Abstract
Exposure to cigarette smoke is known to result in impaired host defense responses and immune suppressive effects. However, the effects of new and emerging tobacco products, such as e-cigarettes, on the immune status of the respiratory epithelium are largely unknown. We conducted a clinical study collecting superficial nasal scrape biopsies, nasal lavage, urine, and serum from nonsmokers, cigarette smokers, and e-cigarette users and assessed them for changes in immune gene expression profiles. Smoking status was determined based on a smoking history and a 3- to 4-wk smoking diary and confirmed using serum cotinine and urine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) levels. Total RNA from nasal scrape biopsies was analyzed using the nCounter Human Immunology v2 Expression panel. Smoking cigarettes or vaping e-cigarettes resulted in decreased expression of immune-related genes. All genes with decreased expression in cigarette smokers (n = 53) were also decreased in e-cigarette smokers. Additionally, vaping e-cigarettes was associated with suppression of a large number of unique genes (n = 305). Furthermore, the e-cigarette users showed a greater suppression of genes common with those changed in cigarette smokers. This was particularly apparent for suppressed expression of transcription factors, such as EGR1, which was functionally associated with decreased expression of 5 target genes in cigarette smokers and 18 target genes in e-cigarette users. Taken together, these data indicate that vaping e-cigarettes is associated with decreased expression of a large number of immune-related genes, which are consistent with immune suppression at the level of the nasal mucosa.
Keywords: e-cigarettes, nasal epithelial cells, gene expression
exposure to cigarette smoke (CS), via active smoking or second-hand smoke (SHS) exposure, continues to be the number one cause of preventable mortality and morbidity worldwide (48a). A large body of clinical and laboratory data supports a significant relationship between CS exposure, immune suppression, and increased risk for respiratory viral or bacterial infection. Even in otherwise healthy subjects, smoking or SHS exposure is associated with enhanced susceptibility to microbial infections as well as enhanced infection-associated severity and morbidity (25, 26). Smoking broadly suppresses multiple host defense mechanisms including epithelial cell responses and recruitment and activation of innate immune cells, such as neutrophils, macrophages, and NK cells. Our previous work shows that, in the context of viral infections, CS exposure modifies the ability of epithelial cells to mount an effective type I interferon response, produce cytokines/chemokines necessary for immune cell activation, and recruit and activate resident immune cells, effectively compromising innate immune host defense responses (14–16, 24, 34, 35, 38).
While smoking rates continue to decline in the United States, the number of e-cigarette users is on the rise. Both the CDC and the FDA consider e-cigarettes as tobacco products, and the recently passed “Deeming Rule” deems e-cigarettes as “tobacco products to be subject to the Federal Food, Drug, and Cosmetic Act” (5, 9). However, e-cigarettes are often advertised as “less harmful” than conventional cigarettes and the effects that vaping e-cigarettes may have on respiratory mucosal immune responses are completely unknown. A few animal studies suggest that inhalation of e-cigarette vapor increases susceptibility to viral and microbial infections (47) or enhances bacterial growth/biofilm formation (20). E-cigarette vapor is a complex mixture derived by aerosolizing e-liquids composed of nicotine, flavoring agents, and humectants, such as propylene glycol and vegetable glycerin. Vaporizing e-liquids, especially at higher temperatures than recommended by the manufacturer, may result in the generation of known pulmonary toxicants such as formaldehyde, acetaldehyde, and acrolein (10, 11). In addition, some of the potential flavorings contained in e-cigarettes, such as diacetyl or benzaldehyde, have known adverse respiratory effects (2, 13, 23, 27, 50). However, the effects of e-cigarettes on innate immune responses in the respiratory mucosa of humans are unknown.
In an initial effort to examine the effects of e-cigarettes on human respiratory innate immune responses, we designed a clinical study collecting nasal scrape biopsies from human smokers, nonsmokers, and e-cigarette users to examine differences in immune gene expression at the level of the epithelium. Previous studies have shown that exposure-related gene expression changes in airway epithelial biopsy samples are potential biomarkers of disease or underlying adverse health effects (3, 4, 42–45). Changes in mRNA expression levels can indicate disturbances in cellular metabolic pathways leading to cell death or disease and as such, are valuable predictors of exposure and/or xenobiotic toxicity. In addition, smoking-induced gene signatures are very similar in bronchial and nasal epithelial cells (45) suggesting that the less invasively obtained nasal tissue is representative and/or an extension of changes induced in the lower airways. Furthermore, we have previously demonstrated that in the context of viral infections, the expression of immune genes was suppressed in nasal epithelial cells obtained from smokers (24, 38). While we have demonstrated that CS exposure can alter gene expression in nasal epithelial cells, our previous studies have been limited to individual gene analysis. The goals of the present study are to provide a more comprehensive profile of CS-induced changes to immune gene expression, a novel assessment of e-cigarettes-induced changes to immune gene expression, and the first comparison of e-cigarette and CS effects on the respiratory innate immune system.
MATERIALS AND METHODS
Subject recruitment and sample collection.
This was a prospective, observational cross-sectional study comparing gene expression profiles in nonsmokers, cigarette smokers, and e-cigarette users. Subjects were healthy young adults 18–50 years of age in three groups: 1) nonsmokers not regularly exposed to SHS (control group); 2) self-described active cigarette smokers (smoker group); and 3) self-described, active e-cigarette users/vapers who had been using e-cigarettes regularly for at least 6 mo. Dual users smoking more than 5 cigarettes/wk in addition to using e-cigarettes were excluded from these studies. The exclusion criteria for this study were current symptoms of allergic rhinitis, diagnosis or symptoms of asthma, forced expiratory volume in 1 s (FEV1) less than 75% of predicted at screen, chronic obstructive pulmonary disorder (COPD), cardiac disease or any chronic cardiorespiratory condition, bleeding disorders, immunodeficiency, recent nasal surgery or nasal steroid use, or current pregnancy. At the initial screen visit, a self-reported smoking/e-cigarette use history was obtained and subjects were asked to complete a 3- to 4-wk smoking and e-cigarette use diary prior to the sample acquisition visit. Subjects were asked to return after 3–4 wk, at which point the smoking diary, vital signs, urine, blood, demographic information, and pregnancy tests (for female subjects) were collected. For the purposes of analysis, individuals were classified according self-reported smoking status as current smokers, nonsmokers, or e-cigarette users. Of the e-cigarette users, n = 9 identified themselves as former cigarette smokers. For the purpose of this study, current e-cigarette use was defined as having exclusively or predominantly vaped e-cigarettes for at least 6 mo. In addition, self-classification was cross-checked against participants' diaries as well as analyses of tobacco and nicotine metabolites in serum and urine to verify smoking status (see Table 1 and Supplemental Table S1; Supplemental Material for this article is available online at the Journal website).
Table 1.
