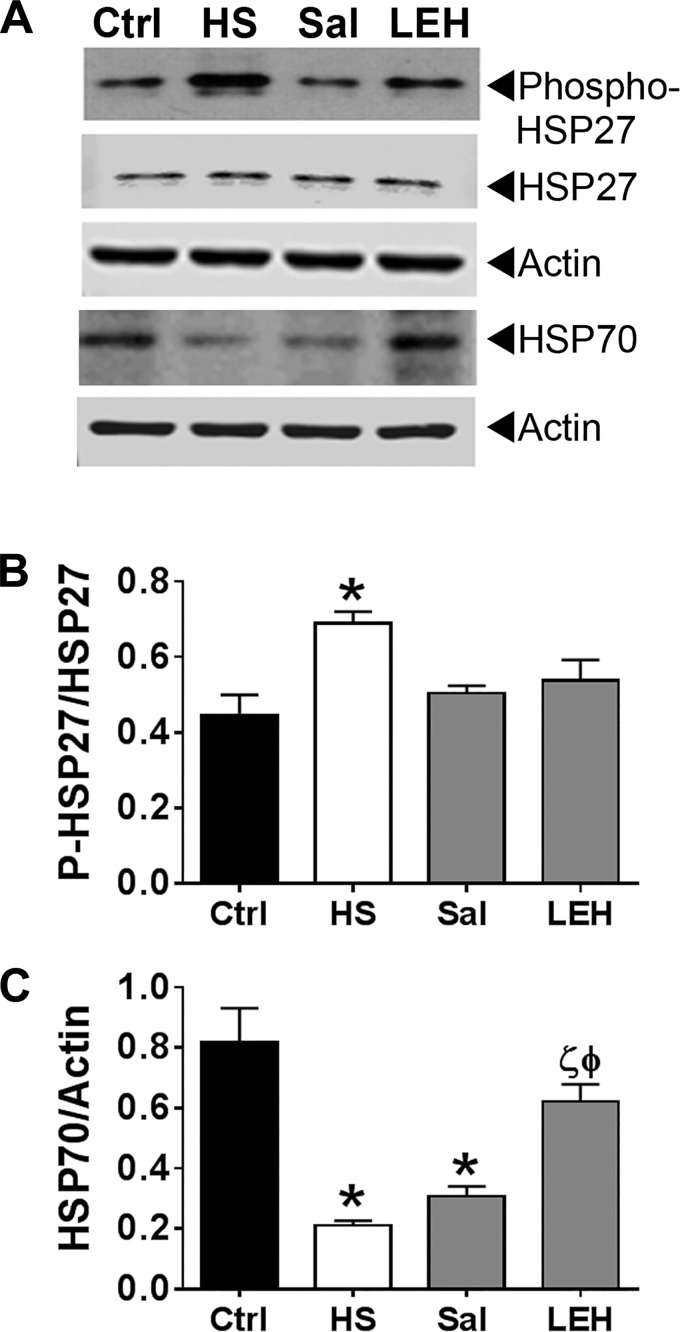
The HSP family of proteins plays an integral part in the ER-localized protein folding mechanisms. First, we monitored the expression of phospho-HSP27 by immunoblotting. Compared with the control samples, hemorrhagic shock significantly increased the phosphorylation of HSP27 without much change in the expression of pan HSP27 (Fig. 3). Both saline and LEH significantly reduced the phosphorylated HSP27, such that the phospho-HSP27 expression in these two groups was not statistically different from that in the control group. The expression of HSP70 also changed significantly in response to the shock and resuscitation. Hemorrhagic shock reduced the expression of HSP70 and the reduction was highly significant (P < 0.0008, HS vs. Ctrl). In the saline group, the HSP70 expression remained low (P < 0.003, Ctrl vs. Sal), but in the LEH group it was not different from that in the control group (P < 0.01, HS vs. LEH, and P < 0.05 Sal vs. LEH). Together with the RT-PCR data, the differential expression of chaperone proteins in gut tissues of the saline- and LEH-treated rats indicates that the two fluids affect ER stress response mechanisms in a different manner.
Fig. 3.
Expression of heat shock proteins in hemorrhagic shock and resuscitation. A: representative immunoblot showing the expression of phospho-HSP27 and HSP70. Actin was used as a loading control. B and C: densitometry of the HSP27 and HSP70 immunoblots, respectively (n = 3 per group). The immunoreactive band density was normalized with the density of actin band. P < 0.05 vs. Ctrl*, HSϕ, and Salζ. Additional immunoblots of phospho-HSP27 and HSP70 are provided in the supporting information.
LEH and saline resuscitation reduce hemorrhagic shock-induced trypsin-like proteasome activity.
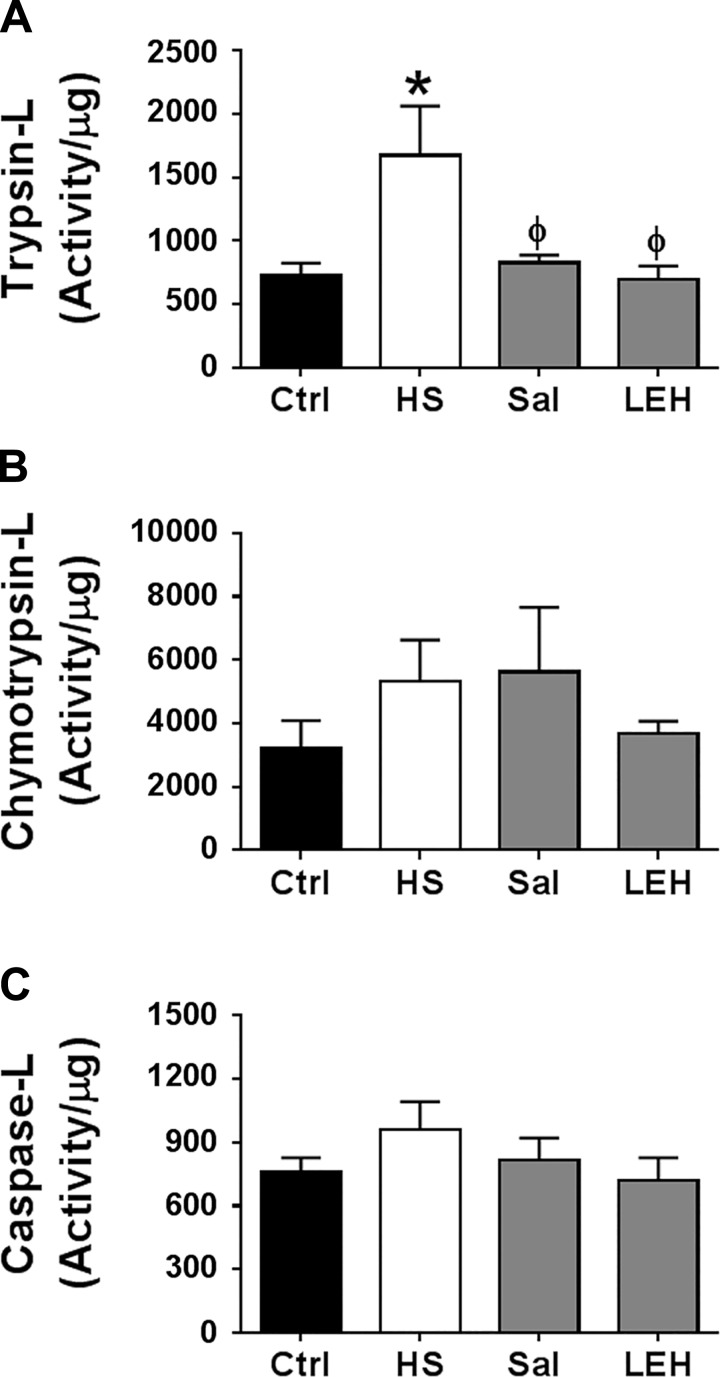
The 26S proteasome-mediated degradation pathway plays an important role in resolution of the ER stress. We examined trypsin-like (trypsin-L), chymotrypsin-like (chymotrypsin-L), and caspase-like (caspase-L) activities in small gut tissue collected after 6 h of hemorrhagic shock (Fig. 4). We found that hemorrhagic shock induced all three types of proteasome activity, but the significant impact was observed