We identified a deleterious effect of chronic administration of metoprolol on microvascular function during handgrip in hypertensive patients, which was not observed with nebivolol. The impaired muscle capillary recruitment during exercise may predispose to development of exercise intolerance.
Keywords: exercise, sympathetic nervous system, muscle blood flow, hypertension
Abstract
Use of β-adrenergic receptor (AR) blocker is associated with increased risk of fatigue and exercise intolerance. Nebivolol is a newer generation β-blocker, which is thought to avoid this side effect via its vasodilating property. However, the effects of nebivolol on skeletal muscle perfusion during exercise have not been determined in hypertensive patients. Accordingly, we performed contrast-enhanced ultrasound perfusion imaging of the forearm muscles in 25 untreated stage I hypertensive patients at rest and during handgrip exercise at baseline or after 12 wk of treatment with nebivolol (5–20 mg/day) or metoprolol succinate (100–300 mg/day), with a subsequent double crossover for 12 wk. Metoprolol and nebivolol each induced a reduction in the resting blood pressure and heart rate (130.9 ± 2.6/81.7 ± 1.8 vs. 131.6 ± 2.7/80.8 ± 1.5 mmHg and 63 ± 2 vs. 64 ± 2 beats/min) compared with baseline (142.1 ± 2.0/88.7 ± 1.4 mmHg and 75 ± 2 beats/min, respectively, both P < 0.01). Metoprolol significantly attenuated the increase in microvascular blood volume (MBV) during handgrip at 12 and 20 repetitions/min by 50% compared with baseline (mixed-model P < 0.05), which was not observed with nebivolol. Neither metoprolol nor nebivolol affected microvascular flow velocity (MFV). Similarly, metoprolol and nebivolol had no effect on the increase in the conduit brachial artery flow as determined by duplex Doppler ultrasound. Thus our study demonstrated a first direct evidence for metoprolol-induced impairment in the recruitment of microvascular units during exercise in hypertensive humans, which was avoided by nebivolol. This selective reduction in MBV without alteration in MFV by metoprolol suggested impaired vasodilation at the precapillary arteriolar level.
NEW & NOTEWORTHY
We identified a deleterious effect of chronic administration of metoprolol on microvascular function during handgrip in hypertensive patients, which was not observed with nebivolol. The impaired muscle capillary recruitment during exercise may predispose to development of exercise intolerance.
previous studies demonstrated impaired muscle capillary recruitment in hypertension (8, 12), possibly related to impaired microvascular function or capillary rarefaction (27, 28). In hypertensive patients, both metoprolol and propranolol can further impair microvascular function by acutely reducing resting capillary blood flow in the skeletal muscle (22, 23), which may predispose to development of exercise intolerance or fatigue. Nebivolol is a third-generation β-adrenergic receptor (AR) blocker that also possesses vasodilator properties (35). The vasodilator effect is attributed to partly due to the antioxidant effect via inhibition of NADPH oxidase activity or reduced expression of the NADPH oxidase subunits p47phox (18, 20, 35). However, the chronic effect of either older or newer generation β-AR blockers on microvascular function during exercise and endothelial cell (EC) expression of the p47phox NADPH oxidase subunit has not been previously assessed in hypertensive patients.
In this study, we used contrast-enhanced ultrasound (CEU) perfusion imaging in hypertensive patients to characterize the skeletal muscle microvascular response to exercise during chronic therapy with β-blockers differing in their vasodilator effects. We conducted a randomized double crossover design study in patients with stage I hypertension to assess the effects of chronic treatment with metoprolol vs. nebivolol in the forearm. We also performed EC collection in the same hypertensive subjects to assess EC expression of the p47phox subunit.
METHODS
Twenty-five subjects with untreated stage I hypertension participated in the study after providing written informed consent. The study was approved by the Institutional Review Board of the University of Texas Southwestern Medical Center at Dallas. All subjects had blood pressures (BP) between 140–159 and 90–99 mmHg on three determinations by oscillometric technique in the seated position. The subjects had no history of heart disease or diabetes mellitus or evidence of target organ damage such as left ventricular hypertrophy by electrocardiography, peripheral vascular disease, or chronic kidney disease. Both treated (n = 13) and untreated patients (n = 12) participated in the study. Patients who had received antihypertensive treatment before study enrollment were asked to stop their antihypertensive drugs for 3 wk before undergoing the experimental procedures. Total of 47 subjects were screened. Among these subjects, 15 decided not to participate due to conflict with work schedule. The remaining 32 subjects were enrolled in the study and 7 were subsequently excluded. One subject became pregnant during the washout phase. Three subjects continued to have normal BP of <120/80 mmHg after the washout period of 3 wk despite multiple BP measurements. Two subjects did not return for study procedures despite multiple phone contact. One subject was excluded from the study after being diagnosed with cervical cancer.
Experimental Procedures
Subjects were studied in the supine position. Heart rate (HR) was recorded continuously by electrocardiography and systolic and diastolic BP were measured by automated oscillometric sphygmomanometry (CE0050; Welch Allyn, Skaneateles Falls, NY). Respiration was monitored with a strain-gauge pneumograph and subjects were instructed to avoid sympathoexcitatory maneuvers including Valsalvas and prolonged expirations. The body weight was measured using a mechanical beam scale (Detecto beam scale model 3P704, Webb City, MO) after an overnight fast and emptying of bladder as previously described (3).
CEU perfusion imaging of skeletal muscle.
