The substrate oxidation and response to I/R are more dependent on diet than on physiologic range changes in circulating substrate and insulin. This was associated with altered insulin sensitivity. After I/R, there was a positive (with ketone) and negative (with FFA) correlation between recovery of function and substrate oxidation.
Keywords: diet, myocardial ischemia-reperfusion injury, insulin, metabolism
Abstract
High-fat, low-carbohydrate Diet (HFLCD) impairs the myocardial response to ischemia-reperfusion, but the underlying mechanisms remain elusive. We sought to determine the magnitude of diet-induced alterations in intrinsic properties of the myocardium (including insulin sensitivity and substrate oxidation) and circulating substrate and insulin differences resulting from diet, leading to this impaired response. Rats were fed HFLCD (60% kcal from fat/30% protein/10% carbohydrate) or control diet (CONT) (16%/19%/65%) for 2 wk. Isolated hearts underwent global low-flow ischemia followed by reperfusion (I/R). Carbon-13 NMR spectroscopy was used to determine myocardial substrate TCA cycle entry. Myocardial insulin sensitivity was assessed as dose-response of Akt phosphorylation. There was a significant effect of HFLCD and I/R with both these factors leading to an increase in free fatty acid (FFA) oxidation and a decrease in carbohydrate or ketone oxidation. Following I/R, HFLCD led to decreased ketone and increased FFA oxidation; the recovery of left ventricular (LV) function was decreased in HFLCD and was negatively correlated with FFA oxidation and positively associated with ketone oxidation. HFLCD also resulted in reduced insulin sensitivity. Under physiologic ranges, there were no direct effects of buffer insulin and ketone levels on oxidation of any substrate and recovery of cardiac function after I/R. An insulin-ketone interaction exists for myocardial substrate oxidation characteristics. We conclude that the impaired recovery of function after ischemia-reperfusion with HFLCD is largely due to intrinsic diet effects on myocardial properties, rather than to diet effect on circulating insulin or substrate levels.
NEW & NOTEWORTHY
The substrate oxidation and response to I/R are more dependent on diet than on physiologic range changes in circulating substrate and insulin. This was associated with altered insulin sensitivity. After I/R, there was a positive (with ketone) and negative (with FFA) correlation between recovery of function and substrate oxidation.
high-fat, low-carbohydrate diets (hflcds) are used by many people in an effort to lose weight. The effect of such diets on cardiovascular health has been the subject of intense investigation. Population studies evaluating the impact of HFLCD on cardiovascular clinical outcomes have provided a range of results, with some finding favorable changes in cardiovascular disease risk factors (9, 35, 37), and others showing an increased risk of adverse outcomes in both animal (29) and human studies, including in the setting of myocardial infarction (23). Consistent with this latter finding, we have previously demonstrated that HFLCD (when eaten prior to experimental infarction) induced lower recovery of cardiac function, larger infarct size, and increased risk of death by pump failure and ventricular arrhythmias following coronary ligation myocardial ischemia-reperfusion (25). However, the mechanisms causing these impaired responses are not fully known. HFLCD resulted in a number of alterations in circulating factors and myocardial properties that may explain poorer outcomes, including lower serum insulin and myocardial glycogen, higher serum ketone, and higher myocardial mitochondrial oxidative stress (24, 45). Therefore, whether the changes of serum insulin and ketone induced by HFLCD play a role, and the combined effects of intrinsic (diet itself) and extrinsic (buffer composition) factors on cardiac function and cardiac energy substrate metabolic responses that underlie ischemia-reperfusion (I/R) injury, need further study.
Cardiomyocytes are capable of oxidizing a variety of substrates to generate high-energy phosphates for cellular needs. Whereas myocardial glucose and free fatty acid (FFA) utilization have been extensively studied and play a role in modulating cardiac function in pathological states, the effect of ketones (the circulating levels of which are increased with HFLCD) on the heart and the importance of ketone body metabolism under both physiological and pathological conditions have not been carefully evaluated. A shift of cardiac energy substrate use from FFA toward carbohydrate (CHO) has been proposed to be beneficial for the ischemic heart, as it results in up to a 12% improvement in “efficiency” of ATP production (30). Insulin at pharmacologically high doses promotes myocardial carbohydrate oxidation, suppresses relative FFA oxidation (26), and improves functional recovery and cardiac efficiency after I/R (44). Thus lower insulin induced by HFLCD could be detrimental by increasing the relative importance of FFA oxidation. These metabolic effects of HFLCD can be mediated through alterations in myocardial properties, as well as because of changes in circulating levels of ketones and insulin, neither of which have been systematically studied. To determine these mechanisms and explore their role in I/R injury, we focused on how HFLCD may alter myocardial oxidative substrate metabolism and recovery of function following I/R. Specifically, we investigated whether any metabolic changes associated with HFLCD were due to intrinsic modifications in the heart developed during feeding with this diet, or were due to altered substrate and hormonal milieu and insulin sensitivity at the time of I/R. Furthermore, we evaluated the relative effect magnitude of both myocardial alterations directly due to the HFLCD diet, as well as the effects of altered substrate presentation that occurs with these diets, on myocardial function and oxidative metabolism following ischemia-reperfusion.
MATERIALS AND METHODS
Animals and diets.
All animal experiments were approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham and followed the Guide for the Care and Use of Laboratory Animals (National Academy of Sciences, 1996). Adult male Sprague-Dawley rats 300 ± 10 g (Taconic Farms, Hudson, NY) were fed one of two diets, each of which was designed to be similar to ones used by humans for weight loss: HFLCD [60% calories from fat/30% from protein/10% from carbohydrate, a relatively high-protein content (30% of kcals), and very high amount of saturated (40%) and monounsaturated (40%) fat designed to be similar to the induction phase of the Atkins diet for humans (10) (TestDiet 5TSY, Richmond, IN)] or a low-fat control diet (CONT) (16/19/65%; TestDiet 5TJM). The detailed components of the diets, including proportions of saturated, mono- and polyunsaturated fats as well as n-3 and n-6 polyunsaturated fatty acids have been described previously (24, 25). Animals were fed the diets for 14 days prior to performing perfusion experiments. The rats were housed at 22°C on a 12-h light-dark cycle. Animals were allowed ad libitum access to food and water.
Isolated heart perfusions.
Isolated hearts (CONT, n = 38; HFLCD, n = 37) were perfused in Langendorff mode with a modified Krebs-Henseleit buffer as described previously (45) (in mM): NaCl 118, KCl 4.8, MgSO4 1.2, CaCl2 1.4, KH2PO4 1.2, Na2HCO3 25, pyruvate 0.1, palmitate 0.3, glucose 5.0, lactate 1.0, glutamine 0.5, and 3% bovine serum albumin with low insulin (LI, 15 μU/ml), high insulin (HI, 30 μU/ml), and ketone (low ketone or LK, 0.6 and high ketone or HK, 1.2 mM), see Table 1 for these designations of perfusion buffers. In vivo, acetoacetate and 3-hydroxybutyrate (3-HB) are the main circulating ketone bodies, typically at a ratio of 1:1 (21). Since the 3-HB concentration and the acetoacetate concentrations are equivalent, we chose to formulate the perfused ketone concentration as twice the measured 3-HB concentration to simulate physiologic conditions and simplify carbon-13 labeling (see below). In our prior work, we found differences only in circulating ketone body and insulin concentrations in serum from animals fed the HFLCD and CONT diets, with no differences in glucose or FFA (45). To enable a comprehensive analysis of the effect of both insulin and ketone in the buffer, including an examination of possible interactions between these two factors, a total of four different buffer compositions were used, independently varying the insulin and ketone composition. We labeled these four combinations as HI-HK, HI-LK, LI-LK, and LI-HK. These four perfusion buffers were supplied to hearts from rats fed HFLCD and CONT diet during the perfusion protocols with normal coronary flow and low-flow ischemia (LFI) (0.3 ml/min) for 60 min followed by 60 min of reperfusion, with details as described previously (45). Heart rate (HR), systolic pressure (SP), left ventricular end diastolic pressure (LVEDP), and peak positive and negative rates of change of pressure (+dP/dt and −dP/dt) were used as indices of ventricular function (DASYLab, Measurement Computing, Norton, MA). From these values, the rate-pressure product [RPP = (SP − LVEDP) × HR] was computed as the index of cardiac power. Recovery during the last 20 min of reperfusion was expressed as % of baseline values.
