This study demonstrates that chronic vagal nerve stimulation slows progression of endothelial dysfunction and aortic stiffening in stroke-prone spontaneously hypertensive rats on high-salt diet. Interleukin-6 and interleukin-10 serum levels correlated with endothelial function, suggesting a mechanistic link between the protective vascular effects of vagal nerve stimulation and inflammation.
Keywords: hypertension, cardiovascular end-organ damage, nitric oxide-dependent relaxation, aortic distensibility, aortic pulse-wave velocity
Abstract
Parasympathetic activity is often reduced in hypertension and can elicit anti-inflammatory mechanisms. Thus we hypothesized that chronic vagal nerve stimulation (VNS) may alleviate cardiovascular end-organ damage in stroke-prone spontaneously hypertensive rats. Vagal nerve stimulators were implanted, a high-salt diet initiated, and the stimulators turned on (VNS, n = 10) or left off (sham, n = 14) for 4 wk. Arterial pressure increased equally in both groups. After 4 wk, endothelial function, assessed by in vivo imaging of the long posterior ciliary artery (LPCA) after stimulation (pilocarpine) and inhibition (Nω-nitro-l-arginine methyl ester) of endothelial nitric oxide synthase (eNOS), had significantly declined (−2.3 ± 1.2 μm, P < 0.05) in sham, but was maintained (−0.7 ± 0.8 μm, nonsignificant) in VNS. Furthermore, aortic eNOS activation (phosphorylated to total eNOS protein content ratio) was greater in VNS (0.83 ± 0.07) than in sham (0.47 ± 0.08, P < 0.05). After only 3 wk, ultrasound imaging of the aorta demonstrated decreased aortic strain (−9.7 ± 2.2%, P < 0.05) and distensibility (−2.39 ± 0.49 1,000/mmHg, P < 0.05) and increased pulse-wave velocity (+2.4 ± 0.7 m/s, P < 0.05) in sham but not in VNS (−3.8 ± 3.8%, −0.70 ± 1.4 1,000/mmHg, and +0.1 ± 0.7 m/s, all nonsignificant). Interleukin (IL)-6 serum concentrations tended to be higher in VNS than in sham (34.3 ± 8.3 vs. 16.1 ± 4.6 pg/ml, P = 0.06), and positive correlations were found between NO-dependent relaxation of the LPCA and serum levels of IL-6 (r = +0.70, P < 0.05) and IL-10 (r = +0.56, P < 0.05) and between aortic eNOS activation and IL-10 (r = +0.48, P < 0.05). In conclusion, chronic VNS prevents hypertension-induced endothelial dysfunction and aortic stiffening in an animal model of severe hypertension. We speculate that anti-inflammatory mechanisms may contribute to these effects.
Listen to this article's corresponding podcast at http://ajpheart.podbean.com/e/chronic-vagal-nerve-stimulation-in-stroke-prone-shr/.
NEW & NOTEWORTHY
This study demonstrates that chronic vagal nerve stimulation slows progression of endothelial dysfunction and aortic stiffening in stroke-prone spontaneously hypertensive rats on high-salt diet. Interleukin-6 and interleukin-10 serum levels correlated with endothelial function, suggesting a mechanistic link between the protective vascular effects of vagal nerve stimulation and inflammation.
hypertension remains a major cause of morbidity and mortality, with over 17 million deaths worldwide per year (World Health Organization data from 2013) and a reduction of average lifespan of 5 years (16). This increased mortality in hypertensive patients is largely attributable to vascular complications, including coronary heart disease and stroke (45). Thus the primary goal of the management of hypertension should be to prevent such vascular complications. However, since the introduction of the first antihypertensive drug (reserpine) into clinical practice in 1950, treatment of hypertension has focused on lowering the elevated blood pressure rather than on primarily preventing cardiovascular end-organ damage. While it is accepted that lowering arterial blood pressure reduces the risk for cardiovascular end-organ damage, it has also been recognized that classes of antihypertensive drugs differ in their effectiveness in preventing end-organ damage even at equipotent antihypertensive doses (35). Thus end-organ protection may not simply be the direct result of lowering arterial blood pressure.
It is widely accepted that the autonomic balance is shifted to higher sympathetic and lower parasympathetic nervous system activity in hypertension, and that the elevated sympathetic tone in hypertension contributes to hypertension-associated cardiovascular end-organ damage (18, 34). Thus lowering sympathetic tone through various measures, such as lifestyle changes (54), sympatholytic drugs (12), or newer interventions such as carotid baroreceptor stimulation and renal denervation (17), have evolved for the treatment of hypertension. Perhaps because the parasympathetic nervous system is thought to play a minor role in blood pressure regulation compared with the sympathetic nervous system, little research has focused on targeting the reduced parasympathetic tone as a treatment strategy for hypertension, even though a decrease in parasympathetic nervous system activity has been documented in patients with hypertension (4). Furthermore, decreased heart rate variability, indicative of reduced parasympathetic modulation, is linked to hypertension-associated cardiovascular diseases, such as myocardial infarction (25), heart failure (23), stroke (8), and kidney disease (46). While increasing parasympathetic tone may have little effect on arterial blood pressure, it may still be effective in reducing hypertension-associated cardiovascular end-organ damage (1, 40). This possibility is particularly relevant, considering that hypertension-associated vascular complications such as coronary heart disease and stroke have a strong inflammatory component (3, 6, 13, 26, 32, 40, 44, 53, 57, 61, 66), and further considering that the parasympathetic nervous system has recently been recognized as an important modulator of inflammation (36, 39, 59–61).
Thus we hypothesized that chronic vagal nerve stimulation (VNS) will alleviate the progression of cardiovascular end-organ damage in severely hypertensive rats. As an experimental model, we used stroke-prone spontaneously hypertensive rats (SHR-SP) on a high-salt diet that develop severe hypertensive end-organ damage within a few weeks after initiation of the high-salt diet (11). To restore parasympathetic nervous system activity, we implanted electrical nerve stimulators to chronically stimulate the cervical vagus nerve. Progression of vascular and cardiac end-organ damage was assessed over a 4-wk observation period by in vivo imaging of the long posterior ciliary artery (LPCA) (56) and aorta and echocardiography, respectively. A secondary aim of the study was to investigate if chronic VNS modulates inflammatory function in this experimental model of severe hypertension. To test this possibility, we measured serum levels of pro- and anti-inflammatory cytokines in SHR-SP on high-salt diet after 4 wk of chronic VNS or sham stimulation.
METHODS
Animals.
Experiments were performed in male SHR-SP rats (SHRSP/A3NCrl, Charles River, Wilmington, MA). Rats were obtained at 10 wk of age, housed at standard housing conditions with controlled temperature and light periods (12:12-h light-dark cycle; light on between 6:00 AM and 6:00 PM). A standard rat chow (NIH-31 Modified Open Formula Mouse/Rat Sterilizable Diet) that contains 0.5% salt and drinking water were provided ad libitum. Starting with the onset of chronic VNS or sham stimulation, 1% NaCl was added to the drinking water. Experiments were approved by the Institutional Animal Care and Use Review Committee of the University of Iowa.
Surgical instrumentation.
All rats were instrumented with telemetric blood pressure sensors and vagal nerve stimulators. After isoflurane anesthesia (induction 5%, maintenance 2–3% in room air), the catheter of a telemetric blood pressure sensor (PA-C40, Data Sciences International, St. Paul, MN) was inserted into the right femoral artery and advanced into the abdominal aorta. The body of the implant was placed subcutaneously in the lower ventral abdominal area. The vagal nerve stimulators (model RNS, Harald Stauss Scientific, Iowa City, IA) were implanted during the same surgical session. The right cervical vagus nerve was separated from the common carotid artery, and bipolar coil electrodes (multistranded stainless steel wires) were wrapped around the nerve. The nerve-electrode preparation was embedded in silicone gel (Kwik-Sil, World Precision Instrument, Sarasota, FL) for electrical insulation and mechanical stability. The body of the vagal nerve stimulator, housing the battery and electronics, was placed subcutaneously on the left side of the thorax.
Experimental protocol.
Two to four days before surgical instrumentation, rats were familiarized with the procedure of in vivo imaging of the LPCA, and aortic strain and cardiac mass were determined by ultrasound imaging of the aorta and echocardiography, respectively. All data obtained before surgical instrumentation are denoted as “week 0” in results. Surgical instrumentations were performed on Thursdays or Fridays. The vagal nerve stimulators remained off (no stimulation) until Monday the following week, when the 4-wk observation period started. Before the vagal nerve stimulators were turned on for the first time, in vivo imaging of the LPCA, ultrasound imaging of the aorta, and echocardiography were performed. Thus these data (denoted “week 1” in results) were obtained before VNS was started and serve as baseline values. Then a high-salt diet was initiated by adding 1% sodium chloride (NaCl) to the drinking water, the vagal nerve stimulators were turned on in rats with VNS and left off in sham-stimulated animals, and a 24-h blood pressure and heart rate recording was obtained using the telemetric blood pressure sensors. These procedures (in vivo imaging of the LPCA, echocardiography, ultrasound imaging of the aorta, and 24-h blood pressure and heart rate monitoring) were repeated weekly for weeks 2, 3, and 4. Body weight was monitored three times a week. Intake of water and food was determined by weighing the water bottles and food containers three times a week (Mondays, Wednesdays, and Fridays) before and after refilling the water bottles and food containers. At the end of the 4-wk observation period, rats were euthanized by an overdose of isoflurane and serum, and organs were harvested.