CEU perfusion imaging was performed using a linear-array transducer at a centerline frequency of 7 MHz (Sequoia 512; Siemens Medical Systems, Malvern, PA) in the fasting state. The nonlinear fundamental signal component for microbubbles was detected using multipulse phase and amplitude modulation at a mechanical index of 0.97 and a dynamic range of 55 dB as previously described (36). The flexor muscles in the forearm were imaged in the transaxial plane during an intravenous infusion of lipid-shelled octafluoropropane microbubbles (Definity; Bristol-Myers Squibb Medical Imaging, at 0.08 to 0.1 ml/min). Imaging was performed first at rest and during handgrip exercise. For the exercise portion, image acquisition was started 1 min into exercise and completed within a total duration of 5 min.
Intermittent imaging was then performed at pulsing intervals (PI) ranging from 1 to 15 s at resting and from 1 to 10 s during handgrip, allowing progressively greater replenishment of the ultrasound beam elevation between destructive pulses until maximal accumulation of microbubbles was achieved. At least three images were acquired at each PI. Videotape images were digitized to an offline image analysis system. Analysis of the data was identical for both at rest and during exercise. Several background frames obtained at a PI of 1 s were averaged and digitally subtracted from similarly averaged frames at each subsequent PI. Background-subtracted video intensity (VI) at each PI was measured from a region of interest placed around the deep forearm flexor muscles. PI vs. VI data were fit to the function: y = A (1 − e−βt), where y is video intensity at PI t, A is plateau video intensity [an index of microvascular blood volume (MBV)], and β is the rate constant, which provides a measure of microvascular flux rate (13, 16, 32). Microvascular blood flow (MBF) was calculated as the product of MBV and microvascular flux rate.
Conduit artery blood flow.
On a separate day, brachial artery diameter and mean brachial artery velocity were measured by Duplex Doppler ultrasonography (Philips ie33, Bothell, WA) using an 11-MHz probe in the same nondominant arm as for the CEU study. The probe was placed in a holder and fixed to the skin over the brachial artery throughout the entire experiment as previously described (34). Diameter measurements were obtained at end diastole. Pulsed-wave Doppler was used to measure centerline velocity using a 60° angle of incidence and angle correction software to generate spectral profiles. Images were stored on DVD discs and were analyzed offline, using edge detection software (Brachial Analyzer; Medical Imaging Applications, Coralville, IA) to measure diameter. Forearm blood flow (ml/min) was calculated as angle-corrected velocity × π (brachial diameter/2)2 × 60. Measurement of forearm blood flow was made at baseline and after occlusion of forearm arterial inflow for 5 min, using a pneumatic cuff inflated at 250 mmHg on the arm distal to the elbow for 5 min.
Handgrip exercise.
Maximal voluntary contraction for each subject was designated as the greatest of at least three maximal squeezes of a handgrip dynamometer (Stoelting, Chicago, IL). Subjects performed intermittent handgrip at 30% maximal voluntary contraction to the rhythm of a metronome at 12 and 20 repetitions/min (rpm) at 50% duty cycle each for 5 min. Force production was displayed on an oscilloscope to provide subjects with visual feedback (11).
Cardiac output.
Cardiac output was measured at rest and during handgrip exercise by thoracic electrical bioimpedance (BioZ; CardioDynamics, San Diego, CA) as previously described (2, 25). Stroke volume was derived from change in impedance/time measured during electrical systole. Cardiac output was determined as the product of stroke volume and HR.
Measurement of asymmetrical dimethylarginine.
Venous blood was collected into ethylenediaminetetraacetic acid tubes and stored at <−70°C. Asymmetrical dimethylarginine (ADMA) was measured using a well-validated high-throughput liquid chromatographic-tandem mass spectrometric (LC-MS/MS) assay as described in detail elsewhere (10).
Experimental Protocols
Protocol 1: effects of nebivolol vs. metoprolol on microvascular function during exercise in hypertensive subjects.
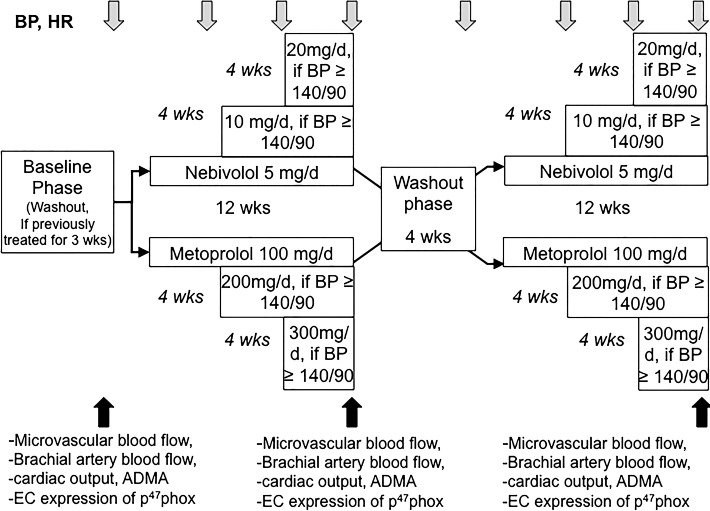
BP and HR were measured in all hypertensive subjects at baseline, every 4 wk during study drug treatment, and after 4 wk of washout before crossover to the second drug (n = 25). Microvascular perfusion of skeletal muscle were measured at rest and during handgrip at baseline, after 12 wk of metoprolol, and after 12 wk of nebivolol (Fig. 1). Handgrip exercise was repeated in the same subjects within a few days to assess conduit brachial artery flow and cardiac output response to exercise at baseline, after 12 wk of metoprolol, and after 12 wk of nebivolol. After the baseline study, subjects were randomized to receive either nebivolol at the dose of 5 mg once daily or metoprolol succinate 100 mg once daily (Fig. 1), using a double-blind crossover design with 4-wk washout between each treatment phase. The investigational drug service at our institution determined the sequence of randomization and ensured that the sequence was balanced before dispensing study drugs to each participant. To ensure that investigators and participants are blinded to the study treatment, the investigational drug pharmacists masked the identity of nebivolol and metoprolol tablets by inserting the tablets into capsule shells with identical appearance. BP was measured using an oscillometric device (CE0050; Welch Allyn) after participants had been sitting quietly for at least 5 min. BP measurements were repeated twice after 1 min between each reading and data were averaged with the first reading. If BP remained above 140/90 mmHg during the first follow-up visit, the dose of nebivolol was increased to 10 mg once daily and metoprolol was increased to 200 mg once daily. If BP remained above 140/90 mmHg during the second follow-up visit 4 wk later, the dose of nebivolol was further increased to 20 mg once daily and metoprolol was increased to 300 mg once daily as shown in Fig. 1. After a 12-wk treatment with each study drug, measurement of BP, HR, forearm blood flow, and microvascular perfusion of skeletal muscle at rest and during handgrip were repeated and these variables were compared with those obtained at baseline.