Table 1.
Insulin and ketone buffer compositions and designations/labels used
| Buffer Composition | Insulin Concentration | 3-Hydroxybutyrate Concentration | Label |
|---|---|---|---|
| High-insulin, high-ketone | 30 μU/ml | 1.2 mM | HI-HK |
| Low-insulin, high-ketone | 15 μU/ml | 1.2 mM | LI-HK |
| High-insulin, low-ketone | 30 μU/ml | 0.6 mM | HI-LK |
| Low-insulin, low-ketone | 15 μU/ml | 0.6 mM | LI-LK |
NMR spectroscopy.
After 30-min equilibration the unlabeled glucose, lactate, palmitate, and ketone in the buffer was switched to 13C-labeled glucose (uniformly labeled at all 6 carbons, designated as [U-13C]glucose), sodium lactate (uniformly labeled at all 3 carbons, designated [U-13C]lactate), sodium pyruvate (uniformly labeled; [U-13C] pyruvate), sodium palmitate (labeled with 13C at the 1-position only; [1-13C]palmitate), and sodium 3-hydroxybutyrate as the ketone (3-HB, labeled at positions 2 and 4; [2,4-13C]3-HB) at the same concentrations as the unlabeled compounds, as described in more detail below. After 30 min of perfusion with labeled substrates, whole hearts were freeze clamped, the frozen hearts pulverized, and the heart tissue was homogenized and extracted with ice-cold 6% perchloric acid. The extract was freeze-dried and redissolved in a potassium phosphate buffer (pH 7.5, 50 mM) with 2H2O solvent (99.9%; Cambridge Isotope Laboratories, Andover, MA).
Proton-decoupled, NOE-enhanced 13C NMR spectra were collected on a Bruker AVANCE 500 NMR spectrometer operating at 11.85 T using a TBI heteronuclear broadband probe. Spectra were collected at 300 K with spectral width 25 kHz and 32k data points. Spectra were collected with summation of scans until adequate signal/noise ratio was achieved (typically 4k–64k scans). Spectra were analyzed with Nuts software (AcornNMR, Livermore, CA). 13C time domain data were processed with 1 to 2 Hz line broadening, zero-filled, Fourier transformed, and referenced to the methyl carbon peak of lactate (at 21.1 ppm). The glutamate 13C NMR multiplet relative areas at carbons 1 through 5, and the integrated areas of the C3 and C4 resonances (corrected for relaxation effects) were measured as described previously (26, 27).
13C-glutamate isotopomer analysis.
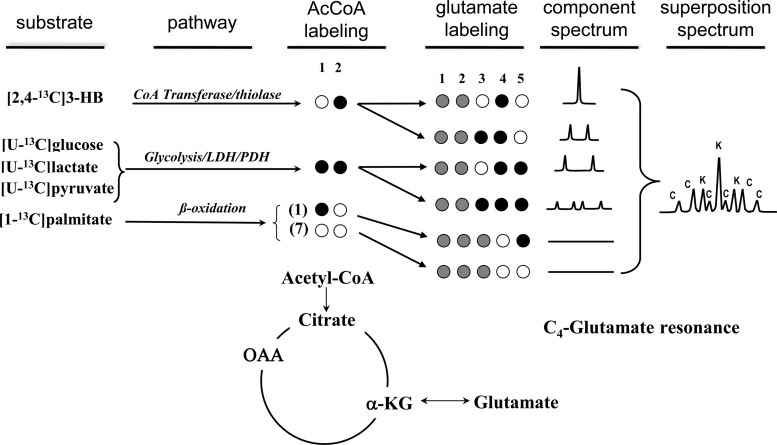
13C-NMR glutamate isotopomer analysis was performed with tcaCALC. (provided courtesy of Dr. Mark Jeffrey, University of Texas Southwestern Medical Center; http://www4.utsouthwestern.edu/rogersnmr/software/index.html), to determine the fraction of total acetyl-CoA entering the TCA cycle that originates from unlabeled, [1,2-13C]-, [2-13C]- and [1-13C]acetyl-CoA (Fig. 1). Data from all glutamate resonances, and the relaxation-corrected C3/C4 spectral area ratio, was used as input. FCHO is the fraction of acetyl-CoA that originated from [U-13C]glucose, [U-13C]lactate, and [U-13C]pyruvate, all of which result in [1,2-13C]-acetyl-CoA. FKB is the fraction of originating from ketones ([2,4-13C] 3-hydroxybutyrate), the metabolism of which results in [2-13C]-acetyl-CoA. Fatty acids are converted to acetyl-CoA via β oxidation, which, in Fig. 1, is represented by Fβ. Given the labeling of palmitate in these experiments ([1-13C]), β oxidation of a single molecule of [1-13C]palmitate produces seven unlabeled acetyl-CoA fragments and a single fragment labeled as [1-13C]acetyl-CoA. Thus the majority of the palmitate-derived TCA cycle entry is unlabeled, with a smaller fraction also appearing as [1-13C]acetyl-CoA. Unlabeled acetyl-CoA could also result from the metabolism of endogenous triglycerides or glycogen; however, earlier studies have shown that under similar conditions, the contribution of endogenous triglyceride (6) and glycogen (27) are negligible. We therefore attributed all unlabeled acetyl-CoA entry to exogenous palmitate, as the main component of Fβ, with all of the [1-13C]acetyl-CoA entry additionally contributing to Fβ. Neglecting any contribution of endogenous glycogen and triglycerides, FCHO + FKB + Fβ = 1.
Fig. 1.
Schematic of labeling from substrates into TCA cycle as used in these experiments and resultant glutamate C-4 spectral isotopomers. Black filled circles represent carbon-13 labeling (98 to 99% enrichment), open circles natural abundance (i.e., 99% carbon-12) labeling, and gray circles may be either carbon-12 or carbon-13 as they do not contribute to the glutamate C-4 spectrum. In the 13C NMR spectrum on the right, K represents isotopomer peaks derived from ketone (3-HB); C represents those derived from carbohydrate (glucose, lactate, and pyruvate). Note that β-oxidation of [1-13C]palmitate results in seven unlabeled acetyl-CoA fragments and one [1-13C]acetyl-CoA fragment, neither of which contributes to the glutamate C4 resonance. OAA, oxaloacetate; α-KG, α-ketoglutarate.
Isolated heart insulin sensitivity measurements.