Chronic VNS.
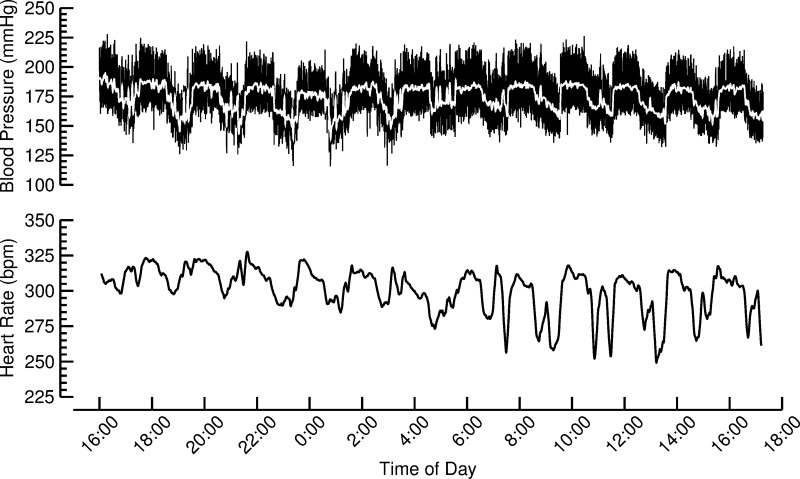
The vagal nerve stimulators can be turned on and off after implantation using an external magnet. The state of the stimulators (on or off) is indicated by a radio signal emitted from the implants every 75 s. The stimulation consisted of charge-balanced impulses of 1-ms duration, 3-V amplitude, and 5-Hz stimulation frequency. The stimulators were programmed for repetitive cycles of 1 h of stimulation followed by 1 h without stimulation. This stimulation protocol resulted in characteristic patterns of heart rate and blood pressure fluctuations with a cycle length of 2 h that became visible during the weekly 24-h blood pressure and heart rate recordings, as illustrated in Fig. 1. These fluctuations in heart rate and blood pressure were used to verify the biological responsiveness to the VNS. We stimulated the right cervical vagus nerve. With regard to a potential anti-inflammatory effect of chronic VNS, it is important to note that both the right and the left vagus nerve can elicit anti-inflammatory responses. Electrical stimulation of the peripheral end of the right cervical vagus nerve reduced the local inflammatory (edema) response to carrageenan injections into the hindpaw of rats (10). Furthermore, electrical stimulation of the left cervical vagus nerve prevented the increase in tumor necrosis factor (TNF)-α in a rat reperfusion injury model (7). In addition, both vagus nerves project to the celiac ganglion from where autonomic innervation of the spleen originates (Fig. 1 in Ref. 52).
Fig. 1.
Twenty-four-hour blood pressure and heart rate recording from a rat with VNS obtained during week 4 of the experimental protocol. The vagal nerve stimulators were programmed to turn on and off every hour, resulting in pronounced oscillations in arterial blood pressure and heart rate with a 2-h cycle length. Presence of such oscillations was used to verify the biological responsiveness to the VNS.
In vivo imaging of the LPCA.
In vivo imaging of the LPCA was performed in conscious animals as described previously (56). This technique allows assessing endothelial function and wall-to-lumen ratio (W/L ratio) of the LPCA of the iris. Briefly, the LPCA of the left eye was imaged through a slit-lamp biomicroscope (Model FS-2; Nikon Instruments, Melville, NY) after corneal application of a 1% solution of pilocarpine (Pilocarpine Hydrochloride Ophthalmic Solution 1%, Sandoz, Princeton, NJ), a muscarinic agonist that causes release of nitric oxide (NO) from vascular endothelial cells, and after corneal application of a 4% organic solvent-free aqueous solution (40 mg/ml) of Nω-nitro-l-arginine methyl ester hydrochloride (l-NAME, item no. 80210, Cayman Chemicals, Ann Arbor, MI) that blocks endothelial NO synthase (eNOS). The same segment of the LPCA was imaged for each pharmacological condition and all weekly imaging sessions. NO-dependent relaxation of the LPCA was calculated as the difference of the lumen diameter measured after pilocarpine application and after l-NAME application. The W/L ratio was estimated by the ratio of the cross-sectional areas of the vascular wall and lumen after vascular relaxation induced by pilocarpine. To calculate the cross-sectional areas of the vascular wall and lumen, the inner and outer diameters of the vessel were used to calculate the inner and outer cross-sectional areas, assuming a circular shape. The inner cross-sectional area corresponded to the lumen area and the difference between the outer and inner cross-sectional areas corresponded to the wall area.
Ultrasound imaging of the aorta and echocardiography.
Ultrasound imaging of the aorta and echocardiography were performed during the same anesthesia (1.0–1.5% isoflurane in room air) using a 7.5-MHz linear ultrasound probe attached to a commercial ultrasound diagnostic system (CMS600B3, Contec Medical Systems, Hebei, China). The time resolution of the ultrasound system was 31 images/s, which allowed imaging of the aorta and heart during systole and diastole. Starting from the cardiac long-axis view, the ultrasound probe was moved to obtain a clear view of the longitudinal axis of the ascending aorta, and a sequence of B- and M-mode images was obtained showing the systolic/diastolic distention and recoil of the aorta. Pulsatile aortic strain was calculated from the systolic (ASYS) and diastolic cross-sectional areas of the aorta (ADIA) using the equation: strain = (ASYS − ADIA)/ADIA. The elastic modulus (κ) was calculated as the ratio of aortic pulse pressure to aortic strain. Aortic distensibility was calculated as 1/κ, and aortic pulse-wave velocity (PWV) was calculated as the square root of the ratio of the elastic module κ and blood mass density (ρ, estimated as 1,060 kg/m3). These equations are based on the Moens-Korteweg equation (21, 28, 37). Since blood pressure was not monitored during ultrasound imaging, the average pulse pressure obtained during the 24-h blood pressure recording that started on the day of the ultrasound imaging was used for calculation of the elastic modulus κ, aortic distensibility, and aortic PWV. Cardiac mass (including the left and right ventricle) was determined from cardiac long-axis and short-axis images obtained during diastole. The average of cardiac mass values calculated by three different equations was used for final statistics. The three equations are based on modeling the heart as a sphere (based on the short-axis view only) or based on the truncated ellipsoid and area length equations provided by Lang et al. (30).
Determination of eNOS protein content in the aorta.
Proteins were isolated from the thoracic aortas through homogenization in a lysis buffer containing the following: 50 mM Tris·Cl pH 7.5, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM PMSF, 1 mM NaVO4, 10 mM NaF, 1 mM sodium pyrophosphate, 0.001% sodium deoxycholic acid (wt/vol), 1% NP-40 (vol/vol), 0.1% SDS (wt/vol), and a protease inhibitor cocktail tablet (Roche). Proteins were then subjected to SDS-PAGE and transferred to polyvinylidene fluoride membranes. Membranes were blocked with 5% nonfat dry milk, followed by incubation with primary antibodies for phospho-eNOS (Ser1177; 1:1,000; Cell Signaling, no. 9571) and total eNOS (1:1,000; BD Transduction Laboratories, no. 610297). Proteins were detected using anti-rabbit (1:5,000; Cell Signaling, no. 7074) or anti-mouse horseradish peroxidase (1:10,000; Abcam no. ab97040) conjugated secondary antibodies. Protein expression was visualized by an ECL Prime chemiluminescent detection kit on film, and densitometry was calculated using ImageJ.
Serum levels of pro- and anti-inflammatory cytokines.
At the time of euthanasia, serum samples were obtained that were used for enzyme-linked immunosorbent assays (ELISA) using commercially available ELISA kits (all from R&D Systems, Minneapolis, MN) to determine the serum concentrations of the proinflammatory cytokines TNF-α (RTA00 kit), interleukin (IL)-1β (RLB00 kit), and IL-6 (R6000B kit), as well as the anti-inflammatory cytokine IL-10 (R1000 kit). All assays were conducted according to the manufacturer's instructions.
Statistics.
All data are presented as means ± SE. Longitudinal data (body weight; food and NaCl intake; heart rate and blood pressure; lumen diameter; NO-dependent relaxation; W/L ratio of the LPCA, aortic strain, distensibility, and PWV; and echocardiographically determined cardiac mass) were analyzed using two-way ANOVA with one repeated (weeks) and one independent (sham vs. VNS or pilocarpine vs. l-NAME for lumen diameter of the LPCA) factor. In case of significance (P < 0.05), post hoc Fisher tests were performed to evaluate time effects and post hoc t-tests were performed to evaluate group or drug effects. End-point measures (cardiac and left and right ventricular masses, cytokine serum concentrations, and aortic eNOS protein content) were analyzed by t-tests for independent measures (sham vs. VNS). Pearson linear correlation analyses were performed to study a potential relationship between cytokine serum levels and NO-dependent relaxation of the LPCA, aortic eNOS protein content and aortic strain, distensibility, and PWV.
RESULTS
Body weight and food and salt intake.