Fig. 1.
Diagram outlining scheduled events in the randomized crossover study: blood pressure (BP) and heart rate (HR) were measured at baseline, every 4 wk during study drug treatment, and after 4 wk of washout before crossover to the second drug. Microvascular blood flow, brachial artery blood flow, cardiac output, asymmetrical dimethylarginine (ADMA), and endothelial cell (EC) expression of p47phox were measured at baseline, after 12 wk of metoprolol, and after 12 wk of nebivolol.
Protocol 2: effects of nebivolol vs. metoprolol on EC cell function.
To evaluate the previously reported effect of β-AR blocker treatment on endogenous nitric oxide (NO) synthase (NOS) inhibitor ADMA in hypertensive patients, we determined circulating ADMA before and after treatment (21). To determine whether nebivolol attenuates impair microvascular flow during exercise by increased EC expression p47phox NADPH oxidase subunit resulting in increased oxidative stress, we performed peripheral venous EC collection in the same subjects (n = 25) as previously described (6). EC collection was performed on a separate day before assessment of microvascular flow in protocol 1 to avoid confounding influence of exercise and octafluoropropane microbubbles. A 20-gauge angiocatheter was inserted into the contralateral forearm vein under sterile conditions. Three J-shaped vascular guidewires (St. Jude, St. Paul, MN) were advanced sequentially into the vein up to 10 cm. ECs were collected by gentle abrasion and placed into a dissociation buffer (0.5% bovine serum albumin, 2 mM EDTA, and 100 ug/ml heparin in PBS). ECs were recovered from the tips of guide wires by repeated washing into collection tubes and subsequent centrifugation. Collected cells were fixed with 3.7% paraformaldehyde in PBS and plated on poly-l-lysine-coated slides (Lab Scientific, Highlands, NJ). The slides were kept at −80° until staining. Protein expression in ECs was assessed using the previously described method (6, 7).
The frozen slides were thawed, rehydrated in PBS, and rendered permeable with 0.1% Triton X-100 in PBS (Alfa Aesar, Ward Hill, MA). After blocking with 5% donkey serum in PBS (Jackson Immunoresearch, West Grove, PA), cells were incubated for 1 h at room temperature with monoclonal antibodies against the polyclonal antibodies against NADPH oxidase p47 subunit (1:50 dilution; Millipore, Billerica, MA). All cells were subsequently incubated with an antibody against von Willebrand factor (vWf; polyclonal, 1:1,000 dilution; Dako, Carpinteria, CA; or monoclonal, 1:1,000 dilution; BD Biosciences, San Diego, CA) for EC identification. Cells were then washed and incubated with the Alexa Fluoro-488-labeled and Alexa Fluor-568-labeled secondary antibodies (1:200 and 1:400 respectively; Invitrogen, Carlsbad, CA). Nuclear integrity was confirmed using DAPI staining (Vector Laboratories, Burlingame, CA). All slides were imaged using a fluorescence microscope (Eclipse 600; Nikon, Melville, NY) at ×60 magnification under identical conditions, and images were digitally captured by a Photometrics CoolSNAPfx camera (Roper Scientific, Tucson, AZ). ImageJ software (26) (National Institutes of Health, Bethesda, MD) was used to quantify the intensity of staining with correction for background fluorescence. Values were averaged from 25 to 35 cells per each slide and reported as ratios of EC protein expression/human umbilical vein EC.
Statistical Analysis
Effects of drug treatment on hemodynamic and microvascular flow responses during exercise were compared with linear mixed-effects model with repeated measures. The analyses included repeated factors to assess treatment phase (baseline, nebivolol, metoprolol), experimental condition (rest, handgrip, 12 rpm and 20 rpm), and the treatment-by-condition interaction. The study subject is modeled as a random effect. In the presence of a statistically significant main effect, planned pairwise comparisons were made from the least squares means contrasts derived from the mixed models. We also compared the difference between the exercise and resting values of the following variables: MBV, microvascular flow velocity, and microvascular flow during baseline and treatment phases, using the mixed models. To test for potential carryover effects between treatment phases on these variables, the randomized sequence was introduced and assigned as a fixed effect. Then, the interaction between sequence and treatment was assessed using the mixed-effects linear model. The magnitude of responses was found to be similar per sequence and the tests of sequence and interaction effects were all nonsignificant, indicating no significant carryover effects (all P > 0.2). Variables with skewed distributions were log or rank transformed before analysis. Statistical analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC). All tests were two-sided and a P < 0.05 was considered statistically significant unless otherwise specified. Data are presented as means ± SE or median and 25th-75th percentile.
