Abstract
Acute lung injury (ALI) and systemic coagulopathy are serious complications of traumatic brain injury (TBI) that frequently lead to poor clinical outcomes. Although the release of tissue factor (TF), a potent initiator of the extrinsic pathway of coagulation, from the injured brain is thought to play a key role in coagulopathy after TBI, its function in ALI following TBI remains unclear. In this study, we investigated whether the systemic appearance of TF correlated with the ensuing coagulopathy that follows TBI in ALI using an anesthetized rat blunt trauma TBI model. Blood and lung samples were obtained after TBI. Compared with controls, pulmonary edema and increased pulmonary permeability were observed as early as 5 min after TBI without evidence of norepinephrine involvement. Systemic TF increased at 5 min and then diminished 60 min after TBI. Lung injury and alveolar hemorrhaging were also observed as early as 5 min after TBI. A biphasic elevation of TF was observed in the lungs after TBI, and TF-positive microparticles (MPs) were detected in the alveolar spaces. Fibrin(ogen) deposition was also observed in the lungs within 60 min after TBI. Additionally, preadministration of a direct thrombin inhibitor, Refludan, attenuated lung injuries, thus implicating thrombin as a direct participant in ALI after TBI. The results from this study demonstrated that enhanced systemic TF may be an initiator of coagulation activation that contributes to ALI after TBI.
Keywords: acute lung injury, traumatic brain injury, tissue factor, coagulopathy, coagulation pathway
traumatic brain injury (TBI) is the leading cause of death and disability in young adults (30). Of the 1.7 million TBIs occurring each year in the United States, ∼16% require hospitalization, and 3% result in death (7). Patients with severe acute brain injury are at risk of developing dysfunction of other organs. These extracranial complications play a critical role in the clinical outcome. Among these, acute lung injury (ALI) and coagulopathy can strongly affect the mortality rate and neurological outcome in patients with TBI (33, 46, 51).
The presence of pulmonary dysfunction after TBI is well recognized, and ALI occurs in 20–25% of TBI patients (21). The development of ALI is an independent predictor of an unfavorable outcome in severe TBI patients (21). In clinical settings, the mechanisms of pulmonary dysfunction in the acute response to TBI are poorly understood. Although the etiology is thought to be the sympathetic response to increased intracranial pressure resulting in cardiopulmonary dysfunction, many reports fail to consistently demonstrate the hypertensive surges and changes in left atrial pressures (9). It has been documented that hemodynamic instability is not necessary for pulmonary edema and ALI after TBI (19, 35). Recent studies demonstrated that a systemic inflammatory response plays an integral role in the development of pulmonary injury associated with brain injury (25, 53). However, this response is mainly related to the secondary injury occurring hours and days after the initial events, while others have reported that the initial insult produced severe pulmonary changes within 3–5 min after cerebral compression in rats (4).
Retrospective and observational studies have consistently shown that coagulopathy is commonly seen in TBI patients (22, 45). Studies in our laboratory have identified platelet dysfunction within 15 min in a rat model of blunt TBI (12). Recent work has shown a correlation between mortality and abnormal hemostasis due to early platelet dysfunction (8). Previous studies have demonstrated that the brain contains a large reserve of tissue factor (TF) (13, 40), which is the initiator of the extrinsic pathway of coagulation, and enhanced systemic levels of TF have been observed in head-injured patients compared with non-head-injured trauma patients (18). In our model, TF was observed in the form of microparticles (MPs) in the paraventricular nucleus after TBI (41). It has also been reported that high levels of procoagulant MPs in the cerebrospinal fluid and in peripheral blood are present at the onset of TBI (39). These results imply that TF released from the injured brain is associated with systemic platelet dysfunction and abnormal hemostasis.
Central to the pathophysiology of ALI/acute respiratory distress syndrome (ARDS) is the presence of fibrin-rich exudates (hyaline membranes) in the lumen of lung alveoli due to activation of the coagulation cascade (44, 52). However, the relationship of ALI and coagulopathy after TBI is poorly understood. TF-MP, which initiates thrombin generation and fibrin deposition, is the most potent candidate for the correlation of both pathological conditions. In previous reports, the presence of red blood cell (RBC) and protein was observed in the alveolar fluid after TBI (1, 49). Catecholamine surges, which can induce hydrostatic pulmonary edema alone cannot explain this phenomenon, while thrombin has direct effects on endothelial permeability (32). In this study, we evaluated the systemic appearance of TF and coagulation pathway activation in the development of ALI after TBI. The results of this study are described herein.
MATERIALS AND METHODS
Brain injury model.
Male Sprague-Dawley rats (245–285 g; Charles River Laboratories, Wilmington, MA) were utilized in a constrained, nonpenetrating brain injury model using the TBI 0310 (Precision Systems and Instrumentation, Fairfax, VA) as previously described (12). For some studies, the direct thrombin inhibitor Refludan [Leu1/Thr2]-63-desulfohirudin (Lepirudin, Berlex, Mortville, NJ) at 2 μg/g weight of rat was injected into the retro-orbital sinus just before injury and the rats were euthanized 5 and 60 min after injury, or in the case of the control (no injury), the injection was given 15 min before harvesting.
All animal studies were performed with prior approval from the University of Notre Dame Institutional Animal Care and Use Committee.
Wet-to-dry weight ratio in lung tissue.
Pulmonary edema was determined using a modified wet/dry method (28). Specifically, 5 and 60 min after injury, rats were anesthetized with isoflurane and the lungs were separated from the thoracic cavity. The lung samples were weighed before and after drying in an oven for 72 h at 80°C. The ratio of wet-to-dry weight was then calculated.
Assessment of pulmonary vascular leakage.
Pulmonary vascular leakage was assessed by quantitating extravasation of Evans blue (EB) into lung parenchyma (28). EB (4%, Sigma-Aldrich, St. Louis, MO) prepared in phosphate-buffered saline (PBS) (2 ml/kg weight of rat) was injected into the retro-orbital sinus of rats 60 min before the animals were euthanized. At 0 (control), 5, and 60 min after injury, rats were anesthetized and perfused with saline to remove the blood from the vasculature, and the lungs were removed and dried at 80°C for 72 h. Noninjured rats were used as controls. The dried lungs were weighed, cut into small pieces, and then incubated in 4 ml formamide (Sigma-Aldrich) at room temperature for 24 h. The supernatant was separated by centrifugation at 5,000 g for 30 min, and then 100 μl of the supernatant were placed in a 96-multiwell plate. The absorbance of the samples was measured at 620 and 740 nm using a spectrophotometer (SoftMax Pro Version 6.2.1; Molecular Devices, Sunnyvale, CA). The concentration of EB in the supernatant was quantitated using the following formula: A620 (correction) = A620 nm − (1.426 × A740 nm + 0.030). The concentration of EB in the lung tissue was determined from an EB standard curve.
Blood collection and measurement of norepinephrine by enzyme immunoassay.
At 0 (control), 5, 15, and 60 min after injury, rats were anesthetized and whole blood was collected from the vena cava in 3.2% sodium citrate Vacutainer (Becton-Dickinson, Franklin Lakes, NJ) or 50 U/ml of a heparin sodium solution (Sagent Pharmaceuticals, Schaumburg, IL). Platelet-poor plasma (PPP) was collected by centrifugation at 3,200 g for 10 min, and the supernatant was removed and stored in individual aliquots at −20°C.
Norepinephrine levels were determined in duplicate using a commercially available rat norepinephrine ELISA kit (MBS269993; MyBio Source, San Diego, CA) per the manufacturer's recommended protocol. The absorbance was read at 450 nm on a spectrophotometer within 10 min of stopping the reactions.
