Abstract
The development of chronic hypoxia (CH)-induced pulmonary hypertension is associated with increased pulmonary arterial smooth muscle cell (PASMC) Ca2+ influx through acid-sensing ion channel-1 (ASIC1) and activation of the Ca2+/calcineurin-dependent transcription factor known as nuclear factor of activated T-cells isoform c3 (NFATc3). Whether Ca2+ influx through ASIC1 contributes to NFATc3 activation in the pulmonary vasculature is unknown. Furthermore, both ASIC1 and calcineurin have been shown to interact with the scaffolding protein known as protein interacting with C kinase-1 (PICK1). In the present study, we tested the hypothesis that ASIC1 contributes to NFATc3 nuclear translocation in PASMC in a PICK1-dependent manner. Using both ASIC1 knockout (ASIC1−/−) mice and pharmacological inhibition of ASIC1, we demonstrate that ASIC1 contributes to CH-induced (1 wk at 380 mmHg) and endothelin-1 (ET-1)-induced (10−7 M) Ca2+ responses and NFATc3 nuclear import in PASMC. The interaction between ASIC1/PICK1/calcineurin was shown using a Duolink in situ Proximity Ligation Assay. Inhibition of PICK1 by using FSC231 abolished ET-1-induced and ionomycin-induced NFATc3 nuclear import, but it did not alter ET-1-mediated Ca2+ responses, suggesting that PICK1 acts downstream of Ca2+ influx. The key findings of the present work are that 1) Ca2+ influx through ASIC1 mediates CH- and ET-1-induced NFATc3 nuclear import and 2) the scaffolding protein PICK1 is necessary for NFATc3 nuclear import. Together, these data provide an essential link between CH-induced ASIC1-mediated Ca2+ influx and activation of the NFATc3 transcription factor. Identification of this ASIC1/PICK1/NFATc3 signaling complex increases our understanding of the mechanisms contributing to the vascular remodeling and increased vascular contractility that are associated with CH-induced pulmonary hypertension.
Keywords: calcineurin, pulmonary hypertension, hypoxia, store-operated calcium, endothelin-1
chronic hypoxia-induced pulmonary hypertension (World Health Organization Group III) is associated with respiratory diseases such as chronic obstructive pulmonary disease, interstitial lung diseases, and sleep-related breathing disorders such as sleep apnea. Chronic hypoxia (CH) results in enhanced vasoconstriction and vascular arterial remodeling due in part to increases in pulmonary vascular intracellular Ca2+ concentration ([Ca2+]i). Acid-sensing ion channel-1 (ASIC1) is expressed in pulmonary artery smooth muscle cells (PASMC); contributes to enhanced store-operated Ca2+ entry (SOCE); and is an important constituent to the active vasoconstriction, vascular remodeling, increased pulmonary arterial pressure, and right ventricular hypertrophy associated with hypoxic pulmonary hypertension (21, 39). ASIC1 is a member of the degenerin/epithelial sodium channel (DEG/ENaC) family and is permeable to both Na+ and Ca2+ (51, 56).
CH additionally activates the Ca2+/calcineurin-dependent transcription factor known as nuclear factor of activated T cells isoform-3 (NFATc3), in mouse PASMC (8, 9). Previous research in our laboratory demonstrated that NFATc3 is required for CH-induced pulmonary arterial remodeling. This process involves an initial proliferation of PASMC (dedifferentiation) followed by differentiation (upregulation of differentiation marker soluble guanylyl cyclase α1) and hypertrophy of PASMC (upregulation of smooth muscle-α-actin) (2, 7, 9). NFATc3 belongs to a family of four transcription factors (NFATc1, NFATc2, NFATc3, and NFATc4) that share the property of Ca2+/calcineurin-dependent nuclear translocation (reviewed in Ref. 15). We have shown that elevated endothelin-1 (ET-1) levels in response to CH contribute to NFATc3 activation through binding to type A ET receptors (8, 9). ET-1 causes release of Ca2+ from intracellular stores and Ca2+ influx through L-type voltage-gated Ca2+ channels, store-operated channels, and receptor-operated cannels (49, 50). Administration of the L-type voltage-gated Ca2+ channel inhibitor diltiazem attenuated but did not abolish CH-induced NFATc3 activation in isolated pulmonary arteries from NFAT-luciferase reporter mice (8). These data are consistent with reports of other Ca2+ entry pathways being involved in NFATc3 activation; namely, SOCE (18, 20, 42, 52, 54). The goal of this study was to determine whether ASIC1-mediated SOCE contributes to CH-induced NFATc3 activation in PASMC.
Most intracellular Ca2+ signaling events take place through the calcium-modulated protein (calmodulin). In response to elevated Ca2+ levels, calmodulin binds to and activates either calmodulin-dependent protein kinases or the serine/threonine protein phosphatase calcineurin. Under resting conditions, NFAT proteins are phosphorylated and reside in the cytoplasm. Upon Ca2+-calmodulin-induced activation, NFAT proteins are dephosphorylated by calcineurin, translocate to the nucleus, and become transcriptionally active (16). We have recently shown that calcineurin limits ASIC1-mediated SOCE through dephosphorylation of the channel in rat PASMC (14). Furthermore, this response is dependent on the PDZ-binding protein known as protein interacting with C kinase (PICK1), which has been shown to bind both ASIC1 (11, 17) and calcineurin B (19). These data are consistent with previous reports demonstrating calcineurin-dependent dephosphorylation and inactivation of ASICs in neuronal cells (4). Together, these studies provide evidence of a functional interaction between ASIC1, PICK1, and calcineurin. Although these studies exhibit regulation of ASIC1 by calcineurin, it is also possible that ASIC1-mediated Ca2+ influx activates calcineurin and thus NFATc3. Therefore, in the present study we tested the hypothesis that ASIC1 contributes to NFATc3 nuclear translocation in PASMC. Furthermore, we hypothesized that ASIC1-stimulated NFATc3 nuclear translocation is dependent on the PDZ-binding protein PICK1.
METHODS
Animals and CH Exposure Protocol
ASIC1-knockout (ASIC1−/−) mice were kindly provided by Drs. M.J. Welsh and J.A. Wemmie [University of Iowa, Iowa City, IA (53)]. Mice were bred on a C57BL/6 background, and ASIC1 wild-type (ASIC1+/+) and ASIC1−/− male and female mice (∼12 wk old) were used equally for each protocol. Disruption of ASIC1 was confirmed by PCR and agarose gel electrophoresis using a three-primer system to detect both wild-type and disrupted alleles (39). Animals designated for exposure to CH were housed in a hypobaric chamber with barometric pressure maintained at ∼380 mmHg for 1 wk [our laboratory had previously demonstrated NFATc3 nuclear accumulation was greatest following 7 day CH (9)]. Age-matched control mice were housed at ambient barometric pressure (∼630 mmHg in Albuquerque, NM). All protocols used in this study were reviewed and approved by the Institutional Animal Care and Use Committee of the University of New Mexico School of Medicine.
Determination of Vasoreactivity and [Ca2+]i in Small Pulmonary Arteries
To determine changes in pulmonary vasoreactivity, [Ca2+]i, and SOCE, small intrapulmonary arteries were cannulated and pressurized for simultaneous dimensional and [Ca2+]i analysis as previously described (39). Briefly, mice were anesthetized with pentobarbital sodium (200 mg/kg ip). The left lung was removed, and small intrapulmonary arteries (fourth to fifth order) were dissected free, transferred to a vessel chamber (Living Systems), and secured to tapered glass pipettes with a single strand of silk ligature. After cannulation, the artery was pressurized with a servo-controlled peristaltic pump (Living Systems) to 12 mmHg. Any artery that failed to maintain pressure upon switching off of the servo-controller was discarded. The vessel chamber was superfused with HEPES-based physiological saline solution (PSS; in mM: 130 NaCl, 4 KC1, 1.2 MgSO4, 4 NaHCO3, 10 HEPES, 1.18 KH2PO4, 1.8 CaCl2, 6 glucose; pH adjusted to 7.4 with NaOH) at 5 ml/min at 37°C. Red-wavelength, bright-field images were obtained using an Eclipse TS100 microscope (Nikon) and IonOptix CCD100M camera to measure inner diameter, and dimensional analysis was performed by IonOptix Ion Wizard software (IonOptix). Arteries were incubated at room temperature with HEPES-PSS containing the cell-permeable ratiometric Ca2+-sensitive fluorescent dye fura-2 acetoxymethyl ester (fura-2 AM, 2 μM; Life Technologies) and 0.02% pluronic acid (Life Technologies) for 45 min, as previously described (39). Fura-2-loaded vessels were alternately excited at 340 and 380 nm at a frequency of 1 Hz with an IonOptix Hyperswitch dual-excitation light source, and the respective 510-nm emissions were collected with a photomultiplier tube (F340/F380). After subtracting background fluorescence, emission ratios were calculated with Ion Wizard software (IonOptix) and recorded continuously throughout the experiment.
Store-operated Ca2+ entry.
The effect of 1 wk of CH on SOCE in isolated pulmonary arteries from ASIC1+/+ and ASIC1−/− mice was assessed as previously described (39). Briefly, fura-2-loaded arteries were superfused with Ca2+-free, HEPES-based PSS (in mM: 130 NaCl, 4 KC1, 1.2 MgSO4, 4 NaHCO3, 10 HEPES, 1.18 KH2PO4, 6 glucose, 3 EGTA; pH adjusted to 7.4 with NaOH) containing 50 μM diltiazem (Sigma-Aldrich) to prevent Ca2+ entry through L-type voltage-gated Ca2+ channels, and 10 μM cyclopiazonic acid (CPA; Calbiochem) to deplete intracellular Ca2+ stores and prevent Ca2+ reuptake through the sarcoplasmic reticulum Ca2+-ATPase. The changes in [Ca2+]i were determined upon repletion of HEPES-based PSS containing 1.8 mM CaCl2 in the continued presence of diltiazem and CPA. Area under the curve (AUC) was calculated as previously described (44).
ET-1 vasoconstrictor reactivity.
ET-1 vasoconstrictor reactivity was assessed by superfusion (5 ml/min at 37°C) of cumulative concentrations of ET-1 (10−10 to 10−8 M; Sigma-Aldrich) in isolated pulmonary arteries from ASIC1+/+ and ASIC1−/− mice exposed to control and CH conditions.
