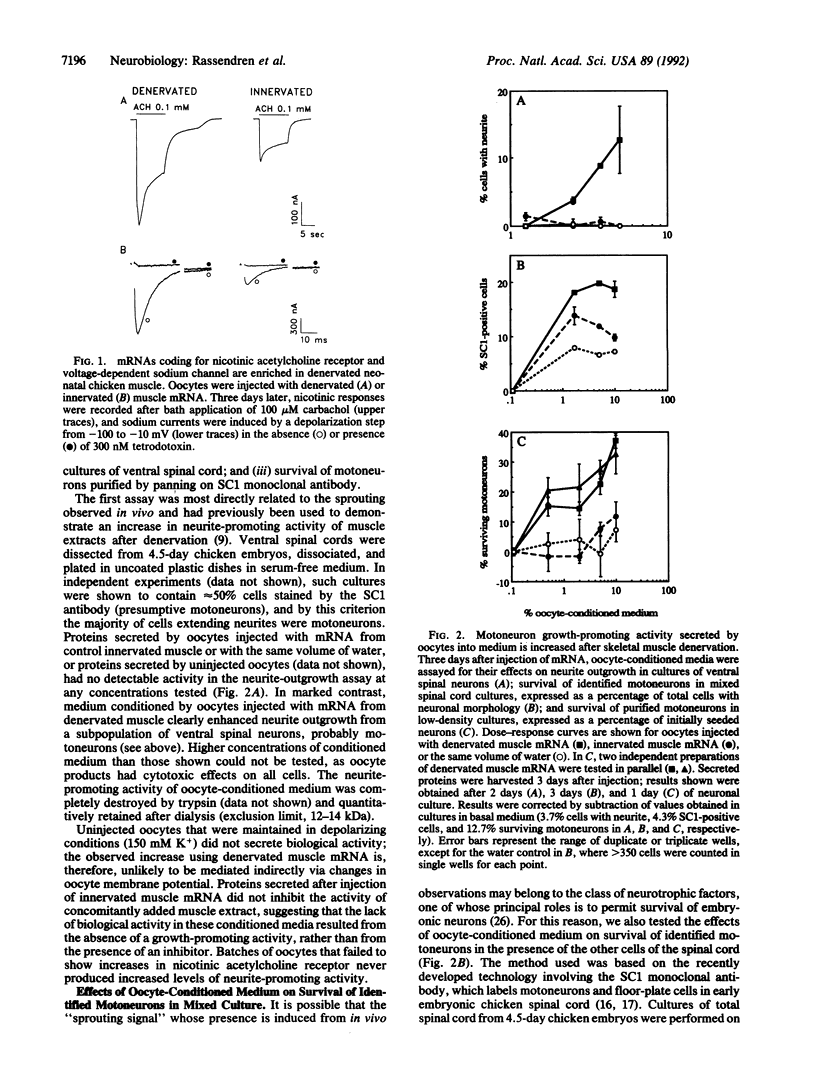
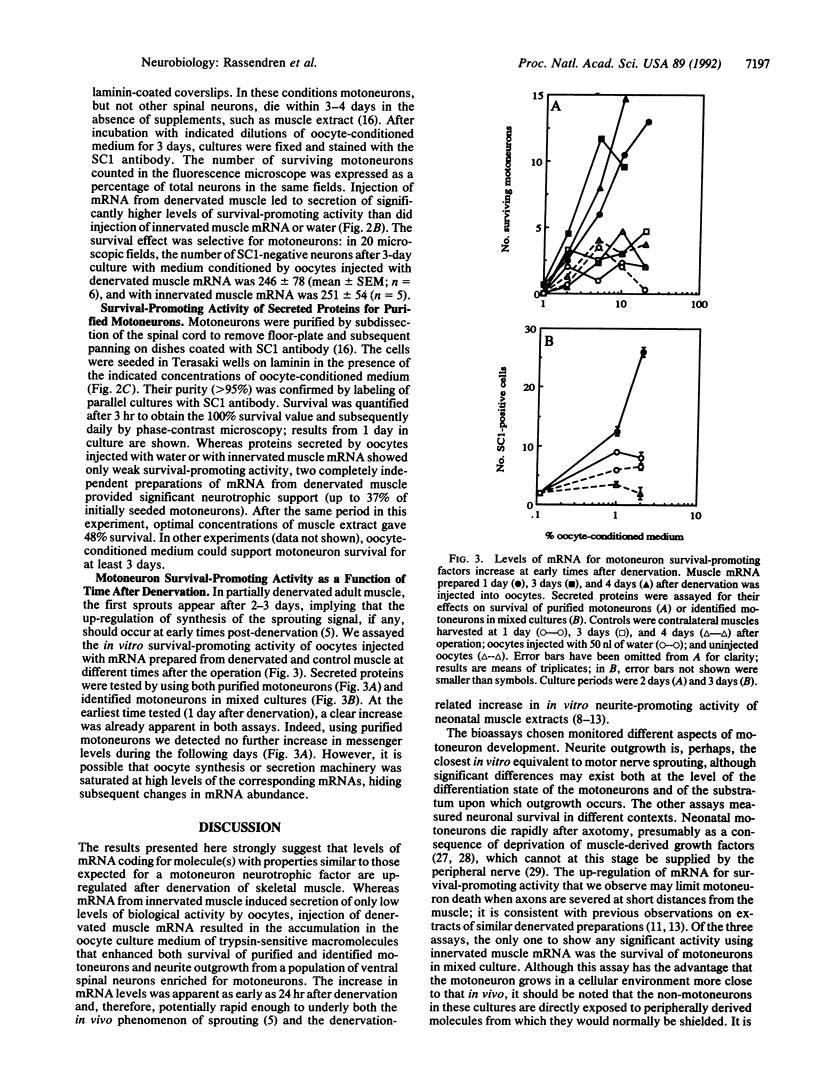
Abstract
Partial denervation of skeletal muscle induces sprouting of axons remaining within the muscle, possibly as a result of increased synthesis by denervated muscle fibers of motoneuron growth-promoting factors. Direct verification of this hypothesis has not been possible because the molecules responsible are not unambiguously characterized. We used Xenopus oocytes as a functional assay for mRNAs coding for secreted growth factors: preparations of mRNA from innervated and denervated neonatal muscle were injected into oocytes. Three days later, oocytes injected with denervated muscle mRNA expressed increased levels of nicotinic acetylcholine receptor and voltage-dependent sodium channels at their membrane. Proteins secreted by the same oocytes were tested for their effects on (i) neurite outgrowth from embryonic chicken ventral spinal cord neurons; (ii) survival in mixed culture of embryonic chicken motoneurons identified using the SC1 antibody; and (iii) survival of embryonic motoneurons purified by panning on SC1 antibody. In all three assays, media conditioned by oocytes injected with mRNA from denervated muscle contained significantly higher levels of biological activity than did those from oocytes injected with innervated muscle mRNA or water. mRNA was prepared from muscle at different times after denervation: a maximal increase was obtained already after 1 day, consistent with an involvement in sprouting. Synthesis of motoneuron growth-promoting factors is thus regulated by denervation in a parallel fashion to that of other key components of the neuromuscular junction.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arakawa Y., Sendtner M., Thoenen H. Survival effect of ciliary neurotrophic factor (CNTF) on chick embryonic motoneurons in culture: comparison with other neurotrophic factors and cytokines. J Neurosci. 1990 Nov;10(11):3507–3515. doi: 10.1523/JNEUROSCI.10-11-03507.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch-Gallego E., Huchet M., el M'Hamdi H., Xie F. K., Tanaka H., Henderson C. E. Survival in vitro of motoneurons identified or purified by novel antibody-based methods is selectively enhanced by muscle-derived factors. Development. 1991 Jan;111(1):221–232. doi: 10.1242/dev.111.1.221. [DOI] [PubMed] [Google Scholar]
