This is the first study that characterizes the novel fluorescent biosensor R-CEPIA1er that allows dynamic measurement of calcium within the sarcoplasmic reticulum in living cardiomyocytes. This novel, powerful tool will be essential in providing important new information regarding calcium regulation in healthy and diseased heart.
Keywords: calcium indicators, confocal microscopy, sarcoplasmic reticulum, excitation-contraction coupling, ventricular myocytes
Abstract
In cardiomyocytes, [Ca] within the sarcoplasmic reticulum (SR; [Ca]SR) partially determines the amplitude of cytosolic Ca transient that, in turn, governs myocardial contraction. Therefore, it is critical to understand the molecular mechanisms that regulate [Ca]SR handling. Until recently, the best approach available to directly measure [Ca]SR was to use low-affinity Ca indicators (e.g., Fluo-5N). However, this approach presents several limitations, including nonspecific cellular localization, dye extrusion, and species limitation. Recently a new genetically encoded family of Ca indicators has been generated, named Ca-measuring organelle-entrapped protein indicators (CEPIA). Here, we tested the red fluorescence SR-targeted Ca sensor (R-CEPIA1er) as a tool to directly measure [Ca]SR dynamics in ventricular myocytes. Infection of rabbit and rat ventricular myocytes with an adenovirus expressing the R-CEPIA1er gene displayed prominent localization in the SR and nuclear envelope. Calibration of R-CEPIA1er in myocytes resulted in a Kd of 609 μM, suggesting that this sensor is sensitive in the whole physiological range of [Ca]SR. [Ca]SR dynamics measured with R-CEPIA1er were compared with [Ca]SR measured with Fluo5-N. We found that both the time course of the [Ca]SR depletion and fractional SR Ca release induced by an action potential were similar between these two Ca sensors. R-CEPIA1er fluorescence did not decline during experiments, indicating lack of dye extrusion or photobleaching. Furthermore, measurement of [Ca]SR with R-CEPIA1er can be combined with cytosolic [Ca] measurements (with Fluo-4) to obtain more detailed information regarding Ca handling in cardiac myocytes. In conclusion, R-CEPIA1er is a promising tool that can be used to measure [Ca]SR dynamics in myocytes from different animal species.
NEW & NOTEWORTHY
This is the first study that characterizes the novel fluorescent biosensor R-CEPIA1er that allows dynamic measurement of calcium within the sarcoplasmic reticulum in living cardiomyocytes. This novel, powerful tool will be essential in providing important new information regarding calcium regulation in healthy and diseased heart.
ca is a universal second messenger that regulates many vital biological processes, including cell contraction, gene expression, synaptic transmission, and cell growth. One of the major pathways by which Ca enters the cytoplasm is by its release from intracellular stores. In the heart, Ca released from the sarcoplasmic reticulum (SR) through ryanodine receptors (RyR) is essential for initiating a robust myocardial contraction. For relaxation to occur efficiently, a major fraction of Ca needs to be pumped from the cytosol back into the SR by the SR Ca-ATPase (SERCA). Thus cardiac pump function critically relies on well-controlled Ca cycling between the cytosol and the SR (3, 16). Defects in SR Ca regulation cause contractile dysfunction in a variety of cardiac pathologies. For example, abnormal SR Ca handling contributes to arrhythmias and contractile dysfunction in infarcted and failing hearts (2, 9, 12, 15, 16, 20, 25). Therefore, accurate measurement of intra-SR [Ca] ([Ca]SR) dynamics is essential for understanding the cellular and molecular mechanisms that cause SR Ca mishandling in different cardiac pathologies.
Currently, [Ca]SR measurement in living cardiomyocytes mainly relies on two experimental approaches. First, the total amount of Ca stored in the SR can be estimated from the amplitude of cytosolic Ca transient (1) as well as from the integral of Na/Ca exchange (NCX) current during RyR activation by a high dose of caffeine (8, 26). However, these indirect approaches to measure [Ca]SR are either not very sensitive because they rely on high-affinity cytosolic Ca indicators (such as Fura-2), which can be easily saturated by a large SR Ca release, or they are technically very challenging because they require a combination of optical and electrophysiological techniques. Moreover, those approaches do not allow for the study of [Ca]SR dynamics and can only estimate SR Ca load at a few time points during an experiment. Finally, very high doses of caffeine used in those methods can affect a variety of cellular processes, such as phosphodiesterase function (5). Recently, another experimental approach has been developed which allows for measuring [Ca]SR directly and continuously (13, 22, 23). This approach is based on the ability of some low-affinity Ca dyes (such as Fluo-5N) to become functional within the SR, that is, when the ester form of Fluo-5N can be properly de-esterified by esterases within the SR. However, this method has also several critical limitations: 1) the low-affinity Ca dye can accumulate in other subcellular compartments (such as mitochondria or cytosol) contaminating the SR Ca signal; 2) the signal from these dyes very often suffers from a decline in fluorescence during the experiment, due to dye extrusion and photobleaching; and 3) most importantly, this approach demonstrates species limitation. For example, while Fluo-5N works well in rabbit cardiomyocytes, it does not work in myocytes isolated from small rodents that are commonly used for transgenic studies.