Contribution of ASIC1 to Hypoxia-Induced NFATc3 Nuclear Import
Isolated lungs were fixed with 4% formaldehyde in PBS, cryoprotected with 30% sucrose in PBS, embedded in optimal cutting temperature (OCT) medium, and frozen. Cryostat sections (10 μm) were stained with primary rabbit polyclonal anti-NFATc3 (1:100, Santa Cruz Biotechnology) and goat anti-α-actin (1:200, Abcam) followed by donkey anti-rabbit DyLight 649 and anti-goat DyLight 549 (Jackson ImmunoResearch Laboratories or Pierce) as previously described (6, 9). Nuclei were stained using SYTOX green (1:10,000, Molecular Probes). Specificity of immune staining was confirmed by the absence of fluorescence in tissues incubated with primary or secondary antibodies alone. Images of small pulmonary arteries were collected using a ×63 glycerol objective on a spectral confocal system (TCS SP5; Leica Microsystems). PASMC were identified by positive α-actin fluorescence. Within these cells, percent colocalization of SYTOX (nuclear) and NFATc3 fluorescence was calculated using Leica software and averaged for all pulmonary artery vascular smooth muscle cells within that artery (8–10 arteries per animal).
Generation of PASMC Culture
ASIC1+/+ and ASIC1−/− mice were anesthetized with pentobarbital sodium (200 mg/kg ip), and the heart and lungs were removed by midline thoracotomy. Intrapulmonary arteries (approximately 2nd to 5th order) were dissected from surrounding lung parenchyma and enzymatically digested in reduced-Ca2+ HBSS containing papain (9.5 U/ml), type-I collagenase (1,750 U/ml), dithiothreitol (1 mg/ml), and BSA (2 mg/ml) at 37°C for 15 min. Single smooth muscle cells were dispersed by gentle trituration with a fire-polished pipette in Ca2+-free HBSS. The cell suspension was plated on gelatin-coated dishes and cultured in Smooth Muscle Cell Medium (Cell Biologics) supplemented with 15% FBS and 1% penicillin/streptomycin in a humidified atmosphere of 5% CO2-95% air at 37°C. Cellular purity was >95% as assessed by morphological appearance under phase-contrast microscopy and positive immunofluorescence for smooth muscle 22α.
Determination of NFAT, TRPC1, and Orai1 Expression in PASMC from ASIC1+/+ and ASIC1−/− Mice
Messenger RNA expression.
Total RNA was isolated from PASMC from ASIC1+/+ and ASIC1−/− mice using TRI Reagent (Zymo Research). RNA extracts were treated with DNase I and RNA cleanup was performed using Zymo-Spin IIICG Columns (Zymo Research) following the manufacturer's recommendations. Messenger RNA was reverse-transcribed to cDNA using a High Capacity Reverse Transcription kit (Life Technologies). Specific primers (Integrated DNA Technologies) and probes (Roche) were used to detect transcripts for NFATc2, NFATc3, TRPC1, and Orai1 by real-time quantitative PCR. Primer sequences and probe number are listed in Table 1. β-Actin was used as the endogenous control and normalizer of gene expression (dCT). Relative quantification was determined by the Comparative CT method (2−ΔΔCT) in which gene expression was determined relative to a single calibrator from an ASIC1+/+ mouse (33).
Table 1.
Primers and probes used for real-time, quantitative PCR
| Gene | Primer Pair Sequence, Sense/Antisense | Probe Number* |
|---|---|---|
| NFATc2 | 5′-GTCAAAGCCCCAACAGGAG-3′ | 45 |
| 5′-TCATCTGCTGTCCCAATGAA-3′ | ||
| NFATc3 | 5′-GGGGCAGTGAAAGCCTCT-3′ | 49 |
| 5′-TCATCGGCTGTTCCAATAAA-3′ | ||
| TRPC1 | 5′-TGAACTTAGTGCTGACTTAAAGGAAC-3′ | 10 |
| 5′-CGGGCTAGCTCTTCATAATCA-3′ | ||
| Orai1 | 5′-TACTTAAGCCGCGCCAAG-3′ | 108 |
| 5′-ACTTCCACCATCGCTACC-3′ | ||
| β-Actin | 5′-CTAAGGCCAACCGTGAAAAG-3′ | 64 |
| 5′-ACCAGAGGCATACAGGGACA-3′ |
Roche.
Protein expression.
PASMC from ASIC1+/+ and ASIC1−/− mice were homogenized in 10 mM Tris·HCl homogenization buffer (containing 255 mM sucrose, 2 mM EDTA, 12 μM leupeptin, 1 μM pepstatin A, and 0.3 μM aprotinin) and centrifuged at 10,000 g for 10 min at 4°C to remove insoluble debris. Sample protein concentrations were determined by the Bradford method (Bio-Rad). PASMC lysates (30 μg) were separated by SDS-PAGE (7.5% or 12% TGX; BioRad) and transferred to polyvinylidene difluoride membranes. Blots were blocked for 1 h with 5% milk and then incubated overnight at 4°C with rabbit anti-TRPC1 (1:250, Abcam) or rabbit anti-Orai1 (1:250, Alomone Labs) and subsequently for 1 h with rabbit anti-GAPDH (1:5,000, Sigma Aldrich). For immunochemical labeling, blots were incubated for 1 h with goat anti-rabbit IgG-horseradish peroxidase (1:3,000, Bio-Rad). Blots were exposed to chemiluminescence-sensitive film (GeneMate) and quantification of TRPC1, Orai1, and GAPDH bands was accomplished by densitometric analysis of scanned images (Sigma Gel).
Determination of ASIC1-PICK1-Calcineurin Colocalization
Protein-protein interactions in PASMC were determined using the Duolink in situ Proximity Ligation Assay (PLA) according to the manufacturer's instructions (Olink Biosciences, Sigma Aldrich) as previously described (14). Briefly, PASMC were plated on 18-well slides (Ibidi) and grown until ∼75% confluent. PASMC were fixed with 2% paraformaldehyde and incubated with Duolink blocking buffer for 30 min at 37°C, then incubated overnight with goat anti-ASIC1 (1:50, Santa Cruz) and rabbit anti-PICK1 (1:100, Abcam), goat anti-ASIC1 and mouse anti-calcineurin B (1:100, Upstate), or rabbit anti-PICK1 and mouse anti-calcineurin B. PASMC were then incubated with the appropriate PLA probes (1:5) for 1 h at 37°C. Samples were amplified with Duolink In Situ Detection Reagent Orange (excitation/emission, 554/579 nm, Sigma Aldrich) for 100 min at 37°C. SYTOX Green (1:5,000, Invitrogen) was used as a nuclear stain and actin was stained with Alexa Fluor 647 phalloidin (1:100, Invitrogen). Samples were mounted with Duolink mounting media and Z-stack images of the PLA interaction were acquired using a confocal microscope (TCS SP5; Leica Microsystems). Negative controls were completed by 1) examining interactions in ASIC1−/− PASMC, 2) omission of primary antibody (not shown), and 3) incubation of each primary antibody individually.
Contribution of ASIC1 and PICK1 to Ca2+ entry in PASMC.
PASMC were incubated with fura-2 AM (2 μM and 0.05% pluronic acid in PSS; Molecular Probes) for 30 min at 32°C and imaged as described above for isolated arteries. To determine the contribution of ASIC1 to ET-1-induced changes in [Ca2+]i, PASMC from ASIC1+/+ and ASIC1−/− mice were superfused with ET-1 (30 nM). Additional experiments were conducted in which PASMC from ASIC1+/+ mice were first pretreated with the ASIC1 inhibitor psalmotoxin 1 (20 nM, PcTX1; Phoenix Peptides). To determine the role of PICK1 in regulating ET-1-induced Ca2+ influx and SOCE, experiments were conducted in PASMC from ASIC1+/+ mice in the absence or presence of the PICK1 inhibitor FSC231 (50 μM, Calbiochem). AUC was calculated as previously described (44).
Determination of NFATc3 Nuclear Import
PASMC from intrapulmonary arteries of ASIC1+/+ and ASIC1−/− mice were cultured until confluent (approximately 5–8 days) at 37°C with 6% CO2. PASMC were electroporated with NFATc3-enhanced green fluorescent protein (EGFP) expression vector using Nucleofector (Lonza). The vector was created by Dr. F. McKeon (Harvard University, Cambridge, MA) and kindly provided by Dr. L.F. Santana (Washington State University, Seattle, WA). NFATc3-EGFP-expressing PASMC were seeded on microscope coverslips and differentiated by culturing for at least 48 h in Serum-Free Smooth Muscle Cell Medium (Cell Biologics). Cells were incubated for 30 min with a myosin light chain peptide inhibitor (1 μM, Peptide 18; Calbiochem) in HEPES-PSS to reduce cell shrinkage in response to constrictors. Experiments were conducted in the presence or absence of the ASIC1 inhibitor PcTX1 (20 nM) or the PICK1 inhibitor FSC231 (50 μM). Treatment was initiated with the addition of vehicle, ET-1 (10−8 M, Sigma Aldrich), or ionomycin (10−7 M, Calbiochem) and the CRM1-exporting inhibitor leptomycin B (4 × 10−8 M, LC Laboratories), which prevents NFATc3-EGFP nuclear export. After 30 min of treatment, cells were fixed with 4% formaldehyde in PBS, washed with PBS, and imaged using a ×63 objective on a Leica TCS SP5 Spectral Confocal System. Nuclear (Fn) and cytosol EGFP fluorescence (Fc) were measured by placing regions of interest in the nucleus and cytosol with the Leica LAS AF Lite software, and fluorescence values were background-corrected and expressed as Fn/Fc. Similarly, NFATc3-EGFP nuclear translocation was determined in PASMC from ASIC1+/+ and ASIC1−/− mice stimulated with ET-1 or ionomycin.
Calculations and Statistical Analyses
Results are expressed as means ± SE. Values of n refer to number of animals in each group unless otherwise stated. Statistical significance was tested at the 95% (P < 0.05) confidence level using an unpaired t-test, one-way ANOVA, or two-way ANOVA followed by a Student-Newman-Keuls posttest. The statistical test performed for each experiment is indicated in the figure legends.
RESULTS
CH-mediated increases in basal [Ca2+]i and SOCE in small pulmonary arteries are dependent on ASIC1.
We have previously demonstrated that the only NFAT isoform activated by CH in mouse pulmonary arteries is NFATc3 (9). NFATc3 activation was time-dependent, with 1 wk of CH being the time with the highest percent of PASMC with nuclear NFATc3 (9). Therefore, we chose this time to examine the contribution of ASIC1 Ca2+ influx to NFATc3 nuclear translocation and activation. Consistent with our previous data following 4 wk of CH (39), we demonstrate that baseline fura-2 ratios (Fig. 1A) and SOCE (Fig. 1, B and C) were greater in arteries isolated from ASIC1+/+ mice exposed to 1 wk of CH compared with control ASIC1+/+ mice. This CH-mediated increase in basal [Ca2+]i and SOCE was not present in arteries isolated from ASIC1−/− mice (Fig. 1). Furthermore, SOCE responses from control and CH ASIC1−/− mice were diminished compared with respective ASIC1+/+ mice (Fig. 1, B and C). These data, along with previous observations from our laboratory (21, 39), demonstrate the involvement of ASIC1 in SOCE in control animals as well as the augmentation of this response after exposure to CH.