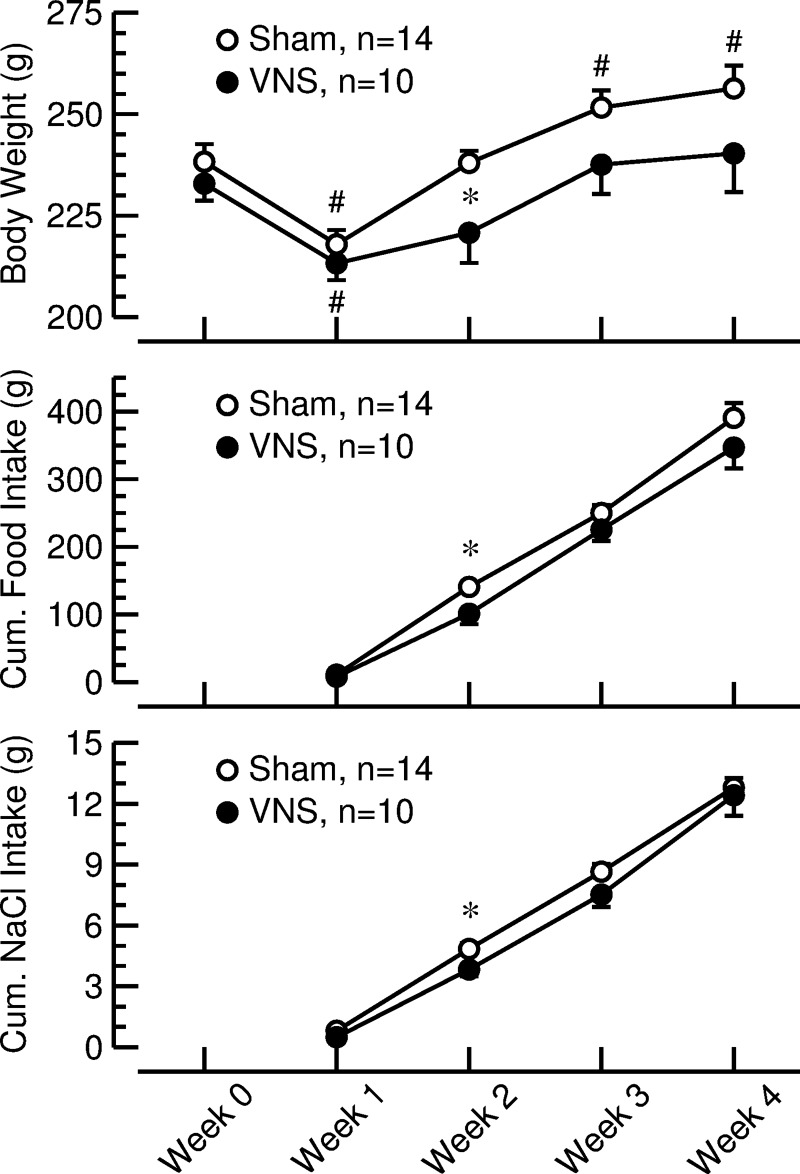
Body weight decreased in both groups of rats following surgical instrumentation (VNS: −19.7 ± 3.7 g, P < 0.05; sham: −20.4 ± 2.9 g, P < 0.05) and returned to presurgical values in rats with VNS but exceeded presurgical weights in rats with sham stimulation (Fig. 2, top). The reduced weight gain in rats with VNS appears to be at least partly mediated by reduced food intake, because 2 wk into the protocol, both body weight (Fig. 2, top) and cumulative food intake (Fig. 2, middle) were significantly less in rats with VNS than in sham-stimulated animals. Cumulative salt intake (sum of salt intake through drinking water and dry food) (Fig. 2, bottom) was similar in both groups, except at week 2, where salt intake was slightly lower in rats with VNS (3.8 ± 0.3 g) than in sham-stimulated animals (4.8 ± 0.3 g, P < 0.05 vs. VNS).
Fig. 2.
Body weight (top) and cumulative food (middle) and salt (NaCl) intake (bottom) in rats with sham stimulation (sham; ○) or vagal nerve stimulation (VNS; ●). Body weight values at week 0 were obtained before anesthesia on the day of surgical instrumentation. Values for weeks 1–4 were obtained after 1, 2, 3, or 4 wk of VNS or sham stimulation. Cumulative NaCl intake includes NaCl consumed with solid food and drinking water. Values are means ± SE. *P < 0.05, sham vs. VNS. #P < 0.05 vs. week 0.
Heart rate and blood pressure.
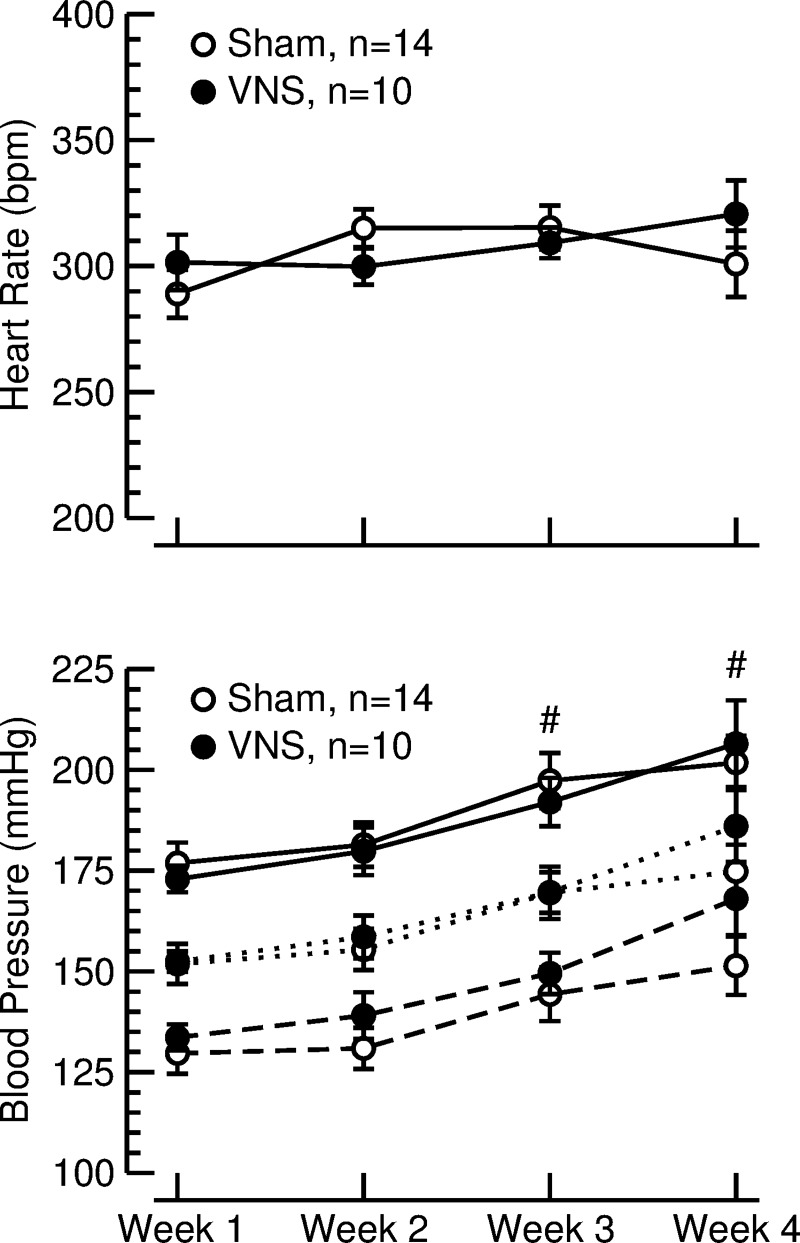
Weekly 24-h recordings of arterial blood pressure and heart rate were obtained by radio telemetry (Fig. 1). The blood pressure and heart rate data from the 24-h recordings were averaged and thus include day- and night-time values and equal periods with and without VNS in the stimulated animals. Twenty-four-hour average heart rate values (Fig. 3, top) remained constant at ∼300 beats/min throughout the 4-wk exposure to high-salt diet and did not differ between rats with VNS and sham-stimulated animals. During the 4 wk of high-salt diet, 24-h average values for blood pressure continuously increased from 173 ± 3 and 177 ± 5 mmHg (systolic, VNS vs. sham, P = 0.52) and 134 ± 3 and 130 ± 5 mmHg (diastolic, VNS vs. sham, P = 0.51) at week 1 to 206 ± 11 and 202 ± 7 mmHg (systolic, VNS vs. sham, P = 0.70) and 168 ± 9 and 151 ± 7 mmHg (diastolic, VNS vs. sham, P = 0.16) at week 4 (Fig. 3, bottom).
Fig. 3.
Heart rate (top) and systolic (solid lines), mean (dotted lines), and diastolic (dashed line) blood pressure (bottom), during 4 wk of sham stimulation (sham; ○) or vagal nerve stimulation (VNS; ●). Values are averages of weekly 24-h recordings (means ± SE). #P < 0.05 vs. week 1 for systolic, mean, and diastolic blood pressure in both groups.
NO-dependent relaxation of the LPCA.
