Abstract
During homeostasis two distinct macrophage (Mø) populations inhabit the lungs: tissue Mø (often called interstitial Mø) and resident alveolar Mø (resAMø). During acute lung inflammation, monocytes from the circulation migrate to areas of injury where they mature into a third Mø population: recruited Mø. Resident AMø uniquely express low levels of CD11b and high levels of CD11c. In comparison, recruited Mø and tissue Mø express high levels of CD11b and low levels of CD11c. It is likely that these three Mø subpopulations play distinct roles in injury and disease states; however, tools with which to individually target or track these populations are lacking. Here we demonstrate the utility of an hCD68-rtTA transgenic system for specific, robust, and inducible targeting of CD11b+ recruited Mø and tissue Mø in the murine lung with negligible activation in resAMø. Using hCD68rtTA-GFP reporter mice, we show both during homeostasis and inflammation that administration of doxycycline induces tet-On reporter expression in recruited Mø and tissue Mø but not in resident AMø. We further demonstrate how hCD68-rtTA can be effectively combined with tet-On Cre to target these same recMø and tissue Mø. Accordingly, the hCD68-rtTA system is a powerful new tool that can be used for lineage tracing, fate mapping, and gene deletion in a variety of murine models, thereby enabling sophisticated investigation of the unique role of these CD11b+ Mø during lung heath and disease.
Keywords: CD11b+ macrophages, hCD68-rtTA, mouse model, recruited macrophages, transgenic mice
discretely targeting macrophage (Mø) subpopulations in the lung has been a challenge, limiting the ability to parse their individual roles. During homeostasis, the lung is populated by two unique sets of Mø that reside in separate compartments (10, 45). The first, resident alveolar Mø (resAMø), reside in the airspaces and airways, and express high levels of CD11c (17). In contrast, tissue Mø (often referred to as interstitial Mø) reside within the lung tissue itself, and although their precise location remains unclear, they do not appear to contact the airways lumen. Unlike resAMø these cells express high levels of CD11b and low levels of CD11c (10, 33). Both resAMø and tissue Mø arise during embryogenesis and populate the lungs at birth (45). ResAMø replicate locally throughout life without significant replacement by cells of postnatal origin (i.e., circulating monocytes) (16, 50). The turnover and replacement of tissue Mø is less clear; both local replication and low-level replacement from circulating monocytes has been suggested, likely indicative of further subtypes within the tissue Mø population (25, 44).
During inflammation, Mø pools in both the luminal and tissue compartments expand, fueled by both local replication of resident Mø, and by recruitment of circulating monocytes that subsequently mature into macrophages (26). Macrophages that are recruited to the alveolus (recMø) initially express high levels of CD11b and CX3CR1, similar to tissue Mø. During this initial period, surface markers are unable to distinguish recMø from tissue Mø. When we cannot define these macrophages by compartment (i.e., all CD11b+Mø in BAL are recMø), we call this combined population of Mø, CD11b+ Mø. However, upon continued exposure to the alveolar environment, recMø undergo significant transcriptional reprogramming, including downregulation of CD11b and upregulation of CD11c to resemble resAMø (14, 17). RecMø achieve 99% transcriptional similarity to resAMø within 8 wk of their arrival in the lungs (14).
Although little is known about the functions of tissue Mø, there is evidence that resAMø and recMø contribute uniquely and at times antagonistically to the progression of infection (20, 36), lung injury (19, 26, 37), and cancer (12, 39). Deeper study of lung Mø requires novel tools to discretely target lung Mø subpopulations in vivo (10, 35).
Murine models remain one of the most powerful and commonly used tools for studying lung disease. Transgenic mice have proven essential in discerning the distinct roles played by various immune cells, including macrophages. Whole body knockout mice continue to be useful, but conditional knockout animals, in which gene deletion is restricted to a specific subset of cells through Cre-loxP strategies, are increasingly required (30, 47). Restriction is achieved through a cell-specific promoter that drives expression of the enzyme, Cre recombinase, only in the cells that activate the chosen promoter. The gene of interest is “floxed” with LoxP sites, allowing Cre-mediated deletion only in cells where Cre is expressed. Several mouse lines have been created that harness this technology to enable deletion of floxed genes in macrophages (22). Those most commonly used employ CD11b, c-fms, and LysM promoters, which are activated in myeloid precursor cells. Deletion of floxed genes occurs as soon as the promoter is activated, even during embryogenesis, which can potentially impact Mø development and organogenesis (1, 3, 5, 8, 22). Another promoter, CD11c-cre, has been used to singly target resAMø during homeostasis (48), although it is likely that recMø would also activate CD11c-cre during inflammation. There are no transgenic systems currently known to target tissue Mø or recMø without also targeting resAMø.
The tet-On Cre system refines this conditional system by adding a layer of inducible, temporal control (52). In the tet-On Cre system, specificity is granted by a reverse tetracycline-controlled transactivator (rtTA) that is constitutively expressed in cells of interest through the selection of a particular promoter (i.e., hCD68-rtTA). rtTA remains inactive in the cell until it binds to doxycycline (generally administered in food or water). The rtTA-doxycycline complex then binds to and activates a tetracycline response element (TRE) that drives expression of Cre recombinase leading to deletion of any floxed gene(s). This inducible tet-On Cre system permits the study of genes whose conditional deletion would normally be embryonic lethal. It similarly provides the ability to assess the adult functions of genes that are not embryonic lethal, but prevent normal development of the immune system. Further, it allows for nuanced dissection of the role of individual genes in time dependent systems, such as the onset of inflammation, the resolution of inflammation, and the progression of fibrosis, through adjusting the time at which doxycycline is administered. The tet-On system can also be used to drive expression of any transgenic protein, not just Cre recombinase, so long as the protein of interest is under control of a TRE. Two lines have been developed to target Mø in this manner: c-fms-rtTA (4) and hCD68-rtTA (38).
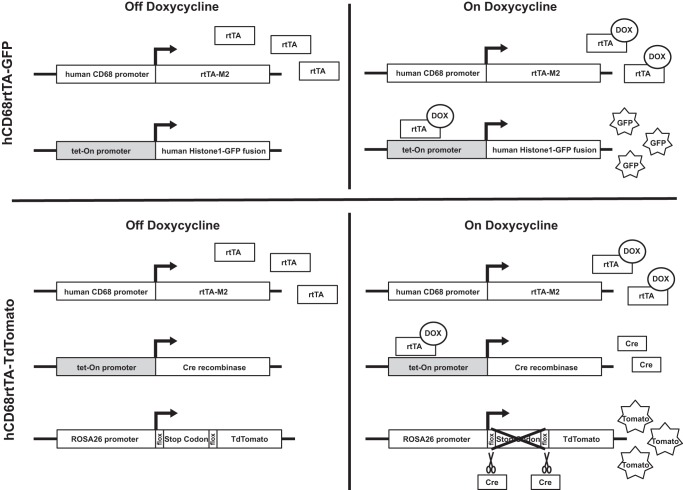
We chose to assess the hCD68-rtTA system for lung studies because the hCD68-rtTA mouse is commercially available and murine CD68 is highly expressed by all lung Mø. We bred two transgenic reporter lines to evaluate hCD68-rtTA activation. The first is a double-transgenic mouse, in which hCD68-rtTA directly drives expression of a tet-On GFP-tagged Histone (hCD68rtTA-GFP) when doxycycline is present. The second is a triple-transgenic mouse, in which hCD68-rtTA drives expression of tet-On Cre in the presence of doxycycline; this then cleaves the floxed stop codon in a CAG promoter-driven TdTomato (hCD68rtTA-TdTomato).
In studies described herein, tissue Mø and recMø showed robust activation of hCD68-rtTA as assessed by inducible GFP and TdTomato reporter expression upon doxycycline administration. Unexpectedly, resAMø did not express GFP or TdTomato during homeostasis or inflammation, making hCD68-rtTA the first described transgene able to specifically target CD11b+ lung tissue Mø and recMø. The hCD68-rtTA system presents a unique and powerful tool that can be used to study the roles that tissue Mø and recMø play in lung biology during homeostasis and disease, applicable to lineage tracing, fate mapping, and targeted gene deletion or overexpression.
METHODS
Animals.
This study was approved and performed in accordance with the ethical guidelines of the Institutional Animal Care and Use Committee at National Jewish Health. hCD68-rtTA mice [B6.Cg-Tg(CD68-rtTA2S*M2)3Mpil/Mmjax)], tet-On GFP mice [Tg(tetO-HIST1H2BJ/GFP)47Efu/J)], tet-On Cre mice (tetO7-Cre), and stop-flox Td Tomato mice [B6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J] were obtained from Jackson Laboratory (Bar Harbor, ME) and crossed at National Jewish Health. hCD68rtTA-GFP mice were on a mixed C57BL/6 and CD-1 background. hCD68rtTA-TdTomato mice were on a mixed C57BL/6 and FVB/N background. Mice were genotyped to confirm at least one allele of each transgene. hCD68rtTA-GFP mice were homozygous for both transgenes. hCD68rtTA-TdTomato mice were hemizygous for all transgenes. Male and female mice between 8 and 16 wk of age were used for these studies, with no discernable sex difference in hCD68-rtTA expression.
LPS and bleomycin instillation.
LPS (Escherichia coli 055:B5; List Biological Laboratories, Campbell, CA) was administered in a dose of 20 μg in 50 μl of PBS. Bleomycin (TEVA Pharmaceuticals, North Wales, PA) was administered in a dose of 3 U/kg, adjusted for the weight of the mouse, in 50 μl of PBS. Both LPS and bleomycin were instilled intratracheally (IT) with a modified gavage needle. Mice were sedated with isoflurane (Baxter, Deerfield, IL) before IT instillations.
Tissue collection.