Some limitations of the previously mentioned approaches have been overcome by developing genetically encoded Ca indicators targeted to the endoplasmic reticulum (ER)/SR (17–19). These Ca indicators contain an endoplasmic reticulum retention sequence which maintains these Ca sensors in the SR lumen. There are two classes of genetically encoded Ca indicators: the Forster resonance energy transfer-based and non-Forster resonance energy transfer-based sensors. The last family consists of circularly permuted green fluorescent protein, the Ca binding protein calmodulin (CaM), and the CaM-interacting M13 peptide. The CaM/M13 complex undergoes a conformational change upon Ca binding, leading to increased brightness (c7, 17). Despite extensive optimization, the majority of these CaM-based sensors still have a relatively high affinity for Ca and therefore are poorly suited to measure [Ca]SR. Furthermore, some of these suffer from a low temporal resolution and a higher photobleaching rate (11, 18, 27). A recent study by Suzuki et al. (24) reported on the generation of R-CEPIA1er: red fluorescence SR-targeted CaM-based indicator with a low affinity to Ca. However, until now this novel Ca sensor has not been tested in cardiomyocytes. In this study, we describe properties of R-CEPIA1er in isolated rabbit and rat ventricular myocytes under various experimental conditions. Our results show that R-CEPIA1er has the appropriate affinity for [Ca]SR and sufficient dynamic range; both properties are necessary to reliably track [Ca]SR with high temporal resolution in cardiac cells. Using R-CEPIA1er simultaneously with another green fluorescent indicator entrapped in the cytosol (such as Fluo-4) can provide more detailed information regarding Ca dynamics in cardiomyocytes. Moreover, this new Ca probe can be employed for the study of [Ca]SR handling in various small rodent, such as different transgenic murine models.
MATERIALS AND METHODS
Myocytes Isolation
The procedures of cell isolation were approved by the Institutional Animal Care and Use Committee and comply with US regulations on animal experimentation. Adult ventricular myocytes were isolated from adult New Zealand White rabbits (10 animals, 2.5 kg; Harlan Laboratories, Indianapolis, IN) or adult Sprague Dawley rats (10 animals, 3 mo old; Harlan Laboratories). Rabbits and rats were anaesthetized with pentobarbital sodium (50 mg/kg iv). Following thoracotomy, hearts were quickly excised, mounted on a Langendorff apparatus, and retrogradely perfused with collagenase-containing solution at 37°C according to the procedure described previously (9, 21, 30). All chemicals and reagents were purchased from Sigma-Aldrich (St. Louis, MO).
Virus Generation
The R-CEPIA1er plasmid was obtained from Addgene (plasmid No. 58216), subcloned into pShuttle-CMV by standard methods, and verified by sequencing. Adenoviral vectors of all the cDNAs were made as per the Stratagene AdEasy XL System. Amplified viruses were purified by cesium chloride gradient ultracentrifugation for 2 h at 22,000 rpm. The resultant adenovirus band was dialyzed and spectrophotometrically read at 260 nm to determine the titer value prior to addition of 10% glycerol by using the calculation OD260 × dilution factor × 1010 = particles/ml. Cells were infected at a multiplicity of infection of 50.
Confocal Microscopy
Experiments were conducted with a Leica SP5 laser scanning confocal microscope equipped with a ×63 water objective lens. Action potentials were induced by electrical field stimulation with a pair of platinum electrodes, which were connected to a Grass stimulator (Astro-Med) set at a voltage ∼50% above the threshold.
Measurement of [Ca]SR with R-CEPIA1er.
Freshly isolated ventricular myocytes from rabbit and rat were infected with an adenovirus (AdV) containing the R-CEPIA1er gene and cultured in PC-1 medium (Life Technologies) supplemented with penicillin and streptomycin (10 mg/ml) (Sigma) at 37°C in air containing 5% CO2. During culturing, adult myocytes were electrically stimulated at a constant frequency (0.2 Hz). Under our experimental conditions (cell quality, temperature, virus concentration, etc.), 48 h were required to induce a sufficient R-CEPIA1er expression level within the SR to allow recording of SR Ca dynamics without significant impact on the SR Ca buffer capacity (Fig. 5). R-CEPIA1er was excited with the 543-nm line of a He-Ne laser, and emitted fluorescence was measured at wavelengths above 580 nm. Images were acquired either in line-scan or 2-D mode. Sarcomere length analysis was performed with a customized version of ImageJ software.
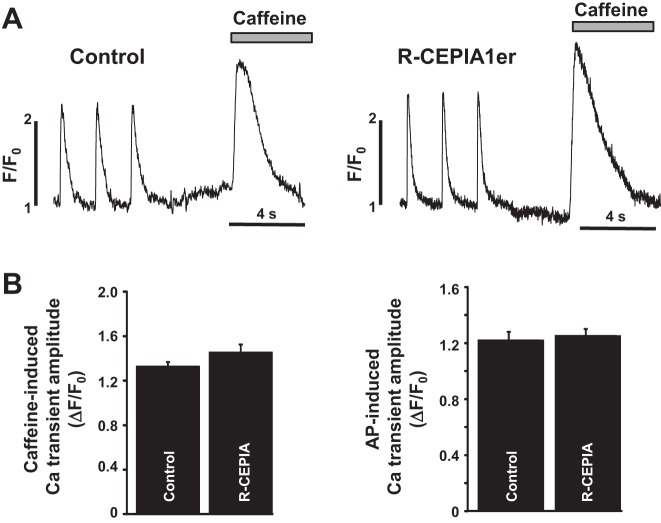
Fig. 5.
Effect of R-CEPIA1er expression on SR Ca content. A: the action potential (AP) and caffeine-induced Ca transients recorded in two different rabbit ventricular myocytes infected with an empty vector (Control) or with a virus containing the R-CEPIA1er gene (R-CEPIA1er). Both myocytes were first field stimulated at 0.75 Hz. Total SR Ca content was estimated from the amplitude of caffeine-induced Ca transient. B: average amplitude of caffeine-induced Ca transients in rabbit ventricular myocytes infected with an empty vector and with the R-CEPIA1er AdV. These data were obtained averaging a total of 18 cells per group from three rabbits.