RESULTS
Baseline characteristics of our hypertensive subjects are shown in Table 1. The average daily dose of nebivolol used in the study was 10 ± 1 mg and the average dose of metoprolol succinate was 152 ± 14 mg. Metoprolol and nebivolol induced a similar reduction in resting BP (131 ± 3/82 ± 2 and 132 ± 3/81 ± 2 mmHg, respectively) compared with respective baseline (Table 2, both P < 0.01). Similarly, metoprolol and nebivolol induced a similar reduction in resting HR (63 ± 2 and 64 ± 2 beats/min, respectively) compared with respective baseline (Table 2, both P < 0.01). Metoprolol and nebivolol significantly increased body weight in the same subjects (P < 0.05 vs. baseline before treatment with metoprolol and nebivolol, Table 3).
Table 1.
Subject characteristics
| Variables | Average ± SE |
|---|---|
| Age, yr | 53 ± 2 |
| Sex (male/female) | 11/14 |
| African Americans, % | 36% |
| Maximal voluntary contraction, kg | 31.8 ± 2 |
| Body mass index, kg/m2 | 30.5 ± 1 |
| Serum creatinine, mg/dl | 1.0 ± 0.04 |
| Total cholesterol, mg/dl | 171 ± 10 |
| Triglycerides, mg/dl | 113 ± 16 |
| Fasting plasma glucose, mg dl | 101 ± 4 |
Values are average ± SE. BP, blood pressure.
Table 2.
Changes in resting BP and heart rate in hypertensive subjects treated with nebivolol vs. metoprolol
| Baseline Before Metoprolol | Metoprolol | Baseline Before Nebivolol | Nebivolol | |
|---|---|---|---|---|
| Systolic BP, mmHg | 140 ± 2 | 131 ± 3* | 140 ± 2 | 132 ± 3* |
| Diastolic BP, mmHg | 87 ± 1 | 82 ± 2 * | 88 ± 1 | 81 ± 2* |
| Heart rate, beats/min | 74 ± 2 | 63 ± 2 * | 77 ± 2 | 64 ± 2* |
Values are means ± SE. BP, blood pressure.
P < 0.01 vs. respective baseline.
Table 3.
Hemodynamic and brachial conduit artery flow responses to handgrip exercise at 20 repetitions per min in hypertensive subjects treated with nebivolol vs. metoprolol
| Baseline (No Drug) | Metoprolol | Nebivolol | |
|---|---|---|---|
| Body weight, kg | 88.9 ± 3.6 | 90.3 ± 3.7† | 89.5 ± 3.5† |
| Resting MAP, mmHg | 105 ± 2 | 95 ± 3† | 97 ± 3† |
| RHG MAP, mmHg | 110 ± 2* | 101 ± 3*† | 103 ± 3*† |
| Resting HR, beat/min | 65 ± 1 | 56 ± 2† | 57 ± 2† |
| RHG HR, beat/min | 71 ± 1* | 60 ± 2*† | 62 ± 2*† |
| Resting FBF, ml/min | 141 ± 18 | 104 ± 19 | 112 ± 13 |
| RHG FBF, ml/min | 469 ± 50* | 398 ± 43* | 427 ± 37* |
| Resting cardiac output, l/min | 5.35 ± 0.4 | 4.83 ± 0.3 | 4.95 ± 0.4 |
| RHG cardiac output, l/min | 5.53 ± 0.4 | 4.82 ± 0.3 | 5.04 ± 0.4 |
| ADMA, μmol/l | 0.43 ± 0.03 | 0.50 ± 0.02 | 0.50 ± 0.03 |
| Ratio human/HUVEC p47Phox expression, arbitrary units | 0.43 ± 0.05 | 0.47 ± 0.12 | 0.44 ± 0.07 |
Values are means ± SE.
ADMA, asymmetrical dimethylarginine; FBF, forearm blood flow; RHG, rhythmic handgrip, EC endothelial cells; MAP, mean arterial pressure; HR, heart rate; HUVEC, human umbilical vein endothelial cell.
P < 0.05 vs rest;
P < 0.05 vs. baseline.