only on the trypsin-like activity, which was more than doubled after hemorrhagic shock (Fig. 4A). The effects on chymotrypsin-like and caspase-like activities were comparatively less pronounced, increasing by 1.7 and 1.3 times, respectively (Fig. 4, B and C, respectively). The treatment of hemorrhaged rats with LEH resuscitation remarkably reduced the trypsin-like activity induced by hemorrhagic shock. Saline resuscitation was also equally effective in recovering the proteasome activity to the basal levels.
Fig. 4.
Proteasome activity in the gut tissue of rats with hemorrhagic shock and resuscitated with saline or LEH. Trypsin-L (A), chymotrypsin-L (B), and caspase-L activity (C). The data are means ± SE of at least 6 samples per group. P < 0.05 vs. Ctrl* and HSϕ.
Hemorrhagic shock does not induce the immunoproteasome phenotype.
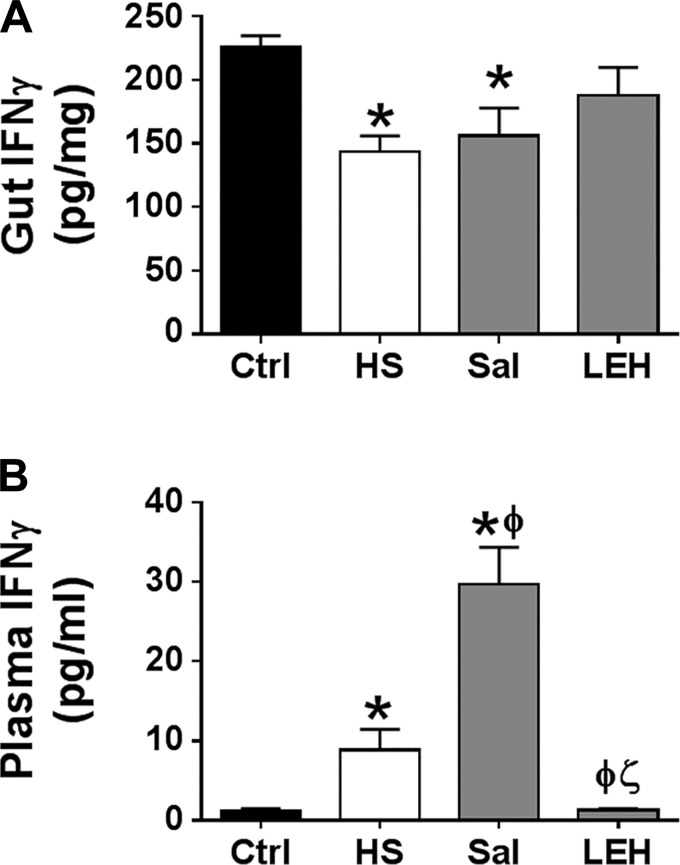
Since proinflammatory cytokine IFNγ is capable of altering the proteasome constitution to generate a highly active immunoproteasome configuration and because gut tissue has IFNγ-producing intraepithelial lymphocytes (9), we determined the concentration of IFNγ in gut tissue (Fig. 5A). We found that, in the HS and saline groups, the gut IFNγ was significantly reduced compared with the control group (P < 0.05 Ctrl vs. HS or Sal). In the LEH group, the gut IFNγ level was not different from that in the control group (226 and 187 pg/mg, respectively). These data at least negated the possibility of gut IFNγ-induced formation of immunoproteasome phenotype. In addition to the gut IFNγ, plasma-borne IFNγ may also induce cellular signaling, culminating into the formation of immunoproteasome. Therefore, we measured the IFNγ concentration in plasma. As shown in Fig. 5B, hemorrhagic shock significantly increased the plasma IFNγ levels (Ctrl 1.1 pg/ml and HS 8.9 pg/ml, P < 0.05) and LEH infusion prevented this increase in a significant manner. Interestingly, the plasma IFNγ was increased in the saline group to highly significant levels compared with the Ctrl, HS, and LEH groups.
Fig. 5.
IFNγ concentration in gut tissue (A) and plasma (B) of rats suffering from hemorrhagic shock and resuscitated with either LEH or saline. P < 0.05 vs. Ctrl*, HSϕ, and Salζ.