Subject demographics
| Nonsmokers (n = 13) | Cigarette Smokers (n = 14) | E-Cigarette Users (n = 12) | |
|---|---|---|---|
| BMI | 28.03 ± 6.48 | 28.15 ± 6.72 | 28.73 ± 9.11 |
| Age | 30.38 ± 6.84 | 30.71 ± 5.64 | 26.33 ± 5.57 |
| Sex, female/male | 8/5 | 8/6 | 5/7 |
| Ethnicity, White/African American/Asian | 9/3/1 | 6/7/1 | 7/2/3 |
| Cigarettes per day | 0 | 11.8 ± 5.49 | 0.21 ± 0.36 |
| E-cigarette puffs per day | 0 | 0 | 200.66 ± 178.26 |
| Serum cotinine, ng/ml | 0.08 ± 0.17 | 158.95 ± 132.27 | 174.19 ± 167.90 |
| Urine NNAL, pg/ml | 1.16 ± 2.73 | 377.14 ± 302.27 | 15.77 ± 18.58 |
| Urine NNAL/creatinine, pg/mg | 0.02 ± 0.07 | 261.76 ± 218.81 | 8.62 ± 12.78 |
Values are mean ± SE.
At that point, superficial scrape biopsies of the epithelium in the inferior surface of the middle nasal turbinate were obtained from each subject, and epithelial RNA was isolated, similar to our previous studies (31, 38). Nasal lavage was carried out similar to our previous studies (16, 33–36) using repetitive spraying of nostrils with sterile normal saline (0.9%) irrigation solution (4 ml per nostril). Cell-free nasal lavage fluid (NLF) was obtained by filtration and centrifugation of the NLF to remove cells and debris as described by us before (13, 29–32). When sufficient NLF cells were available, cytocentrifuge slides were prepared as described by us before (13) and stained using a modified Wright stain for differential cell counts. At least 100 cells were counted on each slide to quantify the percent neutrophils present (16, 33–36).
Informed consent was obtained from all subjects, and the protocol was submitted to and approved by the University of North Carolina at Chapel Hill Biomedical Institutional Review Board.
Assessment of nicotine and tobacco biomarkers.
Serum cotinine and urine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) levels were assayed by liquid chromatography tandem mass spectrometry using published methods (21, 22). Cotinine is the proximate metabolite of nicotine and is a biomarker of daily dose of nicotine. NNAL is a tobacco specific nitrosamine and metabolite of NNK [nicotine-derived nitrosamine ketone (NNK), also known as 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone], which is found in tobacco smoke but not (or in negligible amounts) in e-cigarette vapor. The limits of quantification for serum cotinine were 0.02 ng/ml for nonsmokers and e-cigarette users and 1 ng/ml for cigarette smokers, while the limit for urine NNAL quantification was 0.25 pg/ml for all groups. The limit of quantitation was different for nonsmokers/e-cigarette users and cigarette smokers based on the use of different levels of internal standards. This is necessary because levels in cigarette smokers are so much higher (1–200 ng/ml) than in nonsmokers or e-cigarette users (0.05 to 10 ng/ml).
Nanostring-based gene expression analysis.
Total RNA isolated from superficial scrape biopsies was analyzed by using the nCounter Human Immunology v2 Expression panel from Nanostring (Nanostring, Seattle, WA), which assesses the expression of n = 597 human immunology-related genes. Nanostring data were normalized in a two-step process as per the manufacturer's recommendation and processed using Partek Genomic Suite (St. Louis, MO). First, positive control normalization was performed, where the geometric mean for each sample's positive control was calculated. Then a lane normalization factor was calculated by dividing the positive control geometric mean of each sample by the mean of the geometric means. The lane normalization factors were then used to adjust each sample individually. The same protocol was then repeated for housekeeping genes. Together, these processes control for batch effect and artifact error. Genes that were expressed below the stated manufacturer threshold in more than 25% of subjects were excluded from analysis.
Differential expression was determined between groups using an analysis of covariance (ANCOVA) controlling for age, race, sex, and body mass index (BMI). Specifically, differences were tested between smokers and nonsmokers, e-cigarette users and nonsmokers, and e-cigarette users and cigarette users. Differential expression was defined as an ANCOVA overall P < 0.05 with a false discovery corrected q <0.1.
Pathway analysis and identification of key transcription factors.
Pathway analysis was conducted using Ingenuity Pathway Analysis (IPA) (Ingenuity Systems, Redwood City, CA). Enriched canonical pathways were identified via a right-tailed Fisher's exact test. Statistical significance for canonical pathways was set at P < 0.05. These data were subsequently validated in a separate analysis using DAVID.
In addition to canonical pathway analysis, IPA was used to assess the impact of genes coding for transcription factors that were differentially expressed within our dataset. Specifically, the upstream regulator module was used to determine the number of transcripts that were altered within our dataset and controlled by differentially expressed genes coding for transcription factors. P values for the upstream regulator module were determined by a two-tailed Fisher's exact test and significance was set at P < 0.01. To validate the findings of the IPA analysis, a second analysis was conducted using Genomatix's Overrepresented Transcription Factor Binding Site tool (Genomatix Software, Ann Arbor, MI). This analysis determined the number of genes with binding sites in the promoter region for differentially expressed genes that code for transcription factors. Promoter region sequences were defined as 500 base pairs upstream and 1,000 base pairs downstream of the transcription start site. A z-score was calculated to determine overrepresented transcription factor binding sites, with a z-score ≥ 2 or ≤ −2 corresponding to a P value of 0.05. For this reason statistical significance was set at z > |2|.
ELISA analysis of CSF-1 and CCL26/eotaxin-3.
Cell-free NLF from all subjects were used to analyze Colony Stimulating Factor 1 (CSF-1) and Chemokine (C-C Motif) Ligand 26 (CCL26/eotaxin-3) protein levels via commercially available ELISA kits (Meso Scale Diagnostics, Rockville, MD). Data in the cigarette smokers and e-cigarette users were assessed as picograms per milliliter cell-free NLF and normalized to the average level in nonsmokers. The expression ratio values were converted to fold changes using log2 transformation, similar to the gene expression data and compared with the fold change cutoff for nonsmokers [log2(1) = 0] by the one-sample t-test.
RESULTS
Description of study subjects.
Among the individuals included in this study (n = 39), about an equal number were nonsmokers (n = 13; 33.3%), cigarette smokers (n = 14; 35.8%), and e-cigarette users (n = 12; 30.8%). The average BMI, age, and sex distribution did not significantly differ among the different groups (Table 1). Similarly, the average percent neutrophils present in the NLF did not differ among the three different groups (nonsmokers = 39.66 ± 23.66; cigarette smokers = 52.31 ± 37.72; e-cigarette users = 61.35 ± 24.67). The average number of cigarettes smoked per day in the smokers category was ∼12, ranging from 2.5 to 21 cigarettes/day. In the e-cigarette user category, the average number of puffs inhaled per day was ∼200, ranging from 5.8 to 610.7, illustrating the broad range of users included in this study. Of the e-cigarette users, nine identified themselves as having previously smoked cigarettes, while three indicated no prior cigarette smoking history (Table 1). In addition, five of the subjects reported occasionally smoking cigarettes (Supplemental Table S1).