Protocol 1: Effects of Nebivolol vs. Metoprolol on Microvascular Flow During Exercise in Hypertensive Subjects
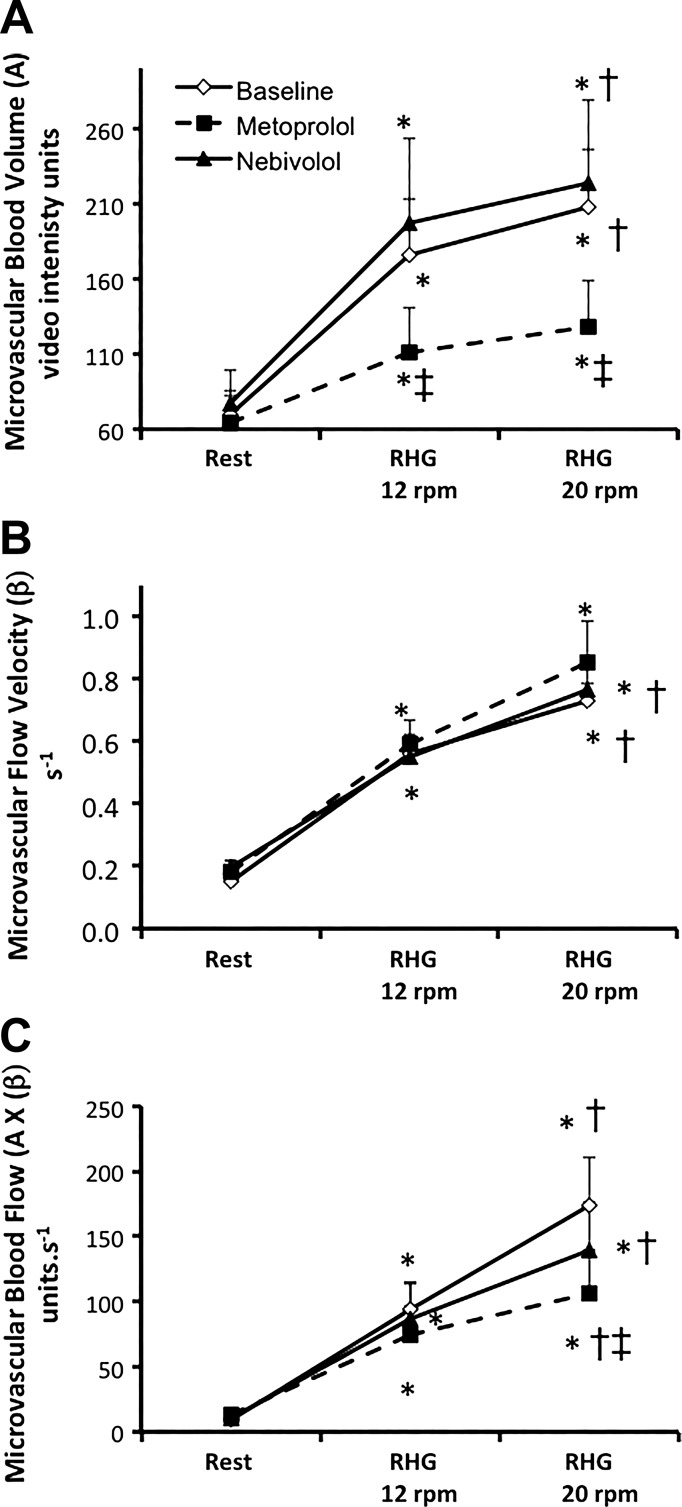
Handgrip exercise induced a significant increase in MBV, microvascular flux rate, and MBF, in a frequency-dependent manner in all variables (P < 0.01. vs. rest, P < 0.05 vs. 12 rpm, Fig. 2) at baseline. Treatment with both metoprolol and nebivolol for 12 wk had no effect on the resting MBV or microvascular flux rate at rest (Fig. 2). However, metoprolol significantly attenuated the increase in MBV during handgrip at 12 and 20 rpm compared with baseline (mixed model P < 0.05, Fig. 2A). Although the MBV during handgrip at 12 and 20 rpm was lower during metoprolol compared with nebivolol treatment, the difference did not reach statistical significance (P = 0.12 and 0.15, respectively). Neither metoprolol nor nebivolol altered microvascular flow velocity during handgrip (Fig. 2B). Metoprolol tended to attenuate the increase in MBF during handgrip at 20 rpm but reduction did not reach significance (mixed model P value = 0.09, pairwise P value < 0.05 metoprolol vs. baseline predrug phase, Fig. 2C). This effect was not observed with nebivolol in the same hypertensive subjects (P > 0.05 vs. baseline, no drug period).
Fig. 2.
Summary data showing changes in microvascular blood volume (A), microvascular flow velocity (B), and microvascular blood flow (C) in hypertensive subjects at rest and during handgrip (RHG) at 12 and 20 repetitions/min. *P < 0.01 vs. rest, †P < 0.05 vs. 12 beats/min, ‡P < 0.05 vs. baseline.
Conduit artery blood flow in the brachial artery also increased significantly with handgrip (Table 3). Metoprolol and nebivolol had no significant effect on the resting brachial artery blood flow (P = 0.43, Table 3). Brachial artery flow during handgrip was also similar between metoprolol and nebivolol (P > 0.05 vs. baseline). There was no significant change in cardiac output during handgrip. Neither metoprolol nor nebivolol treatment altered cardiac output at rest or during mild level exercise (Table 3).
Protocol 2: Effects of Nebivolol vs. Metoprolol on EC Function
Neither metoprolol nor nebivolol treatment affected EC expression of the p47phox subunit in the NADPH oxidase (Table 2). Similarly, circulating ADMA levels were unaffected by nebivolol and metoprolol.
DISCUSSION
The major new findings of the present study are twofold. First, metoprolol impaired the microvascular response to exercise in individuals with mild, uncomplicated hypertension, which was not observed with nebivolol despite a similar reduction in resting BP. Second, this impairment in muscle blood flow regulation during exercise was not explained by impaired vasodilatation of the conduit artery, implicating a direct effect of metoprolol on the microvasculature and specifically the ability to recruit microvascular units during contractile exercise. Taken together, these findings suggested a deleterious effect of conventional β1-adrenergic receptor (AR) blocker on the microcirculatory responses to exercise in hypertensive individuals, which cannot be attributed to its BP-lowering effect or β-AR blockade. In addition, newer generation β-AR blocker nebivolol avoided this adverse effect of Metoprolol.