Mice were euthanized with an intraperitoneal injection of Fatal Plus (Vortech Pharmaceuticals, Dearborn, MI). BAL, blood, and lung tissue were collected on ice in the following order. BAL was collected in 5 ml of PBS containing 0.5 mM EDTA, pelleted, and resuspended in HBSS containing 0.3% BSA and 0.3 mM EDTA. Blood was collected by cardiac puncture with a syringe coated with 50 μl of 100 mM EDTA, then put directly into 10 ml of PharmLyse buffer (BD Biosciences, San Jose, CA) for 10 min on ice. After 10 min, blood cells were washed and resuspended in HBSS containing 0.3% BSA and 0.3 mM EDTA. Lungs were perfused by injecting 10 ml PBS through the right ventricle, after which lung tissue was visibly blanched. Lung tissue was finely chopped with a razor blade, then incubated at 37°C in 1 ml of 27 mg/ml collagenase D (Roche, Indianapolis, IN) in HBSS. Following incubation, tissue was pipetted rapidly up and down to further disaggregate, then filtered through 100-μm filters. Lung cells were pelleted, and residual red blood cells were lysed for 30 s using 1 ml of BD PharmLyse (BD Biosciences). Cells were washed in HBSS and resuspended in HBSS containing 0.3% BSA and 0.3 mM EDTA.
Flow cytometry.
Flow Cytometry was performed on single-cell suspensions. Cells were protected from light, and incubations were performed on ice. Single-cell suspensions were treated with unlabeled CD16/CD32 for 30 min to block nonspecific FcyR-mediated binding. Cells were stained with surface antibody panels to identify myeloid populations for 1 h, then washed. HBSS containing 0.3% BSA and 0.3 mM EDTA was used as a buffer for all incubations of nonpermeabilized cells.
For staining of intracellular antigens (CD68), following the described surface staining, cells were permeabilized, washed, and stained by using the instructions and reagents of the BD Cytofix/Cytoperm fixation/permeabilization kit (BD Biosciences). Single-cell suspensions were analyzed with an LSRII flow cytometer (BD Biosciences) and FlowJo software (Tree Star, Ashland, OR). Flow antibodies used in various combinations are as follows (source/clone): unlabeled CD16/32 (eBioscience/93), Ly6G (BD/IA8), MHCII (BD/114.15.2), F4/80 (ebioscience/BM8), CD45 (BD/30-F11), Ly6C (eBioscience/HK1.4), CD68 (BioLegend/FA-11), SiglecF (BD/E50-2440), CD11c (eBioscience/N418), CD11b (eBioscience/M1/70), CD103 (eBioscience/2E7), and CD115 (eBioscience/AFS98).
Histology.
Dedicated mice were euthanized with intraperitoneal Fatal Plus, and the lungs were perfused with 20 ml of PBS. Lungs were inflated with 1 ml of 1:1 Optimum Cutting Temperature compound (OTC) (VWR International, Radnor, PA): 25% sucrose with 2% PFA in PBS. Inflated lungs were removed from the mouse, embedded in OCT, and frozen at −80°C. Ten-micrometer slices were cut with a cryostat. Sections were air dried, then fixed for 10 min in 1% PFA in PBS. Tissue was blocked with 10% donkey serum (Jackson Immunoresearch) in PBS. For CD68 staining, tissue was permeabilized with 0.2% TritonX-100 (Sigma) in PBS. Fluorescein or Cy5-conjugated tomato lectin (Vector Laboratories, Burlingame, CA) was used at 1:300. Unconjugated CD68 (BioLegend/FA-11) or unconjugated ratIgG2a isotype control (BD) was used at 1:100 for 2 h at 37°C. Donkey antirat Cy3 or Cy5 (Jackson Immunoresearch) secondary antibodies were used at 1:200 for 1 h at room temperature. Slides were mounted with VECTASHIELD containing DAPI (Vector Laboratories).
RESULTS
Techniques to assess hCD68-rtTA activation.
The hCD68-rtTA mouse has been previously explored as a tool to label Mø subsets in the bone marrow and peritoneum (38), but activation of hCD68-rtTA in the lung and other mucosal surfaces has not been defined (22). To investigate the suitability of this model for lung studies, we used hCD68rtTA-GFP and hCD68rtTA-TdTomato reporter mice to track hCD68-rtTA activation (Fig. 1). We first assessed the hCD68-rtTA system in naïve mice in three anatomically distinct compartments: the alveolar space, lung tissue, and circulating blood. All mice were treated the same way: BAL was collected, blood was harvested by cardiac puncture, residual blood was perfused from the lungs, and the remaining lung tissue was digested. We subsequently assessed the hCD68-rtTA system in these same three compartments during inflammation.
Fig. 1.
hCD68-rtTA reporter mice. Two strains of reporter mice were used to assess hCD68-rtTA activation in this study: hCD68rtTA-GFP and hCD68rtTA-TdTomato. Both mice express the hCD68-rtTA transgene, where a fragment of the human CD68 promoter drives expression of rtTA-M2 (rtTA). Even without doxycycline, rtTA is expressed by all cells that activate the human CD68 promoter. When doxycycline is administered, it binds to rtTA and this complex can activate tet-responsive promoters (shown in grey). In hCD68rtTA-GFP mice the combination of rtTA and doxycycline directly activates expression of GFP. In hCD68rtTA-TdTomato mice the combination of rtTA and doxycycline leads to production of the enzyme Cre recombinase (Cre). It indirectly activates expression of TdTomato (tomato); Cre cleaves a floxed stop codon at the start site of TdTomato, after which tomato expression is driven by the ubiquitously activated Rosa26 promoter.
ResAMø do not activate hCD68-rtTA.
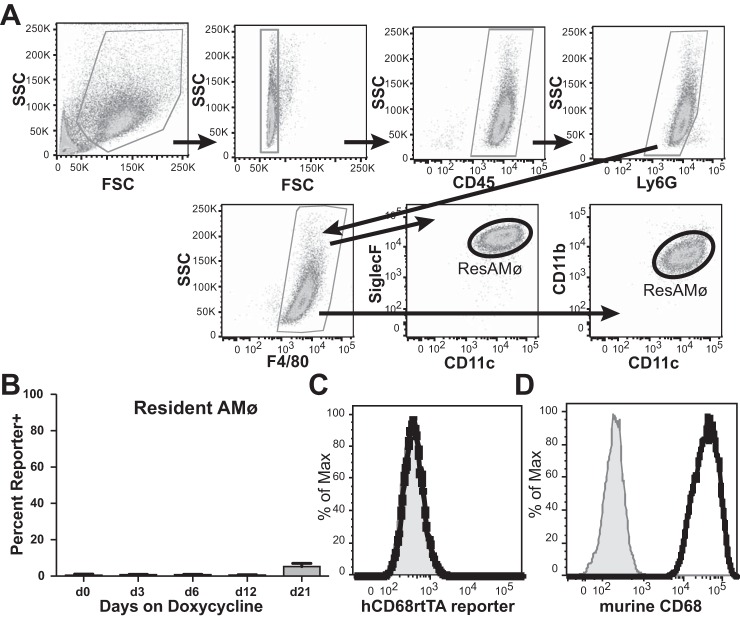
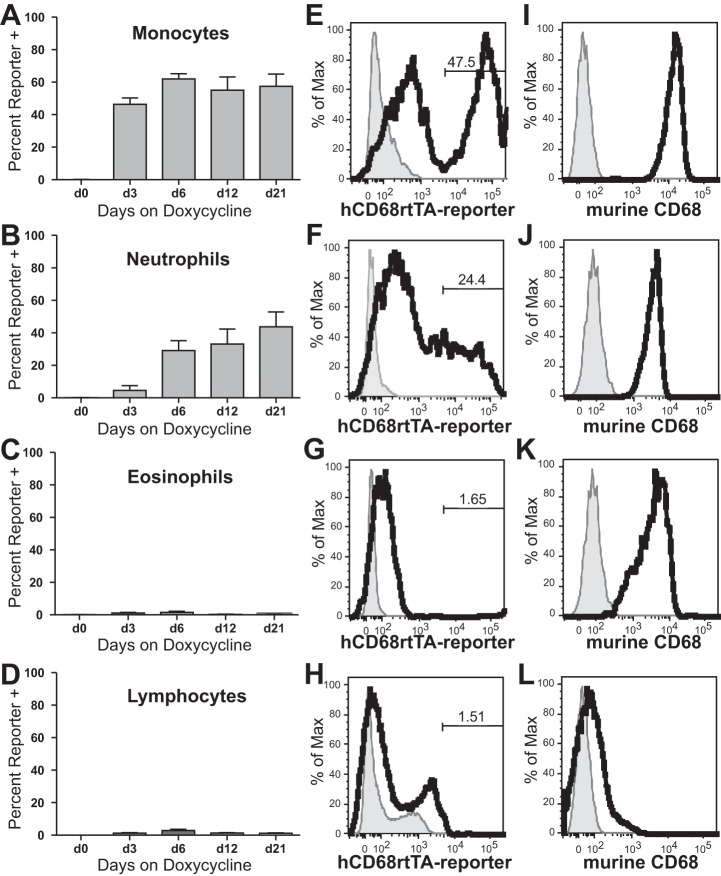
To assess cells within the homeostatic alveolar space we examined the BAL from naïve hCD68rtTA-GFP mice by using flow cytometry (Fig. 2A). A table of all the antibodies used to define leukocyte populations in this study can be referenced for further clarity (Table I). Naïve resAMø showed negligible activation of hCD68-rtTA, evidenced by only minor doxycycline-inducible GFP expression even after mice were fed for 21 days with doxycycline chow (Fig. 2, B and C). This result was unexpected since resAMø express high levels of endogenous murine CD68 (Fig. 2D). To address concerns that doxycycline may be unable to access the alveolar compartment, resAMø from hCD68rtTA-GFP mice were cultured ex vivo in the presence of doxycycline. Ex vivo administration of doxycycline did not induce GFP expression in resAMø (Fig. 3), but led to robust expression in resident peritoneal Mø that were used as a positive control (38). Even with confirmed access to doxycycline, resAMø do not activate hCD68-rtTA, although the reason for this exception remains unclear.