Calibration of R-CEPIA1er.
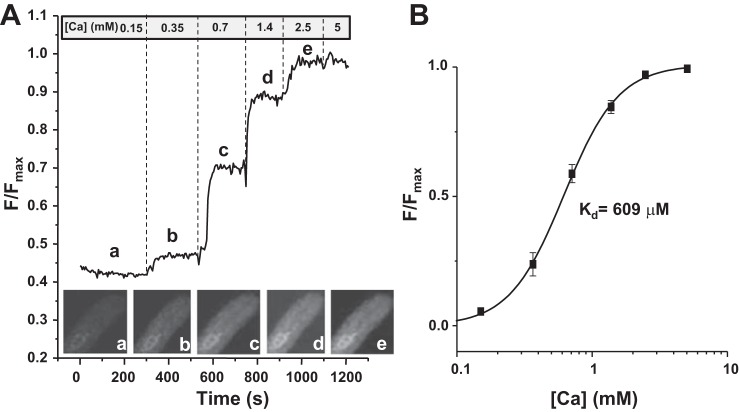
Myocytes infected with the R-CEPIA1er virus were permeabilized with an internal solution containing 1% saponin as previously described (29). After membrane permeabilization, cells were placed in the saponin-free internal solution (in mM): K-aspartate 100, KCl 15, KH2PO4 5, MgATP 5, EGTA 0.35, CaCl2 0.12, MgCl2 0.75, phosphocreatine 10, HEPES 10, creatine phosphokinase 5 U/ml, and dextran (MW: 40,000) 8%; pH 7.2 (KOH). To render the SR membrane permeable to Ca, Ca ionophore ionomycin (2 μM) was introduced in the internal solution. To prevent irreversible contraction during application of high [Ca], cells were pretreated for 5 min with the muscle contraction uncouplers 2,3-butanedione monoxime (10 mM). The free [Ca] in the internal solution was gradually increased from 150 μM to 5 mM, and for each [Ca] the R-CEPIA1er signal was recorded for several minutes. Next, the CEPIA1er fluorescence was plotted as a function of log [Ca]. The apparent Ca binding affinity constant (Kd) of R-CEPIA1er for Ca was calculated by two different methods. First, we calculated the Kd from the Hill curve obtained from data points averaged from 14 myocytes (Fig. 2B). We also calculated the Kd for each individual cell. For each cell, the experimental points were fit to the Hill equation to estimate the Kd. The final Kd was obtained by averaging Kd values obtained from each individual cell. The difference between the Kd values obtained by two different methods was less than 1%.
Fig. 2.
Calibration of R-CEPIA1er in rabbit ventricular myocytes. A: a representative example of the R-CEPIA1er calibration protocol. The signal was normalized to the maximum value of fluorescence obtained at 5 mM Ca (Fmax). On the bottom are images of the rabbit ventricular myocyte at different [Ca]. In the experiments, the R-CEPIA1er fluorescence was collected with an open pinhole and averaged over the entire 2-D image. This was done to improve the signal-to-noise ratio of the R-CEPIA1er signal, particularly at low [Ca]. B: the Hill curve (R-CEPIA1er fluorescence as a function of [Ca]) obtained by averaging experimental points from 14 cells (3 animals). The average Kd estimate for Ca is 609 μM.
Measurement of [Ca]SR by Fluo-5N.
We compared the kinetics of R-CEPIA1er with those derived from Fluo-5N in rabbit ventricular myocytes. Freshly isolated myocytes were split in two groups. In the first group, myocytes were cultured with empty virus (control). In the second group, myocytes were infected with R-CEPIA1er containing AdV. After 48 h in culture, control myocytes were loaded with the low-affinity Ca indicator Fluo-5N. Briefly, myocytes were incubated with 5 μM Fluo-5N/AM for 2.5 h in Tyrode solution at 37°C followed by a 20-min wash as described previously (29). Fluo-5N was excited with the 488-nm line of argon laser and the emission signal collected at wavelengths above 515 nm.
In all experiments (no matter whether Fluo-5N or R-CEPIA1er was used to measure SR Ca) the signal was corrected by subtracting the SR-independent component after depletion of SR Ca with 10 mM of caffeine.
Measurement of cytosolic [Ca].
To record cytosolic [Ca] ([Ca]i), we used the high-affinity Ca indicator Fluo-4 (Molecular Probes/Invitrogen, Carlsbad, CA). To load the cytosol with Fluo-4, control myocytes were incubated at room temperature with 10 μm Fluo-4 AM for 15 min in Tyrode solution (in mM: NaCl 140, KCl 4, CaCl2 2, MgCl2 1, glucose 10, and Hepes 10; pH 7.4), followed by a 20-min wash. Fluo-4 was excited with the 488-nm line of an argon laser, and the emission signal was collected at wavelengths above 515 nm.
Simultaneous measurement of [Ca]SR and [Ca]i.
Rabbit and rat myocytes infected with R-CEPIA1er were loaded with the high-affinity dye Fluo-4/AM. Simultaneous imaging of [Ca]SR and [Ca]i dynamics were performed with a resonance scanner of the Leica SP5 LSM system. Despite the differences in the fluorescent spectra properties of R-CEPIA1er and Fluo-4, these experiments were performed by line sequential scanning to avoid any bleed through in both channels.
Statistics
Data are presented as means ± SE. Statistical comparisons between groups were performed by Student's t-test. Differences were considered statistically significant at P < 0.05.