Complete blood counts, hemostasis parameters, thromboelastography, and platelet mapping.
Complete blood counts (CBCs) were acquired from EDTA-treated blood using a Hemavet 950FS (Drew Scientific, Waterbury, CT). Fibrinogen levels, prothrombin time (PT), and activated partial thromboplastin time (aPTT) values were determined utilizing a Diagnostica STArt4 Hemostasis Analyzer (Diagnostica Stago, Parsnippany, NJ). The details of thromboelastography and platelet mapping have been described previously (12). In brief, thromboelastograms were obtained using a TEG 5000 Thromboelastograph Hemostasis Analyzer System (Haemonetics, Braintree, MA) and analyzed using Haemoscope TAS Version 4.3 Software. The TEG platelet mapping kit (Haemonetics.) was used to assess platelet function. Heparinized blood, arachidonic acid (AA; 1 mM, final concentration), and adenosine diphosphate (ADP; 50 mM, final concentration, Bio/Data, Horsham, PA) were used for the platelet stimulatory assays.
Clotting time measurements.
Clotting times were measured using a mechanical clot detection system (STArt4 Hemostasis Analyzer). Briefly, 50 μl of PPP were diluted 1:1 in PBS and incubated for 1 min at 37°C. The clotting reaction was then initiated by adding 50 μl of 25 mM calcium chloride. Measurements were performed in duplicate.
Immunofluorescence of plasma smears and lung tissue.
For slide preparation of plasma smears, 10 μl PPP were pipetted onto the slides and dispersed. For TF immunofluorescence, the primary antibody was rabbit-anti-human TF monoclonal antibody [EPR8986] (ab151748; Abacm, Cambridge, MA) and the secondary antibody was Alexa Fluor 647-conjugated donkey anti-rabbit IgG (Thermo Fischer Scientific, Waltham, MA). For glial fibrillary acidic protein (GFAP) immunofluorescence, the primary antibody was goat anti-human GFAP polyclonal antibody (ab53554; Abcam) and the secondary antibody was Alexa Fluor 488 conjugated donkey-anti-goat IgG (Thermo Fischer Scientific). The samples were blocked with 10% normal donkey serum (Jackson ImmunoResearch Laboratories, West Grove, PA). The plasma smears were then incubated with the primary antibodies in 1% normal donkey serum overnight at 4°C. After being washed, the samples were exposed to image IT (Thermo Fischer Scientific) for 30 min and incubated with the secondary antibody at room temperature for 1 h. ProLong Gold Antifade Mountant with DAPI (Thermo Fischer Scientific) was used before coverslipping. Fluorescent images were acquired on a Zeiss LSM 710 confocal microscope and image software (Zen 2009; Carl Zeiss, Oberkochen, Germany). An argon laser at 488 nm and a helium/neon laser at 633 nm were used for excitation. Three points were randomly selected from each slide and captured under ×63 (1.4-numerical-aperture) oil objective magnification. Color images were acquired using a sequential scan mode. The images were subsequently analyzed using Imaris 8.0.2 (Bitplane, Zurich, Switzerland) to quantitate the TF- and GFAP-positive surface areas.
For lung tissue studies, the same primary and secondary antibodies were used as described for the plasma smear studies. Periodate-lysine-paraformaldehyde (PLP)-fixed lung tissue was paraffin embedded and then sectioned at 4-μm thickness. Antigen retrieval for all the immunohistochemical stains was accomplished by heat-induced epitope retrieval in citrate buffer, pH 6.0 (Life Technologies, Carlsbad, CA) (microwave 2 × 5 min). The samples were blocked with 10% normal donkey serum (Jackson ImmunoResearch Laboratories, West Grove, PA). The lung samples were then incubated with the primary antibodies in 1% normal donkey serum overnight at 4°C. After being washed, the samples were exposed to image IT (Thermo Fischer Scientific) for 30 min and incubated with the secondary antibody at room temperature for 1 h. ProLong Gold Antifade Mountant with DAPI (Thermo Fischer Scientific) was used before coverslipping. Fluorescent images were acquired on a Zeiss LSM 710 confocal microscope and image software (Zen 2009; Carl Zeiss). An argon laser at 488 nm and a helium/neon laser at 633 nm were used for excitation. Images were captured under ×10 objective magnification. Color images were acquired using a sequential scan mode.
Histology.
At 0 (control), 5, 15, and 60 min after injury, and after blood collection, the rats were perfused with saline, the trachea was cannulated, and the lungs were inflated with PLP. The lungs were removed and fixed with PLP for 24 h at 4°C, embedded in paraffin, and sectioned at 4-μm thickness. After deparaffinization and rehydration, the sections were stained with hematoxylin and eosin (H&E) and scored in a blinded manner. To generate the lung injury score, 14 fields were evaluated on each slide at ×400 magnification. Within each field, points were assigned according to a standardized protocol (34). All of the points for each category were added and weighed and the injury score was calculated according to the following formula: injury score = (alveolar hemorrhage points/number of field) + 2 × (alveolar infiltrate points/number of fields) + 3 × (fibrin points/number of fields) + (alveolar septal congestion/number of fields).
Bronchial alveolar lavages and measurement of tumor necrosis factor alpha (TNF-α) in bronchoalveolar fluid by enzyme immunoassay.
At 0 (control), 5, 15, and 60 min after injury, the rats were anesthetized with isoflurane. The trachea was then exposed and a 18-gauge needle was inserted and tied into the trachea. Lungs were lavaged three times with 2 ml saline and the pooled lavage fluid was recovered. Total leukocyte and red blood cell (RBC) counts were determined using a hemocytometer under a light microscopy and RBC counts were classified according to one of five criteria [1) <10 × 104/ml; 2) 10–35 × 104/ml; 3) 35–60 × 104/ml; 4) 60–85 × 104/ml; and 5) >85 × 104/ml]. The samples were applied to slides using cytospin and stained with Wright-Giemsa (Volu-Sol, Salt Lake City, UT) for cellular differentials. At least 200 leukocytes per sample were identified and counted. Activated macrophages were identified by size and granular appearance (25). The remainder of the lavage fluid was centrifuged at 300 g for 10 min, and the supernatant was removed and stored in individual aliquots at −80°C for enzyme immunoassay. Tumor necrosis factor-α (TNF-α) levels in bronchoalveolar fluid (BALF) were determined in duplicate using a commercially available rat TNF-α ELISA kit (ER3TNFA; Thermo Fischer Scientific) per the manufacturer's recommended protocol. The absorbance was read at 450 nm on a spectrophotometer within 10 min of stopping the reactions.
Immunohistochemistry and quantitation.
For TF immunostains, the primary antibody was rabbit anti-human TF monoclonal antibody [EPR8986] (ab151748; ABCAM; 1:600 dilutions) and the secondary antibody was horseradish peroxidase (HRP)-conjugated donkey anti-rabbit IgG polyclonal antibody (Jackson ImmunoResearch Laboratories; 1:400 dilutions). For fibrin(ogen) immunostains, the primary antibody was goat anti-mouse fibrin(ogen) polyclonal antibody (Nordic MUbio, Susteren, The Netherlands; 1:700 dilutions) and the secondary antibody was HRP-conjugated rabbit anti-goat IgG polyclonal antibody (Bio-Rad, Hercules, CA; 1:500 dilutions). For GFAP immunostains, the primary antibody was goat-anti-human GFAP polyclonal antibody (ab53554; ABCAM; 1:1000 dilutions) and the secondary antibody was HRP-conjugated rabbit anti-goat IgG polyclonal antibody (Bio-Rad; 1:500 dilutions). Antigen-retrieval was as described for the immunofluorescence studies on lung tissue. Endogenous peroxidase activity was blocked using a 3% hydrogen peroxide solution. Sections were blocked with 20% normal donkey serum (Jackson ImmunoResearch Laboratories) for TF immunostains or 20% normal rabbit serum (Jackson ImmunoResearch Laboratories) for fibrin(ogen) and GFAP immunostains. The antigens were labeled with the primary antibodies overnight at 4°C. After being washed, the samples were incubated with the secondary antibody at room temperature for 1 h. The chromogenic substrate for all the immunostains was diaminobenzidine (DAB; Vector Laboratories, Burlingame, CA) and counterstaining was done with hematoxylin.