- Caroni P., Grandes P. Nerve sprouting in innervated adult skeletal muscle induced by exposure to elevated levels of insulin-like growth factors. J Cell Biol. 1990 Apr;110(4):1307–1317. doi: 10.1083/jcb.110.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux J. P. Compartmentalized transcription of acetylcholine receptor genes during motor endplate epigenesis. New Biol. 1991 May;3(5):413–429. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cooperman S. S., Grubman S. A., Barchi R. L., Goodman R. H., Mandel G. Modulation of sodium-channel mRNA levels in rat skeletal muscle. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8721–8725. doi: 10.1073/pnas.84.23.8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniloff J. K., Crossin K. L., Pinçon-Raymond M., Murawsky M., Rieger F., Edelman G. M. Expression of cytotactin in the normal and regenerating neuromuscular system. J Cell Biol. 1989 Feb;108(2):625–635. doi: 10.1083/jcb.108.2.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frelin C., Lombet A., Vigne P., Romey G., Lazdunski M. The appearance of voltage-sensitive Na+ channels during the in vitro differentiation of embryonic chick skeletal muscle cells. J Biol Chem. 1981 Dec 10;256(23):12355–12361. [PubMed] [Google Scholar]
- Gatchalian C. L., Schachner M., Sanes J. R. Fibroblasts that proliferate near denervated synaptic sites in skeletal muscle synthesize the adhesive molecules tenascin(J1), N-CAM, fibronectin, and a heparan sulfate proteoglycan. J Cell Biol. 1989 May;108(5):1873–1890. doi: 10.1083/jcb.108.5.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson C. E., Huchet M., Changeux J. P. Denervation increases a neurite-promoting activity in extracts of skeletal muscle. Nature. 1983 Apr 14;302(5909):609–611. doi: 10.1038/302609a0. [DOI] [PubMed] [Google Scholar]
- Henderson C. E., Huchet M., Changeux J. P. Neurite outgrowth from embryonic chicken spinal neurons is promoted by media conditioned by muscle cells. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2625–2629. doi: 10.1073/pnas.78.4.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson C. E., Huchet M., Changeux J. P. Neurite-promoting activities for embryonic spinal neurons and their developmental changes in the chick. Dev Biol. 1984 Aug;104(2):336–347. doi: 10.1016/0012-1606(84)90089-7. [DOI] [PubMed] [Google Scholar]