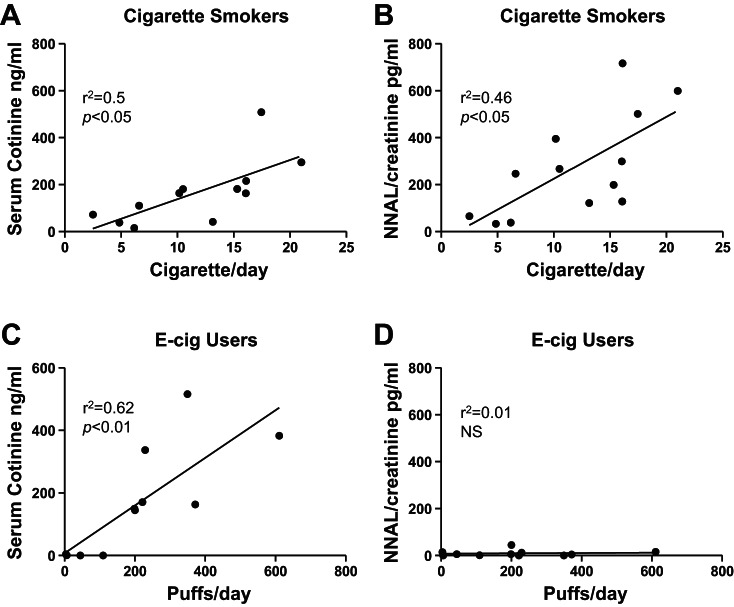
As expected, biochemical markers for nicotine exposure (cotinine) and tobacco-specific NNAL were at or below the detection limit in nonsmokers. In smokers, serum cotinine and urine NNAL levels were significantly correlated with cigarettes smoked per day (P < 0.05). Similarly, serum cotinine levels were significantly correlated with e-cigarette puffs per day (P < 0.01), whereas urine NNAL levels were not. Average urine NNAL levels were significantly lower in e-cigarette users compared with smokers (Fig. 1). Only one subject had urine NNAL levels above the cutoff point distinguishing smokers from nonsmokers of 47 pg/ml (12), while the majority of e-cigarette users had urine NNAL levels comparable to those seen in nonsmokers (Supplemental Table S1), suggesting predominant or exclusive e-cigarette use in these subjects.
Fig. 1.
Correlation between serum cotinine and urine NNAL levels and cigarette/e-cigarette (E-cig) usage in smokers and e-cigarette users. Serum cotinine (A) and urine NNAL levels (B) from smokers were correlated with the average number of cigarettes smoked per day. Serum cotinine (C) and urine NNAL (D) from e-cigarette users were correlated with the average puffs per day. Pearson correlation coefficient and P values are depicted. NS, not significant.
Differential expression of genes in nasal biopsy samples in smokers vs. e-cigarette smokers.
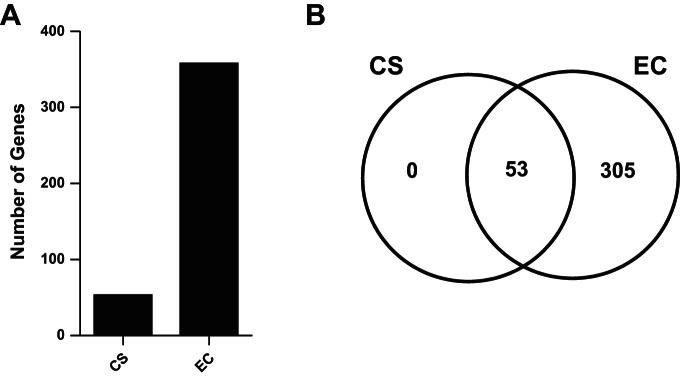
Of the 597 genes tested on the Nanostring nCounter Human Immunology Array, 543 genes were expressed above background in nasal biopsy samples. A total of 358 of the 543 detectable genes were differentially expressed in at least one condition in nasal biopsy samples. Of these, 53 genes were differentially expressed when comparing cigarette smokers and nonsmokers (Fig. 2, A and B). All of the 53 genes that changed in cigarette smokers showed decreased expression (Supplemental Table S2, Fig. 2B). The top five genes with changed expression in cigarette smokers were Early Growth Response 1 (EGR1), Dipeptidyl-Peptidase 4 (DPP4), Chemokine (C-X-C Motif) Ligand 2 (CXCL2), Chemokine (C-X3-C Motif) Receptor 1 (CX3CR1), and CD28 Molecule (CD82). When comparing e-cigarette users with nonsmokers, 358 genes were differentially expressed. As with the cigarette smokers, all 358 genes that were differentially expressed in e-cigarette users also showed decreased expression (Supplemental Table S2). The top five genes with changed expression in e-cigarette users were Zinc Finger And BTB Domain Containing 16 (ZBTB16), EGR1, Polymeric Immunoglobulin Receptor (PIGR), Prostaglandin-Endoperoxide Synthase 2 (PTGS2), and FK506 Binding Protein 5 (FKBP5). All 53 genes changed in the comparison of cigarette smokers with nonsmokers were also changed in the comparison of e-cigarette smokers with nonsmokers (Fig. 2B). The extent of change in gene expression of e-cigarette users was much greater than that of cigarette smokers. The reduction in gene expression is illustrated in the heat map of fold changes induced in e-cigarette users and cigarette smokers compared with nonsmokers (Fig. 3). Together the data indicate that the gene expression changes induced by smoking cigarettes or vaping e-cigarettes are consistent with immune suppression and that e-cigarette users had a large set of unique gene expression changes compared with nonsmokers and cigarette smokers.
Fig. 2.
Number of genes changed in cigarette smokers (CS) and e-cigarette (EC) users. A: total number of genes changed in smokers and e-cigarette users compared with nonsmokers. B: Venn diagram of the genes unique or common to cigarette smokers and e-cigarette users.
Fig. 3.
Comparison of fold change gene expression in cigarette smokers and e-cigarette users. The level of transcriptional changes in the 53 genes common to cigarette smokers and e-cigarette was compared and depicted as the relative fold change in this heatmap.
Pathway analysis of cigarette and e-cigarette smokers.