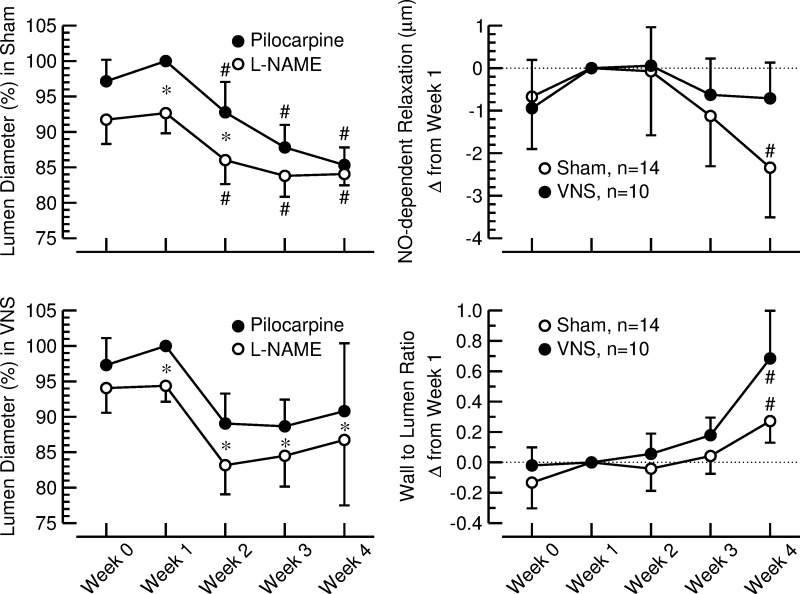
In general (after application of both pilocarpine and l-NAME), lumen diameter of the LPCA decreased during the 4 wk of high-salt diet (Fig. 4, left, top and bottom). The distance between the curves representing the lumen diameters after pilocarpine and l-NAME converged at weeks 3 and 4 in sham-stimulated animals (Fig. 4, left, top). In contrast, in rats with VNS, the lumen diameters after pilocarpine and l-NAME remained significantly different throughout the 4-wk high-salt diet (Fig. 4, left, bottom). In sham-stimulated rats, NO-dependent relaxation assessed by the difference in lumen diameter of the LPCA after application of pilocarpine and l-NAME (Fig. 4, right, top) progressively declined over the 4 wk of high-salt diet and was significantly less at the end (0.51 ± 0.62 μm) compared with the beginning of the protocol (2.85 ± 1.11 μm, P < 0.05). In contrast, in rats with VNS, NO-dependent relaxation was maintained throughout the experimental protocol (2.12 ± 0.84 μm at week 1 vs. 1.41 ± 0.51 μm at week 4, nonsignificant).
Fig. 4.
Left: lumen diameter of the long posterior ciliary artery (LPCA) after corneal application of a 1% pilocarpine solution (●) and after corneal application of a 4% l-NAME solution (○). Values are expressed as a percentage of the values measured after pilocarpine application at week 1 when stimulators were off (means ± SE). Top, sham-stimulated animals (n = 14). Bottom, rats with VNS (n = 10). *P < 0.05, pilocarpine vs. l-NAME. #P < 0.05 vs. week 1. Right: nitric oxide (NO)-dependent relaxation (top) and wall-to-lumen ratio (bottom) of the LPCA assessed by in vivo imaging in sham-stimulated rats (sham; ○) and animals with vagal nerve stimulation (VNS; ●). Values are changes from week 1 (means ± SE). #P < 0.05 vs. week 1.
W/L ratio of the LPCA.
W/L ratio of the LPCA (Fig. 4, right, bottom) increased over the 4-wk observation period from 1.76 ± 0.15 (sham) and 1.59 ± 0.12 (VNS) at the beginning of the protocol to 2.03 ± 0.15 (sham, P < 0.05 vs. week 1) and 2.28 ± 0.31 (VNS, P < 0.05 vs. week 1) at the end of the protocol, with no significant differences between sham-stimulated rats and animals with VNS.
eNOS protein content in the thoracic aorta.
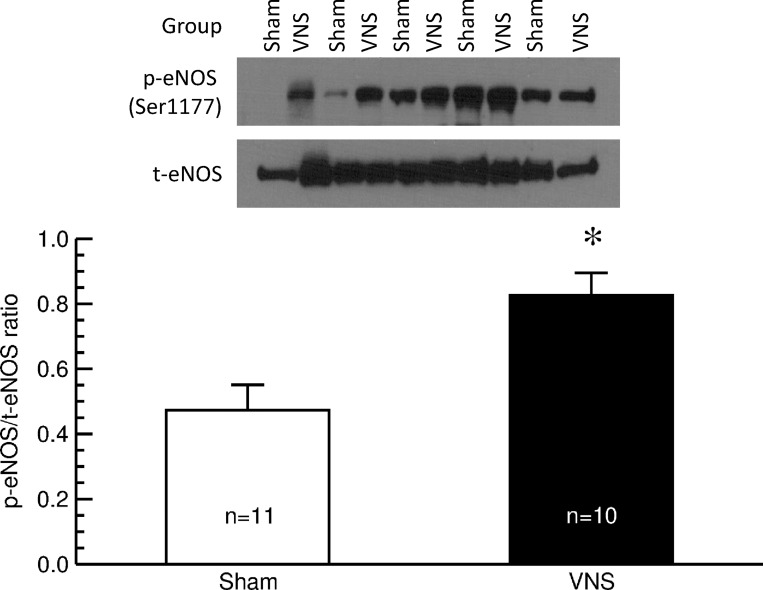
See Fig. 5. After 4 wk of VNS or sham stimulation, the ratio of phosphorylated (active) eNOS to total eNOS protein content was significantly greater in thoracic aortas from rats with VNS than in those from sham-stimulated animals (sham: 0.47 ± 0.08 vs VNS: 0.83 ± 0.07; P < 0.05), confirming the results obtained by in vivo imaging of the LPCA and suggesting that VNS improves endothelial vascular function by preventing the decline in endogenous NO synthesis associated with development of fulminant hypertension in this animal model.
Fig. 5.
Western blot analysis of phosphorylated (p) and total (t) endothelial nitric oxide synthase (eNOS) in the thoracic aorta of sham-stimulated rats (sham; n = 11) and animals with vagal nerve stimulation (VNS; n = 10). Top: original Western blot. Bottom: ratio of p-eNOS to t-eNOS protein content in thoracic aortas. Values are means ± SE. *P < 0.05, sham vs. VNS.
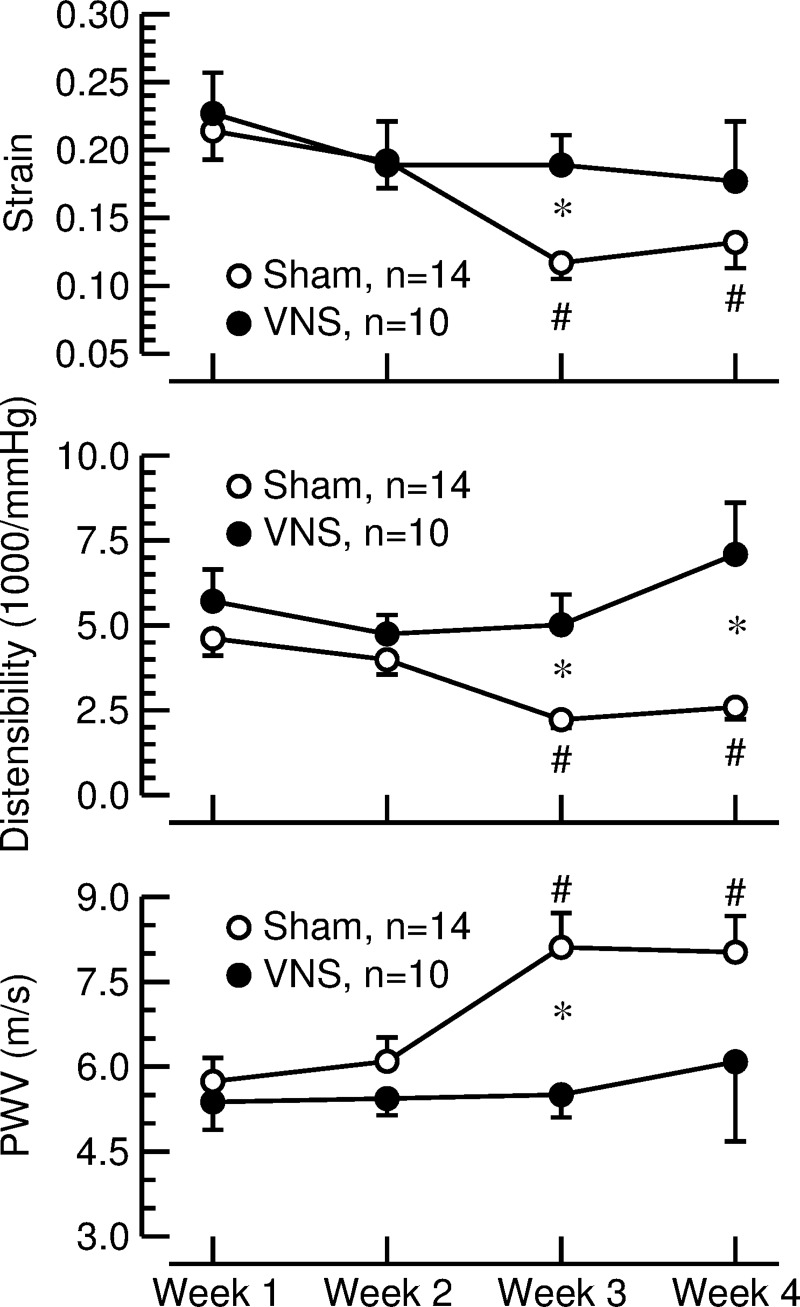
Aortic strain, distensibility, and PWV.