- Heumann R., Korsching S., Bandtlow C., Thoenen H. Changes of nerve growth factor synthesis in nonneuronal cells in response to sciatic nerve transection. J Cell Biol. 1987 Jun;104(6):1623–1631. doi: 10.1083/jcb.104.6.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M. A., Bennett M. R. Motoneurone survival activity in extracts of denervated muscle reduced by prior stimulation of the muscle. Brain Res. 1986 Jan;389(1-2):305–308. doi: 10.1016/0165-3806(86)90201-4. [DOI] [PubMed] [Google Scholar]
- Houenou L. J., McManaman J. L., Prevette D., Oppenheim R. W. Regulation of putative muscle-derived neurotrophic factors by muscle activity and innervation: in vivo and in vitro studies. J Neurosci. 1991 Sep;11(9):2829–2837. doi: 10.1523/JNEUROSCI.11-09-02829.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu L., Natyzak D., Trupin G. L. Neuronotrophic effects of skeletal muscle fractions on neurite development. Muscle Nerve. 1984 Mar-Apr;7(3):211–217. doi: 10.1002/mus.880070305. [DOI] [PubMed] [Google Scholar]
- Ishii D. N. Relationship of insulin-like growth factor II gene expression in muscle to synaptogenesis. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2898–2902. doi: 10.1073/pnas.86.8.2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klarsfeld A., Changeux J. P. Activity regulates the levels of acetylcholine receptor alpha-subunit mRNA in cultured chicken myotubes. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4558–4562. doi: 10.1073/pnas.82.13.4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILEDI R. Properties of regenerating neuromuscular synapses in the frog. J Physiol. 1960 Nov;154:190–205. doi: 10.1113/jphysiol.1960.sp006573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Stefani E., Steinbach A. B. Induction of the action potential mechanism in slow muscle fibres of the frog. J Physiol. 1971 Sep;217(3):737–754. doi: 10.1113/jphysiol.1971.sp009597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyoshi K., Masu M., Ishii T., Shigemoto R., Mizuno N., Nakanishi S. Molecular cloning and characterization of the rat NMDA receptor. Nature. 1991 Nov 7;354(6348):31–37. doi: 10.1038/354031a0. [DOI] [PubMed] [Google Scholar]