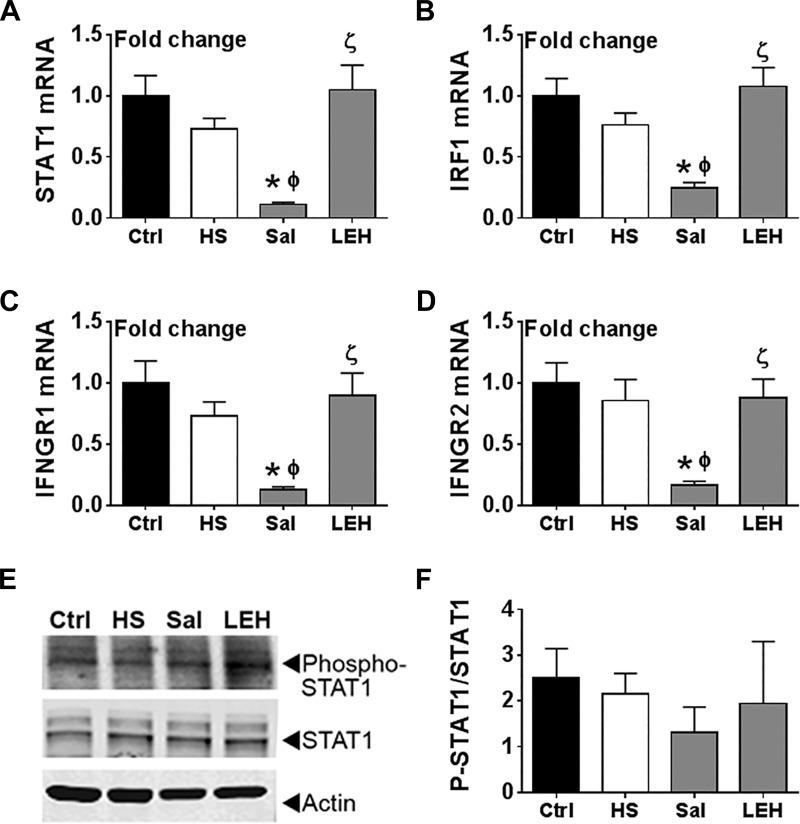
To further investigate IFNγ signaling in gut tissue, we measured the transcript levels of its downstream mediators STAT1 and IRF-1 because IFNγ binding to its receptor complex upregulates the transcription of the STAT1 and IRF-1 genes. As shown in Fig. 6, A and B, we found that the mRNA levels of STAT1 and IRF-1 were essentially unchanged after hemorrhagic shock, but after saline infusion the expression of STAT1 and IRF-1 was significantly reduced compared with both Ctrl and HS groups (P < 0.05). We also examined the expression of IFNGR1 and IFNGR2, which form the IFNγ receptor complex. As shown in Fig. 6, C and D, the expression of both IFNGR1 and IFNGR2 followed a similar profile, i.e., saline infusion led to a significant reduction in their expression compared with Ctrl and HS groups. Notably, hemorrhagic shock by itself did not change the expression of IFNGR1 and IFNGR2. Contrary to the saline group, the LEH group showed normal levels of STAT1, IRF-1, IFNGR1, and IFNGR2 expression (P < 0.05, LEH vs. Sal); the differences in STAT1 and IRF-1 levels between the saline and LEH groups were highly significant (P < 0.005). For further evidence, we assessed the phosphorylation of STAT1 protein in tissue homogenates. As shown in Fig. 6, E and F, the phosphorylation of STAT1 did not significantly change among various groups (P > 0.7 for all comparisons) but was noticeably reduced in the saline group. These results suggested that hemorrhagic shock does not induce IFNγ signaling in gut tissue, but saline infusion may perturb normal IFNγ signaling.
Fig. 6.
Effect of LEH and saline resuscitation on IFNγ signaling in the gut tissues of rats with hemorrhagic shock. Transcript levels of STAT1 (A), IRF1 (B), IFNGR1 (C), and IFNGR2 (D). The mRNA levels were determined in 4 samples per group. E: representative immunoblot showing the expression of phospho-STAT1 in gut tissue. F: densitometric ratio of phospho-STAT1 to total STAT1 expression (n = 3 per group). P < 0.05 vs. Ctrl*, HSϕ, and Salζ.
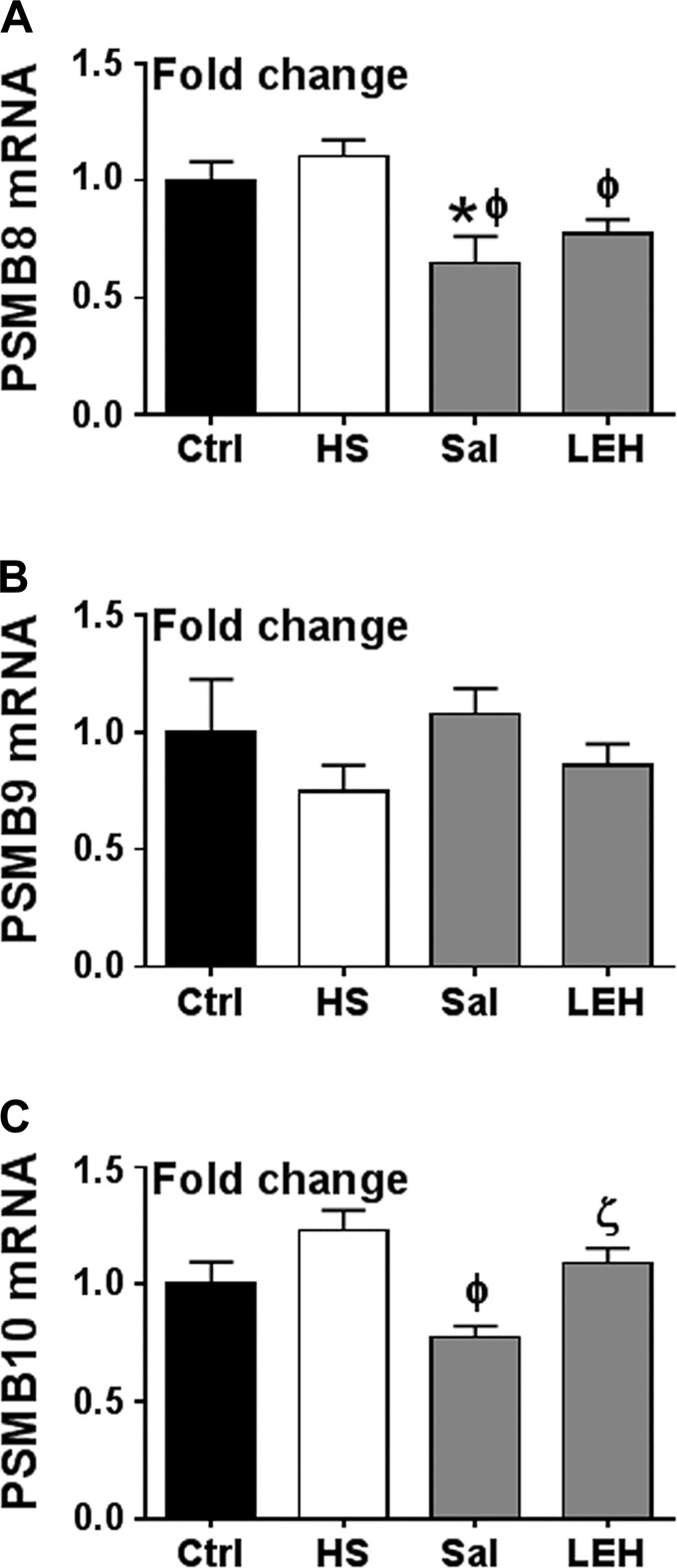
To completely rule out the possibility of hemorrhagic shock-induced immunoproteasome formation, we examined whether the increased plasma IFNγ concentration in shock results in upregulation of PSMB8, PSMB9, and PSMB10 genes, which encode the characteristic β5i, β1i, and β2i catalytic constituents of the immunoproteasome. As shown in Fig. 7, hemorrhagic shock had no effect on PSMB8, PSMB9, and PSMB10 gene transcription. In the saline group, PSMB8 was significantly reduced compared with the control level. The saline group also showed a significant difference for PSMB8 and PSMB10, but not for PSMB9 compared with the HS group. PSMB8 expression was also significantly different in the LEH group vs. the HS group. Moreover, the LEH group showed more PSMB10 mRNA than the saline group. Together with the observations made on IFNγ signaling, these results suggest that the proteasome activation in hemorrhagic shock is not associated with the formation of immunoproteasome.