Fig. 1.
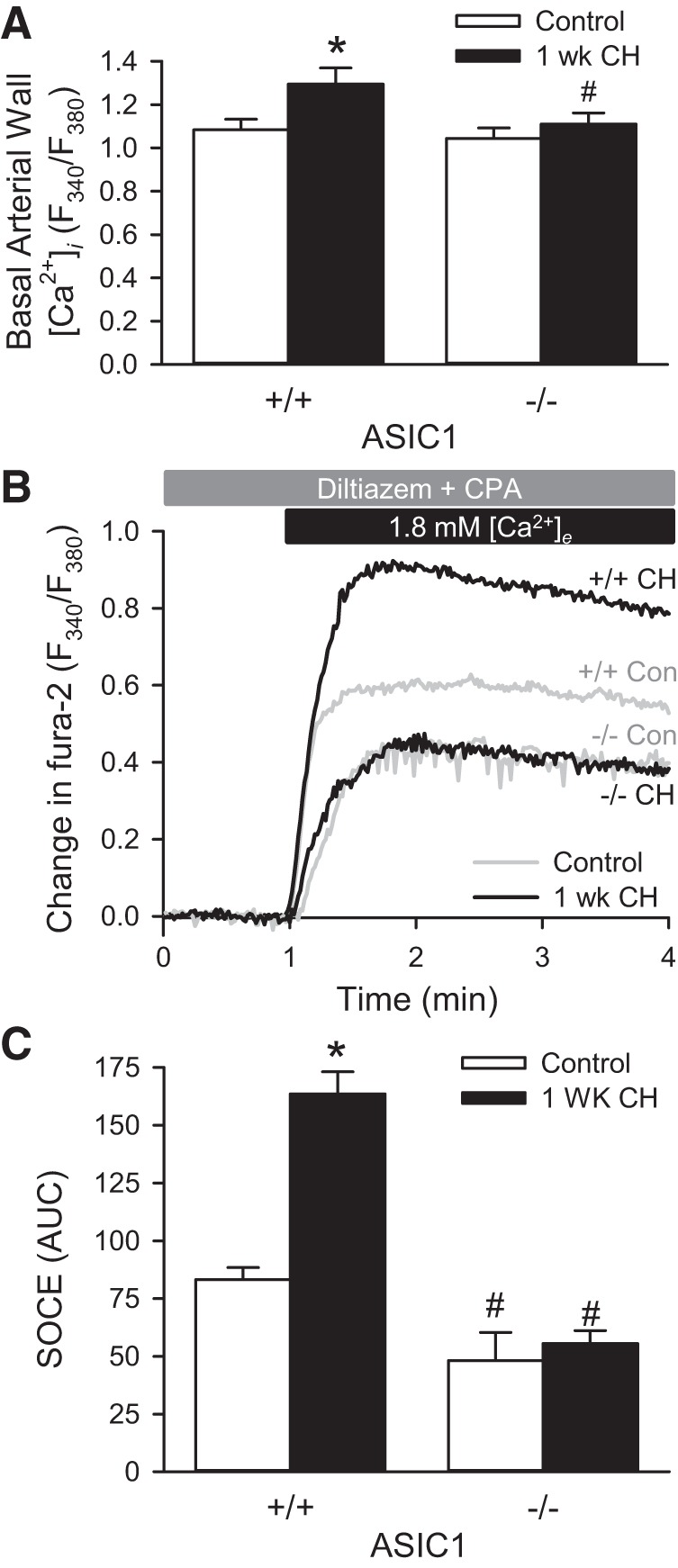
Chronic hypoxia (CH)-induced increases in basal pulmonary arterial intracellular Ca2+ concentration ([Ca2+]i) and store-operated calcium entry (SOCE) are ASIC1 dependent. A: basal arterial wall [Ca2+]i ratios (F340/F380) in small pulmonary arteries from control and CH (1 wk) ASIC1 wild-type (+/+) and knockout (−/−) mice. B: averaged change in fura-2 ratio (F340/F380) upon repletion of 1.8 mM extracellular Ca2+ ([Ca2+]e). C: summary data showing SOCE as area under curve (AUC) in small pulmonary arteries from control and CH-exposed (1 wk) ASIC1 wild-type (+/+) and knockout (−/−) mice. SOCE experiments were performed in the presence of cyclopiazonic acid (CPA, 10 μM) and diltiazem (50 μM). Values are means ± SE; n = 7–10 animals/group. *P < 0.05 vs. control mice, #P <0.05 vs. ASIC1+/+ mice analyzed by a two-way ANOVA and individual groups compared with the Student-Newman-Keuls test.
ASIC1 contributes to CH-induced NFATc3 nuclear accumulation in PASMC.
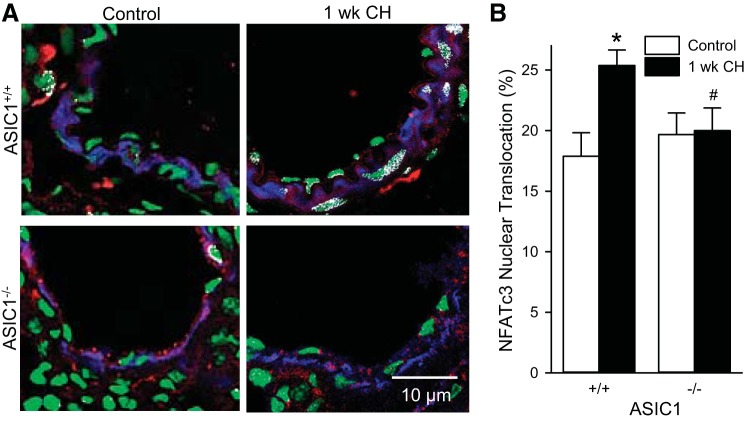
To determine the role of ASIC1 in NFATc3 nuclear translocation in PASMC, we examined the colocalization of anti-NFATc3 and SYTOX. CH exposure increased colocalization of anti-NFATc3 and SYTOX in PASMC from ASIC1+/+ mice but not ASIC1−/− mice (Fig. 2). These data are consistent with our previous report in BALB/c and FVBN mice showing CH-induced increases in NFATc3 nuclear localization (9), and suggest that ASIC1 is important in hypoxia-induced NFATc3 nuclear localization and activation.
Fig. 2.
ASIC1 contributes to CH-induced NFATc3 nuclear accumulation. Representative images (A) and summary data (B) showing NFATc3 nuclear translocation (white) was assessed by % NFATc3 colocalization (red) with SYTOX nuclear stain (green) in smooth muscle α-actin-positive cells (blue) in fixed lung sections from control and CH-exposed (1 wk) ASIC1+/+ and ASIC1−/− mice. Data are expressed as means ± SE, n = 32 arteries from 4 animals per group. *P < 0.05 vs. control mice, #P < 0.05 vs. ASIC1+/+ mice analyzed by a two-way ANOVA and individual groups compared with the Student-Newman-Keuls test.
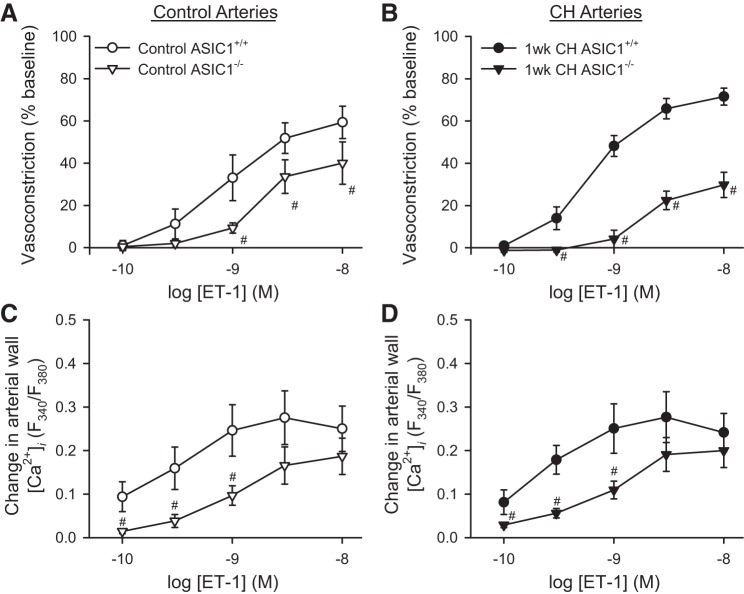
ET-1 vasoconstrictor and arterial wall [Ca2+]i responses are impaired in ASIC1−/− mice.
Activation of the ET-1 system has been demonstrated in patients with pulmonary hypertension and in animal models of pulmonary hypertension (13). We previously demonstrated that ASIC1 contributes to ET-1-induced pulmonary arterial vasoconstriction (21, 39) and that ET-1 contributes to CH-induced NFATc3 activation in mouse pulmonary arteries (8). ET-1-induced vasoconstrictor responses were largely blunted in arteries from both control (Fig. 3A) and CH (Fig. 3B) ASIC1−/− mice when compared with ASIC1+/+ mice. Changes in arterial wall [Ca2+]i were similarly diminished in isolated arteries from ASIC1−/− mice compared with arteries from ASIC1+/+ mice (Fig. 3, C and D). There was no significant difference in ET-1-induced vasoreactivity following 1 wk of CH compared with controls in either group. Although 1 wk of CH exposure tended to increase ET-1 vasoconstrictor reactivity in arteries from ASIC1+/+ mice (Fig. 3, A and B), 1 wk may not be a sufficient length of CH exposure to observe a significant augmentation of ET-1-mediated vasoconstriction as we have previously shown in arteries from ASIC1+/+ mice following 4 wk of CH (39).
Fig. 3.
ASIC1 contributes to ET-1-mediated vasoconstriction in isolated, pressurized small pulmonary arteries. Vasoconstriction (percent baseline diameter, A and B) and changes in arterial wall [Ca2+]i (ΔF340/F380, C and D) in response to endothelin-1 (ET-1, 10−10 to 10−8 M) in small pulmonary arteries from control (A and C) and CH-exposed (1 wk) (B and D) ASIC1+/+ and ASIC1−/− mice. Values are means ± SE, n = 5–7 animals/group. #P <0.05 vs. ASIC1+/+ mice analyzed by a two-way ANOVA and individual groups compared with the Student-Newman-Keuls test.
ASIC1 contributes to ET-1-induced increases in PASMC [Ca2+]i and NFATc3 nuclear import.