Fig. 2.
hCD68-rtTA is not activated by resAMø in the naïve alveolus. A: flow cytometry was used to identify resAMø collected by BAL from hCD68rtTA-GFP mice. ResAMø were identified by the following gating strategy: higher side scatter (SSC) cells were selected, doublets were excluded, CD45+ cells were selected, Ly6G+ neutrophils were excluded, and F4/80+ cells were selected. ResAMø were defined as CD11c+CD11blow or CD11c+SiglecF+. B: GFP reporter expression by resAMø on day 0, 3, 6, 12, or 21 after doxycycline chow. C: representative histogram of GFP expression on day 12 for resAMø; day 0 shown in grey. D: endogenous murine CD68 expression in resAMø; isotype shown in grey. FSC, forward scatter. All data are represented as means ± SE; n = 5–6 mice per group.
Table 1.
Phenotypes of myeloid cells in murine lung and blood
| Lung |
Blood |
|||||||
|---|---|---|---|---|---|---|---|---|
| ResAMø | RecMø | Tissue Mø | CD11b+ DC | CD103+ DC | Eosinophils | Neutrophils | Monocytes | |
| CD45 | + | + | + | + | + | + | + | + |
| Ly6G | − | − | − | − | − | − | + | − |
| Ly6C | − | V | − | − | − | − | − | V |
| CD115 | − | − | − | − | − | − | − | + |
| CD11b | ± | + | + | + | ± | + | + | + |
| CD11c | + | V | ± | + | + | − | − | − |
| MHCII | − | ± | ± | + | + | − | − | V |
| CD103 | − | − | − | − | + | − | − | − |
| F4/80 | + | + | + | − | − | − | − | − |
| Siglec-F | + | ± | − | − | − | + | − | − |
| CD68 | + | + | + | + | + | + | + | + |
+, high expression; ±, low or intermediate expression; −, not expressed; V, expression level varies across population or changes with maturity.
Fig. 3.
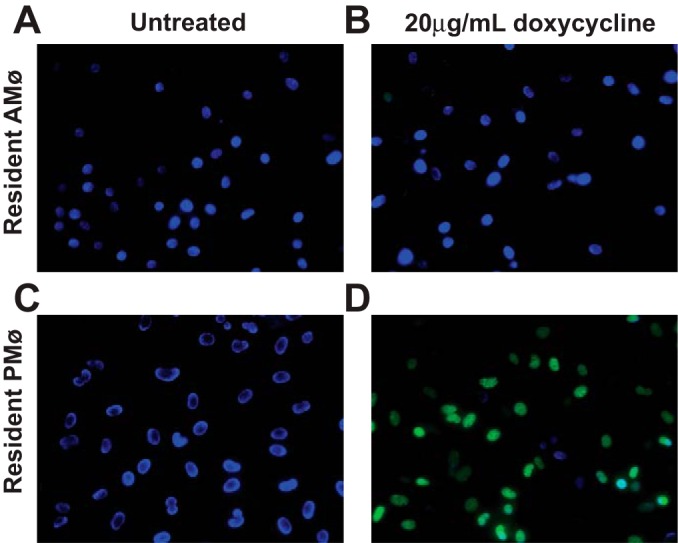
hCD68rtTA-GFP resAMø do not activate GFP even when doxycycline is provided ex vivo. ResAMø and resPMø were isolated by lavage from hCD68rtTA-GFP mice and cultured on glass cover slips in the presence or absence of 20 μg/ml doxycycline for 4 days. Cells were fixed and mounted with DAPI (blue). Tet-On GFP (green) expression was assessed by microscopy. A: resAMø cultured without doxycycline. B: resAMø cultured with doxycycline. C: resPMø cultured without doxycycline. D: resPMø cultured with doxycycline.
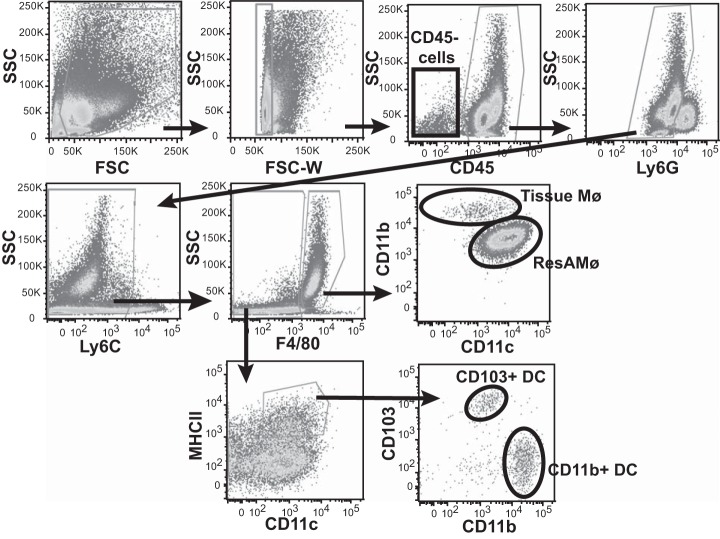
Lung tissue CD11b+ Mø and lung DC activate hCD68-rtTA.
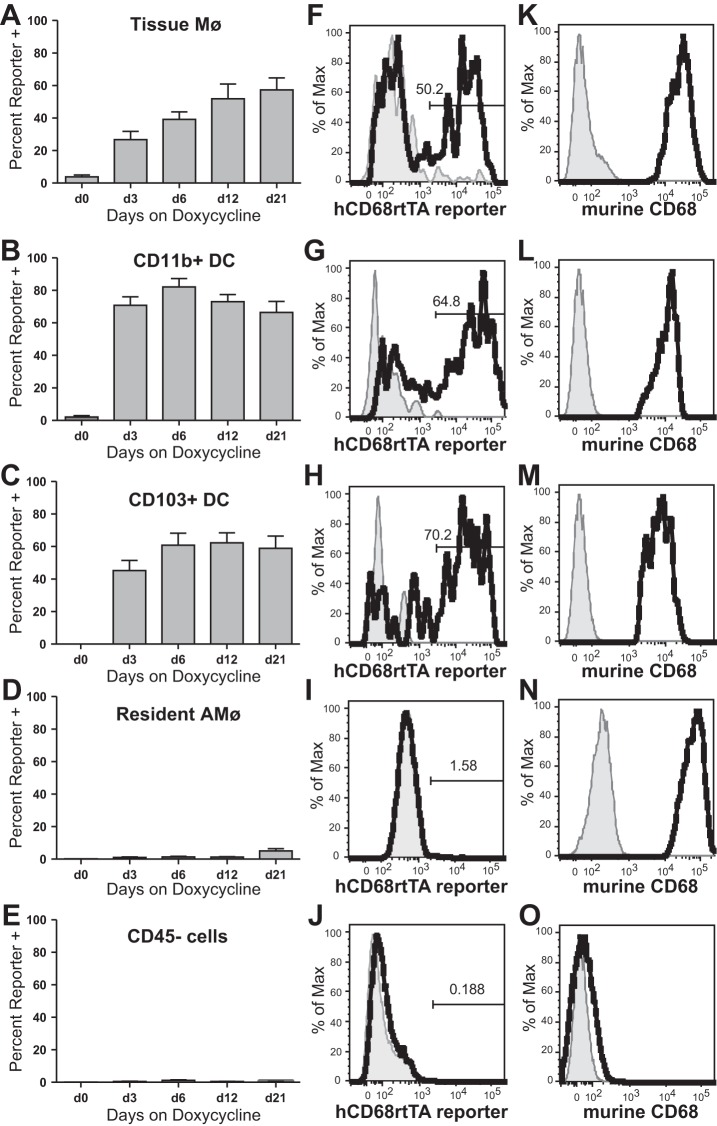
As a next step, we digested lung tissue of hCD68rtTA-GFP mice and assessed activation of the hCD68-rtTA transgene in tissue Mø and DC by using the gating strategy in Fig. 4. Three types of mononuclear phagocytes are found within lung digest: tissue Mø, CD11b+ DC, and CD103+ DC (6, 33, 51). Tissue Mø activate hCD68-rtTA, showing GFP reporter expression in 55% of cells by day 21 of doxycycline chow (Fig. 5, A and F). Both CD11b+ DC and CD103+ DC also show hCD68-rtTA activation, with GFP reporter expression 60–70% of cells by day 21 of doxycycline chow (Fig. 5, B, C, G, and H). However, lymphocytes do not activate hCD68-rtTA (data not shown), nor do CD45− cells (Fig. 5, E and J); GFP reporter expression in these populations was negligible.
Fig. 4.
Flow cytometry gating strategy for lung tissue cells. Flow cytometry was used to identify cells collected from lung digest. Cells of interest were identified by the following gating strategy: higher SSC cells were selected, doublets were excluded, CD45+ cells were selected, Ly6G+ neutrophils were excluded, and high SSC F4/80+ cells were selected. Mø subpopulations were separated into tissue Mø (CD11c−/lowCD11b+) and resAMø (CD11c+CD11blow). DC were identified within SSClowF4/80mid/− cells as Ly6C−CD11c+ MHCIIhigh. Subpopulations of DC were separated into CD11b+DC (CD11b+CD103−) and CD103+DC (CD11b−CD103+).
Fig. 5.
hCD68-rtTA is activated by tissue Mø and DC in the naïve lung tissue. Flow cytometry was used to assess GFP reporter activation in hCD68rtTA-GFP mice fed doxycycline for 0, 3, 6, 12 or 21 days. A–E: GFP reporter expression by tissue Mø, CD11b+ DC, CD103+ DC, resAMø, and CD45− cells. F–J: representative histograms of GFP expression on day 12 for tissue Mø, CD11b+ DC, CD103+ DC, resAMø, and CD45− cells; day 0 shown in grey. K–O: endogenous murine CD68 expression by lung tissue cells; isotype shown in grey. All data are represented as means ± SE; n = 5–6 mice per group.