Hearts isolated from CONT or HFLCD groups were perfused with the four perfusion buffers (HI-HK, HI-LK, LI-LK, and LI-HK) during baseline perfusion and LFI (0.3 ml/min; 60 min duration), followed by 60 min of reperfusion. Frozen whole hearts were powdered, and protein from 50-μg samples was extracted for Western blot. To assess insulin sensitivity, another group of hearts were perfused with four insulin doses: a very low dose (5 μU/ml), a physiologic range dose (15 μU/ml), an intermediate dose (100 μU/ml), and a pharmacologic high dose for maximal stimulation (2,000 μU/ml); N = 5 for each insulin dose and dietary group. After 60-min perfusion, all hearts were quickly frozen, protein from 50-μg samples of whole heart tissue was extracted and Western blots were conducted with commercial antibodies: anti-Akt and anti- phospho-Ser473-Akt (1:2,000 dilution; Abcam, Cambridge, MA). The band intensities were analyzed by ImageJ to quantify Akt and phospho-Akt (p-Akt). The intensity of phospho-Akt was divided by the intensity of total Akt.
Statistical analyses.
Data for comparison of continuous variables between the two groups were analyzed by general linear model multivariate test and linear regression tests (IBM SPSS Statistics, GraphPad Prism). Values were expressed as means ± SE. Differences between groups were regarded as significant at the P < 0.05 probability level by the appropriate statistical test. When general linear model results were used, differences between groups were considered significant if there was no overlap in the 95% confidence limits for the values.
RESULTS
Impact of perfusion buffer composition (insulin and ketone level) and effect of HFLCD on ventricular function following myocardial ischemia-reperfusion.
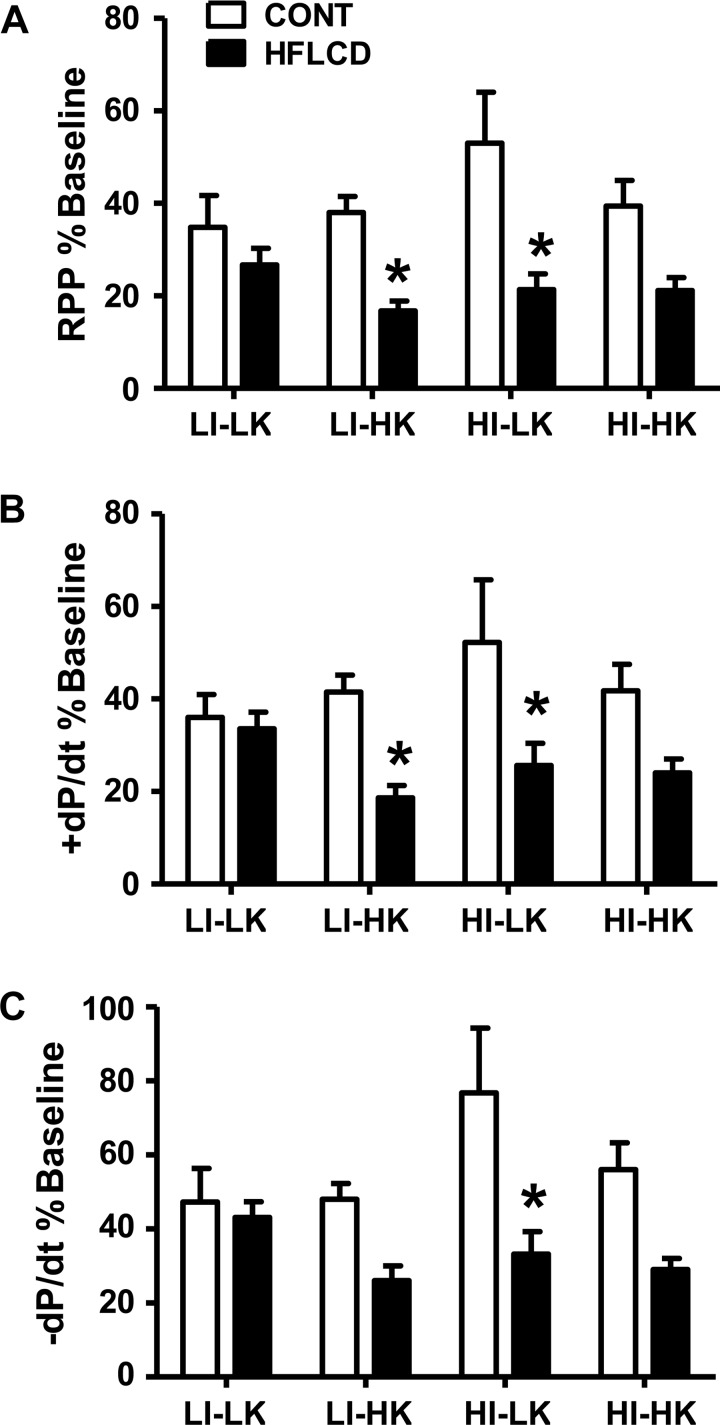
Figure 2 shows the effect of buffer compositions and diet on recovery of function following ischemia-reperfusion (I/R). Overall, there is seen to be a lower recovery of function in HFLCD compared with CONT. Table 2 reveals the results of a general linear model analysis of the recovery data following I/R. As seen in Table 2, there was no effect of perfusion buffer ketone or insulin concentration on the recovery of cardiac function following I/R. However, there was an overall effect of diet which played the major role in determining the recovery of cardiac function following I/R (Table 2). HFLCD reduced recovery of systolic and diastolic function, with the final RPP decreased by 43% and ±dP/dt decreased by 36 to 38%, compared with CONT diet at the end of 60-min reperfusion. There were no two-way or three-way interactions among the experimental variables of diet, insulin, ketone (Table 2).
Fig. 2.
HFLCD increases susceptibility to myocardial ischemic injury. A: % recovery of baseline RPP. B and C: % recovery of baseline ± dP/dt at the end of 60-min LFI and 60-min reperfusion. Data shown for each buffer perfusion combination. Means ± SE, n = 4 to 6 each group, *P < 0.05 vs. CONT.
Table 2.
General linear model determination of magnitude of effects of diet, buffer insulin, buffer ketone, and all possible interactions among these factors on the left ventricular function following ischemia-reperfusion
| RPP |
+dP/dt |
−dP/dt |
||||
|---|---|---|---|---|---|---|
| Factor | Magnitude | P Value | Magnitude | P Value | Magnitude | P Value |
| Base effect | ||||||
| Diet | (−) 7943 | <0.001* | (−) 561 | <0.001* | (−) 494 | <0.001* |
| Buffer insulin | (+) 1031 | 0.53 | (+) 55 | 0.67 | (+) 100 | 0.37 |
| Buffer ketone | (−) 1237 | 0.45 | (−) 99 | 0.44 | (−) 93 | 0.41 |
| interactions | ||||||
| Diet-insulin | — | 0.40 | — | 0.30 | — | 0.10 |
| Diet-ketone | — | 0.55 | — | 0.31 | — | 0.46 |
| Insulin-ketone | — | 0.19 | — | 0.05 | — | 0.06 |
| Diet-insulin-ketone | — | 0.45 | — | 0.14 | — | 0.45 |
Magnitude expressed as the difference in means of final functional parameter between aggregate groups stratified by diet, buffer insulin, and buffer ketone. For diet, (−) means that HFLCD has a lower value than CONT diet; for insulin, (+) means that the higher insulin level results in a greater value; for ketone, (—) means that the higher ketone level results in a lower value.
P < 0.05 for HFLCD compared with CONT diet with same perfusion buffer and perfusion condition.