To better understand the mechanism by which ASIC1 contributes to activation of NFATc3, we examined ET-1-induced NFATc3 nuclear localization in cultured PASMC from ASIC1+/+ and ASIC1−/− mice. We first examined the contribution of ASIC1 to ET-1-induced Ca2+ influx in cultured mouse PASMC. ET-1 resulted in a rapid increase in [Ca2+]i that stabilized at lower levels (Fig. 4A). This initial [Ca2+]i peak amplitude was unaffected by inhibition of ASIC1 and presumably mediated by release of Ca2+ from the sarcoplasmic reticulum (28). However, the duration of this peak and the subsequent sustained Ca2+ influx was blunted by inhibition of ASIC1. When assessing AUC, the ASIC1 inhibitor psalmotoxin I (PcTX1) significantly diminished ET-1-induced increases in [Ca2+]i in PASMC from ASIC1+/+ mice (Fig. 4B). PASMC from ASIC1−/− mice similarly showed reduced ET-1-induced Ca2+ entry (Fig. 4B).
Fig. 4.
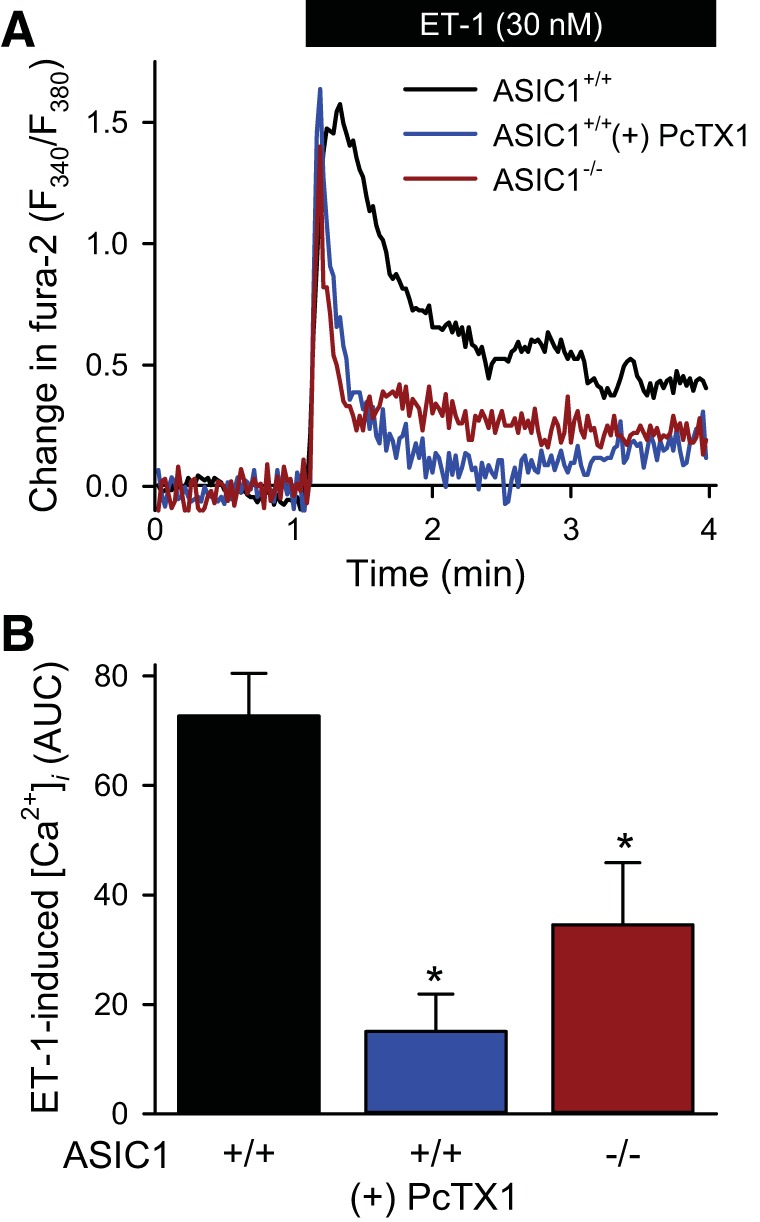
ASIC1 contributes to ET-1-induced Ca2+ influx in mouse PASMC. A: representative traces showing the change in fura-2 ratio. B: summary data of area under the curve (AUC) in response to ET-1 in mouse pulmonary arterial smooth muscle cell (PASMC) from ASIC1+/+ mice in the presence or absence of psalmotoxin 1 (PcTX1, 20 nM) or ASIC1−/− mice. Values are means ± SE, n = 4–6/group. *P < 0.05 vs. ASIC1+/+ mice analyzed by a one-way ANOVA and individual groups compared with the Student-Newman-Keuls test.
Consistent with previous studies (8), NFATc3-EGFP translocated to the nucleus in response to ET-1 in PASMC from ASIC1+/+ mice (Fig. 5, A and B). PcTX1 prevented this ET-1-induced NFATc3 nuclear import in ASIC1+/+ PASMC. We additionally confirmed the data obtained using a pharmacologic ASIC1 inhibitor by showing that ET-1-induced NFATc3 nuclear import was significantly attenuated in cultured PASMC from ASIC1−/− mice compared with PASMC from ASIC1+/+ mice. To specifically determine whether Ca2+ influx through ASIC1 is important in activating NFATc3, we induced NFATc3 nuclear import by increasing [Ca2+]i with ionomycin, a calcium ionophore. Under these experimental conditions in which we have bypassed Ca2+ influx through ASIC1, there was no difference in NFATc3 nuclear import between ASIC1+/+ and ASIC1−/− PASMC (Fig. 5C). These experiments demonstrate that downstream of Ca2+ entry, signaling of NFATc3 nuclear import is intact in PASMC from ASIC1−/− mice and further highlight the essential role for ASIC1-mediated Ca2+ influx in both CH- and ET-1-induced NFATc3 nuclear translocation.
Fig. 5.
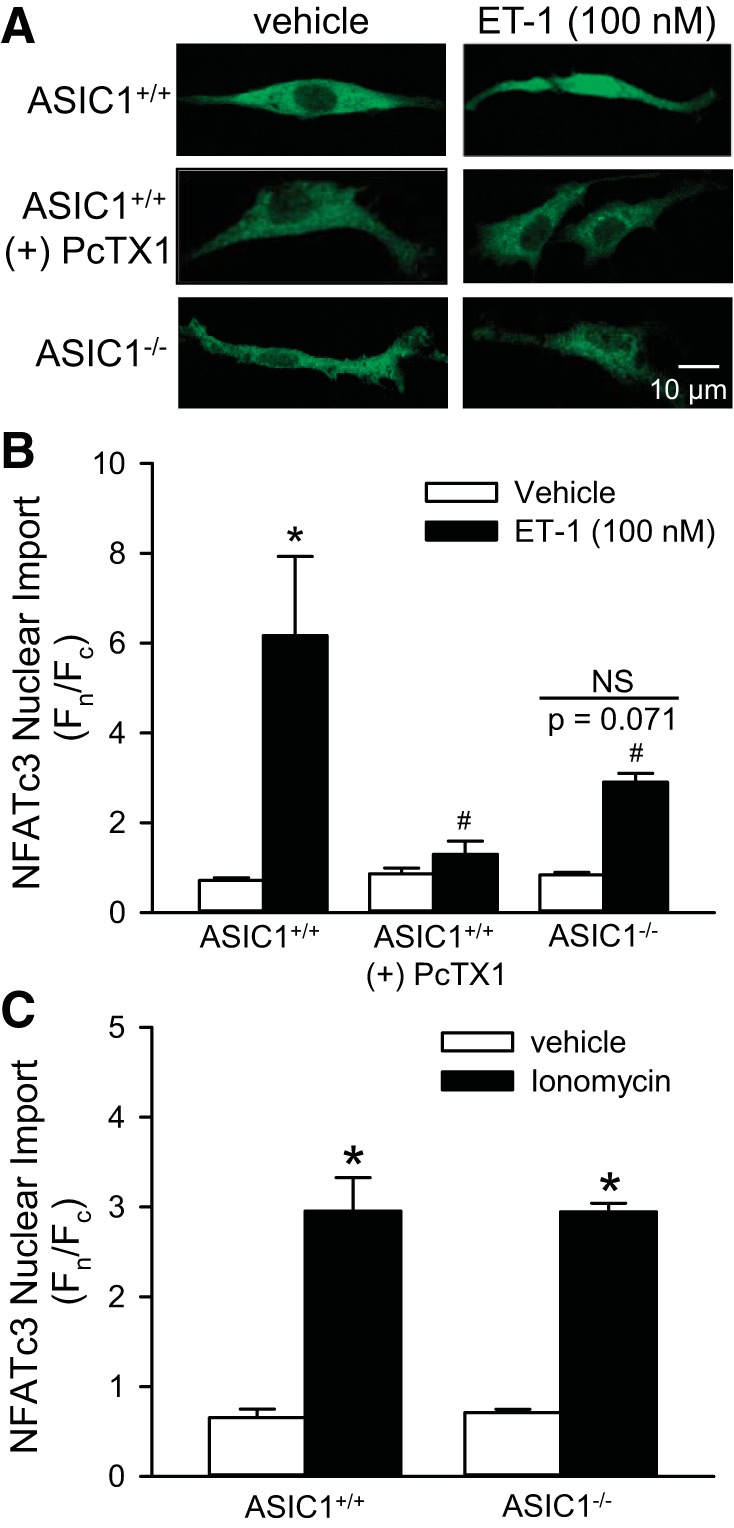
ASIC1 Ca2+ influx contributes to ET-1-induced NFATc3 nuclear import. A: mouse PASMC from ASIC1+/+ or ASIC1−/− mice were transfected with NFATc3-enhanced green fluorescent protein (EGFP). EGFP nuclear (Fn)/cytosolic fluorescence (Fc) was determined in PASMC treated with vehicle, ET-1 (100 nM, B), or ionomycin (0.1 μM, C). PASMC from ASIC1+/+ mice were additionally treated with PcTX1 (20 nM, A and B). Values are means ± SE, n = 3 replicates/group from average of 20–25 cells/each replicate. *P < 0.05 vs. vehicle, #P < 0.05 vs. ASIC1+/+ mice analyzed by a two-way ANOVA and individual groups compared with the Student-Newman-Keuls test.
Knockout of ASIC1 does not alter NFAT, Orai1, or TRPC1 expression in PASMC.