Since residual, nonlavaged resAMø are also observed in the lung digest, intratracheal administration of an anti-CD45 antibody was used in some experiments to label leukocytes in the airspaces and airways. This demonstrated that the CD11c+ Mø present in the digestion are identical to the resAMø removed by BAL (Fig. 6). As was observed in our analysis of BAL, these resAMø do not activate hCD68-rtTA (Fig. 5, D and I). Both Mø and DC populations express endogenous murine CD68 (Fig. 5, K–N).
Fig. 6.
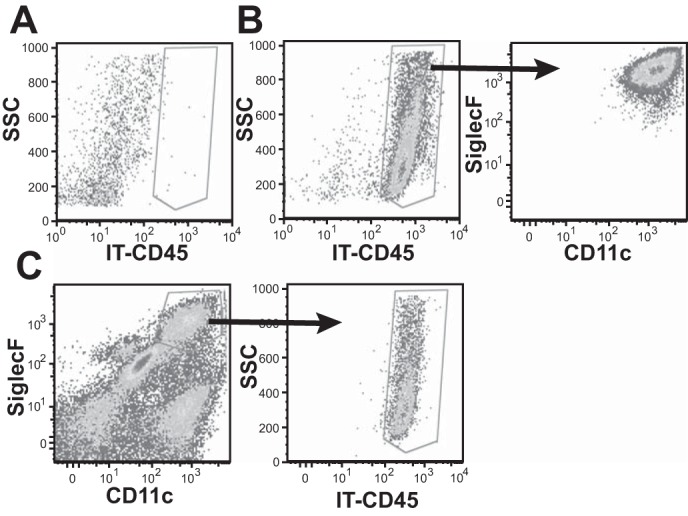
CD11c+ Mø found in lung digest are resAMø, as evidenced by staining with anti-CD45 given IT. A: BAL recovered from a mouse given control saline. B: BAL recovered from a mouse given anti-CD45 IT. Cells are resAMø shown by SiglecF and CD11c expression. C: lung digest from a mouse given anti-CD45 IT. CD11c+SiglecF+ Mø can be detected and are positive for CD45-IT.
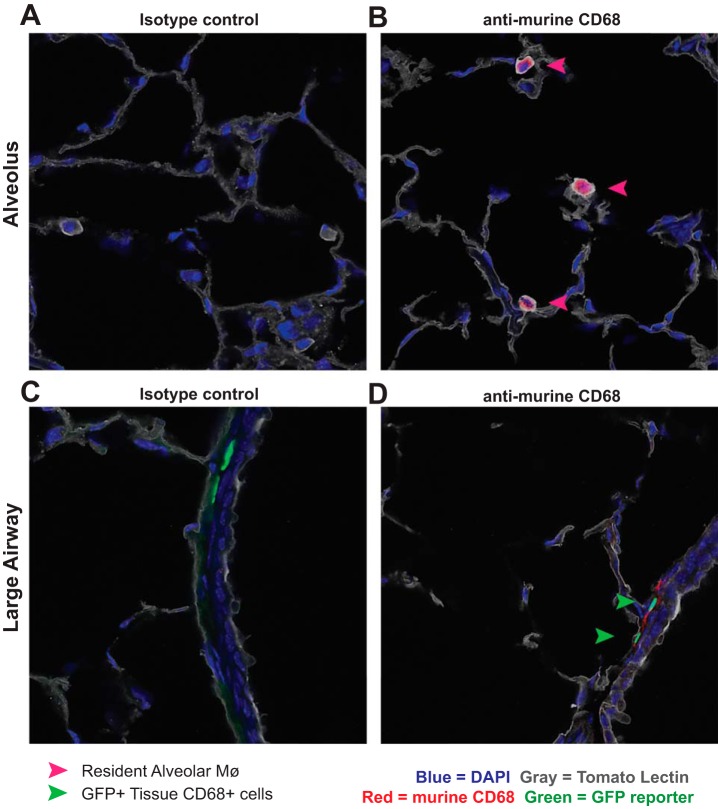
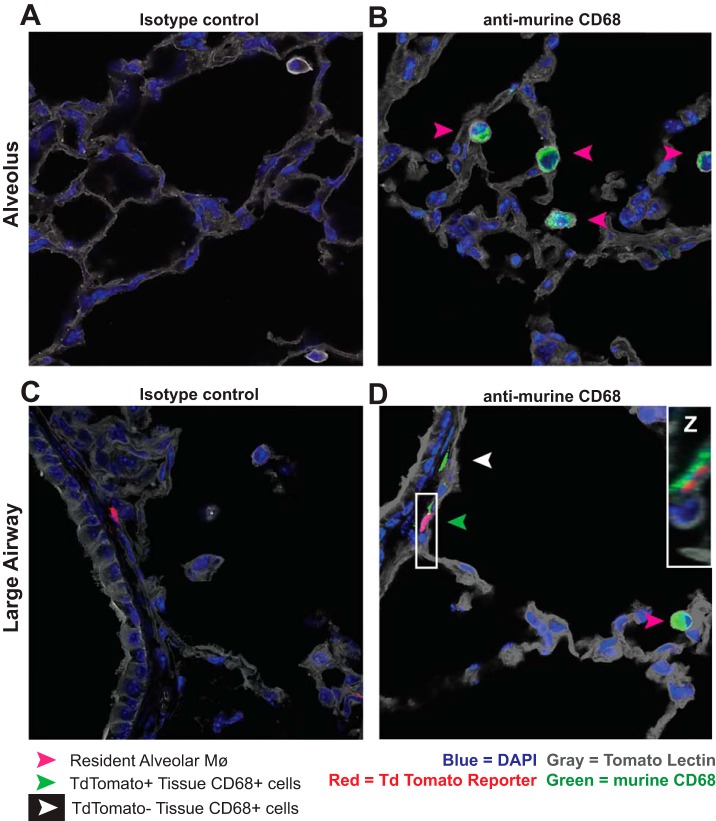
Immunofluorescent histology of lungs from naïve hCD68rtTA-GFP mice further verified that resAMø do not activate hCD68-rtTA; no GFP+ Mø were observed within the alveoli (Fig. 7, A and B). In contrast, GFP+CD68+ cells were observed within the lung tissue, particularly around large airways (Fig. 7, C and D). Taken as a whole, these data demonstrate that when using the hCD68-rtTA system during homeostasis, doxycycline will induce tet-On gene expression in tissue Mø and DC but not resAMø.
Fig. 7.
Immunofluorescence confirms that tissue mononuclear phagocytes express hCD68-rtTA GFP reporter while resAMø do not. Frozen lung sections from naïve hCD68rtTA-GFP mice fed doxycycline for 12 days were cut and stained with tomato lectin (gold), anti-murine CD68 (red), and DAPI (blue). Endogenous hCD68-rtTA GFP reporter expression (green) was preserved through freezing. A and B: representative photos of alveoli containing resAMø but not GFP-reporter+ cells. A: isotype control. B: anti-murine CD68. C and D: representative photos around large airways containing GFP-reporter+ cells within the lung tissue. C: isotype control. D: anti-murine CD68. Pink arrows: ResAMø. Green arrows: tissue CD68+ cells that express GFP.
Blood monocytes and neutrophils activate hCD68-rtTA.
Although resAMø have been shown to have a pure embryonic origin with self-renewing capacity (44, 50), a portion of tissue Mø and all lung DC have adult bone marrow monocyte or cDC precursor origin (7, 45). In addition, undifferentiated monocytes have been shown to patrol the lung during homeostasis (25). Since circulating leukocytes contribute to the lung milieu, we assessed hCD68-rtTA activation in circulating blood by flow cytometry (Fig. 8). Monocytes show hCD68-rtTA activation in 50–60% of cells, as evidenced by GFP reporter expression (Fig. 9, A and E). No difference was observed in GFP reporter expression between Ly6Chi and Ly6Clo monocytes (data not shown). GFP reporter expression by monocytes recovered from lung digest matched the reporter expression by monocytes recovered from circulating blood (data not shown). Neutrophils also show hCD68-rtTA activation, although only in 40% of cells (Fig. 9, B and F). Neither eosinophils nor lymphocytes showed hCD68-rtTA activation (Fig. 9, C, D, G, and H). Monocytes, neutrophils, and eosinophils all express endogenous murine CD68 (Fig. 9, I–K).
Fig. 8.
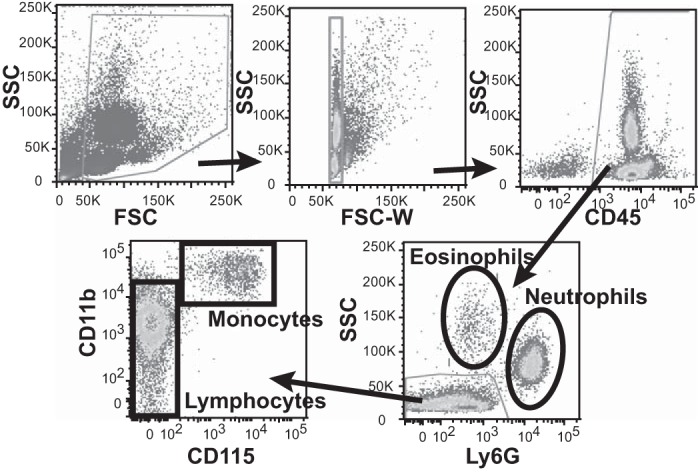
Flow cytometry gating strategy for peripheral blood. Flow cytometry was used to identify cells collected from lysed peripheral blood. Residual RBC and platelets were excluded by gating out low FSC events, CD45+ cells were selected, and Ly6G expression was used to identify neutrophils and eosinophils as the two high SSC populations (eosinophil identity could be confirmed by SiglecF expression, data not shown). Monocytes were identified within Ly6G−SSClow cells as CD11b+CD115+, while remaining cells were lymphocytes.
Fig. 9.
hCD68-rtTA is activated by monocytes and neutrophils in circulating blood. Flow cytometry was used to assess GFP reporter activation in hCD68rtTA-GFP mice fed doxycycline for 0, 3, 6, 12, or 21 days. A–D: GFP reporter expression by monocytes, neutrophils, eosinophils, and lymphocytes. E–H: representative histograms of GFP expression on day 12 for circulating monocytes, neutrophils, eosinophils, and lymphocytes; day 0 shown in grey. I–L: endogenous murine CD68 expression by circulating monocytes, neutrophils, eosinophils, and lymphocytes; isotype shown in grey. All data are represented as means ± SE; n = 5–6 mice per group.