The slides were mounted, digitally scanned at ×40 magnification, and analyzed with an Aperio CS Digital Slide Scanner (Leica Biosystems, Buffalo Grove, IL). Ten areas (800 × 800 μm), which did not contain large vessels and bronchi, were randomly selected from each lung sample (3 from the left lung, 3 from the cardiac lobe, 2 from the diaphragmatic lobe, and 2 from the azygos lobe). The automatic object counting and measuring processes were used to quantitate the immunoreactivity of the sections. These data generated a percentage of positively stained areas, and the values were expressed as a labeling index.
Statistical analysis.
Each experiment represented four to six independent determinations. Injured rats were compared with noninjured control rats for each test using unpaired t-testing. For studies using Refludan, injured rats were compared with injured rats pretreated with Refludan using unpaired t-testing. The data are expressed as the mean ± SE.
RESULTS
TBI-induced pulmonary edema without enhanced norepinephrine production.
Lungs are among the most susceptible organs affected by TBI. Therefore, lung edema was measured to evaluate the systemic effects of TBI. To assess lung water content during the acute phase of TBI-induced lung injury, the lung wet-to-dry weight ratio was measured. Compared with the control group, pulmonary edema increased significantly both at 5 and 60 min after TBI (6.10 ± 0.08 and 6.59 ± 0.30, respectively, vs. 4.89 ± 0.20 for controls; Fig. 1A). To classify the pulmonary edema as hydrostatic or high-permeability edema, the EB technique, which is widely used to study lung permeability and vascular leakage, was performed. An increase in pulmonary capillary permeability was detected at both 5 and 60 min after TBI (38.4 ± 6.5 and 62.3 ± 16.4 μg EB/g dry lung, respectively, vs. 16.4 ± 6.24 μg EB/g dry lung for controls; Fig. 1B). From 5 min following TBI, capillary permeability and pulmonary edema continued to increase through the 60-min time point.
Fig. 1.
Analysis of lung wet/dry weight ratios, pulmonary capillary permeability, and plasma norepinephrine levels after traumatic brain injury (TBI). A: lung wet/dry weight ratio of control (C) and 5 and 60 min after TBI. B: capillary permeability in lungs using the Evans blue assay of C and 5 and 60 min after TBI. C: concentration of plasma norepinephrine of the C and 5, 15, and 60 min after TBI groups. Data are represented as the mean ± SE (n = 4 to 6 per group). *P < 0.05, **P < 0.01, compared with the control.
It is known that norepinephrine increases pulmonary capillary pressure and enhances fluid filtration into the pulmonary interstitium (42). Therefore, plasma norepinephrine levels were measured using an ELISA kit to evaluate whether norepinephrine played a role in this regard. Compared with the control group, the concentration of plasma norepinephrine remained unchanged after TBI (801.2 ± 62.9, 815.4 ± 55.0, and 693.6 ± 69.8 pg/ml, respectively, vs. 862.7 ± 117.0 pg/ml for controls; Fig. 1C). These results demonstrate that the high-permeability pulmonary edema occurred without involving norepinephrine.
TBI-induced systemic platelet dysfunction.
Plasma hemostasis parameters and CBCs were measured 5, 15, and 60 min after TBI. Compared with the control group, PT and aPTT remained unchanged after TBI (Fig. 2, A and B) while the concentration of fibrinogen decreased slightly 5 min after TBI (270.0 ± 9.2 vs. 295.9 ± 7.9 mg/dl; Fig. 2C).
Fig. 2.
Analysis of plasma hemostasis parameters, platelet counts, and platelet mapping after TBI. A, B, and C: hemostasis parameters using a Diagnostica Stago STArt4 Hemostasis Analyzer of control (C) and 5, 15, and 60 min after TBI [A: prothrombin time (PT); B: activated partial thromboplastin time (aPTT); C: fibrinogen]. D: platelet counts of C and 5, 15, and 60 min after TBI groups. E and F: TEG platelet mapping kit was used to assess platelet function. The response of platelets to agonists for C and 5, 15, and 60 min after TBI [E: arachidonic acid (AA); F: adenosine diphosphate (ADP)]. Data are represented as the mean ± SE. *P < 0.05, **P < 0.01, compared with the control.
While platelet counts remained unchanged after TBI (Fig. 2D), a platelet function test was employed using platelet mapping and TEG. The extent that AA (%) and ADP (%) stimulated a platelet response was determined at early time points after TBI. Significant changes were observed for AA stimulation throughout all time points (5, 15, and 60 min) after TBI compared with control rats (17.9 ± 7.2, 31.6 ± 7.9, and 17.5 ± 5.5%, respectively, vs. 58.3 ± 7.4% for controls; Fig. 2E). Significant changes were also observed for ADP stimulation at 15 min after TBI (33.7 ± 7.1 vs. 51.7 ± 3.7%), and the response to ADP recovered 60 min after TBI (Fig. 2F). These results demonstrate that platelet responses to AA and ADP diminished at early time points after TBI.
TBI-induced systemic release of TF.
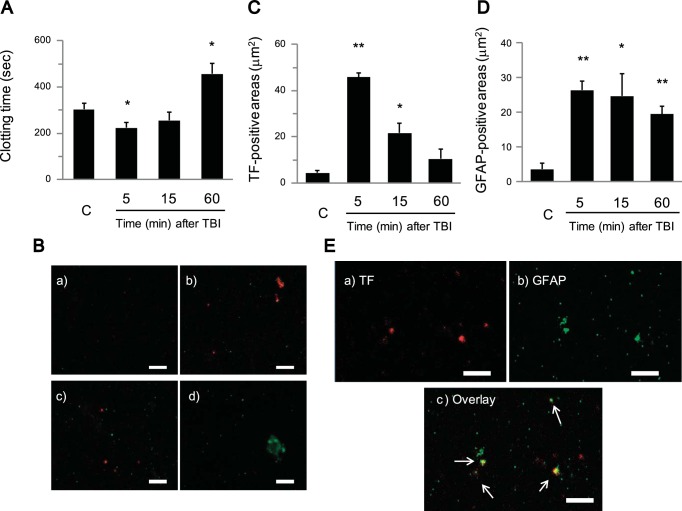
Although plasma hemostasis parameters (PT, aPTT, and fibrinogen) were normal, the responses to platelet stimulation indicated a potential systemic activation of the coagulation cascade leading to platelet dysfunction. Compared with the control group, clotting time (plasma + Ca2+) shortened at 5 min after TBI (224.2 ± 23.9 s vs. 304.1 ± 26.6 s) but became prolonged 60 min after TBI (457.1 ± 44.5 s; Fig. 3A). This result implied that activation of coagulation occurred during early time points after TBI. A potential candidate, based on evidence of vascular damage after TBI, is TF. Therefore, TF in plasma after TBI was evaluated through immunofluorescent techniques using confocal microscopy. TF-positive particles were observed in plasma, especially 5 min after TBI, and GFAP-positive particles, a neuron-specific marker, were also observed after TBI (Fig. 3B). TF-positive areas significantly increased (45.85 ± 1.70 vs. 4.43 ± 1.14 μm2 for controls) 5 min after TBI and then decreased 60 min after TBI (Fig. 3C). On the other hand, GFAP-positive areas increased throughout all time points (5, 15, and 60 min) after TBI (26.43 ± 2.53, 24.72 ± 6.50, 19.50 ± 2.30 μm2, respectively, vs. 3.59 ± 1.69 μm2 for controls; Fig. 3D). TF and GFAP were observed on some of the same particles at 5 min after TBI, which indicates that TF derived from brain tissue was released into the systemic circulation immediately after TBI and was potentially responsible for initiating systemic activation of the coagulation pathway (Fig. 3E).