Effect of diet, perfused ketone, and insulin on the fraction of total acetyl-CoA entering the TCA cycle following I/R.
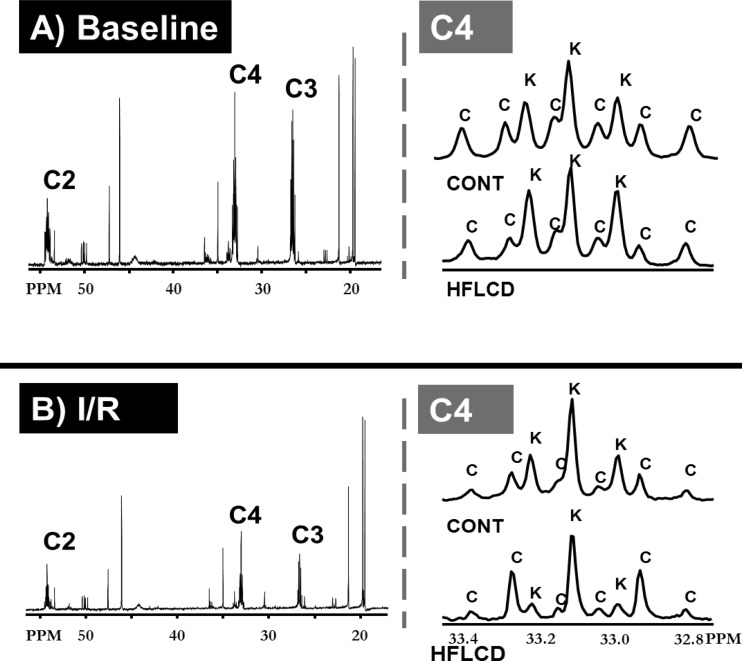
Representative carbon-13 NMR spectra from both diet groups and under both normoxia and I/R conditions are shown in Fig. 3. Using a systematic analysis of all conditions, we found that there was a significant effect of HFLCD (compared with CONT diet) and I/R (compared with normoxia), with both these factors tending to lead to an increase in FFA oxidation and a decrease in CHO oxidation or ketone oxidation (Tables 3 and 4).
Fig. 3.
Representative 13C NMR spectra demonstrating substrate TCA cycle entry under baseline and I/R conditions. Representative 13C NMR spectra in the 15 to 55 ppm (left) and in the neighborhood of 33 ppm (right), focusing on the glutamate C4-resonance, under conditions of baseline (normal flow conditions) (A) or I/R (B). Letter C designates peaks arising from CHO, and K designates peaks arising from ketone incorporation.
Table 3.
Effects of diet, buffer insulin, buffer ketone, and interactions among these factors on TCA cycle substrate entry following ischemia-reperfusion
| FFA |
CHO |
Ketone |
||||
|---|---|---|---|---|---|---|
| Magnitude | P Value | Magnitude | P Value | Magnitude | P Value | |
| Including normoxia and I/R | ||||||
| Diet | (+) 10.17 | <0.001* | (−) 5.19 | <0.01* | (−) 4.99 | 0.05 |
| Buffer insulin | (−) 1.44 | 0.49 | (+) 3.1 | 0.09 | (−) 1.63 | 0.52 |
| Buffer ketone | (−) 3.40 | 0.11 | (+) 2.36 | 0.19 | (+) 1.02 | 0.69 |
| Perfusion | (+) 10.41 | <0.001* | (−) 6.71 | <0.001* | (−) 3.67 | 0.15 |
| I/R considered alone | ||||||
| Diet | (+) 11.31 | <0.01* | (−) 1.04 | 0.69 | (−) 10.27 | <0.05* |
| Buffer insulin | (−) 5.37 | 0.12 | (+) 1.16 | 0.65 | (+) 4.22 | 0.30 |
| Buffer ketone | (+) 0.99 | 0.77 | (+) 1.62 | 0.53 | (−) 2.62 | 0.52 |
| interactions | ||||||
| Insulin-ketone | — | <0.05 | — | 0.88 | — | <0.05* |
Magnitude expressed as the difference in means of TCA cycle substrate entry between aggregate groups stratified by diet, buffer insulin, and buffer ketone. With all data considered as a whole, there was no significant interaction of the experimental variables. For diet, a positive sign means that HFLCD has a greater value than CONT diet; for insulin and ketone, a positive sign means that the higher insulin or ketone level results in a greater value; and for perfusion, positive sign means I/R is greater than normoxia.
P < 0.05 for HFLCD compared with CONT diet with same perfusion buffer and perfusion condition.
Table 4.
Effect of diet, buffer insulin and ketone levels on the fraction of total acetyl-CoA entering the TCA cycle under normoxia and I/R conditions.
| Normoxia |
I/R |
|||||
|---|---|---|---|---|---|---|
| FFA | CHO | Ketone | FFA | CHO | Ketone | |
| Low-insulin/low-ketone | ||||||
| CONT | 37 ± 3 | 26 ± 3 | 37 ± 3 | 45 ± 3 | 19 ± 4 | 36 ± 5 |
| HFLCD | 48 ± 1 | 18 ± 3 | 34 ± 4 | 48 ± 5 | 15 ± 2 | 38 ± 4 |
| Low-insulin/high-ketone | ||||||
| CONT | 26 ± 2 | 25 ± 2 | 49 ± 2 | 45 ± 6# | 18 ± 4 | 38 ± 10 |
| HFLCD | 36 ± 1 | 20 ± 3 | 44 ± 3 | 67 ± 1*#‡ | 19 ± 1 | 14 ± 1*#‡ |
| High-insulin/high-ketone | ||||||
| CONT | 36 ± 2 | 36 ± 6 | 28 ± 4 | 39 ± 7 | 18 ± 5# | 43 ± 3 |
| HFLCD | 39 ± 5 | 25 ± 2 | 37 ± 6 | 45 ± 3*† | 21 ± 5 | 34 ± 5*† |
| High-insulin/low-ketone | ||||||
| CONT | 35 ± 3 | 31 ± 4 | 35 ± 1 | 43 ± 4 | 19 ± 2 | 38 ± 3 |
| HFLCD | 48 ± 2 | 18 ± 2 | 35 ± 3 | 56 ± 3 | 16 ± 2 | 28 ± 3 |
Means ± SE, n = 4 to 6 each group. Values are expressed as % of total TCA cycle entry from the acetyl-CoA pool, derived from each substrate and rounded to the nearest percent.
P < 0.05 for HFLCD compared with CONT diet with same perfusion buffer and perfusion condition.
P < 0.05 for I/R compared with normoxia with same perfusion buffer and diet condition.
P < 0.05 for high-insulin compared with low-insulin with the same ketone level, perfusion condition, and diet.
P < 0.05 for high-ketone compared with low-ketone with the same insulin level, perfusion condition, and diet.
When the I/R perfusion condition was considered separately, we again observed an effect of diet on substrate oxidation, with HFLCD leading to an average 11% increase in FFA oxidation, with a corresponding decrease in ketone oxidation and no effect on CHO oxidation (Table 3). There were no direct effects of buffer insulin and ketone levels on oxidation of any substrate. However, a significant insulin-ketone interaction exists on oxidation of FFA and ketone. The effect of this interaction may be seen in the complete substrate oxidation data, which is listed in Table 4. Under I/R conditions in the HFLCD group, increasing insulin has variable effect on FFA and ketone oxidation, depending on the ketone level: with high ketone buffer, high insulin led to decreased FFA oxidation and upregulated ketone oxidation (P < 0.05 for high insulin compared with low insulin with the same ketone level, perfusion condition, and diet); this effect was not seen with low ketone buffer. At the low level insulin buffer, high ketone upregulated FFA oxidation and decreased ketone oxidation (P < 0.05 in the HFLCD group for high ketone compared with low ketone with the same insulin level, perfusion condition). Surprisingly, there was no overall direct effect of insulin or ketone on CHO oxidation (Table 4). The significance of these interactions is unclear.