We have shown that NFATc3, but not NFATc2, is activated in PASMC by hypobaric CH (8, 9). However, NFATc2 has been shown to be activated in PASMC from patients with primary pulmonary arterial hypertension and in other animal models of pulmonary hypertension in a Ca2+-dependent and independent manner (35, 41). Although the NFATc3 nuclear import pathway in response to ionomycin is not impaired in PASMC from ASIC1−/− mice (Fig. 5C), we further sought to determine whether absence of the ASIC1 gene affects NFATc2 or NFATc3 mRNA expression. There was no difference in NFATc2 (Fig. 6A) or NFATc3 (Fig. 6B) mRNA levels between ASIC1+/+ and ASIC1−/− PASMC. These data further suggest that the underlying mechanism of NFATc3 activation in PASMC is Ca2+ influx, and not a change in NFAT isoform expression.
Fig. 6.
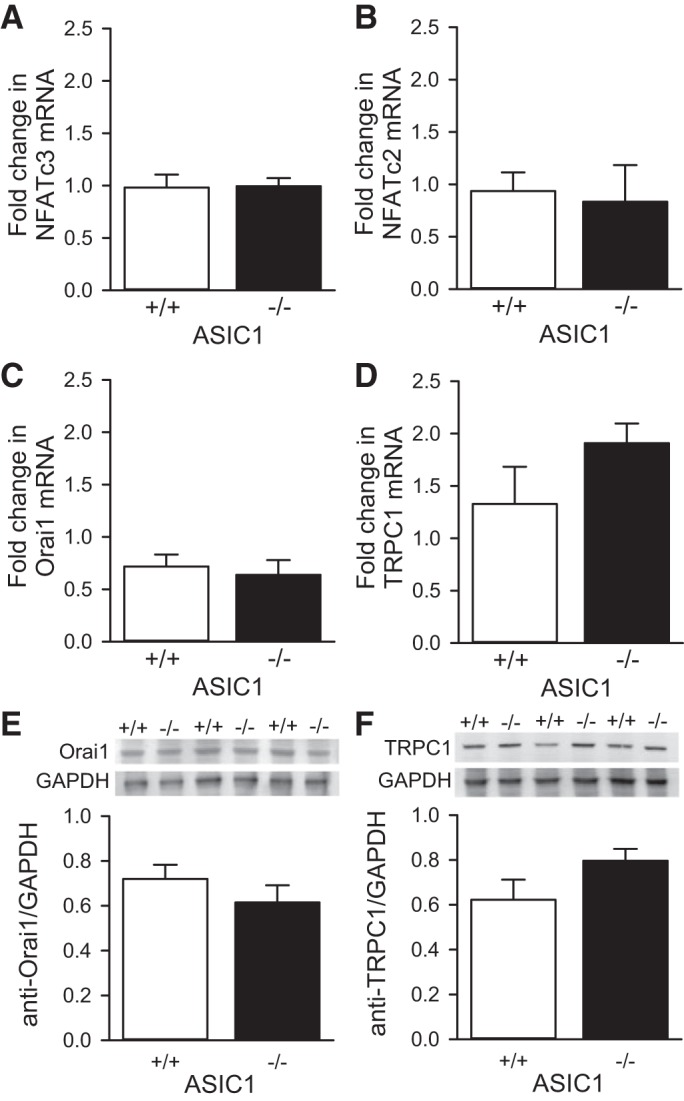
Knockout of ASIC1 does not alter NFAT, Orai1, or TRPC1 mRNA and protein levels in PASMC. Fold change (from calibrator) in NFATc2 (A), NFATc3 (B), Orai1 (C), and TRPC1 (D) mRNA expression in PASMC from ASIC1+/+ and ASIC1−/− mice. β-Actin was used as the endogenous control. Also shown are representative Western blots (top) and summary data (bottom) showing Orai1 (E) and TRPC1 (F) protein expression in PASMC from ASIC1+/+ and ASIC1−/− mice. GAPDH was used as the endogenous control. Values are means ± SE, n = 6 from different passages and at least two different mice, analyzed by unpaired t-test.
Several store-operated channels in PASMC contribute to development of pulmonary hypertension (46). To determine whether the knockout of ASIC1 alters the expression level of other store-operated channels, we examined mRNA and protein expression of Orai1 and TRPC1. There was no difference in Orai1 (Fig. 6, C and E) or TRPC1 (Fig. 6, D and F) mRNA or protein expression between ASIC1+/+ and ASIC1−/− PASMC. Although these data suggest other store-operated channels are not compromised by the absence of ASIC1, we do not currently know whether ASIC1 interacts with these other molecules to form a store-operated signaling complex.
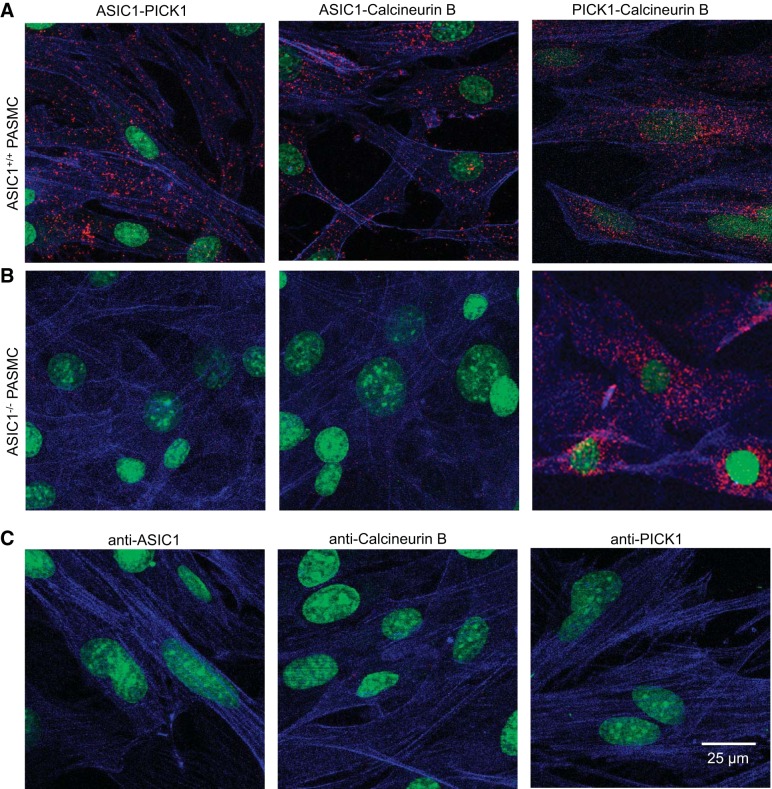
Colocalization between ASIC1, PICK1, and calcineurin B.
NFATc3 is regulated by Ca2+ and calcineurin, a Ca2+/calmodulin-dependent serine phosphatase. In rat PASMC we have recently shown a functional association between ASIC1 and PICK1/calcineurin (14), however, the physical interaction is unknown. Using the Duolink PLA we observe colocalization of ASIC1 with PICK1 and calcineurin B in PASMC from ASIC1+/+ mice, as demonstrated by red puncta (Fig. 7A). This interaction was absent in PASMC from ASIC1−/− mice (Fig. 7B). PICK1 and calcineurin B colocalize in PASMC from both ASIC1+/+ and ASIC1−/− mice (Fig. 7, A and B). As a negative control, either both primary antibodies were omitted (not shown) or one primary antibody was omitted at a time. We observed very few, if any, puncta under these conditions (Fig. 7C).
Fig. 7.
Colocalization of ASIC1, PICK1, and calcineurin B in mouse PASMC. Representative confocal images of the Duolink PLA interaction (red puncta) between ASIC1-PICK1 (left), ASIC1-calcineurin B (middle), and PICK1-calcineurin B (right) in PASMC from ASIC1+/+ mice (A) and ASIC1−/− mice (B). Proximity Ligation Assay (PLA) interactions are not detected for ASIC1-PICK1 or ASIC1-calcineurin B in PASMC from ASIC1−/− mice. For negative controls (C), PASMC were incubated with each primary alone and both PLA probes. Actin is labeled with Alexa Fluor 647 phalloidin (blue) and the nuclei are labeled with SYTOX (green).
PICK1 is essential for NFATc3 nuclear import.
Considering the possibility that PICK1 provides the scaffold that localizes ASIC1 and calcineurin B, we examined the role of PICK1 in NFATc3 nuclear import in PASMC from ASIC1+/+ mice. ET-1 resulted in increased NFATc3 nuclear translocation in PASMC from ASIC1+/+ mice that was prevented by the PICK1 PDZ-domain inhibitor FSC231 (Fig. 8A). FSC231 also diminished NFATc3 nuclear import in response to ionomycin, a calcium ionophore (Fig. 8B), suggesting that PICK1 may contribute to NFATc3 nuclear import downstream of Ca2+ influx. To specifically determine whether PICK1 influences Ca2+ entry in mouse PASMC from ASIC1+/+ mice, we examined the effect of FSC231 on changes in [Ca2+]i in responses to ET-1 and SOCE. FSC231 did not alter Ca2+ influx to either ET-1 (Fig. 9A) or SOCE (Fig. 9B). These data suggest that PICK1 may facilitate NFATc3 nuclear translocation downstream of ASIC1-mediated Ca2+ influx.
Fig. 8.
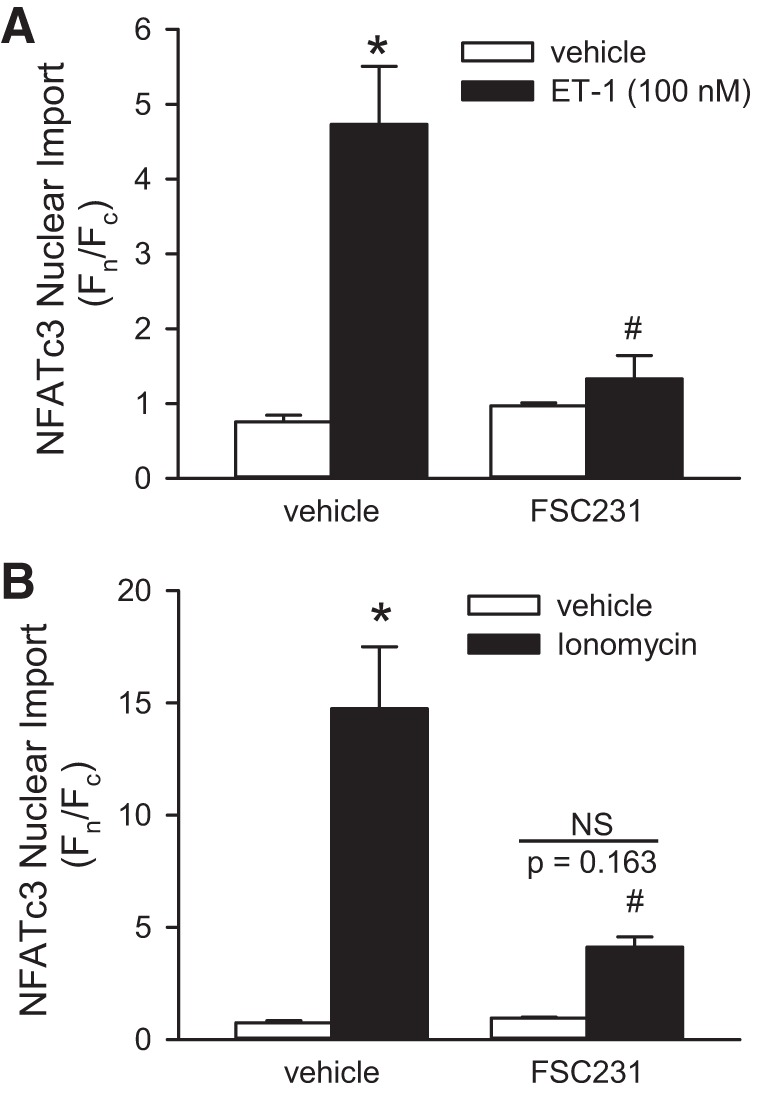
PICK1 contributes to NFATc3 nuclear import downstream Ca2+ influx. Summary data showing EGFP nuclear (Fn)/cytosolic fluorescence (Fc) in mouse PASMC expressing NFATc3-EGFP treated with vehicle, ET-1 (100 nM, A), or ionomycin (0.1 μM, B) in the presence or absence of the PICK1 inhibitor FSC231 (50 μM). Values are means ± SE, n = 5 replicates/group from average of 20–25 cells/each replicate. *P < 0.05 vs. vehicle, #P < 0.05 vs. control mice, analyzed by a two-way ANOVA and individual groups compared with the Student-Newman-Keuls test.