Fig. 3.
Analysis of systemic Tissue Factor (TF) as measured by clotting time and confocal microscopy of plasma after TBI. A: clotting time using plasma samples of control (C) and 5, 15, and 60 min after TBI. Clotting time was determined after recalcification of platelet-poor plasma (PPP). B: representative confocal microscopic images of plasma smears from C and 5, 15, and 60 min after TBI and stained with Alexa Fluor 647 (red) conjugated to anti-human TF and Alexa Fluor 488 (green) conjugated to anti-human glial fibrillary acidic protein (GFAP) (a: C; b: 5 min after TBI; c: 15 min after TBI; d: 60 min after TBI; scale bars = 5 μm). On the plasma smears, TF (red) was observed, especially 5 min after TBI, and GFAP (green) was detected after TBI. C and D: quantitative immunofluorescent analysis of TF (red) (C) and GFAP (green) (D)-positive areas after TBI on plasma smears using the captured confocal microscopic images and analyzed by Imaris software. E: confocal microscopic image of plasma smears 5 min after TBI [a: TF (red); b: GFAP (green); c: overlay; scale bars = 5 μm]. Colocalization of TF with GFAP was observed on some particles (arrows). Data represented as the mean ± SE (n = 5 to 6 per group). *P < 0.05, **P < 0.01, compared with the control.
TBI-induced ALI at early time points after TBI.
Since pulmonary edema was confirmed in the lungs after TBI, histological analysis was performed on the lungs to determine if this led to ALI. Gross observations indicated that there were some bleeding areas on the surface of the lung tissues after TBI (Fig. 4A). Microscopic analysis demonstrated that TBI induced substantial changes in the lung including alveolar hemorrhage, alveolar wall thickening, and exudates in the alveolar spaces (Fig. 4B). The ALI score utilizing a reference scoring system (34) indicated that ALI occurred as early as 5 min after TBI (6.13 ± 0.53 vs. 3.07 ± 0.33; Fig. 4C) and the ALI score remained significantly higher 15 and 60 min after TBI (8.01 ± 0.33 and 8.17 ± 0.68, respectively).
Fig. 4.
Histopathological analysis of the lungs after TBI. A: gross appearance after TBI showing some bleeding on the lung surface (arrows). B: representative photomicrographs of lung tissue stained with hematoxylin and eosin (H&E) (a and b: control; c and d: 15 min after TBI; original magnification a and c: ×100; b and d: ×400). H&E staining of control lung tissue showing normal lung architecture. H&E staining of lung tissue after TBI showing alveolar wall thickening, alveolar hemorrhage, and exudates in the alveolar spaces. C: quantitative analysis of acute lung injury (ALI) using the lung injury score for control (C) and 5, 15, and 60 min after TBI. ALI was scored according to a standardized protocol. Data are represented as the mean ± SE (n = 5 to 6 per group). **P < 0.01, compared with the control.
Alveolar hemorrhage and activated macrophages in BALF after TBI.
Alveolar hemorrhaging and inflammation in the lung were investigated using BALF samples. The RBC score, which reflects alveolar hemorrhage, 5 min after TBI, was significantly higher than the control group (2.82 ± 0.49 vs. 1.20 ± 0.20; Fig. 5A) and the RBC score remained significantly higher 15 and 60 min after TBI (3.07 ± 0.60 and 3.90 ± 0.53, respectively). From Wright-Giemsa staining of BALF, a large number of RBC were observed after TBI, especially in the 60-min sample (Fig. 5B). Alveolar hemorrhaging was also observed at early time points after TBI, and this result confirmed the histological observations (Fig. 4). These results indicate that lung endothelial damage occurred shortly after TBI.
Fig. 5.
Analysis of alveolar hemorrhage and inflammatory cells in bronchoalveolar fluid (BALF) after TBI. Leukocytes and red blood cells (RBCs) in BALF were determined using a hemocytometer under a light microscope, and BALF was centrifuged onto microscope slides using a cytospin. A: Quantitative analysis of alveolar hemorrhage in BALF using the RBC score [1) <10 × 104/ml; 2) 10–35 × 104/ml; 3) 35–60 × 104/ml; 4) 60–85 × 104/ml; and 5) >85 × 104/ml]. B: representative photomicrographs of the BALF stained with Wright-Giemsa (a: control; b: 5 min; c: 60 min after TBI; original magnification a–c: ×200) showing a large number of RBC after TBI. C: Total leukocyte (Total), macrophage (Mac), lymphocyte (Lym), and neutrophil (Neu) counts in BALF. Data are represented as the mean ± SE (n = 5 to 6 per group). *P < 0.05, **P < 0.01, compared with control.
The total number of leukocytes remained unchanged in BALF as well as the number of macrophages, lymphocytes, and neutrophils at any time point within 60 min after TBI (Fig. 5C). While macrophages were the predominant cell type at any time point, neutrophils were ∼1% of the total cell number. The concentration of TNF-α, a mediator of neutrophil infiltration (29, 38), in BALF significantly increased 60 min after TBI compared with the control group (426.2 ± 50.38 pg/ml vs. nondetected controls; Fig. 6A) and may explain why neutrophil levels at these early time points were low. Although the number of alveolar macrophages remained unchanged, the activation status, as determined by the increased size and vacuole quantity (Fig. 6B), increased 5, 15, and 60 min after TBI compared with the control group (43.7 ± 3.42, 47.9 ± 4.13, and 53.8 ± 2.40%, respectively, vs. 30.3 ± 2.20% for controls; Fig. 6C). These results imply that alveolar macrophages are activated after TBI and may be responsible for the observed secondary injury.
Fig. 6.
Analysis of TNF-α levels and activated macrophages in BALF after TBI. A: concentration of TNF-α in BALF of the control (C) and 5, 15, and 60 min after TBI groups. B: photomicrograph of the BALF stained with Wright-Giemsa showing activated macrophages (arrows) 60 min after TBI (original magnification: ×400). C: quantitation of activated macrophages of C and 5, 15, and 60 min after TBI. Activated macrophages were identified by size and granular appearance. Data are represented as the mean ± SE (n = 4 to 6 per group). *P < 0.05, **P < 0.01, compared with control.
TBI-induced TF and fibrin(ogen) deposition in lung tissue.