See Fig. 6. In sham-stimulated animals, aortic strain and distensibility progressively declined, and aortic PWV increased over the 4-wk observation period, indicating increased stiffening of the aorta. In contrast, aortic strain, distensibility, and PWV did not change significantly during the observation period in rats with VNS. As a result, aortic strain, distensibility, and PWV differed significantly between sham-stimulated rats and animals with VNS at week 3 of the protocol. Aortic distensibility also differed between groups at week 4 of the protocol.
Fig. 6.
Aortic strain (top), distensibility (middle), and pulse-wave velocity (PWV; bottom) calculated from measurements of aorta diameter (ultrasound imaging) and aortic pulse pressure (telemetry) in sham-stimulated rats (sham; ○) and animals subjected to vagal nerve stimulation (VNS; ●). Values are means ± SE. *P < 0.05 sham vs. VNS. #P < 0.05 vs. week 1.
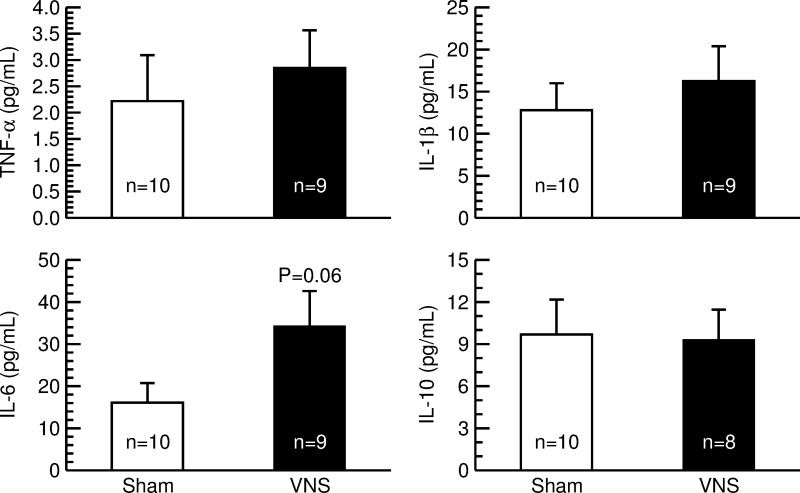
Serum concentrations of cytokines.
No significant differences between rats with sham stimulation and animals with VNS were found for the proinflammatory cytokines TNF-α and IL-1β or the anti-inflammatory cytokine IL-10. However, serum concentrations of IL-6 tended (P = 0.06) to be higher in rats with VNS than in animals with sham stimulation (Fig. 7).
Fig. 7.
Serum concentrations of the cytokines tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6), and interleukin-10 (IL-10) in sham-stimulated rats (sham; n = 10; open bars) and animals with vagal nerve stimulation (VNS; n = 9 for TNF-α, IL-1β, and IL-6; n = 8 for IL-10; solid bars). Serum levels of cytokines that were below the detection threshold of the ELISA assay were assumed to be 0 pg/ml. Values are means ± SE.
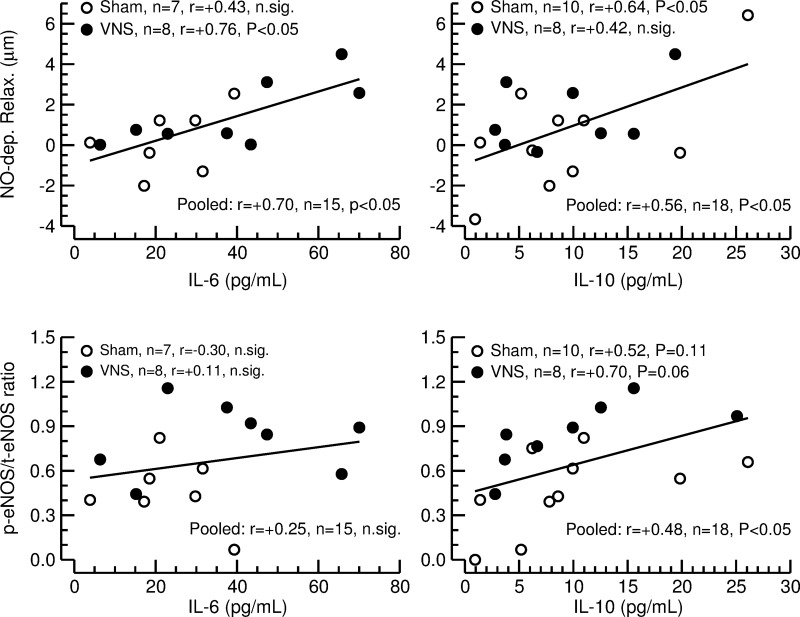
Correlations of serum cytokine concentrations with vascular parameters.
See Fig. 8. No significant correlations between measures of aortic stiffness (strain, distensibility, and PWV) and cytokine serum levels were detected. However, significant correlations were found for NO-dependent relaxation of the LPCA and the cytokines IL-6 and IL-10 (data from sham and VNS pooled), such that higher serum levels of these two cytokines were associated with stronger NO-dependent relaxation. A significant positive correlation was also found between activated (phosphorylated) eNOS protein content in the thoracic aorta and serum levels of the anti-inflammatory cytokine IL-10, but not for IL-6 (data from sham and VNS pooled). Subgroup analyses showed that the correlation for NO-dependent relaxation of the LPCA with IL-6 was significant for animals with VNS, and the correlation with IL-10 was significant for rats with sham stimulation. However, the statistical power of the subgroup analysis is rather low, and the correlation coefficients for the subgroup analyses did not differ significantly between rats with VNS and sham-stimulated animals.
Fig. 8.
Linear correlation analysis between nitric oxide-dependent relaxation (NO-dep. Relax.) of the long posterior ciliary artery (top) and ratio of p-eNOS to t-eNOS protein content in the thoracic aorta (bottom) and serum concentrations of interleukin-6 (IL-6; left) or interleukin-10 (IL-10; right) in sham-stimulated rats (sham; ○) and animals with vagal nerve stimulation (VNS; ●). Linear correlation lines are for the correlations, with the data from sham and VNS pooled. Animals in which IL-6 serum levels were below the detection threshold of the ELISA assay (3 animals in the sham group and 1 animal in the VNS group) were omitted from the correlation analysis.
Cardiac mass.
Cardiac mass was determined weekly by echocardiography and by weighing the heart and ventricles after euthanasia (Table 1). During the 4-wk exposure to high-salt diet, cardiac mass (determined by echocardiography) increased progressively with no significant differences between animals with sham stimulation and rats with VNS (data not shown). This increase in absolute cardiac mass was related to the increase in body weight, since cardiac mass index (cardiac mass-to-body weight ratio) did not change during the 4-wk protocol. At the end of the protocol, absolute and relative (normalized for body weight) cardiac masses and left and right ventricular masses (determined by organ weight) did not differ between rats with sham stimulation and animals with VNS (Table 1).
Table 1.
Cardiac and left and right ventricular masses
| Absolute Mass, mg |
Relative Mass, mg/100 g body wt |
|||
|---|---|---|---|---|
| Organ | Sham | VNS | Sham | VNS |
| n | 12 | 10 | 12 | 10 |
| Cardiac mass | 1,057 ± 27 | 1,039 ± 26 | 410 ± 11 | 442 ± 13 |
| Left ventricle | 872 ± 24 | 846 ± 30 | 338 ± 10 | 358 ± 9 |
| Right ventricle | 174 ± 6 | 173 ± 14 | 68 ± 3 | 74 ± 7 |
Values are means ± SE; n, no. of rats. Cardiac and left and right ventricular masses were determined by weighing after euthanasia. Cardiac mass corresponds to the mass of the whole heart, from which the atrial appendages were dissected off.
DISCUSSION
The most important finding of this study is that chronic VNS significantly slows progression of endothelial dysfunction and aortic stiffening in an animal model of a severe and accelerated form of hypertension. Importantly, 24-h average values of arterial blood pressure were not affected by chronic VNS, suggesting that the beneficial vascular effects of chronic VNS are apparent even without lowering arterial blood pressure. Furthermore, the trend (P = 0.06) toward higher IL-6 serum levels in animals with VNS compared with sham-stimulated animals, together with the significant correlations between NO-dependent relaxation of the LPCA and IL-6 and IL-10 serum levels and between phosphorylated (activated) eNOS content in the aorta and IL-10 serum levels, may potentially suggest that chronic VNS modulates inflammation in this experimental model of severe hypertension.