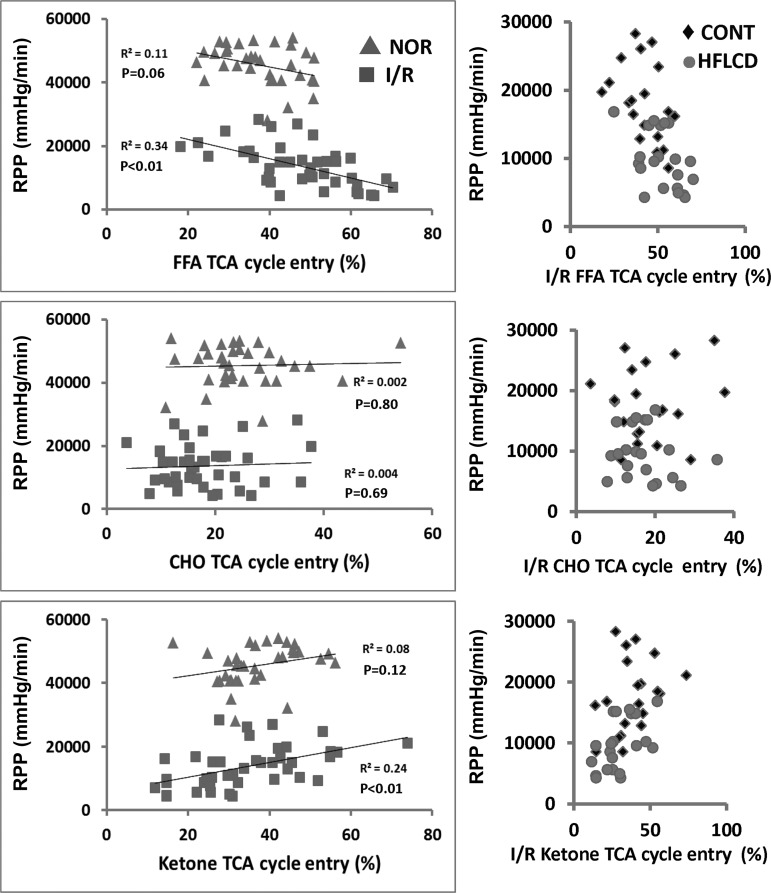
Relationship between TCA cycle substrate entry and cardiac function.
We focused on RPP as the index of cardiac function for this analysis. Because the range of RPP was very different with I/R compared with normoxia flow conditions, we considered these two perfusion conditions separately. With normoxia perfusion conditions, there was no significant relationship between FFA or ketone oxidation and RPP. There was no relationship between CHO oxidation and RPP with either normoxic or I/R conditions. However, under I/R conditions, RPP was negatively correlated with FFA oxidation and positively correlated with ketone oxidation (P < 0.01; Fig. 4, left). Additionally, the HFLCD animals had lower recovery of function across the full range of substrate oxidation proportions compared with CONT diet (Fig. 4, right).
Fig. 4.
Relationship between cardiac function and TCA Cycle Entry of FFA, CHO, and ketone. Left: correlation between rate-pressure produce (RPP) (mmHg/min) and TCA cycle entry by linear regression analysis. Correlation P value < 0.05 for FFA and ketone entry under I/R conditions; no correlation between FFA, CHO, ketone, and RPP under normoxic conditions; n = 34–41. ▲, normoxia; ■ I/R. Right: HFLCD had lower recovery of function across the full range of substrate oxidation proportions comparing to CONT under I/R; n = 20. ⧫ CONT, ● HFLCD.
Effect of diet and ischemia-reperfusion on the insulin signaling in rat myocardium.
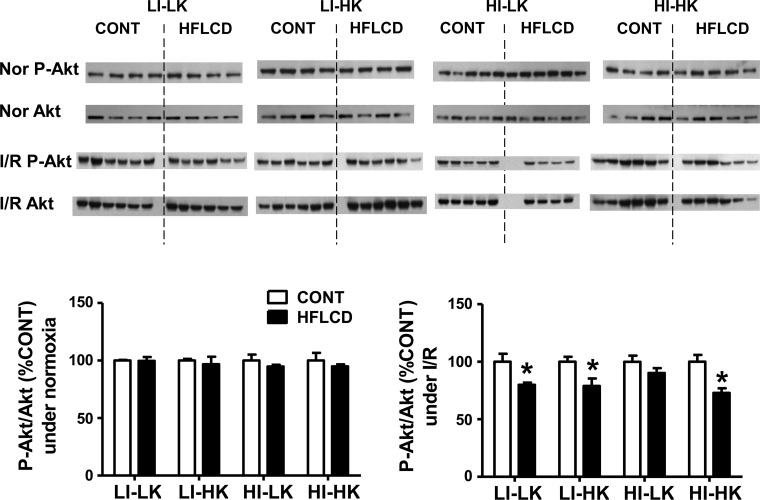
The ratio of phospho-Akt to total Akt was similar between HFLCD and CONT hearts under normoxia. However, under I/R conditions, HFLCD-fed rats generally exhibited a decrease in p-Akt/total Akt across the range of combinations of buffer insulin and ketone, indicating a reduced insulin signaling effect in HFLCD (Fig. 5).
Fig. 5.
Insulin signaling in rat myocardium under normoxic and I/R conditions. Heart tissue samples from CONT and HFLCD perfused with different buffers were subjected to the Western blot analysis detecting p-Ser473-Akt and total Akt. Data shown as the ratio of p-Akt/total Akt, normalized to make the CONT group = 100% under normoxic and I/R conditions; *P < 0.05, n = 4–6 per group.
Insulin sensitivity.
To investigate the impact of HFLCD on intrinsic insulin sensitivity, we assessed the dose-response effect of insulin on Akt phosphorylation. As shown in Fig. 6, Western blot revealed a diet-dependent progression of insulin effect on Akt phosphorylation over the range of insulin concentration from a physiologically low level (5 μU/ml), normal to intermediately high ranges at 15 and 100 μU/ml, to a pharmacologicaly high level (2,000 μU/ml). With HFLCD, relative Akt phosphorylation tended to be higher at lower insulin doses, but exhibited a blunted response to increasing insulin levels compared with CONT diet (which demonstrated the predicted dose-dependent relative increase in p-Akt with increasing insulin dose; Fig. 6).
Fig. 6.
Insulin dose-dependent Akt phosphorylation in rat myocardium of CONT and HFLCD. Western blot analyses of levels of total Akt and phospho-Akt (p-Akt) in CONT and HFLCD hearts. Data shown is p-Akt/total Akt, standardized such that the CONT group is 100% for each condition. Values are expressed as means ± SE; n = 5 each, **P < 0.01 and ***P < 0.001 compared with CONT.