CD11b+ Mø recruited to the alveolar space during LPS or bleomycin-induced inflammation activate hCD68-rtTA.
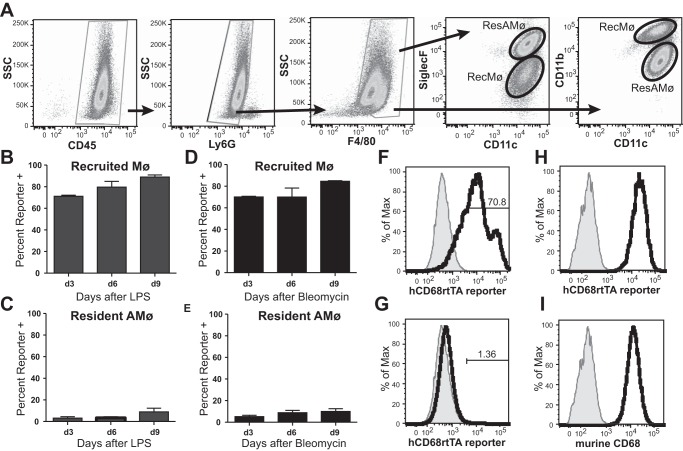
During inflammation, adult-derived recMø immigrate to the alveolar space, joining the embryonic-derived resAMø. To study hCD68-rtTA activation during inflammation, we utilized two common murine models: intratracheal (IT) administration of LPS, a model commonly used to study acute inflammation, and IT administration of bleomycin, a model commonly used to study the development of lung fibrosis. Informed by our studies of GFP expression kinetics we began administration of doxycycline 3 days before injury in both models. ResAMø and recMø were distinguished by their distinctive expression of CD11c, CD11b, and SiglecF (Fig. 10A). Following LPS or bleomycin-induced injury, recMø showed activation of hCD68-rtTA in 70–80% of cells, as evidenced by GFP reporter expression (Fig. 10, B, D, and F). The activation was stable through the course of inflammation. In contrast, resAMø showed negligible hCD68-rtTA activation (Fig. 10, C, E, and G). Both resAMø and recMø express high levels of endogenous murine CD68 (Fig. 10, H and I).
Fig. 10.
hCD68-rtTA is activated in the alveolus by recMø but not by resAMø following LPS or bleomycin lung injury. IT LPS and IT bleomycin were used to model acute lung inflammation and fibrotic lung injury, respectively, in hCD68rtTA-GFP mice. For all experiments, doxycycline diet began 3 days prior to lung injury. A: flow cytometry was used to identify recMø and resAMø collected by BAL. Mø were identified by the following gating strategy: higher SSC cells were selected, doublets were excluded, CD45+ cells were selected, Ly6G+ neutrophils were excluded, and F4/80+ cells were selected. Mø subpopulations were separated by CD11c, CD11b, and SiglecF expression. ResAMø were defined as CD11c+CD11blow or CD11c+SiglecF+. RecMø were defined as CD11clow/+CD11b+ or CD11clow/+SiglecF−. Identical gating strategies were employed for both models of lung injury. Representative gating from d6 post-LPS is shown. B and C: GFP reporter expression by recMø and resAMø after IT LPS. D and E: GFP reporter expression by recMø and resAMø after IT bleomycin. F and G: representative histograms of GFP expression on day 6 LPS for recMø and resAMø; day 0 shown in grey. H and I: endogenous murine CD68 expression by recMø and resAMø; isotype shown in grey. All data are represented as means ± SE; n = 4–5 mice per group.
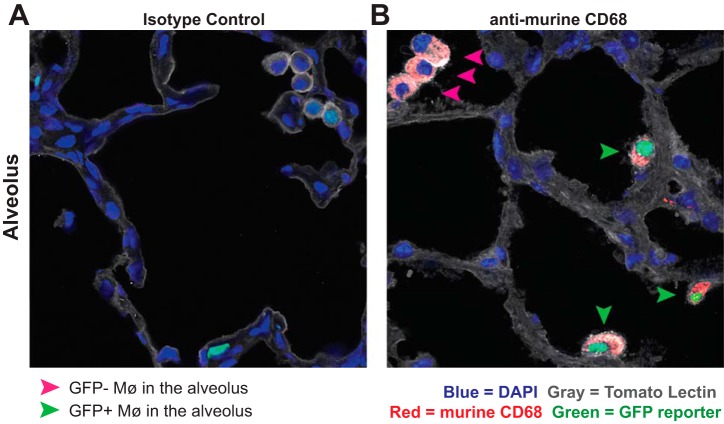
Immunofluorescent histology further verified that recMø activate hCD68-rtTA (Fig. 11). While no GFP+ Mø were found within the alveolar space in naïve lungs (Fig. 7), GFP+ recMø were found in alveoli on day 6 after LPS (Fig. 11). Taken as a whole, these results demonstrate that during acute lung inflammation, hCD68-rtTA targets recMø without affecting resAMø.
Fig. 11.
Immunofluorescence confirms the presence of GFP+ recruited macrophages in the alveoli. Frozen lung sections from hCD68rtTA-GFP mice day 6 after LPS, fed doxycycline for 9 days, were cut and stained with tomato lectin (grey), anti-murine CD68 (red), and DAPI (blue). Endogenous hCD68-rtTA GFP reporter expression (green) was preserved through freezing. A and B: representative photos showing GFP+ Mø in alveoli. A: isotype control. B: anti-murine CD68. Pink arrows: CD68+ Mø that do not express GFP. Green arrows: CD68+ Mø that express GFP.
Lung tissue CD11b+ Mø activate hCD68-rtTA during acute inflammation.
To assess the effect of inflammation on hCD68-rtTA activation in tissue Mø, we examined lung tissue digests of mice treated with LPS or bleomycin. Myeloid populations were identified by the same gating strategy as for naïve lungs (Fig. 4). However, to our knowledge there are no markers that adequately distinguish tissue Mø from recMø. Since both of these cell types express high levels of CD11b, we refer to this group as a whole as CD11b+ Mø. Following either LPS or bleomycin, over 80% of CD11b+ Mø contained in lung digests show hCD68-rtTA activation (Fig. 12, A and F). The level of hCD68-rtTA activation in our mixed population of CD11b+ Mø is identical to that seen in recMø alone (Fig. 9, B and D), suggesting either that CD11b+ Mø isolated from lung digests are predominantly recMø or that tissue Mø and recMø both achieve similar activation. As was observed in BAL, resAMø do not show significant hCD68-rtTA activation (Fig. 12, B and G). Both CD11b+ and CD103+ DC also activate hCD68-rtTA (Fig. 12, C, D, H, and I). In comparison, CD45− cells do not activate hCD68-rtTA, even 14 days post-bleomycin, a time at which fibrosis is maximal (Fig. 12, E and J). Lastly, lymphocytes showed no hCD68-rtTA activation (data not shown). These results demonstrate that hCD68-rtTA effectively targets CD11b+ mononuclear phagocytes, but not resAMø throughout acute lung injury and repair.
Fig. 12.
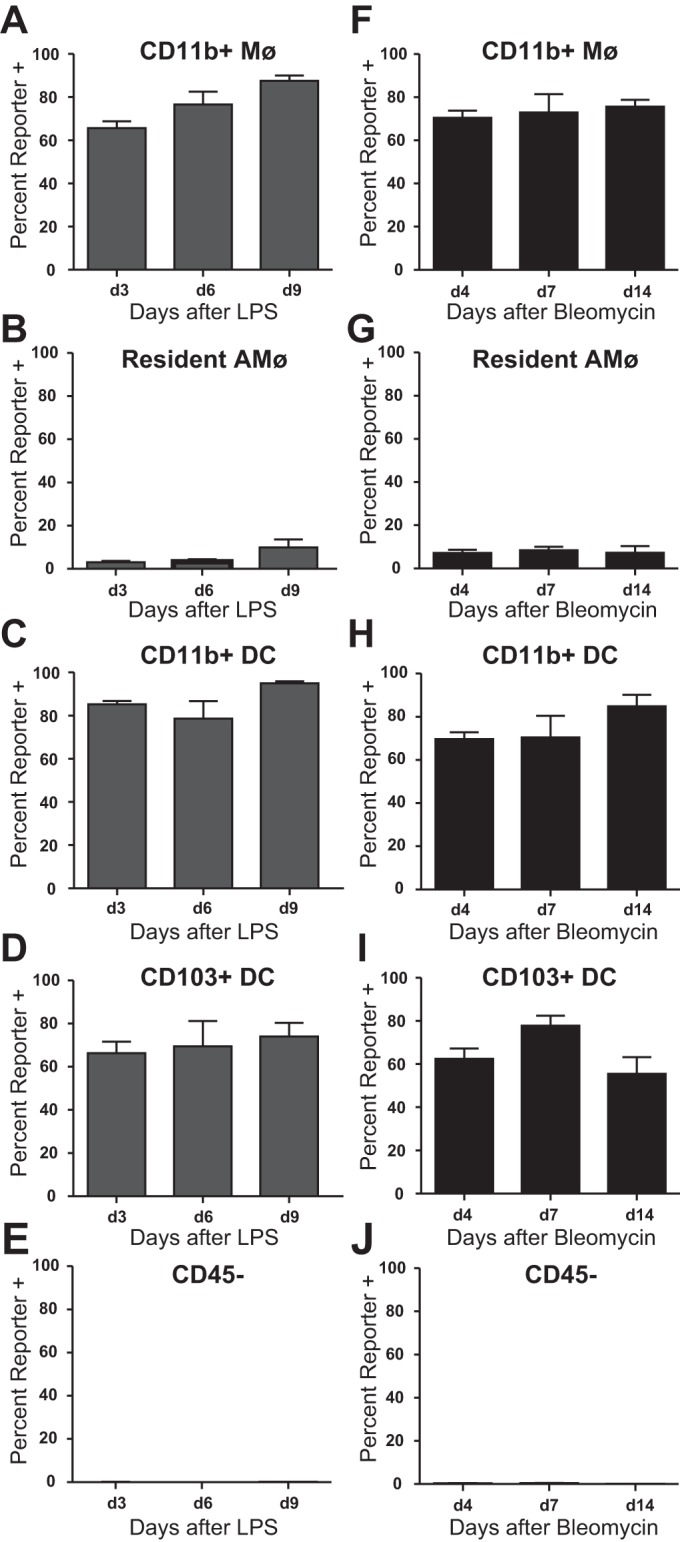
hCD68-rtTA is activated by CD11b+ Mø and DC from lung digest after LPS or bleomycin lung injury. IT LPS and IT bleomycin were used to model acute lung inflammation and fibrotic lung injury, respectively, in hCD68rtTA-GFP mice, and cells from lung digest were examined. For all experiments, doxycycline diet began 3 days prior to lung injury. A–E: GFP reporter expression by tissue Mø, resAMø, CD11b+ DC, CD103+ DC, and CD45− cells after IT LPS. F–J: GFP reporter expression by tissue Mø, resAMø, CD11b+ DC, CD103+ DC, and CD45− cells after IT bleomycin. All data are represented as means ± SE; n = 4–5 mice per group.