Pathway analysis was conducted for three sets of genes using DAVID: the 53 common cigarette and e-cigarette responsive genes (Supplemental Table S2), and the 358 genes representing the entire e-cigarette response (Supplemental Table S2). The top 10 canonical pathways representing the gene expression in each comparison group can be found in Supplemental Table S3. Four pathways, the cytokine-cytokine receptor interaction, apoptosis, Toll-like receptor signaling pathway, and NOD-like receptor signaling pathway, overlapped in both comparison groups [E-cigarette vs. Nonsmokers and Cigarette Smokers vs. Nonsmokers (Supplemental Table S3)]. Outside of these four pathways, there was no overlap in canonical pathways represented in the gene expression changes induced in the two comparison groups.
Transcription factor analysis of cigarette and e-cigarette gene expression changes.
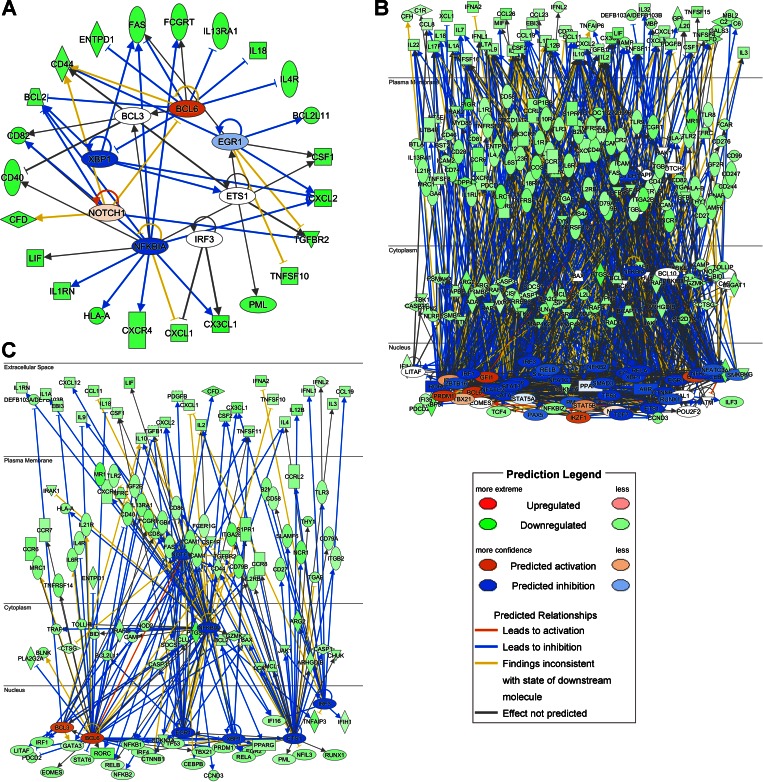
To further understand the difference in gene expression changes induced in smokers and e-cigarette users, we identified functional transcription factor networks based on differentially expressed genes that code for transcription factors using IPA. Additionally, we used Genomatix to determine whether genes within the gene set also contained transcription factor binding sites for these transcription factors. When analyzing the cigarette response, 7 transcription factors were identified as differentially expressed and statistically significant in the upstream regulator analysis: EGR1, V-Ets Avian Erythroblastosis Virus E26 Oncogene Homolog 1 (ETS1), Nuclear Factor Of Kappa Light Polypeptide Gene Enhancer In B-Cells 1 (NFKB1A), NOTCH1 (NOTCH1), X-Box Binding Protein 1 (XBP1), B-Cell CLL/Lymphoma 6 (BCL6), and B-Cell CLL/Lymphoma 3 (BCL3) (Supplemental Table S4). In contrast, 50 transcription factors were identified as differentially expressed in e-cigarette users and were statically significant in the upstream regulator analysis (Supplemental Table S4). This analysis also suggested that the 7 transcription factors regulated 30 of 53 differentially expressed genes in the cigarette smokers, and the 50 transcription factors regulated 262 of 358 genes differentially expressed in the e-cigarette smokers compared with the nonsmokers group (Fig. 4, A and B). Focusing on the 7 transcription factors whose expression was decreased in both cigarette smokers and e-cigarette users, 120 genes were regulated by these transcription factors in e-cigarette users (Fig. 4C) compared with 30 genes regulated in cigarette smokers (Fig. 4A). Genomatix was used to determine whether the gene sets identified as changed in e-cigarette smokers or cigarette smokers compared with nonsmokers were enriched for sequences bound by the predicted transcription factors. Since Genomatix has a more limited database, overlap between the IPA and Genomatix analysis was not complete. In the comparison of cigarette smokers with nonsmokers, 5 of the 7 identified transcription factors were represented within the Genomatix family matrix. Of these 5 transcription factors, 4 were significantly enriched in the comparison of cigarette smokers with nonsmokers. Similarly, in the comparison of e-cigarette users with nonsmokers 33 of 50 identified transcription factors were represented within the Genomatix family matrix, and 31 of these 33 were found to be significant in the transcription factor binding site analysis (Supplemental Table S4). These data suggest that the identified transcription factors likely play an important role in driving the differential responses seen in e-cigarette users and smokers.
Fig. 4.
Functional transcription factor networks in cigarette smokers (A) and e-cigarette users (B).
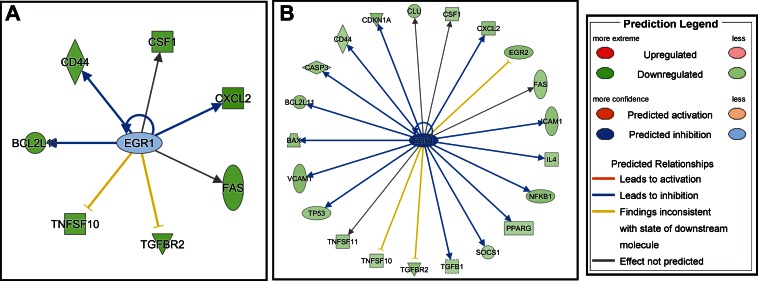
We also found that, for transcription factors whose expression was changed in both the cigarette smoker and e-cigarette user groups, the level of suppression was greater in e-cigarette users than in cigarette smokers for each transcription factor (Table 2). To determine whether this was functionally associated with reduced expression of a greater number of target genes, we focused our analysis on EGR1. EGR1 is an immediate-early gene regulating the transcription of many immune genes, including cytokines/chemokines, adhesion molecules, proteases, and autophagy genes. Using Genomatix and IPA, downregulation of EGR1 was computationally assessed to be functionally associated with reduced expression of 5 target genes in cigarette smokers and 18 target genes in e-cigarette users (Fig. 5, A and B, respectively). Genes functionally associated in both smokers and e-cigarette users with the suppressed EGR1 expression were CD44 Molecule (CD44), Colony Stimulating Factor 1 (CSF1), Chemokine (C-X-C Motif) Ligand 2 (CXCL2), BCL2-Like 11 (BCL2L11), and Fas Cell Surface Death Receptor (FAS).