DISCUSSION
Cardiomyocytes demonstrate considerable metabolic flexibility during dynamic alterations of nutrient state and hemodynamic stress, and this is an important adaptive property (41). Under conditions of dietary variations in carbohydrate and fat, myocardial fuel sources may shift among the possible substrates of glucose, fatty acids, and ketones. It is well known that myocardial tissue is able to extensively metabolize ketones as an energy substrate (17, 46). Oxidation of ketone bodies may be intrinsically more efficient than oxidation of FFA (43), possibly affecting the response to stress including I/R; indeed, we found that under I/R, recovery of LV function was positively associated with ketone oxidation. Furthermore, ketone oxidation was lower in HFLCD than in CONT diet, potentially resulting in decreased energy efficiency and contributing to the worsened post-I/R recovery seen with this diet. Consistent with this concept, deficient myocardial ketone metabolism has been shown to promote accelerated pathological remodeling and impaired cardiac function in response to pressure overload in an animal model (36), and cardioprotective effects have been observed following ischemia-reperfusion in starvation-induced ketosis (39, 49). We found that neither insulin nor buffer ketone composition affected the recovery of left ventricular function after I/R; only the diet itself, and any alterations of myocardial properties due to the diet eaten prior to isolated heart perfusion, played a major role in recovery of function. Interestingly, myocardial ketone oxidation did not seem to be associated with the level of buffer ketone concentration used to perfuse the heart, at least under the physiologic ranges studied.
Examination of the entire data group revealed that both HFLCD and I/R increased FFA oxidation and decreased CHO or ketone oxidation. Other groups have reported that stimulation of glucose oxidation protects against acute myocardial infarction and reperfusion injury (42), and an increased FFA oxidation has been associated with reduced myocardial tolerance to ischemia-reperfusion (13); therefore, any increase in FFA and decrease in CHO or ketone oxidation induced by HFLCD might lead to worsened cardiac function during I/R. This has led to the concept that repressing myocardial FFA oxidation may be a therapeutic target to improve cardiac efficiency in the ischemic heart (40). Furthermore, nutrient excess from high-fat diet may result in the accumulation of toxic intermediates of fatty acid metabolism from incomplete mitochondrial fatty acid oxidation (1), and contribute to insulin resistance, oxidative stress, and uncoupling of oxidative metabolism from electron transfer (15, 20, 28, 38). Indeed, we found the relative degree of FFA oxidation was negatively correlated to recovery of function after I/R.
While our results indicate that feeding with HFLCD prior to I/R is detrimental, in other settings a high-fat diet may be beneficial. Rennison et al. (32) found that high-fat feeding following coronary artery ligation improved mitochondrial and contractile function in chronic heart failure. They also reported that a high-fat diet did not lead to worsened left ventricular dysfunction when eaten after an experimental myocardial infarction; although, in those same series of experiments, administration of high-fat diet for 2 wk before coronary artery ligation resulted in an increased surgical mortality rate (33). Therefore, it seems that eating high-fat diets before a myocardial infarction may be harmful, while eating this diet after an infarction is possibly beneficial.
The specific fatty acid composition may be important in determining the effect on the myocardium. Some studies have shown that high dietary polyunsaturated fatty acids confer cardioprotection and improve cardiac performance through anti-inflammatory and antioxidative effects (11, 47) and by restoring mitochondrial respiratory activities and attenuating lipid peroxidation (11) during or after I/R injury. However, very high saturated and monounsaturated fatty acid-containing diets (as in the HFLCD used by us, with 40% saturated and 40% monounsaturated, reflective of diets commonly eaten by humans) may be harmful because of increased oxidative stress (24).
Studies which directly examined the impact of high-fat, high-protein, low-carbohydrate diets on insulin sensitivity have reported conflicting findings, with some showing that such diets lead to glucose intolerance and insulin resistance (2–4, 19), and others reporting it prevents insulin resistance (22), or causes no change in insulin resistance (5, 8). While we found a steady progression of insulin-dependent Akt phosphorylation (taken as a measure of insulin effect) in hearts of animals fed CONT diet, there was an unusual response to insulin in the HFLCD-fed heart, with an initially high baseline amount of p-Akt at very low insulin levels and a blunted response as the dose was increased. Similar findings by Ouwens et al. (31) showed insulin resistance in high-fat-diet-fed hearts was reflected by impaired activation of the insulin receptor and IRS1-PI3K-PKB/Akt-mediated signaling. While insulin is an important regulator of Akt phosphorylation, other factors, both positive (e.g., mTORC2 and PI3K) and negative regulators (e.g., phosphatases PTEN, PP2A, and PHLPP), are known to affect it as well, and may alter the dynamic range or capacity to activate Akt to the phosphorylated form (16). Alterations in these other regulatory control pathways may be at play to explain the fact that basal (very low insulin) phospho-Akt is higher in HFLCD, though we have not explored this interesting phenomenon further. The impairment of insulin-stimulated Akt signaling in HFLCD with high insulin dose might be associated with fat-induced insulin resistance, which is believed to be fundamental in the development of human Type 2 diabetes.
Insulin may be an important link between myocardial metabolism, function, and response to ischemic injury (14, 18). Insulin reduces ischemia-induced cardiomyocyte necrosis through an Akt/NF-κB-dependent mechanism (7). This protective effect might depend on both the circulating insulin level as well as the specific insulin sensitivity. We showed in this work that HFLCD-fed hearts had lower phosphorylation of Akt after I/R across a physiologic spectrum of perfusion buffer composition (Fig. 5), indicating that the intrinsic myocardial sensitivity to insulin is lower with this diet under these perfusion conditions. This effect is not entirely due to the effect on glucose oxidation, since it is independent of its effect on glucose uptake in the postischemic isolated working rat heart (48). The observed decrease in Akt phosphorylation in HFLCD under I/R, with no change in CHO oxidation compared with CONT diet, is consistent with our previous work finding that HFLCD did not alter expression of glucose uptake (GLUT4) and glucose oxidation genes under I/R (25). Thus the cardioprotective effects of insulin, and its impact on glucose uptake, appear to be at least partly uncoupled.
We note several limitations of the current study. First, the HFLCD and CONT diets we used have different amounts of protein, limiting the ability to determine just how much of the effect was due to fat or carbohydrate alone. However, our intent was not to examine the fat/carbohydrate effect in isolation, but rather to determine the overall effect of a diet similar to the type often used by humans. To further address the separate impact of protein, fat, and carbohydrate in the diets, we plan future experiments with an array of diets controlling for each of these macronutrients. Secondly, although the concentration of FFA we employed is more reflective of physiologic resting levels in humans in the nonischemic, postprandial state, it may be somewhat low compared with levels observed during myocardial ischemic events, at which times the FFA levels may rise to 1 mM or more (34). To truly mimic a physiologic model of I/R, a model could have been employed in which the hearts began by being perfused with the lower level of FFA then switched to higher levels during I/R. To simplify the experimental methods, we did not switch buffers midexperiment in this way. It is unclear whether a higher FFA level during I/R would have affected our comparison of diet, ketone, or insulin effect, but further systematic investigation of this is warranted in future work. We also recognize that the Western blot protein analysis conducted of whole heart samples represent a mixture of both ischemic and infarcted zones, as well as the remote zones which may be less affected by I/R. The global ischemic model utilized here does not lend itself well to separation of these different tissue zones; a regional infarction model, such as with coronary artery ligation, might be helpful to elucidate differences between the zones.