Previous studies in hypertensive patients showed that acute administration of both metoprolol and propranolol reduces resting capillary flow in the forearm muscle as evidenced by 131I clearance (22, 23). In these studies, treatment with propranolol for 4 days was shown to have no effect on skeletal muscle capillary blood flow during exercise (22, 23). In contrast, chronic administration of metoprolol was shown to increase skin capillary flow both at rest and during reactive hyperemia in another study (14). Our new data, using CEU kinetics, show that the skeletal muscle microvascular function during exercise was preserved by the third-generation vasodilating β1-AR blocker nebivolol but impaired by traditional nonvasodilating β1-AR blocker metoprolol. This impairment in microvascular function is driven exclusively by impairment in the increase in the MBV, rather than the increase in microvascular flux rate. Although this abnormality in MBV may be related to subnormal increase in the cardiac output during handgrip, there was no significant difference in cardiac output or brachial artery conduit flow velocity during handgrip after treatment with metoprolol compared with baseline in our study. Thus our data suggested a selective impairment in the precapillary arteriolar dilation induced by metoprolol, which has not been previously described.
Mechanisms underlying our observation are unknown. Previous studies demonstrated a selective increase in the MBV with mild exercise (13, 32) or insulin (5, 17, 33) without alteration in the microvascular flow velocity in normal volunteers. This initial rise in the MBV with insulin was prevented by the NOS inhibitor l-NG-nitroarginine methyl ester (l-NAME) (30, 31). However, l-NAME failed to prevent the increase in MBV during mild exercise (13). Our previous study in hypertensive patients demonstrated an augmented sympathetic mediated vasoconstriction during exercise (34), which was unaltered by metoprolol treatment (24). Nevertheless, assessment of blood flow in our previous studies was limited to the level of conduit (brachial) artery. It is plausible that sympathetic stimulation during mild exercise in our study led to enhanced α-AR-mediated vasoconstriction predominantly at the precapillary arteriolar level after metoprolol treatment. Treatment with metoprolol in our study also induced a small but significant increase in body weight, which may further increase in sympathetic vasoconstrictor activity. However, this is unlikely because the absolute increase in body weight is modest (1.4 kg) and our previous study showed no change in sympathetic nerve activity with the same dose of metoprolol in hypertensive patients (24). Previous studies have demonstrated an association between obesity with impaired microvascular recruitment in response to euglycemic hyperinsulinemia (4) and ingestion of mixed meal in normal volunteers (16). Whether weight gain directly impairs microvascular flow during exercise remains to be determined.
In contrast to metoprolol, nebivolol had no significant effect on microvascular function during handgrip despite inducing a similar increase in body weight. The mechanisms underlying preserved microcirculatory adjustment to exercise induced by nebivolol are unknown but likely reflect its ancillary vasodilating properties. Studies in rodents demonstrated that nebivolol increased NO availability via reducing superoxide formation and inhibition of the p47phox subunit of the NADPH oxidase expression in the vascular cells and myocardium (18, 20, 35). In our study, however, there was no significant change in the EC expression of hypertensive subjects during treatment with either nebivolol or metoprolol. Nebivolol has also been shown to reduce circulating levels of the endogenous NOS inhibitor ADMA in hypertensive patients by increasing the expression and activity of the ADMA-degrading enzyme dimethylarginine dimethylaminohydrolase (21). In another study, however, nebivolol was shown to have no effects on circulating ADMA levels (15), which were similar to our study results. In vitro studies demonstrated that nebivolol dilates human and rodent coronary resistance microarteries through activation of endothelial β3-AR to release NO (9). The contribution of β3-AR on microvascular flow during exercise remains to be determined.
The strength of our study included the use of microbubble contrast enhanced imaging in elucidating pattern of skeletal muscle perfusion in hypertension during exercise and the use of EC collection to directly assess protein expression at the ECs. Our study was limited by lack of a placebo arm for comparison because it is unethical to withhold antihypertensive treatment for an extended duration of 12 wk. Our study is also limited to the arm muscle and it is unclear if our findings will be applicable to the leg muscle. Only venous ECs were collected in our study and the results may not be extrapolated to the arterial ECs. Our small sample size may also limit our ability to detect subtle (< 20–25%) change in p47phox subunit expression. Furthermore, assessment of EC expression of the NADPH oxidase was limited to the p47phox subunit. Other subunits such as NOX1, NOX2, or p22phox may be altered during treatment. Nevertheless, our previous studies demonstrated no significant difference in the plasma levels of oxidative stress marker F2-isoprostanes in response to intravenous angiotensin II infusion in hypertensive patients after nebivolol treatment compared with metoprolol (24).
Perspectives
Treatment with older generation β-AR blockers is associated with exercise intolerance and fatigue (1, 19, 29). Although this is thought to be due to attenuation of the β-AR-mediated increase in HR and cardiac output during exercise, our study demonstrated another potential mechanism of selective impairment in the skeletal muscle microvascular function, which was independent of cardiac output and conduit artery tone. This undesirable side effect of metoprolol was avoided by the newer generation β-blocker nebivolol. Clinical trials with long-term follow-up are needed to determine if nebivolol can improve skeletal muscle perfusion and exercise capacity in hypertensive subjects with or without peripheral arterial disease.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant R01-HL-078782 and grants from the Forest Research Institute and O'Brien Kidney Center (W. Vongpatanasin) and a Clinical and Translational Sciences Award (UL1RR-024982) (to B. Adams-Huet). The Clinical Trial Registration for this study is NCT01501929.