Blood monocytes and neutrophils activate hCD68-rtTA following LPS or bleomycin lung injury.
To assess whether lung inflammation alters hCD68-rtTA activation in circulating blood cells, we isolated leukocytes from peripheral blood and assessed GFP expression in an identical fashion to that employed for naïve blood (Fig. 8). Lung injury did not alter hCD68-rtTA activation by blood leukocytes. Sixty percent of monocytes and thirty percent of neutrophils showed activation of hCD68-rtTA (Fig. 13, A, B, E, and F). Activation was not observed in eosinophils or lymphocytes (Fig. 13, C, D, G, and H).
Fig. 13.
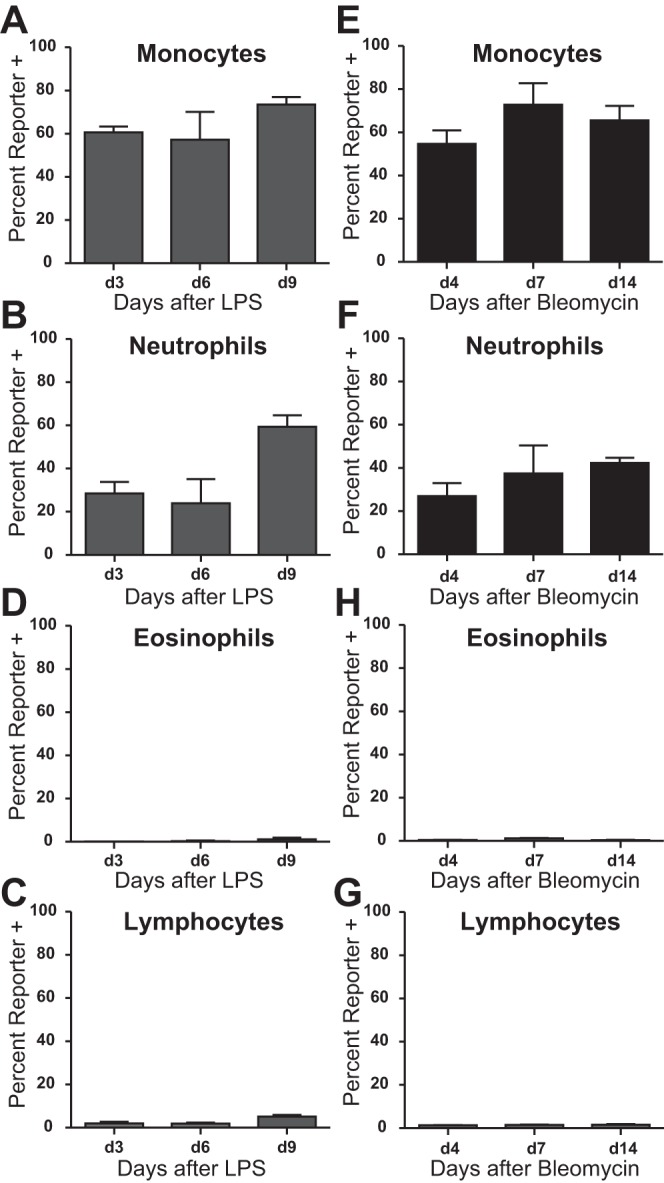
Lung injury does not alter hCD68-rtTA activation in circulating blood. IT LPS and IT bleomycin were used to model acute lung inflammation and fibrotic lung injury, respectively, in hCD68rtTA-GFP mice, and cells from peripheral blood were examined. For all experiments, doxycycline diet began 3 days prior to lung injury. A–D: GFP reporter expression by circulating monocytes, neutrophils, eosinophils, and lymphocytes after IT LPS. E–H: GFP reporter expression by circulating monocytes, neutrophils, eosinophils, and lymphocytes after IT bleomycin. All data are represented as means ± SE; n = 4–5 mice per group.
hCD68-rtTA can be effectively combined with a tetO-cre strategy to target gene deletion to CD11b+ Mø.
To illustrate the utility of hCD68-rtTA for specific and inducible deletion of floxed genes, we used triple-transgenic hCD68rtTA-TdTomato reporter mice in which fluorescent Td Tomato is expressed upon tet-On Cre-driven recombination of a floxed stop codon. Expression of Td Tomato is analogous to deletion of any floxed gene of interest. Immunofluorescent histology of hCD68rtTA-TdTomato reporter mice showed that, as was seen in hCD68rtTA-GFP mice, tissue CD68+ cells express TdTomato, but resAMø do not (Fig. 14). hCD68-rtTA mice can be used to restrict tet-On Cre induction and floxed gene deletion to CD11b+ Mø in the lung.
Fig. 14.
Immunofluorescence confirms that tissue mononuclear phagocytes express hCD68-rtTA Cre-driven TdTomato reporter while resAMø do not. Frozen lung sections from naïve hCD68rtTA-TdTomato mice fed doxycycline for 12 days were cut and stained with tomato lectin (grey), anti-murine CD68 (green), and DAPI (blue). Endogenous hCD68-rtTA TdTomato reporter expression (red) was preserved through freezing. A and B: representative photos of alveoli containing resAMø but not TdTomato-reporter+ cells. A: isotype control. B: anti-murine CD68. C and D: representative photos around large airways containing GFP-reporter+ cells within the lung tissue. C: isotype control. D: anti-murine CD68. Z-stack of the area of interest shows anti-murine CD68 staining colocalizes with hCD68rtTA-TdTomato, inset in white box. Pink arrows: ResAMø. White arrows: tissue CD68+ cells that do not express TdTomato. Green arrows: tissue CD68+ cells that express TdTomato.
DISCUSSION
Recent studies have demonstrated the presence of distinct embryonic and adult-derived Mø subsets in many tissues, including the lung (10, 16, 18). In this study, we show that the hCD68-rtTA mouse robustly targets tissue Mø and recMø subpopulations in the lung while leaving resAMø unaffected. Unexpectedly, although resAMø strongly express endogenous murine CD68, resAMø did not activate hCD68-rtTA during homeostasis or inflammation. In contrast, tissue Mø and recMø showed robust hCD68-rtTA activation in the majority of cells. Only CD11b+ DC and CD103+ DC showed similar levels of hCD68-rtTA activation. Lower levels of activation were observed in blood monocytes and neutrophils and no activation was observed in CD45− cells, lymphocytes, eosinophils, or the aforementioned resAMø during lung homeostasis or injury.
Accordingly, the hCD68-rtTA system has many potential applications for the study of lung Mø biology. For example, hCD68-rtTA reporter mice could be used to define the precise location of tissue Mø during health and disease. Although further work is required, it is interesting that histology of hCD68rtTA-GFP and hCD68rtTA-TdTomato mice show mononuclear phagocytes around large airways and relatively few in the alveolar insterstitium. hCD68-rtTA reporter mice can also be used for lineage tracing and fate mapping of tissue Mø. In this regard, parabiotic studies have been inconsistent, and separate groups have found that tissue Mø exclusively self-renew or partly replenish through bone marrow-derived cells (10, 25, 44). Similar questions remain concerning the origin and fate of recMø. Because recMø adapt resident-like surface markers upon exposure to the alveolar environment, (14, 17, 26) lung-shielded bone marrow transplants with congenic markers have been necessary to track the fate of recMø (26, 27). hCD68-rtTA reporter mice open possibilities for studying recMø without the costly and lengthy use of bone marrow transplants. The inducible kinetics of hCD68-rtTA reporter mice rather than a constitutive hCD68 reporter (i.e., hCD68-GFP mice) are key to their versatile use in studying Mø origin and fate. After withdrawal of doxycycline, computational modeling could be used with hCD68-GFP mice to estimate cell turnover by reporter decay (9) and identify proliferation. Because the reporter is permanently expressed following doxycycline in hCD68-TdTomato mice due to Cre recombination, these mice can be used to track the fate of monocytes or tissue Mø; doxycycline can be administered then withdrawn, and TdTomato-labeled cells can be followed for weeks to months.