RESULTS
Subcellular Localization of R-CEPIA1er in Ventricular Myocytes
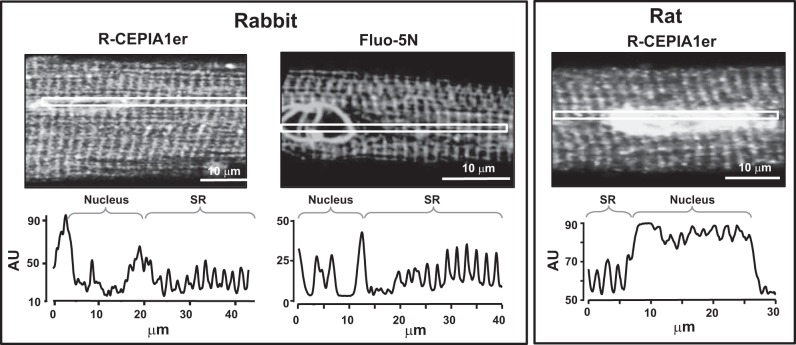
To test whether R-CEPIA1er selectively targets the SR, we analyzed the intracellular distribution of this sensor in ventricular myocytes by using confocal microscopy. After 48 h of culturing rat and rabbit ventricular myocytes with the R-CEPIA1er containing AdV, the subcellular distribution of red fluorescence of R-CEPIA1er was compared with the localization of green fluorescence of Fluo-5N. It has been shown previously that in rabbit ventricular myocytes the Fluo-5N signal originates predominantly from the SR (6, 23). We found that both signals from R-CEPIA1er and Fluo-5N showed periodic peaks of fluorescence approximately every 1.8 μm (Fig. 1), which corresponds to the sarcomere spacing of junctional SR. Because the lumen of the SR forms one common space with the nuclear lumen (10, 28), the nuclear envelope was also labeled with R-CEPIA1er. High levels of R-CEPIA1er and Fluo-5N fluorescence originating from the nuclear envelope could be explained by a larger intraluminal volume of this organelle compared with the SR. These results indicate that in ventricular myocytes R-CEPIA1er is preferentially localized within the lumen of the SR-nuclear network.
Fig. 1.
Subcellular distribution of R-CEPIA1er in rabbit and rat ventricular myocytes. Top: representative confocal images of three different ventricular myocytes (rabbit in panels at left and middle, rat in panel at right) expressing the R-CEPIA1er gene or loaded with Fluo-5N. Bottom: fluorescence intensity of R-CEPIA1er and Fluo-5N in subcellular regions defined by the white squares. The distance between two peaks corresponds to the length of the sarcomere, indicating that R-CEPIA1er localizes in the sarcoplasmic reticulum (SR).
Ca Sensitivity of R-CEPIA1er in Ventricular Myocytes
To investigate whether the Ca sensitivity of R-CEPIA1er is suitable for measurement of physiological changes in [Ca]SR, we calibrated this Ca sensor within the SR of rabbit ventricular myocytes. After sarcolemmal permeabilization with saponin, Ca ionophore ionomycin (2 μM) was introduced to the cytosol to render the SR permeable to Ca. Next, [Ca] in the cytosol was increased stepwise from 0.15 to 5 mM. The stepwise increase in [Ca] increased the R-CEPIA1er signal, also, in a stepwise manner (Fig. 2). The R-CEPIA1er fluorescence as a function of [Ca] was fit to the Hill equation to derive the Kd. The Kd was calculated by two different methods. First, we calculated the Kd from the curve obtained from data points averaged from 14 myocytes (Fig. 2B). The Kd calculated by this method was 609 μM. We also calculated the Kd for each individual cell to obtain the averaged Kd. The Kd calculated by this method was 614 ± 35 μM. The difference between the Kd values obtained by two different methods was less than 1%. However, these values are slightly higher than the value obtained in vitro or ex vivo by Suzuki et al. (24). This discrepancy might be explained by the fact that Suzuki et al. tested the sensitivity of R-CEPIA1er in different cell lines. Because the characteristics of Ca indicators depend on the intracellular environment (e.g., ionic strength, pH), the difference in Kd might reflect a difference in ER/SR properties between cell lines and cardiomyocytes. Regardless, the Ca sensitivity of R-CEPIA1er is in a range that is well suited for the study of [Ca]SR dynamics in ventricular myocytes.