In conclusion, we found that the diets used in our studies, but not insulin or ketone concentration in the perfusion mixtures in our isolated heart experiments, had a large effect on myocardial substrate oxidation and recovery of postischemic function (with poorer recovery of function in animals eating HFLCD). Under I/R conditions, recovery of cardiac function was negatively correlated with FFA oxidation and positively correlated with ketone oxidation. We also demonstrated that HFLCD alters myocardial insulin sensitivity. The derangements in insulin sensitivity and the metabolic substrate oxidation shifts seen with HFLCD are in a direction that may contribute to reduced recovery of systolic function with reperfused myocardial ischemia/infarction.
GRANTS
This work was supported in part by American Heart Association Scientist Development Grant 0735212N and by National Institute of Diabetes and Digestive and Kidney Diseases P30 DK-056336.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.L., P.W., S.L.D., J.M.T., S.S., and S.G.L. performed experiments; J.L., P.W., S.L.D., J.M.T., S.S., and S.G.L. analyzed data; J.L., P.W., and S.G.L. interpreted results of experiments; J.L. and S.G.L. prepared figures; J.L. and S.G.L. drafted manuscript; J.L., P.W., S.L.D., J.M.T., S.S., and S.G.L. edited and revised manuscript; J.L., P.W., S.L.D., J.M.T., S.S., and S.G.L. approved final version of manuscript; P.W. and S.G.L. conception and design of research.
ACKNOWLEDGMENTS
The authors would like to thank Inmaculada Aban, Ph.D. for assistance and recommendations on the statistical analyses, and Ruiqi Wang for proofreading the manuscript.
REFERENCES
- 1.Anderson EJ, Kypson AP, Rodriguez E, Anderson CA, Lehr EJ, Neufer PD. Substrate-specific derangements in mitochondrial metabolism and redox balance in the atrium of the Type 2 diabetic human heart. J Am Coll Cardiol 54: 1891–1898, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Axen KV, Axen K. Longitudinal adaptations to very low-carbohydrate weight-reduction diet in obese rats: body composition and glucose tolerance. Obesity 18: 1538–1544, 2010. [DOI] [PubMed] [Google Scholar]
- 3.Bedarida T, Baron S, Vessieres E, Vibert F, Ayer A, Marchiol-Fournigault C, Henrion D, Paul JL, Noble F, Golmard JL, Beaudeux JL, Cottart CH, Nivet-Antoine V. High-protein-low-carbohydrate diet: deleterious metabolic and cardiovascular effects depend on age. Am J Physiol Heart Circ Physiol 307: H649–H657, 2014. [DOI] [PubMed] [Google Scholar]
- 4.Bielohuby M, Sisley S, Sandoval D, Herbach N, Zengin A, Fischereder M, Menhofer D, Stoehr BJ, Stemmer K, Wanke R, Tschop MH, Seeley RJ, Bidlingmaier M. Impaired glucose tolerance in rats fed low-carbohydrate, high-fat diets. Am J Physiol Endocrinol Metab 305: E1059–E1070, 2013. [DOI] [PubMed] [Google Scholar]
- 5.Bosse JD, Lin HY, Sloan C, Zhang QJ, Abel ED, Pereira TJ, Dolinsky VW, Symons JD, Jalili T. A low-carbohydrate/high-fat diet reduces blood pressure in spontaneously hypertensive rats without deleterious changes in insulin resistance. Am J Physiol Heart Circ Physiol 304: H1733–H1742, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chatham JC, Gao ZP, Forder JR. The impact of 1 week of diabetes on the regulation of myocardial carbohydrate and fatty acid oxidation. Am J Physiol Endocrinol Metab 277: E342–E351, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Diaz A, Humeres C, Gonzalez V, Gomez MT, Montt N, Sanchez G, Chiong M, Garcia L. Insulin/NFkappaB protects against ischemia-induced necrotic cardiomyocyte death. Biochem Biophys Res Commun 467: 451–457, 2015. [DOI] [PubMed] [Google Scholar]
- 8.Fabbrini E, Higgins PB, Magkos F, Bastarrachea RA, Voruganti VS, Comuzzie AG, Shade RE, Gastaldelli A, Horton JD, Omodei D, Patterson BW, Klein S. Metabolic response to high-carbohydrate and low-carbohydrate meals in a nonhuman primate model. Am J Physiol Endocrinol Metab 304: E444–E451, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foster GD, Wyatt HR, Hill JO, Makris AP, Rosenbaum DL, Brill C, Stein RI, Mohammed BS, Miller B, Rader DJ, Zemel B, Wadden TA, Tenhave T, Newcomb CW, Klein S. Weight and metabolic outcomes after 2 years on a low-carbohydrate versus low-fat diet: a randomized trial. Ann Intern Med 153: 147–157, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freedman MR, King J, Kennedy E. Popular diets: a scientific review. Obes Res 9 Suppl 1: 1S–40S, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Gao K, Chen L, Yang M, Han L, Yiguang S, Zhao H, Chen X, Hu W, Liang H, Luo J, Ma J. Marine n-3 PUFA protects hearts from I/R injury via restoration of mitochondrial function. Scand Cardiovasc J 49: 264–269, 2015. [DOI] [PubMed] [Google Scholar]
- 13.Hafstad AD, Khalid AM, Hagve M, Lund T, Larsen TS, Severson DL, Clarke K, Berge RK, Aasum E. Cardiac peroxisome proliferator-activated receptor-alpha activation causes increased fatty acid oxidation, reducing efficiency and post-ischaemic functional loss. Cardiovasc Res 83: 519–526, 2009. [DOI] [PubMed] [Google Scholar]
- 14.Hausenloy DJ, Yellon DM. New directions for protecting the heart against ischaemia-reperfusion injury: targeting the Reperfusion Injury Salvage Kinase (RISK)-pathway. Cardiovasc Res 61: 448–460, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Hendrickson SC, St Louis JD, Lowe JE, Abdel-aleem S. Free fatty acid metabolism during myocardial ischemia and reperfusion. Mol Cell Biochem 166: 85–94, 1997. [DOI] [PubMed] [Google Scholar]
- 16.Hers I, Vincent EE, Tavare JM. Akt signalling in health and disease. Cell Signal 23: 1515–1527, 2011. [DOI] [PubMed] [Google Scholar]
- 17.Janardhan A, Chen J, Crawford PA. Altered systemic ketone body metabolism in advanced heart failure. Tex Heart Inst J 38: 533–538, 2011. [PMC free article] [PubMed] [Google Scholar]
- 18.Jonassen AK, Sack MN, Mjos OD, Yellon DM. Myocardial protection by insulin at reperfusion requires early administration and is mediated via Akt and p70s6 kinase cell-survival signaling. Circ Res 89: 1191–1198, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Kennedy AR, Pissios P, Otu H, Roberson R, Xue B, Asakura K, Furukawa N, Marino FE, Liu FF, Kahn BB, Libermann TA, Maratos-Flier E. A high-fat, ketogenic diet induces a unique metabolic state in mice. Am J Physiol Endocrinol Metab 292: E1724–E1739, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB, Lopaschuk GD, Muoio DM. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 7: 45–56, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Laffel L. Ketone bodies: a review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab Res Rev 15: 412–426, 1999. [DOI] [PubMed] [Google Scholar]
- 22.Leite JO, DeOgburn R, Ratliff JC, Su R, Volek JS, McGrane MM, Dardik A, Fernandez ML. Low-carbohydrate diet disrupts the association between insulin resistance and weight gain. Metabolism 58: 1116–1122, 2009. [DOI] [PubMed] [Google Scholar]