In addition to utility for lineage tracing, hCD68-rtTA can be used to study the function of CD11b+ lung macrophages. hCD68-rtTA can be combined with tet-On Cre systems to specifically delete floxed genes in tissue Mø and recMø without deleting the same gene in resAMø. To our knowledge no other doxycycline-inducible transgenic systems exist that have been shown to distinctly target these CD11b+ lung Mø populations without also targeting resAMø. CX3CR1 ER-Cre inducible mice (50) may also perform similarly, since CX3CR1 is highly expressed by tissue and recMø (49) but not by resAMø (13). However, this has not yet been studied, and there are drawbacks to using a tamoxifen-induced system including substantial gastric toxicity (21). At present, hCD68-rtTA provides the unique opportunity to study tissue Mø and recMø functions in homeostasis and disease. For example, recMø have been suggested to play roles in resolution of inflammation and tissue repair (19, 26, 42). hCD68-rtTA could be used to conditionally delete pro- or antiapoptotic proteins to manipulate recMø survival, then test the effect of a lack or overabundance of recMø on injury and resolution. Alternatively, the timing of doxycycline administration could be adjusted to test the influence of any floxed gene in recMø on the initial progression and resolution of inflammation. We have observed hCD68-rtTA activation as early as 24 h after doxycycline chow is provided (unpublished observation), with stable levels achieved within 3–6 days. hCD68-rtTA could also be used to drive targeted deletion of genes regulating various growth factors and test whether the ability of recMø to produce specific molecules like TGF-β or VEGF is required for resolution and repair. This could be particularly interesting in the study of tumor-associated Møs and the progression of lung cancer.
Cell specificity remains a limitation of most currently available transgenic mice. Heavy redundancy in gene expression between myeloid cell populations has made it particularly difficult to design mice that target only Mø and not additional cell types such as neutrophils and DC. hCD68-rtTA is not exempt from nonspecific myeloid targeting, but represents improvement over LysM and c-fms (CSF1R) driven systems (1, 22, 24, 32, 41, 43), particularly the off-target activation of neutrophils (Table 2). Previous publications show that LysM and c-fms activation occurs in greater than 90% of neutrophils (1, 24, 43), compared with our findings that only 40% of neutrophils activate hCD68-rtTA. LysM-cre activation also occurs in epithelial type II cells, complicating its use in lung studies (34). The specificity of current transgenic mice for Mø remains a limitation of their use and must be considered in experimental design and data interpretation. However, hCD68-rtTA presents Mø specificity that is comparable to or better than LysM and c-fms driven systems in addition to its novel ability to exclude targeting of resAMø. Another consideration is the incomplete activation of hCD68-rtTA observed in monocytes and the targeted CD11b+ Mø populations of interest. Our data suggests that up to 20% of recMø, 30% of tissue Mø, and 30–40% of monocytes do not express Cre recombinase following doxycycline administration. We speculate that this reflects the complexity of an inducible system, where sufficient levels of rtTA or rtTA and Cre must be present to drive expression of a reporter or induce deletion of a floxed gene. Despite this limitation, the specificity of the hCD68-rtTA system for CD11b+ Mø and not resAMø provides a unique advantage not possible with other currently available systems.
Table 2.
Off-target effects of macrophage-targeting transgenic mice shown as percent of cells that express promoter-driven Cre
| LysM | c-fms | hCD68rtTA | |
|---|---|---|---|
| Neutrophils | >90% | >90% | 30–40% |
| Monocytes | 90% | 50–75% | 60–70% |
| Eosinophils | 5% | ND | <1% |
| Dendritic cells | 50% | >90%* | 70–80% |
| Resident AMø | >90% | >90% | 1–8% |
The mechanism by which hCD68-rtTA fails to target resAMø is unclear. Intriguingly, failure to activate hCD68-rtTA in resAMø was noted in both the tet-On-GFP reporter system and a second, unique tet-On-Cre system with TdTomato. We have confirmed that doxycycline administered ex vivo fails to activate tet-On-driven GFP in resAMø, eliminating the possibility that a failure of doxycycline to access the alveolar space is the cause. Supporting this observation, previous studies of human and rabbit cells have shown that doxycycline is internalized by resAMø (28, 29). hCD68-rtTA is driven by the human CD68 promoter, which, while sharing considerable homology to the murine promoter, has been shown to direct uniquely restricted expression compared with a more promiscuous murine promoter (15). However, resAMø from hCD68-GFP mice, in which hCD68 promoter activation leads directly to GFP expression rather than rtTA expression, are GFP positive (23). Vagaries of the human CD68 promoter cannot explain the absence of hCD68-rtTA activation by resAMø. We speculate that the site of hCD68-rtTA transgene insertion may be uniquely methylated in resAMø, preventing promoter access compared with the insertion site of hCD68-GFP. Although we could find no published documentation of cell subset-specific silencing, loss of transgenes through germline silencing can occur in transgenic mice. An alternate possibility is that resAMø are resistant to doxycycline-induced gene expression through inactivation or sequestration of intracellular doxycycline or rtTA. For example, iron has been shown to inhibit doxycycline-mediated tet-On activity in bacteria (46). Iron is an unlikely culprit in the murine hCD68rtTA system since all cells contain iron and we could find no published suggestion that resAMø contain uniquely high amounts. However, resAMø may produce other yet unidentified factors capable of modulating doxycycline or rtTA function. Further experiments are required to explain the lack of hCD68-rtTA activation in resAMø.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-109517 (to W. Janssen), HL-114381 (to W. Janssen), HL-126333 (to M. Mohning), HL-007085-41 (to K. Mould), a Parker B. Francis Foundation Fellowship (to E. Redente), and a Pulmonary Fibrosis Foundation Fellowship (to Redente).
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the author(s).
AUTHOR CONTRIBUTIONS
A.L.M., E.F.R., and W.J.J. conception and design of research; A.L.M., L.B., K.J.M., and M.P.M. performed experiments; A.L.M. analyzed data; A.L.M. interpreted results of experiments; A.L.M. prepared figures; A.L.M. and W.J.J. drafted manuscript; A.L.M., K.J.M., M.P.M., E.F.R., and W.J.J. edited and revised manuscript; A.L.M., L.B., K.J.M., M.P.M., E.F.R., and W.J.J. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Stacey Thomas for sharing her expertise in microscopy and Dr. Peter M. Henson for advice and guidance during the preparation of this manuscript.
REFERENCES
- 1.Abram CL, Roberge GL, Hu Y, Lowell CA. Comparative analysis of the efficiency and specificity of myeloid-Cre deleting strains using ROSA-EYFP reporter mice. J Immunol Methods 408: 89–100, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bedoret D, Wallemacq H, Marichal T, Desmet C, Quesada Calvo F, Henry E, Closset R, Dewals B, Thielen C, Gustin P, de Leval L, Van Rooijen N, Le Moine A, Vanderplasschen A, Cataldo D, Drion PV, Moser M, Lekeux P, Bureau F. Lung interstitial macrophages alter dendritic cell functions to prevent airway allergy in mice. J Clin Invest 119: 3723–3738, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 8: 265–277, 1999. [DOI] [PubMed] [Google Scholar]
- 4.Connelly L, Barham W, Onishko HM, Chen L, Sherrill TP, Zabuawala T, Ostrowski MC, Blackwell TS, Yull FE. NF-kappaB activation within macrophages leads to an anti-tumor phenotype in a mammary tumor lung metastasis model. Breast Cancer Res 13: R83, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deng L, Zhou JF, Sellers RS, Li JF, Nguyen AV, Wang Y, Orlofsky A, Liu Q, Hume DA, Pollard JW, Augenlicht L, Lin EY. A novel mouse model of inflammatory bowel disease links mammalian target of rapamycin-dependent hyperproliferation of colonic epithelium to inflammation-associated tumorigenesis. Am J Pathol 176: 952–967, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desch AN, Gibbings SL, Goyal R, Kolde R, Bednarek J, Bruno T, Slansky JE, Jacobelli J, Mason R, Ito Y, Messier E, Randolph GJ, Prabagar M, Atif SM, Segura E, Xavier RJ, Bratton DL, Janssen WJ, Henson PM, Jakubzick CV. Flow Cytometric Analysis of Mononuclear Phagocytes in Non-diseased Human Lung and Lung-draining Lymph Nodes. Am J Respir Crit Care Med 193: 614–626 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desch AN, Henson PM, Jakubzick CV. Pulmonary dendritic cell development and antigen acquisition. Immunol Res 55: 178–186, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dziennis S, Van Etten RA, Pahl HL, Morris DL, Rothstein TL, Blosch CM, Perlmutter RM, Tenen DG. The CD11b promoter directs high-level expression of reporter genes in macrophages in transgenic mice. Blood 85: 319–329, 1995. [PubMed] [Google Scholar]
- 9.Ensan S, Li A, Besla R, Degousee N, Cosme J, Roufaiel M, Shikatani EA, El-Maklizi M, Williams JW, Robins L, Li C, Lewis B, Yun TJ, Lee JS, Wieghofer P, Khattar R, Farrokhi K, Byrne J, Ouzounian M, Zavitz CC, Levy GA, Bauer CM, Libby P, Husain M, Swirski FK, Cheong C, Prinz M, Hilgendorf I, Randolph GJ, Epelman S, Gramolini AO, Cybulsky MI, Rubin BB, Robbins CS. Self-renewing resident arterial macrophages arise from embryonic CX3CR1(+) precursors and circulating monocytes immediately after birth. Nat Immunol 17: 159–168, 2016. [DOI] [PubMed] [Google Scholar]
- 10.Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity 41: 21–35, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franke-Ullmann G, Pfortner C, Walter P, Steinmuller C, Lohmann-Matthes ML, Kobzik L. Characterization of murine lung interstitial macrophages in comparison with alveolar macrophages in vitro. J Immunol 157: 3097–3104, 1996. [PubMed] [Google Scholar]