To evaluate TF in the lung after TBI, immunohistochemical analysis was performed. In the control sample, TF was observed only on bronchial epithelial regions (Fig. 7A), while in the 60-min sample, TF was stained entirely in the alveolar region, and some macrophages were positive for TF (Fig. 7B). In the 5-min sample, TF-positive granules, potentially microparticles, were observed on the epithelial cells and alveolar macrophages (Fig. 7C). Compared with the control group, a biphasic elevation of TF was observed after TBI. The TF labeling index was elevated 5 and 60 min after TBI (9.13 ± 2.03 and 14.1 ± 2.08%, respectively, vs. 1.84 ± 0.47% for controls; Fig. 7D). Some of the particles were stained with GFAP at 5 min after TBI (Fig. 8A). Fewer GFAP-positive particles were observed in controls and later time points in TBI-challenged rats (15 and 60 min after TBI; Fig. 8B). Confocal microscopy for GFAP and TF in lung tissue demonstrated enhanced GFAP levels at 5 min relative to later time points, and dual appearance of TF and GFAP can be observed on some particles (Fig. 9). TF was also elevated 5 min after TBI and then decreased in plasma (Fig. 3C). However, it increased at 60 min after TBI only in lung tissue (Fig. 7D).
Fig. 7.
Immunohistochemical analysis of TF in the lung after TBI. Representative photomicrographs of lung tissue immunostained for TF after TBI (A: control; B: 60 min after TBI; original magnification: ×100, brown = positive staining). A: in control lung tissue, TF was detected in the bronchial epithelial region; B: while it was detected entirely in the alveolar region 60 min after TBI. Inset: some macrophages stained positive for TF (arrowheads) (original magnification: ×400). C: TF-positive granules (arrows) were observed at 5 min after TBI (original magnification: ×400). D: quantitative immunohistochemical analysis of TF in the lung of control (C) and 5, 15, and 60 min after TBI. Data are represented as the mean ± SE (n = 5 to 6 per group). *P < 0.05, **P < 0.01, compared with the control.
Fig. 8.
Immunohistochemical analysis of GFAP in the lung after TBI. A: photomicrograph of lung tissue immunostained for GFAP 5 min after TBI (original magnification: ×400, brown = positive staining). GFAP stain of the lungs shows some GFAP-positive particles in lung tissues 5 min after TBI (arrows). B: representative photomicrographs of lung tissue immunostained for GFAP after TBI (a: control; b: 5 min; c: 15 min; d: 60 min after TBI; original magnification: ×400). GFAP-positive particles were diminished at later time points (15 and 60 min after TBI).
Fig. 9.
Immunofluorescent analysis of GFAP and TF in lung tissue after TBI. Representative confocal microscopic images of lung tissues from C and 5, 15, and 60 min after TBI and stained with Alexa Fluor 647 (red) conjugated to anti-human TF and Alexa Fluor 488 (green) conjugated to anti-human GFAP (A: C; B: 5 min after TBI; C: 15 min after TBI; D: 60 min after TBI; scale bars = 50 μm). On the lung tissues, TF (red) was observed on the alveolar walls after TBI, and TF-positive particles were detected 5 min after TBI (arrows). GFAP (green)-positive particles were also observed especially 5 min after TBI. Some particles appear to present with both TF and GFAP (arrowheads).
To determine whether fibrin deposition occurred in the alveolar region, fibrin(ogen) immunohistochemistry was performed. Compared with the control group (Fig. 10A), fibrin(ogen) deposition was observed on the alveolar surfaces (Fig. 10B). Five minutes after TBI, light-positive areas, which seemed to be fibrin strands exuding from the vessels, were seen in the alveolar region (Fig. 10C). The fibrin(ogen) labeling index was elevated at 5 min after TBI (2.98 ± 0.91 vs. 0.89 ± 0.15%) and was significantly enhanced at 15 and 60 min after TBI (3.50 ± 0.48 and 2.80 ± 0.38%, respectively; Fig. 10D). These results demonstrate that TF in the alveolar region initiated the extrinsic coagulation cascade resulting in fibrin deposition after TBI (Fig. 10B).
Fig. 10.
Immunohistochemical analysis of fibrino(gen) in the lung after TBI. Representative photomicrographs of lung tissue immunostained for fibrin(ogen) after TBI (A: control; B: 60 min after TBI; original magnification: ×200, brown = positive staining). A: in the control lung sample, fibrin(ogen) was not observed in the alveolar region; B: while fibrin(ogen) deposition was detected in the alveolar wall 60 min after TBI. C: faint fibrin(ogen) staining was identified in the alveolar region 5 min after TBI (original magnification: ×400). D: quantitative immunohistochemical analysis of fibrin(ogen) in the lung of control (C) and 5, 15, and 60 min after TBI. Data are represented as the mean ± SE (n = 5 to 6 per group). **P < 0.01, compared with the control.
The thrombin inhibitor Refludan attenuated lung injury after TBI.
Studies have demonstrated that administration of the thrombin inhibitor Refludan results in a protective effect on platelet function after TBI (12). In the current study, the effects of Refludan on ALI after TBI were evaluated. From gross analysis, only slight bleeding was observed on the lung surface in rats pretreated with Refludan (Fig. 11A). Control lung samples with or without pretreatment with Refludan did not exhibit any abnormalities. However, TBI samples not pretreated with Refludan displayed hemorrhage and alveolar wall thickening, which were attenuated in the lung samples in rats pretreated with Refludan (Fig. 11B). In the control group, the ALI score of lung samples without or with Refludan were almost the same. Compared with TBI rats without Refludan, rats pretreated with Refludan demonstrated significantly lower ALI scores both at 5 and 60 min after TBI (3.81 ± 0.36, vs. 6.13 ± 0.53, 4.44 ± 0.19, vs. 8.17 ± 0.68, respectively; Fig. 11C). Therefore, thrombin inhibition attenuates ALI and diminishes platelet dysfunction after TBI. These results indicate a direct role for excess thrombin production in ALI following TBI.
Fig. 11.
Effects of preadministration of Refludan on ALI after TBI. A: gross appearance of lungs after TBI pretreated with Refludan showing a small blood spot on the lung surface (arrow). B: representative photomicrographs of the lung tissues stained with H&E after TBI with or without pretreatment with Refludan [control (a), control pretreated with Refludan (b), 5 min after TBI (c), 5 min after TBI pretreated with Refludan (d), 60 min after TBI (e), 60 min after TBI pretreated with Refludan (f); original magnification: ×200]. a And b: control lungs from rats with or without pretreatment with Refludan showing no major histological abnormalities. c And e: lung tissue from rats 5 and 60 min after TBI showing alveolar hemorrhage and alveolar wall thickening. d And f: lung tissue from rats 5 and 60 min after TBI pretreated with Refludan showing less hemorrhage and alveolar congestion. C: quantitative analysis of ALI after TBI with or without Refludan pretreament. Data are represented as the mean ± SE (n = 5 to 6 per group). **P < 0.01, compared with injured rats pretreated with Refludan.
DISCUSSION
Acute changes in lungs and coagulopathy associated with TBI were evaluated using a rat blunt TBI model. Early time points after TBI (within 60 min) were analyzed to minimize confounding changes induced by secondary events. The main findings of this study are that TBI is associated with increased pulmonary capillary permeability, alveolar hemorrhage, and enhanced systemic TF and lung TF and fibrin(ogen). These phenomena occurred within 60 min after TBI without affecting norepinephrine elevation and TBI-induced ALI was attenuated by preadministration of the direct thrombin inhibitor, Refludan. To our knowledge, these findings suggest, for the first time, that the release of TF after TBI and sequential systemic activation of the coagulation pathway may induce lung injury after TBI.