Fig. 9.
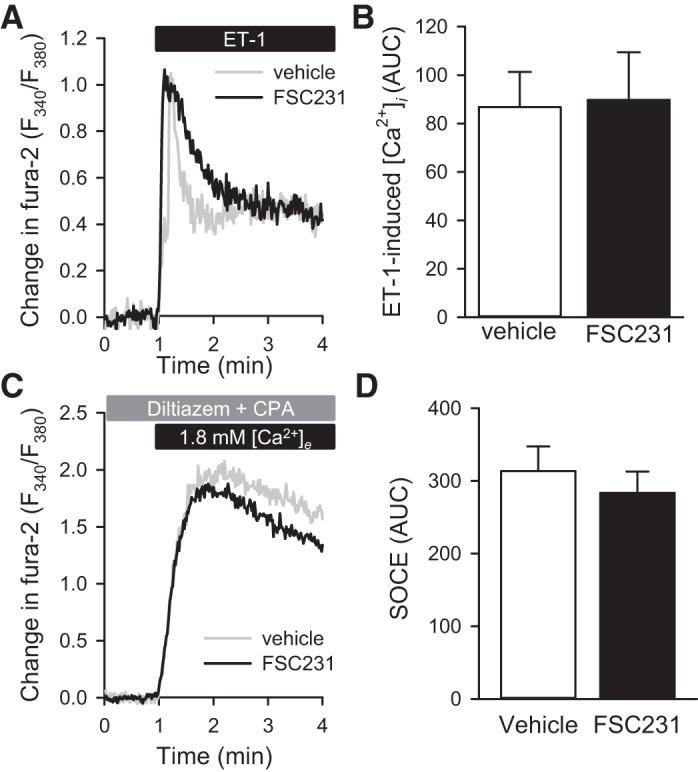
PICK1 does not alter stimulated Ca2+ influx. A: average change in fura-2 ratio (F340/F380) and summary data (B) of area under the curve (AUC) in response to ET-1 (100 nM) in the presence or absence of the PICK1 inhibitor FSC231 (50 μM) in mouse PASMC from ASIC1+/+ mice. Average change in fura-2 ratio (F340/F380) (C) and summary data of AUC (D) following SOCE in the presence or absence of FSC231 (50 μM) in mouse PASMC from ASIC1+/+ mice. Values are means ± SE, n = 4–6/group; analyzed by unpaired t-test.
DISCUSSION
Our research group has previously demonstrated that CH activates both ASIC1 (21, 39) and NFATc3 (8, 9) in PASMC, which contributes to the development of pulmonary hypertension. The goal of this study was to determine whether ASIC1-mediated Ca2+ influx contributes to CH- and ET-1-induced NFATc3 activation in mouse PASMC. Consistent with our previous studies, we found that 1-wk CH-mediated increases in basal [Ca2+]i and SOCE in small pulmonary arteries are dependent on ASIC1. The key findings of the present work are that 1) ASIC1-mediates CH- and ET-1-induced NFATc3 nuclear import, and 2) the scaffolding protein PICK1 is essential for NFATc3 nuclear import. Together, these data indicate that ASIC1 and PICK1 are both major contributors to CH- and ET-1-induced NFATc3 nuclear import. Furthermore, these data provide an essential link between CH-induced ASIC1-mediated Ca2+ influx, activation of the NFATc3 transcription factor, and the development of pulmonary hypertension.
Regulation of NFAT by Ca2+ and the Ca2+/calmodulin-dependent serine phosphatase calcineurin provides a direct link between intracellular Ca2+ signaling and gene expression. Additionally, activation of some NFAT isoforms can occur independent of Ca2+ through regulation of NFAT expression. Both the signal transducers and activators of transcription-3 (STAT3)/Pim-1 and epigenetic reader bromodomain-containing protein-4 (BRD4), which have binding sites on the NFAT promoter region, have been implicated in pulmonary arterial hypertension (35, 41). However, we have previously demonstrated that the only NFAT isoform activated by hypobaric hypoxia or oxidative stress in PASMC is NFATc3, and the mechanism does not involve upregulation of its expression (9, 45). Consistent with our previous findings, NFATc2 and NFATc3 mRNA levels were similar in PASMC from ASIC1+/+ and ASIC1−/− mice, suggesting that decreased NFATc3 nuclear translocation in PASMC from ASIC1−/− mice is not due to downregulation of NFATc3 expression. Moreover, we observe similar inhibition of NFATc3 nuclear import when we acutely inhibit ASIC1 with PcTX1. Therefore, it is unlikely that ASIC1 regulates NFATc3 activation at the level of transcription/translation; rather, our data support a role for ASIC1-dependent Ca2+ influx in activation of NFATc3 in PASMC.
It was recently shown that signaling in specific microdomains is more important for nuclear translocation of NFAT than global increases in [Ca2+]i (26). It is well established that NFAT is activated by cell-surface receptors coupled to SOCE and/or Ca2+-release-activated Ca2+ (CRAC) in a variety of cell types, including vascular smooth muscle (3, 18, 42, 52). In patients with a rare form of hereditary severe combined immunodeficiency, T cells display a pronounced reduction in SOCE, which leads to a selective inability to activate all NFATs (12). SOCE is a dynamic and highly regulated process that is known to be mediated by two distinct types of ionic currents. The CRAC current displays high selectivity for Ca2+ and is mediated by the Orai1 channel (29). Store depletion also activates nonselective cation channel currents (1, 55), believed to be mediated by various transient receptor potential (TRP) channels. The depletion of Ca2+ is sensed by the Ca2+-binding protein stromal interaction molecule-1 (STIM1), which then binds to and activates store-operated channels (32, 48). In PASMC, STIM1 interacts with both Orai1 and the classical/canonical TRP-1 (TRPC1) channel (36, 37). In HEK293 and human submandibular gland cells, Orai1-mediated Ca2+ influx induces nuclear translocation of NFAT and NFAT-dependent gene expression; however, global increases in [Ca2+]i or Ca2+ influx through TRPC1 or TRPC3 was not sufficient to activate NFAT (25, 40). One possible reason for this contrasting response is the association of these ion channels with necessary scaffolding proteins. Following store depletion, Orai1 assembles in a membrane-delimited signaling complex with calcineurin and calmodulin, via the scaffolding protein A kinase-anchoring protein 79/150 [AKAP79/150 (27)]. By contrast, the related Orai3 interacts poorly with AKAP79/150 and fails to activate NFAT (27). Together, these studies demonstrate that discrete Ca2+ events can differentially activate NFAT.
On the basis of current data and previous data from our laboratory (14, 21, 22, 39, 44), ASIC1 appears to be a major component, but unlikely the only component of SOCE in PASMC. How ASIC1 contributes to the SOCE response and its interactions with other known mediators of SOCE such as STIM1, TRPC1, or Orai1 is of interest and an area we are currently investigating. ASIC1 does, however, interact with several scaffolding proteins such as AKAP/150 and PICK1 and with the phosphatase calcineurin (4, 11, 14, 17). Therefore, the goal of this study was to determine whether ASIC1-mediated SOCE is involved in activation of NFATc3 in PASMC.
In our previous studies, administration of the L-type Ca2+ channel inhibitor diltiazem attenuated but did not abolish CH-induced NFATc3 activation, suggesting that other Ca2+-influx pathways contribute to increased NFATc3 nuclear translocation (8). Our first key finding in the present work is that ASIC1-mediated Ca2+ influx is essential for both hypoxia- and ET-1-induced NFATc3 nuclear translocation. The findings supporting Ca2+ influx over other means of NFAT activation are as follows: 1) NFATc3 expression is not altered in PASMC from ASIC1−/− mice compared with ASIC1+/+ mice, 2) CH-mediated increases in basal [Ca2+]i and SOCE in small pulmonary arteries are dependent on ASIC1, 3) inhibition of ASIC1 largely attenuated ET-1-induced Ca2+ responses, and 4) bypassing ASIC1-mediated Ca2+ influx by using ionomycin resulted in similar NFAT nuclear import between ASIC1+/+ and ASIC1−/− PASMC. Previous studies showing that ASIC1 interacts with calcineurin (4), and our current finding of colocalization of ASIC1 and calcineurin B provide additional support for this pathway. Furthermore, ASIC1 is involved in acid-stimulated NFATc1 signaling in osteoclastogenesis. Acid-induced increases in [Ca2+]i to activate calcineurin/NFATc1 signaling is the major stimulus of osteoclast differentiation, and ASIC1a activation in rat osteoclasts induces NFATc1 nuclear translocation and transcriptional activity (31). We anticipate ASIC1 is activated by agonist-induced store-depletion in PASMC, although we cannot currently discount that CH causes localized changes in H+ resulting in activation of ASIC1.