Table 2.
Comparison of mean fold changes in transcription factor genes differentially expressed in both cigarette smokers and e-cigarette users
| Transcription Factor | Cigarette Smokers vs. Nonsmokers | E-Cigarette Users vs. Nonsmokers |
|---|---|---|
| NFKBIA | −1.68542 | −3.03275 |
| ETS1 | −1.73855 | −3.84539 |
| NOTCH1 | −1.78689 | −2.61221 |
| BCL3 | −1.83901 | −3.05216 |
| XBP1 | −1.9088 | −3.00112 |
| BCL6 | −1.93451 | −3.33365 |
| EGR1 | −2.84024 | −9.64874 |
Fig. 5.
Functional networks regulated by the transcription factor EGR1 in cigarette smokers (A) and e-cigarette users (B).
Confirmation of change in CSF-1 and CCL26 levels in smokers and e-cigarette users.
Based on the functional association analyses between suppressed EGR1 expression and target genes, we analyzed the levels of CSF-1 in NLF from the same study subjects as were used in the gene expression analysis. Since CSF-1 is constitutively expressed by a variety of epithelial cell types and has been shown to play important roles in innate immunity, including host defense responses against fungal, bacterial, and viral infections (6, 18) it represented a suitable target for confirmatory studies. CSF-1 expression was significantly decreased in NLF from both cigarette smokers and e-cigarette users compared with nonsmokers (Fig. 6A). We also analyzed the levels of CCL26/eotaxin-3, a chemokine expressed by epithelial cells important for the recruitment of not only for eosinophils, basophils, and T lymphocytes, but also NK cells (7, 29, 37). Similarly to CSF-1, expression of CCL26/eotaxin-3 was significantly decreased in NLF from cigarette smokers, but this suppression did not reach statistical significance in e-cigarette users (Fig. 6B).
Fig. 6.
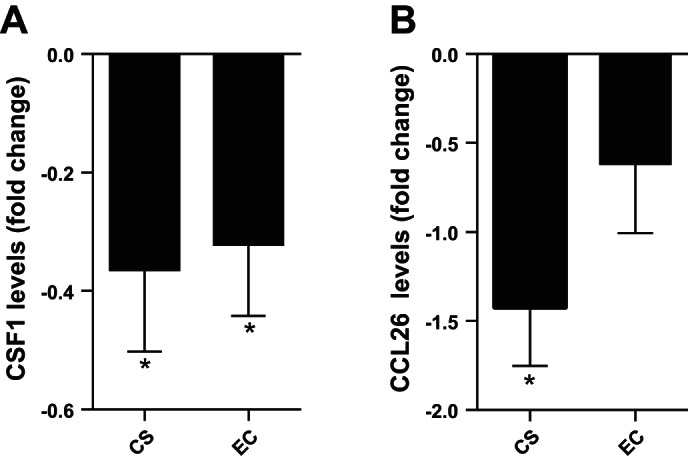
Comparison of fold change CSF-1 levels in cigarette smokers and e-cigarette users. Levels of CSF-1 were analyzed in NLF from cigarette smokers and e-cigarette users and normalized to the average level observed in nonsmokers. Data are expressed as mean ± SE fold change over nonsmokers. *Statistically different from nonsmokers, P < 0.05.
DISCUSSION
In the study described here we compared immune-related gene expression changes induced in the nasal mucosa of cigarette smokers and e-cigarette users, compared with nonsmokers. There were three major observations derived from this study: First, smoking cigarettes or vaping e-cigarettes resulted in decreased expression of a large number of immune-related genes. Second, all of the genes suppressed by cigarette smoking were also suppressed in nasal biopsies from e-cigarette users. Third, vaping e-cigarettes was associated with a much greater number of gene expression changes and, in a gene-by-gene comparison, stronger levels of suppression compared with cigarette smokers. Thus our data indicate that vaping e-cigarettes is associated with broad gene expression changes that are consistent with immune suppression at the level of the nasal mucosa.
The effects of smoking on the respiratory immune response are very complex and include both activation of proinflammatory pathways and suppression of immune responses in the respiratory mucosa, leading to increased tissue injury and enhanced susceptibility to microbial infections (1, 17, 19, 28, 30, 39, 40). The airway epithelium is a key orchestrator of respiratory immune responses through the expression of cytokines/chemokines, adhesion molecules, surface markers/ligands, and antimicrobial peptides/mucins (28). Therefore, we focused our gene array analyses on changes in a defined set of immune response genes in nasal biopsies obtained from nonsmokers, cigarette smokers, and e-cigarette users. Our data demonstrate that immune genes were broadly suppressed in both cigarette smokers and e-cigarette users compared with nonsmokers. Within the 597 immune-related genes tested in our study, the most significantly affected canonical pathway common to both nasal epithelial biopsies obtained from smokers and e-cigarette users was the cytokine-cytokine receptor interaction pathway (Supplemental Table S3). In cigarette smokers this included the suppressed expression of 18 genes and in e-cigarette users 75 genes related to cytokines/chemokines or their receptors, including the two chemokines CSF-1 and CCL26/eotaxin-3. We demonstrate that baseline levels of CSF-1, which is important for the recruitment and activation of innate immune cells, are reduced in the NLF of cigarette smokers and e-cigarette users compared with nonsmokers. CSF-1 is a major chemokine and regulatory factor for mononuclear cells (46, 48). It is constitutively expressed by a variety of epithelial cell types and has been shown to play important roles in innate immunity, including host defense responses against fungal, bacterial, and viral infections (4, 15). Similarly, baseline expression of CCL26/eotaxin-3 was suppressed in NLF from cigarette smokers, but this reduction did not reach statistical significance in NLF from e-cigarette users. CCL26/eotaxin-3 is produced by all epithelial cells lining the respiratory tract (37) and recruits and activates eosinophils in the context of allergic airways disease (29). In addition, CCL26 has been shown to be a potent chemoattractant for nasal NK cells (7), which we have previously demonstrated to be modified in cigarette smokers (16). Even though our data do not demonstrate whether and how the decreased expression of genes associated with cytokine/chemokine signaling is of biological significance, it is likely that the smoking- and vaping-induced reduction of chemokines, such as CSF-1 and CCL26/eotaxin-3, at the level of the epithelium has functional consequences related to orchestrating respiratory immune capabilities.