Fig. 7.
Effect of LEH and saline resuscitation on the transcription of immunoproteasome genes in the gut tissues of rats with hemorrhagic shock. PSMB8 (A), PSMB9 (B), and PSMB10 (C). Each group was represented by 6 samples. P < 0.05 vs. Ctrl*, HSϕ, and Salζ.
Effect of hemorrhagic shock and resuscitation on autophagy.
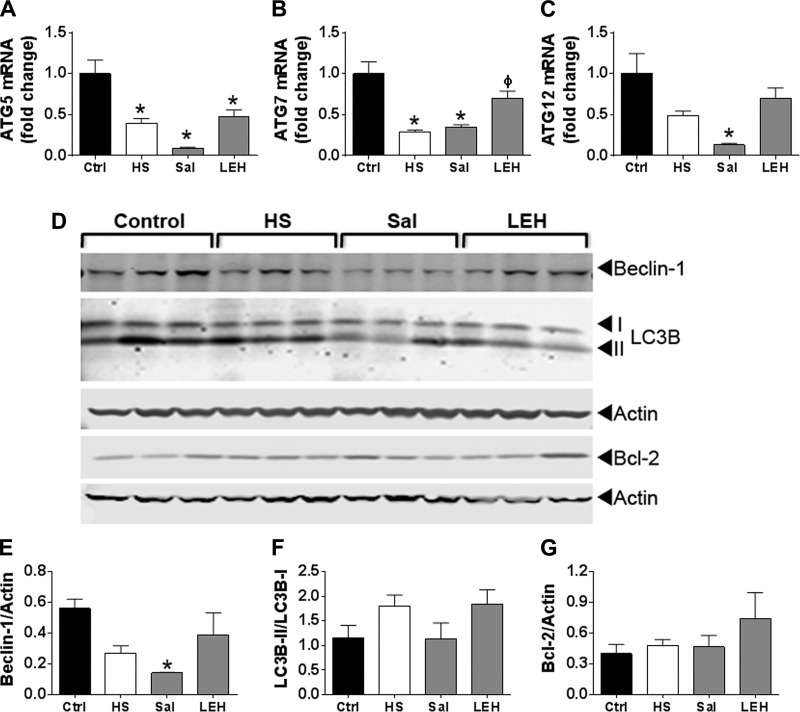
Autophagy is a companion mechanism to the UPS in cellular proteostasis. Since autophagy requires synthesis of several proteins, we first determined the mRNA levels of key autophagy genes (ATG) involved in the autophagy cascade, namely ATG5, ATG7, and ATG12. As shown in Fig. 8A, hemorrhagic shock reduced the levels of ATG transcripts, but only ATG5 and ATG7 were significantly reduced (P < 0.05). Interestingly, saline resuscitation further reduced the levels of ATG mRNAs (P < 0.05, all ATGs vs. Ctrl). In the LEH group, the ATG mRNAs increased, but only ATG7 and ATG12 reached to the control levels (P > 0.05); ATG5 mRNA remained at low levels (P < 0.05, LEH vs. Ctrl). Compared with the HS group, only ATG7 levels significantly increased in the LEH group.
Fig. 8.
Effect of LEH and saline resuscitation on autophagy in the gut tissues of rats with hemorrhagic shock. Messenger RNA levels of ATG5 (A), ATG7 (B), and ATG12 (C). Each group was represented by 4 samples. D: immunoblots showing the expression of beclin-1, LC3B, and Bcl-2 proteins. Actin was used as a loading control. E–G: densitometry of the immunoblots (n = 3 per group). P < 0.05 vs. Ctrl* and HSϕ.
We further studied the expression of beclin-1 and LC3B-II/LC3B-I ratio, which are the definitive biomarkers of autophagy. As shown in Fig. 8, D–F, beclin-1 and LC3B-II/LC3B-I ratios were not significantly altered after hemorrhagic shock or LEH resuscitation. However, the saline group showed a significant reduction in beclin-1 expression. To confirm, we studied the expression of Bcl-2, which is a major regulator of beclin-1; we did not find any change in Bcl-2 expression in various groups (Fig. 8, D and G).
Histopathology.
To assess the overall effect of LEH resuscitation on gut epithelium, we examined the tissue slices after hematoxylin and eosin staining (Fig. 9). The histopathological changes were mild but noticeable [classification 0–1 and occasional 2 under Chiu grading system (10)]. Compared with the control rats, the unresuscitated rats showed significant increase in subepithelial spaces and epithelial denudation. The histopathology in saline group was inconspicuous and was not different from the control group. The shock-induced morphological changes were accompanied by a significant decrease in the thickness of the submucosal and muscularis layers. Saline was able to restore the thickness of muscularis layer, but not the submucosal thickness. On the other hand, LEH resuscitation significantly increased the villus height and thickness of the submucosal and muscularis layers compared with the control and hemorrhaged rats.
Fig. 9.
Histopathology. Control (A), HS (B), saline (C), and LEH (D). Arrows in B point to the subepithelial spaces observed under the microscope. E: depiction of morphometry performed on light microscopic pictures; the thickness of muscularis (mus), submucosa (submuc), and villus are shown. Villus height (F), muscularis thickness (G), and submucosal thickness (H).