The laboratory headed by R. Simon (4) reported that PKA, in association with AKAP/150, is required for full activation of ASICs in neurons and CHO cells. Our recent study using rat PASMC showing that PKA phosphorylates ASIC1 and stimulates channel activity is consistent with this previous study (14). Although the interaction between PICK1 and ASIC1 is well characterized, the functional significance of this interaction is not well understood. PICK1 has been shown to increase ASIC1 surface expression and clustering (11, 17, 24); however, our recent findings suggest that PICK1 interaction with ASIC1 is associated with changes in the phosphorylation state of the channel rather than plasma membrane localization. More specifically, ASIC1 activity was inhibited by a PICK1-dependent and calcineurin-mediated dephosphorylation of ASIC1 (14). Simon's laboratory (4) also reported association with calcineurin resulted in a dephosphorylation and inactivation of ASIC1. Although both AKAP150 and PICK1 also associate with calcineurin (5, 10, 19) and have been shown to promote NFAT activity in various cell types (19, 30, 38), these previous findings regarding reciprocal modulation of ASIC1 activity by PKA/AKAP150 and PICK1/calcineurin directed our focus on PICK1. Interestingly, in the current study we did not observe the same inhibitory effect of PICK1 on ASIC1-mediated Ca2+ influx in mouse PASMC, as we previously had in rat PASMC. Other than species differences, the reason for the disparities is currently unknown. However, the lack of PICK1-mediated inhibition on ASIC1-mediated Ca2+ influx in mouse PASMC allowed us to more accurately study the role of ASIC1 and PICK1 in downstream activation of NFATc3.
The second key finding of the present study is that PICK1 is necessary for ET-1-stimulated NFATc3 nuclear import. The scaffolding protein PICK1 mediates the direct interaction of proteins containing PDZ binding motifs. In addition to the PDZ domain, PICK1 contains a larger Bin/amphiphysin/Rvs (BAR) domain, which binds to lipids to facilitate membrane localization (23). Both the PDZ and BAR domains are essential for vesicular formation and protein trafficking (23, 24, 43). The scaffolding properties of PICK1 enable macromolecular complexes to form, assembling molecules into signaling domains. PICK1 associates with ASIC1 (11, 17) and with calcineurin, through which PICK1 promotes activation of NFAT in PC12 cells (19). Our data suggest that PICK1 has several functions. First, PICK1 provides the necessary scaffolding for ASIC1 and calcineurin, bringing them in close proximity to promote activation of calcineurin by ASIC1-mediated Ca2+ influx, potentially in conjunction with AKAP79/150. Second, PICK1 appears to participate in the Ca2+-independent activation and/or trafficking of calcineurin-NFATc3 to the nucleus. This is supported by the data demonstrating that PICK1 inhibition diminished NFATc3 nuclear accumulation in response to both ET-1 and ionomycin, but did not alter ET-1-induced Ca2+ influx or SOCE in PASMC. Following Ca2+ influx, PICK1 may facilitate the interaction of ASIC1 and calcineurin and downstream activation and nuclear translocation of NFATc3.
In addition to Ca2+-dependent activation of calcineurin, ET-1-induced NFATc3 nuclear translocation requires RhoA/Rho kinase (ROCK) and an intact dynamic actin cytoskeleton (8). PICK1 can interact with cytoskeletal proteins but with some controversial outcomes (34, 47). For example, PICK1 was shown to inhibit actin filament (F-actin) nucleation by the Arp2/3 complex through interactions of the PICK1 BAR domain with F-actin (47). More recently, researchers in the laboratory of R. Dominguez (34) confirmed that PICK1 binds F-actin in vitro; however, in cells, PICK1 did not bind directly to F-actin-rich structures, but they used F-actin indirectly for the fast and nondirectional movement of PICK1-associated vesicles. In these studies, the acidic C-terminal tail (ACT) of PICK1 linked PICK1-associated vesicles to a motility factor such as actin-based myosin motor (34). Together, these data argue that NFATc3 nuclear translocation requires PICK1 association and a properly assembled actin cytoskeleton.
In summary, we observed a complex mechanism of NFATc3 activation in mouse PASMC that involves Ca2+ influx through ASIC1 and downstream regulation by the scaffolding protein PICK1 (Fig. 10). It draws together our previous findings that CH activates both ASIC1 and NFATc3. The contribution of ASIC1 to CH-induced pulmonary vascular remodeling further supports an important role for ASIC1-mediated Ca2+ influx in NFATc3 activation of proliferation and hypertrophy in PASMC and the overall development of hypoxic pulmonary hypertension (2, 7, 9, 39). Our findings suggest that ASIC1 acts as a direct signal transducer and may be a potential therapeutic target for modulation of hypoxia-dependent transcription pathways associated with the pathophysiology of hypoxic pulmonary hypertension.
Fig. 10.
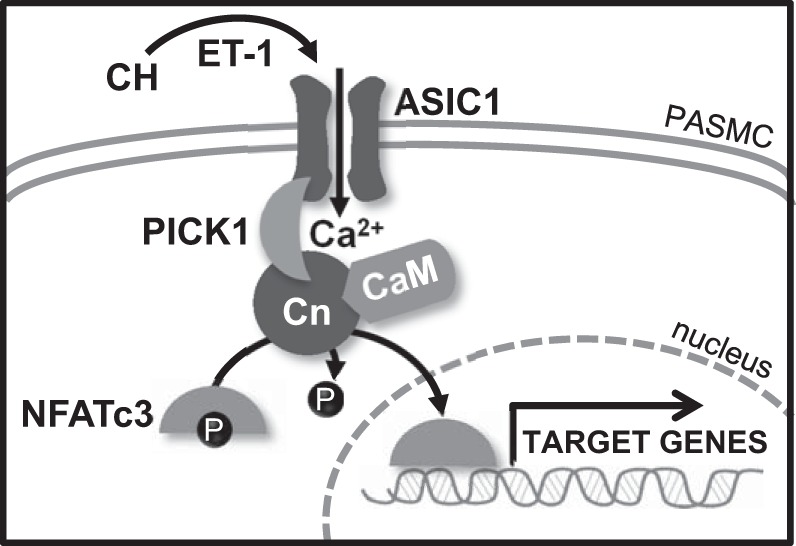
Summary of major findings. CH, presumably through ET-1, increases Ca2+ influx through ASIC1 in PASMC. Elevated intracellular Ca2+ levels leads to calcineurin-mediated dephosphorylation of NFATc3, allowing its nuclear import. PICK1 provides the scaffold for the interaction between ASIC1 and calcineurin, contributing to the NFATc3 activation process without affecting the ability of ASIC1 channels to conduct Ca2+. CaM, calmodulin; Cn, calcineurin.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-111084 to N.L. Jernigan and HL-088151 to L.V. Gonzalez Bosc.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.V.G.B. and N.L.J. conception and design of research; L.V.G.B., D.R.P., L.M.H., W.G., C.B., and N.L.J. performed experiments; L.V.G.B., D.R.P., L.M.H., W.G., and N.L.J. analyzed data; L.V.G.B. and N.L.J. interpreted results of experiments; L.V.G.B. and N.L.J. prepared figures; L.V.G.B. and N.L.J. drafted manuscript; L.V.G.B., D.R.P., and N.L.J. edited and revised manuscript; L.V.G.B., D.R.P., L.M.H., W.G., C.B., and N.L.J. approved final version of manuscript.
ACKNOWLEDGMENTS
ASIC1−/− mice were kindly provided by Drs. M.J. Welsh and J.A. Wemmie (University of Iowa, Iowa City, IA). The NFATc3-EGFP vector was created by Dr. F. McKeon (Harvard University, Cambridge, MA) and kindly provided by Dr. L.F. Santana (Washington State University, Seattle, WA).
REFERENCES
- 1.Albert AP, Large WA. Store-operated Ca2+-permeable non-selective cation channels in smooth muscle cells. Cell Calcium 33: 345–356, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Bierer R, Nitta CH, Friedman J, Codianni S, de Frutos S, Dominguez-Bautista JA, Howard TA, Resta TC, Gonzalez Bosc LV. NFATc3 is required for chronic hypoxia-induced pulmonary hypertension in adult and neonatal mice. Am J Physiol Lung Cell Mol Physiol 301: L872–L880, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bobe R, Hadri L, Lopez JJ, Sassi Y, Atassi F, Karakikes I, Liang L, Limon I, Lompré AM, Hatem SN, Hajjar RJ, Lipskaia L. SERCA2a controls the mode of agonist-induced intracellular Ca2+ signal, transcription factor NFAT and proliferation in human vascular smooth muscle cells. J Mol Cell Cardiol 50: 621–633, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chai S, Li M, Lan J, Xiong Z, Saugstad J, Simon R. A kinase-anchoring protein 150 and calcineurin are involved in regulation of acid-sensing ion channels ASIC1a and ASIC2a. J Biol Chem 282: 22668–22677, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coghlan VM, Perrino BA, Howard M, Langeberg LK, Hicks JB, Gallatin WM, Scott JD. Association of protein kinase A and protein phosphatase 2B with a common anchoring protein. Science 267: 108–111, 1995. [DOI] [PubMed] [Google Scholar]
- 6.de Frutos S, Duling L, Alò D, Berry T, Jackson-Weaver O, Walker M, Kanagy N, González Bosc L. NFATc3 is required for intermittent hypoxia-induced hypertension. Am J Physiol Heart Circ Physiol 294: H2382–H2390, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Frutos S, Nitta CH, Caldwell E, Friedman J, González Bosc LV. Regulation of soluble guanylyl cyclase-α1 expression in chronic hypoxia-induced pulmonary hypertension: role of NFATc3 and HuR. Am J Physiol Lung Cell Mol Physiol 297: L475–L486, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Frutos S, Ramiro Diaz JM, Nitta CH, Sherpa ML, González Bosc LV. Endothelin-1 contributes to increased NFATc3 activation by chronic hypoxia in pulmonary arteries. Am J Physiol Cell Physiol 301: C441–C450, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Frutos S, Spangler R, Alò D, González Bosc LV. NFATc3 mediates chronic hypoxia-induced pulmonary arterial remodeling with α-actin up-regulation. J Biol Chem 282: 15081–15089, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dell'Acqua ML, Dodge KL, Tavalin SJ, Scott JD. Mapping the protein phosphatase-2B anchoring site on AKAP79: binding and inhibition of phosphatase activity are mediated by residues 315–360. J Biol Chem 277: 48796–48802, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duggan A, Garcia-Anoveros J, Corey DP. The PDZ domain protein PICK1 and the sodium channel BNaC1 interact and localize at mechanosensory terminals of dorsal root ganglion neurons and dendrites of central neurons. J Biol Chem 277: 5203–5208, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Feske S, Giltnane J, Dolmetsch R, Staudt LM, Rao A. Gene regulation mediated by calcium signals in T lymphocytes. Nat Immunol 2: 316–324, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Galié N, Manes A, Branzi A. The endothelin system in pulmonary arterial hypertension. Cardiovasc Res 61: 227–237, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Herbert LM, Nitta CH, Yellowhair TR, Browning C, González Bosc LV, Resta TC, Jernigan NL. PICK1/calcineurin suppress ASIC1-mediated Ca2+ entry in rat pulmonary arterial smooth muscle cells. Am J Physiol Cell Physiol 310: C390–C400, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hill-Eubanks DC, Gomez MF, Stevenson AS, Nelson MT. NFAT regulation in smooth muscle. Trends Cardiovasc Med 13: 56–62, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Hogan PG, Chen L, Nardone J, Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev 17: 2205–2232, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Hruska-Hageman A, Wemmie J, Price M, Welsh M. Interaction of the synaptic protein PICK1 (protein interacting with C kinase 1) with the non-voltage gated sodium channels BNC1 (brain Na+ channel 1) and ASIC (acid-sensing ion channel). Biochem J 361: 443–450, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hwang SY, Putney JW. Orai1-mediated calcium entry plays a critical role in osteoclast differentiation and function by regulating activation of the transcription factor NFATc1. FASEB J 26: 1484–1492, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iida T, Egusa H, Saeki M, Yatani H, Kamisaki Y. PICK1 binds to calcineurin B and modulates the NFAT activity in PC12 cells. Biochem Biophys Res Commun 375: 655–659, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Jara E, Hidalgo M, Hancke J, Hidalgo A, Brauchi S, Nuñez L, Villalobos C, Burgos R. Delphinidin activates NFAT and induces IL-2 production through SOCE in T cells. Cell Biochem Biophys 68: 497–509, 2014. [DOI] [PubMed] [Google Scholar]