Despite their increased use and popularity, the effects of e-cigarettes on human health and how they compare with those induced by cigarette smoking is largely unknown. Our data indicate that, compared with nonsmokers, nasal biopsies obtained from cigarette smokers presented an overall suppression of immune-related genes, similar to e-cigarette users. However, the extent of suppression as well as number of immune-related genes whose expression was significantly decreased was six times greater in e-cigarette users than in cigarette smokers (53 vs. 358). Recent studies suggest that, similar to CS, immune suppressive effect and increased susceptibility to microbial infections can also be induced by e-cigarettes. Specifically, mice exposed to e-cigarette vapor showed impaired bacterial clearance and enhanced susceptibility to influenza virus infections (47). In a separate study, exposure to e-cigarette vapor reduced antibacterial host defense responses in mice, resulting in increased bacterial growth and biofilm formation, which was associated with decreased levels of several important chemokines/cytokines in the bronchoalveolar lavage (20). Our data are supportive of the findings in these mouse studies, indicating that, similar to cigarette smoking, e-cigarette use is associated with a large suppressive effect on the expression of innate immune-related genes in the nasal mucosa. Therefore, the decreased ability to fight infection and reduced innate host defense responses associated with cigarette smoking could also be induced by vaping e-cigarettes and may be mediated by reduced expression of key immune genes in the respiratory epithelium.
The majority (n = 9) of our e-cigarette users smoked cigarettes prior to switching to e-cigarettes, while the remainder (n = 3) had no prior history of cigarette smoking. E-cigarette users and cigarette smokers had similar levels of serum cotinine, indicating similar daily levels of nicotine exposure. As expected, e-cigarette users had much lower urine NNAL levels than smokers, but e-cigarette users' levels were also higher than those of nonsmokers. Of the e-cigarette users, seven subjects had urine NNAL levels similar to those seen in nonsmokers (urine NNAL <10 pg/ml; Supplemental Table S1), suggesting that some of the e-cigarette users included in this study occasionally used some form of tobacco. The small sample size used in the data presented here does not allow for a direct comparison among the subgroups of e-cigarette users. However, it is likely that prior cigarette smoking will result in gene expression changes that are maintained for long periods of time after smoking cessation. Previous comparison of gene expression profiles of bronchial epithelial cells from current, never, and former smokers suggest smoking induces a broad range of gene expression changes in bronchial epithelial cells (43) including enhanced expression of genes associated with xenobiotic metabolism or redox stress, while suppressing genes involved in immune responses, such as CX3CL1, which was also suppressed in our study. Follow-up studies also indicated that some smoking-induced gene expression changes are irreversible (45). Thus it is possible that the 53 genes decreased in both cigarette smokers and e-cigarette users are derived from the cigarette smoking history common to both groups, which are not reversed by switching to e-cigarettes. However, beyond the group of genes shared with cigarette smokers, e-cigarette users showed 305 unique genes whose expression was decreased compared with nonsmokers. Based on the similar serum cotinine levels in cigarette smokers and e-cigarette users (Table 1), it does not appear that the overall difference in number and level of gene expression changes is dependent on nicotine. E-cigarette vapors are a chemical mixture, whose complexity varies based on several different factors, including the flavoring of the e-juice or e-liquid. We did not collect detailed information on the preferred flavors used in our e-cigarette user cohort, but it is unlikely that the effects shown here can be attributed to a single flavoring chemical, but possibly a group of flavoring or class of chemicals, like aldehydes, common to many different vapors.
Among the most striking findings presented here is the significantly greater number of genes suppressed in e-cigarette users compared with cigarette smokers, including a greater number of transcription factors. In addition to inducing a broader suppression of immune-related genes, we also observed that the level of suppression of common genes was greater in e-cigarette users compared with cigarette smokers (Fig. 3). This pattern included the 7 common transcription factors whose expression was reduced in both cigarette smokers and e-cigarette users. For example, EGR1, which was the gene with the greatest fold change (FC) observed in nasal biopsies from smokers (FC = −2.84), was decreased almost 10-fold (FC = −9.65) in e-cigarette users (Table 2). A computational prediction model of genes regulated by the transcription factor EGR1 and represented in this gene expression platform indicated that a number of genes were functionally associated with reduced EGR1 expression: specifically, 5 in cigarette smokers and 18 in e-cigarette users. These data suggest that the more enhanced suppression of genes encoding transcription factors in e-cigarette users was also associated with a greater number of downstream affected genes.
Taken together, the data shown here demonstrate that vaping e-cigarettes does not reverse smoking-induced gene expression changes and may result in immunomodulatory effects that go beyond those induced by smoking cigarettes alone. The potential underlying mechanisms of these responses are just starting to emerge as we increase our understanding of the individual components that comprise e-cigarette vapor. The chemical components are varied and dependent on the formulation of the e-liquid, the vaporizing device, and the aerosol generation process itself (8, 10, 11, 49). Recent studies have demonstrated that, depending on the dose, inhalation of the propylene glycol/glycerin vehicle vapor alone can generate proinflammatory responses (49). Vaporization of the humectants contained in e-cigarettes can also lead to the generation of volatile carbonyls, such as formaldehyde, acrolein, and acetaldehyde, which have known adverse pulmonary health effects (10, 11). Emission of the carbonyls is greatly dependent on the device used and increases significantly when the output wattage is increased (10). Interestingly, e-cigarette device wattage can often be manually adjusted, especially in the more recently developed devices, thus potentially enhancing the generation of carbonyls. In addition, the significant number of flavoring chemicals added to e-cigarettes further enhances the complexities of the inhaled mixture. Despite the common perception that vaping e-cigarettes is a safe alternative to cigarettes, the data shown here demonstrate the need for further studies related to changes in respiratory immune health induced by vaping e-cigarettes.
GRANTS
This work was supported by grants from the National Institutes of Health (T32 ES0070108, T32 ES007126, P50 HL120100, P50 CA180890, P42 ES005948). This work was funded by NIH P50 HL120100. Research reported in this publication was in part supported by NIH and the FDA Center for Tobacco Products (CTP). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Food and Drug Administration.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
E.M., P.W.C., M.E.R., E.A.P., and E.E.G.-B. performed experiments; E.M., P.W.C., M.E.R., E.A.P., N.L.B., R.C.F., and I.J. analyzed data; E.M., M.E.R., N.L.B., R.C.F., and I.J. interpreted results of experiments; E.M., M.E.R., E.A.P., and I.J. prepared figures; E.M., P.W.C., M.E.R., E.A.P., and I.J. drafted manuscript; E.M., P.W.C., M.E.R., E.A.P., E.E.G.-B., N.L.B., R.C.F., and I.J. edited and revised manuscript; M.E.R., E.E.G.-B., N.L.B., R.C.F., and I.J. approved final version of manuscript; I.J. conception and design of research.
Supplementary Material
ACKNOWLEDGMENTS
Dr. Glista-Baker is currently at SC Johnson, Racine, Wisconsin.