DISCUSSION
The two immediate consequences of severe blood loss are the volume deficit and oxygen deficit in splanchnic organs. The small intestine is especially sensitive to hypoperfusion and hypoxia because of a high energy demand and turnover of gut epithelium. The gut barrier deterioration, which starts immediately after blood loss, is regarded as a major trigger for the systemic inflammatory response to hemorrhagic shock and multiorgan failure (38, 48). Although the molecular mediators responsible for gut barrier dysfunction in hemorrhagic shock are not fully understood, a suboptimal functioning of the ER is expected because of a known tendency of proteins to misfold or remain unfolded in hypoxic conditions. Gut structural (claudins and occludin), secretory (mucin glycoprotein), as well as regulatory (HIF-1α, NF-κB, etc.) proteins depend on proper folding in the ER lumen. Given the indispensability of oxygen for protein folding, we examined the effect of artificially assembled, oxygen-carrying LEH on proteostatic mechanisms of gut epithelium in a rat model of 45% blood loss. LEH mimics RBCs in isolating hemoglobin and oxygen diffusivity (2, 51, 52), and its nanoparticulate nature enables it to traverse through narrow capillaries and efficiently oxygenate the splanchnic vascular bed. As such, resuscitation with LEH has been shown to maintain hemodynamics and microvascular circulation in the preclinical models of hemorrhagic shock (6, 50).
A successful resuscitation would also reduce stress at cellular level. Nutrient and oxygen deprivation associated with hemorrhagic shock causes the ER stress secondary to a buildup of unfolded or misfolded proteins. The concentration of these damaged proteins can exceed the capacity of chaperones available in the ER to handle them. The UPR is a cellular mechanism to mitigate this ER stress, and it functions in close coordination with the UPS and autophagy to maintain homeostasis (7). In addition to the misfolding of nascent proteins, the ischemic stress may also oxidatively damage the existing proteins resulting in unfolding, misfolding, or acquisition of unstructured regions (46). In our model, the stress caused by hemorrhagic shock resulted in elevated protein oxidation (carbonyl content) and lipid peroxidation (TBARS) in the gut tissue (Fig. 1). LEH was able to reduce both protein and lipid oxidation, whereas saline could only reduce the lipid oxidation. The different effect of saline and LEH on oxidatively damaged proteins suggests that the two fluids would manifest different effects on the ER stress and the UPR. The gut UPR signaling is complex because of its constitutive role in healthy intestinal tissue; the differentiated epithelial cells exhibit higher levels of ER stress than the stem cells in the crypts (22). The UPR transcription factor XBP1 is also involved in the maintenance of secretory cell lineages and its deletion in intestinal epithelial cells has been shown to cause inflammation (27). In addition, because of a high rate of protein synthesis and secretory activity, the small intestine maintains a basal level of UPR to prevent accumulation of misfolded proteins on routine basis. This constitutive UPR resolves the transient stress originating from the normal functioning of cells. We found that in the unresuscitated rats (HS group), the levels of PERK-related UPR indicators (CHOP, GADD34, and ATF4) as well as IRE1-related indicators (sXBP1, GRP78, and GRP94) were not different from those in the control group (Fig. 2). This observation is different from a published study in which ischemia-reperfusion was shown to increase the expression of sXBP1, CHOP, and GADD34 in jejunum samples collected after 1.5 h (18). This discrepancy is most likely due to the difference in tissue collection times (1.5 h vs. our 6 h), because it is quite possible that the UPR is activated early after blood loss and reverts to basal levels during later time points upon resolution of the ER stress. Interestingly, saline infusion, but not LEH, drastically reduced the UPR indicators below the basal levels. This effect of saline could be due to its inability to assist in tissue oxygenation; instead, saline may aggravate the shock-induced tissue hypoxia because of hemodilution. The shutdown of the UPR in saline group appears to be so severe that it hindered the new synthesis of transcription factor encoded by spliced XBP1, resulting in low GRP78 and GRP94 levels, which are necessary for recovery. Interestingly, a sustained stress or a stress due to multiple factors may interfere in the execution of the UPR program (44). For instance, a double stress of primary neuronal cultures by oxidative stress and Ca2+ depletion completely blocked the transcription of GRP78, GRP94, and CHOP (44).
The ER stress marker GRP78 belongs to the ER-resident HSP70 family of proteins (20). The HSP70s (also known as foldases) are involved in the folding and assembly of newly synthesized proteins, refolding of misfolded and aggregated proteins, and intracellular transit of proteins (36). HSP70 is seen as an antiapoptotic protein because it interacts with the intrinsic and extrinsic pathways of apoptosis at several junctions and inhibits cell death through chaperone-dependent as well as -independent activities (12). Unlike HSP70, HSP27 is a small heat shock protein of the holdase class that provides a high-affinity binding platform for unfolded proteins and prevents protein aggregation during stress (11). ER stress has been shown to phosphorylate HSP27 in a cell-dependent manner (23). The activated HSP27 traps the client proteins to prevent their aggregation and delivers them to the foldases (e.g., HSP 70) for refolding. In this context, normal expression of HSP27 and HSP70 in LEH group is a desired outcome in our model of hemorrhagic shock (Fig. 3); however, saline failed to maintain the HSP70 expression. The cellular attempts to refold damaged proteins are not always successful, and the UPR manages the irreversibly damaged proteins by involving the UPS (60). The workhorse of the UPS is the 26S proteasome, which catalyzes the degradation of proteins in an ubiquitin- and ATP-dependent manner. We found that hemorrhagic shock resulted in an increase in the trypsin-L proteasome activity (Fig. 4). Both saline and LEH infusions were able to reduce the shock-induced trypsin-L activity. Since the levels of oxidized protein in saline-infused rats remained significantly high (Fig. 1), it seems that the proteasome function was decoupled from the oxidative stress in these saline-treated animals. Surprisingly, we did not observe any change in the accumulation of ubiquitinated proteins in gut tissue from various groups (immunoblot data not shown), which indicates a greater role of the ubiquitin-independent 20S proteasome in hemorrhagic shock. Elsewhere, others have shown that severe oxidative stress, depletion of the ATP content, or exhaustion of the intracellular NADH result in a disassembly of the 26S proteasome and the liberated 20S core unit functions to degrade proteins in an unregulated fashion (13, 35, 59).