DISCLOSURES
W. Vongpatanasin received research grant from Forest Research Institute.
AUTHOR CONTRIBUTIONS
A.V., E.B.S., A.L.P., Z.W., D.A., and W.V. performed experiments; A.V., E.B.S., A.L.P., Z.W., D.A., G.A., B.A.-H., E.S., J.R.L., and W.V. analyzed data; A.V., E.B.S., Z.W., G.A., B.A.-H., E.S., J.R.L., and W.V. interpreted results of experiments; A.V. and W.V. prepared figures; A.V., E.B.S., A.L.P., Z.W., D.A., G.A., B.A.-H., E.S., J.R.L., and W.V. drafted manuscript; A.V., E.B.S., A.L.P., Z.W., D.A., G.A., B.A.-H., E.S., J.R.L., and W.V. edited and revised manuscript; A.V., E.B.S., A.L.P., Z.W., D.A., G.A., B.A.-H., E.S., J.R.L., and W.V. approved final version of manuscript; W.V. conception and design of research.
ACKNOWLEDGMENTS
We gratefully acknowledge the technical assistance of Robert Ruelas.
REFERENCES
- 1.Anderson RL, Wilmore JH, Joyner MJ, Freund BJ, Hartzell AA, Todd CA, Ewy GA. Effects of cardioselective and nonselective beta-adrenergic blockade on the performance of highly trained runners. Am J Cardiol 55: 149D–154D, 1985. [DOI] [PubMed] [Google Scholar]
- 2.Brillante DG, Johnstone MT, Howes LG. Effects of intravenous PD 123319 on haemodynamic and arterial stiffness indices in healthy volunteers. J Renin Angiotensin Aldosterone Syst 6: 102–106, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Butler SM, Black DR, Blue CL, Gretebeck RJ. Change in diet, physical activity, and body weight in female college freshman. Am J Health Behav 28: 24–32, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Clerk LH, Vincent MA, Jahn LA, Liu Z, Lindner JR, Barrett EJ. Obesity blunts insulin-mediated microvascular recruitment in human forearm muscle. Diabetes 55: 1436–1442, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Coggins M, Lindner J, Rattigan S, Jahn L, Fasy E, Kaul S, Barrett E. Physiologic hyperinsulinemia enhances human skeletal muscle perfusion by capillary recruitment. Diabetes 50: 2682–2690, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Colombo PC, Ashton AW, Celaj S, Talreja A, Banchs JE, Dubois NB, Marinaccio M, Malla S, Lachmann J, Ware JA, Le Jemtel TH. Biopsy coupled to quantitative immunofluorescence: a new method to study the human vascular endothelium. J Appl Physiol 92: 1331–1338, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Colombo PC, Banchs JE, Celaj S, Talreja A, Lachmann J, Malla S, DuBois NB, Ashton AW, Latif F, Jorde UP, Ware JA, LeJemtel TH. Endothelial cell activation in patients with decompensated heart failure. Circulation 111: 58–62, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Debbabi H, Uzan L, Mourad JJ, Safar M, Levy BI, Tibirica E. Increased skin capillary density in treated essential hypertensive patients. Am J Hypertens 19: 477–483, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Dessy C, Saliez J, Ghisdal P, Daneau G, Lobysheva II, Frerart F, Belge C, Jnaoui K, Noirhomme P, Feron O, Balligand JL. Endothelial beta3-adrenoreceptors mediate nitric oxide-dependent vasorelaxation of coronary microvessels in response to the third-generation beta-blocker nebivolol. Circulation 112: 1198–1205, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Gore MO, Luneburg N, Schwedhelm E, Ayers CR, Anderssohn M, Khera A, Atzler D, de Lemos JA, Grant PJ, McGuire DK, Boger RH. Symmetrical dimethylarginine predicts mortality in the general population: observations from the Dallas heart study. Arterioscler Thromb Vasc Biol 33: 2682–2688, 2013. [DOI] [PubMed] [Google Scholar]
- 11.Hansen J, Thomas GD, Harris SA, Parsons WJ, Victor RG. Differential sympathetic neural control of oxygenation in resting and exercising human skeletal muscle. J Clin Invest 98: 584–596, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansen-Smith F, Greene AS, Cowley AW Jr, Lombard JH. Structural changes during microvascular rarefaction in chronic hypertension. Hypertension 15: 922–928, 1990. [DOI] [PubMed] [Google Scholar]
- 13.Inyard AC, Clerk LH, Vincent MA, Barrett EJ. Contraction stimulates nitric oxide independent microvascular recruitment and increases muscle insulin uptake. Diabetes 56: 2194–2200, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Kaiser SE, Sanjuliani AF, Estato V, Gomes MB, Tibirica E. Antihypertensive treatment improves microvascular rarefaction and reactivity in low-risk hypertensive individuals. Microcirculation 20: 703–716, 2013. [DOI] [PubMed] [Google Scholar]