Pulmonary edema following a significant central nervous system insult has been recognized for over a century (9). Activation of the sympathetic nervous system and the release of catecholamines are thought to be the main cause of pulmonary edema after TBI (11, 43). Although hydrostatic pressures may play a role in this pathogenesis, this mechanism alone cannot explain the presence of RBCs and protein observed in the alveolar fluid in subjects after central nervous system insult (1, 49). Indeed, in the current study, pulmonary capillary permeability and pulmonary wet/dry weight ratios were increased as early as 5 min after TBI. Norepinephrine is a catecholamine that increases pulmonary capillary pressure and enhances fluid filtration into the pulmonary interstitium (42). In patients with TBI, plasma norepinephrine levels are predictive of Glasgow Coma Scale at 1 wk, survival, number of ventilator days, and the length of hospital stay (55). In a previous study, plasma norepinephrine levels increased significantly 6 h after brain injury in rats (48). However, plasma norepinephrine levels remained unchanged within 60 min after TBI in the present study. These results indicate that high permeability pulmonary edema occurred without affecting norepinephrine elevation.
Retrospective and observational studies have consistently shown that coagulopathy is common in patients with TBI (18, 45). Additionally, tissue hypoperfusion-mediated activation of protein C has been shown to be a central component in the development of the coagulopathy that follows TBI (5). However, hypoperfusion, manifest by a significant base deficit or hypotension is rare in isolated TBI (2). In fact, there were no rats that were in a state of shock when the samples were harvested in this experiment. On the other hand, other studies implicate TF release from the injured brain, a rich source of TF (10, 15), as a contributor to platelet dysfunction and coagulopathy (27, 31). In an earlier rat blunt trauma brain study, subarachnoid hemorrhage was a consistent finding and systemic platelet responses to agonists were diminished as early as 15 min after TBI (12). In the current study, the calcium-induced clotting time was shortened and systemic TF expression increased 5 min after TBI and recovered 60 min after TBI. These results indicate that TF was released into systemic circulation immediately after blood-brain barrier (BBB) disruption after TBI. In a recent paper, brain-derived MPs reached peak levels 3 h after fluid percussion brain injury. However, the amount of TF and the result within 30 min after injury were not evaluated in that study (47). In an isolated TBI rodent study, we hypothesized that brain-derived TF is largely unsaturated by factor VIIa (20) due to confinement by the BBB (2) and that the release of TF into the circulation, due to disruption of the BBB, results in the interaction with systemic Factor VIIa thereby stimulating the activation of the coagulation cascade and thrombin production. This mechanism appears to be operative in the case of TBI-induced ALI whereby the activation of coagulation is initiated by these early brain-localized events. Therefore, we speculate that the primary pathway in this model is associated with the TF-initiated extrinsic pathway, while components of the intrinsic pathway may be amplified downstream of this effect. Therefore, both coagulation pathways may be activated after TBI in this model but the initiating factor for activation of coagulation is believed to be mediated by TF, which ultimately leads to lung injury. In this study, PT and aPTT were unchanged after TBI. PT and aPTT are insensitive to coagulopathy, except in severe cases, and are unable to assess platelet function status (54). PT and aPTT did not change during the proposed thrombin burst, which suggests that this burst is not enough to alter the plasma-based PT and aPTT. Further, in this study, platelet activation by ADP was diminished 15 min after TBI and recovered 60 min after TBI. This result indicates that platelet mapping is sensitive to this altered thrombin level.
Endothelial cell barrier disruption and increased vascular permeability are hallmark characteristics of ALI. In this study, alveolar hemorrhage and high permeability pulmonary edema occurred immediately after TBI, similarly to the increased presence of systemic TF. An activated complex of TF and Factor VIIa triggers procoagulant activity systemically. In a previous report, a Factor VIIa inhibitor (a competitor for FVIIa/TF interaction) prevented protein leakage, and pulmonary edema and reduced alveolar inflammation and fibrin deposition in a LPS-induced ALI rat model (37). Additionally, in this study a direct inhibitor of thrombin, Hirulog, also inhibited protein leakage in this model. In our study, a direct thrombin inhibitor, Refludan, attenuated the lung injury score as early as 5 min after TBI. Thrombin is generated early during ALI and is thought to contribute to increased lung permeability (16, 26). Thrombin has direct effects on the endothelial barrier via alterations in the cytoskeleton, which has been identified as a critical regulator of vascular barrier integrity (14). In a previous study, thrombin exposure resulted in increased pulmonary edema in vivo (24) and thrombin has direct effects on endothelial permeability (32). These results indicate that systemic thrombin activation alters the pulmonary endothelial barrier, which contributes to ALI. In coagulation, thrombin not only converts fibrinogen to fibrin but also activates platelets (17). Initial increased pulmonary vascular permeability allows influx of fibrinogen and coagulation factors into the airway (50), and the presence of extravascular TF could initiate clotting in the alveolar lesions. In this study, TF-MPs and some fibrin(ogen) were observed in the alveolar regions 5 min after TBI, and fibrin depositions increased 15 min after TBI. Excessive fibrin deposition can elicit respiratory dysfunction and airway obstruction during ALI (23). Studies have shown that intracranial production of proinflammatory mediators is released into the systemic circulation after TBI (36). In particular, high-mobility group box-1 (HMGB1) protein with receptor for advanced glycation end products (RAGE) is a major contributor to pulmonary dysfunction in lung transplantation from TBI donors (53). Although ALI and vascular permeability were confirmed as early as 4 h after brain injury, HMGB1 increased 24 h after TBI (53). These pro-inflammatory mediators might be related to secondary injuries in the subsequent hours and days after TBI. These results imply that TF-MPs influx from injured brain and systemic thrombin activation induce lung injury within minutes as a first wave response and then the entry of proinflammatory mediators into the systemic circulation exacerbate ALI hours and days after the initial BBB disruption.
In this study, biphasic appearance of TF was observed in lung tissue. TF-positive MPs were observed 5 min after TBI and this first phase of TF expression was thought to be MPs, which were shed from brain neurons, platelets, monocyte/macrophages, and endothelial cells in response to the injury (56). Despite systemic TF returning to baseline levels 60 min after TBI, TF in the lung was enhanced. In the lungs, alveolar epithelial cells and macrophages stained positive for TF 60 min after TBI, accompanied by an increase in the levels of activated macrophages and TNF-α in the BALF after TBI. Monocyte and endothelial cells express TF only after induction of stimulating factors such as injury and inflammation (56) and stimulated alveolar macrophages are known to produce and secrete TF (3). This would appear to be an initiator of the secondary phase of injury. Once primed, the system is then vulnerable to normally innocuous secondary inflammatory insults, such as infections, mechanical stress induced by mechanical ventilation, and surgical procedures.
Rats and humans exhibit some physiological differences such as heart rate, respiratory rate, and metabolism. Although the timing of the events following TBI are not completely the same in both species, for the most part the phenomena and results have many similarities between rats and humans. As in humans, neurogenic pulmonary edema has been described with the development of symptoms within minutes to hours following neurologic injury (6, 9). Thrombin-mediated hypercoagulopathy was reported from minutes to up to 4 h after TBI in a human study (22). In the current study, permeability pulmonary edema was observed as early as 5 min after TBI, and preadministration of the thrombin inhibitor Refludan effectively attenuated the early phase of ALI. We speculate that Refludan inhibited the pathological thrombin in the hypercoagulopathy phase after TBI in our model.
In conclusion, the results of the present study indicate that the release of TF and the activation of the coagulation pathways correlated with ALI after TBI. Since a direct thrombin inhibitor attenuates vascular endothelial damage and injury score in the lung as well as platelet dysfunction, this would implicate a direct role for thrombin in these pathophysiologies of ALI following TBI. Results from these studies offer potential targets for preventing pulmonary complications in patients after TBI.