We investigated the effects of chronic VNS on vascular and cardiac parameters. Interestingly, chronic VNS only affected endothelial function and aortic stiffening, but not cardiac or vascular hypertrophy. Specifically, the progressive decline in NO-dependent relaxation of the LPCA observed in sham-stimulated SHR-SP on high-salt diet was prevented in rats with VNS (Fig. 4, right, top), and this improved endothelial function was associated with greater phosphorylated (activated) eNOS protein level in thoracic aortas (Fig. 5). Similarly, aortic stiffening that was apparent 3 and 4 wk after high-salt diet in SHR-SP with sham stimulation was prevented in animals with VNS (Fig. 6). However, vascular hypertrophy assessed by the W/L ratio of the LPCA increased similarly in both groups of rats (Fig. 4, right, bottom), and cardiac mass determined by echocardiography or weighing (Table 1) did not differ significantly between sham-stimulated rats and animals with VNS. It is possible that cardiac and vascular hypertrophy were not affected by VNS, because these parameters may primarily be driven by the level of arterial blood pressure (27) that was not affected by VNS. Since arterial blood pressure was not significantly different in sham-stimulated rats and animals with VNS, it is unlikely that the beneficial effects of VNS on endothelial vascular function and aortic stiffening are secondary to the accelerated hypertension in this animal model (e.g., pressure-induced increase in blood flow leading to increased flow-mediated endothelial NO release or other compensatory vasodilator mechanisms). On the other hand, atherosclerosis, which is the underlying cause of endothelial dysfunction and aortic stiffness, have both been linked to inflammation (33, 47, 62, 65), which is modulated by VNS (36, 39, 40, 59–61). Thus modulation of inflammation may be involved in the mechanisms responsible for the beneficial effects of VNS on measures of endothelial function and aortic stiffness in our study.
To test if chronic VNS in this experimental model of hypertension modulates inflammatory function, we measured serum levels of pro- and anti-inflammatory cytokines and found a strong trend (P = 0.06) for increased IL-6 serum levels in rats with VNS compared with sham-stimulated animals (Fig. 7). This trend is in line with data from our laboratory's previous report (19). In contrast to the powerful anti-inflammatory effect of activating cholinergic nicotinic receptors in normotensive WKY rats, activation of nicotinic receptors markedly enhanced the release of IL-6 induced by ligands of toll-like receptors-7, -8, and -9 in SHR, both from isolated splenocytes and in vivo (19). Thus it is possible that chronic VNS in our study in SHR-SP induced release of IL-6 from macrophages in the spleen through stimulation of nicotinic acetylcholine receptors.
While the trend for higher IL-6 serum levels in animals with VNS compared with sham-stimulated rats suggests a modulatory role of chronic VNS on inflammation in this experimental model of fulminant hypertension, further studies are needed to investigate a possible direct mechanistic link between VNS-induced cardiovascular end-organ damage and VNS-induced modulation of inflammatory function and potential pathways involved. With this regard, the significant positive correlations between NO-dependent relaxation of the LPCA and serum levels of IL-6 and IL-10, and between aortic phosphorylated (activated) eNOS protein content and IL-10 serum levels, indicating that higher serum levels of IL-6 and IL-10 were associated with better endothelial function (Fig. 8), deserve some discussion. IL-10 is generally considered as an anti-inflammatory cytokine (49). Thus the positive correlations between serum levels of IL-10 and NO-dependent relaxation of the LPCA and phosphorylated (activated) eNOS content in the aorta may indicate that IL-10 is endogenously generated in response to chronic inflammation in SHR-SP on high-salt diet and limits the detrimental effect of inflammation on endothelial function. The lack of differences in IL-10 serum levels in animals with VNS compared with sham-stimulated animals suggests that VNS does not alter the endogenous IL-10 response to chronic inflammation in SHR-SP on high-salt diet. In contrast, IL-6 is generally considered as a cytokine that mediates proinflammatory responses via the soluble IL-6 receptor trans-signaling pathway, but can also elicit protective or regenerative responses through the membrane bound (classical) IL-6 receptor pathway (50, 51, 58, 64). Our finding of a positive correlation between IL-6 serum levels and NO-dependent relaxation of the LPCA are in contrast to what was reported by the Multi-Ethnic Study on Atherosclerosis (62) that found an inverse correlation between IL-6 levels and flow-mediated dilation of the brachial artery in a multiethnic cohort of 3,501 participants. The authors of that study concluded that increased levels of IL-6 are associated with impaired endothelial function, and that elevated IL-6 levels may reflect a state that promotes vascular inflammation. However, the trend (P = 0.06) to higher IL-6 serum levels in animals with VNS compared with sham-stimulated rats in our study suggests that chronic VNS may increase IL-6 serum levels beyond the levels induced by chronic inflammation in SHR-SP on high-salt diet, and that these high IL-6 levels may shift the balance from the proinflammatory trans-signaling pathway to the anti-inflammatory membrane bound (classical) pathway and thus contribute to the beneficial effect of VNS on endothelial function. Without VNS, the induction of IL-6 by chronic inflammation in SHR-SP on high-salt diet may not be sufficient to shift the balance from the pro- to the anti-inflammatory pathway.
Another potentially important finding of this study relates to the effect of VNS on body weight regain following surgical instrumentation. Initially, both groups of rats lost weight after surgical implantation of the telemetric blood pressure sensors and vagal nerve stimulators. By the end of the protocol, rats with VNS had returned to their original, presurgical level, while body weight in sham-stimulated animals significantly exceeded the presurgical level (Fig. 2, top). Reduced food intake during the postsurgical period may have contributed to the reduced weight gain in rats with VNS, since their cumulative food intake was significantly less at week 2 of the protocol compared with sham-stimulated animals (Fig. 2, middle). However, we cannot rule out the possibility that other mechanisms, such as increased metabolic rate or reduced intestinal absorption, also contributed to the reduced weight gain in rats with VNS. Our finding of reduced weight gain in SHR-SP with VNS is in line with clinical observations that patients treated with chronic VNS for therapy-refractory major depression or epilepsy lose body weight (2, 42). Since obesity constitutes an important risk factor for hypertension and cardiovascular end-organ damage, including atherosclerosis and endothelial dysfunction (22), VNS may elicit additional protective effects on cardiovascular end-organ damage in obese hypertensive patients through its effects on body weight. As a consequence, VNS has been suggested as a potential treatment for human obesity (38).
An alternative explanation for the reduced weight gain in SHR-SP with VNS may relate to an increase in renal sodium excretion, leading to a reduction in total body water. Since VNS as performed in this study is likely to activate both efferent and afferent fibers within the vagus nerve, it is possible, or even likely, that afferent cardiopulmonary fibers that are part of the so-called venoatrial stretch reflex were also activated. Activation of vagal afferent fibers results in a reflex decrease in renal sympathetic nerve activity (63), leading to a reduction in renal sodium reabsorption and, consequently, increased sodium and water excretion. Interestingly, sodium intake is positively correlated with aortic stiffening (5, 48) and endothelial dysfunction (15, 29). Thus it is possible that increased renal sodium excretion in response to afferent VNS contributed to the improved vascular function in SHR-SP with VNS, especially when considering that the animals were on a high-salt diet. However, further studies are needed to investigate this possibility.
The vagal nerve stimulators were programmed to turn on and off every hour. This stimulation protocol induced fluctuations in heart rate and arterial blood pressure at a 2-h cycle (see Fig. 1). It is possible that this increased cardiovascular variability contributed to improved endothelial vascular function and reduced aortic stiffening observed with VNS in our study. Increased heart rate variability has been associated with improved endothelial vascular function (43) and with reduced aortic stiffening assessed by carotid-femoral PWV (31). However, whether these beneficial vascular effects are directly caused by increased heart rate variability is not known. The increased heart rate variability may result from increased vagal tone, which may improve vascular function independent of heart rate variability, possibly through modulation of inflammation. VNS also induced fluctuations in arterial blood pressure at a 2-h cycle (see Fig. 1). In contrast to heart rate variability, high blood pressure variability has been associated with impaired vascular endothelial function (14, 20) and increased aortic stiffening (41, 55). Despite the VNS-induced increase in blood pressure variability, we found that VNS prevented deterioration of endothelial vascular function and aortic stiffening. Thus a potentially detrimental effect of increased blood pressure variability on vascular endothelial function and aortic stiffening in our study appears to be relatively weak compared with the overall beneficial vascular effects of VNS.
Some limitations of this study need to be considered. First, the validity of SHR-SP on high-salt diet as a model for human hypertension may be questioned due to accelerated development of hypertension and cardiovascular end-organ damage in this animal model compared with human hypertension. However, we specifically selected this animal model because of the rapid progression of fulminant hypertension and cardiovascular end-organ damage. Chronic VNS in rodents is technically challenging, and in our hands chronic VNS is feasible for up to 4 wk in rats. Thereafter, the biological responsiveness to VNS assessed by the heart rate response (as illustrated in Fig. 1) starts to decline, and the batteries of the stimulators start to lose power. Thus selection of an animal model with fulminant hypertension and rapidly progressing cardiovascular end-organ damage was essential for the success of this study. Second, endothelial function was assessed by in vivo imaging of the LPCA in conscious rats that requires manually positioning the animals in front of the lens of the slit-lamp biomicroscope. This procedure may impose a certain level of stress to the animals. To reduce the impact of experimental stress on our results, we familiarized all animals with this procedure before the surgical instrumentation (week 0 values in Fig. 4). The trend toward greater NO-dependent relaxation at week 1 compared with week 0 in both groups of rats may indicate reduced experimental stress at the second imaging session (week 1) compared with the first imaging session (week 0). In addition, we were able to document the expected decline in endothelial function in sham-stimulated rats and, therefore, feel confident that in vivo imaging of the LPCA is a reliable tool to assess endothelial function in conscious rats. Finally, although cumulative NaCl intake was the same at the end of the protocol, it was significantly lower in rats with VNS than in sham-stimulated animals at week 2 (Fig. 2). Furthermore, we did not assess renal sodium excretion. Thus we cannot rule out the possibility that lower NaCl intake at week 2 or possible changes in renal sodium excretion may have contributed to the improved vascular function in animals with VNS.