- 15.Kandavar R, Higashi Y, Chen W, Blackstock C, Vaughn C, Sukhanov S, Sander GE, Roffidal LE, Delafontaine P, Giles TD. The effect of nebivolol versus metoprolol succinate extended release on asymmetric dimethylarginine in hypertension. J Am Soc Hypertens 5: 161–165, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keske MA, Clerk LH, Price WJ, Jahn LA, Barrett EJ. Obesity blunts microvascular recruitment in human forearm muscle after a mixed meal. Diabetes Care 32: 1672–1677, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu J, Jahn LA, Fowler DE, Barrett EJ, Cao W, Liu Z. Free fatty acids induce insulin resistance in both cardiac and skeletal muscle microvasculature in humans. J Clin Endocrinol Metab 96: 438–446, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma L, Gul R, Habibi J, Yang M, Pulakat L, Whaley-Connell A, Ferrario CM, Sowers JR. Nebivolol improves diastolic dysfunction and myocardial remodeling through reductions in oxidative stress in the transgenic (mRen2) rat. Am J Physiol Heart Circ Physiol 302: H2341–H2351, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Messerli FH, Bangalore S, Yao SS, Steinberg JS. Cardioprotection with beta-blockers: myths, facts and Pascal's wager. J Intern Med 266: 232–241, 2009. [DOI] [PubMed] [Google Scholar]
- 20.Oelze M, Daiber A, Brandes RP, Hortmann M, Wenzel P, Hink U, Schulz E, Mollnau H, von Sandersleben A, Kleschyov AL, Mulsch A, Li H, Forstermann U, Munzel T. Nebivolol inhibits superoxide formation by NADPH oxidase and endothelial dysfunction in angiotensin II-treated rats. Hypertension 48: 677–684, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Pasini AF, Garbin U, Stranieri C, Boccioletti V, Mozzini C, Manfro S, Pasini A, Cominacini M, Cominacini L. Nebivolol treatment reduces serum levels of asymmetric dimethylarginine and improves endothelial dysfunction in essential hypertensive patients. Am J Hypertens 21: 1251–1257, 2008. [DOI] [PubMed] [Google Scholar]
- 22.Pechan J, Mikulecky M, Srbecky M. Acute haemodynamic effect of metoprolol in essential hypertension. Cor Vasa 30: 51–59, 1988. [PubMed] [Google Scholar]
- 23.Pechan J, Ondrejicka M, Janotka M, Kellerova E. The effect of guanethidine and propranolol on capillary blood flow in subcutaneous tissue and muscle in essential hypertension. Cardiology 59: 172–183, 1974. [DOI] [PubMed] [Google Scholar]
- 24.Price A, Raheja P, Wang Z, Arbique D, Adams-Huet B, Mitchell JH, Victor RG, Thomas GD, Vongpatanasin W. Differential effects of nebivolol versus metoprolol on functional sympatholysis in hypertensive humans. Hypertension 61: 1263–1269, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenberg P, Yancy CW. Noninvasive assessment of hemodynamics: an emphasis on bioimpedance cardiography. Curr Opin Cardiol 15: 151–155, 2000. [DOI] [PubMed] [Google Scholar]
- 26.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Serne EH, Gans RO, ter Maaten JC, Tangelder GJ, Donker AJ, Stehouwer CD. Impaired skin capillary recruitment in essential hypertension is caused by both functional and structural capillary rarefaction. Hypertension 38: 238–242, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Serne EH, Gans RO, ter Maaten JC, ter Wee PM, Donker AJ, Stehouwer CD. Capillary recruitment is impaired in essential hypertension and relates to insulin's metabolic and vascular actions. Cardiovasc Res 49: 161–168, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Van Bortel LM, van Baak MA. Exercise tolerance with nebivolol and atenolol. Cardiovasc Drugs Ther 6: 239–247, 1992. [DOI] [PubMed] [Google Scholar]
- 30.Vincent MA, Barrett EJ, Lindner JR, Clark MG, Rattigan S. Inhibiting NOS blocks microvascular recruitment and blunts muscle glucose uptake in response to insulin. Am J Physiol Endocrinol Metab 285: E123–E129, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Vincent MA, Clerk LH, Lindner JR, Klibanov AL, Clark MG, Rattigan S, Barrett EJ. Microvascular recruitment is an early insulin effect that regulates skeletal muscle glucose uptake in vivo. Diabetes 53: 1418–1423, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Vincent MA, Clerk LH, Lindner JR, Price WJ, Jahn LA, Leong-Poi H, Barrett EJ. Mixed meal and light exercise each recruit muscle capillaries in healthy humans. Am J Physiol Endocrinol Metab 290: E1191–E1197, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Vincent MA, Dawson D, Clark AD, Lindner JR, Rattigan S, Clark MG, Barrett EJ. Skeletal muscle microvascular recruitment by physiological hyperinsulinemia precedes increases in total blood flow. Diabetes 51: 42–48, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Vongpatanasin W, Wang Z, Arbique D, Arbique G, Adams-Huet B, Mitchell JH, Victor RG, Thomas GD. Functional sympatholysis is impaired in hypertensive humans. J Physiol 589: 1209–1220, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whaley-Connell A, Habibi J, Johnson M, Tilmon R, Rehmer N, Rehmer J, Wiedmeyer C, Ferrario CM, Sowers JR. Nebivolol reduces proteinuria and renal NADPH oxidase-generated reactive oxygen species in the transgenic Ren2 rat. Am J Nephrol 30: 354–360, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu MD, Belcik JT, Qi Y, Zhao Y, Benner C, Pei H, Linden J, Lindner JR. Abnormal regulation of microvascular tone in a murine model of sickle cell disease assessed by contrast ultrasound. J Am Soc Echocardiogr 28: 1122–1128, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]