- Parker I., Sumikawa K., Gundersen C. B., Miledi R. Expression of ACh-activated channels and sodium channels by messenger RNAs from innervated and denervated muscle. Proc R Soc Lond B Biol Sci. 1988 Apr 22;233(1272):235–246. doi: 10.1098/rspb.1988.0021. [DOI] [PubMed] [Google Scholar]
- Rassendren F. A., Lory P., Pin J. P., Nargeot J. Zinc has opposite effects on NMDA and non-NMDA receptors expressed in Xenopus oocytes. Neuron. 1990 May;4(5):733–740. doi: 10.1016/0896-6273(90)90199-p. [DOI] [PubMed] [Google Scholar]
- Rechler M. M., Nissley S. P. The nature and regulation of the receptors for insulin-like growth factors. Annu Rev Physiol. 1985;47:425–442. doi: 10.1146/annurev.ph.47.030185.002233. [DOI] [PubMed] [Google Scholar]
- Rieger F., Grumet M., Edelman G. M. N-CAM at the vertebrate neuromuscular junction. J Cell Biol. 1985 Jul;101(1):285–293. doi: 10.1083/jcb.101.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes J. R. Extracellular matrix molecules that influence neural development. Annu Rev Neurosci. 1989;12:491–516. doi: 10.1146/annurev.ne.12.030189.002423. [DOI] [PubMed] [Google Scholar]
- Sendtner M., Kreutzberg G. W., Thoenen H. Ciliary neurotrophic factor prevents the degeneration of motor neurons after axotomy. Nature. 1990 May 31;345(6274):440–441. doi: 10.1038/345440a0. [DOI] [PubMed] [Google Scholar]
- Sheard P., McCaig C. D., Harris A. J. Critical periods in rat motoneuron development. Dev Biol. 1984 Mar;102(1):21–31. doi: 10.1016/0012-1606(84)90171-4. [DOI] [PubMed] [Google Scholar]
- Slack J. R., Pockett S. Motor neurotrophic factor in denervated adult skeletal muscle. Brain Res. 1982 Sep 9;247(1):138–140. doi: 10.1016/0006-8993(82)91037-x. [DOI] [PubMed] [Google Scholar]
- Tanaka H., Obata K. Developmental changes in unique cell surface antigens of chick embryo spinal motoneurons and ganglion cells. Dev Biol. 1984 Nov;106(1):26–37. doi: 10.1016/0012-1606(84)90057-5. [DOI] [PubMed] [Google Scholar]
- Thoenen H. The changing scene of neurotrophic factors. Trends Neurosci. 1991 May;14(5):165–170. doi: 10.1016/0166-2236(91)90097-e. [DOI] [PubMed] [Google Scholar]
- Yang J. S., Sladky J. T., Kallen R. G., Barchi R. L. TTX-sensitive and TTX-insensitive sodium channel mRNA transcripts are independently regulated in adult skeletal muscle after denervation. Neuron. 1991 Sep;7(3):421–427. doi: 10.1016/0896-6273(91)90294-a. [DOI] [PubMed] [Google Scholar]