[Ca]SR Dynamics Measured with R-CEPIA1er in Ventricular Myocytes
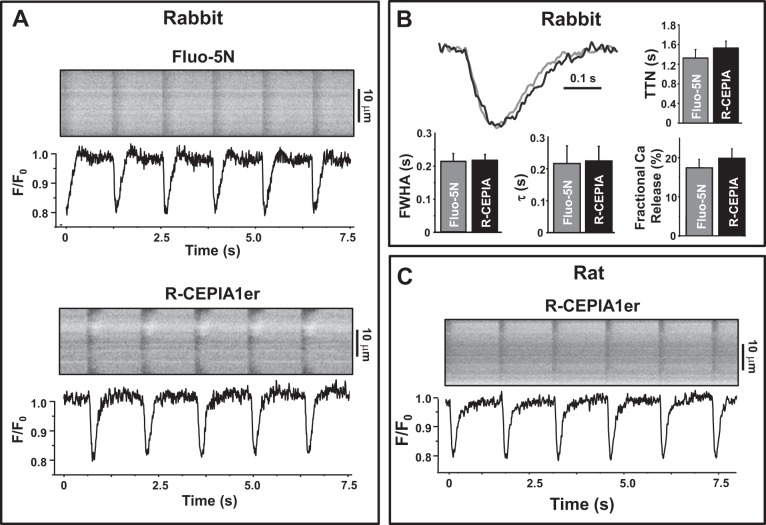
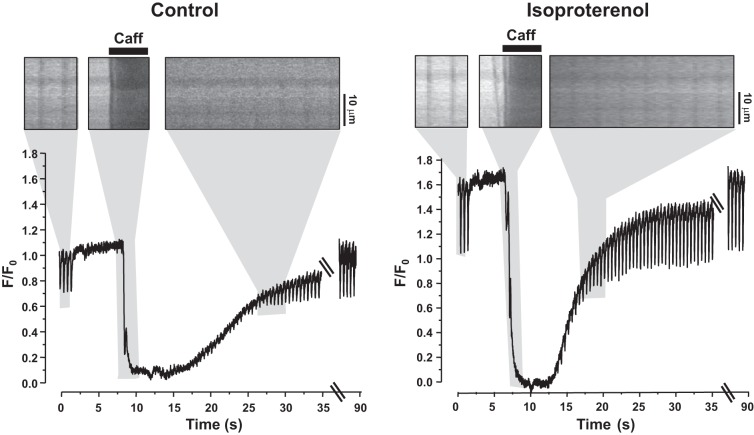
In the next series of experiments, we studied whether R-CEPIA1er is an appropriate Ca sensor to measure the fast changes in [Ca]SR that occur during excitation-contraction coupling. Action potential-induced SR Ca release in rabbit ventricular myocytes expressing R-CEPIA1er was induced by electrical field stimulation. Transient decreases in R-CEPIA1er fluorescence were observed in correspondence to action potentials or application of the RyR agonist caffeine (10 mM; Fig. 3A). Because Fluo-5N is the most common and reliable tool to measure [Ca]SR dynamics in ventricular myocytes (6, 14, 23, 29), we compared the R-CEPIA1er signal with Fluo-5N in rabbit ventricular myocytes. First, we analyzed the kinetics of action potential-induced [Ca]SR depletions measured with these two Ca sensors. Average values of the time to nadir, the rate of SR Ca replenishing, and the full width at half amplitude of [Ca]SR transients are shown on Fig. 3B. We found no significant difference between these parameters measured with Fluo-5N or R-CEPIA1er. These results suggest that this novel Ca sensor has characteristics suitable for the measurement of fast [Ca]SR dynamics in ventricular myocytes. By analyzing [Ca]SR depletions during action potentials and caffeine applications, we also calculated fractional SR Ca release as derived from data obtained with both Ca indicators. Fractional release (FR) was defined as FR = [Ca]SR depletions during action potential/[Ca]SR depletions during caffeine application × 100%. We found that there were no significant differences in FR measured with R-CEPIA1er or Fluo-5N (Fig. 3B). Moreover, we analyzed [Ca]SR dynamics in rat ventricular myocytes during excitation-contraction coupling (Fig. 3C). The kinetics of [Ca]SR depletions and reuptake as well as changes in the [Ca]SR at different pacing frequencies were comparable to that measured in rabbit myocytes. We tested R-CEPIA1er properties in conditions of increased SR Ca load. [Ca]SR dynamics were measured in rat ventricular myocytes in control conditions and during β-adrenergic receptor activation with isoproterenol (0.1 μM; Fig. 4). Myocytes were electrically stimulated at a constant frequency 0.75 Hz. Application of caffeine (10 mM) was used to completely deplete Ca in the SR. When caffeine was washed out, electrical stimulation was resumed, and diastolic [Ca]SR returned to the initial precaffeine level. Isoproterenol increased the R-CEPIA1er signal during diastole more than 50%, suggesting a significant increase in SR Ca load. These results show that R-CEPIA1er is a highly sensitive Ca probe that allows for measurement of [Ca]SR dynamics throughout the entire physiological concentration range. These results also illustrate that R-CEPIA1er can be employed to directly study [Ca]SR dynamics in cardiomyocytes from animal species other than the rabbit, including small rodents that are commonly used for transgenic studies.
Fig. 3.
Spatiotemporal resolution of R-CEPIA1er signal in rabbit and rat ventricular myocytes. A: representative examples of line-scan images and corresponding profiles of Fluo-5N and R-CEPIA1er in rabbit ventricular myocytes. B: averaged profiles of SR Ca transients, time to nadir (TTN), full width at half amplitude (FWHA), rate of SR Ca replenishing (τ), and fractional SR Ca release measured with Fluo-5N and R-CEPIA1er. C: line-scan images and corresponding profiles of R-CEPIA1er in rat ventricular myocyte myocytes. F0 is fluorescence recorded under steady-state conditions during diastole.
Fig. 4.
Sensitivity of R-CEPIA1er at higher [Ca]SR in rat ventricular myocytes. Representative line-scan images and corresponding profiles of R-CEPIA1er fluorescence in control conditions (left) and during application of isoproterenol (100 nM; right). Fragments of line-scan images are shown above the profiles (marked by gray boxes). The line scans are enlarged compared with the profiles. Myocytes were electrically stimulated at 0.75 Hz. When stimulation was stopped, caffeine (Caff, 10 mM) was applied to completely deplete [Ca]SR.