The constitutive catalytic subunits of the 20S core particle of the proteasome, namely β1, β2, and β5, are replaced by β1i, β2i, and β5i subunits under the influence of proinflammatory cytokines, especially IFNγ. The resultant phenotype of immunoproteasome is a highly active configuration of proteasome (30). Like in a published report (16), we found that plasma IFNγ was increased in the hemorrhaged rats. To further examine whether the plasma IFNγ results in an induction of the gut IFNγ signaling, we investigated the expression of elements involved in this pathway. The gut IFNγ signaling involves a receptor complex consisting of IFNGR1 and IFNGR2 subunits (11). Upon stimulation, the IFNGR complex activates the receptor-associated Janus kinases JAK1 and JAK2 and the transcription factor STAT1 in a phosphorylation-dependent manner. The phosphorylated and homodimerized STAT1 binds, together with IFN regulatory factor-1 (IRF-1), to the STAT-binding element and induces β1i, β2i, and β5i genes (17). This IFNγ signaling also upregulates the transcription of STAT1 and IRF-1 (32). Given that hemorrhagic shock had no effect on the transcriptional expression of STAT1, IRF-1, IFNGR1, and IFNGR2 genes (Fig. 6), we hypothesize that the enhanced proteasome activity in the small gut from hemorrhaged rats is not through the activation of IFNγ signaling. Our hypothesis is supported by the different effects of saline and LEH on the expression of STAT1, IRF1, and IFNGR mRNAs (Fig. 6), though both were effective in reducing the proteasome activity (Fig. 4). Moreover, the content of IFNγ in gut tissue was not increased by the shock. On the contrary, the gut IFNγ was reduced in the HS and saline groups. This reduction in gut IFNγ could be due to the loss of gut lymphocytes to apoptosis caused by ischemia. The recoverable lymphocytes from the lamina propria, Peyer's patches, and intraepithelial space are known to be drastically reduced by gut ischemia-reperfusion (15). Yet another indication of the noninvolvement of immunoproteasome came from the lack of induction of β1i, β2i, and β5i transcripts in HS group.
The UPR generated by a cell under stress is also associated with autophagy. It is a catabolic process in which the cytoplasmic contents are sequestered within the autophagolysosomes and degraded in the lysosomes. The autophagic process is especially upregulated by nutrient deprivation. The data in Fig. 8 reveal that hemorrhagic shock and the type of fluid used for resuscitation have significant impact on the expression of ATGs, which is consistent with the report that the UPR players IRE-1, ATF4, and CHOP control several ATG genes (53). The proteins encoded by the conserved ATGs are the core machinery in the autophagy process. An electrophoretic mobility shift of ATG8 orthologue LC3B and a change in expression levels of ATG6 (beclin-1) are regarded as the accepted markers for autophagy. However, the expression of these markers of autophagy was not altered after hemorrhagic shock. The saline group, however, showed significant reduction in beclin-1 expression and a substantial reduction in LC3BII/LCB3I ratio in two-thirds of the rats. Earlier, Yoo et al. (63) have reported that hypoxia downregulates beclin-1 in malignant intestinal epithelial cells. Since autophagy is responsible for removing the misfolded proteins that either form aggregates or are not efficiently processed by the UPS, the suppression of autophagy by saline resuscitation acquires significance, especially when the saline group also showed higher levels of oxidized proteins (Fig. 1). The major link between autophagy and the intestinal barrier function are the Paneth cells. The Paneth cells are highly specialized in the production, packaging and secretion of antimicrobial peptides (AMPs) such as lysozymes. A loss of ATGs has been found to result in defective packaging and secretion of AMPs (45).
Overall, the data from this study show that LEH resuscitation was more effective than saline resuscitation in normalizing the indicators of ER stress and UPR that are perturbed in small gut by hemorrhagic shock. Unlike the LEH-treated rats, the saline-resuscitated group appeared to demonstrate a decoupling between the oxidative stress and UPR signaling. We speculate that the suppressed UPR response after saline infusion may result in an increase in gut epithelial cell death. This speculation is in accordance with the observations that genetic deletion of the UPR factors results in spontaneous intestinal inflammation (26) and a prolonged or unresolved ER stress culminates in cell death (21). We attribute these differences between LEH and saline to the fact that saline only replaces the volume lost in hemorrhagic shock, whereas LEH is also capable of carrying and delivering oxygen to the hypoxic tissue. This capacity of LEH has been shown by molecular imaging to beneficially impact the cerebral oxygen delivery, tissue energetic, and metabolism after hemorrhagic shock (3, 4, 47). Another significant observation in the present study was the IFNγ-independent increase in trypsin-L proteasome activity after hemorrhagic shock. Although the molecular reason for this phenomenon is unclear, we suspect a role of reduced availability of tissue ATP and/or NADH after severe blood loss. Elsewhere, depletion of ATP or NADH has been shown to result in the formation of more active 20S proteasome (35, 59). More work is needed to unravel this pathway in hemorrhagic shock.
GRANTS
The work was supported by National Heart, Lung, and Blood Institute Grant R01HL104286.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
G.R., V.R.Y., and V.A. performed experiments; G.R. and V.A. analyzed data; G.R., S.A., P.R.R., and V.A. interpreted results of experiments; G.R., V.R.Y., and V.A. prepared figures; G.R., P.R.R., and V.A. drafted manuscript; G.R., S.A., P.R.R., and V.A. edited and revised manuscript; G.R., V.R.Y., S.A., P.R.R., and V.A. approved final version of manuscript; V.A. conception and design of research.
ACKNOWLEDGMENTS
The authors acknowledge the technical help from Andria Hedrick, Research Associate in the imaging facility of the OUHSC-College of Pharmacy, Oklahoma City.