- 21.Jernigan NL, Herbert LM, Walker BR, Resta TC. Chronic hypoxia upregulates pulmonary arterial ASIC1: a novel mechanism of enhanced store-operated Ca2+ entry and receptor-dependent vasoconstriction. Am J Physiol Cell Physiol 302: C931–C940, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jernigan NL, Paffett ML, Walker BR, Resta TC. ASIC1 contributes to pulmonary vascular smooth muscle store-operated Ca2+ entry. Am J Physiol Lung Cell Mol Physiol 297: L271–L285, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin W, Ge W, Xu J, Cao M, Peng L, Yung W, Liao D, Duan S, Zhang M, Xia J. Lipid binding regulates synaptic targeting of PICK1, AMPA receptor trafficking, and synaptic plasticity. J Neurosci 26: 2380–2390, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin W, Shen C, Jing L, Zha XM, Xia J. PICK1 regulates the trafficking of ASIC1a and acidotoxicity in a BAR domain lipid binding-dependent manner. Mol Brain 3: 39, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kar P, Nelson C, Parekh AB. Selective activation of the transcription factor NFAT1 by calcium microdomains near Ca2+ release-activated Ca2+ (CRAC) channels. J Biol Chem 286: 14795–14803, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kar P, Parekh Anant B. Distinct spatial Ca2+ signatures selectively activate different NFAT transcription factor isoforms. Mol Cell 58: 232–243, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kar P, Samanta K, Kramer H, Morris O, Bakowski D, Parekh Anant B. Dynamic assembly of a membrane signaling complex enables selective activation of NFAT by Orai1. Curr Biol 24: 1361–1368, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ko EA, Park WS, Ko JH, Han J, Kim N, Earm YE. Endothelin-1 increases intracellular Ca2+ in rabbit pulmonary artery smooth muscle cells through phospholipase C. Am J Physiol Heart Circ Physiol 289: H1551–H1559, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Lewis RS. The molecular choreography of a store-operated calcium channel. Nature 446: 284–287, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Li H, Pink MD, Murphy JG, Stein A, Dell'Acqua ML, Hogan PG. Balanced interactions of calcineurin with AKAP79 regulate Ca2+-calcineurin-NFAT signaling. Nat Struct Mol Biol 19: 337–345, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li X, Xu RS, Jiang DL, He XL, Jin C, Lu WG, Su Q, Yuan FL. Acid-sensing ion channel 1a is involved in acid-induced osteoclastogenesis by regulating activation of the transcription factor NFATc1. FEBS Lett 587: 3236–3242, 2013. [DOI] [PubMed] [Google Scholar]
- 32.Liou J, Kim ML, Do Heo W, Jones JT, Myers JW, Ferrell JJ, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol 15: 1235–1241, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25: 402–408, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Madasu Y, Yang C, Boczkowska M, Bethoney KA, Zwolak A, Rebowski G, Svitkina T, Dominguez R. PICK1 is implicated in organelle motility in an Arp2/3 complex-independent manner. Mol Biol Cell 26: 1308–1222, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meloche J, Potus F, Vaillancourt M, Bourgeois A, Johnson IH, Deschamps L, Chabot S, Ruffenach GN, Henry S, Breuils-Bonnet S, Tremblay È, Nadeau V, Lambert CM, Paradis R, Provencher S, Bonnet S. Bromodomain containing protein-4: the epigenetic origin of pulmonary arterial hypertension. Circ Res 117: 525–535, 2015. [DOI] [PubMed] [Google Scholar]
- 36.Ng LC, McCormack MD, Airey JA, Singer CA, Keller PS, Shen XM, Hume JR. TRPC1 and STIM1 mediate capacitative Ca2+ entry in mouse pulmonary arterial smooth muscle cells. J Physiol 587: 2429–2442, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ng LC, Ramduny D, Airey JA, Singer CA, Keller PS, Shen XM, Tian H, Valencik M, Hume JR. Orai1 interacts with STIM1 and mediates capacitative Ca2+ entry in mouse pulmonary arterial smooth muscle cells. Am J Physiol Cell Physiol 299: C1079–C1090, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nieves-Cintrón M, Hirenallur-Shanthappa D, Nygren PJ, Hinke SA, Dell'Acqua ML, Langeberg LK, Navedo M, Santana LF, Scott JD. AKAP150 participates in calcineurin/NFAT activation during the down-regulation of voltage-gated K+ currents in ventricular myocytes following myocardial infarction. Cell Signal 28: 733–740, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nitta CH, Osmond DA, Herbert LM, Beasley BF, Resta TC, Walker BR, Jernigan NL. Role of ASIC1 in the development of chronic hypoxia-induced pulmonary hypertension. Am J Physiol Heart Circ Physiol 306: H41–H52, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ong HL, Jang SI, Ambudkar IS. Distinct contributions of Orai1 and TRPC1 to agonist-induced [Ca2+]i signals determine specificity of Ca2+-dependent gene expression. PLoS One 7: e47146, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paulin R, Courboulin A, Meloche J, Mainguy V, de la Roque ED, Saksouk N, Côté J, Provencher S, Sussman MA, Bonnet S. Signal transducers and activators of transcription-3/Pim1 axis plays a critical role in the pathogenesis of human pulmonary arterial hypertension. Circulation 123: 1205–1215, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Phuong TT, Yun YH, Kim SJ, Kang TM. Positive feedback control between STIM1 and NFATc3 is required for C2C12 myoblast differentiation. Biochem Biophys Res Commun 430: 722–728, 2013. [DOI] [PubMed] [Google Scholar]
- 43.Pinheiro PS, Jansen AM, de Wit H, Tawfik B, Madsen KL, Verhage M, Gether U, Sørensen JB. The BAR domain protein PICK1 controls vesicle number and size in adrenal chromaffin cells. J Neurosci 34: 10688–10700, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Plomaritas DR, Herbert LM, Yellowhair TR, Resta TC, Gonzalez Bosc LV, Walker BR, Jernigan NL. Chronic hypoxia limits H2O2-induced inhibition of ASIC1-dependent store-operated calcium entry in pulmonary arterial smooth muscle. Am J Physiol Lung Cell Mol Physiol 307: L419–L430, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramiro-Diaz JM, Nitta CH, Maston LD, Codianni S, Giermakowska W, Resta TC, Gonzalez Bosc LV. NFAT is required for spontaneous pulmonary hypertension in superoxide dismutase 1 knockout mice. Am J Physiol Lung Cell Mol Physiol 304: L613–L625, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Redondo PC, Rosado JA. Store-operated calcium entry: unveiling the calcium handling signalplex. In: International Review of Cell and Molecular Biology, edited by Kwang WJ. Cambridge, MA: Academic Press, 2015, p. 183–226. [DOI] [PubMed] [Google Scholar]
- 47.Rocca DL, Martin S, Jenkins EL, Hanley JG. Inhibition of Arp2/3-mediated actin polymerization by PICK1 regulates neuronal morphology and AMPA receptor endocytosis. Nat Cell Biol 10: 259–271, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Velicelebi G, Stauderman KA. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol 169: 435–445, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shimoda LA, Sham JS, Shimoda TH, Sylvester JT. L-type Ca2+ channels, resting [Ca2+]i, and ET-1-induced responses in chronically hypoxic pulmonary myocytes. Am J Physiol Lung Cell Mol Physiol 279: L884–L894, 2000. [DOI] [PubMed] [Google Scholar]
- 50.Shimoda LA, Sylvester JT, Sham JS. Mobilization of intracellular Ca2+ by endothelin-1 in rat intrapulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 278: L157–L164, 2000. [DOI] [PubMed] [Google Scholar]
- 51.Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature 386: 173–177, 1997. [DOI] [PubMed] [Google Scholar]
- 52.Wang C, Li JF, Zhao L, Liu J, Wan J, Wang YX, Wang J, Wang C. Inhibition of SOC/Ca2+/NFAT pathway is involved in the anti-proliferative effect of sildenafil on pulmonary artery smooth muscle cells. Respir Res 10: 123–123, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wemmie J, Chen J, Askwith C, Hruska-Hageman A, Price M, Nolan B, Yoder P, Lamani E, Hoshi T, Freeman J, Welsh M. The acid-activated ion channel ASIC contributes to synaptic plasticity, learning, and memory. Neuron 34: 463–477, 2002. [DOI] [PubMed] [Google Scholar]
- 54.Wong CK, So WY, Law SK, Leung FP, Yau KL, Yao X, Huang Y, Li X, Tsang SY. Estrogen controls embryonic stem cell proliferation via store-operated calcium entry and the nuclear factor of activated T-cells (NFAT). J Cell Physiol 227: 2519–2530, 2012. [DOI] [PubMed] [Google Scholar]
- 55.Xu SZ, Beech DJ. TrpC1 is a membrane-spanning subunit of store-operated Ca2+ channels in native vascular smooth muscle cells. Circ Res 88: 84–87, 2001. [DOI] [PubMed] [Google Scholar]
- 56.Yermolaieva O, Leonard A, Schnizler M, Abboud F, Welsh M. Extracellular acidosis increases neuronal cell calcium by activating acid-sensing ion channel 1a. Proc Natl Acad Sci USA 101: 6752–6757, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]