- 23.Li S, Flint A, Pai JK, Forman JP, Hu FB, Willett WC, Rexrode KM, Mukamal KJ, Rimm EB. Low carbohydrate diet from plant or animal sources and mortality among myocardial infarction survivors. J Am Heart Assoc 3: e001169, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J, Lloyd SG. High-fat, low-carbohydrate diet alters myocardial oxidative stress and impairs recovery of cardiac function after ischemia and reperfusion in obese rats. Nutr Res 33: 311–321, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu J, Wang P, Zou L, Qu J, Litovsky S, Umeda P, Zhou L, Chatham J, Marsh SA, Dell'Italia LJ, Lloyd SG. High-fat, low-carbohydrate diet promotes arrhythmic death and increases myocardial ischemia-reperfusion injury in rats. Am J Physiol Heart Circ Physiol 307: H598–H608, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lloyd S, Brocks C, Chatham JC. Differential modulation of glucose, lactate, and pyruvate oxidation by insulin and dichloroacetate in the rat heart. Am J Physiol Heart Circ Physiol 285: H163–H172, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Lloyd SG, Wang P, Zeng H, Chatham JC. Impact of low-flow ischemia on substrate oxidation and glycolysis in the isolated perfused rat heart. Am J Physiol Heart Circ Physiol 287: H351–H362, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev 90: 207–258, 2010. [DOI] [PubMed] [Google Scholar]
- 29.Muller AP, Dietrich Mde O, Martimbianco de Assis A, Souza DO, Portela LV. High saturated fat and low carbohydrate diet decreases lifespan independent of body weight in mice. Longev Healthspan 2: 10, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Opie LH, Lopaschuk GD. Fuels, aerobic and anaerobic metabolism. In: Heart Physiology: From Cell to Circulation, edited by Opie LH. Philadelphia, PA: Lippincott Williams and Wilkins, chapt. 11, p. 306–354, 2004. [Google Scholar]
- 31.Ouwens DM, Boer C, Fodor M, de Galan P, Heine RJ, Maassen JA, Diamant M. Cardiac dysfunction induced by high-fat diet is associated with altered myocardial insulin signalling in rats. Diabetologia 48: 1229–1237, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Rennison JH, McElfresh TA, Okere IC, Patel HV, Foster AB, Patel KK, Stoll MS, Minkler PE, Fujioka H, Hoit BD, Young ME, Hoppel CL, Chandler MP. Enhanced acyl-CoA dehydrogenase activity is associated with improved mitochondrial and contractile function in heart failure. Cardiovasc Res 79: 331–340, 2008. [DOI] [PubMed] [Google Scholar]
- 33.Rennison JH, McElfresh TA, Okere IC, Vazquez EJ, Patel HV, Foster AB, Patel KK, Chen Q, Hoit BD, Tserng KY, Hassan MO, Hoppel CL, Chandler MP. High-fat diet postinfarction enhances mitochondrial function and does not exacerbate left ventricular dysfunction. Am J Physiol Heart Circ Physiol 292: H1498–H1506, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Roy VK, Kumar A, Joshi P, Arora J, Ahanger AM. Plasma free fatty acid concentrations as a marker for acute myocardial infarction. J Clin Diagn Rese 7: 2432–2434, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruth MR, Port AM, Shah M, Bourland AC, Istfan NW, Nelson KP, Gokce N, Apovian CM. Consuming a hypocaloric high fat low carbohydrate diet for 12 weeks lowers C-reactive protein, and raises serum adiponectin and high density lipoprotein-cholesterol in obese subjects. Metabolism 62: 1779–1787, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schugar RC, Moll AR, Andre d'Avignon D, Weinheimer CJ, Kovacs A, Crawford PA. Cardiomyocyte-specific deficiency of ketone body metabolism promotes accelerated pathological remodeling. Mol Metab 3: 754–769, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shai I, Schwarzfuchs D, Henkin Y, Shahar DR, Witkow S, Greenberg I, Golan R, Fraser D, Bolotin A, Vardi H, Tangi-Rozental O, Zuk-Ramot R, Sarusi B, Brickner D, Schwartz Z, Sheiner E, Marko R, Katorza E, Thiery J, Fiedler GM, Bluher M, Stumvoll M, Stampfer MJ, Dietary Intervention Randomized Controlled Trial Group. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med 359: 229–241, 2008. [DOI] [PubMed] [Google Scholar]
- 38.Sharma S, Adrogue JV, Golfman L, Uray I, Lemm J, Youker K, Noon GP, Frazier OH, Taegtmeyer H. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. Faseb J 18: 1692–1700, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Snorek M, Hodyc D, Sedivy V, Durisova J, Skoumalova A, Wilhelm J, Neckar J, Kolar F, Herget J. Short-term fasting reduces the extent of myocardial infarction and incidence of reperfusion arrhythmias in rats. Physiol Res 61: 567–574, 2012. [DOI] [PubMed] [Google Scholar]
- 40.Stanley WC, Sabbah HN. Metabolic therapy for ischemic heart disease: the rationale for inhibition of fatty acid oxidation. Heart Fail Rev 10: 275–279, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Taegtmeyer H, McNulty P, Young ME. Adaptation and maladaptation of the heart in diabetes: Part I: general concepts. Circulation 105: 1727–1733, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Ussher JR, Wang W, Gandhi M, Keung W, Samokhvalov V, Oka T, Wagg CS, Jaswal JS, Harris RA, Clanachan AS, Dyck JR, Lopaschuk GD. Stimulation of glucose oxidation protects against acute myocardial infarction and reperfusion injury. Cardiovasc Res 94: 359–369, 2012. [DOI] [PubMed] [Google Scholar]
- 43.Veech RL. The therapeutic implications of ketone bodies: the effects of ketone bodies in pathological conditions: ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot Essent Fatty Acids 70: 309–319, 2004. [DOI] [PubMed] [Google Scholar]
- 44.Wang P, Lloyd SG, Chatham JC. Impact of high glucose/high insulin and dichloroacetate treatment on carbohydrate oxidation and functional recovery after low-flow ischemia and reperfusion in the isolated perfused rat heart. Circulation 111: 2066–2072, 2005. [DOI] [PubMed] [Google Scholar]
- 45.Wang P, Tate JM, Lloyd SG. Low carbohydrate diet decreases myocardial insulin signaling and increases susceptibility to myocardial ischemia. Life Sci 83: 836–844, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wentz AE, d'Avignon DA, Weber ML, Cotter DG, Doherty JM, Kerns R, Nagarajan R, Reddy N, Sambandam N, Crawford PA. Adaptation of myocardial substrate metabolism to a ketogenic nutrient environment. J Biol Chem 285: 24447–24456, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xie N, Zhang W, Li J, Liang H, Zhou H, Duan W, Xu X, Yu S, Zhang H, Yi D. α-Linolenic acid intake attenuates myocardial ischemia/reperfusion injury through anti-inflammatory and anti-oxidative stress effects in diabetic but not normal rats. Arch Med Res 42: 171–181, 2011. [DOI] [PubMed] [Google Scholar]
- 48.Zaha V, Francischetti I, Doenst T. Insulin improves postischemic recovery of function through PI3K in isolated working rat heart. Mol Cell Biochem 247: 229–232, 2003. [DOI] [PubMed] [Google Scholar]
- 49.Zou Z, Sasaguri S, Rajesh KG, Suzuki R. dl-3-Hydroxybutyrate administration prevents myocardial damage after coronary occlusion in rat hearts. Am J Physiol Heart Circ Physiol 283: H1968–H1974, 2002. [DOI] [PubMed] [Google Scholar]