REFERENCES
- 1.Agashe H, Lagisetty P, Awasthi S, Awasthi V. Improved formulation of liposome-encapsulated hemoglobin with an anionic non-phospholipid. Colloids Surf B Biointerfaces 75: 573–583, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Awasthi V. Pharmaceutical aspects of hemoglobin-based oxygen carriers. Curr Drug Deliv 2: 133–142, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Awasthi V, Agashe H, Doblas S, Towner R. Magnetic resonance spectroscopy for evaluation of liposome-encapsulated hemoglobin as a resuscitation fluid. Artif Cells Blood Substit Immobil Biotechnol 38: 69–78, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Awasthi V, Yee SH, Jerabek P, Goins B, Phillips WT. Cerebral oxygen delivery by liposome-encapsulated hemoglobin: a positron-emission tomographic evaluation in a rat model of hemorrhagic shock. J Appl Physiol 103: 28–38, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Awasthi VD, Garcia D, Klipper R, Goins BA, Phillips WT. Neutral and anionic liposome-encapsulated hemoglobin: effect of postinserted poly(ethylene glycol)-distearoylphosphatidylethanolamine on distribution and circulation kinetics. J Pharmacol Exp Ther 309: 241–248, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Awasthi VD, Garcia D, Klipper R, Phillips WT, Goins BA. Kinetics of liposome-encapsulated hemoglobin after 25% hypovolemic exchange transfusion. Int J Pharm 283: 53–62, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Benbrook DM, Long A. Integration of autophagy, proteasomal degradation, unfolded protein response and apoptosis. Exp Oncol 34: 286–297, 2012. [PubMed] [Google Scholar]
- 8.Besen BA, Gobatto AL, Melro LM, Maciel AT, Park M. Fluid and electrolyte overload in critically ill patients: an overview. World J Crit Care Med 4: 116–129, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Capaldo CT, Nusrat A. Cytokine regulation of tight junctions. Biochim Biophys Acta 1788: 864–871, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg 101: 478–483, 1970. [DOI] [PubMed] [Google Scholar]
- 11.Chowdhury SR, King DE, Willing BP, Band MR, Beever JE, Lane AB, Loor JJ, Marini JC, Rund LA, Schook LB, Van Kessel AG, Gaskins HR. Transcriptome profiling of the small intestinal epithelium in germfree versus conventional piglets. BMC Genomics 8: 215, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davenport EL, Moore HE, Dunlop AS, Sharp SY, Workman P, Morgan GJ, Davies FE. Heat shock protein inhibition is associated with activation of the unfolded protein response pathway in myeloma plasma cells. Blood 110: 2641–2649, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Davies KJ. Degradation of oxidized proteins by the 20S proteasome. Biochimie 83: 301–310, 2001. [DOI] [PubMed] [Google Scholar]
- 14.DeGracia DJ, Montie HL. Cerebral ischemia and the unfolded protein response. J Neurochem 91: 1–8, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Fukatsu K, Sakamoto S, Hara E, Ueno C, Maeshima Y, Matsumoto I, Mochizuki H, Hiraide H. Gut ischemia-reperfusion affects gut mucosal immunity: a possible mechanism for infectious complications after severe surgical insults. Crit Care Med 34: 182–187, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Griffin TA, Nandi D, Cruz M, Fehling HJ, Kaer LV, Monaco JJ, Colbert RA. Immunoproteasome assembly: cooperative incorporation of interferon gamma (IFN-gamma)-inducible subunits. J Exp Med 187: 97–104, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grimm S, Ott C, Horlacher M, Weber D, Hohn A, Grune T. Advanced-glycation-end-product-induced formation of immunoproteasomes: involvement of RAGE and Jak2/STAT1. Biochem J 448: 127–139, 2012. [DOI] [PubMed] [Google Scholar]
- 18.Grootjans J, Hodin CM, de Haan JJ, Derikx JP, Rouschop KM, Verheyen FK, van Dam RM, Dejong CH, Buurman WA, Lenaerts K. Level of activation of the unfolded protein response correlates with Paneth cell apoptosis in human small intestine exposed to ischemia/reperfusion. Gastroenterology 140: 529–539 e523, 2011. [DOI] [PubMed] [Google Scholar]
- 19.Groschwitz KR, Hogan SP. Intestinal barrier function: molecular regulation and disease pathogenesis. J Allergy Clin Immunol 124: 3–20 quiz 21–22, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haas IG. BiP (GRP78), an essential hsp70 resident protein in the endoplasmic reticulum. Experientia 50: 1012–1020, 1994. [DOI] [PubMed] [Google Scholar]
- 21.Harding HP, Calfon M, Urano F, Novoa I, Ron D. Transcriptional and translational control in the Mammalian unfolded protein response. Annu Rev Cell Dev Biol 18: 575–599, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Heijmans J, van Lidth de Jeude JF, Koo BK, Rosekrans SL, Wielenga MC, van de Wetering M, Ferrante M, Lee AS, Onderwater JJ, Paton JC, Paton AW, Mommaas AM, Kodach LL, Hardwick JC, Hommes DW, Clevers H, Muncan V, van den Brink GR. ER stress causes rapid loss of intestinal epithelial stemness through activation of the unfolded protein response. Cell Rep 3: 1128–1139, 2013. [DOI] [PubMed] [Google Scholar]
- 23.Ito H, Iwamoto I, Inaguma Y, Takizawa T, Nagata K, Asano T, Kato K. Endoplasmic reticulum stress induces the phosphorylation of small heat shock protein, Hsp27. J Cell Biochem 95: 932–941, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Jung T, Hohn A, Grune T. The proteasome and the degradation of oxidized proteins: Part II — protein oxidation and proteasomal degradation. Redox Biol 2: 99–104, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kallakuri S, Ascher E, Pagala M, Gade P, Hingorani A, Scheinman M, Mehraein K, Jacob T. Protective effect of glycine in mesenteric ischemia and reperfusion injury in a rat model. J Vasc Surg 38: 1113–1120, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Kaser A, Adolph TE, Blumberg RS. The unfolded protein response and gastrointestinal disease. Semin Immunopathol 35: 307–319, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaser A, Lee AH, Franke A, Glickman JN, Zeissig S, Tilg H, Nieuwenhuis EE, Higgins DE, Schreiber S, Glimcher LH, Blumberg RS. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell 134: 743–756, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaufman RJ, Scheuner D, Schroder M, Shen X, Lee K, Liu CY, Arnold SM. The unfolded protein response in nutrient sensing and differentiation. Nat Rev Mol Cell Biol 3: 411–421, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Kisselev AF, Goldberg AL. Monitoring activity and inhibition of 26S proteasomes with fluorogenic peptide substrates. Methods Enzymol 398: 364–378, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Kuehn L, Dahlmann B. Structural and functional properties of proteasome activator PA28. Mol Biol Rep 24: 89–93, 1997. [DOI] [PubMed] [Google Scholar]