In conclusion, the results of our study demonstrate that chronic VNS slows the progression of hypertension-associated endothelial dysfunction and aortic stiffening in an experimental model of fulminant hypertension without affecting mean arterial blood pressure. Thus VNS may potentially be considered as an adjunct treatment to mainstream antihypertensive therapy. In addition, the trend (P = 0.06) toward higher serum levels of IL-6 in rats with VNS compared with sham-stimulated animals, together with the significant correlations between endothelial function and serum levels of IL-6 and IL-10, may potentially be seen as indication that chronic VNS modulates inflammatory function in this experimental model of severe hypertension. Future studies are needed to investigate the possibility of a mechanistic link between the protective vascular effects of chronic VNS and its effects on inflammation.
Perspectives.
While this study demonstrates that chronic VNS slows the progression of endothelial dysfunction and aortic stiffening in an animal model of severe hypertension, the mechanisms of this protective effect of VNS remain to be determined. Our data on serum levels of cytokines and their correlations with endothelial function suggest a potential role of cholinergic anti-inflammatory pathways. Based on the increased activation of aortic eNOS observed in the animals with VNS, one may speculate that vascular inflammation, which is known to develop in SHR-SP (9, 24), may inhibit eNOS activation, and that a potential anti-inflammatory effect of VNS may prevent this inhibition of eNOS activation. Thus future studies on the effect of VNS on vascular inflammation and its relation to eNOS activation are warranted. Another important aspect that deserves further investigation relates to the possible contribution of activation of vagal afferent nerves to the beneficial responses to VNS demonstrated in the present study. As outlined in the discussion above, activation of vagal afferents has important effects on renal sodium excretion that may impact on vascular responses. While there are limitations in translating these experimental data obtained in an animal model of hypertension to hypertensive patients, it appears important to explore if chronic VNS used as treatment for major depression or epilepsy elicits anti-inflammatory effects or alters vascular parameters, such as flow-mediated dilation or aortic PWV.
GRANTS
This study was supported by a pilot grant from the University of Iowa Hospitals and Clinics Center for Hypertension Research. K. Rahmouni was also supported by funds from the National Heart, Lung, and Blood Institute (NHLBI) (HL-084207), the American Heart Association (14EIA18860041), and The University of Iowa Fraternal Order of Eagles Diabetes Research Center. M. W. Chapleau was supported by funds from the NHLBI (HL-14388) and the US Department of Veterans Affairs (1BX001414).
DISCLOSURES
H. M. Stauss is the founder of Harald Stauss Scientific. The vagal nerve stimulators used in this study are marketed by this company.
AUTHOR CONTRIBUTIONS
M.W.C., K.R., and H.M.S. conception and design of research; M.W.C., D.L.R., J.J.R., K.R., and H.M.S. interpreted results of experiments; M.W.C., D.L.R., J.J.R., K.R., and H.M.S. edited and revised manuscript; M.W.C., D.L.R., J.J.R., K.R., and H.M.S. approved final version of manuscript; D.L.R., J.J.R., and H.M.S. performed experiments; J.J.R. and H.M.S. analyzed data; J.J.R. and H.M.S. prepared figures; H.M.S. drafted manuscript.
REFERENCES
- 1.Abboud FM, Harwani SC, Chapleau MW. Autonomic neural regulation of the immune system: implications for hypertension and cardiovascular disease. Hypertension 59: 755–762, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abubakr A, Wambacq I. Long-term outcome of vagus nerve stimulation therapy in patients with refractory epilepsy. J Clin Neurosci 15: 127–129, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Ahmad M, Dar NJ, Bhat ZS, Hussain A, Shah A, Liu H, Graham SH. Inflammation in ischemic stroke: mechanisms, consequences and possible drug targets. CNS Neurol Disord Drug Targets 13: 1378–1396, 2014. [DOI] [PubMed] [Google Scholar]
- 4.Amerena J, Julius S. The role of the autonomic nervous system in hypertension. Hypertens Res 18: 99–110, 1995. [DOI] [PubMed] [Google Scholar]
- 5.Avolio AP, Deng FQ, Li WQ, Luo YF, Huang ZD, Xing LF, O'Rourke MF. Effects of aging on arterial distensibility in populations with high and low prevalence of hypertension: comparison between urban and rural communities in China. Circulation 71: 202–210, 1985. [DOI] [PubMed] [Google Scholar]
- 6.Back M, Hansson GK. Anti-inflammatory therapies for atherosclerosis. Nat Rev Cardiol 12: 199–211, 2015. [DOI] [PubMed] [Google Scholar]
- 7.Bernik TR, Friedman SG, Ochani M, DiRaimo R, Susarla S, Czura CJ, Tracey KJ. Cholinergic antiinflammatory pathway inhibition of tumor necrosis factor during ischemia reperfusion. J Vasc Surg 36: 1231–1236, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Binici Z, Mouridsen MR, Kober L, Sajadieh A. Decreased nighttime heart rate variability is associated with increased stroke risk. Stroke 42: 3196–3201, 2011. [DOI] [PubMed] [Google Scholar]
- 9.Bomfim GF, Echem C, Martins CB, Costa TJ, Sartoretto SM, Dos Santos RA, Oliveira MA, Akamine EH, Fortes ZB, Tostes RC, Webb RC, Carvalho MH. Toll-like receptor 4 inhibition reduces vascular inflammation in spontaneously hypertensive rats. Life Sci 122: 1–7, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borovikova LV, Ivanova S, Nardi D, Zhang M, Yang H, Ombrellino M, Tracey KJ. Role of vagus nerve signaling in CNI-1493-mediated suppression of acute inflammation. Auton Neurosci 85: 141–147, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Camargo MJ, von Lutterotti N, Campbell WG Jr, Pecker MS, James GD, Timmermans PB, Laragh JH. Control of blood pressure and end-organ damage in maturing salt-loaded stroke-prone spontaneously hypertensive rats by oral angiotensin II receptor blockade. J Hypertens 11: 31–40, 1993. [DOI] [PubMed] [Google Scholar]
- 12.de Champlain J, Karas M, Toal C, Nadeau R, Larochelle P. Effects of antihypertensive therapies on the sympathetic nervous system. Can J Cardiol 15, Suppl A: 8a–14a, 1999. [PubMed] [Google Scholar]
- 13.Di Napoli M, Papa F. Inflammation, blood pressure, and stroke: an opportunity to target primary prevention? Curr Hypertens Rep 7: 44–51, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Diaz KM, Veerabhadrappa P, Kashem MA, Thakkar SR, Feairheller DL, Sturgeon KM, Ling C, Williamson ST, Kretzschmar J, Lee H, Grimm H, Babbitt DM, Vin C, Fan X, Crabbe DL, Brown MD. Visit-to-visit and 24-h blood pressure variability: association with endothelial and smooth muscle function in African Americans. J Hum Hypertens 27: 671–677, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DuPont JJ, Greaney JL, Wenner MM, Lennon-Edwards SL, Sanders PW, Farquhar WB, Edwards DG. High dietary sodium intake impairs endothelium-dependent dilation in healthy salt-resistant humans. J Hypertens 31: 530–536, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franco OH, Peeters A, Bonneux L, de Laet C. Blood pressure in adulthood and life expectancy with cardiovascular disease in men and women: life course analysis. Hypertension 46: 280–286, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Grassi G, Mark A, Esler M. The sympathetic nervous system alterations in human hypertension. Circ Res 116: 976–990, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grassi G, Seravalle G, Mancia G. Sympathetic activation in cardiovascular disease: evidence, clinical impact and therapeutic implications. Eur J Clin Invest 45: 1367–1375, 2015. [DOI] [PubMed] [Google Scholar]
- 19.Harwani SC, Chapleau MW, Legge KL, Ballas ZK, Abboud FM. Neurohormonal modulation of the innate immune system is proinflammatory in the prehypertensive spontaneously hypertensive rat, a genetic model of essential hypertension. Circ Res 111: 1190–1197, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hodgson JM, Woodman RJ, Croft KD, Ward NC, Bondonno CP, Puddey IB, Lukoshkova EV, Head GA. Relationships of vascular function with measures of ambulatory blood pressure variation. Atherosclerosis 233: 48–54, 2014. [DOI] [PubMed] [Google Scholar]