Effect of R-CEPIA1er Expression on the SR Ca Buffer Capacity
R-CEPIA1er, as a low-affinity Ca indicator, can potentially increase the Ca buffer capacity of the SR, thereby increasing the total amount of Ca stored in the SR. This in turn will modify SR Ca release and uptake (31). Therefore, we measured total [Ca]SR (or SR Ca load) in rabbit ventricular myocytes infected with either R-CEPIA1er or with an empty AdV. The SR Ca load was estimated from the amplitude of the cytosolic Ca transient induced by the rapid application of caffeine (10 mM) measured with the high-affinity Ca dye Fluo-4 (Fig. 5A). Myocytes were electrically stimulated at a constant frequency of 0.75 Hz. Although expression of R-CEPIA1er for 48 h revealed a tendency to increase the SR Ca load compared with myocytes cultured with empty virus (Fig. 5B), the estimated values for SR Ca load in control and R-CEPIA1er-loaded myocytes were not statistically different. Moreover, action potential-induced Ca transients were not significantly different between these two experimental groups (Fig. 5B).
Simultaneous Recording of [Ca]SR and [Ca]i Dynamics
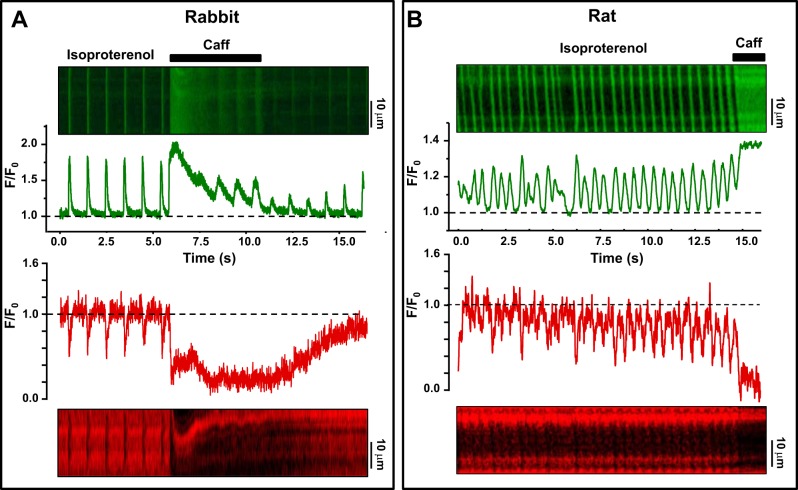
We also tested the feasibility of simultaneous imaging of Ca in the SR and in the cytosol by using R-CEPIA1er and Fluo-4, respectively. Figure 6 shows line-scan images of Fluo-4 (top) and R-CEPIA1er (bottom) from rabbit and rat ventricular myocytes. In these experiments [Ca]SR and [Ca]i dynamics were measured either during action potentials (left) or spontaneous Ca waves (right). In both cases, SR Ca release was studied during activation of β-adrenergic receptors with isoproterenol (0.1 μM). In a rabbit myocyte shown in Fig. 6A, adrenergic stimulation increased cytosolic Ca transients (top) and fractional SR Ca depletions (bottom) induced by electrical pacing (0.75 Hz). In a rat myocytes shown in Fig. 6B, however, adrenergic stimulation induced spontaneous Ca waves (no electrical pacing). These recordings show that changes in [Ca]i mirror changes in [Ca]SR: peaks of cytosolic Ca transients induced by action potentials (left) or Ca waves (right) correspond to nadirs of [Ca]SR depletion. Application of caffeine (10 mM) caused further increase in [Ca]i as a result of total [Ca]SR depletion. Thus in combination with [Ca]i recordings, this novel tool opens a new perspective to study Ca handling at a qualitatively new level in different animal models of heart diseases.
Fig. 6.
Simultaneous recording of [Ca]i and [Ca]SR. Simultaneously recorded [Ca]i and [Ca]SR with Fluo-4 and R-CEPIA1er in rabbit ventricular myocytes (A) and in rat ventricular myocytes (B). Top: line-scan images of Fluo-4 fluorescence with relative profiles recorded during action potential- and caffeine-induced Ca transients. Bottom: line-scan images of R-CEPIA1er fluorescence and corresponding profiles. In both cases, SR Ca release was measured in the presence of isoproterenol (0.1 μM).
DISCUSSION
Ca release from the SR plays a central role in the initiation and regulation of heart contraction. Until recently, real time analysis of [Ca]SR dynamics in cardiomyocytes has not been possible and [Ca]SR measurements relied upon indirect approaches, such as caffeine-induced Ca release or rapid cooling (1, 4). More recently, another approach has been developed to measure [Ca]SR dynamics with the use of a low-affinity Ca dye (i.e., Fluo-5N, Mag-Fura-2) entrapped within the SR (6, 12, 30). Although these approaches represent important tools for studying SR Ca handling in cardiomyocytes, they either lack specificity and sensitivity or are restricted to a few animal species.
In this study we report the characterization of the novel fluorescent biosensor R-CEPIA1er that allows for dynamic measurement of [Ca]SR in living cardiomyocytes with ideal temporal resolution. Recently, the properties of R-CEPIA1er have been characterized in different cell lines and neurons, showing superiority of R-CEPIA1er over other Ca sensors (24). Although the previous study provided essential information about basic properties of this sensor (Ca sensitivity, intracellular distribution, etc.), it remained unclear whether R-CEPIA1er is able to detect fast changes in [Ca]SR dynamics (<100 ms) that occur in cardiomyocytes during response to the action potential or spontaneous SR Ca release. In this first study of R-CEPIA1er in hearts, we found that this sensor is predominantly expressed in the lumen of the SR-nuclear network of ventricular myocytes (Fig. 1). Moreover, the intracellular distribution of the sensor had a similar pattern in both rabbit and rat ventricular myocytes. Our in situ calibration revealed that R-CEPIA1er has an apparent Kd for Ca of ∼600 μM (Fig. 2), which is ideally positioned to measure [Ca]SR dynamics in cardiomyocytes: from the diastolic plateau and the systolic nadir to full depletion (Fig. 3). The sensor is not saturated by Ca during diastole, because the R-CEPIA1er signal can be further increased during β-adrenergic receptor stimulation when SR Ca load is extremely high (Fig. 4). R-CEPIA1er has very similar temporal properties to Fluo-5N (Fig. 3B). Since Fluo-5N has been commonly used to measure fast local and global SR Ca release events (6, 14, 30), it appears that R-CEPIA1er has the appropriate kinetic properties to measure fast SR Ca dynamics during action potentials and spontaneous Ca waves (Fig. 6).