- 31.Lairet JR, Bebarta VS, Burns CJ, Lairet KF, Rasmussen TE, Renz EM, King BT, Fernandez W, Gerhardt R, Butler F, DuBose J, Cestero R, Salinas J, Torres P, Minnick J, Blackbourne LH. Prehospital interventions performed in a combat zone: a prospective multicenter study of 1,003 combat wounded. J Trauma Acute Care Surg 73: S38–S42, 2012. [DOI] [PubMed] [Google Scholar]
- 32.Lehtonen A, Matikainen S, Julkunen I. Interferons up-regulate STAT1, STAT2, and IRF family transcription factor gene expression in human peripheral blood mononuclear cells and macrophages. J Immunol 159: 794–803, 1997. [PubMed] [Google Scholar]
- 33.Li T, Zhu Y, Fang Y, Liu L. Determination of the optimal mean arterial pressure for postbleeding resuscitation after hemorrhagic shock in rats. Anesthesiology 116: 103–112, 2012. [DOI] [PubMed] [Google Scholar]
- 34.Mahambrey T, Pendry K, Nee A, Bonney S, Nee PA. Critical care in emergency department: massive haemorrhage in trauma. Emerg Med J 30: 9–14, 2013. [DOI] [PubMed] [Google Scholar]
- 35.Majetschak M. Regulation of the proteasome by ATP: implications for ischemic myocardial injury and donor heart preservation. Am J Physiol Heart Circ Physiol 305: H267–H278, 2013. [DOI] [PubMed] [Google Scholar]
- 36.Mayer MP, Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci 62: 670–684, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McGuckin MA, Eri RD, Das I, Lourie R, Florin TH. ER stress and the unfolded protein response in intestinal inflammation. Am J Physiol Gastrointest Liver Physiol 298: G820–G832, 2010. [DOI] [PubMed] [Google Scholar]
- 38.Moore FA. The role of the gastrointestinal tract in postinjury multiple organ failure. Am J Surg 178: 449–453, 1999. [DOI] [PubMed] [Google Scholar]
- 39.Nag OK, Yadav VR, Hedrick A, Awasthi V. Post-modification of preformed liposomes with novel non-phospholipid poly(ethylene glycol)-conjugated hexadecylcarbamoylmethyl hexadecanoic acid for enhanced circulation persistence in vivo. Int J Pharm 446: 119–129, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Niederreiter L, Kaser A. Endoplasmic reticulum stress and inflammatory bowel disease. Acta Gastroenterol Belg 74: 330–333, 2011. [PubMed] [Google Scholar]
- 41.Niforou K, Cheimonidou C, Trougakos IP. Molecular chaperones and proteostasis regulation during redox imbalance. Redox Biol 2: 323–332, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oslowski CM, Urano F. Measuring ER stress and the unfolded protein response using mammalian tissue culture system. Methods Enzymol 490: 71–92, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paschen W. Shutdown of translation: lethal or protective? Unfolded protein response versus apoptosis. J Cereb Blood Flow Metab 23: 773–779, 2003. [DOI] [PubMed] [Google Scholar]
- 44.Paschen W, Mengesdorf T, Althausen S, Hotop S. Peroxidative stress selectively down-regulates the neuronal stress response activated under conditions of endoplasmic reticulum dysfunction. J Neurochem 76: 1916–1924, 2001. [DOI] [PubMed] [Google Scholar]
- 45.Patel KK, Stappenbeck TS. Autophagy and intestinal homeostasis. Annu Rev Physiol 75: 241–262, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pickering AM, Davies KJ. Degradation of damaged proteins: the main function of the 20S proteasome. Prog Mol Biol Transl Sci 109: 227–248, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rao G, Hedrick AF, Yadav VR, Xie J, Hussain A, Awasthi V. The brain metabolic activity after resuscitation with liposome-encapsulated hemoglobin in a rat model of hypovolemic shock. J Cereb Blood Flow Metab 35: 1528–1536, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rhodes RS, Depalma RG, Robinson AV. Intestinal barrier function in hemorrhagic shock. J Surg Res 14: 305–312, 1973. [DOI] [PubMed] [Google Scholar]
- 49.Rotstein OD. Pathogenesis of multiple organ dysfunction syndrome: gut origin, protection, and decontamination. Surg Infect (Larchmt) 1: 217–223; discussion 223–215, 2000. [DOI] [PubMed] [Google Scholar]
- 50.Sakai H, Masada Y, Horinouchi H, Yamamoto M, Ikeda E, Takeoka S, Kobayashi K, Tsuchida E. Hemoglobin-vesicles suspended in recombinant human serum albumin for resuscitation from hemorrhagic shock in anesthetized rats. Crit Care Med 32: 539–545, 2004. [DOI] [PubMed] [Google Scholar]
- 51.Sakai H, Sou K, Horinouchi H, Kobayashi K, Tsuchida E. Review of hemoglobin-vesicles as artificial oxygen carriers. Artif Organs 33: 139–145, 2009. [DOI] [PubMed] [Google Scholar]
- 52.Sakai H, Takeoka S, Wettstein R, Tsai AG, Intaglietta M, Tsuchida E. Systemic and microvascular responses to hemorrhagic shock and resuscitation with Hb vesicles. Am J Physiol Heart Circ Physiol 283: H1191–H1199, 2002. [DOI] [PubMed] [Google Scholar]
- 53.Senft D, Ronai ZA. UPR, autophagy, and mitochondria crosstalk underlies the ER stress response. Trends Biochem Sci 40: 141–148, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Senthil M, Brown M, Xu DZ, Lu Q, Feketeova E, Deitch EA. Gut-lymph hypothesis of systemic inflammatory response syndrome/multiple-organ dysfunction syndrome: validating studies in a porcine model. J Trauma 60: 958–965; discussion 965–9572006. [DOI] [PubMed] [Google Scholar]
- 55.Stollings JL, Oyen LJ. Oxygen therapeutics: oxygen delivery without blood. Pharmacotherapy 26: 1453–1464, 2006. [DOI] [PubMed] [Google Scholar]
- 56.Sun Q, Faustman C, Senecal A, Wilkinson AL, Furr H. Aldehyde reactivity with 2-thiobarbituric acid and TBARS in freeze-dried beef during accelerated storage. Meat Sci 57: 55–60, 2001. [DOI] [PubMed] [Google Scholar]
- 57.Tagliavacca L, Caretti A, Bianciardi P, Samaja M. In vivo up-regulation of the unfolded protein response after hypoxia. Biochim Biophys Acta 1820: 900–906, 2012. [DOI] [PubMed] [Google Scholar]
- 58.Tan S, Yokoyama Y, Dickens E, Cash TG, Freeman BA, Parks DA. Xanthine oxidase activity in the circulation of rats following hemorrhagic shock. Free Radic Biol Med 15: 407–414, 1993. [DOI] [PubMed] [Google Scholar]
- 59.Tsvetkov P, Myers N, Eliav R, Adamovich Y, Hagai T, Adler J, Navon A, Shaul Y. NADH binds and stabilizes the 26S proteasomes independent of ATP. J Biol Chem 289: 11272–11281, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vembar SS, Brodsky JL. One step at a time: endoplasmic reticulum-associated degradation. Nat Rev Mol Cell Biol 9: 944–957, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Voges D, Zwickl P, Baumeister W. The 26S proteasome: a molecular machine designed for controlled proteolysis. Annu Rev Biochem 68: 1015–1068, 1999. [DOI] [PubMed] [Google Scholar]
- 62.Yadav VR, Nag O, Awasthi V. Biological evaluation of liposome-encapsulated hemoglobin surface-modified with a novel PEGylated nonphospholipid amphiphile. Artif Organs 38: 625–633, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yoo BH, Wu X, Derouet M, Haniff M, Eskelinen EL, Rosen K. Hypoxia-induced downregulation of autophagy mediator Beclin 1 reduces the susceptibility of malignant intestinal epithelial cells to hypoxia-dependent apoptosis. Autophagy 5: 1166–1179, 2009. [DOI] [PubMed] [Google Scholar]