REFERENCES
- 1.Aligne CA, Stoddard JJ. Tobacco and children. An economic evaluation of the medical effects of parental smoking. Arch Pediatr Adolesc Med 151: 648–653, 1997. [DOI] [PubMed] [Google Scholar]
- 2.Allen JG, Flanigan SS, LeBlanc M, Vallarino J, MacNaughton P, Stewart JH, Christiani DC. Flavoring chemicals in E-cigarettes: diacetyl, 2,3-pentanedione, and acetoin in a sample of 51 products, including fruit-, candy-, and cocktail-flavored E-cigarettes. Environ Health Perspect 124: 733–739, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beane J, Sebastiani P, Liu G, Brody JS, Lenburg ME, Spira A. Reversible and permanent effects of tobacco smoke exposure on airway epithelial gene expression. Genome Biol 8: R201, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell JD, McDonough JE, Zeskind JE, Hackett TL, Pechkovsky DV, Brandsma CA, Suzuki M, Gosselink JV, Liu G, Alekseyev YO, Xiao J, Zhang X, Hayashi S, Cooper JD, Timens W, Postma DS, Knight DA, Lenburg ME, Hogg JC, Spira A. A gene expression signature of emphysema-related lung destruction and its reversal by the tripeptide GHK. Genome Med 4: 67, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control.Emerging tobacco products gaining popularity among youth. 2013. http://www.cdc.gov/media/releases/2013/p1114-emerging-tobacco-products.html [06/June/2016]. [Google Scholar]
- 6.Chitu V, Stanley ER. Colony-stimulating factor-1 in immunity and inflammation. Curr Opin Immunol 18: 39–48, 2006. [DOI] [PubMed] [Google Scholar]
- 7.El-Shazly AE, Doloriert HC, Bisig B, Lefebvre PP, Delvenne P, Jacobs N. Novel cooperation between CX3CL1 and CCL26 inducing NK cell chemotaxis via CX3CR1: a possible mechanism for NK cell infiltration of the allergic nasal tissue. Clin Exp Allergy 43: 322–331, 2013. [DOI] [PubMed] [Google Scholar]
- 8.Flora JW, Meruva N, Huang CB, Wilkinson CT, Ballentine R, Smith DC, Werley MS, McKinney WJ. Characterization of potential impurities and degradation products in electronic cigarette formulations and aerosols. Regul Toxicol Pharmacol 74: 1–11, 2016. [DOI] [PubMed] [Google Scholar]
- 9.Food and Drug Administration. Deeming tobacco products to be subject to the federal food, drug, and cosmetic act, as amended by the Family Smoking Prevention and Tobacco Control Act; Restrictions on the sale and distribution of tobacco products and required warning statements for tobacco products. 21 CFR Parts 1100, 1140, and 1143. Federal Register 81, no. 90 (May 10, 2016): 28974. [PubMed] [Google Scholar]
- 10.Geiss O, Bianchi I, Barrero-Moreno J. Correlation of volatile carbonyl yields emitted by e-cigarettes with the temperature of the heating coil and the perceived sensorial quality of the generated vapours. Int J Hyg Environ Health 219: 268–277, 2016. [DOI] [PubMed] [Google Scholar]
- 11.Gillman IG, Kistler KA, Stewart EW, Paolantonio AR. Effect of variable power levels on the yield of total aerosol mass and formation of aldehydes in e-cigarette aerosols. Regul Toxicol Pharmacol 75: 58–65, 2016. [DOI] [PubMed] [Google Scholar]
- 12.Goniewicz ML, Eisner MD, Lazcano-Ponce E, Zielinska-Danch W, Koszowski B, Sobczak A, Havel C, Jacob P, Benowitz NL. Comparison of urine cotinine and the tobacco-specific nitrosamine metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) and their ratio to discriminate active from passive smoking. Nicotine Tob Res 13: 202–208, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holden VK, Hines SE. Update on flavoring-induced lung disease. Curr Opin Pulm Med 22: 158–164, 2016. [DOI] [PubMed] [Google Scholar]
- 14.Horvath KM, Brighton LE, Herbst M, Noah TL, Jaspers I. Live attenuated influenza virus (LAIV) induces different mucosal T cell function in nonsmokers and smokers. Clin Immunol 142: 232–236, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horvath KM, Brighton LE, Zhang W, Carson JL, Jaspers I. Epithelial cells from smokers modify dendritic cell responses in the context of influenza infection. Am J Respir Cell Mol Biol 45: 237–245, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horvath KM, Herbst M, Zhou H, Zhang H, Noah TL, Jaspers I. Nasal lavage natural killer cell function is suppressed in smokers after live attenuated influenza virus. Respir Res 12: 102, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.HuangFu WC, Liu J, Harty RN, Fuchs SY. Cigarette smoking products suppress anti-viral effects of Type I interferon via phosphorylation-dependent downregulation of its receptor. FEBS Lett 582: 3206–3210, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hubel K, Dale DC, Liles WC. Therapeutic use of cytokines to modulate phagocyte function for the treatment of infectious diseases: current status of granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor, macrophage colony-stimulating factor, and interferon-gamma. J Infect Dis 185: 1490–1501, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Hudy MH, Traves SL, Wiehler S, Proud D. Cigarette smoke modulates rhinovirus-induced airway epithelial cell chemokine production. Eur Respir J 35: 1256–1263, 2010. [DOI] [PubMed] [Google Scholar]
- 20.Hwang JH, Lyes M, Sladewski K, Enany S, McEachern E, Mathew DP, Das S, Moshensky A, Bapat S, Pride DT, Ongkeko WM, Crotty Alexander LE. Electronic cigarette inhalation alters innate immunity and airway cytokines while increasing the virulence of colonizing bacteria. J Mol Med (Berl) 94: 667–679, 2016. [DOI] [PubMed] [Google Scholar]
- 21.Jacob P 3rd, Havel C, Lee DH, Yu L, Eisner MD, Benowitz NL. Subpicogram per milliliter determination of the tobacco-specific carcinogen metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in human urine using liquid chromatography-tandem mass spectrometry. Anal Chem 80: 8115–8121, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacob P 3rd, Yu L, Duan M, Ramos L, Yturralde O, Benowitz NL. Determination of the nicotine metabolites cotinine and trans-3′-hydroxycotinine in biologic fluids of smokers and nonsmokers using liquid chromatography-tandem mass spectrometry: biomarkers for tobacco smoke exposure and for phenotyping cytochrome P450 2A6 activity. J Chromatogr B Analyt Technol Biomed Life Sci 879: 267–276, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jang TY, Park CS, Kim KS, Heo MJ, Kim YH. Benzaldehyde suppresses murine allergic asthma and rhinitis. Int Immunopharmacol 22: 444–450, 2014. [DOI] [PubMed] [Google Scholar]