We also showed the application of R-CEPIA1er as a tool to directly measure [Ca]SR in different animal models, including adult rabbit and rat ventricular myocytes. For example, the previously used Fluo-5N exhibits a poor efficacy to detect changes in [Ca]SR in small rodents. This is a great disadvantage of this Ca indicator as small rodents are commonly used as transgenic models to study cardiac pathologies associated with SR Ca mishandling. As a result, [Ca]SR in transgenic myocytes can be only estimated from the amplitude of cytosolic Ca transient during caffeine application. The main disadvantage of this approach is that [Ca]SR cannot be measured continuously, but rather only at a few time points during an experiment. This approach also does not allow for the study of spatial properties of the SR Ca parameter. In this study, we showed that R-CEPIA1er can overcome these critical limitations. Being targeted to the SR, R-CEPIA1er prevents nonspecific intracellular distribution and dye extrusion, both of which are common drawbacks of low-affinity Ca dyes such as Fluo-5N. Also, CEPIA1er absorbs photons at longer wavelengths (around 543 nm) compared with Fluo-5N (around 488 nm). This significantly minimizes cell photodamage. Moreover, we show here that R-CEPIA1er can be used with Fluo-4 to image Ca dynamics in the SR and the cytosol simultaneously. Such a combination of cytosolic and intra-SR Ca indicators provides more detailed information about cardiac Ca dynamics and excitation-contraction coupling.
The major limitation of R-CEPIA1er is that expression of the indicator requires at least 48 h of myocytes in culture. The culturing procedure significantly alters the structure and function of cardiomyocytes. To overcome this limitation we are currently working on the generation of a method of in vivo gene delivery with an adeno-associated virus that will allow using freshly isolated cardiomyocytes with intact physiological properties that are already expressing R-CEPIA1er.
In summary, R-CEPIA1er is capable of measuring [Ca]SR in living cardiomyocytes with high sensitivity and ideal spatiotemporal resolution. We expect that this novel, powerful tool will be essential in providing important new information regarding SR Ca regulation. Particularly, the ability to directly and continuously measure [Ca]SR in myocardium from transgenic animals will make a significant contribution to our understanding of the molecular mechanisms of SR Ca handling in the healthy and diseased heart.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-130231 (to A. Zima) and HL-062426 (to P. de Tombe) and an American Heart Association Fellowship Grant (to E. Bovo).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
E.B., J.L.M., P.P.d.T., and A.V.Z. conception and design of research; E.B., J.L.M., J.T., and A.V.Z. performed experiments; E.B., J.L.M., J.T., P.P.d.T., and A.V.Z. analyzed data; E.B., J.L.M., J.T., P.P.d.T., and A.V.Z. interpreted results of experiments; E.B., P.P.d.T., and A.V.Z. prepared figures; E.B., J.L.M., P.P.d.T., and A.V.Z. drafted manuscript; E.B., J.L.M., P.P.d.T., and A.V.Z. edited and revised manuscript; E.B., J.L.M., J.T., P.P.d.T., and A.V.Z. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Masamitsu Iino for generating the R-CEPIA1er plasmid and making it commercially available. We thank Dr. Margaret Novak for the Image J plug in.