GRANTS
This study was supported by an Indiana Spinal Cord and Brain Injury Grant from the Indiana State Department of Health (to V. A. Ploplis) and, in part, National Heart, Lung, and Blood Institute Grant HL-019982 (to F. J. C).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
H.Y., M.W., F.J.C., and V.A.P. conception and design of research; H.Y. and D.L.D. performed experiments; H.Y., D.L.D., M.W., F.J.C., and V.A.P. analyzed data; H.Y., D.L.D., M.W., F.J.C., and V.A.P. interpreted results of experiments; H.Y. and V.A.P. prepared figures; H.Y. and V.A.P. drafted manuscript; H.Y., D.L.D., M.W., F.J.C., and V.A.P. edited and revised manuscript; H.Y., D.L.D., M.W., F.J.C., and V.A.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank staff at the Freimann Life Science Center for assistance in the husbandry of the rats used in this study and Haemonetics for platelet mapping reagents.
REFERENCES
- 1.Carlson RW, Schaeffer RC Jr, Michaels SG, Weil MH. Pulmonary edema following intracranial hemorrhage. Chest 75: 731–734, 1979. [DOI] [PubMed] [Google Scholar]
- 2.Castellino FJ, Chapman MP, Donahue DL, Thomas S, Moore EE, Wohlauer MV, Fritz B, Yount R, Ploplis V, Davis P, Evans E, Walsh M. Traumatic brain injury causes platelet adenosine diphosphate and arachidonic acid receptor inhibition independent of hemorrhagic shock in humans and rats. J Trauma Acute Care Surg 76: 1169–1176, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chapman HA Jr, Allen CL, Stone OL, Fair DS. Human alveolar macrophages synthesize factor VII in vitro. Possible role in interstitial lung disease. J Clin Invest 75: 2030–2037, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen HI, Sun SC, Chai CY. Pulmonary edema and hemorrhage resulting from cerebral compression. Am J Physiol 224: 223–229, 1973. [DOI] [PubMed] [Google Scholar]
- 5.Cohen MJ, Brohi K, Ganter MT, Manley GT, Mackersie RC, Pittet JF. Early coagulopathy after traumatic brain injury: the role of hypoperfusion and the protein C pathway. J Trauma 63: 1254–1261; discussion 1261–1252, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Colice GL. Neurogenic pulmonary edema. Clin Chest Med 6: 473–489, 1985. [PubMed] [Google Scholar]
- 7.Coronado VG, Xu L, Basavaraju SV, McGuire LC, Wald MM, Faul MD, Guzman BR, Hemphill JD. Surveillance for traumatic brain injury-related deaths–United States, 1997–2007. MMWR Surveill Summ 60: 1–32, 2011. [PubMed] [Google Scholar]
- 8.Davis PK, Musunuru H, Walsh M, Cassady R, Yount R, Losiniecki A, Moore EE, Wohlauer MV, Howard J, Ploplis VA, Castellino FJ, Thomas SG. Platelet dysfunction is an early marker for traumatic brain injury-induced coagulopathy. Neurocrit Care 18: 201–208, 2013. [DOI] [PubMed] [Google Scholar]
- 9.Davison DL, Terek M, Chawla LS. Neurogenic pulmonary edema. Crit Care 16: 212, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.del Zoppo GJ, Yu JQ, Copeland BR, Thomas WS, Schneiderman J, Morrissey JH. Tissue factor localization in non-human primate cerebral tissue. Thromb Haemost 68: 642–647, 1992. [PubMed] [Google Scholar]
- 11.Demling R, Riessen R. Pulmonary dysfunction after cerebral injury. Crit Care Med 18: 768–774, 1990. [DOI] [PubMed] [Google Scholar]
- 12.Donahue DL, Beck J, Fritz B, Davis P, Sandoval-Cooper MJ, Thomas SG, Yount RA, Walsh M, Ploplis VA, Castellino FJ. Early platelet dysfunction in a rodent model of blunt traumatic brain injury reflects the acute traumatic coagulopathy found in humans. J Neurotrauma 31: 404–410, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drake TA, Morrissey JH, Edgington TS. Selective cellular expression of tissue factor in human tissues. Implications for disorders of hemostasis and thrombosis. Am J Pathol 134: 1087–1097, 1989. [PMC free article] [PubMed] [Google Scholar]
- 14.Dudek SM, Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol (1985) 91: 1487–1500, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Fleck RA, Rao LV, Rapaport SI, Varki N. Localization of human tissue factor antigen by immunostaining with monospecific, polyclonal anti-human tissue factor antibody. Thromb Res 59: 421–437, 1990. [DOI] [PubMed] [Google Scholar]
- 16.Fuchs-Buder T, de Moerloose P, Ricou B, Reber G, Vifian C, Nicod L, Romand JA, Suter PM. Time course of procoagulant activity and D dimer in bronchoalveolar fluid of patients at risk for or with acute respiratory distress syndrome. Am J Respir Crit Care Med 153: 163–167, 1996. [DOI] [PubMed] [Google Scholar]
- 17.Furie B, Furie BC. In vivo thrombus formation. J Thromb Haemost 5, Suppl 1: 12–17, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Harhangi BS, Kompanje EJ, Leebeek FW, Maas AI. Coagulation disorders after traumatic brain injury. Acta Neurochir (Wien) 150: 165–175; discussion 175, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Heuer JF, Selke M, Crozier TA, Pelosi P, Herrmann P, Perske C, Quintel M. Effects of acute intracranial hypertension on extracerebral organs: a randomized experimental study in pigs. J Neurol Surg A Cent Eur Neurosurg 73: 289–295, 2012. [DOI] [PubMed] [Google Scholar]
- 20.Hoffman M, Monroe DM. Tissue factor in brain is not saturated with factor VIIa: implications for factor VIIa dosing in intracerebral hemorrhage. Stroke 40: 2882–2884, 2009. [DOI] [PubMed] [Google Scholar]
- 21.Holland MC, Mackersie RC, Morabito D, Campbell AR, Kivett VA, Patel R, Erickson VR, Pittet JF. The development of acute lung injury is associated with worse neurologic outcome in patients with severe traumatic brain injury. J Trauma 55: 106–111, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Hulka F, Mullins RJ, Frank EH. Blunt brain injury activates the coagulation process. Arch Surg 131: 923–927; discussion 927–928, 1996. [DOI] [PubMed] [Google Scholar]
- 23.Idell S. Coagulation, fibrinolysis, and fibrin deposition in acute lung injury. Crit Care Med 31: S213–220, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Jenkins RG, Su X, Su G, Scotton CJ, Camerer E, Laurent GJ, Davis GE, Chambers RC, Matthay MA, Sheppard D. Ligation of protease-activated receptor 1 enhances alpha(v)beta6 integrin-dependent TGF-beta activation and promotes acute lung injury. J Clin Invest 116: 1606–1614, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalsotra A, Zhao J, Anakk S, Dash PK, Strobel HW. Brain trauma leads to enhanced lung inflammation and injury: evidence for role of P4504Fs in resolution. J Cereb Blood Flow Metab 27: 963–974, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Kipnis E, Guery BP, Tournoys A, Leroy X, Robriquet L, Fialdes P, Neviere R, Fourrier F. Massive alveolar thrombin activation in Pseudomonas aeruginosa-induced acute lung injury. Shock 21: 444–451, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Kutcher ME, Redick BJ, McCreery RC, Crane IM, Greenberg MD, Cachola LM, Nelson MF, Cohen MJ. Characterization of platelet dysfunction after trauma. J Trauma Acute Care Surg 73: 13–19, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Z, Xi R, Zhang Z, Li W, Liu Y, Jin F, Wang X. 4-hydroxyphenylacetic acid attenuated inflammation and edema via suppressing HIF-1alpha in seawater aspiration-induced lung injury in rats. Int J Mol Sci 15: 12861–12884, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lukacs NW, Strieter RM, Chensue SW, Widmer M, Kunkel SL. TNF-alpha mediates recruitment of neutrophils and eosinophils during airway inflammation. J Immunol 154: 5411–5417, 1995. [PubMed] [Google Scholar]