- 24.Jaspers I, Horvath KM, Zhang W, Brighton LE, Carson JL, Noah TL. Reduced expression of IRF7 in nasal epithelial cells from smokers after infection with influenza. Am J Respir Cell Mol Biol 43: 368–375, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kark JD, Lebiush M. Smoking and epidemic influenza-like illness in female military recruits: a brief survey. Am J Public Health 71: 530–532, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kark JD, Lebiush M, Rannon L. Cigarette smoking as a risk factor for epidemic a(h1n1) influenza in young men. N Engl J Med 307: 1042–1046, 1982. [DOI] [PubMed] [Google Scholar]
- 27.Kosmider L, Sobczak A, Prokopowicz A, Kurek J, Zaciera M, Knysak J, Smith D, Goniewicz ML. Cherry-flavoured electronic cigarettes expose users to the inhalation irritant, benzaldehyde. Thorax 71: 376–377, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kulkarni R, Antala S, Wang A, Amaral FE, Rampersaud R, Larussa SJ, Planet PJ, Ratner AJ. Cigarette smoke increases Staphylococcus aureus biofilm formation via oxidative stress. Infect Immun 80: 3804–3811, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larose MC, Chakir J, Archambault AS, Joubert P, Provost V, Laviolette M, Flamand N. Correlation between CCL26 production by human bronchial epithelial cells and airway eosinophils: involvement in patients with severe eosinophilic asthma. J Allergy Clin Immunol 136: 904–913, 2015. [DOI] [PubMed] [Google Scholar]
- 30.Mehta H, Nazzal K, Sadikot RT. Cigarette smoking and innate immunity. Inflamm Res 57: 497–503, 2008. [DOI] [PubMed] [Google Scholar]
- 31.Meyer M, Kesic MJ, Clarke J, Ho E, Simmen RC, Diaz-Sanchez D, Noah TL, Jaspers I. Sulforaphane induces SLPI secretion in the nasal mucosa. Respir Med 107: 472–475, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muller L, Jaspers I. Epithelial cells, the “switchboard” of respiratory immune defense responses: effects of air pollutants. Swiss Med Wkly 142: w13653, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noah TL, Zhang H, Zhou H, Glista-Baker E, Muller L, Bauer RN, Meyer M, Murphy PC, Jones S, Letang B, Robinette C, Jaspers I. Effect of broccoli sprouts on nasal response to live attenuated influenza virus in smokers: a randomized, double-blind study. PLoS One 9: e98671, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noah TL, Zhou H, Jaspers I. Alteration of the nasal responses to influenza virus by tobacco smoke. Curr Opin Allergy Clin Immunol 12: 24–31, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Noah TL, Zhou H, Monaco J, Horvath K, Herbst M, Jaspers I. Tobacco smoke exposure and altered nasal responses to live attenuated influenza virus. Environ Health Perspect 119: 78–83, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noah TL, Zhou H, Zhang H, Horvath K, Robinette C, Kesic M, Meyer M, Diaz-Sanchez D, Jaspers I. Diesel exhaust exposure and nasal response to attenuated influenza in normal and allergic volunteers. Am J Respir Crit Care Med 185: 179–185, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paplinska-Goryca M, Nejman-Gryz P, Chazan R, Grubek-Jaworska H. The expression of the eotaxins IL-6 and CXCL8 in human epithelial cells from various levels of the respiratory tract. Cell Mol Biol Lett 18: 612–630, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rager JE, Bauer RN, Muller LL, Smeester L, Carson JL, Brighton LE, Fry RC, Jaspers I. DNA methylation in nasal epithelial cells from smokers: identification of ULBP3-related effects. Am J Physiol Lung Cell Mol Physiol 305: L432–L438, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Razani-Boroujerdi S, Singh SP, Knall C, Hahn FF, Pena-Philippides JC, Kalra R, Langley RJ, Sopori ML. Chronic nicotine inhibits inflammation and promotes influenza infection. Cell Immunol 230: 1–9, 2004. [DOI] [PubMed] [Google Scholar]
- 40.Robbins CS, Bauer CM, Vujicic N, Gaschler GJ, Lichty BD, Brown EG, Stampfli MR. Cigarette smoke impacts immune inflammatory responses to influenza in mice. Am J Respir Crit Care Med 174: 1342–1351, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Silverman EK, Spira A, Pare PD. Genetics and genomics of chronic obstructive pulmonary disease. Proc Am Thorac Soc 6: 539–542, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spira A, Beane J, Shah V, Liu G, Schembri F, Yang X, Palma J, Brody JS. Effects of cigarette smoke on the human airway epithelial cell transcriptome. Proc Natl Acad Sci USA 101: 10143–10148, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spira A, Beane JE, Shah V, Steiling K, Liu G, Schembri F, Gilman S, Dumas YM, Calner P, Sebastiani P, Sridhar S, Beamis J, Lamb C, Anderson T, Gerry N, Keane J, Lenburg ME, Brody JS. Airway epithelial gene expression in the diagnostic evaluation of smokers with suspect lung cancer. Nat Med 13: 361–366, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Sridhar S, Schembri F, Zeskind J, Shah V, Gustafson A, Steiling K, Liu G, Dumas YM, Zhang X, Brody J, Lenburg M, Spira A. Smoking-induced gene expression changes in the bronchial airway are reflected in nasal and buccal epithelium. BMC Genomics 9: 259, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stanley ER, Guilbert LJ, Tushinski RJ, Bartelmez SH. CSF-1—a mononuclear phagocyte lineage-specific hemopoietic growth factor. J Cell Biochem 21: 151–159, 1983. [DOI] [PubMed] [Google Scholar]
- 47.Sussan TE, Gajghate S, Thimmulappa RK, Ma J, Kim JH, Sudini K, Consolini N, Cormier SA, Lomnicki S, Hasan F, Pekosz A, Biswal S. Exposure to electronic cigarettes impairs pulmonary anti-bacterial and anti-viral defenses in a mouse model. PLoS One 10: e0116861, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tushinski RJ, Stanley ER. The regulation of macrophage protein turnover by a colony stimulating factor (CSF-1). J Cell Physiol 116: 67–75, 1983. [DOI] [PubMed] [Google Scholar]
- 48a.U.S. Department of Health and Human Services.The Health Consequences of Smoking—50 Years of Progress: a report of the Surgeon General. A Report of the Surgeon General, 2014. Atlanta, GA: Department of Health and Human Services, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2014. [Google Scholar]
- 49.Werley MS, Kirkpatrick DJ, Oldham MJ, Jerome AM, Langston TB, Lilly PD, Smith DC, McKinney WJ Jr. Toxicological assessment of a prototype e-cigaret device and three flavor formulations: a 90-day inhalation study in rats. Inhal Toxicol 28: 22–38, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zaccone EJ, Goldsmith WT, Shimko MJ, Wells JR, Schwegler-Berry D, Willard PA, Case SL, Thompson JA, Fedan JS. Diacetyl and 2,3-pentanedione exposure of human cultured airway epithelial cells: ion transport effects and metabolism of butter flavoring agents. Toxicol Appl Pharmacol 289: 542–549, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.