REFERENCES
- 1.Bassani JW, Yuan W, Bers DM. Fractional SR Ca release is regulated by trigger Ca and SR Ca content in cardiac myocytes. Am J Physiol Cell Physiol 268: C1313–C1319, 1995. [DOI] [PubMed] [Google Scholar]
- 2.Belevych AE, Terentyev D, Viatchenko-Karpinski S, Terentyeva R, Sridhar A, Nishijima Y, Wilson LD, Cardounel AJ, Laurita KR, Carnes CA, Billman GE, Gyorke S. Redox modification of ryanodine receptors underlies calcium alternans in a canine model of sudden cardiac death. Cardiovasc Res 84: 387–395, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bers DM. Cardiac excitation-contraction coupling. Nature 415: 198–205, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Bers DM, Bridge JH, Spitzer KW. Intracellular Ca2+ transients during rapid cooling contractures in guinea-pig ventricular myocytes. J Physiol 417: 537–553, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boswell-Smith V, Spina D, Page CP. Phosphodiesterase inhibitors. Br J Pharmacol 147 Suppl 1: S252–S257, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brochet DX, Yang D, Di Maio A, Lederer WJ, Franzini-Armstrong C, Cheng H. Ca2+ blinks: rapid nanoscopic store calcium signaling. Proc Natl Acad Sci U S A 102: 3099–3104, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, Looger LL, Svoboda K, Kim DS. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499: 295–300, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diaz ME, O'Neill SC, Eisner DA. Sarcoplasmic reticulum calcium content fluctuation is the key to cardiac alternans. Circ Res 94: 650–656, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Domeier TL, Blatter LA, Zima AV. Alteration of sarcoplasmic reticulum Ca2+ release termination by ryanodine receptor sensitization and in heart failure. J Physiol 587: 5197–5209, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerasimenko J, Maruyama Y, Tepikin A, Petersen OH, Gerasimenko O. Calcium signalling in and around the nuclear envelope. Biochem Soc Trans 31: 76–78, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Gerasimenko JV, Petersen OH, Gerasimenko OV. Monitoring of intra-ER free Ca2+. WIREs Membr Transp Signal 3: 63–71, 2014. [Google Scholar]
- 12.Gonzalez DR, Treuer AV, Castellanos J, Dulce RA, Hare JM. Impaired S-nitrosylation of the ryanodine receptor caused by xanthine oxidase activity contributes to calcium leak in heart failure. J Biol Chem 285: 28938–28945, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kabbara AA, Allen DG. The use of the indicator fluo-5N to measure sarcoplasmic reticulum calcium in single muscle fibres of the cane toad. J Physiol 534: 87–97, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kubalova Z, Terentyev D, Viatchenko-Karpinski S, Nishijima Y, Gyorke I, Terentyeva R, da Cunha DN, Sridhar A, Feldman DS, Hamlin RL, Carnes CA, Gyorke S. Abnormal intrastore calcium signaling in chronic heart failure. Proc Natl Acad Sci U S A 102: 14104–14109, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kushnir A, Shan J, Betzenhauser MJ, Reiken S, Marks AR. Role of CaMKIIdelta phosphorylation of the cardiac ryanodine receptor in the force frequency relationship and heart failure. Proc Natl Acad Sci U S A 107: 10274–10279, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo M, Anderson ME. Mechanisms of altered Ca(2)(+) handling in heart failure. Circ Res 113: 690–708, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyawaki A, Llopis J, Heim R, McCaffery JM, Adams JA, Ikura M, Tsien RY. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature 388: 882–887, 1997. [DOI] [PubMed] [Google Scholar]
- 18.Palmer AE, Jin C, Reed JC, Tsien RY. Bcl-2-mediated alterations in endoplasmic reticulum Ca2+ analyzed with an improved genetically encoded fluorescent sensor. Proc Natl Acad Sci U S A 101: 17404–17409, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palmer AE, Qin Y, Park JG, McCombs JE. Design and application of genetically encoded biosensors. Trends Biotechnol 29: 144–152, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Respress JL, van Oort RJ, Li N, Rolim N, Dixit SS, deAlmeida A, Voigt N, Lawrence WS, Skapura DG, Skardal K, Wisloff U, Wieland T, Ai X, Pogwizd SM, Dobrev D, Wehrens XH. Role of RyR2 phosphorylation at S2814 during heart failure progression. Circ Res 110: 1474–1483, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sacconi L, Ferrantini C, Lotti J, Coppini R, Yan P, Loew LM, Tesi C, Cerbai E, Poggesi C, Pavone FS. Action potential propagation in transverse-axial tubular system is impaired in heart failure. Proc Natl Acad Sci U S A 109: 5815–5819, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Samtleben S, Jaepel J, Fecher C, Andreska T, Rehberg M, Blum R. Direct imaging of ER calcium with targeted-esterase induced dye loading (TED). J Vis Exp e50317, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shannon TR, Guo T, Bers DM. Ca2+ scraps: local depletions of free [Ca2+] in cardiac sarcoplasmic reticulum during contractions leave substantial Ca2+ reserve. Circ Res 93: 40–45, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki J, Kanemaru K, Ishii K, Ohkura M, Okubo Y, Iino M. Imaging intraorganellar Ca2+ at subcellular resolution using CEPIA. Nat Commun 5: 4153, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Terentyev D, Gyorke I, Belevych AE, Terentyeva R, Sridhar A, Nishijima Y, de Blanco EC, Khanna S, Sen CK, Cardounel AJ, Carnes CA, Gyorke S. Redox modification of ryanodine receptors contributes to sarcoplasmic reticulum Ca2+ leak in chronic heart failure. Circ Res 103: 1466–1472, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trafford AW, Diaz ME, Eisner DA. Measurement of sarcoplasmic reticulum Ca content and sarcolemmal fluxes during the transient stimulation of the systolic Ca transient produced by caffeine. Ann N Y Acad Sci 853: 368–371, 1998. [DOI] [PubMed] [Google Scholar]
- 27.Wu J, Prole DL, Shen Y, Lin Z, Gnanasekaran A, Liu Y, Chen L, Zhou H, Chen SR, Usachev YM, Taylor CW, Campbell RE. Red fluorescent genetically encoded Ca2+ indicators for use in mitochondria and endoplasmic reticulum. Biochem J 464: 13–22, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu X, Bers DM. Sarcoplasmic reticulum and nuclear envelope are one highly interconnected Ca2+ store throughout cardiac myocyte. Circ Res 99: 283–291, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Zima AV, Bovo E, Bers DM, Blatter LA. Ca(2)+ spark-dependent and -independent sarcoplasmic reticulum Ca(2)+ leak in normal and failing rabbit ventricular myocytes. J Physiol 588: 4743–4757, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zima AV, Picht E, Bers DM, Blatter LA. Termination of cardiac Ca2+ sparks: role of intra-SR [Ca2+], release flux, and intra-SR Ca2+ diffusion. Circ Res 103: e105–e115, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zima AV, Qin J, Fill M, Blatter LA. Tricyclic antidepressant amitriptyline alters sarcoplasmic reticulum calcium handling in ventricular myocytes. Am J Physiol Heart Circ Physiol 295: H2008–H2016, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]