- 30.Maas AI, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol 7: 728–741, 2008. [DOI] [PubMed] [Google Scholar]
- 31.Maegele M. Coagulopathy after traumatic brain injury: incidence, pathogenesis, and treatment options. Transfusion 53, Suppl 1: 28S–37S, 2013. [DOI] [PubMed] [Google Scholar]
- 32.Malik AB, Horgan MJ. Mechanisms of thrombin-induced lung vascular injury and edema. Am Rev Respir Dis 136: 467–470, 1987. [DOI] [PubMed] [Google Scholar]
- 33.Mascia L, Zavala E, Bosma K, Pasero D, Decaroli D, Andrews P, Isnardi D, Davi A, Arguis MJ, Berardino M, Ducati A. High tidal volume is associated with the development of acute lung injury after severe brain injury: an international observational study. Crit Care Med 35: 1815–1820, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Matute-Bello G, Winn RK, Jonas M, Chi EY, Martin TR, Liles WC. Fas (CD95) induces alveolar epithelial cell apoptosis in vivo: implications for acute pulmonary inflammation. Am J Pathol 158: 153–161, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McClellan MD, Dauber IM, Weil JV. Elevated intracranial pressure increases pulmonary vascular permeability to protein. J Appl Physiol (1985) 67: 1185–1191, 1989. [DOI] [PubMed] [Google Scholar]
- 36.McKeating EG, Andrews PJ, Signorini DF, Mascia L. Transcranial cytokine gradients in patients requiring intensive care after acute brain injury. Br J Anaesth 78: 520–523, 1997. [DOI] [PubMed] [Google Scholar]
- 37.Miller DL, Welty-Wolf K, Carraway MS, Ezban M, Ghio A, Suliman H, Piantadosi CA. Extrinsic coagulation blockade attenuates lung injury and proinflammatory cytokine release after intratracheal lipopolysaccharide. Am J Respir Cell Mol Biol 26: 650–658, 2002. [DOI] [PubMed] [Google Scholar]
- 38.Ming WJ, Bersani L, Mantovani A. Tumor necrosis factor is chemotactic for monocytes and polymorphonuclear leukocytes. J Immunol 138: 1469–1474, 1987. [PubMed] [Google Scholar]
- 39.Morel N, Morel O, Petit L, Hugel B, Cochard JF, Freyssinet JM, Sztark F, Dabadie P. Generation of procoagulant microparticles in cerebrospinal fluid and peripheral blood after traumatic brain injury. J Trauma 64: 698–704, 2008. [DOI] [PubMed] [Google Scholar]
- 40.Osterud B, Bjorklid E. Sources of tissue factor. Semin Thromb Hemost 32: 11–23, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Ploplis VA, Donahue DL, Sandoval-Cooper MJ, MorenoCaffaro M, Sheets P, Thomas SG, Walsh M, Castellino FJ. Systemic platelet dysfunction is the result of local dysregulated coagulation and platelet activation in the brain in a rat model of isolated traumatic brain injury. J Neurotrauma 31: 1672–1675, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rassler B, Reissig C, Briest W, Tannapfel A, Zimmer HG. Pulmonary edema and pleural effusion in norepinephrine-stimulated rats–hemodynamic or inflammatory effect? Mol Cell Biochem 250: 55–63, 2003. [DOI] [PubMed] [Google Scholar]
- 43.Rogers FB, Shackford SR, Trevisani GT, Davis JW, Mackersie RC, Hoyt DB. Neurogenic pulmonary edema in fatal and nonfatal head injuries. J Trauma 39: 860–866; discussion 866–868, 1995. [DOI] [PubMed] [Google Scholar]
- 44.Sebag SC, Bastarache JA, Ware LB. Therapeutic modulation of coagulation and fibrinolysis in acute lung injury and the acute respiratory distress syndrome. Curr Pharm Biotechnol 12: 1481–1496, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stein SC, Smith DH. Coagulopathy in traumatic brain injury. Neurocrit Care 1: 479–488, 2004. [DOI] [PubMed] [Google Scholar]
- 46.Sun Y, Wang J, Wu X, Xi C, Gai Y, Liu H, Yuan Q, Wang E, Gao L, Hu J, Zhou L. Validating the incidence of coagulopathy and disseminated intravascular coagulation in patients with traumatic brain injury–analysis of 242 cases. Br J Neurosurg 25: 363–368, 2011. [DOI] [PubMed] [Google Scholar]
- 47.Tian Y, Salsbery B, Wang M, Yuan H, Yang J, Zhao Z, Wu X, Zhang Y, Konkle BA, Thiagarajan P, Li M, Zhang J, Dong JF. Brain-derived microparticles induce systemic coagulation in a murine model of traumatic brain injury. Blood 125: 2151–2159, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tumer N, Svetlov S, Whidden M, Kirichenko N, Prima V, Erdos B, Sherman A, Kobeissy F, Yezierski R, Scarpace PJ, Vierck C, Wang KK. Overpressure blast-wave induced brain injury elevates oxidative stress in the hypothalamus and catecholamine biosynthesis in the rat adrenal medulla. Neurosci Lett 544: 62–67, 2013. [DOI] [PubMed] [Google Scholar]
- 49.van der Zee H, Malik AB, Lee BC, Hakim TS. Lung fluid and protein exchange during intracranial hypertension and role of sympathetic mechanisms. J Appl Physiol Respir Environ Exercise Physiol 48: 273–280, 1980. [DOI] [PubMed] [Google Scholar]
- 50.Veress LA, O'Neill HC, Hendry-Hofer TB, Loader JE, Rancourt RC, White CW. Airway obstruction due to bronchial vascular injury after sulfur mustard analog inhalation. Am J Respir Crit Care Med 182: 1352–1361, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wafaisade A, Lefering R, Tjardes T, Wutzler S, Simanski C, Paffrath T, Fischer P, Bouillon B, Maegele M. Acute coagulopathy in isolated blunt traumatic brain injury. Neurocrit Care 12: 211–219, 2010. [DOI] [PubMed] [Google Scholar]
- 52.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 342: 1334–1349, 2000. [DOI] [PubMed] [Google Scholar]
- 53.Weber DJ, Gracon AS, Ripsch MS, Fisher AJ, Cheon BM, Pandya PH, Vittal R, Capitano ML, Kim Y, Allette YM, Riley AA, McCarthy BP, Territo PR, Hutchins GD, Broxmeyer HE, Sandusky GE, White FA, Wilkes DS. The HMGB1-RAGE axis mediates traumatic brain injury-induced pulmonary dysfunction in lung transplantation. Sci Transl Med 6: 252ra124, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 54.Wohlauer MV, Moore EE, Thomas S, Sauaia A, Evans E, Harr J, Silliman CC, Ploplis V, Castellino FJ, Walsh M. Early platelet dysfunction: an unrecognized role in the acute coagulopathy of trauma. J Am Coll Surg 214: 739–746, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Woolf PD, McDonald JV, Feliciano DV, Kelly MM, Nichols D, Cox C. The catecholamine response to multisystem trauma. Arch Surg 127: 899–903, 1992. [DOI] [PubMed] [Google Scholar]
- 56.Zhang J, Jiang R, Liu L, Watkins T, Zhang F, Dong JF. Traumatic brain injury-associated coagulopathy. J Neurotrauma 29: 2597–2605, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]