- 12.Fridlender ZG, Kapoor V, Buchlis G, Cheng G, Sun J, Wang LC, Singhal S, Snyder LA, Albelda SM. Monocyte chemoattractant protein-1 blockade inhibits lung cancer tumor growth by altering macrophage phenotype and activating CD8+ cells. Am J Respir Cell Mol Biol 44: 230–237, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, Helft J, Chow A, Elpek KG, Gordonov S, Mazloom AR, Ma'ayan A, Chua WJ, Hansen TH, Turley SJ, Merad M, Randolph GJ. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol 13: 1118–1128, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibbings SL, Goyal R, Desch AN, Leach SM, Prabagar M, Atif SM, Bratton DL, Janssen W, Jakubzick CV. Transcriptome analysis highlights the conserved difference between embryonic and postnatal-derived alveolar macrophages. Blood 126: 1357–1366, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greaves DR, Quinn CM, Seldin MF, Gordon S. Functional comparison of the murine macrosialin and human CD68 promoters in macrophage and nonmacrophage cell lines. Genomics 54: 165–168, 1998. [DOI] [PubMed] [Google Scholar]
- 16.Guilliams M, De Kleer I, Henri S, Post S, Vanhoutte L, De Prijck S, Deswarte K, Malissen B, Hammad H, Lambrecht BN. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J Exp Med 210: 1977–1992, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guth AM, Janssen WJ, Bosio CM, Crouch EC, Henson PM, Dow SW. Lung environment determines unique phenotype of alveolar macrophages. Am J Physiol Lung Cell Mol Physiol 296: L936–L946, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, Becker CD, See P, Price J, Lucas D, Greter M, Mortha A, Boyer SW, Forsberg EC, Tanaka M, van Rooijen N, Garcia-Sastre A, Stanley ER, Ginhoux F, Frenette PS, Merad M. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 38: 792–804, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herold S, Mayer K, Lohmeyer J. Acute lung injury: how macrophages orchestrate resolution of inflammation and tissue repair. Front Immunol 2: 65, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herold S, Tabar TS, Janssen H, Hoegner K, Cabanski M, Lewe-Schlosser P, Albrecht J, Driever F, Vadasz I, Seeger W, Steinmueller M, Lohmeyer J. Exudate macrophages attenuate lung injury by the release of IL-1 receptor antagonist in gram-negative pneumonia. Am J Respir Crit Care Med 183: 1380–1390, 2011. [DOI] [PubMed] [Google Scholar]
- 21.Huh WJ, Khurana SS, Geahlen JH, Kohli K, Waller RA, Mills JC. Tamoxifen induces rapid, reversible atrophy, and metaplasia in mouse stomach. Gastroenterology 142: 21–24, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hume DA. Applications of myeloid-specific promoters in transgenic mice support in vivo imaging and functional genomics but do not support the concept of distinct macrophage and dendritic cell lineages or roles in immunity. J Leukoc Biol 89: 525–538, 2011. [DOI] [PubMed] [Google Scholar]
- 23.Iqbal AJ, McNeill E, Kapellos TS, Regan-Komito D, Norman S, Burd S, Smart N, Machemer DE, Stylianou E, McShane H, Channon KM, Chawla A, Greaves DR. Human CD68 promoter GFP transgenic mice allow analysis of monocyte to macrophage differentiation in vivo. Blood 124: e33–44, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jakubzick C, Bogunovic M, Bonito AJ, Kuan EL, Merad M, Randolph GJ. Lymph-migrating, tissue-derived dendritic cells are minor constituents within steady-state lymph nodes. J Exp Med 205: 2839–2850, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jakubzick C, Gautier EL, Gibbings SL, Sojka DK, Schlitzer A, Johnson TE, Ivanov S, Duan Q, Bala S, Condon T, van Rooijen N, Grainger JR, Belkaid Y, Ma'ayan A, Riches DW, Yokoyama WM, Ginhoux F, Henson PM, Randolph GJ. Minimal differentiation of classical monocytes as they survey steady-state tissues and transport antigen to lymph nodes. Immunity 39: 599–610, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Janssen WJ, Barthel L, Muldrow A, Oberley-Deegan RE, Kearns MT, Jakubzick C, Henson PM. Fas determines differential fates of resident and recruited macrophages during resolution of acute lung injury. Am J Respir Crit Care Med 184: 547–560, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janssen WJ, Muldrow A, Kearns MT, Barthel L, Henson PM. Development and characterization of a lung-protective method of bone marrow transplantation in the mouse. J Immunol Methods 357: 1–9, 2010. [DOI] [PubMed] [Google Scholar]
- 28.Johnson JD, Hand WL, Francis JB, King-Thompson N, Corwin RW. Antibiotic uptake by alveolar macrophages. J Lab Clin Med 95: 429–439, 1980. [PubMed] [Google Scholar]
- 29.Klimes J, Dohnalova M, Sedlacek J. Microcolumn high-performance liquid chromatographic assay for doxycycline in isolated alveolar macrophages. J Chromatogr A 846: 181–184, 1999. [DOI] [PubMed] [Google Scholar]
- 30.Kos CH. Cre/loxP system for generating tissue-specific knockout mouse models. Nutr Rev 62: 243–246, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Landsman L, Jung S. Lung macrophages serve as obligatory intermediate between blood monocytes and alveolar macrophages. J Immunol 179: 3488–3494, 2007. [DOI] [PubMed] [Google Scholar]
- 32.MacDonald KP, Rowe V, Bofinger HM, Thomas R, Sasmono T, Hume DA, Hill GR. The colony-stimulating factor 1 receptor is expressed on dendritic cells during differentiation and regulates their expansion. J Immunol 175: 1399–1405, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Misharin AV, Morales-Nebreda L, Mutlu GM, Budinger GR, Perlman H. Flow cytometric analysis of macrophages and dendritic cell subsets in the mouse lung. Am J Respir Cell Mol Biol 49: 503–510, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miyake Y, Kaise H, Isono K, Koseki H, Kohno K, Tanaka M. Protective role of macrophages in noninflammatory lung injury caused by selective ablation of alveolar epithelial type II cells. J Immunol 178: 5001–5009, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Murray PJ, Wynn TA. Obstacles and opportunities for understanding macrophage polarization. J Leukoc Biol 89: 557–563, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Osterholzer JJ, Chen GH, Olszewski MA, Zhang YM, Curtis JL, Huffnagle GB, Toews GB. Chemokine receptor 2-mediated accumulation of fungicidal exudate macrophages in mice that clear cryptococcal lung infection. Am J Pathol 178: 198–211, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Osterholzer JJ, Olszewski MA, Murdock BJ, Chen GH, Erb-Downward JR, Subbotina N, Browning K, Lin Y, Morey RE, Dayrit JK, Horowitz JC, Simon RH, Sisson TH. Implicating exudate macrophages and Ly-6C(high) monocytes in CCR2-dependent lung fibrosis following gene-targeted alveolar injury. J Immunol 190: 3447–3457, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pillai MM, Hayes B, Torok-Storb B. Inducible transgenes under the control of the hCD68 promoter identifies mouse macrophages with a distribution that differs from the F4/80 - and CSF-1R-expressing populations. Exp Hematol 37: 1387–1392, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poczobutt JM, Gijon M, Amin J, Hanson D, Li H, Walker D, Weiser-Evans M, Lu X, Murphy RC, Nemenoff RA. Eicosanoid profiling in an orthotopic model of lung cancer progression by mass spectrometry demonstrates selective production of leukotrienes by inflammatory cells of the microenvironment. PLoS One 8: e79633, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prokhorova S, Lavnikova N, Laskin DL. Functional characterization of interstitial macrophages and subpopulations of alveolar macrophages from rat lung. J Leukoc Biol 55: 141–146, 1994. [DOI] [PubMed] [Google Scholar]
- 41.Qian B, Deng Y, Im JH, Muschel RJ, Zou Y, Li J, Lang RA, Pollard JW. A distinct macrophage population mediates metastatic breast cancer cell extravasation, establishment and growth. PLoS One 4: e6562, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Redente EF, Keith RC, Janssen W, Henson PM, Ortiz LA, Downey GP, Bratton DL, Riches DW. Tumor necrosis factor-α accelerates the resolution of established pulmonary fibrosis in mice by targeting profibrotic lung macrophages. Am J Respir Cell Mol Biol 50: 825–837, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sasmono RT, Ehrnsperger A, Cronau SL, Ravasi T, Kandane R, Hickey MJ, Cook AD, Himes SR, Hamilton JA, Hume DA. Mouse neutrophilic granulocytes express mRNA encoding the macrophage colony-stimulating factor receptor (CSF-1R) as well as many other macrophage-specific transcripts and can transdifferentiate into macrophages in vitro in response to CSF-1. J Leukoc Biol 82: 111–123, 2007. [DOI] [PubMed] [Google Scholar]
- 44.Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW, Frampton J, Liu KJ, Geissmann F. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 336: 86–90, 2012. [DOI] [PubMed] [Google Scholar]
- 45.Scott CL, Henri S, Guilliams M. Mononuclear phagocytes of the intestine, the skin, and the lung. Immunol Rev 262: 9–24, 2014. [DOI] [PubMed] [Google Scholar]
- 46.Vogt K, Bhabhra R, Rhodes JC, Askew DS. Doxycycline-regulated gene expression in the opportunistic fungal pathogen Aspergillus fumigatus. BMC Microbiol 5: 1, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang X. Cre transgenic mouse lines. Methods Mol Biol 561: 265–273, 2009. [DOI] [PubMed] [Google Scholar]
- 48.Westphalen K, Gusarova GA, Islam MN, Subramanian M, Cohen TS, Prince AS, Bhattacharya J. Sessile alveolar macrophages communicate with alveolar epithelium to modulate immunity. Nature 506: 503–506, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xiong Z, Leme AS, Ray P, Shapiro SD, Lee JS. CX3CR1+ lung mononuclear phagocytes spatially confined to the interstitium produce TNF-α and IL-6 and promote cigarette smoke-induced emphysema. J Immunol 186: 3206–3214, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, Viukov S, Guilliams M, Misharin A, Hume DA, Perlman H, Malissen B, Zelzer E, Jung S. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 38: 79–91, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zaynagetdinov R, Sherrill TP, Kendall PL, Segal BH, Weller KP, Tighe RM, Blackwell TS. Identification of myeloid cell subsets in murine lungs using flow cytometry. Am J Respir Cell Mol Biol 49: 180–189, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu Z, Zheng T, Lee CG, Homer RJ, Elias JA. Tetracycline-controlled transcriptional regulation systems: advances and application in transgenic animal modeling. Semin Cell Dev Biol 13: 121–128, 2002. [DOI] [PubMed] [Google Scholar]