- 21.Holtz J. Peripheral circulation: fundamental concepts, comparative aspects of control in specific vascular sections, and lymph flow. In: Comprehensive Human Physiology, edited by Greger R and Windhorst U. Berlin: Springer-Verlag, 1996, p. 1865–1915. [Google Scholar]
- 22.Iantorno M, Campia U, Di Daniele N, Nistico S, Forleo GB, Cardillo C, Tesauro M. Obesity, inflammation and endothelial dysfunction. J Biol Regul Homeost Agents 28: 169–176, 2014. [PubMed] [Google Scholar]
- 23.Jiang W, Hathaway WR, McNulty S, Larsen RL, Hansley KL, Zhang Y, O'Connor CM. Ability of heart rate variability to predict prognosis in patients with advanced congestive heart failure. Am J Cardiol 80: 808–811, 1997. [DOI] [PubMed] [Google Scholar]
- 24.Kim HY, Kim HS. IL-10 up-regulates CCL5 expression in vascular smooth muscle cells from spontaneously hypertensive rats. Cytokine 68: 40–49, 2014. [DOI] [PubMed] [Google Scholar]
- 25.Kleiger RE, Miller JP, Bigger JT Jr, Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol 59: 256–262, 1987. [DOI] [PubMed] [Google Scholar]
- 26.Koenig W. Inflammation and coronary heart disease: an overview. Cardiol Rev 9: 31–35, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Korner PI. Cardiovascular hypertrophy and hypertension: causes and consequences. Blood Press Suppl 2: 6–16, 1995. [PubMed] [Google Scholar]
- 28.Korteweg D. Über Die Fortpflanzungsgeschwindigkeit Des Schalles in Elastischen Röhren. Annalen der Physik und Chemie 5: 525–537, 1878. [Google Scholar]
- 29.Kusche-Vihrog K, Schmitz B, Brand E. Salt controls endothelial and vascular phenotype. Pflügers Arch 467: 499–512, 2015. [DOI] [PubMed] [Google Scholar]
- 30.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise J, Solomon S, Spencer KT, St. John Sutton M, Stewart W. Recommendations for chamber quantification. Eur J Echocardiogr 7: 79–108, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Lu DY, Sung SH, Yu WC, Cheng HM, Chuang SY, Chen CH. Wave reflections, arterial stiffness, heart rate variability and orthostatic hypotension. Hypertens Res 37: 1056–1061, 2014. [DOI] [PubMed] [Google Scholar]
- 32.Luft FC. Neural regulation of the immune system modulates hypertension-induced target-organ damage. J Am Soc Hypertens 6: 23–26, 2012. [DOI] [PubMed] [Google Scholar]
- 33.Mahmud A, Feely J. Arterial stiffness is related to systemic inflammation in essential hypertension. Hypertension 46: 1118–1122, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Mancia G, Grassi G. The autonomic nervous system and hypertension. Circ Res 114: 1804–1814, 2014. [DOI] [PubMed] [Google Scholar]
- 35.Mancia G, Grassi G. Management of essential hypertension. Br Med Bull 94: 189–199, 2010. [DOI] [PubMed] [Google Scholar]
- 36.Martelli D, McKinley MJ, McAllen RM. The cholinergic anti-inflammatory pathway: a critical review. Auton Neurosci 182: 65–69, 2014. [DOI] [PubMed] [Google Scholar]
- 37.Moens IA. Der erste Wellengipfel in dem absteigenden Schenkel der Pulskurve. Pflügers Arch 20: 517–533, 1879. [Google Scholar]
- 38.Ogbonnaya S, Kaliaperumal C. Vagal nerve stimulator: evolving trends. J Nat Sci Biol Med 4: 8–13, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olofsson PS, Rosas-Ballina M, Levine YA, Tracey KJ. Rethinking inflammation: neural circuits in the regulation of immunity. Immunol Rev 248: 188–204, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olshansky B. Vagus nerve modulation of inflammation: cardiovascular implications. Trends Cardiovasc Med 26: 1–11, 2016. [DOI] [PubMed] [Google Scholar]
- 41.Omboni S, Posokhov IN, Rogoza AN. Relationship between 24-hour blood pressure variability and 24-hour aortic pressure and stiffness in hypertensive patients. J Hypertens 33, Suppl 1: e41–e42, 2015.26102817 [Google Scholar]
- 42.Pardo JV, Sheikh SA, Kuskowski MA, Surerus-Johnson C, Hagen MC, Lee JT, Rittberg BR, Adson DE. Weight loss during chronic, cervical vagus nerve stimulation in depressed patients with obesity: an observation. Int J Obes (Lond) 31: 1756–1759, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pinter A, Horvath T, Sarkozi A, Kollai M. Relationship between heart rate variability and endothelial function in healthy subjects. Auton Neurosci 169: 107–112, 2012. [DOI] [PubMed] [Google Scholar]
- 44.Plachta DT, Gierthmuehlen M, Cota O, Espinosa N, Boeser F, Herrera TC, Stieglitz T, Zentner J. Blood pressure control with selective vagal nerve stimulation and minimal side effects. J Neural Eng 11: 036011, 2014. [DOI] [PubMed] [Google Scholar]
- 45.Poulter NR, Prabhakaran D, Caulfield M. Hypertension. Lancet 386: 801–812, 2015. [DOI] [PubMed] [Google Scholar]
- 46.Ranpuria R, Hall M, Chan CT, Unruh M. Heart rate variability (HRV) in kidney failure: measurement and consequences of reduced HRV. Nephrol Dial Transplant 23: 444–449, 2008. [DOI] [PubMed] [Google Scholar]
- 47.Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med 340: 115–126, 1999. [DOI] [PubMed] [Google Scholar]
- 48.Safar ME, Temmar M, Kakou A, Lacolley P, Thornton SN. Sodium intake and vascular stiffness in hypertension. Hypertension 54: 203–209, 2009. [DOI] [PubMed] [Google Scholar]
- 49.Saraiva M, O'Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol 10: 170–181, 2010. [DOI] [PubMed] [Google Scholar]
- 50.Schaper F, Rose-John S. Interleukin-6: biology, signaling and strategies of blockade. Cytokine Growth Factor Rev 26: 475–487, 2015. [DOI] [PubMed] [Google Scholar]
- 51.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta 1813: 878–888, 2011. [DOI] [PubMed] [Google Scholar]
- 52.Simons CT, Kulchitsky VA, Sugimoto N, Homer LD, Szekely M, Romanovsky AA. Signaling the brain in systemic inflammation: which vagal branch is involved in fever genesis? Am J Physiol Regul Integr Comp Physiol 275: R63–R68, 1998. [DOI] [PubMed] [Google Scholar]
- 53.Siniscalchi A, Gallelli L, Malferrari G, Pirritano D, Serra R, Santangelo E, De Sarro G. Cerebral stroke injury: the role of cytokines and brain inflammation. J Basic Clin Physiol Pharmacol 25: 131–137, 2014. [DOI] [PubMed] [Google Scholar]
- 54.Slama M, Susic D, Frohlich ED. Prevention of hypertension. Curr Opin Cardiol 17: 531–536, 2002. [DOI] [PubMed] [Google Scholar]
- 55.Song H, Wei F, Liu Z, Zhao Y, Ye L, Lu F, Zhang H, Diao Y, Qi Z, Xu J. Visit-to-visit variability in systolic blood pressure: correlated with the changes of arterial stiffness and myocardial perfusion in on-treated hypertensive patients. Clin Exp Hypertens 37: 63–69, 2015. [DOI] [PubMed] [Google Scholar]
- 56.Stauss HM, Rarick KR, Leick KM, Burkle JW, Rotella DL, Anderson MG. Non-invasive assessment of vascular structure and function in conscious rats based on in vivo imaging of the albino iris. Am J Physiol Regul Integr Comp Physiol 300: R1333–R1343, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sundman E, Olofsson PS. Neural control of the immune system. Adv Physiol Educ 38: 135–139, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tanaka T, Kishimoto T. The biology and medical implications of interleukin-6. Cancer Immunol Res 2: 288–294, 2014. [DOI] [PubMed] [Google Scholar]
- 59.Tracey KJ. The inflammatory reflex. Nature 420: 853–859, 2002. [DOI] [PubMed] [Google Scholar]
- 60.Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest 117: 289–296, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tracy RP. Inflammation markers and coronary heart disease. Curr Opin Lipidol 10: 435–441, 1999. [DOI] [PubMed] [Google Scholar]
- 62.Weiner SD, Ahmed HN, Jin Z, Cushman M, Herrington DM, Nelson JC, Di Tullio MR, Homma S. Systemic inflammation and brachial artery endothelial function in the Multi-Ethnic Study of Atherosclerosis (MESA). Heart 100: 862–866, 2014. [DOI] [PubMed] [Google Scholar]
- 63.Wennergren G, Henriksson BA, Weiss LG, Oberg B. Effects of stimulation of nonmedullated cardiac afferents on renal water and sodium excretion. Acta Physiol Scand 97: 261–263, 1976. [DOI] [PubMed] [Google Scholar]
- 64.Wolf J, Rose-John S, Garbers C. Interleukin-6 and its receptors: a highly regulated and dynamic system. Cytokine 70: 11–20, 2014. [DOI] [PubMed] [Google Scholar]
- 65.Yasmin McEniery CM, Wallace S, Mackenzie IS, Cockcroft JR, Wilkinson IB. C-reactive protein is associated with arterial stiffness in apparently healthy individuals. Arterioscler Thromb Vasc Biol 24: 969–974, 2004. [DOI] [PubMed] [Google Scholar]
- 66.Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis 148: 209–214, 2000. [DOI] [PubMed] [Google Scholar]